Abstract
Objective
In spite of effective antiretroviral therapy (ART), cognition is impaired in upwards of 35% of the HIV-infected population. We investigated a possible link between peripheral immune activation and brain metabolite concentrations.
Design and methods
Thirty-five HIV-seropositive (HIV+) and eight HIV-seronegative adults were recruited to this cross-sectional study. All HIV-positive patients were on ART or a treatment interruption. Participants were evaluated for monocyte gene expression, cognitive status, and brain metabolite concentrations using 4-Tesla short echo-time proton magnetic resonance spectroscopy. Absolute concentrations of brain metabolites in the frontal white matter (FWM), anterior cingulate cortex (ACC), and basal ganglia were derived and related to monocyte gene expression and global deficit scores.
Results
Analysis of monocyte gene arrays revealed an interferon (IFN)-α-induced activation phenotype. Fourteen genes having the greatest fold increase in response to HIV were IFN genes. Monocyte activation as measured by gene expression profiles strongly correlated with lower N-acetylaspartate (NAA) in FWM. The IFN response gene Interferon-gamma inducible protein-10 (IP-10) was activated in monocytes from HIV individuals and strongly correlated with plasma protein levels. Plasma IP-10 correlated significantly and inversely with ACC NAA, which was lower in HIV-positive patients with mild compared to no cognitive impairment.
Conclusion
Chronic peripheral immune activation driven by a type 1 IFN correlates with neuronal injury in FWM and ACC and cognitive dysfunction. Easily measured IFN-induced blood markers may be clinically significant in following early neural cell damage.
Keywords: cognitive symptoms, gene array, interferon, IP-10, magnetic resonance spectroscopy, monocyte, neuroimaging
Introduction
In spite of antiretroviral therapy (ART), individuals chronically infected with HIV-1 (HIV+) continue to show immune activation and neurocognitive dysfunction [1]. Central nervous system (CNS) involvement in the ART era has transitioned from a life-threatening HIV-associated dementia to milder forms of cognitive impairment, which have been collectively named HIV-associated neurocognitive disorder (HAND) [2]. Neurological impairment in chronically infected HIV-positive individuals on ART is associated with measures of brain chemistry obtained by 1H magnetic resonance spectroscopy (MRS) [3,4]. Many years of HIV research show that a decrease of the neuronal marker N-acetylaspartate (NAA) together with an increase in one or more glial activation-related metabolites [i.e., myoinositol (mI) or choline-containing metabolite (Cho)] is a hallmark of HAND with larger metabolic abnormalities generally related to cognitive deficits [3–6]. Recent studies also show that measures of lower cerebral glutamate (Glu) or glutamate and glutamine (Glx) correlate with impaired cognitive performance [4,7,8].
We previously reported a type 1 interferon (IFN)-α-driven monocyte phenotype in individuals with chronic HIV infection that correlated with viral load [9,10]. Here, we show that a monocyte IFN-α activation profile and IFN-gamma-inducible protein-10 (IP-10, CXCL10) in the plasma of HIV-positive individuals correlated with lower concentrations of NAA in the frontal white matter (FWM) measured by 1H MRS.
Methods
Research participants
Participants were recruited from the San Francisco VA Medical Center (SFVAMC) based on their HIV status and viral load; neuropsychological status was not a consideration for enrollment. All participants provided written consent previously approved by the University of California, San Francisco Committee on Human Research. Forty three participants, 35 HIV-positive and eight HIV-negative controls, completed neuroimaging, neuropsychological testing, and blood draws for monocyte gene expression and plasma assays. All HIV-positive individuals were on stable ART or treatment interruption. Neuropsychological testing assessed seven domains of cognitive function and cognitive impairment was defined as more than 1.5 SD below the norm in at least two domains [9]. A global deficit score (GDS) was calculated with 0 being normal and a GDS at least 0.5 defined as mildly impaired [11].
