Abstract
Fast synaptic inhibition in the adult brain is largely mediated by GABAA receptors (GABAAR). GABAAR are anchored to synaptic sites by gephyrin, a scaffolding protein that appears to be assembled as a hexagonal lattice beneath the plasma membrane. Brain derived neurotrophic factor (BDNF) alters the clustering and synaptic distribution of GABAAR but mechanisms behind this regulation are just starting to emerge. The current study was aimed to examine if BDNF alters the protein levels and/or clustering of gephyrin and to investigate whether the modulation of gephyrin is accompanied by changes in the distribution and/or clustering of GABAAR. Exogenous application of BDNF to immature neuronal cultures from rat hippocampus increased the protein levels and clustering of gephyrin. BDNF also augmented the association of gephyrin with GABAAR and promoted the formation of GABAAR clusters. Together, these observations indicate that BDNF might regulate the assembly of GABAergic synapses by promoting the association of GABAAR with gephyrin.
Keywords: BDNF, gephyrin, clustering, GABAAR, Neurons
1. INTRODUCTION
GABAAR are heteropentameric chloride ion channels assembled from a combination of at least 19 homologous subunits: α(1–6), β(1–3), γ(1–3), δ, ε, θ, π and ρ(1–3) (Jacob et al., 2008; Luscher et al., 2011). GABAAR located at synaptic sites mostly contain γ subunits and mediate phasic inhibition whereas the majority of those located at perisynaptic or extrasynaptic sites contain δ subunits and mediate tonic inhibition (Fritschy, 2008; Belelli et al., 2009; Luscher et al., 2011). Targeting of GABAAR to synaptic or extrasynaptic sites is governed by channel composition and protein-protein interactions with scaffolding proteins (Luscher and Keller, 2004; Chen and Olsen, 2007; Jacob et al., 2008; Leidenheimer, 2008). Gephyrin, a 93-kDa polypeptide that forms a hexagonal lattice beneath the plasma membrane, is the main scaffold protein involved in the synaptic anchoring of GABAAR (Jacob et al., 2005; Fritschy et al., 2008; Tretter et al., 2008; Papadopoulos and Soykan, 2011). Removal of gephyrin by gene targeting or siRNA strongly affects GABAAR clustering and results in the loss of inhibitory postsynaptic currents (Essrich et al., 1998; Kneussel et al., 1999; Yu et al., 2007).
The levels of BDNF, a growth factor belonging to the neurotrophin family, increase rapidly during postnatal development (Maisonpierre et al., 1990; Katoh-Semba et al., 1997; Huang et al., 1999). In rodents, the highest levels of BDNF are found in hippocampus where its concentration increases ~20 times during the first weeks of life to reach maximum levels in young adulthood (Maisonpierre et al., 1990; Katoh-Semba et al., 1997). In hippocampal granule cells, a physiological increase in BDNF levels is correlated with increased dendritic spine density (Stranahan, 2011). In vivo overexpression of BNDF during development showed that increased levels of BDNF accelerate GABAergic maturation (Huang et al., 1999) while ablation of BDNF during development showed that a continuous supply of BDNF is essential for the maintenance of dendritic spines in adulthood (Vigers et al., 2012; Zagrebelsky and Korte, 2014).
In vitro studies indicate that BDNF plays a critical role in neuronal growth, maturation, differentiation and formation of synaptic connections (Seil and Drake-Baumann, 2000; Gottmann et al., 2009; Greenberg et al., 2009; Yoshii and Constantine-Paton, 2010). Incubation of immature neurons with BDNF modulates dendritic growth while incubation of mature neurons regulates dendritic spine density and morphology (Zagrebelsky and Korte, 2014). Prolonged exposure of immature cultured hippocampal neurons to exogenous BDNF promotes differentiation, dendritic development and maturation of GABAergic synapses (Rutherford et al., 1997; Vicario-Abejon et al., 1998; Marty et al., 2000; Yamada et al., 2002; Cohen-Cory et al., 2010). Some of the molecular changes associated with GABAergic maturation include increased levels of glutamic acid decarboxylase and accumulation of GABAAR at synaptic sites that result in the enhancement of presynaptic neurotransmission and mIPSC (Rutherford et al., 1997; Vicario-Abejon et al., 1998; Yamada et al., 2002; Palizvan et al., 2004; Swanwick et al., 2006a; Gottmann et al., 2009; Greenberg et al., 2009)”.
Recent studies have demonstrated that the tropomyosin-related kinase B (TrkB) receptor controls assembly and maintenance of GABAergic synapses (Chen et al., 2011; Wuchter et al., 2012). Loss of TrkB function leads to a reduction in gephyrin clustering and mislocalization of GABAAR containing γ 2 subunits (Chen et al., 2011; Wuchter et al., 2012). Since TrkB receptors mediate most of the synaptic effects of BDNF (Nagappan and Lu, 2005), these observations strongly suggest that BDNF-dependent signaling might regulate the number and synaptic localization of GABAAR clusters (Elmariah et al., 2004; Wuchter et al., 2012), however, the mechanisms behind this regulation are not fully understood. Here, I decided to analyze if prolonged incubation of immature neuronal cultures (at 7–8 days in vitro) with exogenously added BDNF regulates the protein levels and/or clustering of gephyrin and whether gephyrin regulation is accompanied by changes in cell surface distribution or clustering of GABAAR. The results obtained suggest that BDNF increased the protein levels and clustering of gephyrin with a concomitant increase in the formation of GABAAR-gephyrin complexes. These observations support the hypothesis that formation of GABAAR-gephyrin complexes is a potential mechanism to explain the BDNF-dependent increase in the formation of GABAAR clusters as previously described both in vivo and in vitro.
2. MATERIALS AND METHODS
2.1. Materials
Cell culture reagents were purchased from Life Technologies (Grand Island, NY). Recombinant human BDNF was purchased from Cell Signaling Technology (Danvers, MA). Recombinant Human TrkB-Fc Chimera was purchased from R&D Systems (Minneapolis, MN). Sulfosuccinimidyl-6-[biotin-amido]hexanoate (Sulfo-NHS-LC-biotin), Ultralink® immobilized monomeric avidin beads, and the bicinchoninic acid (BCA) protein assay reagent kit were purchased from Pierce (Rockford, IL).
2.2. Neuronal cultures
Time pregnant female Sprague-Dawley rats were obtained from Charles River Laboratories (Wilmington, MA, USA). Animal procedures were performed in accordance with Institutional Animal Care and Use Committee regulations and approved protocols by the University of Colorado Anschutz Medical Campus. Neurons were cultured from hippocampal tissue obtained from postnatal day 0–2 (P0–P2) pups as previously described (Gomez et al., 2002; Robertson et al., 2009), with slight modifications. After dissection, hippocampal tissue was dissociated in a papain solution by continuous agitation and mechanical trituration. Cell suspension was plated in MEM media supplemented with 10% fetal bovine serum and the next day switched to Neurobasal media supplemented with B27 and mitotic inhibitors (5-fluorodeoxyuridine and uridine at 10 μM each) to inhibit glial proliferation. Cultures were maintained at 37°C and 5% CO2 in a cell culture incubator. For biochemical experiments, 4 ml of cell suspension (350,000 cells/ml) were plated in 6 cm plates coated with poly-D-lysine/laminin. For imaging studies, 2 ml of cell suspension (65,000 cell/ml) were seeded in 6 well plates containing glass coverslips coated with poly-D-lysine/laminin. Cultures were fed every 3–4 days by exchanging half of the culturing media. BDNF (50 ng/ml) was added daily starting at 7–8 days in vitro (DIV) for a period of 1 or 2 days. TrkB-Fc (100 ng/ml) was added to the culture media along with BDNF in order to sequester BDNF from the extracellular media to compete the interaction of BDNF with endogenous TrkB receptors.
