INTRODUCTION
As an increasing proportion of HIV-infected patients have access to antiretroviral therapy (ART) and achieve virologic suppression, the focus of clinical care is shifting from treating the infectious complications of advanced immunodeficiency to managing and preventing chronic diseases. The increased impact of non-communicable diseases (NCDs) is due to both the aging of the HIV population as a whole and to increased rates of chronic disease complications in the setting of HIV infection. Of these non-infectious complications, coronary heart disease (CHD) is of considerable import. In 2010, ischemic heart disease was the number one cause of disability-adjusted life years (DALYs) globally and the number one cause of deaths, years of life lost, and DALYs in the United States.1 Extensive data over the past decade indicate that HIV infection confers an increased risk of CHD, with greater-than-expected morbidity and mortality from a disease that is already widespread.2 Moreover, risk factors for HIV-associated CHD are thought to differ from those of the general population, with risk mediated by HIV-specific factors including chronic inflammation and immune activation. Therapeutic interventions tailored to traditional CHD risk factors and proven to benefit the general population may therefore not be appropriate in the setting of HIV infection.
In recent years, our understanding of the epidemiology of coronary heart disease in HIV has evolved, reflecting clinical progress both in HIV medicine and in preventative cardiology. Recent studies feature improved characterization of demographic and clinical risk factors which modulate risk for HIV populations. This review will describe the current state of epidemiologic knowledge on coronary heart disease in HIV infection. It will highlight key studies in the field and summarize epidemiologic data with respect to: 1) traditional and novel CHD risk factors; 2) specialized clinical subgroups; and 3) broader cardiovascular outcomes. The review will not focus on mechanistic data or on clinical management, as other recent reviews and articles in this issue summarize these topics.3 Understanding the epidemiology of HIV-associated coronary heart disease has implications for the long-term care of both HIV-infected patients and of other at-risk populations with novel CHD risk factors.
CHD RISK IN HIV INFECTION
HIV confers an increased risk of coronary heart disease across diverse geographic and clinical settings. Large epidemiologic studies spanning the past decade have investigated CHD or myocardial infarction (MI) rates in HIV cohorts compared to appropriate controls and demonstrated consistently increased rates in the HIV groups, with magnitude of risk approximately doubled in the setting of HIV (Table 1).
Table 1. Summary of epidemiologic studies on HIV and CHD.
| Study | Year | N (HIV) | Control group | Female (%) |
Age HIV | Time period |
HIV f/u (yrs) |
Primary Result | Effect Size |
|---|---|---|---|---|---|---|---|---|---|
| Kaiser5 | 2002 | 4159 | Random sample Kaiser members |
0 | Mean 44 | 1996- 2001 |
4.1 | ↑ MI and CHD in HIV vs. controls |
1.5 (MI) 1.7 (CHD) |
| CA Medicaid6 | 2003 | 28513 | Patients enrolled in CA Medicaid > 1 year |
27 | 46% M and 35% F age 35-44 |
1994- 2000 |
2.5 | ↑ CHD in HIV (age 18-33) vs. controls |
2.06 |
| FHDH7 | 2003 | 34976 | Estimated rates from French male general population |
0 | Mean 38 | 1996- 1999 |
2.8 | ↑ MI in HIV with PI exposure >18 months vs. general population |
1.5 (PI 18-29 months) 2.9 (PI ≥ 30 months) |
| Partners HIV cohort8 |
2007 | 3851 | Controls from health care system-based data registry |
30 | Median 38 |
1996- 2004 |
4.5 | ↑ incident MI in HIV vs. controls |
1.75 |
| Danish HIV cohort9 |
2007 | 3953 | Population-based control group matched 95:1 on sex, age, residence |
