Abstract
The objective of the present study was to develop techniques for an abuse-deterrent (AD) platform utilizing hot melt extrusion (HME) process. Formulation optimization was accomplished by utilizing Box-Behnken design of experiments to determine the effect of the three formulation factors: PolyOx™ WSR301, Benecel™ K15M, and Carbopol 71G; each of which was studied at three levels on TR attributes of the produced melt extruded pellets. A response surface methodology was utilized to identify the optimized formulation. Lidocaine Hydrochloride was used as a model drug, and suitable formulation ingredients were employed as carrier matrices and processing aids. All of the formulations were evaluated for the TR attributes such as particle size post-milling, gelling, percentage of drug extraction in water and alcohol. All of the DOE formulations demonstrated sufficient hardness and elasticity, and could not be reduced into fine particles (<150µm), which is a desirable feature to prevent snorting. In addition, all of the formulations exhibited good gelling tendency in water with minimal extraction of drug in the aqueous medium. Moreover, Benecel™ K15M in combination with PolyOx™ WSR301 could be utilized to produce pellets with TR potential. HME has been demonstrated to be a viable technique with a potential to develop novel abuse-deterrent formulations.
Keywords: Abuse-deterrent (AD), Tamper-resistant (TR), Hot melt extrusion (HME), Design of experiments (DOE), Insufflation, Optimization
1. Introduction
In recent years, prescription drug abuse has been identified as source of rapidly growing concern in the United States. Of all the available drugs, Opioids, CNS depressants and stimulants are the three most established drug classes that are abused, next to marijuana (1). Pain, being a potentially debilitating symptom associated with a variety of chronic medical conditions, has necessitated the application of numerous opioids, which have a high potential for abuse. The number of prescriptions for these drugs has also increased considerably over the past two decades (2). Moreover, as per one of the national surveys, more focus on the non-medical use of these opioids has been noticed in all of the different age groups, teenagers being a major constituent amongst those abusers (3, 4). In addition, the number of emergency visits as well as the death rates due to overdose of these medications has increased dramatically over the past few years (5). These drugs, being reasonably accessible to the population, have been largely misused or abused via physical or chemical manipulation in order to obtain high drug concentration in the body to produce euphoric effects, or a significant “high”.
The majority of the opioids/ controlled substances that have shown potential for abuse are available in the form of immediate release (IR) and extended release (ER) tablets and capsule dosage forms, trans-dermal patches, trans-mucosal lozenges etc. The consumption of more number of IR units via oral route results in higher concentrations of the drug in the systemic circulation, producing the desired “high” (6). In addition, there has been a marked interest in manipulating the ER dosage forms due to the large dose of the drug per unit dosage form (7). Apart from the oral route, alternative delivery routes have been explored by the abusers to achieve a similar psychological feel in a short period or with a limited amount of material in hand (8). Since these conventional formulations are not hard enough to resist crushing, the obtained powder-like fine material is easily snorted via nasal route. In the same scenario, if the active is highly soluble in the aqueous medium, it could be extracted in a small volume and injected directly into the blood stream to get an instantaneous “high.” Some of the other drugs with higher vapor pressures are abused or misused by smoking or volatilization (9). An epidemiologic study conducted on the subjects who were addicted to Oxycontin (Oxycodone HCl), have reported that majority of the users have started taking the drugs orally, but over the time have advanced to administering the API through different routes: 62% reported snorting, 26% through injecting, and only 14% by oral route (10).
Due to this alarming issue, the recent interest of several pharmaceutical industries has been shifted towards developing a platform technology, which either prevents or minimizes the abuse of, or tampering with, the prescription opioids. Currently, there are surprisingly few formulations that are either under development or commercially available in the United States that demonstrate such properties (11). Some of the marketed products that are currently approved by United States Food and Drug Administration (FDA) are Oxycontin® OP ER tablets, Oxecta IR tablets, Suboxone sublingual tablets, Nucynta ER tablets, and Opana ER tablets. These emerging opioid containing formulations were produced by novel approaches such as incorporation of gelling agents, nasal irritants, antagonists etc., in the formulations. Thus, they are not only designed to discourage the abusers by reducing the risk of misuse and/or abuse, but are also dually beneficial to physicians in meeting their goals of maximizing pain relief in afflicted populations. However, none of these formulations can guarantee an absolute abuse deterrence/tamper proof. In addition, the majority of these formulations have been produced via conventional methodologies involving several processing steps such as wet/dry granulation, blending, compression, curing, coating, etc., which adds longer time and higher cost to the development process.
Although research in this particular area has gained enormous attention due to the pressure from the law makers and the FDA, there is not much literature published/available, especially on the tamper-resistant (TR) / abuse-deterrent (AD) formulations developed utilizing novel hot melt extrusion (HME) technology. In a recent study by Bartholomaeus et al. the authors produced an ER opioid formulation with crush-resistant features using a combination of a planetary-gear extruder and a tablet press on a laboratory scale, which involved few processing steps (12). However, their primary objective was to stabilize the formulation and be bioequivalent to the marketed ER formulation. In addition, the rationale for their formulation selection does not involve DOE and necessitates further discussion.
The novelty of the current research lies in utilizing HME techniques and enabling a formulation platform technology with possible AD features. Exploration of HME technology in this area of AD is original and has never been published. The primary objective of this underlying research is to demonstrate the potential of HME techniques for producing dosage forms with satisfactory TR properties utilizing a DOE approach. Furthermore, this current work demonstrates that the produced TR formulation displays relatively high resistance to crushing/grinding/milling (to avoid insufflation), and exhibits minimal drug extraction (to avoid abuse via injection). HME is a novel and viable technique that has been effectively utilized in the pharmaceutical industry to improve the bioavailability of the actives, especially those having low aqueous solubility, by formation of molecular dispersions (13–15). This technique is also time-efficient and cost-effective, due to the involvement of fewer processing steps, and utilization of limited excipients. In this study, we have made a sincere attempt to demonstrate the capability of single-step HME technology in producing a TR formulation utilizing appropriate commonly used excipients (16, 17). It should be noted that the terms ‘TR’ and ‘AD’ may be used interchangeably throughout this paper.
2. Materials and Methods
2.1. Materials
Lidocaine HCl (LID), and Vitamin E Succinate (VES) were purchased from spectrum chemicals (Decatur, AL, USA). PolyOx™ WSR 301 (PEO-301), and PolyOx™ N80 (PEO-N80) were kindly gifted from Colorcon (West point, PA, USA). Benecel™ K4M (HPMC K4M), and Benecel™ K15M (HPMC K15M) were kindly gifted from Ashland Inc. (Wilmington, DE, USA). Eudragit® RSPO (EUD) and Carbopol 71G (CBP) were kindly gifted by Evonik (Piscataway, NJ, USA), and Lubrizol (Cleveland, Ohio, USA), respectively. Dibasic potassium phosphate and tribasic sodium phosphate were purchased from sigma-Aldrich (St. Louis, MO, USA). High performance liquid chromatography (HPLC) grade water was freshly prepared in the laboratory by Nanopure systems (Barnstead, Dubuque, IA, USA). All solvents utilized in the study were of analytical grade and obtained from Fisher Scientific (Fair Lawn, NJ, USA).
2.2. Analytical Method
An in-house reverse phase high performance liquid chromatography (HPLC) based analytical method was developed for the determination/quantification of LID. This method was validated according to ICH and FDA guidelines for chromatographic methods. An HPLC equipped with a UV detector, Waters Symmetry shield 5µ C18 column (250×4.6 mm), and an isocratic mode of elution with the mobile phase consisting of 20mM ammonium acetate with 30mM trifluoroacetic acid (TFA), acetonitrile, and methanol (70:10:20) at a flow rate of 1.2 mL/min were employed to quantify the drug at a wavelength (λmax) of 254 nm. The acquired data was processed using Empower 2 build 2154 software (Waters Inc., Mount Holly, NJ, USA).
2.3. Preparation of Physical Mixtures/ Blends
Prior to extrusion, the required materials were sieved through a USP # 30 (600µM) mesh, accurately weighed, and transferred to a twin-shell V-blender (The Patterson-Kelly co., Inc. East Stroudsburg, PA). The materials were then mixed at 25rpm for 10 minutes in the V-blender to obtain a homogeneous blend.
