Abstract
Background
The mouse corneal stroma varies in thickness across its diameter. The purpose of the present study was to explain this variation and to advance our understanding of stromal lamellar architecture in the mammalian cornea.
Methods
Eight C57BL/6 mice were killed, eyes enucleated, immersed in 2% glutaraldehyde fixative, processed and sectioned transversely for light and transmission electron microscopy. Transmission electron micrographs were assembled into montages and printed at 5000× magnification and used for lamellar counts and thickness assessments.
Results
The mouse cornea had an average of 49.8 ± 2.4 lamellae centrally averaging 2.1 µm in thickness versus 35.5 ± 3.0 lamellae, averaging 1.9 µm in thickness peripherally. The central to peripheral decrease in number lamellae and lamellar thickness measured utilizing the transmission electron microscope was statistically significant (P < 0.005).
Conclusions
This study demonstrated that the thickness difference between the thicker central and thinner peripheral mouse cornea is explained primarily by the number of lamellae present and that the peripheral lamellar dropout occurred in the anterior 2/3 of stroma. The decreased lamellar count towards the periphery suggested that not all lamellae cross the cornea limbus to limbus. These findings may be relevant to the thickness variation of the human cornea.
Keywords: cornea, mouse, stromal lamellae, thickness
INTRODUCTION
The structure of the human corneal stroma has many functional implications. It determines corneal refractive power,1 the distribution of water in the presence of stromal oedema,2–7 the response of the cornea to refractive surgery8,9 and the evolution of keratoconus, through a mechanism involving a loss of connective tissue adhesiveness.10,11 The last mentioned underlies the current treatment of keratoconus by cross-linking therapy.12–14 Consequently it is important to understand stromal architecture and in particular, the organization of collagen across the full extent of the cornea.
The first accurate measurements of corneal thickness in the living human eye were made in the 1800s when it was concluded that, across its width, the cornea was thicker in its periphery than at its centre.15 This was supported later by a major study using optical pachymetry16 and has since been confirmed by others, using various techniques – ultrasound,17–19 optical coherence tomography19 and slit-scanning pachymetry.20 None of these reports offered a formal anatomical explanation for this thickness variation although it has been suggested that it may be based on differences between the organization of the anterior and posterior stromal collagen lamellae.21
Kokott,22 who studied the organization of collagen bundles in human cornea and sclera by teasing their fibrils apart, concluded that the lamellae had an orthogonal arrangement in the central cornea and extended without interruption from limbus to limbus, with a circular arrangement at the limbus itself. This view has been adopted in several textbook accounts of corneal structure.1,23–26 However, there is evidence that, for a proportion of collagen bundles at least, this is not the case. Many anterior lamellae, central to the limbus, insert into the anterior limiting lamina (ALL)24,27–31 or terminate focally, subjacent to the ALL, in electron-dense formations.32 These insertions probably make an important contribution to the asphericity of anterior corneal surface and hence to the optical power of the cornea.21 They account too, for the greater antero-posterior interweave in the anterior third of the stroma, which, together with differences in antero-posterior stromal proteoglycan distribution (e.g. in bovine cornea),2,33 is thought to limit the swellability of the anterior stroma considerably.3,4,7 Their existence also indicates that, at least in the anterior third of the stroma, collagen bundles are present, which are not interlimbal. In addition, narrow and wide angle X-ray diffraction studies have indicated that the lamellae of the posterior two-thirds of the stroma have a predominantly orthogonal arrangement, registered on the vertical and horizontal meridian. Those of the anterior stroma are more obliquely aligned. Furthermore, this work, supplemented by more recent microfocus X-ray techniques, has concluded that the annular orientation of limbal and peripheral corneal lamellae is due to the presence of arcs of collagen bundles, which traverse the sclera and enter the limbus tangentially.34–36 In attempting to understand how the organization of collagen lamellae influences the distribution of thickness across the mammalian cornea, we have chosen to study the murine cornea, as it can be obtained in the fresh, unswollen state and has a more simple structure than that of the human cornea. In contrast to the human, we have found, in three different strains of mouse, that the thickness of the peripheral cornea is significantly less (P < 0.001) than that of the central cornea, with up to a 60 % decrease from centre to periphery.37 This attenuation is due to a combined decrease in stromal and epithelial thickness in the periphery. Although the mouse corneal thickness variation across its meridional width is the reverse of that in the human cornea, it may serve as a model for how such a variation is accomplished in the mammalian eye. In the present study the influence of stromal lamellar number on regional thickness variation was examined using transmission electron microscopy (TEM).
