Summary
Generation of CD8+ memory T (TM) cells requires metabolic reprogramming that is characterized by enhanced mitochondrial fatty acid oxidation (FAO). However, where the fatty acids (FA) that fuel this process come from remains unclear. We found that while CD8+ TM cells engaged higher levels of FAO, they acquired substantially fewer long-chain FA from their external environment than CD8+ effector T (TE) cells. Rather than using extracellular FA directly, TM cells used extracellular glucose to support FAO and oxidative phosphorylation (OXPHOS), suggesting that lipids must be synthesized to generate the substrates needed for FAO. We have demonstrated that TM cells rely on cell intrinsic expression of the lysosomal hydrolase LAL (lysosomal acid lipase) to mobilize FA for FAO and TM cell development. Our observations link LAL to metabolic reprogramming in lymphocytes and show that cell intrinsic lipolysis is deterministic for TM cell fate.
Introduction
Upon infection, activated CD8+ T cells undergo a distinct pattern of differentiation, characterized by the proliferation of antigen (Ag)-specific effector T (TE) cells, followed by contraction of these cells and development of long-lived TM cells (Cui and Kaech, 2010; Harty and Badovinac, 2008). During this process, T cells metabolically reprogram to provide for the divergent energetic and functional needs of these distinct cell types. TE cells, which require precursors for biomass accumulation and effector functions, dramatically increase aerobic glycolysis (Caro-Maldonado et al., 2012), while, TM cells use oxidative phosphorylation (OXPHOS) to meet metabolic demands (van der Windt and Pearce, 2012). Although TE cells can engage OXPHOS (Chang et al. 2013; Wang et al. 2011), which is necessary for their Ag driven proliferation (Sena et al. Immunity 2013), TM cells rely on this metabolic pathway, and in particular, the use of fatty acids (FA) to fuel this process (Pearce et al., 2013). We previously demonstrated that fatty acid oxidation (FAO) provides a metabolic advantage for the survival of TM cells and for their rapid recall after re-infection (van der Windt et al., 2012; van der Windt et al., 2013). However, how TM cells access FA to fuel this process remains unclear.
There is a strong association between burning fat and living longer (Hansen et al., 2013; Wang et al., 2008). TM cells are long-lived and previous studies demonstrating that they engage FAO to support survival have helped establish the link between lipid metabolism and cellular longevity in the immune system (Pearce, 2010; van der Windt et al., 2012). Given that long-lived lymphocytes are a goal of vaccination, there is interest in understanding the pathways that regulate their longevity.
Lipolysis is the hydrolysis of stored lipids to liberate FA that can then be used as energy substrates, essential precursors for membrane synthesis, or signaling mediators (Farese Jr and Walther, 2009; Lass et al., 2011; Zechner et al., 2012). Consistent with the importance of lipolysis in energy homeostasis, it is thought to occur in all cell types, but is most abundant in adipose tissue, where the release of stored fats into the vasculature supplies energy substrates to other cells (Lass et al., 2011; Zechner et al., 2012). Several enzymes and regulatory factors, such as adipose triglyceride lipase (ATGL) and hormone-sensitive lipase (HSL), regulate the release of lipids from lipid droplets in response to changes in the nutritional state (Brasaemle, 2007; Farese Jr and Walther, 2009). Other lipases, such as lysosomal acid lipase (LAL) can also contribute to lipolytic processes (Sheriff et al., 1995). Tissues around the body that use FAO, such as cardiac and skeletal muscle, liver, and kidney, acquire FA from the blood and oxidize them in mitochondria to fuel energy production (Kodde et al., 2007; Reddy and Sambasiva Rao, 2006; Weinberg, 2011; Zhang et al., 2010). While lipolysis in adipocytes has been extensively studied, how other cells store, access, or mobilize FA is less well understood (Zechner et al., 2012).
We show that while CD8+ TM cells depend on FAO (van der Windt et al., 2012), they do not acquire appreciable amounts of extracellular free FA to fuel this process, and in contrast to TE cells, do not readily store exogenous long-chain FA in lipid droplets. Instead, TM cells use extracellular glucose to support FAO and OXPHOS, indicating that these cells synthesize FA for mitochondrial FAO. Consistent with the reliance of TM cells on FAO, LAL, an enzyme that hydrolyzes cholesterol esters (CE) and triacylglycerol (TAG) to generate free FA and cholesterol in the lysosomes of cells (Sheriff et al., 1995), is expressed in CD8+ TM cells and supports the metabolic reprogramming necessary for their development.
Results
Unlike TE cells, TM cells do not acquire substantial amounts of extracellular FA
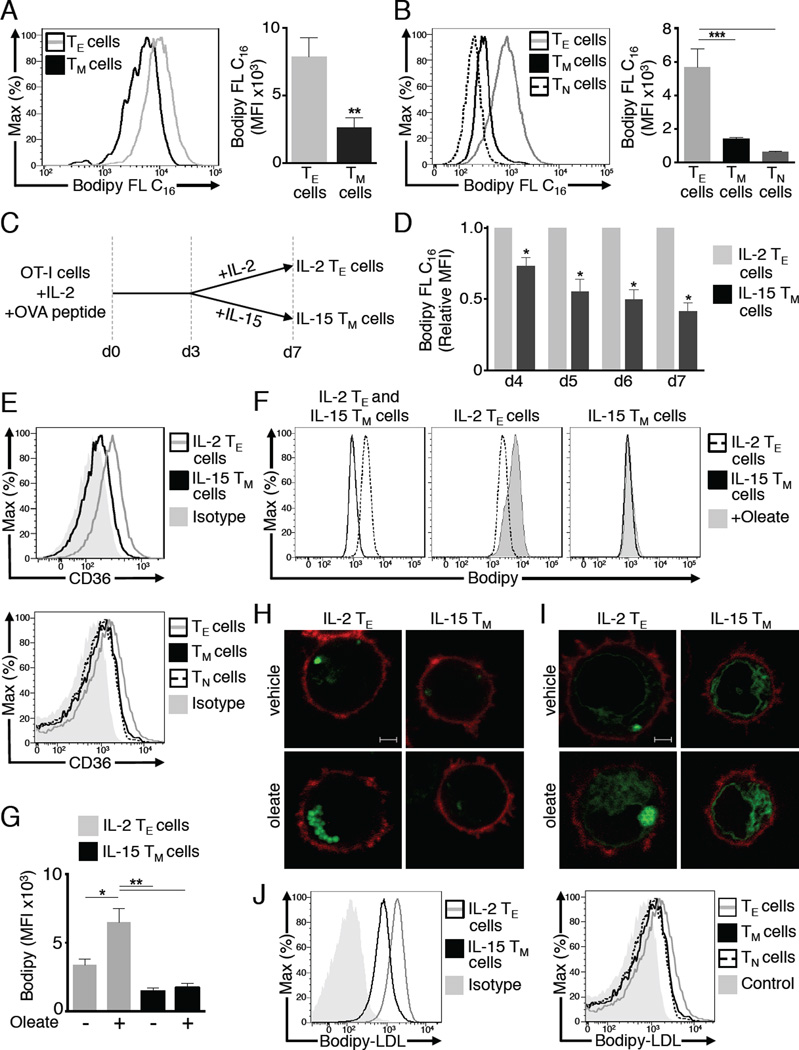
Since TM cells use long-chain FA to fuel FAO (van der Windt et al., 2012), we investigated if these cells, like other cells that use FAO, acquire free FA from their external environment (Kiens, 2006; Koonen et al., 2005). To this end, we isolated CD8+ T cells from OT-I transgenic mice and transferred them into congenic recipients, then infected the mice with Listeria monocytogenes expressing ovalbumin (OVA) (LmOVA) to induce an OVA-specific CD8+ T cell response. We then injected a long-chain FA, fluorescently labeled palmitate (Bodipy FL C16), into the mice 7 days (TE phase) or 21 days (TM phase) after LmOVA infection. One hour after Bodipy FL C16 injection, OVA-specific donor T cells were analyzed for FA uptake. OVA-specific CD8+ TM cells acquired significantly less palmitate compared to OVA-specific CD8+ TE cells (Figure 1A). There was no difference in Bodipy FL C16 uptake between KLRG-1hi and KLRG-1lo Ag-specific TE cells, indicating that increased FA uptake is not specific to short-lived TE cells, but to TE cells as a whole (data not shown) (Sarkar et al., 2008). Since Bodipy FL C16 was injected at distinct time points to assess differences in FA acquisition between TE and TM cells, we also compared cells within an individual animal. We injected Bodipy FL C16 7 days post-infection and 1 hour later assessed its incorporation into CD8+ CD44hiCD62Llo TE cells, and into CD8+ T cells expressing naive (TN; CD44loCD62Lhi) or TM (CD44hiCD62Lhi) markers (Figure 1B, histogram only). We observed the same trend in these polyclonal T cells as compared to day 7 (d7) TE cells and to TM cells and to T cells expressing TN markers d21 post-infection, as TM cells took up less palmitate than TE cells (Figure 1B, bar graph). Although acquisition by TM cells appeared higher than TN cells, the difference was not statistically significant. Consistent with TE cells acquiring more Bodipy FL C16 than TM cells, secondary CD8+ TE cells (2°TE) from mice challenged with LmOVA 4 days prior exhibited increased Bodipy FL C16 uptake relative to TM cells (Figure S1 A). Polyclonal TE cells from these mice, which were comparable in size to TM cells, also acquired more Bodipy FL C16 (Figure S1 A), indicating that differences in FA acquisition are not only due to cell size differences.
Figure 1. Unlike CD8+ TE cells, CD8+ TM cells do not acquire substantial amounts of extracellular fatty acids.
