Abstract
The incidence of esophageal adenocarcinoma has increased dramatically in the United States and Europe since the 1970s without apparent cause. Although specific host factors can affect risk of disease, such a rapid increase in incidence must be predominantly environmental. In the stomach, infection with Helicobacter pylori has been linked to chronic atrophic gastritis, an inflammatory precursor of gastric adenocarcinoma. However, the role of H. pylori in the development of esophageal adenocarcinoma is not well established.
Meanwhile, several studies have established that a complex microbiome in the distal esophagus might play a more direct role. Transformation of the microbiome in precursor states to esophageal adenocarcinoma—reflux esophagitis and Barrett’s metaplasia—from a predominance of gram-positive bacteria to mostly gram-negative bacteria raises the possibility that dysbiosis is contributing to pathogenesis. However, knowledge of the microbiome in esophageal adenocarcinoma itself is lacking. Microbiome studies open a new avenue to the understanding of the etiology and pathogenesis of reflux disorders.
Keywords: GERD, reflux esophagitis, Barrett’s esophagus, adenocarcinoma, microbiome, inflammation, cancer, 16S rRNA gene, sequencing, risk factors
Introduction
Squamous cell carcinoma and adenocarcinoma are the two most common types of esophageal cancer. In the United States and Europe, squamous cell carcinoma made up the vast majority of esophageal cancers until the 1970s, when it was surpassed by adenocarcinoma.1 In recent years, it has become clear that esophageal adenocarcinoma (EA) is a consequence of longstanding reflux esophagitis (RE), an inflammatory condition of the distal esophagus.2 This sequence often progresses through Barrett’s esophagus (BE), a metaplastic malady that may become dysplastic.3,4
The pathophysiology of RE is complex and involves diverse factors, including gastroesophageal reflux, gastric acid secretion, dysfunction of the anti-reflux barrier, gastric emptying disturbances, and abnormalities in esophageal defense mechanisms.5 As with other chronic inflammatory conditions, RE is associated with an increased risk of developing cancer: patients with RE have a 2- to 40-fold increased risk of developing EA. Relative risk increases to 30–400 in patients with BE3.
The incidence of EA in the U.S. has increased 6-fold since the 1970s1. Although host factors can predispose toward disease, such a rapid shift must be predominantly environmental. The current understanding of the etiology of EA is mainly derived from epidemiological studies of risk factors.6 Cigarette smoking, obesity, gastroesophageal reflux disorders (GERD), and low fruit and vegetable consumption account for 39.7%, 41.1%, 29.7%, and 15.3% of EA, respectively, with a combined population attributable risk of 78.7%.7 However, few studies have investigated the factors driving the increase in incidence of EA. Given the advent of widespread antibiotic use preceded the surge of EA, it has been hypothesized that the microbiome in GERD is altered, and that chronic exposure to an abnormal microbiome is carcinogenic.8,9
An enormous number of microorganisms, the vast majority of which are bacterial species, are known to colonize and form complex communities, or microbiota, at various sites within the human body. The human microbiota is estimated to be composed of ~1014 bacterial cells, which is 10 times more than the total number of human cells. The host relationship with these microorganisms can range from mutualism to pathogenicity.10,11 In various diseases, the best example being inflammatory bowel disease, the microbiota appears to play a key pathogenic role.11 On the other hand, bacterial mutualists within the gastrointestinal tract benefit the human host, aiding digestion, assisting in the synthesis of vitamins, promoting the development of the gut immune system, and providing competitive barriers to pathogen invasion. In return, the host provides these bacteria with safe housing and nutrients during lean times. To sustain this symbiotic relationship, the immune system has to balance permissive, tolerogenic responses to food antigens and commensal microbes with potentially damaging inflammatory responses to pathogens.12
Two theories can explain bacterial disease. The classic pathogen theory, attributed to Koch, ascribes the cause of disease to specific pathogens—typically, one or several bacteria. The microecologic disease theory, or the “Pathogenic Microbial Community” theory, is a new model in which the entire community contributes to pathogenicity, with no individual community members being classified as pathogens.13,14 Dysbiosis is a similar concept that refers to an abnormal state of the microbial ecosystem, dividing commensal bacteria into “protective” and “harmful” species. With dysbiosis, the cause of certain chronic diseases is an upset balance between the two groups.13
Recent studies of a small number of hosts have shown that there is a complex microbial community in the distal esophagus.8,9,13,15,16,17,18 Although the exposure and response of the esophageal epithelium to gastric contents has been extensively investigated, little attention has been given to the microbiome, its effect on the esophageal epithelial layer, and its potential changes in GERD.13 In this review, we present and summarize the most recent advances in the field of microbiology pertinent to GERD and EA.