METHODS
All experiments were conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Eight adult C57BL/6 mice, 6–8 weeks of age, were killed. A few drops of 2% glutaraldehyde in 80 mM sodium cacodylate buffer, 330 mOsm/kg fixative38 were immediately applied to the corneas after resection of the eye lids. One intact eye was carefully enucleated from each mouse and immersed in fixative for 4–6 h to ensure adequate tissue preservation. The tissue preparation protocol followed has been published previously.37
Thick, transverse sections (0.5–1 µm) were cut from the central cornea with an ultramicrotome (Research Manufacturing Co. Inc MT-7000, Tucson, Arizona, USA) and stained with 1% toluidine blue for assessment of orientation with an Olympus BX51 (Olympus America, Center Valley, PA, USA) light microscope. For morphological analysis ultrathin sections were obtained and mounted on parallel bar, copper grids (200MP, Cat # G200P, Electron Microscopy Sciences, Hatfield, PA, USA). The sections were double stained, first, in 3.5% uranyl acetate for 20 min at 60°C, followed by Reynold’s lead citrate for 10 min at room temperature. The grids were examined in a Tecnai G2 Bio Twin Spirit (FEI Company, Hillsboro, Oregon, USA) TEM and consecutive slightly overlapping micrographs were captured digitally at 890× magnification across the total thickness of the cornea centrally and peripherally. Micrographs were assembled into montages using image assembly software, PanaVue ImageAssembler 3 (Sharelt Inc, Quebec, Canada) and printed (Roland FJ-52, Roland DGA Corporation, Irvine, CA, USA) at a final magnification of 5000×. All measurements were carried out by one investigator (JTH) following strict, preset criteria. According to these criteria the distinction between adjacent lamellae was based on an obvious difference in contrast between collagen bundles generated by different fibre orientations.39 If obvious branching was noticed within the micrograph care was taken not to double-count such lamellae. Presence of a keratocyte or its cytoplasmic projection as an indication of a lamellar border and lamellae thinner than 0.2 µm were not included in the count.
For analysis, the corneal stroma was divided precisely, into anterior, middle and posterior thirds, to investigate differences in the number of lamellae per unit length between the regions. After the lamellae were counted their individual width was measured using a millimetre ruler.
Peripheral counts were made at the extremity of the cornea, defined histologically as immediately central to the limbal capillaries and to the anterior edge of the trabecular meshwork. The morphometry was conducted in triplicate, at the same location, within a defined anterior-posterior, 10-µm-wide axial band, at the corneal apex and periphery. Central corneal counts were carried out 1300 µm from the peripheral extreme of the cornea and were based on the corneal diameter for this species established and published previously.37 Lamellae counts and individual lamellar thicknesses were measured by a single observer (JTH) using specified criteria to minimize inter-observer differences. A single observer was used, based on a previous study, which showed that the coefficient of variation between trained observers was 1% when determining the number of lamellae present in the human stroma.39 Furthermore, full stromal and epithelial thicknesses were also measured.
A paired t-test was used to compare the average central with the average peripheral corneal counts and measurements. The statistical significance was set to P < 0.05. Data are presented as mean ± SEM.
RESULTS
Under light microscope and TEM, the stroma was found to be composed of collagen fibrils organized into lamellae, most of which were well-demarcated and arranged as flattened sheets running parallel to the surfaces of the cornea (Figs 1,2). As in many other non-primate, mammalian corneas, the ALL was absent. Also different from the human cornea, its most anterior stromal lamellae were disposed in regular layers, without evident interweaving (Fig. 3). Keratocytes were present predominantly between lamellae.
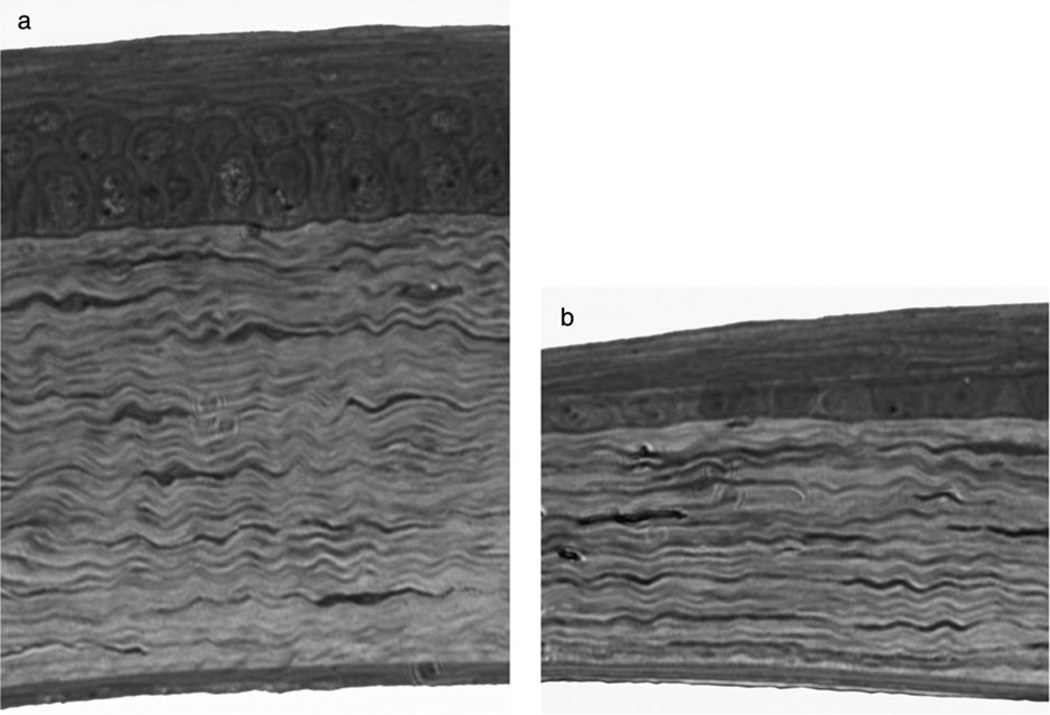
Figure 1.