OT-I cells were injected i.v. into congenic recipients. Mice were infected with LmOVA to generate TE (7 days) and TM (≥ 21 days) cells, and peripheral blood was collected 1h after i.v. Bodipy FL C16 injection and uptake quantified by FACS. (A) Representative plots (histogram) and average MFI (bar graph) of Bodipy FL C16 in TE and TM KbOVA-specific CD8+ OT-I+ cells from 2 experiments (n=7-8 mice/group). Bar graphs show mean ± SEM, **p <0.01. (B) Representative plots (histogram) of Bodipy FL C16 MFI in polyclonal TE (CD44hi CD62Llo), TM (CD44hi CD62Lhi), and TN (CD44lo CD62Lhi) CD8+ T cells 7 days post-infection and average MFI (bar graph) of polyclonal CD8+ T cells from 2 experiments (n=9 mice/group). Bar graphs show mean ± SEM ***p <0.001. (C) OT-I cells were activated with OVA peptide and IL-2 for 3 days and subsequently cultured in IL-15 or IL-2 for 4 more days to generate IL-15 TM and IL-2 TE cells respectively. (D) OTI cells were incubated with Bodipy FL C16 on d4-7 of culture, and uptake was measured. MFIs were normalized to the daily MFI of IL-2 TE cells. Data from 3 experiments shown as mean ± SEM, *p <0.05 by one sample t-test. (E) CD36 expression on d7 IL-2 TE and IL-15 TM cells, or polyclonal T cells. Data represent 3 experiments. (F-H) Day 6 IL-2 TE or IL-15 TM cells were cultured overnight ± oleate then stained with Bodipy. (F) Representative plots showing Bodipy MFI and (G) average Bodipy MFI from 3 experiments show mean ± SEM, *p <0.05, **p <0.01. (H) Images showing Bodipy (green) and CD8 (red) represent 4 experiments. Scale bar = 2 µM (I) OT-I cells expressing a lipid droplet-targeting construct (LD-GFP) were cultured overnight ± oleate. Images show LD-GFP (green) and CD8 (red) and represent 2 experiments. (J) Cells were incubated with Bodipy-LDL or left unstained (Control), data represent 2 experiments.
To further investigate FA acquisition we stimulated CD8+ OT-I cells with OVA peptide and interleukin-2 (IL-2) for 3 days, then differentially cultured the cells in IL-2 or IL-15 for 4 more days (Figure 1C) to approximate in vitro the conditions that program CD8+ TE and TM cells, respectively, after infection (Carrio et al., 2004; van der Windt et al., 2012). Like our in vivo findings, IL-15 differentiated memory-like T (IL-15 TM) cells acquired much less Bodipy FL C16 than IL-2 driven effector-like (IL-2 TE) cells (Figure 1D). As IL-2 TE cells differentiated into IL-15 TM cells, their ability to acquire Bodipy FL C16 decreased. Following overnight incubation with Bodipy FL C16, the difference in uptake between IL-2 TE and IL-15 TM cells remained (Figure S1 B). Surface expression of CD36, a receptor that binds long-chain FA and low-density lipoprotein (LDL) (Silverstein and Febbraio, 2009), was lower on IL-15 TM cells and on in vivo generated TM cells compared to their TE cell counterparts (Figure 1E), consistent with their reduced capacity to acquire FA.
Given the difference in FA uptake between these cells, we assessed their total neutral lipid (indicates stored fat). IL-2 TE cells stained with unconjugated Bodipy had more neutral lipid than IL-15 TM cells (Figure 1F), and when incubated with the FA oleate, only IL-2 TE cells exhibited an increase in Bodipy staining (Figure 1F, G). Furthermore, IL-2 TE cells cultured with oleate formed lipid droplets (Figure 1H). These structures were not evident in IL-15 TM cells. Supporting these observations, Tip47, a lipid droplet protein (Bulankina et al., 2009), was expressed more in IL-2 TE than IL-15 TM cells (Figure S1 C). To confirm that these structures were lipid droplets, we transduced T cells with a retrovirus containing a lipid droplet-targeting construct (LD-GFP), where GFP was fused to the N-terminal hydrophobic region of METTL7B (ALDI), a protein that localizes to lipid droplets (Zehmer et al., 2008), and differentially cultured them in IL-2 or IL-15. LD-GFP localized to lipid droplets in IL-2 TE cells, which became abundant in oleate supplemented IL-2 TE cells (Figure 1I). In contrast, the IL-15 TM cells exhibited diffuse fluorescence, even when supplemented with oleate. We interpret the diffuse fluorescence to indicate retroviral expression of METTL7B-GFP in the cytoplasm. These data show that unlike IL-15 TM cells, IL-2 TE cells acquire long-chain FA and store them as neutral lipid (e.g. TAG and CE) in lipid droplets.
We questioned whether TM cells are able to acquire other forms of lipid produced in vivo, such as those that provide cholesterol. We added Bodipy-conjugated LDL in vitro and found that IL-15 TM cells acquired LDL, although to a lesser extent than IL-2 TE cells (Figure 1J). In addition, TN, as well as TE and TM cells acquired LDL to a somewhat similar extent in vivo (Figure 1J), a pattern distinct from what we observed for Bodipy FL C16. These data, and those shown in Figure 1A and B, indicate that different T cells exhibit distinct preferences for lipid substrates, suggesting that acquisition of lipids that provide cholesterol for membranes and other processes might be different from those that fuel energy metabolism, fitting with published data (Bensinger et al., 2008; Kidani et al., 2013).
Glucose fuels mitochondrial FAO and OXPHOS in IL-15 TM cells
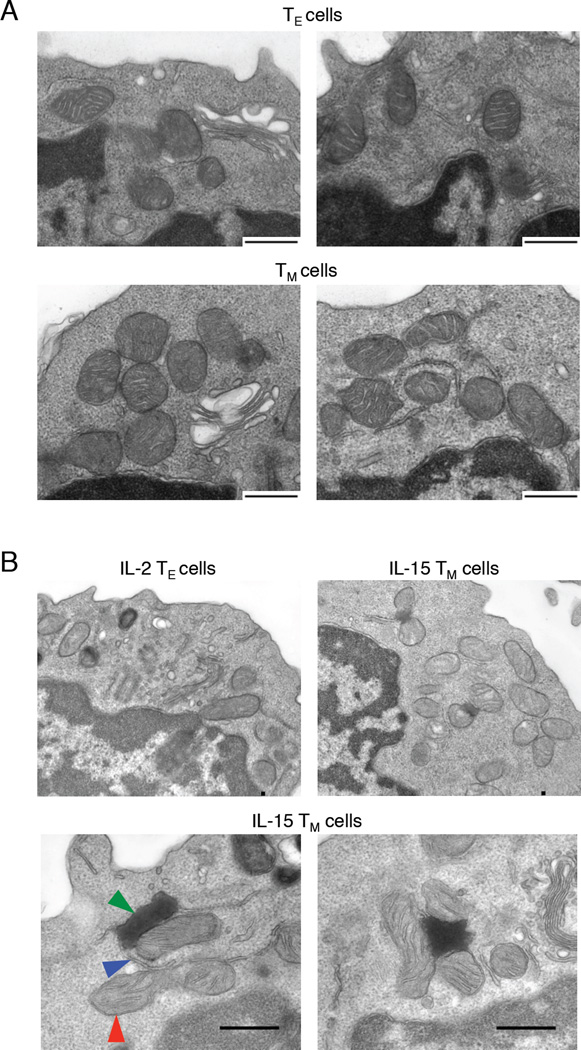
We did not visualize abundant lipid droplets by electron microscopy (EM) in either TE or TM cells. We did, however, observe accumulations of mitochondria in close association with endoplasmic reticulum (ER) in TM cells, but not TE cells (Figure 2A, B). Furthermore, we observed electron dense structures localized to mitochondria and ER in IL-15 TM cells (Figure 2B), which were always proximal to mitochondria and did not appear in other areas of the cytoplasm.
Figure 2. Ultrastructure analysis reveals densely packed mitochondria in close proximity to ER in TM cells.
EM images of mitochondria associated with ER from (A) TE and TM cells and (B) IL-2 TE and IL-15 TM cells; mitochondrion, red arrow; ER, blue arrow; electron dense structure, green arrow. Data represent ≥ 2 experiments.
Lipids imported into the mitochondria can be synthesized in the ER, requiring an interaction between these two compartments (Flis and Daum, 2013), and glycerol-3-phosphate acyltransferases, critical enzymes for glycerolipid synthesis and ultimately TAG production, can associate with ER and mitochondria (Wendel et al., 2009; Wilfling et al., 2013). Given that TM cells engage FAO to fuel OXPHOS (van der Windt et al., 2012), but do not acquire FA to a great extent, nor have appreciable lipid droplets, we wondered whether the localization of ER and mitochondria in TM cells suggested that lipids synthesized in the ER are substrates for FAO. The electron dense structures in IL-15 TM cells might indicate an abundance of synthesized lipid from the ER in nutrient replete culture conditions, whereas in TM cells generated after infection, lipids may be metabolized as they are made, thereby accounting for the absence of these structures in TM cells.
