Abstract
Stem cells have emerged as promising tools for the treatment of incurable neural and heart diseases and tissue damage. However, the survival of transplanted stem cells is reported to be low, reducing their therapeutic effects. The major causes of poor survival of stem cells in vivo are linked to anoikis, potential immune rejection, and oxidative damage mediating apoptosis. This review investigates novel methods and potential molecular mechanisms for stem cell preconditioning in vitro to increase their retention after transplantation in damaged tissues. Microenvironmental preconditioning (e.g., hypoxia, heat shock, and exposure to oxidative stress), aggregate formation, and hydrogel encapsulation have been revealed as promising strategies to reduce cell apoptosis in vivo while maintaining biological functions of the cells. Moreover, this review seeks to identify methods of optimizing cell dose preparation to enhance stem cell survival and therapeutic function after transplantation.
Key words: : aggregate formation, encapsulation, hydrogel, preconditioning, stem cells
Introduction
Heart and neural tissue damage, such as myocardial infraction (MI), stroke, or spinal cord injury, are pathological events for which there are no satisfactory treatments to date.1–3 Stem cell therapy has shown promising results for restoration of tissue homeostasis and regeneration of the damaged tissues.4–6 Stem cells or their derivatives have prolonged proliferation ability, multilineage differentiation potential, and trophic functions, which together enhance tissue repair while being integrated in injured tissues.7–9 Therefore, the use of stem cells in cell therapy has been extensively studied recently.
The site of injured tissue is usually associated with ischemia, extracellular matrix (ECM) degradation, oxidative stress, inflammation, and acute immune response.10 As a consequence, stem cells generally display limited survival and low retention rate in injured tissues, reducing the benefit of their therapeutic effects. In addition, stem cell derivatives may tend to be targeted by innate and adaptive immune responses in the host tissue.11 Thus, increasing stem cell retention is required towards long-term cell efficacy in vivo. The prolonged survival and integration of stem cell derivatives in damaged tissues rely on the prevention of anoikis, minimization of immune rejection, and increased resistance against oxygen and nutrient deprivation and oxidative stress in the ischemic area.
Recent studies indicate that overexpression of anti-apoptotic and antioxidant proteins provides stem cells the resistance against ischemic stresses, thus increasing their retention in vivo.12 However, such methods may have increased risk in long-term integration with the host tissue due to the possibility of tumorigenicity from gene transfection.13 Hence, the development of nongenetic methods is preferred to increase stem cell survival in damaged tissues without further compromising the tissue integrity. It has recently shown that chronic exposure in vitro to stresses that cells experience in damaged tissues, such as hypoxia, can enhance stem cell resistance in vivo.14 Other approaches are also explored such as heat shock treatment and exposure to hydrogen peroxide to induce heat shock proteins (HSPs) or to increase the resistance against oxidative stress.6,15 In addition to the culture environment preconditioning, growing stem cells in three-dimensional (3D) culture such as formation of 3D aggregates (e.g., microtissues) and the use of hydrogels (e.g., resuspended, embedded, or encapsulated) also provide favorable microenvironments that promote stem cell retention and survival under ischemic conditions due to the intimate cell–cell and cell–matrix interactions and the modulation of trophic functions of stem cells.16,17
Hence, this review analyzes current methods and the possible molecular mechanisms for stem cell preconditioning in vitro prior to cell transplantation in injured tissues such as brains and hearts, with the examples of pluripotent stem cell derivatives, cardiac progenitors, neural progenitors, and mesenchymal stem cells. In particular, this work discusses emerging approaches of preconditioning stem cells through 3D aggregate formation or hydrogel encapsulation to modulate their properties for transplantation study. This survey indicates the feasibility of preconditioning stem cells with enhanced retention and survival, as well as the improved therapeutic functions towards long-term restoration of tissue homoeostasis.
Stem Cells for Therapy
Pluripotent stem cells
Pluripotent stem cells (PSCs) including embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) emerge as promising cell sources for tissue engineering and regenerative medicine.18 PSCs have long-term self-renewal ability and a broad potential to differentiate into the cell types of the three germ layers and can in principle provide an unlimited number of cells for transplantation. In particular, iPSCs can be obtained by reprogramming somatic or progenitor cells from the specific patients through the forced expression of pluripotent genes such as KLF-4, c-Myc, Oct-4, and Sox-2,19 enabling possible autologous cell transplantation. While PSCs cannot be directly transplanted in injured tissues due to the risk of teratoma formation,20 tissue-specific progenitor cells or terminally differentiated cells can be derived from PSCs and are more appropriate for cell therapy. To date, oligodendrocyte progenitor cells and retinal pigment epithelium cells are two examples of Phase I clinical trials based on human PSCs for treating patients with spinal cord injury or macular degeneration, respectively.21,22
Mesenchymal stem cells
Many more clinical trials (>300) have been conducted using mesenchymal stem cells (MSCs), which are connective tissue progenitor cells found in perivascular location in vivo (e.g., adventitial reticular cells in bone marrow or satellite cells in muscle).23 MSCs are usually characterized ex vivo by a set of nonspecific markers such as CD73, CD105, and CD90, and the differentiation potential towards osteoblasts, adipocytes, and chondrocytes.24 MSCs can be isolated from various types of tissues including bone marrow, adipose tissue, cartilage, and umbilical cord.25 MSCs have also been derived from PSCs recently through embryoid body (EB) formation and replating in microvascular endothelial cell media.26,27 The derived cells showed the expression of MSC markers and the ability to differentiate into osteocytes, chondrocytes, adipocytes, and myocytes.27 Compared to somatic MSCs, MSCs derived from PSCs have similar biological functions but a reduced telomere shortening process.28 MSCs have been successfully transplanted in vivo, such as in heart tissues in which enhanced cardiac functions were reported.29 The beneficial effects of MSCs mainly rely on the secretion of paracrine factors, such as vascular endothelial growth factor (VEGF), brain-derived neurotrophic factor (BDNF), fibroblast growth factor (FGF), or immune-regulatory molecules such as interleukin (IL)-6, IL-10, and indoleamine 2,3-dioxygenase.16,30 However, MSCs generally have limited survival rate and engraftment in vivo due to the cell loss after injection and the hostile environment of injured tissue.31 Thus, increasing MSC retention in vivo should improve and prolong their therapeutic effects.
Neural progenitor or stem cells
Neural progenitor cells (NPCs) exhibit the tri-lineage neural differentiation potential along neurons, astrocytes, and oligodendrocytes, and are usually characterized by the expression of specific markers such as Nestin, SOX-2, and Musashi-1.32 Somatic NPCs can be isolated from adult and fetal tissues (e.g., the subventricular zone and the dentate gyrus of the brain). In addition, NPCs could be derived from PSCs through EB formation or monolayer induction.2 The comparison of somatic and ESC-derived NPCs showed common differentiation potential and secretory profile, but PSC-derived NPCs displayed enhanced proliferation and were less prone to senescence compared to their somatic counterpart.33 Transplantation of PSC-derived NPCs improved the brain or motor functions after stroke, Alzheimer's disease, Parkinson's disease, amyotrophic lateral sclerosis, and so forth.2,5 The beneficial effects of NPCs in vivo include partial integration with host tissue, the ability to differentiate into neural populations, and the secretion of paracrine factors (such as BDNF) to promote endogenous progenitor differentiation.34 However, the limited engraftment and survival in injured sites are the major hurdles for their therapeutic functions.35
Cardiac progenitor or stem cells
Cardiac progenitor cells (CPCs) can differentiate into cardiomyocytes, smooth muscle cells, and endothelial cells, and are usually characterized by the expression of c-Kit, KDR, PDGFR-α, and Nkx2.5.9 CPCs can be isolated from heart tissues or derived from PSCs.36 While somatic CPCs are prone to senescence associated with aging, PSC-derived CPCs can provide an unlimited number of heart cells and be used for constructing cardiac tissues.36,37 CPCs have been successfully infused after myocardial infarction and are able to reduce scar formation and improve heart function.38 The beneficial effects of CPCs in vivo may be due to their partial differentiation, integration into host tissue, and the paracrine functions of the secreted factors such as VEGF and von Willebrand factor (vWF).38 However, the cell survival and long-term retention of CPCs for prolonged therapeutic effects in injured heart remains challenging.39
Environmental Preconditioning of Stem Cells
Hypoxic, oxidative, or heat shock preconditioning
Ischemic tissue environment, oxidative stress, and loss of ECM are the major challenges of cell survival in vivo. Acute ischemia in injured tissues results from the combination of drastic reduction of oxygen tension and the deprivation of nutrients.40 Hormesis defines the brief exposure of tissues to stresses, which can lead to increased recovery after acute stress.40 The beneficial effect of preconditioning was first demonstrated in treating healthy myocardium with intermittent brief ischemia followed by reperfusion, which protected the heart from acute ischemic episodes.41 Since then, various preconditioning strategies include hypoxic preconditioning, exposure to oxidative stress, and heat shock treatment have been investigated to improve stem cell survival (Table 1).42
Table 1.
