Abstract
Alzheimer’s disease (AD) is a clinically heterogeneous neurodegenerative disease with a strong genetic component. Several genes have been associated with AD risk for nearly twenty years. However, it was not until the recent technological advances that allow for the analysis of millions of polymorphisms in thousands of subjects that we have been able to advance our understanding of the genetic complexity of AD susceptibility. Genome wide association studies and whole exome and whole genome sequencing have revealed more than twenty loci associated with AD risk. These studies have provided insights into the molecular pathways that are altered in AD pathogenesis, which have, in turn, provided insight into novel therapeutic targets.
Keywords: Alzheimer’s disease, genome wide association studies, whole exome sequencing, whole genome sequencing, APP, PSEN1, PSEN2, APOE, CLU, PICALM, CR1, BIN1, CD33, MS4A4A, MS4A6A, CD2AP, EPHA1, ABCA7, CASS4, CELF1, DSG2, FERMT2, HLA-DRB5-DRB1, INPP5D, MEF2C, NME8, PTK2B, SLC24A4, RIN3, SORL1, ZCWPW1, TREM2, PLD3, C9ORF72, MAPT, GRN, ADAM10
Alzheimer’s Disease
Alzheimer’s disease (AD) is defined clinically by a gradual decline in memory and other cognitive functions and neuropathologically by gross atrophy of the brain and the accumulation of extracellular amyloid plaques and intracellular neurofibrillary tangles.
Genetic, biochemical, and neuropathological data suggest that aggregation of β-amyloid (Aβ) is central to initiating AD pathogenesis (Hardy and Selkoe, 2002). Aβ is a proteolytic fragment of the amyloid precursor protein (APP)(Thinakaran and Koo, 2008), generated as a result of sequential cleavage by β- and γ-secretases. Deposition of extracellular amyloid plaques is followed by the accumulation of neurofibrillary tangles in neuronal cell bodies and associated processes (Braak and Braak, 1991). Neurofibrillary tangles are composed of hyperphosphorylated tau aggregates. The presence of neurofibrillary tangles in AD brains is strongly correlated with neuronal dysfunction and disease progression (reviewed in (Holtzman et al., 2011)). The amyloid cascade hypothesis posits that changes in APP and/or Aβ homeostasis lead to the aggregation of Aβ and deposition in plaques and that these events are sufficient to initiate the cascade of pathologic and clinical changes associated with AD, including the aggregation of tau protein in neurofibrillary tangles.
Linkage Studies
Dominantly inherited mutations in β-amyloid precursor protein (APP), presenilin 1 (PSEN1), and presenilin 2 (PSEN2) cause early onset Alzheimer’s disease (AD). These genes as well as APOE were identified through genetic linkage studies in families.
APP
APP is located at chromosome 21q21 and encodes a ubiquitously expressed type 1 transmembrane protein. APP is alternatively spliced to produce three transcripts: APP695, APP751, and APP770. The APP695 isoform is the major APP isoform that is expressed in neurons, while the APP751 isoform is highly expressed in astrocytes (Yoshikai et al., 1990). Proteolytic processing of APP leads to the production of fragments through the non-pathogenic and the amyloidogenic pathways. The majority of APP is proteolyzed by α- and γ-secretases, leading to cleavage of APP within the Aβ domain. The result is non-pathogenic fragments: sAPPα and α-C-terminal fragment (CTF) (Thinakaran and Koo, 2008). Alternatively, APP can be cleaved through sequential proteolytic cleavage by β- and γ-secretases to generate Aβ peptides: sAPPβ, and β-CTF. Cell surface APP is internalized allowing Aβ to be generated in the endocytic pathway and secreted into the extracellular space (Thinakaran and Koo, 2008).
Dominant mutations in APP account for approximately 14% of early onset autosomal dominant cases of AD (reviewed in (Guerreiro et al., 2012a)), with more than 30 mutations described (http://www.molgen.vib-ua.be/ADMutations). Two recessive APP mutations, A673V and E693Δ, also reportedly cause early onset AD (reviewed in (Guerreiro et al., 2012a)). The majority of mutations in APP cluster in the region that is adjacent to or within the Aβ domain; however, early genetic studies only sequenced the exons in APP that encode the Aβ sequence (exons 16 and 17), leaving the possibility that variants may exist elsewhere in APP, that cause or increase risk for AD.
Mutations in APP have revealed many important aspects of the molecular mechanisms underlying AD pathogenesis. The Swedish mutation (KM670/671NL) increases plasma Aβ levels by two-three fold by altering β-secretase cleavage efficiency (Mullan et al., 1992). Duplications of APP and the surrounding sequence are also associated with early onset AD. Families carrying these duplications exhibit classic AD and cerebral amyloid angiopathy (CAA) (reviewed in (Guerreiro et al., 2012a)). The frequency of these copy number mutations among autosomal dominant cases of AD varies based on the population: 18% Japanese, 8% French, 2% Dutch, but none in Swedish/Finnish early onset familial cases (reviewed in (Guerreiro et al., 2012a)). Additionally, individuals with Down syndrome, which results from trisomy of chromosome 21, develop AD neuropathology (reviewed in (Guerreiro et al., 2012a)). Individuals with partial trisomy of chromosome 21, which does not include the APP gene, fail to develop AD neuropathology (reviewed in (Guerreiro et al., 2012a)). Thus, excess Aβ production is sufficient to cause AD. Several APP mutations cluster at or after the C-terminal portion of the Aβ domain. These mutations alter γ-secretase function, leading to a shift in APP processing that increases the highly amyloidogenic Aβ42 fragment while reducing the Aβ40 fragment. The result is an altered Aβ42/Aβ40 ratio without a change in total Aβ levels (Bergmans and De Strooper, 2010). Because Aβ42 is more prone to aggregate than Aβ40, these findings suggest that Aβ aggregation is a critical component of AD pathogenesis. The Arctic mutation (E693G) occurs within the Aβ domain (reviewed in (Guerreiro et al., 2012a)). While this mutation fails to alter absolute Aβ levels or the Aβ42/Aβ40 ratio, the Arctic mutation likely increases the aggregation rate of the mutant peptide (Nilsberth et al., 2001). The Dutch mutation (E693Q) also occurs in the Aβ domain, and reportedly results in accelerated Aβ aggregation (reviewed in (Guerreiro et al., 2012a)). Individuals carrying the Dutch mutation develop hereditary cerebral hemorrhage with amyloidosis, which is characterized by predominant vascular Aβ deposition with diffuse plaques in the parenchymal tissue (reviewed in (Guerreiro et al., 2012a)). These mutations provide further evidence that Aβ aggregation is a critical process in AD pathogenesis. Genetic changes that lead to altered APP processing and Aβ accumulation may produce variable neurological and neurovascular phenotypes.
PSEN1 and PSEN2
PSEN1 is located at chromosome 14q24.3, while its homologue, PSEN2, is located at chromosome 1q31-q42. PSEN1 and PSEN2 are structurally similar integral membrane proteins that contain nine transmembrane domains with a hydrophilic intracellular loop region (reviewed in (Guerreiro et al., 2012a)). PSEN1 and PSEN2 are critical components of the γ-secretase complex, which cleaves APP into Aβ fragments. PSEN1 and PSEN2 localize in the endoplasmic reticulum and Golgi apparatus, where they play an important role in protein processing (De Strooper, 2003; Kovacs et al., 1996). As many as 185 dominant, pathogenic mutations have been identified in PSEN1, accounting for approximately 80% of early onset familial AD cases (reviewed in (Guerreiro et al., 2012a)) (http://www.molgen.vib-ua.be/ADMutations). To date, 13 dominant, pathogenic mutations have been identified in PSEN2, which account for approximately 5% of early onset, familial AD cases (reviewed in (Guerreiro et al., 2012a)) (http://www.molgen.vib-ua.be/ADMutations). PSEN1 and PSEN2 mutations are distributed throughout the protein, with some clustering occurring in the transmembrane domains (Guerreiro et al., 2010). Dominantly inherited mutations in PSEN1, as well as APP, have also been identified in late onset AD cases with a strong family history of disease (see discussion below). These families may carry additional genetic variants that delay age at onset of the normally fully penetrant, disease mutation. Additional variants have been described in PSEN1 and PSEN2 that are non-pathogenic or whose pathogenicity remains unclear. Recent evidence suggests that some of these variants, such as PSEN1 E318G (Benitez et al., 2013) and PSEN2 R62H (Cruchaga et al., 2012a), may represent risk factors for AD (described below).
PSEN1 and PSEN2 with nicastrin, anterior pharynx-defective-1 (APH-1) and presenilin enhancer 2 (PEN2) form the γ-secretase complex, which catalyzes the cleavage of many membrane proteins (Wakabayashi and De Strooper, 2008). Elegant kinetic studies by Chávez-Gutiérrez and colleagues have demonstrated that familial AD mutations in PSEN1 and PSEN2 affect γ-secretase function by three mechanisms (Chavez-Gutierrez et al., 2012). First, there are variable inhibitory effects on the initial endoproteolytic cleavage step that releases the intracellular domain of APP. Second, there is a premature release of intermediary substrates of APP during the consecutive carboxypeptidase-like γ-secretase cleavage, leading to the generation of longer Aβ peptides. Finally, there is an effect on the cleavage site, leading to preferential cleavage of APP at position 49–50 or 51–50. These three mechanisms provide an explanation of the historical observations that PSEN1 and PSEN2 mutations lead to altered Aβ42/ Aβ40 ratios.
ADAM10
Recently, rare coding variants in ADAM10 were found to co-segregate in seven late onset AD families (Kim et al., 2009b). ADAM10 is the major α-secretase involved in cleavage of the APP ectodomain (Jorissen et al., 2010; Kuhn et al., 2010; Postina et al., 2004). ADAM10 risk variants, Q170H and R181G, increase Aβ levels in vitro (Kim et al., 2009b). In Tg2576 mice, ADAM10 Q170H and R181G disrupt α-secretase activity and shift APP processing toward amyloidogenic cleavage, yielding increased plaque load (Suh et al., 2013). These genetic and biological findings provide further support for the hypothesis that alteration of APP processing and Aβ generation is sufficient to cause AD.
APOE
Apolipoprotein E (APOE) is located at chromosome 19q13.2 and encodes a pleiotropic glycoprotein. APOE is highly expressed in liver, brain, and macrophages (Siest et al., 1995), where it plays a role in mobilization and redistribution of cholesterol (Mahley, 1988). APOE has also been implicated in neuronal growth and repair, nerve regeneration, immune response and activation of lipolytic enzymes (Mahley, 1988; Mahley and Rall, 2000). APOE occurs as three possible isoforms that differ based on two amino acid residues (112 and 158): APOEε2, APOEε3, and APOEε4. APOEε3 is the most common APOE isoform, occurring in approximately 72% of the population. Family-based methods originally identified genetic linkage between AD and the region of chromosome 19 that contains the APOE gene (reviewed in (Guerreiro et al., 2012a)). APOEε4 increases risk in familial and sporadic early and late onset AD, increasing risk three-fold for heterozygous carriers and increasing risk 8-10-fold for homozygous carriers (reviewed in (Guerreiro et al., 2012a)). APOEε4 also has a dose-dependent effect on age at onset. Interestingly, APOEε2 decreases risk for late onset AD and delays age at onset (reviewed in (Guerreiro et al., 2012a)). Rare coding variants that affect risk for AD may also occur in APOE (Kamboh et al., 1999; Medway et al., 2014); however, deep sequencing of the APOE gene in large datasets has not been performed. It is also possible that APOE variants exist in regulatory regions that impact APOE expression, which could, in turn, influence plaque accumulation. In mouse models, APOE expression levels influence amyloid plaque accumulation (Kim et al., 2011a).
The role of APOE in AD is complex. APOE binds to Aβ, influencing the clearance of soluble Aβ and Aβ aggregation (Castellano et al., 2011; Kim et al., 2009a). APOE4 binds to Aβ more rapidly than APOE3, resulting in accelerated fibril formation (Sanan et al., 1994; Strittmatter et al., 1993). APOE also regulates Aβ metabolism indirectly by interacting with low-density lipoprotein receptor-related protein 1 receptors (Verghese et al., 2013). In vivo, APOE influences the amount and structure of intraparenchymal Aβ deposits in an isoform-dependent manner (Fagan et al., 2002; Holtzman et al., 2000). Thus, the major risk gene associated with AD likely influences Aβ metabolism as a mechanism of pathogenicity.
Genome-Wide Association Studies
Case/control studies
Only 50% of individuals with AD carry an APOEε4 allele, and only around 2% carry a pathogenic mutation, suggesting that other genetic factors must contribute to risk for disease. Before 2005, the general strategy used to identify novel risk genes was to perform an analysis of one or more polymorphisms in a candidate gene. Despite hundreds of studies, few reported genetic associations were replicated across studies, most likely due to Type 1 error (false positive associations in the discovery dataset) (see http://www.alzgene.org) (Bertram et al., 2010). The main reason for this is that other common genetic risk factors likely have a much smaller impact on risk than APOE. As a result, these studies lacked power to detect novel risk genes because the sample sizes used in earlier studies were too small. Since 2005, several technological advances have transformed studies of the genetics of complex traits such as AD (Wray et al., 2008). The first advance was the development of cheap and comprehensive genome-wide arrays, allowing for the simultaneous evaluation of millions of single-nucleotide polymorphisms (SNPs) in thousands of samples: Genome-Wide Association Studies (GWAS). The first GWAS for AD used modest sample sizes (500–2000 samples) and most studies only detected genome-wide significant evidence of association for APOE (reviewed in (Bertram et al., 2010)). The first studies to use thousands of AD cases and elderly non-demented controls successfully generated replicable associations for several new genetic risk factors including clusterin (CLU), phosphatidylinositol-binding clathrin assembly protein (PICALM), complement receptor 1 (CR1), bridging integrator protein 1 (BIN1), and sialic acid binding immunoglobulin (Ig) like lectin (CD33) (Bertram et al., 2008; Harold et al., 2009; Lambert et al., 2009; Seshadri et al., 2010) (Table 1). Two subsequent studies that each included more than 8000 cases and a similar number of controls identified additional loci with genome-wide significant evidence for association (<5 × 10−8): membrane-spanning 4A gene cluster (MS4A4A, MS4A6A, MS4A6E), CD2-associated protein (CD2AP), Ephrin receptor A1(EPHA1), and ATP-binding cassette transporter (ABCA7) (Hollingworth et al., 2011; Naj et al., 2011) (Table 1).
Table 1.
| SNP | Gene | MAF | Odds Ratio | Known function | Related AD Risk Genes | Potential Effects on APP and Tau | Pathways | Gene Expression |
|---|---|---|---|---|---|---|---|---|
| APP | Neurite outgrowth, adhesion and axonogenesis | Cleavage yields Aβ | APP processing | |||||
| PSEN1 | Component of catalytic subunit of gamma-secretase complex. Proteolytic cleavage of integral membrane proteins | Cleaves APP | APP processing | |||||
| PSEN2 | Component of catalytic subunit of gamma-secretase complex. Proteolytic cleavage of integral membrane proteins | Cleaves APP | APP processing | |||||
| APOE | Mediates binding, internalization and catabolism of lipoproteins | SORL1 | Aβ clearance | Lipid Metabolism | ||||
| ADAM10 | Proteolytic cleavage of integral membrane proteins | Cleaves APP | APP processing | |||||
| rs6656401 | CR1a | 0.197 | 1.18 | Mediates cellular binding of immune complexes that activate complement | Aβ clearance | Immune Response | Increased | |
| rs6733839 | BIN1b | 0.409 | 1.22 | Regulation of endocytosis of synaptic vesicles | SLC24A4 & RIN3 | Mediates tau toxicity | Synapse function | Increased |
| rs10948363 | CD2AP | 0.266 | 1.1 | Scaffold molecule regulating actin cytoskeleton | INPP5D & CASS4 | Mediates tau toxicity | Synapse function & Endocytosis | No change |
| rs11771145 | EPHA1c | 0.338 | 0.9 | Brain and neural development. Angiogenesis, cell proliferation, and apoptosis | N/A | Immune Response & Neural Development | No change | |
| rs9331896 | CLU | 0.379 | 0.86 | Chaperone. Regulation of cell proliferation. | PTK2B | Aβ clearance | Immune Response & Lipid Metabolism | Increased |
| rs983392 | MS4A6Ad | 0.403 | 0.9 | Signal transduction | N/A | Immune Response | No change | |
| rs10792832 | PICALMe | 0.358 | 0.87 | AP2-dependent clathrin mediated endocytosis | APP trafficking & Aβ clearance | Synapse function & Endocytosis | No change | |
| rs4147929 | ABCA7f | 0.19 | 1.15 | Lipid homeostasis. Phagocytosis of apoptotic cells by macrophages | Aβ clearance | Immune Response & Lipid Metabolism | Increased | |
| rs3865444 | CD33 | 0.307 | 0.94 | Mediates sialic acid-dependent binding to cells | Aβ clearance | Immune Response | Increased | |
| rs9271192 | HLA-DRB5 HLA- DRB1g | 0.276 | 1.11 | Immunocompetence and histocompatibility | N/A | Immune Response | N/A | |
| rs28834970 | PTK2B | 0.366 | 1.1 | Induction of long term potentiation in hippocampus | CLU | N/A | Synapse function & Neural Development | N/A |
| rs11218343 | SORL1 | 0.039 | 0.77 | APOE receptor. Binds LDL and RAP and mediates endocytosis of the lipids to which it binds | APOE | APP trafficking | Lipid Metabolism, Synapse Function & Endocytosis | Decreased |
| rs10498633 | SLC24A4 & RIN3 | 0.217 | 0.91 | Brain and neural development (SLC24A4); Stimulates & stabilizes GTP-Rab5 in protein transport from plasma membrane to early endosome (RIN3) | BIN1 | N/A | Neural Development, Synapse Function & Endocytosis | N/A |
| rs8093731 | DSG2h | 0.017 | 0.73 | Mediates cell-cell junctions between epithelial and other cell type. | N/A | N/A | N/A | |
| rs35349669 | INPP5D | 0.488 | 1.08 | Negative regulator of myeloid cell proliferation and survival | CD2AP | N/A | Immune Response | N/A |
| rs190982 | MEF2C | 0.408 | 0.93 | Controls synapse formation during activity-dependent refinement of synaptic connectivity and facilitates hippocampal-dependent learning and memory | N/A | Neural Development, Synapse Function & Immune Response | N/A | |
| rs2718058 | NME8i | 0.373 | 0.93 | Ciliary functions | N/A | N/A | N/A | |
| rs1476679 | ZCWPW1 & NYAP1j | 0.287 | 0.91 | Epigenetic regulation (ZCWPW1); Brain and neural development (NYAP1) | N/A | Neural Development | N/A | |
| rs10838725 | CELF1 & MADDk | 0.316 | 1.08 | Regulates pre-mRNA splicing (CELF1); Long-term neuronal viability (MADD) | Mediates tau toxicity | Neural Development | N/A | |
| rs17125944 | FERMT2 | 0.092 | 1.14 | Actin assembly and cell shape and mediator of angiogenesis | Mediates tau toxicity | Cytoskeleton & Axonal transport | N/A | |
| rs7274581 | CASS4l | 0.083 | 0.88 | Docking protein in tyrosine-kinase signaling involved in cell adhesion and spreading | CD2AP | N/A | Cytoskeleton & Axonal transport | N/A |
| rs75932628 | TREM2 | 0.049 | 2.55 | Immune response, triggers production of inflammatory cytokines | Aβ clearance | Immune Response | N/A | |
| rs145999145 | PLD3 | 0.06 | 2.75 | Unknown | APP trafficking and cleavage | Unknown | Increased |
Additional genes located within GWAS loci:
A, CR2, CR1L
B, CYP27C1
C, LOC285965, TAS2R60
D, MS4A3, MS4A2, MS4A6A, MS4A4A, MS4A6E
E, EED
F, CNN2, POLR3E, GPX4
G, HLA-DRB6, HLA-DQA1, HLA-DQB1
H, GSG2
I, GPR141
J, PMS2P1, PILRB, PILRA, C7ORF61, C7ORF47, MEPCE
K, SLC39A13, PSMC3, NDUFS3, KBTBD4, PTPMT1, MTCH2, AGBL2, FNBP4, NUP160
L, C20ORF43, CSTF1
Several studies have now demonstrated a clear relationship between the size of the study and the number of genome-wide significant signals across a range of traits (Ball, 2013; Lambert et al., 2013). Recently, a meta-analysis of GWAS data from 74,046 individuals from four large consortia, confirmed all of the previous associations except CD33 and reported twelve new susceptibility loci for AD. The signals are close to the genes: Cas scaffolding protein family member 4 (CASS4), CUGBP, Elav-like family member 1 (CELF1), Desmoglein 2 (DSG2), Fermitin family member 2 (FERMT2), Major histocompatibility complex class II, DR beta 5-1 (HLA-DRB5-DRB1), Inositol polyphosphate-5-phosphatase (INPP5D), myocyte enhancer factor 2C (MEF2C), NME/NM23 family member 8 (NME8), Protein tyrosine kinase 2 beta (PTK2B), solute carrier family 24 (SLC24A4)-Ras and rab interactor 3 (RIN3), sortilin-related receptor L, A repeats containing (SORL1), zinc finger, CW type with PWWP domain 1 (ZCWPW1) (Lambert et al., 2013) (Table 1). However, as of today, it is not clear whether variants in these genes or neighboring genes are responsible for the initial association. It is likely that as sample sizes continue to grow, additional risk loci will be revealed. However, until those studies are performed, it is difficult to predict which GWAS signals that are below 10−8 will be “real” AD risk genes.
