Abstract
PURPOSE
To assess the application of near-infrared (NIR) heptamethine carbocyanine dyes, IR-783 and the synthetic analog MHI-148, as optical imaging agents for rapid detection of human kidney cancer.
MATERIALS AND METHODS
The uptake, retention and subcellular localization of these organic dyes were investigated in cultured kidney cancer cells. Tumor specificity of dye uptake and retention was evaluated by whole-body imaging of mice bearing human kidney cancer xenografts or freshly harvested clinical kidney cancer specimens. In addition, dye accumulation at the tissue and cellular levels was confirmed by ex vivo studies with results confirmed by fluorescence imaging of the frozen tissue sections. Peripheral blood spiked with kidney cancer cells was stained to simulate the detection of circulating tumor cells.
RESULTS
Preferential uptake and retention of carbocyanine NIR dyes was observed in cultured human kidney cancer cells, human kidney cancer cell-spiked whole blood, human kidney cancer xenografts and freshly harvested human kidney cancer tissues compared to normal kidney epithelial cells or normal host organs.
CONCLUSIONS
We described a new class of NIR heptamethine carbocyanine dyes showing potential for detecting kidney cancer cells in circulating blood and kidney cancer cells in clinical specimens. NIR carbocyanine dyes can be further developed as dual modality agents for deep-tissue imaging of localized and disseminated kidney cancer in patients.
Keywords: Heptamethine cyanine, near-infrared imaging, kidney cancer, detection, diagnosis
Introduction
Kidney cancer is notorious for its poor prognosis. The survival rate is correlated to early diagnosis. 1 Because kidneys are anatomically amenable to non-invasive diagnosis, optical imaging modalities would be useful for early detection of kidney cancer.
Near-infrared (NIR) tumor imaging is an attractive non-invasive technique. The use of NIR cyanine dyes as contrasting agents has been pursued by several groups, including ours. 2-5 Several unique features of these dyes make them particularly suitable for tumor imaging. These organic dyes are small in size and can easily penetrate tissues and cells. Inside tumor cells these dyes are bound by proteins or nucleic acids. 6, 7 Binding leads to fluorophore rigidization, causing a marked increase of extinction coefficient favoring NIR detection. 8-10 We previously defined two heptamethine carbocyanine dyes, IR-783 and its synthetic analog MHI-148, that display tumor cell specificity, being taken up and retained by human cancer but not non-cancerous cells due to the differential expression of organic anion transporting peptides (OATPs) in cancer cells. 11, 12 These organic dyes could potentially be used directly as optical cancer imaging probes, not requiring additional chemical conjugation to cancer-specific ligands.
To explore the clinical utility of these organic dyes, we tested IR-783 and MHI-148 for the detection of kidney cancer based on the specific uptake and retention of the carbocyanine dyes by cancer cells. The results suggest that this class of NIR dyes can be used as sensitive contrast agents for non-invasive tumor visualization.
Materials and Methods
Chemical reagents
Heptamethine carbocyanine dyes IR-780 and IR-783 were purchased from Sigma-Aldrich (St. Louis, MO). MHI-148 was synthesized and purified as previously reported. 5, 13, 14 Stock solutions (1 mM) in dimethyl sulfoxide (DMSO, Sigma-Aldrich) were diluted with appropriate vehicles, filtered through 0.22 μm filters and stored at 4μC in the dark before use.
Cell lines and cell culture
The source and culture of human embryonic kidney cells (HEK293) has been reported. 7 The source and culture of human kidney cancer cell lines SN12C, ACHN and Caki-1 were previously reported. 15
Cell and tissue uptake studies of heptamethine carbocyanine dyes
The cell staining protocol was previously reported. 7 Briefly, cells (1 × 104/well) grown on vitronectin-coated chamber slides (Nalgen Nunc, Naperville, IL) were exposed to carbocyanine dye (20 μM) in culture medium at 37°C for 30 minutes. The slides were washed twice with phosphate buffered saline (PBS), fixed with 10% paraformaldehyde, and covered with glass coverslips with aqueous mounting medium (Sigma-Aldrich). Images were recorded by confocal laser microscopy using a 633 nm excitation laser and 670-810 nm long pass emission filter. Alternatively, stained cells were imaged under a Nikon Eclipse Ti microscope, excited by a xenon arc light source and imaged through an INDO filter (780 - 840 nm).
The protocol for determining subcellular localization of the dyes was reported previously. 7 Images of mitochondria, lysosome, and the cyanine dye were acquired with confocal imaging using a previously established protocol. 16 Co-localization of the cyanine dye with subcellular organelles was assessed by merging these images.