Environmental Preconditioning of Stem Cells
| Preconditioning methods | Cell source and pretreatment | Animal model | Performance | Reference |
|---|---|---|---|---|
| Hypoxia | PSC-derived NPCs exposed to 1% O2 for 8 h | Rat ischemic brain established through middle cerebral artery occlusion | 30%–40% reduced cell death after transplantation, improved sensorimotor functions compared to normoxic groups | Theus et al.44 |
| Bone marrow–derived MSCs exposed to 0.5% O2 for 24 h | Rat brain subjected to middle cerebral artery occlusion (stroke model) | Increased survival and improved brain functional recovery and motor functions compared to normoxic groups | Wei et al.46 | |
| CPCs derived from adult hearts exposed to 0.1% O2 for 6 h | Mouse heart subjected to coronary ligation (MI model) | Increased survival and heart functions: increased LVS and reduced infarct size | Tang et al.43 | |
| MSCs exposed to 0% O2 for cyclic short-time periods | Rat heart subjected to coronary occlusion (MI model) | 1.5-fold increase in grafted cell number and improved heart functions: reduced LVDd, LVDs, and infarct size, increased LVS | Wang et al.121 | |
| Exposure to low concentration of oxidative stresses | CPCs derived from adult heart tissue exposed to 100 μM H2O2 for 2 days | Rat heart (MI model) | Increased survival and improved heart functions: improved left ventricular cardiac function and reduced scar compared to nonconditioned groups | Pendergrass et al.15 |
| NPCs exposed to 50 μM H2O2 for 24 h | N.A. | Threefold reduced cell death compared to nonconditioned groups | Sharma et al.48 | |
| Wharton Jelly–derived MSCs treated with 200 μM H2O2 for 2 h | Mouse heart subjected to left-sided thoracotomy and left anterior descending coronary artery ligation | Reduced myocardial fibrosis, reduced LVDd, LVDs and increased LVS compared to nonconditioned groups | Zhang et al.49 | |
| Heat shock treatment | hESC-derived cardiomyocytes | Rat heart subjected to thoracotomy and artery ligation | Increased cell engraftment and improved heart functions (increased LVS, reduced LVDd and LVDs) | Laflamme et al.6,52 |
| NPCs treated at 43°C for 3 h | N.A. | Heat shock increased HSP-25 expression, which provides protection against apoptosis | Geum et al.50 | |
| Bone marrow MSCs treated at 42°C for 60 min | N.A. | HSP-20 and -70 expression was increased compared to nontreated groups | Moloney et al.53 | |
| CPCs derived from bone marrow 42°C for 3 h | Mouse heart subjected to left-sided thoracotomy and left anterior descending coronary artery ligation | Twofold increase in cell survival, attenuated fibrosis, and improved ischemic heart functions compared to control groups | Feng et al.51 |
PSC, pluripotent stem cell; NPC, neural progenitor cell; MSC, mesenchymal stem cell; MI, myocardial infraction; CPC, cardiac progenitor cell; LVS, left ventricular shortening; hESC, human embryonic stem cell; LVDd, left ventricular end-diastolic; LVDs, left ventricular end- systolic; HSP, heat shock protein; N.A., not available.
Hypoxia treatment preconditions the cells to adapt to the ischemic environment. Culturing CPCs under hypoxia (2%–5% O2) in vitro was found to enhance cell survival in a mouse model of myocardial ischemia–reperfusion injury.43 Moreover, preconditioning ESC-derived NPCs under hypoxia enhanced cell survival with 30%–40% reduction in cell death after transplantation into the ischemic brain of rats, compared to the groups cultivated under normoxia.44,45 Similarly, MSCs conditioned under hypoxia promoted angiogenesis and neurogenesis in rat ischemic brain models that mimicked stroke.46 MSCs exposed to hypoxia in vitro also showed the enhanced survival through the up-regulation of Bcl-2 and Bcl-xL, leading to the reduced infarct size and the enhanced heart functions.47
Short-term exposure to low concentration of oxidative stresses in vitro also enhances the survival of stem cells. For instance, a 2-day treatment of CPCs with 100 μM H2O2 in vitro improved the left ventricular cardiac function and reduced cardiac fibrosis in ischemia–reperfusion injury of rat hearts after implantation, as compared to the nontreated groups.15 Similarly, NPCs exposed to the repeated low doses of H2O2 (up to 50 μM) showed better resistance to acute oxidative stress.48 The MSCs preconditioned with H2O2 also demonstrated enhanced survival and significant functional improvement of ischemic hearts compared to nontreated groups.49
Heat shock treatment also emerges as an attractive approach to increase the cell survival rate. Heat shock preconditioning of NPCs at 43°C for 3 h was reported to reduce cell apoptosis.50 Similarly, the short-term culture of CPCs at 42°C reduced apoptosis, increased the functionality, and reduced the fibrosis of mouse ischemic myocardium.51 Transplantation of human ESC-derived cardiomyocytes also used the cells treated by 30–60 min of heat shock at 43°C and improved the formation of functional grafts in a rat model of myocardial ischemia–reperfusion injury, possibly due to the up-regulation of HSPs such as HSP-60, -70, and -90.6,52 The exposure of MSCs to high temperature (43°C) was also associated with an increased secretion of HSPs, such as HSP-27 and -70,53 which may contribute to the increased cell survival.
Molecular mechanisms of environmental preconditioning
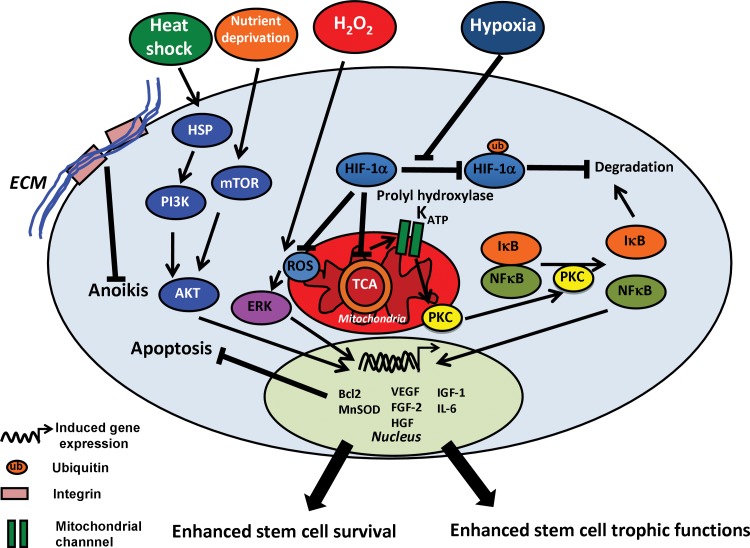
Antioxidative defense mechanisms in the stem cells mediate their survival from acute ischemic stresses.54 Examination of the defense mechanisms against the accumulation of reactive oxygen species (ROS) in human iPSCs showed similar mechanisms compared to human ESCs.55 Preconditioning human PSCs under hypoxia and oxidative stress revealed the induction of anti-apoptotic mechanisms, such as the stabilization of hypoxia-inducible factor (HIF)-2α, which inhibited p53 and increased Bcl-2 expression.56 MSCs cultured under hypoxia promoted the stabilization of HIF-1α, which is normally degraded by HIF prolyl-4-hydroxylases under normoxia.57 HIF-1α acts as a regulatory inducer of glycolytic enzymes and an inhibitor of the pyruvate dehydrogenase, which enables the entry into tricarboxylic acid cycle.58 The metabolic shift from oxidative phosphorylation to glycolysis under hypoxia reduces ROS generation (Fig. 1).58 Similarly, a low glucose concentration prevents excessive ROS generation from stem cells and thus the cells become less prone to oxidative stress–mediated apoptosis.59
FIG. 1.
