Abstract
Airway smooth muscle has classically been of interest for its contractile response linked to bronchoconstriction. However, terminally differentiated smooth muscle cells are phenotypically plastic and have multifunctional capacity for proliferation, cellular hypertrophy, migration, and the synthesis of extracellular matrix and inflammatory mediators. These latter properties of airway smooth muscle are important in airway remodeling which is a structural alteration that compounds the impact of contractile responses on limiting airway conductance. In this overview we describe the important signaling components and the functional evidence supporting a view of smooth muscle cells at the core of fibroproliferative remodeling of hollow organs. Signal transduction components and events are summarized that control the basic cellular processes of proliferation, cell survival, apoptosis and cellular migration. We delineate known intracellular control mechanisms and suggest future areas of interest to pursue to more fully understand factors that regulate normal myocyte function and airway remodeling in obstructive lung diseases.
Keywords: Airway smooth muscle, Apoptosis, Cell proliferation, Chemotaxis, Hypertrophy
Introduction
The classical role of smooth muscle cells in the surrounding muscle layer of hollow organs is to regulate dynamic changes in lumen caliber and wall stiffness. Appreciation of the multifunctional behavior of smooth muscle cells in physiology and pathophysiology has steadily increased since initial insight provided by Wissler’s work on large elastic arteries [1]. In addition to contraction, terminally differentiated smooth muscle cells are also capable of reversibly adopting capacity to express and secrete cytokines, chemokines and extracellular matrix proteins, to proliferate, and to migrate. This has led to current paradigms that place smooth muscle cells at the core of fibroproliferative remodeling of hollow organs in diseases of the vasculature (atherosclerosis and hypertension) and the airways (asthma and chronic obstructive disease).
Remodeling of the airways involves thickening of the bronchial and bronchiolar walls due to multiple events involving multiple cell types. There is epithelial cell denudation, mucus gland hyperplasia, increased smooth muscle mass, thickening of the lamina reticularis and accumulation of sub-epithelial extracellular matrix (ECM), increased numbers of sub-mucosal myofibroblasts, increased vascularization, and development of a chronically healing epithelium [2, 3]. Evidence points to progressive structural change in the airway wall due to rounds of inflammation-driven wound healing as a fundamental component for development of fixed airway narrowing [4, 5]. A significant component of irreversible airway hyperresponsiveness in long-standing asthma excludes the inflammatory response, suggesting that fibro-proliferative changes associated with mesenchymal cell populations in bronchial wall may underpin fixed airway dysfunction [2, 6]. Local inflammation is complex as it is manifest both by recruited leukocytes and mast cells, but also by the intrinsic capacity of airway myocytes to express and release cytokines, chemokines and other pro-inflammatory molecules [7]. Thus airway smooth muscle (ASM) thickening results from a collective of biological signals that induce several trophic myocyte responses.
Though ASM has classically been of interest for its contractile response linked to bronchoconstriction, terminally differentiated smooth muscle cells are phenotypically plastic and have multifunctional capacity for proliferation, cellular hypertrophy, migration, and the synthesis of extracellular matrix and inflammatory mediators [8–11]. It is this property of ASM that positions it as an effector of airway remodeling which is a structural alteration that itself compounds the impact of contractile responses on limiting airway conductance. Understanding airway smooth muscle-associated cellular mechanisms that contribute to airway remodeling is of great relevance for several reasons. First, though remodeling and thickening consists of multiple structural changes, the increased mass of contractile ASM is the most significant causal feature for airway hyperactivity and excessive narrowing that reduces airflow [12–14]. Second, airway remodeling is characterized by increased numbers of myofibroblasts in the submucosal compartment. Their accumulation after allergen challenge is rapid, thus there is growing belief that migration of airway myocytes from the adjacent smooth muscle layer feeds this response [15, 16]. Last, in the preceding decade there has been growing interest in research aimed at developing new therapeutics that target airway smooth muscle to treat asthma [17–19].
As an overarching paradigm for this chapter, Figure 1 provides a schematic model for cellular mechanisms, including migration, proliferation, hypertrophy and apoptosis, which likely support a primary affective and effective role in airway remodeling and hyperresponsiveness linked with obstructive lung disease. This chapter provides an overview of current understanding of ASM proliferation, hypertrophy, apoptosis and migration. Moreover, we delineate intracellular control mechanisms and the repertoire of biological factors that engage them in regulating myocyte function.
Figure 1. Schematic representation of the role of airway smooth muscle cell proliferation, cellular hypertrophy, apoptosis and migration if development of airway remodeling in asthma.
A key local driving force for airway remodeling are cytokines, chemokines, and growth factors released by the epithelium that act on the underlying airway wall (myo)fibroblasts and airway smooth muscle cells. Airway smooth muscle and fibroblasts also release trophic and pro-fibrotic factors that contribute to local inflammation and tissue repair. Central to the initiation and modulation of inflammation, tissue damage and repair is recruitment of active inflammatory cells including Th-2 and Th-1 polarized lymphocytes, eosinophils, neutrophils and mast cells.
Airway Smooth Muscle Proliferation
Airway smooth muscle cells can respond to a variety of mitogenic cues that promote traversing the Gap 1 (G1), S, Gap 2 (G2) and M(itosis) phases of the cell cycle. As an early response to mitogen stimulation, from a quiescent G0 state myocytes enter the G1 phase of the cell cycle coincident with increased expression of specific D-cyclins, such as cyclin D1 [20, 21]. Initially, progression through the G1 phase depends on the binding of one or several D-type cyclins (D1, D2, and/or D3) to existing cyclin dependent kinases (CDK4 and -6), forming active complexes that subsequently activate cyclin E/CDK2. This leads to increased phosphorylation of retinoblastoma protein (Rb), which in turn dissociates from an elongation factor E2F/Rb complex. E2F/Rb is otherwise bound to E2F responsive genes, effectively halting their transcription and creating a cell cycle block; the release of E2F permits the transcription of various genes, including DNA polymerase, essential for effective transit of cells through G1 and into S phase. G1/S transition represents a restriction point (R) past which DNA will be synthesized (S phase), cells will increase in size and synthesize microtubules (G2) and eventually undergo mitosis [22, 23].
This whole process is of course tightly regulated. The activity of CDKs and their effects on cell cycle progression can be negatively regulated by CDK inhibitors during the G1/S transition. In this regard, two principal families of genes have been identified based on their structure and specific CDK targets: 1) the Cip/Kip family (p21Cip1, p27Kip1, and p57Kip2), which interfere with cell cycle in the G1 phase by inactivating cyclin D-, E-and A dependent kinases [22]; and, 2) the INK4/ARF family (Inhibitor of Kinase 4/Alternative Reading Frame; p16INK4a, p15INK4b, p18INK4c, and p19INK4d), which negatively affect the catalytic subunits of CDK4 and -6 and as such prevent interaction with cyclin D1 [22].
During the cell cycle, cells will go through a number of ‘checkpoints’ to ensure that each phase of the cycle has been accurately completed before entering the next one; at each point the cell is screened for DNA integrity, and requires a collective of effective temporal mitogen stimulation. The first cycle checkpoint occurs at the end of the G1 phase, just before entering into S phase, where it is typically decided whether the cell should proceed, enter a resting/repair stage, or exit the cycle via apoptosis. At this checkpoint DNA damage is monitored through a process involving the tumor suppressor protein, p53, which has capacity to arrest cycling of G1 cells by activating transcription of p21Cip1, leading to subsequent CDK inhibition [24]. Depending on the severity of DNA damage, p53 can either activate DNA repair proteins enabling the cell to eventually continue cell cycle or, in cases of irreparable DNA damage, induce apoptosis [24]. A second checkpoint is located at the end of the G2 phase and regulates initiation of M phase. This checkpoint is sub-served by a complex of cyclin B/CDK1 complex (referred to as MPF, maturation promoting factor), which is responsible for essential phosphorylation events in a number of proteins required for mitosis [25]. A third checkpoint (the mitotic spindle checkpoint) occurs during metaphase when chromosomes have aligned at the mitotic plate and are under bipolar tension from the spindle apparatus. The appropriate tension created by this bipolar attachment is necessary to initiate progression to anaphase during which individual chromosomes are segregated and pulled toward opposite poles. Thereafter, cytokinesis proceeds and the original cell spawns two daughter cells that can then continue through G1 phase and another cell cycle, or be diverted to a quiescent state in G0 [24].
Factors controlling airway smooth muscle cell proliferation
Airway smooth muscle proliferation can be affected by at least three groups of mitogens: polypeptide growth factors, G-protein coupled receptor (GPCR) agonists and pro-inflammatory cytokines [20] (Table 1). In addition, extracellular matrix proteins are important regulators of mitogen-induced proliferation [26, 27]. In asthma, excessive accumulation of (contractile) smooth muscle has frequently been described in central and small airways [28–30], and is typically associated with myocyte hyperplasia and hypertrophy. Thus, increased ASM mass may, in part, to be due to cellular proliferation driven by growth factors, inflammatory mediators and neurotransmitters [31, 32].
Table 1.
Primary factors affecting airway smooth muscle cell proliferation in culture.
| Class | Pro-proliferative | Anti-proliferative | References |
|---|---|---|---|
| Receptor tyrosine kinases | PDGF (A, B, C), IGF-1, bFGF, EGF, NGF, insulin | [37, 39, 41, 316–321] | |
| G protein coupled receptors | Histamine, thromboxane A2, endothelin-1, α-adrenergic agonists, cysteinyl leukotrienes, thrombin, tryptase, substance P, sphingosine phosphate, lysophosphatidic acid, muscarinic M3 receptor agonists, 5- hydroxytryptamine, urotensin II, ATP, UTP, bradykinin | PGE2, β-adrenergic agonists, VIP, sphingosine, atrial natriuretic peptide | [31, 42, 48, 49, 54–57, 318, 322–333] |
| Cytokines | IL-1β, TNF-α, TGF-β1, IL-6 | IL-4, TNF-α, TGF-β1, IFNγ, IFNβ | [40, 60, 61, 65, 316, 334–339] |
| Matrix proteins | Fibronectin, collagen I, vitronectin | Laminin, chondroitin sulphate | [26, 27, 67] |
Polypeptide Growth Factors
Polypeptide growth factors induce proliferation by activating receptors with intrinsic protein tyrosine kinase (RTK) activity and are among the most effective inducers of ASM proliferation. This group of mitogens includes for instance basic fibroblast growth factor (bFGF), epidermal growth factor (EGF), platelet-derived growth factor (PDGF), insulin-like growth factor-1 (IGF-1) and insulin, which have all been shown to induce ASM cell proliferation (Table 1). Several RTK growth factors, including EGF, PDGF, and IGF-1, have been implicated in asthma pathogenesis based on either increased immunoreactivity of the growth factor, bioavailability, and/or receptor expression [33–36]. Importantly, some combinations of these growth factors (e.g. EGF + PDGF, insulin + PDGF) can produce synergistic proliferative responses in airway myocytes [37–41]. A number of RTKs, for example the PDGF and EGF receptors, are located in caveolae in the plasma membrane, where they associate with caveolin-1 [42]. This may represent a mechanism for additive or synergistic effects of mitogens. For instance PDGF and EGF receptors uncouple from caveolin-1 in response to mitogen stimulation and thus activated, traffic to peripheral caveolae-free membrane sites, where p42/44 MAPK activation can take place [42, 43].
G-protein Coupled Receptor Agonists
Contractile agonists, such as acetylcholine and cysteinyl leukotrienes, acting via G-protein coupled receptors (GPCRs) have been associated with increased ASM thickening in asthma (Table 1) and in animal models of asthma [44–47]. However, stimulation of muscarinic receptors or cysteinyl leukotriene receptors alone is not sufficient induce ASM cell proliferation. Rather, these GPCR agonists exert profound promitogenic effects in the presence of a peptide growth factor, manifest as a synergistic increase in the proliferative response induced by the growth factor in isolation [31, 48, 49]. In addition to muscarinic M3 and CysLT1 receptor agonists, it has become apparent that these effects are also observed for a number of other contractile agonists, including histamine, bradykinin and thrombin [48, 50–52]. The synergistic effects of contractile agonists on growth factor-induced proliferation are principally mediated through receptors that are coupled to turmeric G-proteins of the Gq subfamily [31, 48, 52]. In addition to Gq-protein coupled receptors, several agonists (e.g. thromboxane, thrombin and lysophosphatidic acid) that mediate effects via Gi-coupled GPCRs also have synergistic effects on growth factor-induced ASM proliferation [51, 53, 54]. Notably, the intracellular mechanisms for this effect differs from that of Gq-coupled receptors, as these agonists do not necessarily require interaction with RTKs. Specifics details on this issue are discussed in a subsequent section describing molecular mechanisms for proliferation.
In contrast to Gq and Gi-coupled receptor agonists, various Gs-protein coupled receptor agonists, including PGE2 and β2-receptor agonists, inhibit ASM cell proliferation [55–57]. These effects appear to rely on the potency of these agonists to induce prolonged cAMP production and subsequent PKA activation [58, 59].
Pro-inflammatory Cytokines
The involvement of pro-inflammatory cytokines, such as TNF, IL-6 and IL1β in ASM cell proliferation is controversial. Several reports suggest modest proliferative effects [60, 61], whereas others demonstrate no effects or even growth inhibition (Table 1) [62, 63]. It has become apparent that for IL-6, IL-1β and TNF that these paradoxical findings might be explained by cytokine-induced production of anti-proliferative mediators such as the cyclooxygenase-2 product prostaglandin E2 or IFNβ, which exert an autocrine effect on the ASM cells [40, 64, 65]. Most of the cytokines of interest exert their effects on gene regulation through cell surface glycoprotein complexes, comprising 2 to 4 receptor chains that couple to several non receptor tyrosine kinases, such as Src family proteins and components of the MAPK and JAK/STAT cascades [20] (Figure 2). The balance between parallel and functionally opposing signaling pathways and unique phenotype of the cell population are ultimately the determinants of the effects of cytokines on ASM proliferation.
Figure 2. Schematic representation of key signaling mechanisms associated with control of airway smooth muscle cell proliferation.
See text for details and Table 1 for list of factors that control activation of these pathways.
Extracellular Matrix Proteins
Several extracellular matrix (ECM) proteins have emerged as regulators of growth factor-induced ASM cell proliferation (Table 1). Cells cultured on monomeric collagen I or fibronectin matrices progress towards a more proliferative phenotype, as evidenced by an augmented basal proliferative response [26, 66] and an augmented mitogenic response towards either RTK or GPCR ligands [26, 27, 66, 67]. Conversely, when cultured on a laminin or laminin-rich Matrigel substrate, growth factor-induced proliferation is markedly suppressed [26, 27, 68]. These observations could be of significant relevance to airway wall remodeling and asthma pathogenesis, as both the quantity and the composition of the ECM is altered in the airways of chronic asthmatics. Deposition of collagen IV and elastin is decreased in the airway wall of asthmatic patients, whereas collagen I, III, V, fibronectin, tenascin, hyaluran, versican and laminin α2/β2 chains are increased [69–72]. Importantly, changes in matrix-composition directly surrounding ASM cells have also been reported: collagen I, hyaluronan and versican increased in patients with asthma [73, 74]. Human ASM cells also secrete ECM proteins in response to asthmatic sera [75] suggesting a cellular source for ECM deposition in airways and implicating a novel mechanism in which ASM cells may modulate autocrine proliferative responses.
ECM proteins interact with smooth muscle cells through integrins, which are heterodimeric glycoproteins consisting of membrane-spanning, non-covalently associated, α and β subunits [76]. Enhancement of growth factor-induced proliferation of ASM cells on a collagen I or fibronectin matrix is dependent on activation of α2β1, α4β1 and α5β1 integrins, of which α5β1 has emerged as a crucial signaling integrin for proliferation both in healthy and asthmatic ASM cells [67]. Laminin most likely exerts its anti-proliferative effects through the α7β1 integrin [77].
Molecular Signaling Pathways in Airway Smooth Muscle Cell Proliferation
Major pathways described below are shown schematically in Figure 2.
MAP Kinases
The mitogen-activated protein (MAP) kinases are a superfamily of serine/threonine directed protein kinases involved in transcriptional regulation in response to a variety of extracellular stimuli, including growth factors [78], thereby being responsible for intracellular transmission of extracellular trophic signals. MAP kinases share a common activation mechanism which involves the phosphorylation of tyrosine and threonine residues in a Thr-X-Tyr (TXY) motif positioned in their activation loop. Based on the identity of the residue between the threonine and tyrosine, the MAP kinase superfamily can be divided into three main groups: ERKs (Thr-Glu-Tyr); Jun amino terminal kinases (JNKs) (Thr-Pro-Tyr); and p38s (Thr-Gly-Tyr). Each MAP kinase is activated by successive activation of a MAP kinase kinase kinase and a MAP kinase kinase. Activation of the ERK pathway constitutes an important regulator of cell cycle entry and G1 progression, and is required for DNA synthesis and proliferation in an extensive variety of mammalian cell systems, including bovine, rat and human ASM [63, 79– 81]. The traditional path to ERK activation is comprised of the growth factor receptor binding protein Grb2, the nucleotide exchange factor Son of sevenless (Sos), the monomeric 21 kDa GTPase Ras, the 74 kDa cytosolic serine/threonine kinase Raf-1, and the 45 kDa dual function kinase MAP kinase/ERK kinase kinase (MEK)-1. Grb2 is found in a stable complex with the nucleotide exchange factor Sos. Docking of Grb2 to a receptor tyrosine kinase causes Sos to bind to and activate Ras. Ras then escorts Raf-1 to the cell membrane, resulting in Raf-1 activation [82]. Raf-1 phosphorylates MEK1 on two serine residues, Ser218 and Ser222 [83] MEK1 phosphorylates tyrosine and threonine residues in the ERK activation loop. Induction of the Ras/Raf1/MEK/ERK1/2 pathway has emerged to be a key pathway in the transcriptional activation of the cyclin D1 promoter, cyclin D1 activity and protein expression [20, 84, 85].
It has been suggested that p21Ras can act as a point of convergence for mitogenic signals induced by different receptor-operated mechanisms [20, 86]. Activation of p21Ras results not only in its binding to Raf-1 but also phosphoinositide 3-kinase (PI-3-kinase) (the latter effect is described in the next section) (Figure 2). Notably, the mechanistic difference between the pro-proliferative effects of Gi and Gq coupled receptors may be explained by the differential involvement of the p42/44 MAPK cascade. Thus, Gi, but not Gq, activates p21Ras in ASM cells [87]. For example, Ras/Raf/MEK/p42/p44MAPK signaling is involved in the mitogenic effects of the Gi-protein coupled receptor agonists thromboxane A2, thrombin and lysophosphatidic acid [88–91]. Gi mediates p42/p44 MAPK activation via its βγ-receptor subunits, which have been shown to increase p21Ras activation through an augmented tyrosine phosphorylation of Shc leading to an increased functional association between Shc, Grb2 and SOS [92, 93] (Figure 2). Along with p42/44 MAPK, p38 MAPK has emerged as a regulator of ASM cell proliferation [94, 95]. However, the involvement of p38MAPK appears to be stimulus dependent, as it is not involved in TGFβ1-induced proliferation of human ASM cells [96]
Ras dependent PI3-kinase pathways
Activation of RTKs results in the intracellular phosphorylation of receptor tyrosine residues (receptor autophosphorylation), which serve as docking sites for other kinases, including Src and phosphatidyl inositol 3 kinase (PI-3-kinase), and mediates p21Ras activation through the guanine nucleotide exchange factor SOS [97]. PI-3-kinase has emerged as a key signaling molecule of proliferation and cellular hypertrophy of ASM [98–100] (Figure 2). Three distinct classes of PI-3-kinase, specifically IA, II and III, have been identified in ASM, of which class IA is primarily involved in cell proliferation, being required for both RTK and GPCR mitogen effects [101]. PI-3-kinase regulates cell function by phosphorylating phosphoinositides (PIP) at the 3-position of the inositol ring. This results in PI3P, PIP2 and PIP3 formation, of which the latter appears to be the most important of these second messengers [102, 103]. Subsequent recruitment of phosphoinositide dependent kinase1 (PDK1) to the cell membrane results in Akt1 activation, which acts as an inhibitor of the constitutively active glycogen synthase kinase 3 (GSK-3) and an activator mTOR and p70 S6 kinase [99, 104–106]. These activities are important for transcriptional activation and protein translation leading to ASM cell proliferation and hypertrophy [106, 107].
A portion of the synergizing effects of GPCRs on growth factor-induced proliferation can be explained by augmented PI3-kinase activity. Together with a peptide growth factor the βγ-subunit derived from a Gq-coupled receptor can synergistically stimulate PI3-kinase, Akt and p70 S6kinase [48, 51, 52, 107], resulting in increased proliferation.
RTK-induced PI3 kinase activity also results in phosphorylation of the non-RTK Src; activation is required for ASM cell proliferation [108], and therefore represents an important pathway by which PI-kinase modulates mitogenesis. Another route through which PI3-kinase affects ASM cell cycle progression is through Rho family GTPases [109]. Indeed, PI3-kinase-dependent activation of Rac1 and Cdc42, but not RhoA, and subsequent induction of cyclin D1 promoter activity has been demonstrated in ASM; importantly, this effect appeared to be independent of ERK1/2, suggesting parallel pathways in the induction of cyclin D1 [110, 111].
Protein Kinase C
In addition to potential synergistic activation of p42/44 MAPK and/or PI3-kinase pathways, as described above, signaling that involves protein kinase C (PKC) can be important in the synergistic effects of GPCRs on RTK-mediated ASM proliferation [50, 107, 112] (Figure 2). It has been postulated that the synergistic effects of PKC activation are mediated through inhibition of GSK-3β. In its unphosphorylated form, GSK-3β is constitutively active and negatively regulates several pro-mitogenic transcription factors and cell cycle regulatory proteins in quiescent cells [113]. Thus far, the involvement of this pathway has been elucidated for muscarinic receptor-mediated synergism only [107]; however, PKC-dependency has also been demonstrated for other Gq-coupled receptor agonists, including bradykinin and endothelin [50, 112]. This indicates that PKC activity, and likely subsequent GSK-3 β inhibition, could represent a general pathway in GPCR-mediated synergism of RTK-induced ASM proliferation.
Reactive Oxygen Species
In parallel with the activation of MAPKs and PI-3-kinase, RTKs can activate a signaling cascade involving the small G protein Rac1, which constitutes part of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex that produces reactive oxygen species (such as H2O2 and O2−). Induction of this pathway is linked to cyclin D1 promoter activity and ASM cell proliferation, likely via the involvement of NF-κB [111, 114, 115]. In addition, a role for Janus kinase 2 (JAK2) and signal transducer and activator of transcription-3 (STAT3) in response to reactive oxygen species that are generated by PDGF stimulation appears to be an important regulatory pathway in the expression of c-myc and cyclin D1, and subsequent DNA-synthesis [116]. In line with these findings, inhibition of p22-and p67phox, subunits of NADPH oxidase, prevents mitogen-induced cyclin D1 promoter activity [109] and DNA synthesis [114] in ASM. Moreover, a role for the nonphagocyte NADPH oxidase catalytic homolog Nox4 in the regulation of TGFβ1-induced mitosis is evident, as silencing of this molecule prevents TGFβ1-induced phosphorylation of Rb and 4E-BP-1 that is essential for ASM cell proliferation [117]. Collectively, these findings implicate an important role for reactive oxygen species in the promotion of growth factor-induced ASM cell proliferation.
