Abstract
The cannabinoid CB1 and CB2 receptors are Class A G protein-coupled receptors (GPCRs). While many Class A GPCRs have endogenous ligands that are hydrophilic cations (e.g., the serotonin and dopamine receptors), the cannabinoid receptors have neutral, highly lipophilic ligands derived from the fatty acid, arachidonic acid. The most well-studied of these are N-arachidonoylethanolamine (anandamide, AEA) and sn-2-arachidonoylglycerol (2-AG). This review focuses on the experimental and computational studies that have been used to probe the nature of endocannabinoid interaction with the cannabinoid receptors. These studies include mutation, SAR and NMR studies, as well as, QSAR, docking and molecular dynamics simulations. Gaps in our knowledge are identified. The review begins more generally, however, by discussing the entire endocannabinoid system, of which the cannabinoid receptors are part. For in order to understand endocannabinoid action, one needs an appreciation for the environments for which these ligands have been designed and the conformational changes these ligands must undergo in order to act on the cannabinoid receptors.
Keywords: Cannabinoid, GPCR, endocannabinoid, anandamide, 2-AG, molecular dynamics, QSAR, mutation
I. THE CANNABINOID RECEPTORS
CB1 Receptor
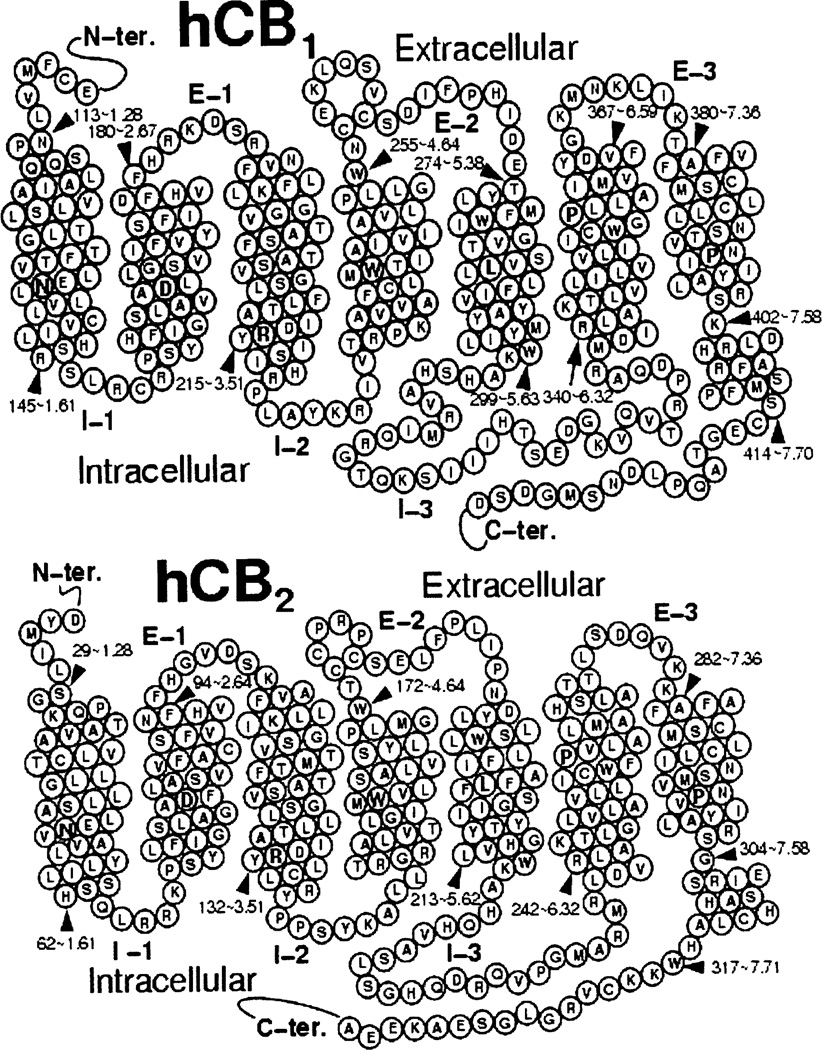
The cannabinoid CB1 receptor (see Fig. 1) belongs to the Class A (rhodopsin (Rho) family) of G-protein coupled receptors (GPCRs). CB1 was initially cloned from a rat cerebral cortex cDNA library [1]. CB1 receptor agonists inhibit forskolin-stimulated adenylyl cyclase by activation of a pertussis toxin-sensitive G-protein [2]. In heterologous cells, CB1 receptors inhibit N-, P-, and Q-type calcium channels and activate inwardly rectifying potassium channels [2–4]. Inhibition of calcium channels and enhancement of inwardly rectifying potassium currents is pertussis toxin-sensitive, but independent of cAMP inhibition, suggestive of a direct G protein mechanism [3]. CB1 receptors are expressed in the central nervous system (CNS)[5, 6] and are particularly rich in certain brain areas such as basal ganglia, cerebellum, and hippocampus [7]. CB1 receptors are also found in the periphery, including human testis [8], retina [9], sperm cells [10], colonic tissues [11], peripheral neurons [12], adipocytes [13] and other organs including human adrenal gland, heart, lung, prostate, uterus, and ovary [14–16].
Fig. (1).
Helix net representations of the amino acid sequences of the CB1 and CB2 receptors are presented here.
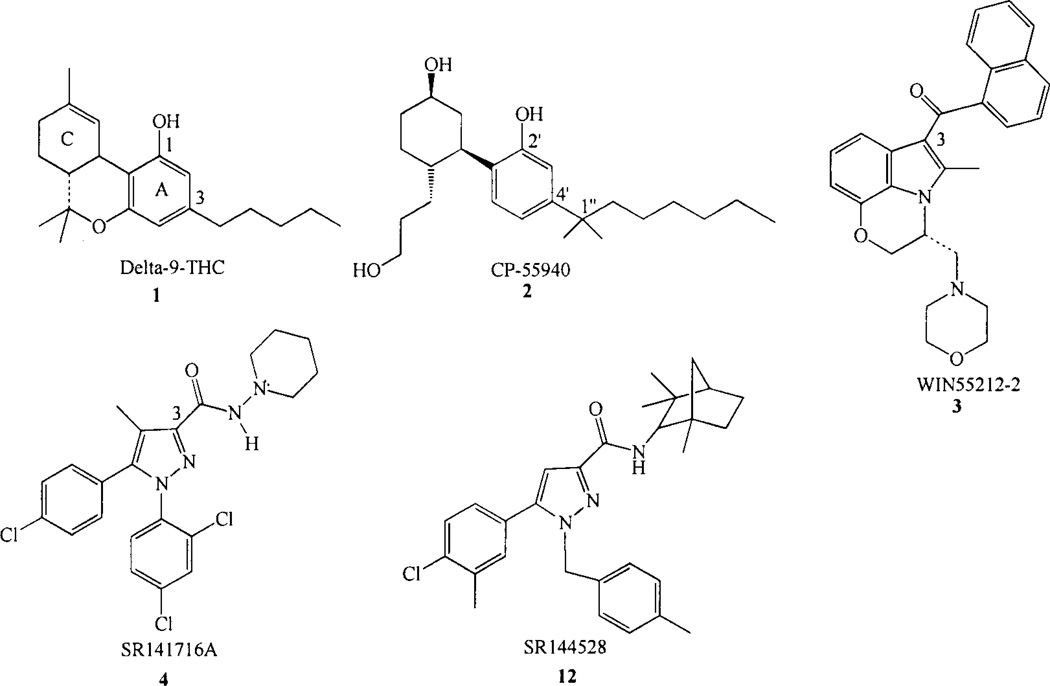
The CB1 receptor transduces signals in response to CNS-active constituents of Cannabis sativa, such as the classical cannabinoid ((−)-Δ9-THC, 1, Chart 1) and to three other structural classes of ligands, the non-classical cannabinoids typified by CP55940 (2, Chart 1) [17, 18], the aminoalkylin-doles (AAIs) typified by WIN55212-2 (3, Chart 1) [19–21] and the endogenous cannabinoids. The non-classical cannabinoids clearly share many structural features with the classical cannabinoids, e.g. a phenolic hydroxyl at C-1 (C2’), and alkyl side chain at C-3 (C-4’), as well as, the ability to adopt the same orientation of the carbocyclic ring as that in classical cannabinoids [22]. The AAIs, on the other hand, bear no obvious structural similarities with the classical/non-classical cannabinoids.
Chart 1.
Cannabinoid receptor agonists and antagonists.
SR141716A, the first reported CB1 antagonist (4, Chart 1) [23] displays nanomolar CB1 affinity (Ki =1.98±0.13nM), but very low affinity for CB2. SR141716A (4) has been shown to act as a competitive antagonist and inverse agonist in host cells transfected with exogenous CB1 receptor, as well as in biological preparations endogenously expressing CB1 [24–26]. Several other CB1 antagonists have been reported, LY-320135 [27], 0–1184 [28], CP-272871 [26], URB447 [29], a class of benzocycloheptapyrazoles [30], a novel series of 3,4-diarylpyrazolines [31] and biarylpyrazolyl oxadiazoles [32]. The first peptide CB1 inverse agonist, hemopressin (HP; PVNFKFLSH), has also been reported [33]. The CB1 antagonist field has been recently reviewed by Jagerovic and co-workers [34],
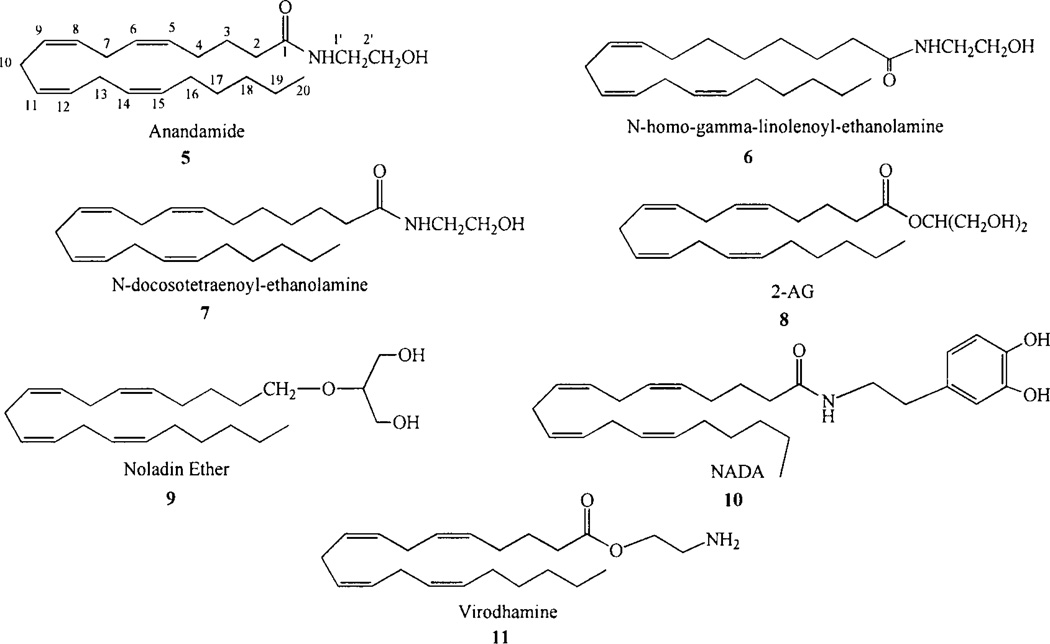
The endogenous cannabinoid ligands are unsaturated fatty-acid ethanolamides, glycerols or glycerol ethers. The first endogenous cannabinoid, N-arachidonoylethanolamine (AEA, also called anandamide, 5, Chart 2), was isolated from porcine brain by Mechoulam and co-workers [35]. Two other polyunsaturated fatty acid ethanolamides, N-homo-y-linolenoylethanolamine (6, Chart 2) and N-docosatetr-aenoylethanolamine (7, Chart 2) have been isolated from porcine brain and shown to bind to the cannabinoid CB1 receptor with high affinity [36]. sn-2-arachidonoylglycerol (2-AG; 8, Chart 2) was isolated from intestinal tissue and shown to be a second endogenous CB ligand [37]. 2-AG has been found present in the brain at concentrations 170 times greater than AEA [38], 2-AG acts as a full agonist and produces the characteristic effects associated with cannabinoid agonists. In addition, 2-eicosa-5',8',H',14'-tetraenylglycerol (2-AG ether, noladin ether, 9, Chart 2), a metabolically stable ether-linked analogue of 2-arachidonoylglycerol (2-AG) has been identified as another endogenous cannabinoid ligand [39], although the presence of this compound in mammalian tissues has been questioned [40].
Chart 2.
Endogenous cannabinoids.
Additional endocannabinoids identified to date include N-arachidonoyl dopamine (NADA) and virodhamine. N-arachidonoyl-dopamine (NADA, 10, Chart 2) is an endogenous “capsaicin-like” substance in mammalian nervous tissues. NADA activates cannabinoid CB1 receptors, but not dopamine Dl and D2 receptors [41, 42]. Virodhamine is arachidonic acid and ethanolamine joined by an ester linkage (11, Chart 2). Felder and co-workers have reported that virodhamine has antagonist properties at the CB1 cannabinoid receptor [43].
CB2 Receptor
The cannabinoid CB2 receptor (see Fig. 1) also belongs to the Class A (rhodopsin (Rho) family) of G protein-coupled receptors (GPCRs). The second cannabinoid receptor sub-type, CB2 was first cloned from a human promyelocytic leukemia cell HL60 cDNA library [44]. The human CB2 receptor exhibits 68% identity to the human CB1 receptor within the transmembrane regions, 44% identity throughout the whole protein [44]. Unlike the CB1 receptor, which is highly conserved across human, rat and mouse, the CB2 receptor is much more divergent. Sequence analysis of the coding region of the rat CB2 genomic clone indicates 93% amino acid identity between rat and mouse and 81% amino acid identity between rat and human.
Intracellular CB2-dependent signaling pathways include Gi/o-dependent inhibition of adenylyl cyclase, stimulation of mitogen-activated protein kinase [45, 46], phosphoinositide 3-kinase pathways [47] and activation of de novo ceramide production or cyclooxygenase-2 (COX-2) induction [48]. The CB2 receptor is highly expressed throughout the immune system [15, 49] and was more recently described in the CNS under both pathological [50–52] and physiological conditions [53]. The quite specific localization in the immune system, as well as the fact that CB2 knock-out mice fail to respond to the immunomodulatory effects of classical cannabinoids [54], suggest that CB2 receptor ligands would have potential therapeutic applications as immunomodulators for the treatment of inflammation and allergy. Several papers report the role of the CB2 receptor in modulating leukocyte migration [55–58], activation [59] and antigen processing [60]. Additional applications could arise from studies on bone physiology, as blockage of CB2 has been reported to protect from bone loss in ovariectomized mice [61]. However, others have reported that CB2 activation is involved in protecting from bone loss [62].
The CB2 receptor recognizes the same structural groups of cannabinoid agonists as CB1, with differing affinities in some cases, for example CB2 has higher affinity for aminoalkylindoles (see 3, Chart 1) [63]. sn-2-arachidonoyl-glycerol (2-AG; 8, Chart 2) was isolated from intestinal tissue and identified to be the CB2 endogenous ligand [37, 64]. While AEA has low CB2 affinity, 2-AG does bind as well to CB1 [37, 64]. The CB1/CB2 classical/non-classical cannabinoid SAR literature clearly shows a difference in recognition of side chains between CB1 and CB2. In contrast to CB1 which shows a strict requirement for pentyl or longer alkyl tails (see 1 and 2, Chart 1), CB2 recognizes classical can-nabinoids with shorter dimethylpropyl [65] and even dimethylethyl [66] side chains. The SAR of the CB2 receptor has suggested that etherification at the C-l position of classical cannabinoids leads to CB2 selective compounds [65, 67, 68].
SRI44528 (12, Chart 1), the first reported CB2 antagonist displays sub-nanomolar affinity for both the rat spleen and cloned human CB2 receptors (Ki = 0.60±0.13nM) and a 700-fold lower affinity for both the rat brain and cloned human CB1 receptors [69]. CB2 receptor-transfected Chinese hamster cells exhibit high constitutive activity. This activity can be blocked by SR144528, working as an inverse agonist [46]. Additional CB2 inverse agonists/antagonists include JTE-907 [70], AM630 [71] and a new class based on a triaryl bissulfone backbone, exemplified by SCH336 [72, 73].