To assess lipid synthesis, we cultured IL-15 TM and IL-2 TE cells with C75, an inhibitor of fatty acid synthase (FASN) (Hansen et al., 2013). Consistent with the idea that IL-15 TM cells synthesize FA for survival, IL-15 TM cell survival decreased when cultured with C75. At the same concentration of C75, IL-2 TE cell survival was unaffected, although proliferation was attenuated (Figure 3A, B), supporting that IL-2 TE cells synthesize lipid for biomass (Wang et al., 2011). However, higher concentrations of C75 reduced IL-2 TE cell survival (Figure S2 A), consistent with a recent finding that proliferating T cells defective in FA synthesis (FAS) have decreased survival (Lee et al., 2014). We also attempted to genetically block FAS by silencing ATP-citrate lyase (ACL), an enzyme that generates cytosolic acetyl-CoA, a substrate needed in FAS (Hatzivassiliou et al., 2005). IL-2 TE cells transduced with a retrovirus expressing shRNA against ACL (hpACL) had no defect in survival, but had attenuated proliferation compared to control shRNA IL-2 TE cells (Figure S2 B-D). Unexpectedly, survival of hpACL IL-15 TM cells was not impaired (Figure S2 B). These data are in contrast to the decreased survival of IL-15 TM cells cultured with C75. This disparity could be due to two possibilities, both of which center on the idea that metabolic and biosynthetic demands are low in TM compared to TE cells. Incomplete silencing of ACL (Figure S2 D) may result in enough acetyl-CoA produced to support FAS needed for IL-15 TM cell survival, but not for the extensive proliferation of IL-2 TE cells. Alternatively, IL-15 TM cells may be able to switch from using ACL derived acetyl-CoA to that produced from acetate via acyl-CoA synthetase, an effect previously observed after ACL deletion (Hatzivassiliou et al., 2005; Zaidi et al., 2012). As C75 inhibits FASN directly, these potential confounding factors are removed. Therefore, the results using C75 indicate that despite IL-15 TM cells having a more quiescent phenotype, these cells are acutely sensitive to inhibition of FAS. Since IL-15 TM cells have a reduced demand for lipid for anabolic processes, as well as reduced extracellular FA uptake, these cells likely use synthesized lipids for catabolic processes such as FAO.
Figure 3. Glucose fuels mitochondrial FAO and OXPHOS in IL-15 TM cells.
(A) IL-2 TE and IL-15 TM cell survival (7-AAD exclusion) ± C75, shown as survival relative to vehicle (Veh.) treated cells. Data from 5 experiments show mean ± SEM, **p <0.01. (B) IL-2 TE proliferation in the presence of C75 or vehicle. Data represent 3 experiments. (C) OCR of IL-2 TE or IL-15 TM cells cultured in TCM (Control) and low glucose TCM (LG) was measured under basal conditions and in response to oligomycin (Oligo), FCCP, etomoxir (Eto) and rotenone + antimycin (Rot+Ant). Data represent 2 experiments. (D) Representative plot (histogram) and average MFI (bar graph) of 2-NBDG uptake in polyclonal TE and TM cells from 1 experiment (n=4 mice/group). Data in bar graph show mean ± SEM, ***p <0.001. (E) OCR represented as % of baseline for IL-15 TM cells cultured with AmA or vehicle (Control) measured in response to indicated drugs. Data represent 2 experiments. (F) OCR of IL-2 TE or IL-15 TM cells cultured in TCM (Control) and lipid depleted TCM (LD), measured under basal conditions and in response to indicated drugs. Data represent 2 experiments.
Most cells can synthesize lipid from glucose carbon that enters the TCA cycle, which is exported from the mitochondria as citrate (Heiden et al., 2009) and is used for FAS (Wakil et al., 1983). To explore if TM cells synthesize lipids from glucose, we cultured IL-2 TE and IL-15 TM cells with 13C-labeled glucose and analyzed 13C-glucose incorporation into the FA chains of PG and PE lipid via mass spectrometry (MS). Both cell types synthesized lipids from glucose; although the overall rate of glucose incorporation into lipid was lower in IL-15 TM cells, a similar relative incorporation of 13C-glucose into these lipids occurred in IL-2 TE and IL-15 TM cells (Figure S2 E). Consistent with active lipid synthesis, FASN, and acetyl-CoA carboxylase 1 and 2 mRNA, were similarly expressed in both cell types (data not shown).
To explore whether IL-15 TM cells use glucose to produce FA for OXPHOS, we assessed O2 consumption rates (OCR, an indicator of OXPHOS) in cells cultured in normal media (11 mM glucose) or media with low (2 mM) glucose (LG) (Figure 3C). The substantial spare respiratory capacity (SRC) prominent in TM cells (van der Windt et al., 2012) (OCR after FCCP/basal OCR) was reduced in LG, indicating that in these cells, glucose contributes to OXPHOS and maximal respiratory capacity (Figure 3C). The precipitous reduction in SRC of cells in LG is consistent with the view that glucose supports FAO, as the majority of the SRC in glucose replete media is derived from long-chain FAO, indicated by the substantial reduction in this parameter in the presence of etomoxir, a specific inhibitor of carnitine palmitoyl transferase 1a (CPT1a) which transports long-chain FA into the mitochondria and is a rate limiting step of FAO (Figure 3C) (van der Windt et al., 2012). IL-15 TM cells in LG also had decreased basal OXPHOS, indicating that glucose supports OXPHOS in the steady state. A similar trend was detected when cells cultured in glucose-replete media were subsequently transferred to LG right before OCR was measured (data not shown), indicating that the decreased OXPHOS in LG was not due to alterations in mitochondrial mass that could occur in prolonged culture. In cancer cells, glutamine provides an alternate carbon source for lipid synthesis in LG (Le et al., 2012), however we found that excess glutamine did not rescue the defects in IL-15 TM cell metabolism (Figure S2 F). In contrast to IL-15 TM cells in LG, OXPHOS in IL-2 TE cells was not impaired (Figure 3C). Consistent with published observations (Buzzai et al., 2005), T cells cultured in IL-2 compensated for reduced glycolysis in LG by increasing FAO supported OXPHOS, as indicated by the reduction in OCR following etomoxir in IL-2 TE cells in LG.
To support that TM cells use glucose for FAO and OXPHOS, we assessed glucose uptake in T cells using the fluorescent glucose analogue 2-NBDG. On day 7 after infection we compared TE cells to TN and TM cells from mice infected one month previously. We found that TN and TM cells acquired more 2-NBDG than TE cells (Figure 3D, Bar graph). This trend was also apparent in polyclonal T cells expressing TN, TE, and TM markers, when analyzed from a single mouse at day 7post infection (Figure 3D, histogram). The increased 2-NBDG uptake in these quiescent cells is consistent with their use of glucose for survival. Importantly, while the data showing that TM and TN cells acquire more 2-NBDG than TE are surprising, they do not argue against a role for glucose and glycolysis in TE cell proliferation or effector function (Chang et al., 2013; Fox et al., 2005; Jacobs et al., 2008; Wang et al., 2011). We find that early TE cells, present d5 after Lm infection, when clonal expansion has yet to peak, have higher ECAR than d7 TE cells (data not shown). We speculate that the later d7 TE cells acquire less glucose than early TE cells, as late TE cells are on the verge of contraction, while early TE cells, which are highly glycolytic, are more similar to in vitro generated IL-2 TE cells. This would be in keeping with the fact that IL-2 TE cells acquire more 2-NBDG than IL-15 TM cells in vitro (Figure S2 G). Also, these data are not inconsistent with a recent paper showing that restraining terminal differentiation of TE cells in vitro by treating with the glycolysis inhibitor 2-DG forms better TM cells in vivo (Sukumar et al., 2013), as 2-DG was administered in the TE phase, but not during TM cell development.
Since intracellular free FA can be toxic, most cells store excess FA in less reactive forms, such as TAG, and then release FA from these stores as needed. (Thiam et al., 2013). To explore if synthesized TAG contributes to FAO in IL-15 TM cells, we inhibited diacylglycerol acyltransferases (DGAT) 1 and 2, critical enzymes for TAG synthesis, with the inhibitor Amidepsine A (AmA) (Tomoda et al., 1995). IL-15 TM cells treated with AmA, exhibited reduced SRC and were insensitive to etomoxir (Figure 3E), indicating that these cells were no longer using long-chain FA to fuel OXPHOS. This effect was specific to IL-15 TM cells, as AmA treated IL-2 TE cells did not exhibit OXPHOS defects (data not shown).
To confirm that IL-15 TM cells synthesize lipids for FAO, we cultured IL-15 TM or IL-2 TE cells in lipid-depleted (LD) media. We observed no defect in basal OXPHOS, SRC, or long-chain FAO in IL-15 TM cells cultured in LD media (Figure 3F). We did find reduced OXPHOS in IL-2 TE cells (Figure 3F), which was accompanied by decreased glycolysis (data not shown), likely due to reduced availability of extracellular lipids impairing anabolic processes and leading to an overall reduction in metabolic activity in these cells. Collectively our data indicate that IL-15 TM cells synthesize lipids and are not dependent on extracellular FA to fuel OXPHOS.