2.4. Preparation of Hot-melt Extrudates
2.4.1. HME: Initial Screening
In order to identify a suitable carrier matrix to produce an AD/TR formulation, different polymers such as HPMC K4M, HPMC K15M, PEO-301, and EUD were evaluated for extrudability at barrel temperatures ranging from 110°C–180°C and a screw speed of 50 rpm in a twin-screw compounder (MiniLab II HAAKE Rheomex CTW5, ThermoFisher Scientific). The polymeric matrices selected from those tested, (i.e. PEO-301 & EUD, and their blends mixed in ratios of 1:1, 2:1, and 1:2 PEO-301 and EUD respectively) were extruded with LID at barrel temperatures ranging from 110°C–130°C and a screw speed of 50 rpm utilizing a lab scale twin-screw hot-melt extruder (Prism 16mm EuroLab, Thermo Fisher Scientific) with 3mm diameter circular die. All of the extrudates contained 10% w/w VES as a processing aid and 20% w/w LID. The obtained extrudates were cut to a length of 3mm using a bench-top pelletizer (Thermo Fisher Scientific) and stored appropriately until further testing. In addition, the API and all components utilized in this study were demonstrated to be stable at extrusion temperatures employed via thermogravimetric analysis and differential scanning calorimetry.
2.4.2. HME: Box-Behnken DOE
Based on the results from the initial screening, PEO-301 was chosen as the carrier matrix, while HPMC K15M and CBP were employed as gelling agents; each of these were designated as a formulation factor. In the current study, the Box-Behnken design was applied for fitting second-order response surface. It is based on the construction of balanced incomplete block design. In this case, there are three independent variables studied at three levels each. Each factor will be fixed at center level (0), while the other two factors are paired with each other in a 22 factorial (−1*−1, −1*1, 1*−1 and 1*1), therefore, there are a total of 12 runs. In addition, 2 center points were conducted individually as control runs to validate the reproducibility and variability of design. The design provided in Table 1 with a total of 14 runs was used to determine the effects of these formulation factors on TR characteristics of the melt-extruded pellets. In addition, it helps in estimating all one-way and two-way effects as well as assist in identifying the interactions and quadratic effects. A response surface methodology was utilized to identify the optimized formulation. The formulations generated by the design were extruded at temperatures ranging from 110°C–130°C and a screw speed of 50 rpm using a twin-screw hot melt extruder (Prism 16mm Eurolab ThermoFisher Scientific). The final extrudates were then cut to 3mm in length using a bench-top pelletizer (ThermoFisher Scientific) and stored appropriately until further testing.
Table 1.
Box-Behnken Design for the three Formulation Factors (PEO-301; HPMC K15M; and CBP) under Study
| Std Order | Run Order | Pt Type | Blocks | PEO-301 | HPMC K15M | CBP |
|---|---|---|---|---|---|---|
| 2 | 1 | 2 | 1 | 40 | 10 | 6 |
| 13 | 2 | 0 | 1 | 30 | 15 | 6 |
| 5 | 3 | 2 | 1 | 20 | 15 | 2 |
| 8 | 4 | 2 | 1 | 40 | 15 | 10 |
| 14 | 5 | 0 | 1 | 30 | 15 | 6 |
| 10 | 6 | 2 | 1 | 30 | 20 | 2 |
| 6 | 7 | 2 | 1 | 40 | 15 | 2 |
| 12 | 8 | 2 | 1 | 30 | 20 | 10 |
| 1 | 9 | 2 | 1 | 20 | 10 | 6 |
| 11 | 10 | 2 | 1 | 30 | 10 | 10 |
| 9 | 11 | 2 | 1 | 30 | 10 | 2 |
| 3 | 12 | 2 | 1 | 20 | 20 | 6 |
| 7 | 13 | 2 | 1 | 20 | 15 | 10 |
| 4 | 14 | 2 | 1 | 40 | 20 | 6 |
The experimental design and data analysis were conducted using Minitab® 15.0. The current response surface methodology DOE was analyzed using multiple regression statistics. A full quadratic model was fitted to the collected responses, and ‘P’ values for each of the factors were used to determine their significance. Highest order, most insignificant terms were sequentially removed until the significant factors were identified. Residual analysis was performed on the final reduced model, and the developed model was validated by executing and analyzing a confirmation run.
2.5. Weight Variation
Twenty pellets from a 100g batch were randomly selected from each of the DOE formulations and weighed using a Sartorius lab scale digital balance. The values were recorded, and the mean ± standard deviation (SD) was calculated.
2.6. Hardness and Friability Testing
The diametric compression strength testing was performed on ten randomly picked pellets per formulation using the Varian tester, and the hardness values were recorded.
The friability testing was performed on a sample of approximately 6.6g of extruded pellets utilizing a Varian friabilator. Pre-weighed pellets were placed in a friabilator and rotated at a speed of 25rpm for 4min. The pellets were then de-dusted, reweighed, and percentage weight loss (friability) was calculated for all of the DOE formulations.
2.7. Fine particle reduction
The obtained pellets equivalent to 200mg of weight from each of these formulations were evaluated for particle size reduction. The particle size reduction of any dosage form could be carried out either by use of mechanical and/or by electrical power-assisted forces. Although utilization of mechanical forces involves low shear, it could be the easiest approach employed to tamper the dosage forms as this can be achieved by applying force using any common household equipment such as hammer or mortar and pestle. If the produced formulations cannot be reduced into fines using the abovementioned tools, power-assisted high shear milling is an alternative approach that could be performed using a coffee grinder, also easily obtainable in the convenience stores. Therefore, in this study pellets were initially subjected to a low-shear grinding in a mortar and pestle for about 5min, followed by milling using a coffee grinder for an additional 5min. The obtained particles (n=3) were passed through a USP # 100 (150µm) mesh, and the fraction retained on the sieve was recorded.
2.8. Drug Extraction in Water
200mg of extruded pellets (equivalent to 40mg of LID) were added to 5ml of de-ionized water in a 20mL scintillation vial. Each of these formulations was vortexed for 2 minutes and kept aside for 30 minutes. At the end point, the samples were further mixed, and an aliquot of 1mL was collected, further diluted with methanol, filtered using 0.2 µm, 13mm PTFE membrane filters (Whatman, Piscataway, NJ). These solutions were analyzed using an HPLC at a wavelength of 254 nm. The test was performed in triplicate and the mean ± SD were recorded.
Additionally, the scintillation vials containing the post-processed dispersions that were kept aside for 30 minutes were slowly tilted upside down and visually observed for the movement of a semi-solid mass or freely available liquid along the inner surface of the vial. The formulation was considered to form a gel when there was a swollen matrix noticed inside the vial exhibiting a very slow and minimal to no movement of the available liquid. Moreover, a 3cc disposable syringe fitted with a 23 gauge, 1” needle was used to visualize if any liquid could be drawn from the vials by manually pulling the plunger. Viscosity of the resultant mixtures was qualitatively assessed depending on their ease of syringeability, as well as the volume of liquid that could be syringed out of the vials. The formulation was considered to be viscous when the formed product was relatively very difficult to be drawn into the syringe in comparison to water, and with a volume of ≤0.1mL in the syringe available for injection.
2.9. Drug Extraction in Alcohol
The extruded pellets (200mg) with a dose containing approximately 40mg drug were taken and dispersed in 60mL of 50% v/v absolute alcohol (simulating one large shot of whiskey). Each of these formulations was vortexed for 2 minutes and kept aside for 30 minutes. At the end point, the samples were further mixed, and an aliquot of 1mL was collected, diluted with methanol, filtered using a 0.2 µm, 13mm PTFE membrane filters (Whatman, Piscataway, NJ), and analyzed utilizing an HPLC at a wavelength of 254 nm. The test was performed in triplicate and the mean ± SD were recorded.
