Summary
Background
Population pharmacokinetic data suggest axitinib plasma exposure correlates with efficacy in metastatic renal-cell carcinoma. Axitinib dose titration might optimise exposure and improve outcomes. We prospectively assessed the efficacy and safety of axitinib dose titration in previously untreated patients with metastatic renal-cell carcinoma.
Methods
In this randomised, double-blind, multicentre, phase 2 study, patients were enrolled from 49 hospitals and outpatient clinics in the Czech Republic, Germany, Japan, Russia, Spain, and USA. Patients with treatment-naive metastatic renal-cell carcinoma received axitinib 5 mg twice daily during a 4 week lead-in period. Those patients with blood pressure 150/90 mm Hg or lower, no grade 3 or 4 treatment-related toxic effects, no dose reductions, and no more than two antihypertensive drugs for 2 consecutive weeks were stratified by Eastern Cooperative Oncology Group performance status (0 vs 1), and then randomly assigned (1:1) to either masked titration with axitinib to total twice daily doses of 7 mg, and then 10 mg, if tolerated, or placebo titration. Patients who did not meet these criteria continued without titration. The primary objective was comparison of the proportion of patients achieving an objective response between randomised groups. Safety analyses were based on all patients who received at least one dose of axitinib. This ongoing trial is registered with ClinicalTrials.gov, number NCT00835978.
Findings
Between Sept 2, 2009, and Feb 28, 2011, we enrolled 213 patients, of whom 112 were randomly assigned to either the axitinib titration group (56 patients) or the placebo titration group (56 patients). 91 were not eligible for titration, and ten withdrew during the lead-in period. 30 patients (54%, 95% CI 40–67) in the axitinib titration group had an objective response, as did 19 patients (34%, 22–48]) in the placebo titration group (one-sided p=0·019). 54 (59%, 95% CI 49–70) of non-randomised patients achieved an objective response. Common grade 3 or worse, all-causality adverse events in treated patients were hypertension (ten [18%] of 56 in the axitinib titration group vs five [9%] of 56 in the placebo titration group vs 45 [49%] of 91 in the non-randomised group), diarrhoea (seven [13%] vs two [4%] vs eight [9%]), and decreased weight (four [7%] vs three [5%] vs six [7%]). One or more all-causality serious adverse events were reported in 15 (27%) patients in the axitinib titration group, 13 (23%) patients in the placebo titration group, and 35 (38%) non-randomised patients. The most common serious adverse events in all 213 patients were disease progression and dehydration (eight each [4%]), and diarrhoea, vomiting, pneumonia, and decreased appetite (four each [2%]).
Interpretation
The greater proportion of patients in the axitinib titration group achieving an objective response supports the concept of individual axitinib dose titration in selected patients with metastatic renal-cell carcinoma. Axitinib shows clinical activity with a manageable safety profile in treatment-naive patients with this disease.
Funding
Pfizer Inc.
Introduction
Metastatic renal-cell carcinoma is characterised by high expression of VEGF, and drugs targeting VEGF, or its receptors, have shown robust clinical activity in this disease.1-5 Axitinib (Pfizer Inc, manufactured in Freiburg, Germany), a potent, selective inhibitor of VEGF receptors,6 has shown efficacy in previously treated patients with metastatic renal-cell carcinoma in phase 2 and 3 trials.7-10 In the phase 3 AXIS trial,9 significantly more patients treated with axitinib achieved an objective response compared with those treated with sorafenib as second-line therapy for metastatic renal-cell carcinoma. Axitinib-treated patients also had prolonged progression-free survival. Subsequently, axitinib was approved in the USA,11 the European Union, Japan, and other countries for second-line treatment of advanced renal-cell carcinoma.
Patients receiving axitinib exhibit variable plasma drug exposure, as is noted with many oral targeted drugs.12 Two phase 1 dose-escalation studies of axitinib in patients with advanced solid tumours12 and in healthy volunteers13 reported dose-proportional pharmacokinetics, suggesting that axitinib dose increases will lead to higher plasma exposure. Population pharmacokinetic analyses using pooled data from phase 2 studies in metastatic renal-cell carcinoma have shown that patients receiving axitinib dose titration have lower plasma concentrations of axitinib at the starting dose of 5 mg twice daily, and that dose titration leads to increased exposures.14 Moreover, higher axitinib exposure was associated with prolonged progression-free and overall survival in previously treated patients with metastatic renal-cell carcinoma.15 Consequently, it was postulated that axitinib dose titration might increase plasma drug exposure in patients who tolerate a starting dose of 5 mg twice daily, resulting in better clinical outcomes. Dose titration based on tolerability has been used in previous clinical studies with axitinib.8-10 However, the axitinib dose titration strategy has not previously been assessed prospectively in a randomised, placebo-controlled trial to our knowledge. This trial was thus designed to prospectively assess the efficacy and safety of axitinib dose titration in previously untreated patients with metastatic renal-cell carcinoma. This study was done in treatment-naive patients to allow for assessment of a population of patients who might better tolerate axitinib dose titration, and to preclude any confounding factors associated with previous systemic therapy.
Methods
Study design and patients
In this randomised, double-blind, multicentre, phase 2 study, patients were enrolled from 49 hospitals and outpatient clinics in six countries (Czech Republic, Germany, Japan, Russia, Spain, and USA). Key eligibility criteria for study participation included: 18 years or older; histologically confirmed metastatic renal-cell carcinoma with a clear cell component; no previous systemic therapy for metastatic renal-cell carcinoma; no radiotherapy within 1 week or surgery within 4 weeks; measurable disease by Response Evaluation Criteria in Solid Tumors (RECIST, version 1.0);16 no uncontrolled hypertension (blood pressure above 140/90 mm Hg); adequate renal, hepatic, and haematological function; Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1; and life expectancy of 12 weeks or more. Major exclusion criteria included: concurrent use of more than two antihypertensive drugs, current use or anticipated need for strong inhibitors or inducers of cytochrome P450 3A4, and a medical history including seizure disorder, evidence of brain or meningeal metastases, spinal cord compression, clinically relevant cardiac disorders within 12 months, deep vein thrombosis or pulmonary embolism within 6 months, or history of malignancy other than renal-cell carcinoma within 2 years.
The trial was done in accordance with the Declaration of Helsinki, the International Conference on Harmonisation guidelines on Good Clinical Practice, and applicable local regulatory requirements and laws. All patients provided written informed consent. The protocol, amendments, and informed consent forms were approved by an institutional review board or independent ethics committee at each study centre.
Randomisation and masking
After a 4 week lead-in period during which all patients were to receive axitinib 5 mg twice daily, we assessed patients for eligibility to be randomly assigned to receive dose titration with axitinib or placebo. Patients were randomly assigned (1:1) to either the axitinib or placebo titration group if the following criteria were met for 2 weeks consecutively during the lead-in period: blood pressure 150/90 mm Hg or lower, no grade 3 or 4 axitinib-related toxic effects, no dose reduction, and use of no more than two concurrent antihypertensive medications. Patients were stratified by ECOG performance status (0 vs 1). Investigators enrolled patients, and patients were randomised using an interactive voice response system which maintained masking by assigning appropriate numbers for a study drug container to every patient. Randomisation lists were generated using a permuted block design of size 2. Patients who were ineligible for dose titration after the lead-in period were not randomised and continued to receive axitinib 5 mg twice daily. Patients and investigators were blinded to dose titration with axitinib versus placebo.