The lipid signature of TM cells suggests active lipolysis
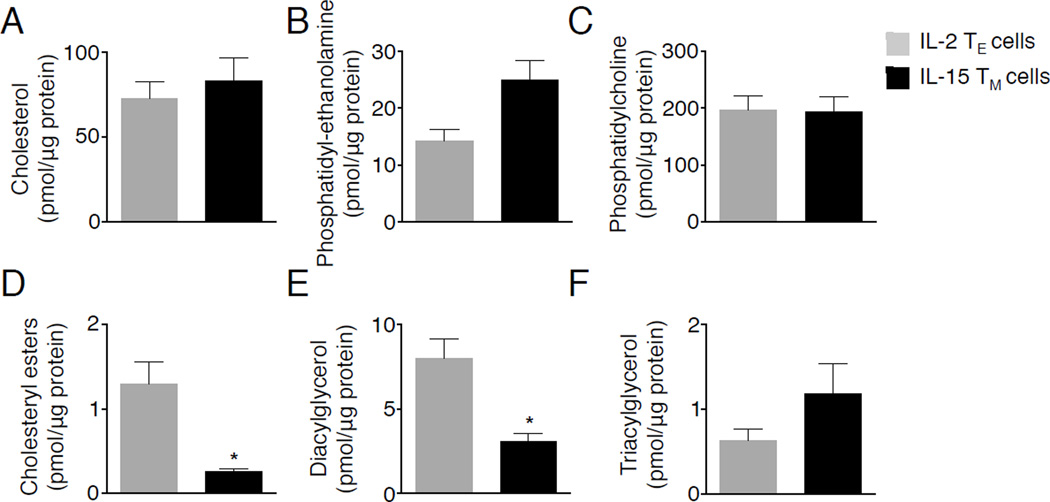
Most cells can store lipids as TAG and CE then subsequently release FA from these stores when needed (Thiam et al., 2013). Since we did not detect lipid droplets in TM cells, we reasoned that they may oxidize fat shortly after its synthesis, and any synthesized TAG could be quickly lipolyzed, for fuel. This would agree with our data demonstrating that inhibiting DGAT impairs FAO in IL-15 TM cells. We next questioned whether we could detect altered concentrations of lipid species that are broken down during lipolysis in TM cells. We assessed the abundance of TAG, diacylglycerol (DAG), and CE, which are targets of lipolysis. Relative abundance of cholesterol, phosphatidylcholine (PC), and phosphatidylethanolamine (PE) were similar between IL-15 TM and IL-2 TE cells (Figure 4A-C). However, consistent with active lipolysis, we found that CE and DAG were significantly decreased in IL-15 TM cells as compared to IL-2 TE cells (Figure 4D, E). In contrast, TAG abundance was similar between these cells (Figure 4F), perhaps suggesting that TAG is actively synthesized in IL-15 TM cells and was evident as the electron dense deposits between mitochondria and ER (Figure 2B). A trend toward decreased neutral lipids was observed in TM compared to TE cells after LmOVA infection, with significantly reduced CE (Figure S3). Overall, the alterations in neutral lipids in TM cells support the idea that these species are hydrolyzed to provide FA for FAO.
Figure 4. The lipid signature of TM cells suggests active lipolysis.
(A-F) Lipids were quantified from d7 cultured IL-2 TE and IL-15 TM cells. Data represent 3 experiments shown as mean ± SEM, *p <0.05.
Lysosomes associate with neutral lipids in TM cells
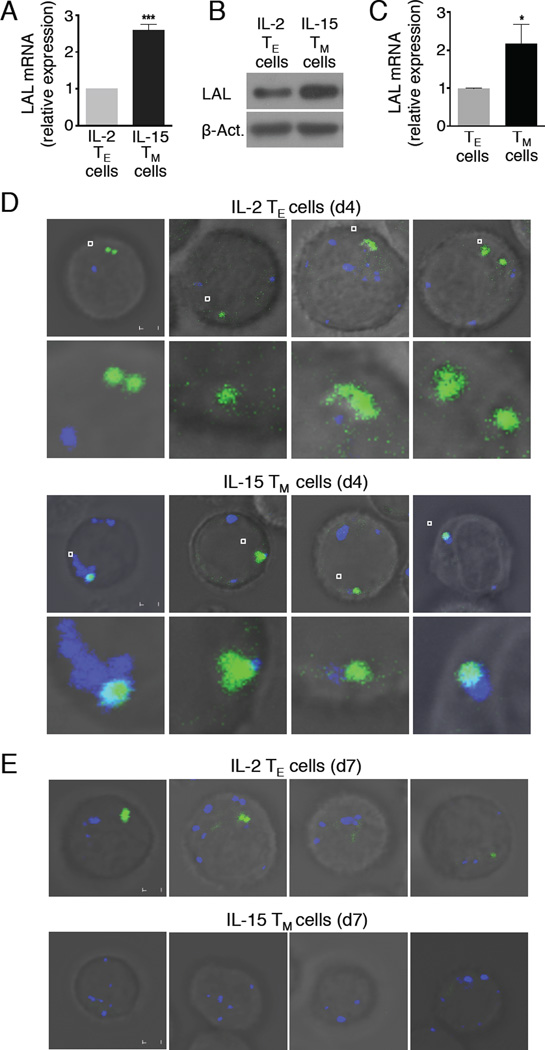
While glucose-derived 13C incorporation into lipids indicated that lipid synthesis occurs in IL-2 TE and IL-15 TM cells, the differences in TAG, CE, and DAG suggested that lipolysis actively occurs in TM cells, which agrees with the fact that they engage FAO (van der Windt et al., 2012). Since we did not find evidence of adipocyte-like lipid droplets in TM cells (Figure 2A, B), we considered the lipolytic machinery might differ from those characterized in adipocytes, such as that mediated by ATGL and HSL (Zechner et al., 2012). LAL is a lipase capable of hydrolyzing TAG, DAG, and CE to generate free FA and cholesterol in lysosomes (Sheriff et al., 1995). We assessed its expression and found that LAL protein and/or mRNA were increased in IL-15 TM and ex vivo TM cells when compared to TE cells, as were TM cells when compared to 2°TE cells (Figure 5A-C and Figure S4).
Figure 5. Lysosomes associate with intracellular neutral lipids during the TM cell transition in vitro.
(A) Relative mRNA expression of LAL in d7 IL-2 TE and IL-15 TM cells. Data from 3 experiments shown as mean ± SEM, ***p <0.001. (B) LAL protein expression compared to β-Actin. Data represent 2 experiments. (C) Relative mRNA expression of LAL in TE and TM cells. Data from 3 experiments shown as mean ± SEM, *p <0.05. IL-2 TE and IL-15 TM cells were stained with LysoTracker (blue) and Bodipy (green) on d4 (D) or d7 (E) of culture. Scale bar = 2 µM. Data represent 3 experiments.
We reasoned that if LAL regulates lipolysis in TM cells, co-localization of neutral lipids and lysosomes may occur shortly after exposure to IL-15, when cells are differentiating toward a TM cell phenotype. IL-15 promotes FAO and the TM cell phenotype in these cells (Carrio et al., 2004; van der Windt et al., 2012). We stained d4 activated IL-2 TE cells, and d4 IL-15 early TM cells (i.e. only 1 day of exposure to IL-15), with Bodipy and LysoTracker, to visualize neutral lipids and lysosomes, respectively. Confocal microscopy revealed that lysosomes associated with neutral lipid in d4 IL-15 TM cells, but not in d4 IL-2 TE cells (Figure 5D). In line with the idea that lysosome association with lipid would lead to lipolysis and subsequent fueling of FAO, the neutral lipid that was evident 1 day after exposure to IL-15 was nearly undetectable after 3 days in culture with IL-15 (Figure 5E). This finding is corroborated by data in Figure S5, showing that LAL mRNA expression increased 1 day following IL-15 exposure, and significant reductions in DAG and CE were detected within 3 days. These data support that TM cells hydrolyze intracellular neutral lipid.
Lysosomal lipolysis metabolically programs T cells toward the TM cell phenotype
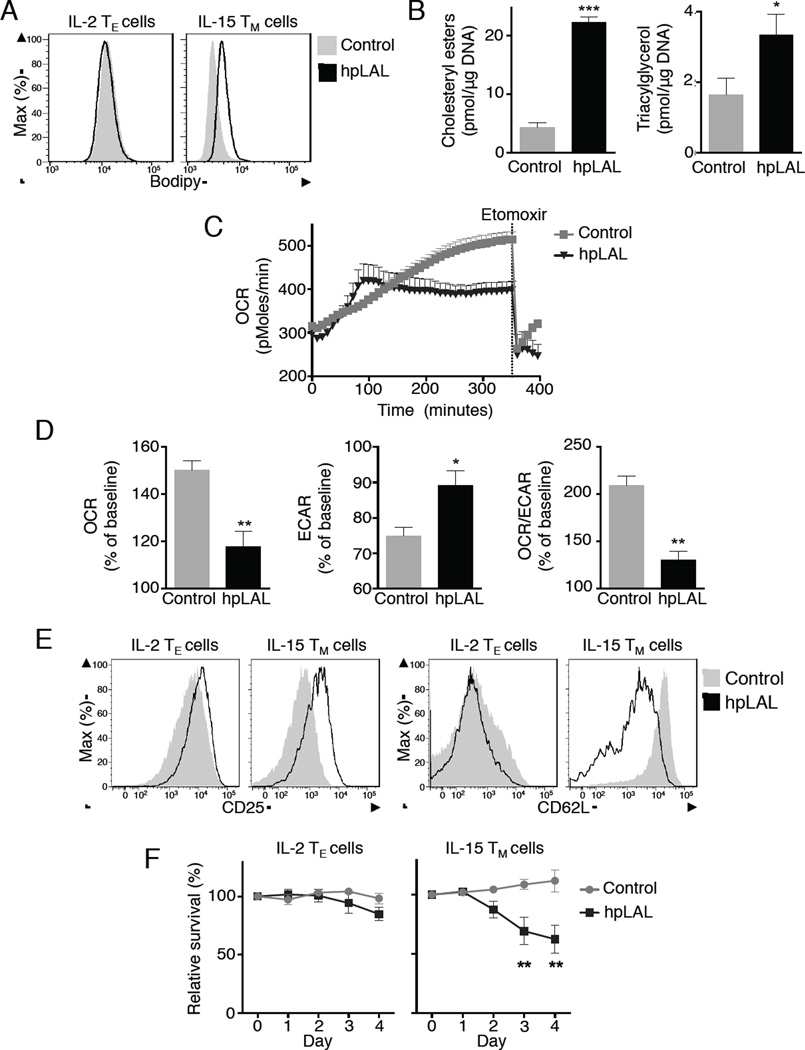
We wondered whether LAL plays an important cell intrinsic role in TM cells. Mice deficient in LAL have defects in thymocyte development and in maturation of peripheral T cells (Qu et al., 2009). Given the defects in T cell development in LAL-deficient mice, this model was not conducive to studying LAL in TM cells. We took a different genetic approach and transduced activated T cells with a retrovirus expressing either shRNA against LAL (hpLAL) or a control shRNA. This allowed us to suppress the expression of LAL (Figure S6 A, B) after the T cells were activated. Neutral lipid was increased in hpLAL IL-15 TM cells compared to controls (Figure 6A), and no increase was observed in hpLAL expressing IL-2 TE cells. These results suggest that LAL actively hydrolyzes lipid in IL-15 TM cells, and silencing LAL blocks lipolysis, resulting in increased neutral lipid. To confirm that hpLAL specifically targets lipolysis, we measured TAG and CE in control and hpLAL IL-15 TM cells and found an increase in these species, indicating that lipolysis was blocked (Figure 6B).