Light micrograph of central (a) and peripheral (b) murine cornea of the same eye. Towards the peripheral cornea the thinning of the stroma is greater in terms of microns than the attenuation of the epithelium. Magnification: ×400.
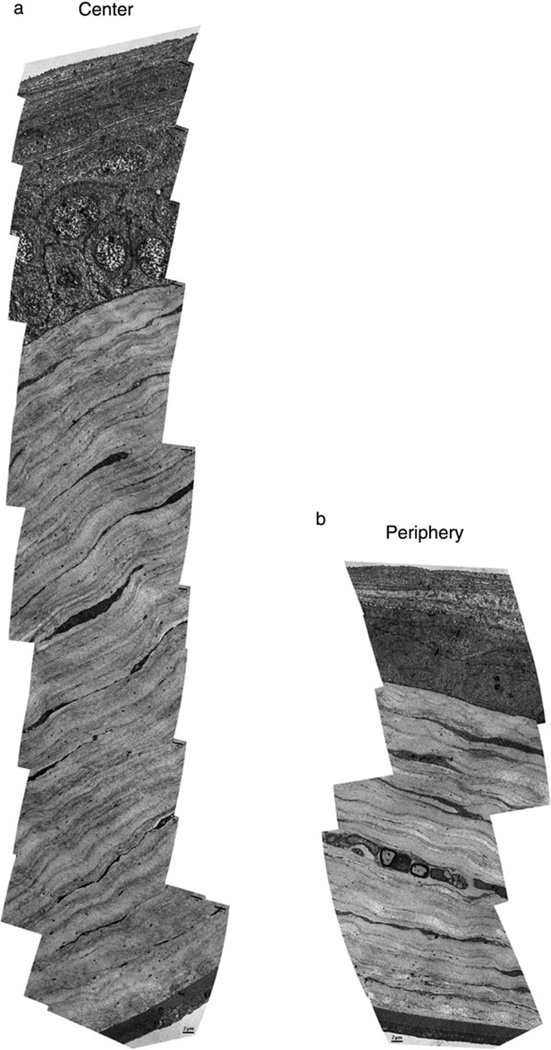
Figure 2.
Transmission electron micrograph montages illustrating transverse section of central (a) and peripheral (b) murine cornea in the same eye. The reduced number of lamellae in the peripheral cornea compared with the central cornea is clearly demonstrated.
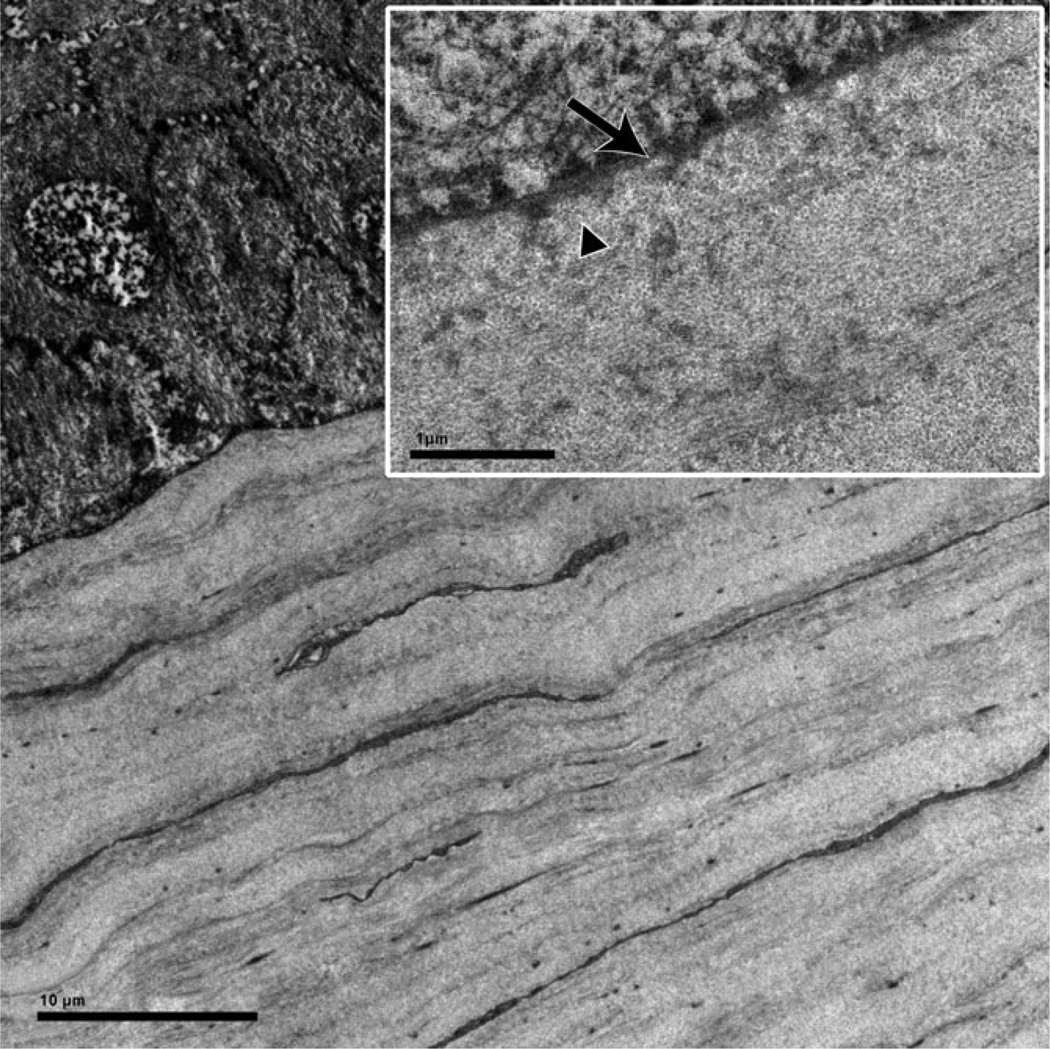
Figure 3.
Ultrastructural detail of anterior stroma and its interface with the epithelium. Lamellae of the anterior stroma are laid down over each other in a uniform manner and the interwoven and thin lamellae of the primate anterior stroma are absent. Inset: High magnification: The epithelial basement membrane (arrow) is lined by collagen fibres (triangle) of parallel orientation. Note that the anterior limiting lamina with its randomly oriented fibrils is not present.
Two assembled montages from each of the eight corneas, one from the centre and one from the periphery, were assessed for the overall corneal stromal thickness from the basement membrane of the epithelium to the posterior limiting lamina, the number of lamellae and individual lamellar and epithelial thickness was measured (Fig. 2). The stroma had an average thickness of 115.9 ± 5.4 µm centrally compared with 72.9 ± 5.7 µm peripherally. The epithelium measured 44.6 ± 1.7 µm in the central cornea compared with 26.9 ± 2.2 µm in the periphery.
