Abstract
Chronic medical conditions and/or their treatments may interact with aging to alter or even accelerate brain senescence. Adult onset cancer, for example, is a disease associated with advanced aging and emerging evidence suggests a profile of subtle but diffuse brain injury following cancer chemotherapy. Breast cancer is currently the primary model for studying these “chemobrain” effects. Given the widespread changes to brain structure and function as well as the common impairment of integrated cognitive skills observed following breast cancer chemotherapy, it is likely that large-scale brain networks are involved. Default mode network (DMN) is a strong candidate considering its preferential vulnerability to aging and sensitivity to toxicity and disease states. Additionally, chemotherapy is associated with several physiologic effects including increased inflammation and oxidative stress that are believed to elevate toxicity in the DMN. Biomarkers of DMN connectivity could aid in the development of treatments for chemotherapy-related cognitive decline. For example, certain nutritional interventions could potentially reduce the metabolic changes (e.g. amyloid beta toxicity) associated with DMN disruption.
Keywords: default mode network, chemotherapy, neuroimaging, resting state fMRI, brain, aging, cognitive decline
1. Brain aging, cancer and chemotherapy
With age, the brain undergoes numerous degenerative changes that tend to result in a decline of cognitive function. Cognitive decline occurs on a continuum with dementia being at the pathological extreme. Age is the most consistent predictor of pathological cognitive decline including mild cognitive impairment (MCI) and dementia (Kravitz, et al., 2012). However, age is also a primary risk factor for several major, non-central nervous system (CNS) medical conditions. Many of these conditions and/or their treatments could potentially trigger an altered or accelerated brain aging process.
Cancer is a common, age-related disease with most diagnoses originating outside the CNS. Approximately 1 in 2 adults will be diagnosed with cancer during their lifetime with a median age at diagnosis of 66 years (Howlader, et al., 2013). Advances in cancer treatments, such as chemotherapy, have resulted in significantly improved survival rates leading to a large and growing cohort of chemotherapy-exposed older adults. Chemotherapy is often associated with persistent cognitive decline affecting an estimated 78% of patients with non-CNS cancer (Wefel and Schagen, 2012). Neuroimaging studies provide insight regarding the effects of chemotherapy on cognition by demonstrating subtle but diffuse brain injury [see reviews by: (de Ruiter and Schagen, 2013;Kaiser, et al., 2014;Koppelmans, et al., 2013;McDonald and Saykin, 2013;Pomykala, et al., 2013a;Scherling and Smith, 2013;Simo, et al., 2013)].
Thus far, most neuroimaging studies have focused on breast cancer, which has become an initial model for investigating chemotherapy-related brain injury in adult onset, non-CNS cancer. Possible mechanisms of brain injury following breast cancer chemotherapy (BCC) include direct toxicity to neural progenitor cells (Monje and Dietrich, 2012), elevation of cytokine release and oxidative stress (Conroy, et al., 2013b;Ganz, et al., 2013;Kesler, et al., 2013a;Pomykala, et al., 2013b;Vardy, et al., 2007), DNA damage and epigenetic alterations (Conroy, et al., 2013b), deficient estrogen-related protection of healthy brain cells (Hogervorst, 2013) following chemotherapy-induced menopause (Conroy, et al., 2013a) and altered cerebral blood supply through blood vessel damage (Seigers, et al., 2010) and/or chemotherapy-induced anemia (O'Shaughnessy, 2003). Chemotherapy-related mechanisms interact with other factors including cancer pathogenesis (Kesler, et al., 2011), allostatic load (Miller, et al., 2008) and genetic variations (Ahles and Saykin, 2007). Therefore, BCC research has broad implications for neuroscience in terms of the effects of various physiologic factors on brain-behavior relationships. This research is also forging new ground in terms of the relevance of cognitive neuroscience for non-CNS medical conditions and provides an opportunity for interdisciplinary approaches to intervention including neuropsychological rehabilitation, physical activity and nutrition, among others.
Many of the candidate mechanisms for BCC-related brain injury overlap significantly with those involved in aging (Ahles, 2012;Koppelmans, et al., 2013;Mandelblatt, et al., 2013). Accordingly, older patients tend to have poorer cognitive outcome following BCC (Ahles, et al., 2010). Gray matter atrophy following BCC is analogous to approximately four years of aging on the brain (Koppelmans, et al., 2012). Roughly 30% of BCC patients demonstrate a new onset of a previously non-existent cognitive deficit at long-term follow up, suggesting possible progressive decline (Wefel, et al., 2010). The presence of the APOE D4 allele is a common risk factor for both dementia and BCC-related cognitive dysfunction (Ahles, et al., 2003).
Aging is associated with microglial activation, causing increased release of pro-inflammatory cytokines (Lynch, 2010) and chronic neuroinflammation is associated with dementia (Herrup, 2010). BCC survivors show elevated neurochemical markers of microglial activity (i.e. myo-inositol) (Kesler, et al., 2013b) and increased peripheral pro-inflammatory cytokines levels (Ganz, et al., 2013;Kesler, et al., 2013a;Kesler, et al., 2013b;Pomykala, et al., 2013b;Vardy, et al.,2007). Compared to healthy women, BCC survivors show increased pro-inflammatory cytokine levels with increased age (Kesler, et al., 2013a). Therefore, BCC is an interesting model for examining the interactions between disease, aging and neurodegeneration.
If chemotherapy accelerates the brain aging process, chemotherapy-treated patients could be at higher risk for dementia. Accordingly, one study suggested that BCC survivors are 20% more likely to be diagnosed with dementia (Heck, et al., 2008). Subsequent reports have not supported this finding (Baxter, et al., 2009;Du, et al., 2010;Raji, et al., 2009) though methodological issues make conclusions difficult (Koppelmans, et al., 2013) and further research in this area is imperative. Dementia risk could be diagnosis-specific given that treatment regimens have been linked with dementia incidence in colon cancer (Gupta and Lamont, 2004) but not breast cancer (Raji, et al., 2009). Neuroimaging biomarkers may offer significantly increased sensitivity and reliability in predicting cognitive outcome following BCC.
2. Aging and the default mode network (DMN)
One of the most promising neuroimaging biomarkers of age- and disease-related cognitive decline is reduction of default mode network (DMN) connectivity (Damoiseaux, 2012). The increasing investigation of large-scale brain networks such as DMN signals a shift beyond theories relying on discrete cortical localization of cognitive function and dysfunction. It is now known that specialized brain regions do not operate in isolation but participate in integrated networks. These networks are dynamically coordinated with specialized regions contributing to different networks in an adaptive, context-specific manner (Hutchison, et al., 2013;Sporns, 2011). Brain networks are organized such that they are simultaneously highly segregated and integrated allowing for optimal efficiency of information processing and learning (Bullmore and Sporns, 2012).
Even at rest, several different large-scale brain networks demonstrate spontaneous, synchronous neuronal activity. These intrinsic, or “resting state” networks show higher activity during rest and tend to be anti-correlated with activity in task-positive networks (Fox, et al., 2005;Raichle, 2011). Intrinsic network regions show attenuation, or “task-induced deactivation” (TID) and are believed to modulate allocation of neural resources to support goal-oriented processes (Sambataro, et al., 2010). The DMN, one of the most commonly observed resting state networks, includes precuneus, posterior cingulate, medial frontal, middle temporal and lateral parietal regions as well as hippocampus (Damoiseaux, et al., 2006). DMN is believed to support processes such as implicit learning, autobiographical memory, prospection, monitoring the external environment, creativity and self-reflection (Abraham, 2013;Agnati, et al., 2013;Qin and Northoff, 2011;Raichle, 2011;Takeuchi, et al., 2012). Both DMN functional connectivity and TID tend to decrease with age and are markedly decreased in individuals with MCI or Alzheimer’s disease (Damoiseaux, et al., 2012;Greicius, et al., 2004;Sheline, et al., 2010). Disruptions of DMN precede amyloid beta toxicity, the hallmark molecular pathology associated with neurodegeneration, identifying individuals who are cognitively normal but at high risk for dementia (Sheline, et al., 2010).
