Abstract
Chagas disease, caused by the protozoan parasite Trypanosoma cruzi (T. cruzi), is characterized by immunopathology driven by IFN-γ secreting Th1-like T cells. T. cruzi has a thick coat of mucin-like glycoproteins covering its surface, which plays an important role in parasite invasion and host immunomodulation. It has been extensively described that T. cruzi or its products—like GPI anchors isolated from GPI-anchored mucins from the trypomastigote life cycle stage (tGPI-mucins)—are potent inducers of proinflammatory responses (i.e., cytokines and NO production) by IFN-γ primed murine macrophages. However, little is known about whether T. cruzi or GPI-mucins exert a similar action in human cells. We therefore decided to further investigate the in vitro cytokine production profile from human mononuclear cells from uninfected donors exposed to T. cruzi as well as tGPI-mucins. We observed that both living T. cruzi trypomastigotes and tGPI-mucins are potent inducers of IL-12 by human peripheral blood monocytes and this effect depends on CD40-CD40L interaction and IFN-γ. Our findings suggest that the polarized T1-type cytokine profile seen in T. cruzi infected patients might be a long-term effect of IL-12 production induced by lifelong exposure to T. cruzi tGPI-mucins.
1. Introduction
The protozoan parasite Trypanosoma cruzi is the causative agent of Chagas disease, which affects approximately 15 million people in South and Central America [1, 2]. It is estimated that about 30% of infected individuals will develop severe chronic forms of the disease, especially the often fatal Chagas disease cardiomyopathy (CCC) [1–4]. Intracellular protozoan parasites are potent stimulators of innate and cell-mediated immunity. The induction of macrophage proinflammatory cytokines by ligands of innate immunity receptors of T. cruzi is considered important in the control of infection and outcome of Chagas disease [5, 6]. It has been extensively described that glycosylphosphatidylinositol-anchored mucins-like glycoproteins from Trypanosoma cruzi trypomastigotes (tGPI-mucins) activate murine macrophages in vitro to produce the proinflammatory cytokines tumor necrosis factor α (TNF-α) and interleukin- (IL-) 12 as well as nitric oxide (NO) [7, 8]. The bulk of evidence establishes that IL-12 and IL-12 driven Th1 cytokines, the ones involved in delayed-type hypersensitivity, are induced during acute infection with T. cruzi in mice, playing an obligatory role in parasite clearance and host survival [9–12]. T. cruzi tGPI-mucins were shown to initiate the inflammatory response through an activation of Toll-like receptors TLR2 [7, 13]. Different components from this parasite are capable of activating TLRs in dendritic cells and macrophages, like the unmethylated CpG motifs present in T. cruzi genome, were identified as a TLR9 agonist [14]. T. cruzi chronically infected Chagas disease patients display a Th1 cytokine profile [15] which is even more pronounced among CCC patients [16, 17]. It has been described that certain infectious agents, like Mycobacterium tuberculosis, possess molecules stimulating innate immunity that can shift the systemic cytokine environment and modify clinical immune profiles [18]. Our group and others have previously reported that heart-infiltrating T cells predominantly produce IFN-γ and TNF-α, suggesting that such Th1 T cells play an important pathogenetic role in heart tissue damage in CCC [16, 19–22]. Even though acute T. cruzi infection induces IL-12 production in mice, little is known about whether T. cruzi or tGPI-mucins exert a similar action in humans. We have previously described the isolation of live T. cruzi trypomastigotes outgrowing from a heart biopsy fragment from a CCC patient [23], routinely cultured for the study of outgrowing heart-infiltrating T cells [16, 24]. In order to study whether T. cruzi and tGPI-mucins could directly induce the production of the Th1-inducing cytokine IL-12 in human cells, we studied the cytokine profile in naturally infected supernatants of heart-infiltrating mononuclear cells. We also assessed the effect of cocultivation of T. cruzi and tGPI-mucins with peripheral blood mononuclear cells and purified monocytes on IL-12 production. Finally, we assessed the role of IFN-γ and CD40L signaling on T. cruzi and tGPI mucin-induced IL-12 production.
2. Methods
2.1. Parasites
The Y strain of T. cruzi was maintained in fibroblast cultures and was used as parasite source for purification of tGPI-mucins. For the trypomastigote culture, L-929 fibroblasts were initially infected with blood trypomastigotes in a ratio of one parasite per cell. The tissue culture trypomastigotes were continuously passed in L-929 fibroblast cultures. The infected cultures were maintained in Dulbecco's modified Eagle's medium (DMEM) containing 5% fetal calf serum (FCS) at 33°C in 5% CO2. After 4 or 5 days of culture, the parasites were collected daily and centrifuged at 40 g at 4°C for 10 min for cellular debris separation, followed by another centrifugation at 700 g at 4°C for 10 min. The resulting pellet containing live trypomastigotes was used to purify GPI-mucins.