These studies employed samples of European descent but two additional GWAS have examined African-American and Asian (Korean and Japanese) case-control series. In addition to APOE, the most significant finding in the African-American study of 5,896 cases and controls was with SNPs in ABCA7, which reached genome-wide significance and exhibited a somewhat larger effect than in European samples (Reitz et al., 2013). Other loci seen in Europeans showed p values <0.05 (BIN1, CR1, EPHA1 and CD33). The study in Japanese and Koreans used a multistage strategy to identify a novel locus, SORL1, which was replicated in a large European American cohort (Lambert et al., 2013; Miyashita et al., 2013). PICALM and BIN1 SNPs also showed association in the Asian case-control series. Together these studies demonstrate that some of these loci show similar effects across populations (e.g. BIN1), while others appear to have a bigger impact in some populations (e.g. ABCA7). As larger datasets become available for other populations, a more complete picture of the population-specific loci and those that are shared across populations will become more fully resolved.
The common SNPs identified in these GWAS alter risk by 10–15% suggesting that the effect of these risk alleles is much smaller than that of APOEε4 unless they are tagging rare alleles of larger effect. For the most part, the functional alleles responsible for each of these associations remain to be determined. For some of the GWAS loci there are several genes within the associated region, any one of which may contain the functional risk variant (see Table 1). However, many of these AD risk loci have putative functions in the immune system (CLU, CR1, ABCA7, CD33, and EPHA1); four are involved in processes at the cell membrane, including endocytosis (PICALM, BIN1, CD33, and CD2AP); and three are involved in lipid biology (APOE, CLU, and ABCA7) (Table 1; Figure 1). Although some of the new genes appear to be involved in Aβ metabolism (for example, CLU and PICALM), the fact that others likely influence inflammation, endocytosis, and lipid biology suggests that targeting specific components of these pathways may lead to novel directions for drug discovery and treatment. It is also possible that some of the new genetic discoveries are not targeting the nearby gene but actually targeting noncoding RNAs. If so, this could also provide new insights.
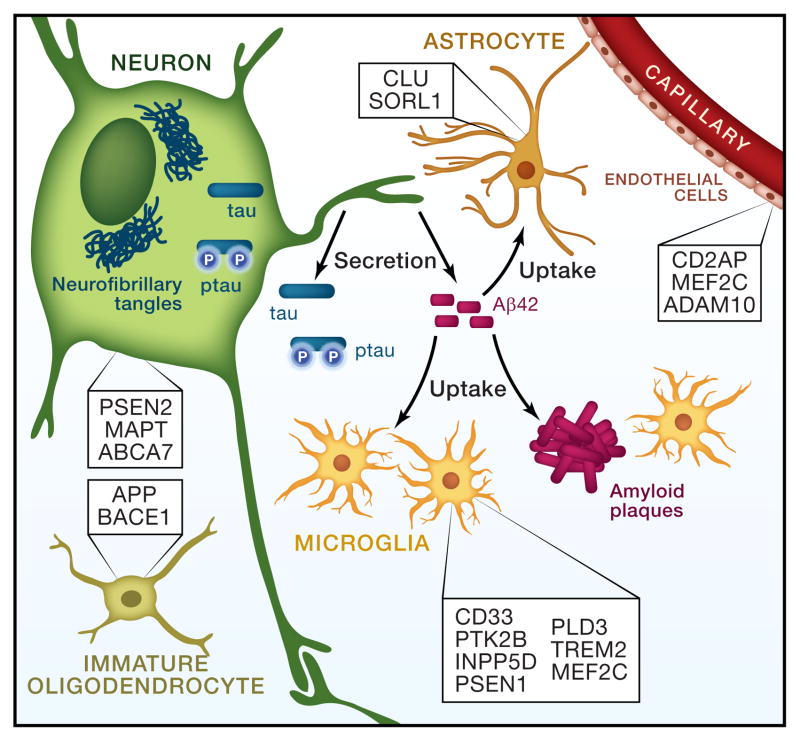
Figure 1. Cell-type expression of Alzheimer’s disease risk genes may influence AD pathogenesis.
The major cell-types present in the brain are depicted. Genes are listed in the cell-type in which they are most highly expressed (Ben Barres, personal communication). GWAS loci that contain multiple genes were excluded from this figure, as it remains unclear which gene is responsible for the signal (CR1, BIN1, PICALM, HLA, SLC24A4, DSG2, ZCWPW1, CELF1, FERMT2, CASS4).
Approaches for identifying new risk factors associated with other aspects of the late onset AD phenotype include genetic studies for elucidating the rate of disease progression, age at onset, cerebrospinal fluid (CSF) or plasma biomarker measurements, and functional and structural imaging measures.
Neuroimaging
Genetic studies using twin and family-based designs have demonstrated that many brain-derived traits, including structural, functional and metabolic measures are significantly heritable (Koten et al., 2009; Thompson et al., 2001). Imaging phenotypes have been used for gene discovery using GWAS approaches and to better understand the phenotypes associated with known genetic risk factors.
The most widely studied candidate gene in imaging studies of AD is APOE. APOEε4 carriers demonstrate accelerated loss of gray matter with age (Lu et al., 2011), decreased connectivity in the default mode network (Damoiseaux et al., 2012), increased hippocampal atrophy (Potkin et al., 2009), increased amyloid deposition, as detected by Aβ ligands (PIB and florbetapir) (Jagust et al., 2012; Ramanan et al., 2014), decreased glucose metabolism (Reiman et al., 2005) and increased numbers of cerebral microbleeds, associated with higher levels of cerebral amyloid angiopathy (Yates et al., 2014). Several studies have shown differences in brain structure and metabolism among individuals carrying APOEε4 (Chen et al., 2010 ; Schraml et al., 2013) and other AD risk alleles in cognitively normal adults decades before the onset of AD (Biffi et al., 2010; Bralten et al., 2011 ; Braskie et al., 2011; Erk et al., 2011 ; Rajagopalan et al., 2013). The late onset AD GWAS SNP in BIN1 has been associated with entorhinal cortex thickness (Biffi et al., 2010), while GWAS SNPs in CR1 have been associated with brain amyloid burden (Thambisetty et al., 2010) and episodic memory decline in healthy and cognitively impaired elders (Keenan et al., 2012), and several PICALM SNPs were associated with entorhinal cortex thickness (Biffi et al., 2010; Furney et al., 2011). The Alzheimer’s disease Neuroimaging Initiative (ADNI) has made both imaging and genetic data publicly available on 1,650 individuals. As a result more than 100 genetic studies have been published using this data (reviewed in (Shen et al., 2013)). A recent GWAS of florbetapir PET signal reported genome-wide significant association with APOE and butyryl cholinesterase (BCHE) SNPs previously associated with BCHE activity (Ramanan et al., 2014). GWAS of hippocampal atrophy implicated additional risk genes, such as FRMD6 (Shen et al., 2013) that have subsequently been shown to be associated with disease in AD cohorts (Hong et al., 2012; Sherva et al., 2014), demonstrating the value of using these endophenotypes to augment genetic studies. The Enhancing Neuroimaging Genetics through Meta-Analysis (ENIGMA) Consortium was formed in 2009 to enhance the replicability of GWAS findings for neuroimaging phenotypes (Thompson et al., 2014). Together with the CHARGE consortium, this group has meta-analyzed tens of thousands of individuals with GWAS and MRI scans. These meta-analyses have provided compelling evidence for novel loci influencing hippocampal volume and intracranial volume (Stein et al., 2012).
Fluid Biomarkers
Several studies have examined the genetics of both plasma and cerebrospinal fluid biomarkers. Most studies have focused on Aβ or tau/ptau, two well-studied and validated AD biomarkers (reviewed in (Craig-Schapiro et al., 2009)). Like the case-control studies, early biomarker studies examined candidate genes and found strong evidence for association between CSF Aβ and APOE genotype, providing further evidence that APOE influences AD through an Aβ-dependent mechanism (Kauwe et al., 2009; Kim et al., 2011b). Several other candidate genes from case-control GWAS have been examined in the fluid biomarker datasets, e.g. variants in SORL1, CLU and MS4A4A have been associated with CSF Aβ levels (Alexopoulos et al., 2011; Elias-Sonnenschein et al., 2013) while others found no evidence for association (Kauwe et al., 2011).
Subsequent studies have taken a genome-wide approach. However, GWAS of CSF Aβ have not observed genome-wide significant evidence of association for any locus other than APOE (Kim et al., 2011b). This suggests that APOE genotype explains a large proportion of the variance in Aβ levels and that no other loci have similarly large effects. It is also likely that some of the problems in these analyses may be due to measurement problems for the Aβ phenotype (reviewed in (Mattsson et al., 2013)). A recent GWAS of plasma Aβ in over 3,500 individuals failed to observe any genome-wide significant signals including APOE (Chouraki et al., 2014). It is unclear whether this reflects differences in systemic versus CNS regulation of Aβ levels by APOE or evidence of differences in measurement of Aβ across studies that can decrease the power of a meta-analysis or both.
In contrast, CSF tau/phospho-tau studies have demonstrated some success. Initial studies using a candidate gene approach reported association between phospho-tau 181/total tau and SNPs in PPP3R1, the regulatory subunit of calcineurin (Cruchaga et al., 2010). The same SNPs were reported to be associated with rate of cognitive decline in this study (Cruchaga et al., 2010) and in an independent cohort (Peterson et al., 2013). Several studies have also reported association between MAPT SNPs and CSF tau levels (Kauwe et al., 2008; Laws et al., 2007). Initial GWAS of CSF tau levels reported association with the APOE region of chromosome 19 but did not identify novel associations at a genome-wide significant level (Han et al., 2010; Kim et al., 2011b). The largest published CSF GWAS examined 1,269 individuals and reported 4 genome-wide significant loci in APOE, between GEMC1 and OSTN on chromosome 3, at 9p24.2 within GLIS3 and at 6p21.1 within the TREM gene cluster (Cruchaga et al., 2013b). The APOE association with CSF tau levels was significant even after inclusion of CSF Aβ levels as a covariate, suggesting that APOE genotype may influence tau levels by both Aβ-dependent and Aβ-independent mechanisms. SNPs in APOE and those between GEMC1 and OSTN also showed association with AD risk, tangle pathology and rate of cognitive decline (Cruchaga et al., 2013b). Cell and animal studies provide some support for Aβ-independent effects of APOE on tau metabolism (Mahley and Huang, 2012; Wolf et al., 2013).
While Aβ and tau are the most well studied AD fluid biomarkers several other proteins implicated in AD GWAS studies have also been examined in genetic studies of AD endophenotypes including CSF and plasma levels of APOE and CLU. SNPs in CLU associated with AD risk were also associated with clusterin protein levels in plasma in several studies (Schurmann et al., 2011 ; Xing et al., 2012). CSF APOE levels appear to exhibit strong genetic control with 72% of the variability in CSF APOE levels explained by SNPs on a GWAS chip (Cruchaga et al., 2012b). The single largest effect was explained by APOEε4 genotype, which explained 8% of the variance. No other genetic variant reached the genome-wide significance threshold, but nine additional variants exhibited a p-value <10−6. Pathway analysis indicated that these loci are involved in lipid metabolism.
Sequencing Studies
The most recent GWAS for late onset AD combined several large datasets and identified more than twenty loci associated with AD risk (Lambert et al., 2013). However, each of these new loci only account for a small percentage of the genetic variance that contributes to AD susceptibility. Thus, a large proportion of the genetic heritability for AD has not been explained by GWAS.
It has been hypothesized that low frequency (MAF 1–5%) and rare variants (MAF <1%) may explain the missing genetic heritability. As was the case with linkage and GWAS, initial sequencing efforts were focused on candidate genes in small populations until the arrival of next-generation sequencing technology, which allowed for low cost, high-throughput sequencing and ushered in the era of whole exome and whole genome sequencing. Even with these new advances, the first studies using this technology were also focused on the pathogenic AD genes. Subsequent genome-wide studies led to the identification of novel variants and genes.
One powerful approach to identify AD risk variants with large effect sizes has been to study families with a strong history of late onset AD. Studies sponsored both by NIMH and by NIA have invested in the collection and characterization of large family datasets with at least two late onset AD cases (Wijsman et al., 2011).
Resequencing of pathogenic AD genes
Although pathogenic mutations in APP, PSEN1 and PSEN2 were originally associated with early onset, familial AD, mutations in these genes have also been reported in families with late onset AD (Devi et al., 2000; Jayadev et al., 2010; Kauwe et al., 2007; Larner et al., 2007; Tomaino et al., 2007). Mutation screening efforts in APP have been historically limited to exons 16 and 17 in early onset AD families (reviewed in (Guerreiro et al., 2012a)). Screening for APP mutations in exons 16–17 in 40 late-onset AD families identified one pathogenic APP mutation (E693G) with incomplete penetrance in a single family (reviewed in (Guerreiro et al., 2012a)). Screening for mutations in PSEN1 has been performed mainly in early-onset AD families, although a small number of late onset AD cases (>60 years; <100 families) were also screened leading to the report of some late onset AD families carrying mutations in PSEN1 (Arango et al., 2001; de Silva et al., 2001; Devi et al., 2000; Kauwe et al., 2007; Larner et al., 2007; Rogaeva et al., 2001; Scacchi et al., 2007; Taddei et al., 2002).
No systematic screen of APP, PSEN1 and PSEN2 in late-onset AD families was published until 2012. Twelve missense, nonsense and splice-site variants were found in 28 families (6.3% of the screened families), including known pathogenic variants in PSEN1 in seven families (Cruchaga et al., 2012a). Likely pathogenic variants, defined as novel variants that segregated with disease status were found in APP. Similar results were reported in an Ibero-American cohort (Jin et al., 2012), where two known and one novel likely pathogenic mutation in PSEN1 were found in three of 176 cases. Together these studies suggest that the impact of mutations in APP, PSEN1, and PSEN2 in late onset AD is higher than previously estimated, affecting between 1.5–2% of all AD cases.
Protective APP variants
It is well known that APOEε4 is associated with increased AD risk and that APOEε2 decreases AD risk, demonstrating that different variants within the same gene may have opposing effects on disease risk. However, most genetic studies of AD have focused on identifying variants that increase AD risk. In fact, until recently, no study had reported a protective variant in APP, PSEN1 or PSEN2. Jonsson et al, reported that rs63750847 (APP A673T) was associated with reduced risk for AD (odds ratio (OR)=0.23) in the Icelandic population (Jonsson et al., 2012). Interestingly, this mutation occurs at the same amino acid residue as a recessive mutation reported to cause early onset AD (APP A673V) (Di Fede et al., 2009). This mutation is near the proteolytic cleavage site of BACE1 (position 2 in the Aβ peptide) and results in impaired BACE1 cleavage of APP in the A673T carriers and reduction of Aβ40 and Aβ42 in vitro (Jonsson et al., 2012). This study provides a proof of principle for the hypothesis that reducing the BACE1 cleavage of APP may protect against AD.
Risk variants in APP, PSEN1 and PSEN2
In addition to identifying known and likely pathogenic mutations in APP and PSEN1, the re-sequencing studies have revealed additional variants that may modify AD risk. Thus far, all of the genetic variants in these genes have been classified as pathogenic or not pathogenic. However, it is likely that rare variants in APP, PSEN1, and PSEN2 could also act as risk factors for AD. For example, the PSEN2 variants, R62H and R71W, failed to show perfect segregation with AD status but were found to be associated with a lower age at onset. The hypothesis that variants in APP, PSEN1 and PSEN2 may serve as risk factors for AD is further supported by a later study in which next-generation sequencing technologies and biomarker levels were combined to identify additional risk variants (Benitez et al., 2013). PSEN1 E318G (rs17125721) was previously classified as non-pathogenic because it does not segregate with disease status in some families (Lindquist et al., 2009; Sandbrink et al., 1996). This coding variant was strongly associated with CSF total Tau and pTau181 levels (Benitez et al., 2013). Analyses in case control series demonstrated that in APOEε4 carriers, PSEN1-E318G is associated with a 10-fold increase in risk of developing AD and an earlier age at onset of AD (Benitez et al., 2013). Together these results suggest that some variants in PSEN1 and PSEN2 are risk factors for AD rather than fully penetrant, causative mutations.
