SUMMARY
The melanocortin 4 receptor (MC4R), well-known for its role in the regulation of energy balance, is widely expressed in stress-regulatory brain regions, including the paraventricular nucleus of the hypothalamus (PVH) and the medial amygdala (MeA). In agreement with this, MC4R has been implicated in hypothalamic-pituitary-adrenocortical axis (HPA) regulation. The present work investigated the role of chronic Mc4r function to modulate basal HPA axis tone and to facilitate acute HPA responses to psychological stress, using a novel rat model with Mc4r loss-of-function. In this study, adult male rats were placed into 3 groups (n=15/group) according to genotype [wild-type (WT); heterozygous mutant (HET); and homozygous mutant (HOM)]. Basal (pre-stress) plasma adrenocorticotropic hormone (ACTH) and corticosterone were measured in the AM and PM, and the HPA axis response to restraint was assessed in the AM. Rats were perfused at 2 hours after restraint to assess the effect of loss of MC4R on stress-induced c-Fos immunolabeling in stress-regulatory brain regions. We find that basal (non-stress) AM and PM plasma ACTH and corticosterone showed a normal diurnal rhythm that was not altered according to genotype. Consistent with this, adrenal and thymus weights were unaffected by genotype. However, the plasma ACTH and corticosterone responses to restraint were significantly reduced by loss of MC4R function. Likewise, stress-induced c-Fos immunolabeling in both PVH and MeA was significantly reduced by loss of Mc4r function. These results support the hypothesis that endogenous MC4R signaling contributes to the HPA axis response to stress. Because MC4R plays a critical role in the regulation of energy balance, the present work suggests that it may also serve as an important communication link between brain metabolic and stress systems.
Keywords: melanocortin, metabolism, stress, HPA, obesity
1. INTRODUCTION
The melanocortin-4 receptor (MC4R) is a G-protein coupled receptor, expressed primarily in the central nervous system (Cone, 2005; Tao, 2010), that plays a key role in the maintenance of energy balance in humans (Vaisse et al., 1998; Yeo et al., 1998; Farooqi and O’Rahilly, 2000; Farooqi et al., 2003) and rodents (Huszar et al., 1997; Mul et al., 2012). MC4R activity is controlled by the opposing effects of its endogenous agonist, α–melanocyte-stimulating hormone (α-MSH), and endogenous antagonist, agouti-related protein (AgRP) (Fong et al., 1997; Ollmann et al., 1997; Shutter et al., 1997). Additionally, MC4R has an intrinsic constitutive activity on which AgRP also acts as an inverse agonist (Srinivasan et al., 2004).
MC4R is expressed in several stress-regulatory brain regions including the paraventricular hypothalamus (PVH) and the medial amygdala (Herman et al., 2003; Balthasar, 2006) (MeA), suggesting that MC4R may play an important role in brain stress integration. In the PVH MC4R co-localizes with corticotropin releasing hormone (CRH), and its pharmacological activation increases CRH mRNA as well as circulating adrenocorticotropic hormone (ACTH) and corticosterone (CORT) (Dhillo et al., 2002; Lu et al., 2003). Pharmacologic activation of MC4R in the MeA also increases circulating CORT (Liu et al., 2013). Moreover, neurons in the arcuate hypothalamus (ARC) that express pro-opiomelanocortin (POMC), the pro-hormone cleaved to produce α-MSH, project to MC4R-expressing neurons in the PVH and MeA (Balthasar, 2006) and are rapidly activated by acute restraint and forced-swim stress (Liu et al., 2007).
Consistent with this neuroanatomy, a growing body of pharmacological evidence suggests that central MC4R activity may play an important role to coordinate behavioral and hormonal responses to psychological stress. Acute intracerebroventricular (ICV) administration of MC4R agonists increases anxiety-like behavior (De Barioglio et al., 1991; Gonzalez et al., 1996; Liu et al., 2013) whereas acute ICV administration of MC4R antagonists is anxiolytic (Chaki et al., 2003; Liu et al., 2007, 2013; Kokare et al., 2010; Serova et al., 2013). Many of these effects can be recapitulated by acute site-specific injections of pharmacological MC4R agonists and antagonists directly into the MeA (Liu et al., 2013). Despite pharmacological evidence that MC4R is involved in the stress response, the effect of chronic Mc4r loss-of-function on basal and stress-induced hypothalamic-pituitary-adrenocortical (HPA) axis tone remains to be determined. In the present study we investigated the hypothesis that tonic MC4R77 signaling facilitates both basal and stress-induced HPA activity, using a functional Mc4r knockout rat model.
2. MATERIALS AND METHODS
2.1 Rats
Age-matched male rats were generated in-house from breeding pairs that were heterozygous for a loss of function mutation (K314×) in Mc4r (Mul et al., 2012). Males either homozygous (HOM, n=15) or heterozygous (HET, n=15) for the mutation, and their wild-type littermate controls (WT, n=15) were identified with the KASPar SNP genotyping system (KBiosciences Hoddesdon, UK). Rats were singly housed in an AAALAC-approved facility with a 12-12 light-dark cycle (lights on at 0600 h), and allowed ad libitum access to water and pelleted chow (Harlan Teklad, Madison WI). The University of Cincinnati IACUC approved all animal protocols.
2.2 HPA axis testing
An ‘unstressed’ tail-clip blood sample was collected in the PM (1600 h) to assess basal HPA axis activity near the peak of the diurnal rhythm. Briefly, rats were quickly placed into well-ventilated restraint tubes, the last ~ 0.5 mm of the tail was removed with a scalpel, and blood (~100–200 µl) was gently milked into an EDTA-coated tube by highly-trained lab personnel. Importantly, collection of this sample was completed within 3 min of first disturbing the rat’s home cage; previous work from our group (Vahl et al., 2005) indicates this quick timeframe provides a snapshot of ‘unstressed’ hormones, as it is completed prior to any increases in ACTH and corticosterone that occur in response to handling of the animal. One week later in the AM (0800 h), another tail-clip blood sample was quickly collected (as described above) except that the rats then remained in the restraint tubes for 30 min, with collection of additional blood samples at 15, 30, 60 and 120 min after the onset of restraint. Plasma ACTH and CORT were measured by radioimmunoassay as described previously (Ulrich-Lai et al., 2010).
