Abstract
Parasitoid wasps are among the most diverse insects on earth with many species causing major mortality in host populations. Parasitoids introduce a variety of factors into hosts to promote parasitism, including symbiotic viruses, venom, teratocytes and wasp larvae. Polydnavirus-carrying wasps use viruses to globally suppress host immunity and prevent rejection of developing parasites. Although prior results provide detailed insights into the genes viruses deliver to hosts, little is known about other products. RNAseq and proteomics were used to characterize the proteins secreted by venom glands, teratocytes and larvae from Microplitis demolitor, which carries M. demolitor bracovirus (MdBV). These data revealed that venom glands and teratocytes secrete large amounts of a small number of products relative to ovaries and larvae. Venom and teratocyte products exhibited almost no overlap with one another or MdBV genes, which suggested that M. demolitor effector molecules are functionally partitioned according to their source. This finding was well illustrated in the case of MdBV and teratocytes. Many viral proteins have immunosuppressive functions that include disruption of antimicrobial peptide production, yet this study showed that teratocytes express high levels of the antimicrobial peptide hymenoptaecin, which likely compensates for MdBV-mediated immunosuppression. A second key finding was the prevalence of duplications among genes encoding venom and teratocyte molecules. Several of these gene families share similarities with proteins from other species, while also showing specificity of expression in venom glands or teratocytes. Overall, these results provide the first comprehensive analysis of the proteins a polydnavirus-carrying wasp introduces into its host.
1. Introduction
Parasitism is an ecological process in which two or more species interact in such a way that one organism, the parasite, derives a fitness benefit at the expense of another organism, the host. Parasitoids are free-living insects as adults whose progeny develop by consuming and usually killing a host, which is generally another arthropod. Estimates suggest up to 20% of all insect species are parasitoids and many species cause high levels of mortality in host populations (Godfray 1994; Pennachio & Strand 2006). Thus, understanding the strategies parasitoids have evolved to successfully parasitize hosts and the counter adaptations hosts have evolved to evade parasitism are issues of broad importance in basic and applied ecology. Most parasitoids are in the order Hymenoptera (wasps, bees, and ants). Within this group microgastroid wasps in the family Braconidae are of interest because they form one of the largest monophyletic assemblages of parasitoids known, many attack economically important hosts, and all rely on symbiotic viruses in the genus Bracovirus (family Polydnaviridae) for successful parasitism (Murphy et al. 2008; Strand 2010).
Bracoviruses (BVs) evolved approximately 100 million years ago (Mya) from a nudivirus that infected the common ancestor of microgastroids (Murphy et al. 2008; Bèzier et al. 2009). Nudiviruses have large (>100 kb), circular double-stranded DNA genomes and are closely related to baculoviruses. All known nudiviruses and baculoviruses are pathogens of insects, which circumstantially suggests the ancestor of BVs was also likely a pathogen. Today, an estimated 20,000 species of microgastroids exist that parasitize one or a few species of hosts, which are primarily larval stage Lepidoptera (moths) (Smith et al. 2008). Each wasp species carries a genetically distinct BV that persists in somatic and germ cells of all individuals as an integrated provirus. Replication in contrast is restricted to only one cell type called calyx cells, which reside in the ovaries of females. The resulting virions package circular doubled-stranded DNAs that wasps inject into a host when laying eggs. Virions rapidly infect host cells followed by expression of viral gene products, which alter immune defences and other processes that are essential for survival of wasp offspring (Strand 2010). BVs never replicate in the hosts of wasps because the portion of the viral genome that is packaged into virions lacks the genes required for replication (Strand 2010, Burke & Strand 2012b, Herniou et al. 2013, Gundersen-Rindal et al. 2013). However, BVs are able to persist in wasps because the viral genome is integrated in the wasp germ line that eggs inherit. BVs have thus evolved into obligate mutualists, which rely on wasps for transmission as proviruses, while wasps rely on BVs as gene delivery vectors to parasitize hosts (Strand & Burke 2012, Strand & Burke 2013, Herniou et al. 2013, Gundersen-Rindal et al. 2013).