23 | Median 37 |
1995- 2004 |
5.2 | ↑ first hospitalization for CHD in HIV on ART vs. controls |
2.12 |
| FHDH10 | 2010 | 74958 | Three population- based French registries |
11 | Median 47 |
2000- 2006 |
-- | ↑ MI in HIV vs. 3 population registries |
1.5 |
| Quebec RAMQ database11 |
2011 | 7053 | Control group on sex, age, entry date, duration insurance |
22 | Median 37 |
1985- 2007 |
4.2 | ↑ MI in HIV vs. 4:1 matched controls |
2.11 |
| VACS cohort13 | 2013 | 27350 | Controls from VACS matched on age, race, site, calendar year |
3 | Median 49 |
2003- 2009 |
5.9 | ↑ MI in HIV vs. 2:1 matched control |
1.48 |
| Kaiser14 | 2013 | 22081 | HIV-uninfected Kaiser members |
9 | 40% age 35-44 |
1996- 2009 |
4.5 | ↑ MI in HIV vs. controls |
1.4 |
Following early case reports of cardiovascular disease in HIV-infected patients and data on protease inhibitor (PI)-induced dyslipidemia,4 several population-based studies investigated associations between HIV, ART and CHD in the early 2000s. In an ongoing study of electronic health record (EHR) data from Kaiser Permanente in Northern California that was recently updated, Klein et al. was one of the first groups to demonstrate significantly higher CHD (6.5 vs. 3.8, p=.003) and MI (4.3 vs. 2.9, p = .07) hospitalization rates comparing HIV-infected men to control patients in the closed health care system.5 These data were soon corroborated by Currier et al., who showed CHD incidence to be significantly increased in an HIV group versus a population-based control group in a study of California Medicaid administrative claims data from more than 3 million individuals.6 The finding of increased risk in HIV was present for both male (RR 6.76; 95% CI, 3.36–13.58 for age 18-24; RR 2.16; 95% CI, 1.81–2.58 for age 25-34) and female (RR 2.47; 95% CI, 1.23–4.95 for age 18-34; RR 1.53; 95% CI, 1.10–2.13 for age 35-34; RR 1.67; 95% CI, 1.41–1.97 for age 35-44) patients although did not persist above age 34 for males or above age 44 for females. Further data from the French Hospital Database on HIV (FHDH) showed validated MI incidence rates to be increased in a large cohort of HIV-infected men exposed to PI therapy for at least 18 months, comparing rates to those calculated for the French general male population (age-adjusted standardized morbidity ratio 0.8, 95% CI, 0.5–1.3 for PI exposure <18 months; 1.5, 95% CI, 0.8–2.5 for PI exposure 18–29 months; 2.9, 95% CI, 1.5–5.0 for PI exposure >30 months).7
Further studies reinforced these early findings, accounting for additional possible confounding factors. A study from the Partners HealthCare System in Boston showed MI incidence rates to be increased in HIV-infected patients versus more than 1 million patients comprising the health care system-based control group. The relative risk for MI was 1.75 (95% CI 1.51-2.02) in a model adjusted for demographics and common CHD risk factors.8 A Danish study compared rates of first hospitalization for ischemic heart disease in HIV-infected patients versus a population-based control group and found patients with HIV to be at significantly increased risk (adjusted RR 2.12, 95% CI 1.62-2.76).9 In updated data from the FHDH cohort, MI incidence was increased for the HIV group compared to sex- and age-standardized rates from the general French population, with a standardized morbidity ratio of 1.5 (95% CI 1.3-1.7).10 Similar results were demonstrated in a Quebec HIV cohort in which MI incidence was elevated for the HIV group compared to a matched control group, with an adjusted incidence ratio of 2.11 (95% CI 1.69-2.63).11 Taken together, these earlier data from multiple discrete cohorts suggested the presence of increased CHD risk in the setting of HIV that is independent of demographics or traditional CHD risk factors.