Rho - Rho kinase signaling
In airway smooth muscle, the Rho-Rho kinase signaling pathway has emerged as an important regulator of many cellular functions [109]. In ASM cell proliferation its involvement is somewhat controversial, with some studies suggesting a rather limited role for the pathway in PDGF and EGF-induced proliferation [89, 118]. In contrast, other studies with human ASM cells suggest a key role for RhoA and Rho kinase, as prevention of RhoA activation and/or pharmacological inhibition of Rho kinase prevent proliferation induced by fetal bovine serum (FBS) [119]. Furthermore, the proliferative response of human ASM cells to the G-protein coupled receptor agonist lysophosphatidic acid (LPA) alone and its strong synergism with EGF can be markedly diminished by Rho inhibition [89]. Parallel effects of Rho kinase inhibition on LPA, LPA/EGF and FBS-induced proliferation likely relates to the fact that LPA is a major component of FBS. The difference in Rho/Rho kinase dependency between FBS and individual RTK mitogens may also be explained by the observation that PDGF induced proliferation relies more on Rac and Cdc42 mediated pathways [110], whereas FBS-induced proliferation of human ASM cells appears independent of Rac and Cdc42 mediated signaling [119]. Thus, Rho/Rho kinase signaling may regulate proliferation of ASM cells; however the level of activation and relative contribution of this pathway is stimulus-dependent.
Integrin-mediated signaling in airway smooth muscle cell proliferation
Integrins mediate signals in response to ECM protein stimulation through (auto)phosphorylation of a number of signaling molecules, including the nonreceptor cytoplasmic tyrosine kinases, focal adhesion kinase (FAK) and c-Src. These kinases subsequently activate other effector proteins, like PI3-kinase, p38 MAPK and ERK 1/2, which are, as described previously, associated with growth factor-induced proliferation [120, 121]. However, the exact mechanisms by which ECM proteins modulate ASM cell proliferation distinct from growth factor-induced signaling are still elusive and might very well be species and stimulus dependent. For instance, in human lung carcinoma cells fibronectin has been shown to affect proliferation by reducing expression of the cell cycle inhibitory protein p21Cip1 in an ERK 1/2- and Rho-kinase-dependent fashion [122]. In contrast, in bovine ASM cells proliferation induced by PDGF has been shown to be dependent on ERK 1/2, p38 MAPK and PI3-kinase, but not Rho-kinase [118].
Regulation of Airway Smooth Muscle Hypertrophy
In asthma, excessive accumulation of contractile smooth muscle in central and small airways is associated not only with myocyte hyperplasia, but also smooth muscle cell hypertrophy [28–30]. With respect to myocyte hypertrophy it is clear that the process require co-ordinated and selective protein synthesis that supports accumulation of contractile proteins. Therefore it is important to understand the signaling pathways that regulate hypertrophic ASM cell growth.
In cell culture, the levels of contractile protein markers vary depending upon cell confluence and the exogenous stimuli provided by the media. Plating ASM cell at low density in the presence of FBS represses expression of contractile proteins such as sm-α-actin, myosin light chain kinase (MLCK), and smooth muscle myosin heavy chain (smMHC) [123–126]. Conversely, long term serum deprivation of confluent myocyte cultures promotes accumulation of contractile proteins and induces the formation of large contractile myocytes [98, 127, 128]. Interestingly the transcriptional activity of contractile protein genes actually peaks whilst myocytes are undergoing proliferation and nearing confluence; this becomes dramatically reduced at confluence when mRNA levels of contractile markers such as SM22 and smMHC reach a maximum and is sustained thereafter during prolonged serum deprivation [98, 127]. During this period of reduced transcription, contractile proteins do, however, accumulate greatly as cells acquire an enlarged, contractile phenotype morphology. Collectively this suggests that accumulation of smooth muscle proteins associated with myocyte enlargement is regulated by critical post-transcriptional mechanisms. Post transcriptional regulation of ASM contractile protein expression is consistent with studies of hypertrophy in other systems including cardiac and skeletal muscles, and vascular smooth muscle [129–135].
The study of smooth muscle cell hypertrophy led to the development of novel cell lines and interventions that enable repression of cell cycle transit and promote myocyte growth. As described in a previous section, the proliferation of eukaryotic cells is tightly regulated through a balance of positive and negative regulatory proteins that exert their effects during the first gap phase (G1) of the cell cycle [23, 136]. Transit through the cell cycle requires accumulation of G1 cyclins that leads to activation of CDKs and phosphorylation of downstream targets that ultimately allows entry into the S phase. The activity of G1 cyclin kinases is modulated by several key proteins, including p21CIP1, p16INK4, and p27Kip1 [137–140]. Based on this paradigm, adenovirus mediated over expression of cell cycle inhibitors p27Kip1 and p21Cip1 has been used as an experimental means of inducing cellular hypertrophy [141]. For cultured human ASM cells, transformation using temperature sensitive simian virus 40 large tumor antigen to induce p21Cip/Waf p57Kip2 expression has been shown to invoke cell cycle arrest in mid-G1 with concomitant accumulation of contractile proteins and an increase in cell size [28, 142]. With cell cycle is blockade, serum induced cell division is prevented however hypertrophic growth appears to continue as contractile protein abundance increases (without affecting mRNA levels).[143] These observations further support the concept that hypertrophic protein accumulation in ASM is regulated in a post-transcriptional manner, likely being under control of effectors that modulate protein translation. This paradigm is consistent with Woodruff and colleagues [30] who reported increased sm-α-actin protein (without any change in mRNA) in airway biopsies from mild asthmatics.
Factors Affecting Airway Smooth Muscle Hypertrophy
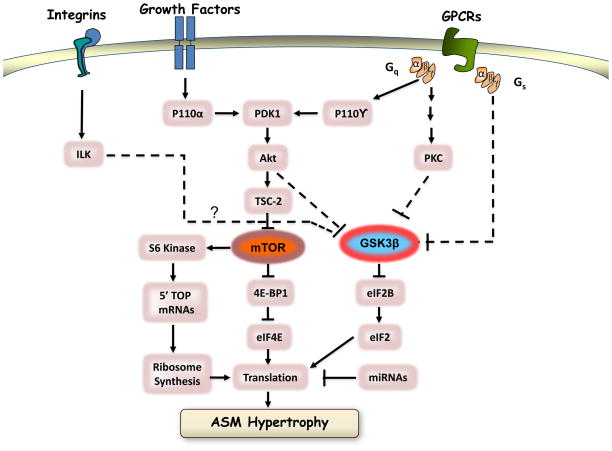
Cellular hypertrophy is largely mediated by signaling through peptide growth factors: insulin-like growth factor (IGF)-1 and growth hormone (GH), the latter acting predominantly via increased production of IGF-1 [144]. Although levels of interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF) are increased in the bronchoalveolar lavage fluid derived from asthmatics [145], whether these cytokines stimulate ASM growth in vitro remains controversial. IL-1β and IL-6 stimulate hyperplasia and hypertrophy of cultured guinea pig ASM cells [61]. Endothelin-1, which is secreted by the epithelium and is elevated in lung lavage fluid from asthmatics [146–148], is also a potent inducer of hypertrophy human ASM cells that is marked by accumulation of contractile phenotype marker proteins such as smMHC, calponin, and α-SMA [149]. When IGF-1, insulin, and other growth factors bind to their membrane tyrosine kinase receptors, a 110-kDa lipid kinase, phosphatidylinositol-3 kinase class IA (also referred to as p110α) is activated [150]. Accumulated data suggest that PI-3-kinase signaling is a critical underpinning for hypertrophy. Gene knockout of p110α is lethal at E9.5–E10.5 in mice (showing a severe proliferative defect) [151]. Indeed, a central role of the p110α pathway in IGF-1 - induced growth and hypertrophy has been demonstrated in different cell systems [98, 150, 152–155]. The details of this signaling cascade are provided in a subsequent section and is outlined in Figure 3.
Figure 3. Schematic representation of key signaling mechanisms associated with control of hypertrophic cell growth.
See text for details.
The extracellular matrix appears to affect the full functional repertoire of smooth muscle cells. Asthmatic airways smooth muscle cells in culture produce increased amounts and an altered composition of extracellular matrix proteins [75, 156]. Airway remodeling is characterized by the deposition of extracellular matrix (ECM) proteins in the airways [71, 157]. ECM proteins (collagen I, III, and V; fibronectin; tenascin; hyaluronan; versican; and laminin 2/β2) are increased in profusion in asthmatic airways [69, 71, 158, 159]. Seeding ASM onto fibronectin or collagen type-1 promotes a proliferative phenotype, whereas laminin-rich matrices promote retention or maturation of a contractile phenotype [27, 160, 161]. Moreover, endogenously expressed laminin-2, which is required for myocyte maturation and hypertrophy [161], is increased in the asthmatic airway. Notably, the ability of laminin-2 to promote maturation and support hypertrophy of human airways smooth muscle cells is mediated selectively via a α7bβ1 integrin heterodimer [160]. Thus an intrinsic autocrine mechanism appears to exist wherein myocytes can express both an ECM element (laminin-2) and requisite receptor (α7β1) to support accumulation of contractile proteins and hypertrophic growth. Given the association of laminin accumulation with airway remodeling, the expression of this glycoprotein and it receptors may be a central intrinsic mechanism regulating ASM hypertrophy in the adult airway.
Signal Transduction Pathways That Regulate ASM Hypertrophy
Pathways discussed in detail below are shown schematically in Figure 3.
PI-3-kinase
Insulin or IGF-I have been proposed to regulate developmental and physiological growth of the cells. Ligand binding to the IGF-I receptor activates PI-3-kinase of the Iα IA subgroup; p110α, which phosphorylates the membrane phospholipid phosphatidylinositol 4,5 bisphosphate at the 3′ position of the inositol ring [150]. Through this mechanism PI 3-kinases thus recruit effector proteins containing PI(3,4,5)P3-binding pleckstrin homology (PH) domains to the plasma membrane [162]. These include Akt (also called PKB), a 57-kD serine/threonine kinase encoded by three genes, and 3-phosphoinositide-dependent protein kinase-1 (PDK1) [163]. This enforced co-localization of Akt and PDK1 causes the latter to phosphorylate the former at its Thr308 residue, a necessary step in for Akt activation [164]. Full Akt1 activation requires membrane localization and phosphorylation at its Thr308 and Ser473 residues. Phosphorylation of Ser473 on Akt is proposed to be mediated by PDK-2, which appears to be identical to the so-called mTOR complex 2 appears (described below) [165, 166].
Downstream targets of PI(3) kinase and Akt that are associated with promoting protein synthesis and accumulation include glycogen synthase kinase-3β (GSK-3 β), p70S6 kinase (p70S6K), and PHAS-1/4E-BP [150, 167] (Figure 3). Akt1 phosphorylates and inhibits GSK-3β resulting in downstream de-inhibition of the translation initiator eIF2 [105, 168, 169]. Akt1 can also phosphorylate, and in part activate, the rapamycin-sensitive threonine/serine kinase, mammalian target of rapamycin (mTOR), a 290-kD protein similar in structure to phosphoinositide kinases, that can be effectively inhibited by the immunosuppressor compound rapamycin when the latter is bound to intracellular FK506-binding protein. Of relevance to a role in cellular hypertrophy, mTOR has downstream targets that include the mitogen- and amino acid–sensitive serine/threonine kinase, p70S6K, and the translation repressor PHAS-1/4E-BP1 [170–172]. mTOR mediated phosphorylation of PHAS-1/4E-BP1 releases the latter from binding to the protein translation mediator, eukaryotic initiation factor 4E (eIF4E), thereby increasing the availability of eIF4E to form an active complex with eIF4F and promote translation of specific sets of mRNA transcripts [170].
Activation of p70S6K activation regulates efficiency of protein translation by phosphorylating of the 40S ribosomal protein S6 [170, 173], and is required for PI-3-kinase mediated differentiation and hypertrophy of skeletal myotubes [150, 152], angiotensin II–induced vascular smooth muscle hypertrophy [174], and autocrine loop mediated ASM cell maturation and hypertrophy [98, 128]. Phosphorylation of ribosomal S6 protein increases translation of mRNAs with 5′ TOP tracts, many of which are involved in mRNA-translation- like elongation factors and ribosomal proteins. Though the principal site required for mTOR dependent activation of p70S6K is Thr389, which resides in a region between catalytic and auto-inhibitory domains [175], full activation of p70S6K is achieved through hierarchical phosphorylation of seven Ser/Thr sites targeted by mTOR, PDK1, and other PI(3) kinase–dependent kinases [176]. As phosphorylation of p70S6K is sensitive to inhibition by both rapamycin and chemical inhibitors of PI-3-kinase, researchers often place PI 3-kinase, mTOR, and S6 kinase into a linear signaling pathway. Such a linear scheme is too simplistic, however, as a rapamycin-resistant mutant of S6 kinase is still sensitive to inhibition by the PI-3-kinase inhibitor wortmanin[177], indicating that mTOR and PI-3-kinase signals to p70S6K can be dissociated. Indeed, S6 kinase may also be phosphorylated by PDK-1 [178, 179], thus providing a mechanism for mTOR-independent, PI-3-kinase-dependent activation. Similarly, mTOR-independent mechanisms of 4E-BP1 phosphorylation may exist. Recent evidence shows that class IA PI-3-kinases may function as 4E-BP1 kinases [180], and ERK can reportedly also phosphorylate 4E-BP1 [181].
Recent studies have elucidated the role of two signaling molecules that link Akt and mTOR in the regulation of cell size. PI-3-kinase may positively regulate cell size via the successive: activation of Akt, inactivation of TSC2, activation of Rheb, and activation of mTOR [182–185]. It is now known that mTOR exists in two distinct multi-protein complexes, one rapamycin-sensitive (mTOR complex 1) and one rapamycin-insensitive (mTOR complex 2) [186]. mTOR complex 1 includes mTOR and Raptor; mTOR complex 2 is comprised of mTOR-Rictor and mammalian stress-activated protein kinase interacting protein. Furthermore, as noted above, mTOR complex 2 appears to be identical to the proposed Akt kinase, PDK-2, which phosphorylates serine 473 on Akt [165, 166]. Thus, Akt acts as both an upstream activator of mTOR complex 1, and is a target for activation by mTOR via mTOR complex 2 to permit high-level PIK/Akt signaling [187].
GSK-3β
GSK-3β is a constitutively active serine/threonine kinase that phosphorylates multiple substrates including eIF2Bε, cyclin D1, and p21 [168, 188–190]. Phosphorylation by Akt inactivates GSK-3β, leading to dephosphorylation and the activation of eIF2B, as well as a general enhancement of ribosomal 43S pre-initiation complex formation [168]. GSK-3β also negatively regulates transcription factors involved in muscle-specific gene expression, including nuclear factors of activated T cells (NFAT), GATA4, and β-catenin [191–195] suggesting a critical role in ASM growth. The phosphorylation of GSK-3β by Akt indicates that PI-3-kinase may regulate mRNA translation via three distinct mechanisms (se Figure 3): (i) regulation of cap-dependent mRNAs via activation of the Akt/TSC2/Rheb/mTOR/4E-BP1 pathway, (ii) regulation of 5′ TOP tract-containing mRNAs via activation of p70S6K (through either mTOR or PDK-1), and (c) a general enhancement of translation initiation via activation of the Akt/GSK-3β/eIF2B pathway. Recent studies also implicate regulation of GSK-3β as a key downstream mechanism for the effects of integrin-mediated effects of extracellular matrix proteins on cell growth; this involving the signaling intermediate, integrin linked kinase (ILK) [196–198].
Despite human studies indicating the presence of ASM hypertrophy and increased contractile protein expression in asthma, little information is available concerning the signaling intermediates and translation initiation factors involved. In confluent serum-deprived canine tracheal myocyte cultures, PI-3-kinase and p70S6K activities are increased five and two days after serum deprivation, respectively, and immunohistochemical studies show selective phosphorylation of Akt and p70S6K in elongated cells expressing smMHC five to seven days after serum deprivation [98]. LY294002 and rapamycin blocked S6 kinase phosphorylation and phenotypic change, implying that PI-3-kinase, mTOR, and p70S6K are responsible for contractile protein accumulation and myocyte hypertrophy. Recently it has been shown by Deng et al [199] that inhibition of GSK-3β (which activates eIF2B) contributes to ASM hypertrophy in vitro and in vivo. More strongly in a mouse model of allergic asthma it has been shown that phosphorylation and inactivation of GSK-3β is associated with ASM hypertrophy [200] while p70S6K alone is responsible for the myocyte enlargement, without changing the contractile protein expression in vitro [201].
Rho GTPases
Rho kinase signaling plays an important role in regulation of smooth muscle gene transcription, which promotes serum response factor (SRF) nuclear localization and increased cytoplasmic actin filaments [202–204]. The ability of the Rho/Rho kinase pathway to promote actin polymerization leads to a loss of globular actin (G-actin), which results in the release of the SRF co-activator MAL, a G-actin binding protein [205]. Thus, SRF, a central regulator of smooth muscle-restricted gene transcription, is under tight control by the Rho/Rho kinase pathway [8, 206]. Rho/Rho kinase activation is regulated by receptor tyrosine kinases and GPCRs through the action of Rho-specific guanine exchange factors (RhoGEFs). Ligand binding to muscarinic M3 receptors coupled to Gαq can induce RhoA activation, likely via p63RhoGEF, and promotes Rho kinase dependent actin polymerization leading to SRF translocation and the induction of smooth muscle specific gene expression. Insulin induced expression of contractile phenotype markers and the induction of a functionally hypercontractile phenotype also requires the Rho/Rho kinase pathway, though the GEFs involved have not yet been identified [41, 207]. It indicates that Rho/Rho kinase signaling plays an important role in the transcription of genes that encode mRNA required for synthesis and accumulation of contractile proteins in hypertrophic ASM.
Protein Kinase C
Protein kinase C (PKC) is a superfamily that includes three classes of isoenzymes. So-called, conventional isoforms (α, β1, β2 and γ) are activated by calcium, phorbol esters and phosphatidylserine; novel isoforms (δ, ε, ι, θ and μ) are calcium-insensitive and activated by phorbol esters and phosphatidylserine; and, atypical isoforms (ζ, τ/λ) are calcium and phorbol ester-insensitive and activated by phosphatidylserine. PKC α, β1, β2, δ, and ζ, but not γ or ι, are expressed in bovine tracheal myocytes [208], whereas PKC α, β1, β2, δ, ε, θ, ι, ζ, τ and μ have each been identified in human tracheal myocytes [209]. There are several studies suggesting the role of PKC’s in ASM proliferation [210–214]. Their role in hypertrophy is not entirely clear but data from other systems indicate that over expression of these select PKC isoforms can induce cardiac hypertrophy in transgenic mice [215]. Moreover, activation of PKC isoenzymes via GPCRs has been linked to GSK-3β phosphorylation, suggesting this class of enzymes could play a permissive role in protein translation via eIF2B [107]. On this basis, future focus on the role of PKCs in airway myocyte hypertrophy appears to be warranted.
Regulation of Airway Smooth Muscle Apoptosis
Tissue development and homeostasis is subject to rounds of cell division and differentiation, but of equal importance is the duration of cell survival and the capacity to orchestrate self-termination to cull infected, damaged, and unwanted cells. Such programmed cell death, dubbed apoptosis, follows specific patterns and includes shrinkage of the cell, margination of chromatin, and nuclear fragmentation [216–218]. Apoptosis occurs in response to environmental or developmental signals, cellular stresses and specific cell death signals. This self-inflicted death involves a number of evolutionarily conserved biochemical pathways that have been intensively studied for over two decades [219].
In mammals, programmed cell death can be initiated by two major pathways: (i) the extrinsic pathway, which can be triggered by ligation of death receptors and subsequent caspase 8 activation; and, (ii) the intrinsic pathway, which is initiated by cellular stress followed by activation of caspase 9 (Figure 4). Each of these pathways converges to a common execution phase that requires the activation of caspases-3 or -7 from their inactive zymogen form to their processed, active form [218, 220–222]. The apical activators (caspase-8 and -9) have a primary specificity for cleavage at Asp297 located in a region that delineates the large and small subunits of active caspases-3 and -7 [218]. Apoptotic cell death is centrally controlled by both caspase activation cascades and/or mitochondrial membrane permeabilization (MMP), processes that are inextricably linked [223–225]. Indeed, MMP itself stimulates caspase activation through the release of several caspase-activating proteins, in particular cytochrome c [222, 224], and caspase activation of proteins such as truncated Bid, Bad, Bcl-XL triggers MMP [226–228]. MMP manifests at the level of the outer membrane, which allows for the release of cytochrome c, as well as at the level of the inner membrane as a loss of the mitochondrial transmembrane potential (Δψm) [223, 224, 229].
Figure 4. Simplified schematic representation of essential pathways for caspase-dependent apoptotic cell death.
Apoptosis is triggered by internal cellular stress (intrinsic pathway) or extracellular signals (extrinsic pathway) that mediate effects via the binding of ligands (eg. Fas, TNFR1, DR5) to cell surface death receptors. Extrinsic pathways directly activate executioner caspases (caspase-3) through initiator caspases (eg. caspase-8 and -9) ultimately leading to cell death. In intrinsic pathways, death signals are conducted through mitochondria, increasing permeability that leads to the release of cytochrome c. Cytosolic cytochrome c binds Apaf-1 to activate the apoptosome and caspase-9 which ultimately leads to downstream activation of executioner caspase-3 [218].
Airway Smooth Muscle Apoptosis and Asthma
Asthma, particularly if severe and/or of long duration, is accompanied by increased ASM mass due both to myocyte hyperplasia and hypertrophy [28–30, 230]. The possible mechanisms of myocyte proliferation to ASM remodeling have been discussed above, but it needs to be pointed out that there is not a compelling accumulation of data from animal models and human specimens that confirm a place for proliferation as the primary underpinning of remodeling. Indeed more recent work suggest that apoptosis may be of equal importance to proliferation in determining the extent of airway remodeling in animal models of asthma [231–233] In another study using rats, reduced ASM apoptosis was shown to contribute to the airway remodeling process [234]. Furthermore, dexamethasone was shown to induce myocyte apoptosis possibly by increasing pro-apoptotic Bax expression and the decreasing anti-apoptotic Bcl-2 expression [234]. Using a rat model for emphysema, it has also been confirmed that that Fas and FasL participate in apoptosis of myocytes in small airways [235]. Interestingly, injection of the Chinese herbal remedy, shenmai, modulated Fas and FasL protein expression and reduced ASM cell apoptosis, likely associated with inhibitory effects on TNF and inflammation [235].
It has been shown that Fas (CD95 – the receptor for FasL) is expressed by ASM tissue in vivo and on the surface of cultured human airway myocytes in vitro [236]. Moreover, cross-linking of surface Fas induces apoptosis in a significant number of cultured myocytes, an effect that is a) potentiated by stimulation with TNF-α, which upregulates surface Fas expression, and b) reduced by prolonged serum deprivation, which, in the absence of TNF-α treatment, reduces surface Fas expression. This effect could be very important considering that even a small sustained level of apoptosis might have a significant impact on smooth muscle accumulation within intact asthmatic airways because the proliferative index of ASM appears is low even in the presence of substantial airway inflammation [237].