Although BVs are the most studied component in parasitism, microgastroid wasps also produce effector molecules from three other sources. The first is venom from the venom gland that wasps inject into hosts at the same time as eggs and virus particles (Asgari & Rivers 2011). The second is teratocytes that form from an extraembryonic membrane surrounding the wasp embryo and are released into the host when wasp eggs hatch (Vinson & Iwantsch 1980; Strand & Pech 1995). The third is the wasp larva itself, which may secrete products while developing (Vinson & Iwantsch 1980; Strand & Pech 1995). Products from these sources collectively represent the ‘parasitism arsenal’ microgastroid wasps introduce into hosts. Since BVs are essential for parasitism in all microgastroids studied to date, previous reviews have suggested that venom glands, teratoctyes and/or larvae could either produce molecules that overlap with viral gene products or in some manner synergize the effects of viral infection (Webb & Strand 2005; Asgari & Rivers 2011). BV genomes from a few microgastroids have been characterized (summarized by Burke & Strand 2012b; Jancek et al. 2013). However, evaluating how viral gene products interact with venom gland, teratocyte, or larval products is unknown because the collective array of secreted proteins BVs, venom glands, teratocytes and larvae produce has never been fully characterized in any species.
Microplitis demolitor carries M. demolitor Bracovirus (MdBV) and parasitizes the larval stage of the moth Chrysodeixis (formerly Pseudoplusia) includens. Previous studies describe the MdBV genome, the activity of the MdBV transcriptome in M. demolitor and C. includens, and the function of several MdBV gene products in parasitism (Webb et al. 2006; Strand 2010; Bitra et al. 2011). Here, we used RNAseq and proteomics to characterize the gene products from M. demolitor venom glands, teratocytes and larvae. Our results indicate that venom glands and teratocytes produce relatively simple sets of proteins that minimally overlap with one another or MdBV, demonstrating functional partitioning of parasitism roles.
2. Materials and methods
(a) Insects
M. demolitor and C. includens were reared at 27° C as previously described (Strand & Noda 1991).
(b) RNA isolation and library preparation
Venom glands were dissected from 100 adult females that were 1-4 days old. Four wasp larvae were collected by dissecting parasitized hosts in physiological saline at 5 days post-parasitism. Teratocytes were collected by dissecting hosts in the same manner at 1 day post-parasitism and removing 10 wasp eggs from the hemocoel. These eggs were placed into serum-free Sf-900II medium (Gibco), which resulted in release of 6000 teratocytes (600 teratocytes per egg) when the eggs hatched 2-4 h later (Strand & Wong 1991). These teratocytes were then maintained in primary culture for 2 days before processing to isolate total RNA.
Total RNA from each sample was prepared using the QIAGEN RNeasy kit with on column DNAse treatment, followed by a second DNAse treatment using Ambion TURBO DNA free reagents. Indexed sequencing libraries were prepared by the University of Georgia Genomics Facility with the Illumina TruSeq DNA sample preparation kit and standard low-throughput protocol as previously described (Burke & Strand 2012a) and sequenced on two lanes with 100 cycles of paired-end sequencing on a Illumina HiSeq system.
(c) Transcriptome sequencing and assembly
Illumina reads were filtered to retain read pairs and single reads with >30 phred score equivalents for >90% of nucleotides. The reads were assembled de novo using Trinity with the jaccard clip option (Grabherr et al. 2011). Reads were re-mapped to the assembled transcripts using bwa bwasw, randomly placing reads mapping to multiple locations (Li & Durbin 2009). The number of reads per kilobase per million reads mapped (RPKM) was calculated per locus by taking the mean of RPKM values for individual transcripts (Mortazavi et al. 2008). Low abundance transcripts from loci with an average RPKM value of <20 among all four tissue types were removed. As RPKM is a metric that depends upon the total number of reads mapped, RPKM values were re-calculated to include only reads mapping to the higher abundance transcripts. Statistical analyses were performed using JMP Pro 10. A value of 0.1 was added to RPKM values that had been rounded to 0 prior to log transformation.