2.10. Drug Release Studies
The in vitro dissolution studies were carried out on the extruded pellets containing PEO, EUD, and their blends as polymeric matrices with approximately 40mg of LID. The pellets are weighed accurately and filled in size#1 gelatin capsules. Dissolution testing (USP XXXI, Apparatus II) was performed utilizing a Hanson SR8-plus™ dissolution test station (Hanson Research Corporation, Chatsworth, CA) operated at 100-rpm paddle speed. The dissolution medium consisted of 750mL of 0.1N hydrochloric acid (pH 1.2), preheated to 37°C. After 2hrs, 250mL of 0.2M tribasic sodium phosphate was added to the existing medium in the dissolution vessel and the pH of the entire medium in the dissolution vessel was adjusted to pH 6.8 by adding either 2N HCl or NaOH. An aliquot of 1.5mL was collected at pre-determined time intervals, filtered, and analyzed using an HPLC at a λmax of 254nm. Drug concentrations were calculated from a standard calibration plot and expressed as cumulative percentage drug dissolved. The release studies were also performed in triplicate and the mean values were compared.
3. Results
3.1. Analytical Method
The validation of the HPLC method employed in this study was carried out as per the ICH and FDA guidelines for chromatographic methods. The linear calibration range for the detection of LID was found to be 5–200 µg/mL, with a coefficient of determination (R2) of 0.999. The limit of detection and quantitation for the drug were 0.3 and 1.0 µg respectively, and the retention time for LID was 8.9 minutes. The percentage relative standard deviation (RSD) within replicates (n=3) was less than 2.0%, which demonstrates the reproducibility of the method. Precision was tested by injecting a single drug concentration (20µg/mL) 10 times, and the peak area was recorded and evaluated. A precision of less than 1.0% of RSD was observed.
3.2. Weight Variation, Hardness and Friability Testing
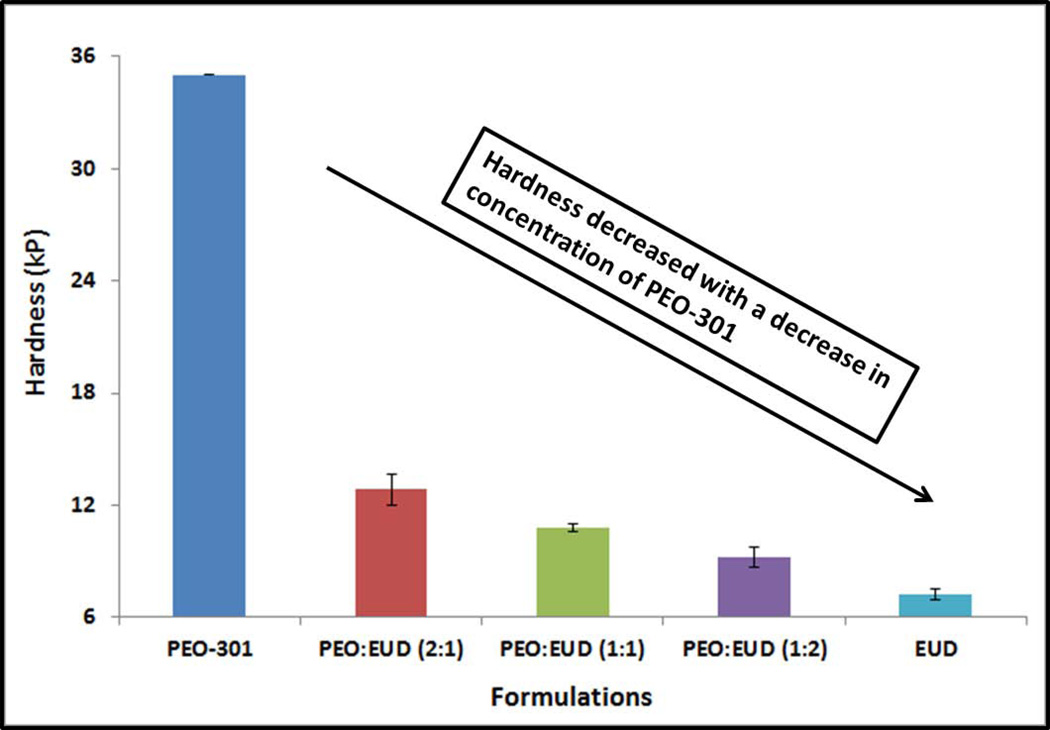
For all of the formulations, the weight and length of the pellets were within 28mg±10% and 3mm±5%, respectively. The hardness of the extruded pellets increased as the concentration of PEO-301 in the extruded pellets increased. This seemed especially true when the concentration of PEO-301 exceeded 50% of the total polymer weight. Moreover, the pellets made of pure PEO-301 could not be broken, but could only be deformed by the apparatus. A maximum hardness of 35kP was displayed on the instrument. This indicates a plastic behavior as a result of thermoplastic nature of the PEO-301 (Figure 1). Friability values were less than 0.1% in all of the DOE batches produced.
Figure 1.
Hardness evaluation of the preliminary melt-extruded pellet formulations. The extrudates prepared from PEO-301 (pure PolyOx WSR 301) could only be deformed, but not broken during the hardness evaluation.
3.3. Fine particle reduction
All of the particles obtained from grinding the extruded pellets in a mortar and pestle, followed by milling in a coffee grinder, were completely retained on the USP # 100 mesh screen (>150µm).
3.4. Drug Extraction in Water
The intact pellet formulation with pure PEO-301 had 27.4% of the drug extracted in water (approximately 11mg out of 40mg of LID) over 30 minutes, whereas all of the other formulations have shown higher drug extraction (>50%) over the same period (data not presented). The formulation with pure PEO-301 visually demonstrated a superior gel formation when compared to the other formulations.
3.5. Drug Extraction in Alcohol
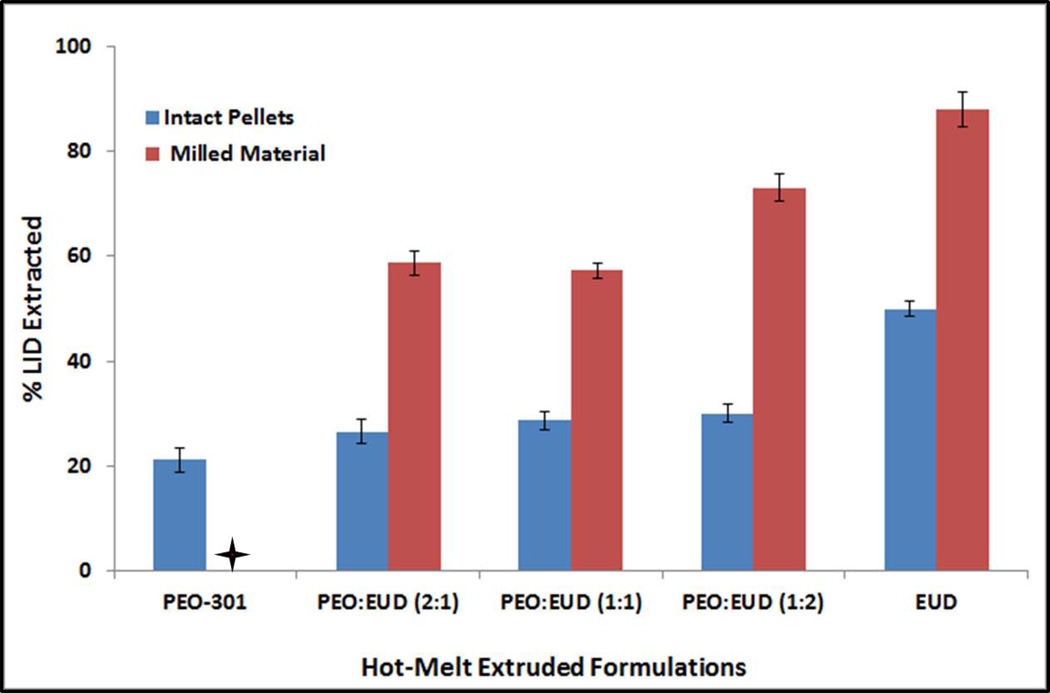
The observed drug extraction in alcohol (ethanol) was only 21.3% from the intact pellets containing pure/unblended PEO-301 in comparison to those made with pure EUD, which was approximately 50% of LID loading. The percentages of drug extracted in alcohol from all of the other intact pellet formulations were found to be between that of the pure polymer compositions. In addition, milled pellets exhibited significantly higher drug extraction compared to the intact pellets as depicted in Figure 2.
Figure 2.