ECM protein alterations are a characteristic feature of asthmatic airway remodeling [238, 239]. These changes include modification such as collagen I, III, and V increase, changes in glycoproteins (fibronectin and tenascin), and alterations in deposition of various proteoglycans (PG) (versican, biglycan, and decorin) [238–243]. It has been reported that culturing cells on different ECM matrices can variably affect ASM number, with laminin in particular imparting a pro-survival response [239, 244]. Culture on decorin resulted in a persistent decrease in cell number via its effects on both proliferation and apoptosis [239], therefore the anti-proliferative and/or proapoptotic effect of decorin could serve to limit the growth of ASM beyond its usual compartment.
The endothelins (ETs) are a family of three isopeptides, acting through two G protein-coupled receptors, ETA and ETB. ET-1 in particular elevates smooth muscle tone [245] and causes a marked potentiation of cholinergic nerve-evoked contraction of ASM [246]. ET-1 expression is increased in asthma and is primarily released from the bronchial epithelium [146–148]. Bronchial smooth muscle cells highly express the ETB receptor which represents about 82–88% of the total ET receptor population [247]. ET-1 is a potent inducer of hypertrophy human ASM cells and at the same time increases the contractile potential of these cells by increasing expression of sm-MHC, calponin, and α-SMA [149]. ET-1-induced-ASM survival has been causally linked with apoptosis inhibition [149], and is a concomitant mechanism leading to increased size and synthetic activity of these cells in primary cell culture [149].
Cigarette smoke has long been considered as a major causative factor for chronic obstructive pulmonary disease (COPD) [248, 249]. A number of mechanisms have been suggested for the pathogenesis of COPD, including disproportionate activities of proteases and antiproteases [250], influx of inflammatory cells into the lung, and oxidative stress [251]. In addition to these mechanisms, gathering evidence suggests that apoptosis may play a significant role in clinical and experimental COPD pathogenesis [249, 252, 253]. It has been reported that cigarette smoke extract (CSE) could induce oxidative stress and apoptosis in ASM cells through activation of both the mitochondrial pathway and death receptor pathway [249]. Neutrophilia is a common feature of smoking induced inflammation and of severe asthma and these cells are a rich source of elastases in the human lung [254]. The degradation of ECM by neutrophil elastases is believed to contribute to decreased airway stability [255]. Neutrophils can also induce apoptosis in ASM, for example, detachment-induced apoptosis (defined as anoikis) [218] with characteristic caspase-3 cleavage [256]. Neutrophil-induced myocyte apoptosis appears to result from the proteolytic activity of proteins released by neutrophils as concomitant fibronectin degradation occurs, and the serine protease inhibitor, α1-antitrypsin, has a protective effect [256].
Most recently it has been reported that simvastatin, an inhibitor of HMG-CoA reductase which is the proximal rate-limiting enzyme in cholesterol biosynthesis [257], can induce apoptosis in primary cultured human airways smooth muscle cells [222]. This effect involves a novel p53-dependent pathway with selective release of mitochondrial protein, Smac and Omi, which inactivate so-called inhibitor of apoptosis protein (eg. XIAP), allowing for cytochrome c independent activation of caspase-9. The pro-apoptosis effects of simvastatin is mainly initiated by depletion of the intracellular pool of cholesterol intermediates called isopernoids [farnesylpyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP)], which are essential for membrane anchoring and activation of small Rho GTPase proteins [222]. This finding suggests there may be means for development of future new asthma therapy to target ASM hyperplasia in asthma.
Airway Smooth Muscle Cell Migration
Airway smooth muscle elongation and smooth muscle differentiation in lung development
Thickening of the airway smooth muscle layer in diseased airways is thought to involve migration of airway smooth muscle cells that recapitulates events of embryonic development. During embryogenesis formation of smooth-muscle containing hollow organs is thought to include migration and reorientation of smooth muscle cells. Cell migration is a common process in formation of blood vessels, the airways and the gastrointestinal system. During lung development migration and differentiation of ASM precursor cells is orchestrated by autocrine and paracrine factors as well as cell-matrix interactions that promote maturation of the airway wall [258, 259]. The molecular and cellular remodeling that occurs during smooth muscle migration may contribute to lung development by mediating elongation of mesenchymal progenitor cells. In the developing airways mesenchymal progenitors differentiate into elongated cells that express smooth muscle-restricted contractile proteins. Elongation is required for differentiation and is associated with activation of the p38 MAP kinase pathway [260], which is known to mediate cell migration[261]. Cell elongation and expression of differentiation marker proteins during development appears to be a mechanical signaling phenomenon because soluble signals that drive smooth muscle differentiation in culture (TGFβ1 and retinoic acid) had no effect on upregulation of smooth muscle marker protein expression [260]. The role of smooth muscle migration in airway development appears to be to more of an effect on cell elongation and orientation rather than a long range chemotactic migration of progenitor cells that occurs in the developing vasculature. In the mouse, the shape change of smooth muscle progenitor cells and ultimately smooth muscle cell differentiation depends critically on expression of laminin 1 and laminin 2 [260, 262]. Both laminins 1 and 2 can ligate integrin α7, and integrin α7 is a protein known to promote vascular and ASM differentiation [263–265]. Disrupting critical changes in cell shape by knocking down laminin 1 and laminin 2 expression results in bronchial smooth muscle hypoplasia [260, 262]. Schuger and colleagues have suggested that mechanical forces in the developing lung that are transmitted through integrin-laminin interactions are critical for upregulation of serum-response factor expression and expression of smooth muscle-restricted genes in differentiated ASM [258]. The hypothesized mechanical signals are integrated with epithelium-derived soluble signals including FGF10, BMP4 and components of the Wnt/catenin and hedgehog signaling families. The combined effect of mechanical signals and biochemical signaling is to drive mesenchymal precursor cells to an elongated, differentiated smooth muscle phenotype. Because cellular processes underlying tube formation are highly conserved from Drosophila to humans [266], it seems reasonable to infer an important role of smooth muscle migration in airway development. However, there are no definitive lineage marker studies of the source of new ASM cells in vivo during lung development. The key question is what percentage of new muscle originates from existing smooth muscle vs. progenitor cells migrating from the surrounding mesenchyme that deposit in the airway wall?
Smooth muscle cell migration and airways remodeling
As discussed above, hyperplasia can result from increased proliferation and diminished apoptosis. In addition, increased cell number could be a result of migration of new cells into the airway wall. There is evidence for two sources of migrating cells in the airways, the lung parenchyma and the blood. Evidence for parenchymal cells as a source of new smooth muscle comes from a structural study of lung biopsies from asthmatics in which lung myofibroblasts were found to migrate in response to allergen challenge [15]. An important question is whether the myofibroblasts are resident cells or are derived from circulating fibrocytes, which are CD34+, collagen I+, α smooth muscle actin+ progenitor cells. These cells are thought to differentiate to myofibroblasts, to contribute to subepithelial fibrosis and possibly to become contractile cells in ASM bundles [35, 267, 268]. A central question for studies of ASM cell migration is whether tube formation during development, wall thickening and epithelial-mesenchymal transformation all require smooth muscle cell migration. An argument can be made for cell migration during tube formation based on analogous events in vascular development. Some interesting questions that need to be tested critically are whether differentiated smooth muscle cells originating in the muscularis migrate in response to cues such as inflammation or lung injury, and does this recapitulate events that occurred during development [269]. Recent evidence for cell migration in remodeling of asthmatic airways is more consistent with immigration of blood-borne fibrocytes [35, 267]. Fibrocytes are present in increased numbers in the lamina propria in patients with asthma [267], the number of fibrocytes in the muscularis increases after allergen challenge [15], and migration of fibrocytes is enhanced by coculture with differentiated ASM cells. While the recent data are quite provocative it remains to be proven by lineage marking approaches that the migrating cells contribute to subepithelial fibrosis, differentiate to contractile airway smooth muscle cells or both. Another important question is whether some fibrocytes remain in the muscle layer as a population of progenitor cells that can be activated to proliferate then differentiate to smooth muscle cells. These are important questions because increased ASM mass and myofibroblast numbers are thought to be major determinants of fixed airway obstruction that is unresponsive to corticosteroid and bronchodilator therapy [28]. Another unresolved issue is what initiates influx of fibrocytes and differentiation of myofibroblasts to smooth muscle cells. As described below many growth factors (eg. PDGF) and proinflammatory signaling proteins (eg. interleukins) stimulate ASM cell migration. Many recent studies of ASM cell migration have focused on the molecular mechanisms that transduce pro-growth and pro-inflammatory signals to cell motion. The following sections summarize conserved features of migration of motile cells, the known promigratory and antimigratory signals affecting ASM migration and some of the key signal transduction pathways that underlie cell migration in ASM and other cell types.
Cellular processes and molecular structures necessary for migration
Cell migration begins with stimulation of receptors that trigger cytoskeletal remodeling and repositioning of organelles as illustrated in Figure 5. There are many receptor systems that sense promigratory stimuli, but we will limit the discussion of these events to the three major classes of receptors involved in cell migration: G protein coupled receptors (GPCR), receptor tyrosine kinases (RTK) and matrix adhesive proteins (integrins). One of the earliest events following receptor ligation and signal transduction is polymerization of actin at the leading edge of a motile cell. This is a fundamentally important process that extends the edge of the cell in the direction of the stimulus during chemotaxis (Figure 5A). For the leading edge of the cell to stick to the substrate and affect forward motion focal contacts must assemble just behind the leading edge (Figure 5B). Myosin II motors bind actin filaments in the body of the cell to generate traction force that moves the cell forward. Myosin I motors at the leading edge are thought to control cortical stiffness and membrane tension. Simultaneously the actin and microtubule cytoskeletal systems remodel and focal contacts at the rear of the cell detach to allow the body of the cell to follow the leading edge towards the stimulus. The nucleus, mitochondria, golgi and endoplasmic reticulum are tethered by adaptor proteins and motors to the cytoskeleton. One role of myosin II motors is to move cellular organelles along with the remodeling cytoskeleton. Depending on the experimental approach cells in vitro will move about randomly in the absence of a chemical gradient (chemokinesis), or move directionally as they follow concentration gradients of soluble attractants (chemotaxis). They may also follow paths of varying matrix adhesiveness and concentrations of bound chemical attractants (haptotaxis), which is a major mechanism of organ formation during embryonic development. A common goal of cell migration studies is to establish the sufficiency and necessity of particular chemicals and signal transduction pathways in migration. Another common goal is to define the cellular machinery necessary for cell movement, and to determine if the function of the machinery is compromised in disease. Studies over the past ten years in ASM muscle have illustrated several important characteristics of migration relevant to airway development and airway remodeling in asthma. The remainder of the chapter summarizes extrinsic molecules that modify ASM migration and the signaling pathways involved in controlling migration. For a more general overview of cell migration and protocols for assaying wound healing and chemotactic migration there are several elegant reviews published by members of the Cell Migration Consortium (www.cellmigration.org). The reader is also referred to previous reviews of smooth muscle cell migration that provide references to methods used in studies of ASM cell migration [270–272].
Figure 5. Schematic model illustrating the prominent features of a migrating cell.
The leading edge of the cell is represented by the cross hatched region on the right. Inset A: The actin polymerization module located at the leading edge is a site of rapid actin polymerization, depolymerization and filament branching. Actin nucleating proteins (mDia1, mDia2, VASP) promote filament formation at the plus (barbed) end. G-actin monomers are added by the action of profilin. Actin filaments are severed by gelsolin and depolymerized by cofilin. Actin branching is regulated by small G proteins acting on WAVE, WASP and proteins of the ARP2/3 complex. The stiffness of the actin gel and traction forces on the matrix are controlled in part myosin II motor proteins that are regulated by activation of multiple kinases (MLCK, PAK, ROCK) and myosin light chain phosphatase (MLCP). Inset B: Signaling and actin attachment modules in the leading edge promote formation of nascent focal contacts (red bars) that rapidly assemble to transiently attach the cell to the matrix. Actin attachment components include integrins, adaptor proteins (talin, vinculin, tensin, paxillin). Signaling module components control assembly and maturation of the focal contact. These include regulatory proteins (Src, CAS, FAK) and proteins controlling actomyosin assembly and myosin II activation and (MLCK, PAK, MLCP and ROCK). As the cell migrates, nascent focal contacts mature and move towards the rear of cell. Focal contacts at the rear of the cell (red bars on the left) are disassembled as the cell advances. Disassembly requires the action of multiprotein complexes that depend on microtubules (gray filaments) emanating from the microtubule organizing center (MTOC). Reprinted from Gerthoffer [270] by permission of the American Thoracic Society.
Conserved biochemical processes known to occur in migrating cells are illustrated in Figure 5. The figure summarizes literature from both nonmuscle and muscle cell motility studies [273, 274]. We will summarize the consensus for how migration occurs in many cell types and then highlight the known and unknown features of ASM migration. In all migrating cells actin polymerization and depolymerization is required. There are numerous actin-associated proteins that coordinate polymerization and depolymerization with some of the best defined proteins being illustrated in the inset Figure 5A. Some of the earliest events in chemotactic cell migration are receptor activation, changes in cell Ca2+ signaling, production of phosphatidyl inositol bis phosphate (PIP2), and activation of monomeric and trimeric G proteins (Figure 6). Each of these proximal signal transduction events can activate multiple signaling cascades. It is impossible to represent all the known signaling mechanisms in a simple schematic, so Figure 6 was designed to make the point that signaling occurs at multiple levels via parallel signaling pathways converging on actin polymerization and myosin II motors, both of which are necessary for traction forces required for cell migration. We will focus on signaling events triggered by platelet-derived growth factor (PDGF) in this chapter because it plays a critical role in smooth muscle cell migration. However, the reader should be aware that numerous promigratory stimuli have been described for ASM (Table 2), and that each stimulus acts via some of the same signaling pathways as well as stimulus-specific pathways not shown in Figure 6. With these limitations in mind we focus on PDGF family members to illustrate the principles of smooth muscle cell migration. PDGF is thoroughly studied in smooth muscle cell migration, and is thought to play a critical role in tube formation during vascular development as well as wound healing in response to injury and inflammation. The β isoform of PDGF receptor (PDGFR-β) is coupled via PI3-K and phospholipase Cγ which elicits changes in myoplasmic calcium, hydrolysis of PIP2 and activation of MAP kinases [261, 275]. These signaling intermediates act together with the small G proteins (Rac and Cdc42) to initiate nucleation of F-actin. Nucleation is promoted in several ways: de novo at the minus (pointed) end, uncapping of plus (barbed) ends by dissociation of actin capping proteins, or by forming new branches (Figure 5A). Nucleation and branching are promoted by proteins of the ARP2/3 complex, profilin and the formins (mDia 1 and 2). The net effect of these proteins is to increase polymerization at the plus ends of existing actin filaments. Profilin is bound to membrane phospholipids in the absence of promigratory stimuli. In the presence of stimuli that activate phospholipases plasma membrane PIP2 levels decrease which releases sequestered profilin. Profilin then enhances adenine nucleotide exchange on G-actin and drives actin polymerization. The formins are activated by binding monomeric G proteins - mDia1 is activated by RhoA, and mDia2 is activated by Cdc42 and Rac. Small G proteins also promote filament branching by activating WASP-family verprolin-homologous protein (WAVE) complex and Wiskott-Aldrich syndrome protein (WASP) respectively. WAVE and WASP proteins activate components of the ARP2/3 complex to increase the number of nucleation sites and the number of sites for branching of filamentous actin. Increased F-actin nucleation, polymerization and branching are all necessary for formation of filopodia and the lamellipodium leading to extension of leading edge of a migrating cell (cross hatched area of the cell in Figure 5). In addition to nucleation and branching, actin filaments must be severed and depolymerized in order to produce effective migration. Actin severing is mediated by several protein including gelsolin and cofilin. Gelsolin is activated by both increased Ca2+ concentrations and by PIP2 (Figure 5A). The number of actin nucleation sites increases when gelsolin is released from the plus end of actin filaments. Migration depends critically on filament growth at the plus ends and filament shrinkage at the minus end. The dynamic behavior of actin filaments is greatly enhanced by cofilin, which promotes depolymerization at the minus end and severs actin filaments thus increasing nucleation sites (Figure 5A). The net effect of all the processes just described is to generate propulsive force at the leading edge of the cell extending filopodia and the lamellipodium towards the stimulus [276]. During the initial stages of lamellipodial extension focal contacts must form between the cell membrane and the extracellular matrix in order for cells to move (Figure 5B). Focal contacts are critically important adhesive structures that are dynamic in a motile cell, forming rapidly, maturing and eventually disassembling at the rear of the cell thus releasing tail of the cell from the matrix.
Figure 6. Signaling pathways that regulate actin polymerization and myosin II motors in smooth muscle cell migration.
Activation of G protein coupled receptors (GPCR) and receptor tyrosine kinases (RTK) initiates activation of parallel signaling cascades that culminate in actin filament remodeling, changes matrix adhesiveness and regulation of myosin II motors that generate traction force. Immediate post-receptor events include activation of trimeric G proteins, Src family tyrosine kinases, phospholipase C (PLC) and PIP2, PI3-kinases (PI3-K) and increased Ca2+. Multiple small G proteins (RhoA, Rac, Cdc42) and calmodulin (CaM) then activate downstream targets that are shown here in darker shades of red. Some targets are effector proteins that regulate actin polymerization including the formins (mDIA1 and mDIA2), WAVE and WASP and the ARP2/3 complex. Other targets include members of the MAP kinase family (p38 MAPK and ERK), Rho kinases (ROCK) and p21-activated protein kinases (PAK). The signaling kinases phosphorylate other protein kinases (MAPKAPK, LIMK) or phosphatases (MLCP) to regulate effector proteins (dark blue ovals) that control actin polymerization and traction forces generated by myosin II. Most of the schematic is organized as sets of parallel linear signaling cascades, which is an oversimplification for the sake of clarity. Pathway convergence and crosstalk are known to occur between the pathways shown. Regulation of MLCK is a good example where both positive and negative inputs are integrated to determine the level of myosin II regulatory light chain phosphorylation and traction force. Reprinted from Gerthoffer271 by permission of the American Heart Association.
Table 2.
Summary of agents that modulate ASM cell migration.
| PROMIGRATORY AGENTS | ANTIMIGRATORY AGENTS |
|---|---|
|
Growth Factors and Cytokines bFGF [340], CXCL10/CXCR3 [267], CC Chemokine ligand 19 (CCL19) [341], IL-1β [261], IL-8 [285], Leukotriene B4 [342], Leukotriene E4 [343], PDGF [261, 344, 345], TGFβ1 [261] |
β-adrenergic Agonists and the PKA pathway Dibutyryl cAMP [313], Formeterol [301], Forskolin [340], Cilomolast [340], Salmeterol [340], Theophylline [313] |
|
Extracellular Matrix Collagens I, III, V [343], Fibronectin [343], Integrins α5, αV [343], Laminin [343], MMP-3 [346] |
Immunomodulating Drugs Fluticasone [340], Pyrimidine synthesis inhibitor, FK778 [347], Sirolimus [347] |
|
Other Promigratory Agents Cyclodextrin [287], Lysophosphatidic acid [313], Thrombin [289], Urokinase plasminogen activator [348] |
Protease Inhibitors 4-(2-Aminoethyl) benzenesulfonylfluoride HCl (AEBSF) [289], Ilomastat [289], Prinomastat [288], TIMPs 1–4 [289] |
|
Protein Kinase and Phosphatase Inhibitors LY294002 [343, 349], PP1 [283], PD98059 [261], SB203580 [261, 343], U-0126 [301, 349], Vanadate [287], Y27632 [313, 343] |
|
|
Other Antimigratory Agents Pertussis toxin [301], Prostaglandin E2 [343], Retinoic acid [349], SB649146 (SP-1 inverse agonist) [345] |
Focal contacts in airway smooth muscle cells and tissues
The protein composition of the focal contact “adhesome” and the function of focal contacts to sense the biochemical and physical environment surrounding a motile cell has been reviewed recently [277, 278]. Geiger and colleagues divided the components and functions of focal contacts into a signaling module, an actin-linking module and an actin-polymerizing module (see Figures 5A and 5B). In this section we will focus on the components of focal contacts that have been described in cultured ASM cells and intact smooth muscle tissue. Several components of the actin linking module have been described including paxillin [279, 280], vinculin [281] and talin [279]. Elements of the signaling module have also been described in ASM including focal adhesion kinase (FAK) [279, 280], Src [108, 282, 283], PI3-kinase [284]; Ca2+ and phospholipase C [285] and several MAP kinases (see below). Signaling module proteins catalyze a variety of reactions, including phospholipid metabolism, protein phosphorylation and dephosphorylation, and increased cell Ca2+, all of which contribute to dynamic formation and degradation of focal contacts during migration. Phosphorylation of focal contact components including FAK, paxillin and talin has been shown in ASM tissue during contraction [280, 286] and following strain of cultured cells [279]. In migrating ASM cells FAK is phosphorylated and degraded during urokinase-stimulated migration [287]. Carlin et al. (2005) also found Src trafficked to the cell membrane during urokinase-induced migration consistent with Src being phosphorylated and activated during ASM cell migration [108, 283]. Components of the signaling module are critical for catalyzing phosphorylation and dephosphorylation events that promote both formation and turnover of the nascent focal contacts at the leading edge.
In addition to an important role for protein phosphorylation there is also a requirement for proteolysis of focal contact proteins by metalloproteinases. Turnover of mature focal contracts is due in part to proteolysis occurring at the trailing edge. In migrating ASM cells, as in many other cell types, upregulation of MMPs 1, 2 and 3 increases during migration. The necessity for MMP activity was demonstrated clearly by the fact that both tissue inhibitors of metalloproteinases (TIMPs) and chemical protease inhibitors reduced or completely blocked AMS cell migration [288, 289]. Protease inhibitors block migration in part because stable focal contacts at the rear of the cell must eventually disassemble for the cell to move forward.
There are some interesting unaddressed questions about the spatial and temporal features of proteins in nascent and mature focal contacts in ASM. It is not clear which components are most sensitive to inflammation, which are altered by mechanical strain during tidal breathing or how the focal adhesion composition and spatial distribution changes as a function of the differentiation state of ASM cells. We assume that many components of focal contacts are similar to those of migrating nonmuscle cells, and evidence to date has largely confirmed this assumption. However, identifying unique protein components of the airway smooth muscle “adhesome” and its constituent modules (signaling, actin binding and polymerization) is important for identifying novel targets for inhibiting or reversing airway remodeling.