Image acquisition
MRS data were obtained on a Bruker MedSpec 4T system with Siemens Trio console. Three-dimensional imaging (T1-weighted sagittal MP-RAGE with TR/TI/TE = 3000/1200/3.4 ms and T2-weighted spin-echo with TR/TI/TE = 5000/1900/355 ms) covered the entire head at 1 mm isotropic resolution. Short echo-time (TE) single volume STEAM spectra (TR/TE/TM = 2000/12/10 ms, spectral width = 2000 Hz, 128 scans = 4 : 24 min) were obtained from three volumes of interest (VOIs) corresponding to different tissue types: mid-frontal centrum semiovale (FWM, 15 × 25 × 20 mm3); anterior cingulate cortex (ACC, cortical gray matter, 20 × 20 × 20 mm3) and basal ganglia (deep gray matter, 17 × 35 × 15 mm3). A STEAM spectrum without water signal suppression was also acquired from the same VOI to aid in calculating absolute metabolite concentrations.
Spectral data processing and metabolite quantitation
All processing methods have been previously described including quantitative spectral processing methods using software tools developed in-house [12]. Metabolite quantification used an approach that has been extensively described, validated, and used in many magnetic resonance laboratories. Cerebrospinal fluid (CSF) contributions to the VOIs were derived from VOI positions overlaid on segmented T1-weighted images, allowing absolute molar quantitation of individual metabolite levels expressed in institutional units (IU).
Monocyte gene microarray
Monocyte isolation, gene expression microarrays, and statistical methods have been previously described [9,10]. Total RNA was isolated and hybridized to Codelink Human Whole Genome Bioarrays (Applied Microarrays, Tempe, Arizona, USA). Microarray data were normalized with Loess normalization using R [13] and Bioconductor package [14]. Differential gene expression, significance, fold changes, and patterns were determined using GeneSpring GX 7.3 software package (Agilent, Santa Clara, California, USA). P values of two-sample t-tests for differential genes of group comparisons were adjusted using Benjamini and Hochberg’s false discovery rate method [15] in GeneSpring.
Plasma IP-10 assay
Plasma IP-10 concentration was quantified using a Bio-Plex assay (Bio-Rad, Luminex, Hercules, California, USA). Data were analyzed using Student’s t-test in SAS 9.1 (SAS Inc., Cary, North Carolina, USA).
Statistical analysis
Two-tailed t-tests with equal variance were used to compare plasma and clinical variables for control and HIV-positive group comparisons. One-tailed t-tests were used for MRS group comparisons of brain metabolite levels as we postulated directionally specific concentration changes with HIV infection based on abundant literature. Spearman’s correlation coefficients were used to describe the relationships between gene expression, metabolite concentrations, and plasma variables. P values were adjusted using false discovery rate multiple testing corrections described by Benjamini and Hochberg [15]. All statistical analyses were performed using SAS 9.1 or R.
Results
Study population and clinical variables
All 43 participants were men, aged 30–66 years (mean 50.2 × 7.5), predominantly white (56%) and African–American (26%), and well educated (mean 14.5 years, range 11–20). The 35 HIV-positive individuals were chronically infected for a mean of 17 years and were on ART or in treatment interruption. Nineteen of the individuals in HIV-positive group had undetectable (<1.7 log10 RNA copies/ml) or low viral loads (LVLs; <4 log10 RNA copies/ml) and 16 had high viral loads (HVLs; ≥4 log10 RNA copies/ml). Based on neuropsychological tests, 16 of the 35 HIV-positive individuals were mildly impaired (mean GDS = 0.49 ± 0.42), whereas 19 of them (54%) showed no impairment [9]. There was no difference in impaired versus nonimpaired groups with respect to current categories of CNS-penetrating antiretrovirals [9,16].
HIV infection alters brain metabolite levels
When comparing HIV-positive individuals with HIV-negative controls, we found infection was linked to higher Cho (P = 0.008) and mI (P = 0.05) concentrations in FWM largely consistent with previous reports (Table 1) [3,5,17]. There was a trend toward reduced Glu concentration in the basal ganglia (P = 0.05), and this reduction did not parallel a corresponding decrease in NAA. As a group, HIV-positive individuals did not show significant NAA decreases in any brain region tested.
