Abstract
Several transcription factors and methods have been used to convert fibroblasts directly to neural fate and have provided insights into molecular mechanisms as to how each of these required factors orchestrate neural fate conversion. Here, we provide evidence and detailed characterization of the direct conversion process of primary adult human fibroblasts (hFib) to neural progenitor cells (NPC) using OCT4 alone. Factors previously associated with neural cell fate conversion were induced during hFib-NPCOCT-4 generation, where OCT-4 alone was sufficient to induce neural fate conversion without the use of promiscuous small-molecule manipulation. Human Fib-NPCOCT-4 proliferate, express neural stem/progenitor markers, and possess developmental potential that gives rise to all three major subtypes of neural cells: astrocytes, oligodendrocytes, and neurons with functional capacity. We propose a de-convoluted reprogramming approach for neural fate conversion in which OCT4 is sufficient for inducing neural conversion from hFib for disease modeling as well as the fundamental study of early neural fate induction.
Introduction
Direct conversion transcription factor reprogramming of somatic cells holds great potential for the generation of patient-specific disease models, and possibly cells for transplantation therapy. Although first reported in the 1980s [1], this reprogramming method has been the basis for a plethora of studies, all of which achieve alteration of cell fate in the absence of establishing pluripotency [2–4]. The catalyzing study which resulted in the revisiting of direct conversion/transdifferentiation methods demonstrated that the expression of neural lineage-associated transcription factors in mouse fibroblasts leads to the activation of endogenous neural genes [5]. Through a process of elimination, Brn-2, Myt1-l, and Ascl-1 were identified as a key set of factors that could initiate a change in cell fate from fibroblast to functional neuron (iN), in the presence of neural supportive culture conditions [5]. Soon after, with the addition of NEUROD1, this same combination of genes was found to induce direct conversion of human fibroblasts (hFib) to neurons. Generation of neural tissue for study or transplantation is of great value, as the physical and ethical barriers regarding biopsies of the brain are numerous. To date, the iN approach has been harnessed to produce multiple neuron subtypes by introduction of additional lineage-specific transcription factors, from both mouse and human starting cell populations of varied tissue origin [6–10]. In addition to directly converting somatic cells to postmitotic neurons, Kim et al. demonstrated the conversion of mouse fibroblasts to neuronal progenitor cells. This transformation was achieved through a brief expression of pluripotency transcription factors followed by the addition of neural supportive culture conditions [11]. Many groups have adapted this strategy by expressing the pluripotency factors alone, or in combination with neural lineage-specific factors to produce both mouse- and human-induced neural progenitor cells (iNPCs) [12–15]. iNPCs are capable of proliferating and differentiating to all three families of neural cells, including neurons, astrocytes, and oligodendrocytes. Despite the large number of studies demonstrating the direct conversion of somatic cells to NPCs, the reprogramming mechanism behind these cellular transformations remains to be completely understood. Here, we demonstrate the direct conversion of adult hFib to NPCs through the use of a single factor OCT4, and provide insights into the minimal essential mechanisms of reprogramming induction.
Materials and Methods
Cell culture
Dermal skin biopsies (5×5 mm) were obtained from the forearm of consenting donors in accordance with Research Ethics Board-approved protocols at McMaster University. Primary hFib cultures were established as described [16]. Human dermal adult fibroblasts (Sciencell and Donor derived) were initially maintained in fibroblast medium: DMEM (Gibco) supplemented with 10% v/v FBS (Neonatal Bovine Serum; HyClone), 1 mM l-glutamine (Gibco), and 1% v/v nonessential amino acids (NEAA; Gibco) before transduction with OCT4 lentivirus vector.
Cell viability
Viable cells were determined by Trypan Blue exclusion assay that was performed using a Countess automated cell counter (Life Technologies).
Lentivirus preparation and transduction
pSIN-EF1α-OCT4-Puro [or pHIV-EF1α-IRES-eGFP (Addgene 21373) subcloned with OCT4 sequence from Addgene 16579] was obtained (Addgene 16579) and co-transfected with pMD2.G (Addgene 12259) and psPAX2 (Addgene 12260) plasmids into the 293-FT cell line (Invitrogen) in order to initiate virus particle production. Viral supernatants were harvested at 48 h after transfection and ultracentrifuged to concentrate the virus. hFib were transduced in 50% Fibroblast medium: 50% reprogramming medium [F12 DMEM 20% Knockout Serum Replacement (Gibco), l-glutamine (Gibco), and 1% v/v nonessential amino acids (NEAA; Gibco), 0.1 mM beta-mercaptoethanol, 16 ng/mL basic fibroblast growth factor (bFGF), 30 ng/mL insulin growth factor 2 (IGF2), supplemented with 8 μg/mL polybrene (SIGMA)].
Reprogramming, neural progenitor and differentiation culture
Adult hFib were seeded on tissue culture-treated plates at ∼2×104 cells per well of a six-well standard plate. Fibroblasts were transduced in 50% fibroblast media: 50% reprogramming media containing 8 μg/mL polybrene with sufficient eGFP or OCT4 expressing lentivirus to achieve ∼20% transduction efficiency as measured by flow cytometry for eGFP or OCT4, respectively. hFib expressing eGFP and/or OCT4 were cultured for 8 days post transduction in reprogramming media before being trypsinzed and seeded as single cells in ultra-low attachment plates at a concentration of 1×105 cells per mL of progenitor culture media: F12:DMEM supplemented with 1×N2 and 1×B27 20 ng/mL bFGF, 20 ng/mL epidermal growth factor. Spheres were fed fresh media and growth factors after 7 days of culture. After 14 days, sphere clusters were dissociated using Accutase (Gibco) and seeded onto POLY-L-ORNATHINE/Mouse laminin-coated tissue culture plates in neural progenitor media for continued maintenance or in differentiation culture media consisting of F12 DMEM 1XN2 1XB27. For best differentiation, cells were cultured adherently in progenitor media until dense colony clusters appeared such as those depicted in Fig. 1K before passaging into differentiation conditions. For neuronal differentiation, media was supplemented with 5 nM forskolin. For Astrocyte differentiation, media was supplemented with 5% FBS. For Oligodendrocyte differentiation, media was supplemented with 100 ng/mL insulin growth factor 1 (IGF1), 200 nm Ascorbic Acid (Sigma), and 5 nM forskolin (Tocris). NSC from H9 were derived and cultured as previously described [17].
