Abstract
Compartmentation of signalling allows multiple stimuli to achieve diverse cellular responses with only a limited pool of second messengers. This spatial control of signalling is achieved, in part, by cellular structures which bring together elements of a particular cascade. One such structure is the caveola, a flask-shaped lipid raft. Caveolae are well-recognised as signalosomes, platforms for assembly of signalling complexes of receptors, effectors and their targets, which can facilitate efficient and specific cellular responses. Here we extend this simple model and present evidence to show how the protein and lipid profiles of caveolae, as well as their characteristic morphology, define their roles in creating local signalling domains in the cardiac myocyte.
Keywords: Caveolae, G protein coupled receptor, cyclic AMP, nitric oxide, calcium, compartmentation
1. Introduction
Compartmentation of signalling allows multiple stimuli to achieve diverse cellular responses with only a limited pool of second messengers. This spatial control of signalling is achieved, in part, by cellular structures which bring together elements of a particular cascade. A prime example in the adult cardiac myocyte is the dyad where juxtaposition of L type Ca2+ channels in the sarcolemmal membrane and RyR in the sarcoplasmic reticular membrane permits efficient and tightly-regulated Ca2+-induced Ca2+ release. Another membrane structure which is of key importance in the spatial control of signalling in the cardiac cell is the caveola. In this review we will consider the evidence for caveolae as local signalling domains, based on their protein and lipid profiles and distinct morphology, with a focus on cAMP, NO and Ca2+.
1.1 Caveolae
Caveolae were first described in endothelial cells by Palade in the 1950s as membrane invaginations and sub-membrane vesicles 50-100 nm in diameter [1]. They are a type of lipid raft, a liquid-ordered domain of the membrane enriched in cholesterol and sphingolipids [2] (see Figure 1). The key feature which distinguishes caveolae from other lipid rafts is the presence of caveolin; caveolins are 18-22 kDa proteins which insert asymmetrically into the plasma membrane in a hairpin-like conformation, with both N and C termini found intracellularly (Figure 1B). Caveolin is responsible, in part, for the typical flask-like morphology of the caveola through this asymmetrical membrane insertion and its tendency to cluster into oligomers, both of which promote membrane curvature [3]. Within the last decade, another group of proteins, the cavins, have been shown to contribute to caveolar biogenesis and function (see [4] for a recent review).
Figure 1. Caveolae and caveolin.
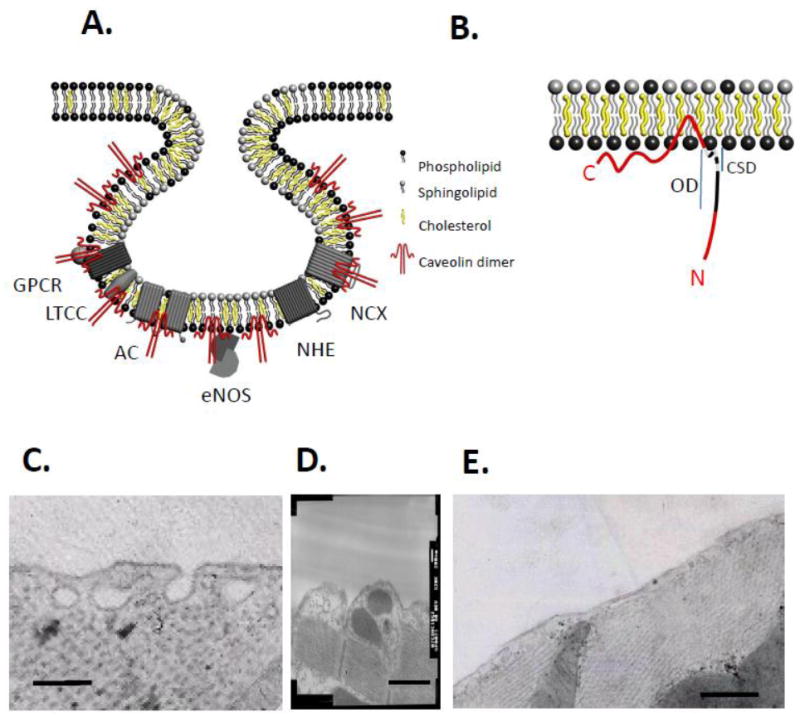
A. Caveolae are invaginated lipid rafts enriched in cholesterol and sphingolipids and lined with the protein caveolin (shown here for simplicity as a dimer, although it normally exists as oligomers of 14-16 caveolins). Within the caveola, some of the key proteins that may be relevant to their function as local signalling domains are shown including G protein coupled receptors (GPCR, with associated G protein), ion channels (e.g. the L type Ca2+ channel, LTCC), eNOS, exchangers (NHE and NCX). B. The caveolin protein inserts into the inner leaflet of the membrane. It oligomerises via its oligomerisation domain (OD) and interacts with its binding partners via the caveolin scaffolding domain (CSD). Figures courtesy of Tim Lee, Faculty of Biological Sciences, University of Leeds. C. Caveolae visualised using transmission EM in the adult rat ventricular myocyte; both open and closed vesicles of a size consistent with caveolae are seen. A proportion of closed vesicles may represent open caveolae sectioned outside the plane of the connecting neck region. D. Caveolae are present in t-tubular openings. E. Treatment of myocytes with the cholesterol-depleting agent methyl-β-cyclodextrin (2 mM for 1 h at 37 °C) results in a marked loss of caveolae. Scale bars represent 200 nm.
Caveolae are important in a variety of cellular processes including endocytosis and cholesterol homeostasis, however one of their best characterized roles is as a signalosome, a platform for pre-assembled complexes of receptors, signal components and their targets (e.g. ion channels), which facilitates fast and specific cellular responses. These complexes can be dynamically regulated on an acute timescale to allow, for example, agonist stimulated access of receptors to their effectors [5, 6].
1.2 Caveolins
A fundamental aspect of the control of signalling by caveolae resides within the caveolin protein itself. Caveolin exists as 3 major isoforms: Cav1, Cav2 (expressed in most cell types) and Cav3 (designated the ‘muscle-specific’ isoform, found only in smooth, skeletal and cardiac muscle). Cav1, 2 and 3 are present at the mRNA and protein level in the adult (rat) ventricular myocyte [7-9] (but see [10]). All caveolins contain a highly conserved 20 residue sequence, the caveolin scaffolding domain (CSD) located in the membrane-proximal N terminus (Figure 1B), which interacts with a complimentary caveolin binding motif (ΦXXXXΦXXΦ or ΦXΦXXXXΦ where is an aromatic amino acid)[11] found in many caveolae-associated proteins. This interaction is generally inhibitory [12], and thereby allows oligomeric caveolin to act as a regulatory macromolecular scaffold [13].
Of the 3 caveolin isoforms, Cav1 and Cav3 share the most sequence homology; human Cav3 is 85% similar to Cav1, but only 58% similar to Cav2 [12]. However, one important distinction between Cav1 and Cav3 is the presence of a phosphorylation site at Tyr14 in Cav1 which is absent in Cav3. In endothelial cells, phosphorylation allows this site to act as an SH2 binding domain that recruits signalling molecules in response to shear or osmotic stress [14]. A similar role for Tyr14 P-Cav1 in recruiting and scaffolding G-protein coupled receptor (GPCR) components in the adult cardiac myocyte has been proposed [15]. Thus, despite sequence similarities, the lack of an equivalent Tyr14 phosphorylation site in Cav3 suggests that these two caveolin isoforms may have functionally distinct roles.
Whilst it lacks a Tyr phosphorylation site, Cav3 has recently been shown to be post-translationally modified by small ubiquitin-like modifier (SUMO) proteins on its N terminus, close to the CSD [16]. This SUMOylation regulates the interaction of Cav3 with some, but not all, of its normal binding partners, and has been implicated in the agonist-induced desensitisation of the β2-adrenoceptor (AR) [16]. No other regulatory post-translational modifications have been identified in Cav3.
1.3 Caveolae and caveolin distribution in the cardiac myocyte
The distribution of caveolae and caveolin within the cardiac myocyte membrane gives limited clues as to the function of caveolae. In the adult ventricular myocyte the main cardiac caveolin isoform, Cav3, is present in both surface and t-tubular sarcolemma [17, 18], and this accords with electron microscopic data showing caveolae in both types of membrane (see Figure 1C,D) [19]. However, within t-tubular membranes, caveolae are excluded from areas that form close connections with junctional sarcoplasmic reticulum [19]. No preferential distribution of caveolae with respect to membrane folds, intracellular structures (e.g. myofibrils) or extracellular structures (e.g. other myocytes, capillaries, nerves) has been detected [19].
There is a large body of literature which describes the role of caveolae in a variety of signalling pathways. Here we will consider how signalling pathways are created and modulated by caveolae from three different perspectives: as protein platforms, as local lipid environments, and as reservoirs of sarcolemmal membrane. We will focus, where possible, on data obtained in the adult cardiac myocyte. Tissue-specific differences in caveolae density, caveolin isoform expression, raft/caveolar localisation of signalling molecules and their targets can limit the value of extrapolating data from non-cardiac cells to cardiac cells. In addition, with regard to compartmentation the neonatal cardiac myocyte does not provide an ideal model of its adult counterpart; it lacks t-tubular membrane structures [20] and shows more global increases in [cAMP] in response to β2-AR stimulation [21].
2. Caveolae as signalling protein platforms
We will begin by considering the role of caveolae in signalling based on their ability to bring together, and regulate, protein elements of signal transduction pathways.
2.1 Cyclic AMP-dependent signaling
In the cardiac cell, a number of different GPCRs signal through the second messenger cAMP, yet they produce diverse functional responses. It is well established that spatial control of cAMP-dependent signalling is responsible for receptor-specific outcomes. The β1- and β2-AR signalling pathways provide an excellent illustration of cAMP compartmentation in the adult ventricular myocyte. β1-AR stimulation produces marked inotropic and lusitropic responses which can be ascribed to protein kinase A (PKA)-dependent phosphorylation of multiple targets throughout the cell - at the sarcolemma (L –type Ca2+ channel) [22], sarcoplasmic reticulum (phospholamban, RyR) [23, 24] and the myofilaments (troponin I) [23]. By contrast, the inotropic and lusitropic effects of β2-AR stimulation are minimal because of limited access of PKA-dependent signals to proteins of the sarcoplasmic reticulum or myofilaments [25, 26]. With the advent of genetically-encoded FRET based biosensors, which allow measurement of cAMP with high spatial and temporal resolution [27], these functional responses to β1- and β2-AR stimulation have been shown to correlate with global and local cAMP signals respectively, e.g. [28].