Early studies of the normal esophageal microflora
The first studies of the esophageal biota were in the field of surgery, where bacteria may play a role in post-operative infection.19,20,21 This work was burdened by the contemporary belief that no indigenous bacteria populated the esophagus. Cultivation of luminal washes was unable to consistently show the presence of bacteria (Table 1). The few microbes isolated by conventional culture were suspected to be transiently deposited, either from the oropharynx by swallowing, or from the stomach by reflux.22 Only a countable few bacterial species were detected—far fewer than the 280 species that have been directly observed in the oral cavity by isolation from culture.23
Table 1.
Summary of culture-based studies on esophageal microbiome (1981–2013)
| Category | Lau19 1981 | Finlay20 1982 | Mannell21 1983 | Gagliardi22 1998 | Macfarlane15 2007 | Blackett16 2013 |
|---|---|---|---|---|---|---|
| Disease | Cancer | Cancer | Cancer | Normal | Normal BE |
Normal GERD BE, EA |
| Specimen | Aspirate | Resection | Aspirate | Aspirate | Biopsy Aspirate |
Biopsy |
| Number of cases | 79 | 12 | 101 | 30 | 14 | 151 |
| Number of isolates | 61 | 85 | 377 | 30 | ND | ND |
| Number of species | 14 | 15 | 32 | 11 | 46 | 111 |
| Mean species per case | 1 | 6 | 4 | 1 | ND | ND |
| % cases positive for bacteria | 64 | 100 | 100 | 67 | 50–71% | 100 |
| % cases positive for Streptococcus | 10 | 92 | ND | ND | 50 | ND |
ND: not determined or reported.
These data reflect the limitations of cultivation as a technique. Cultivation, when combined with selective media and chemical tests, is useful for isolation and characterization of single bacterial pathogens, but is not suitable for use in defining a complex microbial community. Cultivation is unable to identify bacteria in a viable but nonculturable (VBNC) state.24 Further, cultivation introduces bias by selecting for bacteria capable of growing on artificial media and overlooking those whose nutrition needs are undetermined. Uncertainty about the esophageal microbiome was not resolved until cultivation-independent technology became widely applied to human microbiome studies in the early 2000s (Table 2).25
Table 2.
Summary of cultivation-independent studies on esophageal microbiome (2004–2013)
| Category | Pei8 2004 | Narikiyo17 2004 | Pei9 2005 | Yang13 2009 | Liu18 2013 |
|---|---|---|---|---|---|
| Disease | Normal | Cancer | RE BE |
Normal RE, BE |
Normal RE, BE |
| Specimen | Biopsy | Biopsy | Biopsy | Biopsy | Biopsy Aspirate |
| No. cases | 4 | 20 | 24 | 34 | 18 |
| No. clones | 900 | 100 | 147 | 6800 | 424 |
| No. species | 95 | 7 | 39 | 166 | ND |
| Mean species per case | 43 | ≤6 | ND | ND | ND |
| % cases positive for bacteria | 100 | ND | 100 | 100 | 100 |
| % cases positive for Streptococcus | 100 | 87 | ND | 100 | 83.3 |
16S rRNA genes, which are widely-used to estimate the evolutionary history and taxonomic assignment of individual organisms, have become the basis of the most common cultivation-independent technique.26,27,28 This approach began in the 1980s, when it was observed that all bacteria possess one or more 16S ribosomal RNA genes. These genes are key components of protein synthesis and are essential for life. Since all bacteria are descendents of a common ancestor, the regions of the 16S genes most critical for conformation of ribosomal structure and function have remained conserved over billions of years. PCR based on universal primers to these regions can amplify 16S rRNA genes of nearly all bacterial species. The less conserved regions whose sequences recorded the evolutionary history of individual species, meanwhile, serve as signatures for taxonomic classification. It is the combination of PCR, sequencing technology, and comprehensive 16S rRNA gene databases that has made 16S rRNA gene surveys the mainstay of cultivation-independent studies of simple and complex bacterial communities.