2.3 Stress-induced neuronal activation
At 120 min after the onset of restraint, rats were given lethal injections of Pentobarbital and then perfused with normal saline followed by 4% paraformaldehyde in potassium buffered saline (PBS; pH 7.4). Brains were collected for immunohistochemistry, postfixed at 4°C for 24 h in 4.0% paraformaldehyde in PBS, and stored at 4 °C in 30.0% sucrose/PBS.
c-Fos positive cells were identified using immunohistochemistry. Coronal hypothalamic sections (25 µm) were stored in ethylene glycol cryoprotectant at −20 °C until time of use. Briefly, tissue sections were removed from cryoprotectant and washed in 0.1 M potassium phosphate-buffered saline (KPBS). To block endogenous peroxidases, tissue was incubated in 1% hydrogen peroxide solution for 10 min and subsequently rinsed in KPBS. Tissue was then preincubated in blocking buffer (KPBS + 0.2 % triton X-100 + 0.1% bovine serum albumin (BSA)) for 1 hr at room temperature, followed by incubation with c-Fos-specific antibody (1:5000, Santa Cruz Biotechnology, #sc-52) in blocking buffer for 24 h at 4°C. Following washes in KPBS, tissue was incubated for 1 h in biotinylated goat anti-rabbit antibody (1:500 in KPBS + 0.1% BSA; Vector Laboratories), and subsequently washed and incubated in avidin-biotin-peroxidase solution (1:1000 in KPBS + 0.1% BSA; Vector Laboratories) for 30 min. c-Fos immunoreactivity was visualized with 3,3’-diaminobenzidine.
Brightfield images of c-Fos immunolabeling were obtained using an Axio Imager.M2 microscope (Zeiss) equipped with an AxioCam MRm camera (Zeiss). The PVH, ARC, and MeA were identified using a rat brain atlas (Paxinos and Watson, 1998) and standard anatomical landmarks to define nuclear boundaries (third ventricle, fornix, optic tract). An individual blind to the experimental treatment groups counted the number of c-Fos-positive cells in each brain region using Image J software (NIH). Display images were adjusted for brightness and contrast.
2.4 Adrenal and thymus weights
Adrenals and thymus were collected from each rat at sacrifice, cleaned and weighed, providing indirect indices of chronic HPA tone.
2.5 Statistics
Statistical differences were determined using the Kruskal-Wallis test with Dunn’s post-hoc analysis, or by ANOVA (with repeated measures when appropriate) with Tukey’s post-hoc analysis, or by two-tailed t-test as indicated. In instances where the variance was not homogenous, analyses were performed on rank-transformed data, as indicated. α= 0.05.
3. RESULTS
3.1 Energy balance
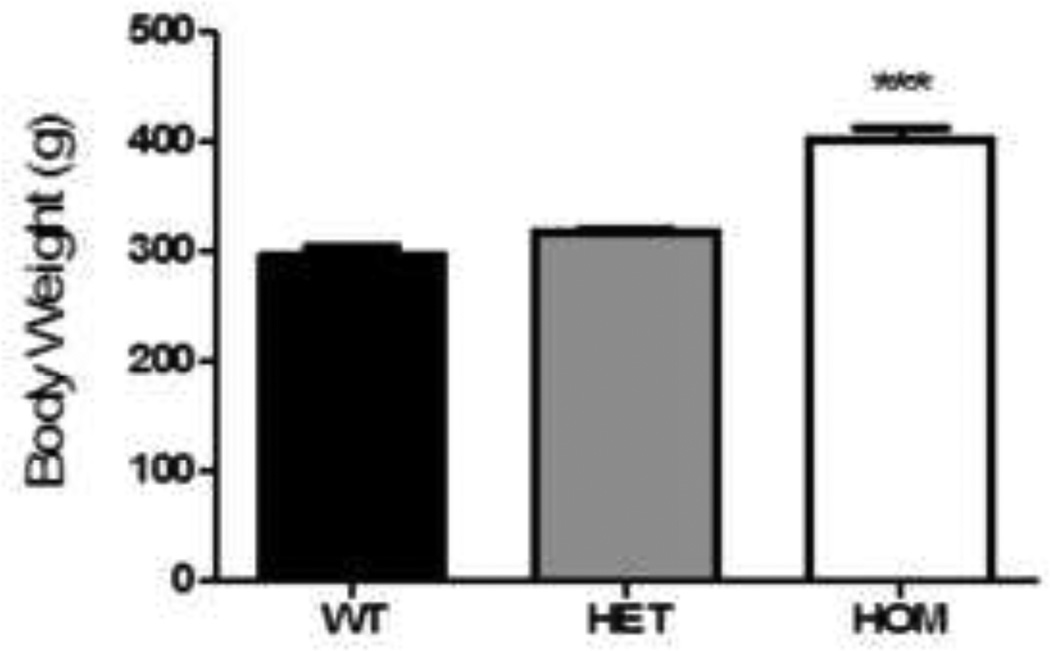
As expected (Mul et al., 2012), HOM rats demonstrated early-onset obesity, with 9-week old HOM rats being 35% heavier than WT littermates (Fig 1, Kruskal-Wallis with Dunn’s posthoc, p< 0.001). However, 9-week old HET rats were not significantly heavier than their WT littermates (Fig 1), confirming late-onset obesity with Mc4r haploinsufficiency (Mul et al., 2012).