Procedures
Axitinib was given orally at a starting dose of 5 mg twice daily with food in 4-week cycles. Results from a food-effect study17 carried out with a previous axitinib formulation in healthy volunteers showed that axitinib given with food provided the most consistent exposures after every dose; however, the new (commercial) axitinib formulation, which was used in this study, showed no clinically meaningful difference between fed and fasted administration.17
After the lead-in period, patients assigned to either titration group were to have their twice daily dose titrated stepwise to 7 mg (5 mg axitinib plus 2 mg axitinib, or placebo), and then to a maximum of 10 mg (5 mg axitinib plus 5 mg axitinib, or placebo) if the criteria for dose titration were met for 2 consecutive weeks. The axitinib dose was reduced stepwise by one dose level in patients with grade 3 adverse events according to Common Terminology Criteria for Adverse Events (CTCAE version 3.0)18 or two readings of systolic blood pressure 150 mm Hg or higher or diastolic blood pressure 100 mm Hg or higher while receiving maximal antihypertensive therapy. A patient given axitinib 5 mg twice daily could have the dose reduced to 3 mg twice daily and then further to 2 mg twice daily. In randomised groups, dose reductions were to begin with masked therapy, followed by reduction of open-label axitinib, if necessary.
Tumours were radiographically assessed by investigators according to RECIST16 at the beginning of the study, after 8, 16, and 24 weeks of treatment, and every 12 weeks thereafter. Physical examinations and assessment of ECOG performance status were done at the beginning of the study, after 2 and 4 weeks of treatment, and every 4 weeks thereafter. Blood pressure was monitored at every clinic visit and twice daily at home by patients. Home blood pressure was measured before every axitinib dose; every patient received an oscillometric blood pressure cuff and a diary to record blood pressure readings. We did laboratory tests for haematology and blood chemistry at baseline and every 4 weeks thereafter. We assessed safety throughout the study period, with adverse events graded according to CTCAE version 3·0.18 In a subset of patients, we did serial 6 h pharmacokinetic sampling (before dose, and 0·5 h, 1 h, 2 h, 4 h, and 6 h after dose) on day 15 of the lead-in period and on day 15 of cycle 2. We measured axitinib plasma concentrations using a validated high-performance liquid chromatography method with tandem mass spectrometry.12
Statistical analysis
The primary objective was to compare the proportion of patients achieving an objective response in the axitinib titrated group to the placebo titrated group. Objective response was investigator-assessed and defined as those patients who achieved a complete or partial response according to RECIST criteria.16 Previous analyses showed that about 10% of patients with sorafenib-refractory or cytokine-refractory metastatic renal-cell carcinoma who did not have diastolic blood pressure of 90 mm Hg or higher achieved an objective response.19 Assuming 15% of patients in the placebo titrated group, and 40% of patients in the axitinib titrated group, achieved an objective response (a 25% absolute improvement), this study had at least 80% power with 10% one-sided type I error to detect a significant improvement in the proportion of patients achieving objective responses between the two groups. To detect this 25% absolute improvement between axitinib and placebo titration groups, a target sample size of about 35 patients in each randomised group was required. 15% and 40% of patients achieving an objective response were a level of efficacy to identify minimum required sample size to detect significant improvement with a level of certainty (80% power). However, due to uncertainty about the final number of patients who would be randomised, it was postulated that a smaller improvement might still be significant and clinically relevant. Assuming that about a third would be eligible for dose titration on the basis of experience from a previous trial,9 a sample size of about 200 patients was initially estimated to ensure that the final number of randomised patients would allow for detection of differences in the primary objective of the study. We used a Cochran-Mantel-Haenszel test stratified by ECOG performance status to compare the proportion of patients achieving objective responses between groups.
Secondary endpoints were progression-free survival, overall survival, duration of response, safety, axitinib plasma pharmacokinetics, blood pressure measurements, and biomarker and pharmacogenomic analyses. We used Kaplan-Meier methods to assess time-to-event endpoints, and one-sided stratified log-rank tests to compare time-to-event endpoints between randomised groups. Additionally, we calculated median progression-free survival according to ECOG performance status 0 versus 1 in the overall population. We obtained the hazard ratio (HR) to compare progression-free survival between randomised groups from a Cox proportional hazard model. We estimated axitinib pharmacokinetic variables using non-compartmental analyses in a target sample size of 75 patients.
Primary efficacy analysis was based on patients in randomised groups. Safety and secondary efficacy analyses were based on all patients who received at least one dose of axitinib. The data cutoff date was Oct 12, 2012. All statistical analyses were done using SAS version 9.2. This trial is registered with ClinicalTrials.gov, number NCT00835978.
Role of the funding source
The study sponsor was involved in trial design, and collection and analysis of data. All authors had access to the data in the report and approved the final content of this report. The corresponding author had full access to all of the data and final responsibility for the decision to submit this report for publication.
Results
Between Sept 2, 2009, and Feb 28, 2011, 213 patients with metastatic renal-cell carcinoma were enrolled (table 1). In the overall population, median age was 62 years (range 28–87); of 213 patients, 143 (67%) were male, 136 (64%) had ECOG performance status of 0, and 183 (86%) had previous nephrectomy (table 1).
Table 1. Patient demographics and baseline characteristics.
| Axitinib titration (n=56) | Placebo titration (n=56) | Non-randomised (n=91) | Withdrew during lead-in period (n=10) | |
|---|---|---|---|---|
|
Age (years)
| ||||
| Median (range) | 60 (33–81) | 62 (28–79) | 63 (43–87) | 62 (49–77) |
|
| ||||
|
Sex
| ||||
| Male | 37 (66%) | 45 (80%) | 55 (60%) | 6 (60%) |
| Female | 19 (34%) | 11 (20%) | 36 (40%) | 4 (40%) |
|
| ||||
|
Ethnic origin
| ||||
| White | 49 (88%) | 49 (88%) | 55 (60%) | 9 (90%) |
| Black | 0 | 0 | 2 (2%) | 0 |
| Asian | 6 (11%) | 6 (11%) | 33 (36%) | 1 (10%) |
| Other | 1 (2%) | 1 (2%) | 1 (1%) | 0 |
|
| ||||
|
ECOG PS*
| ||||
| 0 | 36 (64%) | 34 (61%) | 63 (69%) | 3 (30%) |
| 1 | 20 (36%) | 22 (39%) | 27 (30%) | 7 (70%) |
| 2† | 0 | 0 | 1 (1%) | 0 |
|
| ||||
|
Previous nephrectomy
| ||||
| No | 6 (11%) | 11 (20%) | 10 (11%) | 3 (30%) |
| Yes | 50 (89%) | 45 (80%) | 81 (89%) | 7 (70%) |
|
| ||||
|
LDH‡
| ||||
| >1·5 ULN | 2 (4%) | 3 (5%) | 5 (5%) | 1 (10%) |
| ≤1·5 ULN | 54 (96%) | 53 (95%) | 83 (91%) | 8 (80%) |
|
| ||||
|
Haemoglobin
| ||||
| <LLN | 28 (50%) | 21 (38%) | 40 (44%) | 5 (50%) |
| ≥LLN | 28 (50%) | 35 (63%) | 51 (56%) | 5 (50%) |
|
| ||||
|
Absolute neutrophil count
| ||||
| >ULN | 7 (13%) | 8 (14%) | 7 (8%) | 5 (50%) |
| ≤ULN | 49 (88%) | 48 (86%) | 84 (92%) | 5 (50%) |
|
| ||||
|
Platelets
| ||||
| >ULN | 18 (32%) | 4 (7%) | 13 (14%) | 3 (30%) |
| ≤ULN | 38 (68%) | 52 (93%) | 78 (86%) | 7 (70%) |
Data are n (%) or median (range). ECOG PS=Eastern Cooperative Oncology Group performance status. LDH=lactate dehydrogenase. ULN=upper limit of normal. LLN=lower limit of normal.