Uptake and accumulation of carbocyanine dyes in xenograft kidney tumors
All animal work was conducted following Cedars-Sinai Medical Center institution guidelines (IACUC# 2999). Caki-1 cells were implanted in the subrenal capsules to mimic primary human kidney cancer as previously described. 17 For soft tissue and bone metastases, ACHN and SN12C cells (1 × 106) were inoculated subcutaneously or intraosseously into 4 to 6 week old athymic male mice (Ncr nu/nu).
When tumor sizes reached 2 - 6 mm in diameter as assessed by X-ray radiography or by palpation, 5 mice in each group were injected intravenously (i.v.) or intraperitoneally (i.p.) with carbocyanine dyes at a dose of 11.25 mg/kg. Whole body NIR imaging was taken 24 hours later using a Kodak Imaging Station Imaging System 4000MM (New Haven, CT), following previously established settings. 7 After whole body imaging, organs and tumors were dissected for additional ex vivo imaging by fluorescence microscopy.
NIR imaging of human kidney tumor tissues ex vivo
Human kidney tumor tissue specimens classified as clear cell carcinoma by pathologic evaluation (by AOO) were collected from 5 patients who underwent complete (2 cases) or partial (3 cases) nephrectomy at the Emory University School of Medicine. The use of human specimens was approved (IRB#1214-2003 and IRB#0001-7476) with written informed patient consent. The specimens were placed immediately on ice and transported to the laboratory within 1 hour.
Fresh tissue samples were dissected from the tumor area, normal area and transitional area into 0.5 cm × 0.5 cm × 1.5 cm slices. Slices was split into three and incubated with 20 μM IR-780, IR-783, or the PBS vehicle at 37°C for 30 minutes. 5 Tissues were washed 5 times in PBS to remove free dyes before NIR imaging with the Kodak Imaging Station. To confirm dye uptake by tumor cells, stained samples were frozen at −80°C in OCT and sectioned to 5 μm thickness for pathologic observation under a confocal microscope.
In vivo NIR imaging of freshly harvested human kidney tumor specimens
Kidney cancer specimens from 4 partial nephrectomy patients at Cedars-Sinai Medical Center were used with institutional approval (IRB#Pro00020577) and written informed consent. A 0.6 cm × 0.6 cm × 0.6 cm cube of kidney tumor tissue from each patient was kept on ice for a maximum of 1.5 hours before transport to the laboratory. These specimens were diced into 0.1 cm× 0.2 cm × 0.2 cm sizes and implanted subcutaneously or in the subrenal capsular space of 4 to 6 week old male athymic mice. After recovery from anesthesia for 12 hours, the mice were administered carbocyanine dyes i.p. at a dose of 11.25 mg/kg and subjected to daily fluorescence imaging for 5 days.
Histopathologic analysis
Following carbocyanine dye staining, kidney cancer specimens from either ex vivo incubation or in vivo implantation were recovered for histopathologic analysis. The recovered specimens were frozen in OCT medium, sectioned to 5 μm thickness, mounted in aqueous mounting media containing 4’6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, CA), and subjected to NIR imaging under a fluorescence microscope equipped with a NIR filter (Chroma Technology, Bellows Falls, VT). The same frozen section was then stained in H&E solution and inspected for histopathologic confirmation.
Detection of kidney cancer cells in blood after NIR dye staining and flow cytometry
To determine the feasibility of using NIR dye to detect human renal cancer cells in blood, a known number of SN12C human kidney cancer cells (3 × 103) in single-cell suspension were added to 3 ml citrated whole peripheral blood of healthy donors. The mix was incubated with 20 μM carbocyanine dye at 37°C for 30 minutes. The blood mononuclear cells together with the cancer cells were isolated by gradient centrifugation in Histopaque-1077 (Sigma-Aldrich) and fixed with 0.5 ml of 1% paraformaldehyde. The cells were observed under a confocal microscope and analyzed in a Becton Dickinson LSRII flow cytometer (BD BioSciences, San Jose, CA), with a red laser diode for excitation and Cy7 detector for NIR detection.
Results
We previously reported that a new group of NIR heptamethine carbocyanine dyes, including IR-783 and MHI-148, were preferentially taken up by malignant cells but not normal cells, and could be used in transgenic mice to detect prostate and colon tumors. 5 In this study, we assessed whether this cancer targeting property could be applied to the detection of kidney cancer in different experimental models.
