Abstract
Background
A relation between stress and symptoms of rhinitis has not been established.
Objective
To determine if participants’ reporting of allergy flares correlated with perceived emotional stress, depression, mood, and a biomarker of stress (cortisol).
Methods
This study was a secondary analysis of 179 university employees who participated in a study evaluating the influence of several lifestyle interventions on health symptoms and inflammation. Perceived stress and depressive symptom questionnaires were obtained before each 2-week study period. Online diary entries documenting same-day allergy flares, stressful events, perceived stress, mood, and salivary cortisol levels were collected daily during 2 14-day blocks.
Results
Thirty-nine percent of subjects (n = 69) self-reported allergy symptoms. This allergy flare group had higher perceived stress scores than the group without allergy symptoms. Perceived stress, but not depressive symptoms, positively correlated with allergy flares evaluated during 2 independent 14-day periods. There also was a positive relation between negative mood scores and allergy flares over the course of the study. Cortisol had no association with allergy symptom flares.
Conclusion
These findings suggest that individuals with persistent emotional stress have more frequent allergy flares. Furthermore, those with more flares have greater negative mood.
Introduction
The burden of persistent rhinitis symptoms affects quality of life in allergic individuals,1–4 decreasing daytime arousal,4,5 cognition,6–8 mood,9 and overall social functioning. Furthermore, emotional stress may make allergy symptoms worse. There are limited studies on the connection between stress and worsening allergy symptoms, and they have primarily looked at effects of acute examination stress or a standardized acute stressor.10–12
Cortisol is a biomarker for stress. It participates in bidirectional feedback between the endocrine and immune systems, and it can be activated by psychosocial stress. Salivary cortisol sampling allows for frequent, stress-free self-sampling, approximates mean 24-hour cortisol secretion, and highly correlates with serum cortisol levels.13 In the authors’ laboratory, salivary levels have been more reliable than serum cortisol levels in evaluating immune effects.14
In this study, the authors evaluated the relations between subjects’ self-reported allergy flares and emotional stress, depression, mood, and salivary cortisol. Data were collected over 2 14-day periods within 12 weeks. It is a secondary analysis from a primary investigation, which looked at the role of mindfulness, a meditative practice, for improving health symptoms and biomarkers of inflammation in adults.15 In the present investigation, the authors found significant relations among emotional stress, negative mood, and frequency of allergy flares.
Methods
Study Participants
The study was approved by the institutional review board (The Ohio State University, protocol 2007HO195) and written consent was obtained from all participants. All 179 men and women (age range 35–60 years) were employees of the Ohio State University and from different socioeconomic backgrounds and were recruited by campus-wide e-mail, poster, and radio campaigns.
For this analysis, data from all subjects were used. Two groups were compared on a profile of measurements; one group reported allergies and symptoms of allergy flares and the other reported no symptoms of allergy flares. Within the group that reported symptoms, changes in allergy symptoms over time were related to changes in self-reported stress, negative stressful events, and measurements of negative mood.
Exclusions
Subjects were initially assessed using a screening telephone interview, and exclusions for participating in the study primarily included activities that could significantly and independently alter inflammation or stress levels: (1) pregnancy or amenorrhea (≥4 months) in premenopausal women; (2) a major life stress, such as a family death, in the past 2 months; (3) inadequate reading skills necessary for completing online questionnaires; (4) needle or computer phobia; (5) regular exercise more than 30 minutes per day; (6) excessive daily alcohol intake (>2 1.25-oz shots of liquor, 2 12-oz containers of beer, or 2 6-oz glasses of wine); (7) recreational drug use; (8) vaccination during the past 2 months; (9) any new illness within the past month; (10) body mass index of at least 40 kg/m2; or (11) smoking more than half a pack of cigarettes per day. Subjects were not on glucocorticoid therapy (systemic or topical) during the month before or during the study. No subjects reported daily antihistamine use. Subjects on medications were instructed to notify the authors if there were any changes in their medication regimen during the study. Three subjects made a change in medication (discontinued antidepressant) during the investigation.