Molecular mechanisms of environmental preconditioning of stem cells. Chronic exposure to hypoxia prevents hypoxia-inducible factor (HIF)-1α degradation, by inhibition of its ubiquitination by prolyl hydrolxylase. HIF-1α stabilization reduces oxidative phosphorylation, leading to the opening of mitoKATP channels and consequently the activation of protein kinase C (PKC). PKC activates nuclear factor kappa beta (NFκβ) signaling, leading to the enhanced expression of antioxidant and anti-apoptotic proteins (MnSOD, Bcl-2, etc.). NFκβ also enhances trophic functions of the cells (i.e., secretion of VEGF, FGF, BDNF, etc.). The chronic exposure to oxidative stress (e.g., H2O2) induces a transient release of reactive oxygen species (ROS) from mitochondria, leading to the activation of extracellular signal-regulated kinases (ERK). ERK activation promotes the expression of anti-apoptotic proteins. Heat shock treatment promotes the expression of heat shock proteins (HSPs), which activate the phosphoinositide 3-kinase (PI3K)/AKT signaling. The PI3K/AKT signaling induces the expression of anti-oxidants, anti-apoptotic factors, and trophic factors. Nutrient deprivation activates mammalian target of rapamycin (mTOR) signaling which also leads to the activation of AKT. VEGF, vascular endothelial growth factor; FGF, fibroblast growth factor; BDNF, brain-derived neurotrophic factor; IGF, insulin-like growth factor; HGF, hepatocyte growth factor; TCA cycle, tricarboxylic acid cycle.
Ischemic preconditioning decreases ATP synthesis due to reduced mitochondrial respiration and promotes the opening of mitochondrial ion channels, such as the mitochondrial ATP-sensitive K+ (mitoKATP) channel.60 The mitoKATP opening provokes mild ROS release, which triggers the activation of protein kinase C, leading to the induction of the nuclear factor kappa beta (NFκβ) signaling and the synthesis of antioxidant molecules (e.g., MnSOD).60 Consistently, the treatment of MSCs with diazoxide (a mitoKATP opener) to mimic the effects of ischemia-activated NFκβ signaling led to the increased survival and expression of trophic factors (e.g., FGF-2).61 Similar treatment of PSC-derived CPCs with diazoxide also promoted cell resistance against oxidative stress.62
Ischemic preconditioning enhances tissue survival after acute ischemia through the activation of the cytoplasmic phosphoinositide 3-kinase (PI3K)/AKT signaling pathway.63 The preconditioning-mediated activation of PI3K/AKT triggers mammalian target of rapamycin (mTOR) and NADPH oxidase (NOX) pathways which slow down the transition from early apoptosis to necrosis or apoptosis in vivo.64 In vitro, the activation of mTOR-AKT-STAT3 signaling in MSCs reduces the secretion of proinflammatory molecules and consequently increases their survival against ischemia (Fig. 1).65 In addition, the activation of PI3K/AKT pathway (e.g., using lysophosphatidic acid, angiopoetin-1) has been reported to promote the secretion of Bcl-2, leading to the enhanced resistance to pro-apoptotic stresses.66
The activation of HSPs through temperature increase or incubation with β-mercaptoethanol promotes the survival of stem cells.6,67 Indeed, HSPs trigger the PI3K/AKT and extracellular signal-regulated kinases (ERK) pathway, which leads to the increased secretion of anti-apoptotic proteins (Fig. 1).68 Importantly, the activation of PI3K/AKT, NFκβ, and HSP signaling pathways promotes trophic functions. For instance, enhanced secretion of VEGF, FGF-2, hepatocyte growth factor (HGF), insulin-like growth factor (IGF)-1, and IL-6 has been observed in MSCs upon the activation of PI3K/AKT or NFκβ.69 Hence, reducing mitochondrial respiration and activating the PI3K/AKT pathway in vitro can prevent oxidative damage and increase the survival and trophic functions of stem cells in vivo.
Preconditioning Stem Cells as Aggregates
Structural properties of stem cell aggregates
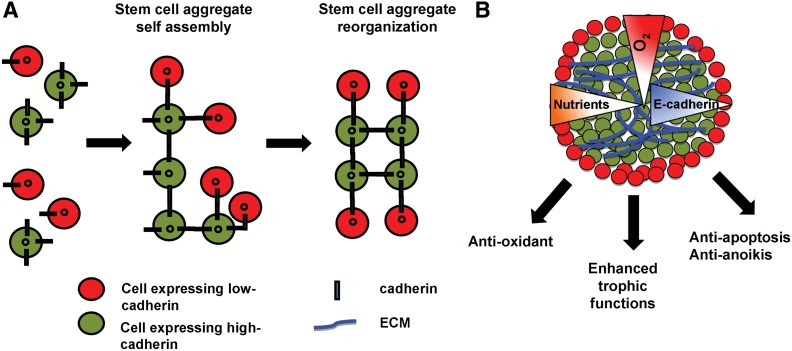
The formation of 3D stem cell aggregates requires both integrin–integrin interactions and homophilic cadherin–cadherin contacts. For instance, N-cadherin expression is required to initiate the spheroid formation from MSCs.16 Similarly, up-regulation of E-cadherin expression was observed in cardiospheres derived from CPCs.70 The formation of neurospheres from NPCs necessitated the expression of neural cell adhesion molecule and N-cadherin.71 Based on the level of cadherin expression, stem cells sort out the aggregate structure according to the differential adhesion hypothesis (Fig. 2). The differential expressions of cell adhesion molecules influence cell surface tension such that cell populations displaying a high expression level of cadherin were found at the interior, whereas cells with high integrin expression were found at the exterior of the aggregates.72 Culturing 3D stem cell aggregates leads to the local gradients of nutrients and oxygen and creates an ischemic-like microenvironment (Fig. 2).73 For instance, MSC spheroids promoted the stabilization of HIF-1α, indicating the formation of a mildly hypoxic environment.74 Similarly, cardiospheres derived from CPCs showed enhanced glycolysis, possibly as a consequence of the formation of hypoxic area.75
FIG. 2.
Stem cell aggregate formation as a preconditioning treatment. (A) Mechanism of stem cell aggregate formation. Stem cells organize and sort out the structure based on the degree of cadherin expression, according to the differential adhesion hypothesis. (B) Formation of stem cell aggregates promotes the secretion of extracellular matrix (ECM) and trophic factors, as well as creating a mildly hypoxic environment. Stem cell aggregation also could avoid anoikis, promote the expression of antioxidant and anti-apoptotic proteins, and enhance the trophic functions.
Aggregation enhances ECM secretion and trophic functions
Promotion of cell–cell contacts in stem cell aggregates can prevent anoikis due to the secretion of endogenous ECMs.76 Cell–cell interactions also increase the expression of gap junction proteins such as connexin-43 which can promote cell survival.16 For example, cell sheet derived from cardiospheres enhanced cell survival possibly due to the expressions of intercellular adhesion molecules and connexin-43.77 Table 2 shows the examples of the secreted endogenous ECM and matrix remodeling proteins in stem cell aggregates,78 which can support integrin signaling and mediate cell proliferation and survival. For MSCs, a higher proliferation rate was observed on vitronectin compared to fibronectin, laminin, and collagen type I or type III.79 NPCs demonstrated the enhanced proliferation on laminin compared to fibronectin, and endogenous fibronectin was found to protect the cells and induce the proliferation of CPCs after myocardial infarction.80,81
Table 2.
Endogenous Extracellular Matrix and Growth Factor Secretion in Stem or Progenitor Cell Aggregates
| Type of stem cell aggregate | Endogenous ECM | Secreted prosurvival factors | Cell source | Reference |
|---|---|---|---|---|
| Cardiosphere | Matrix remodeling, gene expressions of OL14A1, COL7A1, ITGA2, LAMB1, LAMB3, MMP-3, -10, -11, -13, SELE, PECAM1, SPP1 and collagen type IV | VEGF-A, FGF-2, angiopoietin-2, endothelin, receptor type A, E-selectin, CXCL-1, IL-11, GDF-15, gremlin 1, Fms-like tyrosine kinase1 (Flt1), B-cell translocation gene1 | Adult cardiac tissue | Li et al.,70 Cho et al.122 |
| Collagen type I | ND | Pluripotent stem cells | Kensah et al.78 | |
| Mesenchymal stem cell aggregate | Collagens, elastin, tenascin C, fibronectin, and laminin | FGF-2, HGF, and VEGF | Adipose tissue | Amos et al.,123 Bhang et al.74 |
| Fibronectin and laminin | IL-24, CXCR4, PGE2, TSG-6 | Bone marrow | Wang et al.,124 Sart et al.16 |
|
| Neurosphere | Laminin 1, fibronectin | VEGF, PDGFB, TGFA, FGF-5, etc. | Postnatal brain tissues, neural progenitor cells | Campos et al.,125 Lai et al.126 |
| Fibronectin, laminin, collagen type IV, vitronectin | ND | Embryonic stem cells | Sart et al.127 |
VEGF, vascular endothelial growth factor; FGF, fibroblast growth factor; CXCL-1, chemokine (C-X-C motif ) ligand 1; GDF-15, growth differentiation factor 15; Flt1, Fms-like tyrosine kinase 1; PECAM1, platelet endothelial cell adhesion molecule-1; MMP, matrix metalloproteinase; HGF, hepatocyte growth factor; IL, interleukin; CXCR4, chemokine (C-X-C motif ) receptor 4; PGE2, prostaglandin E2; TSG-6, tumor necrosis factor-inducible gene 6 protein; PDGFB, platelet-derived growth factor B; TGFA, transforming growth factor A; ND, not determined.