Mechanics of cell migration
The primary sources of force generation during for cell migration are actin polymerization promoting protrusion of the leading edge and force generated by myosin motors. Myosin II motors produce traction force that is transmitted to the matrix through the focal contacts (Figure 5B). Smooth muscle myosin II is a phosphoprotein that is activated by Ca2+-calmodulin activation of myosin light chain kinase (MLCK). Activated myosin II binds to actin filaments that contain tropomyosins and caldesmon (predominantly l-caldesmon in cultured ASM cells). Phosphorylation of myosin regulatory light chains increases actomyosin ATPase activity and crossbridge cycling, thus generating traction force. In addition to the canonical Ca2+-calmodulin-MLCK activation pathway there may also be an important Ca2+-independent activation of myosin II mediated by RhoA activation of Rho kinases (ROCKs). Rho A and Rho kinases are well known inhibitors of myosin light chain phosphatase via phosphorylation of the myosin binding subunit of the phosphatase [290]. Activation of Rho A and ROCKs increases phosphorylation of myosin II in ASM tissues and in a variety of cultured smooth muscle cells, fibroblasts and cancer cell lines (reviewed by [291, 292]). In addition there is some evidence for direct phosphorylation of myosin regulatory light chains by ROCK in 3T3 fibroblasts [293], but there is no direct evidence yet for this reaction in ASM. Details of myosin II filament assembly and distribution during ASM migration are mostly undefined.
One interesting question that remains unexplored is how myosin motors respond to the physical nature of the matrix. This is potentially important given that there are dynamic changes in matrix composition and stiffness of the lung parenchyma during development and disease. Ingber and coworkers showed that decreasing stiffness of an artificial fibronectin matrix reduced myosin light chain phosphorylation in cultured pulmonary artery smooth muscle cells [294]. In addition, inhibition of myosin ATPase with 2,3-butanedione 2-monoxime reduced myosin light chain phosphorylation, suggesting that traction forces are necessary for proper function of the Ca2+-calmodulin-MLCK signaling pathway. In the same study, disrupting microtubules with nocodazole increased myosin phosphorylation. The authors suggested that decreased adhesiveness, decreased matrix stiffness and reduced force from myosin II motors all reduced the prestress on the cytoskeleton. Reduced prestress then inhibited myosin phosphorylation, possibly by altering proper assembly of the enzymes and other proteins regulating myosin phosphorylation. Ingber et al. have proposed a model where myosin II generates traction force on the matrix, the matrix modifies myosin phosphorylation rate and level, and therefore the activity of actomyosin as a function of matrix stiffness. If this is true several interesting questions arise related to airway remodeling in inflammatory lung diseases. Does inflammation enhance migration of fibrocytes, myofibroblasts or existing smooth muscle cells through increased contractile tone and thus increased prestress? Do anti-inflammatory drugs reverse or inhibit such an effect? Does a decrease in elastic modulus of the lung parenchyma influence migration of these cells in asthmatic airways? Would reversing the changes in parenchymal mechanics prevent immigration of fibrocytes and their subsequent differentiation to myofibroblasts and smooth muscle cells? While there are no direct studies of both matrix composition and migration in asthma, the promigratory influences of collagen, elastin and laminin on ASM cell migration in vitro is consistent with the hypothesis that matrix composition and matrix mechanics could be a key regulator of ASM cell migration in vivo [283, 295]. Further studies of migration of fibrocytes and myofibroblasts on matrices that mimic the remodeled asthmatic airway are warranted.
Microtubules and cell migration
Actin polymerization/depolymerization and focal contact remodeling have justifiably been the focus of many studies of cell migration. However, it is clear that microtubules must also remodel during migration, and that this critical process is not as well defined as remodeling of the actin cytoskeleton. In stationary cells such as ASM embedded in extracellular matrix of the airway walls the microtubule organizing center (MTOC) and the nucleus are centered in the cell. However, during migration the nucleus is relocated toward the trailing edge of the cell. One important mediator of this relocation is Cdc42 regulation of myotonic dystrophy kinase-related Cdc42 binding kinase (MRCK) [296]. Gomes et al. (2005) found that nuclear relocation required phosphorylation myosin II by MRCK. Whether a similar event occurs in ASM cells is unknown, but there is some evidence that dynamic instability of microtubules is required during migration of vascular smooth muscle cells. Paclitaxel (Taxol), which stabilizes microtubules by binding the sides of the tubulin polymer, blocks VSM cell migration [297]. Microtubules clearly affect the degree of prestress in the ASM cytoskeleton and influence traction forces in cultured cells [298, 299], but it is not known to what extent dynamic instability is necessary during ASM migration. In nonmuscle cells dynamic instability of microtubules is important for disassembly of stable focal contacts at the rear of migrating cells, which is required for disengagement of the trailing edge from the matrix [300]. At this time there is only indirect evidence to infer a signaling pathway that would promote dynamic instability in ASM. A study of urokinase-stimulated ASM cell motility showed that urokinase induces ASM migration via a pathway including PI3-kinase [301]. Studies in vascular smooth muscle cell migration indicate urokinase also activates Akt and glycogen synthase kinase 3β (GSK3β) [302]. GSK3β interacts with adenomatous polyposis coli (APC), which is known to regulate cell polarity by interacting with the plus end of microtubules [303]. Whether this signaling model functions in ASM migration is unclear, but it is likely that a functionally analogous system is required for microtubule remodeling during detachment and translocation of the rear of a migrating ASM cell. Blocking detachment of the trailing edge of the cell might in theory be beneficial for blocking remodeling events in the asthmatic airway. Inhibition of Akt and GSK3β signaling might have the appealing feature of reducing hypertrophy as well as reducing immigration of new cells to the muscle layers of diseased airways (see discussion of Akt and GSK3β signaling above).
Soluble and solid state signals that modulate migration
There are many chemically and structurally diverse molecules that enhance or inhibit ASM cell migration (Table 2). Many are soluble signaling molecules, but some are components of the extracellular matrix that are presented to ASM cells as solid-state signals. One of the first soluble promigratory molecules used to stimulate ASM migration in vitro was PDGF [261]. Subsequent studies have identified biogenic amines, lipids, growth factors, cytokines and chemokines as soluble modulators of ASM migration. Many of the promigratory compounds are autocrine or paracrine signaling molecules that are secreted at elevated levels in diseased airways. The earliest studies of solid-state signals that promote migration were by Schuger and colleagues who showed that laminin β1 chain as necessary for migration of smooth muscle cells from mouse lung explants [262]. Later in vitro studies described the promigratory effects of collagens, fibronectin, laminins and matrix metalloproteinases, and antimigratory effects of tissue inhibitors of metaloproteinases and chemical protease inhibitors [283, 288, 289]. Although it is clear that matrix composition changes in the asthmatic airway and that matrix expression by cultured ASM cells changes upon exposure to proinflammatory agents [73, 295], it is not known whether matrix composition alters migration in vivo in the lungs of asthmatic humans or in experimental asthma in mice. Whether a given signaling molecule or pathway is necessary for ASM migration could be tested in knockout and transgenic mouse models using lineage marking strategies similar to those used to define the source of vascular smooth muscle cells in atherosclerotic plaques (reviewed by [304]) and the necessity for PDGF signaling in blood vessel development [305].
Signaling cascades
Multiple highly conserved signal transduction cascades are activated during cell migration. The pathways studied most frequently in both nonmuscle and smooth muscle cell migration are illustrated in Figure 6. In this simplified scheme signal transduction events are shown as cascades beginning with receptor activation. We focus the illustration on three fundamentally important types of receptors: receptor tyrosine kinases (RTK), G protein coupled receptors (GPCR), and integrins, which are each known to promote cell migration. Coupling of early activation to G proteins is common to many promigratory stimuli. Both small G proteins (RhoA, Rac, Cdc42) and trimeric G-proteins are known to participate in promigratory signaling depending of the stimulant used in the experiment. Activated G proteins, Ca2+, and changes in phospholipids including PIP2 and IP3 activate protein kinase cascades that include PI3-kinase, Ca2+-dependent protein kinases, Rho-activated protein kinases (ROCK) and mitogen-activated protein kinases (MAPK) (Figure 6). The substrates for the various protein kinases include other protein kinases (MAPKAP kinase and LIM kinase) as well as proteins that interact with or regulate actin filament formation (HSP27, cofilin, myosin II). The monomeric G proteins (RhoA, rac and cdc42) also frequently regulate proteins that influence F-actin formation (mDia1, WAVE, WASP, ARP2/3). The more distal effector proteins in this scheme (blue ellipses in Figure 6) regulate two critical cellular processes: actin polymerization and activation of myosin II. The proteins required for actin polymerization and coupling of F-actin to the cell membrane integrins are illustrated in Figures 5A and 5B. Stimuli that increase myoplasmic Ca2+ oscillations or mean Ca2+ concentration in a cell activate MLCK which phosphorylates the regulatory light chains of myosin II, which is the protein that generates traction forces necessary to move the cell. Some of these pathways have been described in some detail in airway smooth muscle (Src, ERK, p38 MAPK, PI-3kinase), but other aspects of signaling are less well defined or undefined in ASM migration (Rac, RhoA, ROCK, LIM kinase, cofilin, ARP2/3). The reader is referred to a previous review for more details of signal transduction pathways known to participate in ASM migration [270]. Better understanding of signal transduction processes necessary for ASM migration is significant because migration is a fundamental process in lung development, and migration is presumed to be altered by lung diseases possibly contributing to airway wall thickening in asthmatics.
Modulation of ASM cell migration by drugs
One of the exciting aspects of studies of ASM cell migration is that biochemical processes mediating migration might be novel therapeutic targets for preventing or reversing airway remodeling in asthma. This is frequently cited as a rationale for exploring novel aspects of ASM migration. Identifying novel drug targets has also been a driving force in studies of vascular smooth muscle cell migration. In fact a number of cardiovascular drugs have beneficial effects in reducing atherogenesis and promoting recovery from vascular injury in part by reducing vascular smooth muscle proliferation and cell migration (reviewed by [270]). A clear proof of principle comes from the effects of statins, rapamycin and taxol, which all reduce proliferation, inhibit cell migration and reduce vascular wall remodeling [297, 306, 307]. In the case of statins, the therapeutic goal is to inhibit cholesterol synthesis to reduce serum LDL levels. There may also be a secondary benefit resulting from inhibiting mevalonate synthesis and isoprenylation of small G proteins. As discussed above, statins have been found to reduce airway hyperreactivity in a mouse asthma model [263], reduce cell proliferation [119] and to enhance apoptosis [217]. The pleiotropic effects are very likely due to disrupting signaling via small G proteins that participate in multiple biochemical processes including cell migration (see Figs. 5 and 6). Extended, low dose therapy with statins may well inhibit or reverse pathological airway remodeling by multiple mechanisms including reduced migration of ASM cells, fibrocytes or myofibroblasts. Whether such an effect occurs in the airways is an interesting question that has been raised in recent reviews of ASM as a target for novel asthma therapies [308, 309].
Several established drugs used to treat asthma also have significant antimigratory effects (Table 2). Corticosteriods and β-adrenergic agonists are mainstays of combination therapy for long term asthma control. Both classes of drugs as well as other drugs acting via cAMP have antimigratory effects. This suggests the hypothesis that combination of corticosteroids and long acting β-agonists might act by a combination of reducing ASM proliferation, preventing matrix remodeling and reducing ASM cell migration. Although this is a provocative hypothesis, there is no in vivo animal or human clinical data that critically tests this notion.
Other potential drug targets that should inhibit ASM cell migration include MAP kinases, and Rho kinases. P38 MAP kinases have been targeted for development of drugs to treat inflammatory diseases since the mid-1990s, but development has been limited by toxicity of first generation inhibitors. The advent of second generation inhibitors increases the possibility that expression of numerous contractile, proinflammatory and promigratory signaling proteins might be reduced by blocking p38 MAP kinase signaling [310–312]. In theory, inhibiting p38 MAP kinases could reduce expression of the extracellular signals for cell migration (eg. PDGF, IL1β and IL8) as well as block migration directly [261]. Evidence from asthma models is consistent with this hypothesis [311], but preclinical studies in animal models of asthma models and studies in humans using newer inhibitors are needed to critically test this strategy [310].
Rho kinases (ROCK1 and ROCK2) are also potential antimigratory target proteins. It is clear that Rho kinase inhibitors can inhibit ASM cell migration [313], and a Rho kinase inhibitor (Fasudil) has been tested in humans to reduce cerebral vasospasm and treat angina pectoris. The latter effects are due to vasodilation. Rho kinase inhibitors are also effective bronchodilators in mouse models of asthma [314, 315], but there is no published evidence of clinical benefit to humans with asthma. In addition, there are no data demonstrating a significant effect of Rho kinase inhibition on cell migration in vivo and airway wall remodeling. Nevertheless, Rho kinase inhibitors and p38 MAP kinase inhibitors are mechanistically appealing for modifying multiple aspects of the cell biology of airway dysfunction including smooth muscle contraction, proliferation and cell migration. Off-target effects are the major limitations of protein kinase inhibitors. However, new generation drugs with enhanced selectivity combined with local delivery to the lungs might address this problem and thereby expand the tools available to the pulmonary physician for long term therapy of asthma.
Conclusion
Rapid progress has been made in the past decade in studies of key processes underlying airway remodeling. Based on clinical studies and animal models of asthma it is clear that both airway smooth muscle hyperplasia and hypertrophy occurs. To increase cell number and size in the airway wall cells it is assumed that cells proliferate, survive for longer time periods and increase expression of proteins. In addition it is possible that some immigration of progenitor cells from beyond the muscularis occurs in diseased airways as well as shape changes in resident cells that differentiate to contractile smooth muscle. These notions stimulated a host of studies of biochemical pathways that control the fundamental processes of proliferation, apoptosis and cell migration. Many of the key stimuli, receptors and transduction pathways are conserved molecules known to participate in remodeling of the vasculature and in tumorigenesis. Developing novel drugs or novel uses of existing drugs to modify organ remodeling is one of the compelling reasons for studying many of pathways described. While a comprehensive view of some pathways and processes is emerging there are still basic and applied science questions remaining. Some outstanding basic science questions include the degree to which ASM cells proliferate in vivo in diseased lungs, the source of migrating cells and the potential for novel features of translational control of protein expression and cell survival to be discovered. The latter point is particularly important for developing organ-selective drugs targeting pathways unique in airway remodeling. In addition, even if organ or cell specificity is not possible it is possible that known drugs being tested or used currently in cancer chemotherapy and cardiovascular medicine can be delivered in a lung-restricted manner to alter airway remodeling. Broad-based, multidiscliplinary approaches employing cell, animal and human studies will be required to integrate the basic molecular and cell signaling studies into an effective translational strategy for developing novel therapy of obstructive lung diseases.
Acknowledgments
Supported by NIH grant HL077726 (WTG) and The Canadian Institutes of Health Research (CIHR), GlaxoSmithKline Collaborative Innovation Research Fund, Manitoba Institute of Child Health (MICH), and Canada Foundation for Innovation (AJH). SG is supported by a Parker B. Francis Fellowship in Pulmonary Research. DS is supported by a CIHR Postdoctoral Fellowship. PS is supported by the Manitoba Health Research Council, MICH and CIHR. AJH holds a Canada Research Chair in Airway Cell and Molecular Biology.
Footnotes
Supplementary Information
None
References
- 1.Wissler RW. The arterial medial cell, smooth muscle, or multifunctional mesenchyme? Circulation. 1967;36:1–4. doi: 10.1161/01.cir.36.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Jeffery PK. Remodeling in asthma and chronic obstructive lung disease. Am J Respir Crit Care Med. 2001;164:S28–S38. doi: 10.1164/ajrccm.164.supplement_2.2106061. [DOI] [PubMed] [Google Scholar]
- 3.Holgate ST, Davies DE, Lackie PM, Wilson SJ, Puddicombe SM, Lordan JL. Epithelial-mesenchymal-interactions in the pathogenesis of asthma. J Allergy Clin Immunol. 2000;105:193–204. doi: 10.1016/s0091-6749(00)90066-6. [DOI] [PubMed] [Google Scholar]
- 4.Van Eerdewegh P, Little RD, Dupuis J, Del Mastro RG, Falls K, Simon J, et al. Association of the ADAM33 gene with asthma and bronchial hyperresponsiveness. Nature. 2002;418:426–430. doi: 10.1038/nature00878. [DOI] [PubMed] [Google Scholar]
- 5.Holgate ST. Airway inflammation and remodeling in asthma: current concepts. Mol Biotechnol. 2002;22:179–189. doi: 10.1385/MB:22:2:179. [DOI] [PubMed] [Google Scholar]
- 6.Halayko AJ, Stephens NL. Potential role for phenotypic modulation of bronchial smooth muscle cells in chronic asthma. Can J Physiol Pharmacol. 1994;72:1448–1457. doi: 10.1139/y94-209. [DOI] [PubMed] [Google Scholar]
- 7.Antczak A, Montuschi P, Kharitonov S, Gorski P, Barnes PJ. Increased exhaled cysteinyl-leukotrienes and 8-isoprostane in aspirin-induced asthma. Am J Respir Crit Care Med. 2002;166:301–306. doi: 10.1164/rccm.2101021. [DOI] [PubMed] [Google Scholar]
- 8.Halayko AJ, Tran T, Ji SY, Yamasaki A, Gosens R. Airway smooth muscle phenotype and function: interactions with current asthma therapies. Curr Drug Targets. 2006;7:525–40. doi: 10.2174/138945006776818728. [DOI] [PubMed] [Google Scholar]
- 9.Halayko AJ, Amrani Y. Mechanisms of inflammation-mediated airway smooth muscle plasticity and airways remodeling in asthma. Respir Physiol Neurobiol. 2003;137:209–22. doi: 10.1016/s1569-9048(03)00148-4. [DOI] [PubMed] [Google Scholar]
- 10.Halayko AJ, Solway J. Molecular mechanisms of phenotypic plasticity in smooth muscle cells. J Appl Physiol. 2001;90:358–68. doi: 10.1152/jappl.2001.90.1.358. [DOI] [PubMed] [Google Scholar]
- 11.Halayko AJ, Tran T, Gosens R. Phenotype and functional plasticity of airway smooth muscle: role of caveolae and caveolins. Proc Am Thorac Soc. 2008;5:80–8. doi: 10.1513/pats.200705-057VS. [DOI] [PubMed] [Google Scholar]