1. Preferential uptake of heptamethine carbocyanine dyes by human kidney cancer cells
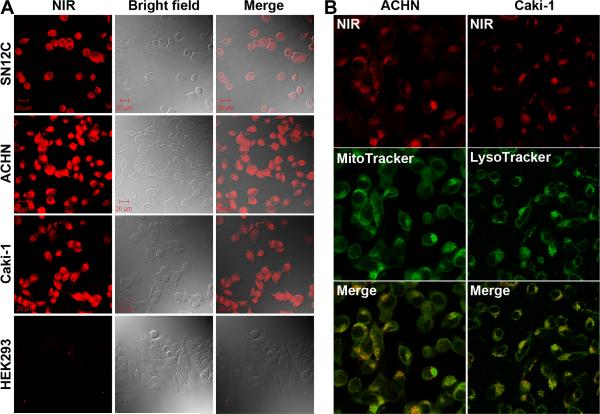
The specific uptake of NIR carbocyanine dyes was assessed in human kidney cancer SN12C, ACHN and Caki-1 cells. After removing free dyes by PBS washing, stained cells were fixed and subjected to NIR fluorescence imaging. Significant and uniform dye uptake was observed in the kidney cancer cell lines (Figure 1A). In contrast, near background uptake of these dyes was observed in the control HEK293 human fetal kidney cells (Figure 1A) and normal endothelial and prostate epithelial cells. 5 The staining was predominantly in cytosols with much weaker signals in the nuclear region, while SN12C, ACHN, and Caki-1 cells appeared to stain equally (Figure 1A). These results support our previous report that this class of dyes is taken up specifically by cancer cells but not by normal cells. 5
Figure 1. Preferential uptake of heptamethine carbocyanine dyes by cultured human kidney cancer cells.
Representative images from three separate staining experiments with IR-783 are shown. A, Photos of NIR imaging (NIR), bright field imaging (Bright field), and superimposed images (Merge) (400 ×). B, Co-localization of NIR and mitochondrial and lysosomal stains by confocal microscope (630 ×).
The cytosolic stains had a grainy appearance, suggesting that the dye bound subcellular organelles. Mitochondrial and lysosomal tracking dyes were used for subcellular localization of the carbocyanine dyes (Figure 1B). The IR-783 stain was almost completely congruent with both MitoTracker and LysoTracker, suggesting that IR-783 stained both mitochondria and lysosomes. Supporting our previous observations with prostate cancer cells, 5 dye uptake by kidney cancer cells was sensitive to blockade by bromosulfophthalein (BSP), a compound known to inhibit the function of OATPs, and only viable cells showed dye uptake and retention (data not shown).
2. Imaging human kidney tumor xenografts with carbocyanine dyes
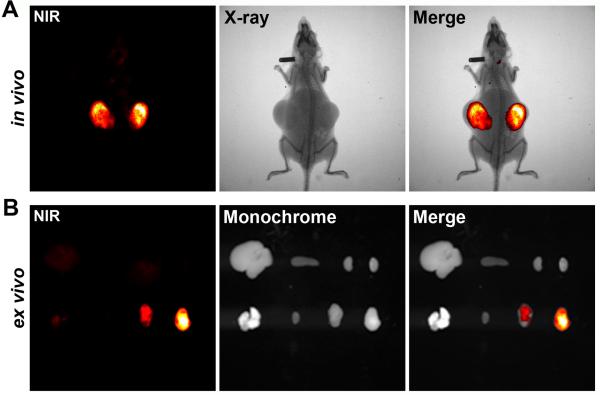
Based on the specific uptake and retention by cultured kidney cancer cells, we observed similar specificity for the uptake and retention of heptamethine cyanine dyes by kidney tumor xenografts in vivo. Athymic mice bearing subcutaneous ACHN tumors were injected with IR-783 dye and subjected to whole body NIR imaging. Time-course studies revealed that this dye initially was distributed in host normal tissues but quickly cleared in most of the cases within 6 hours. Thereafter high signal/noise ratios were detected in the tumors 24 hours after dye administration (Figure 2A). Tumor staining was compared to normal tissues by ex vivo NIR imaging (Figure 2B). We also observed that kidney tumors grown at either intraosseus (SN12C) 5 or subrenal capsule (Caki-1) sites could be readily imaged by IR-783.
Figure 2. Using heptamethine carbocyanine dyes to detect human kidney tumor xenografts.
Representative results detecting bilateral subcutaneous ACHN tumor xenografts (n = 10). A, 24 hours after i.p. administration of IR-783, whole-body (in vivo) NIR imaging (NIR) and X-ray radiography (X-ray) were obtained; NIR-X-ray images are superimposed (Merge) for tumor localization. B, 48 hours after i.p. dye administration, tumors and host organs were dissected and subjected to ex vivo imaging (ex vivo). Top row from left: liver, spleen and kidneys. Bottom row from left: lung, heart and tumors. NIR and monochrome images are superimposed (Merge) to show tumor-specific targeting by NIR dye.
Repeated non-invasive NIR imaging was used to investigate tumor retention of the carbocyanine dyes. In contrary to the fast clearance in host organs, successive imaging revealed that the specific fluorescence signal in tumor xenografts persisted for at least 5 days before gradually fading (data not shown). In many xenograft tumors, repeated imaging was carried out for up to 15 days after dye injection.