The identification of novel variants in genes that are associated with disease may present as a challenge in the clinic. Several groups have developed guidelines for determining whether a novel variant is truly pathogenic (Guerreiro et al., 2010). These guidelines examine segregation and association data. Variants that occur in three or more cases in a single family are classified as pathogenic. Variants occurring in two cases in a single family are probably pathogenic. For variants identified in only one case, variants are classified as probable if present in three cases and absent in at least 100 controls, possible if present in two cases and absent in at least 100 controls, and not pathogenic/risk factor if present in controls. Probable pathogenicity is further confirmed by demonstration of conservation between PSEN1 and PSEN2, presence of pathogenic mutations at the same residue, fits the helix rule, and alters Aβ levels.
Impact of dementia genes in clinical AD
Mutations in progranulin (GRN), microtubule associated protein tau (MAPT) and the hexanucleotide expansion repeat in C9ORF72 are established causes of familial frontotemporal dementia (FTD) (DeJesus-Hernandez et al., 2011; Huey et al., 2006; Van Deerlin et al., 2007). However, several studies have reported individuals carrying these mutations presenting with clinical symptoms that are indistinguishable from AD (Brouwers et al., 2007; Cortini et al., 2008; Kelley et al., 2010; Lindquist et al., 2008; Ludolph et al., 2009; Momeni et al., 2009; Rademakers et al., 2007; Rademakers et al., 2003; Reed et al., 1997). Among those that have come to autopsy, all had a confirmed diagnosis of FTD. Recent studies in which these genes were screened in large clinical series of AD cases suggest that the frequency of pathogenic mutations in GRN, MAPT, and C9ORF72 in clinical AD is similar to the frequency of pathogenic mutations in APP, PSEN1 and PSEN2 (Cruchaga et al., 2012a; Jin et al., 2012; Wojtas et al., 2012). Overall more than 67 GRN and MAPT mutation-carriers with AD-like symptoms of dementia have been described (Brouwers et al., 2007; Brouwers et al., 2008; Carecchio et al., 2009; Cortini et al., 2008; Kelley et al., 2010; Lindquist et al., 2008; Lindquist et al., 2009; Momeni et al., 2009; Ostojic et al., 2004; Rademakers et al., 2007; Rademakers et al., 2003; Tolboom et al., 2010), 80% of which have a clear family history of dementia and 72% showed an age at onset ≥ 60 years. These data suggest that there is substantial heterogeneity in the clinical presentation associated with FTD mutations and that clinical cohorts of AD are frequently contaminated with cases of FTD misdiagnosed as AD.
New AD risk genes: TREM2
Using whole-exome sequencing and whole-genome sequencing strategies, two groups simultaneously reported a low frequency variant (TREM2 R47H; rs75932628) in triggering receptor expressed on myeloid cells 2 protein (TREM2) that was associated with an increase in AD risk (Guerreiro et al., 2013b; Jonsson et al., 2013b). While there has been some disagreement as to the degree to which TREM2 R47H increases AD risk, a meta-analysis of all of the reports describing an association between TREM2 R47H and risk for AD reported that carrying the TREM2 R47H variant increases risk for AD by 3.4 fold (Guerreiro and Hardy, 2013). Guerreiro et al. described several rare variants in TREM2 that were observed more frequently in cases than controls, suggesting that there may be multiple rare variants in TREM2 that increase risk for AD. This is supported by a recent study showing that TREM2 R47H and TREM2 R62H are associated with AD risk (Cuyvers et al., 2014). Several studies also suggest that TREM2 R47H could be associated with Parkinson’s disease, frontotemporal dementia and amyotrophic lateral sclerosis (Guerreiro et al., 2013c; Lattante et al., 2013; Rayaprolu et al., 2013) but this remains controversial. In previous studies, rare homozygous loss-of-function mutations in TREM2 were associated with an autosomal recessive form of early-onset dementia, Polycystic lipomembranous osteodysplasia with sclerosing leukoencephalopathy (PLOSL) (Paloneva et al., 2003). Subsequent studies found TREM2 mutations in three patients with frontotemporal-like dementia without any bone-associated symptoms (Guerreiro et al., 2012b). These observations highlight a recurring theme in neurodegenerative diseases: that heterozygous and homozygous mutations in the same gene can lead to clinically distinct disorders (Hruska et al., 2008; Singleton et al., 2010; Smith et al., 2012; Swan and Saunders-Pullman, 2013).
TREM2 is a type one transmembrane receptor protein expressed on myeloid cells including microglia, monocyte-derived dendritic cells, osteoclasts and bone-marrow derived macrophages (reviewed in (Colonna, 2003)). TREM2 transduces its intracellular signaling through DAP12. Although the natural ligands of TREM2 remain speculative, upon ligand binding TREM2 associates with DAP12 to mediate downstream signaling. In the brain, TREM2 is primarily expressed on microglia and has been shown to control two signaling pathways: regulation of phagocytosis and suppression of inflammation reactivity (reviewed in (Colonna, 2003)). The identification of coding variants in TREM2 that increase risk for AD supports the role of the immune response and inflammation in AD pathogenesis. However, the mechanism by which these variants increase risk for AD remains unknown. Coding variants in other genes in the TREM-gene family may also modify AD disease risk as is the case for a common protective variant in TREML2 (Benitez et al., 2014).
New AD risk genes: PLD3
Applying a family-based design to identify novel AD risk genes revealed several rare, missense and synonymous variants in phospholipase D3 (PLD3) associated with AD risk (Cruchaga et al., 2013a). Whole-exome sequencing identified one variant in PLD3 (PLD3 V232M; rs145999145), which segregated with disease status in two of 14 independent NIA-LOAD families. Follow up studies in several case-control series demonstrated that this variant is associated with a 2–3 fold increase in risk for AD. Re-sequencing of PLD3 in over 4,000 AD cases and controls revealed a significant excess of coding variants in cases compared to controls in both European and African American samples.
PLD3 is a non-classical phospholipase. Little is known regarding the function of PLD3 or its role in the brain. Previous studies have reported a link between PLD1 and PLD2, classical phospholipases, and APP metabolism (Cai et al., 2006a; Cai et al., 2006b). In cell models of APP processing, PLD3 also influences APP metabolism such that overexpression leads to lower Aβ levels while knock-down of PLD3 leads to increased levels of Aβ (Cruchaga et al., 2013a). Additional studies will be needed to elucidate the specific function of PLD3 in the brain and the pathogenic mechanism associated with the risk variants.
Disease mechanisms implicated by studies of genetic risk factors for AD
Early onset AD mutations in APP, PSEN1 and PSEN2 lead to altered production or ratios of Aβ isoforms in the brain, while APOE influences Aβ clearance and aggregation, both observations support the hypothesis that Aβ levels are critical for disease pathogenesis. GWAS have now identified polymorphisms in or near more than twenty genes that are associated with AD risk (Table 1). The identification of common variants that have small effects on AD risk has created a broader picture of the processes and pathways involved in AD risk including lipid metabolism, the inflammatory response, and endocytosis (Figure 1). Whole genome/exome sequencing studies have also identified risk alleles in TREM2 and PLD3. The identification of rare variants in the population that have moderate to large effects on AD risk will be most valuable in identifying pathways that are central to disease pathogenesis. The majority of the genes recently identified affect Aβ production and clearance, highlighting the importance of this pathway in AD pathogenesis.
Lipid Metabolism
APOE and several recent late onset AD GWAS genes are involved in lipid metabolism: CLU, ABCA7, SORL1 (Harold et al., 2009; Hollingworth et al., 2011; Lambert et al., 2009; Lambert et al., 2013; Naj et al., 2011) (Table 1). APOE likely affects Aβ metabolism directly by binding to Aβ or indirectly by binding to lipoprotein receptors that play a role in Aβ clearance (reviewed in (Holtzman et al., 2012)). ABCA7 may influence AD risk via its ability to transfer cholesterol to APOE and modulate Aβ levels or by clearing Aβ aggregates (Chan et al., 2008; Kim et al., 2013). Clusterin (Apolipoprotein J) also plays a role in Aβ clearance. APOE- and Clusterin-deficient APP transgenic mice exhibit earlier and more extensive Aβ deposition than control mice (DeMattos et al., 2004). Genes involved in lipid metabolism share a potential role in clearance of highly amyloidogenic Aβ from the brain. So, polymorphisms in these genes that modify efficiency of Aβ clearance could have deleterious effects in the brain.
Immune Response
Neuroinflammation and dysregulation of the immune response is a central feature of AD (reviewed in (Holtzman et al., 2011)). Common variants have been identified in several genes that are associated with late onset AD in GWAS: CR1, CD33, MS4A, CLU, ABCA7, EPHA1, HLA-DRB5-DRB1, and INPP5D (Bertram et al., 2008; Harold et al., 2009; Hollingworth et al., 2011; Lambert et al., 2009; Lambert et al., 2013; Naj et al., 2011) (Table 1). Rare, coding variants were also identified in TREM2 in sequencing studies of late onset AD cohorts (Guerreiro et al., 2013a; Jonsson et al., 2013a). Elevated complement cascade activity could exacerbate AD pathology; thus, individuals with CR1 variants that dampen the complement response may be at lower risk for developing AD pathology (Liu and Niu, 2009; Shen et al., 2001). CD33 may play a role in Aβ clearance and other neuroinflammatory pathways mediated by microglia in the brain (Griciuc et al., 2013). TREM2 may influence neurodegeneration, possibly through clearance of protein aggregates or via neuroinflammatory mechanisms (Colonna, 2003). Interestingly, TREM2, like APP, is cleaved by γ-secretase (Wunderlich et al., 2013). Extensive evidence from human studies and mouse models demonstrates that amyloid plaques are surrounded by activated immune cells. Interestingly, transcriptomics of unique cell populations in the mouse brain reveal that one third of late onset GWAS genes are differentially expressed at high levels in microglia (Ben Barres, personal communication). Thus, polymorphisms in immune response genes that exacerbate or dampen the immune system’s response to accumulating Aβ may contribute to AD pathogenesis.
Endocytosis
Endocytosis is critical for normal processing of APP, which is central to AD pathogenesis. Furthermore, synaptic activity and neurotransmitter release is disrupted in AD (reviewed in (Holtzman et al., 2011)). Genes associated with endocytosis and synaptic function were identified in several late onset AD GWAS: BIN1, PICALM, CD2AP, EPHA1, SORL1, RIN3, MEF2C, and MADD (Harold et al., 2009; Hollingworth et al., 2011; Lambert et al., 2009; Lambert et al., 2013; Naj et al., 2011) (Table 1). APP, Aβ, and APOE are internalized through the endosomal-lysosomal pathway, in which BIN1 plays a critical role (Chapuis et al., 2013; McMahon et al., 1997). PICALM-mediated Aβ generation and clearance may influence accumulation of Aβ in AD brains (Xiao et al., 2012). CD2AP is required for synapse formation (Dustin et al., 1998), where it associates with Cbl, enodophilin, and synaptojanin. EPHA1 plays roles in cell and axonal guidance and synaptic plasticity (Lai and Ip, 2009). SORL1 directs APP to endocytic pathways for recycling (Rogaeva et al., 2007) and plays an important role in Aβ generation (Schmidt et al., 2007; Spoelgen et al., 2006). Indeed, small molecules that increase the stability and function of the retromer complex, reduce the amount of APP in the endosomes and reduce Aβ levels suggesting this could be a possible therapeutic avenue (Mecozzi et al., 2014). In cell and animal models of AD, synaptic activity alters Aβ (Cirrito et al., 2008; Cirrito et al., 2005); thus, polymorphisms in genes that influence synaptic function and normal processing of APP and Aβ are likely to contribute to AD pathogenesis.
Tau
While all of the AD genes identified by linkage studies and the majority of genes identified by GWAS and sequencing studies have been implicated in pathways related to APP and Aβ metabolism, some genes are also implicated in tau metabolism. Tau is the central component of neurofibrillary tangles, which are highly predictive of AD progression. Recent CSF GWAS have demonstrated that APOE genotype produces an Aβ-independent effect on CSF tau levels, suggesting that APOE could influence tau accumulation in the brain (Cruchaga et al., 2013b). Decreased expression of the Drosophila ortholog of BIN1 is associated with decreased tau-mediated toxicity in a Drosophila model (Chapuis et al., 2013). Similarly, the Drosophila orthologs to CD2AP, FERMT2, and CELF1 were identified as modifiers of tau-mediated toxicity (Shulman et al., 2014). There is increasing evidence that tau is released from the cell and that this release may be modified by synaptic activity (Karch et al., 2012a, b; Pooler et al., 2013; Yamada et al., 2014). Coupled with in vitro and in vivo evidence of spreading of tau aggregates these observations lead us to hypothesize that genetic variants that alter synaptic function, such as BIN1, CD2AP, PTK2B, MEF2C, NYAP1, and MADD may influence tau release and spreading of tau pathology in the brain (Table 1).
Cell-type specific expression of LOAD risk genes
Our understanding of the role that APP processing plays in AD pathogenesis has been informed largely by the familial AD genes, APP, PSEN1, and PSEN2. For these genes, there is a clear link between altered Aβ production by neurons and development of disease. However, with the identification of additional common risk loci by GWAS and rare risk variants by WES and WGS, we are gaining a more complete picture of the important role that supporting cells play in AD pathogenesis (Fig 1). Many genes that fall within the GWAS loci are highly expressed in microglia and astrocytes (Fig. 1). In an unbiased approach, we mined a gene expression dataset from specific cell-types isolated from mouse brains, which included astrocytes, neurons, oligodendrocytes, microglia, and endothelial cells (Ben Barres, personal communication). In searching LOAD risk genes identified by GWAS and sequencing methods, we found that many of these genes were most highly expressed in cells other than neurons. In Fig. 1, we list genes based on the cell-type in which they are most highly expressed in the mouse brain. Considering the expression pattern of these genes in the brain may be very meaningful in determining the mechanisms underlying disease. For example, clearance of Aβ by microglia and astrocytes may play a more important role in LOAD than Aβ production by neurons.
Future directions for genetic studies
Substantial progress has been made during the last five years toward understanding the genetic architecture of AD, because of the technological advances in genotyping and sequencing that have made it feasible to genotype or sequence thousands of individuals. GWAS have now identified more than twenty loci that influence risk for AD. It is clear from these studies that with the exception of APOE, common risk alleles for AD have modest effects on risk individually but they have pointed to a number of important disease pathways. A recent study indicates that common variants explain 33% of total phenotypic variance. APOE alone explained 6% and other known markers 2%, meaning more than 25% of phenotypic variance remains unexplained by known common markers (Ridge et al., 2013). It is expected that studies using even larger sample sizes will identify additional loci, most likely with smaller effect sizes. Identification of additional loci, will likely implicate further biological pathways in disease risk, but it is unlikely that GWAS SNPs will be used to predict individual risk for AD. In other disorders where even larger samples have been genotyped more risk loci have been identified, suggesting that there is some value to continuing to increase the number of samples with GWAS data. While very large sample sizes have been examined for people of European descent this is not true for other populations. Large GWAS in these populations may identify genes not detected in Europeans. In addition, other AD related phenotypes including rate of progression of AD, rate of cognitive decline and age at onset of AD have yet to be examined in the largest datasets.
An alternative approach to increasing sample size is to use gene-based or pathway-based analyses to identify the genes, that while not genome-wide significant are over-represented among the SNPs with low p-values in GWAS. This approach has identified additional significant genes that further implicate immune regulation, energy metabolism and protein degradation in AD risk (Jones et al., 2010). Another approach has compared GWAS data across related disorders to identify common underlying genes demonstrating a genetic link between cardiovascular disease and AD risk (Liu et al., 2014; Moskvina et al., 2013). A challenge for the field remains follow-up of the GWAS loci to identify the specific functional alleles and the specific mechanisms underlying the GWAS signals.
We anticipate that similar success in identifying novel genes will be observed as large-scale sequencing projects begin to yield results. Early studies, in small discovery datasets, have already identified several, promising genes that modify AD risk (Cruchaga et al., 2013a; Guerreiro et al., 2013a; Jonsson et al., 2013a). So far these studies have largely focused on whole-exome sequencing and have identified coding variation that alters risk for AD by as much as two-three fold. This has the advantage of making functional follow up of the genes identified through sequencing substantially easier than those identified in GWAS. Several groups are currently performing whole-exome sequencing or whole-genome sequencing in unrelated AD cases and controls and in families multiply affected by AD. It is anticipated that whole-exome sequencing or whole-genome sequencing will be available on more than twenty thousand well-characterized samples before the end of 2014. Both the GWAS data and the sequencing data will be available through public repositories such as NIAGADS and dbGaP. Combining this data with other large datasets such as transcriptomics from human brain tissue or induced-pluripotent stem cell derived neurons and glia will provide further insight into the disease pathways, and regulatory nodes within these pathways that may provide druggable targets for future therapies. An early application of this integrative network-based approach used brain tissue from over 1,600 AD cases and nondemented individuals to identify an immune- and microglia-specific module that is dominated by genes involved in pathogen phagocytosis (Forabosco et al., 2013; Zhang et al., 2013). This module contains TREM2 (identified by WES) but shows that TYROBP (aka DAP12, which binds to TREM2) is a key regulator of the network, and is upregulated in AD brains. Mouse microglia overexpressing intact or truncated TYROBP revealed expression changes that significantly overlapped the human brain TYROBP network. Interestingly, LOAD GWAS genes MS4A4A, MS4A6A, and CD33 were also identified in the module that contains TYROBP (Zhang et al., 2013). The causal network structure was able to predict response to gene perturbations and thus presents a useful framework to test models of disease mechanisms underlying AD.
Using genetics to provide insights into clinical research
The AD field as a whole has struggled to move drugs and potential druggable targets from mouse models into effective therapies. In part, this may be due to the fundamental study design and patient recruitment of Phase 3 clinical studies (Bateman et al., 2011; Moulder et al., 2013). The majority of Phase 3 clinical trials have used individuals with mild to moderate dementia and a clinical diagnosis of late onset AD, which is known to be a heterogeneous disease. Recent studies have highlighted that many people enrolled in these clinical trials were amyloid negative based on neuroimaging data and therefore unlikely to respond to an anti-amyloid treatment (Vellas et al., 2013). A drug’s impact on halting or slowing disease progression is hard to assess when power is drastically reduced by the presence of non-AD cases among the patients enrolled in the study. A second problem with these trials has been that a drug that targets Aβ may not be effective in symptomatic AD cases because Aβ deposition occurs in the preclinical phase of the disease and thus reversal of Aβ deposition after clinical symptoms appear may have no impact on disease progression. Identifying individuals with presymptomatic disease would require large-scale screening in most populations.