Figure 1. Loss of Mc4r function was associated with increased body weight.
9-week old HOM, but not HET rats were significantly heavier than their WT littermates (Kruskal- Wallis, p < 0.001). Data presented as mean ± S.E.M. *** P < 0.001 compared to both WT and HET littermates. n = 15 per group.
3.2 Basal HPA activity
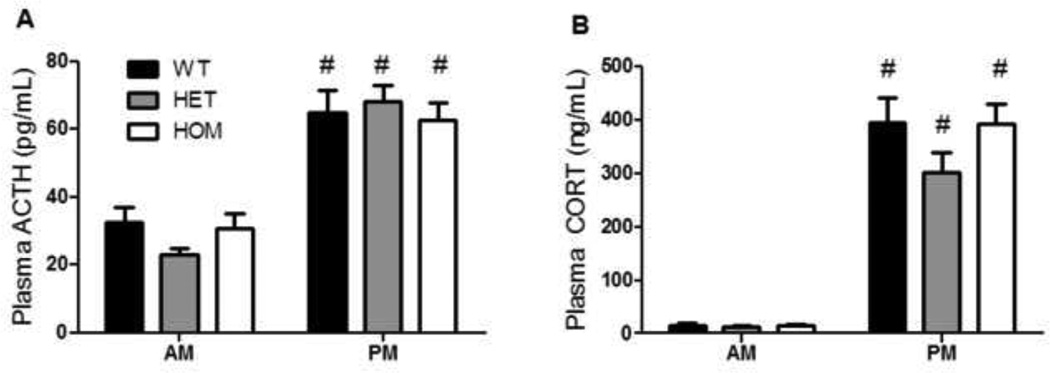
In the absence of stress, basal plasma ACTH and CORT concentrations exhibited a typical diurnal rhythm. Both hormones were lower in the morning (AM) and higher in the late afternoon (PM) (Fig 2a–b, 2-way RM ANOVA, p (time)< 0.001), but there was no significant effect of genotype.
Figure 2. Basal HPA activity was not altered by genotype.
a) ACTH exhibited a normal diurnal rhythm, with significantly greater plasma concentrations in the PM. This was not affected by genotype (2-way RM ANOVA, p (time) < 0.001). b) Corticosterone also exhibited a normal diurnal rhythm, with greater plasma concentrations in the PM. This was not affected by genotype (2-way RM ANOVA, p (time) < 0.001). Data presented as mean ± S.E.M. # P < 0.05 compared to AM. n = 15 per group.
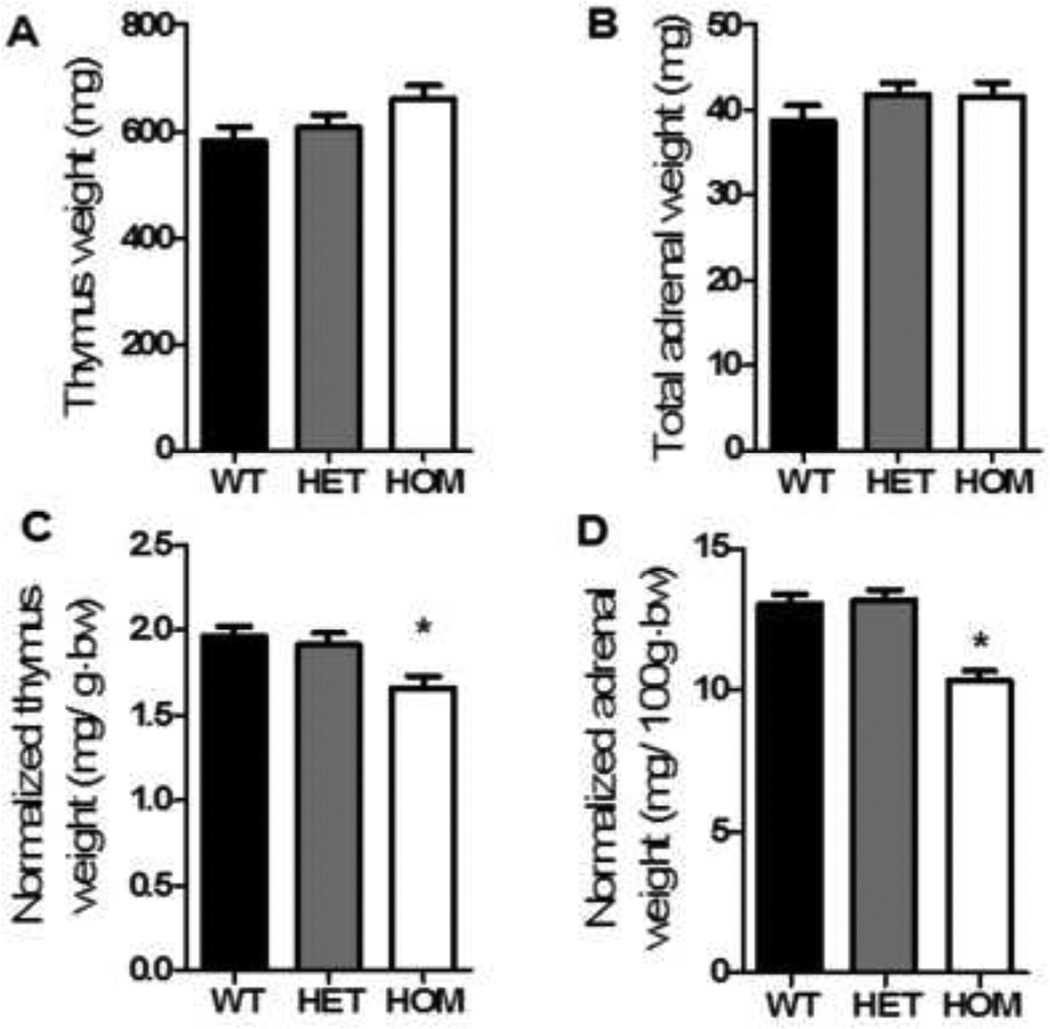
There were no significant differences among the genotypes with regard to either adrenal or thymus weight (Fig 3a–b, ANOVA, ns). However, when adrenal and thymus weights were expressed relative to body weight, both organs were significantly smaller in HOM rats compared to HET and WT littermates (Fig 3c–d, ANOVA with Tukey’s posthoc, p< 0.01), likely as a simple consequence of the different body weights.
