Abstract
The epidemic of chronic kidney disease in Nicaragua (Mesoamerican nephropathy) has been linked with recurrent dehydration. Here we tested whether recurrent dehydration may cause renal injury by activation of the polyol pathway, resulting in the generation of endogenous fructose in the kidney that might subsequently induce renal injury via metabolism by fructokinase. Wild-type and fructokinase-deficient mice were subjected to recurrent heat-induced dehydration. One group of each genotype was provided water throughout the day and the other group was hydrated at night, after the dehydration. Both groups received the same total hydration in 24 h. Wild-type mice that received delayed hydration developed renal injury, with elevated serum creatinine, increased urinary NGAL, proximal tubular injury, and renal inflammation and fibrosis. This was associated with activation of the polyol pathway, with increased renal cortical sorbitol and fructose levels. Fructokinase-knockout mice with delayed hydration were protected from renal injury. Thus, recurrent dehydration can induce renal injury via a fructokinase-dependent mechanism, likely from the generation of endogenous fructose via the polyol pathway. Access to sufficient water during the dehydration period can protect mice from developing renal injury. These studies provide a potential mechanism for Mesoamerican nephropathy.
Keywords: aldose reductase, fructokinase, fructose, hydration, Mesoamerican nephropathy
An epidemic of chronic kidney disease is occurring in the hot coastal communities of Central America, especially among men working in sugarcane fields.1, 2, 3 The cause of the disease, which has been called ‘Mesoamerican nephropathy,'1, 2, 3 has remained a mystery, as it is not associated with the usual causes of chronic kidney disease such as diabetes or hypertension. Studies to date have not identified heavy metals, agrochemicals, or other toxins as likely candidates for causing the epidemic. However, a common predisposing feature appears to be exposure to heat and the development of recurrent dehydration.1, 2, 3
Dehydration is commonly associated with ‘prerenal' dysfunction with retention of urea, but classically this is thought to reflect hemodynamic changes and not to be associated with true renal injury. If dehydration is severe, subjects can develop acute kidney injury from heat stroke, rhabdomyolysis, and hypotension, but such individuals would likely have substantial symptoms and not present with the insidious onset of chronic kidney disease as typically occurs. However, a favorite hypothesis is that recurrent dehydration may lead to low-grade renal injury that over time results in chronic kidney disease. Dehydration from sweat and heat is known to activate the release of vasopressin. In turn, vasopressin has recently been recognized to have a potential role as a mediator of chronic kidney disease.4, 5, 6 However, although a role for vasopressin in increasing the rate of progression in chronic kidney disease has been shown in animals,4, 7 its role in acute renal injury associated with dehydration remains largely unexplored.
Dehydration can also result in an increase in serum osmolarity and in the activation of aldose reductase.8 Activation of aldose reductase is important in the renal medulla, as here it generates sorbitol that can help protect tubular cells from the high osmolarity in the extracellular environment at this site, thus facilitating urinary concentration. However, activation of aldose reductase in the proximal tubule may not be beneficial, as the conversion of glucose to sorbitol may result in its further degradation by sorbitol dehydrogenase to fructose. Although fructose itself may not be toxic, when it is metabolized by fructokinase, it results in transient adenosine triphosphate depletion with the generation of uric acid, oxidants, and inflammatory mediators.9, 10, 11 The proximal tubule is one of the major sites where fructokinase is expressed.12 This raises the hypothesis that recurrent dehydration may result in repeated stimulation of aldose reductase with the generation of fructose in the proximal tubule, leading to tubular injury and inflammation. To test this hypothesis, we designed the following study.
RESULTS
Effect of dehydration on daily weight
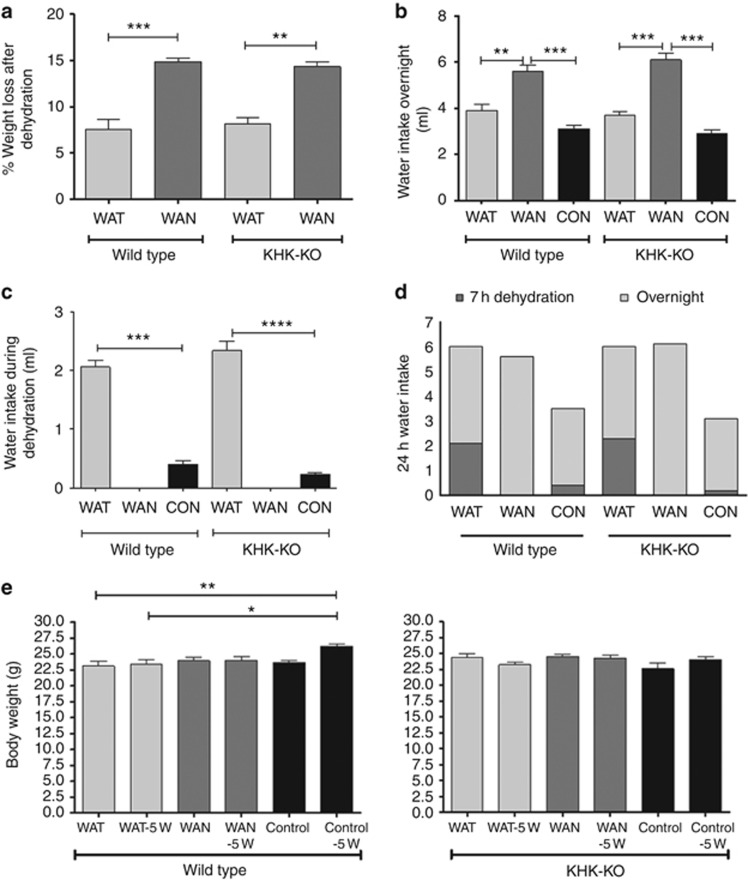
The dehydration protocol resulted in the mice sweating and losing weight acutely. Figure 1a shows the change in mean weight during the 7-h dehydration period. Mice that were not provided water during the day (water at night (WAN)) lost a mean of 15% body weight over the 7-h dehydration period (mean 15.4% for wild-type (WT) mice and 14.6% for the fructokinase (ketohexokinase) A and C knockout (KHK-KO) mice, P=nonsignificant). In contrast, mice who had access to water during the day (water all time (WAT)) showed significantly less weight loss during the day (7.8% and 8.1%, respectively, for the WT and KHK-KO mice, P=nonsignificant). This degree of weight loss was observed both at the beginning and at the end of the study and there was no evidence of adaptation over time (data not shown). Not surprisingly, the mice that had water withheld during the day drank more water at night (Figure 1b), and the mice that had water all the time during the day drank more water compared with the control group in 7 h (Figure 1c), such that total water intake over 24 h was identical in the WAT and WAN groups (Figure 1d).