Impediment of oxygen diffusion in 3D aggregates may lead to the formation of an area of hypoxia that promotes the secretion of antioxidants and anti-apoptotic proteins. For instance, proteomics analysis indicated the increased expression of antioxidant proteins such as glutathione S-transferase, chaperones, HSP, and peroxiredoxin in cardiospheres compared to two-dimensional cultures.82 Similarly, MSC spheroids enhanced the secretion of Bcl-xL and anti-inflammatory molecules (e.g., prostaglandin E2 [PGE2]) compared to monolayers, which is thought to be due to local hypoxia in the core of aggregates.76 The 3D stem cell aggregates also promote the trophic functions of the cells. For instance, MSC spheroids enhanced the secretion of pro-angiogenic factors such as VEGF and FGF-2.74 CPC aggregation also enhanced the secretion of pro-angiogenic factors such as VEGF due to the activation of the HIF signaling pathway.75 While further investigations are required, the stabilization of HIF signaling in neurospheres may enhance the secretion of neurotrophic factors (e.g., BDNF) and pro-angiogenic factors (e.g., VEGF).83 Taken together, stem cell aggregation can promote endogenous ECM secretion, trigger the antioxidative mechanism, and improve trophic functions and the resistance against ischemic damage.
Preconditioning and Encapsulation of Stem Cells in Hydrogels
To avoid anoikis and improve cell survival after in vivo transplantation, various types of hydrogels have been tested during cell preconditioning and in situ delivery. For example, simple resuspension of the cells in an ECM-based hydrogel (e.g., Matrigel) was used during cell dose preparation of human ESC-derived cardiomyocytes to enhance cell survival.6 In particular, the in situ delivery of encapsulated stem cells within hydrogels can reduce invasiveness and improve cell viability (Table 3).17 Specifically, the compositions and physical properties of hydrogels, the bioactive molecules conjugated to hydrogels, and the methods of hydrogel encapsulation can affect the regenerative potential of stem cells.
Table 3.
Hydrogel-Based Methods for Cell Transplantation
| Hydrogel type | Cell source | Animal model | Performance | Reference |
|---|---|---|---|---|
| Agarose | CPCs derived from adult heart tissues | Mouse heart subjected to artery ligation (MI model) | Fivefold increase survival and improved heart functions: twofold reduced infarct size, 10% increase left ventricular ejection fraction compared to non-encapsulated groups | Mayfield et al.104 |
| PGE2-functionalized biodegradable hydrogel | MSCs derived from bone marrow | Rat heart subjected to coronary artery ligation (MI model) | Increased survival decreased the number of CD8+ T cells. Improved heart functions: increased fractional shortening, reduced scar size and reduced LVDs and LVDd compared to non-encapsulated groups | Dhingra et al.106 |
| Gelatin/laminin | Warthon Jelly–derived MSCs | Rat brain subjected to ouabain-mediating excitotoxicity | Improved cell survival post-transplantation, decreased activated microglia/macrophages compared to non-encapsulated groups | Sarnowska et al.107 |
| Hyaluronic acid/gelatin/PEGDA | Immortalized NPCs from fetal tissues | Rat brain striatum; rat spinal cord subjected to laminectomy | Increased cell survival, reduced host immune response compared to non-encapsulated groups | Liang et al.108 |
| Hyaluronan/ heparin sulfate/collagen | Adult tissue and embryonic stem cell-derived NPCs | Mouse brain subjected to cortical photothrombotic stroke | Twofold increase in cell survival, significant reduction of microglia/macrophage infiltration compared to non-encapsulated groups | Zhong et al.103 |
PEGDA, poly(ethylene glycol) diacrylate; LVDd, left ventricular end-diastolic; LVDs, left ventricular end-systolic.
Hydrogels for encapsulation
Two main methodologies have been used for encapsulation: a liquid core containing the cells surrounded by a semisolid membrane, or alternatively, cell embedding within hydrogels either as aggregates or as single cells (Fig. 3).84 Hydrogels and membranes are usually highly hydrophilic, biocompatible, and nonimmunogenic polymeric materials. Various types of materials have been used as hydrogels for encapsulation: natural polymers, such as Matrigel, collagen, gelatin, agarose, and alginate, or synthetic materials, such as poly(ethylene glycol) (PEG).85 Solid capsules and hydrogels generally support mass transport of oxygen and nutrients.86 However, encapsulation can generate the gradients of biomolecules due to diffusion, which may result in the local nutrient and oxygen depletion.87 Hence, a controlled diffusivity is a critical parameter to promote the encapsulated stem cell survival,87 which can be achieved by controlling the polymer concentration.88
FIG. 3.
Stem cell encapsulation as a preconditioning treatment. (A) Liquid core/solid shell encapsulation promotes aggregate formation and provides mass transport of biomolecules and immune isolation. (B) Stem cell encapsulation in nonadhesive hydrogels promotes aggregate formation and provides mass transport of biomolecules and immune isolation. (C) Stem cell encapsulation in adhesive hydrogels (i.e., containing integrin- and MMP-binding sites) promotes stem cell adhesion and provides mass transport of biomolecules and immune isolation.
Encapsulation prevents anoikis and regulates trophic functions
Adhesive hydrogels (i.e., containing or binding ECM proteins) provide 3D substrates for stem cell anchorage and facilitate ECM remodeling, hence limiting the anoikis and promoting cell migration. For instance, collagen or gelatin hydrogels containing RGD sequence and matrix metalloproteinase (MMP) binding sites supported efficient propagation, survival, and migration of stem cells.89 Recent developments of functionalization and bioconjugation methods for nonadhesive hydrogels, such as PEG and hyaluronic acid (HA), with integrin- and MMP-binding domains provide biochemically and biomechanically controlled semisolid scaffolds to regulate cellular behaviors.90,91 For instance, the controlled distribution of RGD sequences on HA-based hydrogels regulated integrin-expression profile, cell shape, proliferation, and survival of MSCs.92 In addition, modulation of the stiffness of alginate or PEG-silica gel functionalized with RGD regulated NPC survival and expansion.93 Moreover, the ECM pattern and stiffness in hydrogels affect trophic functions of stem or progenitor cells. For examples, an increased density of RGD sequence on gellan gum hydrogels promoted the secretion of neurotrophic factors from encapsulated MSCs.94 Cross-linked methacrylate-HA hydrogels with low modulus (1.5 and 2.6 kPa) enhanced the secretion of angiogenic factors from encapsulated stem cells compared to stiffer hydrogels (3.8 and 7.4 kPa).95 Consequently, modulation of hydrogel properties can provide cell delivery vehicles which can enhance the therapeutic functions of stem or progenitor cells in vivo.
Alternatively, nonadhesive hydrogels (such as agarose and PEG) surrounding stem cells in suspension promote the formation and the confinement of stem cell aggregates with controlled size. As demonstrated in PSCs, the aggregate size affected cell survival and the lineage commitment due to diffusion limitation and the size-dependent ratio of outer endoderm cells and inner undifferentiated cells in EBs.96,97 In addition, the degree of spatial confinement may affect local accumulation of paracrine or autocrine factors to regulate the phenotype of human PSC aggregates.98 While further investigations are required to determine the role of compression or confinement on encapsulated stem cell aggregates, the up-regulation of secreted growth factors (e.g., transforming growth factor beta, bone morphogenetic protein) has been observed in MSCs encapsulated in nonadhesive hydrogels with compression force.99
Encapsulation provides immune isolation and enhances cell survival
Stem cells encapsulated in core-shell capsules or embedded within hydrogels demonstrate the limited inflammation and activation of immune responses. For instance, reduced IgG binding to EBs embedded in core-shell alginate-chitosan-alginate capsules was observed compared to non-encapsulated cells.100 Moreover, functionalization of PEG hydrogels with tumor necrosis factor α–antagonizing molecules (e.g., WP9QY peptide) further reduced the immune response.101 CPCs encapsulated in alginate hydrogels that were functionalized with superoxide dismutases demonstrated the enhanced resistance against oxidative stress.102 When encapsulated within cross-linked hyaluronan/heparin/collagen hydrogels, NPCs showed the significantly reduced immune rejection and macrophage infiltration into the infarct zone in a mouse stroke model.103
Besides reducing immune response, hydrogel encapsulation enhances cell survival and functions in vivo. For example, encapsulation of CPCs in agarose hydrogels improved left ventricular functions in mouse heart subjected to MI.104 The MSCs encapsulated in alginate also retained high viability and trophic functions (e.g., secretion of VEGF, IGF-1, FGF-2, and HGF), while minimizing the immune response once injected into hearts.105 The injection of encapsulated MSCs in hydrogel functionalized with PGE2 in an MI model of rat heart reduced scar area and improved left ventricular functions.106 Encapsulated MSCs in gelatin/laminin gels enhanced survival while reducing the neuro-inflammation in ischemic rat hippocampus.107 Similarly, the encapsulation of NPCs in HA/gelatin/PEG diacrylate sustained cell survival after implantation in mouse striatum.108 By modulating the biochemical and biomechanical properties of hydrogels, the trophic function of stem cells and their in vivo retention can be enhanced.