One way to address these issues is to focus trial design on individuals who will develop AD, with a known age at onset and disease progression. Dominantly inherited AD cases meet these criteria since mutation carriers will all develop AD and usually do so with an age at onset similar to that observed in their parents and other family members. Secondly, because the mutations in these families cause disease by increasing Aβ production, a therapy designed to remove Aβ or inhibit Aβ production is directly targeting the disease mechanism. The Dominantly Inherited Alzheimer Network (DIAN) was initiated 6 years ago to recruit families that carry APP, PSEN1, and PSEN2 mutations. Observational studies in these families have demonstrated that biomarker changes can occur 15–20 years prior to the expected age at onset of AD (Fagan et al., 2014; Potter et al., 2013), providing a large window of opportunity for presymptomatic clinical trials. Two clinical trials in presymptomatic, biomarker positive, mutation carriers have started within the last year. The DIAN study is testing an anti-Aβ antibody and a BACE inhibitor, while the Alzheimer’s disease Prevention Initiative is testing a different Aβ monoclonal antibody in presymptomatic individuals from the Colombian kindred carrying the PSEN1 E280A mutation. Initial outcomes in these trials will be a change in fluid and imaging biomarkers, with follow-up studies assessing clinical and cognitive outcomes (Moulder et al., 2013). For the first time, these clinical trials will provide a true test of the β-amyloid cascade hypothesis, more than twenty years after the initial hypothesis. This is perhaps fitting given that the hypothesis arose around initial genetic discoveries in these and other families.
Summary
The identification of common and rare variants that contribute to AD risk has provided many new opportunities to understand the mechanisms underlying AD. GWAS and next-generation sequencing studies have revealed genes that fall into several common pathways that were previously known to be important in AD pathogenesis: lipid metabolism, immune response, and endocytosis/synaptic function. As whole genome and whole exome sequencing studies in large datasets are completed many more genes will likely be added to this list. As we gain a more complete picture of the genes and pathways dysregulated in AD, we will be able to develop better, more targeted therapeutics.
Acknowledgments
CMK is supported by a career development award from NIH (K01 AG046374, AMG received support from Barnes Jewish Foundation and NIH (AG035083); CC received support from AG044546.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexopoulos P, Guo LH, Kratzer M, Westerteicher C, Kurz A, Perneczky R. Impact of SORL1 single nucleotide polymorphisms on Alzheimer’s disease cerebrospinal fluid markers. Dementia and geriatric cognitive disorders. 2011;32:164–170. doi: 10.1159/000332017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arango D, Cruts M, Torres O, Backhovens H, Serrano ML, Villareal E, Montanes P, Matallana D, Cano C, Van Broeckhoven C, Jacquier M. Systematic genetic study of Alzheimer disease in Latin America: mutation frequencies of the amyloid beta precursor protein and presenilin genes in Colombia. American journal of medical genetics. 2001;103:138–143. doi: 10.1002/1096-8628(20011001)103:2<138::aid-ajmg1529>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Ball RD. Designing a GWAS: power, sample size, and data structure. Methods Mol Biol. 2013;1019:37–98. doi: 10.1007/978-1-62703-447-0_3. [DOI] [PubMed] [Google Scholar]
- Bateman RJ, Aisen PS, De Strooper B, Fox NC, Lemere CA, Ringman JM, Salloway S, Sperling RA, Windisch M, Xiong C. Autosomal-dominant Alzheimer’s disease: a review and proposal for the prevention of Alzheimer’s disease. Alzheimers Res Ther. 2011;3:1. doi: 10.1186/alzrt59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitez BA, Jin SC, Guerreiro R, Graham R, Lord J, Harold D, Sims R, Lambert JC, Gibbs JR, Bras J, Sassi C, Harari O, Bertelsen S, Lupton MK, Powell J, Bellenguez C, Brown K, Medway C, Haddick PC, van der Brug MP, Bhangale T, Ortmann W, Behrens T, Mayeux R, Pericak-Vance MA, Farrer LA, Schellenberg GD, Haines JL, Turton J, Braae A, Barber I, Fagan AM, Holtzman DM, Morris JC, Williams J, Kauwe JS, Amouyel P, Morgan K, Singleton A, Hardy J, Goate AM, Cruchaga C C Study Group, tEctAsDGCAsDNItGC. Missense variant in TREML2 protects against Alzheimer’s disease. Neurobiol Aging. 2014;35:1510, e1519–1526. doi: 10.1016/j.neurobiolaging.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitez BA, Karch CM, Cai Y, Jin SC, Cooper B, Carrell D, Bertelsen S, Chibnik L, Schneider JA, Bennett DA, Fagan AM, Holtzman D, Morris JC, Goate AM, Cruchaga C. The PSEN1, p.E318G variant increases the risk of Alzheimer’s disease in APOE-epsilon4 carriers. PLoS Genet. 2013;9:e1003685. doi: 10.1371/journal.pgen.1003685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmans BA, De Strooper B. gamma-secretases: from cell biology to therapeutic strategies. Lancet Neurol. 2010;9:215–226. doi: 10.1016/S1474-4422(09)70332-1. [DOI] [PubMed] [Google Scholar]
- Bertram L, Lange C, Mullin K, Parkinson M, Hsiao M, Hogan MF, Schjeide BM, Hooli B, Divito J, Ionita I, Jiang H, Laird N, Moscarillo T, Ohlsen KL, Elliott K, Wang X, Hu-Lince D, Ryder M, Murphy A, Wagner SL, Blacker D, Becker KD, Tanzi RE. Genome-wide association analysis reveals putative Alzheimer’s disease susceptibility loci in addition to APOE. Am J Hum Genet. 2008;83:623–632. doi: 10.1016/j.ajhg.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram L, Lill CM, Tanzi RE. The genetics of Alzheimer disease: back to the future. Neuron. 2010;68:270–281. doi: 10.1016/j.neuron.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Biffi A, Anderson CD, Desikan RS, Sabuncu M, Cortellini L, Schmansky N, Salat D, Rosand J. Genetic variation and neuroimaging measures in Alzheimer disease. Arch Neurol. 2010;67:677–685. doi: 10.1001/archneurol.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Bralten J, Arias-Vasquez A, Makkinje R, Veltman JA, Brunner HG, Fernandez G, Rijpkema M, Franke B. Association of the Alzheimer’s gene SORL1 with hippocampal volume in young, healthy adults. The American journal of psychiatry. 2011;168:1083–1089. doi: 10.1176/appi.ajp.2011.10101509. [DOI] [PubMed] [Google Scholar]
- Braskie MN, Jahanshad N, Stein JL, Barysheva M, McMahon KL, de Zubicaray GI, Martin NG, Wright MJ, Ringman JM, Toga AW, Thompson PM. Common Alzheimer’s disease risk variant within the CLU gene affects white matter microstructure in young adults. J Neurosci. 2011;31:6764–6770. doi: 10.1523/JNEUROSCI.5794-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwers N, Nuytemans K, van der Zee J, Gijselinck I, Engelborghs S, Theuns J, Kumar-Singh S, Pickut BA, Pals P, Dermaut B, Bogaerts V, De Pooter T, Serneels S, Van den Broeck M, Cuijt I, Mattheijssens M, Peeters K, Sciot R, Martin JJ, Cras P, Santens P, Vandenberghe R, De Deyn PP, Cruts M, Van Broeckhoven C, Sleegers K. Alzheimer and Parkinson diagnoses in progranulin null mutation carriers in an extended founder family. Arch Neurol. 2007;64:1436–1446. doi: 10.1001/archneur.64.10.1436. [DOI] [PubMed] [Google Scholar]
- Brouwers N, Sleegers K, Engelborghs S, Maurer-Stroh S, Gijselinck I, van der Zee J, Pickut BA, Van den Broeck M, Mattheijssens M, Peeters K, Schymkowitz J, Rousseau F, Martin JJ, Cruts M, De Deyn PP, Van Broeckhoven C. Genetic variability in progranulin contributes to risk for clinically diagnosed Alzheimer disease. Neurology. 2008;71:656–664. doi: 10.1212/01.wnl.0000319688.89790.7a. [DOI] [PubMed] [Google Scholar]
- Cai D, Netzer WJ, Zhong M, Lin Y, Du G, Frohman M, Foster DA, Sisodia SS, Xu H, Gorelick FS, Greengard P. Presenilin-1 uses phospholipase D1 as a negative regulator of beta-amyloid formation. Proc Natl Acad Sci U S A. 2006a;103:1941–1946. doi: 10.1073/pnas.0510708103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D, Zhong M, Wang R, Netzer WJ, Shields D, Zheng H, Sisodia SS, Foster DA, Gorelick FS, Xu H, Greengard P. Phospholipase D1 corrects impaired betaAPP trafficking and neurite outgrowth in familial Alzheimer’s disease-linked presenilin-1 mutant neurons. Proc Natl Acad Sci U S A. 2006b;103:1936–1940. doi: 10.1073/pnas.0510710103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carecchio M, Fenoglio C, De Riz M, Guidi I, Comi C, Cortini F, Venturelli E, Restelli I, Cantoni C, Bresolin N, Monaco F, Scarpini E, Galimberti D. Progranulin plasma levels as potential biomarker for the identification of GRN deletion carriers. A case with atypical onset as clinical amnestic Mild Cognitive Impairment converted to Alzheimer’s disease. J Neurol Sci. 2009;287:291–293. doi: 10.1016/j.jns.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Castellano JM, Kim J, Stewart FR, Jiang H, DeMattos RB, Patterson BW, Fagan AM, Morris JC, Mawuenyega KG, Cruchaga C, Goate AM, Bales KR, Paul SM, Bateman RJ, Holtzman DM. Human apoE isoforms differentially regulate brain amyloid-beta peptide clearance. Sci Transl Med. 2011;3:89ra57. doi: 10.1126/scitranslmed.3002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SL, Kim WS, Kwok JB, Hill AF, Cappai R, Rye KA, Garner B. ATP-binding cassette transporter A7 regulates processing of amyloid precursor protein in vitro. J Neurochem. 2008;106:793–804. doi: 10.1111/j.1471-4159.2008.05433.x. [DOI] [PubMed] [Google Scholar]
- Chapuis J, Hansmannel F, Gistelinck M, Mounier A, Van Cauwenberghe C, Kolen KV, Geller F, Sottejeau Y, Harold D, Dourlen P, Grenier-Boley B, Kamatani Y, Delepine B, Demiautte F, Zelenika D, Zommer N, Hamdane M, Bellenguez C, Dartigues JF, Hauw JJ, Letronne F, Ayral AM, Sleegers K, Schellens A, Broeck LV, Engelborghs S, De Deyn PP, Vandenberghe R, O’Donovan M, Owen M, Epelbaum J, Mercken M, Karran E, Bantscheff M, Drewes G, Joberty G, Campion D, Octave JN, Berr C, Lathrop M, Callaerts P, Mann D, Williams J, Buee L, Dewachter I, Van Broeckhoven C, Amouyel P, Moechars D, Dermaut B, Lambert JC. Increased expression of BIN1 mediates Alzheimer genetic risk by modulating tau pathology. Mol Psychiatry. 2013;18:1225–1234. doi: 10.1038/mp.2013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez-Gutierrez L, Bammens L, Benilova I, Vandersteen A, Benurwar M, Borgers M, Lismont S, Zhou L, Van Cleynenbreugel S, Esselmann H, Wiltfang J, Serneels L, Karran E, Gijsen H, Schymkowitz J, Rousseau F, Broersen K, De Strooper B. The mechanism of gamma-Secretase dysfunction in familial Alzheimer disease. Embo J. 2012;31:2261–2274. doi: 10.1038/emboj.2012.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Langbaum JB, Fleisher AS, Ayutyanont N, Reschke C, Lee W, Liu X, Bandy D, Alexander GE, Thompson PM, Foster NL, Harvey DJ, de Leon MJ, Koeppe RA, Jagust WJ, Weiner MW, Reiman EM Alzheimer’s Disease Neuroimaging I. Twelve-month metabolic declines in probable Alzheimer’s disease and amnestic mild cognitive impairment assessed using an empirically predefined statistical region-of-interest: findings from the Alzheimer’s Disease Neuroimaging Initiative. NeuroImage. 2010;51:654–664. doi: 10.1016/j.neuroimage.2010.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouraki V, De Bruijn RF, Chapuis J, Bis JC, Reitz C, Schraen S, Ibrahim-Verbaas CA, Grenier-Boley B, Delay C, Rogers R, Demiautte F, Mounier A, Fitzpatrick AL, Berr C, Dartigues JF, Uitterlinden AG, Hofman A, Breteler M, Becker JT, Lathrop M, Schupf N, Alperovitch A, Mayeux R, van Duijn CM, Buee L, Amouyel P, Lopez OL, Ikram MA, Tzourio C, Lambert JC. A genome-wide association meta-analysis of plasma Abeta peptides concentrations in the elderly. Mol Psychiatry. 2014 doi: 10.1038/mp.2013.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito JR, Kang JE, Lee J, Stewart FR, Verges DK, Silverio LM, Bu G, Mennerick S, Holtzman DM The Alzheimer’s Disease Neuroimaging I. Endocytosis is required for synaptic activity-dependent release of amyloid-beta in vivo. Neuron. 2008;58:42–51. doi: 10.1016/j.neuron.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- Colonna M. TREMs in the immune system and beyond. Nat Rev Immunol. 2003;3:445–453. doi: 10.1038/nri1106. [DOI] [PubMed] [Google Scholar]
- Cortini F, Fenoglio C, Guidi I, Venturelli E, Pomati S, Marcone A, Scalabrini D, Villa C, Clerici F, Dalla Valle E, Mariani C, Cappa S, Bresolin N, Scarpini E, Galimberti D. Novel exon 1 progranulin gene variant in Alzheimer’s disease. Eur J Neurol. 2008;15:1111–1117. doi: 10.1111/j.1468-1331.2008.02266.x. [DOI] [PubMed] [Google Scholar]
- Craig-Schapiro R, Fagan AM, Holtzman DM. Biomarkers of Alzheimer’s disease. Neurobiol Dis. 2009;35:128–140. doi: 10.1016/j.nbd.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruchaga C, Haller G, Chakraverty S, Mayo K, Vallania FL, Mitra RD, Faber K, Williamson J, Bird T, Diaz-Arrastia R, Foroud TM, Boeve BF, Graff-Radford NR, St Jean P, Lawson M, Ehm MG, Mayeux R, Goate AM. Rare variants in APP, PSEN1 and PSEN2 increase risk for AD in late-onset Alzheimer’s disease families. PLoS One. 2012a;7:e31039. doi: 10.1371/journal.pone.0031039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruchaga C, Karch CM, Jin SC, Benitez BA, Cai Y, Guerreiro R, Harari O, Norton J, Budde J, Bertelsen S, Jeng AT, Cooper B, Skorupa T, Carrell D, Levitch D, Hsu S, Choi J, Ryten M, Hardy J, Trabzuni D, Weale ME, Ramasamy A, Smith C, Sassi C, Bras J, Gibbs JR, Hernandez DG, Lupton MK, Powell J, Forabosco P, Ridge PG, Corcoran CD, Tschanz JT, Norton MC, Munger RG, Schmutz C, Leary M, Demirci FY, Bamne MN, Wang X, Lopez OL, Ganguli M, Medway C, Turton J, Lord J, Braae A, Barber I, Brown K, Passmore P, Craig D, Johnston J, McGuinness B, Todd S, Heun R, Kolsch H, Kehoe PG, Hooper NM, Vardy ER, Mann DM, Pickering-Brown S, Kalsheker N, Lowe J, Morgan K, David Smith A, Wilcock G, Warden D, Holmes C, Pastor P, Lorenzo-Betancor O, Brkanac Z, Scott E, Topol E, Rogaeva E, Singleton AB, Kamboh MI, St George-Hyslop P, Cairns N, Morris JC, Kauwe JS, Goate AM. Rare coding variants in the phospholipase D3 gene confer risk for Alzheimer’s disease. Nature. 2013a doi: 10.1038/nature12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruchaga C, Kauwe JS, Harari O, Jin SC, Cai Y, Karch CM, Benitez BA, Jeng AT, Skorupa T, Carrell D, Bertelsen S, Bailey M, McKean D, Shulman JM, De Jager PL, Chibnik L, Bennett DA, Arnold SE, Harold D, Sims R, Gerrish A, Williams J, Van Deerlin VM, Lee VM, Shaw LM, Trojanowski JQ, Haines JL, Mayeux R, Pericak-Vance MA, Farrer LA, Schellenberg GD, Peskind ER, Galasko D, Fagan AM, Holtzman DM, Morris JC, Goate AM. GWAS of cerebrospinal fluid tau levels identifies risk variants for Alzheimer’s disease. Neuron. 2013b;78:256–268. doi: 10.1016/j.neuron.2013.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruchaga C, Kauwe JS, Nowotny P, Bales K, Pickering EH, Mayo K, Bertelsen S, Hinrichs A, Fagan AM, Holtzman DM, Morris JC, Goate AM Alzheimer’s Disease Neuroimaging I. Cerebrospinal fluid APOE levels: an endophenotype for genetic studies for Alzheimer’s disease. Hum Mol Genet. 2012b;21:4558–4571. doi: 10.1093/hmg/dds296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruchaga C, Kauwe JSK, Mayo K, Spiegel N, Bertelsen S, Nowotny P, Shah AR, Abraham R, Hollingworth P, Harold D, Owen MM, Williams J, Lovestone S, Peskind ER, Li G, Leverenz JB, Galasko D, Morris JC, Fagan AM, Holtzman DM, Goate AM Initiative AsDN. SNPs associated with cerebrospinal fluid phospho-tau levels influence rate of decline in Alzheimer’s disease. PLoS Genet. 2010;6 doi: 10.1371/journal.pgen.1001101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuyvers E, Bettens K, Philtjens S, Van Langenhove T, Gijselinck I, van der Zee J, Engelborghs S, Vandenbulcke M, Van Dongen J, Geerts N, Maes G, Mattheijssens M, Peeters K, Cras P, Vandenberghe R, De Deyn PP, Van Broeckhoven C, Cruts M, Sleegers K consortium B. Investigating the role of rare heterozygous TREM2 variants in Alzheimer’s disease and frontotemporal dementia. Neurobiol Aging. 2014;35:726, e711–729. doi: 10.1016/j.neurobiolaging.2013.09.009. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Seeley WW, Zhou J, Shirer WR, Coppola G, Karydas A, Rosen HJ, Miller BL, Kramer JH, Greicius MD Alzheimer’s Disease Neuroimaging I. Gender modulates the APOE epsilon4 effect in healthy older adults: convergent evidence from functional brain connectivity and spinal fluid tau levels. J Neurosci. 2012;32:8254–8262. doi: 10.1523/JNEUROSCI.0305-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Silva R, Weiler M, Morris HR, Martin ER, Wood NW, Lees AJ. Strong association of a novel Tau promoter haplotype in progressive supranuclear palsy. Neurosci Lett. 2001;311:145–148. doi: 10.1016/s0304-3940(01)02109-7. [DOI] [PubMed] [Google Scholar]