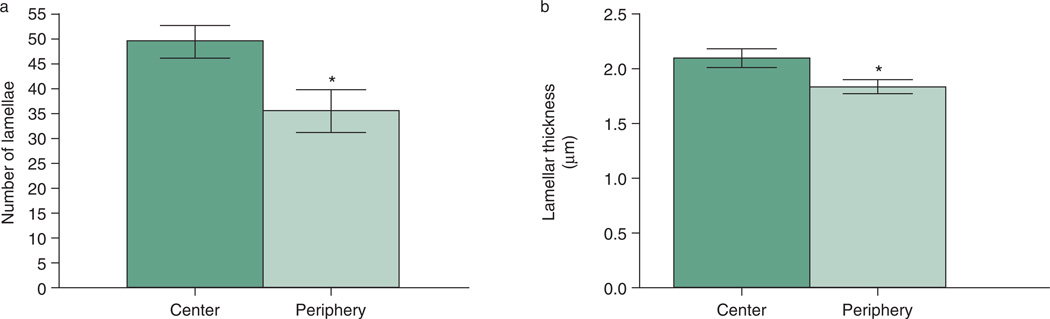
The C57BL/6 mouse cornea had an average of 49.8 ± 2.4 lamellae centrally (range 39–63), compared with an average of 35.5 ± 3.0 (range 26–53) lamellae peripherally (Fig. 4a). The average individual lamellar thickness was measured as 2.10 ± 0.1 µm centrally and 1.86 ± 0.1 µm peripherally (Fig. 4b). A significant decrease in both the average number and average thickness of the lamellae (P < 0.01) was found in the peripheral compared with the central cornea.
Figure 4.
Average number (a) and thickness (b) of lamellae present centrally and peripherally. A match paired t-test was utilized to compare number of lamellae and lamellar thickness between the central and peripheral cornea. Data are presented as mean ± SEM. Asterisk = (P < 0.01).
When comparing the central versus the peripheral lamellar counts, of the three, equal-sized stromal regions, the anterior and mid thirds of the central cornea had a greater number of lamellae compared with the posterior 1/3. In contrast, the peripheral cornea had an almost identical number of lamellae in the three regions (Table 1). The difference in number of lamellae between the central and peripheral cornea was found to be statistically significant for the anterior and mid regions (P < 0.05) but not for the posterior region (P = 0.4) of the cornea.
Table 1.