2.3. Preparation of Whole Cell Lysates
Cultured neurons were washed with ice-cold PBS and scraped in 0.5 ml of RIPA buffer containing protease and phosphatase inhibitors. Following brief sonication cell lysates were centrifuged at 15,000 x g for 20 min at 4°C to remove cell debris. Cell lysates were stored as aliquots at −20°C until analysis. An aliquot of lysate was mixed with an equal volume of 4X Laemmli buffer prior to gel loading. Protein concentration was determined using a BCA protein assay kit.
2.4. Cell Surface Biotinylation
Cell surface levels of GABAAR subunits was measured as previously described (González et al., 2007). All steps were carried out at 4°C. Briefly, neuronal cultures (grown in 6-cm dishes) were rinsed with ice-cold PBS-Ca2+/Mg2+ and incubated in 4 ml of biotin solution (1 mg/ml of sulfo-NH-LC-biotin in PBS Ca2+/Mg2+) for 30 min. Un-reacted biotin was quenched by incubating the cells in PBS Ca2+/Mg2+ containing 100 mM glycine for 30 min. To prepare whole cell lysates, cells were scraped and lysed in RIPA buffer containing protease and phosphatase inhibitors. Lysates were cleared of cell debris by centrifugation at 15,000 x g for 20 min. One aliquot of whole cell lysate (200 μl) was mixed with 200 μl of 4X Laemmli buffer and stored for future analysis. A second aliquot of cell lysate (200 μl) was mixed with an equal volume of Ultralink avidin conjugated beads (200 μl) and stirred overnight. Beads containing biotinylated proteins were washed once with RIPA buffer, twice with a high salt buffer (50 mM Tris, 5 mM EDTA, 500 mM NaCl, 0.1% Triton X-100, pH 7.5) and once with a no-salt buffer (50 mM Tris, pH 7.5). ‘Biotinylated proteins’ were released in 2X Laemmli buffer (400 μl) by incubating the beads at 37°C for 30 min and then recovered and saved. Protein concentration in the whole cell lysate was determined using a BCA protein assay kit. Samples were stored at −20°C until analysis.
2.5. Immunoprecipitation
Neurons were washed with ice-cold PBS and scraped in 0.75 ml of RIPA buffer (modified to contain 0.5% sodium deoxycholate and no SDS). Cell lysates were agitated for 90 min at 4°C and centrifuged at 15,000 x g to remove cell debris. Lysates were pre-cleared for 60 min at 4°C with 40 μl of protein-A agarose beads. Approximately 500 μg of lysate were incubated with 5 μg of α1-subunit antibody (Millipore, Billerica, MA) or control IgG (Santa Cruz Biotechnology, Dallas, TX) for 2 hours at 4°C. Immune complexes were recovered after incubation for 2 h at 4°C with 25 μl of protein-A beads and three washes with RIPA buffer. Immunoisolated proteins were released in 25 μl of 2X Laemmli buffer by boiling at 90–95°C for 3 min.
2.6. Western Blot
15 to 30 μg of protein were used for analysis of whole cell lysates and 30 μg were used for analysis of biotinylation experiments. Samples were separated by SDS-PAGE and transferred to nitrocellulose membranes. Membranes were blocked by incubation in Tris-buffer (50 mM, pH 7.4) containing 5% non-fat milk and 0.1% Tween-20. Blots were incubated with the primary and secondary antibodies in 1% non-fat milk and 0.1% Tween-20. Primary antibodies used included mouse anti-gephyrin and anti-N-ethylmaleimide-sensitive factor (NSF, BD Biosciences, San Jose, CA) and rabbit anti-actin (Sigma-Aldrich, St. Louis, MO). Proteins were visualized using chemiluminescence. Single bands were detected and the size of the bands detected correlated well with the expected molecular weight: 93 kDa for gephyrin, 76 kDa for NSF and 42 kDa for actin. Immunoreactivity was quantified using ImageJ after scanning the films. For the analysis of total protein expression, gephyrin and NSF immunoreactivty were normalized to actin immunoreactivity detected in the corresponding sample. Ratios obtained at 24 and 48 hours were compared to the ratio obtained in the control sample and expressed as a percentage of control. For the biotinylation experiments actin immunoreactivity in the lysate was used to normalize the signal in the biotinylated fraction as previously described (González et al., 2007).
2.7. Immunofluorescence
Cells were fixed with 4% PFA in 0.1 M phosphate buffer (pH 7.4) for 15 min at room temperature. Cells were blocked for at least one hour in PBS containing 5% normal goat serum and 0.3% Triton X-100. Coverslips were incubated overnight in PBS containing 5% goat serum and 0.2% Triton X-100 and a mix of primary antibodies including a purified mouse monoclonal (3B11) antibody (1:500, Synaptic Systems, Goettingen, Germany) and guinea pig polyclonal anti-vesicular GABA transporter (VGAT, 1:300, Synaptic Systems) or rabbit polyclonal anti-α1 subunit (1:75, Millipore). The next day, coverslips were washed and incubated with the appropriate highly cross-adsorbed goat secondary antibodies: Alexa Fluor 488 anti-mouse and Alexa Fluor 568 anti-guinea pig or Alexa Fluor 568 anti-rabbit (1:750, Invitrogen, Grand Island, NY). After washing, coverslips were mounted on slides using Vectashield (Vector Laboratories, Burlingame, CA). Cells from the same culture but different treatment were processed and stained in parallel. Control stainings omitting the primary antibodies were run to confirm that the staining was dependent on the primary antibody. Single plane images were obtained using a Zeiss LSM 510 META confocal microscope: pinhole of 2; ~1.5 μm. Raw images were analyzed using ImageJ. For puncta analysis, raw images were converted to binary images and watershed, puncta were identified as groups of pixels corresponding to 0.1–10.0 μm2 for gephyrin and 0.25–10.0 μm2 for α1-containing GABAAR. Masks generated for puncta quantitation were overlapped and colocalized puncta were determined manually. Puncta measurements were carried out using linearized segments of dendrites (~100 μm for gephyrin and VGAT and ~50 μm for gephyrin or VGAT and α1). Data was obtained from three independent cultures, 4–5 cells per experiment.
2.8. Statistics
Statistical analyses were performed using GraphPad InStat. When comparing two conditions, a two-tailed unpaired Student’s t-test was used. When more than two variables were involved, significance was determined using one-way ANOVA followed by Bonferroni post hoc test.