Inclusions
Subjects in the appropriate age range who were not excluded during the initial telephone screening came to the Clinical Research Center, where they had blood drawn for a serum C-reactive protein. Subjects were asked to participate in the study if the C-reactive protein level was higher than 3 mg/L (upper tertile of risk for cardiovascular disease) and lower than 10 mg/L (levels >10 mg/L suggest acute inflammation).
Data Collection
The study period was 12 weeks long, including 2 weeks of daily diary collection, followed by an 8-week lifestyle intervention, and then another 2 weeks of daily diary collection. Subjects were instructed in the use of the online daily diary, which, for this analysis, included documentation of allergy symptom flares, stressful events, perceived emotional stress, and mood. For completion of their daily diaries, subjects accessed a secure Web site, which consisted of a detailed questionnaire of daily events. They were instructed to fill out the diary questions at bedtime each night. These data were collected and stored using StudyTrax (ScienceTrax, LLC; http://www.sciencetrax.com/Home/default.aspx), a Web-based database maintained by the Clinical Research Center with access limited to the primary investigator and authorized staff. Each daily diary was time-stamped to prevent backfilling responses. Salivary cortisol levels were obtained during the 2 14-day periods of diary collections at 20 minutes after rising, noon, 5 PM, and bedtime. Subjects learned how to properly collect their own saliva specimens, which they returned to the laboratory at the end of each 2-week period. All assays were analyzed in our laboratory, and all samples for a participant were run in the same assay to limit interassay variation. Study participants were asked to fill out several questionnaires at the beginning and end of the study.
Diary Questions
Questions were obtained from the Inventory of Small Life Events.16 The principal diary question related to this report was: “Your allergies flared up” (yes/no). “Allergy flares” were intended to include any typical symptoms of allergic rhino-conjunctivitis, and those responding “yes” were subjects who believed they had allergies. Participants answering “yes” to this question only once during the 12-week study were included in the no allergy flare (NAF) group for statistical analysis. An analysis of the allergy flare (AF) group was performed to look at differences among subjects with self-reported allergies.
Daily stressful events were coded by answering “yes” or “no” to a list of possible positive and negative interpersonal scenarios that might have occurred that day. There were 14 events (8 positive, 6 negative) concerning “friends/acquaintances,” 14 concerning “spouse/partner or ex-spouse/partner” events (7 positive, 7 negative), 11 events (9 positive, 2 negative) concerning “other family members,” and 10 events (4 positive, 6 negative) related to “employment (paid or volunteer) or coworkers,” for a total of 49 events (28 positive, 21 negative). The sum of responses to negative events was divided by the total number of possible negative events to produce a daily stressful event score. A mean score over the 28 days of diary collection was calculated for each subject, producing a mean daily stressful event score.
At the bottom of each of the 4 daily stressful event sections, subjects responded to the question, “Overall, how stressful were your relations with your friends/spouse/family/co-workers today?” Each of these 4 questions was coded on a 5-point scale (not at all; a little; moderately; extremely; no contact today). The sum of responses to all 4 questions was considered the subject’s daily perceived stress score.
There was a question evaluating mood, which stated, “These words describe different feelings and emotions. How much have you felt this way today?” This question was followed by a list of 20 positive and negative mood descriptions, collectively known as the Positive and Negative Affect Schedule (PANAS).17 The 10 negative moods included distressed, upset, guilty, ashamed, hostile, irritable, nervous, jittery, scared, and afraid. Each mood was coded (very slightly or not at all; a little; moderately; quite a bit; extremely) for a sum of positive and negative moods.