Figure 1.
Effects of dehydration. (a) Percent weight loss after 7 h of dehydration. (b) Water intake overnight. (c) Water intake during dehydration. (d) The 24 h water intake (light shade, at night; dark shade, during dehydration). (e) Body weight at baseline and after 5 weeks. Data represent analysis of variance (ANOVA) (Bonferroni post test) *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001. CON, control; KHK-KO, fructokinase knockout; WAN, water at night; WAT, water all time.
The effect of the dehydration procedure on overall changes in morning basal weight was also assessed. At the end of 5 weeks, both WT and KHK-KO mice exposed to dehydration tended to maintain but not increase their weight (Figure 1e). In contrast, control WT and control KHK mice tended to increase their weight over the 5-week period.
Dehydration activates the aldose reductase pathway in the renal cortex of WT mice
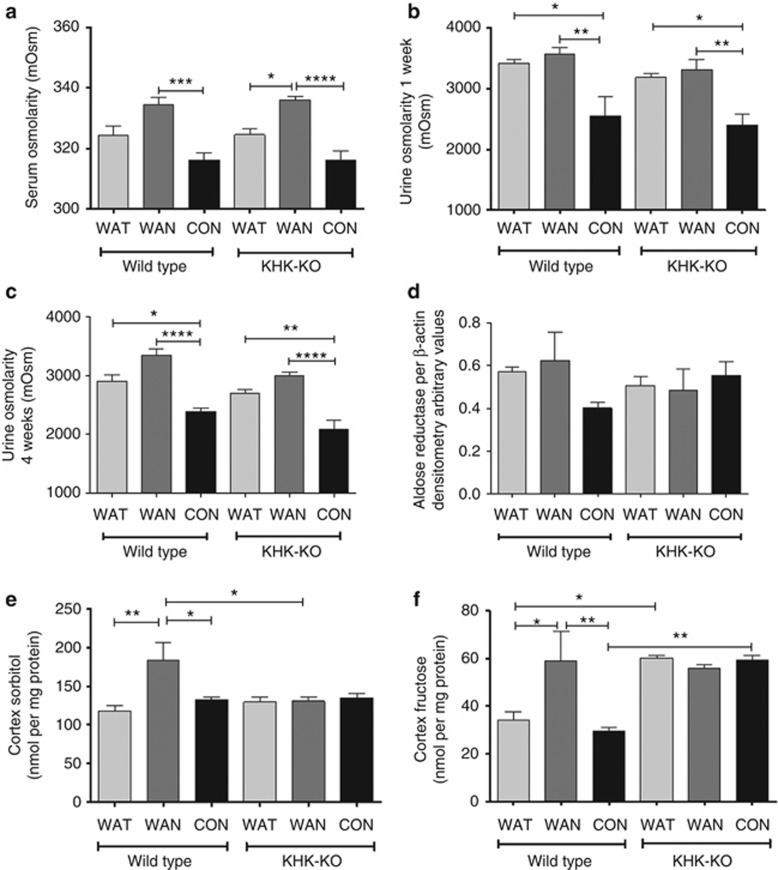
WT and KHK-KO mice that were dehydrated without water (WAN) showed an increase in serum osmolarity at the end of the 7 h dehydration period (Figure 2a). Urinary osmolarity was increased in the mice exposed to heat in samples collected at both week 1 and week 4 (Figure 2b and c). Hyperosmolarity is expected to increase aldose reductase levels, but no differences were observed in aldose reductase protein levels by western blot of the renal cortex at the time of killing, although there was a tendency for higher aldose reductase levels in the WT WAN group (Figure 2d). Nevertheless, both renal cortical sorbitol (Figure 2e) and renal cortical fructose levels (Figure 2f) were increased in WT mice dehydrated without water during the day (WAN group), consistent with an increase in aldose reductase activity. Sorbitol levels were not increased in the KHK-KO mice independent of groups. In contrast, renal fructose levels were elevated in all three groups of KHK-KO mice, consistent with earlier studies showing that fructose levels are high in mice in which fructokinase is absent.13
Figure 2.
Dehydration activates the polyol pathway in the renal cortex. (a) Serum osmolarity at the end of the 7 h dehydration period. Urinary osmolarity after week 1 (b) and week 4 (c). (d) Renal cortical aldose reductase protein level (densitometry of western blot). (e) Renal cortex sorbitol levels. (f) Renal cortex fructose levels. Data represent analysis of variance (ANOVA) (Bonferroni post test) *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001. CON, control; KHK-KO, fructokinase knockout; WAN, water at night; WAT, water all time.