3. RESULTS
3.1. Long-Term Incubation with BDNF Increased the Protein Levels of Gephyrin
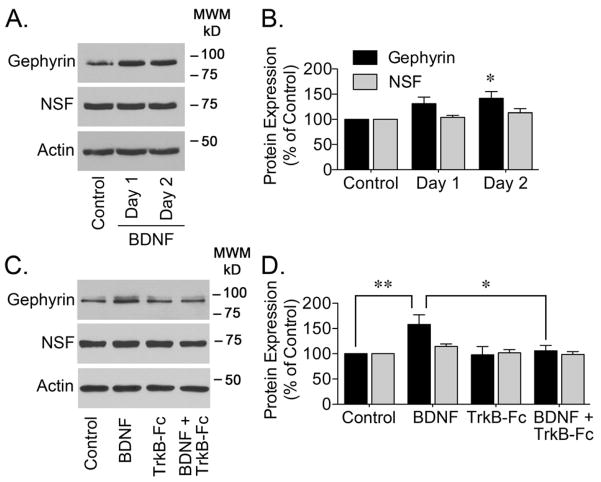
The role of BDNF has been extensively analyzed in vitro upon application of exogenous BDNF to cultured cells (Brunig et al., 2001; Elmariah et al., 2004; Gottmann et al., 2009; Mou et al., 2013). Here, starting at 7–8 DIV, hippocampal neurons were incubated with BDNF (50 ng/ml, added daily) for 1 or 2 days to determine if addition of exogenous BDNF influenced the protein levels of gephyrin. Western blot analysis using an antibody directed towards the E-domain of gephyrin (termed 3B11 antibody) showed a significant increase in gephyrin immunoreactivity in cell lysates obtained from neurons cultured in the presence of BDNF for 2 days (Figure 1A and 1B). No significant change was observed on the levels of the N-ethylmaleimide-sensitive factor (NSF), a protein known to be involved in the regulation of GABAAR (Chou et al., 2010). Since the synaptic effects of BDNF are mainly mediated by TrkB receptors (Nagappan and Lu, 2005), TrkB-Fc (2 μg/ml) was added to the culture media to sequester BDNF and compete the interaction of BDNF with endogenous TrkB receptors (Elmariah et al., 2004; Swanwick et al., 2006a). BDNF scavenging with TrkB-Fc, prevented the increase in gephyrin immunoreactivity (Figure 1C and 1D), strengthening the notion that addition of exogenous BDNF enhances the protein expression of gephyrin. The effects of BDNF on protein levels do not appear to be generalized since analysis of NSF in the same samples showed no change (Figure 1D).
Figure 1. BDNF-Dependent Increase in Gephyrin Protein Expression.
Starting at 7–8 DIV, primary cultured hippocampal neurons were incubated with BDNF (50 ng/ml) for 1 or 2 days to analyze gephyrin protein expression. (A) Representative western blots of gephyrin, NSF, or actin are shown. (B) Densitometry analysis of gephyrin or NSF immunoreactivity is presented as the mean ± S.E.M. of 5 independent experiments. Gephyrin or NSF immunoreactivity was normalized to the corresponding actin signal. BDNF application increased the protein expression of gephyrin (*p<0.05 compared to control by ANOVA) but not that of NSF. (C) To test if the change in gephyrin protein expression was mediated by BDNF, TrkB-Fc was added to sequester BDNF from the extracellular media. Neurons were incubated with BDNF (50 ng/ml) and/or TrkB-Fc (2 μg/ml) for 2 days and gephyrin protein expression was analyzed. Representative blots for gephyrin, NSF, or actin are shown. (D) Gephyrin or NSF immunoreactivity was normalized to actin immunoreactivity and it is presented as the mean ± S.E.M. of 4–7 independent experiments. TrkB-Fc prevented the BDNF-dependent increase in gephyrin protein expression (*p<0.05 or **p<0.01 compared to BDNF treated cultures by ANOVA).
3.2. BDNF-Dependent Increase in Gephyrin Clustering
To determine if the increase in gephyrin protein expression measured by western blot correlates with changes in gephyrin clustering at synaptic sites, neuronal cultures were immunostained with antibodies directed to gephyrin and the vesicular GABA transporter (VGAT) (Figure 2). Images obtained by confocal microscopy following staining of neurons with 3B11 antibodies showed a significant increase in the number of gephyrin clusters following a 2 day incubation with BDNF (Figure 2C); no increase in the size of gephyrin puncta was detected (Figure 2D). Co-staining with an antibody that recognizes the luminal portion of VGAT did not show an increase on the number or size of VGAT clusters after the prolonged treatment with BDNF (Figure 2C and 2D). VGAT staining was used as a reference to analyze the synaptic localization of gephyrin by counting the number of gephyrin punctae that colocalized with VGAT clusters. The number of gephyrin clusters co-localizing with VGAT was boosted by about 40% after a prolonged incubation with BDNF (Figure 2E).
Figure 2. BDNF-Dependent Increase in Gephyrin Clustering.
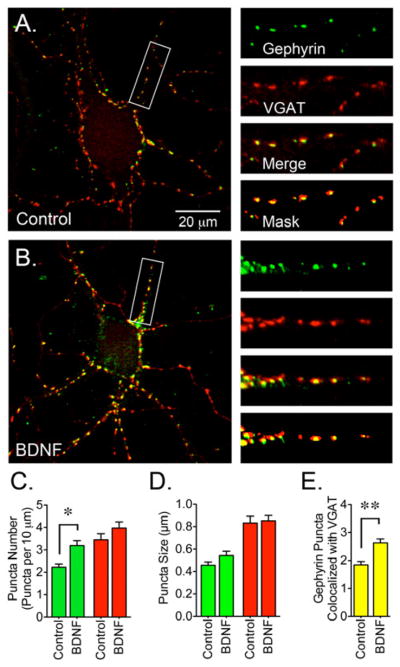
(A) Control or (B) BDNF-treated cultures were co-stained with gephyrin (3B11 antibody, detecting the E-domain, green) and VGAT (red) antibodies. (C) BDNF-treated cultures (50 ng/ml for 2 days starting at 8 DIV) showed an increase in the number of gephyrin clusters. (D) Gephyrin and VGAT puncta size. (E) The number of gephyrin clusters colocalizing with VGAT augmented after BDNF treatment. Data are presented as the mean ± S.E.M. of 15 cells from 3 independent cultures. Green bars represent gephyrin, red bars represent VGAT and yellow bars represent gephyrin clusters that colocalize with VGAT (per 10 μm). BDNF triggers a significant increase in the number and synaptic localization of gephyrin clusters (*p<0.05 or **p<0.01 compared to control cultures by Student’s t-test). Scale bar in A represents 20 μm and the areas within the white boxes are shown at a higher magnification and represent 30 μm.
3.3. BDNF Promotes the Association of GABAAR with Gephyrin
Recently, immunoprecipitation experiments using lysates obtained from cultured neurons provided biochemical evidence that gephyrin and GABAAR are intimately associated and might interact physically (Mukherjee et al., 2011). To evaluate if increased protein expression might promote an increase in the association of GABAAR and gephyrin, GABAAR were immunoisolated with an antibody directed against the α1 subunit of GABAAR and the amount of gephyrin present in the immunoprecipitated receptors was assessed by western blot (Figure 3A). Prolonged incubation of neuronal cultures with BDNF increased the levels of gephyrin that can be detected in α1 immunoprecipitates (Figure 3B), suggesting that BDNF promotes the formation of GABAAR-gephyrin interactions. Complexes isolated from cells co-incubated with TrkB-Fc and BDNF did not contain increased levels of gephyrin, providing evidence that addition of exogenous BDNF might promote the association of GABAAR and gephyrin via activation of TrkB receptors.
Figure 3. BDNF Promotes the Formation of GABAAR-Gephyrin Associations.