Amyloid beta accumulation naturally increases with age and DMN is preferentially vulnerable to this toxicity (Buckner, et al., 2005). DMN regions are hubs – areas that participate in a large number of functional interactions (Cole, et al., 2010b) and therefore have significantly high metabolic demands (Lord, et al., 2013). DMN’s high energy requirements may make it more susceptible to the reduction of physiological resources that occurs with age including reduced metabolic capacity, cerebral blood flow and glucose metabolism (Yao, et al. 2011; Kapogiannis and Mattson, 2011). Additionally, inflammation increases with age and is associated with elevated amyloid beta accumulation (Herrup, 2010). Lower cognitive reserve also appears to degrade the DMN faster by increasing amyloid beta deposition (Bero, et al., 2011).
Alternatively, it has been suggested that amyloid beta toxicity may be a consequence of neurodegeneration rather than a cause (Armstrong, 2011). Amyloid beta is produced from amyloid precursor protein, which shows neuroprotective functions following brain injury (Blennow, et al., 2012). Therefore, the mechanism underlying DMN’s increased vulnerability to age is not entirely clear. DMN susceptibility to amyloid beta accumulation could result from a combination of factors including both metabolic changes and neuroprotective mechanisms that become dysregulated in the aging brain, which has decreased ability to adequately return to homeostasis (Herrup, 2010). Additionally, APOE status influences amyloid beta clearance (Verghese, et al., 2013) indicating genetic moderation of DMN vulnerability to amyloid beta toxicity.
3. Measuring DMN
DMN is primarily measured using functional magnetic resonance imaging (fMRI) (Cole, et al., 2010a;Margulies, et al., 2010;Zhang and Raichle, 2010) but has also been assessed using positron emission tomography (PET) (Buckner, et al., 2008) electroencephalography (Chen, et al., 2013) and near-infrared spectroscopy (Sasai, et al., 2012). Structural connectivity of DMN can be measured using volumetric MRI or diffusion tensor imaging (DTI) (Alexander-Bloch, et al., 2013).
3.1 Functional connectivity
If neural activity in a set of anatomically distinct brain regions is synchronous, these regions are believed to be coordinating into a functional network. Resting state fMRI (rsfMRI) is typically used to assess functional connectivity of DMN and simply requires the participant to lie passively in the scanner. These data ideally should be obtained before task-based scans to reduce the effects of cognitive processes (Northoff, et al., 2010). A minimum duration of 5 minutes is associated with stable correlation strengths (Van Dijk, et al., 2010). However, robust brain network metrics can be achieved using a scan time of as little as 2 minutes if necessary (Whitlow, et al., 2011). RsfMRI is ideally acquired prior to task-based fMRI scans conducted within the same session to reduce the effects of specific cognitive processes on the resting state networks (Waites, et al., 2005). Uncertainty remains as to whether rsfMRI should be acquired with eyes open or closed as the two conditions can yield different results (Liu, et al., 2013). Instructions given to participants during rsfMRI, including whether or not to close the eyes, should be consistent as findings can vary based on instruction content (Benjamin, et al., 2010). There are several other potential participant-related confounds such as body weight and caffeine consumption (Duncan and Northoff, 2013). However, rsfMRI functional connectivity measures show strong stability and test-retest reliability (Van Dijk, et al., 2010).
Pre-processing of rsfMRI data is similar to that of task-based fMRI including motion correction, spatial normalization and smoothing. However, rsfMRI data must be filtered to the <0.1 Hz range of spontaneous activity (Raichle, 2011;Whitfield-Gabrieli and Ford, 2012). Because intrinsic activity occurs in such a low frequency range, rsfMRI signal has increased susceptibility to non-neuronal noise (Hutchison, et al., 2013). Several approaches have been described for minimizing these artifacts including independent component analysis (ICA) (Boubela, et al., 2013), principal component analysis (Behzadi, et al., 2007) and model-based methods (Chang and Glover, 2009).
The two most common statistical approaches for rsfMRI functional connectivity are seed-based correlation (SBC) and ICA. SBC is a model-driven, univariate analysis where the time course of a region of interest, or seed, is correlated with the whole brain in a voxel-wise manner at the individual participant level. The resulting correlation matrix is typically normalized and can then be evaluated at the group level (Whitfield-Gabrieli and Ford, 2012). SBC seed sizes and locations are often ambiguous leading to variability in results (Cole, et al., 2010a). One group recently introduced a data-driven seed selection method that utilizes regional homogeneity (Yan, et al., 2013). Another data driven method - seed-based iterative cross-correlation - uses the voxels from a SBC-generated group level connectivity map as a new seed, repeating this process until the results converge (Yang, et al., 2013).
ICA is a data-driven, multivariate method that works by decomposing the unknown, mixed fMRI signal sources into maximally independent temporal or spatial activation maps (components) (Calhoun and Adali, 2012). Individual components can represent various brain networks as well as non-neuronal noise. Therefore, components associated with DMN must be selected before moving on to group level analyses. One approach is to observe the power spectrum for each component and identify those with a peak frequency in the intrinsic activity range (e.g. 0.008–0.09 Hz). An automatic template matching procedure calculates the “goodness of fit” of a component with a spatial template of interest (Greicius, et al., 2004). Group ICA involves concatenation of individual participants’ components and then back-reconstructing them to ensure that components are consistently ordered and can then be sorted temporally or spatially (Calhoun, et al., 2009). Partner-matching selects components by clustering them based on similarity measures of spatial patterns across multiple within or between participants’ datasets (Wang and Peterson, 2008). Another automatic component selection method combines spatial map filtering, statistical tests and spectral analysis (Storti, et al., 2013).
Intrinsic functional connectivity can also be extracted from task-based block design or event related data (Fair, et al., 2007). These methods include concatenation of interleaved rest epochs or removal of task-induced variance via regression (leaving the underlying spontaneous activity) (Fox, et al., 2006). There are several disadvantages to such emulation of rsfMRI including exclusion of the lower intrinsic frequencies and possible contamination by previous task conditions (Fair, et al., 2007). However, emulation is advantageous for research groups with large existing datasets that did not include an rsfMRI sequence.
Graph theory analysis provides a mathematical representation of brain network topology as a system of interconnected elements defined by nodes (regions) and edges (connections) (Hosseini, et al., 2012a;Sporns, 2011). Brain networks tend to demonstrate an efficient, “small-world” organization where local connectivity (clustering) is high and long-range connections (path lengths) are economical (Sporns, 2011). Both global and local network metrics can be calculated including modularity, which provides an assessment of sub-networks (Stevens, et al., 2012), hub analysis to identify regions that are central to the network’s organization, and attack analyses, which measure the network’s resilience (Hosseini, et al., 2012a). Methodological considerations include parcellation scheme (Shen, et al., 2013), choice of benchmark networks (Hosseini and Kesler, 2013b) and thresholding (van Wijk, et al., 2010).
Multivoxel pattern analysis (MVPA) uses machine learning, a branch of artificial intelligence, to automatically find multivariate patterns of brain structure and/or function that accurately predict categorical or continuous variables (Mahmoudi, et al., 2012;Orru, et al., 2012). MVPA is a highly sensitive method due to its ability to utilize subtle signals across voxels that tend to be undetectable by univariate analyses (Kamitani and Tong, 2005). The functional connectivity correlation matrix or other extracted rsfMRI measures can be used as features (i.e. input variables) for MVPA classification (Craddock, et al., 2009). Linear support vector machine is currently the most common MVPA approach because of its ability to handle large, high-dimensional datasets (Orru, et al., 2012). Like all of the above methods, region of interest, or feature selection, can significantly influence MVPA results (Mahmoudi, et al., 2012).
3.2. Task-induced deactivation (TID)
Intrinsic network activity is attenuated during active tasks in a dose-dependent manner with increased TID occurring with increased task-difficulty (Newton, et al., 2011). Thus, TID can be measured by identifying regions where activation is greater during passive or low-load conditions compared to active or higher-load conditions. In fMRI analyses, statistical modeling is generally accomplished by creating contrasts or subtractions of various task-related conditions or events. The aim is to examine activation patterns associated with the effect of interest after removing irrelevant or nuisance activations. These nuisance activations tend to be accounted for using a control condition such as the 0-back epochs in an n-back paradigm, for example. TID can be measured by subtracting active task conditions from these control conditions (Buckner, et al., 2008). Because DMN TID occurs across tasks (Sambataro, et al., 2010), any fMRI task paradigm could potentially be used.