Table 1.
Brain metabolite concentrations by magnetic resonance spectroscopy (mean ± SD).
| NAA | Glu | Cho | mI | Cr | |
|---|---|---|---|---|---|
| Frontal white matter | |||||
| HIV-negative (n = 7) | 5.68 (± 0.30) | 5.00 (± 0.21) | 1.13 (± 0.22) | 4.90 (± 0.61) | 5.66 (± 0.48) |
| HIV-positive (n = 30) | 5.55 (± 0.69) | 4.91 (± 0.76) | 1.33 (± 0.19) | 5.24 (± 0.50) | 5.63 (± 0.65) |
| P value | 0.224 | 0.299 | 0.008 | 0.053 | 0.454 |
| Anterior cingulate cortex | |||||
| HIV-negative (n = 5) | 7.30 (± 1.24) | 4.68 (± 0.86) | 1.51 (± 0.30) | 5.03 (± 0.33) | 5.85 (± 1.02) |
| HIV-positive (n = 26) | 7.53 (± 0.93) | 4.90 (± 0.80) | 1.69 (± 0.25) | 5.38 (± 0.72) | 6.29 (± 0.87) |
| P value | 0.683 | 0.292 | 0.093 | 0.153 | 0.156 |
| Basal ganglia | |||||
| HIV-negative (n = 6) | 5.73 (± 0.70) | 5.37 (± 0.67) | 1.63 (± 0.22) | 2.73 (± 0.81) | 6.31 (± 0.62) |
| HIV-positive (n = 28) | 5.65 (± 0.88) | 4.86 (± 0.68) | 1.65 (± 0.20) | 2.82 (± 0.61) | 6.10 (± 0.87) |
| P value | 0.409 | 0.051 | 0.380 | 0.389 | 0.279 |
Values are presented as absolute metabolite concentrations (institutional units). P values represent the difference between the HIV-negative controls and HIV-positive groups using one-tailed t-tests. Cho, choline-containing metabolite; Cr, creatine; Glu, glutamate; mI, myoinositol; NAA, N-acetylaspartate.
Peripheral immune activation correlates with brain metabolite concentrations
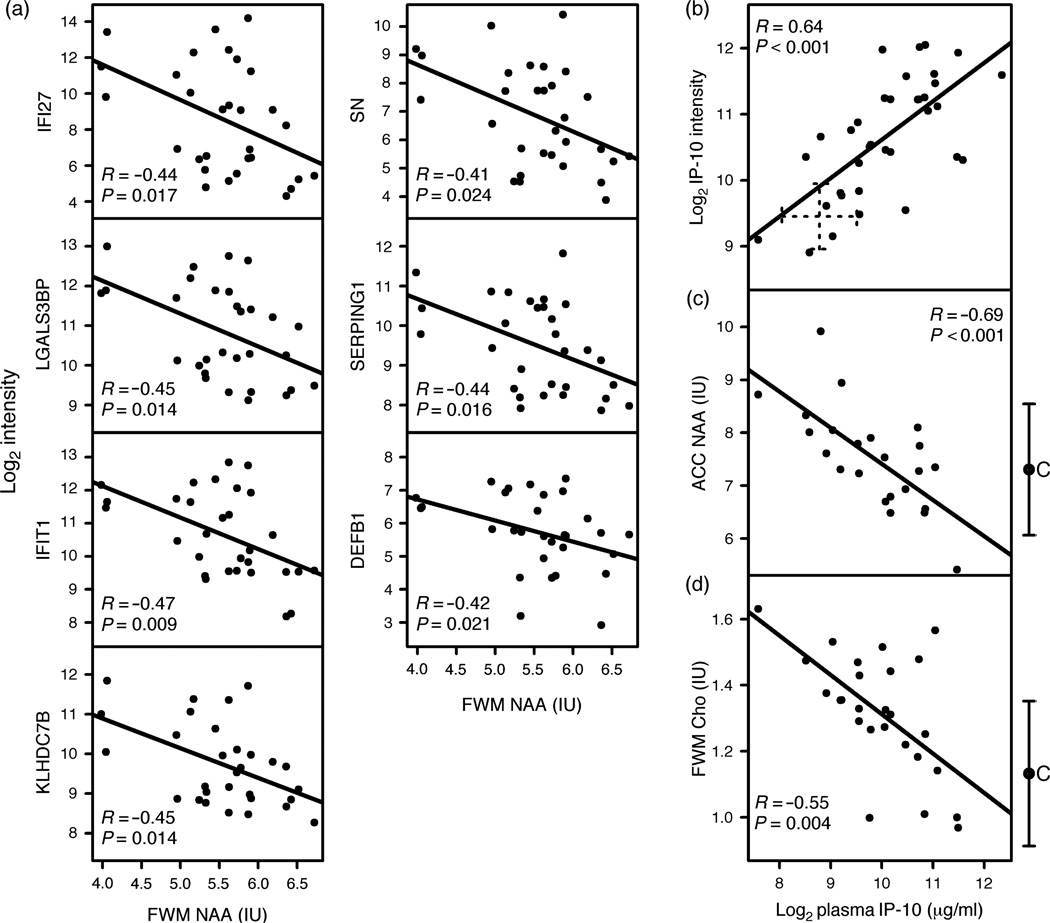
The top 14 differentially expressed monocyte genes with a greater than five-fold increase in activation were all IFN-responsive transcripts. We compared the 14 genes individually with the five metabolite concentrations from the three brain regions. In the FWM, lower NAA correlated significantly with seven IFN genes following correction for multiple comparisons (Fig. 1a).
Fig. 1. Correlations between interferon-α genes, IP-10 plasma levels, and brain metabolites from monocytes of HIV-positive individuals.