Figure 6. Lysosomal lipolysis metabolically programs CD8+ T cells toward the TM cell phenotype.
IL-2 TE or IL-15 TM cells were transduced with retroviral vectors containing shRNA against luciferase (Control) or against LAL (hpLAL). (A) Cells were stained for neutral lipids with Bodipy. Data represent 3 experiments. (B) IL-15 TM cells were analyzed for lipid content by MS. Data from 3 experiments show mean ± SEM, *p <0.05, ***p <0.001. (C-E) On day 3 of culture, IL-2 TE cells were washed and resuspended in media with IL 15. OCR and ECAR were measured. (C) Representative plot showing OCR. (D) Relative change in OCR, ECAR and OCR/ECAR 5 hours after baseline, represented as % change from baseline. Data from 5 experiments show mean ± SEM, *p <0.05 or **p <0.01. (E) Expression of CD25 and CD62L on d7 IL-2 TE or IL-15 TM cells. Data represent 5 experiments. (F) Day 3 IL-2 TE cells were cultured in IL-2 or IL-15 and analyzed daily (d0-4) for survival (7AAD exclusion). Data represented as % survival relative to non-transduced cells. Data from 3 experiments shown as mean ± SEM, **p <0.01 by 2 way ANOVA.
Since IL-15 promotes FAO in T cells (van der Windt et al., 2012) we investigated whether LAL-mediated lipolysis would provide FA for FAO in T cells after IL-15 exposure. As we observed lysosomal association with neutral lipid soon after IL-15 exposure, we expected that any effects on metabolism should be detectable at this time. Therefore, 3 days after activation we exposed control or hpLAL transduced IL-2 TE cells to IL-15 and immediately analyzed OCR in real time. When compared to hpLAL cells, control cells in IL-15 showed a progressive and substantial increase in OCR (Figure 6C, D). The rapid, but blunted increase in IL-15 induced OXPHOS in the hpLAL T cells suggested that the residual amount of LAL expressed (Figure S6A) is sufficient to metabolically reprogram the cells toward lipolysis and FAO under TM cell-inducing conditions (i.e. IL-15 in the absence of IL-2), and that the induction of LAL expression by IL-15 is downstream of this process. When cells were exposed to etomoxir, OCR dropped precipitously, indicating that the increase in OXPHOS following IL-15 exposure was due to FAO. However, the magnitude of this decrease in OCR was substantially reduced in hpLAL cells, indicating that FAO fueled OXPHOS is facilitated by LAL-mediated lipolysis (Figure 6C, D).
As the initial increase in OCR appeared rapid in hpLAL cells and at a slightly different kinetic from the control, it is also possible that a different substrate is fueling OXPHOS shortly after exposure to IL-15 in hpLAL cells, and that FAO only occurs after a few hours, when respiration becomes etomoxir sensitive. Since hpLAL cells remain more glycolytic, as indicated by the increased extracellular acidification rate (ECAR) (Figure 6D), the initial burst in OCR could be fueled by glucose oxidation in the mitochondria. Further experiments will be needed to determine if this is the case. Regardless, the increase in glycolysis also suggested that LAL inhibition impaired the ability of T cells to metabolically transition towards a TM cell phenotype. Consistent with this idea, hpLAL IL-15 TM cells expressed increased CD25 and reduced CD62L compared to control cells, indicating that hpLAL IL-15 TM cells retained a more activated phenotype (Figure 6E).
There is a strong link between FAO and CD8+ T cell longevity (van der Windt et al., 2012). If LAL is important for FAO, reduced LAL expression should limit survival of these cells when cultured in IL-15. We found that while hpLAL IL-2 TE cells exhibited no survival disadvantage compared to controls, hpLAL IL-15 TM cells exhibited a significant survival defect (Figure 6F). A similar decrease in survival was also observed with IL-15 TM cells, but not IL-2 TE cells, when cultured with chloroquine, which prevents LAL activity by inhibiting lysosome acidification (Figure S6 C) (Bowden et al., 2011). These results indicate that LAL supports FAO in T cells, and that lysosomal lipolysis contributes to IL-15-mediated T cell survival in vitro. We considered a role for ATGL in lipolysis in TM cells, despite the fact we could not detect classical lipid droplets in these cells. We cultured activated CD8+ T cells from ATGL-deficient mice (Pnpla2−/−) in IL-15 and found no differences in the expression of T cell activation markers, neutral lipid content, or survival when compared to wild type CD8+ T cells (Figure S7 A, B). Likewise we found no defect in TM cell development by Pnpla2−/− OT-I cells following LmOVA infection (Figure S7 C). These results support that unlike ATGL, LAL has a predominant role in TM cell development.
T cell intrinsic lysosomal lipolysis supports TM cell development after infection
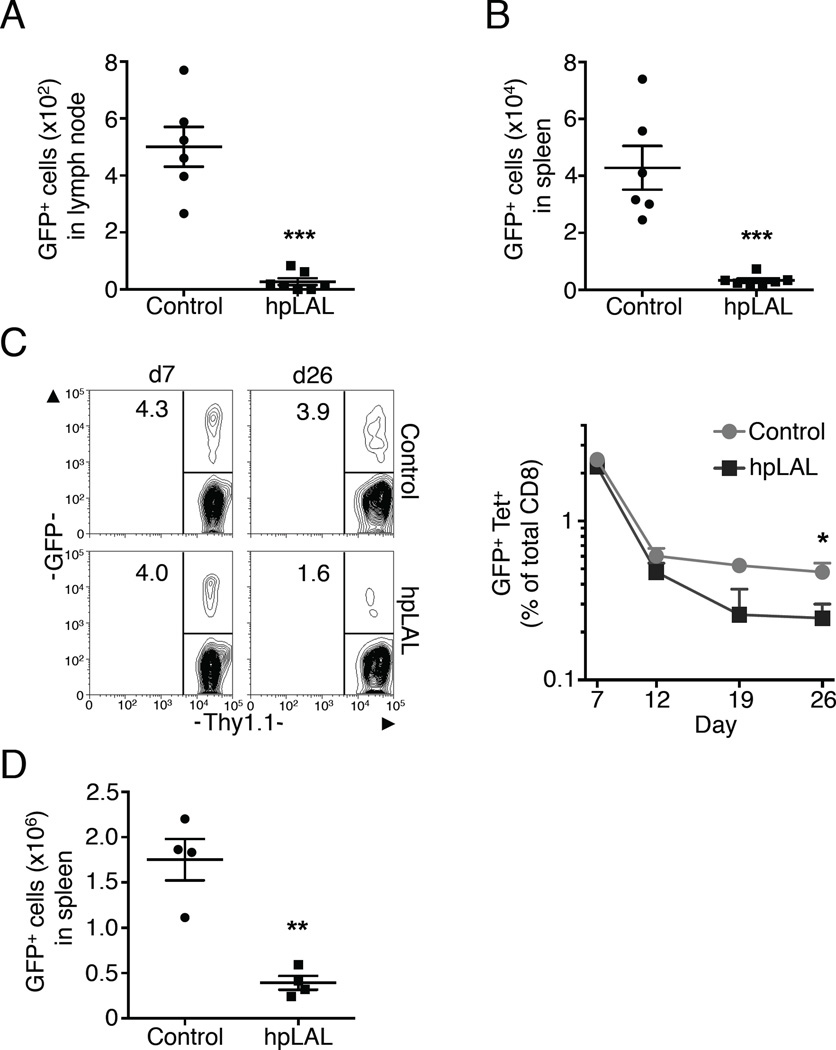
To explore whether LAL, and thus T cell intrinsic lipolysis, supports Ag-specific CD8+ T cell survival in vivo after activation signals are withdrawn, we transferred control or hpLAL transduced congenic IL-2 TE cells into C57BL/6 mice. The number of hpLAL GFP+ donor cells recovered from the spleen and lymph nodes (LN) two days later was dramatically lower than control cells, indicating that LAL regulates the survival of activated CD8+ T cells in vivo after the withdrawal of activation signals (Figure 7A, B). We view this environment to be similar to what occurs after infection, when pathogen is cleared, activation signals wane, and Ag-specific TE cells contract. To establish that LAL is important for TM cell development, we tracked OVA-specific responses of control or hpLAL transduced OT-I cells following immunization with LmOVA. To accomplish this we infected a congenically marked OT-I donor mouse and C57BL/6 recipient mice with LmOVA. One day later, splenocytes from the donor mouse were isolated, transduced, and then transferred into the recipient mice. During the peak of the TE cell response (7 days post-infection), the percentage of control and hpLAL cells (detectable by GFP expression) in the blood were similar, despite efficient silencing of LAL (Figure 7C and Figure S6 B). However, after contraction of the Ag-specific populations (26 days post-infection), the frequency of hpLAL GFP+ cells was significantly less than control GFP+ cells, indicating that hpLAL cells fail to form a robust CD8+ TM cell population (Figure 7C). Illustrating the defect in TM cell development, when the mice were challenged with LmOVA to induce a 2° TE cell response, the number of hpLAL GFP+ cells recovered from the spleen was also significantly less than control GFP+ cells (Figure 7D). Collectively, these results show that LAL regulates CD8+ TM cell development.