FIG. 1.
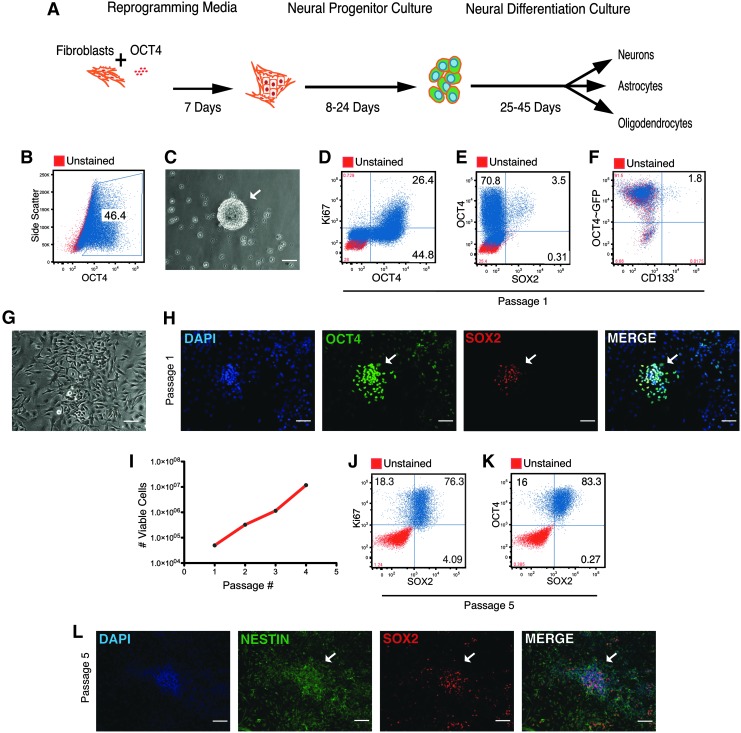
Expression of OCT4 in combination with neural progenitor culture induces conversion of fibroblasts to a proliferating neural progenitor-like cell (hFib-NPCOCT4). (A) Schematic of conversion strategy. (B) Flow cytometric plot of intracellular OCT4 expression in adult human fibroblasts after 8 days of culture in reprogramming media culture (hFibROCT4). (C) Phase-contrast image of hFibROCT4 neural sphere-like clusters after 14 days in neural progenitor culture. (D) Flow cytometric plot of OCT4 versus Ki67 expression in hFibROCT4 sphere-derived cells. (E) Flow cytometric plot of OCT4 versus SOX2 expression in hFibROCT4 sphere-derived cells. (F) Flow cytometric plot of eGFP expression from hFibROCT4 cells generated with pHIV-ef1α-OCT4-IRES-eGFP versus CD133 on live cells. (G) Phase-contrast image of hFibROCT4 sphere-derived cells cultured on poly-ornathine/laminin (POL). (H) Immunofluorescence results for OCT4 and SOX2 expression in hFibROCT4 sphere-derived cells cultured on POL. (I) Cell growth curve illustrating cumulative number of viable trypan blue excluding cells with increasing passage number. (J) Flow cytometric plot of SOX2 versus Ki67 expression in passage 5 POL cultured hFibROCT4 sphere-derived cells. (K) Flow cytometric plot of OCT4 versus SOX2 expression in passage 5 POL cultured hFibROCT4 sphere-derived cells. (L) Immunofluorescence results for NESTIN and SOX2 expression in passage 5 POL cultured hFibROCT4 sphere-derived cells. Representative results from n=4 rounds of hFib-NPCOCT4 conversion. Scale bar=100 μM. White arrows denote areas of interest.
Flow cytometry
Intracellular staining for OCT4 [BD OCT3/4-PE(1:100), OCT3/4-647(1:100)], SOX2 [BD SOX2-647(1:1,000)], Ki67 [BD Ki67-PE(1:400)], and Live Dead Discrimination Dye [BD LiveDeadViolet (1:7,500)] was performed on cells having undergone fixation and permeablization using the BD Fix/Perm kit. Briefly, cells were washed in phosphate-buffered saline (PBS) and stained for 30 min at 4°C in LiveDeadViolet. Cells were then washed in PBS and fixed for 20 min at 4°C in Fix solution. Cells were then washed in Perm solution, and left to block in Perm solution for 1 h at 4°C. Cells were stained overnight at 4°C, before washing in PBS and acquisition. Live cell visualization of eGFP expression was performed on cells treated with 7AAD [BD (1:50)] live dead discriminator. Acquisition was performed using LSRII (BD Biosciences), and analysis was performed using FlowJo 9.2 Software.
