Abstract
Resurgence refers to the reappearance of an extinguished operant behavior when reinforcement for an alternative behavior is also subsequently discontinued. Resurgence has been noted as a source of relapse to problem behavior following interventions involving alternative reinforcement, and has also been recently used as an animal model of relapse to drug seeking induced by reinforcement loss. Existing information about the neuropharmacology of resurgence is scarce, but suggests overlap between relapse observed in the resurgence model and relapse observed in reinstatement and renewal models. In the present experiment rats earned food pellets for pressing a target lever in Phase I. In Phase II lever pressing no longer produced food, but food was delivered for an alterative nose poke response. Finally in Phase III, neither response produced food deliveries. Prior to these Phase III sessions, separate groups of rats were injected with 0, 50, or 100 μg/kg of the dopamine D2 receptor antagonist raclopride or 0, 20, or 40 μg/kg of α2 agonist clonidine. Both doses of raclopride were effective in blocking resurgence, but there was evidence that the higher dose did so via motor rather than motivational impairment. Only the higher dose of clonidine blocked resurgence, but did so with no evidence of motor impairment. Raclopride significantly impacted extinction of the alternative poke at both doses tested, whereas clonidine had no effect at either dose. The results of the present study provide additional information about the neuropharmacology of resurgence, as well as additional evidence of overlap between resurgence, reinstatement, and renewal.
Keywords: Relapse, Resurgence, Dopamine, Norepinephrine, Raclopride, Clonidine
1. INTRODUCTION
Resurgence is a behavioral phenomenon in which extinguished operant behavior reappears when reinforcement is subsequently discontinued for an alternative response (Epstein, 1983). It has more recently been used as an animal model of relapse where extinguished alcohol- (Podlesnik, Jimenez-Gomez, & Shahan, 2006) and cocaine-seeking (Quick, Pyszczynski, Colston, & Shahan, 2011) increase when an alternative response associated with food deliveries is also extinguished (see Marchant, Li, & Shaham, 2013, for review). In addition to drug relapse, increasing interest surrounds resurgence as a more general relapse phenomenon (e.g., Winterbauer & Bouton, 2010, 2011; Winterbauer et al, 2013). Resurgence also has high potential clinical importance as it relates to many widely used behavioral interventions involving differential reinforcement of alternative (DRA) behavior (see Petscher, Rey, & Bailey, 2009). In such interventions, the reinforcing consequences of problem behavior are withheld while appropriate behaviors are reinforced; however, reduction or elimination of reinforcement for the alternative behavior tends to produce increased levels of the problem behavior (e.g., Volkert, Lerman, Call, & Trosclair-Lasserre, 2009).
Although resurgence has been used to model relapse to drug seeking, and may pose a significant risk for relapse within applied settings in which alternative reinforcement is used, little is known about its underlying neuropharmacology. Quick et al. (2011) found that administration of the dopamine D1 receptor antagonist SCH 23390 blocked resurgence of cocaine seeking. Dopamine D1 receptor activation also plays a critical role in various types of reinstatement and renewal of drug seeking (e.g., Alleweireldt, Weber, Kirschner, Bullock, & Neisewander, 2002; Capriles, Rodaros, Sorge, & Stewart, 2003; Crombag, Grimm, & Shaham, 2002; Norman, Norman, Hall, & Tibulsky, 1999), so the Quick et al. results indicate common neuropharmacology among some commonly used models of relapse.
Considerably more is known about the neuropharmacology of relapse observed in reinstatement and renewal models (see Crombag, Bossert, Koya, & Shaham, 2008; Bossert, Ghitza, Lu, Epstein, & Shaham, 2005; Shalev, Grimm, & Shaham, 2002). If resurgence shares common neurobiology with these models, as the Quick et al. (2011) data suggest, then other neural systems that play a role in reinstatement and renewal may also be critical in resurgence. The dopamine D2 receptor is linked to drug-induced reinstatement (e.g., Shaham & Stewart, 1996), cue-induced reinstatement (e.g., Liu & Weiss, 2002; Tobin, Newman, Quinn, & Shalev, 2009), and renewal (Crombag et al., 2002). Thus, the dopamine D2 receptor may play a role in resurgence. Resurgence has been likened to stress-induced relapse (Quick et al., 2011), but the dopamine D2 receptor does not appear to play a role in stress-induced reinstatement (Capriles et al., 2003; Shaham & Stewart, 1996; Tobin et al., 2009). The adrenergic α2 receptor, however, has been heavily implicated in stress-induced reinstatement (Erb et al., 2000; Lê, Harding, Juzytsch, Funk, & Shaham, 2005; Shaham, Highfield, Delfs, Leung, & Stewart, 2000; Zislis, Desai, Prado, Shah, & Bruijnzeel, 2007), but has not yet been examined in resurgence, so it was also a target of the present study.
The role of D2 and α2 receptors in resurgence of food seeking was examined via administration of the dopamine D2 antagonist raclopride and the adrenergic α2 receptor agonist clonidine. Separate groups of rats pressed a target lever for food deliveries during Phase I. In Phase II, the lever press was extinguished while an alternative nose poke response was reinforced. Finally in Phase III, the alternative poke response was also placed on extinction while the target lever press remained on extinction. Prior to Phase III sessions the groups of rats received injections of 0, 50, or 100 μg/kg of raclopride or 0, 20, or 40 μg/kg of clonidine.
2. MATERIAL AND METHODS
2.1 Subjects
Forty-eight experimentally naïve male Long-Evans rats (Charles River, Portage, Michigan, USA) approximately 90 days old upon arrival were used. Free-feeding weights were established over a period of approximately 2 weeks after arrival, and thereafter rats were maintained via supplemental feedings at approximately 80% of their free-feeding weights. Rats were housed individually with free access to water in a temperature-controlled room with a 12:12h light/dark cycle (lights on at 7:00 AM). Experimental sessions took place at approximately the same time each day during the light cycle.