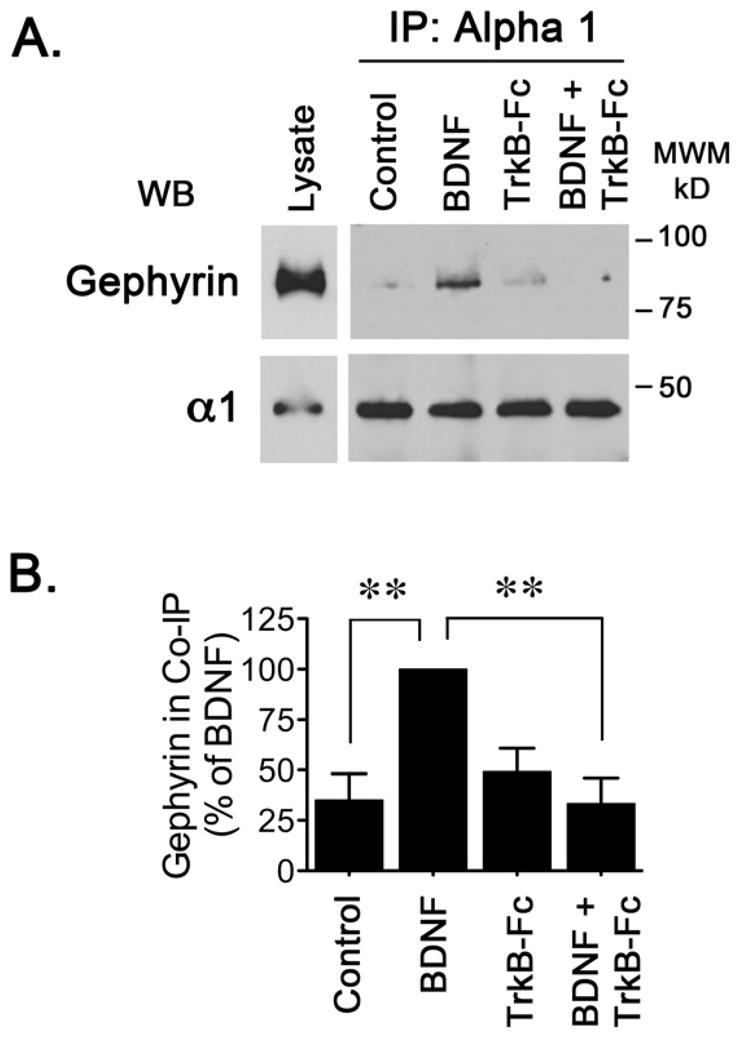
Incubation with BDNF and/or TrkB-Fc was carried out for 2 days. Cell lysates were immunoprecipitated using 5 μg of an antibody directed against the α1 subunit of GABAAR or 5 μg of control IgG. (A) Gephyrin immunoreactivity associated to GABAAR was detected by western blot. (B) Quantitation of 6 independent experiments it is presented as the mean ± S.E.M. BDNF triggers a significant increase in the amount of gephyrin immunoreactivity associated with GABAAR and this effect appears to be dependent on the addition of exogenous BDNF (**p<0.01 compared to BDNF-treated cells by ANOVA).
3.4. No increase in GABAAR Cell Surface Expression After BDNF Treatment
Increased association of GABAAR with gephyrin could result in an increase in the levels of GABAAR present at the plasma membrane. To evaluate this possibility, a cell-surface biotinylation assay was carried out to analyze the plasma membrane levels of GABAAR in control and BDNF-treated cultures (Figure 4). This analysis revealed that BDNF did not increase the plasma membrane levels of GABAAR subunits α1, α4, β2/3 or γ2. This biotinylation assay also showed that most immunoreactivity for the GABAAR subunits analyzed was present in the biotinylated fraction with little to no signal detected in the intracellular fraction (Figure 4A), suggesting that in control cells most GABAAR are already located at the plasma membrane. To evaluate if the biotinylation reagent labeled intracellular proteins, samples were probed to detect actin, an intracellular protein. Actin was not detected in the biotinylated fraction, most actin immunoreactivity was present in the intracellular fraction (Figure 4A), in agreement with the notion that actin was not exposed to the biotinylation reagent. These observations indicate that despite an increase in the formation of GABAAR-gephyrin complexes there is no increase in the accumulation of GABAAR at the plasma membrane.
Figure 4. BDNF Does Not Increase GABAAR Cell Surface Expression.
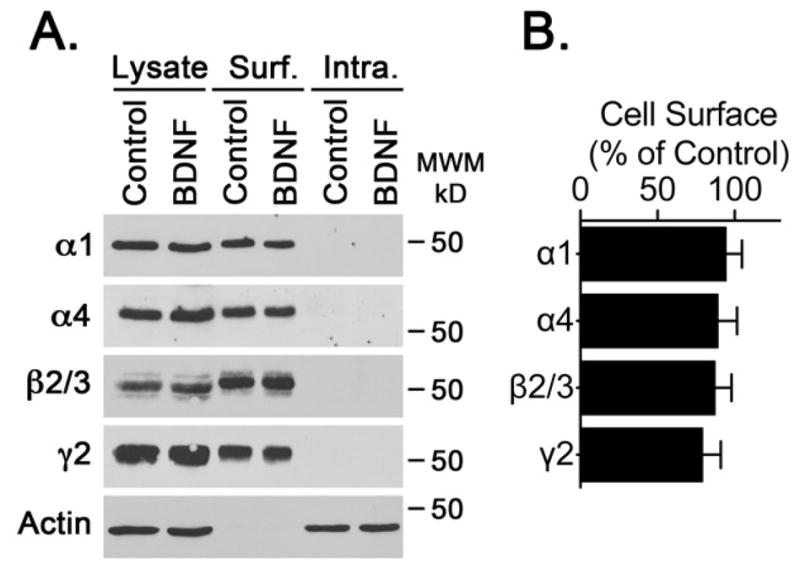
Analysis of the cell surface expression of GABAAR subunits was carried out using a cell-surface biotinylation assay. The levels of biotinylated α1, α4, β2/3 or γ2 subunits were determined by western blot. (A) Representative blots of α1, α4, β2/3, γ2 or actin immunoreactivity in total cell lysate, and cell surface or intracellular fractions. (B) GABAAR immunoreactivity was normalized to the actin signal detected in the corresponding lysate fraction and expressed as percentage of control. Bars represent the normalized values for BDNF treated cells. Control values are 100%. No increase in levels of GABAAR subunits was observed, suggesting that BDNF does not increase the cell surface levels of GABAAR.
3.5. BDNF Promotes Redistribution of GABAAR to Synaptic Sites
Another possible aftereffect of augmented gephyrin protein expression and clustering is the redistribution of GABAAR toward synapses where gephyrin clustering was increased. To investigate this possibility, neurons were co-stained with antibodies to detect gephyrin and GABAAR containing α1 subunits (Figure 5). In control cells, α1 immunofluorescence was diffusely distributed along the neuronal processes and some sparse GABAAR clustering can be detected within the cell body (Figure 5A). In BDNF-treated cells the diffuse α1 staining was reduced and appeared to redistribute into clusters (Figure 5B). Compared to control, the number of α1 clusters was increased in BDNF-treated cells (Figure 5C). Also, the number of GABAAR puncta that colocalized with gephyrin doubled after BDNF incubation (Figure 5E) suggesting that BDNF might coordinately promote the assembly of synaptic sites by increasing the presence of gephyrin at synaptic sites that in turn boosts the incorporation of GABAAR. To determine if GABAAR were redistributed toward synaptic sites, neurons were co-stained with antibodies to detect GABAAR containing α1 subunits and VGAT (Figure 6). The number of GABAAR puncta that colocalized with VGAT doubled in BDNF-treated cells (Figure 6E) suggesting that BDNF might promote the incorporation of GABAAR into synaptic locations.