Immuncytochemistry
For surface marker staining, cells were stained directly in PBS 3% FBS or fixed using BD Fixation buffer for 40 min 4°C. Cells were washed in 3% FBS HBSS (Gibco). For intracellular staining, cells were fixed using BD Fixation/Permeabilization buffer for 40 min at 4°C. Cells were washed in BD 1×Perm solution. Neural lineage cells were identified by staining with monoclonal antibodies OCT4-PE (BD), SOX2-647 (BD), SOX2 anti-human (BD), Nestin anti-Human (R&D), Beta-III Tubulin/Tuj1 anti-Human (R&D), Microtubule-associated protein 2/MAP2 anti-Human (Abcam), Oligodendrocyte marker 4/O4 anti-Human (R&D), Glial fibrilliary acidic protein/GFAP anti-Human (SIGMA), and CD133-PE (Miltenyi). Antibodies were diluted in BD 1×wash buffer and incubated overnight at 4°C. Nonconjugated antibodies were visualized using appropriate Alexa-Flour secondary reagents (Life Technologies). Optimal working dilutions were determined for individual antibodies.
Teratoma assay
5×105 iPSCs or hFib-NPCOCT4 were injected intratesticularly into male NOD/SCID mice. Teratomas/testis were extracted at 10–12 weeks after the injection and analyzed as previously described [17]. Samples were stained with Hematoxylin-Eosin and OCT4, mounted using Permount, and imaged by scanning slides using Aperio Scan Scope. Tissue typing was performed based on stringent histological and morphological criteria that were specific for each germ-layer subtype. The presence of the germ layers and tissue typing was confirmed by the McMaster Pathology department.
Quantitative polymerase chain reaction
Total RNA was isolated using the Qiagen mini RNA isolation kit. RNA was then subjected to cDNA synthesis using superscript III (Invitrogen). Quantitative PCR (qPCR) was performed using Platinum SYBR Green–UDP mix (Invitrogen). Threshold was set to the detection of Gus-B (β-glucuronidase)46 and then normalized to GAPDH. Primer Sequences: ASCL1: 5′ caagagagcgcagccttag, 5′ gcaaaagtcag tgctgaacg BRN-2: 5′ aataaggcaaaaggaaagcaact, 5′ caaaac acatcattacacctgct MYT1L: 5′ caatggaaagggattttaagca, 5′ tttg agattatgtaccaacgttagatg NEUROD1: 5′ gttattgtgttgccttagc acttc, 5′ agtgaaatgaattgctcaaattgt MSI2: 5′ ggcagcaagagga tcagg. 5′ccgtagagatcggcgaca NCAN: 5′ gccttgggccttttgatgc, 5′ ccttggtccactttatccgagg COL1A1: 5′ gagggccaagacgaagac atc, 5′ cagatcacgtcatcgcacaac DKK3: 5′ aggacacgcagcaca aattg, 5′ ccagtctggttgttggttatctt SNAI1: 5′ tcggaagcctaactaca gcga, 5′ agatgagcattggcagcgag.
Cytosolic calcium imaging
Measurement of cytosolic calcium was performed by monitoring Fluo-4 fluorescence of cells, adhered to plastic 35 mm dishes or six-well plastic plates using an Olympus IX81 inverted epi-fluorescence microscope (Olympus) that was coupled to a xenon arc lamp (EXFO). Cells were washed and incubated in Hanks' balanced salt solution HBSS, supplemented with 25 mM HEPES buffer, 5.5 mM Glucose. Indicated agonists, diluted in the aforementioned solution, were washed over the cells at the indicated timepoints, using a custom applicator and an aspirator system. Fluo-4 was loaded into the cells by incubation with 1 μM Fluo-4 (Invitrogen) acetoxymethyl ester (45 min incubation followed by a 45 min period for de-esterification). Fluorescent images were collected using an intensified charge-coupled device video camera (Photometrics) every 2 s through a GFP filter cube (Semrock). Off-line analysis of the intensity pattern of Fluo-4 signal was performed in ImageJ (NIH).
Action potential/patch clamping
To determine whether cells could fire action potentials, the cells were transferred on a coverslip to an upright microscope and were continuously perfused with artificial cerebrospinal fluid (ACSF, pH 7.2) containing (in mM) 125 NaCl, 1 MgSO4, 5 KCl, 1.25 KH2PO4, 10 dextrose, 26 NaHCO3, and 2 CaCl2•2H2O. ACSF was heated to ∼33°C and superfused with 95% O2/5% CO2. Electrodes for cell-attached and whole-cell voltage clamp recordings had resistances of 2–4 MΩ and were filled with a K-gluconate solution (pH 7.3) containing (in mM) 100 K-gluconate, 20 KCl, 10 Na2-phosphocreatine, 10 HEPES, 0.3 GTP-Na, and 4 ATP-Mg•3.5H20. Recordings (MultiClamp 700B or Axopatch 200B amplifier; Molecular Devices) were sampled at 5 or 10 kHz, filtered at 2 or 5 kHz, and saved for offline analysis with Origin or custom Matlab software. Cell-attached and whole-cell recording configuration were achieved in voltage-clamp mode, and recordings were subsequently made in current-clamp mode. Whole-cell recordings were compensated by a minimum of 80% with <10 μs lag, and were discarded if series resistance changed by more than 15% from its initial value. In the attempt to initiate action potentials, current injections were made in six steps (1–6 nA) via the patch electrode. To ensure that voltage-sensitive sodium channels were not in a chronic inactivated state, the recording configuration was periodically turned back to voltage-clamp mode and the cell was held at a potential of −60 mV or less in order to de-inactivate sodium channels. If reliable action potential-like firing was observed in a cell, the broad-spectrum antagonist zonisamide (10 μM; Ascent) or the fast acting sodium channel tetrodotoxin (1 μM) was added to the perfusate.