Average number of lamellae present in three stromal regions
| Number of lamellae | Central (C) | Peripheral (P) | P-value C vs. P |
|---|---|---|---|
| Anterior 1/3 | 18.5 ± 1.0 | 10.6 ± 1.2 | (P = 0.0002) |
| Mid 1/3 | 17.9 ± 1.6 | 13.0 ± 1.3 | (P = 0.016) |
| Posterior 1/3 | 13.4 ± 0.9 | 11.9 ± 1.4 | (P = 0.4) |
| Total number of lamellae | 49.8 ± 2.4 | 35.5 ± 3.0 | (P = 0.0004) |
A matched pair t-test was used to compare between the central and peripheral cornea. Data are presented as mean ± SEM.
DISCUSSION
The current study represents the first systematic attempt to provide anatomical basis for the central to peripheral thickness variation in the mammalian cornea, using the mouse as a model. We employed a fixation protocol designed to maintain osmolarity and pH at approximately physiological levels to minimize shrinkage artefacts.38 The measurement technique utilized provides the observer with well-defined reference points for precise identification of intracorneal interfaces. This is especially true with TEM, which permits lamellae to be clearly distinguished on the basis of fibre direction. Differentiation of lamellae is facilitated in the mouse by the lack of antero-posterior interweave between adjacent lamellae, which complicates measurement in the human cornea. There is some evidence that the lamellae in the mid and posterior regions of the human corneal stroma are of similar antero-posterior width to those of the mouse cornea. The average thickness of the central human cornea is 535 µm,40 which gives a stromal thickness of around 480 µm allowing 55 µm for the combined thickness of the epithelium, posterior limiting lamina and the endothelium. It follows, that the central, human, corneal stroma is four times thicker than that of the C57BL/6 mouse central corneal stroma. Recently, it was established that the human central corneal stroma is formed, on average, by 242 lamellae39 whereas the number calculated in the mouse, in the present study was 50. This suggests that lamellar thickness is comparable between the two species and, most importantly, that the basic building block of the stroma, the lamella, is of the same vertical dimension regardless of differences in corneal size. This similarity supports the use of the mouse cornea as a model for the human one with respect to specific structural arrangements such as thickness variations.
A non-uniform antero-posterior distribution of stromal lamellae has been demonstrated in human central cornea, where around 50% more lamellae are present in the anterior than in the posterior 100 µm of the stroma.39 This emphasizes the value of counting all the lamellae across the entire stromal thickness, rather than extrapolating lamellar counts from regional samples. Using well-defined criteria, it is possible to achieve low inter-observer variation in measuring lamellar number across the thickness of the human cornea39 and we used the same technique here, but with a single trained observer (JTH).
The central thickness of the corneal stroma reported in the present study is comparable to that reported in other studies, using various methods, in mice of different strains.41–43 Although the value of 116 µm found here is approximately 20 µm thicker than that found in our earlier study,37 this is likely to be due to the normal thickness variation encountered in the strain studied. Age, is an unlikely explanation, as the mice were of similar age in both studies.37
The mouse corneal stroma was found to be approximately 60% thicker centrally than peripherally (116 vs. 73 µm). Part of this thickness variation was accounted for by a peripheral thinning of the lamellae and of the epithelium. Thus the average lamellar thickness was 2.1 µm in the central cornea compared with 1.9 µm in the periphery, a reduction of about 11%. Also, the peripheral epithelium was reduced in thickness by 40% (18 µm), compared with the centre. However, the major contributor to the peripheral attenuation of corneal thickness in terms of microns was a significant fall in the average number of lamellae across the thickness of the cornea, from 50 in the centre to 36 in the periphery.
The different lamellar counts reported here for the central and peripheral cornea of the mouse, and perhaps, to a lesser extent a minor reduction in lamellar thickness, explain the stromal contribution to corneal thickness variation. An important conclusion that may be drawn is that a proportion of lamellae in the centre of the mouse cornea do not extend to the limbus. This is implied by the decrease in lamellar number from centre to periphery. The small decrease in lamellar thickness could also imply a decrease in collagen fibre number per lamella, although this apparent thinning could also be explained by a flattening of the lamellae in the plane of section.