(d) Proteomic analysis of venom gland, teratocyte, and larval-secreted factors
Venom glands were collected by dissecting 40 female wasps in physiological saline. Glands were then transferred to a ca. 100 μl drop where the venom reservoir of each was punctured using forceps. The glands were then removed from the droplet resulting in a venom solution. Teratocytes were collected from 100 wasp eggs that hatched in culture medium as described above. The resulting wasp larvae and ca. 60,000 teratocytes were gravitationally separated and collected, gently centrifuged (200 × g), and resuspended in a volume of 60 μl of Sf-900II medium. Conditioned medium from each was then collected after 72 h at 27° C. All wasp larvae were viable after this period, while more than 90% of teratocytes were viable as measured by propidium iodide staining. Sample buffer was added to the venom and teratocyte samples followed by boiling for 10 min. Each sample was then run on either 4–20% or 12.5% Tris-Glycine gels under reducing conditions (Lonza). After electrophoresis, gels were stained with Coomassie InstantBlue. Each lane was then cut into four (venom) or sixteen (teratocytes) pieces to separate proteins by size. Proteomic profiling was performed at the Proteomic and Mass Spectrometry facility at the University of Georgia as described previously (Burke et al. 2013). Proteins were identified from the combined results from all of the gel slices for each sample by searching against a custom database consisting of translated open reading frames (ORFs) greater than 33 amino acids in size from Trinity assembled transcripts described above using the Mascot v2.3 algorithm (Matrix Science Inc.). Only ORFs that were matched by at least two peptide spectra were considered positive identification.
(e) Identification of gene family members and phylogenetic analyses
Gene family member candidates were identified using BLAST with proteins from another species as queries or using HMMER hmmsearch with a specific hmm file against the translated transcriptome database (Finn et al. 2011). Candidates from other insect genomes were identified using BLASTP against the nr database. All candidates were aligned using MUSCLE v3.8.31 and manually edited using Mesquite v2.75 (Edgar 2004; Maddison & Maddison 2011). Candidates that were obviously too divergent or short for robust alignment were excluded. RAxML was used for phylogenetic reconstruction of the sequences with 100 bootstrap replicates using the CIPRES portal (Stamatakis 2006; Miller et al. 2010).
3. Results
(a) Transcriptome analysis of M. demolitor venom glands, teratocytes and larvae
BV genomes consist of two components: 1) a conserved set of nudivirus-like genes required for viral replication, and 2) segments of DNA encoding virulence genes that are packaged into virions (Strand & Burke 2013). The nudivirus-like genes are only transcribed in wasp ovaries (calyx cells) during replication and none are packaged into virions. Reciprocally, almost none of the virulence genes are transcribed in wasps but nearly all are expressed in the hosts wasps parasitize (Strand & Burke 2012). These organizational and functional features of the genome fundamentally distinguish BVs from known nudiviruses. They also underlie why BVs are unable to replicate in the hosts wasps parasitize but are able to deliver virulence genes that wasp offspring depend upon to successfully develop. In the case of MdBV, we previously generated M. demolitor ovary libraries and used RNAseq, proteomics and functional assays to identify the nudivirus-like genes required to assemble MdBV virions (Strand & Burke 2012; 2013). This dataset was used as a baseline to quantify transcription levels of wasp genes whose products are not interacting directly with hosts, with the exception of proteins that comprise MdBV virus particles. We also previously sequenced the DNAs packaged into virions, which identified 51 virulence genes expressed in infected C. includens (Webb et al. 2006; Bitra et al. 2011). In contrast, we had no insights about any of the other virulence factors M. demolitor introduces into hosts.
We therefore began this study by Illumina sequencing M. demolitor venom gland, teratocyte, and larval libraries. This generated quality filtered reads numbering 47.8-68.2 million pairs in addition to 8.2 to 11.0 million single reads (Supplementary Table 1). Trinity assembly of reads from these samples plus the previously generated RNAseq data set for ovaries resulted in 216,988 transcripts from 173,925 loci. From 89-97% of reads for each sample were successfully mapped to assembled transcripts. After removal of loci with an average abundance of <20 RPKM among tissue types, 3894 loci representing 13653 transcripts remained.