Recent studies linking HIV infection to coronary heart disease have further expanded on prior investigations, refining strategies to confirm outcome measures and accounting for additional possible confounding factors. Moreover, recent data reflect contemporary trends in cardiovascular medicine, with decreasing MI rates in the general population, and in HIV medicine, with utilization of new and less metabolically toxic antiretroviral medications. In a recent meta-analysis, HIV-infected patients were shown to have a 61% increased relative risk of cardiovascular disease endpoints.12 Compared to non-HIV-infected patients, the relative risk of CVD was increased among HIV-infected patients not on ART (RR 1.61, 95% CI 1.43-1.81) as well as those on ART (RR 2.00, 95% CI 1.70-2.37). There was a 50% increase in risk comparing HIV-infected patients on ART to those not on ART. Limitations of the analysis reflect those of observational research in general and include unmeasured confounding and inadequate or non-homogenous HIV-uninfected control groups.
In a recent investigation from the Veterans Aging Cohort Study (VACS) Virtual Cohort, more than 27,000 primarily male HIV-infected patients were compared with respect to MI rates to HIV-uninfected patients with similar demographics and behavior patterns.13 Rates of MI were significantly increased for the 40-49 (incidence rate ratio, IRR 1.34, 95% CI 1.04-1.72), 50-59 (IRR 1.80, 95% CI 1.47-1.21), and 60-69 (IRR 1.53, 95% CI 1.03-2.26) age groups. The hazard ratio for MI associated with HIV status was 1.48 (95% CI 1.27-1.72), in modeling adjusted for traditional CHD risk factors, comorbid medical conditions, and substance use. Notably, the association remained significant in patients with undetectable HIV RNA (HR 1.39, 95% CI 1.17-1.66). Adjudicated outcomes, the ability to capture out-of-system events, and ascertainment of many possible confounding factors contribute to the strength of the analysis. Most recently, the Kaiser group published a study updating their original MI data, assessing MI risk in HIV from 1996 to 2009 in more than 250,000 patients including more than 22,000 HIV-infected patients and demographically-matched controls.14 While HIV infection was significantly associated with MI in an analysis of all patients (RR 1.44, 95% CI 1.27-1.64), there was no significant difference in MI rates comparing HIV-infected patients with recent or nadir CD4 cell count ≥500 to HIV negative patients. Strengths of this analysis include long follow-up time, ability to adjust for sociodemographic factors including census-based socioeconomic status, and a closed health delivery system with increased likelihood of capturing complete risk factor and outcome data.
What factors drive the observed increase in CHD risk, and which HIV-infected patients confront the highest risk? What are the implications for the clinical management of CHD in HIV populations? While the summarized studies considered the relative risk of CHD in HIV infection, further epidemiologic data have informed us about 1) traditional and novel CHD risk factors in HIV; 2) clinical and sociodemographic HIV subgroups at heightened risk; and 3) broader cardiovascular outcomes. These data are highlighted below.
ROLE OF CHD RISK FACTORS
Established CHD risk factors
An extensive body of literature has established increased rates of traditional CHD risk factors in HIV-infected patients compared to non-HIV-infected control groups. Dyslipidemia, diabetes, and hypertension have been shown to be more prevalent, with lipid profiles typically characterized by hypertriglyceridemia and low HDL.4 Smoking is also extremely prevalent and poses a significant challenge to HIV-infected patients and their providers.15 While modifying traditional CHD risk factors is an important component of preventative care for HIV-infected populations, these factors have not been shown to account for the entirety of observed increased CHD risk, suggesting that novel factors play a role in mediating risk. Risk factors attributable directly or indirectly to HIV infection are likely to participate in the complex interplay of CHD risk.
HIV-related CHD risk factors
Antiretroviral therapy
Evidence of protease inhibitor (PI)-associated dyslipidemia4 initially prompted consideration of antiretroviral therapy as a contributor to HIV-associated CHD, a complex issue due to the heterogeneity and concomitant use of HIV medications. Early studies on the association of ART and MI conflicted in terms of findings.5-7,16 A large observational study from the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study group, designed to investigate the effects of ART exposure on MI risk, showed an increased relative risk of MI attributable largely to the PI class of medications and independent of lipids (adjusted relative risk of MI 1.16 per year of ART exposure).17 Studies of specific medications implicated several of the older PIs not widely used today,18,19 while newer PIs have not been shown to be associated with increased risk.20 Recently, two meta-analyses based on observational data concluded MI risk to be increased with the PI class of drugs.12,21 The effects of nucleoside reverse transcriptase inhibitors (NRTIs) – particularly abacavir – on CHD risk has prompted extensive investigation and is the subject of an accompanying review by Dr. Mallon.