ECOG PS taken from case report forms.
One patient in the non-randomised group had ECOG PS 1 at screening and ECOG PS 2 on the first day of treatment.
LDH levels missing for three patients in the non-randomised group and one patient who withdrew during the lead-in period.
All enrolled patients received at least one dose of axitinib. In all, 112 patients were eligible for dose titration following the lead-in period and were randomly assigned to axitinib titration or placebo titration groups (n=56 each), 91 were ineligible for randomisation, and ten withdrew during the lead-in period (figure 1). Patient demographics and baseline characteristics were generally well balanced between randomised groups (table 1). The non-randomised group had a higher proportion of Asian patients, most of whom were Japanese, than did the randomised groups (table 1).
Figure 1.
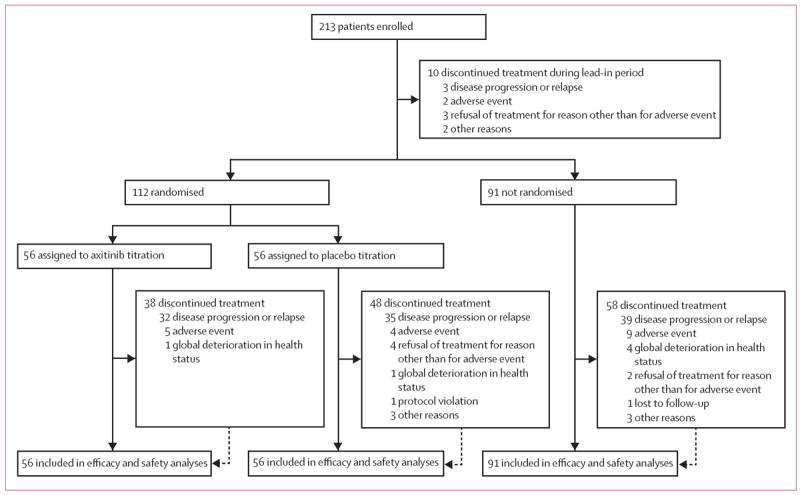
Trial profile
In the primary analysis, 30 patients (54%, 95% CI 40–67) in the axitinib titration group had an objective response, as did 19 patients (34%, 22–48]) in the placebo titration group (risk ratio 1·58 [95% CI 1·02–2·45]; one-sided p=0·019). 54 (59%, 49–70) of non-randomised patients achieved an objective response (table 2). In a pooled analysis of the 213 treated patients, 103 (48%, 95% CI 42–55) patients achieved an objective response. In patients who had on-study tumour measurements, 46 (84%) of 55 patients in the axitinib titration group, 42 (79%) of 53 patients in the placebo titration group, and 85 (96%) of 89 patients in the non-randomised group had tumour shrinkage (appendix). One patient in the axitinib titration group had a confirmed complete response. In the axitinib titration group, median duration of response was not reached, with 13 (43%) of 30 responders progressing on study. Median duration of response was 21·2 months (95% CI 11·1–25·8) in the placebo titration group and 23·3 months (15·7–28·6) in the non-randomised group.
Table 2. Best observed response by RECIST.
| Total*† (N=213) | Axitinib titration (n=56) | Placebo titration† (n=56) | Non-randomised (n=91) | |
|---|---|---|---|---|
| Complete response | 1 (<1%) | 1 (2%) | 0 | 0 |
| Partial response | 102 (48%) | 29 (52%) | 19 (34%) | 54 (59%) |
| Stable disease | 60 (28%) | 13 (23%) | 24 (43%) | 23 (25%) |
| Progressive disease | 38 (18%) | 13 (23%) | 11 (20%) | 11 (12%) |
| Not assessed | 9 (4%) | 0 | 1 (2%) | 2 (2%) |
| Indeterminate | 2 (1%) | 0 | 0 | 1 (1%) |
Data are n (%) unless otherwise stated. RECIST=Response Evaluation Criteria in Solid Tumors.
Includes ten treated patients who withdrew during lead-in period.
One patient in placebo titration group did not have measurable disease at baseline.
Among 112 randomised patients, 73 (65%) experienced progression or death on study. Median follow-up was 26·5 months (IQR 24·3–28·9) for the axitinib titration group and 26·4 months (25·0–28·6) for the placebo titration group. Median progression-free survival for all 213 treated patients was 14·6 months (95% CI 11·5–17·5; figure 2A). The HR for progression-free survival with axitinib titration versus placebo titration was 0·85 (95% CI 0·54–1·35; one-sided stratified p=0·24; figure 2B). Median progression-free survival was 14·5 months (95% CI 9·2–24·5) in the axitinib titration group, 15·7 months (8·3–19·4) in the placebo titration group, and 16·6 months (11·2–22·5) in non-randomised patients. Median progression-free survival was 16·6 months (95% CI 13·0–22·9) versus 10·3 months (4·7–16·6) in patients with ECOG performance status of 0 (n=139) versus 1 (n=74).
Figure 2. Kaplan-Meier estimates of progression-free survival.
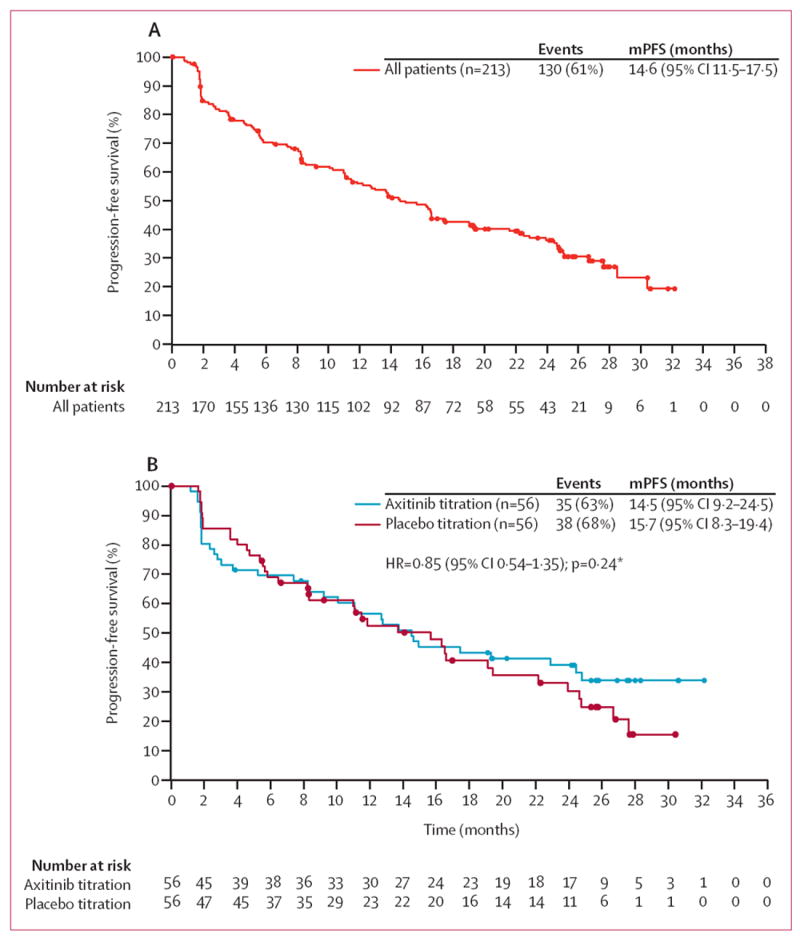
(A) Overall population. (B) Randomised groups. ECOG=Eastern Cooperative Oncology Group. HR=hazard ratio. mPFS=median progression-free survival. *p value is from a one-sided log-rank test stratified by ECOG performance status taken from randomisation system.