GPR55
GPR55 was originally isolated in 1999 as an orphan GPCR with high levels of expression in human striatum [74](Genbank accession # NM_005683). This receptor exhibits low amino acid identity to CB1 (13.5%) or CB2 (14.4%) receptors. The closest related proteins to GPR55 are GPR35 (27%), P2Y (29%), GPR23 (30%), and CCR4 (23%) [74]. GPR55 was first identified as a putative cannabinoid receptor in two patent applications [75, 76]. Drmota and coworkers [76] isolated a variant of GPR55, GPR55a, which contains three amino acid substitutions (F3.33(102)L, G5.52(195)S, C7.47(281)R). The ability of GPR55 to recognize cannabinoids was first described in a yeast expression system, where the CB1 antagonists AM251 and SR141716A acted as agonists at micromolar concentrations [75, 77]. However, Sjogren and co-workers have expressed GPR55 in HEK293 cells; there, nanomolar concentrations of many cannabinoid agonists stimulated GTPγS binding [78]. Most of the endocannabinoids, including anandamide, 2-AG, vi-rodhamine, noladin ether and palmitoylethanolamide, as well as the agonists CP55940 and Δ9-THC, stimulated GTPγS binding, which was not antagonized by AM281, but was blocked with 450 nM Cannabidiol (CBD) [78]. AM251 produced an agonist response in HEK293 cells, similar to that found in the yeast expression system [76, 78]. However, WIN55212-2 did not produce an agonist response at GPR55 [76, 78]. On the other hand, palmitoylethanolamide (PEA), a potent anti-inflammatory, anti-excitotoxic and anti-hyperalgesic compound [79, 80], was a potent agonist at this receptor [78], raising the possibility that GPR55 may be a receptor for this endocannabinoid. More recently, GPR55 has been tested against a number of cannabinoid ligands with mixed results. Observations using a GTPγS functional assay indicate that GPR55 is activated by nanomolar concentrations of the endocannabinoids 2-AG, virodhamine, noladin ether, and palmitoylethanolamine [81]; and the atypical cannabinoids abn-CBD and O-1602 [82], as well as by the classical cannabinoids CP55940, HU210, and (−)-Δ9-THC [83]. Oka et al. [84] reported that GPR55 is not a typical cannabinoid receptor as numerous endogenous and synthetic cannabinoids, including many mentioned above, had no effect on GPR55 activity. They presented compelling data suggesting that the endogenous lipid, lysophosphatidylinositol (LPI) and its 2-arachidonyl analogs are agonists at GPR55 as a result of their abilities to phosphorylate extracellular regulated kinase and induce calcium signaling [85, 86]. Kapur and co-workers recently examined the effects of a representative panel of cannabinoid ligands and LPI on GPR55 using a beta-arrestin-green fluorescent protein biosensor as a direct readout of agonist-mediated receptor activation. Their data demonstrate that AM251 and SR141716A, both cannabinoid antagonists, and the lipid LPI, which is not a cannabinoid receptor ligand, are GPR55 agonists. These ligands possess comparable efficacy in inducing (β-arrestin trafficking, and moreover, activate the G-protein dependent signaling of PKCβII. Conversely, the potent synthetic cannabinoid agonist CP55940 acts as a GPR55 antagonist/partial agonist. CP55940 blocks GPR55 internalization, the formation of β-arrestin GPR55 complexes, and the phosphorylation of ERK1/2 [87]. Kapur and co-workers have concluded that at best, GPR55 is an atypical cannabinoid responder. Because of the clearly controversial identification of GPR55 as a cannabinoid receptor at this time, this review will focus on the CB1 and CB2 receptors and their endogenous mediators only.
Cannabinoid Receptor Structure
The CB1 and CB2 receptors both belong to the Class A family of G protein-coupled receptors (GPCRs). As revealed by the crystal structure of bovine rhodopsin (Rho) at 2.8 Å [88], 2.65 Å [89], 2.6 Å [90] and 2.2 Å resolution [91] and most recently the crystal structures of the carazolol bound β2-AR [92–94], β1-AR [95] and adenosine A2A receptor [96], the general topology of a Class A GPCR includes: (1) an extracellular N terminus; (2) seven transmembrane alpha helices (TMHs) arranged to form a closed bundle; (3) loops connecting TMHs that extend intra- and extracellularly; and, (4) an intracellular C terminus that begins with a short helical segment (Helix 8) oriented parallel to the membrane surface. Ligand binding in GPCRs is thought to occur within the binding site crevice formed by the TMH bundle, to extracellular loops, or to a combination of extracellular loop and binding site crevice residues. Agonists are thought to bind and produce a conformational change which initiates coupling to the G protein which is located inside the cell. An agonist-bound receptor activates an appropriate G protein that promotes dissociation of GDP. Biophysical studies indicate that ligand-induced receptor activation causes a change in the relative orientations of TMHs3 and 6 [97–102], with the intracellular end of TMH6 moving away from TMH3 by hinging and moving up towards lipid [101]. This modification then affects the conformation of the G protein-interacting intracellular loops of the receptor and thus uncovers previously masked G protein-binding sites [103]. No experimental structures for the CB1 and CB2 receptors are yet available. Homology models of CB1 and CB2 have been constructed and have proved to be quite useful in directing mutation and ligand design projects. These models are discussed later in this review.
II. THE ENDOCANNABINOID SIGNALING SYSTEM
The cannabinoid receptors are part of the endocannabinoid signaling system which also includes the enzymes that synthesize and degrade endocannabinoids, as well as possible transporters. The molecular mechanisms for regulating lipid-based signaling events such as cannabinoid receptor signaling are not yet completely understood, although significant progress has been made [104]. Because lipids and their derivatives can readily partition into and diffuse throughout cellular membranes, lipid messengers such as the endocannabinoids are not easily contained by such physical boundaries as those of neurotransmitter membrane vesicles. 2-AG [37, 64], is synthesized on demand from lipid in a two step process in which phospholipase C-β hydrolyses phosphatidylinositol-4,5-bisphosphate to generate diacylglycerol, which is then hydrolyzed by diacylglycerol lipase (DAGL-α) to yield 2-AG [105, 106]. 2-AG has been shown to mediate the retrograde signaling of the endocannabinoid system in the brain [104]. The biosynthetic enzymes for 2-AG are localized on post-synaptic neurons in dendritic spines and somatodendritic compartments. Released 2-AG controls the activity of the complementary pre-synaptic neuron, by binding to the CB1 receptor which is often expressed there [107]. It is still unclear for retrograde signaling how newly synthesized 2-AG is induced to leave the post-synaptic cell plasma membrane to interact with CB1 pre-synaptically. 2-AG may be secreted by simple diffusion; alternatively, passive (energy-independent) carrier proteins may be required to extrude 2-AG. Once 2-AG has reached CB1, it will bind within the binding site crevice formed by the seven transmembrane helices of CB1. The subsequent activation of CB1 by 2-AG results in the inhibition of neurotransmitter release in the presynaptic cell via inhibition of voltage-activated Ca2+ channels and the enhancement of inwardly rectifying K+ channels in the cell [108–114]. Degradation of 2-AG is then accomplished presynaptically, principally by a membrane associated enzyme, monoacylglycerol lipase [115].
In comparison to the state of knowledge concerning 2-AG and its role in retrograde signaling, the molecular and neuroanatomical organization of synaptic AEA signaling has remained largely unknown. Nylias and co-authors have shown that N-acylphosphatidylethanolaminehydrolyzing phospholipase D (NAPE-PLD), a biosynthetic enzyme of AEA [116], is concentrated presynaptically in several types of hippocampal excitatory axon terminals and is associated with intracellular calcium stores. This indicates that, in contrast to 2-AG, endocannabinoids like AEA may have a presynaptic origin and their production may reflect the status of axon terminal [Ca 2+] (in part following release from intracellular stores) [117]. After acting at pre-synaptic CB1 receptors, AEA is taken up by post-synaptic cells via possible transport proteins on both neurons and glia that mediate endocannabinoid uptake [118–120]. After being transported into the cell, AEA is subsequently broken down into arachidonic acid and ethanolamine by a membrane-bound enzyme called fatty-acid amide hydrolase (FAAH) [119, 121, 122] that has been shown by immunohistochemistry to be localized to the endoplasmic reticulum [123, 124].
III. ENDOCANNABINOID SAR
An extensive SAR has been developed for endocannabinoid binding at CB1 and CB2. Compounds that have emerged from this SAR development are important not only as probes for the cannabinoid receptors, but also as valuable tools in the deduction of the binding modes of endocannabinoids at their receptors.
CB1 Receptor
AEA Head Group SAR
In order for high-affinity binding to the CB1 receptor to occur and for agonist binding to activate G-proteins, the carbonyl group of the AEA amide head group must be present [125]. Arachidonamide and simple alkyl esters of arachidonic acid did not show significant CB1 affinity [126]. Cyclization of the head group into an oxazoline ring diminished affinity [127]. Arachidonylethers, carbamates and norarachidonlycarbamates had poor CB1, affinity [128]. However, norarachidonyl ureas showed generally good binding affinities to the CB1 receptor (Ki = 55–746 nM). Some of the weaker affinity analogs in this series, produced potent pharmacological activity. These analogs showed hydrolytic stability toward amidase enzymes [128]. To examine the SAR in the arachidonyl alcohol series, Parkkari and co-workers synthesized several ester, carbonate, and carbamate derivatives of arachidonyl alcohol. The results obtained from their SAR study indicate that among the different analogs that were tested only the ester analogs showed some activity at the CB1 receptor, however even these compounds were found to be less potent and efficacious when compared with AEA [129]. El Fangour and co-workers synthesized AEA analogs in which the amide moiety was replaced by an oxomethylene group or an ester. They found that replacement of the amide NH with a methylene group resulted in an analog with moderate CB1 binding affinity (Ki, = 360 nM) [130],
The Makriyannis group discovered that when the position of the amide nitrogen and carbonyl groups are reversed, the resultant compounds are resistant to enzymatic hydrolysis. This new class of ligands is referred to as retroanandamides (AM1174)[127]. Incorporating a urea functionality into the head group produces ligands that retain CB1 affinity, but are resistant to FAAH breakdown [128], A one carbon homologation of anandamide leads to compounds that are partial agonists for both CB1 and CB2 and are also stable to hydrolysis by FAAH [131].
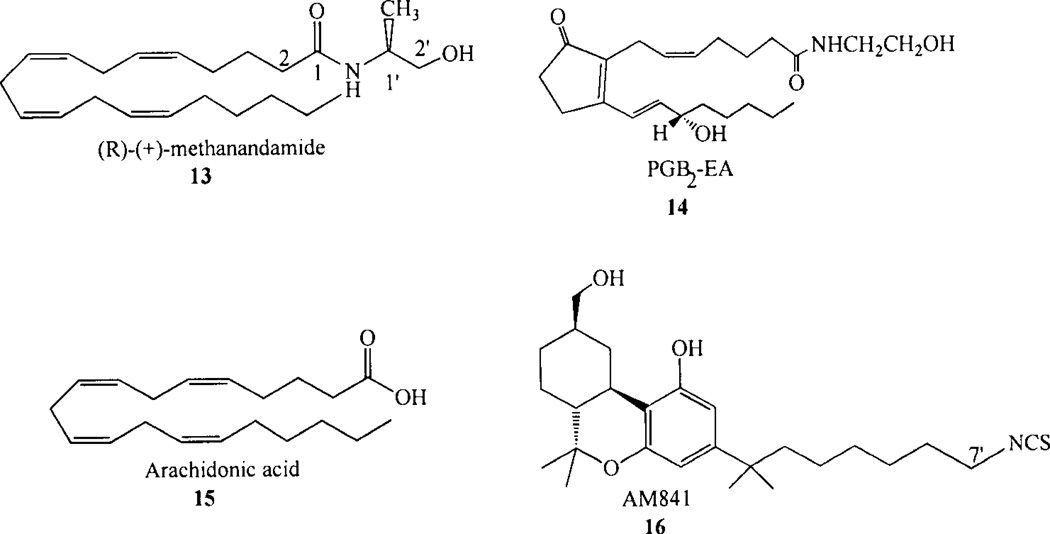
Methylation at the C-1’ position in the AEA (see Chart 2, 5 for numbering system) head group resulted in an 1’-R-methyl isomer (R-methanandamide, 13; Chart 3) which had four-fold higher CB, affinity than AEA, while the 1’-S-methyl isomer had two-fold lower CB1 affinity than AEA. R-methanandamide (13) also was found to be resistant to enzymatic breakdown [132]. Methylation at the 2’ position also produced some stereoselectivity, as the S(+) isomer was found to have 2–5 fold higher CB| affinity than the R(−) -isomer [125, 132]. Introduction of larger alkyl groups had a detrimental effect on CB, affinity [133]. A series of C1’-C2 dimethyl anandamide analogs revealed stereochemical requirements of the CB1 binding pocket, as only the R,R isomer (R)-N-(l-methyl-2-hydroxyethyl)-2-(R)-methyl-arachidon-amide had significant affinity for CB! (K;=7.42 ± 0.86 nM) [134].
Chart 3.
Enlargement of the ethanolamine head group by insertion of methylene groups revealed that the N-propanol analog had slightly higher CB1 affinity than AEA, while higher ho-mologs had reduced CB1 affinity [126, 135]. Alkyl branching of the alcoholic head group lead to lower affinity analogs [135]. N-(propyl) arachidonylamide possessed higher CBI affinity (Ki; = 7.3nM) than anandamide itself (Ki,=22nM) [126, 135]. Substitution of an N-cyclopropyl group for the ethanolamine head group of AEA lead to a very high CB 1 affinity compound [136]. These results suggest that there may exist a hydrophobic sub-site for the AEA head group such that the hydroxyl of AEA may not be necessary for receptor interaction [127]. Replacement of the hydroxyl group of AEA with a halogen such as F or CI increased CBI affinity as well [127, 136, 137]. Substitution of the 2-hydroxyethyl group of AEA with a phenolic group, however, greatly decreased affinity for CBI [138–141].
Taken together, all of these results suggest that the hydroxyl in the anandamide head group is not essential for receptor interaction, but that the cannabinoid receptor can accomodate both hydrophobic and hydrophilic head groups, possibly in two different subsites. The size(s) of the cavity(ies) in which the head group binds, however, is (are) small as only relatively small variations on the head group permit the retention of high affinity binding.
AEA Acyl Chain SAR
Endocannabinoid SAR indicates that the CBI receptor recognizes ethanolamides whose fatty acid acyl chains have 20 or 22 carbons, with at least three homoallylic double bonds and saturation in at least the last five carbons of the acyl chain [135]. Reggio and co-workers have suggested that this acyl chain unsaturation SAR requirement is an outgrowth of the shape of the AEA binding pocket at CBI which may require tightly folded conformations, conformations not possible for AEA analogs with less than three homoallylic double bonds [142].
An analogy has been drawn in the literature between the C16-C20 portion of AEA (see 5, Chart 2) and the C-3 pentyl side chain of the classical cannabinoid, Δ9-THC (see 1, Chart 1). Consistent with this hypothesis, replacement of the pentyl tail of AEA with a dimethylheptyl chain results in enhanced affinity (although not to the same degree as seen in the classical cannabinoids) [143, 144].
Although initially it seemed that the development of rigid anandamide analogs would help to identify the receptor-appropriate conformation of AEA, attempts at rigidifying AEA have been met with little success [126, 145, 146]. Pinto and co-workers investigated a series of arachidonyl amides and esters in addition to a series of “rigid hairpin” conformations typified by N-(2-hydroxyethyl)-prostaglandin amides to determine the structural requirements for binding to the CB1 receptor. Two dimensional drawings of anandamide and PGB2-EA (14, Chart 3) make the shapes of these two compounds look similar. However, all of the rigid prostaglandin analogs synthesized by these investigators [126] failed to alter [3H]CP-55,940 binding to CB1 in concentrations as great as 100 µM. Barnett-Norris and co-workers [147] reported Conformational Memories (CM) results for PGB2-EA (14) which showed an attenuated ability for the prostaglandin ethanolamide to adopt extended conformations or to form U-shaped conformations like AEA and 2-AG. Instead, the CM results showed that the conjugation of the acyl chain with the ring double bond introduces “stiffness” into this part of the molecule, resulting in predominantly in a folded L-shaped conformation.
2-AG SAR at CB1
Sugiura and co-workers have reported that 2-AG (8) and other cannabinoid ligands such as AEA (5) and Δ9 -THC (1) induce rapid transient increases in [Ca2+] in NG108-15 cells through a cannabiniod CB1 receptor-dependent mechanism [148–150]. 2-AG was the most potent compound for inducing these transient increases, as its activity was detectable from as low as 0.3 nM. The maximal response induced by 2-AG exceeded responses induced by other CB1 agonists. Activities of the CB1 agonists, HU-210 and CP 55,940 (2) were also detectable from as low as 0.3 nM, whereas, the maximal responses induced by these compounds were low compared with 2-AG. AEA was also found to act as a partial agonist in this system. Arachidonic acid (15, Chart 3) failed to elicit a response, while noladin ether (9) possessed appreciable activity, although its activity was apparently lower than that of 2-AG [150].
Sugiura and co-workers [150] have found that glycerol is the most suitable head group, and the 2-isomer is preferable over the l(3)-isomer. Parkkari and co-workers also explored the effect of alpha-methylation of 2-AG as a way to improve its enzymatic stability [151]. In addition, the CB1 activity properties of fluoro derivatives of 2-AG were studied. The results indicate that even if the alpha-methylated 2-AG derivatives are slightly weaker CB1 receptor agonists than 2-AG, they are more stable than 2-AG. In addition, the results showed that the replacement of the hydroxyl group(s) of 2-AG by fluorine does not improve the CB1 activity of 2-AG.
Arachidonic acid is the most preferred fatty acid moiety, although the activity of eicosatrienoic acid (n-9)-containing species was almost comparable to that of the arachidonic acid containing species. Because the activities of 2-eico-satrienoy 1(20:3 Δ 8,11,14, n-6) glycerol, 2-eicosatrienoy 1(20:3 Δ11,14,17, n-3) glycerol and 2-docosatetraenoyl(22:4 Δ10,13,16, n-6) glycerol are lower than those of 2-eicosatrienoyl(20:3 Δ5,8,11,n-9) and 2-eicosapentaenoyl(20:5 Δ5,8,11,14,17,n-3) glycerol, it appears that the presence of a double bond at the Δ5 position, rather than further towards the end of the acyl chain, is crucially important, probably for folding or curvature nearer the head group.