(a) Correlation between the top seven overexpressed interferon (IFN)-α genes on monocytes that correlated significantly with a decrease in frontal white matter (FWM) N-acetylaspartate (NAA). (b) Significant correlation between IP-10 monocyte gene expression intensity and its plasma levels from HIV-positive individuals. Cross indicates IP-10 levels (mean ± SD) from controls. (c) Significant inverse correlations were found between plasma IP-10 levels and anterior cingulate cortex (ACC) NAA and (d) FWM Cho. C to the right of panels (c) and (d) are HIV-negative controls (mean ± SD) for corresponding metabolite levels. Spearman’s correlation coefficient [R] and P values are presented in each panel. All correlations remained significant after multiple comparison adjustments.
IP-10 gene expression and plasma levels correlate with brain metabolite levels
In addition to identifying a peripheral mechanism that might correlate with brain metabolites, a goal of this study was to determine whether any IFN-associated blood marker(s) might be related to brain metabolite dysregulation. We chose plasma IP-10 as it was a gene that was overexpressed on monocytes in HIV-positive individuals (elevated 3.8-fold), can be easily measured in blood from HIV-positive individuals [18], and is associated with HIV disease progression [19], neurotoxicity [20], and possibly HIV dementia [19,21]. IP-10 monocyte gene expression correlated with plasma levels (R = 0.61, P < 0.001; Fig. 1b). IP-10 plasma concentrations showed a highly significant inverse correlation with ACC NAA concentrations (R = −0.69, P < 0.001; Fig. 1c) and FWM Cho (R = −0.55, P = 0.004; Fig. 1d). Of all three regions examined and five metabolites, only these two correlations remained significant after corrections for multiple comparisons.
Cognitive function and brain metabolite levels
When comparing HIV-positive individuals for ACC NAA based on GDS, despite a relatively high missing data rate due to MRS acquisition, 11 mildly impaired individuals (mean GDS = 0.91 ± 0.42, mean ACC NAA = 7.15 ± 0.76 IU) had a trend toward lower ACC NAA versus 15 nonimpaired individuals (mean GDS = 0.19 ± 0.16, mean ACC NAA = 7.81 ± 0.98 IU; n = 26, P = 0.031, one-tailed t-test on ACC NAA levels). None of the other metabolite concentrations differed as a function of GDS status.