Figure 3. Basal HPA activity was not altered by genotype.
There was no significant effect of genotype on thymus (a) or total adrenal weight (b). However, when normalized to body weight, HOM rats had significantly lower relative thymus (c, ANOVA, p< 0.01) and total adrenal weights (d, ANOVA, p< 0.001). Data presented as mean ± S.E.M. * P < 0.01 relative to both WT and HET littermates. n = 15 per group.
3.3 HPA response to acute restraint stress
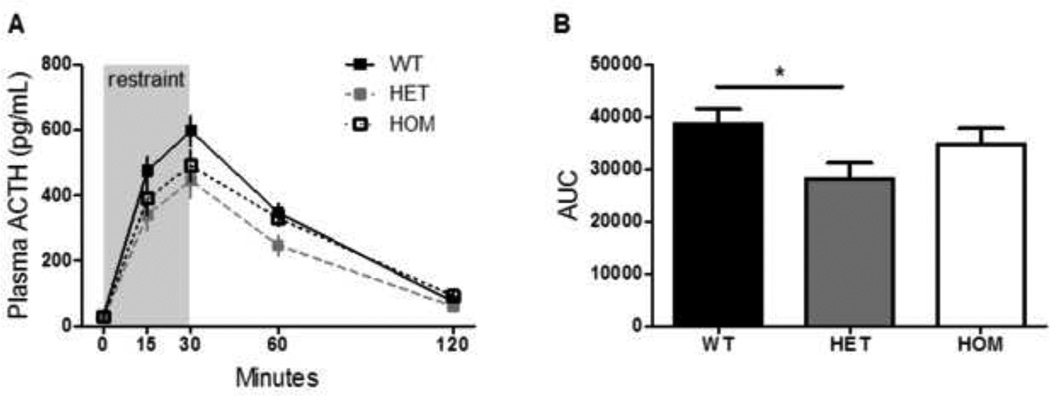
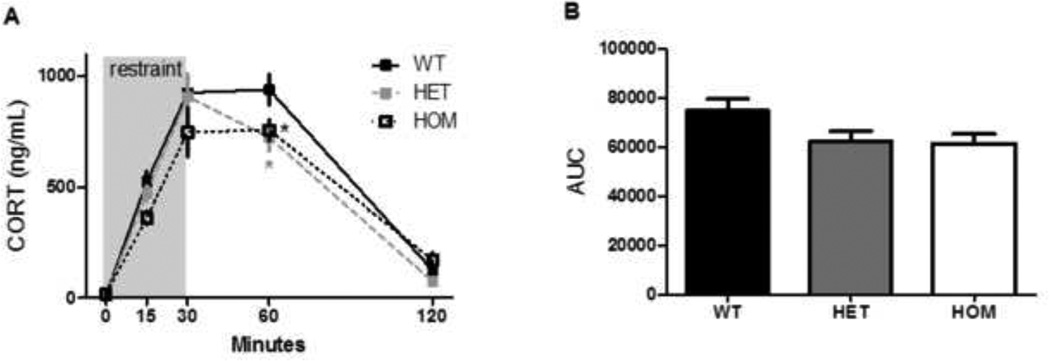
The stress-induced increase in circulating ACTH, as measured by the integrated area under the ACTH: time curve, was significantly blunted among HET rats compared to their WT littermates (Fig 4b, ANOVA with Tukey’s posthoc, p = 0.05). Consistent with this, the stress-induced increase in circulating CORT was also significantly blunted in HET rats as well as HOM rats, relative to their WT littermates (Fig 5a, 2-way RM ANOVA, p (genotype × time) < 0.05). Posthoc analysis using Tukey’s HSD indicated that HET and HOM rats had a lower concentration of plasma CORT at 60 minutes after the onset of restraint (p< 0.05). Furthermore there was a significant effect of genotype on the integrated CORT response to restraint, as calculated by the area under the curve (Fig 5b, Kruskal-Wallis test, p< 0.05), although there were no significantly different posthoc comparisons.
Figure 4. The ACTH response to acute restraint was blunted by Mc4r loss-of-function.
a) Rats were placed in a well-ventilated clear Plexiglas restrainer for 30 minutes. Blood was sampled from the tip of the tail vein and the indicated times. b) The stress-induced increase in circulating ACTH, as measured by the integrated area under the ACTH: time curve, was significantly blunted among HET rats compared to their WT littermates (ANOVA, p = 0.05). Data presented as mean ± S.E.M. * P < 0.05. n = 15 per group.