Figure 5. BDNF Promotes the Colocalization of GABAAR and Gephyrin.
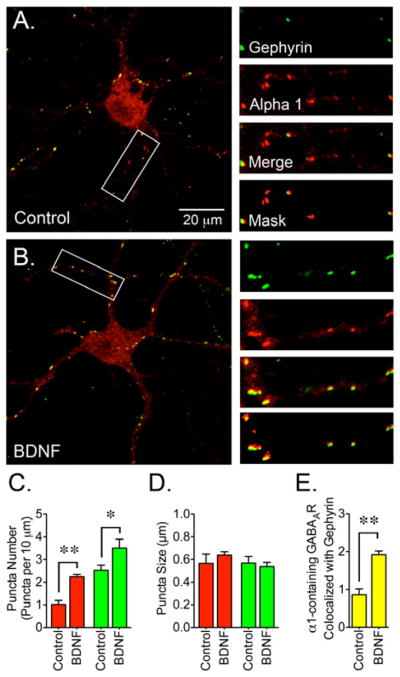
(A) Control or (B) BDNF-treated cells were co-stained with a gephyrin antibody (3B11, green) in combination with an antibody for the α1 subunit (red). BDNF treatment (50 ng/ml for 2 days) resulted in redistribution of α1 immunoreactivity. (C) The number of GABAAR clusters increased after BDNF incubation. (D) Cluster size for α1-containing GABAAR and gephyrin. (E) The number of α1 clusters colocalizing with gephyrin was increased after BDNF treatment. Data presented are the mean ± S.E.M. of 14 cells from 3 independent cultures. Red bars represent α1, green bars represent gephyrin and yellow bars represent GABAAR clusters that colocalize with gephyrin (per 10 μm). BDNF triggers a significant increase in the number of α1 and gephyrin clusters and also increases the colocalization of GABAAR with gephyrin (*p<0.05 or **p<0.01 compared to control cultures using by Student’s t-test). Scale bar in A represents 20 μm and the areas within the white boxes are shown at a higher magnification and represent 30 μm.
Figure 6. BDNF Promotes the Colocalization of GABAAR and VGAT.
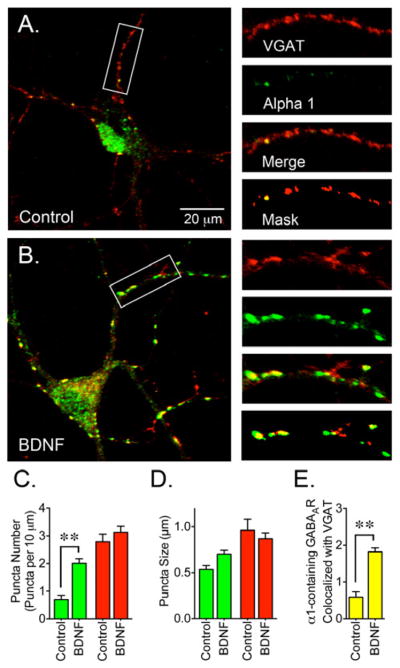
(A) Control or (B) BDNF-treated cells were co-stained with a VGAT antibody (red) in combination with an α1 subunit antibody (green). (C) BDNF treatment (50 ng/ml for 2 days) resulted in a significant increase in the number of GABAAR clusters. (D) Cluster size for α1-containing GABAAR and VGAT. (E) The number of α1 clusters that colocalized with VGAT was increased after BDNF treatment. Data presented are the mean ± S.E.M. of 15 cells from 3 independent cultures. Green bars represent values for the α1 subunit, red bars represent VGAT values and yellow bars represent GABAAR clusters that colocalize with VGAT (per 10 μm). BDNF triggers a significant increase in the number of α1 clusters and also increases the colocalization of GABAAR and VGAT (**p<0.01 compared to control cultures by Student’s t-test). Scale bar represents 20 μm and the areas within the white boxes are shown at a higher magnification and represent 30 μm.
4. DISCUSSION
BDNF/TrkB signaling controls the assembly, maintenance and maturation of GABAergic synapses (Seil and Drake-Baumann, 2000; Yamada et al., 2002; Kuczewski et al., 2010). Exogenous application of BDNF promotes the redistribution and stabilization of GABAAR (Tanaka et al., 1997; Brunig et al., 2001; Elmariah et al., 2004) and loss of BDNF/TrkB receptor signaling results in a reduction in the number of gephyrin and GABAAR clusters (Chen et al., 2011; Wuchter et al., 2012). Despite these advances, the molecular mechanisms underlying the regulation of different components of GABAergic synapses and the role that scaffolding proteins might play in the BDNF-dependent modulation of inhibitory synapses is not fully understood. The current study provides evidence that long-term incubation of immature neuronal cultures with BDNF results in (1) increased protein expression and clustering of gephyrin; (2) increased interaction between gephyrin and α1-containing GABAAR; and (3) increased colocalization of gephyrin and GABAAR at synaptic sites.
During neuronal maturation GABAAR are trapped at synaptic sites by gephyrin and facilitate the formation of postsynaptic structures (Danglot et al., 2003). In vitro, approximately one-third of GABAAR clusters are synaptic by the end of the first week and two thirds are synaptic by the end of the second week (Elmariah et al., 2004; Swanwick et al., 2006b). GABAAR clusters become larger along with the development of presynaptic terminals, bigger cluster size appears to result from increased synaptic GABAAR localization as if extrasynaptic receptors are recruited into synapses (Christie et al., 2002; Danglot et al., 2003; Swanwick et al., 2006b). During development, an escalation in receptor clustering matches the raise in gephyrin concentration at synaptic sites, implying the existence of a developmental pattern that coordinately assembles scaffolding proteins and synaptic receptors.
Gephyrin oligomers are the bases for the formation of a lattice beneath the plasma membrane that anchors receptors to synapses and serves as a hub to facilitate the recruitment of proteins to inhibitory synapses (Papadopoulos and Soykan, 2011; Fritschy et al., 2012; Tretter et al., 2012). This apparently inert structure that helps to stabilize GABAAR is plastic and constantly modified. Phosphorylation and protein-protein interactions directly impact gephyrin clustering and indirectly impacts the function of GABAergic synapses by altering synapse formation and recruitment of GABAAR to synaptic sites (Poulopoulos et al., 2009; Chiou et al., 2011; Tyagarajan et al., 2011; Fritschy et al., 2012; Herweg and Schwarz, 2012; Kuhse et al., 2012). Although much is known about the molecular details involved in the formation of GABAergic synapses less is known about the different factors that might modulate their formation. Long-term exposure of neuronal cultures to BDNF promotes the development and maturation of presynaptic and postsynaptic inhibitory synapses (Vicario-Abejon et al., 1998; Marty et al., 2000; Yamada et al., 2002; Palizvan et al., 2004). BDNF facilitates relocation of GABAAR to postsynaptic structures and formation of receptor clusters that correlate with increases in receptor conductance and elevated mIPSC frequency and amplitude (Rutherford et al., 1997; Li et al., 1998; Bolton et al., 2000; Marty et al., 2000; Elmariah et al., 2004), but the molecular mechanisms involved in the recruitment of GABAAR to synaptic sites are poorly understood.