As of the data cutoff (Oct 12, 2012), more patients were alive and progression-free in the axitinib titration group (17 patients [30%]) than in the placebo titration group (seven patients [13%]). Median overall survival in all patients was 35·2 months (95% CI 29·9–not estimable), with 78 (37%) deaths (22 in the axitinib titration group, 21 in the placebo titration group, 31 in the non-randomised group, and four who withdrew during the lead-in period); no patients died due to study treatment toxic effects on-study or during follow-up. Patients in this study continue to be followed for overall survival, as the data are still immature.
In the subset of 73 patients with serial 6 h pharmacokinetic sampling, mean axitinib drug exposure on day 15 of the lead-in period while receiving the 5 mg twice daily dose was lower in patients eligible for dose titration than in those not eligible (table 3). Axitinib dose increases from 5 mg twice daily (day 15 of the lead-in period) to 7 mg twice daily (cycle 2 day 15) in the axitinib titration group resulted in proportionally increased drug exposure (table 3).
Table 3. Axitinib pharmacokinetic parameters prior to decision for dose titration (day 15 of lead-in period) and after dose titration (cycle 2 day 15).
| Eligible for dose titration* | Not eligible for dose titration* (non-randomised) | ||
|---|---|---|---|
|
|
|||
| Axitinib titration | Placebo titration | ||
| Day 15 of lead-in period, n | 19† | 20‡ | 34 |
| AUC24, ng*h/mL§ | 176 (125–247) | 187 (107–327) | 432 (321–581) |
| Cmax, ng/mL§ | 28·6 (20·5–39·9) | 22·5 (15·1–33·7) | 38·7 (30·2–49·6) |
| Cycle 2 day 15, n | 16¶ | 20¶ | ·· |
| AUC24, ng*h/mL§ | 259 (150–445) | 161 (102–255) | ·· |
| Cmax, ng/mL§ | 31·7 (21·6–46·6) | 23·1 (16·4–32·5) | ·· |
AUC24=area under the plasma concentration-time curve from 0 to 24 h. Cmax=maximum observed plasma concentration.
Subset of patients with serial 6 h pharmacokinetic sampling. Serial pharmacokinetic samples were not collected on cycle 2 day 15 in patients not eligible for dose titration.
Five patients were excluded because of sample collection errors. AUC24 could not be reported for four patients due to non-estimable half-life.
AUC24 could not be reported for 6 patients due to non-estimable half-life.
Geometric mean (95% CI).
Five patients were excluded because of sample collection error. AUC24 could not be reported for 12 patients due to non-estimable half-life.
All patients receiving axitinib or placebo titration had 7 mg treatment doses (5 mg axitinib plus 2 mg axitinib [axitinib titration group] or 5 mg axitinib plus 2 mg placebo [placebo titration group]), but fewer patients in the axitinib titration group had the total twice daily dose further increased (32 [57%] patients had 10 mg axitinib vs 40 [71%] patients had 5 mg axitinib plus 5 mg placebo). In the axitinib titration group, median duration of treatment was 1·8 months (range 0·1–27·6) with 7 mg twice daily axitinib and 3·5 months (<0·1–30·4) with 10 mg twice daily axitinib. Subsequent dose reductions to less than 5 mg twice daily were more common in the axitinib titration group (ten [18%] to 3 mg twice daily, and four [7%] further to 2 mg twice daily, of 56 patients) than in the placebo titration group (five [9%] to 3 mg twice daily, and none to 2 mg twice daily, of 56 patients). In the non-randomised group, 39 (43%) of 91 patients had their dose reduced to 3 mg twice daily and 17 (19%) to 2 mg twice daily. Median relative axitinib dose intensity (100 × [total dose administered / 5 mg twice daily]) was 130% in the axitinib titration group, 99% in the placebo titration group, and 89% in the non-randomised group.
Median treatment duration was 13·3 months (range 1·2–32·5) in the axitinib titration group, 12·0 months (1·4–35·2) in the placebo titration group, and 13·6 months (0·1–35·6) in non-randomised patients. One or more dose interruptions were reported in similar numbers of patients in axitinib titration (43 [77%] of 56 patients) and placebo titration groups (42 [75%] of 56 patients), and in 88 (97%) of 91 patients in the non-randomised group. Adverse events were more frequently the reason for dose interruptions in the axitinib titration group (32 [57%]) than in the placebo titration group (25 [45%]), and were the reason for 71 (78%) dose interruptions in non-randomised patients.
The most frequently reported all-grade, all-causality adverse events in all groups were hypertension, diarrhoea, and fatigue (table 4). Adverse events that were at least 10% more common in patients in the axitinib titration versus placebo titration groups were hypertension, hand–foot syndrome, and vomiting. Adverse events more common in patients in the placebo titration group than in those in the axitinib titration groups were increased levels of aspartate aminotransferase and blood creatinine, and hypotension. Common grade 3 or worse, all-causality adverse events in treated patients were hypertension (10 [18%] in the axitinib titration group vs five [9%] in the placebo titration group vs 45 [49%] in the non-randomised group), diarrhoea (seven [13%] vs two [4%] vs eight [9%]), and decreased weight (four [7%] vs three [5%] vs six [7%]). Common grade 3 or worse all-causality adverse events more frequently reported with axitinib versus placebo titration (5% or greater difference) were hypertension, diarrhoea, decreased appetite, and nausea (table 4). We noted increases in blood pressure after axitinib treatment in the axitinib titration and placebo titration groups, and values at end of treatment and follow-up were similar to those at baseline (table 5).
Table 4. Treatment-emergent, all-causality adverse events reported in ≥10% of patients in the total treated population*.