Questionnaires
Perceived Stress Scale
The Perceived Stress Scale (PSS), developed by Cohen and Williamson,18 is the most widely used psychological instrument for measuring the perception of emotional stress. The PSS has been shown to predict cortisol levels and various immune biomarkers, such as leukocyte subset distributions,19 postvaccination antibody titers,20 and cytokine production.14,21 It is a measurement of the degree to which situations in life are appraised as stressful. Items are designed to evaluate how overloaded, unpredictable, and uncontrollable one finds his or her life. It also queries current levels of experienced stress. In this study, the PSS measured perceived stress in the preceding week.
Center for Epidemiological Studies Depression Scale
The Center for Epidemiological Studies Depression Scale (CES-D) has been used extensively as a brief measurement of depressive symptomatology. Studies have shown acceptable test–retest reliability and excellent construct validity.22 The CES-D also has distinguished depressed from nondepressed participants in community and clinical samples; discriminative validity appears acceptable.22
Assays
Salivary cortisol
Determinations were made using the Cortisol Coat-A-Count Radioimmunoassay (Diagnostic Products Corporation, Los Angeles, California). Intra-assay variation was 4.3% and interassay variation was 5.2%. Sensitivity was adequate for this population at 0.025 μg/dL.
Statistical Analyses
Comparisons of scores for the AF versus the NAF group were done by unpaired t test. The χ2 test was used to assess differences between groups in the number of subjects diagnosed with depression and using antidepressants. A linear mixed model using Toeplitz covariance structure (Proc Mixed; SAS 9.2, SAS Institute, Inc, Cary, North Carolina) was used to analyze repeated measures of allergy flares compared with stress, mood, and cortisol for 28 days of daily reporting within the AF group. Observations from the same subjects were expected to correlate over time (non-0 correlations), with potentially higher correlations for observations closer in time. Toeplitz covariance structure for modeling this correlation was superior to others based on Akaike information criterion.23
To determine directionality between flares and PSS, regression analysis was performed for 28 days of data and 2 separate 14-day cohorts (before and after intervention). This analysis also was used to analyze how flares related to negative mood (mean PANAS negative scores) over 28 days. Scatter and box plots were used to show these linear relations between variables. The authors did not adjust for missing data (4%) because these were less that 10% of the total.
Results
Of the 179 subjects enrolled in the study, 69 subjects (39%) self-reported more than 1 allergy flare during the course of the study. These constituted the AF group. The remaining 110 reported 1 or no symptom of allergy flares and constituted the NAF group. Baseline characteristics of age, gender, body mass index, blood pressure, depression, and antidepressant usage were similar between the 2 groups (Table 1).
Table 1.
Subject characteristicsa
| Characteristic | AF
|
NAF
|
|---|---|---|
| (n = 69) | (n = 110) | |
| Age (y) | 50.1 (0.9) | 50.6 (0.7) |
| Women/men | 60/9 | 97/13 |
| BMI (kg/m2) | 31.8 (0.6) | 33 (0.5) |
| Blood pressure | 122 (1.5)/74 (0.9) | 122 (1.1)/75 (0.6) |
| Depression | 22 (32%) | 33 (30%) |
| Antidepressant usage | 16 (23%) | 28 (26%) |
Abbreviations: AF, allergy flares; BMI, body mass index; NAF, no allergy flares.
Data are normally distributed and presented as mean (standard error of the mean) or number (percentage). All characteristic comparisons had a P value greater than .05.