The mechanisms which are responsible for the creation of distinct cAMP signatures for the β1- and β2-AR have been the subject of intense research over the last decade. Many studies have investigated the role of different phosphodiesterase (PDE) isoforms e.g. [28-31] and most agree that activity of PDE 3 and 4 contributes to the spatial characteristics of the β-AR cAMP signal in the adult myocyte (but see [28]). Local buffering of cAMP by PKA may also play a part [30]. In addition, compartmentation of cAMP-dependent signalling can occur at the level of phosphorylation through the local activity of phosphatases [32, 33]. Spatial control of cAMP signalling is facilitated by the A kinase anchoring proteins (AKAPs) which scaffold elements of the cAMP pathway (including PKA type II, PDEs and phosphatases) at specific cellular locations (see [34] and review by Kritzer et al. in this issue). Interestingly, there is limited evidence that caveolin also acts as a scaffold for PDEs; in adipocytes Cav1 has been shown to interact with PDE3B by co-immunoprecipitation [35] and its activity is dependent on Cav1 expression [36].
Caveolae are important structures for organising GPCRs and their downstream signalling components and several groups have considered the view that distribution of signal components in or outside caveolae is an important factor in determining the functional response to stimulation of a given receptor. Certainly, some work has shown that disruption of caveolae has implications for the response to several cAMP-linked GPCRs (see below). However, the way in which caveolae exert their effects on the cAMP signalling pathway is not fully understood, and to date there has been little reconciliation of data showing spatial control of cAMP-dependent signalling by phosphodiesterase and phosphatase with caveolae-dependent mechanisms.
The first step in understanding how caveolae may impact on cAMP signalling is to consider the differential distribution of receptors and their down-stream signal components in membrane microdomains. For elements of the cAMP cascade, it has been shown that many are located in caveolae and are regulated through their interaction with caveolin. In the adult cardiac myocyte in the absence of agonist stimulation, caveolin-enriched buoyant membrane fractions contain the majority of α1- and β2-ARs, and a proportion of β1-ARs, Gαs, Gαi, adenylyl cyclase (AC) 5/6, PKA RII, protein phosphatase (PP) 2a, although the reported distribution of these latter components varies between investigators [21, 37-42]. By contrast, muscarinic receptors (M2-R), prostaglandin E receptors (EP2-R and EP4-R), and AC 4/7 are excluded from caveolar domains [37, 41, 43]. A number of components physically associate with Cav3 and, in some cases, this has been shown to be associated with a regulation of function. For example, β2-AR, Gαi, Gαs, AC5/6 and PKA RII co-immunprecipitate with Cav3 in the adult cardiac myocyte [39] and the activity of Gαi and Gαs, AC5 and PKA is inhibited when in complex with the CSD domain of caveolin 1 and 3 [44-46].
The membrane distribution of targets of cAMP signalling (e.g. ion channels) is another important factor when considering the role of caveolae in compartmentation in the cardiac cell. Many of the effects of GPCR signalling via PKA are mediated through ICa,L. In the adult ventricular myocyte, the majority of L-type Ca2+ channels are dyadic and, as caveolae are excluded from dyadic couplings of the sarcolemma [19], this implies that the majority of Ca2+ channels are outside caveolae. However, a population of these channels have been shown to be localised to caveolae-containing membrane fractions [39, 47], to interact with Cav3 (Balijepalli et al., 2006), and to cluster with Cav3 in plasma membrane sheets [47] (see review by Best & Kamp in this issue). Other channels which have been linked with cAMP-dependent pathways, including the Na+ channel and Kv4.2/4.3, are also present in both caveolar and non-caveolar fractions of the adult myocyte [38, 40, 47]. By contrast, the HCN4 channel is found exclusively in caveolar fractions of the adult sino-atrial node where it co-immunoprecipitates with Cav3 [48, 49]. As described below, caveolar localisation of ion channels can allow their preferential regulation by cAMP-dependent signalling in caveolae. However, this is not a universal mechanism; in some cases the presence of signal components in caveolae (and their regulation by Cav3) acts to restrict cAMP-dependent regulation of targets.
2.1.1 β1 adrenoceptors
The observation that β1-AR stimulation of cAMP production causes wide-spread phosphorylation of intracellular targets is consistent with the finding that β1-ARs produce an increase in cAMP that is broadcast throughout the entire cell [28, 30, 50]. Given that these receptors are found in both caveolar and non-caveolar fractions of the plasma membrane [37, 41, 51], this raises the question of whether caveolar β1-ARs produce the same responses as non-caveolar receptors. One might predict that β1-ARs found in caveolae are tightly coupled to PKA-dependent regulation of the caveolar population of L-type Ca2+ channels, and that β1-ARs found outside of caveolae regulate non-sarcolemmal targets such as phospholamban and troponin I.
Insight to the answer to this question comes from studies that have examined the effect that manipulating caveolae has on β1-AR responses. Methods used to directly assess the functional contribution of caveolae to signalling generally exploit the dependence of caveolae on cholesterol and caveolin. The cyclic oligosaccharide methly-β-cyclodextrin (MBCD) is an effective cholesterol-depleting agent which is widely used to disrupt caveolae. MBCD causes a translocation of Cav3 from buoyant membrane fractions [25] and a marked reduction in caveolar density [8] in the adult cardiac myocyte (see Figure 1C, E). Agents which target cholesterol will affect caveolae and non-caveolar rafts however it has been argued, based on the protein content of these domains, that caveolae play the more dominant role in signalling in the (neonatal) cardiac cell [52]. Disruption of caveolae-based signalling through caveolin is also common, using knockdown of Cav3 expression [39, 53] or interference of caveolin’s interaction with its normal binding partners by intracellular application of caveolin antibodies [18, 38] or peptides based on the caveolin CSD [54].
In neonatal cells, disrupting caveolae by either depleting cholesterol with MBCD or reducing Cav3 expression with siRNA was found to have no effect on the response of spontaneous beating rate [55] or ICa,L [39] to maximal β1-AR stimulation. We have also reported that MBCD has no significant effect on the inotropic response to β1-AR stimulation in the adult ventricular myocyte [18]. However, in a more recent study, we looked specifically at the effect that cholesterol depletion has on the sensitivity of ICa,L to β1-AR stimulation in the adult myocyte, and found that responses to sub-maximal, but not maximal, β1-AR stimulation were significantly enhanced by MBCD. This suggests that MBCD heightens the sensitivity of receptor-dependent responses to agonist stimulation [41], consistent with previous reports that disrupting caveolae enhances β-AR stimulation of cAMP production [15, 51]. Rybin et al. [51] hypothesized that this type of effect could be a result of removing the inhibitory effect that Cav3 has on adenylyl cyclase.
We have used genetically-encoded FRET-based biosensors, one based on type II PKA which reports on changes in cAMP in PKA II domains of the sarcolemma and SR [41, 56, 57] and another, based on the type 2 exchange protein activated by cAMP (Epac2) without its normal tethering sequence, which is freely diffusible and indexes cAMP in the cytosolic compartment [41, 50, 57]. As illustrated in Figure 2, we found that increases in ICa,L with MBCD treatment correlated with a shift in β1-AR sensitivity of cAMP production in PKA II domains (which include caveolae) [39, 51, 58] but not in the global cytosolic compartment (as indexed by the Epac probe) [41]. These observations indicate that whilst caveolae are associated with compartmentation of cAMP, other factors that limit cAMP diffusion must also be playing a role. In addition, we found that cholesterol depletion not only affected β1-AR regulation of L-type Ca2+ channel function, it also enhanced the rate of [Ca2+]i transient decay and myocyte relaxation consistent with increased phosphorylation of phospholamban at the sarcoplasmic reticulum (SR) [41]. Together these data suggest that caveolae do not serve as a compartment for preferential β1-AR regulation of ICa,L. Rather, caveolae act to restrict β1-AR activation of the L type Ca2+ channels in caveolae (e.g. through Cav3 inhibition of AC) and, importantly, this control extends to other PKA II compartments (which are likely to include the longitudinal SR where phospholamban is located). Thus these data are the first direct evidence that caveolae contribute to compartmentalising β1-AR cAMP signals and restricting these to a local domain.
Figure 2. The caveolae-disrupting agent methyl-β-cyclodextrin (MBCD) increases the sensitivity of β1-AR cAMP generation selectively in a cellular compartment which contains PKA type II.
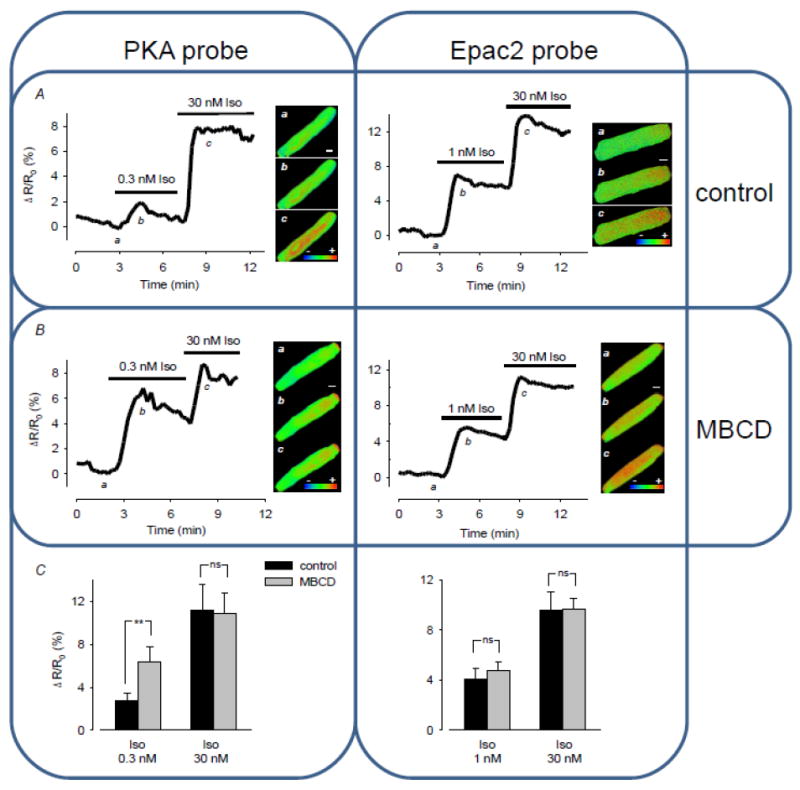
Cyclic AMP responses were measured in adult rat ventricular myocytes using genetically-encoded FRET-based biosensors based on type II PKA which indexes cAMP in type II PKA domains including caveolae (left hand panels) and Epac2 without its tethering sequence which indexes cAMP in the global cytosolic compartment (right hand panels). Time course of changes in FRET response and pseudocolor images recorded under control conditions (a), and following exposure to submaximally (b) and maximally (c) stimulating concentrations of the β1-AR agonist isoproterenol (Iso). Control cells (A); MBCD-treated cells (B); Average changes in FRET responses (C). **P < 0.05, ns = not significant, n = 5-7. All Iso responses were blocked by the β1-AR antagonist CGP20712A (100 nM), but not by the β2-AR antagonist ICI118,551 (100 nM). Adapted from [41].