Figure 5. The corticosterone response to acute restraint was blunted by Mc4r loss-of-function.
a) The corticosterone response was blunted in both HET and HOM rats, relative to WT littermates, at 60 minutes following the onset of acute restraint (RM ANOVA, p (genotype × time) < 0.01). b) There was a significant effect of genotype to reduce the integrated area under the CORT: time curve (Kruskal-Walis, p < 0.05), though none of the posthoc comparisons were significant. Data presented as mean ± S.E.M. * P < 0.05 compared to WT littermates. n = 15 per group.
c-Fos response to acute restraint stress
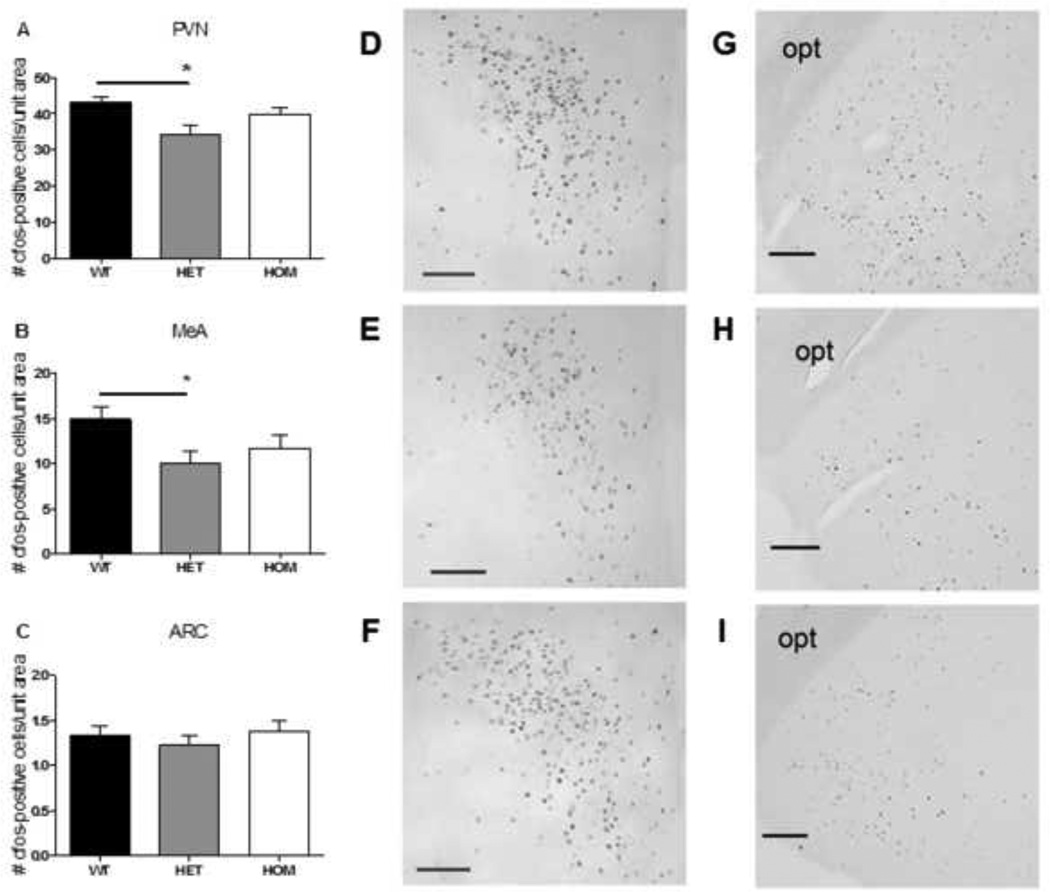
In agreement with the lower ACTH and CORT responses in HET rats, neuronal activation in response to acute restraint stress (indicated by c-Fos positive cells) was blunted by 21% in the PVH (Fig 6a, ANOVA with Tukey posthoc, p< 0.05) and by 33% in the MeA (Fig 6b, ANOVA with Tukey’s posthoc, p= 0.05) of HET rats, relative to WT rats. No significant differences were observed among the genotypes in the ARC (Fig 6c).
Figure 6. The c-Fos response to acute restraint in the PVH and MeA was blunted by Mc4r loss-of-function.
The c-Fos response to acute restraint was significantly blunted in the PVH (a) and MeA (b), but not in the ARC (c), of HET rats compared to their WT littermates (ANOVA with Tukey’s posthoc, p< 0.01). Data presented as mean ± S.E.M. * P < 0.05 relative to WT littermates. n = 15 per group. d–f) representative images of PVH c-Fos in WT (d), HET (e), and HOM (f) rats at 2-h following the onset of restraint; scale bar = 100 µM. g–i) representative images of MeA c-Fos in WT (g), HET (h), and HOM (i) rats at 2-h following the onset of restraint; scale bar = 200 µM; opt = optic tract.
4. DISCUSSION
Our data support the hypothesis that MC4R-signaling facilitates stress-induced, but not basal, HPA activity. Specifically, we found that constitutive Mc4r loss-of-function did not alter basal concentrations of ACTH or CORT, and did not meaningfully alter adrenal or thymus weights; however it did significantly blunt the ACTH and CORT response to an acute psychological stressor. Consistent with this, Mc4r loss-of-function also blunted stress-induced neuronal activity in the PVH and MeA, two key stress-regulatory brain regions that express MC4R and are known to be involved in stress excitation (Balthasar, 2006; Ulrich-Lai and Herman, 2009). The data support that melanocortin-signaling, a well-known pathway important for the regulation of energy homeostasis, also plays a key role to facilitate HPA activity. These data further suggest a possible role for the melanocortin system to link nutrient consumption with stress regulation.
We included rats that were heterozygous for the Mc4r mutation along with their HOM and WT littermates for two reasons. First, heterozygous Mc4r loss-of-function is the most common genetic cause of obesity in humans (Farooqi and O’Rahilly, 2004). Thus the comparison between WT and HET rats was the most clinically relevant comparison. Second, increased adiposity per se may influence both basal and acute HPA function. Unfortunately it was not feasible to account for differences in adiposity between the WT and HOM rats with ‘pair-feeding’ or caloric restriction, since these have significant effects on stress responses (Flak et al., 2011; Guarnieri et al., 2012) and would influence the stress system unevenly across the groups. Importantly however, when we performed these studies at 9 weeks of age, there was no significance difference in the body weights of WT and HET rats (Fig 1). Consequently any differences observed between the WT and HET groups provide clear information about the role of MC4R, whereas comparisons to the HOM group may be confounded by their different body weights.