Drug extraction in alcohol from the preliminary melt-extruded pellet formulations. + indicates that the pellets made of PEO-301 could not be milled into fine powder. Hence, this sample was not tested for drug extraction in alcohol from the hot-melt extruded pellets.
3.6. Drug Release Studies
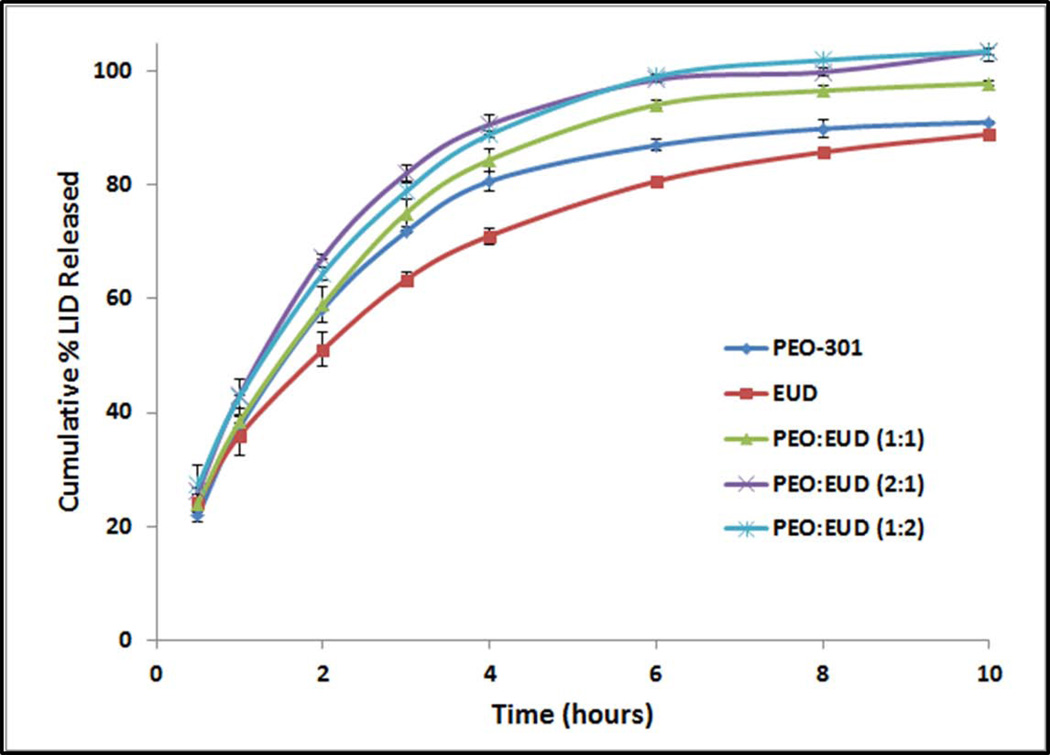
All of the extruded formulations, irrespective of their polymeric compositions (varying PEO:EUD ratios), have demonstrated similarity factor values (f2) of greater than 50. The pellets composed of PEO-301 alone with 20% LID served as the reference (Figure 3).
Figure 3.
Drug release from the preliminary melt-extruded pellet formulations.
4. Discussion
Due to controlled and restricted access to opioids, an active with high water and alcohol solubility (LID) was chosen as a model drug in the current study. Different carrier matrices such as HPMC K4M, HPMC K15M, PEO-301, and EUD were initially screened to identify suitable carriers that exhibit AD/TR properties. When attempting to extrude neat polymers at 70% w/w polymer level, formulations containing HPMC could not be extruded even at temperatures as high as 180°C. Irrespective of its molecular weight, HPMC could not be plasticized under the employed extrusion conditions, and consequently was not pursued further as a carrier matrix. On the other hand, PEO-301 and EUD were easily extruded at a much lower temperature range of 125–140°C. The next level of screening involved studying the effects of chosen polymeric carriers (PEO-301 and EUD) and their combinations at three different ratios to produce formulations with AD/TR features. All of these five formulations could be extruded relatively easily, and without incident. These formulations were subjected to hardness, crushing, extraction and dissolution testing to choose the best carrier among those tested, that is, the one with the most favorable AD/TR properties.
4.1. Fine particle reduction
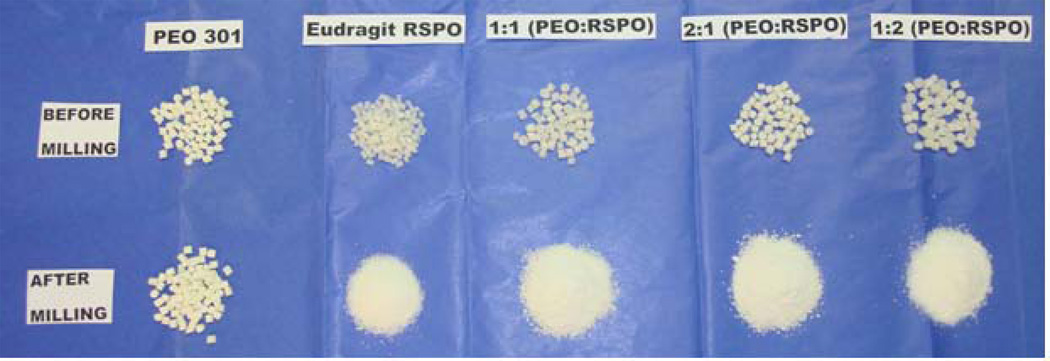
Snorting/insufflation is one of the most convenient and predominant modes of drug abuse, where the tablet formulations are finely crushed and inhaled or “snorted” via nasal route to get rapid onset of action. When inhaled intra-nasally, drugs could reach both the brain (by-passing the blood-brain barrier), and the systemic circulation (by-passing intestinal and hepatic metabolism). Targeting the drugs to the brain would be the most effective approach to get an instant “high” if the particles could get deposited in the olfactory region (required particle size <5 µm), which facilitates the drug’s entrance into the cerebrospinal space (18). However, this small particle size could also result in the drug deposition in the lower airways. Conventional nasal formulations, on the other hand, maintain a particle size of 20–30 µm to facilitate their permeation across the highly vascularized nasal mucosal epithelium (18). However, because of the low volume availability in the nasal environment, the rate-limiting step for drug absorption would be drug dissolution. Therefore, to produce a formulation with TR properties, it is required to alter the formulation such that the particle size could not be minimized to an appreciable extent. This results in a decreased surface area and ensures slower drug dissolution in the available low volume nasal cavity. It has been demonstrated in this study that all of the pellet formulations obtained from either pure EUD or its combination with PEO-301 could be broken, but not reduced into very fine particles as was attempted by the milling process in a coffee grinder (Figure 4).
Figure 4.
Visual comparison of different hot-melt extrusion pellet formulations before and after particle size reduction.
4.2. Drug Extraction in Water
Another route of abuse that has gained considerable attention, and is highly favored among abusers, is through extraction of the drug into aqueous solvent and injecting it directly into the blood stream. To prevent this, a TR formulation should be able to gel sufficiently when in contact with the aqueous media in order to minimize drug extraction. All of the extruded pellet formulations, except the one containing pure EUD, exhibited a reasonable gelling tendency in water. Moreover, the formulation extruded with pure PEO-301 has demonstrated increased swelling and produced a superior viscous gel relative to the other formulations. Although the high molecular weight PEO-301 does hydrate and subsequently forms an extraction preventative gel layer, polymer hydration tends to occur somewhat more slowly than small molecule dissolution. The coupling of these two events could have resulted in the drug’s escape prior to the gel formation event. On the other hand, EUD being a copolymer of ethyl acrylate and methyl methacrylate, and having a low content of the methacrylic acid ester with a quaternary ammonium group, it is less permeable and practically insoluble in water and might have undergone erosion. Hence, as the EUD concentration in the formulation increased, the drug extraction from the intact pellets might also have increased due to both high drug solubility leading to diffusion through any formed channels, and lack of EUD gelling in the aqueous phase.