Figure 7. T cell intrinsic lysosomal lipolysis supports CD8+ TM cell development after infection.
(A-B) Congenic IL-2 TE cells were transduced with control or hpLAL and sorted on GFP. On d6 of culture, 2x106 cells/mouse were injected i.v. and harvested from spleen or LN 2 days later. Data represent 3 experiments, shown as mean ± SEM, ***p <0.001. (C-E) Congenic OT-I control or hpLAL transduced cells were transferred into mice infected 1 day prior with LmOVA. (C-D) Blood was analyzed for CD8, KbOVA, Thy1.1, and GFP. (C) Data shown as % of total CD8+ (line graph); representative plots show % GFP+ of Thy 1.1+ donor cells. Data represent 2 experiments, *p <0.05. (D) Mice were challenged with LmOVA and 5 days later CD8+ KbOVA GFP+ cells from spleen were quantified. Data shown as mean ± SEM and represent 2 experiments, **p <0.01.
Discussion
While our results show that cell intrinsic lipolysis supplies FA for FAO in TM cells, precisely which substrates T cells can use to produce TAG for lipolysis, especially in vivo, is still under investigation. At this time, we infer from our data that glucose is used to synthesize TAG in these cells. We base this on our observations that TM cells do not acquire substantial amounts of free FA, and that glucose supports FAO and OXPHOS in these cells in vitro. Regardless of which substrates these cells use to produce TAG, our data show that the coupling of TAG synthesis and subsequent FAO is how TM cells meet metabolic demands.
Futile metabolic cycling of FAS and FAO has been documented in muscle and adipose tissue (Dulloo et al., 2004; Guan et al., 2002; Xing Xian et al., 2002) and in cells overexpressing DGAT (Liu et al., 2009). How this futile cycling occurs is an intriguing question, as FAO and FAS pathways can negatively regulate each other. For example, malonyl-CoA, a substrate for FAS, is an allosteric inhibitor of CPT1a (McGarry et al., 1977). It remains to be determined if FAS and FAO occur simultaneously, or if cells oscillate between periods of FAS and FAO. If the former occurs, then a mechanism for preventing malonyl-CoA inhibition of CPT1a would be needed. Perhaps cells can partition FAS and FAO into separate areas, or rapidly utilize malonyl-CoA for FAS, while inducing activity of CPT1a. TM cells express more CPT1a than TE cells (van der Windt et al., 2012), suggesting that higher concentrations of malonyl-CoA would be needed for inhibition. However, oscillating cycles could also occur if malonyl-CoA concentrations fluctuate, with higher amounts resulting in CPT1a inhibition and increased FAS, and lower concentrations, due to its consumption through FAS, CPT1a inhibition would be relieved and FAO permitted. Although the coupling of FAS and FAO is not energetically efficient in terms of ATP production, and based on this notion is seemingly paradoxical, it is possible that this process allows TM cells to preserve glycolytic and lipogenic machinery, while maintaining mitochondrial health over long periods of quiescence, rendering these cells ‘primed and ready’ to tap into their substantial metabolic reserve required for rapid reactivation following Ag recognition.
Relatively little is known about lipolysis or lipolytic mechanisms in non-adipose tissues (Zechner et al., 2012). Our results showing that TE cells have the capacity to acquire external FA and store excess in lipid droplets, while TM cells, which rely on FAO for survival, do not, were unexpected. Most cell types are thought to be able to acquire and store excess lipids (Thiam et al., 2013). It has been shown that regulatory T (Treg) cells in adipose tissue express CD36 and have lipid droplets (Cipolletta et al., 2012), and that proliferating T cells during graft versus host disease increase in FA uptake (Byersdorfer et al., 2013). While our data suggest that TM cells do not acquire excess exogenous FA to fuel OXPHOS, more work is needed to determine why these T cells have this unique phenotype. Perhaps TM cells, or even other long-lived immune cells, do not store lipid in lipid droplets as a protective mechanism against viruses, which often use these organelles as sites of replication and persistence (Camus et al., 2013; Miyanari et al., 2007).
We initially hypothesized that since TM cells use FAO, these cells would acquire free FA as fuel, much like in vitro Treg cells and other cells that engage FAO (Dhalla et al., 1992; Kodde et al., 2007; Michalek et al., 2011; Zhang et al., 2010). This idea fits with the fact that CD8+ TM cells reside in lipid-rich niches, such as bone marrow and LN. However, our findings that TE, but not TM cells, acquire substantial amounts of FA raises the possibility that fat around LN supplies substrate for rapidly dividing cells, such as would be required during an immune response. Unlike other cells in the body, lymphocytes divide extremely rapidly following activation, and there could be an advantage to having substrates ‘on hand’, rather than depending on circulating lipids derived from central stores. While FA derived from lipolysis can fuel OXPHOS, lipolysis is also required for the production of lipid signaling molecules, such as lipid ligands that activate the peroxisome proliferator activated receptor (PPAR) pathway (Haemmerle et al., 2011). Future studies are needed to illuminate the precise destinies for all mobilized lipids in T cells.
The fact that we found no evidence that TM cells form lipid droplets agrees with the lack of a role for ATGL in their survival. Our data suggest that TM cells can synthesize FA using glucose-derived carbon, and we speculate that these FA are converted to TAG in the ER, which in turn directly undergo LAL mediated lipolysis to generate FA for FAO. As we do not detect the accumulation of lipid droplets in TM cells, this suggests that lipolysis of TAG would occur shortly after synthesis. Consistent with this model, EM revealed close localization of ER and mitochondria in TM cells. More work is needed to confirm this mechanism and to understand how lysosomes are involved in this process. Of note, in parallel studies, we have found that cell intrinsic expression of LAL is essential for IL-4 driven macrophage alternative activation (Huang et al., unpublished results), suggesting that LAL has broadly assumed a role in coordinating cytokine (IL-4 or IL-15)-induced increases in FAO.
It was shown that autophagy can regulate lipid metabolism, where lipid droplets and autophagic components associate during nutrient deprivation, leading to release of TAG (Singh et al., 2009). A recent study has also connected lysosomal lipolysis and autophagy to nutrient availability, fat storage, and aging in C. elegans (O'Rourke et al., 2013) and showed that lysosomal lipolysis and autophagy are transcriptionally linked to nutrients in mammals. Future studies delineating autophagy and lipolysis in TM cell development and how nutritional status orchestrates these pathways to control cellular lifespan are needed. It has also been shown that the transcription factor forkhead homeobox type protein O1 (FoxO1) is induced by nutrient restriction in adipocytes and exerts transcriptional control of lipid catabolism via the induction of LAL (Lettieri Barbato et al., 2013). Given several papers showing the importance of FoxO1 in the development of TM cells (Rao et al., 2012; Tejera et al., 2013), it is possible this factor promotes TM cell development by promoting lipolysis. Our preliminary results indicate that inhibiting the activity of FoxO1 in IL-15 TM cells blocks LAL mRNA expression. It has also been shown that metformin, a drug used to treat type-2 diabetes, elicited FoxO1-dependent LAL induction and lipophagy in adipocytes (Lettieri Barbato et al., 2013). These results complement our previous findings that metformin promotes FAO in TM cells and enhances their development in vivo (Pearce et al., 2009). Understanding how lipid metabolic programs are enacted in T cells and how these programs can be manipulated to increase cell longevity will be a subject of future study.
Experimental Procedures
See the Supplemental Information for details
Mice and immunizations
C57BL/6, C57BL/6-Tg (TcraTcrb)1100Mjb/J (OT-I), B6.129P2-Pnpla2tm1Rze/J (Pnpla2−/−) and OT-I CD90.1 mice were maintained at Washington University School of Medicine and cared for according to the Animal Care Guidelines. L. monocytogenes deficient for actA and expressing OVA (LmOVA) was used for infections.
Cell culture
T cell media (TCM) contained RPMI 1640 with 10% FCS, 2 mM L-glutamine, 100 U/ml Pen Strep and 55 µM 2-Me. LG TCM contained 2 mM glucose, and excess glutamine was added (8 mM) as indicated. LD TCM contained FCS depleted of lipids using PHM-L Liposorb. For drug cultures, 10 µM chloroquine, 20 or 40 µM C75, 20 µM AmA or respective vehicles (H2O or DMSO) were added on d3 of culture. For oleate cultures cells were incubated overnight in TCM ±100 µM oleate conjugated to bovine serum albumin.
Flow cytometry and microscopy
OVA-specific CD8+ T cells were quantified using H2-KbOVA257–264 (KbOVA) MHC-peptide tetramers. To identify neutral lipids, cells were stained with 500 ng/ml Bodipy 493/503. For lipid uptake in vitro or ex vivo cells were incubated with either 1 µM Bodipy FL C16 or 10 µg/ml Bodipy FL LDL in TCM. For Bodipy FL C16 uptake in vivo mice were injected i.v. with 50 µg/mouse diluted in DPBS. For 2-NBDG uptake in vivo, mice were injected i.v. with 100 µg/mouse diluted in DPBS or in vitro: cells were incubated in media containing 50 µg/ml 2-NBDG. For Transmission EM, cells were fixed in 2% paraformaldehyde 2.5% glutaraldehyde in 100 mM sodium cocodylate containing 0.05% malachite green.