2.2 Apparatus
MED-Associates (1999) programming and interface controlled all experimental events and data recording. Eight MED-Associates modular operant chambers (30 cm × 24 cm × 21 cm) housed in sound-attenuating cubicles were used. Each chamber was equipped with a house light for general illumination. On the back wall of the chambers were five evenly spaced apertures, each containing a yellow light emitting diode (LED), as well as a photobeam capable of detecting head entries (i.e., nose pokes). Centered on the opposite wall was a recessed receptacle (5 cm × 5 cm) in which 45-mg food pellets (Bio-Serv, Frenchtown, New Jersey, USA) were delivered. Pellet deliveries were accompanied by an audible click and lit receptacle. In four of the chambers, the food apertures were divided in half vertically, and pellets were delivered on the right side. These chambers also featured fixed levers to the left and right of the pellet receptacle and above each lever was a series of colored LEDs (red, yellow, green). The four other chambers had retractable levers to the left and right of the pellet receptacle, and above each of those levers was a lamp (2.5 cm diameter).
2.3 Drug
Separate groups of animals (n = 8) received 0 (vehicle), 50, or 100 μg/kg of the dopamine D2 receptor antagonist raclopride or 0 (vehicle), 20, or 40 μg/kg of the α2 adrenergic receptor agonist clonidine. These raclopride doses attenuate renewal (Crombag et al., 2002) and cue-induced reinstatement (Liu & Weiss, 2002; Tobin et al., 2009), but not stress-induced reinstatement (Tobin et al., 2009), while the clonidine doses attenuate shock-induced reinstatement without motor impairment (Erb et al., 2000; Shaham et al., 2000). Both drugs were dissolved in a sterile 0.9% saline solution and all drug doses were prepared at an injection volume of 1 ml/kg. Raclopride was administered subcutaneously 20 minutes prior to experimental sessions, and clonidine was administered via intraperitoneal injection 40 minutes prior to experimental sessions.
2.4 Procedure
2.4.1 Training
Rats experienced a single 30-min session of magazine training in which food pellets were delivered according to a variable time (VT) 60-s schedule. In chambers equipped with retractable levers, the levers were extended into the chambers, but the lever lights and house lights remained off. An audible click and 3-s illumination of the pellet receptacle accompanied pellet deliveries during training and throughout the experiment. In two additional sessions, pellets were delivered for lever pressing according to fixed ratio (FR) 1 schedule. One lever produced pellets when pressed (i.e., the target lever), while the other lever had no programmed consequences (i.e., inactive lever). The light above the target lever was lit and the location of the target lever (left or right) was counterbalanced across subjects.
2.4.2 Phase I
During Phase I, pellets were delivered contingent on target lever presses according to a variable interval (VI) 45-s schedule in 30-min sessions timed exclusive of 3-s pellet deliveries. Phase I lasted 20 sessions. At the end of Phase I, rats were assigned to one of two experimental groups (n = 8 per raclopride dose) or the control group (n = 8) while matching for mean target lever rates during the final 3 sessions of Phase I. Each dose was examined within a separate group of animals based on previous findings demonstrating variations across repeated resurgence tests (Lieving & Lattal, 2003).
2.4.3 Phase II
Lever presses were extinguished and no longer produced pellet deliveries. Occurring in conjunction with extinction of the lever press, the center nose poke at the rear of the chamber was lit. The first head entry into this poke in the first session of Phase II resulted in a pellet delivery, and afterward pellets were delivered according to a VI 10-s schedule. A richer schedule of reinforcement was used in Phase II to produce greater resurgence in Phase III (Shahan & Sweeney, 2011). These contingencies remained in effect for 10 sessions, and were identical for all groups.
2.4.4 Phase III
During the next five sessions, both the lever and poke responses were extinguished and had no programmed consequences for all groups. The groups received subcutaneous injections of 0 (vehicle), 50, or 100 μg/kg raclopride 20 min prior, or intraperitoneal injections of 0 (vehicle), 20, or 40 μg/kg of the α2 receptor agonist clonidine 40 min prior to experimental sessions.
3. RESULTS
3.1 Phase I
All animals acquired the target lever press response during training sessions, and responding proceeded normally throughout Phase I. The top portion of Table 1 displays mean (SEM) target (lever associated with food during Phase I), inactive (lever never associated with food), alternative (center poke associated with food during Phase II), and other (4 pokes never associated with food) response rates (responses per minute), as well as reinforcer rates for the treatment groups across the final 3 Phase I sessions. Rates on the target lever did not vary systematically across groups that received raclopride, F(2,21) = 0.00, p = 1.00, or clonidine, F(2,21) = .01, p = .995, and all other responses occurred similarly at low rates. Rates of food delivery also did not vary systematically across raclopride, F(2,21) = .52, p = .600, or clonidine, F(2,21) = .47, p = .633, groups and were close to the programmed rate of reinforcement of 1.33 foods per minute.
Table 1.