(b) Venom glands and teratocytes abundantly express a small number of unique transcripts
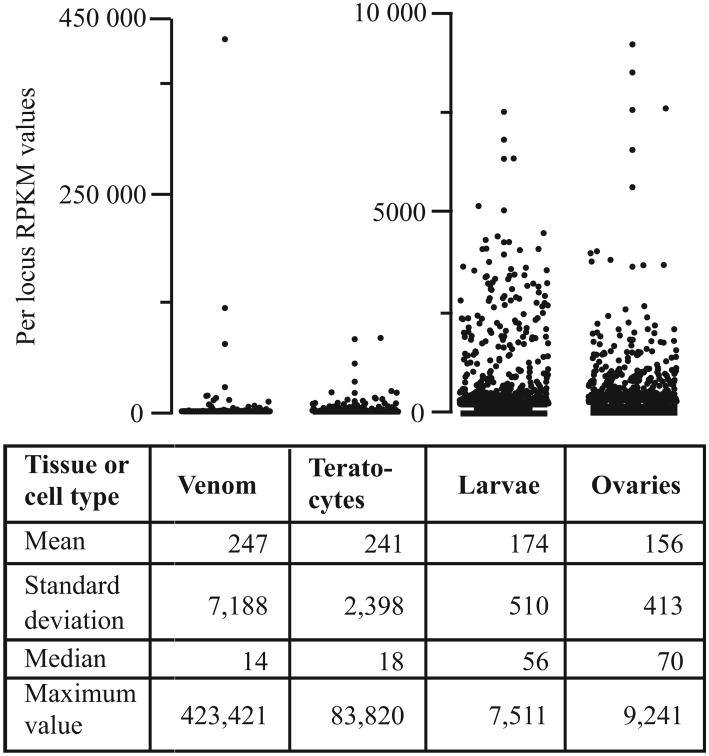
Most female hymenopterans have venom glands that primarily produce proteins wasps inject into hosts, prey, or other organisms using their ovipositor (de Graaf et al. 2010; Asgari & Rivers 2011). Teratocytes are definitively known to be produced by endoparasitoid wasps in the Scelionidae and select subfamilies of Braconidae with a small literature also suggesting their primary function is also secretory (Strand et al. 1986; Strand & Pech 1995; Quicke, 1997). Wasp ovaries and larvae in contrast consist of many cell types with diverse functions. We thus expected the transcriptional profiles from M. demolitor venom glands and teratoctyes to be less complex than those of ovaries and larvae. Among sample distributions of RPKM values fully supported this expectation (Figure 1). Venom gland and teratocyte transcriptomes consisted of a small number of highly abundant transcripts plus a larger number of low abundance transcripts, which resulted in lower medians, higher standard deviations, and higher maximum RPKM values than in the ovary and larval samples (Figure 1). Ovaries and larvae in contrast were distinguished by an abundance of transcripts with intermediate RPKM values, which resulted in higher medians, lower standard deviations and lower maximum RPKM values than in venom glands and teratocytes (Figure 1). Differences in complexity of the data sets, however, resulted in the 10 most highly expressed genes in venom glands and teratocytes accounting for 76.2% and 39.9% of the summed RPKM values for each tissue/cell type, while the 10 most highly expressed genes in ovaries and larvae accounted for only 10.0% and 8.1%, respectively of total RPKM values (Figure 1).
Figure 1.
Distribution and statistics describing locus abundance in RPKM values for teratocytes, venom glands, larvae and ovaries of wasps. Statistics used describe the distribution of RPKM values for all 3894 loci. These include the mean, one standard deviation, the median and the maximum RPKM value obtained for each locus. Note that venom and teratocyte RPKM values are depicted on a different scale from ovaries and larvae.