This observation has a parallel in the human cornea, although operating in the opposite direction. In the human, corneal thickness is greater in the periphery than the centre and there appear to be more collagen lamellae in the periphery than in the central cornea.44 One explanation put forward to explain this is the entry of collagen bundles of scleral origin to contribute to the limbal annulus.35 Further, as noted, many lamellae presumed to originate from the limbus are inserted into the ALL. These insertions have been likened to the sutural fibres found in the dogfish shark,45 which prevent swelling in a cornea that possesses no endothelium.46 Morishige et al. have referred to these lamellae as ‘sutural lamellae’.45,47 Additionally, as noted, anterior lamellae terminate in electron-dense formations subjacent to the ALL.32
A further structural finding in the present study was as follows: a comparison between lamellar counts from the anterior, mid and posterior stromal regions revealed that the posterior count did not show a statistically significant variation between central and peripheral cornea. However, the anterior and mid stromal regions showed a statistically significant lamellar dropout in the peripheral zone. This suggested that the posterior lamellae may indeed all cross the entire corneal width whereas a proportion of the more anterior ones do not. This conclusion is similar to that which has been drawn for the lamellae of the posterior two-thirds of the human cornea.35
Various authors have suggested a relationship between the preferred, orthogonal orientation of collagen bundles in the posterior stroma of the human cornea and the disposition of forces imposed by the rectus muscles.48 This implies either a postnatal role for extraocular muscle contraction in the adaptive modelling of the stromal lamellae or, more likely, the past influence of evolutionary pressures. In the mouse, corneal modelling, including completion of the limbal annulus, is not completed until the 28th postnatal day.49 It may be relevant that in animals, which, like the primate, have frontally placed eyes, eye movements and thus activation of the extraocular muscles, play an important role in the location and tracking of visual targets whereas in animals with laterally placed eyes, such as the mouse, this function is subserved more by head movements. It is therefore of interest that the central region of the mouse cornea, unlike that of the human cornea, does not contain a significant amount of preferentially aligned, orthogonally disposed collagen.50 It could be that the contribution to peripheral corneal thickness of arcing fibres of scleral origin, thought to exist in the human cornea35 is lacking in those species in which eye movements in the horizontal plane are limited.
In conclusion, it may be summarized that the stroma of mouse cornea differs from that of the human cornea in several respects. The human cornea possesses an ALL, and an anterior stroma with a marked lamellar interweave. The corneal thickness in the murine eye is modulated primarily by the number of lamellae present in the anterior 2/3 of the stroma. This intracorneal axial lamellar count variation suggested that not all stromal lamellae bridge from limbus to limbus. These principals of stromal architecture may also apply to the human cornea, although its thickness variation is the reverse of that in the mouse.
ACKNOWLEDGEMENTS
The authors would like to thank Dr Alan Burns for providing the C57BL/6 mice utilized for this experiment and Dr William Miller for constructive comments on the manuscript. We would also like to thank Dr Deborah Otteson for excellent technical advice and support. We gratefully recognize the funding from NEI Core Grant P30 EY007551 to University of Houston College of Optometry. Last but not least we wish to acknowledge the generous graduate student stipends by Optikbranschen (Stockholm, Sweden), the 2009 William C Ezell Fellowship sponsored by Bausch and Lomb and the 2010 William C Ezell Fellowship sponsored by Vistakon to Ms Johanna Tukler Henriksson.
Funding sources: NEI Core Grant P30 EY007551 to University of Houston College of Optometry.
Footnotes
Conflict/competing interest: No stated conflict of interest.
REFERENCES
- 1.Edelhauser HF, Ubels JL. The Cornea. In: Kaufman PL, Alm A, editors. Adler’s Physiology of the Eye. 10th edn. Vol. 56. St Louis, MO: CV Mosby; 2003. [Google Scholar]
- 2.Bettelheim FA, Plessy B. The hydration of proteoglycans of bovine cornea. Biochim Biophys Acta. 1975;381:203–214. doi: 10.1016/0304-4165(75)90202-0. [DOI] [PubMed] [Google Scholar]
- 3.Cristol SM, Edelhauser HF, Lynn MJ. A comparison of corneal stromal edema induced from the anterior or the posterior surface. Refract Corneal Surg. 1992;8:224–229. [PubMed] [Google Scholar]
- 4.Edelhauser HF. Endothelial and stromal response to injury: corneal biophysics workshop. Corneal Biomechanics Wound Healing NIH. 1989:191–194. [Google Scholar]