4.3. Drug Extraction in Alcohol
Apart from the aqueous media, extraction studies were also carried out in hydro-alcoholic solutions. This increased drug extraction from EUD pellets was attributed to the higher solubility of both the EUD polymer and drug in alcohol. Apart from testing the whole pellets, all of the extrudates were subjected to milling and the milled portions were also exposed to alcohol for LID extraction. As expected, due to an increase in surface area as a result of milling, all of these formulations under investigation have shown much greater drug release in alcohol (60–90%) when compared to the intact pellets (21–50%). The percent drug extracted between intact and milled pellets for all of the tested formulations was statistically significantly different (p<0.05). However, the drug extraction from the milled PEO-301 pellets could not be performed as the pure PEO compacts were very plastic in nature and, as previously mentioned, very difficult to crush.
4.4. Drug Release Studies
The obtained dissolution data was fitted to different release models, and the drug release mechanism from all of these formulations was established using the Korsmeyer-Peppas model. The high coefficient of determination (R2) values were observed when plotted as per the Higuchi equation, which indicates that, the regression line fits the data well. Additionally, the calculated ‘n’ values for all of these five preliminary formulations were in the range of 0.28–0.37, which indicates that the drug’s release is mainly by fickian diffusion.
PEO-301, being a hydrophilic polymer, demonstrates rapid hydration and swelling of the polymer when in contact with the aqueous media resulting in the formation of a uniform gel layer on the surface of the matrix. Since the model drug under investigation is highly water-soluble, it quickly gets dissolved in the media and diffuses out through the channels formed due to polymer swelling (19). However, the molecular weight of the polymer under discussion, PEO-301, is relatively high (4 million), resulting in a slower gel erosion and a prolonged drug release behavior, which translates to reduced extractability. On the other hand, although the EUD matrices hydrate to a lesser extent, the drug release follows a similar mechanism of diffusion. This could be attributed to the high aqueous solubility of the LID that diffuses through the channels formed due to EUD erosion, or dissolution of other soluble formulation components. The study results were in accordance with the previously reported data in the literature (20).
4.5. Functionality of Utilized Excipients
Based on the preliminary results obtained from the above studies, it has been concluded that the pure PEO-301 extrudates exhibited the most suitable properties as an AD/TR carrier matrix when processed by hot-melt extrusion. However, these pellets need further improvement to minimize the drug’s extraction in aqueous media, thereby preventing intravenous drug abuse. In order to improve the formulation further, excipients such as CBP and HPMC K15M were employed as gelling agents, and Vitamin E Succinate (VES) was used a processing aid in all of the formulations. Both of these polymers, each at three different levels, were studied as gelling agents to increase the viscosity of the formulation when in contact with water.
CBP was studied in a broad range of concentrations (2–10% w/w). In general, if the concentration of CBP in the formulation is beyond 3%, it could potentially exhibit pH dependent drug release, which is not desirable. However, the grade of CBP chosen is a granular form, and requires a high concentration (~10% w/w or higher) in the formulation to demonstrate gelling and pH dependency. For CBP to act as a gelling agent, an alkaline environment is required to ionize the polymeric chains and induce swelling for gel formation. Therefore, k2HPO4 was added as a buffering agent in the formulation. Furthermore, researchers have utilized HPMC up to 30% w/w in controlled release tablet formulations to provide sufficient gelling. However, when used at high levels, there is a potential for the development of a brittle formulation that could be crushed easily and compromise the insufflation inhibiting feature. When a minimal amount of HPMC is used, there is a possibility of insufficient gel formation in water leading to enhance drug extraction. As our primary intent was to improve the extraction in water (learning from the preliminary experiments), 10–20% w/w range was assumed to be reasonable to study. With several ingredients present in the formulation, a DOE was performed to optimize the levels of PEO-301, HPMC K15M and CBP to produce a formulation with better TR/AD features keeping all of the other components unchanged except for PEO-N80, which was used as filler. The base formulation composition utilized for DOE and its excipient functionality is presented in Table 2.
Table 2.
Composition and Functionality of Hot-Melt Extruded Base Pellet Formulation Utilized in Design of Experiments
| Formulation Components | Concentration (% w/w) | Functionality |
|---|---|---|
| Lidocaine HCl (LID) | 20 | Active |
| PEO-301 | 20 – 40 | Gelling Polymeric Matrix |
| HPMC K15M | 10 – 20 | Gelling Polymeric Matrix |
| Carbopol 71G (CBP) | 2 – 10 | Gelling Agent |
| Vitamin E Succinate | 10 | Process Aid |
| K2HPO4 | 1 | Base/Gel Promoter |
| PEO-N80 | 3 – 33 | Polymeric Filler |
4.6. Evaluation of DOE Formulations and Interpretation of Results
All of the extruded formulations produced under DOE were evaluated for various TR tests and the obtained results were considered responses for optimization. The TR tests performed were: (1) hardness testing using a Varian hardness tester; (2) fine particle reduction using a mortar & pestle and a coffee grinder, followed by passing the milled extrudates through a 100 mesh screen; (3) extraction of the active pharmaceutical ingredient (API) in water; and (4) extraction of the API in 60mL of 50% v/v absolute ethanol.
A total of 14 formulations produced under the Box-Behnken design, including two center points, were easily extruded at the employed conditions. Hardness testing revealed that all of the produced formulations were plastic in nature and deformed. A maximum hardness value of 35kP was recorded on the instrument. None of the pellets subjected to testing could be broken. LID pellet formulations demonstrated good post-processing drug content (approximately 99.0% and a relative standard deviation of 2.0%).
A low-shear pulverization using mortar & pestle, followed by milling in a coffee grinder generated particles with reduced size in all of the DOE formulations. However, none of these formulations passed through mesh # 100 when subjected to sieve analysis, and the particle size of all of the milled portions were larger than 150µm, which is indicative of the formulations’ resistance to snorting/insufflation. As mentioned earlier, the larger the particle size, the smaller the surface area, which could lead to a relatively slower dissolution and lower absorption across the nasal environment.
All of the formulations exhibited an appreciable gelling tendency in water with only 8–12% of drug being extracted. Good gelling behavior in water is attributed to the water uptake potential of the employed hydrophilic polymers present in the formulation and their ionizable groups, due to the micro-environmental pH, resulting in an increase in the viscosity of the aqueous medium. Studies in the literature have reported that HPMC in combination with CBP demonstrated a synergistic effect with a stronger cross linking between the polymers leading to the formation of a more firm gel structure because of the hydrogen bonding between the anionic CBP and the HPMC. Additionally, interaction between non-ionic polymers has also been well established in the literature. Some reports have mentioned that PEO in combination with HPMC might be favorable when slower initial release is required from the matrices. Both PEO and HPMC, being hydrophilic and non-ionic polymers, are dually advantageous in that they can facilitate a faster hydration of PEO-301, and could also result in the formation of a stronger gel layer. This ultimately results in minimizing the drug diffusion from the swollen matrix while simultaneously decreasing the erosion rate of the formed gel layer (21). Similar behavior was also reported with HPMC in the presence of non-ionic hydrophilic polymer, hydroxylpropyl cellulose (HPC).