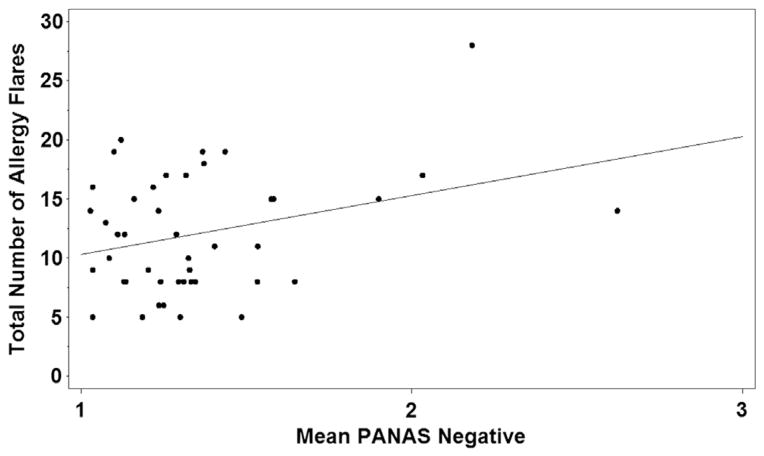
Within the AF group, the number of allergy flares reported during the 2 14-day reporting periods ranged from 2 to 28. Sixty-four percent had more than 4 flares. A highly significant positive relation was found between perceived stress (PSS) and allergy flares experienced over the course of the study (P = .0003; Fig 1). When analyzing preintervention and postintervention periods as 2 separate 14-day cohorts, a significant positive correlation between stress (PSS) and number of allergy flares remained evident (P = .001 and .008, respectively). There also was a strong positive relation between negative mood (subject’s average PANAS negative score) and the number of flares a subject reported over 28 days (P = .004; Fig 2).
Figure 1.
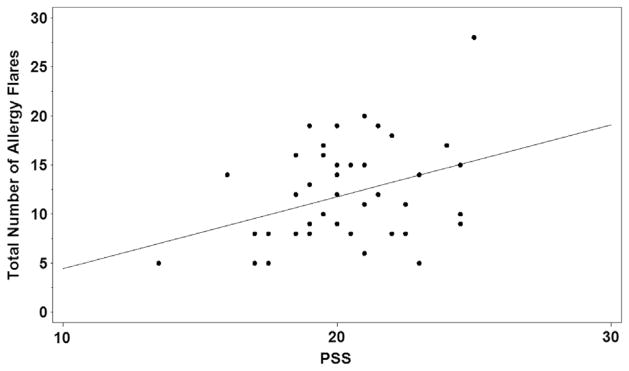
Distribution of Perceived Stress Scale (PSS) scores for subjects reporting at least 5 days of allergy flare.
Figure 2.
Distribution of averaged negative mood scores (Positive and Negative Affect Schedule [PANAS]) for subjects reporting 5 or more flares.
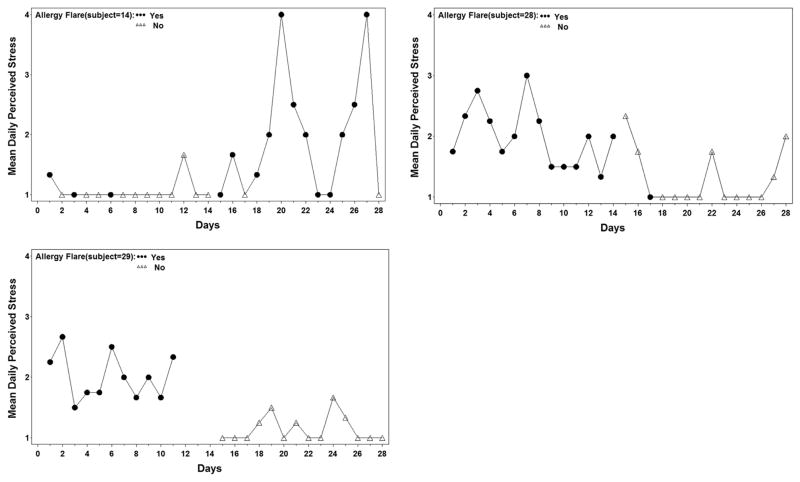
There were no significant same-day correlations between allergy flares and stress or mood (daily perceived stress, P = .79; negative daily stressful events, P = .96; PANAS negative scores, P = .25). However, there were some subjects who clearly exhibited patterns of flares correlating with days of increased daily perceived stress (Fig 3). Patient 14 had low levels of perceived stress each day during the initial 2-week reporting period and noted only 3 days of allergy flare during that time. During the last 2 weeks of reporting, when daily perceived stress was higher, this subject had allergy flare symptoms on most days (12 of 14 days). Similarly, subjects 28 and 29 showed a pattern of frequent allergy flares reported during the high-stress period and no symptoms when stress levels decreased.