(c) Venom glands, teratocytes larvae and ovaries differentially express a number of genes
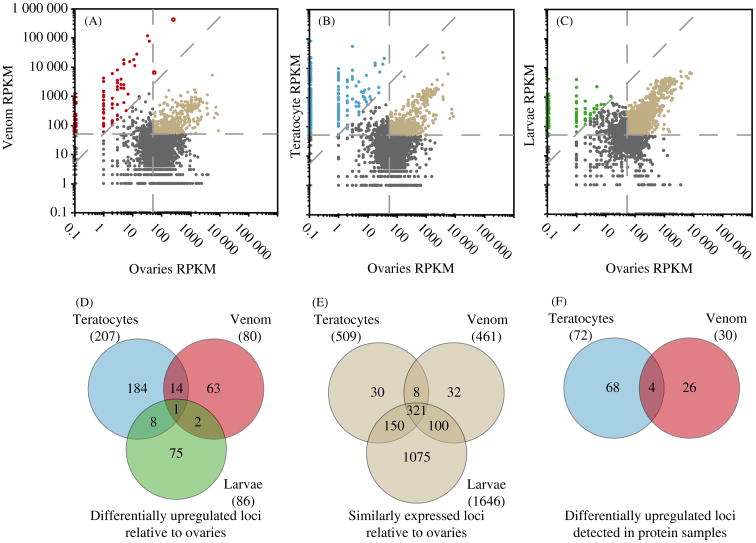
Among the largest deficiencies in the parasitoid literature is the lack of knowledge about the molecules wasps produce from different sources (venom, virus/ovary secretions, teratocytes, larvae) and introduce into hosts. As a first step to addressing this issue in M. demolitor, we used simple pairwise comparisons to identify abundant, differentially upregulated transcripts in the venom gland, teratocyte, and larval transcriptomes compared to those in MdBV-producing ovaries. Transcripts were classified as differentially upregulated if they had RPKM values of >50 in the query sample and were also 50-fold more abundant relative to ovaries, whereas they were classified as similarly expressed if RPKM values were >50 in both the query sample and ovaries. By these criteria, 80 venom gland, 207 teratocyte, and 86 larval loci were upregulated compared to ovaries, while 509 venom gland, 461 teratocyte, and 1646 larval loci were similarly expressed (Figure 2A-C). Strikingly, only 25 (7.2%) of the 347 loci classified as differentially expressed had RPKM values of >50 in two or more of the query samples (Figure 2D). Thus, most of these genes were highly expressed in only venom glands, teratocytes, or larvae. In contrast, we found substantial overlap (579 of the 1716 genes or 33.7%) in similarly expressed loci between ovaries and venom, teratocytes or larvae, suggestive of constitutive expression among tissues and functions of general importance to multiple cell types (Figure 2E).
Figure 2.
Pairwise comparisons of gene expression in venom glands (A), teratocytes (B) or larvae (C) relative to ovaries. Loci classified as differentially expressed in venom glands (red), teratocytes (blue) or larvae (green) are indicated in the upper left of each graph while loci classified as similarly expressed in both the query and ovaries are indicated in the upper right (light grey). All other loci are indicated as dark grey. Overlap between (D) loci differentially expressed between venom glands, teratocytes, and larvae, (E) loci similarly expressed between venom glands, teratocytes and larvae, and (F) differentially expressed loci whose corresponding proteins were detected in venom gland and teratocyte secretions. Colouration in D-E is the same as in A-C. Two loci from venom glands, a plancitoxin (a deoxyribonuclease) and an uncharacterized member of the conotoxin superfamily, met the criteria (see results) for both the differential and similar expression categories (Shiomi et al. 2004).
(d) Several venom and teratocyte secretory products correspond to abundant transcripts
Venom glands consist of a reservoir that stores the secretion products from cells located in one or more filamentous structures that extend from the reservoir (Quicke 1997). To determine which genes in the venom gland transcriptome code for proteins in venom, samples were separated by SDS-PAGE, in-gel trypsin digested, and then analysed using an Orbitrap Elite mass spectrometer. We identified proteins corresponding to 30 of the 80 loci classified as differentially expressed, which we named the venom data set (Supplementary Table 2). Twenty-three (77%) of the differentially expressed loci had predicted signal peptides as expected for venom products, while only 54% (27/50) of the differentially expressed loci not detected in venom had signal peptides (Likelihood Ratio X2 test p = 0.04).