Parkkari and co-workers explored the effect of incorporation of a dimethyl heptyl (DMH) tail in 2-AG and noladin ether (9) at the CB1 receptor. The importance of the chain length was also explored by synthesizing 2-AG and noladin ether derivatives possessing the chain length C21 instead of C22. Replacement of the pentyl end chain with the DMH resulted in distinct potency decrease as compared to the reference compounds. The modification did not have such a strong impact on the efficacy values. In fact, the efficacy of the derivatives of noladin ether was comparable or even slightly improved. Introducing a more stable and hydrophilic urea bond led to a dramatic decrease in biological activity [152].
CB2 Receptor
2-AG SAR at CB2
Sugiura and co-workers [153] have also found that 2-AG can induce rapid transient increases in [Ca2+] in HL-60 cells. It was evident in this case that the response was mediated by the CB2 receptor, but not the CB1 receptor, because the CB2 antagonist, SR144528 (12), but not the CB1 antagonist, SR141716A (4), blocked the response. 2-AG was found to have more activity than its l(3)-isomer. Ester and ether analogs, including nolandin ether (9) showed appreciable activity, albeit less than that of 2-AG. Anandamide was found to be a weak partial agonist toward the CB2 receptor. The cannabinoid agonist Δ9 -THC (1) exhibited weak agonistic activity for the CB2 receptor in the transient [Ca2+] assay.
The Sugiura group has also reported that a hydroxy group-containing 2-AG analog, a ketone group-containing analog, and a methylene-linked analog exhibited only weak agonistic activities toward either the CB1 receptor or the CB2 receptor [154]. The Makriyannis group has reported a series of head group constrained and conformationally restricted analogues of 2-AG which are 2-AG esters of 1,2,3-cyclohexanetriol. Resolution of one of these (AM5503) into stereoisomers yielded (+)AM4434 and (−)AM4435 both of which exhibited CB1 Ki,= 360 nM and CB2 Ki =770nM binding [155].
The Sugiura group has reported that 2-glycerols with fatty acid chains of 20–22 carbons showed the strongest CB2 agonist activity. For C2o fatty acids, activity was best for the 20:3 Δ5,8,11 and 20:4 Δ5,8,11,14 (2-AG, 8), suggesting that like the 2-AG SAR generated for CB1, a double bond at Δ5 was important [153]. More recently, the Sugiura group has reported a series of 2-AG analogs with additional variations in the fatty acid moiety [154]. These include an analog containing an isomer of arachidonic acid with migrated olefins, an analog containing a one-carbon shortened fatty acyl moiety and an analog containing a one-carbon elongated fatty acyl moiety. These analogs exhibited only weak agonistic activities toward either the CB1 receptor or the CB2 receptor, which is in good contrast to 2-AG which acted as a full agonist at these cannabinoid receptors [154].
IV. ENDOCANNABINOID CONFORMATION
Relationship Between Endocannabinoid Conformation and Productive Receptor Interaction: Dynamic Plasticity Appears Key
One of the striking facts that emerges from endocannabinoid SAR for CB1 and CB2 is that for AEA as well as 2-AG, the moiety with the smallest number of viable substitutions is the fatty acid moiety. The arachidonic acid (AA, 15) moiety in AEA, 2-AG and congeners confers on the molecule “dynamic plasticity”. The arachidonic acid acyl chain contains four homoallylic double bonds (i.e. cis double bonds separated by methylene carbons). Rabinovich and Ripatti [156] reported that polyunsaturated acyl chains in which double bonds are separated by one methylene group are characterized by the highest equilibrium flexibility compared with other unsaturated acyl chains. Rich [157] reported that a broad domain of low-energy conformational freedom exists for these C-C bonds. Results of the Biased Sampling phase from Conformational Memories calculations of AA are consistent with Rich’s and with Rabinovich and Ripatti’s results [147], as they revealed a relatively broad distribution of populated torsional space about the classic skew angles of 119°(s) and −119°(s’) for the C8-C9-C10-C11 torsion angle in AEA, for example (see Chart 2, 5 for numbering system).
While the fatty acid literature indicates that unsaturated fatty acids that possess multiple homoallylic double bonds, such as AA, exhibit a high degree of flexibility, this literature also indicates that saturated fatty acids tend to be significantly less flexible and adopt primarily extended conformations. Fatty acids with decreasing amounts of unsaturation tend to show a decreasing tendency to form folded structures, but still tend to curve in acyl chain regions in which unsaturation is present [158]. A correlation has been drawn between this acyl chain conformation trend and the SAR of the anandamide (AEA) acyl chain [142].
CoMFA QSAR pharmacophore models for AEA and its analogs have focused on folded conformations, such as a J-shape [159] or a helical shape [160]. Di Marzo and coworkers designed, synthesized and evaluated the CB1 binding affinity of a number of new conformationally restricted lipopeptides. All of them present some of the AEA key structural elements incorporated in a hairpinlike peptide framework. Some of the analogues showed CB1 affinity, albeit SO-SO fold less than AEA [161]. Barnett-Norris and co-workers [147] performed Monte Carlo/ simulated annealing studies of anandamide, 2-AG, a dimethylheptyl analog of AEA with higher CB1 affinity (16,16-dimethyldocosa-cis-5,8,11,14-tetraenoylethanolamine), and N-(2-hydroxyethyl)prostagl-andin-B2-ethanolamide (PGB2-EA, 14), a prostanoid ligand which does not bind to the CB1 receptor. They found that the highest conformer populations for AEA and 2-AG were angle-iron (extended) and U shaped conformations, with the predominant population influenced by the environment (aqueous vs. CHC13). For the dimethylheptyl analog of AEA, a U shape and an angle-iron (extended) conformation was favored, however, the inactive PGB2-EA was found to be incapable of forming angle-iron (extended conformations). It instead favored an L shaped conformation. The investigator’s concluded that the low probability of PGB2-EA adopting an extended conformation may be why PGB2-EA shows such low affinity for the CB1 receptor.
A susequent Conformational Memories study of AEA (20:4, n-6 (Ki =39.2 ± 5.7 nM) and its 22:4, n-6 (Ki = 34.4 ± 3.2 nM); 20:3, n-6 (Ki = 53.4± 5.5 nM); and 20:2, n-6 (Ki > 1500 nM)[135] congeners indicated that each analogue could form both extended and U/J-shaped families of conformers. However, for the low affinity 20:2, n-6 ethanolamide, the higher populated family was the extended conformer family, while for the other analogues in the series, the U/J-shaped family had the higher population. In addition, the 20:2, n-6 ethanolamide U-shaped family was not as tightly curved as were those of the other analogues studied. The average radii of curvature (in the C-3 to C-17 region, see 5, Chart 2 for numbering system) (with their 95% confidence intervals) were found to be 5.8 Å (5.3–6.2) for 20:2, n-6; 4.4 Å (4.1– 4.7) for 20:3, n-6; 4.0 Å (3.7–4.2) for 20:4, n-6; and 4.0 Å (3.6–4.5) for 22:4, n-6. These results suggest that higher CB1 affinity is associated with endocannabinoids that can form tightly curved structures [142].
The important, take-home message from these conformational analyses of AEA and its analogs is that dynamic plasticity appears to be key, as it is important for these analogs to be able to (1) extend [147], but also (2) to form tightly curved conformations [142, 159, 160]. Only analogs that are capable of these two extremes in conformation are recognized at CB1. It is possible that one of these conformations is important for getting to the receptor and the other is important for getting into the receptor.
Does the Lipid Bilayer Orient Endocannabinoids for Productive Receptor Interaction?
As discussed above, the phospholipid bilayer plays a central role in the lifecycle of the endogenous cannabinoids, AEA and 2-AG. While each has its own set of synthetic and degradative enzmes and system architecture [107], both endocannabinoids are synthesized on demand in the lipid bilayer; they interact with the membrane-embedded cannabinoid receptors; subsequently they are degraded by membrane associated enzymes that each endocannabinoid likely accesses via the bilayer. Because the endocannabinoid system is intimately associated with the lipid milieu, information concerning endocannabinoid location in the phospholipid bilayer and the conformations they can adopt is important to our understanding of the mechanism of cannabinoid action at the molecular level. Two techniques have been used to obtain such information about the endocannabinoids: moelcular dynamics simulations and NMR spectroscopy.
Molecular Dynamics Simulations in Lipid
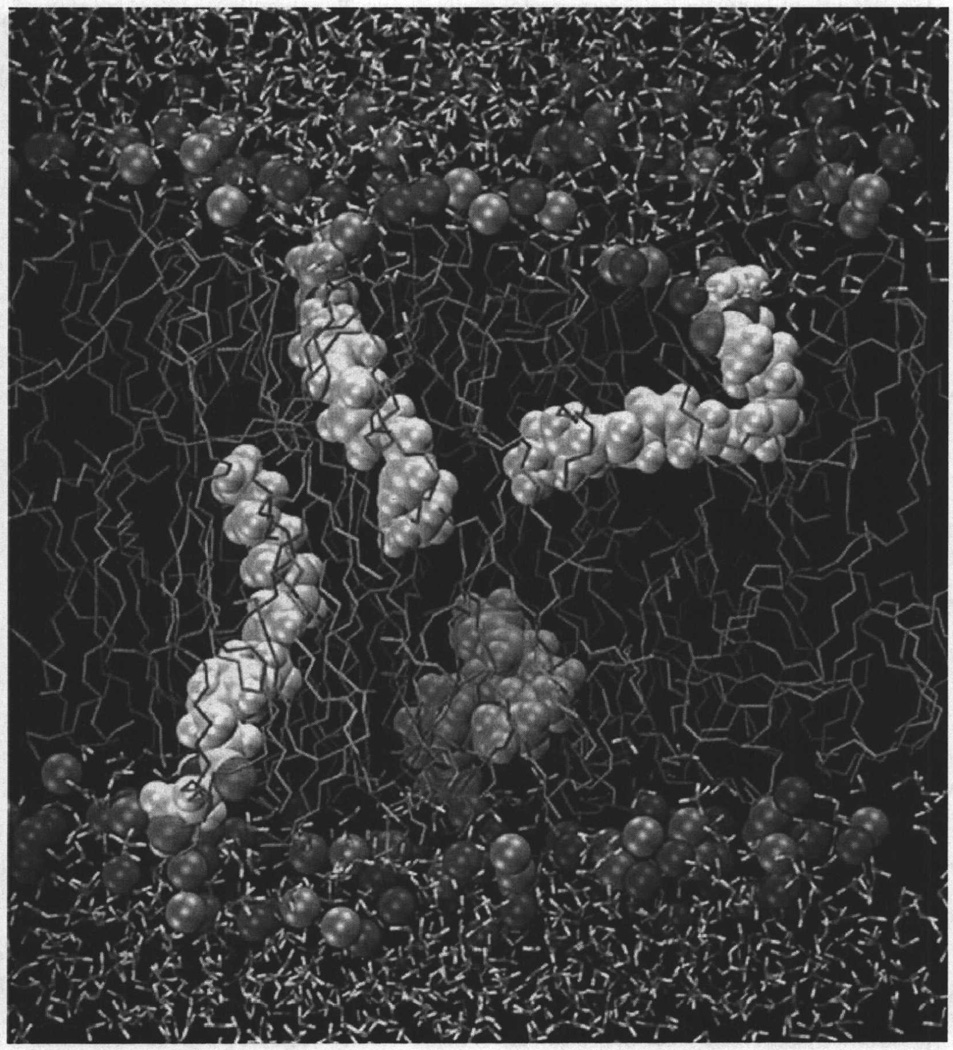
Multi-nanosecond molecular dynamics simulations of AEA in a 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) phospholipid bilayer have suggested that the AEA polar headgroup resides at the lipid-water interface, specifically in the polar phospholipid headgroup region, whereas the AEA nonpolar acyl chain extends into the hydrocarbon core of the membrane. The analysis also indicated that (i) an AEA elongated conformation is preferred in the DOPC bilayer environment; however, many other AEA conformations are observed. These included angle-iron/extended/helical conformers, as well as J and U shaped conformations; (ii) hydrogenbonding between the lipid (DOPC) and the AEA headgroup, although extensive, is quite short-lived; and (iii) the C-H bond order parameters for the AEA acyl chain are low compared to order parameters typically seen for saturated acyl chains of fatty acids, and these order parameters decrease toward the bilayer center. The orientation of AEA in DOPC is illustrated in Fig. (2).
Fig. (2).
A simulation cell containing four molecules of N-arachidonoylethanolamine (anandamide, AEA, 5, Chart 2), in a fully hydrated 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) bilayer is illustrated here. Spheres represent the DOPC choline group nitrogen and phosphate group phosphorous atoms. The DOPC acyl chains are shown in tube display. Molecules of 5 are contoured at their Van der Waals radii, while water is depicted here in tube display. This figure shows that when 5 adopts an extended conformation in the bilayer, its terminal methyl group is at the center of the bilayer. This figure also shows that 5 is capable of forming more compact shapes while in the bilayer [210].
NMR Spectroscopic Studies in Lipid
The Makriyannis lab has reported an NMR study of AEA conformation, location, and dynamic properties in a dipalmitoylphosphatidylcholine multilamellar model membrane bilayer system [162]. Their results demonstrated that anandamide adopts an extended conformation within the membrane with its headgroup at the level of the phospholipid polar group and its terminal methyl group near the bilayer center. Parallel static 2H NMR experiments further confirmed these findings and provided evidence that AEA experiences dynamic properties similar to those of the membrane phospholipids and produces no perturbation to the bilayer. Thus, the emerging picture from experimental and MD studies of AEA behavior in the membrane is that AEA tends to assume an extended shape in the bilayer, but dynamically explores other conformations.
V. ENDOCANNABINOID INTERACTION WITH THE CANNABINOID RECEPTORS
Interaction via the Lipid Bilayer
Because of the high lipophilicity of the cannabinoids and the endocannabinoids, one of the hypotheses being probed in the cannabinoid literature is that cannabinoids enter the cannabinoid receptors via the lipid bilayer. The Reggio group has identified a βXXβ motif (formed by beta branching amino acids, V6.43 and 16.46) on the lipid face of the cannabinoid CB1 receptor in its inactive state that may serve as an initial CB1 interaction site for AEA. Early Conformational Memories calculations on CB1 TMH6 revealed that interaction of an alkyl chain with this groove can induce an activated TMH6 conformation [163]. Molecular dynamics simulations of AEA conducted in a model system composed of CB1 transmembrane helix 6 (TMH6) in a 1,2-dioleoyl-sn-glycero-3 phosphocholine (DOPC) bilayer revealed that AEA exhibited a higher incidence of V6.43/I6.46 groove insertion than its low CB1 affinity (20:2, n–6) analog. In certain cases, AEA established a high energy of interaction with TMH6 by first associating with the V6.43/I6.46 groove and then molding itself to the lipid face of TMH6 to establish a hydrogen bonding interaction with the exposed backbone carbonyl of P6.50. Based upon these results, the investigators proposed that the formation of this hydrogen bonded AEA/ TMH6 complex may be the initial step in CB1 recognition of AEA in the lipid bilayer [164].
If AEA gains access to the CB1 receptor via the lipid bilayer in the manner suggested by the study discussed above, the ligand entry point would be between TMH6 and TMH7, with the ligand acyl tail at the level of the CWXP motif on TMH6. To test this hypothesis, the Makriyannis lab has created an isothiocyanate labelled classical cannabinoid ligand, AM841 (16, Chart 3), to serve as a covalent probe of cannabinoid interaction with their receptors. In this ligand, the isothiocyanate functional group is attached to the last carbon of the 1’,1’-dimethylheptyl (DMH) side chain. The CB1 and the CB2 receptors each possess Cys residues that face into the ligand binding pocket (CB1: C7.38(382) and C7.42(386); CB2: C7.38(284) and C7.42(288)). For both CB1 [165] and CB2 [166], AM841 was found to specifically label C6.47, a residue in the TMH6 CWXP hinge motif that is located near the floor of the binding pocket (near bilayer center) in the TMH6/7 interface in the inactive state of both CB1 and CB2. A recent NMR study of CP55940 in POPC bilayers has shown that the DMH sidechain of CP55940 orients itself parallel to the phospholipid acyl chains [167]. A similar membrane orientation is likely for the AM841 DMH side chain. The selective labelling of C6.47 by the AM841 DMH alkyl chain is consistent with ligand entry between TMH6/7 from the lipid bilayer, as C6.47 would be the first Cys residue seen by the ligand if it entered from the lipid bilayer. The Makriyannis group has reported the synthesis of AEA analogs labelled at the end of their fatty acid chain with either an azido or isothiocyanate group [168]. Results of labelling studies with this ligand are much anticipated.