This study provides an initial insight into a possible mechanism underlying the effects of BDNF on the maturation of GABAergic synapses. Prolonged incubation of immature neuronal cultures with exogenously added BDNF increased the protein expression and clustering of gephyrin and boosted the formation of GABAAR-gephyrin interactions. Stimulation of neurons by addition of exogenous BDNF also promoted the augmentation of receptor clustering at synaptic sites. These observations indicate that BDNF might stimulate synapse maturation by coordinately upregulating the recruitment of gephyrin and GABAAR to newly formed synaptic sites. These results are in agreement with a previous report describing that escalation of GABAAR clustering requires 36–48 hours to develop (Elmariah et al., 2004). In addition, they suggest that a response to BDNF application is delayed because a time lag is necessary to increase the protein expression and assembly of gephyrin into newly formed clusters. Studies in organotypic hippocampal slices further support this hypothesis since during normal development the escalation of inhibitory synaptic strength is accompanied by a concomitant increase in gephyrin clustering and stability at synaptic sites (Vlachos et al., 2013).
BDNF-dependent induced changes in GABAergic neurotransmission may differ across brain regions, cell types and culture age (Brunig et al., 2001; Jovanovic et al., 2004; Mou et al., 2011). In particular, the maturation stage of the cell culture might provide an explanation for the differential effects observed after BDNF treatment. BDNF application to cells cultured for 14–21 days induced a rapid internalization of GABAAR containing α1 subunits (Mou et al., 2011) along with a decrease in mIPSC currents (Brunig et al., 2001). In neurons isolated from postnatal amygdala, BDNF treatment triggers a rapid reduction in gephyrin protein expression that leads to a decrease in cell surface GABAAR (Mou et al., 2013). In this case, while the total protein expression of gephyrin is reduced, a greater proportion of gephyrin forms complexes with GABAAR, as if compensatory mechanisms were activated to recruit receptors back to the plasma membrane (Mou et al., 2013). The factors involved in the maturational effects of BDNF on neurons in culture are unknown but recent evidence demonstrates that younger cultures display more dynamic responses to exogenous BDNF than mature cultures (Zhou et al., 2011).
The status of gephyrin phosphorylation directly impacts synaptic GABAergic function by allowing formation of synapses and recruitment of GABAAR to synapses (Tyagarajan et al., 2011; Kuhse et al., 2012; Tyagarajan et al., 2013). Analysis of the effects of gephyrin phosphorylation on the function of GABAergic neurotransmission using phosphorylation deficient mutants or pharmacological inhibition of protein kinases has provided evidence for the role of gephyrin phosphorylation on gephyrin clustering (Tyagarajan et al., 2011; Kuhse et al., 2012; Tyagarajan et al., 2013). Pharmacological inhibition of kinases resulted in a reduction on phosphorylated gephyrin whereas the levels of total gephyrin were not significantly different, suggesting the possible dissociation between the formation of gephyrin clusters and the levels of gephyrin phosphorylation (Kuhse et al., 2012). The impact of BDNF-mediated signaling on gephyrin phosphorylation and its impact on the formation of synapses during development remains to be investigated.
Several lines of evidence suggest that BDNF accelerates the rate of GABAergic maturation, promotes GABAAR expression and enhances postsynaptic currents in immature neurons (Mizoguchi et al., 2003; Palizvan et al., 2004). The current study provides the key observation that gephyrin regulation (and possibly regulation of other scaffolding proteins) might underlie the molecular mechanisms behind the formation of new synapses triggered by exogenous application of BDNF. The impact of gephyrin regulation onto the localization and distribution GABAAR represents another level of complexity that needs to be explored in order to get a better understanding of the mechanisms involved in the formation and maturation of GABAergic synapses.
Highlights.
In immature neuronal cultures, long-term incubation with BDNF increased the protein expression and clustering of gephyrin,
BDNF treatment promoted the interaction of GABAAR with gephyrin,
GABAAR clustering at synaptic sites increased after long-term incubation with BDNF.
Acknowledgments
A National Institute of Neurological Disorders and Stroke grant to MIG, K01NS069583, supported this work. Imaging experiments were performed at the University of Colorado Anschutz Medical Campus Advance Light Microscopy Core supported in part by NIH/NCRR Colorado CTSI Grant Number UL1 RR025780. The author appreciates the technical support of Amelia Zommer and Philip Lam.
Abbreviations
- GABAAR
gamma-aminobutyric acid type A (GABAA) receptors
- BDNF
brain derived neurotrophic factor
- TrkB
tropomyosin-related kinase B receptor
- VGAT
vesicular GABA transporter
- NSF
N-ethylmaleimide-sensitive factor
- PSD-95
PDZ domain, postsynaptic density 95
- Dlg
discs large
- ZO-1
zonula occludens-1
Footnotes
Conflict of Interest: The author declares no conflict of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Belelli D, Harrison NL, Maguire J, Macdonald RL, Walker MC, Cope DW. Extrasynaptic GABAA receptors: form, pharmacology, and function. J Neurosci. 2009;29:12757–12763. doi: 10.1523/JNEUROSCI.3340-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton MM, Pittman AJ, Lo DC. Brain-derived neurotrophic factor differentially regulates excitatory and inhibitory synaptic transmission in hippocampal cultures. J Neurosci. 2000;20:3221–3232. doi: 10.1523/JNEUROSCI.20-09-03221.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunig I, Penschuck S, Berninger B, Benson J, Fritschy JM. BDNF reduces miniature inhibitory postsynaptic currents by rapid downregulation of GABA(A) receptor surface expression. Eur J Neurosci. 2001;13:1320–1328. doi: 10.1046/j.0953-816x.2001.01506.x. [DOI] [PubMed] [Google Scholar]
- Chen AI, Nguyen CN, Copenhagen DR, Badurek S, Minichiello L, Ranscht B, Reichardt LF. TrkB (tropomyosin-related kinase B) controls the assembly and maintenance of GABAergic synapses in the cerebellar cortex. J Neurosci. 2011;31:2769–2780. doi: 10.1523/JNEUROSCI.4991-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZW, Olsen RW. GABAA receptor associated proteins: a key factor regulating GABAA receptor function. J Neurochem. 2007;100:279–294. doi: 10.1111/j.1471-4159.2006.04206.x. [DOI] [PubMed] [Google Scholar]
