Summary
The intensity and prevalence of viral infections are typically higher in males, whereas disease outcome can be worse for females. Females mount higher innate and adaptive immune responses than males, which can result in faster clearance of viruses, but also contributes to increased development of immunopathology. In response to viral vaccines, females mount higher antibody responses and experience more adverse reactions than males. The efficacy of antiviral drugs at reducing viral load differs between the sexes, and the adverse reactions to antiviral drugs are typically greater in females than males. Several variables should be considered when evaluating male/female differences in responses to viral infection and treatment: these include hormones, genes, and gender-specific factors related to access to, and compliance with, treatment. Knowledge that the sexes differ in their responses to viruses and to treatments for viral diseases should influence the recommended course of action differently for males and females.
Keywords: antiviral drugs, estrogen, hepatitis, herpes simplex virus, HIV, influenza, sex difference, testosterone, vaccine
Males and females differ in the intensity, prevalence, and pathogenesis of viral infections (Table 1). Although behavioral factors can influence exposure to viruses, several studies illustrate that physiological differences between males and females cause differential responses to infection. Females display reduced susceptibility to viral infections because they often mount stronger immune responses than males (Figure 1). The innate recognition and response to viruses as well as downstream adaptive immune responses differ between males and females during viral infections. As a result of heightened immunity to viruses, both the intensity (i.e., viral load within an individual) and prevalence (i.e., number of infected individuals within a population) of viral infections are often lower for females than males (Table 1). There is growing awareness, however, that much of the disease attributed to viral infection results from aberrant host inflammatory responses [1,2]. Consequently, heightened antiviral, inflammatory, and cellular immune responses in females, though essential for virus clearance, might underlie increased development of symptoms of disease among females as compared with males following infection (Table 1).
Table 1.
Sex differences in the intensity (I), prevalence (P), severity of disease (D), or mortality (M) following viral infections in humans.
| Virus | Dependent Measure | Sex-specific difference | Reference |
|---|---|---|---|
| Cytomegalovirus | P | M < F | [14] |
| Dengue Virus | P | M > F | [106] |
| Epstein Barr virus | D | M > F | [107] |
| Hantaviruses (multiple species) | P M |
M > F M < F |
[108] |
| Hepatitis B virus | I, P, D | M > F | [61,64,65,67] |
| Hepatitis C virus | P, I | M > F | [70,71] |
| Herpes simplex virus type 2 | I, P | M < F | [48,109] |
| Human immunodeficiency virus | I D |
M > F M < F |
[32,33,37] |
| Human T-cell Leukemia Virus Type 1 | P | M < F | [110] |
| Influenza A viruses | D, M | M < F | [86,88,89] |
| Measles | M | M < F | [111] |
| West Nile virus | I | M > F | [112] |
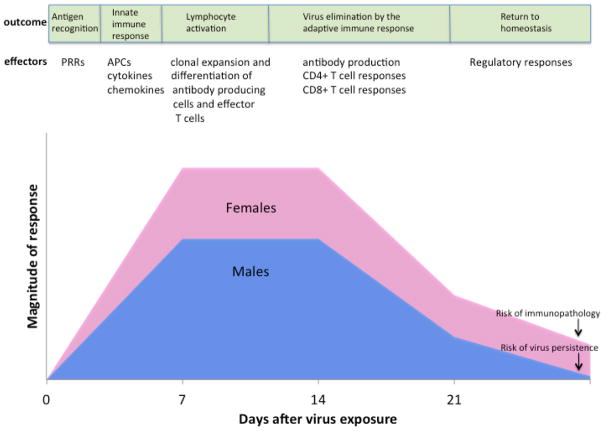
Figure 1. Sex-based differences in immune responses to viruses.
Following exposure to a virus, antigen recognition by pattern recognition receptors (PRRs) and induction of innate immune responses, including the activity of antigen presenting cells (APCs) (e.g., dendritic cells and macrophages) and production of inflammatory cytokines (e.g., IFN-β, IFN-γ, and TNF-α) and chemokines (e.g., CCL2) are higher in females than males. Induction of the adaptive immune response, including the activation of lymphocytes, production of antibodies by B cells, and activity of CD4+ and CD8+ T cells also are higher in females than males. Following clearance of virus, the immune systems of both males and females return to homeostasis. During the return to homeostasis, females can maintain elevated immune responses resulting in a greater risk of developing immunopathology (i.e., tissue damage caused by excessively high or prolonged activation of immune responses) than males. In contrast, during the return to homeostasis in males, lower antiviral immune responses can result in an increased risk of virus persistence in males as compared with females.
Although sex and gender differences have been well documented for viral infections, considerably less attention has been paid to the differences between males and females in prophylaxis and therapeutic treatments for viral diseases. Vaccines are the principle preventative treatment for viral diseases and have successfully reduced many diseases in both males and females. The efficacy of vaccines relies on their ability to induce protective immunity in either the short-term (e.g., influenza) or long-term (e.g., measles). There is growing evidence that protective immune responses and adverse reactions to viral vaccines are higher in females than males. Most antiviral drugs are used as a therapeutic treatment for specific viral infections, with these drugs typically inhibiting the replication of viruses in host cells. Only recently has it been documented that the pharmacokinetics (i.e., absorption, distribution, metabolism, and excretion of drug) and pharmacodynamics (i.e., the effect of the drug on physiological and biochemical processes, both therapeutic and adverse) differ between the sexes. The goals of this review will be to: 1) document the immunological differences that exist between males and females and the factors that mediate these differences; 2) examine how immunological differences between males and females contribute to dimorphic disease pathogenesis and responses to vaccines; and 3) evaluate how the efficacy of antiviral drug treatments differs between the sexes. The contribution of gender-related differences in access to, compliance with, and acceptance of treatments for viral diseases will be addressed where data exist; the primary focus throughout this review, however, will be on the biological differences between males and females and how these sex differences contribute to differential responses to treatments for disease. All too often sex differences are either ignored or understudied in clinical and basic biomedical research. As a result, significant gaps exist in our understanding of how the profound biological differences between males and females affect the efficacy of treatments for viral infections.
Innate and adaptive immune responses differ between the sexes
Males and females differ in their innate immune responses to viruses (Figure 1). Innate detection of viral and bacterial nucleic acids by pattern recognition receptors (PRRs) differs between the sexes [3,4]. There are differences between the sexes in the induction of genes associated with toll-like receptor (TLR) pathways and antiviral type I interferon (IFN) responses [1,5,6]; cells from females can show a 10-fold greater level of expression than cells from males [6]. Studies of both humans and rodents illustrate that the number and activity of innate immune cells, including monocytes, macrophages, and dendritic cells (DCs) as well as inflammatory immune responses in general are higher in females than males [7–9].
In rodents and humans, females exhibit greater humoral and cell-mediated immune responses to antigenic stimulation, vaccination, and infection than do males (Figure 1)[6]. Both basal levels of immunoglobulin [10] as well as antibody responses to viruses and vaccines are consistently higher in females than males [6,11,12]. Clinical studies reveal that males have lower CD3+ and CD4+ cell counts, CD4+: CD8+ cell ratios, and inflammatory helper T cell type 1 (Th1) responses than females[13–16]. Studies in mice further reveal that cytokine responses of CD4+ T cells often differ between males and females with females reportedly exhibiting higher inflammatory Th1 (i.e., IFN-γ), anti-inflammatory Th2 (i.e., IL-4), and regulatory T cell (i.e., IL-10) responses than males, depending on stage of infection or type of antigen encountered [17–20]. Female mice also have higher proportions of regulatory T cells than males, at least in response to certain viruses [21]. Females exhibit higher cytotoxic T cell activity along with upregulated expression of antiviral and proinflammatory genes, many of which have estrogen response elements in their promoters[22].