HIV disease parameters
Further adding to the complexity of the interplay of risk factors for CHD in HIV populations is HIV disease stage. Clinically measured HIV disease parameters including CD4 cell count, which represents the degree of immune reconstitution, and HIV RNA or viral load, which indicates the level of viremia, have been correlated with CHD risk. Low recent22,23 and nadir14 CD4 cell count have been associated with CVD outcomes in several studies. Cardiovascular outcomes have also been shown to relate to increased or detectable HIV RNA across several studies.13,24,25 These data support the hypothesis that MI risk is mediated at least in part by degree of control of HIV. Patients who have uncontrolled viremia, are more immunosuppressed, or reached a more profound degree of immunosuppression prior to ART initiation appear to be at heightened CHD risk. These data suggest that initiation of ART earlier in the course of disease would be beneficial from a CHD standpoint, a hypothesis which will be tested through the ongoing Strategic Timing of Antiretroviral Treatment (START) trial.
CHD RISK IN HIV SUBGROUPS
Women
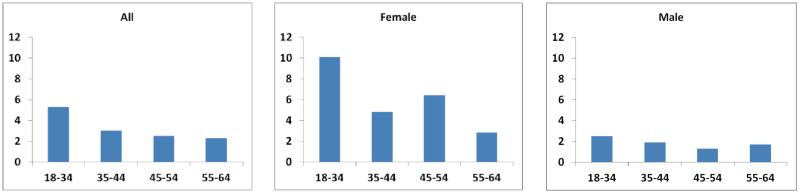
HIV may uniquely impact women with respect to CHD risk. Women differ from men on the basis of both CHD risk factors and HIV-related factors, yet are historically underrepresented in both HIV clinic trials as well as observational studies (Table 1). Available observational data suggest a striking difference in relative risk of CHD for HIV-infected women, with HIV conferring a risk increase (relative to non-HIV counterparts) of double for women compared to men in several studies (Figure 1).8,10 For example, the relative risk of MI conferred by HIV status was 3 for women versus 1.4 for men, in the Partners HIV cohort.8 Results from the FHDH cohort were strikingly similar, with sex- and age-standardized morbidity ratios of 2.7 for women and 1.4 for men.10 This increased relative risk has been shown to translate into increased mortality attributable to CVD.26 The gender-specific determinants of HIV-associated CHD are an important priority for future study.
Figure 1. Incidence rate ratios comparing relative rates of myocardial infarction in HIV-infected versus control patients according to age group and gender (based on data from Partners HIV cohort8).
Resource-limited settings
The effect of HIV on coronary heart disease in resource-limited settings is a significant yet understudied area. As a vast number of patients will be living with treated HIV infection in a region with escalating levels of cardiovascular disease, the dual impact of these epidemics is likely to be substantial. While data suggest that CHD risk factors are indeed prevalent in HIV populations resource-limited settings,27 data on cardiovascular outcomes are scarce,28 with no studies to date comparing MI rates in HIV versus matched control patients. A complete discussion of this important topic is beyond the scope of this review. An enhanced understanding of this area will have important implications for developing strategies for CHD risk factor modification in HIV groups and for considering ways to integrate HIV and NCD health care delivery systems globally.