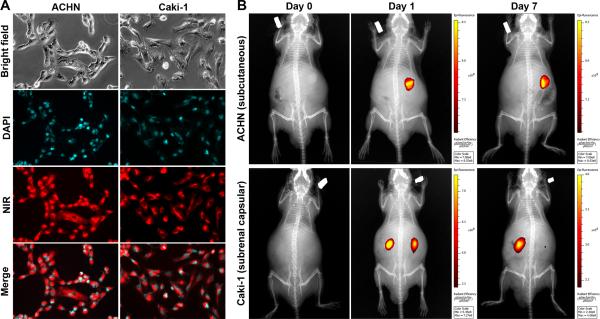
In parallel studies of synthetic MHI-148, rapid uptake of the dye was observed in ACHN and Caki-1 cells after being stained with MHI-148 (Figure 3A), and xenograft tumors could be sensitively detected by a single i.v. administration of this dye (Figure 3B). While MHI-148 in subcutaneous tumors was stable for as long as 7 days, subrenal capsular tumor staining was stable for 4 days before the fluorescence signal decayed. In mice bearing subrenal capsular Caki-1 tumors (Figure 3B), 5 of the 8 tumors could still be seen by NIR imaging 7 days after dye administration.
Figure 3. Retention of synthetic MHI-148 in kidney tumor.
A, Substantial uptake of MHI-148 dye during 30-minute staining (200 ×). B, mice bearing small (< 0.5 cm in diameter) xenograft tumors were injected i.v. with MHI-148 at day 0 and subjected to repeated NIR imaging and X-ray radiography from day 1 to day 7. Merged images are shown. Top panels: imaging of mouse bearing unilateral subcutaneous ACHN tumor. Bottom panels: imaging of mouse bearing bilateral subranal capsular Caki-1 tumors, in which one of the two capsular tumors remained fluorescent 7 days after initial staining. Each result is representative of studies of 4 mice (n = 4).
3. Ex vivo NIR imaging of freshly dissected human kidney tumors
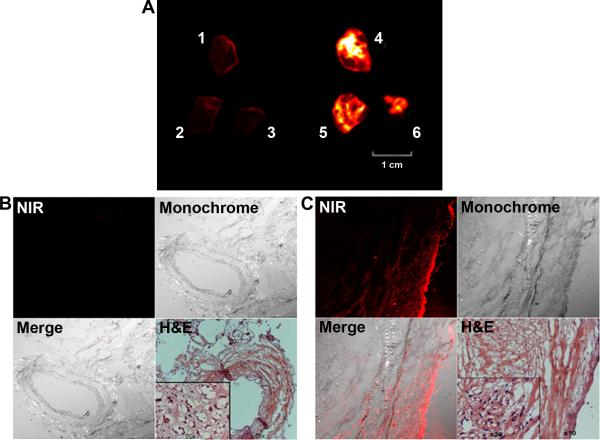
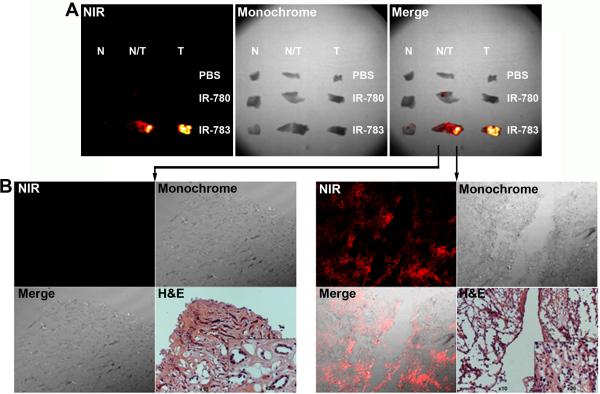
We used human kidney cancer surgical specimens to evaluate if carbocyanine dyes could detect kidney tumors from the clinic. From a complete nephrectomy, multiple samples of normal and kidney tumor portions were stained by IR-783 and subjected to ex vivo NIR imaging (Figure 4A). Marked differences in dye uptake and retention were noted between normal (sample #1, 2 and 3) and kidney cancer tissues (sample #4, 5, and 6). Tumor staining was confirmed in frozen sections of the stained specimens, which revealed no significant signals from the normal tissue (Figure 4B) but substantial dye infiltration in the tumor (Figure 4C). In another nephrectomy specimen, we tested if IR-783 and its less active structural analog IR-780 can differentiate normal (N), tumor (T) and mixed normal and tumor (N/T) regions. IR-780 was less stable and can be washed off (Figure 5A). IR-783 staining in the kidney cancer was confirmed by NIR fluorescence imaging of frozen sections (Figure 5B). IR-783 tumor specificity was confirmed in all 5 human kidney tumors examined; higher staining was always associated with tumors, compared to normal kidney tissues, although connective tissues surrounding the tumors may also yield positive albeit weaker staining.