Mutation Studies of the CB1 and CB2 Receptors
Mutation and chimera studies are excellent ways to gather information on ligand binding sites. Although the CB1 binding sites for SR141716A [169–177], WIN55212-2 [169, 172, 176, 178–180] and CP55940 [176, 177, 179, 180]/HU-210 [172, 174, 175, 178, 180] and the CB2 binding site for WIN55212-2 [63, 181–183], CP55940 [182, 183], HU-210 [182] and SR144528 [184, 185] have been explored (many quite extensively) via mutation studies, very few mutations have shed light on the endocannabinoid binding sites at CB1 or CB2. Song and Bonner reported that a K3.28A mutation in CB1 leads to severe loss of binding for anan-damide, HU-210 and CP-55940 [178]. Mutations of aromatic residues on TMH3,4,5,6 of CB1 revealed that the binding of anandamide was affected by the mutation of one aromatic on TMH3, F3.25 [169, 176]. Recently, the Kendall lab has reported that mutations of F268W, P269A and 1271A in the EC-2 loop of CB1 have a profound effect on the binding of R-methanandamide (13, Chart 3) at CB1. These results are suggestive of a steric effect on R-methanandamide binding and imply that anandamide binds high enough in the CB1 binding pocket to be impacted by changes in the EC-2 loop [175]. Surprisingly, there have been no mutation studies that explored the 2-AG binding pocket at CB1 or CB2.
Computer Models of the Cannabinoid CB1 and CB2 Receptors
Rhodopsin based models of the CB1 [186–191] and CB2 [184, 188, 191–194] receptors have been published by several research groups and new CB1 [195, 196] and CB2 [195] models based on the β2-AR crystal structure have recently been described. In their recent comparison of the engineered β2-AR crystal structure to Rho based models, Cherezov and co-workers remarked that most published β2-AR computer models were more similar to Rho than to the β2-AR structure [93]. This reflects the bias introduced by the template structure used for model development. There are significant sequence divergences between CB1/CB2 and Rho that need to be taken into account during model development. These include the absence of helix kinking proline residues in TMH1 and TMH5, the lack of a GG motif in TMH2, the lack of a PP at the EC end of TMH4 (CB1/CB2 have only one P), as well as, the presence of extra flexibility in TMH6, particularly in CB1. Another significant sequence divergence between Rho and CB1/CB2 is in the second extracellular (EC-2) loop region. This loop in CB1/CB2 is shorter than in Rho and is missing the conserved disulfide bridge between the cysteine in EC-2 and Cys3.25 in TMH3 of Rho. Instead, in CB1 there is a Cys residue at the TMH4 EC end (CB1 C4.66(257); CB2 C4.66(174)) and a Cys near the middle of the EC-2 loop (CB1 C(264); CB2 C(179)) that experiments suggest may form a disulfide bridge [173, 184]. Consequently, the position of the EC-2 loop with respect to the binding site crevice in CB1/CB2 around TMHs 3–4–5 is likely to be quite different from that in Rho.
While most CB models are homology models based strictly on a crystal structure template, some groups have used crystal structure information as a starting point, with refinements made to reflect the important sequence divergences in CB1/CB2 from a Rho template. The Reggio group has constructed and refined models of the CB1[172, 197, 198] and CB2 [199] receptor inactive and activated states. These models, initially built based upon a Rho template, have undergone refinements via biased Monte Carlo/ simulated annealing calculations on individual helices [200, 201]. Important loop regions have been modeled using a novel Scaled Collective Variable in Monte Carlo loop modeling method [202, 203] with an implicit solvent model that is based on a screened Coulomb potential formulation (the SCP-ISM) [204, 205]. These calculations on helices and loops were undertaken so that the models reflect the sequence dictated differences between CB1/CB2 and Rho.
Modelling Studies of Endocannabinoid Binding to the CB1 and CB2 Receptors
The binding of AEA at CB1 and CB2 has been studied by several groups. The first endocannabinoid docking studies in the CB1 receptor were reported by Barnett-Norris and coworkers [142] who used a refined rhodopsin homology model of the CB1 activated state (R*) to study the binding of a series of dimethyl anandamide analogs, for which the CB1 affinity of the R,R analog ((R)-N-(l-methyl-2-hydroxyethyl)-2-(R)-rnethyl-arachidonamide) is quite high (Ki = 7.42 ± 0.86 nM). These studies suggested that the congeners adopt tightly curved U/J-shaped conformations at CB1 and that the high CB1 affinity of the R,R stereoisomer is due to the ability of the headgroup to form an intramolecular hydrogen bond between the carboxamide oxygen and the headgroup hydroxyl that orients the C2 and C1’ methyl groups (see 5, Chart 1 for numbering system) to have hydrophobic interactions with V3.32(196), while the carboxamide oxygen forms a hydrogen bond with K3.28(192) at CB1. In this position in the CB1 binding pocket, the acyl chain has hydrophobic and C-H•••π interactions with residues in the transmembrane helix (TMH) 2–3–7 region.
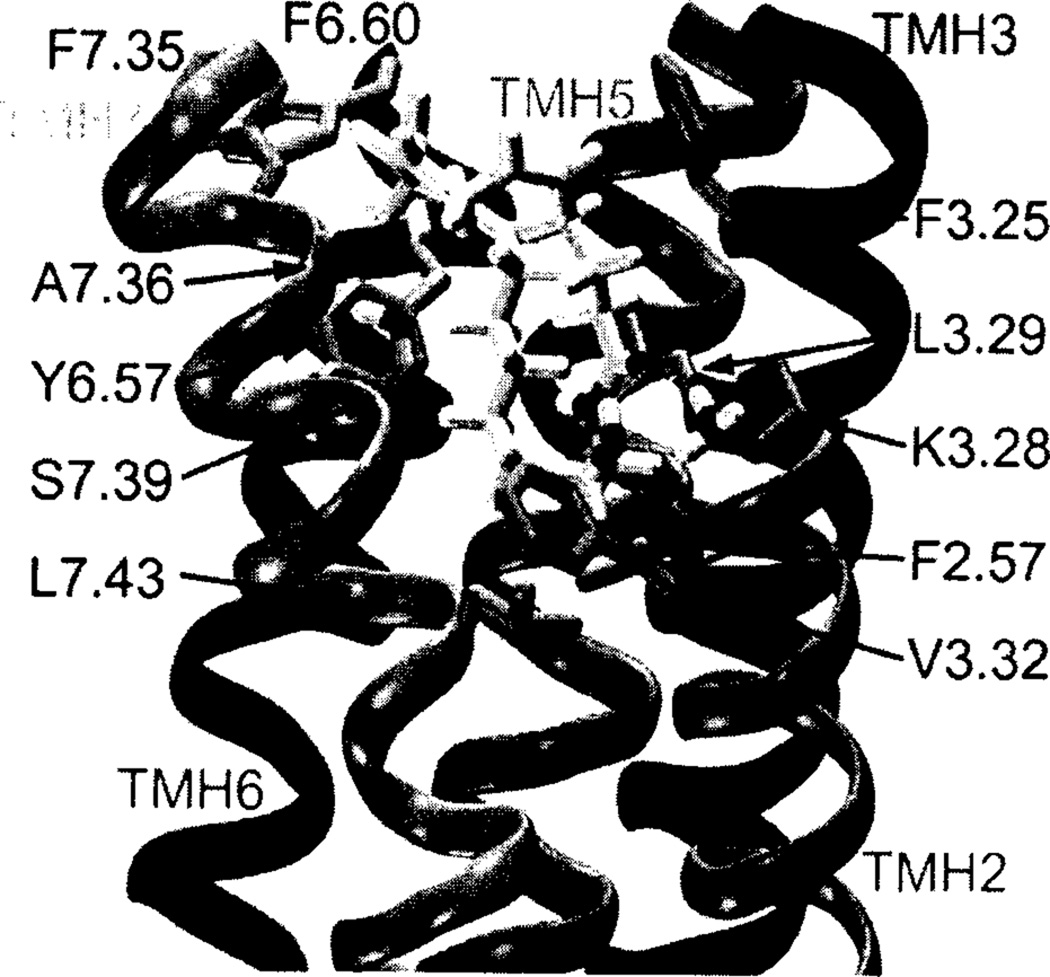
A subsequent study of anandamide binding at CB1 using the same CB1 R* model, suggested that K3.28(192) forms a hydrogen bond with the amide oxygen of anandamide [169]. At the same time, the headgroup hydroxyl of anandamide is engaged in an intramolecular hydrogen bond with its amide oxygen (see Fig. 3). The anandamide binding pocket is lined with residues that are largely hydrophobic, including L3.29, V3.32, F6.60, F7.35, A7.36, Y6.57, S7.39 (hydrogen bonded back to its own backbone carbonyl oxygen), and L7.43. F3.25 has a C-H•••π interaction with the C5-C6 double bond of anandamide, while F2.57 has an interaction with the amide oxygen of anandamide. The binding-site interactions identified for anandamide agree with results first reported by Pinto and co-workers [126], which showed that the hydroxyl group in the headgroup region of anandamide could be replaced by a methyl group without a loss in CB1 affinity. This result suggested that the hydroxyl group is not essential for anandamide binding and also that this hydroxyl may exist in a hydrophobic region of CB1. This result has been echoed in later endocannabinoid structure-activity relationship studies that showed, for example, that a cyclopropyl headgroup results in a very high CB1 affinity ligand [136]. In the binding site, identified for anandamide in the model, the headgroup hydroxyl is located in a hydrophobic pocket and satisfies its hydrogen-bonding potential by forming an intramolecular hydrogen bond with the amide oxygen. This result is consistent with NMR solution studies of anandamide reported by Bonechi and co-workers [206], who found that this intramolecular hydrogen bond in anandamide persists in solution. These results are also consistent with CB1 mutation studies which have indicated that K3.28(192) is a primary interaction site for AEA [178] and separate mutation studies in which the binding of AEA was reduced 6-fold by a F3.25A mutation [169].
Fig. (3).
The AEA/R* complex in the TMH2-3-6-7 region of CB1 R* is illustrated here. AEA (5) is shown in tube display. K3.28 forms a hydrogen bond with the amide oxygen of 5. At the same time, the head group hydroxyl of 5 is engaged in an intramolecular hydrogen bond with its amide oxygen. The AEA binding pocket is lined with residues that are largely hydrophobic, including L3.29, V3.32, F6.60, F7.35, A7.36, Y6.57, S7.39 (hydrogen bonded back to its own backbone carbonyl oxygen) and L7.43. F3.25 has a C-H•••π interaction with the C5-C6 double bond of 5; while, F2.57 has an interaction with the amide oxygen of 5 [169].
In their rhodopsin based homology model of CB1, Tuc-cinardi and co-workers [188] found that AEA binds in the TM2–3–6–7 region of CB1, adopting a U-shaped molecular conformation. The amide oxygen atom of the ligand interacts with K3.28(192), in agreement with site-directed mutagenesis studies [178], and the hydroxy group forms a hydrogen bond with S7.39(383). The residues that delimit the AEA binding pocket are principally hydrophobic, including F2.57(170), F3.25(189), L3.29(193), V3.32(196), F3.36 (200), and F7.35(379), in agreement with the CB1 model proposed by McAllister et al. [169]. F2.57(170) interacts with the aliphatic chain of AEA through a C-H•••π interaction, whereas F3.25(189) has an interaction with the amide oxygen atom.
In Tuccinardi and co-workers [188] CB2 receptor model, AEA binds in the TM3–4–5–6 region. It does not interact with K.3.28(109), but it forms a H-bond with S3.31(112) through the amide oxygen atom, and this is in agreement with mutagenesis studies [183]. Moreover, the hydroxy group interacts with the oxygen backbone of L3.27(108). The AEA aliphatic chain interacts principally with W5.43(194) and W6.48(25 8). The AEA docking results seem to support the validity of these CB1 and CB2 models since they are in good agreement with the main mutagenesis data available for this ligand. Brizzi and co-workers recently used these models to analyze the binding of hybrid resorcinol/anandamide hybids [207].
Padgett and co-workers recently explored the binding of ligands designed to mimic possible anandamide conformations. The most potent and efficacious of the ligands adopted conformations characterized by interactions with both the K3.28 and hydrophobic residues that interact with the non-classical cannabinoid, CP55244. The other five compounds formed fewer or less energetically favorable interactions with these critical residues [189].
Salo and co-workers [190] reported docking results for both AEA and 2-AG in their rhodpsin based CB1 receptor model. Both the carbonyl and hydroxyl groups of AEA acted as hydrogen bond acceptors with K3.28. In addition, the carbonyl oxygen of AEA could serve as a hydrogen bond acceptor with S7.39. For 2-AG, the carbonyl oxygen acted as a hydrogen bond acceptor with K3.28 and the ester oxygen of 2-AG acted as a hydrogen bond acceptor with S7.39. Both endocannabinoids adopt a U-shaped molecular conformations, with their aliphatic tails turning to the lipophilic region of the binding site. In this conformation, the ligand acyl chain is located in the vicinity of residues such as F3.36(200), W5.43(279), and L5.40(276). However, no specific C-H•••π interactions were recognized between the hydrogens of the aromatic rings and the double bonds of AEA in this binding model. In a subsequent study, Salo and coworkers [208] used both manual techniques and automated docking at rhodopsin-based CB1 receptor models to obtain a common alignment of endocannabinoid and classical cannabinoid derivatives. In their final alignment models, the endocannabinoid headgroup occupies a unique region distinct from the classical cannabinoid structures, supporting the hypothesis that these structurally diverse molecules overlap only partially within the receptor binding site.
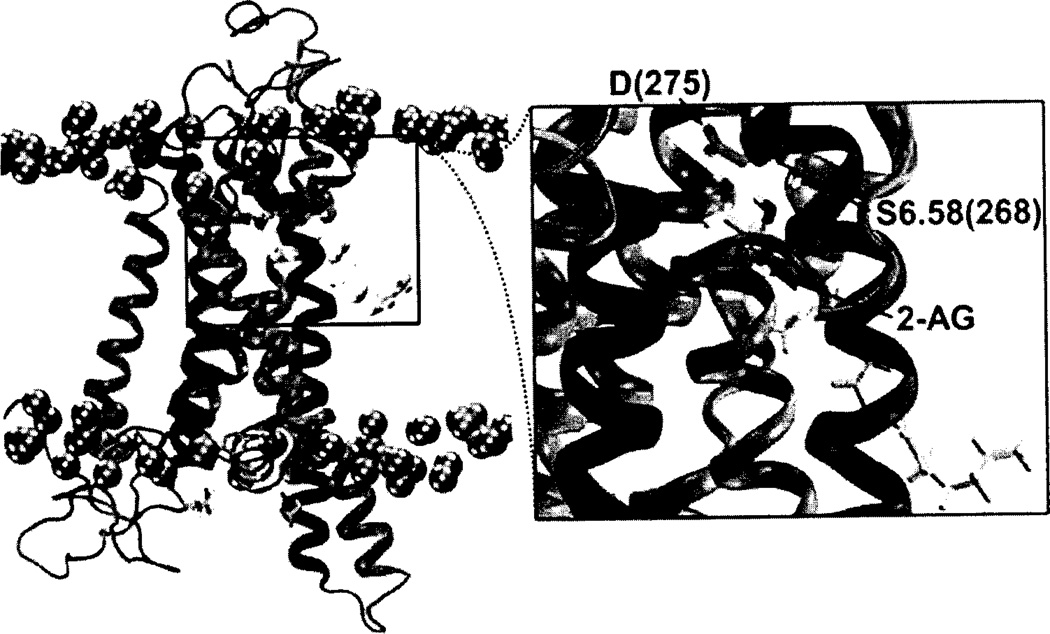
A very recent, microsecond timescale molecular dynamics study of 2-AG interaction with the CB2 receptor in a palmitoyl-oleoylphosphatidylcholine (POPC) lipid bilayer has suggested that (1) 2-AG first partitions out of bulk lipid at the CB2 TMH6/7 interface; (2) 2-AG then enters the CB2 receptor binding pocket by passing between TMH6/7; and (3) the entrance of the 2-AG head group into the CB2 binding pocket is sufficient to trigger conformational changes associated with the initial stages of GPCR activation, such as breaking of the intracellular (IC) TMH3/TMH6 ionic lock and the movement of the TMH6 IC end away from TMH3. Upon entry into the CB2 bundle, one 2-AG hydroxyl group interacts with S7.39(285), while the second hydroxyl forms an intramolecular hydrogen bond with the first hydroxyl. Ultimately, one 2-AG hydroxyl group establishes a long lasting interaction with D(275) in the EC-3 loop, while the other forms a hydrogen bond with S6.58(268). This set of interactions remains throughout the rest of the simulation (nearly 2 us; see Fig. 4) [209]. One of the interesting outcomes of this study was that receptor activation was triggered by head group entry into CB2. As is clear in Fig. (4), a portion of the fatty acid chain remains in lipid. In fact, only the first double bond of 2-AG resides in the binding pocket. While this may simply mean that fatty acid chain entry requires additional time to pull into the binding pocket, it is interesting that Sugiura’s SAR studies for 2-AG in CB2 indicated that the Δ5 (first) double bond in the fatty acid chain of 2-AG was crucial for CB2 activity [153].
Fig. (4).
A simulation cell containing a model of the CB2 receptor [211] immersed in a fully hydrated palmitoyl-oleoyl-phosphatidylcholine (POPC) lipid bilayer is illustrated here from a microsecond timescale NAMD2 MD simulation [209], The spheres represent the phosphorous atoms of the POPC head-groups. Water and the fatty acid acyl chains of the bilayer have been turned off for simplicity. 2-AG is contoured at its Van der Waals’ radius. In this figure, a 2-AG molecule has entered the CB2 receptor by passing between TMH6 and TMH7. Inset. Subsequent to entry, 2-AG establishes a long lasting interaction with D(275) in the EC-3 loop, a hydrogen bond with S6.58(268), and maintains an intramolecular hydrogen bond.