Discussion
Our results demonstrated a strong association between high IFN-α-driven peripheral monocyte transcripts and lower concentrations of the neuronal marker NAA in the FWM. This has also been observed in simian immunodeficiency virus (SIV)-macaque models in which immune responses in the periphery directly influence CNS damage [22]. In humans, an activated peripheral phenotype correlated with neuronal dysfunction related to lower NAA levels in the cortex [23]. Several studies including our own are consistent with IFN-α driving immune activation in the periphery [10,24,25] and the brain [26]. These reports underscore the significance of cognition and NAA loss in the FWM and the correlations with several blood and CSF markers. Our findings suggest that an increase in peripheral activation that accompanies an increase in viral load correlates with a decrease in NAA.
The IFN-driven plasma protein IP-10 has previously been reported to be elevated in HIV and SIV infection [10,21,27]; however, this is the first report of an inverse correlation with ACC NAA concentrations. In a reported post-hoc analysis, higher CSF levels of IP = 10 correlated with lower FWM NAA/Cr ratios (Spearman’s correlation R = −0.43 and P = 0.003, not adjusted for multiple comparisons) [28]. Our study shows higher IP-10 in the plasma inversely correlating with ACC NAA (R = −0.69, P < 0.001 with adjustment for multiple comparisons) suggesting that the peripheral IFN response is a robust measure of neuronal changes.
Whereas we examined the relationship between periphery and brain metabolites, others have compared metabolites with CSF markers. Macrophage colony-stimulating factor (M-CSF) was followed as a marker of activation before and during ART and correlated with MRS [29]. At 3 months after treatment initiation, reduced CSF M-CSF and viral load were observed concurrent with increased NAA in several brain regions. In another study, CSF macrophage chemoattractant protein (MCP-1, CCL2) levels inversely correlated with MRS-derived Glx (glutamate and glutamine together) levels in the basal ganglia [30]. Also, higher CSF MCP-1 levels correlated with cognitive impairment that returned to normal after therapy; however, sustained high CSF MCP-1 correlated with a glial response [31].
In a recent study, low Glu in the FWM or parietal gray matter was associated with cognitive deficits [8] or HIV dementia [4] and may indicate mitochondrial and glial injury as well as altered glutamate neurotransmission [7,8]. Here, we report a trend to lower Glu in the basal ganglia of HIV-positive individuals versus HIV-negative controls. Taken together, glutamatergic dysregulation appears to be present in anterior brain regions and may be of clinical significance.
Our finding that monocyte gene expression and IP-10 levels in the periphery are linked to FWM NAA suggests these would be useful biomarkers for individuals at risk for cognitive impairment. Also, by targeting IFN-α activation with adjunctive therapies, it might be possible to reduce neurocognitive impairment associated with infection in today’s aging HIV population.
Acknowledgements
The authors thank their research participants, Sandra Charles R.N, Linda Adams, R.N, and Dr Harry Lampiris, MD at the SFVAMC for recruiting participants into the study. The authors acknowledge Dr John Kornak at the Clinical and Translational Science Institute (CTSI) for statistical advice. The study is funded by the National Institute of Mental Health, (RO1 MH073478, L.P., D.J.M.).