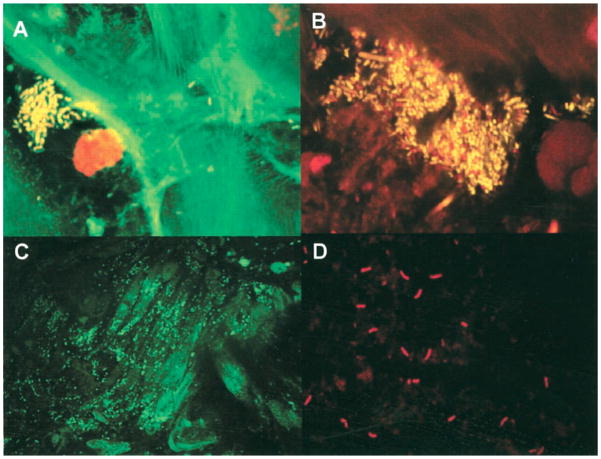
Using broad-range 16S rRNA gene PCR, coupled with cloning and Sanger sequencing and histology technique, Pei and colleagues examined mucosal biopsies from the distal esophagus of four normal human adults.8 From analysis of 900 recovered clones, they made three key observations. First, an esophageal microbiome is invariably present (Table 2). Second, the esophageal microbiome is comparable to the oral microbiome, as they share the same six major phyla (Firmicutes, Bacteroides Actinobacteria, Proteobacteria, Fusobacteria, and TM7) and are both uneven communities dominated by the genus Streptococcus. Third, bacteria are visible on the mucosal surface by tissue gram-stain. Later, Macfarlane and colleagues demonstrated viable bacteria on normal esophageal mucosa in microcolonies/aggregates (Fig. 1).15 These observations established the existence of an esophageal microbiome and laid the foundation for later studies of its role in disease.
Figure 1.
In situ visualization of bacteria in esophageal mucosal biopsies. A and B: Confocal sections of mucosal biopsy specimens stained for cell viability, containing mixtures of living (yellow) and dead (red) organisms. Microcolony and aggregate formation can be seen in mucosal samples from control subjects (A) and from patients with Barrett’s esophagus (B). C and D: Fluorescence light micrographs of transverse sections of Barrett’s esophageal mucosae showing colonization by streptococci (C) and Campylobacter species (D), using 16S ribosomal RNA oligonucleotide probes labeled with FITC and cy3. Figure originally published in Journal of Clinical Infectious Diseases15 and permission to reuse of the figure is granted by the Journal.