Results
OCT4 direct conversion reprogramming facilitates generation of a proliferative neural progenitor phenotype from fibroblasts
In an effort to assess the functional conversion capacity of OCT4 expressing cells, we devised a culture strategy incorporating elements from our previous direct conversion approach using OCT4, and the derivation of neural progenitors (NPCs) from pluripotent stem cells (Fig. 1A) [18,19]. Adult hFib transduced with lentivirus expressing OCT4 were cultured in reprogramming media (R) (hFibROCT4) for 1 week to enable sufficient expansion of transduced cells (Fig. 1B). hFibROCT4 were then trypsinized to form a single-cell suspension and seeded in neural progenitor culture media. Single-cell suspensions of hFibROCT4 maintained in neural progenitor culture formed neural sphere-like clusters similar to those described from pluripotent cells (Fig. 1C) [19]. To address whether these spheres were a result of proliferation rather than coalescence of cells, we dissociated the spheres and performed cell-cycle analysis using Ki67, a marker of all stages of the active cell cycle. Ki67 expression was evident in a defined subset of sphere-derived cells, of which nearly all cells co-expressed OCT4 (Fig. 1D). Since proliferation is a property of NPCs (Supplementary Fig. S1A; Supplementary Data are available online at www.liebertpub.com/scd), we asked whether hFibROCT4 sphere-derived cells also expressed key neural progenitor regulators. Given that recent reports suggest SOX2, a key regulator of NPCs (Supplementary Fig. S1A), is sufficient to induce direct conversion of mouse and human fetal fibroblasts to neural progenitors [20], we analyzed adult-derived hFibROCT4 cells for SOX2 expression. A small but distinct population of cells expressed SOX2, of which nearly all co-expressed OCT4, indicating that OCT4 expression in conjunction with neural progenitor culture can induce hallmarks of a neural progenitor phenotype (Fig. 1E). Further analysis of hFibROCT4 for the expression of additional neural progenitor markers revealed a subset of OCT4-positive cells that co-expressed the cell surface marker CD133 (Fig. 1F). In addition to these protein-level analyses, hFibROCT4 also displayed up-regulation of MSI2 and NCAN transcript compared with hFibs (Supplementary Fig. S1C). Importantly, hFibROCT4 down-regulated key fibroblast genes COL1A1, DKK3, and SNAI1 in response to the reprogramming process (Supplementary Fig. S1D). Taken together, these results demonstrate that hFibROCT4 sphere-derived cells have initiated a molecular shift from fibroblastic programs toward that which reflects well-characterized primary human NPCs [21].
To further characterize these emerging neural-like cells from hFibROCT4 cells, we cultured them on the neural substrate poly-ornathine/laminin (POL). hFibROCT4 sphere-derived cells cultured on POL displayed a similar morphology to NPCs derived from pluripotent cells (Fig. 1F and Supplementary Fig. S1B) [22]. In agreement with flow cytometric analysis, immunofluorescence for OCT4 and SOX2 revealed co-expressing populations of cells organized in colony-like regions consistent with an emerging progenitor phenotype (Fig. 1G). Continued passaging on laminin resulted in linear growth on an exponential scale, indicating that the proliferating population of cells had become abundant (Fig. 1H). Consistent with this observation as well as with expansion of the neural progenitor phenotype, nearly all of the cells in culture expressed the proliferation marker Ki67 in combination with SOX2 (Fig. 1I). Moreover, the vast majority of cells co-expressed OCT4 and SOX2, a clear indication that passaging of hFibROCT4 on laminin had enriched for this phenotype (Fig. 1J). Despite being highly proliferative and expressing the pluripotency-associated transcription factors OCT4 and SOX2, hFibROCT4 cells failed to produce teratomas (or tumors of any kind) when injected into immune-deficient mice at cell concentrations well above the tumor initiating requirement of iPSC (Supplementary Fig. S2A, B). Having verified the absence of pluripotency, we assessed whether the expansion of this phenotype occurred alongside up-regulation of additional neural stem/progenitor marks. Accordingly, we performed immunofluorescence for SOX2 in combination with the neural filament NESTIN, a marker of primitive neural stem/progenitor cells. This analysis revealed co-expression of both SOX2 and NESTIN within hFibROCT4 cells (Fig. 1K). Cumulatively, these results demonstrate that culturing of hFibROCT4 cells in neural progenitor culture is sufficient to confer both growth and phenotypic properties of neural stem/progenitor cells. As such, we termed these cells hFib-NPCOCT4.
To determine whether OCT4 expression was required to induce direct conversion of adult hFib to NPCs, we cultured eGFP expressing fibroblasts using the strategy highlighted in Fig. 1A. Similar to hFibROCT4, hFibReGFP were propagated in reprogramming media to expand eGFP expressing cells (Supplementary Fig. S3A). hFibReGFP were then seeded as single cells in progenitor culture media and analyzed for sphere formation. Surprisingly, hFibReGFP in these conditions formed sphere-like clusters similar in morphology to hFibROCT4 (Supplementary Fig. S3B). However, cell-cycle analysis of the resulting spheres revealed a comparatively small number of Ki67-positive cells to that of hFibROCT4 spheres (Supplementary Fig. S3C). In addition to the lack of proliferation, hFibReGFP sphere-derived cells failed to up-regulate the neural stem/progenitor transcription factor SOX2 (Supplementary Fig. S3D), and they displayed fibroblastic morphology after culturing on laminin (Supplementary Fig. S3E). Together, these results indicate that culturing of adult hFib in neural progenitor media is insufficient to sustain growth and phenotypic characteristics of induced human neural progenitors.