The prevailing hypothesis for immunological differences between the sexes is that sex steroids, particularly testosterone (T), estradiol (E2), and progesterone (P4), influence the functioning of immune cells (Figure 2). Sex steroids alter the functioning of immune cells by binding to specific receptors, expressed in many immune cells, including lymphocytes, macrophages, and DCs[23]. The binding of sex steroids to their respective steroid receptors directly influences cell signaling pathways, including NF-κB, cJun, and interferon regulatory factor (IRF) 1, resulting in differential production of cytokines and chemokines[23].
Although direct effects of gonadal steroids cause many sex differences in immune function, some sex differences might be caused by the inherent imbalance in the expression of genes encoded on the X and Y chromosomes [24]. Several immune-related genes are encoded on the X chromosome and there is some evidence of greater activation of X-linked genes in immune cells from females than males [25]. The expression of X-linked genes might be affected by X-linked microRNAs (miRNAs), which are small noncoding RNAs that regulate gene expression at a post-transcriptional level and play a role in maintaining immunological homeostasis[26]. There are disproportionately more miRNAs located on the X chromosome than on any autosomal chromosome [26]. Sex chromosomal complement (i.e., XX or XY) can also differentially affect susceptibility to some autoimmune diseases and viral infections [27,28]. Lastly, polymorphisms in sex chromosomal and autosomal genes that encode for immunological proteins can contribute to sex differences in immune responses [29], antibody responses to vaccination [30], and susceptibility to infection with viruses, including human immunodeficiency virus (HIV)[31].
The responses to and outcome of viral infection, antiviral treatment, and vaccination differ between the sexes
1. HIV load is higher in males and adverse reactions to antiretroviral therapy is greater in females
HIV pathogenesis
Human immunodeficiency virus replication exhibits a sexually dimorphic pattern. HIV RNA levels are consistently lower in women than men [32]. A meta-analysis of published studies revealed that women have approximately 41% less HIV RNA in circulation than do men [32]. In addition to having lower HIV loads, women have higher antiviral responses to HIV than men. Plasmacytoid DCs (pDCs)are significant initiators of antiviral immunity and these cells produce more IFN-α in response to HIV-1 encoded TLR7 ligands when derived from women compared with men [1]. The higher pDC responses appear to result in higher levels of CD8+ T cell activation in women than men[1]. HIV-infected women also have higher baseline CD4+ T cell counts than males [33]. Progesterone can modulate the function of pDCs and women with higher plasma P4 concentrations have greater numbers of IFN-α producing pDCs in response to the HIV TLR7 ligand than women with lower P4 concentrations [1]. Use of P4-based hormone contraceptives also is associated with increased acquisition of HIV-1, loss of CD4+ T cells, disease progression, and death rates among women [34].
In men, HIV infection causes hypogonadism (i.e., reduced androgen concentrations), which is associated with wasting syndrome, loss of bone mass, and depression [35]. Treatment of patients with anabolic steroids improves muscle mass, bone density, and quality of life in both men and women [35]; the immunological consequence of androgen treatment, however, has not been reported. In parallel with reduced androgen concentrations, estrone and E2 concentrations increase with the progression of HIV in men[36]. Consequently, E2 augments transcription of HIV in vitro, and this effect can be reversed by exposure to an estrogen receptor (ER) antagonist [37].
Antivirals
Women are less likely to receive ART and there is some indication that initiation of antiretroviral therapies (ART) is lower in women than men, but this observation may involve racial and geographic variables as well [33,38]. There is growing knowledge that women experience more adverse reactions to antiretroviral drugs, including nonnucleoside reverse transcriptase inhibitors and protease inhibitors, than men [39](Table 2). In particular, treatment with antiretroviral drugs results in more frequent rashes, lactic acidosis, gastrointestinal intolerance, metabolic disorders, and abnormal fat distribution in women[39,40]. As a result of more frequent adverse reactions to antiretroviral drugs, women are more likely than men to reduce drug dosage or discontinue use [41]. To illustrate the magnitude of this problem, in one trial of nevirapine, a nonnucleoside reverse transcriptase inhibitor, women were 7 times more likely to develop a rash and 3–5 times more likely to discontinue drug therapy than men [42]. With antiretroviral therapies containing protease inhibitors, gastrointestinal intolerance is 2–3 times higher among women than men [40]. The mechanisms that result in greater adverse reactions to antiretroviral drugs in women compared with men are not known. However, they might include body mass, fat distribution, drug metabolism, drug bioavailability, and gastric motility [39]. Pharmacokinetic studies suggest that antiretroviral drugs, including zidovudine and lamivudine triphosphate, reach higher intracellular concentrations in women than men, which might explain the higher rate of antiretroviral drug toxicity in women [43]. Disease progression, however, is reportedly 4 times lower for women than men on antiretroviral therapy [41]. Virologic suppression also is more rapid in women than men on antiretroviral drug therapy [44]. Future studies must determine how to reduce the adverse reactions and still maintain low disease progression and viral loads in HIV-infected women on ART. There also is interest, but no available data, in the role that sex hormones play in modulating the pharmacokinetics of antiretroviral drugs in women and men [39].
Table 2.
Sex differences in adverse reactions, immune responses, and efficacy of vaccines and antiviral drugs in humans1.
| Virus | Antiviral drug/vaccine | Sex-specific differences | Comments | References |
|---|---|---|---|---|
| HIV | HAART | M < F | CD4+ T cell count; adverse reactions; fat accumulation; drug concentration; virus clearance; hepatitis | [40,42,43,113–115] |
| HAART | M > F | Fat loss; survival | [114,116] | |
| HSV-2 | HSV-2 gD vaccine | M < F | Humoral immune responses; cell-mediated immune responses; vaccine efficacy | [47,117,118] |
| Acyclovir | M < F | Frequency of prescription; adverse reaction | [59,60] | |
| Acyclovir | M > F | Reduction of virus shedding | [59] | |
| HBV | HBV vaccine | M < F | Humoral immune responses | [78,79,81] |
| HCV | pegylated interferon alpha/ribavirin | M < F | Adverse reaction; sustained virologic response2 | [83–85] |
| Seasonal Influenza viruses | TIV vaccine | M < F | Humoral immune responses; adverse reactions | [99,119] |
| Oseltamivir | M < F | Drug clearance and metabolism3 | [105] | |
| Oseltamivir | M > F | Alleviation of symptoms; reduction of viral load | [104] | |
| Zanamivir | M = F | Alleviation of symptoms; reduction of viral load | [104] |
Abbreviations: HAART: highly-active antiretroviral therapy; HBV: hepatitis B virus; HCV: hepatitis C virus; HIV: human immunodeficiency virus; HSV: herpes simplex virus; TIV: trivalent inactivated influenza virus
premenopausal females only
tested in neonates only
There have been several trials of candidate HIV vaccines, all of which enrolled both women and men, but none of which have adequately analyzed sex differences in the immunogenicity, adverse events, or prevention of HIV-1 by vaccination [45,46]. The STEP Study, for example, which evaluated the efficacy of a cell-mediated immunity vaccine, reported that antibody responses to the vaccine were similar between the sexes, but did not show these data [46]. The same study also did not report whether adverse events or infection following vaccination differed between the sexes. Based on data from trials of herpes simplex virus (HSV) vaccines[47], not partitioning and analyzing data by sex can be a major flaw in study design.
2. Susceptibility to HSV, responses to vaccines, and the efficacy of antiviral treatments differ between the sexes
HSV pathogenesis
Herpes simplex virus-type 2 (HSV-2)infects the reproductive tract and is influenced by E2 and P4. The prevalence of HSV-2 typically is higher in women than men [48]. In HSV-2 seropositive women, oral contraceptive use is associated with increased genital tract shedding of HSV-2 [49]. Ex vivo E2 treatment of primary genital epithelial cells co-cultured with stromal cells increases HSV-2 shedding, whereas pretreatment of cells with P4 decreases HSV-2 shedding [50]. In female mice, susceptibility to HSV-2 varies with stage of the estrous cycle [51]. High concentrations of P4 are associated with reduced survival, increased viral titers in the vagina, inflammation, infiltration of neutrophils, and the expression of chemokines and chemokine receptors in vaginal tissue from mice [52]. Conversely, administration of E2 increases survival and reduces signs of inflammation and vaginal pathology during primary HSV-2 infection, at least in mice[52].