Effect of dehydration on blood pressure, renal function, and renal histology
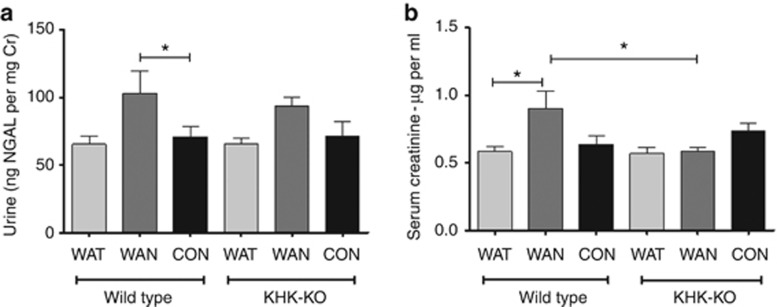
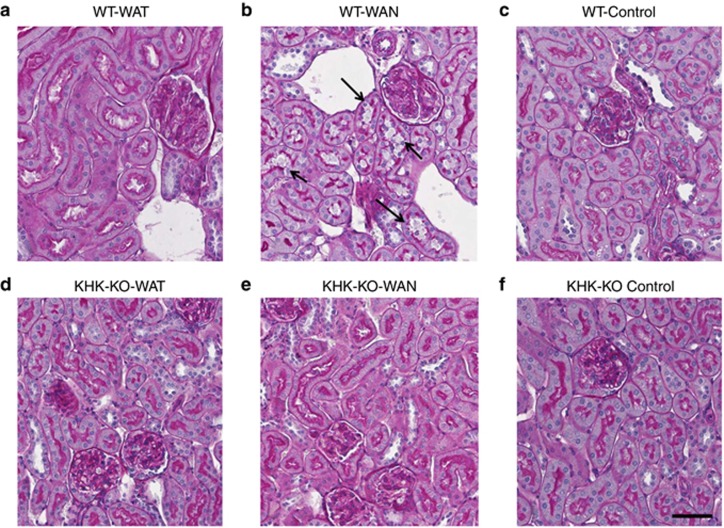
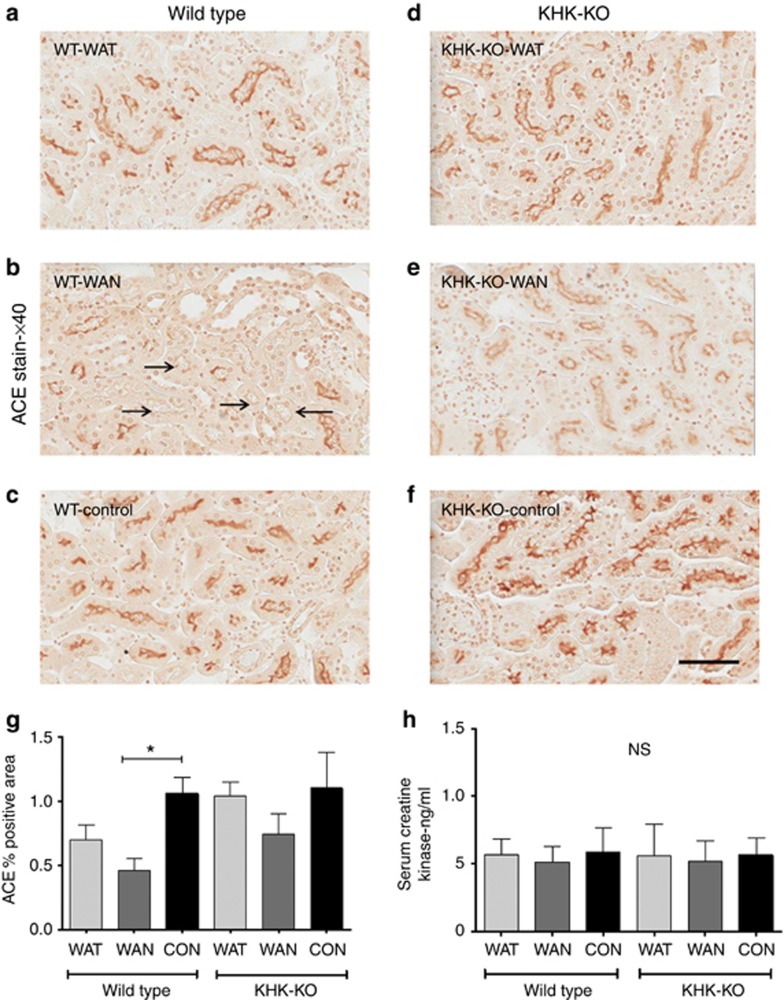
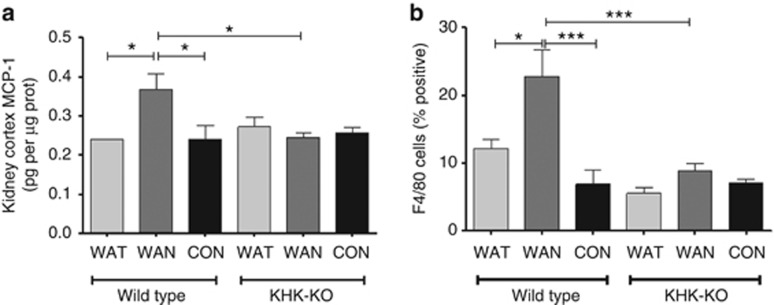
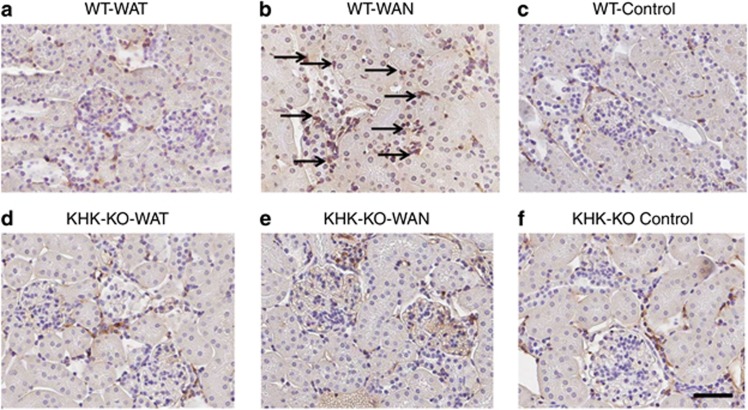
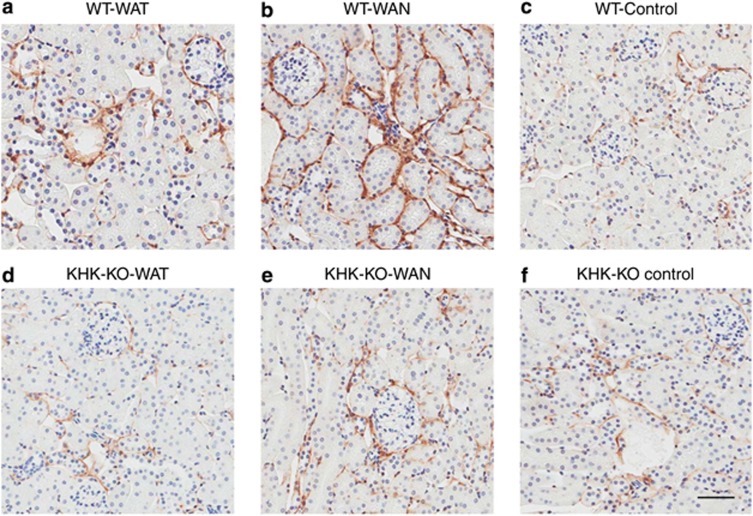
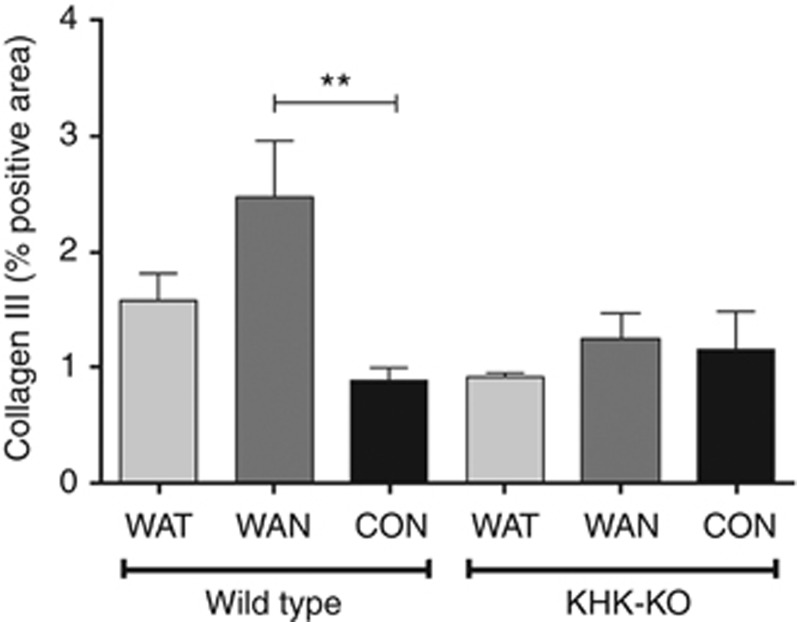
As shown in Figure 2 the WT mice that were dehydrated during the day without water (the WAN group) constituted the group that showed evidence for an increase in aldose reductase activity, as noted by an increase in renal sorbitol and fructose levels. This was also the only group that showed evidence of renal injury, as noted by an increase in urinary neutrophil gelatinase-associated lipocalin (Figure 3a), serum creatinine (Figure 3b), and evidence of proximal tubular injury by renal histology (Figure 4), and with a loss of proximal tubular brush border based on staining for angiotensin-converting enzyme using computer image analysis (Figure 5). Injury was present in both proximal tubules in the renal cortex and in the outer stripe. The injury was not due to rhabdomyolysis, as serum creatine phosphokinase levels at the time of killing were normal in all mice regardless of group (creatine phosphokinase levels <10 ng/ml). Consistent with the known effects of fructose to stimulate the chemokine monocyte chemotactic protein-1 (MCP-1) in proximal tubular cells,10 we also found both an increase in renal cortical MCP-1 levels (Figure 6a) and an increase in infiltrating macrophages in the WAN group compared with the other groups of WT mice (Figure 6b) as we can see in the F4/80 stain (Figure 7). An increase in interstitial fibrosis (collagen III staining) was also observed in the WT WAN group (Figures 8 and 9).
Figure 3.