OCT4-NPC differentiate to all three neural subfamilies, including iNs
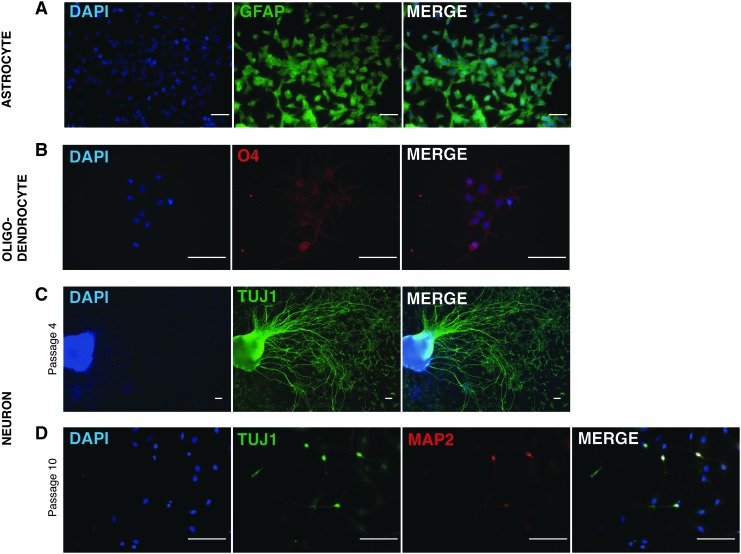
Although neural progenitor phenotype is defined by the expression of specific transcription factors and structural proteins, progenitor function is assessed in part by the ability to differentiate to the three major families of neural cells: neurons, astrocytes, and oligodendrocytes. Differentiation of neural stem/progenitors can be achieved by withdrawal of factors shown to support proliferation and addition of those that support the generation of specific lineages of neural cells (Supplementary Fig. S4) [22]. Accordingly, the culturing of hFib-NPCOCT4 in differentiation conditions shown to support the generation of glial cells produced glial fibrilliary acidic protein expressing cells resembling astrocytes (Fig. 2A) and O4 expressing cells resembling oligodendrocytes (Fig. 2B). Moreover, culturing of hFib-NPCOCT4 in neuronal differentiation media was able to induce the generation of a neuronal phenotype, marked by expression of the neuron-specific class III beta-tubulin protein or TUJ1 (Fig. 2C). Further exposure of hFib-NPCOCT4-derived neurons to differentiation media resulted in the formation of a subset of TUJ1-positive neurons that up-regulated the mature neuronal marker MAP2 (Fig. 2D). Importantly, cells which expressed high levels of TUJ1 also demonstrated little to no expression of OCT4, suggesting that differentiation toward mature functional cell types occurs concomitantly with silencing of OCT4 expression (Supplementary Fig. S5A, B).
FIG. 2.
hFib-NPCOCT4 display tri-potent differentiation potential. (A–D) Immunofluorescence images of neural lineage protein expression (A), GFAP expressing astrocytes. (B) O4 expressing oligodendrocytes. (C) TUJ1 expressing neurons. (D) TUJ1 MAP2 co-expression in a subset of neurons. Representative images from n=4 rounds of hFib-NPCOCT4 conversion. Scale bar=100 μM.
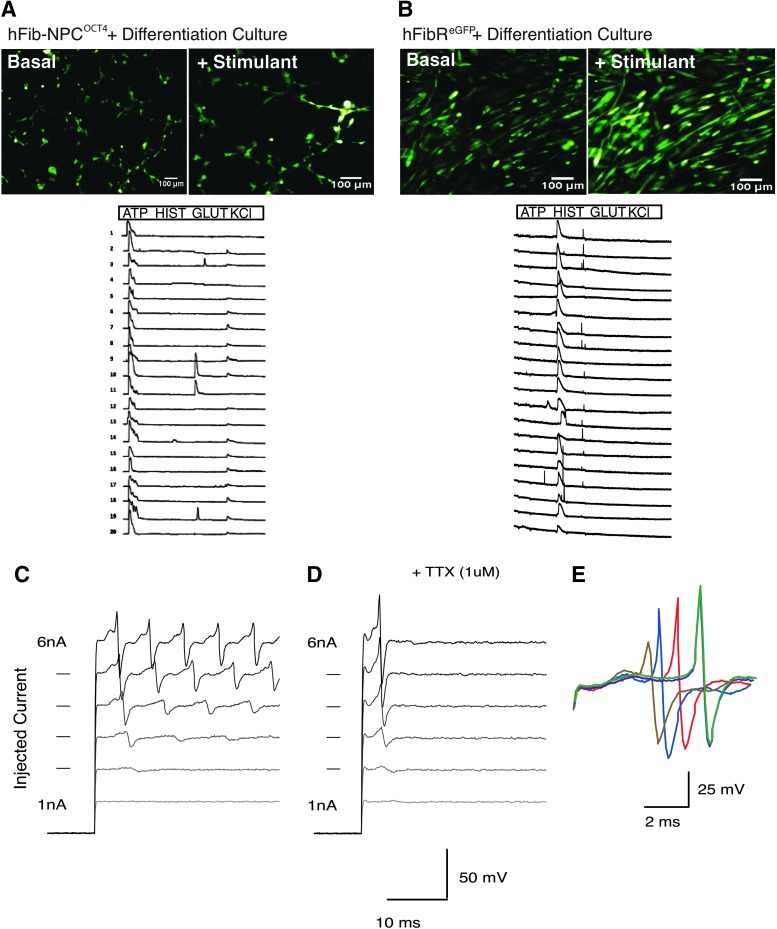
To assess the functional capacity of hFib-NPCOCT4-derived mature neurons, we evaluated their response to the neurotransmitters ATP and glutamate via calcium transient formation using a FLUO-4 fluorescence assay. Nearly all hFib-NPCOCT4-derived neurons produced calcium transients in response to ATP, while a distinct subset responded to glutamate. Importantly, hFib-NPCOCT4 neurons did not respond to histamine, a known inducer of calcium release in fibroblasts (Fig. 3A, B). Furthermore, hFib-NPCOCT4-derived neurons fired repetitive action potentials in response to a current injection, and exhibited sensitivity to a sodium channel inhibitor (Fig. 3C–E). These collective results suggest that hFib-NPCOCT4 possess all of the defining features of tri-potent NPCs, confirming successful cell fate transition through the expression of OCT4.