4. DMN and chemotherapy
BCC-related cognitive dysfunction may represent a brain network disorder given the widespread brain alterations that are noted even decades after treatment has ended (Pomykala, et al., 2013a). The coordinated, dynamic brain network response that supports cognitive function depends critically on stable structural networks (Sporns, 2011). Accordingly, intrinsic functional connectivity is dependent on underlying structural connectivity (Damoiseaux, et al., 2012;Hosseini and Kesler, 2013a). BCC is associated with widespread reductions in white matter pathway integrity in regions including cingulum and superior frontal occipital fasciculus (Deprez, et al., 2013), which connect DMN regions. Deficits in white matter structure reduce functional integration. BCC survivors also demonstrate reduced gray matter volumes of DMN regions including precuneus, cingulate, lateral parietal cortex, medial frontal gyrus and hippocampus (Pomykala, et al., 2013a). Alterations in gray matter structure reduce functional specialization.
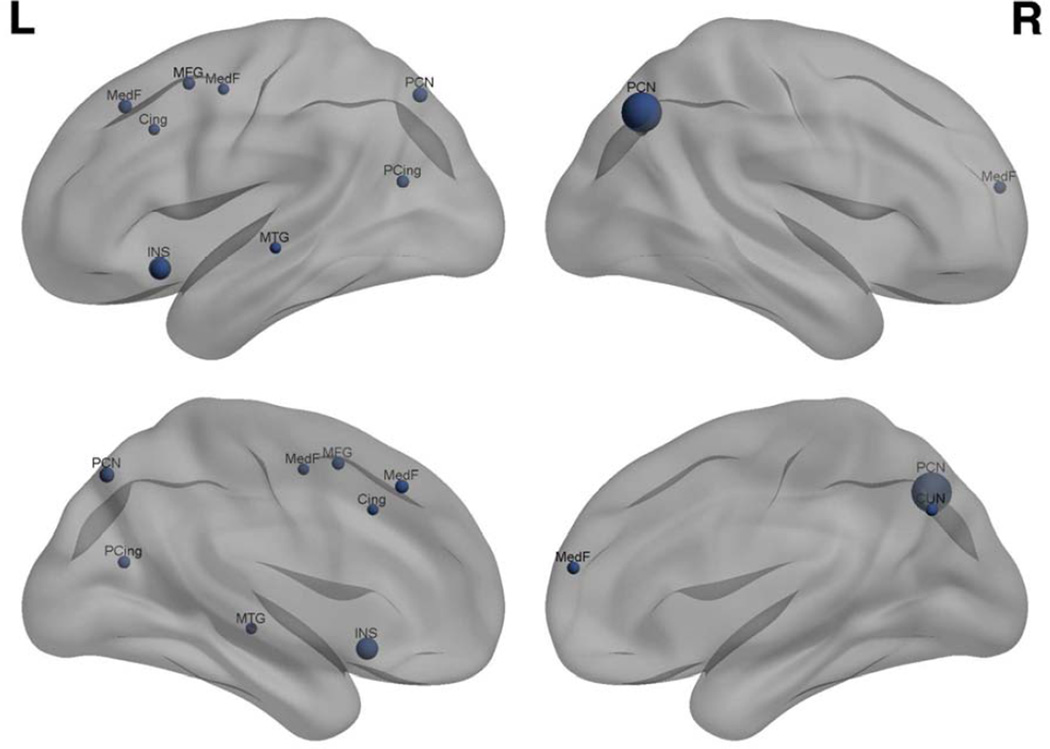
Most neuroimaging studies of BCC to date have involved task-based fMRI methods. Although these studies did not aim to examine DMN, they may collectively suggest reduced task-related response of DMN regions. To investigate this possibility, a quantitative, anatomic likelihood estimate meta-analysis was performed using GingerALE software (http://www.brainmap.org/ale/) (Laird, et al., 2011). A threshold of p < 0.05, false discovery rate (nonparametric p-value) corrected with a 40mm minimum cluster volume was used. All studies that employed task-based fMRI to study BCC and neurobiologic status were included. PubMed searches included the keywords “cancer and chemotherapy and brain” or “cancer and chemotherapy and brain and MRI”. Studies were also identified using recent reviews (de Ruiter and Schagen, 2013;Pomykala, et al., 2013a;Scherling and Smith, 2013;Simo, et al., 2013). Six studies were identified (36 total foci, 258 total participants) (Conroy, et al., 2013b;de Ruiter, et al., 2011;Kesler, et al., 2009;Kesler, et al., 2011;Lopez Zunini, et al., 2012;McDonald, et al., 2012). Meta-analysis indicated that abnormality of regions involved in DMN was the most consistent finding, particularly in the precuneus and medial frontal gyrus (Figure 1). It is important to note that DMN activity is reduced during tasks, not extinguished. Task-related functional activation is believed to reflect a combination of spontaneous intrinsic network activity and response to cognitive load (Fox, et al., 2006). The present meta-analysis suggests disrupted modulation of the DMN during various executive and memory tasks following BCC. However, these findings are an indirect assessment of DMN function and precuneus and medial frontal gyrus participate in other brain networks as well.
Figure 1.
Meta-analysis results showing regions of significant (p < 0.05 corrected) overlap across fMRI studies of BCC-related brain activation abnormality. Increased region marker size indicates increased cluster volume. PCN: precuneus (MNI coordinates: 40,−68,42; −16,−72,48), INS: insula (−32,18,−12), MedF: medial frontal gyrus (−6,30,44; 4,56,16; −16,−4,50), PCing: posterior cingulate (−28,−66,18), CUN: cuneus (8,−68,36), MFG: middle frontal gyrus (−26,8,52), MTG: middle temporal gyrus (−56,−22,−5), Cing: cingulate (−10,20,36). Figure created using BrainNet Viewer (http://www.nitrc.org/projects/bnv/).
Application of graph theory in neuroimaging provides novel insight regarding the topology of large-scale brain networks. Increased age tends to be associated with reduced global efficiency of large-scale brain networks, which is believed to represent a high risk for dysconnectivity syndromes such as MCI and dementia (Wu, et al., 2013). Altered organization of large-scale structural and intrinsic functional brain networks has been demonstrated in patients with brain tumor (Bartolomei, et al., 2006;Bosma, et al., 2009;Heimans and Reijneveld, 2012) and patients exposed to intrathecal chemotherapy (Hosseini, et al., 2012a) as well as following BCC.
Hosseini et al. examined organization of the large-scale gray matter network in 37 BCC survivors compared to 38 healthy female controls. Breast cancer survivors had completed chemotherapy an average of 4.5 years prior to study enrollment. Using graph theoretical analysis of 90 anatomic regions of interest, a structural correlational brain network was constructed for each group. Brain network statistics were computed, including mean clustering coefficient, a measure of network segregation, characteristic path length, a measure of network integration and small-worldness, a measure of the network’s ability to balance segregation and integration. A permutation distribution was created based on 1000 benchmark networks to determine the significance of between group differences in network measures. Results indicated that the BCC group demonstrated significantly reduced mean clustering (p = 0.03) and marginally reduced small-worldness (p = 0.08). Further, the control group demonstrated expected hub regions in DMN including precuneus and middle temporal gyrus whereas the BCC group did not (Hosseini, et al., 2012b). These findings suggest that chemotherapy may disrupt participation of DMN regions in the structural brain network.
A follow up study of the same cohort, demonstrated that organization of the global resting state functional brain network is also disrupted following chemotherapy (Bruno, et al., 2012). Consistent with the previous study, mean clustering was significantly reduced in BCC (p = 0.03) with a marginal reduction in small-worldness (p = 0.06). Additionally, characteristic path length was decreased in the BCC group (p = 0.05) indicating inefficient functional network integration. Again, the BCC group showed an altered hub profile with hippocampus not participating effectively in the functional brain network. There were no significant correlations between network measures and cognitive or demographic factors. Although neither of these brain network studies examined DMN specifically, they provide evidence that BCC negatively impacts large-scale brain networks, particularly with respect to DMN connectivity. However, without a non-chemotherapy comparison group, it is not possible to conclude from these studies that alterations in DMN are specific to chemotherapy.
Altered brain network organization not only results in cognitive impairment but also reduces the brain’s resilience (Achard, et al., 2006;He, et al., 2008;Hosseini, et al., 2012a;Rubinov, et al., 2009) consistent with the frail phenotype of aging and reduced cognitive reserve, both significant predictors of pathologic cognitive decline (Mandelblatt, et al., 2013;Steffener and Stern, 2012;Stern, 2012;Whalley, et al., 2004). Thus, even if a patient does not demonstrate symptoms of chemotherapy-related cognitive dysfunction at the time of initial evaluation, she may have increased vulnerability for cognitive decline due to later disease, injury and aging. Decreased brain resilience may help explain the new onset of previously non-existent cognitive impairment and progressive worsening of existing symptoms that have been noted in some chemotherapy-treated breast cancer survivors (Wefel, et al., 2010).