- 5.Kikkawa Y, Hirayama K. Uneven swelling of the corneal stroma. Invest Ophthalmol. 1970;9:735–741. [PubMed] [Google Scholar]
- 6.Lee D, Wilson G. Non-uniform swelling properties of the corneal stroma. Curr Eye Res. 1981;1:457–461. doi: 10.3109/02713688109019986. [DOI] [PubMed] [Google Scholar]
- 7.Müller LJ, Pels E, Vrensen GF. The specific architecture of the anterior stroma accounts for maintenance of corneal curvature. Br J Ophthalmol. 2001;85:437–443. doi: 10.1136/bjo.85.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang SS, Maurice DM, Ramirez-Florez S. Quantitative measurement of corneal haze after myopic PRK. J Refract Surg. 1996;12:412–416. doi: 10.3928/1081-597X-19960301-16. [DOI] [PubMed] [Google Scholar]
- 9.Ramirez-Florez S, Maurice DM. Inflammatory cells, refractive regression, and haze after excimer laser PRK. J Refract Surg. 1996;12:370–381. doi: 10.3928/1081-597X-19960301-12. [DOI] [PubMed] [Google Scholar]
- 10.Meek KM, Tuft SJ, Huang Y, et al. Changes in collagen orientation and distribution in keratoconus corneas. Invest Ophthalmol Vis Sci. 2005;46:1948–1956. doi: 10.1167/iovs.04-1253. [DOI] [PubMed] [Google Scholar]
- 11.Bron AJ. Keratoconus – the disease. Br J Contact Lens Assoc (Proc Ruben Symp London 1983) 1984;7:57–62. [Google Scholar]
- 12.Koller T, Seiler T. [Therapeutic cross-linking of the cornea using riboflavin/UVA] Klin Monbl Augenheilkd. 2007;224:700–706. doi: 10.1055/s-2007-963492. [DOI] [PubMed] [Google Scholar]
- 13.Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135:620–627. doi: 10.1016/s0002-9394(02)02220-1. [DOI] [PubMed] [Google Scholar]
- 14.McCall AS, Kraft S, Edelhauser HF, et al. Mechanisms of corneal tissue cross-linking in response to treatment with topical riboflavin and long-wavelength ultraviolet radiation (UVA) Invest Ophthalmol Vis Sci. 2010;51:129–138. doi: 10.1167/iovs.09-3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerlach J. Von der Cornea. Handbuch der allegemeinen und speciellen gewebelehre des menschlichen körpers. Mainz: Verlag von Eduard Janitsch; 1854. [Google Scholar]
- 16.Martola EL, Baum JL. Central and peripheral corneal thickness. A clinical study. Arch Ophthalmol. 1968;79:28–30. doi: 10.1001/archopht.1968.03850040030009. [DOI] [PubMed] [Google Scholar]
- 17.Avitabile T, Marano F, Uva MG, Reibaldi A. Evaluation of central and peripheral corneal thickness with ultrasound biomicroscopy in normal and keratoconic eyes. Cornea. 1997;16:639–644. [PubMed] [Google Scholar]
- 18.Gromacki SJ, Barr JT. Central and peripheral corneal thickness in keratoconus and normal patient groups. Optom Vis Sci. 1994;71:437–441. doi: 10.1097/00006324-199407000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Prospero Ponce CM, Rocha KM, Smith SD, Krueger RR. Central and peripheral corneal thickness measured with optical coherence tomography, Scheimpflug imaging, and ultrasound pachymetry in normal, keratoconus-suspect, and post-laser in situ keratomileusis eyes. J Cataract Refract Surg. 2009;35:1055–1062. doi: 10.1016/j.jcrs.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 20.Jonuscheit S, Doughty MJ. Discrepancy between central and midperipheral corneal thickness measurements obtained with slit-scanning pachymetry and noncontact specular microscopy. J Cataract Refract Surg. 2009;35:2127–2135. doi: 10.1016/j.jcrs.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 21.Bron AJ. The architecture of the corneal stroma. Br J Ophthalmol. 2001;85:379–381. doi: 10.1136/bjo.85.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kokott W. Über mechanisch-funktionelle Strukturen des Auges. Albrecht Von Graefes Arch Ophthalmol. 1938;138:424–485. [Google Scholar]
- 23.Duke-Elder S, Wybar KC. Cornea. In: Duke-Elder S, editor. System of Ophthalmology, Vol 2. The Anatomy of the Visual System. St Louis, MO: Henry Kimpton; 1961. pp. 92–131. [Google Scholar]
- 24.Hogan MJ, Alvarado JA, Weddell JE. Histology of the Human Eye. Philadelphia, PA: WB Saunders; 1971. [Google Scholar]
- 25.Kaufman PL, Alm A. Adler’s Physiology of the Eye. 10th edn. St Louis, MO: CV Mosby; 2003. [Google Scholar]
- 26.Klyce SD, Beuerman RW. Structure and function of the cornea. In: Kaufman HE, Barron BA, McDonald MB, editors. The Cornea. 2nd edn. Boston, MA: Butterworth-Heinemann; 1998. pp. 3–50. [Google Scholar]
- 27.Bron AJ, Tripathi RC. The anterior corneal mosaic. Br J Physiol Opt. 1970;25:8–13. [PubMed] [Google Scholar]
- 28.McTigue JW. The human cornea: a light and electron microscopic study of the normal cornea and its alterations in various dystrophies. Trans Am Ophthalmol Soc. 1967;65:591–660. [PMC free article] [PubMed] [Google Scholar]
- 29.Polack FM. Morphology of the cornea. I. Study with silver stains. Am J Ophthalmol. 1961;51:1051–1056. doi: 10.1016/0002-9394(61)91794-9. [DOI] [PubMed] [Google Scholar]