CONCLUSIONS
In addition to summarizing what is known about endocannabinoid interaction with the cannabinoid receptors, this review has pointed to areas in which more research needs to be done. Endocannabinoid SAR studies have largely focused on anandamide SAR. Given the importance of 2-AG as the mediator of cannabinoid retrograde signaling in the brain, more attention needs to be focused on 2-AG SAR. 2-AG binds to both CB1 and CB2, yet 2-AG is rarely used to evaluate mutations of either receptor. As a result, there is little mutational data that can be used to deduce where 2-AG binds in either CB1 or CB2. There are still major question marks associated with the issue of how newly synthesized endocannabinoids get to their targets (CB1 or CB2) and then ultimately reach the enzymes that degrade them. It has been a long-held assumption in the field that due to the high lipophilicity of the endocannabinoids, approach to their receptors may be via the lipid bilayer. Recent experimental and computational results discussed in this review have suggested that this may be true. However, more research needs to be done in this area. Finally, our current thinking about the location of key components of the endocannabinoid system (synthesizing enzymes, receptor protein and degrading enzymes), as described in this review still involves endocannabinoids crossing the synaptic cleft. Given endocannabinoid high lipophilicity, the deduction of how such a movement across the cleft occurs will be a very great contribution to the field.
ACKNOWLEDGEMENTS
Research support from National Institute on Drug Abuse grants RO1 DA003934 and K05 DA021358 is gratefully acknowledged.
REFERENCES
- 1.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 2.Felder CC, Joyce KE, Briley EM, Mansouri J, Mackie K, Blond O, Lai Y, Ma AL, Mitchell RL. Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Mol. Pharmacol. 1995;48:443–450. [PubMed] [Google Scholar]
- 3.Mackie K, Lai Y, Westenbroek R, Mitchell R. Cannabinoids activate an inwardly rectifying potassium conductance and inhibit Q-type calcium currents in AtT20 cells transfected with rat brain cannabinoid receptor. J. Neurosci. 1995;15:6552–6561. doi: 10.1523/JNEUROSCI.15-10-06552.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan X, Ikeda SR, Lewis DL. Rat brain cannabinoid receptor modulates N-type Ca2+ channels in a neuronal expression system. Mol Pharmacol. 1996;49:707–714. [PubMed] [Google Scholar]
- 5.Glass M, Dragunow M, Faull RL. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. 1997;77:299–318. doi: 10.1016/s0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- 6.Westlake TM, Howlett AC, Bonner TI, Matsuda LA, Herk-enham M. Cannabinoid receptor binding and messenger RNA expression in human brain: an in vitro receptor autoradiography and in situ hybridization histochemistry study of normal aged and Alzheimer’s brains. Neuroscience. 1994;63:637–652. doi: 10.1016/0306-4522(94)90511-8. [DOI] [PubMed] [Google Scholar]
- 7.Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol. Ther. 1997;74:129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- 8.Gerard CM, Mollereau C, Vassart G, Parmentier M. Molecular cloning of a human cannabinoid receptor which is also expressed in testis. Biochem. J. 1991;279(Pt 1):129–134. doi: 10.1042/bj2790129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Straiker AJ, Maguire G, Mackie K, Lindsey J. Localization of cannabinoid CB1 receptors in the human anterior eye and retina. Invest. Ophthalmol. Vis. Sci. 1999;40:2442–2448. [PubMed] [Google Scholar]
- 10.Schuel H, Chang MC, Burkman LJ, Picone RP, Makriyan-nis A, Zimmerman AM, Zimmerman S. Cannabinoid Receptors in Sperm in Marijuana and Medicine. Humana Press; Totowa: 1999. pp. 335–345. [Google Scholar]
- 11.Wright K, Rooney N, Feeney M, Tate J, Robertson D, Wel-ham M, Ward S. Differential expression of cannabinoid receptors in the human colon: cannabinoids promote epithelial wound healing. Gastroenterology. 2005;129:437–453. doi: 10.1016/j.gastro.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 12.Ishac EJ, Jiang L, Lake KD, Varga K, Abood ME, Kunos G. Inhibition of exocytotic noradrenaline release by presynaptic cannabinoid CB1 receptors on peripheral sympathetic nerves. Br. J. Pharmacol. 1996;118:2023–2028. doi: 10.1111/j.1476-5381.1996.tb15639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roche R, Hoareau L, Bes-Houtmann S, Gonthier MP, Laborde C, Baron JF, Haffaf Y, Cesari M, Festy F. Presence of the cannabinoid receptors, CB1 and CB2, in human omental and subcutaneous adipocytes. Histochem. Cell Biol. 2006;126:177–187. doi: 10.1007/s00418-005-0127-4. [DOI] [PubMed] [Google Scholar]
- 14.Bouaboula M, Rinaldi M, Carayon P, Carillon C, Delpech B, Shire D, Le Fur G, Casellas P. Cannabinoid-receptor expression in human leukocytes. Eur. J. Biochem. 1993;214:173–180. doi: 10.1111/j.1432-1033.1993.tb17910.x. [DOI] [PubMed] [Google Scholar]
- 15.Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, Bouaboula M, Shire D, Le Fur G, Casellas P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur. J. Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- 16.Rice W, Shannon JM, Burton F, Fiedeldey D. Expression of a brain-type cannabinoid receptor (CB1) in alveolar Type II cells in the lung: regulation by hydrocortisone. Eur. J. Pharmacol. 1997;327:227–232. doi: 10.1016/s0014-2999(97)89665-3. [DOI] [PubMed] [Google Scholar]
- 17.Devane WA, Dysarz FA, 3rd; Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol. Pharmacol. 1988;34:605–613. [PubMed] [Google Scholar]
- 18.Melvin LS, Milne GM, Johnson MR, Wilken GH, Howlett AC. Structure-activity relationships defining the ACD-tricyclic cannabinoids: cannabinoid receptor binding and analgesic activity. Drug Pes. Discov. 1995;13:155–166. [PubMed] [Google Scholar]
- 19.D’Ambra TE, Estep KG, Bell MR, Eissenstat MA, Josef KA, Ward SJ, Haycock DA, Baizman ER, Casiano FM, Beglin NC, Chippari SM, Grego JD, Kullnig RK, Daley GT. Conformationally restrained analogues of pravadoline: nanomolar potent, enantioselective, (aminoalkyl)indole agonists of the cannabinoid receptor. J. Med. Chem. 1992;35:124–135. doi: 10.1021/jm00079a016. [DOI] [PubMed] [Google Scholar]
- 20.Ward SJ, Baizman E, Bell M, Childers S, D’Ambra T, Eissenstat M, Estep K, Haycock D, Howlett A, Luttinger D, Miller M, Pacheco M. Aminoalkylindoles (AAIs): a new route to the cannabinoid receptor? N1DA Res. Monogr. 1991;105:425–426. [PubMed] [Google Scholar]
- 21.Compton DR, Gold LH, Ward SJ, Balster RL, Martin BR. Aminoalkylindole analogs: cannabimimetic activity of a class of compounds structurally distinct from delta 9-tetrahydrocannabinol. J. Pharmacol. Exp. Ther. 1992;263:1118–1126. [PubMed] [Google Scholar]
- 22.Reggio PH, Panu AM, Miles S. Characterization of a region of steric interference at the cannabinoid receptor using the active analog approach. J. Med. Chem. 1993;36:1761–1771. doi: 10.1021/jm00064a010. [DOI] [PubMed] [Google Scholar]
- 23.Rinaldi-Carmona M, Barth F, Heaulme M, Shire D, Calandra B, Congy C, Martinez S, Maruani J, Neliat G, Caput D, et al. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- 24.Bouaboula M, Perrachon S, Milligan L, Canat X, RinaldiCarmona M, Portier M, Barth F, Calandra B, Pecceu F, Lupker J, Maffrand JP, Le Fur G, Casellas P. A selective inverse agonist for central cannabinoid receptor inhibits mitogen-activated protein kinase activation stimulated by insulin or insulin-like growth factor 1. Evidence for a new model of receptor/ligand interactions. J.Biol. Chem. 1997;272:22330–22339. doi: 10.1074/jbc.272.35.22330. [DOI] [PubMed] [Google Scholar]
- 25.Pan X, Ikeda SR, Lewis DL. SR 141716A acts as an inverse agonist to increase neuronal voltage-dependent Ca2+ currents by reversal of tonic CB1 cannabinoid receptor activity. Mol. Pharmacol. 1998;54:1064–1072. doi: 10.1124/mol.54.6.1064. [DOI] [PubMed] [Google Scholar]
- 26.Meschler JP, Kraichely DM, Wilken GH, Howlett AC. Inverse agonist properties of N-(piperidin-l-yl)-5-(4-chloro-phenyl)-l-(2,4-dichlorophenyl)-4-methyl-lH-pyrazole-3-carboxa-mide HC1 (SR141716A) and l-(2-chlorophenyl)-4-cyano-5-(4-methoxyphenyl)-lH-pyrazole-3-carboxyl ic acid phenylamide (CP-272871) for the CB(1) cannabinoid receptor. Biochem. Pharmacol. 2000;60:1315–1323. doi: 10.1016/s0006-2952(00)00447-0. [DOI] [PubMed] [Google Scholar]
- 27.Felder CC, Joyce KE, Briley EM, Glass M, Mackie KP, Fahey KJ, Cullinan GJ, Hunden DC, Johnson DW, Chaney MO, Koppel GA, Brownstein M. LY320135, a novel cannabinoid CB1 receptor antagonist, unmasks coupling of the CB1 receptor to stimulation of cAMP accumulation. J. Pharmacol. Exp. Ther. 1998;284:291–297. [PubMed] [Google Scholar]
- 28.Ross RA, Brockie HC, Fernando SR, Sana B, Razdan RK, Pertwee RG. Comparison of cannabinoid binding sites in guinea-pig forebrain and small intestine. Br. J. Pharmacol. 1998;125:1345–1351. doi: 10.1038/sj.bjp.0702204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.LoVerme J, Duranti A, Tontini A, Spadoni G, Mor M, Ri-vara S, Stella N, Xu C, Tarzia G, Piomelli D. Synthesis and characterization of a peripherally restricted CB1 cannabinoid antagonist, URB447, that reduces feeding and body-weight gain in mice. Bioorg. Med. Chem. Lett. 2009;19:639–643. doi: 10.1016/j.bmcl.2008.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stoit AR, Lange JHM, den Hartog AP, Ronken E, Tipker K, van Stuivenberg HH, Dijksman JAR, Wals HC, Kruse CG. Design, synthesis and biological activity of rigid cannabinoid CB1 receptor antagonists. Chem. Pharm. Bull. 2002;50:1109–1113. doi: 10.1248/cpb.50.1109. [DOI] [PubMed] [Google Scholar]
- 31.Lange JH, Coolen HK, van Stuivenberg HH, Dijksman JA, Herremans AH, Ronken E, Keizer HG, Tipker K, McCreary AC, Veerman W, Wals HC, Stork B, Verveer PC, den Hartog AP, de Jong NM, Adolfs TJ, Hoogendoorn J, Kruse CG. Synthesis, biological properties, and molecular modeling investigations of novel 3,4-diarylpyrazolines as potent and selective CB(1) cannabinoid receptor antagonists. J. Med. Chem. 2004;47:627–643. doi: 10.1021/jm031019q. [DOI] [PubMed] [Google Scholar]
- 32.Lee SH, Seo HJ, Lee SH, Jung ME, Park JH, Park HJ, Yoo J, Yun H, Na J, Kang SY, Song KS, Kim MA, Chang CH, Kim J, Lee J. Biarylpyrazolyl oxadiazole as potent, selective, orally bioavailable cannabinoid-1 receptor antagonists for the treatment of obesity. J. Med. Chem. 2008;51:7216–7233. doi: 10.1021/jm800843r. [DOI] [PubMed] [Google Scholar]
- 33.Heimann AS, Gomes I, Dale CS, Pagano RL, Gupta A, de Souza LL, Luchessi AD, Castro LM, Giorgi R, Rioli V, Ferro ES, Devi LA. Hemopressin is an inverse agonist of CB1 cannabinoid receptors. Proc. Natl. Acad. Sci. U.S.A. 2007;104:20588–20593. doi: 10.1073/pnas.0706980105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jagerovic N, Fernandez-Fernandez C, Goya P. CB1 cannabinoid antagonists: structure-activity relationships and potential therapeutic applications. Curr. Top. Med. Chem. 2008;8:205–230. doi: 10.2174/156802608783498050. [DOI] [PubMed] [Google Scholar]
- 35.Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 36.Hanus L, Gopher A, Almog S, Mechoulam R. Two new unsaturated fatty acid ethanolamides in brain that bind to the cannabinoid receptor. J. Med. Chem. 1993;36:3032–3034. doi: 10.1021/jm00072a026. [DOI] [PubMed] [Google Scholar]
- 37.Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kamin-ski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR, Pertwee RG, Griffin G, Bayewitch M, Barg J, Vogel Z. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- 38.Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- 39.Hanus L, Abu-Lafi S, Fride E, Breuer A, Vogel Z, Shalev DE, Kustanovich I, Mechoulam R. 2-arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor. Proc. Natl Acad. Sci. U.S.A. 2001;98:3662–3665. doi: 10.1073/pnas.061029898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oka S, Tsuchie A, Tokumura A, Muramatsu M, Suhara Y, Takayama H, Waku K, Sugiura T. Ether-linked analogue of 2-arachidonoylglycerol (noladin ether) was not detected in the brains of various mammalian species. J. Neurochem. 2003;85:1374–1381. doi: 10.1046/j.1471-4159.2003.01804.x. [DOI] [PubMed] [Google Scholar]
- 41.Bezuglov V, Bobrov M, Gretskaya N, Gonchar A, Zinchenko G, Melck D, Bisogno T, Di Marzo V, Kuklev D, Rossi JC, Vidal JP, Durand T. Synthesis and biological evaluation of novel amides of polyunsaturated fatty acids with dopamine. Bioorg. Med. Chem. Lett. 2001;11:447–449. doi: 10.1016/s0960-894x(00)00689-2. [DOI] [PubMed] [Google Scholar]
- 42.Bisogno T, Melck D, Bobrov M, Gretskaya NM, Bezuglov VV, De Petrocellis L, Di Marzo V. N-acyl-dopamines: novel synthetic CB(1) cannabinoid-receptor ligands and inhibitors of anandamide inactivation with cannabimimetic activity in vitro and in vivo. Biochem. J. 2000;351(Pt3):817–824. [PMC free article] [PubMed] [Google Scholar]
- 43.Porter AC, Sauer JM, Knierman MD, Becker GW, Bema MJ, Bao J, Nomikos GG, Carter P, Bymaster FP, Leese AB, Felder CC. Characterization of a novel endocannabinoid, vi-rodhamine, with antagonist activity at the CB1 receptor. J. Pharmacol. Exp. Ther. 2002;301:1020–1024. doi: 10.1124/jpet.301.3.1020. [DOI] [PubMed] [Google Scholar]
- 44.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 45.Bouaboula M, Poinot-Chazel C, Marchand J, Canat X, Bour-rie B, Rinaldi-Carmona M, Calandra B, Le Fur G, Casellas P. Signaling pathway associated with stimulation of CB2 peripheral cannabinoid receptor. Involvement of both mitogen-activated protein kinase and induction of Krox-24 expression. Eur. J. Biochem. 1996;237:704–711. doi: 10.1111/j.1432-1033.1996.0704p.x. [DOI] [PubMed] [Google Scholar]
- 46.Bouaboula M, Desnoyer N, Carayon P, Combes T, Casellas P. Gi protein modulation induced by a selective inverse agonist for the peripheral cannabinoid receptor CB2: implication for intracellular signalization cross-regulation. Mol Pharmacol. 1999;55:473–480. [PubMed] [Google Scholar]
- 47.Sanchez MG, Ruiz-Llorente L, Sanchez AM, Diaz-Laviada I. Activation of phosphoinositide 3-kinase/PKB pathway by CB(1), CB(2) cannabinoid receptors expressed in prostate PC-3 cells. Involvement in Raf-1 stimulation and NGF induction. Cell Signal. 2003;15:851–859. doi: 10.1016/s0898-6568(03)00036-6. [DOI] [PubMed] [Google Scholar]
- 48.Guzman M, Galve-Roperh I, Sanchez C. Ceramide: a new second messenger of cannabinoid action. Trends Pharmacol. Sci. 2001;22:19–22. doi: 10.1016/s0165-6147(00)01586-8. [DOI] [PubMed] [Google Scholar]