Proteomic analysis of teratocyte-conditioned medium detected proteins corresponding to 72 of 207 differentially expressed loci, 107 of the similarly expressed loci, and 71 previously unclassified loci (Supplementary Table 3, 4, and 5). Sixty of the differentially expressed loci (83%) had predicted signal peptides, whereas only 55% (74/135) of differentially expressed loci absent from conditioned medium did (Likelihood Ratio X2 test p < 0.0001). Most of the similarly expressed loci detected in medium had predicted functions in protein biosynthesis or metabolism and lacked signal peptides, which strongly suggested most were present due to low level teratocyte mortality during culture (Supplementary Table 4). Likewise, the previously unclassified loci detected in medium had average RPKM values of 37 with low (1.9) average fold change values compared to ovaries, which suggested they too were not secretory products (Supplementary Table 5). We thus considered the 72 differentially expressed loci detected in medium as the teratocyte data set (Supplementary Table 3). Comparing the teratocyte and venom data sets to one another identified four overlapping loci, including a) two metalloprotease-like loci, with both biased transcriptionally towards venom glands, and b) a serine protease similar to snake from Drosophila melanogaster (Figure 2F). Two additional loci (comp40703_c0 and comp35200_c0) were detected in both venom and teratocyte protein samples, but did not meet the expression abundance or fold-change requirements for membership in the teratocyte or venom data sets.
We did not conduct a proteomic analysis of larva-conditioned medium because no proteins were detected after SDS-PAGE analysis and Coomassie staining (Supplementary Figure 1). Annotation of the 86 differentially expressed loci from larvae showed that most were related to genes in databases with unknown functions (15) or hypotheticals (34) (Supplementary Table 6). The few with homology to known genes consisted of predicted cuticular proteins, hexamerins, apolipoproteins or carboxypeptidases.
(e) Some M. demolitor venom components belong to functional classes detected in other wasps
Eighteen of the loci detected in M. demolitor venom shared homology with genes of known or predicted function, 6 shared homology with genes in databases of unknown function, and 6 were unique hypotheticals (Supplementary Table 2). Transcriptome and/or proteomic data for venom glands are available for only a few other parasitoid wasps. These include three braconids, C. inanitus and two species in the genus Microctonus (Euphorinae) (Crawford et al. 2008; Vincent et al. 2010), plus a small number of species in other families (Periquet et al. 1997; Richards & Edwards 1999; Asgari et al. 2003; Crawford et al. 2008; Richards & Dani 2008; de Graaf et al. 2010; Vincent et al. 2010; Zhu et al. 2010; Dorémus et al. 2013; Colinet et al. 2013; Goecks et al. 2013; Heavner et al. 2013). We identified direct orthologs of three venom proteins from C. inanitus: Ci-300 which is a predicted reprolysin-like metalloprotease, Ci-50 which is a predicted lipase, and Ci-48a which shares some homology with imaginal disc growth factor proteins (Vincent et al. 2010). We identified 5 Ci-48a-like proteins in M. demolitor venom and noted that homologs of this protein have been detected in venom from other wasps (Supplementary Table 2). M. demolitor venom also contained two cysteine-rich proteins (eg. VG3) and an esterase/lipase previously detected in Microctonus sp. although sequence analysis suggested neither were direct orthologs (Supplementary Table 2) (Crawford et al. 2008). Other products belonging to functional classes reported from venom in other parasitoids included proteases, serpins, cysteine-rich proteins of unknown function, and glycosyl hydrolases (Periquet et al. 1997; Richards & Edwards 1999; Asgari et al. 2003; Richards & Dani 2008; Colinet et al. 2009; de Graaf et al. 2010; Zhu et al. 2010; Dorémus et al. 2013). In contrast, a plancitoxin with predicted function in DNA degradation, a bifunctional polynucleotide kinase/phosphatase, and two baculovirus-like loci with uncharacterized functions (Shiomi et al. 2004; Punta et al. 2012) had not previously been identified as hymenopteran venom components. Several up-regulated transcripts absent from M. demolitor venom but detected in venom glands also shared homology with venom gland transcripts from other species. However, most if not all of these factors have predicted maintenance functions and are likely not products wasps inject into hosts.