HCV co-infection
Patients co-infected with Hepatitis C virus (HCV) represent an important clinical subgroup that may differ in terms of risk factor distribution and inflammatory profile compared with patients infected with HIV alone. HCV itself may increase CHD risk, and the effect of co-infection on cardiovascular risk has begun to be delineated. While two studies have demonstrated increased risk of cardiovascular outcomes in co-infected patients compared to HIV mono-infected patients,29,30 another study did not find a significant difference between the two groups.31 A finding of heightened risk in this subgroup would enable CHD preventative efforts to be further tailored to HIV-infected patients with clinical comorbidities which place them at the highest risk.
CARDIOVASCULAR OUTCOMES
HIV appears to exert a wide range of effects on the heart and vasculature. In the early era of ART, HIV-infected patients were found to have increased rates of cardiomyopathy thought related to direct viral effects. As the data on coronary heart disease has continued to accumulate, broader cardiovascular endpoints have been examined and have been shown to be similarly modulated by HIV and its presumed accompanying inflammatory and immunologic effects. Specifically, HIV infection has been linked with ischemic stroke,25,32 peripheral arterial disease,33 congestive heart failure,34 and sudden cardiac death.35
IMPLICATIONS OF EPIDEMIOLOGIC DATA
Taken together, epidemiologic studies investigating the risk of coronary heart disease in HIV populations suggest that being infected with HIV confers increased risk, with an effect 1.5- to 2-fold higher than baseline risk, comparable to that observed in other inflammatory disorders. This effect persists in the current era characterized by decreasing incidence of atherosclerotic cardiovascular disease. Interestingly, the impact of HIV on coronary heart disease has been shown to be most pronounced in demographic groups typically considered low risk. While the absolute risk of MI within HIV populations is higher among men, the relative increase in risk comparing HIV-infected to non-HIV-infected populations is higher among women, with several studies showing double the relative risk for HIV-infected women versus HIV-infected men.8,10 A similar effect is true for age. The highest absolute risk of MI among HIV-infected patients occurs in the oldest age groups, yet the relative risk, comparing each age strata with appropriately matched control patients, is highest in the youngest age groups and decreases with increasing age.6,8 These data suggest that our typical conception of a “low risk” patient is likely not to be applicable in the setting of HIV, thereby necessitating increased vigilance on the part of HIV-infected patients and providers with respect to cardiovascular risk.
Coupled with rapidly accumulating mechanistic data, epidemiologic data suggest that CHD risk is modulated by HIV disease characteristics. The link between CHD risk and higher levels of viremia and more profound immunosuppression observed in epidemiologic studies reinforces the hypothesis that the degree of underlying inflammation and immune activation affect an individual’s cardiovascular risk. These data suggest that earlier treatment of HIV – endorsed by the most recent HIV treatment guidelines – could decrease cardiovascular risk. Yet several recent studies indicate that increased cardiovascular risk persists even in the setting of controlled HIV disease as evidenced by undetectable HIV RNA,12,13 postulated to be due to ongoing low levels of viral replication and associated inflammation. This finding suggests that cardiovascular risk will continue to be an important consideration for an aging HIV population, even with health optimized from an HIV and from a cardiovascular risk factor perspective. Tailored cardiovascular interventions are needed – beyond traditional risk factor modification and virological suppression with ART – to eliminate this residual risk for a vast group of at-risk patients.
Observational research provides invaluable data by investigating large patient groups and long-term outcomes which cannot be studied in clinical trials. Yet such studies must always be considered in the context of limitations intrinsic to observational cohort research. As with clinical trials, observational studies are not necessarily broadly representative, depending on the study population. For example, the two most recent studies of CHD risk in HIV were largely limited to men (97% and 91% men),13,14 making generalizability to HIV-infected women, who may differ in terms of CHD and HIV risk factors, problematic. All studies are subject to the possibility of unmeasured confounding, although recent studies have used rigorous approaches to account for a broad range of sociodemographic and clinical factors which might affect risk.13,14 Finally, the problem of capturing out-of-system outcome events can impact study results; recent studies utilizing multiple data sources or being conducted in closed systems have attempted to minimize this limitation.13,14 Methods to optimize use of electronic health record data, including selection of appropriate populations and control groups, improved characterization of risk factor data, and enhanced methods to control for confounding will be extremely important clinical research tools as EHR sources of data are increasingly widespread.