Dehydration causes renal dysfunction. (a) Urinary neutrophil gelatinase-associated lipocalin (NGAL)/creatinine (Cr) ratio. (b) Serum creatinine (measured by high-performance liquid chromatography (HPLC)). Data represent analysis of variance (ANOVA) (Bonferroni post test) *P<0.05. CON, control; KHK-KO, fructokinase knockout; WAN, water at night; WAT, water all time.
Figure 4.
Renal histology. Proximal tubular injury (loss of brush border with tubular dilation; arrows) is observed in wild-type mice that were dehydrated without water (WAN) (b). Comparing with the groups WT-WAT (a); WT-Control (c); KHK-KO-WAT (d); KHK-KO-WAN (e); and KHK-KO Control (f). Scale bar=50 μm. KHK-KO, fructokinase-knockout; WAN, water at night; WAT, water all time; WT, wild type.
Figure 5.
Proximal tubular brush border staining using angiotensin-converting enzyme (ACE) antibody. Proximal tubular brush border is decreased in the renal cortex (black arrows) of wild-type mice with dehydration-associated kidney injury (b) compared with other groups (a, c–f). Percentage of positively stained area is shown in (g). Creatine kinase levels are normal and similar among groups (h). Scale bar=50 μm. KHK-KO, fructokinase knockout; WAN, water at night; WAT, water all time; WT, wild type.
Figure 6.
Inflammation occurs with chronic dehydration. (a) Renal cortical MCP-1 protein levels; (b) infiltrating macrophages F4/80 stain. Data represent analysis of variance (ANOVA) (Bonferroni post test) *P<0.05; ***P<0.001. CON, control; KHK-KO, fructokinase knockout; MCP-1, monocyte chemotactic protein-1; WAN, water at night; WAT, water all time.
Figure 7.
Macrophage (F4/80) infiltration with chronic dehydration. Wild-type mice with water provided only at night show increased macrophage infiltration (arrows) (b) compared with other groups WT-WAT (a); WT-Control (c); KHK-KO-WAT (d); KHK-KO-WAN (e); and KHK-KO Control (f). Scale bar=50 μm. KHK-KO, fructokinase knockout; WAN, water at night; WAT, water all time; WT, wild type.
Figure 8.
Interstitial collagen III deposition with chronic dehydration. Wild-type mice with water provided only at night show increased interstitial fibrosis (b), compared with other groups. WT-WAT (a); WT-Control (c); KHK-KO, WAT (d); WAN (e); and Control (f). Scale bar=50 mm. KHK-KO, fructokinase knockout; WAN, water at night; WAT, water all time; WT, wild type.
Figure 9.
Interstitial fibrosis (type III collagen staining) occurs with chronic dehydration. Data represent analysis of variance (ANOVA) (Bonferroni post test) **P<0.01. CON, control; KHK-KO, fructokinase knockout; WAN, water at night; WAT, water all time.
Importantly, KHK-KO mice were protected from renal injury. Despite the KHK-KO WAN group having the same degree of weight loss and increase in serum osmolarity, the KHK-KO mice did not show an increase in serum creatinine, tubular injury, inflammation, or fibrosis by histology, or increased expression of the chemokine MCP-1 (Figures 6, 7, 8, 9).
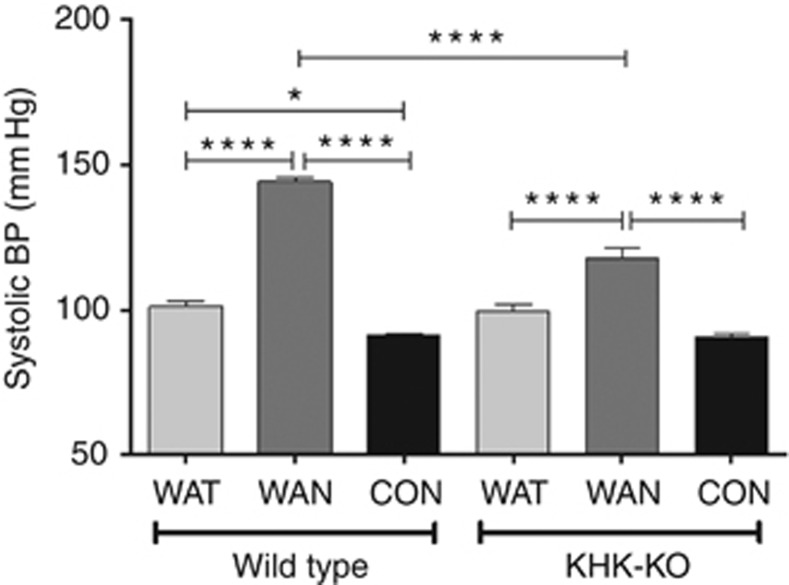
Blood pressure was also measured in both WT and KHK-KO mice at the end of a dehydration period at week 4 (Figure 10). Blood pressure was increased in the WT WAN mice compared with all other groups. KHK-KO mice that were dehydrated without water (WAN) also showed a higher blood pressure, but it was significantly less than that of the WAN WT mice (Figure 10).
Figure 10.
Systolic blood pressure (BP) at 4 weeks. Systolic blood pressure was increased in the WT WAN mice compared with all other groups. KHK-KO mice that were dehydrated without water (WAN) also showed a higher BP, but it was significantly less than that in WAN WT mice. Data represent analysis of variance (ANOVA) (Bonferroni post test) *P<0.05; ****P<0.0001. CON, control; KHK-KO, fructokinase knockout; WAN, water at night; WAT, water all time.