Mean (SEM) Response and Food Rates Averaged Over the Final Three Sessions of Phase I and Phase II
| Phase I | Raclopride
|
Clonidine
|
||||
|---|---|---|---|---|---|---|
| 0 μg | 50 μg | 100 μg | 0 μg | 20 μg | 40 μg | |
| Target | 38.03 (10.31) | 38.35 (6.54) | 38.22 (7.04) | 32.77 (8.26) | 33.07 (9.19) | 33.93 (7.55) |
| Alt | 0.02 (0.01) | 0.06 (0.04) | 0.06 (0.03) | 0.12 (0.03) | 0.08 (0.03) | 0.12 (0.04) |
| Inactive | 1.15 (0.58) | 1.38 (0.43) | 1.02 (0.23) | 0.99 (0.42) | 2.75 (1.67) | 1.28 (0.44) |
| Other | 0.11 (0.04) | 0.25 (0.17) | 0.33 (0.19) | 0.73 (0.20) | 0.44 (0.18) | 0.57 (0.21) |
| Foods | 1.24 (0.02) | 1.22 (0.01) | 1.23 (0.01) | 1.19 (0.03) | 1.22 (0.02) | 1.19 (0.03) |
|
| ||||||
| Phase II | ||||||
| Target | 2.53 (0.79) | 2.29 (0.49) | 1.63 (0.25) | 2.74 (0.76) | 2.22 (0.29) | 3.87 (1.45) |
| Alt | 50.93 (15.02) | 65.61 (18.39) | 49.14 (14.37) | 48.54 (11.42) | 52.81 (11.98) | 36.75 (5.40) |
| Inactive | 0.86 (0.33) | 0.55 (0.13) | 0.48 (0.13) | 0.32 (0.15) | 0.69 (0.34) | 1.91 (1.38) |
| Other | 7.43 (2.36) | 4.49 (1.21) | 6.25 (1.87) | 4.98 (1.51) | 3.79 (2.38) | 7.44 (2.41) |
| Foods | 4.70 (0.25) | 4.57 (0.32) | 4.62 (0.26) | 4.62 (0.40) | 4.79 (0.21) | 4.66 (0.17) |
3.2 Phase II
One rat belonging to the 0 μg clonidine group experienced an additional Phase II session because of a substantial decrease in response rates during session 10. Response rates nearly recovered to their prior levels in the following session, so he proceeded to Phase III and his data (with the exception of session 10) were included in all data analyses. The bottom portion of Table 1 shows response and reinforcer rates averaged over the last 3 sessions of Phase II. The extinction contingencies in effect on the target lever reduced response rates to similarly low levels across raclopride, F(2,21) = .71, p = .502, and clonidine, F(2,21) = .77, p = .477, groups. Reinforcement of the alternative poke response was effective in increasing rates of poke responding, which were not significantly different across raclopride, F(2,21) = .32, p = .731, or clonidine, F(2,21) = .69, p = .515, groups. Phase II was also associated with decreased rates of responding on the inactive lever, and increased rates of response in the other pokes. The richer VI (10-s in Phase II versus 45-s in Phase I) produced more frequent reinforcement than in Phase I, and although food delivery rates fell short of the programmed reinforcement rate of 6 pellets per minute, animals earned comparable rates of pellet deliveries within the raclopride, F(2,21) = .04, p = .943, and clonidine, F(2,21) = .10, p = .904, groups.
3.3 Phase III
3.3.1 Resurgence in first Phase III session
Figures 1 and 2 show the effects of raclopride and clonidine on response rates in the first session of Phase III relative to the last session of Phase II. For each drug, separate mixed model ANOVAs were conducted for each response with Phase as a within-subjects factor and Dose as a between-subjects factor.
Figure 1.
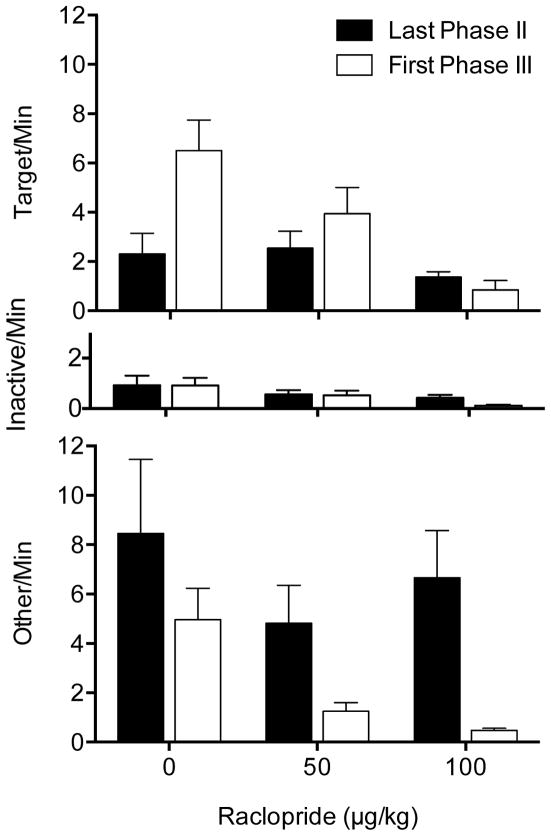
Effects of raclopride on mean (±SEM) response rates on the target lever (top panel), inactive lever (middle panel), and other pokes (bottom panel) within the last session of Phase II and first session of Phase III
Figure 2.
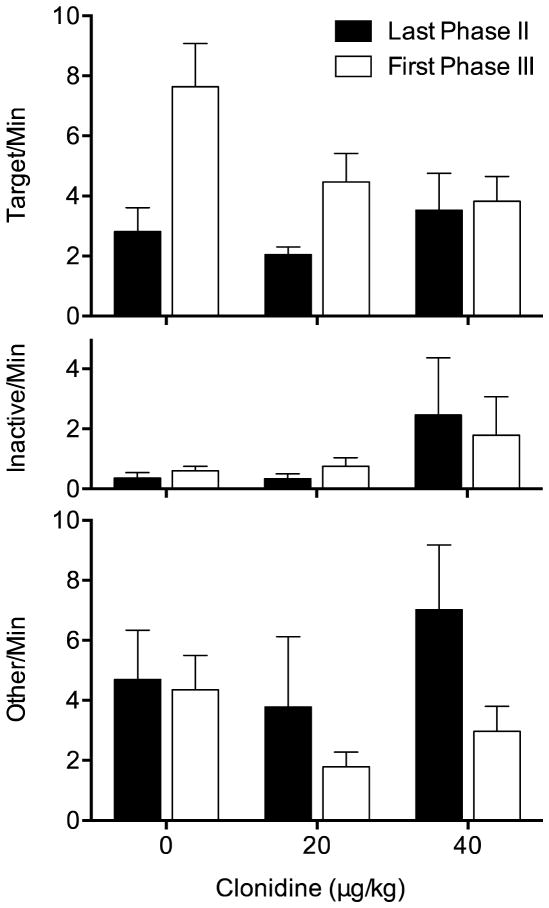
Effects of clonidine on mean (±SEM) response rates on the target lever (top panel), inactive lever (middle panel), and other pokes (bottom panel) within the last session of Phase II and first session of Phase III
3.3.1.1 Raclopride
The top panel of Figure 1 shows response rates on the target lever. Raclopride dose-dependently reduced the amount of resurgence on the target lever as indicated by the mixed ANOVA yielding significant main effects of Phase, F(1,21) = 11.61, p = .003, and Dose, F(2,21) = 5.75, p = .010, as well as a significant interaction, F(2,21) = 7.72, p = .003. Fisher’s Least Significant Difference (LSD) test indicated that the 0 and 100 μg groups differed significantly. To follow-up the significant interaction, paired t-tests comparing the effect of Phase at each Dose indicated that the significant interaction arose from significant resurgence occurring in the 0 μg group, t(7) = −5.35, p = .022, but neither the 50 μg group, t(7) = −1.16, p = .286, nor the 100 μg group, t(7) = 1.59, p = .157. Thus, elimination of alternative reinforcement produced resurgence on the target lever, but only in the saline group, indicating that raclopride suppressed resurgence.