Microbiome in GERD
The first study to apply cultivation-independent technique to the microbiome in esophageal disease was reported in 2005, with the goal of demonstrating feasibility.9 Two 16S rRNA gene clones were recovered and examined from each of the esophageal biopsies taken from 24 subjects (9 with normal mucosa, 12 with GERD, and 3 with BE). As expected, bacterial signals were successfully detected in all biopsies, and the overall diversity and community membership resembled those of the normal esophageal microbiome. A large scale survey to compare the esophageal microbiome among subjects with RE, BE, and healthy esophagus was performed by Yang and coworkers in 2009.13 It was one of the largest human microbiome studies to date, with a total of 6,800 16S rRNA gene clones from 34 subjects analyzed by Sanger sequencing. Using unsupervised cluster analysis and phenotype-guided analyses, samples were found to contain one of two distinct microbiomes. Microbiome type I was mainly associated with a normal esophagus and was dominated by gram-positive bacteria from the Firmicutes phylum, of which Streptococcus was the most dominant genus. Microbiome type II had greater proportion of gram-negative anaerobes/microaerophiles (phyla Bacteroidetes, Proteobacteria, Fusobacteria, and Spirochaetes) and primarily correlated with RE (odds ratio, 15.4) and BE (odds ratio, 16.5). The microbiome did not differ between GERD and BE patients. Recently, a study in Japan used cloning and Sanger sequencing to analyze the esophageal microbiome of 18 subjects (6 each with normal esophagus, RE, and BE).18 A unique test performed was quantification of total bacterial loads by quantitative 16S rRNA gene PCR. Notably, the three groups harbored similar numbers of bacteria, equivalent to 106–107 colony forming units per sample. Thus, changes in the relative abundance of taxa, rather than absolute bacterial loads, are likely more relevant to esophageal diseases. Although far from comprehensive (only approximately 24 clones were sequenced per sample), the study found Veillonella (19%), Prevotella (12%), Neisseria (4%), and Fusobacterium (9%) to be more prevalent in patients with RE and BE than in controls. These observations support those published by Yang and Pei, confirming that the esophageal microbiome is reliably altered in reflux disorders.9,13
Osias et al. quantified bacteria by staining of biopsies from esophageal diseases.29 Bacteria were detected more often in BE than non-BE, and increasing bacterial stain scores were associated both with metaplasia and increasing dysplasia. Interestingly, Macfarlane and colleagues found Campylobacter species (C. concisus and C. rectus)—which have been implicated in the pathogenesis of enteritis, periodontal diseases, and tumorigenesis in animal models—in the majority of BE patients but none of the controls (Figure 1).15 Fluorescence in situ hybridization using specific 16S ribosomal RNA oligonucleotide probes revealed colonization of Barrett’s esophageal mucosae by Campylobacter species (Fig. 1).
Microbiome in gastroesophageal adenocarcinoma
Nearly all studies of the local microbiome associated with esophageal cancer do not distinguish between adenocarcinoma and squamous cell carcinoma (Table 1).17,19,20,21 Because these two cancers have marked differences in epidemiology and etiology, these studies cannot be assumed to be applicable to EA and thus will not be discussed here. To date, there has been only one published study to address the link between the microbiome and EA, in which Blackett and colleagues compared 30 cases of EA with 39 cases of controls using culture analysis.16 The study recovered more species (n=73) in EA than controls (n=56). However, no statistical difference in specific taxa was reported.
Role of Helicobacter pylori
Case-control studies have suggested that H. pylori gastritis may play a protective role in the development of GERD and associated EA. However, eradication of H. pylori does not increase new GERD cases or worsen GERD symptoms (except in patients with hiatal hernia and corpus gastritis).30 The role of H. pylori in the pathogenesis of GERD, BE and EA remains an unclear and controversial topic that has been extensively reviewed elsewhere.31
Perspectives
Esophageal microbiology is an understudied field, especially in EA. Data from the few available studies have established a convincing association between an altered microbiome and the reflux disorders that precede EA. A large scale study of the microbiome in the development of EA has been funded under the NIH Human Microbiome Project and may fill the knowledge gap.32, 33 Prospective studies to explore whether the microbiome changes before or after onset of disease are the next logical step to evaluate causality. Large cohorts such as the National Cancer Institute-Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial Cohort (NCI-PLCO) and the American Cancer Society Cancer Prevention Study II Cohort (ACS-CPS-II) provide access to tissue samples collected prior to cancer diagnosis and therefore should prove invaluable to further characterize the role of the esophageal microbiome in carcinogenesis.34,35
Acknowledgments
This work was supported in part by grants U01CA18237, UH3CA140233, R03CA159414, and R01CA159036 from the National Cancer Institute and NIH Human Microbiome Project and by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development.
Contributor Information
Liying Yang, Research Assistant Professor, Department of Medicine, New York University School of Medicine, New York, NY 10016.
Noami Chaudhary, Clinical Instructor, Department of Medicine, New York University School of Medicine, New York, NY 10016.
Jonathan Baghdadi, Clinical Instructor, Department of Medicine, New York University School of Medicine, New York, NY 10016.
Zhiheng Pei, Staff Physician, Department of Veterans Affairs New York Harbor Healthcare System, New York, NY 10010; Associate Professor, Departments of Medicine and Pathology, New York University School of Medicine, New York, NY 10016.