Figure 4. Ex vivo imaging of surgically removed kidney tumor specimens.
A, differential IR-783 uptake and retention by kidney tumor samples. B, frozen section of stained normal kidney tissue (from sample # 1) subjected to NIR imaging (100 ×). An adjacent section was subjected to H&E staining (H&E) to show the histomorphology of the area, with inset displaying the normal histomorphology of the tissue (200 ×). C, the same setting was used to show the strong NIR uptake and retention in sample #4 which contains live kidney cancer cells.
Figure 5. Differential heptamethine carbocyanine staining in the transitional region of surgical kidney tumor specimens.
Representative results from 5 human kidney specimens. A, fluorescence image (NIR) and monochrome image (Monochrome) of the same view show specific staining in the tumor area by IR-783. B, frozen section of the stained specimen was cut and subjected to NIR imaging under a fluorescence microscope. Kidney cancer in the specimens was confirmed by histopathological analysis (H&E, 100 ×). Inset shows representative cancer tissue (400 ×).
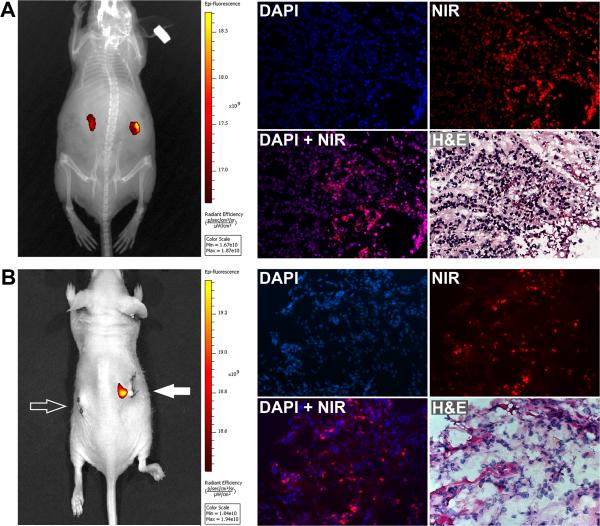
4. In vivo NIR fluorescence imaging of freshly implanted human kidney tumors
To assess whether freshly-derived kidney tumors can be detected in vivo, we implanted surgical kidney tumor specimens in athymic mice, and stained the tumor tissues with i.p. MHI-148. Whole body NIR imaging revealed that tumor specimens as small as 0.1 cm × 0.2 cm × 0.2 cm could be detected. In animals with subcutaneous implants, the implantation-inflicted wounds were detected with signals slightly above background, while the tumor implants showed strong staining (data not shown). We observed that subrenal capsular implants could be distinguished from the host tissue (Figure 6A) for as long as 4 days. Likewise, human SN12C, but not normal mouse kidney xenograft, when implanted at subrenal sites, could also be clearly detected (Figure 6B); histomorphologic and NIR dye images confirmed the presence of tumor cells within tumor specimens.
Figure 6. Using heptamethine carbocyanines to detect freshly harvested kidney tumors as renal xenograft implant.
Dices of implanted kidney tumor specimens were subjected to NIR imaging following MHI-148 administration. A, left panel shows NIR imaging results of human kidney tumor dices 2 days after implanting to subrenal capsular space. After imaging, tumor dices were removed and processed into frozen sections for in vitro validation. Right panel shows the NIR stain in the frozen tissue section. The same section was then processed for H&E to confirm the location of normal and cancer cells (200 ×). B, same procedures were used to detect human kidney tumor xenograft from an SN12C tumor and background signal in subrenal implanted normal mouse kidney. Left panel shows no background signal from NIR imaging of a subrenal capsular implanted normal nude mouse kidney (open arrow) as opposed to a strong NIR signal from subrenal implant of a SH12C tumor (solid arrow).
To confirm uptake by kidney tumor cells, we retrieved the implanted specimens 48 hours after dye administration to examine stained cancer cells. The frozen sections contained clusters of cells showing cytoplasmic staining. In the same sections, H&E staining confirmed the presence of kidney cancer cells (Figure 6A). We noted the heterogeneity of MHI-148 staining, which could be due to the viability of the kidney cancer cells and their metabolic activity status. In parallel control studies the host liver, kidney, spleen, and skeletal muscle (vastus medialis) did not shown any detectable NIR signals (data not shown), illustrating the specificity of MHI-148 uptake and retention by kidney cancer tissues.