Uric acid levels
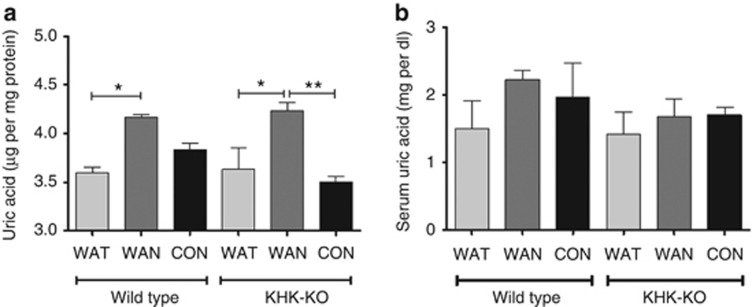
Previous studies have found that uric acid generated during fructose metabolism may be partially responsible for the stimulation of MCP-1.10 Consistent with this hypothesis, an increase in kidney cortical uric acid was observed in the WT WAN mice (Figure 11a). However, renal cortical uric acid levels were also high in the KHK-KO WAN mice despite no stimulation of MCP-1 (Figure 11a). We also noted no difference in serum uric acid levels among all groups (Figure 11b). These results suggest that uric acid may not be the primary mechanism by which KHK mediates renal injury.
Figure 11.
Uric acid levels after 7 h of dehydration. (a) Renal cortical uric acid levels. (b) Serum uric acid levels. Data represent analysis of variance (ANOVA) (Bonferroni post test) *P<0.05; **P<0.01. CON, control; KHK-KO, fructokinase knockout; WAN, water at night; WAT, water all time.
DISCUSSION
The purpose of this study was to explore the potential mechanisms by which chronic or recurrent dehydration could cause kidney injury. The hypothesis was that dehydration-associated hyperosmolarity may activate the aldose reductase pathway in the renal cortex and thus lead to the generation of fructose during the reclamation of glucose by the proximal tubule. In turn, the metabolism of fructose by fructokinase in the proximal tubule would lead to the generation of oxidants and chemokines (MCP-1) that would result in local tubular injury and inflammation.
To test this hypothesis, we developed a model of recurrent dehydration by placing mice in heated chambers for a total of 3.5 h per day, for 5 days per week, for a total of 5 weeks. In the absence of being provided water, the average mouse lost ∼15% of their body weight, only to fully hydrate itself during the night in preparation for the next day. A second group was dehydrated but allowed access to water both during the day and during the night. These mice lost ∼8% of their body weight during the day. Importantly, this group also hydrated themselves during the evening, such that both groups recovered their normal weight and drank the same total amount of water in the 24 h period.
The first major finding was that the mice that were severely dehydrated during the day and had delayed rehydration activated the aldose reductase pathway in their kidneys, as noted by an increase in renal sorbitol and fructose levels, and this was associated with the development of renal injury, as noted by an increase in urinary neutrophil gelatinase-associated lipocalin, an increase in serum creatinine, proximal tubular injury by biopsy, an increase in renal MCP-1 and macrophage infiltration, and early renal fibrosis. A key finding was that the mice that were exposed to the same heat but who hydrated during the day were protected. The observation that only the group that was not well hydrated during the day developed renal injury, despite both groups receiving the same amount of total hydration in a 24-h period, emphasizes the importance of adequate hydration during heat exposure to prevent renal damage.
The second major finding was that mice lacking fructokinase were protected from renal injury despite similar degrees of dehydration. In particular, these mice did not show an increase in serum creatinine or the presence of renal injury, inflammation, or fibrosis. These studies strongly suggest that it is the metabolism of endogenous fructose that is driving the renal injury, and this also implicates an important role for fructokinase. These data are consistent with recent studies that suggest that high dietary intake of fructose may also cause tubulointerstitial disease in the mouse and rat.14, 15
An interesting aspect was that renal sorbitol was not elevated in the WAN KHK-KO mice despite a similar increase in serum osmolarity. This suggests that the aldose reductase pathway was inhibited in the setting in which fructokinase is blocked. The mechanism is not evident, but could potentially relate to a negative feedback pathway from fructose itself that accumulates in mice with fructokinase deficiency. Further studies for exploring this pathway are in order. We also noted an increase in renal cortical uric acid in both WT and fructokinase-KO mice that were dehydrated without access to water during the day. We had postulated that the increase in renal uric acid may be limited to the WT mice as they would have an intact fructokinase system that would be expected to generate uric acid during fructose metabolism. However, increased proximal tubular uptake of uric acid also occurs during dehydration, and this likely accounts for the lack of difference between the groups. It does suggest, however, that the renal injury observed may not be due to the uric acid, as the fructokinase-knockout mice were protected. We previously reported that lowering uric acid was not completely effective at blocking oxidants in isolated proximal tubules exposed to fructose.10
These findings could be relevant to the etiology of the epidemic of kidney disease in Central America, which also appears clinically and histologically to be a type of primary tubulointerstitial injury, although with some global glomerulosclerosis.16 To date, the only common risk factor seems to be exposure to heat and recurrent dehydration. Many of the workers also hydrate themselves with fructose-rich juices or beverages that might compound the problem. Thus, we would like to suggest that repeated renal injury may be occurring from the effects of endogenously generated fructose and that this might be potentiated by fructose provided in the drinks.
We recognize that other mechanisms are also likely important and could be acting synergistically with the consequences of activation of the aldose reductase pathway. Dehydration and volume depletion, for example, are known to activate a variety of mediators that are capable of inducing renal injury, including the renin–angiotensin–aldosterone system, the sympathetic nervous system, and vasopressin. Dehydration may lead to increased reabsorption of toxins into the kidney, such as heavy metals and agrochemicals.