- 49.Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- 50.Benito C, Nunez E, Tolon RM, Carrier EJ, Rabano A, Hillard CJ, Romero J. Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaque-associated glia in Alzheimer’s disease brains. J. Neurosci. 2003;23:11136–11141. doi: 10.1523/JNEUROSCI.23-35-11136.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ashton JC, Glass M. The Cannabinoid CB2 receptor as a target for inflammation-dependent neurodegeneration. Curr. Neuropharmacol. 2007;5:73–80. doi: 10.2174/157015907780866884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Onaivi ES, Ishiguro H, Gong JP, Patel S, Meozzi PA, Myers L, Perchuk A, Mora Z, Tagliaferro PA, Gardner E, Brusco A, Akinshola BE, Hope B, Lujilde J, Inada T, Iwa-saki S, Macharia D, Teasenfitz L, Arinami T, Uhl GR. Brain neuronal CB2 cannabinoid receptors in drug abuse and depression: from mice to human subjects. PLoS One. 2008;3:el640. doi: 10.1371/journal.pone.0001640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Ur-bani P, Mackie K, Stella N, Makriyannis A, Piomelli D, Davison JS, Marnett LJ, Di Marzo V, Pittman QJ, Patel KD, Sharkey KA. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- 54.Buckley NE, McCoy KL, Mezey E, Bonner T, Zimmer A, Felder CC, Glass M. Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB(2) receptor. Eur. J. Pharmacol. 2000;396:141–149. doi: 10.1016/s0014-2999(00)00211-9. [DOI] [PubMed] [Google Scholar]
- 55.Massi P, Fuzio D, Vigano D, Sacerdote P, Parolaro D. Relative involvement of cannabinoid CB(1) and CB(2) receptors in the Delta(9)-tetrahydrocannabinol-induced inhibition of natural killer activity. Eur. J. Pharmacol. 2000;387:343–347. doi: 10.1016/s0014-2999(99)00860-2. [DOI] [PubMed] [Google Scholar]
- 56.Jorda MA, Verbakel SE, Valk PJ, Vankan-Berkhoudt YV, Maccarrone M, Finazzi-Agro A, Lowenberg B, Delwel R. Hematopoietic cells expressing the peripheral cannabinoid receptor migrate in response to the endocannabinoid 2-arachidonoyl-glycerol. Blood. 2002;99:2786–2793. doi: 10.1182/blood.v99.8.2786. [DOI] [PubMed] [Google Scholar]
- 57.Kishimoto S, Gokoh M, Oka S, Muramatsu M, Kajiwara T, Waku K, Sugiura T. 2-arachidonoylglycerol induces the migration of HL-60 cells differentiated into macrophage-like cells and human peripheral blood monocytes through the cannabinoid CB2 receptor-dependent mechanism. J. Biol. Chem. 2003;278:24469–24475. doi: 10.1074/jbc.M301359200. [DOI] [PubMed] [Google Scholar]
- 58.Franklin A, Stella N. Arachidonylcyclopropylamide increases microglial cell migration through cannabinoid CB2 and abnormal-cannabidiol-sensitive receptors. Eur. J. Pharmacol. 2003;474:195–198. doi: 10.1016/s0014-2999(03)02074-0. [DOI] [PubMed] [Google Scholar]
- 59.KrsTTrmoto S, Kobayashi Y, Oka S, Gokoh M, Waku K, Sugiura T. 2-Arachidonoylglycerol, an endogenous cannabinoid receptor ligand, induces accelerated production of chemokines in HL-60 cells. J. Biochem. (Tokyo) 2004;135:517–524. doi: 10.1093/jb/mvh063. [DOI] [PubMed] [Google Scholar]
- 60.McCoy KL, Matveyeva M, Carlisle SJ, Cabral GA. Cannabinoid inhibition of the processing of intact lysozyme by macrophages: evidence for CB2 receptor participation. J. Pharmacol. Exp. Ther. 1999;289:1620–1625. [PubMed] [Google Scholar]
- 61.Idris AI, van ‘t Hof RJ, Greig IR, Ridge SA, Baker D, Ross RA, Ralston SH. Regulation of bone mass, bone loss and osteoclast activity by cannabinoid receptors. Nat. Med. 2005;11:774–779. doi: 10.1038/nm1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ofek O, Karsak M, Leclerc N, Fogel M, Frenkel B, Wright K, Tarn J, Attar-Namdar M, Kram V, Shohami E, Mechou-lam R, Zimmer A, Bab I. Peripheral cannabinoid receptor, CB2, regulates bone mass. Proc. Natl. Acad. Sci. U.S.A. 2006;103:696–701. doi: 10.1073/pnas.0504187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Song ZH, Slowey CA, Hurst DP, Reggio PH. The difference between the CB(1) and CB(2) cannabinoid receptors at position 5.46 is crucial for the selectivity of WIN55212-2 for CB(2) Mol. Pharmacol. 1999;56:834–840. [PubMed] [Google Scholar]
- 64.Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem. Biophys. Res. Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- 65.Huffman JW, Liddle J, Yu S, Aung MM, Abood ME, Wiley JL, Martin BR. 3-(l’,l’-Dimethylbutyl)-l-deoxy-delta8-THC and related compounds: synthesis of selective ligands for the CB2 receptor. Bioorg. Med. Chem. 1999;7:2905–2914. doi: 10.1016/s0968-0896(99)00219-9. [DOI] [PubMed] [Google Scholar]
- 66.Huffman JW, Bushell SM, Miller JR, Wiley JL, Martin BR. 1-Methoxy-, 1-deoxy-1 1-hydroxy- and 11-Hydroxy-l-methoxy-Delta(8)-tetrahydrocannabinols: new selective ligands for the CB(2) receptor. Bioorg. Med. Chem. 2002;10:4119–4129. doi: 10.1016/s0968-0896(02)00331-0. [DOI] [PubMed] [Google Scholar]
- 67.Gareau Y, Dufresne C, Gallant M, Rochette C, Sawyer N, Slipetz DM, Tremblay N, Weech PK, Metters KM, Labelle M. Structure activity relationships of tetrahydrocannabinol analogues on human cannabinoid receptors. Bioorg. Med. Chem. 1996;5:186–194. [Google Scholar]
- 68.Huffman JW, Yu S, Showalter V, Abood ME, Wiley JL, Compton DR, Martin BR, Bramblett RD, Reggio PH. Synthesis and pharmacology of a very potent cannabinoid lacking a phenolic hydroxyl with high affinity for the CB2 receptor. J. Med. Chem. 1996;39:3875–3877. doi: 10.1021/jm960394y. [DOI] [PubMed] [Google Scholar]
- 69.Rinaldi-Carmona M, Barth F, Millan J, Derocq JM, Casellas P, Congy C, Oustric D, Sarran M, Bouaboula M, Calandra B, Portier M, Shire D, Breliere JC, Le Fur GL. SR 144528, the first potent and selective antagonist of the CB2 cannabinoid receptor. J. Pharmacol. Exp. Ther. 1998;284:644–650. [PubMed] [Google Scholar]
- 70.Iwamura H, Suzuki H, Ueda Y, Kaya T, Inaba T. In vitro and in vivo pharmacological characterization of JTE-907, a novel selective ligand for cannabinoid CB2 receptor. J. Pharmacol. Exp. Ther. 2001;296:420–425. [PubMed] [Google Scholar]
- 71.Ross RA, Brockie HC, Stevenson LA, Murphy VL, Tem-pleton F, Makriyannis A, Pertwee RG. Agonist-inverse agonist characterization at CB1 and CB2 cannabinoid receptors of L759633, L759656, and AM630. Br. J. Pharmacol. 1999;126:665–672. doi: 10.1038/sj.bjp.0702351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lavey BJ, Kozlowski JA, Hipkin RW, Gonsiorek W, Lun-dell DJ, Piwinski JJ, Narula S, Lunn CA. Triaryl bis-sulfones as a new class of cannabinoid CB2 receptor inhibitors: identification of a lead and initial SAR studies. Bioorg. Med. Chem. Lett. 2005;15:783–786. doi: 10.1016/j.bmcl.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 73.Shankar BB, Lavey BJ, Zhou G, Spitler JA, Tong L, Rizvi R, Yang DY, Wolin R, Kozlowski JA, Shih NY, Wu J, Hipkin RW, Gonsiorek W, Lunn CA. Triaryl bis-sulfones as cannabinoid-2 receptor ligands: SAR studies. Bioorg. Med. Chem. Lett. 2005;75:4417–4420. doi: 10.1016/j.bmcl.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 74.Sawzdargo M, Nguyen T, Lee DK, Lynch KR, Cheng R, Heng HH, George SR, O’Dowd BF. Identification and cloning of three novel human G protein-coupled receptor genes GPR52, PsiGPR53 and GPR55: GPR55 is extensively expressed in human brain. Brain Res Mol Brain Res. 1999;64:193–198. doi: 10.1016/s0169-328x(98)00277-0. [DOI] [PubMed] [Google Scholar]
- 75.Brown A, Wise A. Identification of modulators of GPR55 activity. WO0186305. GlaxoSmithKline: USPTO; 2003. [Google Scholar]
- 76.Drmota T, Greasley P, Groblewski T. Screening assays for cannabinoid-ligand type modulators of GPR55. Astrazeneca; USA: 2004. [Google Scholar]
- 77.Brown A, Ueno S, Suen K, Dowell S, Wise A. Molecular identification of GPR55 as a third G-protein coupled receptor responsive to cannabinoid ligands. in 2005 Symposium of the Cannabinoids. Clearwater, FL: International Cannabinoid Research Society; 2005. [Google Scholar]
- 78.Sjogren S, Ryberg E, Lindblom A, Larsson N, Astrand A, Hjorth S, Andersson A, Groblewski T, Greasley P. A new receptor for cannabinoid ligands in 2005 Symposium on the Cannabinoids. Clearwater, FA: International Cannabinoid Research Society; 2005. [Google Scholar]
- 79.Skaper SD, Buriani A, Toso RD, Petrelli L, Romanello S, Facci L, Leon A. The ALIAmide palmitoylethanolamide and cannabinoids, but not anandamide, are protective in a delayed postglutamate paradigm of excitotoxic death in cerebellar granule neurons. Proc. Natl. Acad. Sci. USA. 1996;93:3984–3989. doi: 10.1073/pnas.93.9.3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jaggar S, Sellaturay S, Rice A. The endogenous cannabinoid anandamide, but not the CB2 ligand palmitoylethanolamide, prevents the viscero-visceral hyper-reflexia associated with inflammation of the rat urinary bladder. Neurosci. Lett. 1998;253:123–126. doi: 10.1016/s0304-3940(98)00621-1. [DOI] [PubMed] [Google Scholar]
- 81.Ryberg E, Larsson N, Sjogren S, Hjorth S, Hermansson NO, Leonova J, Elebring T, Nilsson K, Drmota T, Greasley PJ. The orphan receptor GPR55 is a novel cannabinoid receptor. Br. J. Pharmacol. 2007;152:1092–1101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Johns DG, Behm DJ, Walker DJ, Ao Z, Shapland EM, Daniels DA, Riddick M, Dowell S, Staton PC, Green P, Shabon U, Bao W, Aiyar N, Yue TL, Brown AJ, Morrison AD, Douglas SA. The novel endocannabinoid receptor GPR55 is activated by atypical cannabinoids but does not mediate their vasodilator effects. Br. J. Pharmacol. 2007;152:825–831. doi: 10.1038/sj.bjp.0707419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lauckner JE, Jensen JB, Chen HY, Lu HC, Hille B, Mackie K. GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc. Natl Acad. Sci U.S.A. 2008;105:2699–2704. doi: 10.1073/pnas.0711278105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Oka S, Nakajima K, Yamashita A, Kishimoto S, Sugiura T. Identification of GPR55 as a lysophosphatidylinositol receptor. Biochem. Biophys. Res. Commun. 2007;362:928–934. doi: 10.1016/j.bbrc.2007.08.078. [DOI] [PubMed] [Google Scholar]
- 85.Oka S, Nakajima K, Yamashita A, Kishimoto S, Sugiura T. Identification of GPR55 as a lysophosphatidylinositol receptor. Biochem. Biophys. Res. Commun. 2007;362:928–934. doi: 10.1016/j.bbrc.2007.08.078. [DOI] [PubMed] [Google Scholar]
- 86.Oka S, Toshida T, Maruyama K, Nakajima K, Yamashita A, Sugiura T. 2-Arachidonoyl-sn-glycero-3-phosphoinositoI: a possible natural ligand forGPR55. J. Biochem. 2009;145:13–20. doi: 10.1093/jb/mvn136. [DOI] [PubMed] [Google Scholar]
- 87.Kapur A, Zhao P, Sharir H, Bai Y, Caron MG, Barak LS, Abood ME. Atypical responsiveness of the orphan receptor GPR55 to cannabinoid ligands. J. Biol. Chem. 2009;284:29817–29827. doi: 10.1074/jbc.M109.050187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, Yamamoto M, Miyano M. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 89.Li J, Edwards PC, Burghammer M, Villa C, Schertler GF. Structure of bovine rhodopsin in a trigonal crystal form. J. Mol. Biol. 2004;343:1409–1438. doi: 10.1016/j.jmb.2004.08.090. [DOI] [PubMed] [Google Scholar]
- 90.Okada T, Fujiyoshi Y, Silow M, Navarro J, Landau EM, Shichida Y. Functional role of internal water molecules in rhodopsin revealed by X-ray crystallography. Proc. Natl. Acad. Sci. U.S.A. 2002;99:5982–5987. doi: 10.1073/pnas.082666399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Okada T, Sugihara M, Bondar AN, Elstner M, Entel P, Buss V. The retinal conformation and its environment in rhodopsin in light of a new 2.2 A crystal structure. J. Mol. Biol. 2004;342:571–583. doi: 10.1016/j.jmb.2004.07.044. [DOI] [PubMed] [Google Scholar]
- 92.Rasmussen SG, Choi HJ, Rosenbaum DM, Kobilka TS, Thian FS, Edwards PC, Burghammer M, Ratnala VR, Sanishvili R, Fischetti RF, Schertler GF, Weis WI, Kobilka BK. Crystal structure of the human beta(2) adrenergic G-proteincoupled receptor. Nature. 2007;450:383–387. doi: 10.1038/nature06325. [DOI] [PubMed] [Google Scholar]
- 93.Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Kuhn P, Weis WI, Kobilka BK, Stevens RC. High-Resolution Crystal Structure of an Engineered Human {beta}2-Adrenergic G Protein Coupled Receptor. Science. 2007 doi: 10.1126/science.1150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rosenbaum DM, Cherezov V, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Yao XJ, Weis WI, Stevens RC, Kobilka BK. GPCR Engineering Yields HighResolution Structural Insights into {beta}2 Adrenergic Receptor Function. Science. 2007 doi: 10.1126/science.1150609. [DOI] [PubMed] [Google Scholar]
- 95.Warne T, Serrano-Vega MJ, Baker JG, Moukhametzianov R, Edwards PC, Henderson R, Leslie AG, Tate CG, Schertler GF. Structure of a beta 1-adrenergic G-protein-coupled receptor. Nature. 2008;454:486–491. doi: 10.1038/nature07101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jaakola VP, Griffith MX, Hanson MA, Cherezov V, Chien EY, Lane JR, Ijzerman AP, Stevens RC. The 2.6 angstrom crystal structure of a human A2A adenosine receptor bound to an antagonist. Science. 2008;322:1211–1217. doi: 10.1126/science.1164772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Farrens D, Altenbach C, Ynag K, Hubbell W, Khorana H. Requirement of rigid-body motion of transmembrane helices for light activation of rhodopsin. Science. 1996;274:768–770. doi: 10.1126/science.274.5288.768. [DOI] [PubMed] [Google Scholar]
- 98.Ghanouni P, Steenhuis JJ, Farrens DL, Kobilka BK. Agonist-induced conformational changes in the G-protein-coupling domain of the beta 2 adrenergic receptor. Proc. Natl. Acad. Sci. U.S.A. 2001;98:5997–6002. doi: 10.1073/pnas.101126198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lin SW, Sakmar TP. Specific tryptophan UV-absorbance changes are probes of the transition of rhodopsin to its active state. Biochemistry. 1996;35:11149–11159. doi: 10.1021/bi960858u. [DOI] [PubMed] [Google Scholar]
- 100.Javitch JA, Fu D, Liapakis G, Chen J. Constitutive activation of the beta2 adrenergic receptor alters the orientation of its sixth membrane-spanning segment. J. Biol. Chem. 1997;272:18546–18549. doi: 10.1074/jbc.272.30.18546. [DOI] [PubMed] [Google Scholar]
- 101.Jensen AD, Guarnieri F, Rasmussen SG, Asmar F, Ballesteros JA, Gether U. Agonist-induced conformational changes at the cytoplasmic side of transmembrane segment 6 in the beta 2 adrenergic receptor mapped by site-selective fluorescent labeling. J. Biol. Chem. 2001;276:9279–9290. doi: 10.1074/jbc.M004871200. [DOI] [PubMed] [Google Scholar]
- 102.Nakanishi J, Takarada T, Yunoki S, Kikuchi Y, Maeda M. FRET-based monitoring of conformational change of the beta2 adrenergic receptor in living cells. Biochem. Biophys. Res. Commun. 2006;343:1191–1196. doi: 10.1016/j.bbrc.2006.03.064. [DOI] [PubMed] [Google Scholar]