FIG. 3.
Differentiated hFib-NPCOCT4-derived neurons demonstrate functional response to neurotransmitters and current injection. (A) hFib-NPCOCT4 differentiated cells treated with calcium reactive compound FLUO-4 and various calcium release stimuli. Representative fluorescent microscopy images of FLUO-4 dye reacting with calcium after administration with neurotransmitter glutamate. Fluorescent intensity cell trace diagrams of individual cells and cell–cell junctions during treatment with intracellular calcium release stimulants. (B) Fibroblasts cultured in neural progenitor and neural differentiation medium treated with FLUO-4 and assayed for calcium release using stimulants. Representative fluorescent microscopy images of FLUO-4 dye reacting with calcium after administration with histamine. Fluorescent intensity cell trace diagrams of individual cells and cell–cell junctions during treatment with intracellular calcium release stimulants. (C) Action potential measurements of hFib-NPCOCT4-derived neurons. (D) Action potential measurement during administration of fast acting sodium channel inhibitor tetrodotoxin (TTX). (E) Averaged traces of evoked repetitive action potentials in four separate cells.
OCT4 expression activates a hierarchy of neural conversion transcription factors during reprogramming of adult hFib to NPC
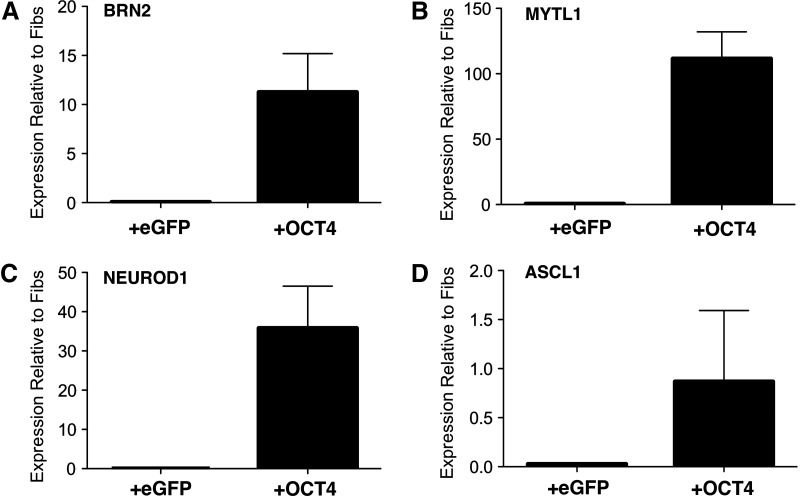
Having confirmed that hFib-NPCOCT4 possess both phenotypic and developmental characteristics of NPCs, we next aimed at better understanding the molecular events underlying the reprogramming process. Given that hFib-NPCOCT4 express SOX2, a neural fate conversion factor, we investigated whether other genes shown to induce neural fate were similarly activated during the reprogramming process. We performed qRT-PCR on Fibs, hFibReGFP, and hFibROCT4 sphere-derived cells to measure the expression of genes shown to induce the neural fate from fibroblasts: BRN-2, ASCL1, MYT1L, and NEUROD1 (Fig. 4A–D). With the exception of ASCL1, hFibROCT4 progenitor culture demonstrated elevated expression of neural conversion genes compared with naïve fibroblasts and hFibReGFP progenitor culture. Based on these results and the induced expression of SOX2, we hypothesized that the combination of OCT4 and neural progenitor culture may regulate a hierarchy of established transcriptional networks for converting fibroblasts to the neural lineage. Considering recent reports, we postulated that SOX2 should act downstream of OCT4, and, therefore, should be able to convert adult hFib to neural progenitors in the absence of OCT4. To test this hypothesis, we transduced adult hFib with lentivirus expressing SOX2 and cultured them according to the strategy in Fig. 1A. hFibRSOX2 were cultured in reprogramming media to enable expansion of SOX2 expressing cells (Fig. 5A). hFibRSOX2 cells were seeded as single cells in neural progenitor culture media and analyzed for sphere formation. After 2 weeks in neural progenitor culture, hFibRSOX2 single cells had given rise to neural sphere-like clusters similar to hFibROCT4 (Fig. 5B). Although forced expression of OCT4 was able to up-regulate expression of SOX2 (Fig. 1E), forced expression of SOX2 did not up-regulate OCT4 (Fig. 5C). Similar to hFibReGFP, hFibRSOX2 sphere-like clusters contained low percentages of Ki67-positive proliferating cells (Fig. 5D). Moreover, propagation of hFibRSOX2 sphere-derived cells on the neural substrate laminin resulted in the generation of cells with fibroblastic morphologies (Fig. 5E), and a sharp decline in cell viability (Fig. 5F). Despite the failure of hFibRSOX2 to propagate in neural supportive culture conditions, qRT-PCR analysis displayed a similar pattern to hFibROCT4 regarding the up-regulation of BRN-2, MYT1L, and NEUROD1 (Fig. 5G–J). These results suggest that SOX2 expression is activated in response to OCT4 expression, and that SOX2 expression is sufficient to activate neural fate conversion factors BRN-2, MYT1L, ASCL1, and NEUROD1. However, forced expression of SOX2 alone is not sufficient to induce neural progenitor fate from adult hFib, suggesting a unique additional role for OCT4 during the reprogramming process.
FIG. 4.
hFib-NPCOCT4 activate neural conversion transcription factors. (A–D) qRT-PCR results for expression of neural conversion transcription factors in hFibReGFP cultured in neural progenitor culture, and hFibROCT4 cultured in neural progenitor culture. Expression levels are normalized to expression in hFibs.
FIG. 5.