- 30.Salzmann M. Anatomie und Histologie des menschlichen Augapfels im Normalzustande seine Entwicklung und sein Altern. Vienna: Franz Deuticke Verlag; 1912. [Google Scholar]
- 31.Maurice DM. The cornea and sclera. In: Davson H, editor. The Eye. New York and London: Academic Press; 1969. pp. 489–600. [Google Scholar]
- 32.Mathew JH, Bergmanson JP, Doughty MJ. Fine structure of the interface between the anterior limiting lamina and the anterior stromal fibrils of the human cornea. Invest Ophthalmol Vis Sci. 2008;49:3914–3918. doi: 10.1167/iovs.07-0707. [DOI] [PubMed] [Google Scholar]
- 33.Castoro JA, Bettelheim AA, Bettelheim FA. Water gradients across bovine cornea. Invest Ophthalmol Vis Sci. 1988;29:963–968. [PubMed] [Google Scholar]
- 34.Meek KM, Boote C. The organization of collagen in the corneal stroma. Exp Eye Res. 2004;78:503–512. doi: 10.1016/j.exer.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Meek KM, Boote C. The use of X-ray scattering techniques to quantify the orientation and distribution of collagen in the corneal stroma. Prog Retin Eye Res. 2009;28:369–392. doi: 10.1016/j.preteyeres.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 36.Newton RH, Meek KM. Circumcorneal annulus of collagen fibrils in the human limbus. Invest Ophthalmol Vis Sci. 1998;39:1125–1134. [PubMed] [Google Scholar]
- 37.Henriksson JT, McDermott AM, Bergmanson JP. Dimensions and morphology of the cornea in three strains of mice. Invest Ophthalmol Vis Sci. 2009;50:3648–3654. doi: 10.1167/iovs.08-2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doughty MJ, Bergmanson JP, Blocker Y. Shrinkage and distortion of the rabbit corneal endothelial cell mosaic caused by a high osmolality glutaraldehyde-formaldehyde fixative compared to glutaraldehyde. Tissue Cell. 1997;29:533–547. doi: 10.1016/s0040-8166(97)80054-7. [DOI] [PubMed] [Google Scholar]
- 39.Bergmanson JP, Horne J, Doughty MJ, Garcia M, Gondo M. Assessment of the number of lamellae in the central region of the normal human corneal stroma at the resolution of the transmission electron microscope. Eye Contact Lens. 2005;31:281–287. doi: 10.1097/01.icl.0000165280.94927.0d. [DOI] [PubMed] [Google Scholar]
- 40.Doughty MJ, Zaman ML. Human corneal thickness and its impact on intraocular pressure measures: a review and meta-analysis approach. Surv Ophthalmol. 2000;44:367–408. doi: 10.1016/s0039-6257(00)00110-7. [DOI] [PubMed] [Google Scholar]
- 41.Jester JV, Ghee Lee Y, Li J, et al. Measurement of corneal sublayer thickness and transparency in transgenic mice with altered corneal clarity using in vivo confocal microscopy. Vision Res. 2001;41:1283–1290. doi: 10.1016/s0042-6989(00)00222-4. [DOI] [PubMed] [Google Scholar]
- 42.Schulz D, Iliev ME, Frueh BE, Goldblum D. In vivo pachymetry in normal eyes of rats, mice and rabbits with the optical low coherence reflectometer. Vision Res. 2003;43:723–728. doi: 10.1016/s0042-6989(03)00005-1. [DOI] [PubMed] [Google Scholar]
- 43.Song J, Lee YG, Houston J, et al. Neonatal corneal stromal development in the normal and lumicandeficient mouse. Invest Ophthalmol Vis Sci. 2003;44:548–557. doi: 10.1167/iovs.02-0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamada R, Giraud JP, Graf B, Pouliquen Y. [Analytical and statistical study of the lamellae, keratocytes and collagen fibrils of the central region of the normal human cornea. (Light and electron microscopy)] Arch Ophtalmol Rev Gen Ophtalmol. 1972;32:563–570. [PubMed] [Google Scholar]
- 45.Morishige N, Wahlert AJ, Kenney MC, et al. Second-harmonic imaging microscopy of normal human and keratoconus cornea. Invest Ophthalmol Vis Sci. 2007;48:1087–1094. doi: 10.1167/iovs.06-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goldman JN, Benedek GB, Dohlman CH, Kravitt B. Structural alterations affecting transparency in swollen human corneas. Invest Ophthalmol. 1968;7:501–519. [PubMed] [Google Scholar]
- 47.Morishige N, Takagi Y, Chikama TI, Takahara A, Nishida T. Three-dimensional analysis of collagen lamellae in the anterior stroma of the human cornea visualized by second harmonic generation imaging microscopy. Invest Ophthalmol Vis Sci. 2010;52:911–915. doi: 10.1167/iovs.10-5657. [DOI] [PubMed] [Google Scholar]
- 48.Daxer A, Fratzl P. Collagen fibril orientation in the human corneal stroma and its implication in keratoconus. Invest Ophthalmol Vis Sci. 1997;38:121–129. [PubMed] [Google Scholar]
- 49.Sheppard J, Hayes S, Boote C, Votruba M, Meek KM. Changes in corneal collagen architecture during mouse postnatal development. Invest Ophthalmol Vis Sci. 2010;51:2936–2942. doi: 10.1167/iovs.09-4612. [DOI] [PubMed] [Google Scholar]
- 50.Quantock AJ, Dennis S, Adachi W, et al. Annulus of collagen fibrils in mouse cornea and structural matrix alterations in a murine-specific keratopathy. Invest Ophthalmol Vis Sci. 2003;44:1906–1911. doi: 10.1167/iovs.02-0884. [DOI] [PubMed] [Google Scholar]