2.1.2 β2 adrenoceptors
A role for caveolae in creating the local β2-AR cAMP signal was first suggested by Rybin et al. who showed that cholesterol depletion with cyclodextrin enhances cAMP accumulation in response to β2-AR stimulation in the neonatal cardiac myocyte [51]. Later, we confirmed a similar enhancement of ICa,L, [Ca2+]i transient and contractile responses to the β2-AR agonist salbutamol in MBCD-treated adult ventricular myocytes, effects which could be mimicked by intracellular dialysis with an antibody against Cav3 [18]. We have further shown that potentiation of the inotropic response to β2-AR stimulation when Gi coupling is abolished with pertussis toxin (PTX) is mimicked by caveolar disruption with MBCD, and that these effects are not additive [18], suggesting that caveolar control is mediated through β2-AR-Gi coupling. Certainly some studies have found the majority of Gi, along with β2-ARs, in buoyant caveolar fractions of the adult cardiac myocyte [21, 37]. This dependence on Gi could account for some differences reported in the literature regarding caveolar control of β2-AR responses. For example, in a study by Kamp and co-workers, the β2-AR response of ICa,L in neonatal myocytes was shown to be markedly attenuated by cholesterol depletion and siRNA against Cav3 [39]. This elegant work also showed evidence of caveolar complexes containing Cav3, β2-AR, the pore-forming subunit of the Ca2+ channel, Gαs, AC and PKA, consistent with the view that caveolae are required for β2-AR regulation of ICa,L. However, this work was carried out in the presence of PTX, and therefore reports specifically on effects of caveolar disruption on the Gs component of the β2-AR response. Our work in the adult myocyte suggests that the role of caveolae in facilitating β2-AR-Gi coupling may normally predominate when both Gs and Gi systems are intact.
We have shown that effects of caveolar disruption on β2-AR responses are associated with changes in the spatial characteristics of cAMP-dependent signalling. Following MBCD treatment, β2-AR stimulation promotes phosphorylation of PLB, an effect which is absent in control cells [25], suggesting that disruption of caveolae allows the β2-AR cAMP signal access to the SR compartment. These data are consistent with the view that the cAMP signal from β2-ARs is normally confined and curtailed by a caveolae-dependent mechanism. Over the years, a number of mechanisms have been suggested to account for the constraints on the β2-AR cAMP signal including a switch in β2-AR coupling from Gs to Gi (which inhibits AC 5/6 and enhances phosphatase activity)[26, 32], recruitment of PDEs to the β2-AR complex [59], fast desensitisation of the β2-AR upon agonist stimulation [60] and local PKA buffering of cAMP [30]. How these mechanisms relate to the caveolar-dependence of compartmentation is a point of debate.
One recent suggestion by Xiang and co-workers is that it is the transient nature of β2-AR-dependent PKA activation that determines lack of propagation of the signal to sites distant from the sarcolemma [29]. One mechanism which limits cAMP generation and facilitates local degradation of cAMP, thereby defining the kinetic characteristics of the signal, is β2-AR phosphorylation. Receptors can be phosphorylated by PKA [61] and G protein receptor kinase (GRK) [62]. PKA and GRK phosphorylation of the β2-AR shifts coupling from Gs to Gi so limiting cAMP production through inhibition of AC activity [61, 63]. GRK-dependent phosphorylation also recruits β-arrestin to the receptor [64, 65] which prevents receptor-G protein coupling and triggers receptor internalisation, so curtailing cAMP production. Furthermore, β arrestin recruits PDE4D3/5 to the receptor complex, promoting local cAMP degradation [64, 65]. The relative contribution of these different mechanisms to β2-AR cAMP production in the adult cardiac myocyte is not known; most studies use neonatal myocytes and/or heterologous receptor over-expression systems. However, one attractive proposition is that the caveolar compartment defines the small transient β2-AR cAMP signal by facilitating PKA- and/or GRK-dependent phosphorylation of the receptor. Modelling compartmentalised β-AR signalling in the canine ventricular myocyte has highlighted a pivotal role of PKA-mediated feedback loops in restricting cAMP in a localised manner [66]. Indeed, our recent work (MacDougall et al., this issue) suggests that caveolar compartmentation of β2-AR signalling arises through facilitation of receptor phosphorylation and receptor- Gi coupling, and that this has implications for cAMP production within the caveolar domain and for phosphatase activity in the SR compartment of the cell.
It is important not to lose sight of the fact that the distribution of receptors in the membrane can be dynamically regulated (e.g. by agonist stimulation). Insel and co-workers have shown that β2-ARs are lost from Cav3 immunoprecipitates following 10 min stimulation with 1 μM isoproterenol in the neonatal myocyte [6]. One interpretation of this data is that translocation of β2-ARs from the caveolar compartment is responsible for curtailing coupling between receptor and AC, thereby defining the brief β2-AR cAMP signal. In this study, receptor translocation was abolished by βARKct, which inhibits GRK activation by sequestering Gβγ subunits, consistent with GRK-dependent receptor phosphorylation acting as the trigger for receptor translocation. However, this must be reconciled with data which shows that GRK2 is largely absent from the caveolar compartment in these cells [51]. Further work defining the contribution of β2-AR phosphorylation in the adult cardiac myocyte is necessary to resolve the mechanisms underlying the spatial characteristics of the cAMP signal.
Although PKA is the most common effector of changes in cAMP mediated through GPCR stimulation, β-AR regulation of HCN4, the molecular correlate of If which contributes to sino-atrial node automaticity, is regulated by direct binding of cAMP to the channel [67] (but see [68]). Functionally, these channels are preferentially regulated by β2-, rather than β1-, AR stimulation [48] which reflects the established caveolar location of both HCN4 and the β2-AR. These data illustrate an example of caveolae acting as a local cAMP compartment which facilitates the access of cAMP produced via β2-AR stimulation of AC to the HCN4 channel. Indeed, it could be argued that the role of caveolae as a local signalling domain is more important for a mechanism which relies on direct binding of cAMP, than for one mediated by phosphorylation in which further specificity of signalling can be achieved by AKAP-tethering of PKA.
2.1.3 α1 adrenoceptors
α1-ARs have been linked with both Gs and Gq signalling pathways (see Section 3). Regarding the former, α1-AR attenuation of the transient outward current (Ito) via Gαs stimulation of a PKA-dependent pathway has been described [69], although the mechanism for α1-AR regulation of Ito is controversial [70] and α1-AR stimulation has been shown to inhibit ICa,L by reducing cAMP-dependent signalling [71]. α1-AR are found predominantly in caveolar fractions of the adult myocardium [40, 42] and it has recently been proposed that caveolae represent an α1-AR-Ito regulatory domain in the adult ventricular myocyte, based on findings of 2 caveolar-based complexes, one of PKA tethered to the KV4.2/KV4.3 channel via AKAP100 and another containing α1-AR, Gαs and AC. These workers suggest that the population of Ito channels present in Cav3-containing membrane domains is rapidly and specifically regulated by α1-AR stimulation of PKA; channels outside caveolae are still accessible to α1-AR signalling, but regulation is less efficient [40].
2.1.4 Muscarinic receptors
The M2 muscarinic receptor (M2-R) is the primary subtype of muscarinic acetylcholine receptor in the heart, where it produces diverse functional responses in pacemaker, atrial and ventricular cells [72]. Some of the M2-R effects are mediated through changes in cAMP production and PKA activity, and this plays an important role in regulating both electrical and mechanical activity in the heart [73]. M2-R activation can regulate cAMP production directly through Gi-dependent modulation of adenylyl cyclase, or indirectly through NO which, via cGMP, activates PDE2 and inhibits PDE3 (see [73]).
In the adult ventricular myocyte, M2-Rs are associated primarily with non-caveolar fractions of the plasma membrane in unstimulated conditions [5, 37], but translocate to caveolar fractions following exposure to high concentrations of agonist [5]. Feron et al. concluded that this translocation is necessary for the receptor to regulate the activity of eNOS which is found exclusively in caveolae [5, 74] (see Section 2.2). Indeed, in agreement with this hypothesis, in atrial myocytes MBCD treatment abolishes NO production in response to acetylcholine [75]. In the ventricular myocyte, the role of NO in mediating the effects of M2-R activation on cAMP production is controversial [73], however, current data are consistent with the idea that muscarinic inhibition of cAMP production occurs in a caveolar membrane domain. This is supported by work done in our laboratory using a FRET-based biosensor to demonstrate that M2-R activation inhibits cAMP production in locations where type II PKA is located [56], which includes caveolar fractions of the plasma membrane [39, 51, 58].