The middle and bottom panels of Figure 1 show response rates on the inactive lever and all other nose pokes, respectively. These response rates were analyzed to rule out the possibility that resurgence on the target lever was accompanied by increases in responses with no history of reinforcement, indicative of general activation. Although inactive pressing was suppressed in the 100 μg group, a mixed model ANOVA indicated that these differences were not significant, Phase F(1,21) = 2.52, p = .127, Dose F(2,21) = 2.30, p = .125, Interaction F(2,21) = 1.44, p = .260. Other nose pokes decreased from Phase II to III (Phase, F(1,21) = 19.31, p < .001), and although raclopride appeared to suppress rates of other pokes in the 50 and 100 μg groups relative to the 0 μg group, the main effect of Dose, F(2,21) = 1.93, p = .170, and the interaction, F(2,21) = .77, p = .474, did not reach significance. Thus, resurgence during Phase III occurred only on the target lever, and only in the group of animals that did not receive raclopride injections. Therefore, raclopride effectively attenuated resurgence at both doses tested, 50 and 100 μg/kg.
3.3.1.2 Clonidine
Response rates on the target lever in the last session of Phase II and the first of Phase III are shown in the top panel of Figure 2. As in the group of animals treated with raclopride, responding was generally higher during Phase III, and varied according to clonidine treatment. The mixed ANOVA yielded a significant main effect of Phase, F(1,21) = 20.39, p < .001, and significant interaction, F(2,21) = 5.55, p = .012, but the main effect of Dose failed to reach significance, F(2,21) = 1.43, p = .261. Follow-up paired t-tests indicated significant resurgence in the 0 μg, t(7) = −4.12, p = .004, and 20 μg, t(7) = −2.43, p = .045, but not 40 μg group, t(7) = −.44, p = .672. Thus, clonidine attenuated resurgence of the target lever at both doses tested, but only significantly at the highest dose tested, 40 μg.
The middle panel of Figure 2 shows response rates on the inactive lever in the last session of Phase II and the first of Phase III. Inactive lever pressing was lower in the 0 and 20 μg groups than in the 40 μg group across phases. The 0 and 20 μg groups also showed negligible increases in inactive lever pressing, whereas the 40 μg group showed a slight decrease. However, the mixed ANOVA indicated nonsignificant effects of Phase, F(1,21) = .001, p = .970, and Dose, F(2,21) = 1.00, p = .383, and interaction, F(2,21) = 2.15, p = .142. The bottom panel of Figure 2 shows response rates in the other nose pokes. Like the inactive lever presses, these pokes tended to occur less frequently in the 0 and 20 μg groups as compared to the 40 μg group. Both groups treated with clonidine showed decreased levels of other poking in Phase III, whereas other pokes in the 0 μg group were roughly the same. Significantly fewer other pokes occurred in Phase III as evidenced by a significant effect of Phase, F(1,21) = 6.32, p = .020. No other differences were significantly different, as indicated by the main effect of Dose, F(2,21) = .69, p = .514, and the interaction, F(2,21) = 1.61, p = .223, failing to reach significance.
Resurgence specific to the target lever occurred in the 0 and 20 μg groups, but was blocked by 40 μg of clonidine. Although responses to the other nose pokes decreased in Phase III as in the animals treated with raclopride, this decrease was observed only in the groups treated with clonidine. Whereas in in the raclopride groups the other pokes decreased across all groups, which was attributed to the experimental contingencies, direct drug effects cannot be ruled out in the animals treated with clonidine.
3.3.2 Across Phase III sessions
Drug injections were administered before each of the five Phase III sessions, allowing examination of repeated raclopride and clonidine administration over the course of resurgence for the target lever, as well as the course of extinction for the alterative poke. Figures 3 and 4 show mean (±SEM) response rates for the target and alternative responses across all five sessions of Phase III.
Figure 3.
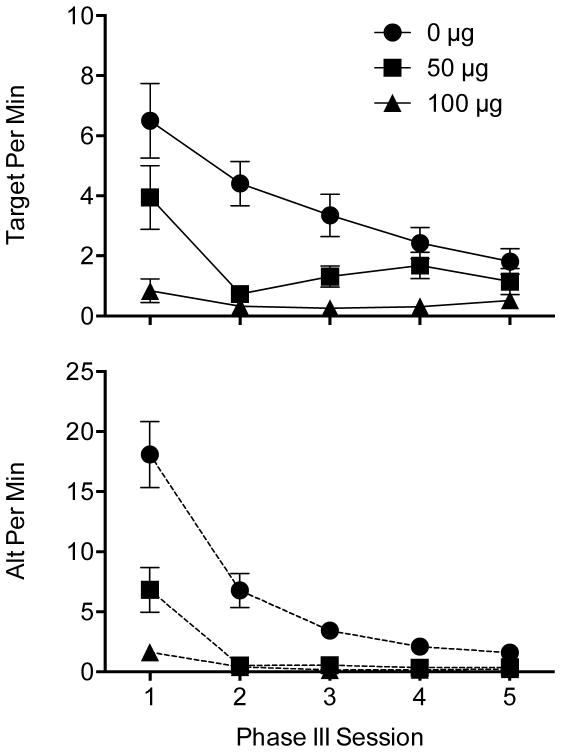
Effects of raclopride on mean (±SEM) response rates for the target lever (top panel) and alternative poke (bottom panel) across Phase III sessions
Figure 4.