Retroviral transduction
Activated OT-I splenocytes were transduced with human CD8 or GFP expressing control (virus expressing shRNA against luciferase), hpLAL (virus expressing shRNA aganst LAL) or LD-GFP (virus expressing the sequence of the 38 AA N-terminal of METTL7B fused to eGFP).
Adoptive transfers
For in vivo TM cell experiments, donor and recipient mice were infected i.v. with 5x106 CFU of LmOVA. The following day, splenocytes from donor mice were transduced with control or hpLAL. Following transduction, cells were injected i.v. (5x105 CD8+ cells/mouse) into recipient mice. For in vivo survival experiments, OT-I cells transduced with control or hpLAL and sorted on GFP expression. On d6 of culture, 2x106 CD8+ cells/mouse were injected i.v into naive C57BL/6 mice.
Metabolism assays
Cells were plated in XF media ± 10% FCS added as indicated. For LG conditions, 2 mM glucose XF media was prepared ± L-glutamine (8 mM) as indicated. For LD conditions, XF media was prepared without FCS. OCR and ECAR were measured under basal conditions, and following the addition of IL-15 (10 ng/ml) or the following drugs: 1 µM oligomycin, 1.5 µM FCCP, 200 µM etomoxir, and 100 nM rotenone + 1 µM antimycin A using an Extracellular Flux Analyzer.
Lipidomics and glucose tracing
For glucose tracing, cells were cultured into IL-2 TE and IL-15 TM. On d6 cells were cultured for 24 hours in TCM containing only D-[U-13C] labeled glucose and analyzed by MS.
Supplementary Material
Highlights.
Unlike TE cells, TM cells do not acquire substantial amounts of long-chain FA
Glucose supports mitochondrial FAO and OXPHOS in TM cells
TM cells use LAL-mediated cell intrinsic lipolysis to mobilize FA for FAO
T cell intrinsic lysosomal lipolysis is important for TM cell development
Acknowledgements
We thank Guenther Haemmerle and Rudolf Zechner for the Pnpla2−/− mice, Nathan Wolins for reagents and advice, Babak Razani for helpful discussion, Wandy Beatty (Molecular Microbiology Imaging Facility), Erica Lantelme, Dorjan Brinja, Hui Jiang, Dave Scherrer and the Endocrinology, Metabolism and Lipid Research Mass Spectrometry Resource for technical support. The work was supported by NIH grant AI091965 (to E.L.P). D.O., G.v.d.W., S.H., C-H.C., M.D.B., W.Y.L., L.M.D., M.J.B., E.L.P designed research. D.O., G.v.d.W., J.D.C., C-H.C., J.Q., F-F.H., and E.L.P. analyzed data. D.O., G.v.d.W., J.D.C., M.D.B., A.M.S., L.M.D., performed experiments. D.O., G.v.d.W., M.D.B., J.Q., M.J.B., F-F.H., E.J.P., and E.L.P. contributed to manuscript preparation. D.O. and E.L.P. wrote the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bensinger SJ, Bradley MN, Joseph SB, Zelcer N, Janssen EM, Hausner MA, Shih R, Parks JS, Edwards PA, Jamieson BD, Tontonoz P. LXR Signaling Couples Sterol Metabolism to Proliferation in the Acquired Immune Response. Cell. 2008;134:97–111. doi: 10.1016/j.cell.2008.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden KL, Bilbey NJ, Bilawchuk LM, Boadu E, Sidhu R, Ory DS, Du H, Chan T, Francis GA. Lysosomal Acid Lipase Deficiency Impairs Regulation of ABCA1 Gene and Formation of High Density Lipoproteins in Cholesteryl Ester Storage Disease. Journal of Biological Chemistry. 2011;286:30624–30635. doi: 10.1074/jbc.M111.274381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasaemle DL. Thematic review series: Adipocyte Biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. Journal of Lipid Research. 2007;48:2547–2559. doi: 10.1194/jlr.R700014-JLR200. [DOI] [PubMed] [Google Scholar]
- Bulankina AV, Deggerich A, Wenzel D, Mutenda K, Wittmann JG, Rudolph MG, Burger KNJ, Höning S. TIP47 functions in the biogenesis of lipid droplets. Journal of Cell Biology. 2009;185:641–655. doi: 10.1083/jcb.200812042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzzai M, Bauer DE, Jones RG, DeBerardinis RJ, Hatzivassiliou G, Elstrom RL, Thompson CB. The glucose dependence of Akt-transformed cells can be reversed by pharmacologic activation of fatty acid [beta]-oxidation. Oncogene. 2005;24:4165–4173. doi: 10.1038/sj.onc.1208622. [DOI] [PubMed] [Google Scholar]
- Byersdorfer CA, Tkachev V, Opipari AW, Goodell S, Swanson J, Sandquist S, Glick GD, Ferrara JLM. Effector T cells require fatty acid metabolism during murine graft-versus-host disease. Blood. 2013;122:3230–3237. doi: 10.1182/blood-2013-04-495515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camus G, Vogt DA, Kondratowicz AS, Ott M. Lipid Droplets and Viral Infections. 2013:167–190. doi: 10.1016/B978-0-12-408051-5.00009-7. [DOI] [PubMed] [Google Scholar]
- Caro-Maldonado A, Gerriets VA, Rathmell JC. Matched and mismatched metabolic fuels in lymphocyte function. Seminars in Immunology. 2012;24:405–413. doi: 10.1016/j.smim.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrio R, Bathe OF, Malek TR. Initial antigen encounter programs CD8+ T cells competent to develop into memory cells that are activated in an antigen-free, IL-7- and IL-15-rich environment. Journal of Immunology. 2004;172:7315–7323. doi: 10.4049/jimmunol.172.12.7315. [DOI] [PubMed] [Google Scholar]
- Chang C-H, Curtis Jonathan D, Maggi Leonard B, Jr, Faubert B, Villarino Alejandro V, O’Sullivan D, Huang Stanley C-C, van der Windt Gerritje JW, Blagih J, Qiu J, et al. Posttranscriptional Control of T Cell Effector Function by Aerobic Glycolysis. Cell. 2013;153:1239–1251. doi: 10.1016/j.cell.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolletta D, Feuerer M, Li A, Kamei N, Lee J, Shoelson SE, Benoist C, Mathis D. PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue T reg cells. Nature. 2012;486:549–553. doi: 10.1038/nature11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui W, Kaech SM. Generation of effector CD8+ T cells and their conversion to memory T cells. Immunological Reviews. 2010;236:151–166. doi: 10.1111/j.1600-065X.2010.00926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhalla NS, Elimban V, Rupp H. Paradoxical role of lipid metabolism in heart function and dysfunction. Molecular and Cellular Biochemistry. 1992;116:3–9. doi: 10.1007/978-1-4615-3514-0_1. [DOI] [PubMed] [Google Scholar]
- Dulloo AG, Gubler M, Montani JP, Seydoux J, Solinas G. Substrate cycling between de novo lipogenesis and lipid oxidation: A thermogenic mechanism against skeletal muscle lipotoxicity and glucolipotoxicity. International Journal of Obesity. 2004;28:S29–S37. doi: 10.1038/sj.ijo.0802861. [DOI] [PubMed] [Google Scholar]
- Farese RV, Jr, Walther TC. Lipid Droplets Finally Get a Little R-E-S-P-EC- T. Cell. 2009;139:855–860. doi: 10.1016/j.cell.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox CJ, Hammerman PS, Thompson CB. Fuel feeds function: energy metabolism and the T-cell response. Nat Rev Immunol. 2005;5:844–852. doi: 10.1038/nri1710. [DOI] [PubMed] [Google Scholar]
- Guan HP, Yong L, Jensen MV, Newgard CB, Steppan CM, Lazar MA. A futile metabolic cycle activated in adipocytes by antidiabetic agents. Nature Medicine. 2002;8:1122–1128. doi: 10.1038/nm780. [DOI] [PubMed] [Google Scholar]
- Haemmerle G, Moustafa T, Woelkart G, Buttner S, Schmidt A, van de Weijer T, Hesselink M, Jaeger D, Kienesberger PC, Zierler K, et al. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-[alpha] and PGC-1. Nat Med. 2011;17:1076–1085. doi: 10.1038/nm.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M, Flatt T, Aguilaniu H. Reproduction, fat metabolism, and life span: What is the connection? Cell Metabolism. 2013;17:10–19. doi: 10.1016/j.cmet.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harty JT, Badovinac VP. Shaping and reshaping CD8+ T-cell memory. Nature Reviews Immunology. 2008;8:107–119. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- Hatzivassiliou G, Zhao F, Bauer DE, Andreadis C, Shaw AN, Dhanak D, Hingorani SR, Tuveson DA, Thompson CB. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell. 2005;8:311–321. doi: 10.1016/j.ccr.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Heiden MGV, Cantley LC, Thompson CB. Understanding the warburg effect: The metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs SR, Herman CE, MacIver NJ, Wofford JA, Wieman HL, Hammen JJ, Rathmell JC. Glucose Uptake Is Limiting in T Cell Activation and Requires CD28-Mediated Akt-Dependent and Independent Pathways. The Journal of Immunology. 2008;180:4476–4486. doi: 10.4049/jimmunol.180.7.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidani Y, Elsaesser H, Hock MB, Vergnes L, Williams KJ, Argus JP, Marbois BN, Komisopoulou E, Wilson EB, Osborne TF, et al. Sterol regulatory element-binding proteins are essential for the metabolic programming of effector T cells and adaptive immunity. Nat Immunol. 2013;14:489–499. doi: 10.1038/ni.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiens B. Skeletal Muscle Lipid Metabolism in Exercise and Insulin Resistance. Physiological Reviews. 2006;86:205–243. doi: 10.1152/physrev.00023.2004. [DOI] [PubMed] [Google Scholar]