Mortality rates following exposure to HSV-type 1 (HSV-1) also differ between the sexes. Male mice exhibit more severe pathology following corneal infection and are more likely to die from infection than are females[53]. Treatment of female mice with dihydrotestosterone prior to corneal infection significantly worsens periocular eye disease, but not corneal disease[53]. Intraperitoneal infection of mice with HSV-1 results in higher virus loads in the central nervous system and greater dissemination of virus to peripheral tissues, including the gonads, in females compared with males [54]. Intraperitoneal infection with HSV-1 also results in greater mortality in females than males; an outcome that associated with signaling through DAP12, a molecule that negatively regulates TLR signaling and antiviral responses to HSV-1 [55].
Vaccines and antivirals
Responses to vaccines against HSV-2 differ between the sexes. The HSV-2 vaccine provides protection against the development of symptoms associated with genital herpes in women, but not in men [56]. For example, in phase 1 and 2 studies of a recombinant glycoprotein D (gD)-based HSV-2 vaccine, there was no significant protection from acquisition of HSV-2 infection in HSV-1 and HSV-2 seronegative subjects when data were combined for males and females (overall efficacy 38%). When data were partitioned by sex, a significant sex-bias in protection was observed, in which the efficacy was 73% in females and only 11% in males [47].
In ovariectomized female mice, immunization with an attenuated strain of HSV-2 only protects against challenge with wild-type HSV-2 when females are treated with P4, but not E2. Progesterone, administered either alone or in combination with E2, reduces HSV-2 replication in the reproductive tract by increasing the number of DCs and T cells in the vaginal lamina propria and increasing titers of gB-specific vaginal IgA [57]. Cessation of E2 treatment for 5 days, but not 1–3 days, prior to challenge with HSV-2 can increase protection in ovariectomized mice suggesting that the effects of E2 can be reversed and are dependent on E2 clearance[58]. Immunization of female mice with regular estrous cycles with a recombinant adenovirus vector expressing HSV gB results in higher titers of gB-specific vaginal IgA during estrus than during either diestrus or proestrus and exogenous administration of P4 to female mice at the time of immunization protects females from lethal intravaginal HSV-2 challenge [51]. These data indicate that sex steroids affect induction of protective immunity following vaccination against HSVs.
Despite significant differences between males and females in the prevalence, shedding, and healing of lesions caused by HSV [48,59], there is a paucity of data pertaining to sex differences in the efficacy of antiviral drugs (Table 2). Acyclovir (Zovirax) is a synthetic nucleoside analogue administered orally or topically to reduce pain and increase healing of herpes sores and blisters. The available data suggest that topical treatment of HSV-2 sores with acyclovir reduces virus shedding in male, but not female, patients [59]. In some regions of the world, oral acyclovir is prescribed more frequently for female than male HSV patients [60]. Considerably more research is required to determine whether the efficacy of acyclovir treatment consistently differs between the sexes. From the available data, vaccines may be more efficacious in females, whereas antiviral drugs may be more effective in males at reducing symptoms of HSV disease.
3. The outcome of hepatitis B and C virus (HBV and HCV) infections, responses to vaccines, and the efficacy of antiviral treatments differ between the sexes
HBV/HCV pathogenesis
Hepatitis B and C viruses cause chronic infections and are a major risk factor for the development of liver cancer and hepatocellular carcinoma. The prevalence of serum HBV surface antigen (HBsAg) is consistently higher in men than in women [61]. Male sex also is an independent factor associated with elevated HBV DNA titers [62]. Development of hepatocellular carcinoma occurs at a 2:1 to 4:1 ratio of males to females [63]. Increased rates of exposure to HBV in males do not completely explain why men are more likely to develop liver cancer than women. Among HBsAg positive individuals, males are more than twice as likely to experience mortality from liver cancer as are females[64]. Men might be more sensitive to the effect of HBV infection on the development of liver cancer [64].
Hormones mediate sex differences in susceptibility to liver cancer following infection with HBV. Among HBsAg positive men, elevated concentrations of T and the expression of certain androgen receptor (AR) gene alleles (SRD5A2 and V89L) correlate with increased risk of hepatocellular carcinoma [65]. The development of chemically-induced hepatocellular carcinoma is delayed in AR male knockout mice as compared with wild-type male mice [66]. In HBV transgenic mice, castration of males reduces serum HBsAg concentrations; replacement of Tin castrated males increases serum HBsAg concentrations [67]. The effect of androgens on HBV is mediated by the AR because male Tfm mice (i.e., mice with a mutation in the AR) do not show elevated concentrations of HBsAg as do wild-type males following HBV infection [68]. One mechanism by which androgens affect HBV replication is through direct binding to androgen response elements that have been identified in the enhancer I of HBV [64]. In addition to direct modulation of virus transcription, hormones can alter host immune responses to infection. For example, chemically-induced hepatocellular carcinoma is more severe in male than female mice—an effect that is ascribed to the greater IL-6 production by Kupffer cells in the livers of male mice[69]. These studies further reveal that E2 reduces the synthesis of IL-6 by Kupffer cells through inhibition of Myd 88-dependent induction of NF-κB [69]. Thus, sex steroids modulate sex differences in the prevalence of HBV and development of liver cancer through effects on the transcription of virus and host immune responses to HBV.
For HCV, injection drug use is the single most important risk factor for acquiring HCV. Male sex is an independent risk factor for HCV prevalence as measured by either antibody or detection of HCV RNA [70]. The odds ratio of being HCV positive is 3.47 (95% confidence interval 2.48–4.87) for males compared with females [71]. Females are more likely to spontaneously clear HCV than males [72]. When risk factors, such as injection drug use, are considered, then females are at an increased risk of exposure to HCV because they appear to be more like to share needles and other drug equipment than males[73]. In people chronically infected with HCV, the risk of developing cirrhosis is higher for males than females [74]. The time to onset of cirrhosis also is shorter for males than females by 8–11 years [74]. Sex differences in chronic HCV disease appear to be mitigated after menopause; postmenopausal women show accelerated rates of cirrhosis and fibrotic progression, which can be reversed by hormone replacement therapy [75]. Genetic variation can impact the outcome of HCV infection. Male HCV patients are more likely to carry detrimental IL-6 promoter polymorphisms associated with development of chronic HCV infection [76]. CTLA4 is an inhibitory T cell receptor. Certain polymorphisms in Ctla4 are associated with resolution of HCV infection and are more common in women than men[77].
Vaccines and antivirals
Following vaccination against HBV, among both children and adults, anti-HBV antibody titers are higher in females than males [78–80]. In multivariate analyses, being male is a significant predictor of being ‘non-responsive’ to the HBV vaccine; thus, adult females show higher rates of seroconversion following exposure to the HBV vaccine than do males [81]. Greater efficacy of the HBV vaccine in females also may contribute to reduced prevalence of HBV and development of liver cancer among females compared with males.
HCV infections are treated with antiviral drugs. Sex differences in the efficacy and responses to antiviral treatment, including the standard combined treatment with pegylated interferon alpha and ribavirin, are only consistently observed between men and premenopausal women (Table 2). Among patients of reproductive ages, females experience more adverse reactions (e.g., anemia, weight loss, nausea, vision impairment, reduced thyroid function, and infections) to the combined antiviral drug treatment, are more likely to modify the doses of the antiviral drugs, and are more likely to interrupt or suspend treatment than males [82,83]. In HCV patients of reproductive ages who maintain at least 80% of the planned antiviral drug doses, the sustained virologic response is greater for females than males [84]. Sex differences in the sustained virologic response to antiviral therapy are not apparent in analyses that include women who have entered menopause [85]. Among HCV-infected women who have entered menopause, baseline liver inflammation, fibrosis, and proinflammatory cytokine concentrations are significantly elevated as compared with premenopausal women and might explain why antiviral therapy is less effective following menopause [85].