Although previous studies (Liu et al., 2007, 2013) have used acute pharmacological manipulations to interrogate a potential role for MC4R-signaling in acute HPA responses to stress, the effect of Mc4r function on basal HPA activity has not yet been reported. Here we find that constitutive Mc4r loss-of-function does not alter basal HPA tone. Specifically, we observed a normal circadian rhythm for circulating ACTH and CORT, with equivalent plasma levels regardless of genotype at both the nadir (AM) and peak (PM) of the circadian rhythm (Fig 2a–b). Other indices of tonic HPA activity include adrenal hypertrophy, commonly observed as a consequence of chronic stress-induced ACTH release, and thymic atrophy, triggered by prolonged glucocorticoid exposure. Consistent with the basal hormones, we did not observe any meaningful differences in adrenal or thymus weights. Specifically, when we compared absolute adrenal and thymus weights at sacrifice, there were no significant differences among the genotypes (Fig 3a–b). However, when normalized to total body weight, both organ weights were reduced in HOM rats (Fig 3 c–d). We interpret this as an artifact of the greater adiposity induced by the HOM mutation. Indeed, if Mc4r loss-of-function influences basal HPA tone, we would expect adrenal and thymus weights to be affected in opposite directions.
Consistent with previous acute pharmacological experiments (Liu et al., 2013), constitutive loss of Mc4r-function was associated with blunted HPA responses to acute psychological stress. Specifically, we observed that, compared to WT littermates, HET rats exhibited a significantly blunted ACTH and CORT response, and HOM rats exhibited a significantly blunted CORT response to restraint. These data support the hypothesis that endogenous MC4R-signaling facilitates the HPA response to psychological stress. In agreement with this, a recent study using human genetic loci to understand the relationship between adiposity and psychological distress in a Danish population concluded that, when Mc4r genotype was used as an instrumental variable, adiposity was inversely associated with distress (Lawlor et al., 2011). This suggests that diminished Mc4r function associates with both increased body weight and diminished stress. Intriguingly, when these authors applied conventional multivariable analyses to the same dataset, adiposity was positively associated with distress (Lawlor et al., 2011), suggesting there may be additional opposing effects of body fat compared to Mc4r genotype per se on these endpoints. Consistent with this, there is some evidence to suggest that increased adiposity is associated with an exaggerated cortisol response to psychological stress (Francis et al., 2013; Lu et al., 2014).
The apparent discrepancy observed by Lawlor et al (2011) is consistent with the present findings. That is, our work supports a model in which loss of Mc4r function per se is associated with blunted indices of psychological distress, which is partially masked by much greater adiposity in HOM individuals. In contrast to the “dose dependent” effect of the Mc4r gene on obesity (Huszar et al., 1997; Vaisse et al., 1998; Farooqi and O’Rahilly, 2000; Mul et al., 2012), the effect of Mc4r function on stress reactivity in this study was generally more apparent in HET compared to HOM rats. For example, we compared the activation of neuronal populations in key stress-regulatory regions in response to restraint by immunolabeling for the immediate-early protein, Fos. In the MeA, we observed a significant effect of genotype to blunt the Fos response, and this was most apparent in the HET group relative to WT littermates (Fig. 6a). The MeA is essential for processing emotions, including anxiety, and is especially sensitive to stressors with a predominantly emotional component, such as restraint (Ulrich-Lai and Herman, 2009). Lesions of the MeA attenuate the HPA response to stress (Dayas et al., 1999). Conversely, direct stimulation of the MeA, either globally via electrophysiology (Dunn and Whitener, 1986; Feldman et al., 1990), or via direct pharmacological stimulation of MC4R-expressing MeA neurons (Liu et al., 2013), increases plasma CORT. Our data suggest, therefore, that constitutive loss of Mc4r function reduces stress-induced neuronal activity in the MeA, likely contributing to the blunted hormonal response to restraint.
Intriguingly, MeA lesion not only attenuates the HPA response to stress, but also is remarkably effective to attenuate stress-induced c-fos in the PVH (Dayas et al., 1999). In agreement with this, and with the blunted ACTH and CORT response to restraint, we also observed a significant effect of genotype to blunt the Fos response to restraint in the PVH. Also consistent with the previous findings, and with a potentially confounding role of excess adiposity per se, this was more apparent among the HET rats compared to their WT littermates. The PVH is a key region for integrating the HPA response to stress, and it receives inputs from the MeA indirectly via the bed nucleus of the stria terminalis (Ulrich-Lai and Herman, 2009). Additionally, the PVH itself expresses MC4R, and these neurons receive direct inputs from ARC α-MSH and/or AgRP neurons. In this way, melanocortin signaling directly in the PVH may also contribute to HPA activation following stress (Dhillo et al., 2002; Lu et al., 2003). The pronounced reduction in c-fos induction in the MeA and PVH, relative to the somewhat more modest reduction in the HPA hormonal response, likely reflects that the MeA and PVH are just two important players among a complex HPA regulatory system that includes numerous other neurotransmitter systems acting across multiple brain regions (Ulrich-Lai and Herman, 2009).
Importantly, metabolic diseases like anorexia, obesity, and diabetes, and stress-associated psychological disorders like anxiety and depression, have a high incidence of co-morbidity (Dixon, 2010). This relationship likely arises from complex interactions, and overlap, between brain stress and feeding circuitry, as well as associated receptor-signaling pathways (Ulrich-Lai and Ryan, 2014). Arcuate melanocortinergic neurons expressing AgRP or α-MSH play a well-described, critical role to integrate peripheral signals regarding energy needs and availability (Schwartz et al., 2000; Ryan et al., 2012). These neurons signal via MC4R in the PVH, MeA and elsewhere to regulate feeding behavior and energy expenditure. Additionally, α-MSH neurons are activated in response to psychological stress. The present findings emphasize that MC4R signaling facilitates stress-induced, but not basal, HPA axis activity, and suggest a potentially key role for MC4R-signaling to integrate and coordinate energy balance and stress regulatory responses.