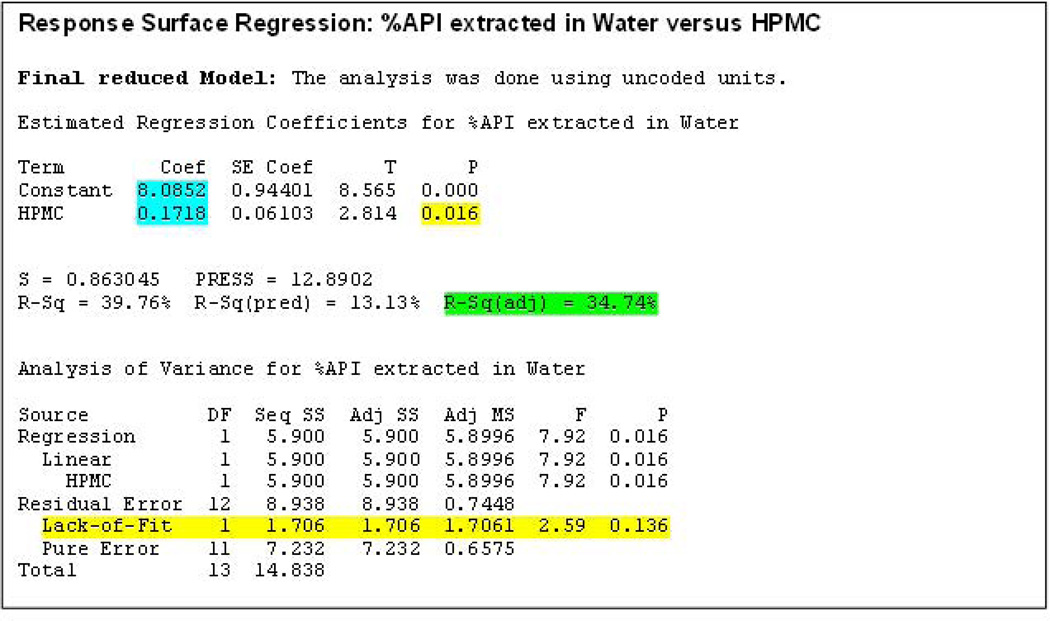
When analyzing the percentage of the API extracted in water, the percentage of HPMC K15M was the only factor that demonstrated a significant influence on the response (P<0.05). The R-Sq (adjusted) value in the final reduced model indicated that the model explained 35% of the data variability (Figure 5). In addition, the P value for ‘Lack-of-Fit’ was found to be 0.136 (P>0.05). Therefore, the null hypothesis was accepted i.e., the model developed is not different from the proper fitting model. The reduced final model for this response can be provided as follows:
Percentage API extracted in water = 8.085 + 0.172 ((% of HPMC)
Figure 5.
Response surface regression after model reduction obtained using ‘percent active pharmaceutical ingredient extracted in water’ as a response.
The drug extraction in alcohol was another response that ranged from 17–45%, amongst different formulations tested. Similar results were observed in a study conducted by Robert et al. who studied the release of aspirin from HPMC matrix in hydro-alcoholic media (22). The authors noticed an initial sudden burst release of the drug without causing any dose dumping, which was attributed to the delayed hydration of HPMC in the alcoholic medium. Therefore, in the present study both PEO and HPMC being insoluble in alcohol, high drug extraction in alcohol was accounted for by the high solubility of LID as well as the delayed hydration of the HPMC polymeric matrix in the presence of ethanol.
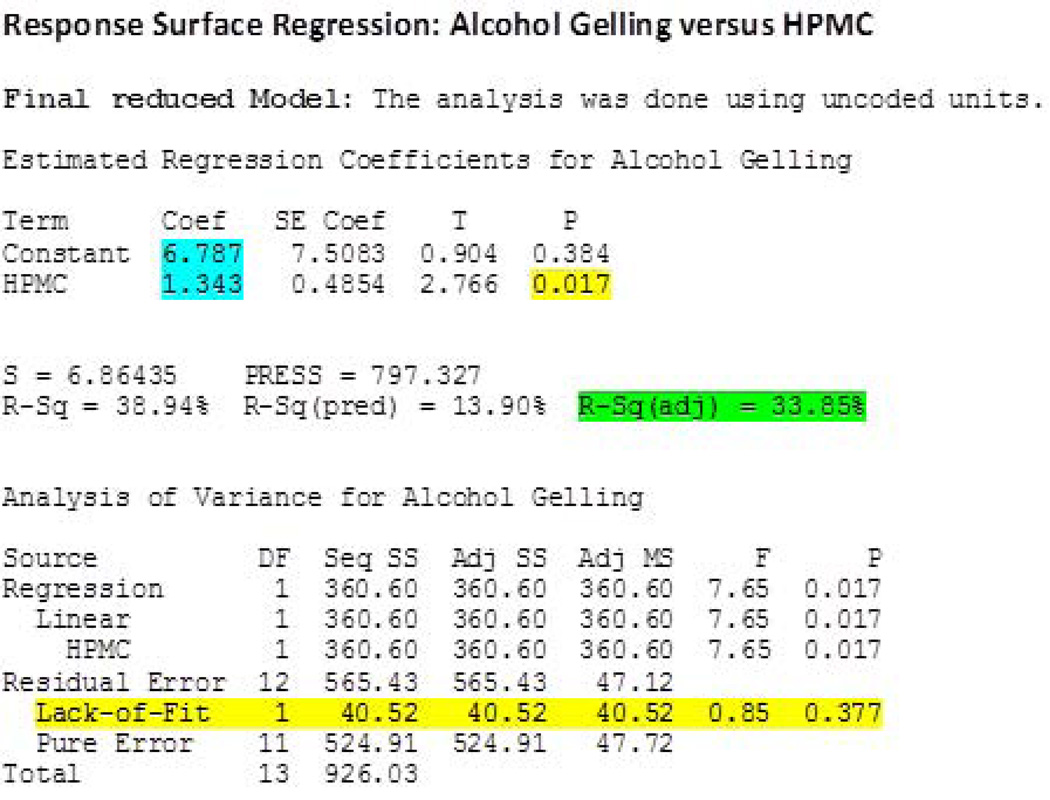
‘Percentage API extracted in alcohol’ was also significantly influenced (P<0.05) by the percentage of HPMC K15M employed in the formulation. When the obtained data was fitted to the model, the R-Sq (adjusted) value in the final reduced model indicated that the model explained 34% of the data variability (Figure 6). In addition, the P value for ‘Lack-of-Fit’ was found to be 0.377 (P>0.05). Hence, the null hypothesis was accepted i.e., the model developed is not different from the proper fitting model. The reduced final model for this response can be provided as follows:
Percentage API extracted in alcohol = 6.787 + 1.343 ((% of HPMC)
Figure 6.
Response surface regression after model reduction obtained using ‘percent active pharmaceutical ingredient extracted in alcohol’ as a response.
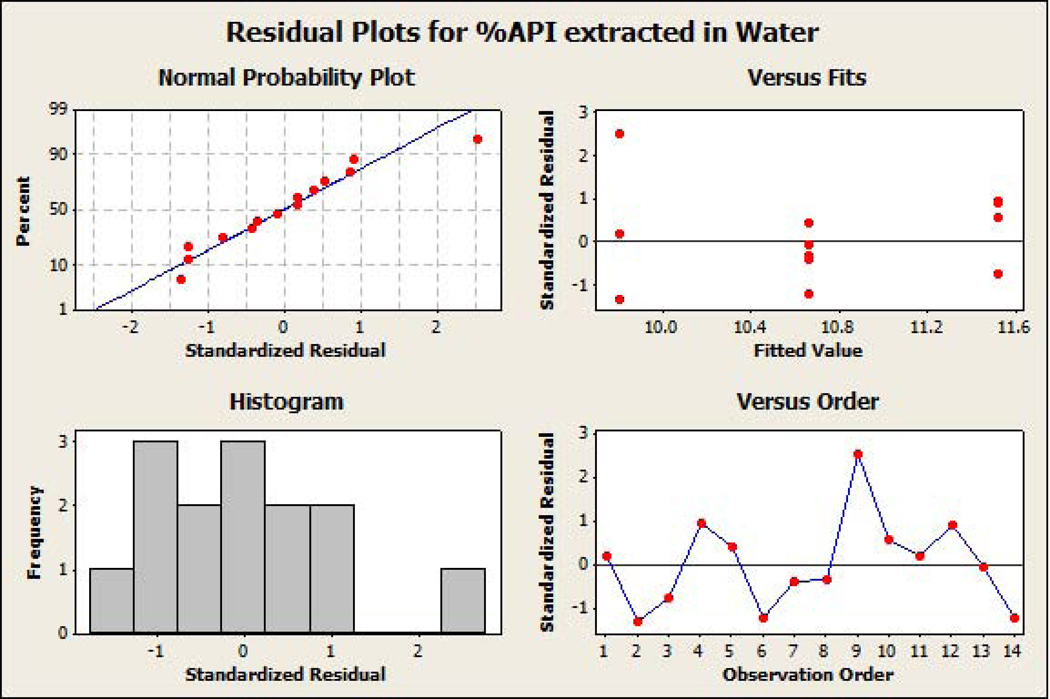
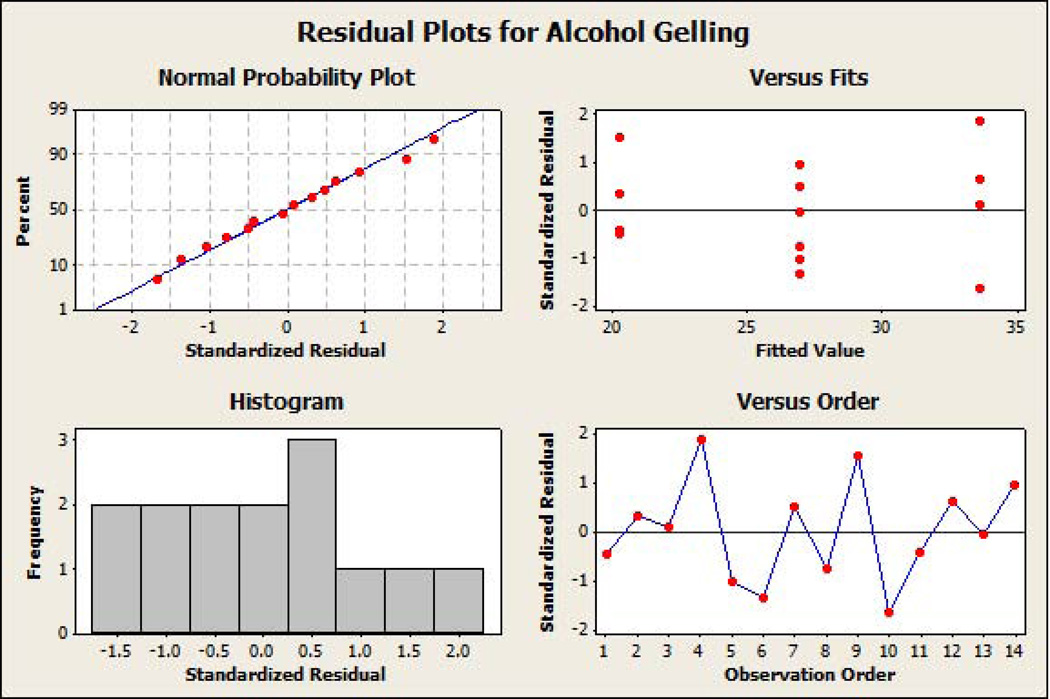
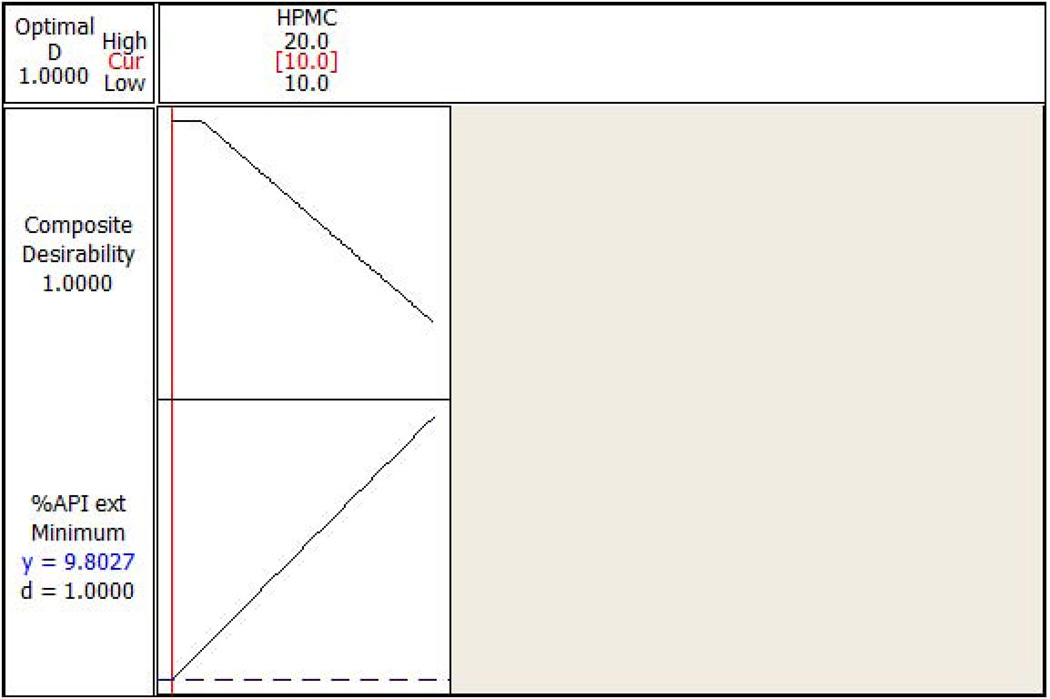
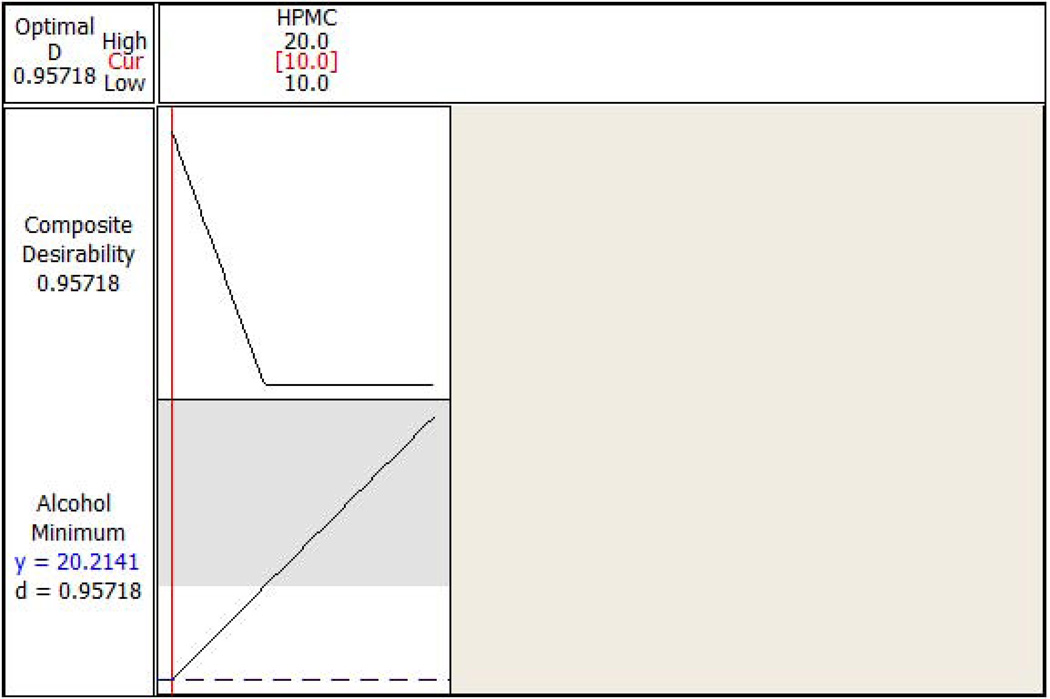
The standardized residual plots (Figure 7A and Figure 7B) of the final reduced models for the responses ‘percentage API extracted in water’ and ‘percentage API extracted in alcohol’ meet all of the basic assumptions of a good model fit. The figures indicate that the residuals are normally distributed around approximately the mean of zero. Moreover, the residuals are homoscedastic, also known as homogeneity of variance (residuals have constant variability and are independent of fitted value), and do not show any trending (independent of observation order). Response optimization plots (Figure 8A and Figure 8B) indicated that lower extraction of API in water (9.8%) and alcohol (20.2%) could be achieved by using HPMC even at lower concentrations (10% w/w level), when other factors are utilized at appropriate concentrations in the formulation.
Figure 7.
(a) Standardized residual plots on the final reduced models for percent active pharmaceutical ingredient extracted in water as the response. (b) Standardized residual plots on the final reduced models for percent active pharmaceutical ingredient extracted in alcohol as the response.
Figure 8.
(a) Response optimization plot for the HPMC (the only significant factor) with percent active pharmaceutical ingredient extracted in water as a response. (b) Response optimization plot for the HPMC (the only significant factor) with percent active pharmaceutical ingredient extracted in alcohol as a response.