Two PET studies from the same research group examined both task-related and resting state cerebral metabolism in BCC (Pomykala, et al., 2013b;Silverman, et al., 2007). The earlier study involved 16 BCC survivors 5–10 years post-chemotherapy and 8 healthy female controls (Silverman, et al., 2007) while the later study involved 23 chemotherapy treated and 10 non-chemotherapy treated breast cancer patients assessed after completing chemotherapy and 1 year later (Pomykala, et al., 2013b). Differences in PET activation were measured using whole brain voxel-wise as well as region of interest analyses. There were no between group or longitudinal differences in resting metabolism in either study even though regions of interest included some DMN regions. However, the study did not actually aim to measure DMN and did not utilize TID or functional connectivity methods. Therefore, it is not likely that DMN would have been detected. PET studies provided the original evidence of DMN through meta-analysis of TID (Buckner, et al., 2008). PET’s limited spatial and temporal resolution prevents functional connectivity analysis at the individual participant level (Greicius, et al., 2007) and may result in inconsistent DMN results compared with fMRI (Di, et al., 2012).
Very few direct investigations of DMN in BCC have been conducted to date. A case study by LaViolette et al. examined change in DMN functional connectivity of a patient with breast cancer from pre-chemotherapy to 3 months post-chemotherapy compared to 5 control participants. RsfMRI was used to measure functional connectivity and arterial spin labeling was used to measure cerebral blood flow. DMN connectivity was defined via SBC of posterior cingulate. Functional connectivity between posterior cingulate and medial temporal cortex and hippocampus were significantly reduced post-chemotherapy (p < 0.005). Additionally, cerebral blood flow was decreased in the lateral parietal cortex and precuneus (p < 0.001) (LaViolette, 2009). This study preliminarily demonstrates that BCC-related DMN changes can be measured at the individual level, potentially providing valuable patient-specific information.
In a longitudinal study, Conroy and colleagues investigated the effect of chemotherapy-induced amenorrhea (CIA) on task-induced activation as well as TID during an n-back working memory task in 9 post-menopausal BC patients undergoing chemotherapy, 9 pre- or peri-menopausal BC patients undergoing chemotherapy and 6 healthy female controls. TID was used to define DMN. There were no significant differences in activation or deactivation among the groups. However, the CIA group showed a significant increase in the summed magnitude of activation and deactivation from pre-chemotherapy to one month post-chemotherapy (p = 0.011) (Conroy, et al., 2013a). This study suggests that alteration in DMN TID may play a role in working memory dysfunction following BCC and that menopausal status is an important factor to consider when evaluating the effects of BCC on DMN. However, the study was very limited by the small sample size and lack of separation between the contribution of task-induced activation versus DMN TID to the overall findings.
Dumas and colleagues conducted an SBC analysis using an fMRI n-back task in 9 patients with breast cancer prior to chemotherapy, 1 month and 1 year after completion of chemotherapy. Task-related activation was removed via regression. An intraparietal sulcus seed was used to interrogate dorsal attention network connectivity and a posterior cingulate seed was used to investigate DMN. Dorsal attention network connectivity was decreased at 1 month with partial recovery at 1 year (p < 0.001, uncorrected). DMN connectivity was also decreased at 1 month, specifically in the precuneus, and did not recover at 1 year (p = 0.001, uncorrected). N-back performance did not change over time but self-rated memory difficulties decreased across all 3 time points (Dumas, et al., 2013). The authors did not correlate fMRI and cognitive-behavioral data so it is unknown if changes in connectivity were related to changes in memory complaints. This study was limited by the emulation method of resting state connectivity, small sample size, lack of control group and uncorrected statistical threshold. However, it provides further preliminary evidence that DMN may show increased vulnerability to chemotherapy characterized by limited recovery compared to other brain networks.
To examine the specificity of BCC on DMN, Kesler and colleagues conducted MVPA of DMN functional connectivity in 30 BCC and 27 non-chemotherapy treated breast cancer survivors (4.9 ± 3.4 years post-chemotherapy) as well as 24 healthy female controls. RsfMRI temporal correlations were calculated between 19 functional regions of interest. Data were corrected for age, education, psychiatric symptoms and gray matter volume. The MVPA classifiers were tested for each group pair as well disease stage (breast cancer group only) using leave-one-out cross-validation. Permutation analysis was conducted to determine classifier significance at an alpha level adjusted for multiple comparisons.
Patterns of DMN connectivity significantly distinguished chemotherapy from both non-chemotherapy survivors and healthy females with 90–91% accuracy [p < 0.0001, receiver operating characteristic (ROC) effect sizes: 0.97–0.98]. The non-chemotherapy group could not be distinguished from the healthy control group and disease stage classifiers also were not significantly better than chance. Connectivity within DMN regions as well as connectivity between DMN and prefrontal regions contributed the most to the classifiers indicating that these connections were the most important for distinguishing the groups. The classifiers were significantly correlated with memory complaints (p < 0.002) indicating that the more similar a participant’s DMN connectivity to that of the chemotherapy group, the lower the memory function (Kesler, et al., 2013c). This study was limited by its cross-sectional design but provides evidence that DMN may be preferentially vulnerable to chemotherapy.
There are multiple different brain networks and chemotherapy could potentially affect several of these. In fact, the profile of BCC-related cognitive impairments strongly suggests deficit of central executive network and several studies have demonstrated altered prefrontal cortex structure and function following chemotherapy (de Ruiter and Schagen, 2013). Hosseini and Kesler examined prefrontal functional connectivity in 27 chemotherapy treated and 29 non-chemotherapy treated breast cancer survivors and 30 healthy females. fMRI data were obtained during an executive function task. Functional connectivity was calculated between 12 frontal and parietal executive network regions of interest. MVPA was conducted as described above.
The MVPA classifiers significantly distinguished between the chemotherapy group and both the non-chemotherapy and health female group with 71–72% accuracy (p < 0.01, ROC: 0.71–0.72). The classifier for non-chemotherapy and healthy females was not significant. Connections between prefrontal and parietal regions contributed the most in discriminating the chemotherapy group from healthy females while connections within prefrontal regions had the greatest weight for discriminating the two breast cancer groups (Hosseini and Kesler, 2013c). ROC effect sizes were much lower than those obtained for the DMN classifier (Kesler, et al., 2013c). Additionally, the prefrontal classifier was associated with disease stage (p < 0.05) whereas the DMN classifier was not. These findings provide further evidence that DMN may be preferentially vulnerable to chemotherapy versus disease severity or alternate treatments (e.g. radiation) compared to other networks. However, prospective, longitudinal studies are needed to further evaluate these findings.
5. Conclusions and future directions
Thus far, studies of DMN in BCC have been very limited. Many have involved cross-sectional designs, small sample sizes and/or lacked appropriate comparison groups. However, indirect as well as direct neuroimaging evidence suggests that DMN represents a promising potential biomarker of chemotherapy-related brain injury. The inclusion of rsfMRI in prospective study designs will allow investigation of longitudinal changes in DMN connectivity. Thus far, the majority of neuroimaging studies of BCC have involved middle aged women (Ahles, 2012). Future efforts should focus on longitudinal evaluation of very long-term survivors and/or older patients in order to examine how BCC may moderate the effect of age on DMN status.
To address the issue of small sample sizes, a standard neuroimaging battery analogous to that proposed by the International Cognition and Cancer Task Force for harmonizing studies of cognitive status (Wefel, et al., 2011) is needed. This would allow multi-site research groups to combine data and increase statistical power. This review suggests that the inclusion of rsfMRI is indicated. RsfMRI can be used to assess multiple brain systems using one brief acquisition (Fox and Greicius, 2010). Resting state networks closely correspond to a variety of active networks (Smith, et al., 2009) and rsfMRI has been used to decode networks associated with various cognitive states including memory, executive control and salience, among others (Shirer, et al., 2011). The simplicity of rsfMRI makes it easy to standardize across sites and more feasible for a wider range of participants, including older adults. Additionally, task-related changes in brain metabolism tend to be quite small compared to the brain’s large resting state energy consumption (Raichle and Mintun, 2006). RsfMRI may therefore provide a more robust source of disease-related signal change (Fox and Greicius, 2010;Raichle and Mintun, 2006).