CONCLUSION
The intersection of coronary heart disease and HIV represents a significant clinical challenge from both the cardiovascular and HIV medicine standpoints. HIV appears to nearly double cardiovascular risk, yet a “high-risk” HIV patient differs from what we might expect clinically and demographically. The effect of HIV on CHD persists over time, is independent of traditional cardiovascular risk factors and of antiretroviral medications, and is likely due in large part to the chronic inflammation and immune activation underlying HIV infection. Salient gaps in our current knowledge include the relative determinants of CHD in HIV populations, the specific effects of HIV on CHD outcomes in women, and the association between HIV and cardiovascular disease in resource-limited settings. A fundamental question that remains to be definitely answered is whether HIV should be considered a cardiovascular risk factor that increases an individual’s risk, meriting aggressive intervention as in the case of diabetes. Available epidemiologic data suggesting this to be the case has spurred ongoing mechanistic studies and clinical trials to solidify our understanding. Findings from these studies will enable the development of tailored and evidence-based clinical strategies to reduce CHD in the HIV population. Moreover, HIV-infected patients could serve as a prototype of a patient population with CHD risk based on non-traditional factors, whose optimal management is not necessarily being captured by the most recent general population guidelines and paradigms on cardiovascular risk reduction. Continued expansion of knowledge of this important convergence of diseases will have important implications for both HIV and cardiovascular medicine.
REFERENCES
- 1.Murray CJ, Lopez AD. Measuring the global burden of disease. N Engl J Med. 2013 Aug 1;369(5):448–457. doi: 10.1056/NEJMra1201534. [DOI] [PubMed] [Google Scholar]
- 2.Causes of death in HIV-1-infected patients treated with antiretroviral therapy, 1996-2006: collaborative analysis of 13 HIV cohort studies. Clin Infect Dis. 2010 May 15;50(10):1387–1396. doi: 10.1086/652283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boccara F, Lang S, Meuleman C, et al. HIV and Coronary Heart Disease: Time for a Better Understanding. J Am Coll Cardiol. 2013 Feb 5;61(5):511–523. doi: 10.1016/j.jacc.2012.06.063. [DOI] [PubMed] [Google Scholar]
- 4.Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med. 2005 Jan 6;352(1):48–62. doi: 10.1056/NEJMra041811. [DOI] [PubMed] [Google Scholar]
- 5.Klein D, Hurley LB, Quesenberry CP, Jr., Sidney S. Do protease inhibitors increase the risk for coronary heart disease in patients with HIV-1 infection? J Acquir Immune Defic Syndr. 2002 Aug 15;30(5):471–477. doi: 10.1097/00126334-200208150-00002. [DOI] [PubMed] [Google Scholar]
- 6.Currier JS, Taylor A, Boyd F, et al. Coronary heart disease in HIV-infected individuals. J Acquir Immune Defic Syndr. 2003 Aug 1;33(4):506–512. doi: 10.1097/00126334-200308010-00012. [DOI] [PubMed] [Google Scholar]
- 7.Mary-Krause M, Cotte L, Simon A, Partisani M, Costagliola D. Increased risk of myocardial infarction with duration of protease inhibitor therapy in HIV-infected men. Aids. 2003 Nov 21;17(17):2479–2486. doi: 10.1097/00002030-200311210-00010. [DOI] [PubMed] [Google Scholar]
- 8.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007 Jul;92(7):2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Obel N, Thomsen HF, Kronborg G, et al. Ischemic heart disease in HIV-infected and HIV-uninfected individuals: a population-based cohort study. Clin Infect Dis. 2007 Jun 15;44(12):1625–1631. doi: 10.1086/518285. [DOI] [PubMed] [Google Scholar]