Nevertheless, the importance of this study is that it identifies a new potential mediator of chronic kidney disease that is activated by heat and dehydration. This could be important as global warming leads to the emergence of new diseases. Besides Central America, there is concern that kidney diseases are increasing in other hot agricultural communities, such as in Sri Lanka, India, Mexico, Ecuador, and Egypt. Clearly, more studies are needed. However, we would like to suggest that the activation of fructokinase could represent one new player in driving acute and chronic kidney disease.
MATERIALS AND METHODS
Animals
KHK-KO mice were provided by Dr David Bonthron (Leeds, UK) and bred in our vivarium for this study. The experimental protocols were approved by the University of Colorado Animal Care and Use Committee. Seven-week-old male KHK-KO and WT mice from Jackson Laboratory (Bar Harbor, ME) of the same C57BL/6 background were used. Mice were kept under temperature- and humidity-controlled specific pathogen-free conditions and maintained on a 14-h dark/10-h light cycle. Mice were allowed ad libitum access to normal laboratory chow (Harlan Teklad; 2920X, Madison, WI) and given water ad libitum until the start of the study. This chow is a starch-based diet and does not contain any fructose. Mice were housed three per cage and all were treated simultaneously under equivalent conditions.
Study design
The dehydration protocol consists of placing mice in a heated environment (Isotemp-Fisher Scientific incubator, Dubuque, IA) at 39.5 °C for 30 min each hour for a total of 7 h, 5 days a week, for a total duration of 5 weeks. This procedure results in the animals sweating (primarily in the foot pads) and losing ∼15% of body weight per day in the absence of hydration (see Results).
Mice were divided into three groups. Group I received daily dehydration but with water provided during the periods between the heat exposure (WAT). Group 2 were dehydrated but provided water only for 12 h each night (WAN). Group 3 consisted of mice that were never dehydrated and water was provided ad libitum (Control). Each group consisted of six KHK-KO and six WT mice for a total of 36 mice. Standard mouse chow (Harlan Teklad, 2920X) was provided both day and night (except during the periods of heat exposure).
Blood testing
Mice were killed at the end of the dehydration period (that is, after the 7 h dehydration protocol at 5 weeks) by anesthesia and cardiac exasanguination. Serum uric acid was measured by a modified carbonate-phosphotungstate method.17 Serum fructose levels were measured using an assay kit (EnzyChrom Fructose assay kit, BioAssay System, Hayward, CA). Serum creatinine concentration was analyzed with the high-performance liquid chromatography–tandem mass spectrometry method.18 Serum creatine kinase was measured using the enzyme-linked immunosorbent assay (ELISA) kit for Creatine Kinase, Muscle (CKM; USCN Life Science, Houston TX). Serum osmolarity was measured using The Advance Micro Osmometer (Model 3300, Advanced Instruments, Norwood, MA).
Urine testing
Urine was collected at 1 and 4 weeks at the end of the dehydration period by stimulating excretion by holding the mice over a collection plate, and were analyzed for urine osmolarity (Advance Micro Osmometer), urinary neutrophil gelatinase-associated lipocalin (mouse neutrophil gelatinase-associated lipocalin ELISA Kit, Immunology Consultants Laboratory, Portland, OR), and urinary creatinine (by high-performance liquid chromatography–tandem mass spectrometry method).17
Renal histology
One kidney was removed and tissue sections were fixed in 10% formalin or methyl Carnoy's and embedded in paraffin. Sections of 3 μm thickness were stained with periodic acid–Schiff reagent. Macrophage infiltration was assessed by immunostaining with a monoclonal anti-mouse monocyte–macrophage marker F4/80 diluted 1:50 (Serotec, Oxford, UK). The number of positive cells for F4/80 was counted using an Aperio scanner (Aperio Technologies, Vista, CA). The software allows color recognition and positive cells were identified as % positive color saturation at × 20 magnification in a blinded manner using at least 15 fields for each biopsy sample. Proximal tubular brush border loss was assessed by immunostaining for angiotensin-converting enzyme (anti-murine angiotensin-converting enzyme antibody; R&D, Minneapolis, MN) and digital images were quantified at × 20 magnification using Image scope software (Aperio Technologies, Vista, CA). Renal fibrosis was determined by staining for type III collagen with a goat anti-type III collagen antibody diluted 1:100 (Southern Biotech, Birmingham, AL) and digital images at × 20 magnification were analyzed using Image scope software. The percentage of positive area was determined as the 3,3-diaminobenzidine-positive pixel values per examined interest area in each section.
Renal tissue analysis
The second kidney was removed, the renal cortex was separated from the medulla, and the cortical tissues were lysed with lysis buffer and used for tissue and western blot analyses. Western blot for aldose reductase was performed using a rabbit polyclonal mouse antibody to aldose reductase (provided by Dr Mark Petrash at the University of Colorado Denver, Denver, CO), and corrected for β-actin. Renal MCP-1 was measured on cortical lysates by ELISA (BD Biosciences Pharmingen, San Diego, CA) and corrected for total protein (Pierce, Rockford, IL). Renal fructose content was measured with the EnzyChrom fructose assay kit (BioAssay Systems, Houston, TX) and renal sorbitol was measured using the Biovision (Mountain View, CA) enzymatic assay based on the conversion of sorbitol to fructose with the generation of NADH (color) by recombinant sorbitol dehydrogenase. Renal cortical uric acid was measured by a modified carbonate-phosphotungstate method.18
Blood pressure
Systolic blood pressure was measured in conscious mice using tail-cuff sphygmomanometer (Visitech BP2000; Apex, NC,) at week 4 at the end of the dehydration period. For each mouse, five measurements were recorded and the mean was obtained. Preconditioning was not necessary as the mice remained docile throughout the procedure.