- De Strooper B. Aph-1, Pen-2, and Nicastrin with Presenilin generate an active gamma-Secretase complex. Neuron. 2003;38:9–12. doi: 10.1016/s0896-6273(03)00205-8. [DOI] [PubMed] [Google Scholar]
- DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, Nicholson AM, Finch NA, Flynn H, Adamson J, Kouri N, Wojtas A, Sengdy P, Hsiung GY, Karydas A, Seeley WW, Josephs KA, Coppola G, Geschwind DH, Wszolek ZK, Feldman H, Knopman DS, Petersen RC, Miller BL, Dickson DW, Boylan KB, Graff-Radford NR, Rademakers R. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72:245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMattos RB, Cirrito JR, Parsadanian M, May PC, O’Dell MA, Taylor JW, Harmony JA, Aronow BJ, Bales KR, Paul SM, Holtzman DM. ApoE and clusterin cooperatively suppress Abeta levels and deposition: evidence that ApoE regulates extracellular Abeta metabolism in vivo. Neuron. 2004;41:193–202. doi: 10.1016/s0896-6273(03)00850-x. [DOI] [PubMed] [Google Scholar]
- Devi G, Fotiou A, Jyrinji D, Tycko B, DeArmand S, Rogaeva E, Song YQ, Medieros H, Liang Y, Orlacchio A, Williamson J, St George-Hyslop P, Mayeux R. Novel presenilin 1 mutations associated with early onset of dementia in a family with both early-onset and late-onset Alzheimer disease. Arch Neurol-Chicago. 2000;57:1454–1457. doi: 10.1001/archneur.57.10.1454. [DOI] [PubMed] [Google Scholar]
- Di Fede G, Catania M, Morbin M, Rossi G, Suardi S, Mazzoleni G, Merlin M, Giovagnoli AR, Prioni S, Erbetta A, Falcone C, Gobbi M, Colombo L, Bastone A, Beeg M, Manzoni C, Francescucci B, Spagnoli A, Cantu L, Del Favero E, Levy E, Salmona M, Tagliavini F. A recessive mutation in the APP gene with dominant-negative effect on amyloidogenesis. Science. 2009;323:1473–1477. doi: 10.1126/science.1168979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin ML, Olszowy MW, Holdorf AD, Li J, Bromley S, Desai N, Widder P, Rosenberger F, van der Merwe PA, Allen PM, Shaw AS. A novel adaptor protein orchestrates receptor patterning and cytoskeletal polarity in T-cell contacts. Cell. 1998;94:667–677. doi: 10.1016/s0092-8674(00)81608-6. [DOI] [PubMed] [Google Scholar]
- Elias-Sonnenschein LS, Helisalmi S, Natunen T, Hall A, Paajanen T, Herukka SK, Laitinen M, Remes AM, Koivisto AM, Mattila KM, Lehtimaki T, Verhey FR, Visser PJ, Soininen H, Hiltunen M. Genetic loci associated with Alzheimer’s disease and cerebrospinal fluid biomarkers in a Finnish case-control cohort. PLoS One. 2013;8:e59676. doi: 10.1371/journal.pone.0059676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erk S, Meyer-Lindenberg A, Opitz von Boberfeld C, Esslinger C, Schnell K, Kirsch P, Mattheisen M, Muhleisen TW, Cichon S, Witt SH, Rietschel M, Nothen MM, Walter H. Hippocampal function in healthy carriers of the CLU Alzheimer’s disease risk variant. J Neurosci. 2011;31:18180–18184. doi: 10.1523/JNEUROSCI.4960-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan AM, Watson M, Parsadanian M, Bales KR, Paul SM, Holtzman DM. Human and murine ApoE markedly alters A beta metabolism before and after plaque formation in a mouse model of Alzheimer’s disease. Neurobiol Dis. 2002;9:305–318. doi: 10.1006/nbdi.2002.0483. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Xiong C, Jasielec MS, Bateman RJ, Goate AM, Benzinger TL, Ghetti B, Martins RN, Masters CL, Mayeux R, Ringman JM, Rossor MN, Salloway S, Schofield PR, Sperling RA, Marcus D, Cairns NJ, Buckles VD, Ladenson JH, Morris JC, Holtzman DM Dominantly Inherited Alzheimer N. Longitudinal Change in CSF Biomarkers in Autosomal-Dominant Alzheimer’s Disease. Sci Transl Med. 2014;6:226ra230. doi: 10.1126/scitranslmed.3007901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forabosco P, Ramasamy A, Trabzuni D, Walker R, Smith C, Bras J, Levine AP, Hardy J, Pocock JM, Guerreiro R, Weale ME, Ryten M. Insights into TREM2 biology by network analysis of human brain gene expression data. Neurobiol Aging. 2013;34:2699–2714. doi: 10.1016/j.neurobiolaging.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furney SJ, Simmons A, Breen G, Pedroso I, Lunnon K, Proitsi P, Hodges A, Powell J, Wahlund LO, Kloszewska I, Mecocci P, Soininen H, Tsolaki M, Vellas B, Spenger C, Lathrop M, Shen L, Kim S, Saykin AJ, Weiner MW, Lovestone S, AddNeuroMed C Alzheimer’s Disease Neuroimaging I. Genome-wide association with MRI atrophy measures as a quantitative trait locus for Alzheimer’s disease. Mol Psychiatry. 2011;16:1130–1138. doi: 10.1038/mp.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griciuc A, Serrano-Pozo A, Parrado AR, Lesinski AN, Asselin CN, Mullin K, Hooli B, Choi SH, Hyman BT, Tanzi RE. Alzheimer’s disease risk gene CD33 inhibits microglial uptake of amyloid beta. Neuron. 2013;78:631–643. doi: 10.1016/j.neuron.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro R, Hardy J. TREM2 and neurodegenerative disease. N Engl J Med. 2013;369:1569–1570. doi: 10.1056/NEJMc1306509. [DOI] [PubMed] [Google Scholar]
- Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, Cruchaga C, Sassi C, Kauwe JS, Younkin S, Hazrati L, Collinge J, Pocock J, Lashley T, Williams J, Lambert JC, Amouyel P, Goate A, Rademakers R, Morgan K, Powell J, St George-Hyslop P, Singleton A, Hardy J. TREM2 variants in Alzheimer’s disease. N Engl J Med. 2013a;368:117–127. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, Cruchaga C, Sassi C, Kauwe JS, Younkin S, Hazrati L, Collinge J, Pocock J, Lashley T, Williams J, Lambert JC, Amouyel P, Goate A, Rademakers R, Morgan K, Powell J, St George-Hyslop P, Singleton A, Hardy J Alzheimer Genetic Analysis G. TREM2 variants in Alzheimer’s disease. N Engl J Med. 2013b;368:117–127. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro RJ, Baquero M, Blesa R, Boada M, Brás JM, Bullido MJ, Calado A, Crook R, Ferreira C, Frank A, Gómez-Isla T, Hernández I, Lleó A, Machado A, Martínez-Lage P, Masdeu J, Molina-Porcel L, Molinuevo JL, Pastor P, Pérez-Tur J, Relvas R, Oliveira CR, Ribeiro MH, Rogaeva E, Sa A, Samaranch L, Sánchez-Valle R, Santana I, Tàrraga L, Valdivieso F, Singleton A, Hardy J, Clarimón J. Genetic screening of Alzheimer’s disease genes in Iberian and African samples yields novel mutations in presenilins and APP. Neurobiol Aging. 2010;31:725–731. doi: 10.1016/j.neurobiolaging.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro RJ, Gustafson DR, Hardy J. The genetic architecture of Alzheimer’s disease: beyond APP, PSENs and APOE. Neurobiol Aging. 2012a;33:437–456. doi: 10.1016/j.neurobiolaging.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro RJ, Lohmann E, Bras JM, Gibbs JR, Rohrer JD, Gurunlian N, Dursun B, Bilgic B, Hanagasi H, Gurvit H, Emre M, Singleton A, Hardy J. Using Exome Sequencing to Reveal Mutations in TREM2 Presenting as a Frontotemporal Dementia-like Syndrome Without Bone Involvement. Arch Neurol. 2012b:1–7. doi: 10.1001/jamaneurol.2013.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro RJ, Lohmann E, Bras JM, Gibbs JR, Rohrer JD, Gurunlian N, Dursun B, Bilgic B, Hanagasi H, Gurvit H, Emre M, Singleton A, Hardy J. Using exome sequencing to reveal mutations in TREM2 presenting as a frontotemporal dementia-like syndrome without bone involvement. JAMA neurology. 2013c;70:78–84. doi: 10.1001/jamaneurol.2013.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han MR, Schellenberg GD, Wang LS Alzheimer’s Disease Neuroimaging I. Genome-wide association reveals genetic effects on human Abeta42 and tau protein levels in cerebrospinal fluids: a case control study. BMC neurology. 2010;10:90. doi: 10.1186/1471-2377-10-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Williams A, Jones N, Thomas C, Stretton A, Morgan AR, Lovestone S, Powell J, Proitsi P, Lupton MK, Brayne C, Rubinsztein DC, Gill M, Lawlor B, Lynch A, Morgan K, Brown KS, Passmore PA, Craig D, Mcguinness B, Todd S, Holmes C, Mann D, Smith AD, Love S, Kehoe PG, Hardy J, Mead S, Fox N, Rossor M, Collinge J, Maier W, Jessen F, Schürmann B, Van Den Bussche H, Heuser I, Kornhuber J, Wiltfang J, Dichgans M, Frölich L, Hampel H, Hüll M, Rujescu D, Goate AM, Kauwe JSK, Cruchaga C, Nowotny P, Morris JC, Mayo K, Sleegers K, Bettens K, Engelborghs S, De Deyn PP, Van Broeckhoven C, Livingston G, Bass NJ, Gurling H, Mcquillin A, Gwilliam R, Deloukas P, Al-Chalabi A, Shaw CE, Tsolaki M, Singleton AB, Guerreiro R, Mühleisen TW, Nöthen MM, Moebus S, Jöckel KH, Klopp N, Wichmann HE, Carrasquillo MM, Pankratz VS, Younkin SG, Holmans PA, O’donovan M, Owen MJ, Williams J. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet. 2009;41:1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingworth P, Harold D, Sims R, Gerrish A, Lambert JC, Carrasquillo MM, Abraham R, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Jones N, Stretton A, Thomas C, Richards A, Ivanov D, Widdowson C, Chapman J, Lovestone S, Powell J, Proitsi P, Lupton MK, Brayne C, Rubinsztein DC, Gill M, Lawlor B, Lynch A, Brown KS, Passmore PA, Craig D, McGuinness B, Todd S, Holmes C, Mann D, Smith AD, Beaumont H, Warden D, Wilcock G, Love S, Kehoe PG, Hooper NM, Vardy ERLC, Hardy J, Mead S, Fox NC, Rossor M, Collinge J, Maier W, Jessen F, Rüther E, Schürmann B, Heun R, Kölsch H, Van Den Bussche H, Heuser I, Kornhuber J, Wiltfang J, Dichgans M, Frölich L, Hampel H, Gallacher J, Hüll M, Rujescu D, Giegling I, Goate AM, Kauwe JSK, Cruchaga C, Nowotny P, Morris JC, Mayo K, Sleegers K, Bettens K, Engelborghs S, De Deyn PP, Van Broeckhoven C, Livingston G, Bass NJ, Gurling H, Mcquillin A, Gwilliam R, Deloukas P, Al-Chalabi A, Shaw CE, Tsolaki M, Singleton AB, Guerreiro R, Mühleisen TW, Nöthen MM, Moebus S, Jöckel KH, Klopp N, Wichmann HE, Pankratz VS, Sando SB, Aasly JO, Barcikowska M, Wszolek ZK, Dickson DW, Graff-Radford NR, Petersen RC, van Duijn CM, Breteler MMB, Ikram MA, Destefano AL, Fitzpatrick AL, Lopez O, Launer LJ, Seshadri S, consortium C, Berr C, Campion D, Epelbaum J, Dartigues JF, Tzourio C, Alpérovitch A, Lathrop M, consortium E, Feulner TM, Friedrich P, Riehle C, Krawczak M, Schreiber S, Mayhaus M, Nicolhaus S, Wagenpfeil S, Steinberg S, Stefansson H, Stefansson K, Snædal J, Björnsson S, Jonsson PV, Chouraki V, Genier-Boley B, Hiltunen M, Soininen H, Combarros O, Zelenika D, Delepine M, Bullido MJ, Pasquier F, Mateo I, Frank-Garcia A, Porcellini E, Hanon O, Coto E, Alvarez V, Bosco P, Siciliano G, Mancuso M, Panza F, Solfrizzi V, Nacmias B, Sorbi S, Bossù P, Piccardi P, Arosio B, Annoni G, Seripa D, Pilotto A, Scarpini E, Galimberti D, Brice A, Hannequin D, Licastro F, Jones L, Holmans PA, Jonsson T, Riemenschneider M, Morgan K, Younkin SG, Owen MJ, O’donovan M, Amouyel P, Williams J Initiative t.A.s.D.N. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat Genet. 2011;43:429–435. doi: 10.1038/ng.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman DM, Bales KR, Tenkova T, Fagan AM, Parsadanian M, Sartorius LJ, Mackey B, Olney J, McKeel D, Wozniak D, Paul SM. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2000;97:2892–2897. doi: 10.1073/pnas.050004797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman DM, Herz J, Bu G. Apolipoprotein E and apolipoprotein E receptors: normal biology and roles in Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:a006312. doi: 10.1101/cshperspect.a006312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman DM, Morris JC, Goate AM. Alzheimer’s disease: the challenge of the second century. Sci Transl Med. 2011;3:77sr71. doi: 10.1126/scitranslmed.3002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong MG, Reynolds CA, Feldman AL, Kallin M, Lambert JC, Amouyel P, Ingelsson E, Pedersen NL, Prince JA. Genome-wide and gene-based association implicates FRMD6 in Alzheimer disease. Human mutation. 2012;33:521–529. doi: 10.1002/humu.22009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruska KS, LaMarca ME, Scott CR, Sidransky E. Gaucher disease: mutation and polymorphism spectrum in the glucocerebrosidase gene (GBA) Human mutation. 2008;29:567–583. doi: 10.1002/humu.20676. [DOI] [PubMed] [Google Scholar]
- Huey ED, Grafman J, Wassermann EM, Pietrini P, Tierney MC, Ghetti B, Spina S, Baker M, Hutton M, Elder JW, Berger SL, Heflin KA, Hardy J, Momeni P. Characteristics of frontotemporal dementia patients with a Progranulin mutation. Ann Neurol. 2006;60:374–380. doi: 10.1002/ana.20969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagust WJ, Landau SM Alzheimer’s Disease Neuroimaging I. Apolipoprotein E, not fibrillar beta-amyloid, reduces cerebral glucose metabolism in normal aging. J Neurosci. 2012;32:18227–18233. doi: 10.1523/JNEUROSCI.3266-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayadev S, Leverenz JB, Steinbart E, Stahl J, Klunk W, Yu CE, Bird TD. Alzheimer’s disease phenotypes and genotypes associated with mutations in presenilin 2. Brain : a journal of neurology. 2010;133:1143–1154. doi: 10.1093/brain/awq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SC, Pastor P, Cooper B, Cervantes S, Benitez BA, Razquin C, Goate A, Cruchaga C. Pooled-DNA sequencing identifies novel causative variants in PSEN1, GRN and MAPT in a clinical early-onset and familial Alzheimer’s disease Ibero-American cohort. Alzheimers Res Ther. 2012;4:34. doi: 10.1186/alzrt137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L, Holmans PA, Hamshere ML, Harold D, Moskvina V, Ivanov D, Pocklington A, Abraham R, Hollingworth P, Sims R, Gerrish A, Pahwa JS, Jones N, Stretton A, Morgan AR, Lovestone S, Powell J, Proitsi P, Lupton MK, Brayne C, Rubinsztein DC, Gill M, Lawlor B, Lynch A, Morgan K, Brown KS, Passmore PA, Craig D, McGuinness B, Todd S, Holmes C, Mann D, Smith AD, Love S, Kehoe PG, Mead S, Fox N, Rossor M, Collinge J, Maier W, Jessen F, Schürmann B, Van Den Bussche H, Heuser I, Peters O, Kornhuber J, Wiltfang J, Dichgans M, Frölich L, Hampel H, Hüll M, Rujescu D, Goate AM, Kauwe JSK, Cruchaga C, Nowotny P, Morris JC, Mayo K, Livingston G, Bass NJ, Gurling H, Mcquillin A, Gwilliam R, Deloukas P, Al-Chalabi A, Shaw CE, Singleton AB, Guerreiro R, Mühleisen TW, Nöthen MM, Moebus S, Jöckel KH, Klopp N, Wichmann HE, Rüther E, Carrasquillo MM, Pankratz VS, Younkin SG, Hardy J, O’Donovan MC, Owen MJ, Williams J. Genetic evidence implicates the immune system and cholesterol metabolism in the aetiology of Alzheimer’s disease. PLoS One. 2010;5:e13950. doi: 10.1371/journal.pone.0013950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson T, Atwal JK, Steinberg S, Snaedal J, Jonsson PV, Bjornsson S, Stefansson H, Sulem P, Gudbjartsson D, Maloney J, Hoyte K, Gustafson A, Liu Y, Lu Y, Bhangale T, Graham RR, Huttenlocher J, Bjornsdottir G, Andreassen OA, Jonsson EG, Palotie A, Behrens TW, Magnusson OT, Kong A, Thorsteinsdottir U, Watts RJ, Stefansson K. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature. 2012;488:96–99. doi: 10.1038/nature11283. [DOI] [PubMed] [Google Scholar]
- Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, Bjornsson S, Huttenlocher J, Levey AI, Lah JJ, Rujescu D, Hampel H, Giegling I, Andreassen OA, Engedal K, Ulstein I, Djurovic S, Ibrahim-Verbaas C, Hofman A, Ikram MA, van Duijn CM, Thorsteinsdottir U, Kong A, Stefansson K. Variant of TREM2 associated with the risk of Alzheimer’s disease. N Engl J Med. 2013a;368:107–116. doi: 10.1056/NEJMoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, Bjornsson S, Huttenlocher J, Levey AI, Lah JJ, Rujescu D, Hampel H, Giegling I, Andreassen OA, Engedal K, Ulstein I, Djurovic S, Ibrahim-Verbaas C, Hofman A, Ikram MA, van Duijn CM, Thorsteinsdottir U, Kong A, Stefansson K. Variant of TREM2 associated with the risk of Alzheimer’s disease. N Engl J Med. 2013b;368:107–116. doi: 10.1056/NEJMoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorissen E, Prox J, Bernreuther C, Weber S, Schwanbeck R, Serneels L, Snellinx A, Craessaerts K, Thathiah A, Tesseur I, Bartsch U, Weskamp G, Blobel CP, Glatzel M, De Strooper B, Saftig P. The disintegrin/metalloproteinase ADAM10 is essential for the establishment of the brain cortex. J Neurosci. 2010;30:4833–4844. doi: 10.1523/JNEUROSCI.5221-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamboh MI, Aston CE, Perez-Tur J, Kokmen E, Ferrell RE, Hardy J, DeKosky ST. A novel mutation in the apolipoprotein E gene (APOE*4 Pittsburgh) is associated with the risk of late-onset Alzheimer’s disease. Neurosci Lett. 1999;263:129–132. doi: 10.1016/s0304-3940(99)00129-9. [DOI] [PubMed] [Google Scholar]
- Karch CM, Jeng AT, Goate AM. Calcium phosphatase calcineurin influences tau metabolism. Neurobiol Aging. 2012a doi: 10.1016/j.neurobiolaging.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch CM, Jeng AT, Goate AM. Extracellular tau levels are influenced by variability in tau that is associated with tauopathies. J Biol Chem. 2012b doi: 10.1074/jbc.M112.380642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauwe JS, Jacquart S, Chakraverty S, Wang J, Mayo K, Fagan AM, Holtzman DM, Morris JC, Goate AM. Extreme cerebrospinal fluid amyloid beta levels identify family with late-onset Alzheimer’s disease presenilin 1 mutation. Ann Neurol. 2007;61:446–453. doi: 10.1002/ana.21099. [DOI] [PubMed] [Google Scholar]
- Kauwe JSK, Cruchaga C, Karch CM, Sadler B, Lee M, Mayo K, Latu W, Su’a M, Fagan AM, Holtzman DM, Morris JC, Goate AM Initiative A.s.D.N. Fine mapping of genetic variants in BIN1, CLU, CR1 and PICALM for association with cerebrospinal fluid biomarkers for Alzheimer’s disease. PLoS One. 2011;6:e15918. doi: 10.1371/journal.pone.0015918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauwe JSK, Cruchaga C, Mayo K, Fenoglio C, Bertelsen S, Nowotny P, Galimberti D, Scarpini E, Morris JC, Fagan AM, Holtzman DM, Goate AM. Variation in MAPT is associated with cerebrospinal fluid tau levels in the presence of amyloid-beta deposition. Proceedings of the National Academy of Sciences. 2008;105:8050–8054. doi: 10.1073/pnas.0801227105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauwe JSK, Wang J, Mayo K, Morris JC, Fagan AM, Holtzman DM, Goate AM. Alzheimer’s disease risk variants show association with cerebrospinal fluid amyloid beta. Neurogenetics. 2009;10:13–17. doi: 10.1007/s10048-008-0150-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan BT, Shulman JM, Chibnik LB, Raj T, Tran D, Sabuncu MR, Allen AN, Corneveaux JJ, Hardy JA, Huentelman MJ, Lemere CA, Myers AJ, Nicholson-Weller A, Reiman EM, Evans DA, Bennett DA, De Jager PL Alzheimer’s Disease Neuroimaging I. A coding variant in CR1 interacts with APOE-epsilon4 to influence cognitive decline. Hum Mol Genet. 2012;21:2377–2388. doi: 10.1093/hmg/dds054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley BJ, Haidar W, Boeve BF, Baker M, Shiung M, Knopman DS, Rademakers R, Hutton M, Adamson J, Kuntz KM, Dickson DW, Parisi JE, Smith GE, Petersen RC. Alzheimer disease-like phenotype associated with the c.154delA mutation in progranulin. Arch Neurol. 2010;67:171–177. doi: 10.1001/archneurol.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer’s disease. Neuron. 2009a;63:287–303. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Jiang H, Park S, Eltorai AE, Stewart FR, Yoon H, Basak JM, Finn MB, Holtzman DM. Haploinsufficiency of human APOE reduces amyloid deposition in a mouse model of amyloid-beta amyloidosis. J Neurosci. 2011a;31:18007–18012. doi: 10.1523/JNEUROSCI.3773-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Suh J, Romano D, Truong MH, Mullin K, Hooli B, Norton D, Tesco G, Elliott K, Wagner SL, Moir RD, Becker KD, Tanzi RE. Potential late-onset Alzheimer’s disease-associated mutations in the ADAM10 gene attenuate {alpha}-secretase activity. Hum Mol Genet. 2009b;18:3987–3996. doi: 10.1093/hmg/ddp323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Swaminathan S, Shen L, Risacher SL, Nho K, Foroud T, Shaw LM, Trojanowski JQ, Potkin SG, Huentelman MJ, Craig DW, DeChairo BM, Aisen PS, Petersen RC, Weiner MW, Saykin AJ Alzheimer’s Disease Neuroimaging I. Genome-wide association study of CSF biomarkers Abeta1–42, t-tau, and p-tau181p in the ADNI cohort. Neurology. 2011b;76:69–79. doi: 10.1212/WNL.0b013e318204a397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WS, Li H, Ruberu K, Chan S, Elliott DA, Low JK, Cheng D, Karl T, Garner B. Deletion of Abca7 increases cerebral amyloid-beta accumulation in the J20 mouse model of Alzheimer’s disease. J Neurosci. 2013;33:4387–4394. doi: 10.1523/JNEUROSCI.4165-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koten JW, Jr, Wood G, Hagoort P, Goebel R, Propping P, Willmes K, Boomsma DI. Genetic contribution to variation in cognitive function: an FMRI study in twins. Science. 2009;323:1737–1740. doi: 10.1126/science.1167371. [DOI] [PubMed] [Google Scholar]
- Kovacs DM, Fausett HJ, Page KJ, Kim TW, Moir RD, Merriam DE, Hollister RD, Hallmark OG, Mancini R, Felsenstein KM, Hyman BT, Tanzi RE, Wasco W. Alzheimer-associated presenilins 1 and 2: neuronal expression in brain and localization to intracellular membranes in mammalian cells. Nat Med. 1996;2:224–229. doi: 10.1038/nm0296-224. [DOI] [PubMed] [Google Scholar]
- Kuhn PH, Wang H, Dislich B, Colombo A, Zeitschel U, Ellwart JW, Kremmer E, Rossner S, Lichtenthaler SF. ADAM10 is the physiologically relevant, constitutive alpha-secretase of the amyloid precursor protein in primary neurons. Embo J. 2010;29:3020–3032. doi: 10.1038/emboj.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai KO, Ip NY. Synapse development and plasticity: roles of ephrin/Eph receptor signaling. Curr Opin Neurobiol. 2009;19:275–283. doi: 10.1016/j.conb.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Lambert JC, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, Combarros O, Zelenika D, Bullido MJ, Tavernier B, Letenneur L, Bettens K, Berr C, Pasquier F, Fiévet N, Barberger-Gateau P, Engelborghs S, De Deyn P, Mateo I, Franck A, Helisalmi S, Porcellini E, Hanon O, De Pancorbo MM, Lendon C, Dufouil C, Jaillard C, Leveillard T, Alvarez V, Bosco P, Mancuso M, Panza F, Nacmias B, Bossù P, Piccardi P, Annoni G, Seripa D, Galimberti D, Hannequin D, Licastro F, Soininen H, Ritchie K, Blanché H, Dartigues JF, Tzourio C, Gut I, Van Broeckhoven C, Alpérovitch A, Lathrop M, Amouyel P Investigators E.A.s.D.I. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat Genet. 2009;41:1094–1099. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, Jun G, Destefano AL, Bis JC, Beecham GW, Grenier-Boley B, Russo G, Thornton-Wells TA, Jones N, Smith AV, Chouraki V, Thomas C, Ikram MA, Zelenika D, Vardarajan BN, Kamatani Y, Lin CF, Gerrish A, Schmidt H, Kunkle B, Dunstan ML, Ruiz A, Bihoreau MT, Choi SH, Reitz C, Pasquier F, Hollingworth P, Ramirez A, Hanon O, Fitzpatrick AL, Buxbaum JD, Campion D, Crane PK, Baldwin C, Becker T, Gudnason V, Cruchaga C, Craig D, Amin N, Berr C, Lopez OL, De Jager PL, Deramecourt V, Johnston JA, Evans D, Lovestone S, Letenneur L, Moron FJ, Rubinsztein DC, Eiriksdottir G, Sleegers K, Goate AM, Fievet N, Huentelman MJ, Gill M, Brown K, Kamboh MI, Keller L, Barberger-Gateau P, McGuinness B, Larson EB, Green R, Myers AJ, Dufouil C, Todd S, Wallon D, Love S, Rogaeva E, Gallacher J, St George-Hyslop P, Clarimon J, Lleo A, Bayer A, Tsuang DW, Yu L, Tsolaki M, Bossu P, Spalletta G, Proitsi P, Collinge J, Sorbi S, Sanchez-Garcia F, Fox NC, Hardy J, Naranjo MC, Bosco P, Clarke R, Brayne C, Galimberti D, Mancuso M, Matthews F, Moebus S, Mecocci P, Del Zompo M, Maier W, Hampel H, Pilotto A, Bullido M, Panza F, Caffarra P, Nacmias B, Gilbert JR, Mayhaus M, Lannfelt L, Hakonarson H, Pichler S, Carrasquillo MM, Ingelsson M, Beekly D, Alvarez V, Zou F, Valladares O, Younkin SG, Coto E, Hamilton-Nelson KL, Gu W, Razquin C, Pastor P, Mateo I, Owen MJ, Faber KM, Jonsson PV, Combarros O, O’Donovan MC, Cantwell LB, Soininen H, Blacker D, Mead S, Mosley TH, Jr, Bennett DA, Harris TB, Fratiglioni L, Holmes C, de Bruijn RF, Passmore P, Montine TJ, Bettens K, Rotter JI, Brice A, Morgan K, Foroud TM, Kukull WA, Hannequin D, Powell JF, Nalls MA, Ritchie K, Lunetta KL, Kauwe JS, Boerwinkle E, Riemenschneider M, Boada M, Hiltunen M, Martin ER, Schmidt R, Rujescu D, Wang LS, Dartigues JF, Mayeux R, Tzourio C, Hofman A, Nothen MM, Graff C, Psaty BM, Jones L, Haines JL, Holmans PA, Lathrop M, Pericak-Vance MA, Launer LJ, Farrer LA, van Duijn CM, Van Broeckhoven C, Moskvina V, Seshadri S, Williams J, Schellenberg GD, Amouyel P. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet. 2013 doi: 10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larner AJ, Ray PS, Doran M. The R269H mutation in presenilin-1 presenting as late-onset autosomal dominant Alzheimer’s disease. J Neurol Sci. 2007;252:173–176. doi: 10.1016/j.jns.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Lattante S, Le Ber I, Camuzat A, Dayan S, Godard C, Van Bortel I, De Septenville A, Ciura S, Brice A, Kabashi E French Research Network on FTD and Ftd ALS. TREM2 mutations are rare in a French cohort of patients with frontotemporal dementia. Neurobiol Aging. 2013;34:2443 e2441–2442. doi: 10.1016/j.neurobiolaging.2013.04.030. [DOI] [PubMed] [Google Scholar]
- Laws SM, Friedrich P, Diehl-Schmid J, Muller J, Eisele T, Bauml J, Forstl H, Kurz A, Riemenschneider M. Fine mapping of the MAPT locus using quantitative trait analysis identifies possible causal variants in Alzheimer’s disease. Mol Psychiatry. 2007;12:510–517. doi: 10.1038/sj.mp.4001935. [DOI] [PubMed] [Google Scholar]
- Lindquist SG, Holm IE, Schwartz M, Law I, Stokholm J, Batbayli M, Waldemar G, Nielsen JE. Alzheimer disease-like clinical phenotype in a family with FTDP-17 caused by a MAPT R406W mutation. European journal of neurology : the official journal of the European Federation of Neurological Societies. 2008;15:377–385. doi: 10.1111/j.1468-1331.2008.02069.x. [DOI] [PubMed] [Google Scholar]
- Lindquist SG, Schwartz M, Batbayli M, Waldemar G, Nielsen JE. Genetic testing in familial AD and FTD: mutation and phenotype spectrum in a Danish cohort. Clin Genet. 2009;76:205–209. doi: 10.1111/j.1399-0004.2009.01191.x. [DOI] [PubMed] [Google Scholar]
- Liu D, Niu ZX. The structure, genetic polymorphisms, expression and biological functions of complement receptor type 1 (CR1/CD35) Immunopharmacol Immunotoxicol. 2009;31:524–535. doi: 10.3109/08923970902845768. [DOI] [PubMed] [Google Scholar]
- Liu G, Yao L, Liu J, Jiang Y, Ma G, Chen Z, Zhao B, Li K Genetic Environmental Risk for Alzheimer’s disease C. Cardiovascular disease contributes to Alzheimer’s disease: evidence from large-scale genome-wide association studies. Neurobiol Aging. 2014;35:786–792. doi: 10.1016/j.neurobiolaging.2013.10.084. [DOI] [PubMed] [Google Scholar]
- Lu PH, Thompson PM, Leow A, Lee GJ, Lee A, Yanovsky I, Parikshak N, Khoo T, Wu S, Geschwind D, Bartzokis G. Apolipoprotein E genotype is associated with temporal and hippocampal atrophy rates in healthy elderly adults: a tensor-based morphometry study. J Alzheimers Dis. 2011;23:433–442. doi: 10.3233/JAD-2010-101398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludolph AC, Kassubek J, Landwehrmeyer BG, Mandelkow E, Mandelkow EM, Burn DJ, Caparros-Lefebvre D, Frey KA, de Yebenes JG, Gasser T, Heutink P, Hoglinger G, Jamrozik Z, Jellinger KA, Kazantsev A, Kretzschmar H, Lang AE, Litvan I, Lucas JJ, McGeer PL, Melquist S, Oertel W, Otto M, Paviour D, Reum T, Saint-Raymond A, Steele JC, Tolnay M, Tumani H, van Swieten JC, Vanier MT, Vonsattel JP, Wagner S, Wszolek ZK. Tauopathies with parkinsonism: clinical spectrum, neuropathologic basis, biological markers, and treatment options. Eur J Neurol. 2009;16:297–309. doi: 10.1111/j.1468-1331.2008.02513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- Mahley RW, Huang Y. Apolipoprotein e sets the stage: response to injury triggers neuropathology. Neuron. 2012;76:871–885. doi: 10.1016/j.neuron.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley RW, Rall SC., Jr Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genomics Hum Genet. 2000;1:507–537. doi: 10.1146/annurev.genom.1.1.507. [DOI] [PubMed] [Google Scholar]
- Mattsson N, Andreasson U, Persson S, Carrillo MC, Collins S, Chalbot S, Cutler N, Dufour-Rainfray D, Fagan AM, Heegaard NH, Robin Hsiung GY, Hyman B, Iqbal K, Lachno DR, Lleo A, Lewczuk P, Molinuevo JL, Parchi P, Regeniter A, Rissman R, Rosenmann H, Sancesario G, Schroder J, Shaw LM, Teunissen CE, Trojanowski JQ, Vanderstichele H, Vandijck M, Verbeek MM, Zetterberg H, Blennow K, Kaser SA Alzheimer’s Association QCPWG. CSF biomarker variability in the Alzheimer’s Association quality control program. Alzheimers Dement. 2013;9:251–261. doi: 10.1016/j.jalz.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon HT, Wigge P, Smith C. Clathrin interacts specifically with amphiphysin and is displaced by dynamin. FEBS Lett. 1997;413:319–322. doi: 10.1016/s0014-5793(97)00928-9. [DOI] [PubMed] [Google Scholar]