Cell Dose Preparation for In Vivo Study
For the safe and efficient transplantation of preconditioned stem cells in injured sites, accurate characterization of cells and hydrogels with minimal invasiveness are required. The parameters of cell dose preparation include cell concentration, volume, and buffer formulation. Also, the cell delivery methods impact the cell viability postinjection, where injectable hydrogels may protect the cells from the extensional flow during injection.109 These parameters have to be optimized based on specific cell types and animal models.
Cell dose preparation
The environmental conditioning of stem cells with ischemic, hypoxic, and oxidative treatments could be performed before cell harvesting, while the cells need additional steps for aggregate formation or formulation in hydrogels after the harvesting. For high dose study (∼107–108 cells per dose) such as in heart diseases, the cells are highly concentrated compared to the low dose (∼106–107 cells per dose) that is usually used in neural diseases.6,110,111 For the dose formulation, prosurvival factors may also be added to enhance cell survival in addition to hydrogels, such as Bcl-xL peptide to block mitochondrial death pathways, IGF-1 to activate cytoprotective AKT pathways, and the caspase inhibitor ZvAD-fmk to reduce apoptosis.6,8 The accurate characterizations of biochemical, biomechanical, immunological, and diffusion properties of the prosurvival factors and hydrogels have to be provided. For example, it has been shown that mechanical properties of alginate gel contributed to cell protection rather than the biochemical properties of alginate biopolymers.109 PEG-based microcryogels with predefined size and shape have also been shown to augment the paracrine functions and the tissue integration as injectable cell delivery vehicles due to the cell protection from shear-induced damage and the controlled cell localization.112
To ensure proper stem cell retention and function after transplantation, the controlled functional properties of stem cells in vitro in terms of trophic factor secretion, immunogenicity, and the expressions of antioxidants and anti-apoptotic proteins may need to be performed. The control of stem cell aggregate size, which regulates stem cell properties and viability, has to be rigorously achieved as well. The specifications that are used for quality control are mainly established based on the experimental results. In this regard, the in vitro measurements that can correlate with the in vivo behaviors are critical, yet challenging.
Cell dose delivery methods
Stem cells are usually delivered via injection through systemic infusion (i.e., intravenous or intra-arterial) or local transplantation at the injured sites. More tissue-specific routes are also used such as intraventricular delivery for neural tissue and transendocardial delivery for cardiovascular diseases.113 These different cell dose delivery routes can affect the transplantation outcome. For examples, transendocardial route showed higher cell retention and increased vascularity than intracoronary delivery.114 Transplantation of MSCs through endocardium showed enhanced cell retention and improved left ventricular ejection fraction compared to intravascular infusion.115 For stroke, stem cell delivery through the intracerebral route was reported as the most invasive method.116 Alternatively, intracisternal/cerebroventricular and intravascular delivery routes showed less invasiveness, but cell migration to the injured site was limited.116 Similarly, for the treatment of spinal cord injury, intralesion delivery displayed enhanced cell retention and facilitated secretion of neurotrophic factors while intravascular infusion had limited cell engraftment, which reduced the therapeutic functions.117
The cell delivery methods affect cell viability and the actual dose administered due to flow stress, backpressure, tissue density, and so forth. To reduce the variations in manual injection, automated methods (e.g., computer-controlled syringe pump) have also been developed that show similar outcomes for cell viability with the controlled cell volume and flow rate.118 Among various parameters, length and diameter of the tubing as well as cell density have been shown to be critical factors for better survival and retention of therapeutic cells.119 The automated methods may be used when systemic delivery of single cells is required. For example, single cells of MSCs can transmigrate through the endothelial barrier via paracellular or transcellular diapedesis.120 For large cellular constructs such as stem cell aggregates or encapsulated cells, the cell delivery methods still need to be better designed to control the cell dose and maintain the structure integrity.
Conclusions
Stem cell preconditioning through chronic environmental stresses, aggregate formation, and hydrogel encapsulation improves cell survival and biological functions of transplanted cells in vivo. The selection of preconditioning strategy must consider the cell delivery route and the specific clinical application. For example, intravenous or intra-arterial injections are incompatible with the transplantation of encapsulated cells or aggregates. In these cases, environmental preconditioning, which enables the injection of single cell suspension, is more suitable for cell survival and function. In contrast, the local stem cell injection to the injury site can enhance stem cell retention in situ. Thus, stem cell preconditioning as aggregates and/or encapsulated cells within hydrogels is more suitable for cell survival and maintaining biological activity and for restoring the damaged tissue's integrity. However, the molecular mechanisms of environmental preconditioning need to be further elucidated. Further investigations are also required to understand the structural and biological properties of stem cell aggregates in order to enhance cell survival and trophic functions. In addition, the influence of biochemical and biophysical properties of 3D hydrogels on stem cell behaviors merits further study towards the increased functionality in vivo. Preparation of cell dose needs to be carefully designed based on cell types, animal models, and the delivery methods. Taken together, stem cell preconditioning can be applied prior to transplantation towards the safe long-term efficacy of stem cell–based therapy.
Abbreviations Used
- 3D
three-dimensional
- BDNF
brain-derived neurotrophic factor
- CPC
cardiac progenitor cell
- EB
embryoid body
- ECM
extracellular matrix
- ERK
extracellular signal-regulated kinases
- ESC
embryonic stem cell
- FGF
fibroblast growth factor
- HA
hyaluronic acid
- HGF
hepatocyte growth factor
- HIF
hypoxia-inducible factor
- HSP
heat shock protein
- IGF
insulin-like growth factor
- IL
interleukin
- iPSC
induced pluripotent stem cell
- MI
myocardial infraction
- MMP
matrix metalloproteinase
- MSC
mesenchymal stem cell
- mTOR
mammalian target of rapamycin
- NFκβ
nuclear factor kappa beta
- NPC
neural progenitor cell
- PEG
poly(ethylene glycol)
- PGE2
prostaglandin E2
- PI3K
phosphoinositide 3-kinase
- PSC
pluripotent stem cell
- ROS
reactive oxygen species
- VEGF
vascular endothelial growth factor
- vWF
von Willebrand factor
Acknowledgments
ERC starting grant (to S.S.), FSU start up fund (to Y.L.), partial support from National Science Foundation (1342192 to Y.L.), and partial support from the James King Biomedical Research Program (4KB09 to T.M.) are acknowledged. We thank Chunhui Xu at the Department of Pediatrics at Emory University for comments on the manuscript.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Sanganalmath SK, Bolli R. Cell therapy for heart failure: a comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ Res. 2013;113:810–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu DX, Marchetto MC, Gage FH. Therapeutic translation of iPSCs for treating neurological disease. Cell Stem Cell. 2013;12:678–688 [DOI] [PubMed] [Google Scholar]
- 3.Li J, Lepski G. Cell transplantation for spinal cord injury: a systematic review. Biomed Res Int. 2013;2013:786475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandenberger R, Burger S, Campbell A, et al. . Cell therapy bioprocessing. BioProcess Int. 2011;9:30–37 [Google Scholar]
- 5.Kriks S, Shim JW, Piao J, et al. . Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson's disease. Nature. 2011;480:547–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laflamme MA, Chen KY, Naumova AV, et al. . Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024 [DOI] [PubMed] [Google Scholar]