5. Detection of circulating kidney tumor cells in blood
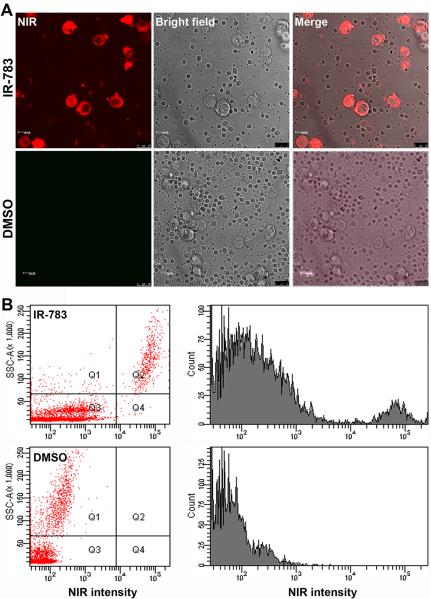
Kidney cancer cells may metastasize to distant organs via the systemic circulation. We assessed whether heptamethine carbocyanine dyes could differentiate kidney cancer cells from peripheral blood mononuclear cells using a flow cytometric assay. Whole blood from healthy donors was spiked with known numbers of cultured human kidney cancer cells. The blood sample was then subjected to a brief staining with MHI-148 dye, followed by isolation of nucleated cells by a gradient centrifugation method.
Under a fluorescence microscope, cancer cells in the blood could be clearly visualized in the preparation, with much larger sizes and higher fluorescence intensities than the mononuclear blood cells (Figure 7A). Detected by flow cytometric method (Figure 7B), the stained cancer cells showing strong NIR fluorescence could be segregated from blood mononuclear cells (Figure 7B). These analyses suggested that the tumor specificity of the heptamethine carbocyanine dye could be exploited for the detection of CTCs in peripheral blood.
Fig. 7. Using heptamethine carbocyanines to detect kidney cancer cells in human blood.
MHI-148 was used to stain human peripheral blood samples mixed with SN12C kidney cancer cells. The diluent (DMSO) was used as control. A, stained samples were subjected to NIR imaging (400 ×). B, the stained samples were subjected to flow cytometric detection of cancer cells in the blood. Both dot plot and histogram analysis demonstrated a clear segregation of the cancer cells from mononucleated blood cells based on NIR fluorescence imaging.
Discussion
Near-infrared optical imaging is a powerful tool for cancer research and tumor detection. 18 Various cyanine derivatives have been investigated as contrast agents for detecting cancer cells. 4, 19 We previously identified two NIR fluorescence heptamethine carbocyanine dyes as dual imaging and targeting agents. 5 In the current study, we critically evaluated their applicability for detecting human kidney cancer in freshly dissected kidney tumors and human kidney cancer cells in blood.
Both in vitro and in vivo experiments demonstrated that these dyes were taken up specifically by kidney cancer cells. The uptake was substantial not only in cultured cancer cells (Figure 1) but also in experimental tumor xenografts (Figures 2 and 3) and freshly acquired human kidney tumor specimens (Figures 4-6). In addition, these NIR dyes were taken up by an experimental model in which live human kidney cancer cells were added to human blood (Figure 7). These study designs represent the closest approximation to different clinical settings, and the results from this study confirmed the potential clinical utility of heptamethine carbocyanine dyes for the detection of human kidney cancer cells.
In agreement with our previous findings, 5 this study determined that heptamethine carbocyanine dyes can be retained in tumors for a prolonged period. Carbocyanine dyes are bound by proteins and nucleic acids. 6, 7 It is possible that the binding to these macromolecules causes the retention. This unique feature of carbocyanine dye staining will facilitate the noninvasive detection of tumors with a high signal/noise ratio.
In comparison to other carbocyanine dyes such as indocyanine green (ICG) or rhodamine 123 that lack tumor cell specificity, 20, 21 MHI-148 and IR-783 exhibit tumor-specific uptake and retention and can detect cancer cells in blood or in tissues directly without chemical conjugation. This provides the unique advantage of identifying heterogeneous populations of kidney cancer cells in clinical specimens despite heterogeneous expression of surface markers. Kidney cancer cells may escape detection by surface marker-based methods, such as those based on epithelial cell adhesion molecule (EpCAM). 22 The conundrum of surface marker-based detection is especially conspicuous in kidney cancer, which is of mesodermal origin and expresses intrinsically low or no EpCAM. A number of other cancer types, including transitional cell carcinoma of the urinary bladder, squamous carcinoma of the skin, and hepatocellular carcinoma, have been known to express little EpCAM. 23, 24 In addition, many epithelial tumors may undergo mesenchymal transition, which rids them of characteristic surface EpCAM. 25 Carbocyanine dyes offer the advantage of being able to detect all of these cancer types because of their unique ability to stain viable cancer cells.