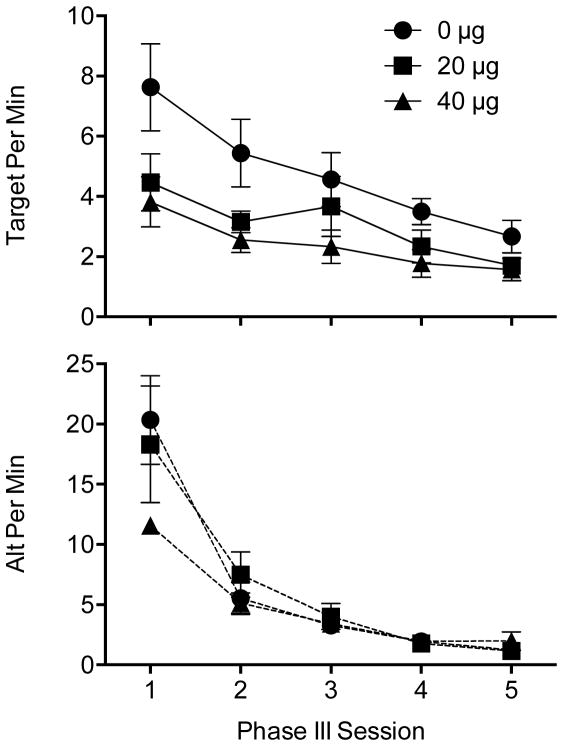
Effects of clonidine on mean (±SEM) response rates for the target lever (top panel) and alternative poke (bottom panel) across Phase III sessions
3.3.2.1 Raclopride
The top panel of Figure 3 shows the course of resurgence across Phase III sessions on the target lever, and shows that raclopride dose-dependently reduced resurgence across sessions. A mixed ANOVA with Dose as a between-subjects factor and Session as a repeated factor supported this interpretation, yielding significant main effects of Dose, F(2,21) = 16.99, p < .001, and Session, F(4,84) =14.92, p < .001, as well a significant interaction, F(8,84) = 4.34, p < .001. Fisher’s LSD test indicated each pair of groups differed significantly. The significant interaction arose from the effect of Session reaching significance within the 0 [F(4,28) = 10.28, p < .001] and 50 [F(4,28) = 6.33, p = .001], but not 100 μg group [F(4,28) = 1.07, p = .391]. Furthermore, the effect of Dose was significant in sessions 1–4 (all p values < .01), but not session 5 [F(2,23) = 2.83, p = .08].
Rates of nose poking across Phase III sessions are depicted in the bottom panel of Figure 3. Raclopride dose-dependently reduced nose poking during the first session of Phase III, but appeared to suppress responding almost completely throughout the remaining sessions in both groups treated with raclopride. A mixed ANOVA supported this interpretation, producing significant main effects of Dose, F(2,21) = 81.99, p < .001, and Session, F(4,84) = 43.28, p < .001, as well as a significant interaction, F(8,84) = 12.62, p < .001. Fisher’s test indicated that rates of the alternative poke response were significantly suppressed in both raclopride groups relative to the saline control, but did not differ from one another. The significant interaction arose because Dose was significant at each session (all p values < .01) due to both groups treated with raclopride differing significantly from the 0 μg control group.
Thus, raclopride suppressed resurgence of the target lever and hastened extinction of the alternative nose poke across Phase III sessions at both doses tested. Slightly different patterns emerged between the two responses in the 50 μg group. Both responses fell to very low levels during the second Phase III session, but there was a subsequent increase in the target response, whereas the alternative poke remained suppressed almost completely.
3.3.2.2 Clondine
The top panel of Figure 4 shows target lever rates across Phase III sessions for rats treated with clonidine. A mixed ANOVA showed that response rates decreased across sessions as indicated by a significant main effect of Session, F(4,84) = 19.26, p < .001, and were impacted by clonidine as indicated by a significant main effect of Dose, F(2,21) = 4.04, p = .033. Fisher’s test showed that responding was significantly lower in the 40 μg group than the 0 μg group, but no other comparisons reached significance. Rate of decline was similar across groups as indicated by a nonsignificant interaction, F(8,84) = 1.506, p = .167. Thus, 40 μg clonidine reduced target responding across Phase III sessions, but did not appear to hasten the rate of decline across those sessions.
The bottom panel of Figure 4 shows response rates on the alternative poke across Phase III sessions. Although responding in the 40 μg group was somewhat suppressed in the first session, rates of the alternative poke decreased similarly across sessions in all groups. A mixed ANOVA supported this interpretation yielding a significant main effect of Session, F(4,84) = 51.43, p < .001, but not Dose, F(2,21) = .76, p = .478, nor interaction, F(8,84) = 1.97, p = .060. Although the interaction nearly reached statistical significance, the effect of Dose did not reach significance within any of the Phase III sessions (all p values > .20) indicating that clonidine did not significantly impact extinction of the alternative poke response across Phase III sessions. Thus, clonidine reduced activity at the target lever across Phase III sessions while leaving activity at the alternative poke relatively intact.
4. DISCUSSION
The present study examined resurgence of a previously food-maintained response as a preliminary investigation into whether D2 and α2 receptors play a role in resurgence. Raclopride and clonidine both attenuated resurgence of the target response, but showed different patterns with respect to the levels of attenuation, as well as effects on the alternative response. Treatment with 50 and 100 μg/kg of the D2 antagonist raclopride significantly reduced resurgence of food maintained behavior, but also reduced the alternative poke response. Raclopride also hastened the rate of decline for both the target and alternative responses across Phase III sessions at both doses tested. At least one previous study has reported motor impairment at the doses tested in the present study (Crombag et al., 2002); however, previous studies on various types of reinstatement have used the same or higher doses of raclopride and reported little or no evidence of motor impairment (Cervo, Carnovali, Stark, & Mennini, 2003; Shaham & Stewart, 1996; Tobin et al., 2009). There is some evidence of raclopride having differential effects on ongoing food-and drug-maintained behavior (Caine & Koob, 1994; Weissenborn, Deroche, Koob, & Weiss, 1996), but it is not clear whether these results apply to relapse of extinguished behavior.