4. The outcome of Influenza A virus infection, responses to vaccines, and the efficacy of oral antiviral treatments differ between the sexes
Influenza pathogenesis
Sex differences in the incidence influenza A viruses have been documented in humans[86]. Although exposure rates are often higher in men, fatality following exposure to pathogenic influenza A viruses is reportedly higher in women[86]. Hospitalization with severe disease from 2009 H1N1 was higher in young women[87], with data from Canada and the US indicating that females had an over 2-fold higher risk of death than males [88,89]. In addition to pregnancy, which is a critical factor associated with increased severity of disease, many cases involve comorbid conditions, including chronic lung disease (e.g., asthma), which is typically more severe in females than males[90].
Although sex differences in the incidence of influenza virus infection may reflect differences in exposure to these viruses, differential disease severity between the sexes might involve biological differences in response to infection. The extent to which immune responses differ between males and females during influenza virus infection requires assessment as this might contribute to differential severity of disease between the sexes. Disease associated with highly pathogenic influenza viruses is hypothesized to be mediated by the profound proinflammatory cytokine and chemokine response (referred to as the ‘cytokine storm’) initiated by the host in response to infection [91]. Humans, macaques, and mice infected with highly pathogenic strains of influenza virus, including the 1918 H1N1 or avian H5N1, produce excessively high concentrations of proinflammatory cytokines and chemokines, which correlate with elevated mortality [91–93]. Studies in mice reveal that adult females experience greater morbidity and mortality in response to H1N1 infection than males and this appears to be mediated, not by altered levels of virus replication, but by greater pulmonary proinflammatory immune responses[2]. Administration of E2 or an ERα agonist to females reduces virus-induced lung proinflammatory immune responses, morbidity, and mortality[2]. Elevated immunity in females against influenza A viruses might represent a delicate balance between immune responses conferring protection through clearance of virus or causing pathology through increased production of proteins and an influx of immune cells into the lungs.
Vaccines and antivirals
In addition to influenza virus pathogenesis, males and females differ in response to influenza virus vaccines(Table 2). Rates of immunization against influenza are reportedly either similar between the sexes or lower in women [94,95] and may be influenced by greater negative beliefs about the risks of vaccination [96] and lower acceptance of vaccines [97,98] among women. Antibody responses to the seasonal trivalent inactivated vaccines (TIV) are higher in women than men [12,99]. Whether antibody responses to the live attenuated influenza vaccine differ between the sexes has not been reported, to date. Women also report more frequent and severe local and systemic reactions to the seasonal TIV than men[99].
As is the case in women [12,99], female mice mount higher neutralizing and total antibody responses against a sublethal primary infection/vaccination with influenza viruses than males[100]. Following vaccination, female mice are better protected against lethal challenge with heterosubtypic (i.e., novel) strains of influenza viruses than males[100]. Although elevated immunity afforded females greater protection than males against lethal challenge with heterosubtypic viruses, both sexes are equally protected against lethal challenge with homologous virus (i.e., the strain of virus in the vaccine) [100]. Estradiol, at physiological concentrations, can stimulate antibody production by B cells [101], including antibody responses to an inactivated influenza vaccine administered in mice [102].
Following infection, neuraminidase inhibitors can be administered to alleviate symptoms of disease and virus shedding[103]. Oseltamivir (Tamiflu) is administered orally, absorbed in the gastrointestinal tract, and converted to the active metabolite, oseltamivir carboxylate, by an esterase in the liver [103]. Zanamivir (Relenza) is an inhaled powder delivered as the active compound directly into the respiratory tract [103]. In patients with confirmed influenza A virus infection and treated with oseltamivir, alleviation of symptoms of disease is faster and the reduction of nasal virus load is greater among males than females [104]. In contrast, in influenza A virus-infected patients treated with zanamivir, no sex differences in either alleviation of symptoms or virus load are observed, suggesting that male-female differences in drug absorption or metabolism may contribute to the dimorphic outcome of treatment with oseltamivir, but not zanamivir [104]. Data also suggest that females clear oseltamivir more rapidly than males, at least in newborns [105]. Male-female differences in the outcome of oseltamivir treatment do not appear to be due to gender differences in treatment compliance [104]. Future clinical studies must continue to partition and analyze antiviral outcome data by sex and establish the biological mechanisms mediating how oseltamivir is more effective in males than females.
Conclusions and clinical implications
Adverse reactions to both vaccines and antiviral drug treatments are consistently greater for females than males (Table 2). Male/female differences in the pharmacological effects of antiviral drugs do not solely involve differences in body mass or fat distribution, but also involve differences in the pharmacokinetics and pharmacodynamics of these drugs. There is growing evidence that the absorption, metabolism, and clearance of antiviral drugs differ between the sexes. The importance of sex differences in pharmacokinetics is highlighted by the observation that the efficacy of anti-influenza drugs that are processed and metabolized in the liver result in sex differences in clinical and virological responses, whereas the efficacy of anti-influenza drugs delivered directly to the lungs is not significantly different between the sexes [104]. Sex differences in antiviral drug delivery should be further evaluated in other systems.
The beneficial effects of prophylaxis and therapeutic treatments for viral disease might differ by sex. For example, antiviral treatments for infection with HSVs or influenza A viruses result in better clinical and virological outcomes in males. In contrast, vaccines against these viruses are more efficacious in females. These observations are based on a limited number of studies; thus, restraint must be shown before drawing strong conclusions. There is a significant need for additional basic biomedical research in this area.
Sex steroid hormones influence the outcome of infection and the efficacy of antiviral drug treatments and possibly vaccines. Future studies should continue to consider the age and reproductive status of females as well as whether females are using exogenous hormones (either through contraceptives or replacement therapy) at the time of infection, drug treatment, or both. The observation that sex differences in the outcome of infection and in responses to antiviral treatments for hepatitis depend on the hormonal status, and not merely the age, of women [85]is an important observation that should be pursued for other diseases [39]. Additionally, whether the hormonal milieu at the time of vaccination influences immune responses and long-term protection against disease should be examined.
The recommendation of funding agencies, universities, and journals should be that clinicians, epidemiologists, and basic biomedical scientists design experiments that include both males and females, develop a priori hypotheses that the sexes will differ in their responses to and the outcome of infection and treatments, and statistically analyze outcome data by sex. The end goal should be that clinicians and researchers alike consider the sex of their patients or animals when designing and administering treatments for viral diseases because the outcomes will likely differ.
Acknowledgments
NIH AI079342 and AI090344 and a Medtronic SWHR award provided support for this work.
Abbreviations
- E2
estradiol
- HIV
human immunodeficiency virus
- HSV
herpes simplex virus
- HBV/HCV
hepatitis B or C virus
- P4
progesterone
- T
testosterone
Footnotes
No conflict of interest declared.