- 7.Pourrajab F, Babaei Zarch M, Baghi Yazdi M, et al. . Application of stem cell/growth factor system, as a multimodal therapy approach in regenerative medicine to improve cell therapy yields. Int J Cardiol. 2014;173:12–19 [DOI] [PubMed] [Google Scholar]
- 8.Wollert KC, Drexler H. Cell therapy for the treatment of coronary heart disease: a critical appraisal. Nat Rev Cardiol. 2010;7:204–215 [DOI] [PubMed] [Google Scholar]
- 9.Xu C. Differentiation and enrichment of cardiomyocytes from human pluripotent stem cells. J Mol Cell Cardiol. 2012;52:1203–1212 [DOI] [PubMed] [Google Scholar]
- 10.Lo EH, Wang X, Cuzner ML. Extracellular proteolysis in brain injury and inflammation: role for plasminogen activators and matrix metalloproteinases. J Neurosci Res. 2002;69:1–9 [DOI] [PubMed] [Google Scholar]
- 11.de Almeida PE, Ransohoff JD, Nahid A, et al. . Immunogenicity of pluripotent stem cells and their derivatives. Circ Res. 2013;112:549–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakata H, Niizuma K, Wakai T, et al. . Neural stem cells genetically modified to overexpress Cu/Zn-superoxide dismutase enhance amelioration of ischemic stroke in mice. Stroke. 2012;43:2423–2429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lan F, Liu J, Narsinh KH, et al. . Safe genetic modification of cardiac stem cells using a site-specific integration technique. Circulation. 2012;126:S20–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsiao ST, Dilley RJ, Dusting GJ, et al. . Ischemic preconditioning for cell-based therapy and tissue engineering. Pharmacol Ther. 2014;142:141–153 [DOI] [PubMed] [Google Scholar]
- 15.Pendergrass KD, Boopathy AV, Seshadri G, et al. . Acute preconditioning of cardiac progenitor cells with hydrogen peroxide enhances angiogenic pathways following ischemia-reperfusion injury. Stem Cells Dev. 2013;22:2414–2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sart S, Tsai A-C, Li Y, et al. . Three-dimensional aggregates of mesenchymal stem cells: cellular mechanisms, biological properties, and applications. Tissue Eng Part B Rev. 2013; [Epub ahead of print]; DOI: 10.1089/ten.teb.2013.0537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye Z, Zhou Y, Cai H, et al. . Myocardial regeneration: roles of stem cells and hydrogels. Adv Drug Deliv Rev. 2011;63:688–697 [DOI] [PubMed] [Google Scholar]
- 18.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. . Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147 [DOI] [PubMed] [Google Scholar]
- 19.Takahashi K, Tanabe K, Ohnuki M, et al. . Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872 [DOI] [PubMed] [Google Scholar]
- 20.Fong CY, Gauthaman K, Bongso A. Teratomas from pluripotent stem cells: a clinical hurdle. J Cell Biochem. 2010;111:769–781 [DOI] [PubMed] [Google Scholar]
- 21.Abbasalizadeh S, Baharvand H. Technological progress and challenges towards cGMP manufacturing of human pluripotent stem cells based therapeutic products for allogeneic and autologous cell therapies. Biotechnol Adv. 2013;31:1600–1623 [DOI] [PubMed] [Google Scholar]
- 22.Maher KO, Xu C. Marching towards regenerative cardiac therapy with human pluripotent stem cells. Discov Med. 2013;15:349–356 [PMC free article] [PubMed] [Google Scholar]
- 23.da Silva Meirelles L, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008;26:2287–2299 [DOI] [PubMed] [Google Scholar]
- 24.Dominici M, Le Blanc K, Mueller I, et al. . Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317 [DOI] [PubMed] [Google Scholar]
- 25.Pozzobon M, Piccoli M, De Coppi P. Sources of mesenchymal stem cells: current and future clinical use. Adv Biochem Eng Biotechnol. 2013;130:267–286 [DOI] [PubMed] [Google Scholar]
- 26.Kimbrel EA, Kouris NA, Yavanian G, et al. . Mesenchymal stem cell population derived from human pluripotent stem cells displays potent immunomodulatory and therapeutic properties. Stem Cells Dev. 2014; [Epub ahead of print]; DOI: 10.1089/scd.2013.0554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee EJ, Lee HN, Kang HJ, et al. . Novel embryoid body-based method to derive mesenchymal stem cells from human embryonic stem cells. Tissue Eng Part A. 2010;16:705–715 [DOI] [PubMed] [Google Scholar]
- 28.Lian Q, Zhang Y, Zhang J, et al. . Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation. 2010;121:1113–1123 [DOI] [PubMed] [Google Scholar]
- 29.Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95:9–20 [DOI] [PubMed] [Google Scholar]
- 30.Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9:11–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toma C, Wagner WR, Bowry S, et al. . Fate of culture-expanded mesenchymal stem cells in the microvasculature: in vivo observations of cell kinetics. Circ Res. 2009;104:398–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Feo D, Merlini A, Laterza C, et al. . Neural stem cell transplantation in central nervous system disorders: from cell replacement to neuroprotection. Curr Opin Neurol. 2012;25:322–333 [DOI] [PubMed] [Google Scholar]
- 33.Colombo E, Giannelli SG, Galli R, et al. . Embryonic stem-derived versus somatic neural stem cells: a comparative analysis of their developmental potential and molecular phenotype. Stem Cells. 2006;24:825–834 [DOI] [PubMed] [Google Scholar]
- 34.Martino G, Pluchino S. The therapeutic potential of neural stem cells. Nat Rev Neurosci. 2006;7:395–406 [DOI] [PubMed] [Google Scholar]
- 35.Bakshi A, Keck CA, Koshkin VS, et al. . Caspase-mediated cell death predominates following engraftment of neural progenitor cells into traumatically injured rat brain. Brain Res. 2005;1065:8–19 [DOI] [PubMed] [Google Scholar]
- 36.Christoforou N, Liau B, Chakraborty S, et al. . Induced pluripotent stem cell-derived cardiac progenitors differentiate to cardiomyocytes and form biosynthetic tissues. PLoS One. 2013;8:e65963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burridge PW, Keller G, Gold JD, et al. . Production of de novo cardiomyocytes: human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell. 2012;10:16–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chimenti I, Smith RR, Li TS, et al. . Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res. 2010;106:971–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu S, Huang M, Nguyen PK, et al. . Novel microRNA prosurvival cocktail for improving engraftment and function of cardiac progenitor cell transplantation. Circulation. 2011;124:S27–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calabrese EJ, Bachmann KA, Bailer AJ, et al. . Biological stress response terminology: Integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicol Appl Pharmacol. 2007;222:122–128 [DOI] [PubMed] [Google Scholar]
- 41.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136 [DOI] [PubMed] [Google Scholar]
- 42.Haider H, Ashraf M. Preconditioning and stem cell survival. J Cardiovasc Transl Res. 2010;3:89–102 [DOI] [PubMed] [Google Scholar]
- 43.Tang YL, Zhu W, Cheng M, et al. . Hypoxic preconditioning enhances the benefit of cardiac progenitor cell therapy for treatment of myocardial infarction by inducing CXCR4 expression. Circ Res. 2009;104:1209–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Theus MH, Wei L, Cui L, et al. . In vitro hypoxic preconditioning of embryonic stem cells as a strategy of promoting cell survival and functional benefits after transplantation into the ischemic rat brain. Exp Neurol. 2008;210:656–670 [DOI] [PubMed] [Google Scholar]
- 45.Francis KR, Wei L. Human embryonic stem cell neural differentiation and enhanced cell survival promoted by hypoxic preconditioning. Cell Death Dis. 2010;1:e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wei L, Fraser JL, Lu ZY, et al. . Transplantation of hypoxia preconditioned bone marrow mesenchymal stem cells enhances angiogenesis and neurogenesis after cerebral ischemia in rats. Neurobiol Dis. 2012;46:635–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu X, Yu SP, Fraser JL, et al. . Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thorac Cardiovasc Surg. 2008;135:799–808 [DOI] [PubMed] [Google Scholar]
- 48.Sharma RK, Zhou Q, Netland PA. Effect of oxidative preconditioning on neural progenitor cells. Brain Res. 2008;1243:19–26 [DOI] [PubMed] [Google Scholar]
- 49.Zhang J, Chen GH, Wang YW, et al. . Hydrogen peroxide preconditioning enhances the therapeutic efficacy of Wharton's Jelly mesenchymal stem cells after myocardial infarction. Chin Med J (Engl). 2012;125:3472–3478 [PubMed] [Google Scholar]