References
- 1.Pohl H, Sirovich B, Welch HG. Esophageal adenocarcinoma incidence: are we reaching the peak? Cancer Epidemiol Biomarkers Prev. 2010;19:1468–70. doi: 10.1158/1055-9965.EPI-10-0012. [DOI] [PubMed] [Google Scholar]
- 2.Lagergren J, Bergstrom R, Lindgren A, Nyren O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;340:825–31. doi: 10.1056/NEJM199903183401101. [DOI] [PubMed] [Google Scholar]
- 3.Lagergren J. Adenocarcinoma of oesophagus: what exactly is the size of the problem and who is at risk? Gut. 2005;54 (Suppl 1):i1–5. doi: 10.1136/gut.2004.041517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson LA, Murphy SJ, Johnston BT, et al. Relationship between Helicobacter pylori infection and gastric atrophy and the stages of the oesophageal inflammation, metaplasia, adenocarcinoma sequence: results from the FINBAR case-control study. Gut. 2008;57:734–9. doi: 10.1136/gut.2007.132662. [DOI] [PubMed] [Google Scholar]
- 5.Rieder F, Biancani P, Harnett K, et al. Inflammatory mediators in gastroesophageal reflux disease: impact on esophageal motility, fibrosis, and carcinogenesis. Am J Physiol Gastrointest Liver Physiol. 2010;298:G571–81. doi: 10.1152/ajpgi.00454.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reid BJ, Li X, Galipeau PC, et al. Barrett’s oesophagus and oesophageal adenocarcinoma: time for a new synthesis. Nat Rev Cancer. 2010;10:87–101. doi: 10.1038/nrc2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engel LS, Chow WH, Vaughan TL, et al. Population attributable risks of esophageal and gastric cancers. J Natl Cancer Inst. 2003;95:1404–13. doi: 10.1093/jnci/djg047. [DOI] [PubMed] [Google Scholar]
- 8.Pei Z, Bini EJ, Yang L, et al. Bacterial biota in the human distal esophagus. Proc Natl Acad Sci USA. 2004;101:4250–4255. doi: 10.1073/pnas.0306398101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pei Z, Yang L, Peek RM, et al. Bacterial biota in reflux esophagitis and Barrett’s esophagus. World Journal of Gastroenterology. 2005;11:7277–7283. doi: 10.3748/wjg.v11.i46.7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115–1118. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- 11.Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577–594. doi: 10.1053/j.gastro.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 12.Yang L, Fritz Francois, Pei Z. Molecular Pathways: Pathogenesis and clinical implications of microbiome alteration in esophagitis and Barrett’s esophagus. Clin Cancer Res. 2012;18:2138–44. doi: 10.1158/1078-0432.CCR-11-0934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang L, Lu X, Nossa CW, et al. Inflammation and intestinal metaplasia of the distal esophagus are associated with alterations in the microbiome. Gastroenterology. 2009;137:588–597. doi: 10.1053/j.gastro.2009.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ley RE, Turnbaugh PJ, Klein S, et al. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–3. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 15.Macfarlane S, Furrie E, Macfarlane, et al. Microbial colonization of the upper gastrointestinal tract in patients with Barrett’s esophagus. Clin Infect Dis. 2007;45:29–38. doi: 10.1086/518578. [DOI] [PubMed] [Google Scholar]
- 16.Blackett KL, Siddhi SS, Cleary S, et al. Oesophageal bacterial biofilm changes in gastro-oesophageal reflux disease, Barrett’s and oesophageal carcinoma: association or causality. Aliment Pharmacol Ther. 2013;37:1084–92. doi: 10.1111/apt.12317. [DOI] [PubMed] [Google Scholar]