As noted above, amyloid beta deposition is a key element in the pathology of age-related cognitive decline and neurodegeration (Herrup, 2010) but it is unknown if this toxicity plays a role in BCC-related brain injury. Chemotherapy may theoretically increase amyloid beta accumulation through elevation of inflammation and oxidative stress (Cai, et al., 2011), altered glucose metabolism (Baudino, et al., 2011) and/or other factors. Future studies combining APOE status, PET-based amyloid beta markers (i.e. Florbetapir) and rsfMRI are required to examine the interactions between aging, amyloid accumulation, genetic risk, intrinsic brain networks and chemotherapy.
The increased application of multivariate neuroimaging analyses to the study of chemobrain would significantly advance this field of research. Most studies thus far have employed mass univariate methods, which are very limited with respect to evaluation of large-scale networks as well as prediction of individual patient outcome. Chemotherapy treatment is the only situation where it is known in advance that a potential brain injury is about to occur. With further refinement of MVPA algorithms, baseline neuroimaging could be used to predict which patients are at highest risk for persistent chemotherapy-related brain injury. This information could potentially inform treatment regimen decision-making and prioritize patients for early interventions. The inclusion of multimodal DMN biomarkers, such as a combination of rsfMRI and DTI, may also improve prediction accuracy. MVPA methods could help separate the neurobiological effects of different treatments (e.g. chemotherapy vs. endocrine therapy) as well as psychiatric symptoms (e.g. depression, fatigue vs. chemotherapy).
Finally, further study of DMN’s role in chemotherapy-related cognitive decline could aid in the development of interventions. Both cognitive and physical exercise have been shown to increase DMN functional connectivity in healthy adults (Takeuchi, et al., 2013,Voss, et al., 2010). Medication that has shown promise in improving chemobrain symptoms (i.e. modafinil) improves DMN TID in healthy adults (Minzenberg, et al., 2011). Additionally, given the strong link between DMN decline and metabolic processes, DMN biomarkers associated with chemotherapy may provide an important opportunity for assessing nutritional interventions. For example, regulators of mitochondrial metabolic activity (e.g. B- vitamins) and dietary energy restrictions may reduce or prevent amyloid beta toxicity in the brain (Yao, et al., 2011,Kapogiannis and Mattson, 2011).
Acknowledgements
This work was supported by the National Institutes of Health New Innovator Award (1DP2OD004445) and the National Cancer Institute (1R01CA172145).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement
The author has no conflicts of interest.
References
- Abraham A. The world according to me: personal relevance and the medial prefrontal cortex. Front Hum Neurosci. 2013;7:341. doi: 10.3389/fnhum.2013.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achard S, Salvador R, Whitcher B, Suckling J, Bullmore E. A resilient, low-frequency, small- world human brain functional network with highly connected association cortical hubs. J Neurosci. 2006;26(1):63–72. doi: 10.1523/JNEUROSCI.3874-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnati LF, Guidolin D, Battistin L, Pagnoni G, Fuxe K. The neurobiology of imagination: possible role of interaction-dominant dynamics and default mode network. Frontiers in psychology. 2013;4:296. doi: 10.3389/fpsyg.2013.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles TA. Brain vulnerability to chemotherapy toxicities. Psychooncology. 2012;21:1141–1148. doi: 10.1002/pon.3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer. 2007;7(3):192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ, McDonald BC, Li Y, Furstenberg CT, Hanscom BS, Mulrooney TJ, Schwartz GN, Kaufman PA. Longitudinal Assessment of Cognitive Changes Associated With Adjuvant Treatment for Breast Cancer: Impact of Age and Cognitive Reserve. Journal of Clinical Oncology. 2010;28(29):4434–4440. doi: 10.1200/JCO.2009.27.0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ, Noll WW, Furstenberg CT, Guerin S, Cole B, Mott LA. The relationship of APOE genotype to neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy. Psychooncology. 2003;12(6):612–619. doi: 10.1002/pon.742. [DOI] [PubMed] [Google Scholar]
- Alexander-Bloch A, Giedd JN, Bullmore E. Imaging structural co-variance between human brain regions. Nat Rev Neurosci. 2013;14(5):322–336. doi: 10.1038/nrn3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong RA. The pathogenesis of Alzheimer's disease: a reevaluation of the "amyloid cascade hypothesis". International journal of Alzheimer's disease. 2011;2011:630865. doi: 10.4061/2011/630865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomei F, Bosma I, Klein M, Baayen JC, Reijneveld JC, Postma TJ, Heimans JJ, van Dijk BW, de Munck JC, de Jongh A, Cover KS, Stam CJ. Disturbed functional connectivity in brain tumour patients: evaluation by graph analysis of synchronization matrices. Clin Neurophysiol. 2006;117(9):2039–2049. doi: 10.1016/j.clinph.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Baudino B, D’Agata F, Castellano G, Caroppo P, Cauda S, Parente A, Manfredi M, Geda E, Orsi L, Cauda F, Castelli L, Sacco K, Ardito R, Torta R, Bisi G. Chemotherapy effects on brain glucose metabolism at rest. 2011 Nat Precedings DOI: hdl:10101/npre.2011.5637.1.
- Baxter NN, Durham SB, Phillips KA, Habermann EB, Virning BA. Risk of dementia in older breast cancer survivors: a population-based cohort study of the association with adjuvant chemotherapy. Journal of the American Geriatrics Society. 2009;57(3):403–411. doi: 10.1111/j.1532-5415.2008.02130.x. [DOI] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage. 2007;37(1):90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin C, Lieberman DA, Chang M, Ofen N, Whitfield-Gabrieli S, Gabrieli JD, Gaab N. The influence of rest period instructions on the default mode network. Front Hum Neurosci. 2010;4:218. doi: 10.3389/fnhum.2010.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bero AW, Yan P, Roh JH, Cirrito JR, Stewart FR, Raichle ME, Lee JM, Holtzman DM. Neuronal activity regulates the regional vulnerability to amyloid-beta deposition. Nat Neurosci. 2011;14(6):750–756. doi: 10.1038/nn.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow K, Hardy J, Zetterberg H. The neuropathology and neurobiology of traumatic brain injury. Neuron. 2012;76(5):886–899. doi: 10.1016/j.neuron.2012.11.021. [DOI] [PubMed] [Google Scholar]
- Bosma I, Reijneveld JC, Klein M, Douw L, van Dijk BW, Heimans JJ, Stam CJ. Disturbed functional brain networks and neurocognitive function in low-grade glioma patients: a graph theoretical analysis of resting-state MEG. Nonlinear Biomed Phys. 2009;3(1):9. doi: 10.1186/1753-4631-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boubela RN, Kalcher K, Huf W, Kronnerwetter C, Filzmoser P, Moser E. Beyond Noise: Using Temporal ICA to Extract Meaningful Information from High-Frequency fMRI Signal Fluctuations during Rest. Front Hum Neurosci. 2013;7:168. doi: 10.3389/fnhum.2013.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno J, Hosseini SM, Kesler S. Altered resting state functional brain network topology in chemotherapy-treated breast cancer survivors. Neurobiol Dis. 2012;48(3):329–338. doi: 10.1016/j.nbd.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25(34):7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. The economy of brain network organization. Nat Rev Neurosci. 2012;13(5):336–349. doi: 10.1038/nrn3214. [DOI] [PubMed] [Google Scholar]