2.2. Purification of tGPI-Mucins
The GPI-mucins were isolated from T. cruzi trypomastigotes as described previously [7, 8] using sequential organic extraction followed by hydrophobic-interaction chromatography in an Octyl-Sepharose column (Amersham Pharmacia Biotech, Uppsala, Sweden) and elution with a propan-1-ol gradient (5–60%).
2.3. Heart-Infiltrating T Cell Lines
T cell lines were established from endomyocardial biopsy explants from CCC patients as described [16]. Briefly, biopsy tissue was minced and seeded on to 96-well flat bottom plates in the presence of IL-2 and irradiated peripheral blood mononuclear cells (PBMC) until lymphoblast outgrowth was observed; T cell lines were expanded by restimulation every two weeks with 5 μg phytohemagglutinin (PHA) and irradiated PBMC. PBMC were obtained from blood of healthy donors and separated by density gradient centrifugation with Ficoll-HypaqueR. All cells were cultured in Dulbecco's modified Eagle's medium supplemented with 2 mM L-glutamine, 1 mM sodium pyruvate, MEM's nonessential amino acids and MEM's vitamins (all from GIBCO, Grand Island, NY, USA), 50 μg/mL gentamicin, 10 mM HEPES buffer, and 10% normal human serum (complete medium). This protocol has been approved by the Institutional Review Board of the University of São Paulo School of Medicine and all subjects provided informed consent.
2.4. T. cruzi Coculture/GPI Treatment
Ten to 12 days after the last PHA stimulation, heart-infiltrating T cell lines (from four different individuals, in separate experiments) were stimulated in the presence of irradiated PBMC (5 × 105/well) plus 5 μg/mL PHA and supernatants were obtained after 48 h incubation. In another set of experiments, culture conditions included variable components: irradiated PBMC, heart-infiltrating T cell lines (from four different individuals), 5 μg/mL PHA, 5 × 104 Y strain T. cruzi trypomastigotes obtained from LLC-MK2 monolayer cell culture, or 10 pmol/mL of T. cruzi tGPI-mucins. Blocking/neutralizing monoclonal antibodies against CD28, CD40, and IFN-γ (Pharmingen, La Jolla, CA) were employed in selected experiments.
2.5. Human Monocytes
Human monocytes were obtained by leukapheresis of normal volunteers at the blood bank of National Institutes of Health (Bethesda, MD). After density sedimentation of the mononuclear cells with lymphocyte separation medium (Organon, Teknika, Durham, NC), the monocytes were purified by counterflow centrifugal elutriation, as described previously [25], except that pyrogen-free PBS was used in the elutriation procedure. Monocytes were enriched >90% as determined by morphology, non-specific esterase staining, and flow cytometry. The purification procedure did not activate the monocytes, as shown by the fact that, after overnight incubation at 37°C in suspension, 4% of the cells were IL-12R positive or spontaneously secreted any of the cytokines measured. After purification, monocytes were left at 4°C overnight and then transferred to 5 mL polystyrene Falcon tubes (Becton Dickson Labware, Lincoln Park, NJ) and cultured for 24 h in the presence or absence of 10 pmol/mL tGPI-mucins, in the presence or absence of 100 units/mL of human IFN-γ (Genetech), as indicated. Culture supernatants were collected 48 h after stimulation for IL-12 determination.
2.6. Cytokine Measurements
Cytokines IFN-γ, IL-4, IL-2, IL-10, IL-12, and TNF-α were measured by double sandwich ELISA using the anti-human cytokine antibody pairs (R&D Systems, Minneapolis).
3. Statistical Analysis
Groups were compared by a nonparametrical test (Mann-Whitney Rank Sum Test) with GraphPad InStat software (version 5.0; GraphPad). Results were expressed as medians and interquartile ranges. P values were considered significant if <0.05.