- 103.Wess J. G-protein-coupled receptors: Molecular mechanisms involved in receptor activation and selectivity of G-protein recognition. FASEB J. 1997;11:346–354. [PubMed] [Google Scholar]
- 104.Katona L, Freund TF. Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat. Med. 2008;14:923–930. doi: 10.1038/nm.f.1869. [DOI] [PubMed] [Google Scholar]
- 105.Piomelli D. The molecular logic of endocannabinoid signalling. Nat. Rev. Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- 106.Di Marzo V. Targeting the endocannabinoid system: to enhance or reduce? Nat. Rev. Drug Discov. 2008;7:438–455. doi: 10.1038/nrd2553. [DOI] [PubMed] [Google Scholar]
- 107.Di Marzo V. The endocannabinoid system: its general strategy of action, tools for its pharmacological manipulation and potential therapeutic exploitation. Pharmacol. Res. 2009;60:77–84. doi: 10.1016/j.phrs.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 108.Levenes C, Daniel H, Soubrie P, Crepel F. Cannabinoids decrease excitatory synaptic transmission and impair long-term depression in rat cerebellar Purkinje cells. J. Physiol. 1998;570(Pt 3):867–879. doi: 10.1111/j.1469-7793.1998.867bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hoffman AF, Lupica CR. Mechanisms of cannabinoid inhibition of GABA(A) synaptic transmission in the hippocampus. J. Neurosci. 2000;20:2470–2479. doi: 10.1523/JNEUROSCI.20-07-02470.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 2001;29:717–727. doi: 10.1016/s0896-6273(01)00246-x. [DOI] [PubMed] [Google Scholar]
- 111.Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron. 2001;29:729–738. doi: 10.1016/s0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- 112.Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- 113.Takahashi KA, Linden DJ. Cannabinoid receptor modulation of synapses received by cerebellar Purkinje cells. J. Neurophysiol. 2000;83:1167–1180. doi: 10.1152/jn.2000.83.3.1167. [DOI] [PubMed] [Google Scholar]
- 114.Mackie K. Signaling via CNS cannabinoid receptors. Mol. Cell Endocrinol. 2008;286:S60–S65. doi: 10.1016/j.mce.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, Piomelli D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc. Natl. Acad. Sci. U.S.A. 2002;99:10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Okamoto Y, Wang J, Morishita J, Ueda N. Biosynthetic pathways of the endocannabinoid anandamide. Chem. Biodivers. 2007;4:1842–1857. doi: 10.1002/cbdv.200790155. [DOI] [PubMed] [Google Scholar]
- 117.Nyilas R, Dudok B, Urban GM, Mackie K, Watanabe M, Cravatt BF, Freund TF, Katona I. Enzymatic machinery for endocannabinoid biosynthesis associated with calcium stores in glutamatergic axon terminals. J. Neurosci. 2008;28:1058–1063. doi: 10.1523/JNEUROSCI.5102-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, Piomelli D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;572:686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- 119.Piomelli D, Beltramo M, Giuffrida A, Stella N. Endogenous cannabinoid signaling. Neurobiol. Pis. 1998;5:462–473. doi: 10.1006/nbdi.1998.0221. [DOI] [PubMed] [Google Scholar]
- 120.Kaczocha M, Glaser ST, Deutsch DG. Identification of intracellular carriers for the endocannabinoid anandamide. Proc. Natl. Acad. Sci. U.S.A. 2009;106:6375–6380. doi: 10.1073/pnas.0901515106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Di Marzo V, Melck D, Bisogno T, De Petrocellis L. Endocannabinoids: endogenous cannabinoid receptor ligands with neuromodulatory action. Trends Neurosci. 1998;21:521–528. doi: 10.1016/s0166-2236(98)01283-1. [DOI] [PubMed] [Google Scholar]
- 122.Bracey MH, Hanson MA, Masuda KR, Stevens RC, Cravatt BF. Structural adaptations in a membrane enzyme that terminates endocannabinoid signaling. Science. 2002;298:1793–1786. doi: 10.1126/science.1076535. [DOI] [PubMed] [Google Scholar]
- 123.Giang DK, Cravatt BF. Molecular characterization of human and mouse fatty acid amide hydrolases. Proc. Natl. Acad. Sci. U.S.A. 1997;94:2238–2242. doi: 10.1073/pnas.94.6.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Arreaza G, Deutsch DG. Deletion of a proline-rich region and a transmembrane domain in fatty acid amide hydrolase. FEBS Lett. 1999;454:57–60. doi: 10.1016/s0014-5793(99)00774-7. [DOI] [PubMed] [Google Scholar]
- 125.Berglund BA, Boring DL, Wilken GH, Makriyannis A, Howlett AC, Lin S. Structural requirements for arachidonyletha-nolamide interaction with CB1 and CB2 cannabinoid receptors: pharmacology of the carbonyl and ethanolamide groups. Prosta-glandins Leukot Essent Fatty Acids. 1998;59:111–118. doi: 10.1016/s0952-3278(98)90089-8. [DOI] [PubMed] [Google Scholar]
- 126.Pinto JC, Potie F, Rice KC, Boring D, Johnson MR, Evans DM, Wilken GH, Cantrell CH, Howlett AC. Cannabinoid receptor binding and agonist activity of amides and esters of arachi-donic acid. Mol. Pharmacol. 1994;46:516–522. [PubMed] [Google Scholar]
- 127.Lin S, Khanolkar AD, Fan P, Goutopoulos A, Qin C, Papahadjis D, Makriyannis A. Novel analogues of arachidonylethanolamide (anandamide): affinities for the CB1 and CB2 cannabinoid receptors and metabolic stability. J. Med. Chem. 1998;41:5353–5361. doi: 10.1021/jm970257g. [DOI] [PubMed] [Google Scholar]
- 128.Ng EW, Aung MM, Abood ME, Martin BR, Razdan RK. Unique analogues of anandamide: arachidonyl ethers and carbamates and norarachidonyl carbamates and ureas. J. Med. Chem. 1999;42:1975–1981. doi: 10.1021/jm980711w. [DOI] [PubMed] [Google Scholar]
- 129.Parkkari T, Savinainen JR, Rauhala AL, Tolonen TL, Ne-valainen T, Laitinen JT, Gynther J, Jarvinen T. Synthesis and CB1 receptor activities of novel arachidonyl alcohol derivatives. Bioorg. Med. Chem. Lett. 2004;14:3231–3234. doi: 10.1016/j.bmcl.2004.03.093. [DOI] [PubMed] [Google Scholar]
- 130.El Fangour S, Balas L, Rossi JC, Fedenyuk A, Gretskaya N, Bobrov M, Bezuglov V, Hillard CJ, Durand T. Hemisynthesis and preliminary evaluation of novel endocannabinoid analogues. Bioorg. Med. Chem. Lett. 2003;13:1977–1980. doi: 10.1016/s0960-894x(03)00348-2. [DOI] [PubMed] [Google Scholar]
- 131.Parkkari T, Savinainen JR, Raitio KH, Saario SM, Matilainen L, Sirvio T, Laitinen JT, Nevalainen T, Niemi R, Jarvinen T. Synthesis, cannabinoid receptor activity, and enzymatic stability of reversed amide derivatives of arachidonoyl ethanolamide. Bioorg. Med. Chem. 2006;14:5252–5258. doi: 10.1016/j.bmc.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 132.Abadji V, Lin S, Taha G, Griffin G, Stevenson LA, Pertwee RG, Makriyannis A. (R)-methanandamide: a chiral novel anandamide possessing higher potency and metabolic stability. J. Med. Chem. 1994;37:1889–1893. doi: 10.1021/jm00038a020. [DOI] [PubMed] [Google Scholar]
- 133.Adams IB, Ryan W, Singer M, Razdan RK, Compton DR, Martin BR. Pharmacological and behavioral evaluation of alkylated anandamide analogs. Life Sci. 1995;56:2041–2048. doi: 10.1016/0024-3205(95)00187-b. [DOI] [PubMed] [Google Scholar]
- 134.Goutopoulos A, Fan P, Khanolkar AD, Xie XQ, Lin S, Makriyannis A. Stereochemical selectivity of methanandamides for the CB1 and CB2 cannabinoid receptors and their metabolic stability. Bioorg. Med. Chem. 2001;9:1673–1684. doi: 10.1016/s0968-0896(01)00088-8. [DOI] [PubMed] [Google Scholar]
- 135.Sheskin T, Hanus L, Slager J, Vogel Z, Mechoulam R. Structural requirements for binding of anandamide-type compounds to the brain cannabinoid receptor. J. Med. Chem. 1997;40:659–667. doi: 10.1021/jm960752x. [DOI] [PubMed] [Google Scholar]
- 136.Hillard CJ, Manna S, Greenberg MJ, DiCamelli R, Ross RA, Stevenson LA, Murphy V, Pertwee RG, Campbell WB. Synthesis and characterization of potent and selective agonists of the neuronal cannabinoid receptor (CB1) J. Pharmacol. Exp. Ther. 1999;289:1427–1433. [PubMed] [Google Scholar]
- 137.Adams IB, Ryan W, Singer M, Thomas BF, Compton DR, Razdan RK, Martin BR. Evaluation of cannabinoid receptor binding and in vivo activities for anandamide analogs. J. Pharmacol. Exp. Ther. 1995;27J:1172–1181. [PubMed] [Google Scholar]
- 138.Lang W, Qin C, Lin S, Khanolkar AD, Goutopoulos A, Fan P, Abouzid K, Meng Z, Biegel D, Makriyannis A. Substrate specificity and stereoselectivity of rat brain microsomal anandamide amidohydrolase. J. Med. Chem. 1999;42:896–902. doi: 10.1021/jm980461j. [DOI] [PubMed] [Google Scholar]
- 139.Khanolkar AD, Abadjl V, Lin 5, Hill WA, Taha G, Abouzid K, Meng Z, Fan P, Makriyannis A. Head group analogs of arachidonylethanolamide, the endogenous cannabinoid ligand. J. Med. Chem. 1996;39:4515–4519. doi: 10.1021/jm960152y. [DOI] [PubMed] [Google Scholar]
- 140.Edgemond WS, Hillard CJ, Falck JR, Kearn CS, Campbell WB. Human platelets and polymorphonuclear leukocytes synthesize oxygenated derivatives of arachidonylethanolamide (anandamide): their affinities for cannabinoid receptors and pathways of inactivation. Mol. Pharmacol. 1998;54:180–188. doi: 10.1124/mol.54.1.180. [DOI] [PubMed] [Google Scholar]
- 141.Edgemond WS, Campbell WB, Hillard CJ. The binding of novel phenolic derivatives of anandamide to brain cannabinoid receptors. Prostaglandins Leukot. Essent. Fatty Acids. 1995;52:83–86. doi: 10.1016/0952-3278(95)90002-0. [DOI] [PubMed] [Google Scholar]
- 142.Barnett-Norris J, Hurst DP, Lynch DL, Guarnieri F, Makriyannis A, Reggio PH. Conformational memories and the endo-cannabinoid binding site at the cannabinoid CB1 receptor. J. Med. Chem. 2002;45:3649–3659. doi: 10.1021/jm0200761. [DOI] [PubMed] [Google Scholar]
- 143.Seltzman HH, Fleming DN, Thomas BF, Gilliam AF, McCallion DS, Pertwee RG, Compton DR, Martin BR. Synthesis and pharmacological comparison of dimethylheptyl and pentyl analogs of anandamide. J. Med. Chem. 1997;40:3626–3634. doi: 10.1021/jm9702950. [DOI] [PubMed] [Google Scholar]
- 144.Ryan WJ, Banner WK, Wiley JL, Martin BR, Razdan RK. Potent anandamide analogs: the effect of changing the length and branching of the end pentyl chain. J. Med. Chem. 1997;40:3617–3625. doi: 10.1021/jm970212f. [DOI] [PubMed] [Google Scholar]
- 145.Berglund BA, Boring DL, Howlett AC. Investigation of structural analogs of prostaglandin amides for binding to and activation of CB1 and CB2 cannabinoid receptors in rat brain and human tonsils. Adv. Exp. Med. Biol. 1999;469:527–533. doi: 10.1007/978-1-4615-4793-8_77. [DOI] [PubMed] [Google Scholar]
- 146.Berglund BA, Fleming PR, Rice KC, Shim JY, Welsh WJ, Howlett AC. Development of a novel class of monocyclic and bicyclic alkyl amides that exhibit CB1 and CB2 cannabinoid receptor affinity and receptor activation. Drug Pes. Discov. 2000;76:281–294. [PubMed] [Google Scholar]
- 147.Barnett-Norris J, Guamieri F, Hurst DP, Reggio PH. Exploration of biologically relevant conformations of anandamide, 2-arachidonylglycerol, and their analogues using conformational memories. J. Med. Chem. 1998;41:4861–4872. doi: 10.1021/jm9803471. [DOI] [PubMed] [Google Scholar]
- 148.Sugiura T, Kodaka T, Kondo S, Tonegawa T, Nakane S, Kishimoto S, Yamashita A, Waku K. 2-Arachidonoylglycerol, a putative endogenous cannabinoid receptor ligand, induces rapid, transient elevation of intracellular free Ca2+ in neuroblastoma x glioma hybrid NG108-15 cells. Biochem. Biophys. Res. Commun. 1996;229:58–64. doi: 10.1006/bbrc.1996.1757. [DOI] [PubMed] [Google Scholar]
- 149.Sugiura T, Kodaka T, Kondo S, Nakane S, Kondo H, Waku K, Ishima Y, Watanabe K, Yamamoto I. Is the cannabinoid CB1 receptor a 2-arachidonoylglycerol receptor? Structural requirements for triggering a Ca2+ transient in NG108-15 cells. J. Biochem. (Tokyo) 1997;122:890–895. doi: 10.1093/oxfordjournals.jbchem.a021838. [DOI] [PubMed] [Google Scholar]
- 150.Sugiura T, Kodaka T, Nakane S, Miyashita T, Kondo S, Suhara Y, Takayama H, Waku K, Seki C, Baba N, Ishima Y. Evidence that the cannabinoid CB1 receptor is a 2-arachidonoylglycerol receptor. Structure-activity relationship of 2-arachidonoylglycerol, ether-linked analogues, and related compounds. J. Biol. Chem. 1999;274:2794–2801. doi: 10.1074/jbc.274.5.2794. [DOI] [PubMed] [Google Scholar]
- 151.Parkkari T, Myllymaki M, Savinainen JR, Saario SM, Casti-llo-Melendez JA, Laitinen JT, Nevalainen T, Koskinen AM, Jarvinen T. Alpha-methylated derivatives of 2-arachidonoyl glycerol: synthesis, CB1 receptor activity, and enzymatic stability. Bioorg. Med. Chem. Lett. 2006;16:2437–2440. doi: 10.1016/j.bmcl.2006.01.101. [DOI] [PubMed] [Google Scholar]
- 152.Parkkari T, Salo OM, Huttunen KM, Savinainen JR, Laitinen JT, Poso A, Nevalainen T, Jarvinen T. Synthesis and CB1 receptor activities of dimethylheptyl derivatives of 2-arachidonoyl glycerol (2-AG) and 2-arachidonyl glyceryl ether (2-AGE) Bioorg. Med Chem. 2006;14:2850–2858. doi: 10.1016/j.bmc.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 153.Sugiura T, Kondo S, Kishimoto S, Miyashita T, Nakane S, Kodaka T, Suhara Y, Takayama H, Waku K. Evidence that 2-arachidonoylglycerol but not N-palmitoylethanolamine or anandamide is the physiological ligand for the cannabinoid CB2 receptor. Comparison of the agonistic activities of various cannabinoid receptor ligands in HL-60 cells. J. Biol. Chem. 2000;275:605–612. doi: 10.1074/jbc.275.1.605. [DOI] [PubMed] [Google Scholar]
- 154.Suhara Y, Oka S, Kittaka A, Takayama H, Waku K, Sugiura T. Synthesis and biological evaluation of several structural analogs of 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand. Bioorg. Med. Chem. 2007;15:854–867. doi: 10.1016/j.bmc.2006.10.049. [DOI] [PubMed] [Google Scholar]
- 155.Vadivel SK, Vardarajan S, Duclos RI, Jr., Wood JT, Guo J, Makriyannis A. Conformationally constrained analogues of 2-arachidonoylglycerol. Bioorg. Med. Chem. Lett. 2007;17:5959–5963. doi: 10.1016/j.bmcl.2007.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rabinovich AL, Ripatti PO. On the conformational, physical properties and function of polyunsaturated acylchains. Biochim. Biophys. Acta. 1991;1085:53–56. doi: 10.1016/0005-2760(91)90231-6. [DOI] [PubMed] [Google Scholar]
- 157.Rich MR. Conformational analysis of arachidonic and related fatty acids using molecular dynamics simulations. Biochim. Biophys. Acta. 1993;1178:87–96. doi: 10.1016/0167-4889(93)90113-4. [DOI] [PubMed] [Google Scholar]
- 158.Reggio PH, Traore H. Conformational requirements for endocannabinoid interaction with the cannabinoid receptors, the anandamide transporter and fatty acid amidohydrolase. Chem Phys Lipids. 2000;108:15–35. doi: 10.1016/s0009-3084(00)00185-7. [DOI] [PubMed] [Google Scholar]
- 159.Thomas BF, Adams IB, Mascarella SW, Martin BR, Razdan RK. Structure-activity analysis of anandamide analogs: relationship to a cannabinoid pharmacophore. J. Med. Chem. 1996;39:471–479. doi: 10.1021/jm9505167. [DOI] [PubMed] [Google Scholar]