- Cai Z, Zhao B, Ratka A. Oxidative stress and beta-amyloid protein in Alzheimer's disease. Neuromolecular medicine. 2011;13(4):223–250. doi: 10.1007/s12017-011-8155-9. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T. Multisubject independent component analysis of fMRI: a decade of intrinsic networks, default mode, and neurodiagnostic discovery. IEEE reviews in biomedical engineering. 2012;5:60–73. doi: 10.1109/RBME.2012.2211076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Liu J, AdalI Tl. A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. NeuroImage. 2009;45(1, Supplement 1):S163–S172. doi: 10.1016/j.neuroimage.2008.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Glover GH. Effects of model-based physiological noise correction on default mode network anti-correlations and correlations. NeuroImage. 2009;47(4):1448–1459. doi: 10.1016/j.neuroimage.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JL, Ros T, Gruzelier JH. Dynamic changes of ICA-derived EEG functional connectivity in the resting state. Hum Brain Mapp. 2013;34(4):852–868. doi: 10.1002/hbm.21475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DM, Smith SM, Beckmann CF. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Front Syst Neurosci. 2010a;4:8. doi: 10.3389/fnsys.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Pathak S, Schneider W. Identifying the brain’s most globally connected regions. NeuroImage. 2010b;49(4):3132–3148. doi: 10.1016/j.neuroimage.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Conroy SK, McDonald BC, Ahles TA, West JD, Saykin AJ. Chemotherapy-induced amenorrhea: a prospective study of brain activation changes and neurocognitive correlates. Brain Imaging Behav. 2013a;7(4):491–500. doi: 10.1007/s11682-013-9240-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy SK, McDonald BC, Smith DJ, Moser LR, West JD, Kamendulis LM, Klaunig JE, Champion VL, Unverzagt FW, Saykin AJ. Alterations in brain structure and function in breast cancer survivors: effect of post-chemotherapy interval and relation to oxidative DNA damage. Breast Cancer Res Treat. 2013b;137(2):493–502. doi: 10.1007/s10549-012-2385-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock RC, Holtzheimer PE, 3rd, Hu XP, Mayberg HS. Disease state prediction from resting state functional connectivity. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2009;62(6):1619–1628. doi: 10.1002/mrm.22159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS. Resting-state fMRI as a biomarker for Alzheimer’s disease? Alzheimer's research & therapy. 2012;4(2):8. doi: 10.1186/alzrt106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Prater KE, Miller BL, Greicius MD. Functional connectivity tracks clinical deterioration in Alzheimer's disease. Neurobiol Aging. 2012;33(4):e19–e30. doi: 10.1016/j.neurobiolaging.2011.06.024. 828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(37):13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ruiter MB, Reneman L, Boogerd W, Veltman DJ, van Dam FS, Nederveen AJ, Boven E, Schagen SB. Cerebral hyporesponsiveness and cognitive impairment 10 years after chemotherapy for breast cancer. Hum Brain Mapp. 2011;32(8):1206–1219. doi: 10.1002/hbm.21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ruiter MB, Schagen SB. Functional MRI studies in non-CNS cancers. Brain Imaging Behav. 2013;7(4):388–408. doi: 10.1007/s11682-013-9249-9. [DOI] [PubMed] [Google Scholar]
- Deprez S, Billiet T, Sunaert S, Leemans A. Diffusion tensor MRI of chemotherapy-induced cognitive impairment in non-CNS cancer patients: a review. Brain Imaging Behav. 2013;7(4):409–435. doi: 10.1007/s11682-012-9220-1. [DOI] [PubMed] [Google Scholar]
- Di X, Biswal BB, Alzheimer’s Disease Neuroimaging. I Metabolic brain covariant networks as revealed by FDG-PET with reference to resting-state fMRI networks. Brain connectivity. 2012;2(5):275–283. doi: 10.1089/brain.2012.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du XL, Xia R, Hardy D. Relationship between chemotherapy use and cognitive impairments in older women with breast cancer: findings from a large population-based cohort. Am J Clin Oncol. 2010;33(6):533–543. doi: 10.1097/COC.0b013e3181b9cf1b. [DOI] [PubMed] [Google Scholar]
- Dumas JA, Makarewicz J, Schaubhut GJ, Devins R, Albert K, Dittus K, Newhouse PA. Chemotherapy altered brain functional connectivity in women with breast cancer: a pilot study. Brain Imaging Behav. 2013;7(4):524–532. doi: 10.1007/s11682-013-9244-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan NW, Northoff G. Overview of potential procedural and participant-related confounds for neuroimaging of the resting state. Journal of psychiatry & neuroscience : JPN. 2013;38(2):84–96. doi: 10.1503/jpn.120059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Schlaggar BL, Cohen AL, Miezin FM, Dosenbach NU, Wenger KK, Fox MD, Snyder AZ, Raichle ME, Petersen SE. A method for using blocked and event-related fMRI data to study "resting state" functional connectivity. NeuroImage. 2007;35(1):396–405. doi: 10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Greicius M. Clinical applications of resting state functional connectivity. Front Syst Neurosci. 2010;4:19. doi: 10.3389/fnsys.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Zacks JM, Raichle ME. Coherent spontaneous activity accounts for trial-to-trial variability in human evoked brain responses. Nat Neurosci. 2006;9(1):23–25. doi: 10.1038/nn1616. [DOI] [PubMed] [Google Scholar]
- Ganz PA, Bower JE, Kwan L, Castellon SA, Silverman DHS, Geist C, Breen EC, Irwin MR, Cole SW. Does tumor necrosis factor-alpha (TNF-α) play a role in post-chemotherapy cerebral dysfunction? Brain, Behavior, and Immunity. 2013;30:S99–S108. doi: 10.1016/j.bbi.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Reiss AL, Schatzberg AF. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62(5):429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(13):4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SK, Lamont EB. Patterns of presentation, diagnosis, and treatment in older patients with colon cancer and comorbid dementia. J Am Geriatr Soc. 2004;52(10):1681–1687. doi: 10.1111/j.1532-5415.2004.52461.x. [DOI] [PubMed] [Google Scholar]
- He Y, Chen Z, Evans A. Structural insights into aberrant topological patterns of large-scale cortical networks in Alzheimer's disease. J Neurosci. 2008;28(18):4756–4766. doi: 10.1523/JNEUROSCI.0141-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck JE, Albert SM, Franco R, Gorin SS. Patterns of dementia diagnosis in surveillance, epidemiology, and end results breast cancer survivors who use chemotherapy. Journal of the American Geriatrics Society. 2008;56(9):1687–1692. doi: 10.1111/j.1532-5415.2008.01848.x. [DOI] [PubMed] [Google Scholar]
- Heimans JJ, Reijneveld JC. Factors affecting the cerebral network in brain tumor patients. J Neurooncol. 2012;108(2):231–237. doi: 10.1007/s11060-012-0814-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrup K. Reimagining Alzheimer's disease--an age-based hypothesis. J Neurosci. 2010;30(50):16755–16762. doi: 10.1523/JNEUROSCI.4521-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogervorst E. Effects Of Gonadal Hormones On Cognitive Behavior In Elderly Men And Women. Journal of neuroendocrinology. 2013;25(11):1182–1195. doi: 10.1111/jne.12080. [DOI] [PubMed] [Google Scholar]
- Hosseini SM, Hoeft F, Kesler SR. GAT: A Graph-Theoretical Analysis Toolbox for Analyzing Between-Group Differences in Large-Scale Structural and Functional Brain Networks. PLoS ONE. 2012a;7(7):e40709. doi: 10.1371/journal.pone.0040709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini SM, Kesler SR. Comparing connectivity pattern and small-world organization between structural correlation and resting-state networks in healthy adults. NeuroImage. 2013a;78:402–414. doi: 10.1016/j.neuroimage.2013.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini SM, Kesler SR. Influence of choice of null network on small-world parameters of structural correlation networks. PLoS ONE. 2013b;8(6):e67354. doi: 10.1371/journal.pone.0067354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini SM, Kesler SR. Multivariate Pattern Analysis of fMRI in Breast Cancer Survivors and Healthy Women. J Int Neuropsychol Soc. 2013c;19:1–11. doi: 10.1017/S1355617713001173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini SM, Koovakkattu D, Kesler SR. Altered small-world properties of gray matter networks in breast cancer. BMC Neurol. 2012b;12(1):28. doi: 10.1186/1471-2377-12-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlader N, Noone A, Krapcho M, Garshell J, Neyman N, Altekruse S, Kosary C, Yu M, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Lewis D, Chen H, Feuer E, Cronin K. SEER Cancer Statistics Review. 2013 1975–2010 National Cancer Institute. [Google Scholar]