- 17.Narikiyo M, Tanabe C, Yamada Y, et al. Frequent and preferential infection of Treponema denticola, Streptococcus mitis, and Streptococcus anginosus in esophageal cancers. Cancer Sci. 2004;95:569–74. doi: 10.1111/j.1349-7006.2004.tb02488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu N, Ando T, Ishiguro K, et al. Characterization of bacterial biota in the distal esophagus of Japanese patients with reflux esophagitis and Barrett’s esophagus. BMC Infect Dis. 2013;13:130. doi: 10.1186/1471-2334-13-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lau WF, Wong J, Lam KH, et al. Oesophageal microbial flora in carcinoma of the oesophagus. Aust N Z J Surg. 1981;51:52–55. doi: 10.1111/j.1445-2197.1981.tb05905.x. [DOI] [PubMed] [Google Scholar]
- 20.Finlay IG, Wright PA, Menzies T, et al. Microbial flora in carcinoma of oesophagus. Thorax. 1982;37:181–184. doi: 10.1136/thx.37.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mannell A, Plant M, Frolich J. The microflora of the oesophagus. Ann R Coll Surg Engl. 1983;65:152–4. 40. [PMC free article] [PubMed] [Google Scholar]
- 22.Gagliardi D, Makihara S, Corsi PR, et al. Microbial flora of the normal esophagus. Dis Esophagus. 1998;11:248–250. doi: 10.1093/dote/11.4.248. [DOI] [PubMed] [Google Scholar]
- 23.Dewhirst FE, Chen T, Izard J, et al. The human oral microbiome. J Bacteriol. 2010;192:5002–17. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oliver JD. Recent findings on the viable but nonculturable state in pathogenic bacteria. FEMS Microbiol Rev. 2010;34:415–25. doi: 10.1111/j.1574-6976.2009.00200.x. [DOI] [PubMed] [Google Scholar]
- 25.Nossa CW, Tang Y-W, Pei Z. Pearls and Pitfalls of Genomics Based Microbiome Analysis. Emerging Microbes & Infections. 2012;1:e45. doi: 10.1038/emi.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woese CR. Bacterial evolution. Microbiol Rev. 1987;51:221–71. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stackebrandt E. Taxonomic note: a place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int J Syst Bacteriol. 1994;44:846–849. [Google Scholar]
- 28.Pei AY, Oberdorf WE, Nossa CW, et al. Diversity of 16S rRNA genes within individual prokaryotic genomes. Appl Environ Microbiol. 2010;76:3886–97. doi: 10.1128/AEM.02953-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osias GL, Bromer MQ, Thomas RM, et al. Esophageal bacteria and Barrett’s esophagus: a preliminary report. Dig Dis Sci. 2004;49:228–36. doi: 10.1023/b:ddas.0000017443.44802.4b. [DOI] [PubMed] [Google Scholar]
- 30.Hamada H, Haruma K, Mihara M, et al. High incidence of reflux oesophagitis after eradication therapy for Helicobacter pylori: impacts of hiatal hernia and corpus gastritis. Aliment Pharmacol Ther. 2000;14:729–35. doi: 10.1046/j.1365-2036.2000.00758.x. [DOI] [PubMed] [Google Scholar]
- 31.Peek RM. Helicobacter pylori and Gastroesophageal Reflux Disease. Curr Treat Options Gastroenterol. 2004;7:59–70. doi: 10.1007/s11938-004-0026-0. [DOI] [PubMed] [Google Scholar]
- 32.Yang L, Oberdorf W, Gerz E, et al. Foregut microbiome in development of esophageal adenocarcinoma. Nature Proceedings. 2010 http://dx.doi.org/10.1038/npre.2010.5026.1.
- 33.IH HMP Working Group. Peterson J, Garges S, Giovanni M, et al. The NIH Human Microbiome Project. Genome Res. 2009;19:2317–23. doi: 10.1101/gr.096651.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayes RB, Reding D, Kopp W, et al. Etiologic and early marker studies in the prostate, lung, colorectal and ovarian (PLCO) cancer screening trial. Control Clin Trials. 2000;21:349S–55S. doi: 10.1016/s0197-2456(00)00101-x. [DOI] [PubMed] [Google Scholar]
- 35.Calle EE, Rodriguez C, Jacobs EJ, et al. The American Cancer Society Cancer Prevention Study II Nutrition Cohort: rationale, study design, and baseline characteristics. Cancer. 2002;94:500–11. doi: 10.1002/cncr.10197. [DOI] [PubMed] [Google Scholar]