References
- 1.Meier A, Chang JJ, Chan ES, Pollard RB, Sidhu HK, et al. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med. 2009;15:955–9. doi: 10.1038/nm.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robinson DP, Lorenzo ME, Jian W, Klein SL. Elevated 17 beta-estradiol protects females from influenza a virus pathogenesis by suppressing inflammatory responses. PLoS Pathog. 2011;7:e1002149. doi: 10.1371/journal.ppat.1002149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berghofer B, Frommer T, Haley G, Fink L, Bein G, et al. TLR7 ligands induce higher IFN-alpha production in females. J Immunol. 2006;177:2088–96. doi: 10.4049/jimmunol.177.4.2088. [DOI] [PubMed] [Google Scholar]
- 4.Marriott I, Huet-Hudson YM. Sexual dimorphism in innate immune responses to infectious organisms. Immunol Res. 2006;34:177–92. doi: 10.1385/IR:34:3:177. [DOI] [PubMed] [Google Scholar]
- 5.Hannah MF, Bajic VB, Klein SL. Sex differences in the recognition of and innate antiviral responses to Seoul virus in Norway rats. Brain Behav Immun. 2008;22:503–16. doi: 10.1016/j.bbi.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein SL, Jedlicka A, Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect Dis. 2010;10:338–49. doi: 10.1016/S1473-3099(10)70049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boissier J, Chlichlia K, Digon Y, Ruppel A, Mone H. Preliminary study on sex-related inflammatory reactions in mice infected with Schistosoma mansoni. Parasitol Res. 2003;91:144–50. doi: 10.1007/s00436-003-0943-1. [DOI] [PubMed] [Google Scholar]
- 8.Xia HJ, Zhang GH, Wang RR, Zheng YT. The influence of age and sex on the cell counts of peripheral blood leukocyte subpopulations in Chinese rhesus macaques. Cell Mol Immunol. 2009;6:433–40. doi: 10.1038/cmi.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melgert BN, Oriss TB, Qi Z, Dixon-McCarthy B, Geerlings M, et al. Macrophages: regulators of sex differences in asthma? Am J Respir Cell Mol Biol. 2010;42 :595–603. doi: 10.1165/rcmb.2009-0016OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butterworth M, McClellan B, Allansmith M. Influence of sex in immunoglobulin levels. Nature. 1967;214:1224–5. doi: 10.1038/2141224a0. [DOI] [PubMed] [Google Scholar]
- 11.Klein SL, Huber S. Sex differences in susceptibility to viral infection. In: Klein SL, Roberts CW, editors. Sex hormones and immunity to infection. Berlin: Springer-Verlag; 2010. pp. 93–122. [Google Scholar]
- 12.Cook IF. Sexual dimorphism of humoral immunity with human vaccines. Vaccine. 2008;26 :3551–5. doi: 10.1016/j.vaccine.2008.04.054. [DOI] [PubMed] [Google Scholar]
- 13.Wikby A, Mansson IA, Johansson B, Strindhall J, Nilsson SE. The immune risk profile is associated with age and gender: findings from three Swedish population studies of individuals 20–100 years of age. Biogerontology. 2008;9:299–308. doi: 10.1007/s10522-008-9138-6. [DOI] [PubMed] [Google Scholar]
- 14.Villacres MC, Longmate J, Auge C, Diamond DJ. Predominant type 1 CMV-specific memory T-helper response in humans: evidence for gender differences in cytokine secretion. Hum Immunol. 2004;65:476–85. doi: 10.1016/j.humimm.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 15.Amadori A, Zamarchi R, De Silvestro G, Forza G, Cavatton G, et al. Genetic control of the CD4/CD8 T-cell ratio in humans. Nat Med. 1995;1:1279–83. doi: 10.1038/nm1295-1279. [DOI] [PubMed] [Google Scholar]
- 16.Das BR, Bhanushali AA, Khadapkar R, Jeswani KD, Bhavsar M, et al. Reference ranges for lymphocyte subsets in adults from western India: influence of sex, age and method of enumeration. Indian J Med Sci. 2008;62:397–406. [PubMed] [Google Scholar]
- 17.Roberts CW, Walker W, Alexander J. Sex-associated hormones and immunity to protozoan parasites. Clin Microbiol Rev. 2001;14:476–88. doi: 10.1128/CMR.14.3.476-488.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Araneo BA, Dowell T, Diegel M, Daynes RA. Dihydrotestosterone exerts a depressive influence on the production of interleukin-4 (IL-4), IL-5, and gamma-interferon, but not IL-2 by activated murine T cells. Blood. 1991;78:688–99. [PubMed] [Google Scholar]
- 19.Barrat F, Lesourd B, Boulouis HJ, Thibault D, Vincent-Naulleau S, et al. Sex and parity modulate cytokine production during murine ageing. Clin Exp Immunol. 1997;109:562–8. doi: 10.1046/j.1365-2249.1997.4851387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinzan CF, Ruas LP, Casabona-Fortunato AS, Carvalho FC, Roque-Barreira MC. Immunological basis for the gender differences in murine Paracoccidioides brasiliensis infection. PLoS ONE. 2010;5:e10757. doi: 10.1371/journal.pone.0010757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frisancho-Kiss S, Davis SE, Nyland JF, Frisancho JA, Cihakova D, et al. Cutting edge: cross-regulation by TLR4 and T cell Ig mucin-3 determines sex differences in inflammatory heart disease. J Immunol. 2007;178:6710–4. doi: 10.4049/jimmunol.178.11.6710. [DOI] [PubMed] [Google Scholar]
- 22.Hewagama A, Patel D, Yarlagadda S, Strickland FM, Richardson BC. Stronger inflammatory/cytotoxic T-cell response in women identified by microarray analysis. Genes Immun. 2009;10:509–16. doi: 10.1038/gene.2009.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kovats S, Carreras E, Agrawal H. Sex steroid receptors in immune cells. In: Klein SL, Roberts CW, editors. Sex hormones and immunity to infection. Berlin: Springer-Verlag; 2010. pp. 53–92. [Google Scholar]
- 24.Arnold AP, Chen X. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol. 2009;30:1–9. doi: 10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stamova B, Tian Y, Jickling G, Bushnell C, Zhan X, et al. The X-Chromosome Has a Different Pattern of Gene Expression in Women Compared With Men With Ischemic Stroke. Stroke. 2011 doi: 10.1161/STROKEAHA.111.629337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pinheiro I, Dejager L, Libert C. X-chromosome-located microRNAs in immunity: might they explain male/female differences? The X chromosome-genomic context may affect X-located miRNAs and downstream signaling, thereby contributing to the enhanced immune response of females. BioEssays. 2011;33:791–802. doi: 10.1002/bies.201100047. [DOI] [PubMed] [Google Scholar]
- 27.Smith-Bouvier DL, Divekar AA, Sasidhar M, Du S, Tiwari-Woodruff SK, et al. A role for sex chromosome complement in the female bias in autoimmune disease. J Exp Med. 2008;205:1099–108. doi: 10.1084/jem.20070850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson DP, Huber SA, Moussawi M, Roberts B, Teuscher C, et al. Sex chromosome complement contributes to sex differences in Coxsackievirus B3 but not Influenza A virus pathogenesis. Biol Sex Differ. 2011;2:8. doi: 10.1186/2042-6410-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poland GA, Ovsyannikova IG, Jacobson RM. Personalized vaccines: the emerging field of vaccinomics. Expert Opin Biol Ther. 2008;8:1659–67. doi: 10.1517/14712598.8.11.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gordeeva LA, Shabaldin AV, Semenova EM, Glushkov AN. Influence of genetic and phenotypical factors on the efficiency of the vaccination of young children against diphtheria and measles. Zh Mikrobiol Epidemiol Immunobiol. 2006:42–6. [PubMed] [Google Scholar]