Statistical analysis
Statistical analysis was performed using one-way analysis of variance followed by t-tests with the Bonferroni correction. All values presented are expressed as mean±s.d. Significance was defined as P⩽0.05.
Acknowledgments
We thank Danone Research for providing the funding for this investigator-initiated trial. This study was supported by generous funds from Danone Research, Palaiseau, France.
RJJ and members of his laboratory (TI, MAL, CJR, and CARJ) have patent applications related to blocking fructose metabolism as a means to prevent acute and chronic kidney disease. RJJ also has funding from Amway and is on the Scientific Board of Amway. RJJ also has grants from Questcor, the NIH, and the State of Colorado. All the other authors declared no competing interests.
References
- Torres C, Aragon A, Gonzalez M, et al. Decreased kidney function of unknown cause in Nicaragua: a community-based survey. Am J Kidney Dis. 2010;55:485–496. doi: 10.1053/j.ajkd.2009.12.012. [DOI] [PubMed] [Google Scholar]
- O'Donnell JK, Tobey M, Weiner DE, et al. Prevalence of and risk factors for chronic kidney disease in rural Nicaragua. Nephrol Dial Transplant. 2011;26:2798–2805. doi: 10.1093/ndt/gfq385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peraza S, Wesseling C, Aragon A, et al. Decreased kidney function among agricultural workers in El Salvador. Am J Kidney Dis. 2012;59:531–540. doi: 10.1053/j.ajkd.2011.11.039. [DOI] [PubMed] [Google Scholar]
- Bouby N, Fernandes S. Mild dehydration, vasopressin and the kidney: animal and human studies. Eur J Clin Nutr. 2003;57 (Suppl 2:S39–S46. doi: 10.1038/sj.ejcn.1601900. [DOI] [PubMed] [Google Scholar]
- Bolignano D, Zoccali C. Vasopressin beyond water: implications for renal diseases. Curr Opin Nephrol Hypertens. 2010;19:499–504. doi: 10.1097/MNH.0b013e32833d35cf. [DOI] [PubMed] [Google Scholar]
- Bankir L, Bouby N, Ritz E. Vasopressin: a novel target for the prevention and retardation of kidney disease. Nat Rev Nephrol. 2013;9:223–239. doi: 10.1038/nrneph.2013.22. [DOI] [PubMed] [Google Scholar]
- Bouby N, Bachmann S, Bichet D, et al. Effect of water intake on the progression of chronic renal failure in the 5/6 nephrectomized rat. Am J Physiol. 1990;258:F973–F979. doi: 10.1152/ajprenal.1990.258.4.F973. [DOI] [PubMed] [Google Scholar]
- Ruepp B, Bohren KM, Gabbay KH. Characterization of the osmotic response element of the human aldose reductase gene promoter. Proc Natl Acad Sci USA. 1996;93:8624–8629. doi: 10.1073/pnas.93.16.8624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glushakova O, Kosugi T, Roncal C, et al. Fructose induces the inflammatory molecule ICAM-1 in endothelial cells. J Am Soc Nephrol. 2008;19:1712–1720. doi: 10.1681/ASN.2007121304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo P, Gersch MS, Mu W, et al. Ketohexokinase-dependent metabolism of fructose induces proinflammatory mediators in proximal tubular cells. J Am Soc Nephrol. 2009;20:545–553. doi: 10.1681/ASN.2008060576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanaspa MA, Sanchez-Lozada LG, Choi YJ, et al. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: potential role in fructose-dependent and -independent fatty liver. J Biol Chem. 2012;287:40732–40744. doi: 10.1074/jbc.M112.399899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diggle CP, Shires M, Leitch D, et al. Ketohexokinase: expression and localization of the principal fructose-metabolizing enzyme. J Histochem Cytochem. 2009;57:763–774. doi: 10.1369/jhc.2009.953190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimoto T, Lanaspa MA, Le MT, et al. Opposing effects of fructokinase C and A isoforms on fructose-induced metabolic syndrome in mice. Proc Natl Acad Sci USA. 2012;109:4320–4325. doi: 10.1073/pnas.1119908109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama T, Kosugi T, Gersch M, et al. Dietary fructose causes tubulointerstitial injury in the normal rat kidney. Am J Physiol Renal Physiol. 2010;298:F712–F720. doi: 10.1152/ajprenal.00433.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama M, Isshiki K, Kume S, et al. Fructose induces tubulointerstitial injury in the kidney of mice. Biochem Biophys Res Commun. 2012;419:244–249. doi: 10.1016/j.bbrc.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Wijkstrom J, Leiva R, Elinder CG, et al. Clinical and pathological characterization of Mesoamerican nephropathy: a new kidney disease in Central America. Am J Kidney Dis. 2013;62:908–918. doi: 10.1053/j.ajkd.2013.05.019. [DOI] [PubMed] [Google Scholar]
- Henry RJ, Sobel C, Kim J. A modified carbonate phosphotungstate method for the determination of uric acid and comparison with the spectrophoto-metric uricase method. Am J Clin Pathol. 1957;28:152–160. doi: 10.1093/ajcp/28.2.152. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Boysen G, Li F, et al. Tandem mass spectrometry measurements of creatinine in mouse plasma and urine for determining glomerular filtration rate. Kidney Int. 2007;71:266–271. doi: 10.1038/sj.ki.5002033. [DOI] [PubMed] [Google Scholar]