In addition to inhibiting cAMP production, M2-R activation can also enhance cAMP production, resulting in a complex biphasic response [73]. In ventricular myocytes, these opposing actions have been attributed to the ability of M2-R activation of Gi to inhibit cAMP production by AC type 5 and/or 6, while at the same time facilitating cAMP production by AC 4/7 [76]. It has been hypothesized that differences in the distribution of these AC isoforms may play a role in producing the complex M2-R response [77]. While AC 5/6 is found in caveolar membrane fractions of cardiac myocytes [37, 51], AC 4/7 is consistently excluded from caveolar fractions wherever it has been examined [43]. Computational modelling suggests that M2-R inhibition of cAMP production in caveolar domains and facilitation of cAMP production in extracaveolar domains is a feasible explanation for the biphasic response to M2-R stimulation in the adult ventricular myocyte [50, 77].
2.1.5 Prostaglandin receptors
Prostaglandin receptor signalling provided the first example of cAMP compartmentation in the heart (see [78]). In adult cardiac myocytes, activation of E-type prostaglandin receptors (e.g. EP2-R and EP4-R) stimulates cAMP production [41, 57, 78], yet this does not increase ICa,L or contractility [31, 41, 57], suggesting that the increase in cAMP is taking place in a compartment which does not contain L-type Ca2+ channels, phospholamban or RyR. Using FRET based cAMP biosensors, we have recently shown that disrupting caveolae with MBCD in adult rat myocytes has no impact on cAMP in either cytosolic or PKA II compartments following prostaglandin E1 (PGE1) stimulation [41]. Furthermore, MBCD does not reveal inotropic effects of PGE1 in these cells [41]. These data suggest that it is actually exclusion from caveolae that contributes to compartmentation of the cAMP produced by prostaglandin receptors. Consistent with this idea, we only find prostaglandin receptors in non-caveolar fractions of the plasma-membrane from adult myocytes [41]. The fact that disrupting caveolae does not eliminate compartmentation of prostaglandin receptor responses is further evidence that other factors must also be involved in defining the spatial distribution of cAMP. We previously demonstrated that, as far as PGE1 responses are concerned, this does not include PDE activity or Gi signalling [57].
To summarise, the cAMP signalling pathway provides a very clear illustration of the way in which caveolar distribution of receptors, key downstream signal components and their targets determines the profile of the response to receptor stimulation. This can be achieved through facilitation of the access of receptors to effectors (e.g. M2-R to eNOS and AC 5/6) and second messengers to targets (e.g. cAMP to HCN4). In addition, the caveolar organisation (and regulation) of elements responsible for the production and degradation of cAMP provides a potential framework for understanding how cAMP compartmentation is achieved following β1-and β2-AR stimulation.
2.2 Nitric oxide
The major constitutively expressed isoform of NOS in the heart, eNOS, is exquisitely dependent on caveolae. Its dual palmitoylation directs it to caveolae, where interaction with caveolin maintains it in an inactive state (see [79]). Caveolar derived NO production can regulate cAMP-dependent signalling via effects on PDE activity as already discussed for M2-R regulation of NO production (section 2.1.4). However, a role for caveolae in regulation of NO-dependent signalling extends beyond the M2-R pathway.
NO regulates protein function independently of cGMP- and cAMP-dependent pathways via direct S-nitrosylation. One example of this in the adult cardiac cell is progesterone regulation of Kv7.1 channels, which are responsible for the slow delayed rectifier current (IKs). These channels are found in caveolar membrane fractions [80, 81] along with the progesterone receptor, c-Src, phosphoinositide 3-kinse (PI3K), Akt and eNOS [81]. Nakamura et al. suggested that the presence of Kv7.1 channels in caveolae plays an important role in stimulation of IKs by progesterone, through PI3K/Akt-dependent activation of eNOS and subsequent S-nitrosylation of the channel [81]. This study also showed inhibition of ICa,L by progesterone stimulation of NO production (by a separate cGMP-dependent mechanism). These authors proposed that co-ordinated regulation of multiple caveolar ion channels by a NO-dependent signalling pathway could explain the complex changes in action potential repolarisation that are thought to provide protection from ventricular arrhythmia in certain hormonal states.
So far in this review, discussion has focused on the way in which the caveolar environment affects the regulation of proteins within caveolae. However, caveolar NO production provides a good illustration of the way in which a caveolar-derived signalling component has the potential to affect proteins outside caveolae. In neonatal and adult cardiac myocytes, MBCD treatment reduces SR Ca2+ release without effects on SR Ca2+ load or trigger (ICa,L) [18, 82], suggesting that caveolae can regulate Ca2+ release from the SR, likely through direct effects on the RyR. A simple explanation for the effects of disrupting caveolae in the adult ventricular myocyte is that caveolae constitutively regulate SR Ca2+ release by local production of diffusible mediators such as NO. We know that eNOS is located exclusively in caveolae in the adult myocardium [5, 74] and that Cav3 co-localises with extra-dyadic RyR in the cell interior by confocal microscopy [17], suggesting that t-tubular caveolae are close to corbular SR compartments. NO-dependent S-nitrosylation acts to increase RyR open probability [83] which would enhance SR Ca2+ release for a given ICa,L trigger (but another SR loading mechanism would need to operate in parallel in order for this to result in a maintained change in the [Ca2+]i transient [84]).
2.3 Caveolae as a Ca2+ compartment
As described in sections 2.1 and 2.2, caveolae regulate ionic fluxes primarily through their ability to modulate access of signal components to ion channels. However, a body of evidence suggest that caveolae are local ion compartments independent of ion channel regulation by GPCRs and NO. This is particularly true for Ca2+. Caveolae were first proposed as Ca2+ compartments by Popescu et al. [85] who showed that these are sites of Ca2+ entry in smooth muscle. In the cardiac cell, caveolae contain a proportion of L type Ca2+ channels as well as other Ca2+-regulatory proteins. In the bovine heart, the Na-Ca exchanger (NCX1) is enriched in Cav3-containing buoyant membrane fractions, and shows positive regulation by interaction with Cav3 [86]. NCX has also been found in a molecular complex with Cav3 in the human heart [87]. Although studies that have attempted to index the proportion of NCX which colocalises with Cav3 using immunofluorescence with image deconvolution suggest that only a small proportion of NCX is in caveolae [17, 88], it is tempting to speculate that caveolar-based NCX may be important for providing efficient coupling between Na+ and Ca2+ fluxes. For example, NCX is important for stretch- and AT1 receptor-induced elevations in [Ca2+]i secondary to increased [Na+]i via the Na-H exchanger (NHE) and TRPC3 [89, 90]. In various non-cardiac tissues, (including endothelial, vascular smooth muscle, and skeletal muscle cells), TRPC 1, 3 and 4 are found in caveolin-containing membrane fractions [91] and TRPC1 binds Cav3 (probably through a CSD binding sequence in its C terminus) [92]. Likewise, NHE1 is found exclusively in caveolae fractions in the adult myocardium [74]. Thus co-localisation of ion channels/transporters in a restricted diffusional space represented by caveolae may link ionic fluxes. This may have implications for local regulation of Ca2+ that contributes to contractility, regulation of Ca2+ -sensitive proteins resident in caveolae (AC, eNOS) [93, 94], and prohypertrophic signalling (see review in this issue by Goonasekera & Molkentin).
3. Caveolae as lipid-dependent signalling compartments
As described in section 2, the vast majority of work which links caveolae with the control of signalling in the cardiac cell is based on the role of caveolae in bringing together protein components of signal cascades. However, there is evidence that certain signalling pathways exploit the local lipid environment of caveolae. This concept was proposed by Pike and co-workers in the 1990s who observed, in a range of cultured cell lines, that as much as half of the cellular phosphatidylinositol 4,5 bisphosphate (PIP2) was present in caveolin-enriched buoyant fractions [95-97]. This caveolar pool of PIP2 was selectively depleted by epidermal growth factor (EGF) or bradykinin in A431 cells [97]; effects which were absent following disruption of the caveolar domain with MBCD [98]. More recently, these findings have been extended to the cardiac cell. The α1-AR is found in caveolar fractions of the adult myocardium, along with its main transducing G protein, Gq [40, 42]. Whilst Gs/Gi-linked signalling relies on the protein constituents of caveolae, evidence suggests that Gq coupled cascades utilise specific changes in the lipid components of caveolae to create local signal domains. In the neonatal cardiac cell, caveolar and non-caveolar rafts are enriched in PIP2, and α1-AR stimulation has been shown to selectively deplete PIP2 from caveolar fractions [52]. Hydrolysis of PIP2 to diacylglycerol (DAG) by phospholipase C will have consequences for the activity of PIP2-sensitive ion channels and exchangers (e.g. TRP and NCX), and DAG-activated channels (e.g. TRPC) [99-101]. Therefore this work illustrates a mechanism which allows selective regulation of the activity of the caveolar population of PIP2/DAG- sensitive ion channels/exchangers [52].
4. Caveolae as a membrane reservoir
Caveolar regulation of signalling relies on the distinct protein and lipid composition of caveolar membranes. However, there is an additional property of caveolae which can likewise contribute to their role in signal transduction; this is their morphology. The invaginated structure of caveolae may permit them to function as a reservoir of ion channels, which can be recruited as required, and to act as a buffer of membrane tension with implications for the activity of mechano-gated ion channels.
4.1 Caveolae as a recruitable reservoir of ion channels
A proportion of Na+ channels are localised to caveolar fractions in the adult ventricular myocyte, interact with Cav3 (as shown by co-immunoprecipitation), and cluster with Cav3 in plasma membrane sheets [38, 47]. β-AR stimulation increases INa via Gαs–dependent mechanisms; one which involves PKA-dependent phosphorylation (with consequences for single channel voltage characteristics) and another which is independent of PKA and appears to increase the number of functional channels at the cell membrane (see [38]). Perhaps surprisingly, given the wealth of evidence for caveolardependent regulation of PKA, for INa it is the PKA-independent pathway that has received attention as one that depends on caveolae. Shibata and co-workers have proposed that populations of ‘closed’ caveolae act as a reservoir of electrically inaccessible Na channels that can be recruited by Gαs-Cav3 dependent opening of the caveolar neck, as required [38, 47, 102]. Of course, such a model would predict effects of β-AR stimulation on the plasma membrane presentation of other ion channels normally located in caveolae and, as such, would be expected to play a major role in sympathetic regulation of myocyte excitability. The physiological relevance of this mechanism requires further investigation. However, the finding of Cav3 mutations in patients with long QT syndrome has been tentatively linked with an increase in late INa through increased caveolar Na+ channel accessibility [103].