Figure 3.
Correlation of increased daily perceived stress with days of allergy flares in 3 subjects.
The AF and NAF groups had a similar number of negative daily stressful events and occurrences of negative and positive mood feelings (PANAS). There was a difference between groups, however, with the AF group trending toward higher perceived stress scores (PSS) than the NAF group (difference in means AF–NAF = 0.85, P = .02). Note that PSS scores evaluating weekly perceived stress levels for all subjects did not differ significantly between their initial baseline evaluation and their score obtained 10 weeks into the study (baseline–10 weeks = 0.35, P = .59). There were no significant differences in depressive symptoms (CES-D scores) or salivary cortisol levels between the AF and NAF groups (Table 2) or relations between these factors and allergy flares within the AF group. The 2 groups had mean CES-D scores of 16. Values of 16 and above suggest the presence of clinical depression.
Table 2.
Questionnaire and biomarker resultsa
| Test | AF
|
NAF
|
|---|---|---|
| (n = 69) | (n = 110) | |
| PSS scoreb | 20.3 (0.3) | 19.5 (0.2) |
| CES-D score | 16.3 (0.5) | 16.3 (0.4) |
| Salivary cortisol | 0.4 (0.01) | 0.3 (0.01) |
Abbreviations: AF, allergy flares; CES-D, Center for Epidemiological Studies Depression Scale; NAF, no allergy flares; PSS, Perceived Stress Scale.
Data are normally distributed and presented as mean (standard error of the mean).
P < .05. All other test comparisons had a P value greater than .05.
Discussion
This study is the first that the authors are aware of to establish a link between emotional stress and allergy flares. When recalling higher emotional stress levels from the preceding week, the group of self-reported allergy participants had more days of allergy flares on 2 occasions, each 14 days in duration.
This same association of stress and allergy flares was not observed when stress was reported on the same day of a flare. These findings indicate that perceived stress over time may have an additive effect and is more important in predicting allergy flares than a single day of high stress. This latter finding is consistent with a previous study that did not find an increase in allergic symptoms after acute stressors.10
Contrary to the trend, 3 subjects showed a pattern of increased allergy symptom flares on stressful days (Fig 3). The authors speculate that some individuals may possess a more sensitive neuroimmunologic trigger in response to stress and/or experience higher persistent stress levels.
In this study, a significant relation was observed between negative mood over the 28 reporting days and rhinitis symptoms. A relation between negative mood and allergy symptoms has been noted previously.9,24 Similar to the present findings with stress, there was not an association between a single day of negative mood and an allergy flare on that day. Overall, participants felt more upset, irritable, or afraid when their allergy symptoms were active over time, similar to a previous report.24 Although it is generally accepted that negative mood is a result of discomfort related to allergy flare, one cannot rule out that it plays some role in precipitating the flare. The authors were unable to determine directionality in this study.
The authors found that AF and NAF participants had higher mean depressive symptom scores (CES-D) than reported in the general population.25 Using different screening tools, cross-sectional studies have associated allergic rhinitis with increased rates of depressive symptoms.26 Differences between the present findings and those of other studies may be due to use of different screening instruments for depressive symptoms or a higher level of depressive symptoms in the present NAF subjects.
Although there was no reported use of allergy medications during the study period, the authors cannot exclude the possibility that a subject may have taken an antihistamine during the study and not remembered to report it. Because symptom frequency, rather than severity, was measured, the authors expect that if symptoms were bothersome enough to warrant use of antihistamine, subjects still would have reported a flare on the day they took “as-needed” medication. Furthermore, if subjects regularly used antihistamines during this study without reporting it, then the effects of stress on symptom flares could be greater than reported.