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.Harezlak J, Buchthal S, Taylor M, Schifitto G, Zhong J, Daar E, et al. Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. AIDS. 2011;25:625–633. doi: 10.1097/QAD.0b013e3283427da7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paul RH, Yiannoutsos CT, Miller EN, Chang L, Marra CM, Schifitto G, et al. Proton MRS and neuropsychological correlates in AIDS dementia complex: evidence of subcortical specificity. J Neuropsychiatry Clin Neurosci. 2007;19:283–292. doi: 10.1176/jnp.2007.19.3.283. [DOI] [PubMed] [Google Scholar]
- 4.Mohamed MA, Barker PB, Skolasky RL, Selnes OA, Moxley RT, Pomper MG, et al. Brain metabolism and cognitive impairment in HIV infection: a 3-T magnetic resonance spectroscopy study. Magn Reson Imaging. 2010;28:1251–1257. doi: 10.1016/j.mri.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyerhoff DJ, Bloomer C, Cardenas V, Norman D, Weiner MW, Fein G. Elevated subcortical choline metabolites in cognitively and clinically asymptomatic HIV+ patients. Neurology. 1999;52:995–1003. doi: 10.1212/wnl.52.5.995. [DOI] [PubMed] [Google Scholar]
- 6.Chang L, Lee PL, Yiannoutsos CT, Ernst T, Marra CM, Richards T, et al. A multicenter in vivo proton-MRS study of HIV-associated dementia and its relationship to age. Neuroimage. 2004;23:1336–1347. doi: 10.1016/j.neuroimage.2004.07.067. [DOI] [PubMed] [Google Scholar]
- 7.Sailasuta N, Shriner K, Ross B. Evidence of reduced glutamate in the frontal lobe of HIV-seropositive patients. NMR Biomed. 2009;22:326–331. doi: 10.1002/nbm.1329. [DOI] [PubMed] [Google Scholar]
- 8.Ernst T, Jiang CS, Nakama H, Buchthal S, Chang L. Lower brain glutamate is associated with cognitive deficits in HIV patients: a new mechanism for HIV-associated neurocognitive disorder. J Magn Reson Imaging. 2010;32:1045–1053. doi: 10.1002/jmri.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun B, Abadjian L, Rempel H, Calosing C, Rothlind J, Pulliam L. Peripheral biomarkers do not correlate with cognitive impairment in highly active antiretroviral therapy-treated subjects with human immunodeficiency virus type 1 infection. J Neurovirol. 2010;16:115–124. doi: 10.3109/13550280903559789. [DOI] [PubMed] [Google Scholar]
- 10.Rempel H, Sun B, Calosing C, Pillai SK, Pulliam L. Interferon-alpha drives monocyte gene expression in chronic unsuppressed HIV-1 infection. AIDS. 2010;24:1415–1423. doi: 10.1097/QAD.0b013e32833ac623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, et al. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol. 2004;26:307–319. doi: 10.1080/13803390490510031. [DOI] [PubMed] [Google Scholar]
- 12.Gazdzinski S, Millin R, Kaiser LG, Durazzo TC, Mueller SG, Weiner MW, et al. BMI and neuronal integrity in healthy, cognitively normal elderly: a proton magnetic resonance spectroscopy study. Obesity (Silver Spring) 2010;18:743–748. doi: 10.1038/oby.2009.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ihaka R, Gentleman R. R: a language for data analysis and graphics. J Cmp Gr St. 1996;5:299–314. [Google Scholar]
- 14.Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57:125–133. [Google Scholar]
- 16.Koopmans PP, Ellis R, Best BM, Letendre S. Should antiretroviral therapy for HIV infection be tailored for intracerebral penetration? Neth J Med. 2009;67:206–211. [PMC free article] [PubMed] [Google Scholar]
- 17.Chang L, Ernst T, Witt MD, Ames N, Gaiefsky M, Miller E. Relationships among brain metabolites, cognitive function, and viral loads in antiretroviral-naive HIV patients. Neuroimage. 2002;17:1638–1648. doi: 10.1006/nimg.2002.1254. [DOI] [PubMed] [Google Scholar]