- 12.Affonce DA, Lutchen KR. New perspectives on the mechanical basis for airway hyperreactivity and airway hypersensitivity in asthma. J Appl Physiol. 2006;101:1710–9. doi: 10.1152/japplphysiol.00344.2006. [DOI] [PubMed] [Google Scholar]
- 13.Lambert RK, Wiggs BR, Kuwano K, Hogg JC, Pare PD. Functional significance of increased airway smooth muscle in asthma and COPD. J Appl Physiol. 1993;74:2771–81. doi: 10.1152/jappl.1993.74.6.2771. [DOI] [PubMed] [Google Scholar]
- 14.Wiggs BR, Moreno R, Hogg JC, Hilliam C, Pare PD. A model of the mechanics of airway narrowing. J Appl Physiol. 1990;69:849–60. doi: 10.1152/jappl.1990.69.3.849. [DOI] [PubMed] [Google Scholar]
- 15.Gizycki MJ, Adelroth E, Rogers AV, O’Byrne PM, Jeffery PK. Myofibroblast involvement in the allergen-induced late response in mild atopic asthma. Am J Respir Cell Mol Biol. 1997;16:664–73. doi: 10.1165/ajrcmb.16.6.9191468. [DOI] [PubMed] [Google Scholar]
- 16.Kelly MM, O’Connor TM, Leigh R, Otis J, Gwozd C, Gauvreau GM, et al. Effects of budesonide and formoterol on allergen-induced airway responses, inflammation, and airway remodeling in asthma. J Allergy Clin Immunol. 2010;125:349–356. e13. doi: 10.1016/j.jaci.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Chung KF. Should treatments for asthma be aimed at the airway smooth muscle? Expert Rev Respir Med. 2007;1:209–17. doi: 10.1586/17476348.1.2.209. [DOI] [PubMed] [Google Scholar]
- 18.Baroffio M, Crimi E, Brusasco V. Airway smooth muscle as a model for new investigative drugs in asthma. Ther Adv Respir Dis. 2008;2:129–39. doi: 10.1177/1753465808091154. [DOI] [PubMed] [Google Scholar]
- 19.Hirst SJ, Lee TH. Airway smooth muscle as a target of glucocorticoid action in the treatment of asthma. Am J Respir Crit Care Med. 1998;158:S201–6. doi: 10.1164/ajrccm.158.supplement_2.13tac190. [DOI] [PubMed] [Google Scholar]
- 20.Hirst SJ, Martin JG, Bonacci JV, Chan V, Fixman ED, Hamid QA, et al. Proliferative aspects of airway smooth muscle. J Allergy Clin Immunol. 2004;114:S2–17. doi: 10.1016/j.jaci.2004.04.039. [DOI] [PubMed] [Google Scholar]
- 21.Xiong W, Pestell RG, Watanabe G, Li J, Rosner MR, Hershenson MB. Cyclin D1 is required for S phase traversal in bovine tracheal myocytes. Am J Physiol. 1997;272:L1205–10. doi: 10.1152/ajplung.1997.272.6.L1205. [DOI] [PubMed] [Google Scholar]
- 22.Sherr CJ, Roberts JM. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–63. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 23.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–12. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 24.Elledge SJ. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274:1664–72. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- 25.Lindqvist A, Rodriguez-Bravo V, Medema RH. The decision to enter mitosis: feedback and redundancy in the mitotic entry network. J Cell Biol. 2009;185:193–202. doi: 10.1083/jcb.200812045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dekkers BG, Schaafsma D, Nelemans SA, Zaagsma J, Meurs H. Extracellular matrix proteins differentially regulate airway smooth muscle phenotype and function. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1405–13. doi: 10.1152/ajplung.00331.2006. [DOI] [PubMed] [Google Scholar]
- 27.Hirst SJ, Twort CH, Lee TH. Differential effects of extracellular matrix proteins on human airway smooth muscle cell proliferation and phenotype. Am J Respir Cell Mol Biol. 2000;23:335–44. doi: 10.1165/ajrcmb.23.3.3990. [DOI] [PubMed] [Google Scholar]
- 28.Benayoun L, Druilhe A, Dombret MC, Aubier M, Pretolani M. Airway structural alterations selectively associated with severe asthma. Am J Respir Crit Care Med. 2003;167:1360–8. doi: 10.1164/rccm.200209-1030OC. [DOI] [PubMed] [Google Scholar]
- 29.Ebina M, Takahashi T, Chiba T, Motomiya M. Cellular hypertrophy and hyperplasia of airway smooth muscles underlying bronchial asthma. A 3-D morphometric study. Am Rev Respir Dis. 1993;148:720–6. doi: 10.1164/ajrccm/148.3.720. [DOI] [PubMed] [Google Scholar]
- 30.Woodruff PG, Dolganov GM, Ferrando RE, Donnelly S, Hays SR, Solberg OD, et al. Hyperplasia of smooth muscle in mild to moderate asthma without changes in cell size or gene expression. Am J Respir Crit Care Med. 2004;169:1001–6. doi: 10.1164/rccm.200311-1529OC. [DOI] [PubMed] [Google Scholar]
- 31.Gosens R, Nelemans SA, Grootte Bromhaar MM, McKay S, Zaagsma J, Meurs H. Muscarinic M3-receptors mediate cholinergic synergism of mitogenesis in airway smooth muscle. Am J Respir Cell Mol Biol. 2003;28:257–62. doi: 10.1165/rcmb.2002-0128OC. [DOI] [PubMed] [Google Scholar]
- 32.Naureckas ET, I, Ndukwu M, Halayko AJ, Maxwell C, Hershenson MB, Solway J. Bronchoalveolar lavage fluid from asthmatic subjects is mitogenic for human airway smooth muscle. Am J Respir Crit Care Med. 1999;160:2062–6. doi: 10.1164/ajrccm.160.6.9903131. [DOI] [PubMed] [Google Scholar]
- 33.Ruocco S, Lallemand A, Tournier JM, Gaillard D. Expression and localization of epidermal growth factor, transforming growth factor-alpha, and localization of their common receptor in fetal human lung development. Pediatr Res. 1996;39:448–55. doi: 10.1203/00006450-199603000-00012. [DOI] [PubMed] [Google Scholar]
- 34.Rajah R, Nachajon RV, Collins MH, Hakonarson H, Grunstein MM, Cohen P. Elevated levels of the IGF-binding protein protease MMP-1 in asthmatic airway smooth muscle. Am J Respir Cell Mol Biol. 1999;20:199–208. doi: 10.1165/ajrcmb.20.2.3148. [DOI] [PubMed] [Google Scholar]
- 35.Aubert JD, Hayashi S, Hards J, Bai TR, Pare PD, Hogg JC. Platelet-derived growth factor and its receptor in lungs from patients with asthma and chronic airflow obstruction. Am J Physiol. 1994;266:L655–63. doi: 10.1152/ajplung.1994.266.6.L655. [DOI] [PubMed] [Google Scholar]
- 36.Amishima M, Munakata M, Nasuhara Y, Sato A, Takahashi T, Homma Y, et al. Expression of epidermal growth factor and epidermal growth factor receptor immunoreactivity in the asthmatic human airway. Am J Respir Crit Care Med. 1998;157:1907–12. doi: 10.1164/ajrccm.157.6.9609040. [DOI] [PubMed] [Google Scholar]
- 37.Hirst SJ, Barnes PJ, Twort CH. Quantifying proliferation of cultured human and rabbit airway smooth muscle cells in response to serum and platelet-derived growth factor. Am J Respir Cell Mol Biol. 1992;7:574–81. doi: 10.1165/ajrcmb/7.6.574. [DOI] [PubMed] [Google Scholar]
- 38.Ediger TL, Toews ML. Synergistic stimulation of airway smooth muscle cell mitogenesis. J Pharmacol Exp Ther. 2000;294:1076–82. [PubMed] [Google Scholar]
- 39.Noveral JP, Bhala A, Hintz RL, Grunstein MM, Cohen P. Insulin-like growth factor axis in airway smooth muscle cells. Am J Physiol. 1994;267:L761–5. doi: 10.1152/ajplung.1994.267.6.L761. [DOI] [PubMed] [Google Scholar]
- 40.Stewart AG, Tomlinson PR, Fernandes DJ, Wilson JW, Harris T. Tumor necrosis factor alpha modulates mitogenic responses of human cultured airway smooth muscle. Am J Respir Cell Mol Biol. 1995;12:110–9. doi: 10.1165/ajrcmb.12.1.7529028. [DOI] [PubMed] [Google Scholar]
- 41.Gosens R, Nelemans SA, Hiemstra M, Grootte Bromhaar MM, Meurs H, Zaagsma J. Insulin induces a hypercontractile airway smooth muscle phenotype. Eur J Pharmacol. 2003;481:125–31. doi: 10.1016/j.ejphar.2003.08.081. [DOI] [PubMed] [Google Scholar]
- 42.Gosens R, Stelmack GL, Dueck G, McNeill KD, Yamasaki A, Gerthoffer WT, et al. Role of caveolin-1 in p42/p44 MAP kinase activation and proliferation of human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2006;291:L523–34. doi: 10.1152/ajplung.00013.2006. [DOI] [PubMed] [Google Scholar]
- 43.Gosens R, Dueck G, Gerthoffer WT, Unruh H, Zaagsma J, Meurs H, et al. p42/p44 MAP kinase activation is localized to caveolae-free membrane domains in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1163–72. doi: 10.1152/ajplung.00471.2006. [DOI] [PubMed] [Google Scholar]
- 44.Bos IS, Gosens R, Zuidhof AB, Schaafsma D, Halayko AJ, Meurs H, et al. Inhibition of allergen-induced airway remodelling by tiotropium and budesonide: a comparison. Eur Respir J. 2007;30:653–61. doi: 10.1183/09031936.00004907. [DOI] [PubMed] [Google Scholar]
- 45.Gosens R, I, Bos S, Zaagsma J, Meurs H. Protective effects of tiotropium bromide in the progression of airway smooth muscle remodeling. Am J Respir Crit Care Med. 2005;171:1096–102. doi: 10.1164/rccm.200409-1249OC. [DOI] [PubMed] [Google Scholar]
- 46.Henderson WR, Jr, Tang LO, Chu SJ, Tsao SM, Chiang GK, Jones F, et al. A role for cysteinyl leukotrienes in airway remodeling in a mouse asthma model. Am J Respir Crit Care Med. 2002;165:108–16. doi: 10.1164/ajrccm.165.1.2105051. [DOI] [PubMed] [Google Scholar]
- 47.Wang CG, Du T, Xu LJ, Martin JG. Role of leukotriene D4 in allergen-induced increases in airway smooth muscle in the rat. Am Rev Respir Dis. 1993;148:413–7. doi: 10.1164/ajrccm/148.2.413. [DOI] [PubMed] [Google Scholar]
- 48.Krymskaya VP, Orsini MJ, Eszterhas AJ, Brodbeck KC, Benovic JL, Panettieri RA, Jr, et al. Mechanisms of proliferation synergy by receptor tyrosine kinase and G protein-coupled receptor activation in human airway smooth muscle. Am J Respir Cell Mol Biol. 2000;23:546–54. doi: 10.1165/ajrcmb.23.4.4115. [DOI] [PubMed] [Google Scholar]
- 49.Panettieri RA, Tan EM, Ciocca V, Luttmann MA, Leonard TB, Hay DW. Effects of LTD4 on human airway smooth muscle cell proliferation, matrix expression, and contraction In vitro: differential sensitivity to cysteinyl leukotriene receptor antagonists. Am J Respir Cell Mol Biol. 1998;19:453–61. doi: 10.1165/ajrcmb.19.3.2999. [DOI] [PubMed] [Google Scholar]
- 50.Gosens R, Grootte Bromhaar MM, Maarsingh H, ten Damme A, Meurs H, Zaagsma J, et al. Bradykinin augments EGF-induced airway smooth muscle proliferation by activation of conventional protein kinase C isoenzymes. Eur J Pharmacol. 2006;535:253–62. doi: 10.1016/j.ejphar.2006.01.065. [DOI] [PubMed] [Google Scholar]
- 51.Billington CK, Kong KC, Bhattacharyya R, Wedegaertner PB, Panettieri RA, Jr, Chan TO, et al. Cooperative regulation of p70S6 kinase by receptor tyrosine kinases and G protein-coupled receptors augments airway smooth muscle growth. Biochemistry. 2005;44:14595–605. doi: 10.1021/bi0510734. [DOI] [PubMed] [Google Scholar]
- 52.Kong KC, Billington CK, Gandhi U, Panettieri RA, Jr, Penn RB. Cooperative mitogenic signaling by G protein-coupled receptors and growth factors is dependent on G(q/11) FASEB J. 2006;20:1558–60. doi: 10.1096/fj.05-5622fje. [DOI] [PubMed] [Google Scholar]
- 53.Capra V, Habib A, Accomazzo MR, Ravasi S, Citro S, Levy-Toledano S, et al. Thromboxane prostanoid receptor in human airway smooth muscle cells: a relevant role in proliferation. Eur J Pharmacol. 2003;474:149–59. doi: 10.1016/s0014-2999(03)02014-4. [DOI] [PubMed] [Google Scholar]
- 54.Cerutis DR, Nogami M, Anderson JL, Churchill JD, Romberger DJ, Rennard SI, et al. Lysophosphatidic acid and EGF stimulate mitogenesis in human airway smooth muscle cells. Am J Physiol. 1997;273:L10–5. doi: 10.1152/ajplung.1997.273.1.L10. [DOI] [PubMed] [Google Scholar]
- 55.Tomlinson PR, Wilson JW, Stewart AG. Inhibition by salbutamol of the proliferation of human airway smooth muscle cells grown in culture. Br J Pharmacol. 1994;111:641–7. doi: 10.1111/j.1476-5381.1994.tb14784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Florio C, Martin JG, Styhler A, Heisler S. Antiproliferative effect of prostaglandin E2 in cultured guinea pig tracheal smooth muscle cells. Am J Physiol. 1994;266:L131–7. doi: 10.1152/ajplung.1994.266.2.L131. [DOI] [PubMed] [Google Scholar]
- 57.Young PG, Skinner SJ, Black PN. Effects of glucocorticoids and beta-adrenoceptor agonists on the proliferation of airway smooth muscle. Eur J Pharmacol. 1995;273:137–43. doi: 10.1016/0014-2999(94)00679-2. [DOI] [PubMed] [Google Scholar]
- 58.Guo M, Pascual RM, Wang S, Fontana MF, Valancius CA, Panettieri RA, Jr, et al. Cytokines regulate beta-2-adrenergic receptor responsiveness in airway smooth muscle via multiple PKA- and EP2 receptor-dependent mechanisms. Biochemistry. 2005;44:13771–82. doi: 10.1021/bi051255y. [DOI] [PubMed] [Google Scholar]
- 59.Tomlinson PR, Wilson JW, Stewart AG. Salbutamol inhibits the proliferation of human airway smooth muscle cells grown in culture: relationship to elevated cAMP levels. Biochem Pharmacol. 1995;49:1809–19. doi: 10.1016/0006-2952(94)00532-q. [DOI] [PubMed] [Google Scholar]
- 60.De S, Zelazny ET, Souhrada JF, Souhrada M. Interleukin-1 beta stimulates the proliferation of cultured airway smooth muscle cells via platelet-derived growth factor. Am J Respir Cell Mol Biol. 1993;9:645–51. doi: 10.1165/ajrcmb/9.6.645. [DOI] [PubMed] [Google Scholar]
- 61.De S, Zelazny ET, Souhrada JF, Souhrada M. IL-1 beta and IL-6 induce hyperplasia and hypertrophy of cultured guinea pig airway smooth muscle cells. J Appl Physiol. 1995;78:1555–63. doi: 10.1152/jappl.1995.78.4.1555. [DOI] [PubMed] [Google Scholar]
- 62.McKay S, Bromhaar MM, de Jongste JC, Hoogsteden HC, Saxena PR, Sharma HS. Pro-inflammatory cytokines induce c-fos expression followed by IL-6 release in human airway smooth muscle cells. Mediators Inflamm. 2001;10:135–42. doi: 10.1080/09629350124155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Orsini MJ, V, Krymskaya P, Eszterhas AJ, Benovic JL, Panettieri RA, Jr, Penn RB. MAPK superfamily activation in human airway smooth muscle: mitogenesis requires prolonged p42/p44 activation. Am J Physiol. 1999;277:L479–88. doi: 10.1152/ajplung.1999.277.3.L479. [DOI] [PubMed] [Google Scholar]
- 64.Misior AM, Yan H, Pascual RM, Deshpande DA, Panettieri RA, Penn RB. Mitogenic effects of cytokines on smooth muscle are critically dependent on protein kinase A and are unmasked by steroids and cyclooxygenase inhibitors. Mol Pharmacol. 2008;73:566–74. doi: 10.1124/mol.107.040519. [DOI] [PubMed] [Google Scholar]
- 65.Tliba O, Tliba S, Da Huang C, Hoffman RK, DeLong P, Panettieri RA, Jr, et al. Tumor necrosis factor alpha modulates airway smooth muscle function via the autocrine action of interferon beta. J Biol Chem. 2003;278:50615–23. doi: 10.1074/jbc.M303680200. [DOI] [PubMed] [Google Scholar]
- 66.Bonacci JV, Harris T, Stewart AG. Impact of extracellular matrix and strain on proliferation of bovine airway smooth muscle. Clin Exp Pharmacol Physiol. 2003;30:324–8. doi: 10.1046/j.1440-1681.2003.03838.x. [DOI] [PubMed] [Google Scholar]
- 67.Nguyen TT, Ward JP, Hirst SJ. beta1-Integrins mediate enhancement of airway smooth muscle proliferation by collagen and fibronectin. Am J Respir Crit Care Med. 2005;171:217–23. doi: 10.1164/rccm.200408-1046OC. [DOI] [PubMed] [Google Scholar]
- 68.Dekkers BG, Schaafsma D, Tran T, Zaagsma J, Meurs H. Insulin-induced laminin expression promotes a hypercontractile airway smooth muscle phenotype. Am J Respir Cell Mol Biol. 2009;41:494–504. doi: 10.1165/rcmb.2008-0251OC. [DOI] [PubMed] [Google Scholar]
- 69.Altraja A, Laitinen A, Virtanen I, Kampe M, Simonsson BG, Karlsson SE, et al. Expression of laminins in the airways in various types of asthmatic patients: a morphometric study. Am J Respir Cell Mol Biol. 1996;15:482–8. doi: 10.1165/ajrcmb.15.4.8879182. [DOI] [PubMed] [Google Scholar]
- 70.Laitinen A, Altraja A, Kampe M, Linden M, Virtanen I, Laitinen LA. Tenascin is increased in airway basement membrane of asthmatics and decreased by an inhaled steroid. Am J Respir Crit Care Med. 1997;156:951–8. doi: 10.1164/ajrccm.156.3.9610084. [DOI] [PubMed] [Google Scholar]
- 71.Laitinen LA, Laitinen A. Inhaled corticosteroid treatment and extracellular matrix in the airways in asthma. Int Arch Allergy Immunol. 1995;107:215–6. doi: 10.1159/000236981. [DOI] [PubMed] [Google Scholar]
- 72.Roche WR, Beasley R, Williams JH, Holgate ST. Subepithelial fibrosis in the bronchi of asthmatics. Lancet. 1989;1:520–4. doi: 10.1016/s0140-6736(89)90067-6. [DOI] [PubMed] [Google Scholar]
- 73.Roberts CR, Walker DC, Schellenberg RR. Extracellular matrix. Clin Allergy Immunol. 2002;16:143–78. [PubMed] [Google Scholar]
- 74.Wilson JW, Li X. The measurement of reticular basement membrane and submucosal collagen in the asthmatic airway. Clin Exp Allergy. 1997;27:363–71. [PubMed] [Google Scholar]
- 75.Johnson PRA, Black JL, Carlin S, Ge Q, Underwood PA. The production of extracellular matrix proteins by human passively sensitized airway smooth-muscle cells in culture - The effect of beclomethasone. Am J Respir Crit Care Med. 2000;162:2145–2151. doi: 10.1164/ajrccm.162.6.9909111. [DOI] [PubMed] [Google Scholar]
- 76.Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–32. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 77.Tran T, Ens-Blackie K, Rector ES, Stelmack GL, McNeill KD, Tarone G, et al. Laminin-binding integrin alpha7 is required for contractile phenotype expression by human airway myocytes. Am J Respir Cell Mol Biol. 2007;37:668–80. doi: 10.1165/rcmb.2007-0165OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhou L, Hershenson MB. Mitogenic signaling pathways in airway smooth muscle. Respir Physiol Neurobiol. 2003;137:295–308. doi: 10.1016/s1569-9048(03)00154-x. [DOI] [PubMed] [Google Scholar]
- 79.Karpova AY, Abe MK, Li J, Liu PT, Rhee JM, Kuo WL, et al. MEK1 is required for PDGF-induced ERK activation and DNA synthesis in tracheal myocytes. Am J Physiol. 1997;272:L558–65. doi: 10.1152/ajplung.1997.272.3.L558. [DOI] [PubMed] [Google Scholar]
- 80.Lew DB, Dempsey BK, Zhao Y, Muthalif M, Fatima S, Malik KU. beta-hexosaminidase-induced activation of p44/42 mitogen-activated protein kinase is dependent on p21Ras and protein kinase C and mediates bovine airway smooth-muscle proliferation. Am J Respir Cell Mol Biol. 1999;21:111–8. doi: 10.1165/ajrcmb.21.1.3542. [DOI] [PubMed] [Google Scholar]
- 81.Whelchel A, Evans J, Posada J. Inhibition of ERK activation attenuates endothelin-stimulated airway smooth muscle cell proliferation. Am J Respir Cell Mol Biol. 1997;16:589–96. doi: 10.1165/ajrcmb.16.5.9160841. [DOI] [PubMed] [Google Scholar]
- 82.Stokoe D, Macdonald SG, Cadwallader K, Symons M, Hancock JF. Activation of Raf as a result of recruitment to the plasma membrane. Science. 1994;264:1463–7. doi: 10.1126/science.7811320. [DOI] [PubMed] [Google Scholar]
- 83.Yan M, Templeton DJ. Identification of 2 serine residues of MEK-1 that are differentially phosphorylated during activation by raf and MEK kinase. J Biol Chem. 1994;269:19067–73. [PubMed] [Google Scholar]
- 84.Ammit AJ, Panettieri RA., Jr Invited review: the circle of life: cell cycle regulation in airway smooth muscle. J Appl Physiol. 2001;91:1431–7. doi: 10.1152/jappl.2001.91.3.1431. [DOI] [PubMed] [Google Scholar]
- 85.Page K, Li J, Hershenson MB. Platelet-derived growth factor stimulation of mitogen-activated protein kinases and cyclin D1 promoter activity in cultured airway smooth-muscle cells. Role of Ras. Am J Respir Cell Mol Biol. 1999;20:1294–302. doi: 10.1165/ajrcmb.20.6.3597. [DOI] [PubMed] [Google Scholar]
- 86.Ammit AJ, Kane SA, Panettieri RA., Jr Activation of K-p21ras and N-p21ras, but not H-p21ras, is necessary for mitogen-induced human airway smooth-muscle proliferation. Am J Respir Cell Mol Biol. 1999;21:719–27. doi: 10.1165/ajrcmb.21.6.3731. [DOI] [PubMed] [Google Scholar]
- 87.Emala CW, Liu F, Hirshman CA. Gialpha but not gqalpha is linked to activation of p21(ras) in human airway smooth muscle cells. Am J Physiol. 1999;276:L564–70. doi: 10.1152/ajplung.1999.276.4.L564. [DOI] [PubMed] [Google Scholar]
- 88.Citro S, Ravasi S, Rovati GE, Capra V. Thromboxane prostanoid receptor signals through Gi protein to rapidly activate extracellular signal-regulated kinase in human airways. Am J Respir Cell Mol Biol. 2005;32:326–33. doi: 10.1165/rcmb.2004-0356OC. [DOI] [PubMed] [Google Scholar]
- 89.Ediger TL, Schulte NA, Murphy TJ, Toews ML. Transcription factor activation and mitogenic synergism in airway smooth muscle cells. Eur Respir J. 2003;21:759–69. doi: 10.1183/09031936.03.00075702. [DOI] [PubMed] [Google Scholar]
- 90.Lin CC, Shyr MH, Chien CS, Wang CC, Chiu CT, Hsiao LD, et al. Mechanisms of thrombin-induced MAPK activation associated with cell proliferation in human cultured tracheal smooth muscle cells. Cell Signal. 2001;13:257–67. doi: 10.1016/s0898-6568(01)00134-6. [DOI] [PubMed] [Google Scholar]
- 91.Lin CC, Shyr MH, Chien CS, Wang CC, Chiu CT, Hsiao LD, et al. Thrombin-stimulated cell proliferation mediated through activation of Ras/Raf/MEK/MAPK pathway in canine cultured tracheal smooth muscle cells. Cell Signal. 2002;14:265–75. doi: 10.1016/s0898-6568(01)00249-2. [DOI] [PubMed] [Google Scholar]
- 92.van Biesen T, Hawes BE, Luttrell DK, Krueger KM, Touhara K, Porfiri E, et al. Receptor-tyrosine-kinase- and G beta gamma-mediated MAP kinase activation by a common signalling pathway. Nature. 1995;376:781–4. doi: 10.1038/376781a0. [DOI] [PubMed] [Google Scholar]