4. Results
4.1. T. cruzi Outgrowth from Endomyocardial Biopsies from CCC Patients Induces the Production of T1-Type Cytokine Profile
We routinely cultured T cell lines from endomyocardial biopsies from CCC patients for the isolation of T cell lines. In one of these biopsy explants, highly motile T. cruzi trypomastigotes were observed in some of the seeded wells, indicating that the tissue fragments in those wells probably contained a T. cruzi pseudocyst. We therefore compared PHA-stimulated cytokine production in the supernatant from the T cell line established from wells containing live T. cruzi parasites (with T. cruzi trypomastigote growth) with the cell line derived from wells of the same biopsy devoid of T. cruzi (no T. cruzi trypomastigote growth). Figures 1(a) and 1(b) depict the cytokine profile of the T cell line obtained from the T. cruzi-positive wells as compared to the T cell line of the same sample, obtained from wells where no T. cruzi trypomastigotes were observed. As can be seen, T. cruzi trypomastigotes outgrowth induced the production of IL-12, TNF-α, and IFN-γ, with undetectable levels of IL4. The presence of T. cruzi strongly reduced the levels of IL-2 and mildly reduced IL-10 levels.
Figure 1.
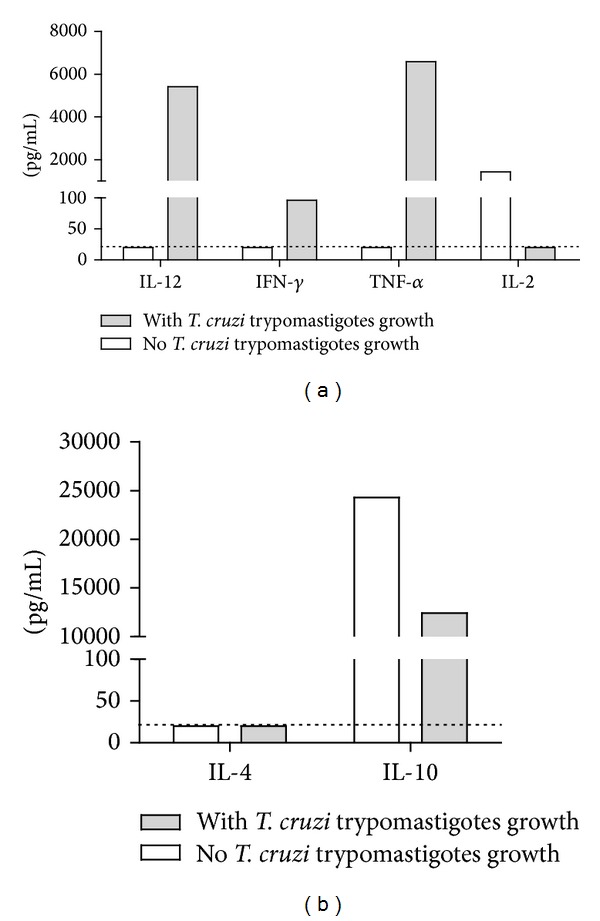
T. cruzi trypomastigotes outgrowth from endomyocardial biopsies from CCC patients induces the production of T1-type cytokine profile.
4.2. T. cruzi Trypomastigotes Induce IL-12 Production by Human PBMC, Which Is Potentiated by Activated T Cells
To further investigate the phenomenon observed in the endomyocardial biopsies wells we assayed cytokine production in supernatants of human PBMC in the presence of living T. cruzi and/or PHA-activated T cells. As shown in Figure 2, T. cruzi trypomastigotes can induce moderate production of IL-12 directly on irradiated PBMC or in cocultures of PBMC and T cells. However, coculture with PHA-activated T cell lines induced a 10- to 100-fold increase in IL-12 production by irradiated PBMC.
Figure 2.
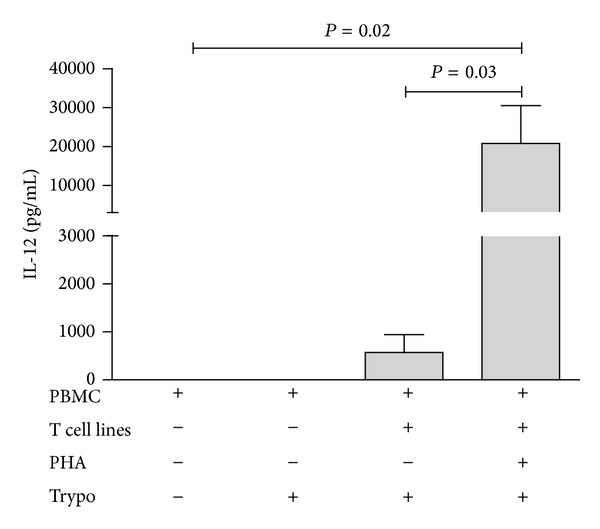
T. cruzi-induced IL-12 production is potentiated by activated T cells. Results come from 4 distinct experiments. Groups were compared by a nonparametrical test (Mann-Whitney Rank Sum Test) with GraphPad InStat software (version 5.0; GraphPad). Results were expressed as medians and interquartile ranges. P values were considered significant if <0.05.