- 18.Kolb SA, Sporer B, Lahrtz F, Koedel U, Pfister HW, Fontana A. Identification of a T cell chemotactic factor in the cerebrospinal fluid of HIV-1-infected individuals as interferon-gamma inducible protein 10. J Neuroimmunol. 1999;93:172–181. doi: 10.1016/s0165-5728(98)00223-9. [DOI] [PubMed] [Google Scholar]
- 19.Cinque P, Bestetti A, Marenzi R, Sala S, Gisslen M, Hagberg L, et al. Cerebrospinal fluid interferon-gamma-inducible protein 10 (IP-10, CXCL10) in HIV-1 infection. J Neuroimmunol. 2005;168:154–163. doi: 10.1016/j.jneuroim.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Sui Y, Stehno-Bittel L, Li S, Loganathan R, Dhillon NK, Pinson D, et al. CXCL10-induced cell death in neurons: role of calcium dysregulation. Eur J Neurosci. 2006;23:957–964. doi: 10.1111/j.1460-9568.2006.04631.x. [DOI] [PubMed] [Google Scholar]
- 21.Durudas A, Milush JM, Chen HL, Engram JC, Silvestri G, Sodora DL. Elevated levels of innate immune modulators in lymph nodes and blood are associated with more-rapid disease progression in simian immunodeficiency virus-infected monkeys. J Virol. 2009;83:12229–12240. doi: 10.1128/JVI.01311-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams K, Westmoreland S, Greco J, Ratai E, Lentz M, Kim WK, et al. Magnetic resonance spectroscopy reveals that activated monocytes contribute to neuronal injury in SIV neuroAIDS. J Clin Invest. 2005;115:2534–2545. doi: 10.1172/JCI22953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lentz MR, Kim WK, Lee V, Bazner S, Halpern EF, Venna N, et al. Changes in MRS neuronal markers and T cell phenotypes observed during early HIV infection. Neurology. 2009;72:1465–1472. doi: 10.1212/WNL.0b013e3181a2e90a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bosinger SE, Li Q, Gordon SN, Klatt NR, Duan L, Xu L, et al. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. J Clin Invest. 2009;119:3556–3572. doi: 10.1172/JCI40115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mandl JN, Barry AP, Vanderford TH, Kozyr N, Chavan R, Klucking S, et al. Divergent TLR7 and TLR9 signaling and type I interferon production distinguish pathogenic and non-pathogenic AIDS virus infections. Nat Med. 2008;14:1077–1087. doi: 10.1038/nm.1871. [DOI] [PubMed] [Google Scholar]
- 26.Sas AR, Bimonte-Nelson HA, Tyor WR. Cognitive dysfunction in HIV encephalitic SCID mice correlates with levels of Interferon-alpha in the brain. AIDS. 2007;21:2151–2159. doi: 10.1097/QAD.0b013e3282f08c2f. [DOI] [PubMed] [Google Scholar]
- 27.Jacquelin B, Mayau V, Targat B, Liovat AS, Kunkel D, Petitjean G, et al. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J Clin Invest. 2009;119:3544–3555. doi: 10.1172/JCI40093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Letendre SL, Zheng JC, Kaul M, Yiannoutsos CT, Ellis RJ, Taylor MJ, et al. Chemokines in cerebrospinal fluid correlate with cerebral metabolite patterns in HIV-infected individuals. J Neurovirol. 2011;17:63–69. doi: 10.1007/s13365-010-0013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lentz MR, Degaonkar M, Mohamed MA, Kim H, Conant K, Halpern EF, et al. Exploring the relationship of macrophage colony-stimulating factor levels on neuroaxonal metabolism and cognition during chronic human immunodeficiency virus infection. J Neurovirol. 2010;16:368–376. doi: 10.3109/13550284.2010.513029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang L, Ernst T, St Hillaire C, Conant K. Antiretroviral treatment alters relationship between MCP-1 and neurometabolites in HIV patients. Antivir Ther. 2004;9:431–440. doi: 10.1177/135965350400900302. [DOI] [PubMed] [Google Scholar]
- 31.Mohamed MA, Lentz MR, Lee V, Halpern EF, Sacktor N, Selnes O, et al. Factor analysis of proton MR spectroscopic imaging data in HIV infection: metabolite-derived factors help identify infection and dementia. Radiology. 2010;254:577–586. doi: 10.1148/radiol.09081867. [DOI] [PMC free article] [PubMed] [Google Scholar]