- 160.Tong W, Collantes ER, Welsh WJ, Berglund BA, Howlett AC. Derivation of a pharmacophore model for anandamide using constrained conformational searching and comparative molecular field analysis. J. Med. Chem. 1998;41:4207–4215. doi: 10.1021/jm970239z. [DOI] [PubMed] [Google Scholar]
- 161.Di Marzo M, Casapullo A, Bifulco G, Cimino P, Ligresti A, Di Marzo V, Riccio R, Gomez-Paloma L. Synthesis, conformational analysis and CB1 binding affinity of hairpin-like anandamide pseudopeptide mimetics. J. Pept. Sci. 2006;12:575–591. doi: 10.1002/psc.754. [DOI] [PubMed] [Google Scholar]
- 162.Tian X, Guo J, Yao F, Yang DP, Makriyannis A. The conformation, location, and dynamic properties of the endocannabinoid ligand anandamide in a membrane bilayer. J. Biol. Chem. 2005;280:29788–29795. doi: 10.1074/jbc.M502925200. [DOI] [PubMed] [Google Scholar]
- 163.Barnett-Norris J, Hurst DP, Buehner K, Ballesteros JA, Guarnieri F, Reggio PH. Agonist alkyl tail interaction with cannabinoid CB1 receptor V6.43/I6.46 groove induces a Helix 6 active conformation. Int. J. Quantum Chem. 2002;88:76–86. [Google Scholar]
- 164.Lynch DL, Reggio PH. Cannabinoid CB1 receptor recognition of endocannabinoids via the lipid bilayer: molecular dynamics simulations of CB1 transmembrane helix 6 and anandamide in a phospholipid bilayer. J. Comput. Aided Mol. Pes. 2006;20:495–509. doi: 10.1007/s10822-006-9068-9. [DOI] [PubMed] [Google Scholar]
- 165.Picone RP, Khanolkar AD, Xu W, Ayotte LA, Thakur GA, Hurst DP, Abood ME, Reggio PH, Fournier DJ, Makriyannis A. (-)-7’-Isothiocyanato-l 1-hydroxy-l’.l’-dimethylheptylhexahydrocannabinol (AM841), a high-affinity electrophilic ligand, interacts covalently with a cysteine in helix six and activates the CB1 cannabinoid receptor. Mol. Pharmacol. 2005;68:1623–1635. doi: 10.1124/mol.105.014407. [DOI] [PubMed] [Google Scholar]
- 166.Pei Y, Mercier RW, Anday JK, Thakur GA, Zvonok AM, Hurst D, Reggio PH, Janero DR, Makriyannis A. Ligandbinding architecture of human CB2 cannabinoid receptor: evidence for receptor subtype-specific binding motif and modeling GPCR activation. Chem. Biol. 2008;75:1207–1219. doi: 10.1016/j.chembiol.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kimura T, Cheng K, Rice KC, Gawrisch K. Location, structure, and dynamics of the synthetic cannabinoid ligand CP-55,940 in lipid bilayers. Biophys. J. 2009;96:4916–4924. doi: 10.1016/j.bpj.2009.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Li C, Xu W, Vadivel SK, Fan P, Makriyannis A. High affinity electrophilic and photoactivatable covalent endocannabinoid probes for the CB1 receptor. J. Med. Chem. 2005;48:6423–6429. doi: 10.1021/jm050272i. [DOI] [PubMed] [Google Scholar]
- 169.McAllister SD, Rizvi G, Anavi-Goil’er S, Hurst DP, Barnett-Norris J, Lynch DL, Reggio PH, Abood ME. An aromatic microdomain at the cannabinoid CB(1) receptor constitutes an agonist/inverse agonist binding region. J. Med. Chem. 2003;46:5139–5152. doi: 10.1021/jm0302647. [DOI] [PubMed] [Google Scholar]
- 170.Hurst DP, Lynch DL, Bamett-Norris J, Hyatt SM, Seltzman HH, Zhong M, Song ZH, Nie J, Lewis D, Reggio PH. N-(piperidin-l-yl)-5-(4-chlorophenyl)-l-(2,4-dichlorophenyl)-4-methyl-IH-p yrazole-3-carboxamide (SR141716A) interaction with LYS 3.28(192) is crucial for its inverse agonism at the cannabinoid CB1 receptor. Mol. Pharmacol. 2002;62:1274–1287. doi: 10.1124/mol.62.6.1274. [DOI] [PubMed] [Google Scholar]
- 171.Hurst D, Umejiego U, Lynch D, Seltzman H, Hyatt S, Roche M, McAllister S, Fleischer D, Kapur A, Abood M, Shi S, Jones J, Lewis D, Reggio P. Biarylpyrazole inverse agonists at the cannabinoid CB1 receptor: Importance of the C-3 carboxamide oxygen/lysine3.28(192) interaction. J. Med. Chem. 2006 doi: 10.1021/jm060446b. in press. [DOI] [PubMed] [Google Scholar]
- 172.Kapur A, Hurst DP, Fleischer D, Whitnell R, Thakur GA, Makriyannis A, Reggio PH, Abood ME. Mutation studies of Ser7.39 and Ser2.60 in the human CB1 cannabinoid receptor: evidence for a serine-induced bend in CB1 transmembrane helix 7. Mol. Pharmacol. 2007;71:1512–1524. doi: 10.1124/mol.107.034645. [DOI] [PubMed] [Google Scholar]
- 173.Fay JF, Dunham TD, Farrens DL. Cysteine residues in the human cannabinoid receptor: only C257 and C264 are required for a functional receptor, and steric bulk at C386 impairs antagonist SR141716A binding. Biochemistry. 2005;44:8757–8769. doi: 10.1021/bi0472651. [DOI] [PubMed] [Google Scholar]
- 174.Shire D, Calandra B, Delpech M, Dumont X, Kaghad M, Le Fur G, Caput D, Ferrara P. Structural features of the central cannabinoid CB1 receptor involved in the binding of the specific CB1 antagonist SR 141716A. J. Biol. Chem. 1996;271:6941–6946. doi: 10.1074/jbc.271.12.6941. [DOI] [PubMed] [Google Scholar]
- 175.Ahn KH, Bertalovitz AC, Mierke DF, Kendall DA. Dual role of the second extracellular loop of the cannabinoid receptor 1: ligand binding and receptor localization. Mol. Pharmacol. 2009;76:833–842. doi: 10.1124/mol.109.057356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.McAllister SD, Hurst DP, Bamett-Norris J, Lynch D, Reggio PH, Abood ME. Structural mimicry in class A G protein-coupled receptor rotamer toggle switches: the importance of the F3.36(201 )/W6.48(357) interaction in cannabinoid CB1 receptor activation. J. Biol. Chem. 2004;279:48024–48037. doi: 10.1074/jbc.M406648200. [DOI] [PubMed] [Google Scholar]
- 177.Murphy JW, Kendall DA. Integrity of extracellular loop 1 of the human cannabinoid receptor 1 is critical for high-affinity binding of the ligand CP 55,940 but not SR 141716A. Biochem. Pharmacol. 2003;65:1623–1631. doi: 10.1016/s0006-2952(03)00155-2. [DOI] [PubMed] [Google Scholar]
- 178.Song ZH, Bonner TI. A lysine residue of the cannabinoid receptor is critical for receptor recognition by several agonists but not WIN55212-2. Mol. Pharmacol. 1996;49:891–896. [PubMed] [Google Scholar]
- 179.Chin C, Lucas-Lenard J, Abadji V, Kendall DA. Ligand Binding and Modulation of Cyclic AMP Levels Depend on the Chemical Nature of Residue 192 of the Human Cannabinoid Receptor 1. J. Neurochem. 1998;70:366–373. doi: 10.1046/j.1471-4159.1998.70010366.x. [DOI] [PubMed] [Google Scholar]
- 180.Shen CP, Xiao JC, Armstrong H, Hagmann W, Fong TM. F200A substitution in the third transmembrane helix of human cannabinoid CB1 receptor converts AM2233 from receptor agonist to inverse agonist. Eur. J. Pharmacol. 2006;531:41–46. doi: 10.1016/j.ejphar.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 181.Chin CN, Murphy JW, Huffman JW, Kendall DA. The third transmembrane helix of the cannabinoid receptor plays a role in the selectivity of aminoalkylindoles for CB2, peripheral cannabinoid receptor. J. Pharmacol. Exp. Ther. 1999;291:837–844. [PubMed] [Google Scholar]
- 182.Rhee MH. Functional role of serine residues of transmembrane dopamin VII in signal transduction of CB2 cannabinoid receptor. J. Vet. Sci. 2002;3:185–191. [PubMed] [Google Scholar]
- 183.Tao Q, McAllister SD, Andreassi J, Nowell KW, Cabral GA, Hurst DP, Bachtel K, Ekman MC, Reggio PH, Abood ME. Role of a conserved lysine residue in the peripheral cannabinoid receptor (CB2): evidence for subtype specificity. Mol Pharmacol. 1999;55:605–613. [PubMed] [Google Scholar]
- 184.Gouldson P, Calandra B, Legoux P, Kerneis A, Rinaldi-Carmona M, Barth F, Le Fur G, Ferrara P, Shire D. Mutational analysis and molecular modelling of the antagonist SR 144528 binding site on the human cannabinoid CB(2) receptor. Eur. J. Pharmacol. 2000;401:17–25. doi: 10.1016/s0014-2999(00)00439-8. [DOI] [PubMed] [Google Scholar]
- 185.Shire D, Calandra B, Bouaboula M, Barth F, Rinaldi-Carmona M, Casellas P, Ferrara P. Cannabinoid receptor interactions with the antagonists SR 141716A and SR 144528. Life Sci. 1999;65:627–635. doi: 10.1016/s0024-3205(99)00285-4. [DOI] [PubMed] [Google Scholar]
- 186.Shim JY, Welsh WJ, Howlett AC. Homology model of the CB1 cannabinoid receptor: Sites critical for nonclassical cannabinoid agonist interaction. Biopolymers. 2003;71:169–189. doi: 10.1002/bip.10424. [DOI] [PubMed] [Google Scholar]
- 187.Shim JY, Howlett AC. WIN55212-2 docking to the CB1 cannabinoid receptor and multiple pathways for conformational induction. J. Chem. Inf. Model. 2006;46:1286–1300. doi: 10.1021/ci0504824. [DOI] [PubMed] [Google Scholar]
- 188.Tuccinardi T, Ferrarini PL, Manera C, Ortore G, Saccomanni G, Martinelli A. Cannabinoid CB2/CB1 selectivity. Receptor modeling and automated docking analysis. J. Med. Chem. 2006;49:984–994. doi: 10.1021/jm050875u. [DOI] [PubMed] [Google Scholar]
- 189.Padgett LW, Howlett AC, Shim JY. Binding mode prediction of conformationally restricted anandamide analogs within the CB1 receptor. J. Mol. Signal. 2008;3:5. doi: 10.1186/1750-2187-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Salo OM, Lahtela-Kakkonen M, Gynther J, Jarvinen T, Poso A. Development of a 3D model for the human cannabinoid CB1 receptor. J. Med. Chem. 2004;47:3048–3057. doi: 10.1021/jm031052c. [DOI] [PubMed] [Google Scholar]
- 191.Montero C, Campillo NE, Goya P, Paez JA. Homology models of the cannabinoid CB1 and CB2 receptors. A docking analysis study. Eur. J. Med. Chem. 2005;40:75–83. doi: 10.1016/j.ejmech.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 192.Xie XQ, Chen JZ, Billings EM. 3D structural model of the G-protein-coupled cannabinoid CB2 receptor. Proteins. 2003;53:307–319. doi: 10.1002/prot.10511. [DOI] [PubMed] [Google Scholar]
- 193.Salo OM, Raitio KH, Savinainen JR, Nevalainen T, Lahtela-Kakkonen M, Laitinen JT, Jarvinen T, Poso A. Virtual screening of novel CB2 ligands using a comparative model of the human cannabinoid CB2 receptor. J. Med. Chem. 2005;48:7166–7171. doi: 10.1021/jm050565b. [DOI] [PubMed] [Google Scholar]
- 194.Chen JZ, Wang J, Xie XQ. GPCR structure-based virtual screening approach for CB2 antagonist search. J. Chem. Inf. Model. 2007;47:1626–1637. doi: 10.1021/ci7000814. [DOI] [PubMed] [Google Scholar]
- 195.Dainese E, Oddi S, Maccarrone M. Lipid-mediated dimerization of beta2-adrenergic receptor reveals important clues for cannabinoid receptors. Cell Mol Life Sci. 2008;65:2277–2279. doi: 10.1007/s00018-008-8139-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Shim JY. Transmembrane helical domain of the cannabinoid CB1 receptor. Biophys. J. 2009;96:3251–3262. doi: 10.1016/j.bpj.2008.12.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hurst DP, L D, Bamett-Norris J, Hyatt SM, Seltzman HH, Zhong M, Song ZH, Nie J, Lewis D, Reggio PH. N-(piperidin-l-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methy 1-1 H-pyrazole-3-carboxamide (SR141716A) interaction with LYS 3.28(192) is crucial for its inverse agonism at the cannabinoid CB1 receptor. Mol. Pharmacol. 2002;62:1274–1287. doi: 10.1124/mol.62.6.1274. [DOI] [PubMed] [Google Scholar]
- 198.Hurst D, Umejiego U, Lynch D, Seltzman H, Hyatt S, Roche M, McAllister S, Fleischer D, Kapur A, Abood M, Shi S, Jones J, Lewis D, Reggio P. Biarylpyrazole inverse agonists at the cannabinoid CB1 receptor: importance of the C-3 carboxamide oxygen/lysine3.28(192) interaction. J. Med. Chem. 2006;49:5969–5987. doi: 10.1021/jm060446b. [DOI] [PubMed] [Google Scholar]
- 199.Zhang R, Hurst DP, Bamett-Norris J, Reggio PH, Song ZH. Cysteine 2.59(89) in the second transmembrane domain of human CB2 receptor is accessible within the ligand binding crevice: evidence for possible CB2 deviation from a rhodopsin template. Mol. Pharmacol. 2005;68:69–83. doi: 10.1124/mol.104.007823. [DOI] [PubMed] [Google Scholar]
- 200.Guarnieri F, Weinstein H. Conformational memories and the Exploration of biologically relevant peptide conformations: an illustration for the gonadotropin-releasing hormone. J. Amer. Chem. Soc. 1996;118:5580–5589. [Google Scholar]
- 201.Whitnell RM, Hurst DP, Reggio PH, Guarnieri F. Conformational memories with variable bond angles. J. Comput. Chem. 2007 doi: 10.1002/jcc.20822. [DOI] [PubMed] [Google Scholar]
- 202.Barnett-Norris J, Hurst DP, Reggio PH. The influence of cannabinoid receptor second extracellular loop conformation on the binding of CP55940. in 2003 Symposium on the Cannabinoids. Cornwall, Ontario: Internatiobnal Cannabinoid Research Society; 2003. [Google Scholar]
- 203.Hassan SA, Mehler EL, Weinstein H. In: Structure calculation of protein segments connecting domains with defined secondary structure: A simulated annealing Monte Carlo combined with biased scaled collective variables technique, in Computational Methods for Macromolecules: Challenges and Applications. Schlick T, Gan HH, editors. New York: Springer Verlag; 2002. pp. 197–231. [Google Scholar]
- 204.Hassan SA, Guarnieri F, Mehler EL. A General treatment of solvent effects based on screened coulomb potentials. J. Phys. Chem. B. 2000;104:6478–6489. [Google Scholar]
- 205.Hassan SA, Guarnieri F, Mehler EL. Characterization of hydrogen bonding in a continuum solvent model. J. Phys. Chem. B. 2000;104:6490–6498. [Google Scholar]
- 206.Bonechi C, Brizzi A, Brizzi V, Francoli M, Donati A, Rossi C. Conformational analysis of N-arachidonylethanolamide (anan-damide) using nuclear magnetic resonance and theoretical calculations. Magn. Reson. Chem. 2001;39:432–437. [Google Scholar]
- 207.Brizzi A, Brizzi V, Cascio MG, Corelli F, Guida F, Ligresti A, Maione S, Martinelli A, Pasquini S, Tuccinardi T, Di Marzo V. New resorcinol-anandamide “hybrids” as potent cannabinoid receptor ligands endowed with antinociceptive activity in vivo . J. Med. Chem. 2009;52:2506–2514. doi: 10.1021/jm8016255. [DOI] [PubMed] [Google Scholar]
- 208.Salo OM, Savinainen JR, Parkkari T, Nevalainen T, Lahtela-Kakkonen M, Gynther J, Laitinen JT, Jarvinen T, Poso A. 3D–QSAR studies on cannabinoid CB1 receptor agonists: G-protein activation as biological data. J. Med. Chem. 2006;49:554–566. doi: 10.1021/jm0505157. [DOI] [PubMed] [Google Scholar]
- 209.Lynch DL, Hurst DP, Reggio PH, Grossfield A, Gawrisch K, Feller S, Pitman M. Biophysical Society. Boston, MA: 2009. Atomic Level Description ofGPCR Activation Revealed by Microsecond Timescale MD. [Google Scholar]
- 210.Lynch DL, Reggio PH. Molecular dynamics simulations of the endocannabinoid, N-arachidonoylethanolamine (anandamide) in a phospholipid bilayer: probing structure and dynamics. J. Med. Chem. 2005;48:4824–4833. doi: 10.1021/jm058185d. [DOI] [PubMed] [Google Scholar]
- 211.Nebane NM, Hurst DP, Carrasquer CA, Qiao Z, Reggio PH, Song ZH. Residues accessible in the binding-site crevice of transmembrane helix 6 of the CB2 cannabinoid receptor. Biochemistry. 2008;47:13811–13821. doi: 10.1021/bi8007802. [DOI] [PMC free article] [PubMed] [Google Scholar]