4.3. GPI-Mucins from T. cruzi Trypomastigotes Induce IL-12 Production by Human Monocytes
We also tested if purified tGPI-mucins could activate isolated PBMC-derived monocytes in vitro to produce IL-12. As shown in Figure 3, tGPI-mucins induce significant production of IL-12 by human monocytes, which is further potentiated after IFNγ priming of cells.
Figure 3.
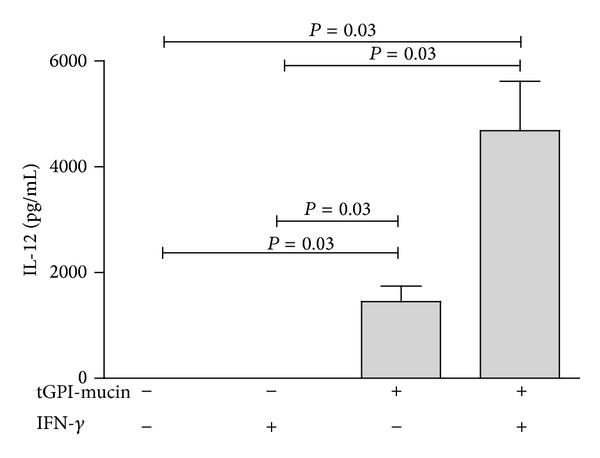
tGPI-mucins from T. cruzi induce IL-12 production by human monocytes. Results come from 3 distinct experiments. Groups were compared by a nonparametrical test (Mann-Whitney Rank Sum Test) with GraphPad InStat software (version 5.0; GraphPad). Results were expressed as medians and interquartile ranges. P values were considered significant if <0.05.
4.4. Induction of IL-12 Production by T. cruzi or tGPI-Mucins Is Dependent on IFN-γ and CD40-CD40L Interactions
In an attempt to study the mechanisms underlying T. cruzi-induced potentiation of IL-12 production by human monocytes, we cocultured these cells with PHA-activated T cell lines, 5 × 104 T. cruzi Y strain living trypomastigotes, or tGPI-mucins and added neutralizing/blocking antibodies to human IFN-γ, CD40, and CD28. Results indicated that blocking IFN-γ or CD40 individually caused approximately 50% and 35% of inhibition of IL-12 production, respectively, while anti-CD28 showed negligible inhibition. The combined effect of the three antibodies induced 85% of inhibition, suggesting that most of the IL-12-inducing effects of PHA-activated T cell lines are due to IFN-γ production and CD40-CD40L interactions (Figure 4(a)). Similar results were obtained when tGPI mucin was used as stimulus (Figure 4(b)) suggesting that these molecules may be the effectors in the T. cruzi-induced IL-12 production in humans, as has been previously described in mice [8].
Figure 4.
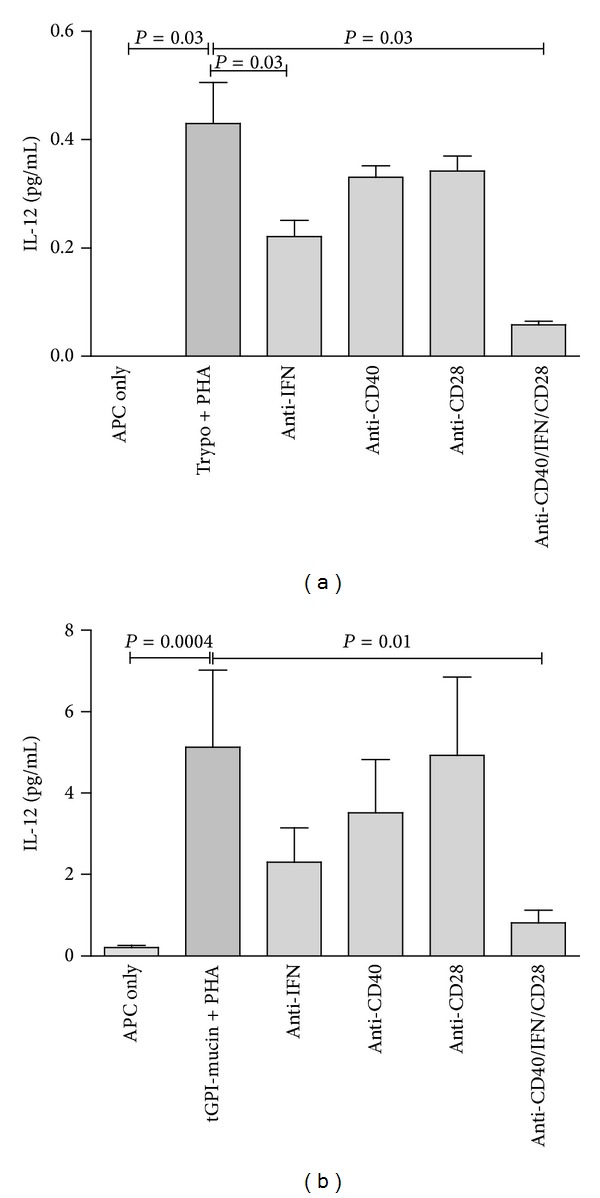
Potentiation of IL-12 production induced by (a) live T. cruzi trypomastigotes or (b) tGPI-mucins is dependent on IFN-γ and CD40. Irradiated PBMC were incubated with T cell lines, PHA (5 ug/mL), and different blocking antibodies (anti-IFN-γ, anti-CD40, anti-CD28, and anti-IFN/CD40/CD28). Results come from 4 distinct experiments. Groups were compared by a nonparametrical test (Mann-Whitney Rank Sum Test) with GraphPad InStat software (version 5.0; GraphPad). Results were expressed as medians and interquartile ranges. P values were considered significant if <0.05. Significance bars are shown in comparison with trypo + PHA or tGPI-mucin + PHA.