In order to validate the developed model, a confirmation batch utilizing PEO-301, HPMC K15M, and CBP at 20%, 10% and 6% w/w, respectively, was produced and analyzed. The predicted values obtained from the response optimizer were found to be similar to the observed values of 9.4% and 19.5% respectively, for percentage API extracted in water, and percentage API extracted in alcohol demonstrating the validity of the developed model. This final formulation was studied for drug release along with other TR properties and found that almost 60–70% of the drug was released in 2–3hrs (data not presented). Although extended release polymers such as PEO-301 and HPMC K15M were used as carrier matrices in the present study, the observed faster dissolution rate in the initial hours could be attributed to the high water solubility of the LID, as well as utilization of lower molecular weight PEO (PEO-N80) as a filler in the formulation, which does not swell to a great extent and compromises the integrity of the gel network relatively quickly as opposed to high molecular weight grade polyethylene oxide polymers.
5. Conclusion
HME was demonstrated as a viable technique for the development of much needed abuse-deterrent formulations. Moreover, high molecular weight grade polyethylene oxide was shown to be utilized as a TR matrix, while HPMC K15M in combination with CBP significantly improved the gelling characteristics in water and alcohol to limit or minimize API extraction. The optimized response models created for ‘percentage API extracted in water’ and ‘percentage API extracted in alcohol’ also correlate well with the data generated, confirming its validity. Our future studies will be focused to work with controlled substances as model drugs, and evaluate newer excipients in conjunction with appropriate processing conditions to further improve this developed formulation and fully characterize its TR, dissolution and stability characteristics. Although these formulations are termed as AD/TR dosage forms, such formulations may never be completely abuse-free. One may not totally stop a determined person from abusing drugs. However, these developed formulations would most likely serve a significant purpose as the abusers would need to perform an enormous amount of work, and/or exhibit uncharacteristic patience, to use them in an unintended way. Development of such AD formulations may perhaps also significantly reduce the deaths due to overdose, as well as decrease the percentage of young children and patients with acute or chronic pain who might convert into potential abusers.
Acknowledgements
This work was supported by the Pii Center for Pharmaceutical Technology and HRSA/OFAM/DGMO grant #D1DHP20294. This publication was also made possible by Grant Number P20GM104931 from the National Institute of General Medical Sciences (NIGMS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIGMS or NIH. In addition, the authors would also like to thank Ashland Inc., the Dow Chemical Company, Evonik, and Lubrizol for their generous supply of polymers.
References
- 1.Mastropietro DJ, Omidian H. Current approaches in tamper-resistant and abuse-deterrent formulations. [accessed 21 December 2012];Drug Dev Ind Pharm. 2012 Apr 26; doi: 10.3109/03639045.2012.680468. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 2.Manchikanti L, Singh A. Therapeutic opioids: a ten-year perspective on the complexities and complications of the escalating use, abuse, and nonmedical use of opioids. Pain Physician. 2008 Mar;11(2 Suppl):S63–S88. [PubMed] [Google Scholar]
- 3.Boyd CJ, McCabe SE, Cranford JA, Young A. Prescription drug abuse and diversion among adolescents in a southeast Michigan school district. Arch Pediatr Adolesc Med. 2007 Mar;161(3):276–281. doi: 10.1001/archpedi.161.3.276. [DOI] [PubMed] [Google Scholar]
- 4.Butler SF, Black RA, Cassidy TA, Dailey TM, Budman SH. Abuse risks and routes of administration of different prescription opioid compounds and formulations. Harm Reduct J. 2011;8:29. doi: 10.1186/1477-7517-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall AJ, Logan JE, Toblin RL, Kaplan JA, Kraner JC, Bixler D, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008 Dec 10;300(22):2613–2620. doi: 10.1001/jama.2008.802. [DOI] [PubMed] [Google Scholar]
- 6.Cone EJ. Ephemeral profiles of prescription drug and formulation tampering: evolving pseudoscience on the Internet. Drug Alcohol Depend. 2006 Jun;83(Suppl 1):S31–S39. doi: 10.1016/j.drugalcdep.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 7.Sloan P, Babul N. Extended-release opioids for the management of chronic non-malignant pain. Expert Opin Drug Deliv. 2006 Jul;3(4):489–497. doi: 10.1517/17425247.3.4.489. [DOI] [PubMed] [Google Scholar]
- 8.Passik SD, Hays L, Eisner N, Kirsh KL. Psychiatric and pain characteristics of prescription drug abusers entering drug rehabilitation. J Pain Palliat Care Pharmacother. 2006;20(2):5–13. [PubMed] [Google Scholar]
- 9.Meng Y, Lichtman AH, Bridgen DT, Martin BR. Inhalation studies with drugs of abuse. NIDA Res Monogr. 1997;173:201–224. [PubMed] [Google Scholar]
- 10.Hays LR. A profile of OxyContin addiction. J Addict Dis. 2004;23(4):1–9. doi: 10.1300/J069v23n04_01. [DOI] [PubMed] [Google Scholar]
- 11.Webster L. Update on abuse-resistant and abuse-deterrent approaches to opioid formulations. Pain Med. 2009 Jul;10(Suppl 2):S124–S133. doi: 10.1111/j.1526-4637.2009.00672.x. [DOI] [PubMed] [Google Scholar]
- 12.Bartholomaeus JH, Arkenau-Maric E, Galia E. Opioid extended-release tablets with improved tamper-resistant properties. Expert Opin Drug Deliv. 2012 Aug;9(8):879–891. doi: 10.1517/17425247.2012.698606. [DOI] [PubMed] [Google Scholar]
- 13.Repka MA, Battu SK, Upadhye SB, Thumma S, Crowley MM, Zhang F, et al. Pharmaceutical applications of hot-melt extrusion: Part II. Drug Dev Ind Pharm. 2007 Oct;33(10):1043–1057. doi: 10.1080/03639040701525627. [DOI] [PubMed] [Google Scholar]
- 14.Repka MA, Majumdar S, Kumar Battu S, Srirangam R, Upadhye SB. Applications of hot-melt extrusion for drug delivery. Expert Opin Drug Deliv. 2008 Dec;5(12):1357–1376. doi: 10.1517/17425240802583421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palem CR, Kumar Battu S, Maddineni S, Gannu R, Repka MA, Yamsani MR. Oral transmucosal delivery of domperidone from immediate release films produced via hot-melt extrusion technology. Pharm Dev Technol. 2013 Feb;18(1):186–195. doi: 10.3109/10837450.2012.693505. [DOI] [PubMed] [Google Scholar]
- 16.Crowley MM, Zhang F, Repka MA, Thumma S, Upadhye SB, Battu SK, et al. Pharmaceutical applications of hot-melt extrusion: part I. Drug Dev Ind Pharm. 2007 Sep;33(9):909–926. doi: 10.1080/03639040701498759. [DOI] [PubMed] [Google Scholar]
- 17.Repka MA, Shah S, Lu J, Maddineni S, Morott J, Patwardhan K, et al. Melt extrusion: process to product. Expert Opin Drug Deliv. Jan;9(1):105–125. doi: 10.1517/17425247.2012.642365. [DOI] [PubMed] [Google Scholar]
- 18.Keldmann T. Advanced simplification of nasal delivery technology: anatomy + innovative device = added value opportunity, in Nasal Drug Delivery: Rapid Onset via Convenient Route. ONdrugDelivery. 2005:4–7. [Google Scholar]
- 19.Palmer D, Levina M, Farrell TP, Rajabi-Siahboomi AR. The influence of hydro-alcoholic media on drug release from polyethylene oxide extended-release matrix tablets. PharmTech. 2011;35(7):50–58. [Google Scholar]
- 20.Apu AS, Pathan AH, Kibria G, Jalil R. In vitro Release Kinetic Study of Theophylline from Eudragit RS PO and Eudragit RL PO Matrix Tablets. Dhaka Univ J Pharm Sci. 2009;8(1):1–6. [Google Scholar]
- 21.Tiwari SB, Rajabi-Siahboomi AR. Modulation of drug release from hydrophilic matrices. PharmTech Eur. 2008 Sep;:1–8. [Google Scholar]
- 22.Roberts M, Cespi M, Ford JL, Dyas AM, Downing J, Martini LG, et al. Influence of ethanol on aspirin release from hypromellose matrices. Int. J. Pharm. 2007;332(1–2):31–37. doi: 10.1016/j.ijpharm.2006.09.055. [DOI] [PubMed] [Google Scholar]