ACKNOWLEDGEMENTS
The authors thank Dr. William C. Engeland (University of Minnesota) for providing antiserum for the ACTH radioimmunoassay. This work was supported by the National Institutes of Health: DK082173 and HL111319 to KKR, DK091425 to YMU, MH069860 to JPH, T32NS00743 to AEE. CC was supported by the UNIK: Food, Fitness & Pharma for Health and Disease research program.
JDM is a paid speaker for Taconic. RJS has received research support from Ethicon Surgical Care, Novo Nordisk, Ablaris, Roche, Boehringer Ingelheim and Zealand. He has served as a consultant or paid speaker for Ethicon Surgical Care, Novo Nordisk, Merck, Angiochem, Zealand, Takeda, Boehringer Ingelheim, Eissai, Forrest and Givaudan. He has a small equity position in Zafgen.
ROLE OF THE FUNDING SOURCE
The funding sources supported the authors and funded the science, but had no influence over experimental design, analysis or reporting.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The other authors have nothing to declare.
K.K.R. and Y.M.U conceptualized, designed, performed and analyzed the experiments and wrote the manuscript. J.D.M., C.C., A.E.E., D.P.B., and K.H. performed experiments and edited the manuscript. R.J.S. and J.P.H. designed experiments and edited the manuscript.
REFERENCES
- Balthasar N. Genetic dissection of neuronal pathways controlling energy homeostasis. Obesity (Silver Spring) 2006;14(Suppl 5):222S–227S. doi: 10.1038/oby.2006.313. [DOI] [PubMed] [Google Scholar]
- Chaki S, Ogawa S, Toda Y, Funakoshi T, Okuyama S. Involvement of the melanocortin MC4 receptor in stress-related behavior in rodents. Eur. J. Pharmacol. 2003;474:95–101. doi: 10.1016/s0014-2999(03)02033-8. [DOI] [PubMed] [Google Scholar]
- Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Day TA. Neuroendocrine responses to an emotional stressor: evidence for involvement of the medial but not the central amygdala. Eur. J. Neurosci. 1999;11:2312–2322. doi: 10.1046/j.1460-9568.1999.00645.x. [DOI] [PubMed] [Google Scholar]
- De Barioglio SR, Lezcano N, Celis ME. Alpha MSH-induced excessive grooming behavior involves a GABAergic mechanism. Peptides. 1991;12:203–205. doi: 10.1016/0196-9781(91)90189-v. [DOI] [PubMed] [Google Scholar]
- Dhillo WS, Small CJ, Seal LJ, Kim M-S, Stanley SA, Murphy KG, Ghatei MA, Bloom SR. The hypothalamic melanocortin system stimulates the hypothalamo-pituitary-adrenal axis in vitro and in vivo in male rats. Neuroendocrinology. 2002;75:209–216. doi: 10.1159/000054712. [DOI] [PubMed] [Google Scholar]
- Dixon JB. The effect of obesity on health outcomes. Mol. Cell. Endocrinol. 2010;316:104–108. doi: 10.1016/j.mce.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Dunn JD, Whitener J. Plasma corticosterone responses to electrical stimulation of the amygdaloid complex: cytoarchitectural specificity. Neuroendocrinology. 1986;42:211–217. doi: 10.1159/000124442. [DOI] [PubMed] [Google Scholar]
- Farooqi IS, O’Rahilly S. Recent advances in the genetics of severe childhood obesity. Arch Dis Child. 2000;83:31–34. doi: 10.1136/adc.83.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqi IS, O’Rahilly S. Monogenic human obesity syndromes. Recent Prog. Horm. Res. 2004;59:409–424. doi: 10.1210/rp.59.1.409. [DOI] [PubMed] [Google Scholar]
- Farooqi IS, Yeo GS, O’Rahilly S. Binge eating as a phenotype of melanocortin 4 receptor gene mutations. N Engl J Med. 2003;349:606–609. [PubMed] [Google Scholar]
- Feldman S, Conforti N, Saphier D. The preoptic area and bed nucleus of the stria terminalis are involved in the effects of the amygdala on adrenocortical secretion. Neuroscience. 1990;37:775–779. doi: 10.1016/0306-4522(90)90107-f. [DOI] [PubMed] [Google Scholar]
- Flak JN, Jankord R, Solomon MB, Krause EG, Herman JP. Opposing effects of chronic stress and weight restriction on cardiovascular, neuroendocrine and metabolic function. Physiol. Behav. 2011;104:228. doi: 10.1016/j.physbeh.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong TM, Mao C, MacNeil T, Kalyani R, Smith T, Weinberg D, Tota MR, Van, der PLH. ART (protein product of agouti-related transcript) as an antagonist of MC-3 and MC-4 receptors. Biochem. Biophys. Res. Commun. 1997;237:629–631. doi: 10.1006/bbrc.1997.7200. [DOI] [PubMed] [Google Scholar]
- Francis La, Granger Da, Susman EJ. Adrenocortical regulation, eating in the absence of hunger and BMI in young children. Appetite. 2013;64:32–38. doi: 10.1016/j.appet.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez MI, Vaziri S, Wilson CA. Behavioral effects of alpha-MSH and MCH after central administration in the female rat. Peptides. 1996;17:171–177. doi: 10.1016/0196-9781(95)02092-6. [DOI] [PubMed] [Google Scholar]