The mechanism by which carbocyanine dyes are specifically taken up by malignant cells remains to be elucidated. Although water-soluble carbocyanine dyes may diffuse across cytoplasmic membranes, the uptake and retention of IR-783 and MHI-148 has been found to be tumor-specific. These dyes have been found co-localizing with mitochondrial and lysosomal organelles which are functional constituents of both normal and cancerous cells. With their structural similarities to indocyanine, IR-783 and MHI-148 uptake could be mediated by transmembrane proteins of the OATP family. 26 We have determined that the uptake of IR-783 is an active process requiring energy consumption. 5 We detected differential expression of certain OATP proteins in prostate and kidney cancer cell lines (data not shown) in agreement with previous reports. 27-30 Further investigation is warranted to identify the OATP member that mediates specific uptake and retention in malignant cells.
In summary, heptamethine carbocyanine dyes offer an effective tool to detect kidney cancers with high sensitivity. This class of dyes is especially useful for detecting solid tumors that do not express the classic EpCAM epithelial marker. IR-783, MHI-148 and other dyes with similar properties should be explored as generalized vehicles for both cancer detection and targeting.
Acknowledgements
This study was supported by National Institutes of Health research grants 2P01CA98912 and 1P50CA128301-01A1 and the Board of Governors Endowed Cancer Research Chair, Cedars-Sinai Medical Center (LWKC). We dedicate this work to the late Dr. Fray F. Marshall, educator, clinician and researcher, who inspired us to pursue translational research passionately to improve the care of patients with urologic diseases.
References
- 1.National Cancer Institute, U. S. N. I. o. H. Kidney cancer, estimated new cases and deaths from kidney (renal cell and renal pelvis) cancer in the United State in 2010. 2010 http://www.cancer.gov/cancertopics/types/kidney.
- 2.Ballou B, Fisher GW, Waggoner AS, et al. Tumor labeling in vivo using cyanine-conjugated monoclonal antibodies. Cancer Immunol Immunother. 1995;41:257. doi: 10.1007/BF01517001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weissleder R, Tung CH, Mahmood U, et al. In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat Biotechnol. 1999;17:375. doi: 10.1038/7933. [DOI] [PubMed] [Google Scholar]
- 4.Licha K, Riefke B, Ntziachristos V, et al. Hydrophilic cyanine dyes as contrast agents for near-infrared tumor imaging: synthesis, photophysical properties and spectroscopic in vivo characterization. Photochem Photobiol. 2000;72:392. doi: 10.1562/0031-8655(2000)072<0392:hcdaca>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 5.Yang X, Shi C, Tong R, et al. Near IR heptamethine cyanine dye-mediated cancer imaging. Clin Cancer Res. 2010;16:2833. doi: 10.1158/1078-0432.CCR-10-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stadler AL, Renikuntla BR, Yaron D, et al. Substituent effects on the assembly of helical cyanine dye aggregates in the minor groove of a DNA template. Langmuir. 2011;27:1472. doi: 10.1021/la104329c. [DOI] [PubMed] [Google Scholar]
- 7.Silva GL, Ediz V, Yaron D, et al. Experimental and computational investigation of unsymmetrical cyanine dyes: understanding torsionally responsive fluorogenic dyes. J Am Chem Soc. 2007;129:5710. doi: 10.1021/ja070025z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frangioni JV. In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol. 2003;7:626. doi: 10.1016/j.cbpa.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Hawrysz DJ, Sevick-Muraca EM. Developments toward diagnostic breast cancer imaging using near-infrared optical measurements and fluorescent contrast agents. Neoplasia. 2000;2:388. doi: 10.1038/sj.neo.7900118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ntziachristos V, Bremer C, Weissleder R. Fluorescence imaging with near-infrared light: new technological advances that enable in vivo molecular imaging. Eur Radiol. 2003;13:195. doi: 10.1007/s00330-002-1524-x. [DOI] [PubMed] [Google Scholar]
- 11.Zhang C, Liu T, Su Y, et al. A near-infrared fluorescent heptamethine indocyanine dye with preferential tumor accumulation for in vivo imaging. Biomaterials. 2010;31:6612. doi: 10.1016/j.biomaterials.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Chung LW, Wang R, Zhau HE, et al. Cyanine-containing compounds for cancer imaging and treatment. 2007 USPTO Application #20110262354. [Google Scholar]