- Mecozzi VJ, Berman DE, Simoes S, Vetanovetz C, Awal MR, Patel VM, Schneider RT, Petsko GA, Ringe D, Small SA. Pharmacological chaperones stabilize retromer to limit APP processing. Nature chemical biology. 2014 doi: 10.1038/nchembio.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medway CW, Abdul-Hay S, Mims T, Ma L, Bisceglio G, Zou F, Pankratz S, Sando SB, Aasly JO, Barcikowska M, Siuda J, Wszolek ZK, Ross OA, Carrasquillo M, Dickson DW, Graff-Radford N, Petersen RC, Ertekin-Taner N, Morgan K, Bu G, Younkin SG. ApoE variant p.V236E is associated with markedly reduced risk of Alzheimer’s disease. Mol Neurodegener. 2014;9:11. doi: 10.1186/1750-1326-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita A, Koike A, Jun G, Wang LS, Takahashi S, Matsubara E, Kawarabayashi T, Shoji M, Tomita N, Arai H, Asada T, Harigaya Y, Ikeda M, Amari M, Hanyu H, Higuchi S, Ikeuchi T, Nishizawa M, Suga M, Kawase Y, Akatsu H, Kosaka K, Yamamoto T, Imagawa M, Hamaguchi T, Yamada M, Moriaha T, Takeda M, Takao T, Nakata K, Fujisawa Y, Sasaki K, Watanabe K, Nakashima K, Urakami K, Ooya T, Takahashi M, Yuzuriha T, Serikawa K, Yoshimoto S, Nakagawa R, Kim JW, Ki CS, Won HH, Na DL, Seo SW, Mook-Jung I, St George-Hyslop P, Mayeux R, Haines JL, Pericak-Vance MA, Yoshida M, Nishida N, Tokunaga K, Yamamoto K, Tsuji S, Kanazawa I, Ihara Y, Schellenberg GD, Farrer LA, Kuwano R Alzheimer Disease Genetics C. SORL1 is genetically associated with late-onset Alzheimer’s disease in Japanese, Koreans and Caucasians. PLoS One. 2013;8:e58618. doi: 10.1371/journal.pone.0058618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momeni P, Pittman A, Lashley T, Vandrovcova J, Malzer E, Luk C, Hulette C, Lees A, Revesz T, Hardy J, de Silva R. Clinical and pathological features of an Alzheimer’s disease patient with the MAPT Delta K280 mutation. Neurobiology of aging. 2009;30:388–393. doi: 10.1016/j.neurobiolaging.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskvina V, Harold D, Russo G, Vedernikov A, Sharma M, Saad M, Holmans P, Bras JM, Bettella F, Keller MF, Nicolaou N, Simon-Sanchez J, Gibbs JR, Schulte C, Durr A, Guerreiro R, Hernandez D, Brice A, Stefansson H, Majamaa K, Gasser T, Heutink P, Wood N, Martinez M, Singleton AB, Nalls MA, Hardy J, Owen MJ, O’Donovan MC, Williams J, Morris HR, Williams NM Ipdgc, Investigators G. Analysis of genome-wide association studies of Alzheimer disease and of Parkinson disease to determine if these 2 diseases share a common genetic risk. JAMA Neurol. 2013;70:1268–1276. doi: 10.1001/jamaneurol.2013.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder KL, Snider BJ, Mills SL, Buckles VD, Santacruz AM, Bateman RJ, Morris JC. Dominantly Inherited Alzheimer Network: facilitating research and clinical trials. Alzheimers Res Ther. 2013;5:48. doi: 10.1186/alzrt213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullan M, Crawford F, Axelman K, Houlden H, Lilius L, Winblad B, Lannfelt L. A pathogenic mutation for probable Alzheimer’s disease in the APP gene at the N-terminus of beta-amyloid. Nat Genet. 1992;1:345–347. doi: 10.1038/ng0892-345. [DOI] [PubMed] [Google Scholar]
- Naj AC, Jun G, Beecham GW, Wang LS, Vardarajan BN, Buros J, Gallins PJ, Buxbaum JD, Jarvik GP, Crane PK, Larson EB, Bird TD, Boeve BF, Graff-Radford NR, De Jager PL, Evans D, Schneider JA, Carrasquillo MM, Ertekin-Taner N, Younkin SG, Cruchaga C, Kauwe JSK, Nowotny P, Kramer P, Hardy J, Huentelman MJ, Myers AJ, Barmada MM, Demirci FY, Baldwin CT, Green RC, Rogaeva E, George-Hyslop PS, Arnold SE, Barber R, Beach T, Bigio EH, Bowen JD, Boxer A, Burke JR, Cairns NJ, Carlson CS, Carney RM, Carroll SL, Chui HC, Clark DG, Corneveaux J, Cotman CW, Cummings JL, Decarli C, Dekosky ST, Diaz-Arrastia R, Dick M, Dickson DW, Ellis WG, Faber KM, Fallon KB, Farlow MR, Ferris S, Frosch MP, Galasko DR, Ganguli M, Gearing M, Geschwind DH, Ghetti B, Gilbert JR, Gilman S, Giordani B, Glass JD, Growdon JH, Hamilton RL, Harrell LE, Head E, Honig LS, Hulette CM, Hyman BT, Jicha GA, Jin LW, Johnson N, Karlawish J, Karydas A, Kaye JA, Kim R, Koo EH, Kowall NW, Lah JJ, Levey AI, Lieberman AP, Lopez OL, Mack WJ, Marson DC, Martiniuk F, Mash DC, Masliah E, McCormick WC, McCurry SM, McDavid AN, McKee AC, Mesulam M, Miller BL, Miller CA, Miller JW, Parisi JE, Perl DP, Peskind E, Petersen RC, Poon WW, Quinn JF, Rajbhandary RA, Raskind M, Reisberg B, Ringman JM, Roberson ED, Rosenberg RN, Sano M, Schneider LS, Seeley W, Shelanski ML, Slifer MA, Smith CD, Sonnen JA, Spina S, Stern RA, Tanzi RE, Trojanowski JQ, Troncoso JC, Van Deerlin VM, Vinters HV, Vonsattel JP, Weintraub S, Welsh-Bohmer KA, Williamson J, Woltjer RL, Cantwell LB, Dombroski BA, Beekly D, Lunetta KL, Martin ER, Kamboh MI, Saykin AJ, Reiman EM, Bennett DA, Morris JC, Montine TJ, Goate AM, Blacker D, Tsuang DW, Hakonarson H, Kukull WA, Foroud TM, Haines JL, Mayeux R, Pericak-Vance MA, Farrer LA, Schellenberg GD. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nat Genet. 2011;43:436–441. doi: 10.1038/ng.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsberth C, Westlind-Danielsson A, Eckman CB, Condron MM, Axelman K, Forsell C, Stenh C, Luthman J, Teplow DB, Younkin SG, Naslund J, Lannfelt L. The ‘Arctic’ APP mutation (E693G) causes Alzheimer’s disease by enhanced Abeta protofibril formation. Nat Neurosci. 2001;4:887–893. doi: 10.1038/nn0901-887. [DOI] [PubMed] [Google Scholar]
- Ostojic J, Elfgren C, Passant U, Nilsson K, Gustafson L, Lannfelt L, Froelich Fabre S. The tau R406W mutation causes progressive presenile dementia with bitemporal atrophy. Dement Geriatr Cogn Disord. 2004;17:298–301. doi: 10.1159/000077158. [DOI] [PubMed] [Google Scholar]
- Paloneva J, Mandelin J, Kiialainen A, Bohling T, Prudlo J, Hakola P, Haltia M, Konttinen YT, Peltonen L. DAP12/TREM2 deficiency results in impaired osteoclast differentiation and osteoporotic features. J Exp Med. 2003;198:669–675. doi: 10.1084/jem.20030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson D, Munger C, Crowley J, Corcoran C, Cruchaga C, Goate AM, Norton MC, Green RC, Munger RG, Breitner JC, Welsh-Bohmer KA, Lyketsos C, Tschanz J, Kauwe JS Alzheimer’s Disease Neuroimaging I. Variants in PPP3R1 and MAPT are associated with more rapid functional decline in Alzheimer’s disease: The Cache County Dementia Progression Study. Alzheimers Dement. 2013 doi: 10.1016/j.jalz.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooler AM, Phillips EC, Lau DH, Noble W, Hanger DP. Physiological release of endogenous tau is stimulated by neuronal activity. EMBO reports. 2013;14:389–394. doi: 10.1038/embor.2013.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postina R, Schroeder A, Dewachter I, Bohl J, Schmitt U, Kojro E, Prinzen C, Endres K, Hiemke C, Blessing M, Flamez P, Dequenne A, Godaux E, van Leuven F, Fahrenholz F. A disintegrin-metalloproteinase prevents amyloid plaque formation and hippocampal defects in an Alzheimer disease mouse model. J Clin Invest. 2004;113:1456–1464. doi: 10.1172/JCI20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potkin SG, Guffanti G, Lakatos A, Turner JA, Kruggel F, Fallon JH, Saykin AJ, Orro A, Lupoli S, Salvi E, Weiner M, Macciardi F Alzheimer’s Disease Neuroimaging I. Hippocampal atrophy as a quantitative trait in a genome-wide association study identifying novel susceptibility genes for Alzheimer’s disease. PLoS One. 2009;4:e6501. doi: 10.1371/journal.pone.0006501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter R, Patterson BW, Elbert DL, Ovod V, Kasten T, Sigurdson W, Mawuenyega K, Blazey T, Goate A, Chott R, Yarasheski KE, Holtzman DM, Morris JC, Benzinger TL, Bateman RJ. Increased in vivo amyloid-beta42 production, exchange, and loss in presenilin mutation carriers. Sci Transl Med. 2013;5:189ra177. doi: 10.1126/scitranslmed.3005615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademakers R, Baker M, Gass J, Adamson J, Huey ED, Momeni P, Spina S, Coppola G, Karydas AM, Stewart H, Johnson N, Hsiung GY, Kelley B, Kuntz K, Steinbart E, Wood EM, Yu CE, Josephs K, Sorenson E, Womack KB, Weintraub S, Pickering-Brown SM, Schofield PR, Brooks WS, Van Deerlin VM, Snowden J, Clark CM, Kertesz A, Boylan K, Ghetti B, Neary D, Schellenberg GD, Beach TG, Mesulam M, Mann D, Grafman J, Mackenzie IR, Feldman H, Bird T, Petersen R, Knopman D, Boeve B, Geschwind DH, Miller B, Wszolek Z, Lippa C, Bigio EH, Dickson D, Graff-Radford N, Hutton M. Phenotypic variability associated with progranulin haploinsufficiency in patients with the common 1477C-->T (Arg493X) mutation: an international initiative. Lancet Neurol. 2007;6:857–868. doi: 10.1016/S1474-4422(07)70221-1. [DOI] [PubMed] [Google Scholar]
- Rademakers R, Dermaut B, Peeters K, Cruts M, Heutink P, Goate A, Van Broeckhoven C. Tau (MAPT) mutation Arg406Trp presenting clinically with Alzheimer disease does not share a common founder in Western Europe. Human mutation. 2003;22:409–411. doi: 10.1002/humu.10269. [DOI] [PubMed] [Google Scholar]
- Rajagopalan P, Hibar DP, Thompson PM. TREM2 and neurodegenerative disease. N Engl J Med. 2013;369:1565–1567. doi: 10.1056/NEJMc1306509#SA3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanan VK, Risacher SL, Nho K, Kim S, Swaminathan S, Shen L, Foroud TM, Hakonarson H, Huentelman MJ, Aisen PS, Petersen RC, Green RC, Jack CR, Koeppe RA, Jagust WJ, Weiner MW, Saykin AJ. APOE and BCHE as modulators of cerebral amyloid deposition: a florbetapir PET genome-wide association study. Mol Psychiatry. 2014;19:351–357. doi: 10.1038/mp.2013.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayaprolu S, Mullen B, Baker M, Lynch T, Finger E, Seeley WW, Hatanpaa KJ, Lomen-Hoerth C, Kertesz A, Bigio EH, Lippa C, Josephs KA, Knopman DS, White CL, 3rd, Caselli R, Mackenzie IR, Miller BL, Boczarska-Jedynak M, Opala G, Krygowska-Wajs A, Barcikowska M, Younkin SG, Petersen RC, Ertekin-Taner N, Uitti RJ, Meschia JF, Boylan KB, Boeve BF, Graff-Radford NR, Wszolek ZK, Dickson DW, Rademakers R, Ross OA. TREM2 in neurodegeneration: evidence for association of the p.R47H variant with frontotemporal dementia and Parkinson’s disease. Mol Neurodegener. 2013;8:19. doi: 10.1186/1750-1326-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed LA, Grabowski TJ, Schmidt ML, Morris JC, Goate A, Solodkin A, Van Hoesen GW, Schelper RL, Talbot CJ, Wragg MA, Trojanowski JQ. Autosomal dominant dementia with widespread neurofibrillary tangles. Ann Neurol. 1997;42:564–572. doi: 10.1002/ana.410420406. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, Saunders AM, Hardy J. Correlations between apolipoprotein E epsilon4 gene dose and brain-imaging measurements of regional hypometabolism. Proc Natl Acad Sci U S A. 2005;102:8299–8302. doi: 10.1073/pnas.0500579102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitz C, Tosto G, Vardarajan B, Rogaeva E, Ghani M, Rogers RS, Conrad C, Haines JL, Pericak-Vance MA, Fallin MD, Foroud T, Farrer LA, Schellenberg GD, George-Hyslop PS, Mayeux R Alzheimer’s Disease Genetics C. Independent and epistatic effects of variants in VPS10-d receptors on Alzheimer disease risk and processing of the amyloid precursor protein (APP) Transl Psychiatry. 2013;3:e256. doi: 10.1038/tp.2013.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridge PG, Mukherjee S, Crane PK, Kauwe JS Alzheimer’s Disease Genetics C. Alzheimer’s disease: analyzing the missing heritability. PLoS One. 2013;8:e79771. doi: 10.1371/journal.pone.0079771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogaeva E, Meng Y, Lee JH, Gu Y, Kawarai T, Zou F, Katayama T, Baldwin CT, Cheng R, Hasegawa H, Chen F, Shibata N, Lunetta KL, Pardossi-Piquard R, Bohm C, Wakutani Y, Cupples LA, Cuenco KT, Green RC, Pinessi L, Rainero I, Sorbi S, Bruni A, Duara R, Friedland RP, Inzelberg R, Hampe W, Bujo H, Song YQ, Andersen OM, Willnow TE, Graff-Radford N, Petersen RC, Dickson D, Der SD, Fraser PE, Schmitt-Ulms G, Younkin S, Mayeux R, Farrer LA, St George-Hyslop P. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat Genet. 2007;39:168–177. doi: 10.1038/ng1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogaeva EA, Fafel KC, Song YQ, Medeiros H, Sato C, Liang Y, Richard E, Rogaev EI, Frommelt P, Sadovnick AD, Meschino W, Rockwood K, Boss MA, Mayeux R, St George-Hyslop P. Screening for PS1 mutations in a referral-based series of AD cases: 21 novel mutations. Neurology. 2001;57:621–625. doi: 10.1212/wnl.57.4.621. [DOI] [PubMed] [Google Scholar]
- Sanan DA, Weisgraber KH, Russell SJ, Mahley RW, Huang D, Saunders A, Schmechel D, Wisniewski T, Frangione B, Roses AD, et al. Apolipoprotein E associates with beta amyloid peptide of Alzheimer’s disease to form novel monofibrils. Isoform apoE4 associates more efficiently than apoE3. J Clin Invest. 1994;94:860–869. doi: 10.1172/JCI117407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandbrink R, Zhang D, Schaeffer S, Masters CL, Bauer J, Forstl H, Beyreuther K. Missense mutations of the PS-1/S182 gene in German early-onset Alzheimer’s disease patients. Ann Neurol. 1996;40:265–266. doi: 10.1002/ana.410400225. [DOI] [PubMed] [Google Scholar]
- Scacchi R, Gambina G, Moretto G, Corbo RM. A mutation screening by DHPLC of PSEN1 and APP genes reveals no significant variation associated with the sporadic late-onset form of Alzheimer’s disease. Neuroscience letters. 2007;418:282–285. doi: 10.1016/j.neulet.2007.03.035. [DOI] [PubMed] [Google Scholar]
- Schmidt V, Sporbert A, Rohe M, Reimer T, Rehm A, Andersen OM, Willnow TE. SorLA/LR11 regulates processing of amyloid precursor protein via interaction with adaptors GGA and PACS-1. J Biol Chem. 2007;282:32956–32964. doi: 10.1074/jbc.M705073200. [DOI] [PubMed] [Google Scholar]
- Schraml F, Chen K, Ayutyanont N, Auttawut R, Langbaum JB, Lee W, Liu X, Bandy D, Reeder SQ, Alexander GE, Caselli RJ, Fleisher AS, Reiman EM the Alzheimer’s Disease Neuroimaging I. Association between an Alzheimer’s Disease-Related Index and Gene Dose. PLoS One. 2013;8:e67163. doi: 10.1371/journal.pone.0067163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurmann B, Wiese B, Bickel H, Weyerer S, Riedel-Heller SG, Pentzek M, Bachmann C, Williams J, van den Bussche H, Maier W, Jessen F. Association of the Alzheimer’s disease clusterin risk allele with plasma clusterin concentration. J Alzheimers Dis. 2011;25:421–424. doi: 10.3233/JAD-2011-110251. [DOI] [PubMed] [Google Scholar]
- Seshadri S, Fitzpatrick AL, Ikram MA, DeStefano AL, Gudnason V, Boada M, Bis JC, Smith AV, Carassquillo MM, Lambert JC, Harold D, Schrijvers EM, Ramirez-Lorca R, Debette S, Longstreth WT, Jr, Janssens AC, Pankratz VS, Dartigues JF, Hollingworth P, Aspelund T, Hernandez I, Beiser A, Kuller LH, Koudstaal PJ, Dickson DW, Tzourio C, Abraham R, Antunez C, Du Y, Rotter JI, Aulchenko YS, Harris TB, Petersen RC, Berr C, Owen MJ, Lopez-Arrieta J, Varadarajan BN, Becker JT, Rivadeneira F, Nalls MA, Graff-Radford NR, Campion D, Auerbach S, Rice K, Hofman A, Jonsson PV, Schmidt H, Lathrop M, Mosley TH, Au R, Psaty BM, Uitterlinden AG, Farrer LA, Lumley T, Ruiz A, Williams J, Amouyel P, Younkin SG, Wolf PA, Launer LJ, Lopez OL, van Duijn CM, Breteler MM. Genome-wide analysis of genetic loci associated with Alzheimer disease. Jama. 2010;303:1832–1840. doi: 10.1001/jama.2010.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Thompson PM, Potkin SG, Bertram L, Farrer LA, Foroud TM, Green RC, Hu X, Huentelman MJ, Kim S, Kauwe JS, Li Q, Liu E, Macciardi F, Moore JH, Munsie L, Nho K, Ramanan VK, Risacher SL, Stone DJ, Swaminathan S, Toga AW, Weiner MW, Saykin AJ for the Alzheimer’s Disease Neuroimaging I. Genetic analysis of quantitative phenotypes in AD and MCI: imaging, cognition and biomarkers. Brain imaging and behavior. 2013 doi: 10.1007/s11682-013-9262-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Lue L, Yang L, Roher A, Kuo Y, Strohmeyer R, Goux WJ, Lee V, Johnson GV, Webster SD, Cooper NR, Bradt B, Rogers J. Complement activation by neurofibrillary tangles in Alzheimer’s disease. Neurosci Lett. 2001;305:165–168. doi: 10.1016/s0304-3940(01)01842-0. [DOI] [PubMed] [Google Scholar]
- Sherva R, Tripodis Y, Bennett DA, Chibnik LB, Crane PK, de Jager PL, Farrer LA, Saykin AJ, Shulman JM, Naj A, Green RC, Consortium G Alzheimer’s Disease Neuroimaging I, Alzheimer’s Disease Genetics C. Genome-wide association study of the rate of cognitive decline in Alzheimer’s disease. Alzheimers Dement. 2014;10:45–52. doi: 10.1016/j.jalz.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman JM, Imboywa S, Giagtzoglou N, Powers MP, Hu Y, Devenport D, Chipendo P, Chibnik LB, Diamond A, Perrimon N, Brown NH, De Jager PL, Feany MB. Functional screening in Drosophila identifies Alzheimer’s disease susceptibility genes and implicates Tau-mediated mechanisms. Hum Mol Genet. 2014;23:870–877. doi: 10.1093/hmg/ddt478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siest G, Pillot T, Regis-Bailly A, Leininger-Muller B, Steinmetz J, Galteau MM, Visvikis S. Apolipoprotein E: an important gene and protein to follow in laboratory medicine. Clin Chem. 1995;41:1068–1086. [PubMed] [Google Scholar]