- 50.Geum D, Son GH, Kim K. Phosphorylation-dependent cellular localization and thermoprotective role of heat shock protein 25 in hippocampal progenitor cells. J Biol Chem. 2002;277:19913–19921 [DOI] [PubMed] [Google Scholar]
- 51.Feng Y, Huang W, Meng W, et al. . Heat shock improves Sca-1+stem cell survival and directs ischemic cardiomyocytes toward a prosurvival phenotype via exosomal transfer: a critical role for HSF1/miR-34a/HSP70 pathway. Stem Cells. 2014;32:462–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laflamme MA, Gold J, Xu C, et al. . Formation of human myocardium in the rat heart from human embryonic stem cells. Am J Pathol. 2005;167:663–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moloney TC, Hoban DB, Barry FP, et al. . Kinetics of thermally induced heat shock protein 27 and 70 expression by bone marrow-derived mesenchymal stem cells. Protein Sci. 2012;21:904–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang K, Zhang T, Dong Q, et al. . Redox homeostasis: the linchpin in stem cell self-renewal and differentiation. Cell Death Dis. 2013;4:e537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Armstrong L, Tilgner K, Saretzki G, et al. . Human induced pluripotent stem cell lines show stress defense mechanisms and mitochondrial regulation similar to those of human embryonic stem cells. Stem Cells. 2010;28:661–673 [DOI] [PubMed] [Google Scholar]
- 56.Das B, Bayat-Mokhtari R, Tsui M, et al. . HIF-2alpha suppresses p53 to enhance the stemness and regenerative potential of human embryonic stem cells. Stem Cells. 2012;30:1685–1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muscari C, Giordano E, Bonafè F, et al. . Priming adult stem cells by hypoxic pretreatments for applications in regenerative medicine. J Biomed Sci. 2013;20:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sart S, Agathos SN, Li Y. Process engineering of stem cell metabolism for large scale expansion and differentiation in bioreactors. Biochem Eng J. 2014;84:74–82 [Google Scholar]
- 59.Choudhery MS, Khan M, Mahmood R, et al. . Mesenchymal stem cells conditioned with glucose depletion augments their ability to repair-infarcted myocardium. J Cell Mol Med. 2012;16:2518–2529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Otani H. Reactive oxygen species as mediators of signal transduction in ischemic preconditioning. Antioxid Redox Signal. 2004;6:449–469 [DOI] [PubMed] [Google Scholar]
- 61.Afzal MR, Haider H, Idris NM, et al. . Preconditioning promotes survival and angiomyogenic potential of mesenchymal stem cells in the infarcted heart via NF-kappaB signaling. Antioxid Redox Signal. 2010;12:693–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim JH, Oh AY, Choi YM, et al. . Isoflurane decreases death of human embryonic stem cell-derived, transcriptional marker Nkx2.5(+) cardiac progenitor cells. Acta Anaesthesiol Scand. 2011;55:1124–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hausenloy DJ, Yellon DM. Survival kinases in ischemic preconditioning and postconditioning. Cardiovasc Res. 2006;70:240–253 [DOI] [PubMed] [Google Scholar]
- 64.Zhang Q, Yang YJ, Wang H, et al. . Autophagy activation: a novel mechanism of atorvastatin to protect mesenchymal stem cells from hypoxia and serum deprivation via AMP-activated protein kinase/mammalian target of rapamycin pathway. Stem Cells Dev. 2012;21:1321–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fan W, Cheng K, Qin X, et al. . mTORC1 and mTORC2 play different roles in the functional survival of transplanted adipose-derived stromal cells in hind limb ischemic mice via regulating inflammation in vivo. Stem Cells. 2013;31:203–214 [DOI] [PubMed] [Google Scholar]
- 66.Bai Y, Meng Z, Cui M, et al. . An Ang1-Tie2-PI3K axis in neural progenitor cells initiates survival responses against oxygen and glucose deprivation. Neuroscience. 2009;160:371–381 [DOI] [PubMed] [Google Scholar]
- 67.Cizkova D, Rosocha J, Vanicky I, et al. . Induction of mesenchymal stem cells leads to HSP72 synthesis and higher resistance to oxidative stress. Neurochem Res. 2006;31:1011–1020 [DOI] [PubMed] [Google Scholar]
- 68.Gao F, Hu XY, Xie XJ, et al. . Heat shock protein 90 protects rat mesenchymal stem cells against hypoxia and serum deprivation-induced apoptosis via the PI3K/Akt and ERK1/2 pathways. J Zhejiang Univ Sci B. 2010;11:608–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen J, Crawford R, Chen C, et al. . The key regulatory roles of the PI3K/Akt signaling pathway in the functionalities of mesenchymal stem cells and applications in tissue regeneration. Tissue Eng Part B Rev. 2013;19:516–528 [DOI] [PubMed] [Google Scholar]
- 70.Li TS, Cheng K, Lee ST, et al. . Cardiospheres recapitulate a niche-like microenvironment rich in stemness and cell-matrix interactions, rationalizing their enhanced functional potency for myocardial repair. Stem Cells. 2010;28:2088–2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ladiwala U, Basu H, Mathur D. Assembling neurospheres: dynamics of neural progenitor/stem cell aggregation probed using an optical trap. PLoS One. 2012;7:e38613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Foty RA, Steinberg MS. The differential adhesion hypothesis: a direct evaluation. Dev Biol. 2005;278:255–263 [DOI] [PubMed] [Google Scholar]
- 73.Van Winkle AP, Gates ID, Kallos MS. Mass transfer limitations in embryoid bodies during human embryonic stem cell differentiation. Cells Tissues Organs. 2012;196:34–47 [DOI] [PubMed] [Google Scholar]
- 74.Bhang SH, Cho SW, La WG, et al. . Angiogenesis in ischemic tissue produced by spheroid grafting of human adipose-derived stromal cells. Biomaterials. 2011;32:2734–2747 [DOI] [PubMed] [Google Scholar]
- 75.Kawaguchi N, Machida M, Hatta K, et al. . Cell shape and cardiosphere differentiation: a revelation by proteomic profiling. Biochem Res Int. 2013;2013:730874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bartosh TJ, Ylostalo JH, Mohammadipoor A, et al. . Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc Natl Acad Sci U S A. 2010;107:13724–13729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zakharova L, Mastroeni D, Mutlu N, et al. . Transplantation of cardiac progenitor cell sheet onto infarcted heart promotes cardiogenesis and improves function. Cardiovasc Res. 2010;87:40–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kensah G, Roa Lara A, Dahlmann J, et al. . Murine and human pluripotent stem cell-derived cardiac bodies form contractile myocardial tissue in vitro. Eur Heart J. 2013;34:1134–1146 [DOI] [PubMed] [Google Scholar]
- 79.Mathews S, Bhonde R, Gupta PK, et al. . Extracellular matrix protein mediated regulation of the osteoblast differentiation of bone marrow derived human mesenchymal stem cells. Differentiation. 2012;84:185–192 [DOI] [PubMed] [Google Scholar]
- 80.Flanagan LA, Rebaza LM, Derzic S, et al. . Regulation of human neural precursor cells by laminin and integrins. J Neurosci Res. 2006;83:845–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Konstandin MH, Toko H, Gastelum GM, et al. . Fibronectin is essential for reparative cardiac progenitor cell response after myocardial infarction. Circ Res. 2013;113:115–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bauer M, Kang L, Qiu Y, et al. . Adult cardiac progenitor cell aggregates exhibit survival benefit both in vitro and in vivo. PLoS One. 2012;7:e50491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cunningham LA, Candelario K, Li L. Roles for HIF-1alpha in neural stem cell function and the regenerative response to stroke. Behav Brain Res. 2012;227:410–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Radhakrishnan J, Krishnan UM, Sethuraman S. Hydrogel based injectable scaffolds for cardiac tissue regeneration. Biotechnol Adv. 2014;32:449–461 [DOI] [PubMed] [Google Scholar]
- 85.Murua A, Portero A, Orive G, et al. . Cell microencapsulation technology: towards clinical application. J Control Release. 2008;132:76–83 [DOI] [PubMed] [Google Scholar]
- 86.Uludag H, De Vos P, Tresco PA. Technology of mammalian cell encapsulation. Adv Drug Deliv Rev. 2000;42:29–64 [DOI] [PubMed] [Google Scholar]
- 87.Colton CK. Oxygen supply to encapsulated therapeutic cells. Adv Drug Deliv Rev. 2014; [Epub ahead of print]; DOI: 10.1016/j.addr.2014.02.007 [DOI] [PubMed] [Google Scholar]