- 10.Lang S, Mary-Krause M, Cotte L, et al. Increased risk of myocardial infarction in HIV-infected patients in France, relative to the general population. AIDS. 2010 May 15;24(8):1228–1230. doi: 10.1097/QAD.0b013e328339192f. [DOI] [PubMed] [Google Scholar]
- 11.Durand M, Sheehy O, Baril JG, Lelorier J, Tremblay CL. Association between HIV infection, antiretroviral therapy, and risk of acute myocardial infarction: a cohort and nested case-control study using Quebec’s public health insurance database. J Acquir Immune Defic Syndr. 2011 Jul 1;57(3):245–253. doi: 10.1097/QAI.0b013e31821d33a5. [DOI] [PubMed] [Google Scholar]
- 12.Islam FM, Wu J, Jansson J, Wilson DP. Relative risk of cardiovascular disease among people living with HIV: a systematic review and meta-analysis. HIV Med. 2012 Sep;13(8):453–468. doi: 10.1111/j.1468-1293.2012.00996.x. [DOI] [PubMed] [Google Scholar]
- 13.Freiberg MS, Chang CC, Kuller LH, et al. HIV Infection and the Risk of Acute Myocardial Infarction. JAMA Intern Med. 2013 Mar 4;:1–9. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silverberg M, Leyden W, Xu L, et al. Immunodeficiency and Risk of Myocardial Infarction among HIV-positive Individuals with Access to Care. J Acquir Immune Defic Syndr. 2013 doi: 10.1097/QAI.0000000000000009. [DOI] [PubMed] [Google Scholar]
- 15.Petrosillo N, Cicalini S. Smoking and HIV: time for a change? BMC Med. 2013;11:16. doi: 10.1186/1741-7015-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bozzette SA, Ake CF, Tam HK, Chang SW, Louis TA. Cardiovascular and cerebrovascular events in patients treated for human immunodeficiency virus infection. N Engl J Med. 2003 Feb 20;348(8):702–710. doi: 10.1056/NEJMoa022048. [DOI] [PubMed] [Google Scholar]
- 17.Friis-Moller N, Reiss P, Sabin CA, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007 Apr 26;356(17):1723–1735. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 18.Worm SW, Sabin C, Weber R, et al. Risk of myocardial infarction in patients with HIV infection exposed to specific individual antiretroviral drugs from the 3 major drug classes: the data collection on adverse events of anti-HIV drugs (D:A:D) study. J Infect Dis. 2010 Feb 1;201(3):318–330. doi: 10.1086/649897. [DOI] [PubMed] [Google Scholar]
- 19.Lang S, Mary-Krause M, Cotte L, et al. Impact of Individual Antiretroviral Drugs on the Risk of Myocardial Infarction in Human Immunodeficiency Virus-Infected Patients: A Case-Control Study Nested Within the French Hospital Database on HIV ANRS Cohort CO4. Arch Intern Med. 2010 Jul 26;170(14):1228–1238. doi: 10.1001/archinternmed.2010.197. [DOI] [PubMed] [Google Scholar]
- 20.Monforte A, Reiss P, Ryom L, et al. Atazanavir is not associated with an increased risk of cardio or cerebrovascular disease events. AIDS. 2013 Jan 28;27(3):407–415. doi: 10.1097/QAD.0b013e32835b2ef1. [DOI] [PubMed] [Google Scholar]
- 21.Bavinger C, Bendavid E, Niehaus K, et al. Risk of Cardiovascular Disease from Antiretroviral Therapy for HIV: A Systematic Review. PloS one. 2013;8(3):e59551. doi: 10.1371/journal.pone.0059551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lichtenstein KA, Armon C, Buchacz K, et al. Low CD4+ T cell count is a risk factor for cardiovascular disease events in the HIV outpatient study. Clin Infect Dis. 2010 Aug 15;51(4):435–447. doi: 10.1086/655144. [DOI] [PubMed] [Google Scholar]