| Total† (N=213)
|
Axitinib titration (n=56)
|
Placebo titration (n=56)
|
Non-randomised (n=91)
|
|||||
|---|---|---|---|---|---|---|---|---|
| Grades 1–2 | Grade 3 | Grades 1–2 | Grade 3 | Grades 1-2 | Grade 3 | Grades 1–2 | Grade 3 | |
| Hypertension | 75 (35%) | 63 (30%) | 24 (43%) | 10 (18%) | 19 (34%) | 5 (9%) | 30 (33%) | 45 (49%) |
|
| ||||||||
| Diarrhoea | 110 (52%) | 16 (8%) | 27 (48%) | 6 (11%) | 33 (59%) | 2 (4%) | 49 (54%) | 8 (9%) |
|
| ||||||||
| Fatigue | 90 (42%) | 13 (6%) | 22 (39%) | 3 (5%) | 24 (43%) | 2 (4%) | 42 (46%) | 6 (7%) |
|
| ||||||||
| Dysphonia | 83 (39%) | 2 (1%) | 17 (30%) | 1 (2%) | 20 (36%) | 0 | 44 (48%) | 0 |
|
| ||||||||
| Decreased appetite | 69 (32%) | 7 (3%) | 18 (32%) | 3 (5%) | 17 (30%) | 0 | 31 (34%) | 4 (4%) |
|
| ||||||||
| Hypothyroidism | 74 (35%) | 0 | 18 (32%) | 0 | 13 (23%) | 0 | 41 (45%) | 0 |
|
| ||||||||
| Nausea | 68 (32%) | 5 (2%) | 18 (32%) | 3 (5%) | 19 (34%) | 0 | 30 (33%) | 1 (1%) |
|
| ||||||||
| Hand-foot syndrome | 60 (28%) | 8 (4%) | 16 (29%) | 2 (4%) | 9 (16%) | 1 (2%) | 35 (38%) | 5 (5%) |
|
| ||||||||
| Proteinuria | 60 (28%) | 3 (1%) | 9 (16%) | 2 (4%) | 11 (20%) | 0 | 39 (43%) | 0 |
|
| ||||||||
| Vomiting | 49 (23%) | 4 (2%) | 14 (25%) | 3 (5%) | 11 (20%) | 0 | 22 (24%) | 1 (1%) |
|
| ||||||||
| Weight decreased | 40 (19%) | 13 (6%) | 11 (20%) | 4 (7%) | 9 (16%) | 3 (5%) | 19 (21%) | 6 (7%) |
|
| ||||||||
| Constipation | 47 (22%) | 0 | 10 (18%) | 0 | 8 (14%) | 0 | 27 (30%) | 0 |
|
| ||||||||
| Headache | 46 (22%) | 2 (1%) | 8 (14%) | 0 | 12 (21%) | 1 (2%) | 26 (29%) | 1 (1%) |
|
| ||||||||
| Dyspnoea | 34 (16%) | 5 (2%) | 4 (7%) | 1 (2%) | 5 (9%) | 2 (4%) | 25 (27%) | 1 (1%) |
|
| ||||||||
| Back pain | 29 (14%) | 8 (4%) | 10 (18%) | 3 (5%) | 7 (13%) | 1 (2%) | 12 (13%) | 4 (4%) |
|
| ||||||||
| Pain in extremity | 35 (16%) | 2 (1%) | 6 (11%) | 1 (2%) | 5 (9%) | 0 | 23 (25%) | 0 |
|
| ||||||||
| Arthralgia | 31 (15%) | 5 (2%) | 10 (18%) | 1 (2%) | 7 (13%) | 2 (4%) | 14 (15%) | 2 (2%) |
|
| ||||||||
| Dysgeusia | 35 (16%) | 0 | 9 (16%) | 0 | 5 (9%) | 0 | 20 (22%) | 0 |
|
| ||||||||
| Cough | 31 (15%) | 1 (<1%) | 7 (13%) | 0 | 8 (14%) | 0 | 16 (18%) | 1 (1%) |
|
| ||||||||
| Dizziness | 26 (12%) | 5 (2%) | 7 (13%) | 1 (2%) | 8 (14%) | 2 (4%) | 10 (11%) | 2 (2%) |
|
| ||||||||
| Mucosal inflammation | 27 (13%) | 4 (2%) | 9 (16%) | 2 (4%) | 7 (13%) | 0 | 11 (12%) | 2 (2%) |
|
| ||||||||
| Rash | 31 (15%) | 0 | 4 (7%) | 0 | 8 (14%) | 0 | 19 (21%) | 0 |
|
| ||||||||
| Stomatitis | 29 (14%) | 1 (<1%) | 9 (16%) | 0 | 4 (7%) | 0 | 16 (18%) | 1 (1%) |
|
| ||||||||
| Abdominal pain | 27 (13%) | 2 (1%) | 9 (16%) | 0 | 6 (11%) | 1 (2%) | 11 (12%) | 1 (1%) |
|
| ||||||||
| Dyspepsia | 29 (14%) | 0 | 6 (11%) | 0 | 5 (9%) | 0 | 18 (20%) | 0 |
|
| ||||||||
| Musculoskeletal pain | 25 (12%) | 1 (<1%) | 7 (13%) | 0 | 4 (7%) | 0 | 14 (15%) | 1 (1%) |
|
| ||||||||
| ALT increased | 20 (9%) | 5 (2%) | 3 (5%) | 1 (2%) | 7 (13%) | 2 (4%) | 10 (11%) | 2 (2%) |
|
| ||||||||
| AST increased | 22 (10%) | 3 (1%) | 3 (5%) | 1 (2%) | 10 (18%) | 0 | 9 (10%) | 2 (2%) |
|
| ||||||||
| Blood creatinine increased | 23 (11%) | 1 (<1%) | 1 (2%) | 0 | 7 (13%) | 1 (2%) | 14 (15%) | 0 |
|
| ||||||||
| Blood TSH increased | 24 (11%) | 0 | 8 (14%) | 0 | 7 (13%) | 0 | 9 (10%) | 0 |
|
| ||||||||
| Blood glucose increased | 23 (11%) | 0 | 4 (7%) | 0 | 7 (13%) | 0 | 10 (11%) | 0 |
|
| ||||||||
| Nasopharyngitis | 22 (10%) | 0 | 4 (7%) | 0 | 3 (5%) | 0 | 15 (16%) | 0 |
|
| ||||||||
| Peripheral oedema | 21 (10%) | 1 (<1%) | 3 (5%) | 1 (2%) | 4 (7%) | 0 | 14 (15%) | 0 |
|
| ||||||||
| Thrombocytopenia | 21 (10%) | 0 | 4 (7%) | 0 | 3 (5%) | 0 | 14 (15%) | 0 |
Data are n (%). ALT=alanine aminotransferase. AST=aspartate aminotransferase. TSH=thyroid stimulating hormone.
Of the listed adverse events, those of grade 4 severity were diarrhoea and dyspnoea (n=1 each [2%] of 56 patients in the axitinib titration group), fatigue (n=1 [1%] of 91 non-randomised patients), and constipation (n=1 [10%] of ten patients who withdrew during lead-in period).
Includes ten treated patients who withdrew during lead-in period.
Table 5. Blood pressure measurements in randomised groups.
| Axitinib titration
|
Placebo titration
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | dBP | ΔdBP | sBP | ΔsBP | n | dBP | ΔdBP | sBP | ΔsBP | |
| Baseline | 56 | 76·7 (9·6) | ·· | 127·0 (13·6) | ·· | 56 | 77·9 (8·0) | ·· | 126·5 (13·1) | ·· |
|
| ||||||||||
| Cycle 1 day 1 | 52 | 75·0 (8·1) | –1·6 (8·2) | 123·1 (13·3) | –4·3 (11·1) | 51 | 76·0 (7·8) | –2·6 (7·4) | 123·6 (12·6) | –2·9 (9·2) |
|
| ||||||||||
| Cycle 1 day 15 | 56 | 81·5 (9·9) | 4·8 (8·5) | 130·9 (16·7) | 3·8 (12·2) | 56 | 81·0 (7·5) | 3·0 (7·7) | 130·5 (13·1) | 4·1 (12·5) |
|
| ||||||||||
| Cycle 2 day 1 | 56 | 80·9 (7·9) | 4·2 (9·0) | 129·0 (12·5) | 1·9 (12·4) | 56 | 81·5 (7·4) | 3·5 (8·0) | 127·3 (12·7) | 0·9 (13·6) |
|
| ||||||||||
| Cycle 2 day 15 | 55 | 82·4 (10·7) | 5·5 (10·5) | 130·7 (13·3) | 3·6 (13·8) | 55 | 82·3 (10·0) | 4·4 (10·7) | 129·2 (14·5) | 2·7 (16·6) |
|
| ||||||||||
| Cycle 3 day 1 | 48 | 83·9 (8·1) | 6·6 (8·3) | 131·5 (12·8) | 3·5 (15·1) | 49 | 83·9 (9·2) | 5·9 (9·3) | 134·1 (13·9) | 8·4 (15·4) |
|
| ||||||||||
| Cycle 4 day 1 | 45 | 85·0 (10·8) | 7·4 (8·2) | 132·3 (13·5) | 4·3 (12·7) | 48 | 82·5 (9·6) | 4·6 (8·5) | 129·2 (10·6) | 3·5 (13·0) |
|
| ||||||||||
| End of treatment | 35 | 75·2 (13·1) | 0·6 (10·8) | 127·6 (17·5) | 2·4 (17·0) | 44 | 81·1 (10·1) | 3·5 (6·3) | 128·5 (15·7) | 1·7 (14·0) |
|
| ||||||||||
| Follow-up | 16 | 72·5 (13·0) | –4·5 (10·1) | 124·2 (17·1) | –3·6 (16·9) | 25 | 77·8 (11·0) | –1·3 (8·5) | 127·2 (14·7) | –0·4 (16·3) |
Data are mean blood pressure, mm Hg (SD). SD=standard deviation. dBP=diastolic blood pressure. ΔdBP=change in diastolic blood pressure from baseline. sBP=systolic blood pressure;. ΔsBP=change in systolic blood pressure from baseline.