- 31.Siddiqui RA, Sauermann U, Altmuller J, Fritzer E, Nothnagel M, et al. X chromosomal variation is associated with slow progression to AIDS in HIV-1-infected women. Am J Hum Genet. 2009;85:228–39. doi: 10.1016/j.ajhg.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Napravnik S, Poole C, Thomas JC, Eron JJ., Jr Gender difference in HIV RNA levels: a meta-analysis of published studies. J Acquir Immune Defic Syndr. 2002;31:11–9. doi: 10.1097/00126334-200209010-00002. [DOI] [PubMed] [Google Scholar]
- 33.Meditz AL, MaWhinney S, Allshouse A, Feser W, Markowitz M, et al. Sex, race, and geographic region influence clinical outcomes following primary HIV-1 infection. J Infect Dis. 2011;203:442–51. doi: 10.1093/infdis/jiq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hel Z, Stringer E, Mestecky J. Sex steroid hormones, hormonal contraception, and the immunobiology of human immunodeficiency virus-1 infection. Endocr Rev. 2010;31 :79–97. doi: 10.1210/er.2009-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grinspoon S. Androgen deficiency and HIV infection. Clin Infect Dis. 2005;41:1804–5. doi: 10.1086/498320. [DOI] [PubMed] [Google Scholar]
- 36.Teichmann J, Schmidt A, Lange U, Stracke H, Discher T, et al. Longitudinal evaluation of serum estradiol and estrone in male patients infected with the human immunodeficiency virus. Eur J Med Res. 2003;8:77–80. [PubMed] [Google Scholar]
- 37.Katagiri D, Hayashi H, Victoriano AF, Okamoto T, Onozaki K. Estrogen stimulates transcription of human immunodeficiency virus type 1 (HIV-1) Int Immunopharmacol. 2006;6:170–81. doi: 10.1016/j.intimp.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 38.Gebo KA, Fleishman JA, Conviser R, Reilly ED, Korthuis PT, et al. Racial and gender disparities in receipt of highly active antiretroviral therapy persist in a multistate sample of HIV patients in 2001. J Acquir Immune Defic Syndr. 2005;38:96–103. doi: 10.1097/00126334-200501010-00017. [DOI] [PubMed] [Google Scholar]
- 39.Ofotokun I. Sex differences in the pharmacologic effects of antiretroviral drugs: potential roles of drug transporters and phase 1 and 2 metabolizing enzymes. Top HIV Med. 2005;13:79–83. [PubMed] [Google Scholar]
- 40.Ofotokun I, Pomeroy C. Sex differences in adverse reactions to antiretroviral drugs. Top HIV Med. 2003;11:55–9. [PubMed] [Google Scholar]
- 41.Currier JS, Spino C, Grimes J, Wofsy CB, Katzenstein DA, et al. Differences between women and men in adverse events and CD4+ responses to nucleoside analogue therapy for HIV infection. The Aids Clinical Trials Group 175 Team. J Acquir Immune Defic Syndr. 2000;24:316–24. doi: 10.1097/00126334-200008010-00003. [DOI] [PubMed] [Google Scholar]
- 42.Bersoff-Matcha SJ, Miller WC, Aberg JA, van Der Horst C, Hamrick HJ, Jr, et al. Sex differences in nevirapine rash. Clin Infect Dis. 2001;32:124–9. doi: 10.1086/317536. [DOI] [PubMed] [Google Scholar]
- 43.Anderson PL, Kakuda TN, Kawle S, Fletcher CV. Antiviral dynamics and sex differences of zidovudine and lamivudine triphosphate concentrations in HIV-infected individuals. AIDS. 2003;17:2159–68. doi: 10.1097/00002030-200310170-00003. [DOI] [PubMed] [Google Scholar]
- 44.Moore AL, Mocroft A, Madge S, Devereux H, Wilson D, et al. Gender differences in virologic response to treatment in an HIV-positive population: a cohort study. J Acquir Immune Defic Syndr. 2001;26:159–63. doi: 10.1097/00042560-200102010-00008. [DOI] [PubMed] [Google Scholar]
- 45.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–20. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 46.Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–93. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stanberry LR, Spruance SL, Cunningham AL, Bernstein DI, Mindel A, et al. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N Engl J Med. 2002;347:1652–61. doi: 10.1056/NEJMoa011915. [DOI] [PubMed] [Google Scholar]
- 48.Wald A. Herpes simplex virus type 2 transmission: risk factors and virus shedding. Herpes. 2004;11(Suppl 3):130A–7A. [PubMed] [Google Scholar]
- 49.Cherpes TL, Melan MA, Kant JA, Cosentino LA, Meyn LA, et al. Genital tract shedding of herpes simplex virus type 2 in women: effects of hormonal contraception, bacterial vaginosis, and vaginal group B Streptococcus colonization. Clin Infect Dis. 2005;40:1422–8. doi: 10.1086/429622. [DOI] [PubMed] [Google Scholar]
- 50.MacDonald EM, Savoy A, Gillgrass A, Fernandez S, Smieja M, et al. Susceptibility of human female primary genital epithelial cells to herpes simplex virus, type-2 and the effect of TLR3 ligand and sex hormones on infection. Biol Reprod. 2007;77 :1049–59. doi: 10.1095/biolreprod.107.063933. [DOI] [PubMed] [Google Scholar]
- 51.Gallichan WS, Rosenthal KL. Effects of the estrous cycle on local humoral immune responses and protection of intranasally immunized female mice against herpes simplex virus type 2 infection in the genital tract. Virology. 1996;224:487–97. doi: 10.1006/viro.1996.0555. [DOI] [PubMed] [Google Scholar]
- 52.Gillgrass AE, Fernandez SA, Rosenthal KL, Kaushic C. Estradiol regulates susceptibility following primary exposure to genital herpes simplex virus type 2, while progesterone induces inflammation. J Virol. 2005;79:3107–16. doi: 10.1128/JVI.79.5.3107-3116.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Han X, Lundberg P, Tanamachi B, Openshaw H, Longmate J, et al. Gender influences herpes simplex virus type 1 infection in normal and gamma interferon-mutant mice. J Virol. 2001;75:3048–52. doi: 10.1128/JVI.75.6.3048-3052.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burgos JS, Ramirez C, Sastre I, Alfaro JM, Valdivieso F. Herpes simplex virus type 1 infection via the bloodstream with apolipoprotein E dependence in the gonads is influenced by gender. J Virol. 2005;79:1605–12. doi: 10.1128/JVI.79.3.1605-1612.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Geurs TL, Hill EB, Lippold DM, French AR. Sex differences in murine susceptibility to systemic viral infections. J Autoimmun. 2012;38:J245–53. doi: 10.1016/j.jaut.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stephenson J. Genital herpes vaccine shows limited promise. Jama. 2000;284:1913–4. [PubMed] [Google Scholar]
- 57.Gillgrass AE, Tang VA, Towarnicki KM, Rosenthal KL, Kaushic C. Protection against genital herpes infection in mice immunized under different hormonal conditions correlates with induction of vagina-associated lymphoid tissue. J Virol. 2005;79:3117–26. doi: 10.1128/JVI.79.5.3117-3126.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gillgrass A, Chege D, Bhavanam S, Kaushic C. Estradiol limits viral replication following intravaginal immunization leading to diminished mucosal IgG response and non-sterile protection against genital herpes challenge. Am J Reprod Immunol. 2010;63:299–309. doi: 10.1111/j.1600-0897.2009.00796.x. [DOI] [PubMed] [Google Scholar]
- 59.Reichman RC, Badger GJ, Guinan ME, Nahmias AJ, Keeney RE, et al. Topically administered acyclovir in the treatment of recurrent herpes simplex genitalis: a controlled trial. J Infect Dis. 1983;147:336–40. doi: 10.1093/infdis/147.2.336. [DOI] [PubMed] [Google Scholar]
- 60.Theng TS, Chan RK. Genital herpes in a sexually-transmitted infection clinic in Singapore: a 1-year retrospective study. Ann Acad Med Singapore. 2004;33:200–3. [PubMed] [Google Scholar]