5. Discussion
In this paper, we observed that both living trypomastigotes and tGPI-mucins are potent inducers of IL-12 production in human monocytes and that this effect depends on CD40-CD40L interaction and IFN-γ signaling. The finding that spontaneous outgrowth of parasites in culture cells derived from chronically infected myocardium induced the production of T1-type proinflammatory cytokines, like IL-12, TNFα, and IFNγ, is in accordance with data from murine models from other studies [26–30]. These cytokines, which are induced during acute infection with T. cruzi in mice, play an obligatory role in parasite clearance and host survival [26, 29]. However, the same immunological pattern may participate in mechanisms of tissue damage in Chagas disease, indicating that protective and pathological responses must share important characteristics in this context [31]. When parasites were deliberately added to cocultures of irradiated PBMC and activated T cell lines, we observed again high levels of IL-12 expression in PBMC, although the T. cruzi stimulus itself was capable of inducing some IL-12 expression by PBMC in the absence of activated T cells. This corroborates the findings obtained with cultures with spontaneous outgrowth of T. cruzi trypomastigotes, where we had PHA-activated cell lines and T. cruzi trypomastigotes. Our results indicate that PBMC-derived monocytes are the cell population responding to tGPI-mucins with in vitro IL-12 production. Although we already observed induction of IL-12 production by monocytes using tGPI-mucins as a first signal (microbial stimulus via TLR-2), maximal levels of IL-12 are reached only after a second signal through the presence of IFN-γ, as it has been reported by other studies [32, 33]. The alkylacylglycerolipid component of tGPI-mucins [7] is capable of triggering Toll-like receptors-2 at subnanomolar concentrations [13]. Moreover, macrophages derived from Tlr2−/− or Myd88−/− mice are less responsive to tGPI-mucins, further confirming the possible role of the TLR pathway in this process [34]. Our findings that anti-IFN-γ and anti-CD40L neutralizing antibodies were able to significantly reduce IL-12 production indicate this phenomenon is mediated by IFN-γ and CD40-CD40L interactions. This can be explained by the fact that, in this context, T cells are likely to be the major source of IFN-γ and membrane CD40L, activators of macrophages involved in many aspects of parasite control [11, 35]. As previously described by Chaussabel et al. CD40 ligation in T. cruzi-infected mice has a protective effect because it is related to upregulation of IL-12 as well as NO by a direct stimulation of INF-γ activated macrophages [36]. Previous studies also showed that the CD40-CD40L signaling pathway mediated protective effect with other pathogens such as Leishmania [37], Schistosoma mansoni [38], Cryptococcus neoformans [39], Cryptosporidium parvum [40], and Pneumocystis carinii [41]. The enhanced production of IFN-γ, TNF-α, and nitric oxide associated with CD40/CD40L signaling is thought to be responsible for this protective effect. It was shown that IFNγ stimulus also upregulates the transcription factor T-bet [42], which in turn maintains IL-12Rβ chain expression [43], possibly resulting in a positive feedback loop that, consequently, keeps the shift towards a Th1 response in Chagas disease. In summary, our data suggest that the T1-type cytokine profile found in the peripheral blood and among heart-infiltrating T cells is related to previous or ongoing encounters with IL-12 generated as a response to T. cruzi GPI-anchored mucin-like glycoproteins.