- Guarnieri DJ, Brayton CE, Richards SM, Maldonado-Aviles J, Trinko JR, Nelson J, Taylor JR, Gourley SL, DiLeone RJ. Gene profiling reveals a role for stress hormones in the molecular and behavioral response to food restriction. Biol. Psychiatry. 2012;71:358–365. doi: 10.1016/j.biopsych.2011.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front. Neuroendocrinol. 2003;24:151. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Huszar D, Lynch CAVF-H, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- Kokare DM, Dandekar MP, Singru PS, Gupta GL, Subhedar NK. Involvement of alpha-MSH in the social isolation induced anxiety- and depression-like behaviors in rat. Neuropharmacology. 2010;58:1009–1018. doi: 10.1016/j.neuropharm.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Lawlor DA, Harbord RM, Tybjaerg-Hansen A, Palmer TM, Zacho J, Benn M, Timpson NJ, Smith GD, Nordestgaard BG. Using genetic loci to understand the relationship between adiposity and psychological distress: a Mendelian Randomization study in the Copenhagen General Population Study of 53,221 adults. J. Intern. Med. 2011;269:525–537. doi: 10.1111/j.1365-2796.2011.02343.x. [DOI] [PubMed] [Google Scholar]
- Liu J, Garza JC, Li W, Lu X-Y. Melanocortin-4 receptor in the medial amygdala regulates emotional stress-induced anxiety-like behaviour, anorexia and corticosterone secretion. Int. J. Neuropsychopharmacol. 2013;16:105–120. doi: 10.1017/S146114571100174X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Garza JC, Truong HV, Henschel J, Zhang W, Lu X-Y. The melanocortinergic pathway is rapidly recruited by emotional stress and contributes to stress-induced anorexia and anxiety-like behavior. Endocrinology. 2007;148:5531–5540. doi: 10.1210/en.2007-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q, Tao F, Hou F, Zhang Z, Sun Y, Xu Y, Xu S, Zhao Y. Cortisol reactivity, delay discounting and percent body fat in Chinese urban young adolescents. Appetite. 2014;72:13–20. doi: 10.1016/j.appet.2013.09.019. [DOI] [PubMed] [Google Scholar]
- Lu X-Y, Barsh GS, Akil H, Watson SJ. Interaction between alpha-melanocyte-stimulating hormone and corticotropin-releasing hormone in the regulation of feeding and hypothalamo-pituitary-adrenal responses. J. Neurosci. 2003;23:7863–7872. doi: 10.1523/JNEUROSCI.23-21-07863.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mul JD, van Boxtel R, Bergen DJ, Brans MA, Brakkee JH, Toonen PW, Garner KM, Adan RA, Cuppen E. Melanocortin receptor 4 deficiency affects body weight regulation, grooming behavior, and substrate preference in the rat. Obes. (Silver Spring) 2012;20:612–621. doi: 10.1038/oby.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. (80-.). [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Fourth. ed. San Diego, CA: Academic Press; 1998. [Google Scholar]
- Ryan KK, Woods SC, Seeley RJ. Central nervous system mechanisms linking the consumption of palatable high-fat diets to the defense of greater adiposity. Cell Metab. 2012;15:137–149. doi: 10.1016/j.cmet.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG, Porte DJ. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- Serova LI, Laukova M, Alaluf LG, Sabban EL. Intranasal infusion of melanocortin receptor four (MC4R) antagonist to rats ameliorates development of depression and anxiety related symptoms induced by single prolonged stress. Behav. Brain Res. 2013;250:139–147. doi: 10.1016/j.bbr.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Shutter JR, Graham M, Kinsey AC, Scully S, Luthy R, Stark KL. Hypothalamic expression of ART, a novel gene related to agouti, is up-regulated in obese and diabetic mutant mice. Genes Dev. 1997;11:593–602. doi: 10.1101/gad.11.5.593. [DOI] [PubMed] [Google Scholar]
- Srinivasan S, Lubrano-Berthelier C, Govaerts C, Picard F, Santiago P, Conklin BR, Vaisse C. Constitutive activity of the melanocortin-4 receptor is maintained by its N-terminal domain and plays a role in energy homeostasis in humans. J. Clin. Invest. 2004;114:1158–1164. doi: 10.1172/JCI21927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y-X. The melanocortin-4 receptor: physiology, pharmacology, and pathophysiology. Endocr. Rev. 2010;31:506–543. doi: 10.1210/er.2009-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Christiansen AM, Ostrander MM, Jones AA, Jones KR, Choi DC, Krause EG, Evanson NK, Furay AR, Davis JF, Solomon MB, de Kloet AD, Tamashiro KL, Sakai RR, Seeley RJ, Woods SC, Herman JP. Pleasurable behaviors reduce stress via brain reward pathways. Proc. Natl. Acad. Sci. 2010;107:20529–20534. doi: 10.1073/pnas.1007740107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Ryan KK. Neuroendocrine circuits governing energy balance and stress regulation: functional overlap and therapuetic implications. Cell Metab. 2014 doi: 10.1016/j.cmet.2014.01.020. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahl TP, Ulrich-Lai YM, Ostrander MM, Dolgas CM, Elfers EE, Seeley RJ, D’Alessio DA, Herman JP. Comparative analysis of ACTH and corticosterone sampling methods in rats. Am. J. Physiol. Endocrinol. Metab. 2005;289:E823–E828. doi: 10.1152/ajpendo.00122.2005. [DOI] [PubMed] [Google Scholar]
- Vaisse C, Clement KBG-G, Froguel P. A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nat. Genet. 1998;20:113–114. doi: 10.1038/2407. [DOI] [PubMed] [Google Scholar]
- Yeo GS, Farooqi IS, Aminian S, Halsall DJ, Stanhope RG, O’Rahilly S. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nat Genet. 1998;20:111–112. doi: 10.1038/2404. [DOI] [PubMed] [Google Scholar]