- 93.Stoyanov B, Volinia S, Hanck T, Rubio I, Loubtchenkov M, Malek D, et al. Cloning and characterization of a G protein-activated human phosphoinositide-3 kinase. Science. 1995;269:690–3. doi: 10.1126/science.7624799. [DOI] [PubMed] [Google Scholar]
- 94.Fernandes DJ, Ravenhall CE, Harris T, Tran T, Vlahos R, Stewart AG. Contribution of the p38MAPK signalling pathway to proliferation in human cultured airway smooth muscle cells is mitogen-specific. Br J Pharmacol. 2004;142:1182–90. doi: 10.1038/sj.bjp.0705809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nath P, Leung SY, Williams A, Noble A, Chakravarty SD, Luedtke GR, et al. Importance of p38 mitogen-activated protein kinase pathway in allergic airway remodelling and bronchial hyperresponsiveness. Eur J Pharmacol. 2006;544:160–7. doi: 10.1016/j.ejphar.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 96.Xie S, Sukkar MB, Issa R, Khorasani NM, Chung KF. Mechanisms of induction of airway smooth muscle hyperplasia by transforming growth factor-beta. Am J Physiol Lung Cell Mol Physiol. 2007;293:L245–53. doi: 10.1152/ajplung.00068.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zwick E, Hackel PO, Prenzel N, Ullrich A. The EGF receptor as central transducer of heterologous signalling systems. Trends Pharmacol Sci. 1999;20:408–12. doi: 10.1016/s0165-6147(99)01373-5. [DOI] [PubMed] [Google Scholar]
- 98.Halayko AJ, Kartha S, Stelmack GL, McConville J, Tam J, Camoretti-Mercado B, et al. Phophatidylinositol-3 kinase/mammalian target of rapamycin/p70S6K regulates contractile protein accumulation in airway myocyte differentiation. Am J Respir Cell Mol Biol. 2004;31:266–75. doi: 10.1165/rcmb.2003-0272OC. [DOI] [PubMed] [Google Scholar]
- 99.Krymskaya VP, Penn RB, Orsini MJ, Scott PH, Plevin RJ, Walker TR, et al. Phosphatidylinositol 3-kinase mediates mitogen-induced human airway smooth muscle cell proliferation. Am J Physiol. 1999;277:L65–78. doi: 10.1152/ajplung.1999.277.1.L65. [DOI] [PubMed] [Google Scholar]
- 100.Walker TR, Moore SM, Lawson MF, Panettieri RA, Jr, Chilvers ER. Platelet-derived growth factor-BB and thrombin activate phosphoinositide 3-kinase and protein kinase B: role in mediating airway smooth muscle proliferation. Mol Pharmacol. 1998;54:1007–15. doi: 10.1124/mol.54.6.1007. [DOI] [PubMed] [Google Scholar]
- 101.Krymskaya VP, Ammit AJ, Hoffman RK, Eszterhas AJ, Panettieri RA., Jr Activation of class IA PI3K stimulates DNA synthesis in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2001;280:L1009–18. doi: 10.1152/ajplung.2001.280.5.L1009. [DOI] [PubMed] [Google Scholar]
- 102.Toker A, Cantley LC. Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature. 1997;387:673–6. doi: 10.1038/42648. [DOI] [PubMed] [Google Scholar]
- 103.Wymann MP, Zvelebil M, Laffargue M. Phosphoinositide 3-kinase signalling--which way to target? Trends Pharmacol Sci. 2003;24:366–76. doi: 10.1016/S0165-6147(03)00163-9. [DOI] [PubMed] [Google Scholar]
- 104.Burgering BM, Coffer PJ. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- 105.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–9. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 106.Scott PH, Belham CM, al-Hafidh J, Chilvers ER, Peacock AJ, Gould GW, et al. A regulatory role for cAMP in phosphatidylinositol 3-kinase/p70 ribosomal S6 kinase-mediated DNA synthesis in platelet-derived-growth-factor-stimulated bovine airway smooth-muscle cells. Biochem J. 1996;318(Pt 3):965–71. doi: 10.1042/bj3180965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gosens R, Dueck G, Rector E, Nunes RO, Gerthoffer WT, Unruh H, et al. Cooperative regulation of GSK-3 by muscarinic and PDGF receptors is associated with airway myocyte proliferation. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1348–58. doi: 10.1152/ajplung.00346.2007. [DOI] [PubMed] [Google Scholar]
- 108.Krymskaya VP, Goncharova EA, Ammit AJ, Lim PN, Goncharov DA, Eszterhas A, et al. Src is necessary and sufficient for human airway smooth muscle cell proliferation and migration. FASEB J. 2005;19:428–30. doi: 10.1096/fj.04-2869fje. [DOI] [PubMed] [Google Scholar]
- 109.Schaafsma D, Roscioni SS, Meurs H, Schmidt M. Monomeric G-proteins as signal transducers in airway physiology and pathophysiology. Cell Signal. 2008;20:1705–14. doi: 10.1016/j.cellsig.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 110.Bauerfeld CP, Hershenson MB, Page K. Cdc42, but not RhoA, regulates cyclin D1 expression in bovine tracheal myocytes. Am J Physiol Lung Cell Mol Physiol. 2001;280:L974–82. doi: 10.1152/ajplung.2001.280.5.L974. [DOI] [PubMed] [Google Scholar]
- 111.Page K, Li J, Hodge JA, Liu PT, Vanden Hoek TL, Becker LB, et al. Characterization of a Rac1 signaling pathway to cyclin D(1) expression in airway smooth muscle cells. J Biol Chem. 1999;274:22065–71. doi: 10.1074/jbc.274.31.22065. [DOI] [PubMed] [Google Scholar]
- 112.Yahiaoui L, Villeneuve A, Valderrama-Carvajal H, Burke F, Fixman ED. Endothelin-1 regulates proliferative responses, both alone and synergistically with PDGF, in rat tracheal smooth muscle cells. Cell Physiol Biochem. 2006;17:37–46. doi: 10.1159/000091462. [DOI] [PubMed] [Google Scholar]
- 113.Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci. 2003;116:1175–86. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Brar SS, Kennedy TP, Sturrock AB, Huecksteadt TP, Quinn MT, Murphy TM, et al. NADPH oxidase promotes NF-kappaB activation and proliferation in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2002;282:L782–95. doi: 10.1152/ajplung.00206.2001. [DOI] [PubMed] [Google Scholar]
- 115.Brar SS, Kennedy TP, Whorton AR, Murphy TM, Chitano P, Hoidal JR. Requirement for reactive oxygen species in serum-induced and platelet-derived growth factor-induced growth of airway smooth muscle. J Biol Chem. 1999;274:20017–26. doi: 10.1074/jbc.274.28.20017. [DOI] [PubMed] [Google Scholar]
- 116.Simon AR, Takahashi S, Severgnini M, Fanburg BL, Cochran BH. Role of the JAK-STAT pathway in PDGF-stimulated proliferation of human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2002;282:L1296–304. doi: 10.1152/ajplung.00315.2001. [DOI] [PubMed] [Google Scholar]
- 117.Sturrock A, Huecksteadt TP, Norman K, Sanders K, Murphy TM, Chitano P, et al. Nox4 mediates TGF-beta1-induced retinoblastoma protein phosphorylation, proliferation, and hypertrophy in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1543–55. doi: 10.1152/ajplung.00430.2006. [DOI] [PubMed] [Google Scholar]
- 118.Gosens R, Schaafsma D, Meurs H, Zaagsma J, Nelemans SA. Role of Rho-kinase in maintaining airway smooth muscle contractile phenotype. Eur J Pharmacol. 2004;483:71–8. doi: 10.1016/j.ejphar.2003.10.027. [DOI] [PubMed] [Google Scholar]
- 119.Takeda N, Kondo M, Ito S, Ito Y, Shimokata K, Kume H. Role of RhoA inactivation in reduced cell proliferation of human airway smooth muscle by simvastatin. Am J Respir Cell Mol Biol. 2006;35:722–9. doi: 10.1165/rcmb.2006-0034OC. [DOI] [PubMed] [Google Scholar]
- 120.Gerthoffer WT, Gunst SJ. Invited review: focal adhesion and small heat shock proteins in the regulation of actin remodeling and contractility in smooth muscle. J Appl Physiol. 2001;91:963–72. doi: 10.1152/jappl.2001.91.2.963. [DOI] [PubMed] [Google Scholar]
- 121.Wozniak MA, Modzelewska K, Kwong L, Keely PJ. Focal adhesion regulation of cell behavior. Biochim Biophys Acta. 2004;1692:103–19. doi: 10.1016/j.bbamcr.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 122.Han S, Sidell N, Roman J. Fibronectin stimulates human lung carcinoma cell proliferation by suppressing p21 gene expression via signals involving Erk and Rho kinase. Cancer Lett. 2005;219:71–81. doi: 10.1016/j.canlet.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 123.Panettieri RA, Murray RK, DePalo LR, Yadvish PA, Kotlikoff MI. A human airway smooth muscle cell line that retains physiological responsiveness. Am J Physiol. 1989;256:C329–35. doi: 10.1152/ajpcell.1989.256.2.C329. [DOI] [PubMed] [Google Scholar]
- 124.Halayko AJ, Salari H, Ma X, Stephens NL. Markers of airway smooth muscle cell phenotype. Am J Physiol. 1996;270:L1040–51. doi: 10.1152/ajplung.1996.270.6.L1040. [DOI] [PubMed] [Google Scholar]
- 125.Mitchell RW, Halayko AJ, Kahraman S, Solway J, Wylam ME. Selective restoration of calcium coupling to muscarinic M(3) receptors in contractile cultured airway myocytes. Am J Physiol Lung Cell Mol Physiol. 2000;278:L1091–100. doi: 10.1152/ajplung.2000.278.5.L1091. [DOI] [PubMed] [Google Scholar]
- 126.Blank RS, Thompson MM, Owens GK. Cell cycle versus density dependence of smooth muscle alpha actin expression in cultured rat aortic smooth muscle cells. J Cell Biol. 1988;107:299–306. doi: 10.1083/jcb.107.1.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Camoretti-Mercado B, Liu HW, Halayko AJ, Forsythe SM, Kyle JW, Li B, et al. Physiological control of smooth muscle-specific gene expression through regulated nuclear translocation of serum response factor. J Biol Chem. 2000;275:30387–93. doi: 10.1074/jbc.M000840200. [DOI] [PubMed] [Google Scholar]
- 128.Halayko AJ, Camoretti-Mercado B, Forsythe SM, Vieira JE, Mitchell RW, Wylam ME, et al. Divergent differentiation paths in airway smooth muscle culture: induction of functionally contractile myocytes. Am J Physiol. 1999;276:L197–206. doi: 10.1152/ajplung.1999.276.1.L197. [DOI] [PubMed] [Google Scholar]
- 129.Ivester CT, Tuxworth WJ, Cooper Gt, McDermott PJ. Contraction accelerates myosin heavy chain synthesis rates in adult cardiocytes by an increase in the rate of translational initiation. J Biol Chem. 1995;270:21950–7. doi: 10.1074/jbc.270.37.21950. [DOI] [PubMed] [Google Scholar]
- 130.Nikcevic G, Heidkamp MC, Perhonen M, Russell B. Mechanical activity in heart regulates translation of alpha-myosin heavy chain mRNA but not its localization. Am J Physiol. 1999;276:H2013–9. doi: 10.1152/ajpheart.1999.276.6.H2013. [DOI] [PubMed] [Google Scholar]
- 131.Morgan HE, Gordon EE, Kira Y, Chua HL, Russo LA, Peterson CJ, et al. Biochemical mechanisms of cardiac hypertrophy. Annu Rev Physiol. 1987;49:533–43. doi: 10.1146/annurev.ph.49.030187.002533. [DOI] [PubMed] [Google Scholar]
- 132.Sartorelli V, Fulco M. Molecular and cellular determinants of skeletal muscle atrophy and hypertrophy. Sci STKE. 2004;2004:re11. doi: 10.1126/stke.2442004re11. [DOI] [PubMed] [Google Scholar]
- 133.Glass DJ. Molecular mechanisms modulating muscle mass. Trends Mol Med. 2003;9:344–50. doi: 10.1016/s1471-4914(03)00138-2. [DOI] [PubMed] [Google Scholar]
- 134.Hesketh JE, Campbell GP, Lobley GE, Maltin CA, Acamovic F, Palmer RM. Stimulation of actin and myosin synthesis in rat gastrocnemius muscle by clenbuterol; evidence for translational control. Comp Biochem Physiol C. 1992;102:23–7. doi: 10.1016/0742-8413(92)90037-8. [DOI] [PubMed] [Google Scholar]
- 135.Geisterfer AA, Peach MJ, Owens GK. Angiotensin II induces hypertrophy, not hyperplasia, of cultured rat aortic smooth muscle cells. Circ Res. 1988;62:749–56. doi: 10.1161/01.res.62.4.749. [DOI] [PubMed] [Google Scholar]
- 136.Hunter T, Pines J. Cyclins and cancer. II: Cyclin D and CDK inhibitors come of age. Cell. 1994;79:573–82. doi: 10.1016/0092-8674(94)90543-6. [DOI] [PubMed] [Google Scholar]
- 137.el-Deiry WS, Harper JW, O’Connor PM, Velculescu VE, Canman CE, Jackman J, et al. WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res. 1994;54:1169–74. [PubMed] [Google Scholar]
- 138.Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993;366:701–4. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- 139.Serrano M, Hannon GJ, Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993;366:704–7. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- 140.Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–16. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 141.Terada Y, Nakashima O, Inoshita S, Yamada T, Sasaki S, Marumo F. Overexpression of cell cycle inhibitors (p27(Kip1) and p21(cip1)) using adenovirus vector causes hypertrophy in LLCPK1 cells. Journal of the American Society of Nephrology. 1997;8:A1989–A1989. doi: 10.1681/ASN.V8151. [DOI] [PubMed] [Google Scholar]
- 142.Zhou L, Li J, Goldsmith AM, Newcomb DC, Giannola DM, Vosk RG, et al. Human bronchial smooth muscle cell lines show a hypertrophic phenotype typical of severe asthma. Am J Respir Crit Care Med. 2004;169:703–11. doi: 10.1164/rccm.200307-964OC. [DOI] [PubMed] [Google Scholar]
- 143.Fingar DC, Salama S, Tsou C, Harlow E, Blenis J. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev. 2002;16:1472–87. doi: 10.1101/gad.995802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lupu F, Terwilliger JD, Lee K, Segre GV, Efstratiadis A. Roles of growth hormone and insulin-like growth factor 1 in mouse postnatal growth. Dev Biol. 2001;229:141–62. doi: 10.1006/dbio.2000.9975. [DOI] [PubMed] [Google Scholar]
- 145.Broide DH, Lotz M, Cuomo AJ, Coburn DA, Federman EC, Wasserman SI. Cytokines in symptomatic asthma airways. J Allergy Clin Immunol. 1992;89:958–67. doi: 10.1016/0091-6749(92)90218-q. [DOI] [PubMed] [Google Scholar]
- 146.Redington AE, Springall DR, Ghatei MA, Madden J, Bloom SR, Frew AJ, et al. Airway endothelin levels in asthma: influence of endobronchial allergen challenge and maintenance corticosteroid therapy. Eur Respir J. 1997;10:1026–32. doi: 10.1183/09031936.97.10051026. [DOI] [PubMed] [Google Scholar]
- 147.Rozengurt N, Springall DR, Polak JM. Localization of endothelin-like immunoreactivity in airway epithelium of rats and mice. J Pathol. 1990;160:5–8. doi: 10.1002/path.1711600104. [DOI] [PubMed] [Google Scholar]
- 148.Springall DR, Howarth PH, Counihan H, Djukanovic R, Holgate ST, Polak JM. Endothelin immunoreactivity of airway epithelium in asthmatic patients. Lancet. 1991;337:697–701. doi: 10.1016/0140-6736(91)90279-x. [DOI] [PubMed] [Google Scholar]
- 149.McWhinnie R, Pechkovsky DV, Zhou D, Lane D, Halayko AJ, Knight DA, et al. Endothelin-1 induces hypertrophy and inhibits apoptosis in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2007;292:L278–86. doi: 10.1152/ajplung.00111.2006. [DOI] [PubMed] [Google Scholar]
- 150.Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, et al. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol. 2001;3:1009–13. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- 151.Bi L, Okabe I, Bernard DJ, Wynshaw-Boris A, Nussbaum RL. Proliferative defect and embryonic lethality in mice homozygous for a deletion in the p110alpha subunit of phosphoinositide 3-kinase. J Biol Chem. 1999;274:10963–8. doi: 10.1074/jbc.274.16.10963. [DOI] [PubMed] [Google Scholar]
- 152.Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3:1014–9. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- 153.Hayashi K, Saga H, Chimori Y, Kimura K, Yamanaka Y, Sobue K. Differentiated phenotype of smooth muscle cells depends on signaling pathways through insulin-like growth factors and phosphatidylinositol 3-kinase. J Biol Chem. 1998;273:28860–7. doi: 10.1074/jbc.273.44.28860. [DOI] [PubMed] [Google Scholar]
- 154.Hayashi K, Takahashi M, Kimura K, Nishida W, Saga H, Sobue K. Changes in the balance of phosphoinositide 3-kinase/protein kinase B (Akt) and the mitogen-activated protein kinases (ERK/p38MAPK) determine a phenotype of visceral and vascular smooth muscle cells. J Cell Biol. 1999;145:727–40. doi: 10.1083/jcb.145.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7:261–9. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 156.Johnson PRA. Role of human airway smooth muscle in altered extracellular matrix production in asthma. Clinical and Experimental Pharmacology and Physiology. 2001;28:233–236. doi: 10.1046/j.1440-1681.2001.03426.x. [DOI] [PubMed] [Google Scholar]
- 157.Roberts CR. Is asthma a fibrotic disease? Chest. 1995;107:111S–117S. doi: 10.1378/chest.107.3_supplement.111s. [DOI] [PubMed] [Google Scholar]
- 158.Bousquet J, Vignola AM, Chanez P, Campbell AM, Bonsignore G, Michel FB. AIRWAYS REMODELING IN ASTHMA - NO DOUBT, NO MORE. International Archives of Allergy and Immunology. 1995;107:211–214. doi: 10.1159/000236980. [DOI] [PubMed] [Google Scholar]
- 159.Roberts CR, Burke AK. Remodelling of the extracellular matrix in asthma: proteoglycan synthesis and degradation. Can Respir J. 1998;5:48–50. [PubMed] [Google Scholar]
- 160.Tran T, Gosens R, Halayko AJ. Effects of extracellular matrix and integrin interactions in airway smooth muscle phenotype and function: it takes two to tango! Curr Respir Med Rev. 2007 In Press. [Google Scholar]
- 161.Tran T, McNeill KD, Gerthoffer WT, Unruh H, Halayko AJ. Endogenous laminin is required for human airway smooth muscle cell maturation. Respir Res. 2006;7:117. doi: 10.1186/1465-9921-7-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lemmon MA, Ferguson KM, O’Brien R, Sigler PB, Schlessinger J. Specific and high-affinity binding of inositol phosphates to an isolated pleckstrin homology domain. Proc Natl Acad Sci U S A. 1995;92:10472–6. doi: 10.1073/pnas.92.23.10472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Brazil DP, Yang ZZ, Hemmings BA. Advances in protein kinase B signalling: AKTion on multiple fronts. Trends Biochem Sci. 2004;29:233–42. doi: 10.1016/j.tibs.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 164.Stephens L, Anderson K, Stokoe D, Erdjument-Bromage H, Painter GF, Holmes AB, et al. Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science. 1998;279:710–4. doi: 10.1126/science.279.5351.710. [DOI] [PubMed] [Google Scholar]
- 165.Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, et al. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–37. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 166.Yang Q, Inoki K, Ikenoue T, Guan KL. Identification of Sin1 as an essential TORC2 component required for complex formation and kinase activity. Genes Dev. 2006;20:2820–32. doi: 10.1101/gad.1461206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Brazil DP, Hemmings BA. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem Sci. 2001;26:657–64. doi: 10.1016/s0968-0004(01)01958-2. [DOI] [PubMed] [Google Scholar]
- 168.Welsh GI, Miller CM, Loughlin AJ, Price NT, Proud CG. Regulation of eukaryotic initiation factor eIF2B: glycogen synthase kinase-3 phosphorylates a conserved serine which undergoes dephosphorylation in response to insulin. FEBS Lett. 1998;421:125–30. doi: 10.1016/s0014-5793(97)01548-2. [DOI] [PubMed] [Google Scholar]
- 169.Welsh GI, Stokes CM, Wang X, Sakaue H, Ogawa W, Kasuga M, et al. Activation of translation initiation factor eIF2B by insulin requires phosphatidyl inositol 3-kinase. FEBS Lett. 1997;410:418–22. doi: 10.1016/s0014-5793(97)00579-6. [DOI] [PubMed] [Google Scholar]
- 170.Brunn GJ, Hudson CC, Sekulic A, Williams JM, Hosoi H, Houghton PJ, et al. Phosphorylation of the translational repressor PHAS-I by the mammalian target of rapamycin. Science. 1997;277:99–101. doi: 10.1126/science.277.5322.99. [DOI] [PubMed] [Google Scholar]
- 171.von Manteuffel SR, Dennis PB, Pullen N, Gingras AC, Sonenberg N, Thomas G. The insulin-induced signalling pathway leading to S6 and initiation factor 4E binding protein 1 phosphorylation bifurcates at a rapamycin-sensitive point immediately upstream of p70s6k. Mol Cell Biol. 1997;17:5426–36. doi: 10.1128/mcb.17.9.5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Burnett PE, Barrow RK, Cohen NA, Snyder SH, Sabatini DM. RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc Natl Acad Sci U S A. 1998;95:1432–7. doi: 10.1073/pnas.95.4.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Dufner A, Thomas G. Ribosomal S6 kinase signaling and the control of translation. Exp Cell Res. 1999;253:100–9. doi: 10.1006/excr.1999.4683. [DOI] [PubMed] [Google Scholar]
- 174.Giasson E, Meloche S. Role of p70 S6 protein kinase in angiotensin II-induced protein synthesis in vascular smooth muscle cells. J Biol Chem. 1995;270:5225–31. doi: 10.1074/jbc.270.10.5225. [DOI] [PubMed] [Google Scholar]
- 175.Pearson RB, Dennis PB, Han JW, Williamson NA, Kozma SC, Wettenhall RE, et al. The principal target of rapamycin-induced p70s6k inactivation is a novel phosphorylation site within a conserved hydrophobic domain. EMBO J. 1995;14:5279–87. doi: 10.1002/j.1460-2075.1995.tb00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rhoads RE. Signal transduction pathways that regulate eukaryotic protein synthesis. J Biol Chem. 1999;274:30337–40. doi: 10.1074/jbc.274.43.30337. [DOI] [PubMed] [Google Scholar]
- 177.Cheatham L, Monfar M, Chou MM, Blenis J. Structural and functional analysis of pp70S6k. Proc Natl Acad Sci U S A. 1995;92:11696–700. doi: 10.1073/pnas.92.25.11696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Toker A, Newton AC. Cellular signaling: pivoting around PDK-1. Cell. 2000;103:185–8. doi: 10.1016/s0092-8674(00)00110-0. [DOI] [PubMed] [Google Scholar]
- 179.Vanhaesebroeck B, Alessi DR. The PI3K-PDK1 connection: more than just a road to PKB. Biochem J. 2000;346(Pt 3):561–76. [PMC free article] [PubMed] [Google Scholar]