One or more all-causality serious adverse events were reported in similar numbers of patients in axitinib titration (15 [27%] of 56 patients) and placebo titration (13 [23%] of 56 patients) groups, and in 35 (38%) of 91 non-randomised patients. The most common serious adverse events in all 213 patients were disease progression and dehydration (eight each [4%]), and diarrhoea, vomiting, pneumonia, and decreased appetite (four each [2%]). Four (7%) of 56 patients in the axitinib titration group, two (4%) of 56 patients in the placebo titration group, and six (7%) of 91 patients in the non-randomised group discontinued treatment because of treatment-related adverse events. Frequencies of dose reductions and dose interruptions due to treatment-related adverse events were higher in the axitinib titration versus placebo titration group (data not shown).
Discussion
In this randomised phase 2 trial, a significantly higher proportion of treatment-naive patients with metastatic renal-cell carcinoma who initially tolerated a twice daily 5 mg dose of axitinib achieved an objective response with axitinib titration than with placebo titration. These results thus support the concept of individual axitinib dose titration. These data further demonstrate that axitinib has clinical activity in first-line treatment of patients with metastatic renal-cell carcinoma (panel). Clinical activity of axitinib in this setting is also supported by results from a phase 3 trial of axitinib versus sorafenib in treatment-naive patients with metastatic renal-cell carcinoma, where patients treated with axitinib had a median progression-free survival of 10·1 months, and around a third of patients achieved an objective response.20 By contrast with the population in the present study, most patients in the phase 3 study were recruited from eastern Europe and India; geographical variation between the two studies might have translated into disparities in patient-specific characteristics or supportive care, or both. Consistent with results reported in the phase 3 trial,20 ECOG performance status in the present trial had an effect on median progression-free survival in the overall population of patients treated with axitinib, with longer median progression-free survival noted in those with ECOG performance status 0 versus 1. Inferences from cross-trial comparisons should be made cautiously because of potentially biasing features,21 but median progression-free survival in the overall population as well as in individual treatment groups in the present study is longer than in the previous phase 2 and 3 studies of other antiangiogenic drugs in first-line metastatic renal-cell carcinoma settings.2-5,22,23
By contrast with other targeted drugs currently approved for metastatic renal-cell carcinoma, dose titration has been used across axitinib clinical trials. Until now, this approach was supported only by retrospective population pharmacokinetic analyses.15 To our knowledge, the present trial is the first to prospectively assess the efficacy and safety of the axitinib dose titration scheme in a randomised, placebo-controlled manner. Dose titration of sorafenib has been assessed in previous phase 2 studies with conflicting results regarding feasibility.22,24-26 As a result, sorafenib dose titration is not routinely used in clinical practice. The pharmacokinetic data reported here indicate that the clinical criteria used in this study to select patients for dose titration were generally successful in the identification of those with lower mean axitinib plasma exposure at the 5 mg twice daily starting dose. Furthermore, axitinib exposure increased proportionally with dose increases from 5 mg twice daily to 7 mg twice daily in the axitinib titration group. 53% of patients were eligible for dose titration (112 of 213 patients), which was higher than the predicted 33%, an assumption based on data from the phase 3 AXIS trial.9 One possible explanation for the discrepancy could be variation in the dose titration scheme between these two studies: in the AXIS trial,9 patients were not eligible for dose titration if they were receiving any antihypertensive medication, whereas the present study allowed patients to receive a maximum of two antihypertensive medications. Additionally, the timing of assessment for dose titration eligibility was standardised in the current study but not in the AXIS trial.9 The increased number of patients eligible for titration led to there being 21 more patients in each titration group than minimally required, and so the power of the study was increased by at least 10%, allowing a smaller treatment effect to be found significant. The 20% increase in the proportion of patients achieving an objective response in the axitinib titration group compared with the placebo titration group is still deemed clinically relevant.
Importantly, of the 56 patients titrated to axitinib doses over 5 mg twice daily, ten (18%) had to have dose reductions to below this previously tolerated level. This suggests that meeting clinical criteria does not necessarily guarantee patients will tolerate axitinib titration. Here, axitinib titration from 5 to 7 mg twice daily was over-titration, and led to adverse events, as well as dose reductions to potentially subtherapeutic levels, or dose interruptions. This phenomenon could plausibly explain the discrepancy between the objective response advantage but lack of progression-free survival advantage noted with axitinib titration. That is, brief exposure to higher axitinib doses could drive more immediate tumour shrinkage (reflected in the number of patients who had a tumour response), but subsequently the substantial proportion of patients who then had to reduce to sub-optimum axitinib dosing could have led to a reduction in longer-term disease control (as shown in the similar progression-free survival to the placebo titration group). It is important to note that our data do not suggest that the patients with progressive disease in the axitinib titration group were necessarily those who underwent dose reduction to below 5 mg twice daily. However, given the small size of this patient subset it is not possible to draw definitive conclusions about the effect of dose reductions on clinical outcomes. The titration scheme might benefit from further refinement, including exploring modification of the study’s dose titration criteria, and the use of intermediate doses (eg 6 mg twice daily), to optimise axitinib exposure, minimise excess toxic effects in individual patients, and perhaps better balance tumour reduction with control against toxic effects.
The suitability of measuring the proportion of patients achieving an objective response (the primary objective of this study) as an efficacy endpoint in oncology trials has been challenged by the introduction of targeted antiangiogenic drugs. These drugs do not necessarily yield sufficient tumour shrinkage to obtain a categorically defined objective response. As a result, use of continuous time-to-event endpoints (progression-free survival and overall survival) in clinical trials of targeted drugs has been advocated.27 Nevertheless, there is some evidence suggesting that the proportion of patients achieving objective responses correlates with overall survival, and that extent of tumour shrinkage might have prognostic relevance in patients with renal-cell carcinoma.28,29 The overall progression-free survival curve, as reflected by the HR, favoured axitinib titration but differences were not significant; however, the study was not powered to detect a difference in progression-free survival between randomised groups. Longer follow-up will determine whether axitinib titration is associated with a greater proportion of durable responses.