Raclopride also appeared to have a larger impact on the alternative poke, nearly eliminating it at both doses of raclopride tested, whereas the same suppressive effect was only observed on the target response at the 100 μg/kg dose. Such differential effects may be attributable to the rates at which the responses occurred prior to raclopride administration (i.e., the target occurred at a much lower rate); however, it could also be a matter of relapse versus extinction (i.e., reappearance of an extinguished response versus elimination of a previously reinforced response). The near elimination of target and alternative responding in the 100 μg/kg group is highly suggestive of motor impairment, and despite the fact that some target responding occurred in the group that received 50 μg/kg, alternative responding within that group was also almost eliminated. Thus, attenuation of resurgence via motor impairment cannot be ruled out at either dose.
Administration of 20 and 40 μg/kg of the α2-adrenergic agonist clonidine reduced resurgence; however, only the highest dose tested effectively reduced resurgence to levels similar to extinction. Unlike raclopride, clonidine reduced responding on the target lever across Phase III sessions without impacting the rate of extinction of the alternative poke. Thus, the clonidine results provide better evidence of reduced resurgence in the absence of motor impairment.
While the present study examined resurgence of food seeking, the majority of the literature that informed the present design has examined relapse to drug seeking. Although few studies have examined the effects of these drugs on relapse to food seeking, some discrepancies have been noted between the effects of these drugs on drug and food seeking. For instance, administration of D2 antagonists has previously potentiated (Ball, Combs, & Beyer, 2011) or had no effect (Gál & Gyertyán, 2006) on cue-induced reinstatement of food seeking, whereas these drugs typically reduce cue-induced drug seeking (Gál & Gyertyán, 2006; Liu et al., 2010; Tobin et al., 2009). Also, adrenergic α2 agonists do not reduce yohimbine-induced reinstatement of food seeking (Lê et al., 2011; Nair, Adams-Deutsch, Epstein, & Shaham, 2009), whereas these drugs readily block yohimbine-induced drug seeking (Lê, Funk, Harding, Juzytsch, & Fletcher, 2009; Lee, Tiefenbacher, Platt, & Spealman, 2003). Therefore, future studies will need to assess the effects of these drugs on resurgence of drug seeking, in which the target response is associated with drug deliveries and the alternative is associated with a nondrug reinforcer (e.g., Podlesnik et al., 2006; Quick et al., 2011).
The fact that both the target and alternative response produced the same nondrug reinforcer in the present experiment may also limit the treatment implications of the findings. Dissociation of effects on drug versus food seeking is important for behavioral interventions that attempt to curb drug taking. However, not all behavioral interventions aim to reduce drug-maintained behavior. For instance, behavioral interventions attempting to replace maladaptive behaviors in children with developmental disabilities with more functional behavior often result in the same consequence (e.g., Volkert et al., 2009).
Within the present experiment the target response was always a lever press and the alternative response was always a nose poke response. A potential criticism of the present study is that response type was confounded with whether the response was the target or alternative. There is some evidence that these response may be differentially impacted by pharmacological manipulation; however, not necessarily by the drugs examined in the present experiments (Gerhardt & Leibman, 1981). Future studies might counterbalance response type across the target and alternative responses, or use two responses that are not as readily emitted by rats (e.g., lever press and chain pull).
Raclopride and clonidine both reduced resurgence of the target lever press, but raclopride also suppressed the alternative response, while clonidine had minimal impact. In DRA-based treatments for problem behavior for which results of resurgence research are most applicable, the alternative response is typically a functional one that would be undesirable to reduce. Based on the present results, clonidine appears to be a more viable option as a pharmacological treatment to supplement behavioral interventions. Interestingly, previous studies have examined the effects of both D2 antagonists and α2 agonists on drug craving in human participants, and their results appear to be consistent with the present experiments with respect to undesirable motor side effects. Multiple studies have found that treatment with antipsychotic medications with action at dopamine D2 receptors reduce cue-elicited craving in abstinent cocaine (Berger et al., 1996; De La Garza, Newton, & Kalechstein, 2005; Smelson et al., 2004) and cigarette users (see Matthews, Wilson, & Mitchell, 2011; but see Mahler & de Wit, 2005). However, atypical antipsychotic drugs are typically favored in this research (Hutchison et al., 2004; Rohsenow et al., 2008) because traditional antipsychotics with higher affinities for dopamine D2 receptors (e.g., haloperidol) tend to produce greater motor disturbances, making their use less practical (see Shirzadi & Ghaemi, 2006). There is also evidence that α2 agonists have therapeutic value with regard to craving induced by cues, as well as stress (Jobes et al., 2011; Sinha, Kimmerling, Doebrick, & Kosten, 2007).
5. CONCLUSIONS
In conclusion, the present experiments shed additional light on the neuropharmacology of resurgence, implicating dopamine D2 and adrenergic α2 receptors. These results also provide evidence of further overlap among the commonly used models of relapse, and may have implications in applied settings that employ DRA-based treatments for problem behavior or for human drug users in which stressful situations characterized by significant loss exacerbate drug use or induce relapse to maladaptive behaviors.
Research Highlights.
We examined the role of two receptor types in resurgence of food seeking
Dopamine D2 receptors were examined via administration of raclopride
Adrenergic α2 receptors were examined via administration of clonidine
Both drugs effectively attenuated resurgence of the target response
Clonidine did so with less impact on the alternative response
Acknowledgments
Funding
This research was supported by NIH grant R01AA016786 to Timothy A. Shahan.