- 180.Foukas LC, Shepherd PR. eIF4E binding protein 1 and H-Ras are novel substrates for the protein kinase activity of class-I phosphoinositide 3-kinase. Biochem Biophys Res Commun. 2004;319:541–9. doi: 10.1016/j.bbrc.2004.04.191. [DOI] [PubMed] [Google Scholar]
- 181.Herbert TP, Tee AR, Proud CG. The extracellular signal-regulated kinase pathway regulates the phosphorylation of 4E-BP1 at multiple sites. J Biol Chem. 2002;277:11591–6. doi: 10.1074/jbc.M110367200. [DOI] [PubMed] [Google Scholar]
- 182.Gao X, Pan D. TSC1 and TSC2 tumor suppressors antagonize insulin signaling in cell growth. Genes Dev. 2001;15:1383–92. doi: 10.1101/gad.901101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Goncharova EA, Goncharov DA, Eszterhas A, Hunter DS, Glassberg MK, Yeung RS, et al. Tuberin regulates p70 S6 kinase activation and ribosomal protein S6 phosphorylation. A role for the TSC2 tumor suppressor gene in pulmonary lymphangioleiomyomatosis (LAM) J Biol Chem. 2002;277:30958–67. doi: 10.1074/jbc.M202678200. [DOI] [PubMed] [Google Scholar]
- 184.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–57. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 185.Potter CJ, Huang H, Xu T. Drosophila Tsc1 functions with Tsc2 to antagonize insulin signaling in regulating cell growth, cell proliferation, and organ size. Cell. 2001;105:357–68. doi: 10.1016/s0092-8674(01)00333-6. [DOI] [PubMed] [Google Scholar]
- 186.Jacinto E, Loewith R, Schmidt A, Lin S, Ruegg MA, Hall A, et al. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol. 2004;6:1122–8. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- 187.Hietakangas V, Cohen SM. Re-evaluating AKT regulation: role of TOR complex 2 in tissue growth. Genes Dev. 2007;21:632–7. doi: 10.1101/gad.416307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Alt JR, Cleveland JL, Hannink M, Diehl JA. Phosphorylation-dependent regulation of cyclin D1 nuclear export and cyclin D1-dependent cellular transformation. Genes Dev. 2000;14:3102–14. doi: 10.1101/gad.854900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Rossig L, Badorff C, Holzmann Y, Zeiher AM, Dimmeler S. Glycogen synthase kinase-3 couples AKT-dependent signaling to the regulation of p21Cip1 degradation. J Biol Chem. 2002;277:9684–9. doi: 10.1074/jbc.M106157200. [DOI] [PubMed] [Google Scholar]
- 191.Haq S, Choukroun G, Kang ZB, Ranu H, Matsui T, Rosenzweig A, et al. Glycogen synthase kinase-3beta is a negative regulator of cardiomyocyte hypertrophy. J Cell Biol. 2000;151:117–30. doi: 10.1083/jcb.151.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Antos CL, McKinsey TA, Frey N, Kutschke W, McAnally J, Shelton JM, et al. Activated glycogen synthase-3 beta suppresses cardiac hypertrophy in vivo. Proc Natl Acad Sci U S A. 2002;99:907–12. doi: 10.1073/pnas.231619298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Morisco C, Seta K, Hardt SE, Lee Y, Vatner SF, Sadoshima J. Glycogen synthase kinase 3beta regulates GATA4 in cardiac myocytes. J Biol Chem. 2001;276:28586–97. doi: 10.1074/jbc.M103166200. [DOI] [PubMed] [Google Scholar]
- 194.Haq S, Michael A, Andreucci M, Bhattacharya K, Dotto P, Walters B, et al. Stabilization of beta-catenin by a Wnt-independent mechanism regulates cardiomyocyte growth. Proc Natl Acad Sci U S A. 2003;100:4610–5. doi: 10.1073/pnas.0835895100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Gosens R, Baarsma HA, Heijink IH, Oenema TA, Halayko AJ, Meurs H, et al. De novo synthesis of {beta}-catenin via H-Ras and MEK regulates airway smooth muscle growth. FASEB J. 24:757–68. doi: 10.1096/fj.09-136325. [DOI] [PubMed] [Google Scholar]
- 196.Cordes N, van Beuningen D. Cell adhesion to the extracellular matrix protein fibronectin modulates radiation-dependent G2 phase arrest involving integrin-linked kinase (ILK) and glycogen synthase kinase-3beta (GSK-3beta) in vitro. Br J Cancer. 2003;88:1470–9. doi: 10.1038/sj.bjc.6600912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Delcommenne M, Tan C, Gray V, Rue L, Woodgett J, Dedhar S. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc Natl Acad Sci U S A. 1998;95:11211–6. doi: 10.1073/pnas.95.19.11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Maydan M, McDonald PC, Sanghera J, Yan J, Rallis C, Pinchin S, et al. Integrin-linked kinase is a functional Mn2+-dependent protein kinase that regulates glycogen synthase kinase-3beta (GSK-3beta) phosphorylation. PLoS One. 5:e12356. doi: 10.1371/journal.pone.0012356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Deng H, Dokshin GA, Lei J, Goldsmith AM, Bitar KN, Fingar DC, et al. Inhibition of glycogen synthase kinase-3beta is sufficient for airway smooth muscle hypertrophy. J Biol Chem. 2008;283:10198–207. doi: 10.1074/jbc.M800624200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Bentley JK, Deng H, Linn MJ, Lei J, Dokshin GA, Fingar DC, et al. Airway smooth muscle hyperplasia and hypertrophy correlate with glycogen synthase kinase-3(beta) phosphorylation in a mouse model of asthma. Am J Physiol Lung Cell Mol Physiol. 2009;296:L176–84. doi: 10.1152/ajplung.90376.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Deng H, Hershenson MB, Lei J, Bitar KN, Fingar DC, Solway J, et al. p70 Ribosomal S6 Kinase is Required for Airway Smooth Muscle Cell Size Enlargement but not Increased Contactile Protein Expression. Am J Respir Cell Mol Biol. 2009 doi: 10.1165/rcmb.2009-0037OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Mack CP, Somlyo AV, Hautmann M, Somlyo AP, Owens GK. Smooth muscle differentiation marker gene expression is regulated by RhoA-mediated actin polymerization. J Biol Chem. 2001;276:341–7. doi: 10.1074/jbc.M005505200. [DOI] [PubMed] [Google Scholar]
- 203.Liu HW, Halayko AJ, Fernandes DJ, Harmon GS, McCauley JA, Kocieniewski P, et al. The RhoA/Rho kinase pathway regulates nuclear localization of serum response factor. Am J Respir Cell Mol Biol. 2003;29:39–47. doi: 10.1165/rcmb.2002-0206OC. [DOI] [PubMed] [Google Scholar]
- 204.Wang DZ, Olson EN. Control of smooth muscle development by the myocardin family of transcriptional coactivators. Curr Opin Genet Dev. 2004;14:558–66. doi: 10.1016/j.gde.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 205.Miralles F, Posern G, Zaromytidou AI, Treisman R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell. 2003;113:329–42. doi: 10.1016/s0092-8674(03)00278-2. [DOI] [PubMed] [Google Scholar]
- 206.Gosens R, Schaafsma D, Nelemans SA, Halayko AJ. Rho-kinase as a drug target for the treatment of airway hyperrespon-siveness in asthma. Mini Rev Med Chem. 2006;6:339–48. doi: 10.2174/138955706776073402. [DOI] [PubMed] [Google Scholar]
- 207.Schaafsma D, McNeill KD, Stelmack GL, Gosens R, Baarsma HA, Dekkers BG, et al. Insulin increases expression of contractile phenotypic markers in airway smooth muscle. Am J Physiol Cell Physiol. 2007 doi: 10.1152/ajpcell.00502.2006. [DOI] [PubMed] [Google Scholar]
- 208.Webb BLJ, Lindsay MA, Barnes PJ, Giembycz MA. Protein kinase C isoenzymes in airway smooth muscle. Biochemical Journal. 1997;324:167–175. doi: 10.1042/bj3240167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Carlin S, Yang KXF, Donnelly R, Black JL. Protein kinase C isoforms in human airway smooth muscle cells: activation of PKC-zeta during proliferation. American Journal of Physiology-Lung Cellular and Molecular Physiology. 1999;276:L506–L512. doi: 10.1152/ajplung.1999.276.3.L506. [DOI] [PubMed] [Google Scholar]
- 210.Gosens R, Bromhaar MMG, Maarsingh H, ten Damme A, Meurs H, Zaagsma J, et al. Bradykinin augments EGF-induced airway smooth muscle proliferation by activation of conventional protein kinase C isoenzymes. European Journal of Pharmacology. 2006;535:253–262. doi: 10.1016/j.ejphar.2006.01.065. [DOI] [PubMed] [Google Scholar]
- 211.Gosens R, Meurs H, Bromhaar MMG, Zaagsma J, Nelemans SA. Role of PKC and p42/p44 MAP kinase in synergism of airway smooth muscle proliferation induced by G-protein coupled receptor agonists and growth factors. Naunyn-Schmiedebergs Archives of Pharmacology. 2004;369:18. [Google Scholar]
- 212.Lin J, Xu Y, Zhang Z, Ni W, Chen S. Effect of cigarette smoke extract on the role of protein kinase C in the proliferation of passively sensitized human airway smooth muscle cells. J Huazhong Univ Sci Technolog Med Sci. 2005;25:269–73. doi: 10.1007/BF02828139. [DOI] [PubMed] [Google Scholar]
- 213.Xu SY, Xu YJ, Zhang ZX, Ni W, Chen SX. Contribution of protein kinase C to passively sensitized human airway smooth muscle cells proliferation. Chinese Medical Journal. 2004;117:30–36. [PubMed] [Google Scholar]
- 214.Xu SY, Xu YJ, Zhang ZX, Ni W, Chen SX. A study of protein kinase C signal pathway in regulating airway smooth muscle cell proliferation in asthmatic rats. Zhonghua Jiehe He Huxi Zazhi. 2003;26:756–760. [PubMed] [Google Scholar]
- 215.Dorn GW, 2nd, Force T. Protein kinase cascades in the regulation of cardiac hypertrophy. J Clin Invest. 2005;115:527–37. doi: 10.1172/JCI24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–57. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ghavami S, Eshraghi M, Kadkhoda K, Mutawe MM, Maddika S, Bay GH, et al. Role of BNIP3 in TNF-induced cell death--TNF upregulates BNIP3 expression. Biochim Biophys Acta. 2009;1793:546–60. doi: 10.1016/j.bbamcr.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 218.Ghavami S, Hashemi M, Ande SR, Yeganeh B, Xiao W, Eshraghi M, et al. Apoptosis and cancer: mutations within caspase genes. J Med Genet. 2009;46:497–510. doi: 10.1136/jmg.2009.066944. [DOI] [PubMed] [Google Scholar]
- 219.Degterev A, Yuan J. Expansion and evolution of cell death programmes. Nat Rev Mol Cell Biol. 2008;9:378–90. doi: 10.1038/nrm2393. [DOI] [PubMed] [Google Scholar]
- 220.Salvesen GS, V, Dixit M. Caspases: intracellular signaling by proteolysis. Cell. 1997;91:443–6. doi: 10.1016/s0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- 221.Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–6. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 222.Ghavami S, Mutawe MM, Hauff K, Stelmack GL, Schaafsma D, Sharma P, et al. Statin-triggered cell death in primary human lung mesenchymal cells involves p53-PUMA and release of Smac and Omi but not cytochrome c. Biochim Biophys Acta. 2010;1803:452–467. doi: 10.1016/j.bbamcr.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 223.Green D, Kroemer G. The central executioners of apoptosis: caspases or mitochondria? Trends Cell Biol. 1998;8:267–71. doi: 10.1016/s0962-8924(98)01273-2. [DOI] [PubMed] [Google Scholar]
- 224.Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15:2922–33. [PubMed] [Google Scholar]
- 225.Ghavami S, Eshragi M, Ande SR, Chazin WJ, Klonisch T, Halayko AJ, et al. S100A8/A9 induces autophagy and apoptosis via ROS-mediated cross-talk between mitochondria and lysosomes that involves BNIP3. Cell Res. 2010;20:314–31. doi: 10.1038/cr.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Brenner D, Mak TW. Mitochondrial cell death effectors. Curr Opin Cell Biol. 2009;21:871–7. doi: 10.1016/j.ceb.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 227.Li X, Marani M, Mannucci R, Kinsey B, Andriani F, Nicoletti I, et al. Overexpression of BCL-X(L) underlies the molecular basis for resistance to staurosporine-induced apoptosis in PC-3 cells. Cancer Res. 2001;61:1699–706. [PubMed] [Google Scholar]
- 228.Kim TH, Zhao Y, Barber MJ, Kuharsky DK, Yin XM. Bid-induced cytochrome c release is mediated by a pathway independent of mitochondrial permeability transition pore and Bax. J Biol Chem. 2000;275:39474–81. doi: 10.1074/jbc.M003370200. [DOI] [PubMed] [Google Scholar]
- 229.Ghavami S, Kerkhoff C, Chazin WJ, Kadkhoda K, Xiao W, Zuse A, et al. S100A8/9 induces cell death via a novel, RAGE-independent pathway that involves selective release of Smac/DIABLO and Omi/HtrA2. Biochim Biophys Acta. 2008;1783:297–311. doi: 10.1016/j.bbamcr.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 230.Bai TR, Cooper J, Koelmeyer T, Pare PD, Weir TD. The effect of age and duration of disease on airway structure in fatal asthma. Am J Respir Crit Care Med. 2000;162:663–9. doi: 10.1164/ajrccm.162.2.9907151. [DOI] [PubMed] [Google Scholar]
- 231.Ramos-Barbon D, Presley JF, Hamid QA, Fixman ED, Martin JG. Antigen-specific CD4+ T cells drive airway smooth muscle remodeling in experimental asthma. J Clin Invest. 2005;115:1580–9. doi: 10.1172/JCI19711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Herszberg B, Ramos-Barbon D, Tamaoka M, Martin JG, Lavoie JP. Heaves, an asthma-like equine disease, involves airway smooth muscle remodeling. J Allergy Clin Immunol. 2006;118:382–8. doi: 10.1016/j.jaci.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 233.Leclere M, Lavoie-Lamoureux A, Gelinas-Lymburner E, David F, Martin JG, Lavoie JP. Effect of Antigen Exposure on Airway Smooth Muscle Remodeling in an Equine Model of Chronic Asthma. Am J Respir Cell Mol Biol. doi: 10.1165/rcmb.2010-0300OC. [DOI] [PubMed] [Google Scholar]
- 234.Ding MJ, Wang LX, Dai YR. Changes of airway smooth muscle cell apoptosis in asthmatic airway remodeling and the effect of dexamethasone in rats. Zhonghua Jie He He Hu Xi Za Zhi. 2008;31:607–10. [PubMed] [Google Scholar]
- 235.Niu RJ, Fu J, Liu HG. Effect of shenmai injection and aminophylline on small airway smooth muscle cell apoptosis and related gene expression in rats with emphysema. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2002;22:40–2. [PubMed] [Google Scholar]
- 236.Hamann KJ, Vieira JE, Halayko AJ, Dorscheid D, White SR, Forsythe SM, et al. Fas cross-linking induces apoptosis in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2000;278:L618–24. doi: 10.1152/ajplung.2000.278.3.L618. [DOI] [PubMed] [Google Scholar]
- 237.Panettieri RA, Jr, Murray RK, Bilgen G, Eszterhas AJ, Martin JG. Repeated allergen inhalations induce DNA synthesis in airway smooth muscle and epithelial cells in vivo. Chest. 1995;107:94S–95S. doi: 10.1378/chest.107.3_supplement.94s. [DOI] [PubMed] [Google Scholar]
- 238.Jeffery PK. Remodeling in asthma and chronic obstructive lung disease. Am J Respir Crit Care Med. 2001;164:S28–38. doi: 10.1164/ajrccm.164.supplement_2.2106061. [DOI] [PubMed] [Google Scholar]
- 239.D’Antoni ML, Torregiani C, Ferraro P, Michoud MC, Mazer B, Martin JG, et al. Effects of decorin and biglycan on human airway smooth muscle cell proliferation and apoptosis. Am J Physiol Lung Cell Mol Physiol. 2008;294:L764–71. doi: 10.1152/ajplung.00436.2007. [DOI] [PubMed] [Google Scholar]
- 240.de Kluijver J, Schrumpf JA, Evertse CE, Sont JK, Roughley PJ, Rabe KF, et al. Bronchial matrix and inflammation respond to inhaled steroids despite ongoing allergen exposure in asthma. Clin Exp Allergy. 2005;35:1361–9. doi: 10.1111/j.1365-2222.2005.02334.x. [DOI] [PubMed] [Google Scholar]
- 241.Huang J, Olivenstein R, Taha R, Hamid Q, Ludwig M. Enhanced proteoglycan deposition in the airway wall of atopic asthmatics. Am J Respir Crit Care Med. 1999;160:725–9. doi: 10.1164/ajrccm.160.2.9809040. [DOI] [PubMed] [Google Scholar]
- 242.de Medeiros Matsushita M, da Silva LF, dos Santos MA, Fernezlian S, Schrumpf JA, Roughley P, et al. Airway proteoglycans are differentially altered in fatal asthma. J Pathol. 2005;207:102–10. doi: 10.1002/path.1818. [DOI] [PubMed] [Google Scholar]
- 243.Pini L, Hamid Q, Shannon J, Lemelin L, Olivenstein R, Ernst P, et al. Differences in proteoglycan deposition in the airways of moderate and severe asthmatics. Eur Respir J. 2007;29:71–7. doi: 10.1183/09031936.00047905. [DOI] [PubMed] [Google Scholar]
- 244.Freyer AM, Johnson SR, Hall IP. Effects of growth factors and extracellular matrix on survival of human airway smooth muscle cells. Am J Respir Cell Mol Biol. 2001;25:569–76. doi: 10.1165/ajrcmb.25.5.4605. [DOI] [PubMed] [Google Scholar]
- 245.Chalmers GW, Little SA, Patel KR, Thomson NC. Endothelin-1-induced bronchoconstriction in asthma. Am J Respir Crit Care Med. 1997;156:382–8. doi: 10.1164/ajrccm.156.2.9702066. [DOI] [PubMed] [Google Scholar]
- 246.Goldie RG, D’Aprile AC, Self GJ, Rigby PJ, Henry PJ. Influence of endothelin-1(1–31) on smooth muscle tone and cholinergic nerve-mediated contraction in rat isolated trachea. J Cardiovasc Pharmacol. 2000;36:S228–31. doi: 10.1097/00005344-200036051-00068. [DOI] [PubMed] [Google Scholar]
- 247.Goldie RG, Henry PJ. Endothelins and asthma. Life Sci. 1999;65:1–15. doi: 10.1016/s0024-3205(98)00614-6. [DOI] [PubMed] [Google Scholar]
- 248.Bartal M. COPD and tobacco smoke. Monaldi Arch Chest Dis. 2005;63:213–25. doi: 10.4081/monaldi.2005.623. [DOI] [PubMed] [Google Scholar]
- 249.Hu W, Xie J, Zhao J, Xu Y, Yang S, Ni W. Involvement of Bcl-2 Family in Apoptosis and Signal Pathways Induced by Cigarette Smoke Extract in the Human Airway Smooth Muscle Cells. DNA Cell Biol. 2008 doi: 10.1089/dna.2008.0782. [DOI] [PubMed] [Google Scholar]
- 250.Demedts IK, Brusselle GG, Bracke KR, Vermaelen KY, Pauwels RA. Matrix metalloproteinases in asthma and COPD. Curr Opin Pharmacol. 2005;5:257–63. doi: 10.1016/j.coph.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 251.Kirkham P, Rahman I. Oxidative stress in asthma and COPD: antioxidants as a therapeutic strategy. Pharmacol Ther. 2006;111:476–94. doi: 10.1016/j.pharmthera.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 252.Yokohori N, Aoshiba K, Nagai A. Increased levels of cell death and proliferation in alveolar wall cells in patients with pulmonary emphysema. Chest. 2004;125:626–32. doi: 10.1378/chest.125.2.626. [DOI] [PubMed] [Google Scholar]
- 253.Imai K, Mercer BA, Schulman LL, Sonett JR, D’Armiento JM. Correlation of lung surface area to apoptosis and proliferation in human emphysema. Eur Respir J. 2005;25:250–8. doi: 10.1183/09031936.05.00023704. [DOI] [PubMed] [Google Scholar]
- 254.Sibille Y, Marchandise FX. Pulmonary immune cells in health and disease: polymorphonuclear neutrophils. Eur Respir J. 1993;6:1529–43. [PubMed] [Google Scholar]
- 255.Bousquet J, Lacoste JY, Chanez P, Vic P, Godard P, Michel FB. Bronchial elastic fibers in normal subjects and asthmatic patients. Am J Respir Crit Care Med. 1996;153:1648–54. doi: 10.1164/ajrccm.153.5.8630616. [DOI] [PubMed] [Google Scholar]
- 256.Oltmanns U, Sukkar MB, Xie S, John M, Chung KF. Induction of human airway smooth muscle apoptosis by neutrophils and neutrophil elastase. Am J Respir Cell Mol Biol. 2005;32:334–41. doi: 10.1165/rcmb.2004-0321OC. [DOI] [PubMed] [Google Scholar]
- 257.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–30. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 258.Badri KR, Zhou Y, Schuger L. Embryological origin of airway smooth muscle. Proc Am Thorac Soc. 2008;5:4–10. doi: 10.1513/pats.200704-049VS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Shannon JM, Hyatt BA. Epithelial-mesenchymal interactions in the developing lung. Annu Rev Physiol. 2004;66:625–45. doi: 10.1146/annurev.physiol.66.032102.135749. [DOI] [PubMed] [Google Scholar]
- 260.Beqaj S, Jakkaraju S, Mattingly RR, Pan D, Schuger L. High RhoA activity maintains the undifferentiated mesenchymal cell phenotype, whereas RhoA down-regulation by laminin-2 induces smooth muscle myogenesis. J Cell Biol. 2002;156:893–903. doi: 10.1083/jcb.200107049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Hedges JC, Dechert MA, Yamboliev IA, Martin JL, Hickey E, Weber LA, et al. A role for p38(MAPK)/HSP27 pathway in smooth muscle cell migration. J Biol Chem. 1999;274:24211–9. doi: 10.1074/jbc.274.34.24211. [DOI] [PubMed] [Google Scholar]
- 262.Zhang J, O’Shea S, Liu J, Schuger L. Bronchial smooth muscle hypoplasia in mouse embryonic lungs exposed to a laminin beta1 chain antisense oligonucleotide. Mech Dev. 1999;89:15–23. doi: 10.1016/s0925-4773(99)00198-7. [DOI] [PubMed] [Google Scholar]
- 263.Chiba Y, Arima J, Sakai H, Misawa M. Lovastatin inhibits bronchial hyperresponsiveness by reducing RhoA signaling in rat allergic asthma. Am J Physiol Lung Cell Mol Physiol. 2008;294:L705–13. doi: 10.1152/ajplung.00531.2007. [DOI] [PubMed] [Google Scholar]
- 264.Welser JV, Lange N, Singer CA, Elorza M, Scowen P, Keef KD, et al. Loss of the alpha7 integrin promotes extracellular signal-regulated kinase activation and altered vascular remodeling. Circ Res. 2007;101:672–81. doi: 10.1161/CIRCRESAHA.107.151415. [DOI] [PubMed] [Google Scholar]
- 265.Yao CC, Breuss J, Pytela R, Kramer RH. Functional expression of the alpha 7 integrin receptor in differentiated smooth muscle cells. J Cell Sci. 1997;110(Pt 13):1477–87. doi: 10.1242/jcs.110.13.1477. [DOI] [PubMed] [Google Scholar]
- 266.Duvernelle C, Freund V, Frossard N. Transforming growth factor-beta and its role in asthma. Pulm Pharmacol Ther. 2003;16:181–96. doi: 10.1016/S1094-5539(03)00051-8. [DOI] [PubMed] [Google Scholar]
- 267.Saunders R, Siddiqui S, Kaur D, Doe C, Sutcliffe A, Hollins F, et al. Fibrocyte localization to the airway smooth muscle is a feature of asthma. J Allergy Clin Immunol. 2009;123:376–84. doi: 10.1016/j.jaci.2008.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol. 2003;171:380–9. doi: 10.4049/jimmunol.171.1.380. [DOI] [PubMed] [Google Scholar]