Acknowledgments
This work has been supported by grants of the Brazilian National Research Council (CNPq), São Paulo State Foundation for Scientific Research (FAPESP), National Institute of Allergy and Infectious Disease (Grant no. 1P50AI098461-01), and Instituto Nacional de Ciência e Tecnologia de Vacinas (INCT/Vacinas). LRPF is recipient of a CNPq fellowship; ICN and MAB are recipients of a FAPESP fellowship. LVR, ECN, and JK are recipients of Brazilian Council for Scientific and Technological Development-CNPq productivity award. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the paper.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contribution
Lúcia Cristina Jamli Abel and Ludmila Rodrigues Pinto Ferreira are equally contributing authors.
References
- 1.Hotez PJ, Dumonteil E, Woc-Colburn L, et al. Chagas disease: ‘the new HIV/AIDS of the Americas’. PLOS Neglected Tropical Diseases. 2012;6(5) doi: 10.1371/journal.pntd.0001498.e1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rassi A, Jr., Marcondes de Rezende J. American trypanosomiasis (Chagas disease) Infectious Disease Clinics of North America. 2012;26(2):275–291. doi: 10.1016/j.idc.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Machado FS, Jelicks LA, Kirchhoff LV, et al. Chagas heart disease: report on recent developments. Cardiology in Review. 2012;20(2):53–65. doi: 10.1097/CRD.0b013e31823efde2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bilate AM, Cunha-Neto E. Chagas disease cardiomyopathy: current concepts of an old disease. Revista do Instituto de Medicina Tropical de São Paulo. 2008;50(2):67–74. doi: 10.1590/s0036-46652008000200001. [DOI] [PubMed] [Google Scholar]
- 5.Gazzinelli RT, Denkers EY. Protozoan encounters with Toll-like receptor signalling pathways: implications for host parasitism. Nature Reviews Immunology. 2006;6(12):835–906. doi: 10.1038/nri1978. [DOI] [PubMed] [Google Scholar]
- 6.Golgher D, Gazzinelli RT. Innate and acquired immunity in the pathogenesis of Chagas disease. Autoimmunity. 2004;37(5):399–409. doi: 10.1080/08916930410001713115. [DOI] [PubMed] [Google Scholar]
- 7.Almeida IC, Camargo MM, Procopio DO, et al. Highly purified glycosylphosphatidylinositols from Trypanosoma cruzi are potent proinflammatory agents. The EMBO Journal. 2000;19(7):1476–1485. doi: 10.1093/emboj/19.7.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camargo MM, Almeida IC, Pereira ME, Ferguson MA, Travassos LR, Gazzinelli RT. Glycosylphosphatidylinositol-anchored mucin-like glycoproteins isolated from Trypanosoma cruzi trypomastigotes initiate the synthesis of proinflammatory cytokines by macrophages. The Journal of Immunology. 1997;158(12):5890–5901. [PubMed] [Google Scholar]
- 9.Hoft DF, Eickhoff CS. Type 1 immunity provides both optimal mucosal and systemic protection against a mucosally invasive, intracellular pathogen. Infection and Immunity. 2005;73(8):4934–4940. doi: 10.1128/IAI.73.8.4934-4940.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graefe SE. Interleukin-12 but not interleukin-18 is required for immunity to Trypanosoma cruzi in mice. Microbes and Infection. 2003;5(10):833–839. doi: 10.1016/s1286-4579(03)00176-x. [DOI] [PubMed] [Google Scholar]
- 11.Michailowsky V, Silva NM, Rocha CD, Vieira LQ, Lannes-Vieira J, Gazzinelli RT. Pivotal role of interleukin-12 and interferon-gamma axis in controlling tissue parasitism and inflammation in the heart and central nervous system during Trypanosoma cruzi infection. American Journal of Pathology. 2001;159(5):1723–1733. doi: 10.1016/s0002-9440(10)63019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Talvani A, Ribeiro CS, Aliberti JC, et al. Kinetics of cytokine gene expression in experimental chagasic cardiomyopathy: tissue parasitism and endogenous IFN-gamma as important determinants of chemokine mRNA expression during infection with Trypanosoma cruzi . Microbes and Infection. 2000;2(8):851–866. doi: 10.1016/s1286-4579(00)00388-9. [DOI] [PubMed] [Google Scholar]
- 13.Campos MA, Almeida IC, Takeuchi O, et al. Activation of Toll-like receptor-2 by glycosylphosphatidylinositol anchors from a protozoan parasite. The Journal of Immunology. 2001;167(1):416–423. doi: 10.4049/jimmunol.167.1.416. [DOI] [PubMed] [Google Scholar]