- 13.Strekowski L, Lipowska M, Patonay G. Substitution reaction of a nucleofugal group in heptamethine cyanine dyes. Synthesis of an isothiocyanate derivatives for labeling of proteins with a near-infrared chromophore. J. Org. Chem. 1992;57:4578. [Google Scholar]
- 14.Narayanan N, Patonay G. A new method for the synthesis of heptamethine cyanine dyes: Synthesis of new near-infrared fluorescent labels. J. Org. Chem. 1995;60:2391. [Google Scholar]
- 15.Nomura T, Huang WC, Seo S, et al. Targeting beta2-microglobulin mediated signaling as a novel therapeutic approach for human renal cell carcinoma. J Urol. 2007;178:292. doi: 10.1016/j.juro.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Moreno RD, Ramalho-Santos J, Chan EK, et al. The Golgi apparatus segregates from the lysosomal/acrosomal vesicle during rhesus spermiogenesis: structural alterations. Dev Biol. 2000;219:334. doi: 10.1006/dbio.2000.9606. [DOI] [PubMed] [Google Scholar]
- 17.Guthrie PD, Freeman MR, Liao ST, et al. Regulation of gene expression in rat prostate by androgen and beta-adrenergic receptor pathways. Mol Endocrinol. 1990;4:1343. doi: 10.1210/mend-4-9-1343. [DOI] [PubMed] [Google Scholar]
- 18.Pu Y, Wang WB, Tang GC, et al. Spectral polarization imaging of human prostate cancer tissue using a near-infrared receptor-targeted contrast agent. Technol Cancer Res Treat. 2005;4:429. doi: 10.1177/153303460500400410. [DOI] [PubMed] [Google Scholar]
- 19.Bugaj JE, Achilefu S, Dorshow RB, et al. Novel fluorescent contrast agents for optical imaging of in vivo tumors based on a receptor-targeted dye-peptide conjugate platform. J Biomed Opt. 2001;6:122. doi: 10.1117/1.1352748. [DOI] [PubMed] [Google Scholar]
- 20.Nakajima T, Mitsunaga M, Bander NH, et al. Targeted, activatable, in vivo fluorescence imaging of prostate-specific membrane antigen (PSMA) positive tumors using the quenched humanized J591 antibody-indocyanine green (ICG) conjugate. Bioconjug Chem. 2011;22:1700. doi: 10.1021/bc2002715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen LB, Summerhayes IC, Johnson LV, et al. Probing mitochondria in living cells with rhodamine 123. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):141. doi: 10.1101/sqb.1982.046.01.018. [DOI] [PubMed] [Google Scholar]
- 22.Criscitiello C, Sotiriou C, Ignatiadis M. Circulating tumor cells and emerging blood biomarkers in breast cancer. Curr Opin Oncol. 2010;22:552. doi: 10.1097/CCO.0b013e32833de186. [DOI] [PubMed] [Google Scholar]
- 23.Went P, Dirnhofer S, Salvisberg T, et al. Expression of epithelial cell adhesion molecule (EpCam) in renal epithelial tumors. Am J Surg Pathol. 2005;29:83. doi: 10.1097/01.pas.0000.146028.70868.7a. [DOI] [PubMed] [Google Scholar]
- 24.Seligson DB, Pantuck AJ, Liu X, et al. Epithelial cell adhesion molecule (KSA) expression: pathobiology and its role as an independent predictor of survival in renal cell carcinoma. Clin Cancer Res. 2004;10:2659. doi: 10.1158/1078-0432.ccr-1132-03. [DOI] [PubMed] [Google Scholar]
- 25.van der Gun BT, Melchers LJ, Ruiters MH, et al. EpCAM in carcinogenesis: the good, the bad or the ugly. Carcinogenesis. 2010;31:1913. doi: 10.1093/carcin/bgq187. [DOI] [PubMed] [Google Scholar]
- 26.Cui Y, Konig J, Leier I, et al. Hepatic uptake of bilirubin and its conjugates by the human organic anion transporter SLC21A6. J Biol Chem. 2001;276:9626. doi: 10.1074/jbc.M004968200. [DOI] [PubMed] [Google Scholar]
- 27.Al Sarakbi W, Mokbel R, Salhab M, et al. The role of STS and OATP-B mRNA expression in predicting the clinical outcome in human breast cancer. Anticancer Res. 2006;26:4985. [PubMed] [Google Scholar]
- 28.Ballestero MR, Monte MJ, Briz O, et al. Expression of transporters potentially involved in the targeting of cytostatic bile acid derivatives to colon cancer and polyps. Biochem Pharmacol. 2006;72:729. doi: 10.1016/j.bcp.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 29.Marzolini C, Tirona RG, Kim RB. Pharmacogenomics of the OATP and OAT families. Pharmacogenomics. 2004;5:273. doi: 10.1517/phgs.5.3.273.29831. [DOI] [PubMed] [Google Scholar]
- 30.Mikkaichi T, Suzuki T, Tanemoto M, et al. The organic anion transporter (OATP) family. Drug Metab Pharmacokinet. 2004;19:171. doi: 10.2133/dmpk.19.171. [DOI] [PubMed] [Google Scholar]