Relatively high proportions of patients achieving an objective response, and a long progression-free survival were noted in the placebo titration group, despite predictions that patients were only exposed to a sub-therapeutic axitinib dose. These data suggest the therapeutic threshold for axitinib exposure varies between patients. Moreover, initial dose titration in this study was done in the absence of tumour response information. Although frequent CT scans to assess response are limited by safety and practicality, such information would help to refine the titration scheme. Importantly, a substantial percentage of patients randomly assigned to placebo titration, and with lower axitinib exposure at the 5 mg twice daily dose, showed an objective response; thus the true benefit of axitinib titration in this subset is unclear. Therefore, it is not currently possible to provide axitinib dosing recommendations based solely on axitinib pharmacokinetics in treated patients, and further study could develop a better individualised dosing strategy. Moreover, the data suggest that axitinib exposure is not the sole driver of clinical outcome in patients with metastatic renal-cell carcinoma. Pharmacodynamic factors and patient-specific or tumour-specific characteristics probably also contribute to axitinib efficacy, an idea supported by studies showing an association between treatment-related increases in blood pressure and axitinib or sunitinib activity.19,30,31 Some patients with disease biology with little to no susceptibility to VEGF inhibition (presumably those patients whose best response was progressive disease) might not benefit even with maximum axitinib titration. However, patients who achieve stable disease might have at least some susceptibility to VEGF inhibition. Consequently optimum dosing, potentially achieved with axitinib titration, could result in greater tumour shrinkage and allow those patients to achieve an objective response. Analysis of secondary endpoints from this study, including blood pressure measurements and biomarker and pharmacogenomic analyses, might elucidate underlying patient-specific characteristics that contribute to axitinib efficacy, and potentially identify predictive biomarkers. There is also a possibility that adherence to medication and dose titration influenced efficacy in the randomised groups. We assessed treatment compliance during the study using patient-maintained diaries, which listed missed or changed doses of study drug, and pill counts were done on returned study drug bottles. Overall compliance was high, as reflected in the median relative dose intensity, which was higher in the axitinib titration group than in the placebo titration group.
Patients in this study who were not eligible for dose titration presumably achieved therapeutic exposure at the 5 mg twice daily starting dose. Consistent with previous retrospective analyses correlating exposure and efficacy,15 more patients in the non-randomised group achieved an objective response than did those in the randomised groups, and had the longest median progression-free survival. The higher rate of progressive disease as best response in patients in the titration groups compared with those patients in the non-randomised group might have been due to suboptimum dosing of axitinib during the interval between baseline and first on-treatment scans. The value of axitinib titration at the time of disease progression is not addressed by the current study.
The overall safety profile in the present study was manageable, and concordant with data for axitinib from the AXIS trial9 (axitinib as second-line therapy for patients with metastatic renal-cell carcinoma), as well as reports of other approved VEGF-pathway targeted drugs in treatment-naive patients with this disease.2-5,22 The relatively high incidence of hypertension in this study might, partly, result from a reporting bias due to intensive monitoring of blood pressure. Notably, the increases in blood pressure seen during axitinib treatment returned to baseline by end of treatment in patients in titration groups, presumably because of active management of blood pressure while on study. The proportions of patients without previous nephrectomy were similar in the randomised versus non-randomised groups (17 [15%] of 112 patients vs ten [11%] of 91 patients respectively), suggesting that this characteristic did not affect treatment-induced hypertension. The incidence of hypothyroidism and dysphonia during treatment with axitinib was higher here than in the AXIS trial (74 [35%] vs 69 [19%] for hypothyroidism and 85 [40%] vs 111 [31%] for dysphonia), and might have been due to more patients receiving higher axitinib doses in the present study than in the AXIS trial. Toxic effects reported at a higher incidence with axitinib versus placebo titration, such as hypertension and hand–foot syndrome, are considered class effects of VEGF pathway tyrosine kinase inhibitors,32 and thus were expected to be more common in patients receiving higher axitinib doses. Similarly, higher axitinib exposure at the 5 mg twice daily starting dose might have resulted in higher incidences of all-grade hypertension, dysphonia, hypothyroidism, and hand–foot syndrome in patients in the non-randomised group. It is important to note that patients in the non-randomised group were preselected for greater numbers of grade 3 or worse adverse events, since patients who experienced these events during the trial lead-in period were not eligible for either titration group. The proportion of patients who required axitinib dose interruptions in the titrated groups was consistent with that reported in the phase 3 AXIS trial (77%);9 the higher rate of dose interruptions in the non-randomised group might have been due to patients having higher axitinib exposure at the 5 mg twice daily starting dose, resulting in an increased incidence of adverse events.
We did not assess patient quality-of-life in this study. This represents a limitation because these data might affect the decision for axitinib dose titration in clinical practice. Another limitation of the study is that we did not obtain calcium data in patients, precluding analyses of efficacy by Memorial Sloan-Kettering Cancer Center33 or Heng risk groups.34
In conclusion, present data show axitinib has clinical activity in the first-line setting of metastatic renal-cell carcinoma, and axitinib dose titration is associated with a greater proportion of patients achieving an objective response versus those who underwent placebo titration, balanced against the potential for increased toxic effects. The effect of axitinib titration on other clinical outcome measures, such as progression-free survival or overall survival, as well as the optimum method for patient selection for titration and the titration scheme, require further study.
Supplementary Material
Panel: Research in context.
Systematic review
We did not do a systematic literature review as part of the planning for this trial. Population pharmacokinetic data from previous phase 2 studies of axitinib in previously treated patients with metastatic renal-cell carcinoma showed that higher axitinib exposure, and the occurrence of diastolic blood pressure 90 mm Hg or higher, are independently associated with improved clinical outcomes.15 These findings suggested that axitinib dose titration in patients who tolerate the starting dose of 5 mg twice daily might improve efficacy, and provided the rationale for conducting the present trial. To our knowledge, no other studies had prospectively assessed the efficacy and safety of axitinib dose titration. Therefore, we carried out a randomised, double-blind phase 2 study to compare axitinib versus placebo titration in previously untreated patients with metastatic renal-cell carcinoma.
Interpretation
The practice of axitinib dose titration is supported by a significantly greater proportion of patients achieving an objective response in the axitinib titrated group compared with this in the placebo titrated group. These data challenge the concept of flat dosing of targeted drugs, and show that axitinib has clinical activity as first-line treatment of metastatic renal-cell carcinoma. Axitinib exposure might not be the only driver of clinical outcome, and patient-specific pharmacodynamic factors are likely to contribute to axitinib efficacy in metastatic renal-cell carcinoma. The optimum method for patient selection for axitinib titration and the titration scheme require further study.
Acknowledgments
This study was sponsored by Pfizer Inc. Medical writing support was provided by Joanna Bloom of Engage Scientific Solutions, and was funded by Pfizer Inc. We thank Geraldine Quigley, Yoshiko Umeyama, and Yoichi Kamei of Pfizer Inc for data collection, and Ying Chen and Paul Bycott of Pfizer Inc for data analysis. EJ acknowledges funding from NIH/NCI (award number P30CA016672) to MD Anderson Cancer Center and use of the Clinical Trials Support Resource.
Footnotes
Contributors
BIR, MNF, AHB, YKP, GIA, and SK participated in study conception and design. BIR, BM, TU, VG, JAA, AHB, DP, SK, and EJ participated in collection and assembly of data. BIR, BM, TU, MNF, JAA, AHB, YKP, GIA, DP, SK, and EJ participated in data analysis and interpretation. BIR, BM, TU, VG, MNF, JAA, and EJ participated in provision of patients. All authors participated in writing of the paper and approved the final version of the paper.
Conflicts of interest
BIR has served as an adviser for and received research funding from Pfizer. BM has served an as adviser for Roche and Astellas, and has received lecture fees from Roche, Novartis, Pfizer, and GlaxoSmithKline. TU has received lecture fees from Pfizer. VG has served as an adviser for and received lecture fees from Pfizer, Novartis, GlaxoSmithKline, Astellas, Bayer, and Roche. MNF has received research funding from Amgen, Altor Biosciences, Aveo, Bayer, Bristol-Myers Squibb, Eisai, Genentech, GlaxoSmithKline, and Pfizer, has received honoraria from Aveo, Altor Biosciences, Bayer, Genentech, GlaxoSmithKline, Prometheus, and Pfizer, and has served on speakers bureaus for Bayer, GlaxoSmithKline, Novartis, Pfizer, and Prometheus. JAA has served as an adviser for Pfizer. AHB, YKP, GIA, and DP are employees of and own stock in Pfizer. SK, employed at Pfizer at the time of the study described here, is currently employed by Mirna Therapeutics and owns stock in Pfizer and Mirna Therapeutics. EJ has served as an adviser for and received research funding from Pfizer.