- Chiou TT, Bonhomme B, Jin H, Miralles CP, Xiao H, Fu Z, Harvey RJ, Harvey K, Vicini S, De Blas AL. Differential regulation of the postsynaptic clustering of gamma-aminobutyric acid type A (GABAA) receptors by collybistin isoforms. J Biol Chem. 2011;286:22456–22468. doi: 10.1074/jbc.M111.236190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou WH, Wang D, McMahon T, Qi ZH, Song M, Zhang C, Shokat KM, Messing RO. GABAA receptor trafficking is regulated by protein kinase C(epsilon) and the N-ethylmaleimide-sensitive factor. J Neurosci. 2010;30:13955–13965. doi: 10.1523/JNEUROSCI.0270-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie SB, Miralles CP, De Blas AL. GABAergic innervation organizes synaptic and extrasynaptic GABAA receptor clustering in cultured hippocampal neurons. J Neurosci. 2002;22:684–697. doi: 10.1523/JNEUROSCI.22-03-00684.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Cory S, Kidane AH, Shirkey NJ, Marshak S. Brain-derived neurotrophic factor and the development of structural neuronal connectivity. Dev Neurobiol. 2010;70:271–288. doi: 10.1002/dneu.20774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danglot L, Triller A, Bessis A. Association of gephyrin with synaptic and extrasynaptic GABAA receptors varies during development in cultured hippocampal neurons. Mol Cell Neurosci. 2003;23:264–278. doi: 10.1016/s1044-7431(03)00069-1. [DOI] [PubMed] [Google Scholar]
- Elmariah SB, Crumling MA, Parsons TD, Balice-Gordon RJ. Postsynaptic TrkB-mediated signaling modulates excitatory and inhibitory neurotransmitter receptor clustering at hippocampal synapses. J Neurosci. 2004;24:2380–2393. doi: 10.1523/JNEUROSCI.4112-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essrich C, Lorez M, Benson JA, Fritschy JM, Luscher B. Postsynaptic clustering of major GABAA receptor subtypes requires the gamma 2 subunit and gephyrin. Nat Neurosci. 1998;1:563–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- Fritschy JM. Epilepsy, E/I Balance and GABA(A) Receptor Plasticity. Front Mol Neurosci. 2008;1:5. doi: 10.3389/neuro.02.005.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy JM, Harvey RJ, Schwarz G. Gephyrin: where do we stand, where do we go? Trends Neurosci. 2008;31:257–264. doi: 10.1016/j.tins.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Panzanelli P, Tyagarajan SK. Molecular and functional heterogeneity of GABAergic synapses. Cell Mol Life Sci. 2012;69:2485–2499. doi: 10.1007/s00018-012-0926-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez LL, Alam S, Smith KE, Horne E, Dell’Acqua ML. Regulation of A-kinase anchoring protein 79/150-cAMP-dependent protein kinase postsynaptic targeting by NMDA receptor activation of calcineurin and remodeling of dendritic actin. J Neurosci. 2002;22:7027–7044. doi: 10.1523/JNEUROSCI.22-16-07027.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González MI, Krizman-Genda E, Robinson MB. Caveolin-1 regulates the delivery and endocytosis of the glutamate transporter, excitatory amino acid carrier 1. J Biol Chem. 2007;282:29855–29865. doi: 10.1074/jbc.M704738200. [DOI] [PubMed] [Google Scholar]
- Gottmann K, Mittmann T, Lessmann V. BDNF signaling in the formation, maturation and plasticity of glutamatergic and GABAergic synapses. Exp Brain Res. 2009;199:203–234. doi: 10.1007/s00221-009-1994-z. [DOI] [PubMed] [Google Scholar]
- Greenberg ME, Xu B, Lu B, Hempstead BL. New insights in the biology of BDNF synthesis and release: implications in CNS function. J Neurosci. 2009;29:12764–12767. doi: 10.1523/JNEUROSCI.3566-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herweg J, Schwarz G. Splice-specific glycine receptor binding, folding, and phosphorylation of the scaffolding protein gephyrin. J Biol Chem. 2012;287:12645–12656. doi: 10.1074/jbc.M112.341826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- Jacob TC, Moss SJ, Jurd R. GABA(A) receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci. 2008;9:331–343. doi: 10.1038/nrn2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob TC, Bogdanov YD, Magnus C, Saliba RS, Kittler JT, Haydon PG, Moss SJ. Gephyrin regulates the cell surface dynamics of synaptic GABAA receptors. J Neurosci. 2005;25:10469–10478. doi: 10.1523/JNEUROSCI.2267-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic JN, Thomas P, Kittler JT, Smart TG, Moss SJ. Brain-derived neurotrophic factor modulates fast synaptic inhibition by regulating GABA(A) receptor phosphorylation, activity, and cell-surface stability. J Neurosci. 2004;24:522–530. doi: 10.1523/JNEUROSCI.3606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh-Semba R, Takeuchi IK, Semba R, Kato K. Distribution of brain-derived neurotrophic factor in rats and its changes with development in the brain. J Neurochem. 1997;69:34–42. doi: 10.1046/j.1471-4159.1997.69010034.x. [DOI] [PubMed] [Google Scholar]
- Kneussel M, Brandstatter JH, Laube B, Stahl S, Muller U, Betz H. Loss of postsynaptic GABA(A) receptor clustering in gephyrin-deficient mice. J Neurosci. 1999;19:9289–9297. doi: 10.1523/JNEUROSCI.19-21-09289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczewski N, Porcher C, Gaiarsa JL. Activity-dependent dendritic secretion of brain-derived neurotrophic factor modulates synaptic plasticity. Eur J Neurosci. 2010;32:1239–1244. doi: 10.1111/j.1460-9568.2010.07378.x. [DOI] [PubMed] [Google Scholar]
- Kuhse J, Kalbouneh H, Schlicksupp A, Mukusch S, Nawrotzki R, Kirsch J. Phosphorylation of gephyrin in hippocampal neurons by cyclin-dependent kinase CDK5 at Ser-270 is dependent on collybistin. J Biol Chem. 2012;287:30952–30966. doi: 10.1074/jbc.M112.349597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leidenheimer NJ. Regulation of excitation by GABA(A) receptor internalization. Results Probl Cell Differ. 2008;44:1–28. doi: 10.1007/400_2007_039. [DOI] [PubMed] [Google Scholar]
- Li YX, Zhang Y, Lester HA, Schuman EM, Davidson N. Enhancement of neurotransmitter release induced by brain-derived neurotrophic factor in cultured hippocampal neurons. J Neurosci. 1998;18:10231–10240. doi: 10.1523/JNEUROSCI.18-24-10231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher B, Keller CA. Regulation of GABAA receptor trafficking, channel activity, and functional plasticity of inhibitory synapses. Pharmacol Ther. 2004;102:195–221. doi: 10.1016/j.pharmthera.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Luscher B, Fuchs T, Kilpatrick CL. GABAA receptor trafficking-mediated plasticity of inhibitory synapses. Neuron. 2011;70:385–409. doi: 10.1016/j.neuron.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonpierre PC, Belluscio L, Friedman B, Alderson RF, Wiegand SJ, Furth ME, Lindsay RM, Yancopoulos GD. NT-3, BDNF, and NGF in the developing rat nervous system: parallel as well as reciprocal patterns of expression. Neuron. 1990;5:501–509. doi: 10.1016/0896-6273(90)90089-x. [DOI] [PubMed] [Google Scholar]