- 14.Gravina HD, Antonelli L, Gazzinelli RT, Ropert C. Differential use of TLR2 and TLR9 in the regulation of immune responses during the infection with Trypanosoma cruzi . PLoS ONE. 2013;8(5) doi: 10.1371/journal.pone.0063100.e63100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ribeirao M, Pereira-Chioccola VL, Rénia L, Augusto Fragata Filho A, Schenkman S, Rodrigues MM. Chagasic patients develop a type 1 immune response to Trypanosoma cruzi trans-sialidase. Parasite Immunology. 2000;22(1):49–53. doi: 10.1046/j.1365-3024.2000.00260.x. [DOI] [PubMed] [Google Scholar]
- 16.Abel LC. Chronic Chagas' disease cardiomyopathy patients display an increased IFN-γ response to Trypanosoma cruzi infection. Journal of Autoimmunity. 2001;17(1):99–107. doi: 10.1006/jaut.2001.0523. [DOI] [PubMed] [Google Scholar]
- 17.Gomes JA, Bahia-Oliveira LM, Rocha MO, Martins-Filho OA, Gazzinelli G, Correa-Oliveira R. Evidence that development of severe cardiomyopathy in human Chagas' disease is due to a Th1-specific immune response. Infection and Immunity. 2003;71(3):1185–1193. doi: 10.1128/IAI.71.3.1185-1193.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shirakawa T, Enomoto T, Shimazu S, Hopkin JM. The inverse association between tuberculin responses and atopic disorder. Science. 1997;275(5296):77–79. doi: 10.1126/science.275.5296.77. [DOI] [PubMed] [Google Scholar]
- 19.Reis DD, Jones EM, Tostes S., Jr. Characterization of inflammatory infiltrates in chronic chagasic myocardial lesions: presence of tumor necrosis factor-alpha+ cells and dominance of granzyme A+, CD8+ lymphocytes. The American Journal of Tropical Medicine and Hygiene. 1993;48(5):637–644. doi: 10.4269/ajtmh.1993.48.637. [DOI] [PubMed] [Google Scholar]
- 20.Reis MM, Higuchi Mde L, Benvenuti LA, et al. An in situ quantitative immunohistochemical study of cytokines and IL-2R+ in chronic human chagasic myocarditis: correlation with the presence of myocardial Trypanosoma cruzi antigens. Clinical Immunology and Immunopathology. 1997;83(2):165–172. doi: 10.1006/clin.1997.4335. [DOI] [PubMed] [Google Scholar]
- 21.Rodrigues DBR, dos Reis MA, Romano A, et al. In situ expression of regulatory cytokines by heart inflammatory cells in Chagas' disease patients with heart failure. Clinical and Developmental Immunologyl. 2012;2012:7 pages. doi: 10.1155/2012/361730.361730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cunha-Neto E, Dzau VJ, Allen PD, et al. Cardiac gene expression profiling provides evidence for cytokinopathy as a molecular mechanism in Chagas' disease cardiomyopathy. American Journal of Pathology. 2005;167(2):305–313. doi: 10.1016/S0002-9440(10)62976-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teixeira MM, da Silva FM, Marcili A, et al. Short communication: trypanosoma cruzi lineage I in endomyocardial biopsy from a north-eastern Brazilian patient at end-stage chronic Chagasic cardiomyopathy. Tropical Medicine & International Health. 2006;11( 3):294–298. doi: 10.1111/j.1365-3156.2006.01575.x. [DOI] [PubMed] [Google Scholar]
- 24.Cunha-Neto E, Coelho V, Guilherme L, Fiorelli A, Stolf N, Kalil J. Autoimmunity in Chagas’ disease. Identification of cardiac myosin-B13 Trypanosoma cruzi protein crossreactive T cell clones in heart lesions of a chronic Chagas’ cardiomyopathy patient. The Journal of Clinical Investigation. 1996;98(8):1709–1712. doi: 10.1172/JCI118969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyên P, Broussas M, Cornillet-Lefèbvre P, Potron G. Coexpression of tissue factor and tissue factor pathway inhibitor by human monocytes purified by leukapheresis and elutriation. Response of nonadherent cells to lipopolysaccharide. Transfusion. 1999;39(9):975–982. doi: 10.1046/j.1537-2995.1999.39090975.x. [DOI] [PubMed] [Google Scholar]
- 26.Aliberti JC, Cardoso MA, Martins GA, et al. Interleukin-12 mediates resistance to Trypanosoma cruzi in mice and is produced by murine macrophages in response to live trypomastigotes. Infection and Immunity. 1996;64(6):1961–1967. doi: 10.1128/iai.64.6.1961-1967.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frosch S, Kraus S, Fleischer B. Trypanosoma cruzi is a potent inducer of interleukin-12 production in macrophages. Medical Microbiology and Immunology. 1996;185(3):189–193. doi: 10.1007/s004300050030. [DOI] [PubMed] [Google Scholar]