4.2 Caveolae as a buffer of membrane tension
In addition to recruitment via β-AR opening of caveolar necks, caveolar membranes can also be incorporated into surface sarcolemma when extra surface membrane is required during cell stretch or swelling. This property of caveolae has led over the years to suggestions that caveolae are cellular mechanosensors and/or mechanotransducers (e.g. [104, 105]). Initial studies of a role for caveolae in mechanotransduction proposed a model whereby caveolae simply act as a platform for co-ordination of mechanotransductive signalling complexes [106]. This work focused on the swelling-activated chloride current (ICl,swell). The ICl,swell channel responds to changes in membrane tension during cell swelling and is an important regulator of cell volume. In the cardiac cell, the electrophysiological effects of ICl, Swell activation can pre-dispose to arrhythmia during episodes of ischaemia-reperfusion [107]. In an epithelial cell line, the requirement of caveolin for swelling-induced activation of ICl,swell has been clearly demonstrated [106]. Eggermont and co-workers proposed that caveolar colocalisation of the ICl, Swell channel with tyrosine kinases that regulate its activity is essential for channel activation in response to stretch [106, 108]. However, our data in the adult cardiac myocyte suggest that this is not a universal mechanism; in cardiac cells we have shown that caveolae provide a means of buffering changes in membrane tension and it is this effect which predominates in the process of (limiting) channel activation during swelling [8]. Similar evidence for caveolae as a reserve of membrane during swelling and stretch has recently been presented for HeLa and endothelial cells [109]. These findings have wide implications for caveolae in regulation of the many channels that are gated by changes in membrane tension (see [74] for a recent review). Indeed it has been suggested that membrane reserves such as caveolae, which provide a means of maintaining membrane tension during acute mechanical perturbations, may account for the difficulty in activating mechanosensitive channels in some mammalian cells [110].
5. Conclusions and outlook
Caveolae are protein compartments that serve as signalling platforms allowing receptors and their downstream signal cascades to efficiently and selectively access their targets, and functionally link ionic fluxes by providing a restricted diffusional space. However, caveolae’s local control extends beyond the caveolae itself through compartmentalisation of cAMP signalling and by the action of NO produced specifically in the caveolar domain. In addition to their role as protein compartments, the lipid environment of caveolae and their distinct flask-shaped morphology may have implications for the function of ion channels and exchangers located within and outside the caveolar domain.
Whilst the concept of local control by caveolae is well-accepted, in recent years the idea of heterogeneous populations of caveolae has added something to our understanding of how caveolae can co-ordinate myriad signalling pathways within a single cell. There is evidence (from non-cardiac myocytes) that differences in caveolin isoform, cavin isoform and cholesterol content can create morphologically and/or functionally distinct populations of caveolae within a tissue (e.g. [111-113]). Furthermore, the size of caveolae means that they are likely to contain only a limited number of proteins [114, 115]. Dart and co-workers proposed the concept of subsets of caveolae containing different complements of signalling molecules and their targets, based on a study of protein kinase C regulation of KATP channels in vascular smooth muscle [116]. Functional heterogeneity of caveolae may be more likely in cardiac and vascular smooth muscle cells that have been shown to express all 3 isoforms of caveolin.
The necessity of maintaining caveolin expression within normal limits is clearly demonstrated by studies of transgenic mice with knockdown or over-expression of Cav3; both develop cardiomyopathy [53, 117]. Diverse changes in caveolae, caveolin and cavin expression have been documented in experimental models of cardiac hypertrophy and failure [118-120], and in patients with long QT syndrome [103, 121]). We anticipate that the future direction of this field will focus on caveolae as a mechanism and target for cardiac dysfunction in disease.
Highlights.
Caveolae are protein compartments that control the production of cAMP and NO
Some signalling pathways exploit the lipid (PIP2) environment of caveolae
Caveolae can buffer increases in membrane tension during stretch
Caveolae are key components of GPCR and mechanotransductive signalling in the heart
Acknowledgments
Sarah Calaghan and Robert Harvey’s work is sponsored by the Medical Research Council (SC, RH), British Heart Foundation (SC), National Institutes of Health (RH), and American Heart Association (RH). We thank John Colyer for helpful discussion of this manuscript.
Footnotes
Disclosures
None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Palade GE. Fine structure of blood capillaries. J Appl Physiol. 1953;24:1424–1436. [Google Scholar]
- 2.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–72. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 3.Sens P, Turner MS. The forces that shape caveolae. In: Fielding CJ, editor. Lipid Rafts and Caveolae. Weinheim: WILEY-VCH Verlag GmbH & Co. KGaA; 2007. pp. 25–44. [Google Scholar]
- 4.Hansen CG, Nichols BJ. Exploring the caves: cavins, caveolins and caveolae. Trends Cell Biol. 2010;20:177–86. doi: 10.1016/j.tcb.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Feron O, Smith TW, Michel T, Kelly RA. Dynamic targeting of the agonist-stimulated m2 muscarinic acetylcholine receptor to caveolae in cardiac myocytes. J Biol Chem. 1997;272:17744–8. doi: 10.1074/jbc.272.28.17744. [DOI] [PubMed] [Google Scholar]
- 6.Ostrom RS, Gregorian C, Drenan RM, Xiang Y, Regan JW, Insel PA. Receptor number and caveolar co-localization determine receptor coupling efficiency to adenylyl cyclase. J Biol Chem. 2001;276:42063–9. doi: 10.1074/jbc.M105348200. [DOI] [PubMed] [Google Scholar]
- 7.Patel HH, Tsutsumi YM, Head BP, Niesman IR, Jennings M, Horikawa Y, et al. Mechanisms of cardiac protection from ischemia/reperfusion injury: a role for caveolae and caveolin-1. FASEB J. 2007;21:1565–74. doi: 10.1096/fj.06-7719com. [DOI] [PubMed] [Google Scholar]
- 8.Kozera L, White E, Calaghan S. Caveolae act as membrane reserves which limit mechanosensitive I(Cl,swell) channel activation during swelling in the rat ventricular myocyte. PLoS One. 2009;4:e8312. doi: 10.1371/journal.pone.0008312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graham G, Stones R, White E, Harrison S, Gilbert S, Billeter R, et al. Microarray analysis of gene expression during adult ventricular myocye culture. Biophys J. 2008:1491–Pos. [Google Scholar]
- 10.Balijepalli RC, Kamp TJ. Caveolae, ion channels and cardiac arrhythmias. Prog Biophys Mol Biol. 2008;98:149–60. doi: 10.1016/j.pbiomolbio.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Couet J, Li S, Okamoto T, Ikezu T, Lisanti MP. Identification of peptide and protein ligands for the caveolin-scaffolding domain. Implications for the interaction of caveolin with caveolae-associated proteins. J Biol Chem. 1997;272:6525–33. doi: 10.1074/jbc.272.10.6525. [DOI] [PubMed] [Google Scholar]
- 12.Razani B, Woodman SE, Lisanti MP. Caveolae: from cell biology to animal physiology. Pharmacol Rev. 2002;54:431–67. doi: 10.1124/pr.54.3.431. [DOI] [PubMed] [Google Scholar]
- 13.Sargiacomo M, Scherer PE, Tang Z, Kubler E, Song KS, Sanders MC, et al. Oligomeric structure of caveolin: implications for caveolae membrane organization. Proc Natl Acad Sci U S A. 1995;92:9407–11. doi: 10.1073/pnas.92.20.9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Radel C, Rizzo V. Integrin mechanotransduction stimulates caveolin-1 phosphorylation and recruitment of Csk to mediate actin reorganization. Am J Physiol Heart Circ Physiol. 2005;288:H936–H945. doi: 10.1152/ajpheart.00519.2004. [DOI] [PubMed] [Google Scholar]
- 15.Head BP, Patel HH, Roth DM, Murray F, Swaney JS, Niesman IR, et al. Microtubules and actin microfilaments regulate lipid raft/caveolae localization of adenylyl cyclase signaling components. J Biol Chem. 2006;281:26391–9. doi: 10.1074/jbc.M602577200. [DOI] [PubMed] [Google Scholar]
- 16.Fuhs SR, Insel PA. Caveolin-3 Undergoes SUMOylation by the SUMO E3 Ligase PIASy: SUMOYLATION AFFECTS G-PROTEIN-COUPLED RECEPTOR DESENSITIZATION. J Biol Chem. 2011;286:14830–41. doi: 10.1074/jbc.M110.214270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scriven DR, Klimek A, Asghari P, Bellve K, Moore ED. Caveolin-3 is adjacent to a group of extradyadic ryanodine receptors. Biophys J. 2005;89:1893–901. doi: 10.1529/biophysj.105.064212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calaghan S, White E. Caveolae modulate excitation-contraction coupling and beta2-adrenergic signalling in adult rat ventricular myocytes. Cardiovasc Res. 2006;69:816–24. doi: 10.1016/j.cardiores.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Levin KR, Page E. Quantitative studies on plasmalemmal folds and caveolae of rabbit ventricular myocardial cells. Circ Res. 1980;46:244–55. doi: 10.1161/01.res.46.2.244. [DOI] [PubMed] [Google Scholar]