Expression of SOX2 in combination with neural progenitor culture fails to induce conversion of adult fibroblasts to neural progenitors, despite successfully activating downstream neural conversion transcription factors. (A) Flow cytometric plot of intracellular SOX2 expression in adult human fibroblasts after 8 days in reprogramming media (hFibRSOX2). (B) Phase-contrast image of hFibRSOX2 sphere-like clusters after 14 days in neural progenitor culture. (C) Flow cytometric plot of SOX2 versus OCT4 expression in hFibRSOX2 sphere-derived cells. (D) Flow cytometric plot of SOX2 versus Ki67 expression in hFibRSOX2 sphere-derived cells. (E) Phase-contrast image of hFibRSOX2 sphere-derived cells cultured on POL. (F) Cell growth curve illustrating cumulative number of viable trypan blue excluding cells with increasing passage number. (G–J) qRT-PCR results for expression of neural conversion transcription factors in hFibeGFP cultured in neural progenitor culture, and hFibRSOX2 cultured in neural progenitor culture. Expression levels are normalized to expression in hFibs (n=2). Representative results from n=3 attempts of hFibRSOX2 conversion. White arrows indicate areas of interest.
Reprogramming technologies are seen to hold great potential for the construction of patient-specific disease models and cells for drug screening. However, in order to ensure applicability of a given reprogramming technology to patient-specific endeavors, freshly derived cell cultures from donors should prove to be a suitable starting material for successful cell fate transition. To ensure that hFib-NPCOCT4 reprogramming was applicable to donor-derived fibroblasts, we generated hFib-NPCOCT4 cells from a freshly isolated skin biopsy [hFib(2)]. hFibR(2)OCT4 formed spheres in neural progenitor culture media, and on dissociation were able to propagate on the neural substrate laminin (Supplementary Fig. S6A). Laminin-adapted hFibR(2) sphere-derived cells displayed a similar morphology to hFibROCT4 sphere-derived cells and NPCs derived from ESCs (Fig. 1G and Supplementary Fig. S1B). Importantly, hFibR(2)OCT4 sphere-derived cells activated established neural conversion factors in an OCT4-dependent manner, suggesting that they had established similar neural conversion regulatory networks as hFib-NPCOCT4 (Supplementary Fig. S6B). Furthermore, when cultured in neural differentiation conditions, hFibR(2)OCT4 cells successfully differentiated to all three major subfamilies of neural cells, including astrocytes, oligodendrocytes, and neurons (Supplementary Fig. S6C). Taken together, these results confirm that hFib-NPCOCT4 reprogramming is applicable to multiple lines of adult fibroblasts, illustrating the robust nature of OCT4 to alter cell fate.
Discussion
Derivation of hFib-NPCOCT4 is simple, rapid, and produces a proliferative, nontumorgenic population of progenitor cells that are capable of differentiating to all three major neural subtypes: neurons, astrocytes, and oligodendrocytes. Importantly, hFib-NPCOCT4-derived neurons functionally respond to both current injection and neurotransmitters, positioning hFib-NPCOCT4 as an appealing reprogramming tool for the generation of neuronal-based disease models in a manner that is not convoluted by the use of other factors associated with stages of neural development [23] or promiscuous chemical manipulation in vitro [24]. This reprogramming technology is sufficiently robust and enables the use of donor-derived adult fibroblasts as a starting material for cell fate conversion, positioning it as a candidate for the construction of relevant patient-specific disease models for use in drug screening.
Our study represents the only example where a single exogenous factor, OCT4, has directly reprogrammed adult human somatic cells to tri-potent neural progenitors without other manipulations. Although co-expression of SOX2 hallmarks the emergence of hFib-NPCOCT4, expression of SOX2 alone in adult hFib using our culture system does not result in successful reprogramming to neural progenitors. However, forced expression of SOX2 in the presence of a feeder support layer has been shown to induce direct conversion of human fetal fibroblasts to tri-potent NPCs [20]. Therefore, it is likely that the required (yet undefined) regulation bestowed by the feeder layer on cells undergoing SOX2 neural cell fate conversion is intrinsic to cells undergoing OCT4-induced neural cell fate conversion. The up-regulation of key neural conversion factors during reprogramming of fibroblasts to hFib-NPCOCT4 in this study is not unlike the up-regulation of key hematopoietic transcription factors reported during the conversion of fibroblasts to multipotent blood progenitors using OCT-4 alone [18]. Together, these findings highlight the acquisition of crucial fate-inducing transcriptional programs as a result of forced OCT-4 expression in hFib that may also enable other cell fates to be induced. These and other lineage-based studies are currently ongoing in our lab.
Supplementary Material
Acknowledgments
The authors would like to thank Tony Collins and Luca Orlando for a critical review of this article as well as Nick Holzapfel and Kristin Hope for MSI2 primer sequences. R.R.M is funded by CIHR (Alexander Graham Bell Graduate Scholarship). E.S. is funded by MITACS during her involvement with these studies. Y.D.B is funded by CIHR. J.A. is funded by CONACyT fellowship. D.C.G. is funded by CIHR and Ontario Early Researcher Award. M.B. is funded by CIHR operating grants and Boris Foundation. Portions of this material were presented at ISSCR Roddenberry International Symposium on Cellular Reprogramming October 2012.
Author Disclosure Statement
The authors declare that there are no conflicts of interest.