- 28.Torrico F, Heremans H, Rivera MT, van Marck E, Billiau A, Carlier Y. Endogenous IFN-gamma is required for resistance to acute Trypanosoma cruzi infection in mice. The Journal of Immunology. 1991;146(10):3626–3632. [PubMed] [Google Scholar]
- 29.Cardillo F, Voltarelli JC, Reed SG, Silva JS. Regulation of Trypanosoma cruzi infection in mice by γ interferon and interleukin 10: role of NK cells. Infection and Immunity. 1996;64(1):128–34. doi: 10.1128/iai.64.1.128-134.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abrahamsohn IA, Coffman RL. Trypanosoma cruzi: IL-10, TNF, IFN-γ, and IL-12 regulate innate and acquired immunity to infection. Experimental Parasitology. 1996;84(2):231–244. doi: 10.1006/expr.1996.0109. [DOI] [PubMed] [Google Scholar]
- 31.Cunha-Neto E, Rizzo LV, Albuquerque F, et al. Cytokine production profile of heart-infiltrating T cells in Chagas' disease cardiomyopathy. Brazilian Journal of Medical and Biological Research. 1998;31(1):133–137. doi: 10.1590/s0100-879x1998000100018. [DOI] [PubMed] [Google Scholar]
- 32.Snijders A, Kalinski P, Hilkens CM, Kapsenberg ML. High-level IL-12 production by human dendritic cells requires two signals. International Immunology. 1998;10(11):1593–1598. doi: 10.1093/intimm/10.11.1593. [DOI] [PubMed] [Google Scholar]
- 33.Schulz O, Edwards AD, Schito M, et al. CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity. 2000;13(4):453–462. doi: 10.1016/s1074-7613(00)00045-5. [DOI] [PubMed] [Google Scholar]
- 34.Campos MA, Closel M, Valente EP. Impaired production of proinflammatory cytokines and host resistance to acute infection with Trypanosoma cruzi in mice lacking functional myeloid differentiation factor 88. Journal of Immunology. 2004;172(3):1711–1718. doi: 10.4049/jimmunol.172.3.1711. [DOI] [PubMed] [Google Scholar]
- 35.Marinho CR, Nuñez-Apaza LN, Bortoluci KR. Infection by the Sylvio X10/4 clone of Trypanosoma cruzi: relevance of a low-virulence model of Chagas' disease. Microbes and Infection. 2009;11(13):1037–1045. doi: 10.1016/j.micinf.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 36.Chaussabel D, Jacobs F, de Jonge J, et al. CD40 ligation prevents Trypanosoma cruzi infection through interleukin-12 upregulation. Infection and Immunity. 1999;67(4):1929–1934. doi: 10.1128/iai.67.4.1929-1934.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferlin WG, von der Weid T, Cottrez F, Ferrick DA, Coffman RL, Howard MC. The induction of a protective response in Leishmania major-infected BALB/c mice with anti-CD40 mAb. European Journal of Immunology. 1998;28(2):525–531. doi: 10.1002/(SICI)1521-4141(199802)28:02<525::AID-IMMU525>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 38.MacDonald AS, Patton EA, La Flamme AC, et al. Impaired Th2 development and increased mortality during Schistosoma mansoni infection in the absence of CD40/CD154 interaction. The Journal of Immunology. 2002;168(9):4643–4649. doi: 10.4049/jimmunol.168.9.4643. [DOI] [PubMed] [Google Scholar]
- 39.Chen GH, Osterholzer JJ, Choe MY, et al. Dual roles of CD40 on microbial containment and the development of immunopathology in response to persistent fungal infection in the lung. The American Journal of Pathology. 2010;177(5):2459–2471. doi: 10.2353/ajpath.2010.100141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cosyns M. Requirement of CD40-CD40 ligand interaction for elimination of Cryptosporidium parvum from mice. Infection and Immunity. 1998;66(2):603–607. doi: 10.1128/iai.66.2.603-607.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wiley JA, Harmsen AG. CD40 ligand is required for resolution of Pneumocystis carinii pneumonia in mice. Journal of Immunology. 1995;155(7):3525–3229. [PubMed] [Google Scholar]
- 42.Lighvani AA, Frucht DM, Jankovic D, et al. T-bet is rapidly induced by interferon-gamma in lymphoid and myeloid cells. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(26):15137–15142. doi: 10.1073/pnas.261570598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Afkarian M, Sedy JR, Yang J, et al. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. Nature Immunology. 2002;3(6):549–557. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]