See Online for appendix
References
- 1.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–34. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 2.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–24. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 3.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2007;370:2103–11. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 4.Rini BI, Halabi S, Rosenberg JE, et al. Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J Clin Oncol. 2008;26:5422–28. doi: 10.1200/JCO.2008.16.9847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–68. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 6.Hu-Lowe DD, Zou HY, Grazzini ML, et al. Nonclinical antiangiogenesis and antitumor activities of axitinib (AG-013736), an oral, potent, and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinases 1, 2, 3. Clin Cancer Res. 2008;14:7272–83. doi: 10.1158/1078-0432.CCR-08-0652. [DOI] [PubMed] [Google Scholar]
- 7.Rixe O, Bukowski RM, Michaelson MD, et al. Axitinib treatment in patients with cytokine-refractory metastatic renal-cell cancer: a phase II study. Lancet Oncol. 2007;8:975–84. doi: 10.1016/S1470-2045(07)70285-1. [DOI] [PubMed] [Google Scholar]
- 8.Rini BI, Wilding G, Hudes G, et al. Phase II study of axitinib in sorafenib-refractory metastatic renal cell carcinoma. J Clin Oncol. 2009;27:4462–68. doi: 10.1200/JCO.2008.21.7034. [DOI] [PubMed] [Google Scholar]
- 9.Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378:1931–39. doi: 10.1016/S0140-6736(11)61613-9. [DOI] [PubMed] [Google Scholar]
- 10.Tomita Y, Uemura H, Fujimoto H, et al. Key predictive factors of axitinib (AG-013736)-induced proteinuria and efficacy: a phase II study in Japanese patients with cytokine-refractory metastatic renal cell carcinoma. Eur J Cancer. 2011;47:2592–602. doi: 10.1016/j.ejca.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 11. [Aug 29, 2013];INLYTA (axitinib) prescribing information. http://labeling.pfizer.com/ShowLabeling.aspx?id=759.
- 12.Rugo HS, Herbst RS, Liu G, et al. Phase I trial of the oral antiangiogenesis agent AG-013736 in patients with advanced solid tumors: pharmacokinetic and clinical results. J Clin Oncol. 2005;23:5474–83. doi: 10.1200/JCO.2005.04.192. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y, Jiang J, Zhang J, et al. A Phase I study to evaluate the pharmacokinetics of axitinib (AG-13736) in healthy Chinese volunteers. Int J Clin Pharmacol Ther. 2011;49:679–87. doi: 10.5414/cp201570. [DOI] [PubMed] [Google Scholar]
- 14.Rini BI, Grünwald V, Fishman MN, et al. Axitinib for first-line metastatic renal cell carcinoma (mRCC): overall efficacy and pharmacokinetic (PK) analyses from a randomized phase II study. Proc Am Soc Clin Oncol. 2012;30(suppl) abstr 4503. [Google Scholar]
- 15.Rini BI, Garrett M, Poland B, et al. Axitinib in metastatic renal cell carcinoma: results of a pharmacokinetic and pharmacodynamic analysis. J Clin Pharmacol. 2013;53:491–504. doi: 10.1002/jcph.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 17.Pithavala YK, Chen Y, Toh M, et al. Evaluation of the effect of food on the pharmacokinetics of axitinib in healthy volunteers. Cancer Chemother Pharmacol. 2012;70:103–12. doi: 10.1007/s00280-012-1888-9. [DOI] [PubMed] [Google Scholar]
- 18.Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–81. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 19.Rini BI, Schiller JH, Fruehauf JP, et al. Diastolic blood pressure as a biomarker of axitinib efficacy in solid tumors. Clin Cancer Res. 2011;17:3841–49. doi: 10.1158/1078-0432.CCR-10-2806. [DOI] [PubMed] [Google Scholar]
- 20.Hutson TE, Lesovoy V, Al-Shukri S, et al. Axitinib versus sorafenib as first-line therapy in patients with metastatic renal-cell carcinoma: a randomised, open-label, phase 3 trial. Lancet Oncol. 2013 doi: 10.1016/S1470-2045(13)70465-0. in press. [DOI] [PubMed] [Google Scholar]
- 21.Rubinstein L, Crowley J, Ivy P, Leblanc M, Sargent D. Randomized phase II designs. Clin Cancer Res. 2009;15:1883–90. doi: 10.1158/1078-0432.CCR-08-2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Escudier B, Szczylik C, Hutson TE, et al. Randomized phase II trial of first-line treatment with sorafenib versus interferon Alfa-2a in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:1280–89. doi: 10.1200/JCO.2008.19.3342. [DOI] [PubMed] [Google Scholar]
- 23.Motzer RJ, Nosov D, Eisen T, et al. Tivozanib versus sorafenib as initial targeted therapy for patients with metastatic renal cell carcinoma: results from a phase III trial. J Clin Oncol. 2013 doi: 10.1200/JCO.2012.47.4940. published online Sept 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amato RJ, Harris P, Dalton M, et al. A phase II trial of intra-patient dose-escalated sorafenib in patients (pts) with metastatic renal cell cancer (MRCC) Proc Am Soc Clin Oncol. 2007;25(suppl) abstr 5026. [Google Scholar]
- 25.Amato RJ, Jac J, Harris P, et al. A phase II trial of intra-patient dose escalated-sorafenib in patients (pts) with metastatic renal cell cancer (MRCC) Proc Am Soc Clin Oncol. 2008;26(suppl) abstr 5122. [Google Scholar]
- 26.Gore ME, Jones RJ, Ravaud A, et al. Efficacy and safety of intrapatient dose escalation of sorafenib as first-line treatment for metastatic renal cell carcinoma (mRCC) Proc Am Soc Clin Oncol. 2011;29(suppl) abstr 4609. [Google Scholar]
- 27.Adjei AA, Christian M, Ivy P. Novel designs and end points for phase II clinical trials. Clin Cancer Res. 2009;15:1866–72. doi: 10.1158/1078-0432.CCR-08-2035. [DOI] [PubMed] [Google Scholar]
- 28.Molina AM, Zhang J, Lin X, et al. Sunitinib objective response (OR) in metastatic renal cell carcinoma (mRCC): analysis of 1,059 patients treated on clinical trials. Proc Am Soc Clin Oncol. 2012;30(suppl) abstr 4542. [Google Scholar]
- 29.Gruenwald V, Busch J, Weikert S, Seidel C. Tumor shrinkage during VEGF inhibitor therapy as an independent predictor of PFS and OS in renal cell carcinoma (RCC) Proc Am Soc Clin Oncol. 2013;31(suppl) abstr 423. [Google Scholar]
- 30.Motzer RJ, Escudier B, Tomczak P, et al. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol. 2013;14:552–62. doi: 10.1016/S1470-2045(13)70093-7. [DOI] [PubMed] [Google Scholar]
- 31.Rini BI, Cohen DP, Lu DR, et al. Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst. 2011;103:763–73. doi: 10.1093/jnci/djr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen RB, Oudard S. Antiangiogenic therapy for advanced renal cell carcinoma: management of treatment-related toxicities. Invest New Drugs. 2012;30:2066–79. doi: 10.1007/s10637-012-9796-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol. 2002;20:289–96. doi: 10.1200/JCO.2002.20.1.289. [DOI] [PubMed] [Google Scholar]
- 34.Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27:5794–99. doi: 10.1200/JCO.2008.21.4809. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