References
- 1.Weintraub H, Tapscott SJ, Davis RL, Thayer MJ, Adam MA, Lassar AB. and Miller AD. (1989). Activation of muscle-specific genes in pigment, nerve, fat, liver, and fibroblast cell lines by forced expression of MyoD. Proc Natl Acad Sci U S A 86:5434–5438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sancho-Martinez I, Baek SH. and Izpisua Belmonte JC. (2012). Lineage conversion methodologies meet the reprogramming toolbox. Nat Cell Biol 14:892–899 [DOI] [PubMed] [Google Scholar]
- 3.Vierbuchen T. and Wernig M. (2012). Molecular roadblocks for cellular reprogramming. Mol Cell 47:827–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orkin SH. and Hochedlinger K. (2011). Chromatin connections to pluripotency and cellular reprogramming. Cell 145:835–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC. and Wernig M. (2010). Direct conversion of fibroblasts to functional neurons by defined factors. Nature 463:1035–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qiang L, Fujita R, Yamashita T, Angulo S, Rhinn H, Rhee D, Doege C, Chau L, Aubry L, et al. (2011). Directed conversion of Alzheimer's disease patient skin fibroblasts into functional neurons. Cell 146:359–371 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Pfisterer U, Kirkeby A, Torper O, Wood J, Nelander J, Dufour A, Bjorklund A, Lindvall O, Jakobsson J. and Parmar M. (2011). Direct conversion of human fibroblasts to dopaminergic neurons. Proc Natl Acad Sci U S A 108:10343–10348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Son EY, Ichida JK, Wainger BJ, Toma JS, Rafuse VF, Woolf CJ. and Eggan K. (2011). Conversion of mouse and human fibroblasts into functional spinal motor neurons. Cell Stem Cell 9:205–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marro S, Pang ZP, Yang N, Tsai MC, Qu K, Chang HY, Sudhof TC. and Wernig M. (2011). Direct lineage conversion of terminally differentiated hepatocytes to functional neurons. Cell Stem Cell 9:374–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caiazzo M, Dell'Anno MT, Dvoretskova E, Lazarevic D, Taverna S, Leo D, Sotnikova TD, Menegon A, Roncaglia P, et al. (2011). Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature 476:224–227 [DOI] [PubMed] [Google Scholar]
- 11.Kim J, Efe JA, Zhu S, Talantova M, Yuan X, Wang S, Lipton SA, Zhang K. and Ding S. (2011). Direct reprogramming of mouse fibroblasts to neural progenitors. Proc Natl Acad Sci U S A 108:7838–7843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thier M, Worsdorfer P, Lakes YB, Gorris R, Herms S, Opitz T, Seiferling D, Quandel T, Hoffmann P, et al. (2012). Direct conversion of fibroblasts into stably expandable neural stem cells. Cell Stem Cell 10:473–479 [DOI] [PubMed] [Google Scholar]
- 13.Sheng C, Zheng Q, Wu J, Xu Z, Wang L, Li W, Zhang H, Zhao XY, Liu L, et al. (2012). Direct reprogramming of Sertoli cells into multipotent neural stem cells by defined factors. Cell Res 22:208–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lujan E, Chanda S, Ahlenius H, Sudhof TC. and Wernig M. (2012). Direct conversion of mouse fibroblasts to self-renewing, tripotent neural precursor cells. Proc Natl Acad Sci U S A 109:2527–2532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han DW, Tapia N, Hermann A, Hemmer K, Hoing S, Arauzo-Bravo MJ, Zaehres H, Wu G, Frank S, et al. (2012). Direct reprogramming of fibroblasts into neural stem cells by defined factors. Cell Stem Cell 10:465–472 [DOI] [PubMed] [Google Scholar]
- 16.Villegas J. and McPhaul M. (2005). Establishment and culture of human skin fibroblasts. Curr Protoc Mol Biol Chapter 28:Unit 283. [DOI] [PubMed] [Google Scholar]
- 17.Werbowetski-Ogilvie TE, Bosse M, Stewart M, Schnerch A, Ramos-Mejia V, Rouleau A, Wynder T, Smith MJ, Dingwall S, et al. (2009). Characterization of human embryonic stem cells with features of neoplastic progression. Nat Biotechnol 27:91–97 [DOI] [PubMed] [Google Scholar]
- 18.Szabo E, Rampalli S, Risueno RM, Schnerch A, Mitchell R, Fiebig-Comyn A, Levadoux-Martin M. and Bhatia M. (2010). Direct conversion of human fibroblasts to multilineage blood progenitors. Nature 468:521–526 [DOI] [PubMed] [Google Scholar]
- 19.Carpenter MK, Inokuma MS, Denham J, Mujtaba T, Chiu CP. and Rao MS. (2001). Enrichment of neurons and neural precursors from human embryonic stem cells. Exp Neurol 172:383–397 [DOI] [PubMed] [Google Scholar]
- 20.Ring KL, Tong LM, Balestra ME, Javier R, Andrews-Zwilling Y, Li G, Walker D, Zhang WR, Kreitzer AC. and Huang Y. (2012). Direct reprogramming of mouse and human fibroblasts into multipotent neural stem cells with a single factor. Cell Stem Cell 11:100–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwartz PH, Bryant PJ, Fuja TJ, Su H, O'Dowd DK. and Klassen H. (2003). Isolation and characterization of neural progenitor cells from post-mortem human cortex. J Neurosci Res 74:838–851 [DOI] [PubMed] [Google Scholar]
- 22.Werbowetski-Ogilvie TE, Morrison LC, Fiebig-Comyn A. and Bhatia M. (2012). In vivo generation of neural tumors from neoplastic pluripotent stem cells models early human pediatric brain tumor formation. Stem Cells 30:392–404 [DOI] [PubMed] [Google Scholar]
- 23.Wapinski OL, Vierbuchen T, Qu K, Lee QY, Chanda S, Fuentes DR, Giresi PG, Ng YH, Marro S, et al. (2013). Hierarchical mechanisms for direct reprogramming of fibroblasts to neurons. Cell 155:621–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu S, Ambasudhan R, Sun W, Kim HJ, Talantova M, Wang X, Zhang M, Zhang Y, Laurent T, et al. (2014). Small molecules enable OCT4-mediated direct reprogramming into expandable human neural stem cells. Cell Res 24:126–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.