- Singleton AB, Hardy J, Traynor BJ, Houlden H. Towards a complete resolution of the genetic architecture of disease. Trends in genetics : TIG. 2010;26:438–442. doi: 10.1016/j.tig.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KR, Damiano J, Franceschetti S, Carpenter S, Canafoglia L, Morbin M, Rossi G, Pareyson D, Mole SE, Staropoli JF, Sims KB, Lewis J, Lin WL, Dickson DW, Dahl HH, Bahlo M, Berkovic SF. Strikingly different clinicopathological phenotypes determined by progranulin-mutation dosage. Am J Hum Genet. 2012;90:1102–1107. doi: 10.1016/j.ajhg.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoelgen R, von Arnim CA, Thomas AV, Peltan ID, Koker M, Deng A, Irizarry MC, Andersen OM, Willnow TE, Hyman BT. Interaction of the cytosolic domains of sorLA/LR11 with the amyloid precursor protein (APP) and beta-secretase beta-site APP-cleaving enzyme. J Neurosci. 2006;26:418–428. doi: 10.1523/JNEUROSCI.3882-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JL, Medland SE, Vasquez AA, Hibar DP, Senstad RE, Winkler AM, Toro R, Appel K, Bartecek R, Bergmann O, Bernard M, Brown AA, Cannon DM, Chakravarty MM, Christoforou A, Domin M, Grimm O, Hollinshead M, Holmes AJ, Homuth G, Hottenga JJ, Langan C, Lopez LM, Hansell NK, Hwang KS, Kim S, Laje G, Lee PH, Liu X, Loth E, Lourdusamy A, Mattingsdal M, Mohnke S, Maniega SM, Nho K, Nugent AC, O’Brien C, Papmeyer M, Putz B, Ramasamy A, Rasmussen J, Rijpkema M, Risacher SL, Roddey JC, Rose EJ, Ryten M, Shen L, Sprooten E, Strengman E, Teumer A, Trabzuni D, Turner J, van Eijk K, van Erp TG, van Tol MJ, Wittfeld K, Wolf C, Woudstra S, Aleman A, Alhusaini S, Almasy L, Binder EB, Brohawn DG, Cantor RM, Carless MA, Corvin A, Czisch M, Curran JE, Davies G, de Almeida MA, Delanty N, Depondt C, Duggirala R, Dyer TD, Erk S, Fagerness J, Fox PT, Freimer NB, Gill M, Goring HH, Hagler DJ, Hoehn D, Holsboer F, Hoogman M, Hosten N, Jahanshad N, Johnson MP, Kasperaviciute D, Kent JW, Jr, Kochunov P, Lancaster JL, Lawrie SM, Liewald DC, Mandl R, Matarin M, Mattheisen M, Meisenzahl E, Melle I, Moses EK, Muhleisen TW, Nauck M, Nothen MM, Olvera RL, Pandolfo M, Pike GB, Puls R, Reinvang I, Renteria ME, Rietschel M, Roffman JL, Royle NA, Rujescu D, Savitz J, Schnack HG, Schnell K, Seiferth N, Smith C, Steen VM, Valdes Hernandez MC, Van den Heuvel M, van der Wee NJ, Van Haren NE, Veltman JA, Volzke H, Walker R, Westlye LT, Whelan CD, Agartz I, Boomsma DI, Cavalleri GL, Dale AM, Djurovic S, Drevets WC, Hagoort P, Hall J, Heinz A, Jack CR, Jr, Foroud TM, Le Hellard S, Macciardi F, Montgomery GW, Poline JB, Porteous DJ, Sisodiya SM, Starr JM, Sussmann J, Toga AW, Veltman DJ, Walter H, Weiner MW, Bis JC, Ikram MA, Smith AV, Gudnason V, Tzourio C, Vernooij MW, Launer LJ, DeCarli C, Seshadri S, Andreassen OA, Apostolova LG, Bastin ME, Blangero J, Brunner HG, Buckner RL, Cichon S, Coppola G, de Zubicaray GI, Deary IJ, Donohoe G, de Geus EJ, Espeseth T, Fernandez G, Glahn DC, Grabe HJ, Hardy J, Hulshoff Pol HE, Jenkinson M, Kahn RS, McDonald C, McIntosh AM, McMahon FJ, McMahon KL, Meyer-Lindenberg A, Morris DW, Muller-Myhsok B, Nichols TE, Ophoff RA, Paus T, Pausova Z, Penninx BW, Potkin SG, Samann PG, Saykin AJ, Schumann G, Smoller JW, Wardlaw JM, Weale ME, Martin NG, Franke B, Wright MJ, Thompson PM Cohorts for H, Aging Research in Genomic Epidemiology C. Enhancing Neuro Imaging Genetics through Meta-Analysis C. Alzheimer’s Disease Neuroimaging I Consortium E, Consortium I Saguenay Youth Study G. Identification of common variants associated with human hippocampal and intracranial volumes. Nat Genet. 2012;44:552–561. doi: 10.1038/ng.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter WJ, Weisgraber KH, Huang DY, Dong LM, Salvesen GS, Pericak-Vance M, Schmechel D, Saunders AM, Goldgaber D, Roses AD. Binding of human apolipoprotein E to synthetic amyloid beta peptide: isoform-specific effects and implications for late-onset Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90:8098–8102. doi: 10.1073/pnas.90.17.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh J, Choi SH, Romano DM, Gannon MA, Lesinski AN, Kim DY, Tanzi RE. ADAM10 missense mutations potentiate beta-amyloid accumulation by impairing prodomain chaperone function. Neuron. 2013;80:385–401. doi: 10.1016/j.neuron.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan M, Saunders-Pullman R. The association between ss-glucocerebrosidase mutations and parkinsonism. Current neurology and neuroscience reports. 2013;13:368. doi: 10.1007/s11910-013-0368-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei K, Fisher C, Laws SM, Martins G, Paton A, Clarnette RM, Chung C, Brooks WS, Hallmayer J, Miklossy J, Relkin N, St George-Hyslop PH, Gandy SE, Martins RN. Association between presenilin-1 Glu318Gly mutation and familial Alzheimer’s disease in the Australian population. Molecular Psychiatry. 2002;7:776–781. doi: 10.1038/sj.mp.4001072. [DOI] [PubMed] [Google Scholar]
- Thambisetty M, Simmons A, Velayudhan L, Hye A, Campbell J, Zhang Y, Wahlund LO, Westman E, Kinsey A, Güntert A, Proitsi P, Powell J, Causevic M, Killick R, Lunnon K, Lynham S, Broadstock M, Choudhry F, Howlett DR, Williams RJ, Sharp SI, Mitchelmore C, Tunnard C, Leung R, Foy C, O’Brien D, Breen G, Furney SJ, Ward M, Kloszewska I, Mecocci P, Soininen H, Tsolaki M, Vellas B, Hodges A, Murphy DGM, Parkins S, Richardson JC, Resnick SM, Ferrucci L, Wong DF, Zhou Y, Muehlboeck S, Evans A, Francis PT, Spenger C, Lovestone S. Association of plasma clusterin concentration with severity, pathology, and progression in Alzheimer disease. Arch Gen Psychiatry. 2010;67:739–748. doi: 10.1001/archgenpsychiatry.2010.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thinakaran G, Koo EH. Amyloid precursor protein trafficking, processing, and function. J Biol Chem. 2008;283:29615–29619. doi: 10.1074/jbc.R800019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Cannon TD, Narr KL, van Erp T, Poutanen VP, Huttunen M, Lonnqvist J, Standertskjold-Nordenstam CG, Kaprio J, Khaledy M, Dail R, Zoumalan CI, Toga AW. Genetic influences on brain structure. Nat Neurosci. 2001;4:1253–1258. doi: 10.1038/nn758. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Stein JL, Medland SE, Hibar DP, Vasquez AA, Renteria ME, Toro R, Jahanshad N, Schumann G, Franke B, Wright MJ, Martin NG, Agartz I, Alda M, Alhusaini S, Almasy L, Almeida J, Alpert K, Andreasen NC, Andreassen OA, Apostolova LG, Appel K, Armstrong NJ, Aribisala B, Bastin ME, Bauer M, Bearden CE, Bergmann O, Binder EB, Blangero J, Bockholt HJ, Boen E, Bois C, Boomsma DI, Booth T, Bowman IJ, Bralten J, Brouwer RM, Brunner HG, Brohawn DG, Buckner RL, Buitelaar J, Bulayeva K, Bustillo JR, Calhoun VD, Cannon DM, Cantor RM, Carless MA, Caseras X, Cavalleri GL, Chakravarty MM, Chang KD, Ching CR, Christoforou A, Cichon S, Clark VP, Conrod P, Coppola G, Crespo-Facorro B, Curran JE, Czisch M, Deary IJ, de Geus EJ, den Braber A, Delvecchio G, Depondt C, de Haan L, de Zubicaray GI, Dima D, Dimitrova R, Djurovic S, Dong H, Donohoe G, Duggirala R, Dyer TD, Ehrlich S, Ekman CJ, Elvsashagen T, Emsell L, Erk S, Espeseth T, Fagerness J, Fears S, Fedko I, Fernandez G, Fisher SE, Foroud T, Fox PT, Francks C, Frangou S, Frey EM, Frodl T, Frouin V, Garavan H, Giddaluru S, Glahn DC, Godlewska B, Goldstein RZ, Gollub RL, Grabe HJ, Grimm O, Gruber O, Guadalupe T, Gur RE, Gur RC, Goring HH, Hagenaars S, Hajek T, Hall GB, Hall J, Hardy J, Hartman CA, Hass J, Hatton SN, Haukvik UK, Hegenscheid K, Heinz A, Hickie IB, Ho BC, Hoehn D, Hoekstra PJ, Hollinshead M, Holmes AJ, Homuth G, Hoogman M, Hong LE, Hosten N, Hottenga JJ, Hulshoff Pol HE, Hwang KS, Jack CR, Jr, Jenkinson M, Johnston C, Jonsson EG, Kahn RS, Kasperaviciute D, Kelly S, Kim S, Kochunov P, Koenders L, Kramer B, Kwok JB, Lagopoulos J, Laje G, Landen M, Landman BA, Lauriello J, Lawrie SM, Lee PH, Le Hellard S, Lemaitre H, Leonardo CD, Li CS, Liberg B, Liewald DC, Liu X, Lopez LM, Loth E, Lourdusamy A, Luciano M, Macciardi F, Machielsen MW, Macqueen GM, Malt UF, Mandl R, Manoach DS, Martinot JL, Matarin M, Mather KA, Mattheisen M, Mattingsdal M, Meyer-Lindenberg A, McDonald C, McIntosh AM, McMahon FJ, McMahon KL, Meisenzahl E, Melle I, Milaneschi Y, Mohnke S, Montgomery GW, Morris DW, Moses EK, Mueller BA, Munoz Maniega S, Muhleisen TW, Muller-Myhsok B, Mwangi B, Nauck M, Nho K, Nichols TE, Nilsson LG, Nugent AC, Nyberg L, Olvera RL, Oosterlaan J, Ophoff RA, Pandolfo M, Papalampropoulou-Tsiridou M, Papmeyer M, Paus T, Pausova Z, Pearlson GD, Penninx BW, Peterson CP, Pfennig A, Phillips M, Pike GB, Poline JB, Potkin SG, Putz B, Ramasamy A, Rasmussen J, Rietschel M, Rijpkema M, Risacher SL, Roffman JL, Roiz-Santianez R, Romanczuk-Seiferth N, Rose EJ, Royle NA, Rujescu D, Ryten M, Sachdev PS, Salami A, Satterthwaite TD, Savitz J, Saykin AJ, Scanlon C, Schmaal L, Schnack HG, Schork AJ, Schulz SC, Schur R, Seidman L, Shen L, Shoemaker JM, Simmons A, Sisodiya SM, Smith C, Smoller JW, Soares JC, Sponheim SR, Sprooten E, Starr JM, Steen VM, Strakowski S, Strike L, Sussmann J, Samann PG, Teumer A, Toga AW, Tordesillas-Gutierrez D, Trabzuni D, Trost S, Turner J, Van den Heuvel M, van der Wee NJ, van Eijk K, van Erp TG, van Haren NE, van ‘t Ent D, van Tol MJ, Valdes Hernandez MC, Veltman DJ, Versace A, Volzke H, Walker R, Walter H, Wang L, Wardlaw JM, Weale ME, Weiner MW, Wen W, Westlye LT, Whalley HC, Whelan CD, White T, Winkler AM, Wittfeld K, Woldehawariat G, Wolf C, Zilles D, Zwiers MP, Thalamuthu A, Schofield PR, Freimer NB, Lawrence NS, Drevets W the Alzheimer’s Disease Neuroimaging Initiative ECICSYSG. The ENIGMA Consortium: large-scale collaborative analyses of neuroimaging and genetic data. Brain imaging and behavior. 2014 doi: 10.1007/s11682-013-9269-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolboom N, Koedam EL, Schott JM, Yaqub M, Blankenstein MA, Barkhof F, Pijnenburg YA, Lammertsma AA, Scheltens P, van Berckel BN. Dementia mimicking Alzheimer’s disease Owing to a tau mutation: CSF and PET findings. Alzheimer disease and associated disorders. 2010;24:303–307. doi: 10.1097/WAD.0b013e3181cf35ec. [DOI] [PubMed] [Google Scholar]
- Tomaino C, Bernardi L, Anfossi M, Costanzo A, Ferrise F, Gallo M, Geracitano S, Maletta R, Curcio SA, Mirabelli M, Colao R, Frangipane F, Puccio G, Calignano C, Muraca MG, Paonessa A, Smirne N, Leotta A, Bruni AC. Presenilin 2 Ser130Leu mutation in a case of late-onset “sporadic” Alzheimer’s disease. Journal of Neurology. 2007;254:391–393. doi: 10.1007/s00415-006-0373-y. [DOI] [PubMed] [Google Scholar]
- Van Deerlin VM, Wood EM, Moore P, Yuan W, Forman MS, Clark CM, Neumann M, Kwong LK, Trojanowski JQ, Lee VM, Grossman M. Clinical, genetic, and pathologic characteristics of patients with frontotemporal dementia and progranulin mutations. Arch Neurol. 2007;64:1148–1153. doi: 10.1001/archneur.64.8.1148. [DOI] [PubMed] [Google Scholar]
- Vellas B, Carrillo MC, Sampaio C, Brashear HR, Siemers E, Hampel H, Schneider LS, Weiner M, Doody R, Khachaturian Z, Cedarbaum J, Grundman M, Broich K, Giacobini E, Dubois B, Sperling R, Wilcock GK, Fox N, Scheltens P, Touchon J, Hendrix S, Andrieu S, Aisen P, Members EUCTF. Designing drug trials for Alzheimer’s disease: what we have learned from the release of the phase III antibody trials: a report from the EU/US/CTAD Task Force. Alzheimers Dement. 2013;9:438–444. doi: 10.1016/j.jalz.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Verghese PB, Castellano JM, Garai K, Wang Y, Jiang H, Shah A, Bu G, Frieden C, Holtzman DM. ApoE influences amyloid-beta (Abeta) clearance despite minimal apoE/Abeta association in physiological conditions. Proc Natl Acad Sci U S A. 2013;110:E1807–1816. doi: 10.1073/pnas.1220484110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi T, De Strooper B. Presenilins: members of the gamma-secretase quartets, but part-time soloists too. Physiology (Bethesda) 2008;23:194–204. doi: 10.1152/physiol.00009.2008. [DOI] [PubMed] [Google Scholar]
- Wijsman EM, Pankratz ND, Choi Y, Rothstein JH, Faber KM, Cheng R, Lee JH, Bird TD, Bennett DA, Diaz-Arrastia R, Goate AM, Farlow M, Ghetti B, Sweet RA, Foroud TM, Mayeux R, Group NLNFS. Genome-wide association of familial late-onset Alzheimer’s disease replicates BIN1 and CLU and nominates CUGBP2 in interaction with APOE. PLoS Genet. 2011;7:e1001308. doi: 10.1371/journal.pgen.1001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtas A, Heggeli KA, Finch N, Baker M, Dejesus-Hernandez M, Younkin SG, Dickson DW, Graff-Radford NR, Rademakers R. C9ORF72 repeat expansions and other FTD gene mutations in a clinical AD patient series from Mayo Clinic. American journal of neurodegenerative disease. 2012;1:107–118. [PMC free article] [PubMed] [Google Scholar]
- Wolf AB, Valla J, Bu G, Kim J, Ladu MJ, Reiman EM, Caselli RJ. Apolipoprotein E as a beta-amyloid-independent factor in alzheimer’s disease. Alzheimers Res Ther. 2013;5:38. doi: 10.1186/alzrt204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray NR, Goddard ME, Visscher PM. Prediction of individual genetic risk of complex disease. Current opinion in genetics & development. 2008;18:257–263. doi: 10.1016/j.gde.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Wunderlich P, Glebov K, Kemmerling N, Tien NT, Neumann H, Walter J. Sequential proteolytic processing of the triggering receptor expressed on myeloid cells-2 (TREM2) by ectodomain shedding and gamma-secretase dependent intramembranous cleavage. J Biol Chem. 2013 doi: 10.1074/jbc.M113.517540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Q, Gil SC, Yan P, Wang Y, Han S, Gonzales E, Perez R, Cirrito JR, Lee JM. Role of phosphatidylinositol clathrin assembly lymphoid-myeloid leukemia (PICALM) in intracellular amyloid precursor protein (APP) processing and amyloid plaque pathogenesis. J Biol Chem. 2012;287:21279–21289. doi: 10.1074/jbc.M111.338376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing YY, Yu JT, Cui WZ, Zhong XL, Wu ZC, Zhang Q, Tan L. Blood clusterin levels, rs9331888 polymorphism, and the risk of Alzheimer’s disease. J Alzheimers Dis. 2012;29:515–519. doi: 10.3233/JAD-2011-111844. [DOI] [PubMed] [Google Scholar]
- Yamada K, Holth JK, Liao F, Stewart FR, Mahan TE, Jiang H, Cirrito JR, Patel TK, Hochgrafe K, Mandelkow EM, Holtzman DM. Neuronal activity regulates extracellular tau in vivo. J Exp Med. 2014;211:387–393. doi: 10.1084/jem.20131685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates PA, Desmond PM, Phal PM, Steward C, Szoeke C, Salvado O, Ellis KA, Martins RN, Masters CL, Ames D, Villemagne VL, Rowe CC For the ARG. Incidence of cerebral microbleeds in preclinical Alzheimer disease. Neurology. 2014 doi: 10.1212/WNL.0000000000000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikai S, Sasaki H, Doh-ura K, Furuya H, Sakaki Y. Genomic organization of the human amyloid beta-protein precursor gene. Gene. 1990;87:257–263. doi: 10.1016/0378-1119(90)90310-n. [DOI] [PubMed] [Google Scholar]
- Zhang B, Gaiteri C, Bodea LG, Wang Z, McElwee J, Podtelezhnikov AA, Zhang C, Xie T, Tran L, Dobrin R, Fluder E, Clurman B, Melquist S, Narayanan M, Suver C, Shah H, Mahajan M, Gillis T, Mysore J, MacDonald ME, Lamb JR, Bennett DA, Molony C, Stone DJ, Gudnason V, Myers AJ, Schadt EE, Neumann H, Zhu J, Emilsson V. Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer’s disease. Cell. 2013;153:707–720. doi: 10.1016/j.cell.2013.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]