There were some limitations to this study. The large number of inclusion and exclusion criteria may limit application to the general population. Also, the authors used self-report of allergy flare, and cohorts were run throughout the academic year, September through May, which may not have aligned with a subject’s allergy season.
In summary, the present findings suggest that allergic individuals with persistent emotional stress have more frequent allergy symptoms. These data also show positive relations over time between allergy flares and negative mood. There were no significant relations between allergy flares and acute emotional stress, depression, or salivary cortisol levels. Current diagnostic guidelines for allergic rhinitis include assessment of quality-of-life measurements, but they do not include recommendations for objectively evaluating an individual’s stress level. The present data suggest that certain allergic individuals may benefit from developing awareness of their stress levels. Prospective studies would need to confirm this before making recommendations to screen all allergy patients with the PSS or any other stress instrument.
Acknowledgments
Funding Sources: This work was supported by National Institutes of Health grant R21AT003670-01A2 (Dr Marlarkey) and Clinical and Translational Science Award UL1RR0025755 (Dr Malarkey).
The authors thank David Jarjoura, PhD, for statistical guidance and interpretation in the Center for Biostatistics at the Ohio State University Medical Center.
Footnotes
Disclosures: Authors have nothing to disclose.
References
- 1.Meltzer EO. Quality of life in adults and children with allergic rhinitis. J Allergy Clin Immunol. 2001;108(suppl):S45–S53. doi: 10.1067/mai.2001.115566. [DOI] [PubMed] [Google Scholar]
- 2.Borres MP. Allergic rhinitis: more than just a stuffy nose. Acta Paediatr. 2009;98:1088–1092. doi: 10.1111/j.1651-2227.2009.01304.x. [DOI] [PubMed] [Google Scholar]
- 3.Leynaert B, Neukirch C, Liard R, Bousquet J, Neukirch F. Quality of life in allergic rhinitis and asthma. A population-based study of young adults. Am J Respir Crit Care Med. 2000;162:1391–1396. doi: 10.1164/ajrccm.162.4.9912033. [DOI] [PubMed] [Google Scholar]
- 4.Stuck BA, Czajkowski J, Hagner AE, et al. Changes in daytime sleepiness, quality of life, and objective sleep patterns in seasonal allergic rhinitis: a controlled clinical trial. J Allergy Clin Immunol. 2004;113:663–668. doi: 10.1016/j.jaci.2003.12.589. [DOI] [PubMed] [Google Scholar]
- 5.Spaeth J, Klimek L, Mosges R. Sedation in allergic rhinitis is caused by the condition and not by antihistamine treatment. Allergy. 1996;51:893–906. doi: 10.1111/j.1398-9995.1996.tb04490.x. [DOI] [PubMed] [Google Scholar]
- 6.Blaiss MS. Pediatric allergic rhinitis: physical and mental complications. Allergy Asthma Proc. 2008;29:1–6. doi: 10.2500/aap2008.29.3072. [DOI] [PubMed] [Google Scholar]
- 7.Meltzer EO, Nathan R, Derebery J, et al. Sleep, quality of life, and productivity impact of nasal symptoms in the United States: findings from the Burden of Rhinitis in America survey. Allergy Asthma Proc. 2009;30:244–254. doi: 10.2500/aap.2009.30.3230. [DOI] [PubMed] [Google Scholar]
- 8.Hartgerink-Lutgens I, Vermeeren A, Vuurman E, Kremer B. Disturbed cognitive functions after nasal provocation in patients with seasonal allergic rhinitis. Clin Exp Allergy. 2009;39:500–508. doi: 10.1111/j.1365-2222.2009.03200.x. [DOI] [PubMed] [Google Scholar]
- 9.Marshall PS, O’Hara C, Steinberg P. Effects of seasonal allergic rhinitis on fatigue levels and mood. Psychosom Med. 2002;64:684–691. doi: 10.1097/01.psy.0000021944.35402.44. [DOI] [PubMed] [Google Scholar]