- Marty S, Wehrle R, Sotelo C. Neuronal activity and brain-derived neurotrophic factor regulate the density of inhibitory synapses in organotypic slice cultures of postnatal hippocampus. J Neurosci. 2000;20:8087–8095. doi: 10.1523/JNEUROSCI.20-21-08087.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi Y, Ishibashi H, Nabekura J. The action of BDNF on GABA(A) currents changes from potentiating to suppressing during maturation of rat hippocampal CA1 pyramidal neurons. J Physiol. 2003;548:703–709. doi: 10.1113/jphysiol.2003.038935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou L, Heldt SA, Ressler KJ. Rapid brain-derived neurotrophic factor-dependent sequestration of amygdala and hippocampal GABA(A) receptors via different tyrosine receptor kinase B-mediated phosphorylation pathways. Neuroscience. 2011;176:72–85. doi: 10.1016/j.neuroscience.2010.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou L, Dias BG, Gosnell H, Ressler KJ. Gephyrin plays a key role in BDNF-dependent regulation of amygdala surface GABARs. Neuroscience. 2013;255:33–44. doi: 10.1016/j.neuroscience.2013.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee J, Kretschmannova K, Gouzer G, Maric HM, Ramsden S, Tretter V, Harvey K, Davies PA, Triller A, Schindelin H, Moss SJ. The residence time of GABA(A)Rs at inhibitory synapses is determined by direct binding of the receptor alpha1 subunit to gephyrin. J Neurosci. 2011;31:14677–14687. doi: 10.1523/JNEUROSCI.2001-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagappan G, Lu B. Activity-dependent modulation of the BDNF receptor TrkB: mechanisms and implications. Trends Neurosci. 2005;28:464–471. doi: 10.1016/j.tins.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Palizvan MR, Sohya K, Kohara K, Maruyama A, Yasuda H, Kimura F, Tsumoto T. Brain-derived neurotrophic factor increases inhibitory synapses, revealed in solitary neurons cultured from rat visual cortex. Neuroscience. 2004;126:955–966. doi: 10.1016/j.neuroscience.2004.03.053. [DOI] [PubMed] [Google Scholar]
- Papadopoulos T, Soykan T. The role of collybistin in gephyrin clustering at inhibitory synapses: facts and open questions. Front Cell Neurosci. 2011;5:11. doi: 10.3389/fncel.2011.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulopoulos A, Aramuni G, Meyer G, Soykan T, Hoon M, Papadopoulos T, Zhang M, Paarmann I, Fuchs C, Harvey K, Jedlicka P, Schwarzacher SW, Betz H, Harvey RJ, Brose N, Zhang W, Varoqueaux F. Neuroligin 2 drives postsynaptic assembly at perisomatic inhibitory synapses through gephyrin and collybistin. Neuron. 2009;63:628–642. doi: 10.1016/j.neuron.2009.08.023. [DOI] [PubMed] [Google Scholar]
- Robertson HR, Gibson ES, Benke TA, Dell’Acqua ML. Regulation of postsynaptic structure and function by an A-kinase anchoring protein-membrane-associated guanylate kinase scaffolding complex. J Neurosci. 2009;29:7929–7943. doi: 10.1523/JNEUROSCI.6093-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford LC, DeWan A, Lauer HM, Turrigiano GG. Brain-derived neurotrophic factor mediates the activity-dependent regulation of inhibition in neocortical cultures. J Neurosci. 1997;17:4527–4535. doi: 10.1523/JNEUROSCI.17-12-04527.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seil FJ, Drake-Baumann R. TrkB receptor ligands promote activity-dependent inhibitory synaptogenesis. J Neurosci. 2000;20:5367–5373. doi: 10.1523/JNEUROSCI.20-14-05367.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM. Physiological variability in brain-derived neurotrophic factor expression predicts dendritic spine density in the mouse dentate gyrus. Neurosci Lett. 2011;495:60–62. doi: 10.1016/j.neulet.2011.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanwick CC, Murthy NR, Kapur J. Activity-dependent scaling of GABAergic synapse strength is regulated by brain-derived neurotrophic factor. Mol Cell Neurosci. 2006a;31:481–492. doi: 10.1016/j.mcn.2005.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanwick CC, Murthy NR, Mtchedlishvili Z, Sieghart W, Kapur J. Development of gamma-aminobutyric acidergic synapses in cultured hippocampal neurons. J Comp Neurol. 2006b;495:497–510. doi: 10.1002/cne.20897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Saito H, Matsuki N. Inhibition of GABAA synaptic responses by brain-derived neurotrophic factor (BDNF) in rat hippocampus. J Neurosci. 1997;17:2959–2966. doi: 10.1523/JNEUROSCI.17-09-02959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter V, Jacob TC, Mukherjee J, Fritschy JM, Pangalos MN, Moss SJ. The clustering of GABA(A) receptor subtypes at inhibitory synapses is facilitated via the direct binding of receptor alpha 2 subunits to gephyrin. J Neurosci. 2008;28:1356–1365. doi: 10.1523/JNEUROSCI.5050-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter V, Mukherjee J, Maric HM, Schindelin H, Sieghart W, Moss SJ. Gephyrin, the enigmatic organizer at GABAergic synapses. Front Cell Neurosci. 2012;6:23. doi: 10.3389/fncel.2012.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagarajan SK, Ghosh H, Yevenes GE, Imanishi SY, Zeilhofer HU, Gerrits B, Fritschy JM. Extracellular signal-regulated kinase and glycogen synthase kinase 3beta regulate gephyrin postsynaptic aggregation and GABAergic synaptic function in a calpain-dependent mechanism. J Biol Chem. 2013;288:9634–9647. doi: 10.1074/jbc.M112.442616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagarajan SK, Ghosh H, Yevenes GE, Nikonenko I, Ebeling C, Schwerdel C, Sidler C, Zeilhofer HU, Gerrits B, Muller D, Fritschy JM. Regulation of GABAergic synapse formation and plasticity by GSK3beta-dependent phosphorylation of gephyrin. Proc Natl Acad Sci U S A. 2011;108:379–384. doi: 10.1073/pnas.1011824108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicario-Abejon C, Collin C, McKay RD, Segal M. Neurotrophins induce formation of functional excitatory and inhibitory synapses between cultured hippocampal neurons. J Neurosci. 1998;18:7256–7271. doi: 10.1523/JNEUROSCI.18-18-07256.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigers AJ, Amin DS, Talley-Farnham T, Gorski JA, Xu B, Jones KR. Sustained expression of brain-derived neurotrophic factor is required for maintenance of dendritic spines and normal behavior. Neuroscience. 2012;212:1–18. doi: 10.1016/j.neuroscience.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlachos A, Reddy-Alla S, Papadopoulos T, Deller T, Betz H. Homeostatic regulation of gephyrin scaffolds and synaptic strength at mature hippocampal GABAergic postsynapses. Cereb Cortex. 2013;23:2700–2711. doi: 10.1093/cercor/bhs260. [DOI] [PubMed] [Google Scholar]
- Wuchter J, Beuter S, Treindl F, Herrmann T, Zeck G, Templin MF, Volkmer H. A comprehensive small interfering RNA screen identifies signaling pathways required for gephyrin clustering. J Neurosci. 2012;32:14821–14834. doi: 10.1523/JNEUROSCI.1261-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada MK, Nakanishi K, Ohba S, Nakamura T, Ikegaya Y, Nishiyama N, Matsuki N. Brain-derived neurotrophic factor promotes the maturation of GABAergic mechanisms in cultured hippocampal neurons. J Neurosci. 2002;22:7580–7585. doi: 10.1523/JNEUROSCI.22-17-07580.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii A, Constantine-Paton M. Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity, and disease. Dev Neurobiol. 2010;70:304–322. doi: 10.1002/dneu.20765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Jiang M, Miralles CP, Li RW, Chen G, de Blas AL. Gephyrin clustering is required for the stability of GABAergic synapses. Mol Cell Neurosci. 2007;36:484–500. doi: 10.1016/j.mcn.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagrebelsky M, Korte M. Form follows function: BDNF and its involvement in sculpting the function and structure of synapses. Neuropharmacology. 2014;76:628–638. doi: 10.1016/j.neuropharm.2013.05.029. [DOI] [PubMed] [Google Scholar]
- Zhou X, Xiao H, Wang H. Developmental changes of TrkB signaling in response to exogenous brain-derived neurotrophic factor in primary cortical neurons. J Neurochem. 2011;119:1205–1216. doi: 10.1111/j.1471-4159.2011.07528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]