- 88.Wilson JL, McDevitt TC. Stem cell microencapsulation for phenotypic control, bioprocessing, and transplantation. Biotechnol Bioeng. 2013;110:667–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chan BP, Hui TY, Yeung CW, et al. . Self-assembled collagen-human mesenchymal stem cell microspheres for regenerative medicine. Biomaterials. 2007;28:4652–4666 [DOI] [PubMed] [Google Scholar]
- 90.Zhu J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials. 2010;31:4639–4656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huang G, Wang L, Wang S, et al. . Engineering three-dimensional cell mechanical microenvironment with hydrogels. Biofabrication. 2012;4:042001. [DOI] [PubMed] [Google Scholar]
- 92.Lam J, Segura T. The modulation of MSC integrin expression by RGD presentation. Biomaterials. 2013;34:3938–3947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Banerjee A, Arha M, Choudhary S, et al. . The influence of hydrogel modulus on the proliferation and differentiation of encapsulated neural stem cells. Biomaterials. 2009;30:4695–4699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Silva NA, Moreira J, Ribeiro-Samy S, et al. . Modulation of bone marrow mesenchymal stem cell secretome by ECM-like hydrogels. Biochimie. 2013;95:2314–2319 [DOI] [PubMed] [Google Scholar]
- 95.Marklein RA, Soranno DE, Burdick JA. Magnitude and presentation of mechanical signals influence adult stem cell behavior in 3-dimensional macroporous hydrogels. Soft Matter. 2012;8:8113–8120 [Google Scholar]
- 96.Khoury M, Bransky A, Korin N, et al. . A microfluidic traps system supporting prolonged culture of human embryonic stem cells aggregates. Biomed Microdevices. 2010;12:1001–1008 [DOI] [PubMed] [Google Scholar]
- 97.Bauwens CL, Peerani R, Niebruegge S, et al. . Control of human embryonic stem cell colony and aggregate size heterogeneity influences differentiation trajectories. Stem Cells. 2008;26:2300–2310 [DOI] [PubMed] [Google Scholar]
- 98.Giobbe GG, Zagallo M, Riello M, et al. . Confined 3D microenvironment regulates early differentiation in human pluripotent stem cells. Biotechnol Bioeng. 2012;109:3119–3132 [DOI] [PubMed] [Google Scholar]
- 99.Haudenschild AK, Hsieh AH, Kapila S, et al. . Pressure and distortion regulate human mesenchymal stem cell gene expression. Ann Biomed Eng. 2009;37:492–502 [DOI] [PubMed] [Google Scholar]
- 100.Zhang W, Zhao S, Rao W, et al. . A novel core-shell microcapsule for encapsulation and 3D culture of embryonic stem cells. J Mater Chem B Mater Biol Med. 2013;2013:1002–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lin CC, Metters AT, Anseth KS. Functional PEG-peptide hydrogels to modulate local inflammation induced by the pro-inflammatory cytokine TNFalpha. Biomaterials. 2009;30:4907–4914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu TC, Ismail S, Brennan O, et al. . Encapsulation of cardiac stem cells in superoxide dismutase-loaded alginate prevents doxorubicin-mediated toxicity. J Tissue Eng Regen Med. 2013;7:302–311 [DOI] [PubMed] [Google Scholar]
- 103.Zhong J, Chan A, Morad L, et al. . Hydrogel matrix to support stem cell survival after brain transplantation in stroke. Neurorehabil Neural Repair. 2010;24:636–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mayfield AE, Tilokee EL, Latham N, et al. . The effect of encapsulation of cardiac stem cells within matrix-enriched hydrogel capsules on cell survival, post-ischemic cell retention and cardiac function. Biomaterials. 2014;35:133–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ceccaldi C, Fullana SG, Alfarano C, et al. . Alginate scaffolds for mesenchymal stem cell cardiac therapy: influence of alginate composition. Cell Transplant. 2012;21:1969–1984 [DOI] [PubMed] [Google Scholar]
- 106.Dhingra S, Li P, Huang XP, et al. . Preserving prostaglandin E2 level prevents rejection of implanted allogeneic mesenchymal stem cells and restores postinfarction ventricular function. Circulation. 2013;128:S69–78 [DOI] [PubMed] [Google Scholar]
- 107.Sarnowska A, Jablonska A, Jurga M, et al. . Encapsulation of mesenchymal stem cells by bioscaffolds protects cell survival and attenuates neuroinflammatory reaction in injured brain tissue after transplantation. Cell Transplant. 2013;22(Suppl 1):S67–S82 [DOI] [PubMed] [Google Scholar]
- 108.Liang Y, Walczak P, Bulte JW. The survival of engrafted neural stem cells within hyaluronic acid hydrogels. Biomaterials. 2013;34:5521–5529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Aguado BA, Mulyasasmita W, Su J, et al. . Improving viability of stem cells during syringe needle flow through the design of hydrogel cell carriers. Tissue Eng Part A. 2012;18:806–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Oki K, Tatarishvili J, Woods J, et al. . Human induced pluripotent stem cells form functional neurons and improve recovery after grafting in stroke-damaged brain. Stem Cells. 2012;30:1120–1133 [DOI] [PubMed] [Google Scholar]
- 111.Sharma S, Raju R, Sui S, et al. . Stem cell culture engineering—process scale up and beyond. Biotechnol J. 2011;6:1317–1329 [DOI] [PubMed] [Google Scholar]
- 112.Liu W, Li Y, Zeng Y, et al. . Microcryogels as injectable 3-D cellular microniches for site-directed and augmented cell delivery. Acta Biomater. 2014;10:1864–1875 [DOI] [PubMed] [Google Scholar]
- 113.O'Cearbhaill ED, Ng KS, Karp JM. Emerging medical devices for minimally invasive cell therapy. Mayo Clin Proc. 2014;89:259–273 [DOI] [PubMed] [Google Scholar]
- 114.Perin EC, Silva GV, Assad JA, et al. . Comparison of intracoronary and transendocardial delivery of allogeneic mesenchymal cells in a canine model of acute myocardial infarction. J Mol Cell Cardiol. 2008;44:486–495 [DOI] [PubMed] [Google Scholar]
- 115.Campbell NG, Suzuki K. Cell delivery routes for stem cell therapy to the heart: current and future approaches. J Cardiovasc Transl Res. 2012;5:713–726 [DOI] [PubMed] [Google Scholar]
- 116.Misra V, Ritchie MM, Stone LL, et al. . Stem cell therapy in ischemic stroke: role of IV and intra-arterial therapy. Neurology. 2012;79:S207–S212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kang ES, Ha KY, Kim YH. Fate of transplanted bone marrow derived mesenchymal stem cells following spinal cord injury in rats by transplantation routes. J Korean Med Sci. 2012;27:586–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gobbel GT, Kondziolka D, Fellows-Mayle W, et al. . Manual vs automated delivery of cells for transplantation: accuracy, reproducibility, and impact on viability. Neurosurgery. 2010;67:1662–1668; discussion 1668 [DOI] [PubMed] [Google Scholar]
- 119.Kondziolka D, Gobbel GT, Fellows-Mayle W, et al. . Injection parameters affect cell viability and implant volumes in automated cell delivery for the brain. Cell Transplant. 2011;20:1901–1906 [DOI] [PubMed] [Google Scholar]
- 120.Teo GS, Ankrum JA, Martinelli R, et al. . Mesenchymal stem cells transmigrate between and directly through tumor necrosis factor-alpha-activated endothelial cells via both leukocyte-like and novel mechanisms. Stem Cells. 2012;30:2472–2486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang JA, He A, Hu X, et al. . Anoxic preconditioning: a way to enhance the cardioprotection of mesenchymal stem cells. Int J Cardiol. 2009;133:410–412 [DOI] [PubMed] [Google Scholar]
- 122.Cho HJ, Lee HJ, Youn SW, et al. . Secondary sphere formation enhances the functionality of cardiac progenitor cells. Mol Ther. 2012;20:1750–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Amos PJ, Kapur SK, Stapor PC, et al. . Human adipose-derived stromal cells accelerate diabetic wound healing: impact of cell formulation and delivery. Tissue Eng Part A. 2010;16:1595–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang CC, Chen CH, Hwang SM, et al. . Spherically symmetric mesenchymal stromal cell bodies inherent with endogenous extracellular matrices for cellular cardiomyoplasty. Stem Cells. 2009;27:724–732 [DOI] [PubMed] [Google Scholar]
- 125.Campos LS, Leone DP, Relvas JB, et al. . Beta1 integrins activate a MAPK signalling pathway in neural stem cells that contributes to their maintenance. Development. 2004;131:3433–3444 [DOI] [PubMed] [Google Scholar]
- 126.Lai Y, Asthana A, Cheng K, et al. . Neural cell 3D microtissue formation is marked by cytokines' up-regulation. PLoS One. 2011;6:e26821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sart S, Liu Y, Ma T, et al. . Microenvironment regulation of pluripotent stem cell-derived neural progenitor aggregates by human mesenchymal stem cell secretome. Tissue Eng Part A. 2014; [Epub ahead of print]; DOI: 10.1089/ten.tea.2013.0437 [DOI] [PubMed] [Google Scholar]