- 20.Haddock PS, Coetzee WA, Cho E, Porter L, Katoh H, Bers DM, et al. Subcellular [Ca2+]i gradients during excitation-contraction coupling in newborn rabbit ventricular myocytes. Circ Res. 1999;85:415–27. doi: 10.1161/01.res.85.5.415. [DOI] [PubMed] [Google Scholar]
- 21.Rybin VO, Pak E, Alcott S, Steinberg SF. Developmental changes in beta2-adrenergic receptor signaling in ventricular myocytes: the role of Gi proteins and caveolae microdomains. Mol Pharmacol. 2003;63:1338–48. doi: 10.1124/mol.63.6.1338. [DOI] [PubMed] [Google Scholar]
- 22.Hulme JT, Westenbroek RE, Scheuer T, Catterall WA. Phosphorylation of serine 1928 in the distal C-terminal domain of cardiac CaV1.2 channels during beta1-adrenergic regulation. Proc Natl Acad Sci U S A. 2006;103:16574–9. doi: 10.1073/pnas.0607294103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sulakhe PV, Vo XT. Regulation of phospholamban and troponin-I phosphorylation in the intact rat cardiomyocytes by adrenergic and cholinergic stimuli: roles of cyclic nucleotides, calcium, protein kinases and phosphatases and depolarization. Mol Cell Biochem. 1995;149-150:103–26. doi: 10.1007/BF01076569. [DOI] [PubMed] [Google Scholar]
- 24.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–76. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 25.Calaghan S, Kozera L, White E. Compartmentalisation of cAMP-dependent signalling by caveolae in the adult cardiac myocyte. J Mol Cell Cardiol. 2008;45:88–92. doi: 10.1016/j.yjmcc.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Xiao RP, Cheng H, Zhou YY, Kuschel M, Lakatta EG. Recent advances in cardiac beta(2)-adrenergic signal transduction. Circ Res. 1999;85:1092–100. doi: 10.1161/01.res.85.11.1092. [DOI] [PubMed] [Google Scholar]
- 27.Zaccolo M, De GF, Cho CY, Feng L, Knapp T, Negulescu PA, et al. A genetically encoded, fluorescent indicator for cyclic AMP in living cells. Nat Cell Biol. 2000;2:25–9. doi: 10.1038/71345. [DOI] [PubMed] [Google Scholar]
- 28.Nikolaev VO, Bunemann M, Schmitteckert E, Lohse MJ, Engelhardt S. Cyclic AMP Imaging in Adult Cardiac Myocytes Reveals Far-Reaching {beta}1-Adrenergic but Locally Confined {beta}2-Adrenergic Receptor-Mediated Signaling. Circ Res. 2006;99:1084–91. doi: 10.1161/01.RES.0000250046.69918.d5. [DOI] [PubMed] [Google Scholar]
- 29.Soto D, De AV, Zhang J, Xiang Y. Dynamic protein kinase a activities induced by beta-adrenoceptors dictate signaling propagation for substrate phosphorylation and myocyte contraction. Circ Res. 2009;104:770–9. doi: 10.1161/CIRCRESAHA.108.187880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nikolaev VO, Moshkov A, Lyon AR, Miragoli M, Novak P, Paur H, et al. Beta2-adrenergic receptor redistribution in heart failure changes cAMP compartmentation. Science. 2010;327:1653–7. doi: 10.1126/science.1185988. [DOI] [PubMed] [Google Scholar]
- 31.Rochais F, bi-Gerges A, Horner K, Lefebvre F, Cooper DM, Conti M, et al. A specific pattern of phosphodiesterases controls the cAMP signals generated by different Gs-coupled receptors in adult rat ventricular myocytes. Circ Res. 2006;98:1081–8. doi: 10.1161/01.RES.0000218493.09370.8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuschel M, Zhou YY, Cheng H, Zhang SJ, Chen Y, Lakatta EG, et al. G(i) protein-mediated functional compartmentalization of cardiac beta(2)-adrenergic signaling. J Biol Chem. 1999;274:22048–52. doi: 10.1074/jbc.274.31.22048. [DOI] [PubMed] [Google Scholar]
- 33.Jo SH, Leblais V, Wang PH, Crow MT, Xiao RP. Phosphatidylinositol 3-kinase functionally compartmentalizes the concurrent G(s) signaling during beta2-adrenergic stimulation. Circ Res. 2002;91:46–53. doi: 10.1161/01.res.0000024115.67561.54. [DOI] [PubMed] [Google Scholar]
- 34.Dodge-Kafka KL, Bauman A, Kapiloff MS. A-kinase anchoring proteins as the basis for cAMP signaling. Handb Exp Pharmacol. 2008:3–14. doi: 10.1007/978-3-540-72843-6_1. [DOI] [PubMed] [Google Scholar]
- 35.Nilsson R, Ahmad F, Sward K, Andersson U, Weston M, Manganiello V, et al. Plasma membrane cyclic nucleotide phosphodiesterase 3B (PDE3B) is associated with caveolae in primary adipocytes. Cell Signal. 2006;18:1713–21. doi: 10.1016/j.cellsig.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 36.Berger K, Lindh R, Wierup N, Zmuda-Trzebiatowska E, Lindqvist A, Manganiello VC, et al. Phosphodiesterase 3B is localized in caveolae and smooth ER in mouse hepatocytes and is important in the regulation of glucose and lipid metabolism. PLoS One. 2009;4:e4671. doi: 10.1371/journal.pone.0004671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Head BP, Patel HH, Roth DM, Lai NC, Niesman IR, Farquhar MG, et al. G-protein-coupled receptor signaling components localize in both sarcolemmal and intracellular caveolin-3-associated microdomains in adult cardiac myocytes. J Biol Chem. 2005;280:31036–44. doi: 10.1074/jbc.M502540200. [DOI] [PubMed] [Google Scholar]
- 38.Yarbrough TL, Lu T, Lee HC, Shibata EF. Localization of cardiac sodium channels in caveolin-rich membrane domains: regulation of sodium current amplitude. Circ Res. 2002;90:443–9. doi: 10.1161/hh0402.105177. [DOI] [PubMed] [Google Scholar]
- 39.Balijepalli RC, Foell JD, Hall DD, Hell JW, Kamp TJ. Localization of cardiac L-type Ca(2+) channels to a caveolar macromolecular signaling complex is required for beta(2)-adrenergic regulation. Proc Natl Acad Sci U S A. 2006;103:7500–5. doi: 10.1073/pnas.0503465103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alday A, Urrutia J, Gallego M, Casis O. alpha1-adrenoceptors regulate only the caveolae-located subpopulation of cardiac K(V)4 channels. Channels (Austin) 2010;4 doi: 10.4161/chan.4.3.11479. [DOI] [PubMed] [Google Scholar]
- 41.Agarwal SR, Macdougall DA, Tyser R, Pugh SD, Calaghan SC, Harvey RD. Effects of cholesterol depletion on compartmentalized cAMP responses in adult cardiac myocytes. J Mol Cell Cardiol. 2011;50:500–9. doi: 10.1016/j.yjmcc.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fujita T, Toya Y, Iwatsubo K, Onda T, Kimura K, Umemura S, et al. Accumulation of molecules involved in alpha1-adrenergic signal within caveolae: caveolin expression and the development of cardiac hypertrophy. Cardiovasc Res. 2001;51:709–16. doi: 10.1016/s0008-6363(01)00348-0. [DOI] [PubMed] [Google Scholar]
- 43.Willoughby D, Cooper DM. Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiol Rev. 2007;87:965–1010. doi: 10.1152/physrev.00049.2006. [DOI] [PubMed] [Google Scholar]
- 44.Toya Y, Schwencke C, Couet J, Lisanti MP, Ishikawa Y. Inhibition of adenylyl cyclase by caveolin peptides. Endocrinology. 1998;139:2025–31. doi: 10.1210/endo.139.4.5957. [DOI] [PubMed] [Google Scholar]
- 45.Razani B, Rubin CS, Lisanti MP. Regulation of cAMP-mediated signal transduction via interaction of caveolins with the catalytic subunit of protein kinase A. J Biol Chem. 1999;274:26353–60. doi: 10.1074/jbc.274.37.26353. [DOI] [PubMed] [Google Scholar]
- 46.Li S, Okamoto T, Chun M, Sargiacomo M, Casanova JE, Hansen SH, et al. Evidence for a regulated interaction between heterotrimeric G proteins and caveolin. J Biol Chem. 1995;270:15693–701. doi: 10.1074/jbc.270.26.15693. [DOI] [PubMed] [Google Scholar]
- 47.Shibata EF, Brown TL, Washburn ZW, Bai J, Revak TJ, Butters CA. Autonomic regulation of voltagegated cardiac ion channels. J Cardiovasc Electrophysiol. 2006;17(Suppl 1):S34–S42. doi: 10.1111/j.1540-8167.2006.00387.x. [DOI] [PubMed] [Google Scholar]
- 48.Barbuti A, Terragni B, Brioschi C, Difrancesco D. Localization of f-channels to caveolae mediates specific beta2-adrenergic receptor modulation of rate in sinoatrial myocytes. J Mol Cell Cardiol. 2007;42:71–8. doi: 10.1016/j.yjmcc.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 49.Barbuti A, Gravante B, Riolfo M, Milanesi R, Terragni B, Difrancesco D. Localization of pacemaker channels in lipid rafts regulates channel kinetics. Circ Res. 2004;94:1325–31. doi: 10.1161/01.RES.0000127621.54132.AE. [DOI] [PubMed] [Google Scholar]
- 50.Iancu RV, Ramamurthy G, Warrier S, Nikolaev VO, Lohse MJ, Jones SW, et al. Cytoplasmic cAMP concentrations in intact cardiac myocytes. Am J Physiol Cell Physiol. 2008;295:C414–C422. doi: 10.1152/ajpcell.00038.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rybin VO, Xu X, Lisanti MP, Steinberg SF. Differential targeting of beta -adrenergic receptor subtypes and adenylyl cyclase to cardiomyocyte caveolae. A mechanism to functionally regulate the cAMP signaling pathway. J Biol Chem. 2000;275:41447–57. doi: 10.1074/jbc.M006951200. [DOI] [PubMed] [Google Scholar]