- 269.Warburton D, Tefft D, Mailleux A, Bellusci S, Thiery JP, Zhao J, et al. Do lung remodeling, repair, and regeneration recapitulate respiratory ontogeny? Am J Respir Crit Care Med. 2001;164:S59–62. doi: 10.1164/ajrccm.164.supplement_2.2106064. [DOI] [PubMed] [Google Scholar]
- 270.Gerthoffer WT. Migration of airway smooth muscle cells. Proc Am Thorac Soc. 2008;5:97–105. doi: 10.1513/pats.200704-051VS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Goncharova EA, Goncharov DA, Krymskaya VP. Assays for in vitro monitoring of human airway smooth muscle (ASM) and human pulmonary arterial vascular smooth muscle (VSM) cell migration. Nat Protoc. 2006;1:2933–9. doi: 10.1038/nprot.2006.434. [DOI] [PubMed] [Google Scholar]
- 272.Madison JM. Migration of airway smooth muscle cells. Am J Respir Cell Mol Biol. 2003;29:8–11. doi: 10.1165/rcmb.F272. [DOI] [PubMed] [Google Scholar]
- 273.Vicente-Manzanares M, Webb DJ, Horwitz AR. Cell migration at a glance. J Cell Sci. 2005;118:4917–9. doi: 10.1242/jcs.02662. [DOI] [PubMed] [Google Scholar]
- 274.Zaidel-Bar R, Cohen M, Addadi L, Geiger B. Hierarchical assembly of cell-matrix adhesion complexes. Biochem Soc Trans. 2004;32:416–20. doi: 10.1042/BST0320416. [DOI] [PubMed] [Google Scholar]
- 275.Bornfeldt KE, Raines EW, Graves LM, Skinner MP, Krebs EG, Ross R. Platelet-derived growth factor. Distinct signal transduction pathways associated with migration versus proliferation. Ann N Y Acad Sci. 1995;766:416–30. doi: 10.1111/j.1749-6632.1995.tb26691.x. [DOI] [PubMed] [Google Scholar]
- 276.Prass M, Jacobson K, Mogilner A, Radmacher M. Direct measurement of the lamellipodial protrusive force in a migrating cell. J Cell Biol. 2006;174:767–72. doi: 10.1083/jcb.200601159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol. 2009;10:21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- 278.Zaidel-Bar R, Itzkovitz S, Ma’ayan A, Iyengar R, Geiger B. Functional atlas of the integrin adhesome. Nat Cell Biol. 2007;9:858–67. doi: 10.1038/ncb0807-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Smith AF, Bigsby RM, Word RA, Herring BP. A 310-bp minimal promoter mediates smooth muscle cell-specific expression of telokin. Am J Physiol. 1998;274:C1188–95. doi: 10.1152/ajpcell.1998.274.5.C1188. discussion C1187. [DOI] [PubMed] [Google Scholar]
- 280.Tang D, Mehta D, Gunst SJ. Mechanosensitive tyrosine phosphorylation of paxillin and focal adhesion kinase in tracheal smooth muscle. Am J Physiol. 1999;276:C250–8. doi: 10.1152/ajpcell.1999.276.1.C250. [DOI] [PubMed] [Google Scholar]
- 281.Opazo Saez A, Zhang W, Wu Y, Turner CE, Tang DD, Gunst SJ. Tension development during contractile stimulation of smooth muscle requires recruitment of paxillin and vinculin to the membrane. Am J Physiol Cell Physiol. 2004;286:C433–47. doi: 10.1152/ajpcell.00030.2003. [DOI] [PubMed] [Google Scholar]
- 282.Hirshman CA, Zhu D, Pertel T, Panettieri RA, Emala CW. Isoproterenol induces actin depolymerization in human airway smooth muscle cells via activation of an Src kinase and GS. Am J Physiol Lung Cell Mol Physiol. 2005;288:L924–31. doi: 10.1152/ajplung.00463.2004. [DOI] [PubMed] [Google Scholar]
- 283.Parameswaran K, Radford K, Zuo J, Janssen LJ, O’Byrne PM, Cox PG. Extracellular matrix regulates human airway smooth muscle cell migration. Eur Respir J. 2004;24:545–51. doi: 10.1183/09031936.04.00113103. [DOI] [PubMed] [Google Scholar]
- 284.Ammit AJ, Lazaar AL, Irani C, O’Neill GM, Gordon ND, Amrani Y, et al. Tumor necrosis factor-alpha-induced secretion of RANTES and interleukin-6 from human airway smooth muscle cells: modulation by glucocorticoids and beta-agonists. Am J Respir Cell Mol Biol. 2002;26:465–74. doi: 10.1165/ajrcmb.26.4.4681. [DOI] [PubMed] [Google Scholar]
- 285.Govindaraju V, Michoud MC, Al-Chalabi M, Ferraro P, Powell WS, Martin JG. Interleukin-8: novel roles in human airway smooth muscle cell contraction and migration. Am J Physiol Cell Physiol. 2006;291:C957–65. doi: 10.1152/ajpcell.00451.2005. [DOI] [PubMed] [Google Scholar]
- 286.Pavalko FM, Adam LP, Wu MF, Walker TL, Gunst SJ. Phosphorylation of dense-plaque proteins talin and paxillin during tracheal smooth muscle contraction. Am J Physiol. 1995;268:C563–71. doi: 10.1152/ajpcell.1995.268.3.C563. [DOI] [PubMed] [Google Scholar]
- 287.Carlin SM, Resink TJ, Tamm M, Roth M. Urokinase signal transduction and its role in cell migration. FASEB J. 2005;19:195–202. doi: 10.1096/fj.04-1644com. [DOI] [PubMed] [Google Scholar]
- 288.Hasaneen NA, Zucker S, Cao J, Chiarelli C, Panettieri RA, Foda HD. Cyclic mechanical strain-induced proliferation and migration of human airway smooth muscle cells: role of EMMPRIN and MMPs. FASEB J. 2005;19:1507–9. doi: 10.1096/fj.04-3350fje. [DOI] [PubMed] [Google Scholar]
- 289.Henderson N, Markwick LJ, Elshaw SR, Freyer AM, Knox AJ, Johnson SR. Collagen I and thrombin activate MMP-2 by MMP-14-dependent and - independent pathways: implications for airway smooth muscle migration. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1030–8. doi: 10.1152/ajplung.00317.2006. [DOI] [PubMed] [Google Scholar]
- 290.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–58. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- 291.Clark K, Langeslag M, Figdor CG, van Leeuwen FN. Myosin II and mechanotransduction: a balancing act. Trends Cell Biol. 2007;17:178–86. doi: 10.1016/j.tcb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 292.Narumiya S, Tanji M, Ishizaki T. Rho signaling, ROCK and mDia1, in transformation, metastasis and invasion. Cancer Metastasis Rev. 2009;28:65–76. doi: 10.1007/s10555-008-9170-7. [DOI] [PubMed] [Google Scholar]
- 293.Totsukawa G, Yamakita Y, Yamashiro S, Hartshorne DJ, Sasaki Y, Matsumura F. Distinct roles of ROCK (Rho-kinase) and MLCK in spatial regulation of MLC phosphorylation for assembly of stress fibers and focal adhesions in 3T3 fibroblasts. J Cell Biol. 2000;150:797–806. doi: 10.1083/jcb.150.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Polte TR, Eichler GS, Wang N, Ingber DE. Extracellular matrix controls myosin light chain phosphorylation and cell contractility through modulation of cell shape and cytoskeletal prestress. Am J Physiol Cell Physiol. 2004;286:C518–28. doi: 10.1152/ajpcell.00280.2003. [DOI] [PubMed] [Google Scholar]
- 295.Parameswaran K, Willems-Widyastuti A, Alagappan VK, Radford K, Kranenburg AR, Sharma HS. Role of extracellular matrix and its regulators in human airway smooth muscle biology. Cell Biochem Biophys. 2006;44:139–46. doi: 10.1385/CBB:44:1:139. [DOI] [PubMed] [Google Scholar]
- 296.Gomes ER, Jani S, Gundersen GG. Nuclear movement regulated by Cdc42, MRCK, myosin, and actin flow establishes MTOC polarization in migrating cells. Cell. 2005;121:451–63. doi: 10.1016/j.cell.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 297.Pauly RR, Bilato C, Sollott SJ, Monticone R, Kelly PT, Lakatta EG, et al. Role of calcium/calmodulin-dependent protein kinase II in the regulation of vascular smooth muscle cell migration. Circulation. 1995;91:1107–15. doi: 10.1161/01.cir.91.4.1107. [DOI] [PubMed] [Google Scholar]
- 298.Amrani Y, Tliba O, Deshpande DA, Walseth TF, Kannan MS, Panettieri RA., Jr Bronchial hyperresponsiveness: insights into new signaling molecules. Curr Opin Pharmacol. 2004;4:230–4. doi: 10.1016/j.coph.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 299.Stamenovic D, Mijailovich SM, Tolic-Norrelykke IM, Chen J, Wang N. Cell prestress. II. Contribution of microtubules. Am J Physiol Cell Physiol. 2002;282:C617–24. doi: 10.1152/ajpcell.00271.2001. [DOI] [PubMed] [Google Scholar]
- 300.Kaverina I, Krylyshkina O, Small JV. Microtubule targeting of substrate contacts promotes their relaxation and dissociation. J Cell Biol. 1999;146:1033–44. doi: 10.1083/jcb.146.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Carlin SM, Roth M, Black JL. Urokinase potentiates PDGF-induced chemotaxis of human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2003;284:L1020–6. doi: 10.1152/ajplung.00092.2002. [DOI] [PubMed] [Google Scholar]
- 302.Galaria II, Nicholl SM, Roztocil E, Davies MG. Urokinase-induced smooth muscle cell migration requires PI3-K and Akt activation. J Surg Res. 2005;127:46–52. doi: 10.1016/j.jss.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 303.Etienne-Manneville S, Hall A. Cdc42 regulates GSK-3beta and adenomatous polyposis coli to control cell polarity. Nature. 2003;421:753–6. doi: 10.1038/nature01423. [DOI] [PubMed] [Google Scholar]
- 304.Orlandi A, Bennett M. Progenitor cell-derived smooth muscle cells in vascular disease. Biochem Pharmacol. 79:1706–13. doi: 10.1016/j.bcp.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 305.Betsholtz C, Lindblom P, Gerhardt H. Role of pericytes in vascular morphogenesis. EXS. 2005:115–25. doi: 10.1007/3-7643-7311-3_8. [DOI] [PubMed] [Google Scholar]
- 306.Corsini A, Raiteri M, Soma MR, Bernini F, Fumagalli R, Paoletti R. Pathogenesis of atherosclerosis and the role of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors. Am J Cardiol. 1995;76:21A–28A. doi: 10.1016/s0002-9149(05)80011-6. [DOI] [PubMed] [Google Scholar]
- 307.Poon M, Marx SO, Gallo R, Badimon JJ, Taubman MB, Marks AR. Rapamycin inhibits vascular smooth muscle cell migration. J Clin Invest. 1996;98:2277–83. doi: 10.1172/JCI119038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Camoretti-Mercado B. Targeting the airway smooth muscle for asthma treatment. Transl Res. 2009;154:165–74. doi: 10.1016/j.trsl.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Royce SG, Tang ML. The effects of current therapies on airway remodeling in asthma and new possibilities for treatment and prevention. Curr Mol Pharmacol. 2009;2:169–81. doi: 10.2174/1874467210902020169. [DOI] [PubMed] [Google Scholar]
- 310.Birrell MA, Wong S, McCluskie K, Catley MC, Hardaker EL, Haj-Yahia S, et al. Second-generation inhibitors demonstrate the involvement of p38 mitogen-activated protein kinase in post-transcriptional modulation of inflammatory mediator production in human and rodent airways. J Pharmacol Exp Ther. 2006;316:1318–27. doi: 10.1124/jpet.105.093310. [DOI] [PubMed] [Google Scholar]
- 311.Duan W, Wong WS. Targeting mitogen-activated protein kinases for asthma. Curr Drug Targets. 2006;7:691–8. doi: 10.2174/138945006777435353. [DOI] [PubMed] [Google Scholar]
- 312.Hope HR, Anderson GD, Burnette BL, Compton RP, Devraj RV, Hirsch JL, et al. Anti-inflammatory properties of a novel N-phenyl pyridinone inhibitor of p38 mitogen-activated protein kinase: preclinical-to-clinical translation. J Pharmacol Exp Ther. 2009;331:882–95. doi: 10.1124/jpet.109.158329. [DOI] [PubMed] [Google Scholar]
- 313.Hirakawa M, Karashima Y, Watanabe M, Kimura C, Ito Y, Oike M. Protein kinase A inhibits lysophosphatidic acid-induced migration of airway smooth muscle cells. J Pharmacol Exp Ther. 2007;321:1102–8. doi: 10.1124/jpet.106.118042. [DOI] [PubMed] [Google Scholar]
- 314.Henry PJ, Mann TS, Goldie RG. A rho kinase inhibitor, Y-27632 inhibits pulmonary eosinophilia, bronchoconstriction and airways hyperresponsiveness in allergic mice. Pulm Pharmacol Ther. 2005;18:67–74. doi: 10.1016/j.pupt.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 315.Taki F, Kume H, Kobayashi T, Ohta H, Aratake H, Shimokata K. Effects of Rho-kinase inactivation on eosinophilia and hyper-reactivity in murine airways by allergen challenges. Clin Exp Allergy. 2007;37:599–607. doi: 10.1111/j.1365-2222.2007.02693.x. [DOI] [PubMed] [Google Scholar]
- 316.Bosse Y, Thompson C, Stankova J, Rola-Pleszczynski M. Fibroblast growth factor 2 and transforming growth factor beta1 synergism in human bronchial smooth muscle cell proliferation. Am J Respir Cell Mol Biol. 2006;34:746–53. doi: 10.1165/rcmb.2005-0309OC. [DOI] [PubMed] [Google Scholar]
- 317.Freund-Michel V, Bertrand C, Frossard N. TrkA signalling pathways in human airway smooth muscle cell proliferation. Cell Signal. 2006;18:621–7. doi: 10.1016/j.cellsig.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 318.Gosens R, Roscioni SS, Dekkers BG, Pera T, Schmidt M, Schaafsma D, et al. Pharmacology of airway smooth muscle proliferation. Eur J Pharmacol. 2008;585:385–97. doi: 10.1016/j.ejphar.2008.01.055. [DOI] [PubMed] [Google Scholar]
- 319.Hirst SJ, Barnes PJ, Twort CH. PDGF isoform-induced proliferation and receptor expression in human cultured airway smooth muscle cells. Am J Physiol. 1996;270:L415–28. doi: 10.1152/ajplung.1996.270.3.L415. [DOI] [PubMed] [Google Scholar]
- 320.Krymskaya VP, Hoffman R, Eszterhas A, Kane S, Ciocca V, Panettieri RA., Jr EGF activates ErbB-2 and stimulates phosphatidylinositol 3-kinase in human airway smooth muscle cells. Am J Physiol. 1999;276:L246–55. doi: 10.1152/ajplung.1999.276.2.L246. [DOI] [PubMed] [Google Scholar]
- 321.Stewart AG, Fernandes D, Tomlinson PR. The effect of glucocorticoids on proliferation of human cultured airway smooth muscle. Br J Pharmacol. 1995;116:3219–26. doi: 10.1111/j.1476-5381.1995.tb15127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Berger P, Perng DW, Thabrew H, Compton SJ, Cairns JA, McEuen AR, et al. Tryptase and agonists of PAR-2 induce the proliferation of human airway smooth muscle cells. J Appl Physiol. 2001;91:1372–9. doi: 10.1152/jappl.2001.91.3.1372. [DOI] [PubMed] [Google Scholar]
- 323.Chen YH, Zhao MW, Yao WZ, Pang YZ, Tang CS. The signal transduction pathway in the proliferation of airway smooth muscle cells induced by urotensin II. Chin Med J (Engl) 2004;117:37–41. [PubMed] [Google Scholar]
- 324.Cohen P, Noveral JP, Bhala A, Nunn SE, Herrick DJ, Grunstein MM. Leukotriene D4 facilitates airway smooth muscle cell proliferation via modulation of the IGF axis. Am J Physiol. 1995;269:L151–7. doi: 10.1152/ajplung.1995.269.2.L151. [DOI] [PubMed] [Google Scholar]
- 325.Delamere F, Holland E, Patel S, Bennett J, Pavord I, Knox A. Production of PGE2 by bovine cultured airway smooth muscle cells and its inhibition by cyclo-oxygenase inhibitors. Br J Pharmacol. 1994;111:983–8. doi: 10.1111/j.1476-5381.1994.tb14840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Maruno K, Absood A, Said SI. VIP inhibits basal and histamine-stimulated proliferation of human airway smooth muscle cells. Am J Physiol. 1995;268:L1047–51. doi: 10.1152/ajplung.1995.268.6.L1047. [DOI] [PubMed] [Google Scholar]
- 327.Michoud MC, Napolitano G, Maghni K, Govindaraju V, Cogo A, Martin JG. Effects of extracellular triphosphate nucleotides and nucleosides on airway smooth muscle cell proliferation. Am J Respir Cell Mol Biol. 2002;27:732–8. doi: 10.1165/rcmb.4768. [DOI] [PubMed] [Google Scholar]
- 328.Noveral JP, Grunstein MM. Role and mechanism of thromboxane-induced proliferation of cultured airway smooth muscle cells. Am J Physiol. 1992;263:L555–61. doi: 10.1152/ajplung.1992.263.5.L555. [DOI] [PubMed] [Google Scholar]
- 329.Noveral JP, Grunstein MM. Adrenergic receptor-mediated regulation of cultured rabbit airway smooth muscle cell proliferation. Am J Physiol. 1994;267:L291–9. doi: 10.1152/ajplung.1994.267.3.L291. [DOI] [PubMed] [Google Scholar]
- 330.Noveral JP, Grunstein MM. Tachykinin regulation of airway smooth muscle cell proliferation. Am J Physiol. 1995;269:L339–43. doi: 10.1152/ajplung.1995.269.3.L339. [DOI] [PubMed] [Google Scholar]
- 331.Panettieri RA, Yadvish PA, Kelly AM, Rubinstein NA, Kotlikoff MI. Histamine stimulates proliferation of airway smooth muscle and induces c-fos expression. Am J Physiol. 1990;259:L365–71. doi: 10.1152/ajplung.1990.259.6.L365. [DOI] [PubMed] [Google Scholar]
- 332.Panettieri RA, Jr, Hall IP, Maki CS, Murray RK. alpha-Thrombin increases cytosolic calcium and induces human airway smooth muscle cell proliferation. Am J Respir Cell Mol Biol. 1995;13:205–16. doi: 10.1165/ajrcmb.13.2.7626288. [DOI] [PubMed] [Google Scholar]
- 333.Pyne S, Pyne NJ. The differential regulation of cyclic AMP by sphingomyelin-derived lipids and the modulation of sphingolipid-stimulated extracellular signal regulated kinase-2 in airway smooth muscle. Biochem J. 1996;315(Pt 3):917–23. doi: 10.1042/bj3150917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Amrani Y, Panettieri RA, Jr, Frossard N, Bronner C. Activation of the TNF alpha-p55 receptor induces myocyte proliferation and modulates agonist-evoked calcium transients in cultured human tracheal smooth muscle cells. Am J Respir Cell Mol Biol. 1996;15:55–63. doi: 10.1165/ajrcmb.15.1.8679222. [DOI] [PubMed] [Google Scholar]
- 335.Amrani Y, Tliba O, Choubey D, Huang CD, Krymskaya VP, Eszterhas A, et al. IFN-gamma inhibits human airway smooth muscle cell proliferation by modulating the E2F-1/Rb pathway. Am J Physiol Lung Cell Mol Physiol. 2003;284:L1063–71. doi: 10.1152/ajplung.00363.2002. [DOI] [PubMed] [Google Scholar]
- 336.Chen G, Khalil N. TGF-beta1 increases proliferation of airway smooth muscle cells by phosphorylation of map kinases. Respir Res. 2006;7:2. doi: 10.1186/1465-9921-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Cohen MD, Ciocca V, Panettieri RA., Jr TGF-beta 1 modulates human airway smooth-muscle cell proliferation induced by mitogens. Am J Respir Cell Mol Biol. 1997;16:85–90. doi: 10.1165/ajrcmb.16.1.8998083. [DOI] [PubMed] [Google Scholar]
- 338.Hawker KM, Johnson PR, Hughes JM, Black JL. Interleukin-4 inhibits mitogen-induced proliferation of human airway smooth muscle cells in culture. Am J Physiol. 1998;275:L469–77. doi: 10.1152/ajplung.1998.275.3.L469. [DOI] [PubMed] [Google Scholar]
- 339.Okona-Mensah KB, Shittu E, Page C, Costello J, Kilfeather SA. Inhibition of serum and transforming growth factor beta (TGF-beta1)-induced DNA synthesis in confluent airway smooth muscle by heparin. Br J Pharmacol. 1998;125:599–606. doi: 10.1038/sj.bjp.0702046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Goncharova EA, Billington CK, Irani C, Vorotnikov AV, Tkachuk VA, Penn RB, et al. Cyclic AMP-mobilizing agents and glucocorticoids modulate human smooth muscle cell migration. Am J Respir Cell Mol Biol. 2003;29:19–27. doi: 10.1165/rcmb.2002-0254OC. [DOI] [PubMed] [Google Scholar]
- 341.Kaur D, Saunders R, Berger P, Siddiqui S, Woodman L, Wardlaw A, et al. Airway smooth muscle and mast cell-derived CC chemokine ligand 19 mediate airway smooth muscle migration in asthma. Am J Respir Crit Care Med. 2006;174:1179–88. doi: 10.1164/rccm.200603-394OC. [DOI] [PubMed] [Google Scholar]
- 342.Watanabe S, Yamasaki A, Hashimoto K, Shigeoka Y, Chikumi H, Hasegawa Y, et al. Expression of functional leukotriene B4 receptors on human airway smooth muscle cells. J Allergy Clin Immunol. 2009;124:59–65. e1–3. doi: 10.1016/j.jaci.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Parameswaran K, Cox G, Radford K, Janssen LJ, Sehmi R, O’Byrne PM. Cysteinyl leukotrienes promote human airway smooth muscle migration. Am J Respir Crit Care Med. 2002;166:738–42. doi: 10.1164/rccm.200204-291OC. [DOI] [PubMed] [Google Scholar]
- 344.Irani C, Goncharova EA, Hunter DS, Walker CL, Panettieri RA, Krymskaya VP. Phosphatidylinositol 3-kinase but not tuberin is required for PDGF-induced cell migration. Am J Physiol Lung Cell Mol Physiol. 2002;282:L854–62. doi: 10.1152/ajplung.00291.2001. [DOI] [PubMed] [Google Scholar]
- 345.Waters CM, Long J, Gorshkova I, Fujiwara Y, Connell M, Belmonte KE, et al. Cell migration activated by platelet-derived growth factor receptor is blocked by an inverse agonist of the sphingosine 1-phosphate receptor-1. FASEB J. 2006;20:509–11. doi: 10.1096/fj.05-4810fje. [DOI] [PubMed] [Google Scholar]
- 346.Ito I, Fixman ED, Asai K, Yoshida M, Gounni AS, Martin JG, et al. Platelet-derived growth factor and transforming growth factor-beta modulate the expression of matrix metalloproteinases and migratory function of human airway smooth muscle cells. Clin Exp Allergy. 2009;39:1370–80. doi: 10.1111/j.1365-2222.2009.03293.x. [DOI] [PubMed] [Google Scholar]
- 347.Deuse T, Schrepfer S, Koch-Nolte F, Haddad M, Schafer H, Detter C, et al. Sirolimus and FK778: a comparison of two anti-proliferative immunosuppressants for prevention of experimental obliterative airway disease. Transpl Int. 2006;19:310–8. doi: 10.1111/j.1432-2277.2006.00277.x. [DOI] [PubMed] [Google Scholar]
- 348.Goncharova EA, Vorotnikov AV, Gracheva EO, Wang CL, Panettieri RA, Jr, Stepanova VV, et al. Activation of p38 MAP-kinase and caldesmon phosphorylation are essential for urokinase-induced human smooth muscle cell migration. Biol Chem. 2002;383:115–26. doi: 10.1515/BC.2002.012. [DOI] [PubMed] [Google Scholar]
- 349.Day RM, Lee YH, Park AM, Suzuki YJ. Retinoic acid inhibits airway smooth muscle cell migration. Am J Respir Cell Mol Biol. 2006;34:695–703. doi: 10.1165/rcmb.2005-0306OC. [DOI] [PMC free article] [PubMed] [Google Scholar]