- Kodde IF, van der Stok J, Smolenski RT, de Jong JW. Metabolic and genetic regulation of cardiac energy substrate preference. Comparative Biochemistry and Physiology - A Molecular and Integrative Physiology. 2007;146:26–39. doi: 10.1016/j.cbpa.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Koonen DPY, Glatz JFC, Bonen A, Luiken JJFP. Long-chain fatty acid uptake and FAT/CD36 translocation in heart and skeletal muscle. Biochimica et Biophysica Acta - Molecular and Cell Biology of Lipids. 2005;1736:163–180. doi: 10.1016/j.bbalip.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Lass A, Zimmermann R, Oberer M, Zechner R. Lipolysis - A highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Progress in Lipid Research. 2011;50:14–27. doi: 10.1016/j.plipres.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le A, Lane Andrew N, Hamaker M, Bose S, Gouw A, Barbi J, Tsukamoto T, Rojas Camilio J, Slusher Barbara S, Zhang H, et al. Glucose-Independent Glutamine Metabolism via TCA Cycling for Proliferation and Survival in B Cells. Cell Metabolism. 2012;15:110–121. doi: 10.1016/j.cmet.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Walsh MC, Hoehn KL, James DE, Wherry EJ, Choi Y. Regulator of fatty acid metabolism, acetyl coenzyme a carboxylase 1, controls T cell immunity. Journal of Immunology. 2014;192:3190–3199. doi: 10.4049/jimmunol.1302985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lettieri Barbato D, Tatulli G, Aquilano K, Ciriolo MR. FoxO1 controls lysosomal acid lipase in adipocytes: Implication of lipophagy during nutrient restriction and metformin treatment. Cell Death and Disease. 2013;4 doi: 10.1038/cddis.2013.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Shi X, Choi CS, Shulman GI, Klaus K, Nair KS, Schwartz GJ, Zhang Y, Goldberg IJ, Yu YH. Paradoxical Coupling of Triglyceride Synthesis and Fatty Acid Oxidation in Skeletal Muscle Overexpressing DGAT1. Diabetes. 2009;58:2516–2524. doi: 10.2337/db08-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry JD, Mannaerts GP, Foster DW. A possible role for malonyl CoA in the regulation of hepatic fatty acid oxidation and ketogenesis. Journal of Clinical Investigation. 1977;60:265–270. doi: 10.1172/JCI108764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, Sullivan SA, Nichols AG, Rathmell JC. Cutting Edge: Distinct Glycolytic and Lipid Oxidative Metabolic Programs Are Essential for Effector and Regulatory CD4+ T Cell Subsets. The Journal of Immunology. 2011;186:3299–3303. doi: 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyanari Y, Atsuzawa K, Usuda N, Watashi K, Hishiki T, Zayas M, Bartenschlager R, Wakita T, Hijikata M, Shimotohno K. The lipid droplet is an important organelle for hepatitis C virus production. Nat Cell Biol. 2007;9:1089–1097. doi: 10.1038/ncb1631. [DOI] [PubMed] [Google Scholar]
- O’Rourke EJ, Kuballa P, Xavier R, Ruvkun G. ω-6 Polyunsaturated fatty acids extend life span through the activation of autophagy. Genes & Development. 2013;27:429–440. doi: 10.1101/gad.205294.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EL. Metabolism in T cell activation and differentiation. Current Opinion in Immunology. 2010;22:314–320. doi: 10.1016/j.coi.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EL, Poffenberger MC, Chang C-H, Jones RG. Fueling Immunity: Insights into Metabolism and Lymphocyte Function. Science. 2013;342 doi: 10.1126/science.1242454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu P, Du H, Wilkes DS, Yan C. Critical roles of lysosomal acid lipase in t cell development and function. American Journal of Pathology. 2009;174:944–956. doi: 10.2353/ajpath.2009.080562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RR, Li Q, Bupp M, Shrikant P. Transcription Factor Foxo1 Represses T-bet-Mediated Effector Functions and Promotes Memory CD8 + T Cell Differentiation. Immunity. 2012;36:374–387. doi: 10.1016/j.immuni.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy JK, Sambasiva Rao M. Lipid Metabolism and Liver Inflammation. II. Fatty liver disease and fatty acid oxidation. American Journal of Physiology -Gastrointestinal and Liver Physiology. 2006;290:G852–G858. doi: 10.1152/ajpgi.00521.2005. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S, Ahmed R. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. The Journal of Experimental Medicine. 2008;205:625–640. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sena LA, Li S, Jairaman A, Prakriya M, Ezponda T, Hildeman DA, Wang CR, Schumacker PT, Licht JD, Perlman H, Bryce PJ, Chandel NS. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity. 2013 Feb 21;38(2):225–236. doi: 10.1016/j.immuni.2012.10.020. Epub 2013 Feb 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheriff S, Du H, Grabowski GA. Characterization of Lysosomal Acid Lipase by Site-directed Mutagenesis and Heterologous Expression. Journal of Biological Chemistry. 1995;270:27766–27772. doi: 10.1074/jbc.270.46.27766. [DOI] [PubMed] [Google Scholar]
- Silverstein RL, Febbraio M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Science Signaling. 2009;2 doi: 10.1126/scisignal.272re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumar M, Liu J, Ji Y, Subramanian M, Crompton JG, Yu Z, Roychoudhuri R, Palmer DC, Muranski P, Karoly ED, et al. Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. The Journal of Clinical Investigation. 2013;123:4479–4488. doi: 10.1172/JCI69589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejera MM, Kim EH, Sullivan JA, Plisch EH, Suresh M. FoxO1 Controls Effector-to-Memory Transition and Maintenance of Functional CD8 T Cell Memory. The Journal of Immunology. 2013;191:187–199. doi: 10.4049/jimmunol.1300331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiam AR, Farese Jr RV, Walther TC. The biophysics and cell biology of lipid droplets. Nat Rev Mol Cell Biol. 2013;14:775–786. doi: 10.1038/nrm3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomoda H, Ito M, Tabata N, Masuma R, Yamaguchi Y, Omura S. Amidepsines, inhibitors of diacylglycerol acyltransferase produced by Humicola sp. FO-2942. I. Production, isolation and biological properties. Journal of Antibiotics. 1995;48:937–941. doi: 10.7164/antibiotics.48.937. [DOI] [PubMed] [Google Scholar]
- van der Windt GJW, Everts B, Chang CH, Curtis JD, Freitas TC, Amiel E, Pearce EJ, Pearce EL. Mitochondrial Respiratory Capacity Is a Critical Regulator of CD8 + T Cell Memory Development. Immunity. 2012;36:68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Windt GJW, O’Sullivan D, Everts B, Huang SCC, Buck MD, Curtis JD, Chang CH, Smith AM, Ai T, Faubert B, et al. CD8 memory T cells have a bioenergetic advantage that underlies their rapid recall ability. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:14336–14341. doi: 10.1073/pnas.1221740110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Windt GJW, Pearce EL. Metabolic switching and fuel choice during T-cell differentiation and memory development. Immunological Reviews. 2012;249:27–42. doi: 10.1111/j.1600-065X.2012.01150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakil SJ, Stoops JK, Joshi VC. Fatty Acid Synthesis and its Regulation. Annual Review of Biochemistry. 1983;52:537–579. doi: 10.1146/annurev.bi.52.070183.002541. [DOI] [PubMed] [Google Scholar]
- Wang MC, O’Rourke EJ, Ruvkun G. Fat metabolism links germline stem cells and longevity in Celegans. Science. 2008;322:957–960. doi: 10.1126/science.1162011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Dillon CP, Shi LZ, Milasta S, Carter R, Finkelstein D, McCormick LL, Fitzgerald P, Chi H, Munger J, Green DR. The Transcription Factor Myc Controls Metabolic Reprogramming upon T Lymphocyte Activation. Immunity. 2011;35:871–882. doi: 10.1016/j.immuni.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg JM. Mitochondrial Biogenesis in Kidney Disease. Journal of the American Society of Nephrology. 2011;22:431–436. doi: 10.1681/ASN.2010060643. [DOI] [PubMed] [Google Scholar]
- Xing Xian YU, Lewin DA, Forrest W, Adams SH. Cold elicits the simultaneous induction of fatty acid synthesis and β-oxidation in murine brown adipose tissue: Prediction from differential gene expression and confirmation in vivo. FASEB Journal. 2002;16:155–168. doi: 10.1096/fj.01-0568com. [DOI] [PubMed] [Google Scholar]
- Zaidi N, Royaux I, Swinnen JV, Smans K. ATP Citrate Lyase Knockdown Induces Growth Arrest and Apoptosis through Different Cell- and Environment-Dependent Mechanisms. Molecular Cancer Therapeutics. 2012;11:1925–1935. doi: 10.1158/1535-7163.MCT-12-0095. [DOI] [PubMed] [Google Scholar]
- Zechner R, Zimmermann R, Eichmann TO, Kohlwein SD, Haemmerle G, Lass A, Madeo F. FAT SIGNALS - Lipases and lipolysis in lipid metabolism and signaling. Cell Metabolism. 2012;15:279–291. doi: 10.1016/j.cmet.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Keung W, Samokhvalov V, Wang W, Lopaschuk GD. Role of fatty acid uptake and fatty acid β-oxidation in mediating insulin resistance in heart and skeletal muscle. Biochimica et Biophysica Acta - Molecular and Cell Biology of Lipids. 2010;1801:1–22. doi: 10.1016/j.bbalip.2009.09.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.