- 10.Jernelov S, Hoglund CO, Axelsson J, et al. Effects of examination stress on psychological responses, sleep and allergic symptoms in atopic and non-atopic students. Int J Behav Med. 2009;16:305–310. doi: 10.1007/s12529-008-9020-6. [DOI] [PubMed] [Google Scholar]
- 11.Hoglund CO, Axen J, Kemi C, et al. Changes in immune regulation in response to examination stress in atopic and healthy individuals. Clin Exp Allergy. 2006;36:982–992. doi: 10.1111/j.1365-2222.2006.02529.x. [DOI] [PubMed] [Google Scholar]
- 12.Buske-Kirschbaum A, Ebrecht M, Hellhammer DH. Blunted HPA axis responsiveness to stress in atopic patients is associated with the acuity and severeness of allergic inflammation. Brain Behav Immun. 2010;24:1347–1353. doi: 10.1016/j.bbi.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 13.Estrada YMRM, Orlander PR. Salivary cortisol can replace free serum cortisol measurements in patients with septic shock. Chest. 2011;140:1216–1222. doi: 10.1378/chest.11-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glaser R, Kiecolt-Glaser JK, Marucha PT, MacCallum RC, Laskowski BF, Malarkey WB. Stress-related changes in proinflammatory cytokine production in wounds. Arch Gen Psychiatry. 1999;56:450–456. doi: 10.1001/archpsyc.56.5.450. [DOI] [PubMed] [Google Scholar]
- 15.Malarkey WB, Jarjoura D, Klatt M. Workplace based mindfulness practice and inflammation: a randomized trial. Brain Behav Immun. 2013;27:145–154. doi: 10.1016/j.bbi.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zautra AJ, Guarnaccia CA, Dohrenwend BP. Measuring small life events. Am J Community Psychol. 1986;14:629–655. doi: 10.1007/BF00931340. [DOI] [PubMed] [Google Scholar]
- 17.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Person Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 18.Cohen S, Williamson G. Perceived stress in a probability sample of the United States. In: Spacapan S, Oskamp S, editors. The Social Psychology of Health. Newbury Park, CA: Sage Publications; 1988. pp. 31–67. [Google Scholar]
- 19.Maes M, Van Bockstaele DR, Gastel A, et al. The effects of psychological stress on leukocyte subset distribution in humans: evidence of immune activation. Neuropsychobiology. 1999;39:1–9. doi: 10.1159/000026552. [DOI] [PubMed] [Google Scholar]
- 20.Burns VE, Drayson M, Ring C, Carroll D. Perceived stress and psychological well-being are associated with antibody status after meningitis C conjugate vaccination. Psychosom Med. 2002;64:963–970. doi: 10.1097/01.psy.0000038936.67401.28. [DOI] [PubMed] [Google Scholar]
- 21.Cohen S, Doyle WJ, Skoner DP. Psychological stress, cytokine production, and severity of upper respiratory illness. Psychosom Med. 1999;61:175–180. doi: 10.1097/00006842-199903000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Basco M, Krebaum S, Rush A. Outcome measures of depression. In: Strupp H, Horowitz L, Lambert M, editors. Measuring Patient Changes in Mood, Anxiety, and Personality Disorders. Washington, DC: American Psychological Association; 1997. pp. 207–245. [Google Scholar]
- 23.Burnham KP, Anderson DR. Model Selection and Multi-Model Inference. 2. XXVI. New York: Springer; 2002. [Google Scholar]
- 24.Sharp TJ, Seeto C. The psychosocial impact of self-reported morning allergy symptoms: findings from an Australian internet-based survey. J Allergy. 2010;2010:710926. doi: 10.1155/2010/710926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Measure. 1977;1:385–401. [Google Scholar]
- 26.Lv X, Xi L, Han D, Zhang L. Evaluation of the psychological status in seasonal allergic rhinitis patients. ORL J Otorhinolaryngol Relat Spec. 2010;72:84–90. doi: 10.1159/000297576. [DOI] [PubMed] [Google Scholar]