- 61.Tsay PK, Tai DI, Chen YM, Yu CP, Wan SY, et al. Impact of gender, viral transmission and aging in the prevalence of hepatitis B surface antigen. Chang Gung Med J. 2009;32:155–64. [PubMed] [Google Scholar]
- 62.Chen CJ, Yang HI, Su J, Jen CL, You SL, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 63.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–76. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 64.Wang SH, Yeh SH, Lin WH, Wang HY, Chen DS, et al. Identification of androgen response elements in the enhancer I of hepatitis B virus: a mechanism for sex disparity in chronic hepatitis B. Hepatology. 2009;50:1392–402. doi: 10.1002/hep.23163. [DOI] [PubMed] [Google Scholar]
- 65.Yu MW, Cheng SW, Lin MW, Yang SY, Liaw YF, et al. Androgen-receptor gene CAG repeats, plasma testosterone levels, and risk of hepatitis B-related hepatocellular carcinoma. J Natl Cancer Inst. 2000;92:2023–8. doi: 10.1093/jnci/92.24.2023. [DOI] [PubMed] [Google Scholar]
- 66.Ma WL, Hsu CL, Wu MH, Wu CT, Wu CC, et al. Androgen receptor is a new potential therapeutic target for the treatment of hepatocellular carcinoma. Gastroenterology. 2008;135:947–55. 55 e1–5. doi: 10.1053/j.gastro.2008.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Farza H, Salmon AM, Hadchouel M, Moreau JL, Babinet C, et al. Hepatitis B surface antigen gene expression is regulated by sex steroids and glucocorticoids in transgenic mice. Proc Natl Acad Sci U S A. 1987;84:1187–91. doi: 10.1073/pnas.84.5.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Breidbart S, Burk RD, Saenger P. Hormonal regulation of hepatitis B virus gene expression: influence of androgen receptor. Pediatr Res. 1993;34:300–2. doi: 10.1203/00006450-199309000-00012. [DOI] [PubMed] [Google Scholar]
- 69.Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–4. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 70.Burguete-Garcia AI, Conde-Gonzalez CJ, Jimenez-Mendez R, Juarez-Diaz Y, Meda-Monzon E, et al. Hepatitis C seroprevalence and correlation between viral load and viral genotype among primary care clients in Mexico. Salud Publica Mex. 2011;53(Suppl 1):S7–12. [PubMed] [Google Scholar]
- 71.Balogun MA, Vyse AJ, Hesketh LM, Kafatos G, Parry JV, et al. Estimating hepatitis C infection acquired in England, 1986–2000. Epidemiol Infect. 2009;137:1249–54. doi: 10.1017/S0950268809002143. [DOI] [PubMed] [Google Scholar]
- 72.Grebely J, Raffa JD, Lai C, Krajden M, Conway B, et al. Factors associated with spontaneous clearance of hepatitis C virus among illicit drug users. Can J Gastroenterol. 2007;21:447–51. doi: 10.1155/2007/796325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Iversen J, Wand H, Gonnermann A, Maher L. Gender differences in hepatitis C antibody prevalence and risk behaviours amongst people who inject drugs in Australia 1998–2008. Int J Drug Policy. 2010;21:471–6. doi: 10.1016/j.drugpo.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 74.Rodriguez-Torres M, Rios-Bedoya CF, Rodriguez-Orengo J, Fernandez-Carbia A, Marxuach-Cuetara AM, et al. Progression to cirrhosis in Latinos with chronic hepatitis C: differences in Puerto Ricans with and without human immunodeficiency virus coinfection and along gender. J Clin Gastroenterol. 2006;40:358–66. doi: 10.1097/01.mcg.0000210105.66994.dc. [DOI] [PubMed] [Google Scholar]
- 75.Di Martino V, Lebray P, Myers RP, Pannier E, Paradis V, et al. Progression of liver fibrosis in women infected with hepatitis C: long-term benefit of estrogen exposure. Hepatology. 2004;40:1426–33. doi: 10.1002/hep.20463. [DOI] [PubMed] [Google Scholar]
- 76.Cussigh A, Falleti E, Fabris C, Bitetto D, Cmet S, et al. Interleukin 6 promoter polymorphisms influence the outcome of chronic hepatitis C. Immunogenetics. 2011;63 :33–41. doi: 10.1007/s00251-010-0491-7. [DOI] [PubMed] [Google Scholar]
- 77.Schott E, Witt H, Hinrichsen H, Neumann K, Weich V, et al. Gender-dependent association of CTLA4 polymorphisms with resolution of hepatitis C virus infection. J Hepatol. 2007;46:372–80. doi: 10.1016/j.jhep.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 78.Fang JW, Lai CL, Chung HT, Wu PC, Lau JY. Female children respond to recombinant hepatitis B vaccine with a higher titre than male. J Trop Pediatr. 1994;40:104–7. doi: 10.1093/tropej/40.2.104. [DOI] [PubMed] [Google Scholar]
- 79.Hess G, Hingst V, Cseke J, Bock HL, Clemens R. Influence of vaccination schedules and host factors on antibody response following hepatitis B vaccination. Eur J Clin Microbiol Infect Dis. 1992;11:334–40. doi: 10.1007/BF01962073. [DOI] [PubMed] [Google Scholar]
- 80.Bock HL, Kruppenbacher J, Sanger R, Hobel W, Clemens R, et al. Immunogenicity of a recombinant hepatitis B vaccine in adults. Arch Intern Med. 1996;156:2226–31. [PubMed] [Google Scholar]
- 81.Zeeshan M, Jabeen K, Ali AN, Ali AW, Farooqui SZ, et al. Evaluation of immune response to Hepatitis B vaccine in health care workers at a tertiary care hospital in Pakistan: an observational prospective study. BMC Infect Dis. 2007;7:120. doi: 10.1186/1471-2334-7-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bhattacharya D, Umbleja T, Carrat F, Chung RT, Peters MG, et al. Women experience higher rates of adverse events during hepatitis C virus therapy in HIV infection: a meta-analysis. J Acquir Immune Defic Syndr. 2010;55:170–5. doi: 10.1097/QAI.0b013e3181e36420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Narciso-Schiavon JL, Schiavon Lde L, Carvalho-Filho RJ, Sampaio JP, Batah PN, et al. Gender influence on treatment of chronic hepatitis C genotype 1. Rev Soc Bras Med Trop. 2010;43:217–23. doi: 10.1590/s0037-86822010000300001. [DOI] [PubMed] [Google Scholar]
- 84.Yu JW, Sun LJ, Zhao YH, Kang P, Yan BZ. Impact of sex on virologic response rates in genotype 1 chronic hepatitis C patients with peginterferon alpha-2a and ribavirin treatment. Int J Infect Dis. 2011;15:e740–6. doi: 10.1016/j.ijid.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 85.Villa E, Karampatou A, Camma C, Di Leo A, Luongo M, et al. Early menopause is associated with lack of response to antiviral therapy in women with chronic hepatitis C. Gastroenterology. 2011;140:818–29. doi: 10.1053/j.gastro.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 86.Klein SL, Pekosz A, Passaretti C, Anker M, Olukoya P. Sex, gender and influenza. Geneva: World Health Organization; 2010. pp. 1–58. [Google Scholar]
- 87.Klein SL, Passaretti C, Anker M, Olukoya P, Pekosz A. The impact of sex, gender and pregnancy on 2009 H1N1 disease. Biol Sex Differ. 2010;1:5. doi: 10.1186/2042-6410-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Randolph AG, Vaughn F, Sullivan R, Rubinson L, Thompson BT, et al. Critically ill children during the 2009–2010 influenza pandemic in the United States. Pediatrics. 2011;128:e1450–8. doi: 10.1542/peds.2011-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zarychanski R, Stuart TL, Kumar A, Doucette S, Elliott L, et al. Correlates of severe disease in patients with 2009 pandemic influenza (H1N1) virus infection. CMAJ. 2010;182:257–64. doi: 10.1503/cmaj.091884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tam A, Morrish D, Wadsworth S, Dorscheid D, Man SP, et al. The role of female hormones on lung function in chronic lung diseases. BMC Womens Health. 2011;11 :24. doi: 10.1186/1472-6874-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.de Jong MD, Simmons CP, Thanh TT, Hien VM, Smith GJ, et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med. 2006;12:1203–7. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Szretter KJ, Gangappa S, Lu X, Smith C, Shieh WJ, et al. Role of host cytokine responses in the pathogenesis of avian H5N1 influenza viruses in mice. J Virol. 2007;81 :2736–44. doi: 10.1128/JVI.02336-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kobasa D, Jones SM, Shinya K, Kash JC, Copps J, et al. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature. 2007;445:319–23. doi: 10.1038/nature05495. [DOI] [PubMed] [Google Scholar]