(f) Teratocytes produce unique proteins and products related to venom components
Among the 72 loci in the teratocyte data set, 38 shared homology with genes of known function, 13 shared homology with genes of unknown function, and 21 were unique hypotheticals (Supplementary Table 3). No transcriptome or proteome data sets have previously been generated for teratocytes. Similar to venom, however, we noted that a number of products were related and belonged to gene families. These included hymenoptaecin-like antimicrobial peptides, flavin reductase-like proteins, growth factor receptor-like proteins, and phospholipase-like proteins. In addition, while few teratocyte loci directly overlapped with venom components (see above) teratocytes produced Ci-48a-like proteins, reprolysin-like metalloproteases, esterase/lipases, glycosylases, peptidases, and serine proteases that were related to gene family members in venom (Supplementary Table 3). The abundant production of hymenoptaecin-like antimicrobial peptides (the second- and seventh-most abundant products in the teratocyte set, Supplementary Table 3) is interesting given the immunosuppressive effects of MdBV upon hosts that include disrupted expression of some antimicrobial peptide (AMP) genes (Strand 2010). Thus, teratocyte produced AMPs could represent a strategy of replacing host defence molecules with wasp factors. Other teratocyte loci of note were an ortholog of TSP14 previously identified from Microplitis croceipes teratocytes and a carboxylesterase from teratocytes of Dinocampus coccinellae (Braconidae) (Okuda & Kadono-Okuda 1995; Hoy & Dahlman 2002; Dahlman et al. 2003; Gopalapillai et al. 2005). Our proteomic analysis also identified a chitinase with similarities to a chitinase produced by teratocytes of the microgastroid Toxoneuron nigriceps (Consoli et al. 2007). This product was absent, however, from our differentially upregulated data set (Supplementary Table 3).
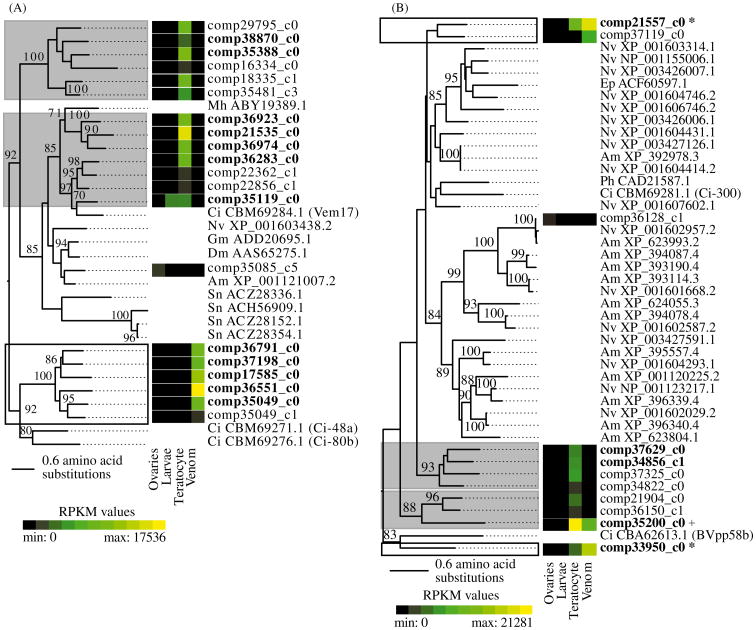
We further analysed the Ci-48a and reprolysin-like metalloprotease genes to assess the evolutionary history and tissue specificity of these two very large gene families detected in venom and teratocyte secretions. A total of 20 Ci-48a-like loci were identified in our M. demolitor transcript database of which 12 were venom or teratocyte products (Supplementary Table 2, 3). Phylogenetic reconstruction showed that these loci segregated into three well-supported groups that were strongly biased toward either teratocyte or venom gland expression (Figure 3A). Ci-48a-like loci from M. demolitor were closely related to other insect genes not expressed in venom or teratocytes, suggesting they have been co-opted multiple times to become venom or teratocyte proteins from genes with other physiological functions. PFAM searches against the M. demolitor transcriptome database identified a total of 11 loci with reprolysin-like metalloprotease domains of which 5 were venom or teratocyte products that were also likely co-opted from genes involved in functions unrelated to parasitism (Figure 3B).
Figure 3.