- Hutchison RM, Womelsdorf T, Allen EA, Bandettini PA, Calhoun VD, Corbetta M, Della Penna S, Duyn JH, Glover GH, Gonzalez-Castillo J, Handwerker DA, Keilholz S, Kiviniemi V, Leopold DA, de Pasquale F, Sporns O, Walter M, Chang C. Dynamic functional connectivity: Promise, issues, and interpretations. NeuroImage. 2013;80:360–378. doi: 10.1016/j.neuroimage.2013.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser J, Bledowski C, Dietrich J. Neural correlates of chemotherapy-related cognitive impairment. Cortex. 2014 doi: 10.1016/j.cortex.2014.01.010. [DOI] [PubMed] [Google Scholar]
- Kamitani Y, Tong F. Decoding the visual and subjective contents of the human brain. Nat Neurosci. 2005;8(5):679–685. doi: 10.1038/nn1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler S, Janelsins M, Koovakkattu D, Palesh O, Mustian K, Morrow G, Dhabhar FS. Reduced hippocampal volume and verbal memory performance associated with interleukin-6 and tumor necrosis factor-alpha levels in chemotherapy-treated breast cancer survivors. Brain Behav Immun. 2013a;(30 Suppl):S109–S116. doi: 10.1016/j.bbi.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Bennett FC, Mahaffey ML, Spiegel D. Regional brain activation during verbal declarative memory in metastatic breast cancer. Clin Cancer Res. 2009;15(21):6665–6673. doi: 10.1158/1078-0432.CCR-09-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Kent JS, O’Hara R. Prefrontal cortex and executive function impairments in primary breast cancer. Archives of neurology. 2011;68(11):1447–1453. doi: 10.1001/archneurol.2011.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Watson C, Koovakkattu D, Lee C, O’Hara R, Mahaffey ML, Wefel JS. Elevated prefrontal myo-inositol and choline following breast cancer chemotherapy. Brain Imaging Behav. 2013b;7(4):501–510. doi: 10.1007/s11682-013-9228-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Wefel JS, Hosseini SM, Cheung M, Watson CL, Hoeft F. Default mode network connectivity distinguishes chemotherapy-treated breast cancer survivors from controls. Proceedings of the National Academy of Sciences of the United States of America. 2013c;110(28):11600–11605. doi: 10.1073/pnas.1214551110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppelmans V, Breteler MM, Boogerd W, Seynaeve C, Schagen SB. Late effects of adjuvant chemotherapy for adult onset non-CNS cancer; cognitive impairment, brain structure and risk of dementia. Crit Rev Oncol Hematol. 2013;88:87–101. doi: 10.1016/j.critrevonc.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Koppelmans V, de Ruiter MB, van der Lijn F, Boogerd W, Seynaeve C, van der Lugt A, Vrooman H, Niessen WJ, Breteler MM, Schagen SB. Global and focal brain volume in long-term breast cancer survivors exposed to adjuvant chemotherapy. Breast Cancer Res Treat. 2012;132(3):1099–1106. doi: 10.1007/s10549-011-1888-1. [DOI] [PubMed] [Google Scholar]
- Kravitz E, Schmeidler J, Beeri MS. Cognitive decline and dementia in the oldest-old. Rambam Maimonides medical journal. 2012;3(4):e0026. doi: 10.5041/RMMJ.10092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Fox PM, Uecker AM, Ray KL, Saenz JJ, Jr, McKay DR, Bzdok D, Laird RW, Robinson JL, Turner JA, Turkeltaub PE, Lancaster JL, Fox PT. The BrainMap strategy for standardization, sharing, and meta-analysis of neuroimaging data. BMC Res Notes. 2011;4:349. doi: 10.1186/1756-0500-4-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaViolette P, Collier W, Schmainda KM, Piacentine L, Douville KL, Chitambar CR, Tran A, Claesges SA, Durgerian SJ, Bloom AS. Functional Connectivity and Arterial Spin Labeling in Chemotherapy Induced Cognitive Impairment ("Chemobrain") Honolulu, HI, USA: International Society for Magnetic Resonance Medicine (ISMRM); 2009. URL: http://cds.ismrm.org/protected/09MProceedings/files/02231.pdf. [Google Scholar]
- Liu D, Dong Z, Zuo X, Wang J, Zang Y. Eyes-Open/Eyes-Closed Dataset Sharing for Reproducibility Evaluation of Resting State fMRI Data Analysis Methods. Neuroinformatics. 2013;11(4):469–476. doi: 10.1007/s12021-013-9187-0. [DOI] [PubMed] [Google Scholar]
- Lopez Zunini RA, Scherling C, Wallis N, Collins B, Mackenzie J, Bielajew C, Smith AM. Differences in verbal memory retrieval in breast cancer chemotherapy patients compared to healthy controls: a prospective fMRI study. Brain Imaging Behav. 2012;7(4):460–477. doi: 10.1007/s11682-012-9213-0. [DOI] [PubMed] [Google Scholar]
- Lord LD, Expert P, Huckins JF, Turkheimer FE. Cerebral energy metabolism and the brain’s functional network architecture: an integrative review. J Cereb Blood Flow Metab. 2013;33(9):1347–1354. doi: 10.1038/jcbfm.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch MA. Age-related neuroinflammatory changes negatively impact on neuronal function. Front Aging Neurosci. 2010;1:6. doi: 10.3389/neuro.24.006.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudi A, Takerkart S, Regragui F, Boussaoud D, Brovelli A. Multivoxel pattern analysis for FMRI data: a review. Computational and mathematical methods in medicine. 2012;2012:961257. doi: 10.1155/2012/961257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelblatt JS, Hurria A, McDonald BC, Saykin AJ, Stern RA, VanMeter JW, McGuckin M, Traina T, Denduluri N, Turner S, Howard D, Jacobsen PB, Ahles T Thinking, Living With Cancer, S. Cognitive effects of cancer and its treatments at the intersection of aging: what do we know; what do we need to know? Semin Oncol. 2013;40(6):709–725. doi: 10.1053/j.seminoncol.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies DS, Bottger J, Long X, Lv Y, Kelly C, Schafer A, Goldhahn D, Abbushi A, Milham MP, Lohmann G, Villringer A. Resting developments: a review of fMRI post-processing methodologies for spontaneous brain activity. MAGMA. 2010;23(5–6):289–307. doi: 10.1007/s10334-010-0228-5. [DOI] [PubMed] [Google Scholar]
- McDonald BC, Conroy SK, Ahles TA, West JD, Saykin AJ. Alterations in Brain Activation During Working Memory Processing Associated With Breast Cancer and Treatment: A Prospective Functional Magnetic Resonance Imaging Study. J Clin Oncol. 2012;30(20):2500–2508. doi: 10.1200/JCO.2011.38.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald BC, Saykin AJ. Alterations in brain structure related to breast cancer and its treatment: chemotherapy and other considerations. Brain Imaging Behav. 2013;7(4):374–387. doi: 10.1007/s11682-013-9256-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-Immune Mechanisms of Behavioral Comorbidities in Patients With Cancer. Journal of Clinical Oncology. 2008;26(6):971–982. doi: 10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje M, Dietrich J. Cognitive side effects of cancer therapy demonstrate a functional role for adult neurogenesis. Behav Brain Res. 2012;227(2):376–379. doi: 10.1016/j.bbr.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton AT, Morgan VL, Rogers BP, Gore JC. Modulation of steady state functional connectivity in the default mode and working memory networks by cognitive load. Hum Brain Mapp. 2011;32(10):1649–1659. doi: 10.1002/hbm.21138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Qin P, Nakao T. Rest-stimulus interaction in the brain: a review. Trends Neurosci. 2010;33(6):277–284. doi: 10.1016/j.tins.2010.02.006. [DOI] [PubMed] [Google Scholar]
- O’Shaughnessy JA. Chemotherapy-induced cognitive dysfunction: a clearer picture. Clin Breast Cancer. 2003;2(4 Suppl.):S89–S94. doi: 10.3816/cbc.2003.s.021. [DOI] [PubMed] [Google Scholar]
- Orru G, Pettersson-Yeo W, Marquand AF, Sartori G, Mechelli A. Using Support Vector Machine to identify imaging biomarkers of neurological and psychiatric disease: a critical review. Neurosci Biobehav Rev. 2012;36(4):1140–1152. doi: 10.1016/j.neubiorev.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Pomykala KL, de Ruiter MB, Deprez S, McDonald BC, Silverman DH. Integrating imaging findings in evaluating the post-chemotherapy brain. Brain Imaging Behav. 2013a;7(4):436–452. doi: 10.1007/s11682-013-9239-y. [DOI] [PubMed] [Google Scholar]
- Pomykala KL, Ganz PA, Bower JE, Kwan L, Castellon SA, Mallam S, Cheng I, Ahn R, Breen EC, Irwin MR, Silverman DH. The association between pro-inflammatory cytokines, regional cerebral metabolism, and cognitive complaints following adjuvant chemotherapy for breast cancer. Brain Imaging Behav. 2013b;7(4):511–523. doi: 10.1007/s11682-013-9243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin P, Northoff G. How is our self related to midline regions and the default-mode network? NeuroImage. 2011;57(3):1221–1233. doi: 10.1016/j.neuroimage.2011.05.028. [DOI] [PubMed] [Google Scholar]