Footnotes
Disclosure
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alleweireldt AT, Weber SM, Kirschner KF, Bullock BL, Neisewander JL. Blockade or stimulation of D1 dopamine receptors attenuates cue reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology. 2002;159(3):284–293. doi: 10.1007/s002130100904. [DOI] [PubMed] [Google Scholar]
- Ball KT, Combs TA, Beyer DN. Opposing roles for dopamine D1- and D2-like receptors in discrete cue-induced reinstatement of food seeking. Behavioural Brain Research. 2011;222(2):390–393. doi: 10.1016/j.bbr.2011.03.064. [DOI] [PubMed] [Google Scholar]
- Berger SP, Reid MS, Delucchi K, Hall S, Hall S, Mickalian JD, Crawford CA. Haloperidol antagonism of cue-elicited cocaine craving. The Lancet. 1996;347:504–508. doi: 10.1016/S0140-6736(96)91139-3. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: An update and clinical implications. European Journal of Pharmacology. 2005;526:36–50. doi: 10.1016/j.ejphar.2005.09.030. [DOI] [PubMed] [Google Scholar]
- Caine SB, Koob GF. Effects of dopamine D-1 and D-2 antagonists on cocaine self-administration under different schedules of reinforcement in the rat. Journal of Pharmacology and Experimental Therapeutics. 1994;270(1):209–218. [PubMed] [Google Scholar]
- Capriles N, Rodaros D, Sorge RE, Stewart J. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2003;168:66–74. doi: 10.1007/s00213-002-1283-z. [DOI] [PubMed] [Google Scholar]
- Cervo L, Carnovali F, Stark JA, Mennini T. Cocaine-seeking behavior in response to drug-associated stimuli in rats: involvement of D3 and D2 dopamine receptors. Neuropsychopharmacology. 2003;28:1150–1159. doi: 10.1038/sj.npp.1300169. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Bossert JM, Koya E, Shaham Y. Context-induced relapse to drug seeking: A review. Philosophical Transactions of the Royal Society of London, Biological Sciences. 2008;363:3233–3243. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Grimm JW, Shaham Y. Effect of dopamine receptor antagonists on renewal of cocaine seeking by reexposure to drug-associated contextual cues. Neuropsychopharmacology. 2002;27:1006–1015. doi: 10.1016/S0893-133X(02)00356-1. [DOI] [PubMed] [Google Scholar]
- De La Garza R, Newton TF, Kalechstein AD. Risperidone diminishes cocaine-induced craving. Psychopharmacology. 2005;178(2):347–350. doi: 10.1007/s00213-004-2010-8. [DOI] [PubMed] [Google Scholar]
- Epstein R. Resurgence of previously reinforced behavior during extinction. Behaviour Analysis Letters. 1983;3:391–397. [Google Scholar]
- Erb S, Shaham Y, Stewart J. The role of corticotropin-releasing factor and corticosterone in stress-and cocaine-induced relapse to cocaine seeking in rats. The Journal of Neuroscience. 1998;18(14):5529–5536. doi: 10.1523/JNEUROSCI.18-14-05529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gál K, Gyertyán I. Dopamine D3 as well as D2 receptor ligands attenuate the cue-induced cocaine-seeking in a relapse model in rats. Drug and Alcohol Dependence. 2006;81:63–70. doi: 10.1016/j.drugalcdep.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Gerhardt S, Liebman JM. Differential effects of drug treatments on nose-poke and bar-press self-stimulation. Pharmacology Biochemistry and Behavior. 1981;15(5):767–771. doi: 10.1016/0091-3057(81)90020-4. [DOI] [PubMed] [Google Scholar]
- Hutchison KE, Rutter MC, Niaura R, Swift RM, Pickworth WB, Sobik L. Olanzapine attenuates cue-elicited craving for tobacco. Psychopharmacology. 2004;175(4):407–413. doi: 10.1007/s00213-004-1837-3. [DOI] [PubMed] [Google Scholar]
- Jobes ML, Ghitza UE, Epstein DH, Phillips KA, Heishman SJ, Preston KL. Clonidine blocks stress-induced craving in cocaine users. Psychopharmacology. 2011;218(1):83–88. doi: 10.1007/s00213-011-2230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê AD, Funk D, Harding S, Juzytsch W, Fletcher PJ. The role of noradrenaline and 5-hydroxytryptamine in yohimbine-induced increases in alcohol-seeking in rats. Psychopharmacology. 2009;204(3):477–488. doi: 10.1007/s00213-009-1481-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê AD, Funk D, Juzytsch W, Coen K, Navarre BM, Cifani C, Shaham Y. Effect of prazosin and guanfacine on stress-induced reinstatement of alcohol and food seeking in rats. Psychopharmacology. 2011;218(1):89–99. doi: 10.1007/s00213-011-2178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê AD, Harding S, Juzytsch W, Funk D, Shaham Y. Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology. 2005;179(2):366–373. doi: 10.1007/s00213-004-2036-y. [DOI] [PubMed] [Google Scholar]
- Lee B, Tiefenbacher S, Platt DM, Spealman RD. Role of the hypothalamic-pituitary-adrenal axis in reinstatement of cocaine-seeking behavior in squirrel monkeys. Psychopharmacology. 2003;168:177–183. doi: 10.1007/s00213-003-1391-4. [DOI] [PubMed] [Google Scholar]
- Lieving GA, Lattal KA. Recency, repeatability, and reinforcer retrenchment: An experimental analysis of resurgence. Journal of the Experimental Analysis of Behavior. 2003;80:217–233. doi: 10.1901/jeab.2003.80-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Jernigan C, Gharib M, Booth S, Caggiula AR, Sved AF. Effects of dopamine antagonists on drug cue-induced reinstatement of nicotine-seeking behavior in rats. Behavioural Pharmacology. 2010;21:153–160. doi: 10.1097/FBP.0b013e328337be95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Weiss F. Reversal of ethanol-seeking behavior by D1 and D2 antagonists in an animal model of relapse: Differences in antagonist potency in previously ethanol-dependent versus nondependent rats. The Journal of Pharmacology and Experimental Therapeutics. 2002;300:882–889. doi: 10.1124/jpet.300.3.882. [DOI] [PubMed] [Google Scholar]
- Mahler SV, De Wit H. Effects of haloperidol on reactions to smoking cues in humans. Behavioural Pharmacology. 2005;16(2):123–126. doi: 10.1097/00008877-200503000-00008. [DOI] [PubMed] [Google Scholar]