- 94.Merrill RM, Beard JD. Influenza vaccination in the United States, 2005–2007. Med Sci Monit. 2009;15:PH92–100. [PubMed] [Google Scholar]
- 95.Endrich MM, Blank PR, Szucs TD. Influenza vaccination uptake and socioeconomic determinants in 11 European countries. Vaccine. 2009;27:4018–24. doi: 10.1016/j.vaccine.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 96.Santibanez TA, Mootrey GT, Euler GL, Janssen AP. Behavior and beliefs about influenza vaccine among adults aged 50–64 years. Am J Health Behav. 2010;34:77–89. doi: 10.5993/ajhb.34.1.10. [DOI] [PubMed] [Google Scholar]
- 97.Schwarzinger M, Flicoteaux R, Cortarenoda S, Obadia Y, Moatti JP. Low acceptability of A/H1N1 pandemic vaccination in French adult population: did public health policy fuel public dissonance? PLoS One. 2010;5:e10199. doi: 10.1371/journal.pone.0010199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chor JS, Ngai KL, Goggins WB, Wong MC, Wong SY, et al. Willingness of Hong Kong healthcare workers to accept pre-pandemic influenza vaccination at different WHO alert levels: two questionnaire surveys. British Medical Journal. 2009;339:b3391. doi: 10.1136/bmj.b3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cook IF, Barr I, Hartel G, Pond D, Hampson AW. Reactogenicity and immunogenicity of an inactivated influenza vaccine administered by intramuscular or subcutaneous injection in elderly adults. Vaccine. 2006;24:2395–402. doi: 10.1016/j.vaccine.2005.11.057. [DOI] [PubMed] [Google Scholar]
- 100.Lorenzo ME, Hodgson A, Robinson DP, Kaplan JB, Pekosz A, et al. Antibody responses and cross protection against lethal influenza A viruses differ between the sexes in C57BL/6 mice. Vaccine. 2011;29:9246–55. doi: 10.1016/j.vaccine.2011.09.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lu FX, Abel K, Ma Z, Rourke T, Lu D, et al. The strength of B cell immunity in female rhesus macaques is controlled by CD8+ T cells under the influence of ovarian steroid hormones. Clin Exp Immunol. 2002;128:10–20. doi: 10.1046/j.1365-2249.2002.01780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nguyen DC, Masseoud F, Lu X, Scinicariello F, Sambhara S, et al. 17beta-Estradiol restores antibody responses to an influenza vaccine in a postmenopausal mouse model. Vaccine. 2011;29:2515–8. doi: 10.1016/j.vaccine.2011.01.080. [DOI] [PubMed] [Google Scholar]
- 103.De Clercq E. Antiviral agents active against influenza A viruses. Nat Rev Drug Discov. 2006;5:1015–25. doi: 10.1038/nrd2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Blanchon T, Mentre F, Charlois-Ou C, Dornic Q, Mosnier A, et al. Factors associated with clinical and virological response in patients treated with oseltamivir or zanamivir for influenza A during the 2008–2009 winter. Clin Microbiol Infect. 2011 doi: 10.1111/j.1469-0691.2011.03751.x. [DOI] [PubMed] [Google Scholar]
- 105.Maltezou HC, Drakoulis N, Siahanidou T, Karalis V, Zervaki E, et al. Safety and Pharmacokinetics of Oseltamivir for Prophylaxis of Neonates Exposed to Influenza H1N1. Pediatr Infect Dis J. 2011 doi: 10.1097/INF.0b013e3182472f28. [DOI] [PubMed] [Google Scholar]
- 106.Guha-Sapir D, Schimmer B. Dengue fever: new paradigms for a changing epidemiology. Emerg Themes Epidemiol. 2005;2:1. doi: 10.1186/1742-7622-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Murphy G, Pfeiffer R, Camargo MC, Rabkin CS. Meta-analysis shows that prevalence of Epstein-Barr virus-positive gastric cancer differs based on sex and anatomic location. Gastroenterology. 2009;137:824–33. doi: 10.1053/j.gastro.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Klein SL, Marks MA, Li W, Glass GE, Fang LQ, et al. Sex differences in the incidence and case fatality rates from hemorrhagic Fever with renal syndrome in china, 2004–2008. Clin Infect Dis. 2011;52:1414–21. doi: 10.1093/cid/cir232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fleming DT, McQuillan GM, Johnson RE, Nahmias AJ, Aral SO, et al. Herpes simplex virus type 2 in the United States, 1976 to 1994. N Engl J Med. 1997;337:1105–11. doi: 10.1056/NEJM199710163371601. [DOI] [PubMed] [Google Scholar]
- 110.Eshima N, Iwata O, Iwata S, Tabata M, Higuchi Y, et al. Age and gender specific prevalence of HTLV-1. J Clin Virol. 2009;45:135–8. doi: 10.1016/j.jcv.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 111.Garenne M. Sex differences in measles mortality: a world review. Int J Epidemiol. 1994;23 :632–42. doi: 10.1093/ije/23.3.632. [DOI] [PubMed] [Google Scholar]
- 112.Jean CM, Honarmand S, Louie JK, Glaser CA. Risk factors for West Nile virus neuroinvasive disease, California, 2005. Emerg Infect Dis. 2007;13:1918–20. doi: 10.3201/eid1312.061265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hawkins C, Chalamilla G, Okuma J, Spiegelman D, Hertzmark E, et al. Sex differences in antiretroviral treatment outcomes among HIV-infected adults in an urban Tanzanian setting. AIDS. 2011;25:1189–97. doi: 10.1097/QAD.0b013e3283471deb. [DOI] [PubMed] [Google Scholar]
- 114.Emery J, Pick N, Mills EJ, Cooper CL. Gender differences in clinical, immunological, and virological outcomes in highly active antiretroviral-treated HIV-HCV coinfected patients. Patient Prefer Adherence. 2010;4:97–103. doi: 10.2147/ppa.s9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ofotokun I, Chuck SK, Hitti JE. Antiretroviral pharmacokinetic profile: a review of sex differences. Gend Med. 2007;4:106–19. doi: 10.1016/s1550-8579(07)80025-8. [DOI] [PubMed] [Google Scholar]
- 116.Andany N, Raboud JM, Walmsley S, Diong C, Rourke SB, et al. Ethnicity and gender differences in lipodystrophy of HIV-positive individuals taking antiretroviral therapy in Ontario, Canada. HIV Clin Trials. 2011;12:89–103. doi: 10.1310/hct1202-89. [DOI] [PubMed] [Google Scholar]
- 117.Straus SE, Corey L, Burke RL, Savarese B, Barnum G, et al. Placebo-controlled trial of vaccination with recombinant glycoprotein D of herpes simplex virus type 2 for immunotherapy of genital herpes. Lancet. 1994;343:1460–3. doi: 10.1016/s0140-6736(94)92581-x. [DOI] [PubMed] [Google Scholar]
- 118.Zhang X, Castelli FA, Zhu X, Wu M, Maillere B, et al. Gender-dependent HLA-DR-restricted epitopes identified from herpes simplex virus type 1 glycoprotein D. Clin Vaccine Immunol. 2008;15:1436–49. doi: 10.1128/CVI.00123-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Engler RJ, Nelson MR, Klote MM, VanRaden MJ, Huang CY, et al. Half-vs full-dose trivalent inactivated influenza vaccine (2004–2005): age, dose, and sex effects on immune responses. Arch Intern Med. 2008;168:2405–14. doi: 10.1001/archinternmed.2008.513. [DOI] [PubMed] [Google Scholar]