- Raichle ME. The restless brain. Brain connectivity. 2006;1(1):3–12. doi: 10.1089/brain.2011.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Mintun MA. Brain work and brain imaging. Annu Rev Neurosci. 2006;29:449–76. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- Raji MA, Tamborello LP, Kuo YF, Ju H, Freeman JL, Zhang DD, Giordano SH, Goodwin JS. Risk of subsequent dementia diagnoses does not vary by types of adjuvant chemotherapy in older women with breast cancer. Medical oncology. 2009;26(4):452–459. doi: 10.1007/s12032-008-9145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M, McIntosh AR, Valenzuela MJ, Breakspear M. Simulation of neuronal death and network recovery in a computational model of distributed cortical activity. Am J Geriatr Psychiatry. 2009;17(3):210–217. doi: 10.1097/JGP.0b013e318187137a. [DOI] [PubMed] [Google Scholar]
- Sambataro F, Murty VP, Callicott JH, Tan HY, Das S, Weinberger DR, Mattay VS. Age-related alterations in default mode network: impact on working memory performance. Neurobiol Aging. 2010;31(5):839–852. doi: 10.1016/j.neurobiolaging.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai S, Homae F, Watanabe H, Sasaki AT, Tanabe HC, Sadato N, Taga G. A NIRS-fMRI study of resting state network. NeuroImage. 2012;63(1):179–193. doi: 10.1016/j.neuroimage.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Scherling CS, Smith A. Opening up the window into “chemobrain”: a neuroimaging review. Sensors. 2013;13(3):3169–3203. doi: 10.3390/s130303169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seigers R, Timmermans J, van der Horn HJ, de Vries EF, Dierckx RA, Visser L, Schagen SB, van Dam FS, Koolhaas JM, Buwalda B. Methotrexate reduces hippocampal blood vessel density and activates microglia in rats but does not elevate central cytokine release. Behav Brain Res. 2010;207(2):265–272. doi: 10.1016/j.bbr.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Raichle ME, Snyder AZ, Morris JC, Head D, Wang S, Mintun MA. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol Psychiatry. 2010;67(6):584–587. doi: 10.1016/j.biopsych.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, Tokoglu F, Papademetris X, Constable RT. Groupwise whole-brain parcellation from resting-state fMRI data for network node identification. NeuroImage. 2013;82C:403–415. doi: 10.1016/j.neuroimage.2013.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD. Decoding Subject-Driven Cognitive States with Whole-Brain Connectivity Patterns. Cerebral Cortex. 2011;22:158–165. doi: 10.1093/cercor/bhr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman DH, Dy CJ, Castellon SA, Lai J, Pio BS, Abraham L, Waddell K, Petersen L, Phelps ME, Ganz PA. Altered frontocortical, cerebellar, and basal ganglia activity in adjuvant-treated breast cancer survivors 5–10 years after chemotherapy. Breast Cancer Res Treat. 2007;103(3):303–311. doi: 10.1007/s10549-006-9380-z. [DOI] [PubMed] [Google Scholar]
- Simo M, Rifa-Ros X, Rodriguez-Fornells A, Bruna J. Chemobrain: A systematic review of structural and functional neuroimaging studies. Neurosci Biobehav Rev. 2013;37(8):1311–1321. doi: 10.1016/j.neubiorev.2013.04.015. [DOI] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF. Correspondence of the brain's functional architecture during activation and rest. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(31):13040–13055. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O. The human connectome: a complex network. Ann N Y Acad Sci. 2011;1224:109–125. doi: 10.1111/j.1749-6632.2010.05888.x. [DOI] [PubMed] [Google Scholar]
- Steffener J, Stern Y. Exploring the neural basis of cognitive reserve in aging. Biochimica et biophysica acta. 2012;1822(3):467–473. doi: 10.1016/j.bbadis.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve in ageing and Alzheimer's disease. The Lancet Neurology. 2012;11(11):1006–1012. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens AA, Tappon SC, Garg A, Fair DA. Functional brain network modularity captures inter- and intra-individual variation in working memory capacity. PLoS One. 2012;7(1):e30468. doi: 10.1371/journal.pone.0030468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storti SF, Formaggio E, Nordio R, Manganotti P, Fiaschi A, Bertoldo A, Toffolo GM. Automatic selection of resting-state networks with functional magnetic resonance imaging. Front Neurosci. 2013;7:72. doi: 10.3389/fnins.2013.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Hashizume H, Sassa Y, Nagase T, Nouchi R, Kawashima R. The association between resting functional connectivity and creativity. Cereb Cortex. 2012;22(12):2921–2929. doi: 10.1093/cercor/bhr371. [DOI] [PubMed] [Google Scholar]
- Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol. 2010;103(1):297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijk BCM, Stam CJ, Daffertshofer A. Comparing Brain Networks of Different Size and Connectivity Density Using Graph Theory. PLoS ONE. 2010;5(10):e13701. doi: 10.1371/journal.pone.0013701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardy JL, Booth C, Pond GR, Zhang H, Galica J, Dhillon H, Clarke SJ, Tannock IF. Cytokine levels in patients (pts) with colorectal cancer and breast cancer and their relationship to fatigue and cognitive function. J Clin Oncol (Meeting Abstracts) 2007;25(18_suppl):9070. [Google Scholar]
- Verghese PB, Castellano JM, Garai K, Wang Y, Jiang H, Shah A, Bu G, Frieden C, Holtzman DM. ApoE influences amyloid-beta (Abeta) clearance despite minimal apoE/Abeta association in physiological conditions. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(19):E1807–E1816. doi: 10.1073/pnas.1220484110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waites AB, Stanislavsky A, Abbott DF, Jackson GD. Effect of prior cognitive state on resting state networks measured with functional connectivity. Hum Brain Mapp. 2005;24(1):59–68. doi: 10.1002/hbm.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Peterson BS. Partner-matching for the automated identification of reproducible ICA components from fMRI datasets: algorithm and validation. Hum Brain Mapp. 2008;29(8):875–893. doi: 10.1002/hbm.20434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wefel JS, Saleeba AK, Buzdar AU, Meyers CA. Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer. 2010;116(14):3348–3356. doi: 10.1002/cncr.25098. [DOI] [PubMed] [Google Scholar]
- Wefel JS, Schagen SB. Chemotherapy-related cognitive dysfunction. Current neurology and neuroscience reports. 2012;12(3):267–275. doi: 10.1007/s11910-012-0264-9. [DOI] [PubMed] [Google Scholar]
- Wefel JS, Vardy J, Ahles T, Schagen SB. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. The lancet oncology. 2011;12(7):703–708. doi: 10.1016/S1470-2045(10)70294-1. [DOI] [PubMed] [Google Scholar]
- Whalley LJ, Deary IJ, Appleton CL, Starr JM. Cognitive reserve and the neurobiology of cognitive aging. Ageing research reviews. 2004;3(4):369–382. doi: 10.1016/j.arr.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- Whitlow CT, Casanova R, Maldjian JA. Effect of resting-state functional MR imaging duration on stability of graph theory metrics of brain network connectivity. Radiology. 2011;259(2):516–524. doi: 10.1148/radiol.11101708. [DOI] [PubMed] [Google Scholar]
- Wu K, Taki Y, Sato K, Qi H, Kawashima R, Fukuda H. A longitudinal study of structural brain network changes with normal aging. Front Hum Neurosci. 2013;7:113. doi: 10.3389/fnhum.2013.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan FX, Wu CW, Cheng SY, Lim KE, Hsu YY, Liu HL. Resting-State Functional Magnetic Resonance Imaging Analysis with Seed Definition Constrained by Regional Homogeneity. Brain connectivity. 2013;3(4):438–449. doi: 10.1089/brain.2013.0164. [DOI] [PubMed] [Google Scholar]
- Yang L, Lin F, Zhou Y, Xu J, Yu C, Pan WJ, Lei H. Iterative cross-correlation analysis of resting state functional magnetic resonance imaging data. PLoS One. 2013;8(3):e58653. doi: 10.1371/journal.pone.0058653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Raichle ME. Disease and the brain's dark energy. Nat Rev Neurol. 2010;6(1):15–28. doi: 10.1038/nrneurol.2009.198. [DOI] [PubMed] [Google Scholar]