- 23.Triant VA, Regan S, Lee H, Sax PE, Meigs JB, Grinspoon SK. Association of immunologic and virologic factors with myocardial infarction rates in a US healthcare system. J Acquir Immune Defic Syndr. 2010 Dec 15;55(5):615–619. doi: 10.1097/QAI.0b013e3181f4b752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lang S, Mary-Krause M, Simon A, et al. HIV Replication and Immune Status are Independent Predictors of the Risk of Myocardial Infarction in HIV-Infected Individuals. Clin Infect Dis. 2012 May 18; doi: 10.1093/cid/cis489. [DOI] [PubMed] [Google Scholar]
- 25.Chow FC, Regan S, Feske S, Meigs JB, Grinspoon SK, Triant VA. Comparison of ischemic stroke incidence in HIV-infected and non-HIV-infected patients in a US health care system. J Acquir Immune Defic Syndr. 2012 Aug 1;60(4):351–358. doi: 10.1097/QAI.0b013e31825c7f24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.French AL, Gawel SH, Hershow R, et al. Trends in mortality and causes of death among women with HIV in the United States: a 10-year study. J Acquir Immune Defic Syndr. 2009 Aug 1;51(4):399–406. doi: 10.1097/QAI.0b013e3181acb4e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Julius H, Basu D, Ricci E, et al. The burden of metabolic diseases amongst HIV positive patients on HAART attending The Johannesburg Hospital. Curr HIV Res. 2011 Jun 1;9(4):247–252. doi: 10.2174/157016211796320360. [DOI] [PubMed] [Google Scholar]
- 28.Wester CW, Koethe JR, Shepherd BE, et al. Non-AIDS-defining events among HIV-1-infected adults receiving combination antiretroviral therapy in resource-replete versus resource-limited urban setting. AIDS. 2011 Jul 31;25(12):1471–1479. doi: 10.1097/QAD.0b013e328347f9d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bedimo R, Westfall AO, Mugavero M, Drechsler H, Khanna N, Saag M. Hepatitis C virus coinfection and the risk of cardiovascular disease among HIV-infected patients. HIV medicine. 2010 Aug;11(7):462–468. doi: 10.1111/j.1468-1293.2009.00815.x. [DOI] [PubMed] [Google Scholar]
- 30.Freiberg MS, Chang CC, Skanderson M, et al. The risk of incident coronary heart disease among veterans with and without HIV and hepatitis C. Circ Cardiovasc Qual Outcomes. 2011 Jul;4(4):425–432. doi: 10.1161/CIRCOUTCOMES.110.957415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weber R, Sabin C, Reiss P, et al. HBV or HCV coinfections and risk of myocardial infarction in HIV-infected individuals: the D:A:D Cohort Study. Antivir Ther. 2010;15(8):1077–1086. doi: 10.3851/IMP1681. [DOI] [PubMed] [Google Scholar]
- 32.Rasmussen LD, Engsig FN, Christensen H, et al. Risk of cerebrovascular events in persons with and without HIV: a Danish nationwide population-based cohort study. AIDS. 2011 Aug 24;25(13):1637–1646. doi: 10.1097/QAD.0b013e3283493fb0. [DOI] [PubMed] [Google Scholar]
- 33.Ye Y, Zeng Y, Li X, et al. HIV infection: an independent risk factor of peripheral arterial disease. J Acquir Immune Defic Syndr. 2010 Feb;53(2):276–278. doi: 10.1097/QAI.0b013e3181ba1c31. [DOI] [PubMed] [Google Scholar]
- 34.Butt AA, Chang CC, Kuller L, et al. Risk of heart failure with human immunodeficiency virus in the absence of prior diagnosis of coronary heart disease. Arch Intern Med. 2011 Apr 25;171(8):737–743. doi: 10.1001/archinternmed.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tseng ZH, Secemsky EA, Dowdy D, et al. Sudden cardiac death in patients with human immunodeficiency virus infection. J Am Coll Cardiol. 2012 May 22;59(21):1891–1896. doi: 10.1016/j.jacc.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]