- Marchant NJ, Li X, Shaham Y. Recent developments in animal models of drug relapse. Current Opinion in Neurobiology. 2013;23(4):675–683. doi: 10.1016/j.conb.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews AM, Wilson VB, Mitchell SH. The role of antipsychotics in smoking and smoking cessation. CNS Drugs. 2011;25(4):299–315. doi: 10.2165/11588170-000000000-00000. [DOI] [PubMed] [Google Scholar]
- MED-Associates. Med-PC for Windows. St. Albans, VT: Author; 1999. [Google Scholar]
- Nair SG, Adams-Deutsch T, Epstein DH, Shaham Y. The neuropharmacology of relapse to food seeking: Methodology, main findings, and comparison with relapse to drug seeking. Progress in Neurobiology. 2009;89:18–45. doi: 10.1016/j.pneurobio.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman AB, Norman MK, Hall JF, Tsibulsky VL. Priming threshold: A novel quantitative measure of the reinstatement of cocaine self-administration. Brain Research. 1999;831:165–174. doi: 10.1016/S0006-8993(99)01423-7. [DOI] [PubMed] [Google Scholar]
- Petscher ES, Rey C, Bailey JS. A review of empirical support for differential reinforcement of alternative behavior. Research of Developmental Disabilities. 2009;30:409–425. doi: 10.1016/j.ridd.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Podlesnik CA, Jimenez-Gomez C, Shahan TA. Resurgence of alcohol seeking produced by discontinuing nondrug reinforcement as an animal model of drug relapse. Behavioural Pharmacology. 2006;17:369–374. doi: 10.1097/01.fbp.0000224385.09486.ba. [DOI] [PubMed] [Google Scholar]
- Quick SL, Pyszczynski AD, Colston KA, Shahan TA. Loss of alternative non-drug reinforcement induces relapse of cocaine-seeking in rats: Role of dopamine D1 receptors. Neuropsychopharmacology. 2011;36:1015–1020. doi: 10.1038/npp.2010.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohsenow DJ, Tidey JW, Miranda R, Jr, McGeary JE, Swift RM, Hutchison KE, Monti PM. Olanzapine reduces urge to smoke and nicotine withdrawal symptoms in community smokers. Experimental and Clinical Psychopharmacology. 2008;16(3):215–222. doi: 10.1037/1064-1297.16.3.215. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Highfield D, Delfs J, Leung S, Stewart J. Clonidine blocks stress-induced reinstatement of heroin seeking in rats: an effect independent of locus coeruleus noradrenergic neurons. European Journal of Neuroscience. 2000;12(1):292–302. doi: 10.1046/j.1460-9568.2000.00899.x. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Stewart J. Effects of opioid and dopamine receptor antagonists on relapse induced by stress and re-exposure to heroin in rats. Psychopharmacology. 1996;125:385–391. doi: 10.1007/BF02246022. [DOI] [PubMed] [Google Scholar]
- Shahan TA, Sweeney MM. A model of resurgence based on behavioral momentum theory. Journal of the Experimental Analysis of Behavior. 2011;95(1):91–108. doi: 10.1901/jeab.2011.95-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: A review. Pharmacological Reviews. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Shirzadi AA, Ghaemi SN. Side effects of atypical antipsychotics: Extrapyramidal symptoms and the metabolic syndrome. Harvard Review of Psychiatry. 2006;14(3):152–164. doi: 10.1080/10673220600748486. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Annals of the New York Academy of Sciences. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Kimmerling A, Doebrick C, Kosten TR. Effects of lofexidine on stress-induced and cue-induced opioid craving and opioid abstinence rates: Preliminary findings. Psychopharmacology. 2007;190(4):569–574. doi: 10.1007/s00213-006-0640-8. [DOI] [PubMed] [Google Scholar]
- Smelson DA, Williams J, Ziedonis D, Sussner BD, Losonczy MF, Engelhart C, Kaune M. A double-blind placebo-controlled pilot study of risperidone for decreasing cue-elicited craving in recently withdrawn cocaine dependent patients. Journal of Substance Abuse Treatment. 2004;27(1):45–49. doi: 10.1016/j.jsat.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Tobin S, Newman AH, Quinn T, Shalev U. A role for dopamine D1-like receptors in acute food deprivation- induced reinstatement of heroin seeking in rats. International Journal of Neuropsychopharmacology. 2009;12:217–226. doi: 10.1017/S1461145708008778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkert VM, Lerman DC, Call NA, Trosclair-Lasserre N. An evaluation of resurgence during treatment with functional communication training. Journal of Applied Behavior Analysis. 2009;42:145–160. doi: 10.1901/jaba.2009.42-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenborn R, Deroche V, Koob GF, Weiss F. Effects of dopamine agonists and antagonists on cocaine-induced operant responding for a cocaine-associated stimulus. Psychopharmacology. 1996;126(4):311–322. doi: 10.1007/BF02247382. [DOI] [PubMed] [Google Scholar]
- Winterbauer NE, Bouton ME. Mechanisms of resurgence of an extinguished instrumental behavior. Journal of Experimental Psychology: Animal Behavior Processes. 2010;36(3):343. doi: 10.1037/a0017365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbauer NE, Bouton ME. Mechanisms of resurgence II: Response-contingent reinforcers can reinstate a second extinguished behavior. Learning and Motivation. 2011;42(2):154–164. doi: 10.1016/j.lmot.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbauer NE, Lucke S, Bouton ME. Some factors modulating the strength of resurgence after extinction of an instrumental behavior. Learning and Motivation. 2013;44(1):60–71. doi: 10.1016/j.lmot.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zislis G, Desai TV, Prado M, Shah HP, Bruijnzeel AW. Effects of the CRF receptor antagonist D-Phe CRF (12–41) and the α2-adrenergic receptor agonist clonidine on stress-induced reinstatement of nicotine-seeking behavior in rats. Neuropharmacology. 2007;53(8):958. doi: 10.1016/j.neuropharm.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]