- 52.Morris JB, Huynh H, Vasilevski O, Woodcock EA. Alpha1-adrenergic receptor signaling is localized to caveolae in neonatal rat cardiomyocytes. J Mol Cell Cardiol. 2006;41:17–25. doi: 10.1016/j.yjmcc.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 53.Woodman SE, Park DS, Cohen AW, Cheung MW, Chandra M, Shirani J, et al. Caveolin-3 knock-out mice develop a progressive cardiomyopathy and show hyperactivation of the p42/44 MAPK cascade. J Biol Chem. 2002;277:38988–97. doi: 10.1074/jbc.M205511200. [DOI] [PubMed] [Google Scholar]
- 54.Feron O, Dessy C, Opel DJ, Arstall MA, Kelly RA, Michel T. Modulation of the endothelial nitric-oxide synthase-caveolin interaction in cardiac myocytes. Implications for the autonomic regulation of heart rate. J Biol Chem. 1998;273:30249–54. doi: 10.1074/jbc.273.46.30249. [DOI] [PubMed] [Google Scholar]
- 55.Xiang Y, Rybin VO, Steinberg SF, Kobilka B. Caveolar localization dictates physiologic signaling of beta 2-adrenoceptors in neonatal cardiac myocytes. J Biol Chem. 2002;277:34280–6. doi: 10.1074/jbc.M201644200. [DOI] [PubMed] [Google Scholar]
- 56.Warrier S, Belevych AE, Ruse M, Eckert RL, Zaccolo M, Pozzan T, et al. Beta-adrenergic- and muscarinic receptor-induced changes in cAMP activity in adult cardiac myocytes detected with FRETbased biosensor. Am J Physiol Cell Physiol. 2005;289:C455–C461. doi: 10.1152/ajpcell.00058.2005. [DOI] [PubMed] [Google Scholar]
- 57.Warrier S, Ramamurthy G, Eckert RL, Nikolaev VO, Lohse MJ, Harvey RD. cAMP microdomains and L-type Ca2+ channel regulation in guinea-pig ventricular myocytes. J Physiol. 2007;580:765–76. doi: 10.1113/jphysiol.2006.124891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nichols CB, Rossow CF, Navedo MF, Westenbroek RE, Catterall WA, Santana LF, et al. Sympathetic stimulation of adult cardiomyocytes requires association of AKAP5 with a subpopulation of L-type calcium channels. Circ Res. 2010;107:747–56. doi: 10.1161/CIRCRESAHA.109.216127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xiang Y, Naro F, Zoudilova M, Jin SL, Conti M, Kobilka B. Phosphodiesterase 4D is required for beta2 adrenoceptor subtype-specific signaling in cardiac myocytes. Proc Natl Acad Sci U S A. 2005;102:909–14. doi: 10.1073/pnas.0405263102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Violin JD, DiPilato LM, Yildirim N, Elston TC, Zhang J, Lefkowitz RJ. beta2-adrenergic receptor signaling and desensitization elucidated by quantitative modeling of real time cAMP dynamics. J Biol Chem. 2008;283:2949–61. doi: 10.1074/jbc.M707009200. [DOI] [PubMed] [Google Scholar]
- 61.Zamah AM, Delahunty M, Luttrell LM, Lefkowitz RJ. Protein kinase A-mediated phosphorylation of the beta 2-adrenergic receptor regulates its coupling to Gs and Gi. Demonstration in a reconstituted system. J Biol Chem. 2002;277:31249–56. doi: 10.1074/jbc.M202753200. [DOI] [PubMed] [Google Scholar]
- 62.Seibold A, Williams B, Huang ZF, Friedman J, Moore RH, Knoll BJ, et al. Localization of the sites mediating desensitization of the beta(2)-adrenergic receptor by the GRK pathway. Mol Pharmacol. 2000;58:1162–73. doi: 10.1124/mol.58.5.1162. [DOI] [PubMed] [Google Scholar]
- 63.Wang Y, De AV, Gao X, Ramani B, Jung YS, Xiang Y. Norepinephrine- and epinephrine-induced distinct beta2-adrenoceptor signaling is dictated by GRK2 phosphorylation in cardiomyocytes. J Biol Chem. 2008;283:1799–807. doi: 10.1074/jbc.M705747200. [DOI] [PubMed] [Google Scholar]
- 64.Baillie GS, Sood A, McPhee I, Gall I, Perry SJ, Lefkowitz RJ, et al. beta-Arrestin-mediated PDE4 cAMP phosphodiesterase recruitment regulates beta-adrenoceptor switching from Gs to Gi. Proc Natl Acad Sci U S A. 2003;100:940–5. doi: 10.1073/pnas.262787199. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 65.Perry SJ, Baillie GS, Kohout TA, McPhee I, Magiera MM, Ang KL, et al. Targeting of cyclic AMP degradation to beta 2-adrenergic receptors by beta-arrestins. Science. 2002;298:834–6. doi: 10.1126/science.1074683. [DOI] [PubMed] [Google Scholar]
- 66.Heijman J, Volders PG, Westra RL, Rudy Y. Local control of beta-adrenergic stimulation: Effects on ventricular myocyte electrophysiology and Ca(2+)-transient. J Mol Cell Cardiol. 2011;50:863–71. doi: 10.1016/j.yjmcc.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Difrancesco D, Tortora P. Direct activation of cardiac pacemaker channels by intracellular cyclic AMP. Nature. 1991;351:145–7. doi: 10.1038/351145a0. [DOI] [PubMed] [Google Scholar]
- 68.Liao Z, Lockhead D, Larson ED, Proenza C. Phosphorylation and modulation of hyperpolarization-activated HCN4 channels by protein kinase A in the mouse sinoatrial node. J Gen Physiol. 2010;136:247–58. doi: 10.1085/jgp.201010488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gallego M, Setien R, Puebla L, Boyano-Adanez MC, Arilla E, Casis O. alpha1-Adrenoceptors stimulate a Galphas protein and reduce the transient outward K+ current via a cAMP/PKA-mediated pathway in the rat heart. Am J Physiol Cell Physiol. 2005;288:C577–C585. doi: 10.1152/ajpcell.00124.2004. [DOI] [PubMed] [Google Scholar]
- 70.Fedida D, Braun AP, Giles WR. Alpha 1-adrenoceptors in myocardium: functional aspects and transmembrane signaling mechanisms. Physiol Rev. 1993;73:469–87. doi: 10.1152/physrev.1993.73.2.469. [DOI] [PubMed] [Google Scholar]
- 71.Belevych AE, Nulton-Persson A, Sims C, Harvey RD. Role of tyrosine kinase activity in alpha-adrenergic inhibition of the beta-adrenergically regulated L-type Ca(2+) current in guinea-pig ventricular myocytes. J Physiol. 2001;537:779–92. doi: 10.1111/j.1469-7793.2001.00779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dhein S, van Koppen CJ, Brodde OE. Muscarinic receptors in the mammalian heart. Pharmacol Res. 2001;44:161–82. doi: 10.1006/phrs.2001.0835. [DOI] [PubMed] [Google Scholar]
- 73.Harvey RD, Belevych AE. Muscarinic regulation of cardiac ion channels. Br J Pharmacol. 2003;139:1074–84. doi: 10.1038/sj.bjp.0705338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Calaghan S. Role of caveolae in stretch-sensing: Implications for mechano-electric coupling. In: Kohl P, Sachs F, Franz MR, editors. Cardiac Mechano-Electric Feedback and Arrhythmias. Oxford: OUP; 2011. pp. 50–6. [Google Scholar]
- 75.Dedkova EN, Ji X, Wang YG, Blatter LA, Lipsius SL. Signaling mechanisms that mediate nitric oxide production induced by acetylcholine exposure and withdrawal in cat atrial myocytes. Circ Res. 2003;93:1233–40. doi: 10.1161/01.RES.0000106133.92737.27. [DOI] [PubMed] [Google Scholar]
- 76.Belevych AE, Sims C, Harvey RD. ACh-induced rebound stimulation of L-type Ca(2+) current in guinea-pig ventricular myocytes, mediated by Gbetagamma-dependent activation of adenylyl cyclase. J Physiol. 2001;536:677–92. doi: 10.1111/j.1469-7793.2001.00677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Iancu RV, Jones SW, Harvey RD. Compartmentation of cAMP signaling in cardiac myocytes: a computational study. Biophys J. 2007;92:3317–31. doi: 10.1529/biophysj.106.095356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Buxton IL, Brunton LL. Compartments of cyclic AMP and protein kinase in mammalian cardiomyocytes. J Biol Chem. 1983;258:10233–9. [PubMed] [Google Scholar]
- 79.Balligand JL, Feron O, Dessy C. eNOS activation by physical forces: from short-term regulation of contraction to chronic remodeling of cardiovascular tissues. Physiol Rev. 2009;89:481–534. doi: 10.1152/physrev.00042.2007. [DOI] [PubMed] [Google Scholar]
- 80.Balijepalli RC, Delisle BP, Balijepalli SY, Foell JD, Slind JK, Kamp TJ, et al. Kv11.1 (ERG1) K+ channels localize in cholesterol and sphingolipid enriched membranes and are modulated by membrane cholesterol. Channels (Austin) 2007;1:263–72. doi: 10.4161/chan.4946. [DOI] [PubMed] [Google Scholar]
- 81.Nakamura H, Kurokawa J, Bai CX, Asada K, Xu J, Oren RV, et al. Progesterone regulates cardiac repolarization through a nongenomic pathway: an in vitro patch-clamp and computational modeling study. Circulation. 2007;116:2913–22. doi: 10.1161/CIRCULATIONAHA.107.702407. [DOI] [PubMed] [Google Scholar]
- 82.Lohn M, Furstenau M, Sagach V, Elger M, Schulze W, Luft FC, et al. Ignition of calcium sparks in arterial and cardiac muscle through caveolae. Circ Res. 2000;87:1034–9. doi: 10.1161/01.res.87.11.1034. [DOI] [PubMed] [Google Scholar]
- 83.Xu L, Eu JP, Meissner G, Stamler JS. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science. 1998;279:234–7. doi: 10.1126/science.279.5348.234. [DOI] [PubMed] [Google Scholar]
- 84.Trafford AW, Diaz ME, Sibbring GC, Eisner DA. Modulation of CICR has no maintained effect on systolic Ca2+: simultaneous measurements of sarcoplasmic reticulum and sarcolemmal Ca2+ fluxes in rat ventricular myocytes. J Physiol. 2000;522(Pt 2):259–70. doi: 10.1111/j.1469-7793.2000.t01-2-00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Popescu LM, Diculescu I, Zelck U, Ionescu N. Ultrastructural distribution of calcium in smooth muscle cells of guinea-pig taenia coli. A correlated electron microscopic and quantitative study. Cell Tissue Res. 1974;154:357–78. doi: 10.1007/BF00223732. [DOI] [PubMed] [Google Scholar]
- 86.Bossuyt J, Taylor BE, James-Kracke M, Hale CC. The cardiac sodium-calcium exchanger associates with caveolin-3. Ann N Y Acad Sci. 2002;976:197–204. doi: 10.1111/j.1749-6632.2002.tb04741.x. [DOI] [PubMed] [Google Scholar]
- 87.Camors E, Charue D, Trouve P, Monceau V, Loyer X, Russo-Marie F, et al. Association of annexin A5 with Na+/Ca2+ exchanger and caveolin-3 in non-failing and failing human heart. J Mol Cell Cardiol. 2006;40:47–55. doi: 10.1016/j.yjmcc.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 88.Cavalli A, Eghbali M, Minosyan TY, Stefani E, Philipson KD. Localization of sarcolemmal proteins to lipid rafts in the myocardium. Cell Calcium. 2007;42:313–22. doi: 10.1016/j.ceca.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Perez NG, de Hurtado MC, Cingolani HE. Reverse mode of the Na+-Ca2+ exchange after myocardial stretch: underlying mechanism of the slow force response. Circ Res. 2001;88:376–82. doi: 10.1161/01.res.88.4.376. [DOI] [PubMed] [Google Scholar]
- 90.Eder P, Probst D, Rosker C, Poteser M, Wolinski H, Kohlwein SD, et al. Phospholipase C-dependent control of cardiac calcium homeostasis involves a TRPC3-NCX1 signaling complex. Cardiovasc Res. 2007;73:111–9. doi: 10.1016/j.cardiores.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 91.Yao X, Garland CJ. Recent developments in vascular endothelial cell transient receptor potential channels. Circ Res. 2005;97:853–63. doi: 10.1161/01.RES.0000187473.85419.3e. [DOI] [PubMed] [Google Scholar]
- 92.Gervasio OL, Whitehead NP, Yeung EW, Phillips WD, Allen DG. TRPC1 binds to caveolin-3 and is regulated by Src kinase - role in Duchenne muscular dystrophy. J Cell Sci. 2008;121:2246–55. doi: 10.1242/jcs.032003. [DOI] [PubMed] [Google Scholar]
- 93.Crossthwaite AJ, Seebacher T, Masada N, Ciruela A, Dufraux K, Schultz JE, et al. The cytosolic domains of Ca2+-sensitive adenylyl cyclases dictate their targeting to plasma membrane lipid rafts. J Biol Chem. 2005;280:6380–91. doi: 10.1074/jbc.M411987200. [DOI] [PubMed] [Google Scholar]
- 94.Michel JB, Feron O, Sacks D, Michel T. Reciprocal regulation of endothelial nitric-oxide synthase by Ca2+-calmodulin and caveolin. J Biol Chem. 1997;272:15583–6. doi: 10.1074/jbc.272.25.15583. [DOI] [PubMed] [Google Scholar]
- 95.Hope HR, Pike LJ. Phosphoinositides and phosphoinositide-utilizing enzymes in detergent- insoluble lipid domains. Mol Biol Cell. 1996;7:843–51. doi: 10.1091/mbc.7.6.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu Y, Casey L, Pike LJ. Compartmentalization of phosphatidylinositol 4,5-bisphosphate in low-density membrane domains in the absence of caveolin. Biochem Biophys Res Commun. 1998;245:684–90. doi: 10.1006/bbrc.1998.8329. [DOI] [PubMed] [Google Scholar]
- 97.Pike LJ, Casey L. Localization and turnover of phosphatidylinositol 4,5-bisphosphate in caveolin-enriched membrane domains. J Biol Chem. 1996;271:26453–6. doi: 10.1074/jbc.271.43.26453. [DOI] [PubMed] [Google Scholar]
- 98.Pike LJ, Miller JM. Cholesterol depletion delocalizes phosphatidylinositol bisphosphate and inhibits hormone-stimulated phosphatidylinositol turnover. J Biol Chem. 1998;273:22298–304. doi: 10.1074/jbc.273.35.22298. [DOI] [PubMed] [Google Scholar]
- 99.Suh BC, Hille B. PIP2 is a necessary cofactor for ion channel function: how and why? Annu Rev Biophys. 2008;37:175–95. doi: 10.1146/annurev.biophys.37.032807.125859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sharif-Naeini R, Folgering JH, Bichet D, Duprat F, Delmas P, Patel A, et al. Sensing pressure in the cardiovascular system: Gq-coupled mechanoreceptors and TRP channels. J Mol Cell Cardiol. 2009 doi: 10.1016/j.yjmcc.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 101.Beech DJ. Integration of transient receptor potential canonical channels with lipids. Acta Physiol (Oxf) 2011 doi: 10.1111/j.1748-1716.2011.02311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Palygin OA, Pettus JM, Shibata EF. Regulation of caveolar cardiac sodium current by a single Gsα histidine residue. American Journal of Physiology - Heart and Circulatory Physiology. 2008;294:H1693–H1699. doi: 10.1152/ajpheart.01337.2007. [DOI] [PubMed] [Google Scholar]
- 103.Vatta M, Ackerman MJ, Ye B, Makielski JC, Ughanze EE, Taylor EW, et al. Mutant caveolin-3 induces persistent late sodium current and is associated with long-QT syndrome. Circulation. 2006;114:2104–12. doi: 10.1161/CIRCULATIONAHA.106.635268. [DOI] [PubMed] [Google Scholar]
- 104.Prescott L, Brightman MW. The sarcolemma of Aplysia smooth muscle in freeze-fracture preparations. Tissue Cell. 1976;8:248–58. [PubMed] [Google Scholar]
- 105.Kawamura S, Miyamoto S, Brown JH. Initiation and transduction of stretch-induced RhoA and Rac1 activation through caveolae: cytoskeletal regulation of ERK translocation. J Biol Chem. 2003;278:31111–7. doi: 10.1074/jbc.M300725200. [DOI] [PubMed] [Google Scholar]
- 106.Trouet D, Nilius B, Jacobs A, Remacle C, Droogmans G, Eggermont J. Caveolin-1 modulates the activity of the volume-regulated chloride channel. J Physiol. 1999;520(Pt 1):113–9. doi: 10.1111/j.1469-7793.1999.t01-1-00113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Baumgarten CM, Clemo HF. Swelling-activated chloride channels in cardiac physiology and pathophysiology. Prog Biophys Mol Biol. 2003;82:25–42. doi: 10.1016/s0079-6107(03)00003-8. [DOI] [PubMed] [Google Scholar]
- 108.Trouet D, Carton I, Hermans D, Droogmans G, Nilius B, Eggermont J. Inhibition of VRAC by c-Src tyrosine kinase targeted to caveolae is mediated by the Src homology domains. Am J Physiol Cell Physiol. 2001;281:C248–C256. doi: 10.1152/ajpcell.2001.281.1.C248. [DOI] [PubMed] [Google Scholar]
- 109.Sinha B, Koster D, Ruez R, Gonnord P, Bastiani M, Abankwa D, et al. Cells respond to mechanical stress by rapid disassembly of caveolae. Cell. 2011;144:402–13. doi: 10.1016/j.cell.2010.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Groulx N, Boudreault F, Orlov SN, Grygorczyk R. Membrane reserves and hypotonic cell swelling. J Membr Biol. 2006;214:43–56. doi: 10.1007/s00232-006-0080-8. [DOI] [PubMed] [Google Scholar]
- 111.Fujimoto T, Kogo H, Nomura R, Une T. Isoforms of caveolin-1 and caveolar structure. J Cell Sci. 2000;113(Pt 19):3509–17. doi: 10.1242/jcs.113.19.3509. [DOI] [PubMed] [Google Scholar]
- 112.Hansen CG, Bright NA, Howard G, Nichols BJ. SDPR induces membrane curvature and functions in the formation of caveolae. Nat Cell Biol. 2009;11:807–14. doi: 10.1038/ncb1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ortegren U, Yin L, Ost A, Karlsson H, Nystrom FH, Stralfors P. Separation and characterization of caveolae subclasses in the plasma membrane of primary adipocytes; segregation of specific proteins and functions. FEBS J. 2006;273:3381–92. doi: 10.1111/j.1742-4658.2006.05345.x. [DOI] [PubMed] [Google Scholar]
- 114.Simons K, Ikonen E. How cells handle cholesterol. Science. 2000;290:1721–6. doi: 10.1126/science.290.5497.1721. [DOI] [PubMed] [Google Scholar]
- 115.Feron O, Dessy C, Desager JP, Balligand JL. Hydroxy-methylglutaryl-coenzyme A reductase inhibition promotes endothelial nitric oxide synthase activation through a decrease in caveolin abundance. Circulation. 2001;103:113–8. doi: 10.1161/01.cir.103.1.113. [DOI] [PubMed] [Google Scholar]
- 116.Sampson LJ, Standen NB, Dart C. Vasocontrictor-induced targeting of PKC isozymes to smooth muscle caveolae. Biophys J. 2007:605–Pos. [Google Scholar]
- 117.Aravamudan B, Volonte D, Ramani R, Gursoy E, Lisanti MP, London B, et al. Transgenic overexpression of caveolin-3 in the heart induces a cardiomyopathic phenotype. Hum Mol Genet. 2003;12:2777–88. doi: 10.1093/hmg/ddg313. [DOI] [PubMed] [Google Scholar]
- 118.Ratajczak P, Damy T, Heymes C, Oliviero P, Marotte F, Robidel E, et al. Caveolin-1 and -3 dissociations from caveolae to cytosol in the heart during aging and after myocardial infarction in rat. Cardiovasc Res. 2003;57:358–69. doi: 10.1016/s0008-6363(02)00660-0. [DOI] [PubMed] [Google Scholar]
- 119.Hare JM, Lofthouse RA, Juang GJ, Colman L, Ricker KM, Kim B, et al. Contribution of caveolin protein abundance to augmented nitric oxide signaling in conscious dogs with pacing-induced heart failure. Circ Res. 2000;86:1085–92. doi: 10.1161/01.res.86.10.1085. [DOI] [PubMed] [Google Scholar]
- 120.Ogata T, Ueyama T, Isodono K, Tagawa M, Takehara N, Kawashima T, et al. MURC, a Muscle- Restricted Coiled-Coil Protein That Modulates the Rho/ROCK Pathway, Induces Cardiac Dysfunction and Conduction Disturbance. Mol Cell Biol. 2008;28:3424–36. doi: 10.1128/MCB.02186-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rajab A, Straub V, McCann LJ, Seelow D, Varon R, Barresi R, et al. Fatal cardiac arrhythmia and long- QT syndrome in a new form of congenital generalized lipodystrophy with muscle rippling (CGL4) due to PTRF-CAVIN mutations. PLoS Genet. 2010;6:e1000874. doi: 10.1371/journal.pgen.1000874. [DOI] [PMC free article] [PubMed] [Google Scholar]