Phylogenetic relationships of multi-gene families expressed in parasitism-specific tissues. Relatedness of (A) Ci-48a-like proteins and (B) reprolysin-like metalloprotease domain-containing proteins and their expression in M. demolitor tissues. Groups of teratocyte-specialized loci are indicated in grey shaded boxes, and venom-specialized groups are in white. Venom or teratocyte data sets are indicated in bold type, asterisks indicate detection in both. The plus sign indicates a gene product detected in both venom and teratocytes but does not belong to the venom set due to not meeting expression requirements. Support values for 100 bootstrap replicates are shown at nodes in the tree for clades supported by >80 replicates. Expression values (RPKM) for each locus in ovaries, larvae, teratocytes or venom glands are shown using a heat-map. Other species in the phylogeny are hymenopteran species Am: Apis mellifera, Ci: Chelonus inanitus, Ep: Eulophus pennicornis, Md: Microctonus hyperodae, Nv: Nasonia vitripennis, and Ph: Pimpla hypochondriaca, and dipteran species Dm: Drosophila melanogaster, Gm: Glossina moristans, and Sn: Simulium nigrimanum. Not enough sequence was available for H. didymator Hd-Ven1 and Hd-Ven2 metalloproteases or Microctonus aethipoides Ci-48a to be included. Microctonus and Venturia canescens peptidases also belong to a different family of proteases to those featured here (Crawford et al. 2003; Asgari et al. 2002).
(g) Venom glands, teratocytes and larvae express few MdBV-like genes
As previously noted, all of the nudivirus-like genes of the MdBV genome are expressed in ovaries during replication. None of these genes were detected in the teratocyte or larval transcriptomes but one, odv-e56 (=pif-5), was abundantly expressed in venom glands (Supplementary Table 7). Since ODV-E56 is a likely envelope component of MdBV virions (Burke et al. 2013), its expression in venom glands suggests it may be a plasma membrane component in one or more venom gland cell types. While most virulence genes packaged into MdBV virions are not expressed in wasps, we had previously detected low level expression of a few in ovaries and in RNA pools from whole wasps (Bitra et al. 2011). We searched for these genes and related homologs against all assembled M. demolitor transcripts using BLAST and MdBV genes as queries. The only direct match was egf0.4, a small serine protease inhibitor family member in the MdBV genome that was expressed at intermediate levels in venom glands, teratocytes, larvae and ovaries (Supplementary Table 8). We also detected several other small serine protease inhibitor family members plus divergent homologs related to the MdBV ptp and ank gene families (Supplementary Table 8). None of these loci were components of venom or teratocyte secretory products but their presence in our transcriptome data set suggested several genes related to MdBV virulence genes reside elsewhere in the M. demolitor genome. Small serine protease inhibitor family members have been identified from several invertebrate species. Phylogenetic analysis, however, showed that the egf genes in the MdBV genome were most closely related to comp38084_c0 which is a small serine protease family member in M. demolitor expressed at moderate levels in larvae and teratocytes (Supplementary Figure 2).
4. Discussion
The Hymenoptera is divided into several lineages of herbivores (sawflies and woodwasps) and the monophyletic Apocrita, which consists of several large groups and contains more than 95% of the estimated total number of species in the order (Pennacchio & Strand 2006). The common ancestor of the Apocrita was almost certain a parasitoid, and outside of aculeates all of the remaining apocritan groups consist predominantly or entirely of parasitoids. All parasitoids are broadly divided into externally feeding ectoparasitoids and internally feeding endoparasitoids. Ectoparasitism and endoparasitism has independently evolved on multiple occasions within different apocritan lineages. However, endoparasitoid-host associations are almost always more complex than those of ectoparasitoids because wasps must maintain the viability of hosts for long periods while simultaneously disabling immune defences and manipulating growth (Pennacchio & Strand 2006; Asgari & Rivers 2011).
Quicke (1997) describes several adaptations in the Hymenoptera that have facilitated the parasitoid lifestyle including the evolution of several sources of virulence molecules and a flexible ovipositor that wasps use to inject these factors into hosts. Phylogenetic and morphological data point to the venom gland as the most likely source of virulence molecules that the most basal apocritans relied upon to parasitize hosts (Pennacchio & Strand 2006). Thereafter other sources of virulence molecules including teratocytes and associations with different types of microbial symbionts evolved in particular wasp lineages (Quicke 1997; Pennacchio & Strand 2006; Kaltenpoth & Engl 2013). In the case of BV-carrying microgastroids, venom plus ovary secretions are the first sources of molecules wasps introduce into hosts but as proteins most of these products only transiently persist. BVs are thus thought to have greatly contributed to the evolutionary success of microgastroid braconids because they provide wasps the means to deliver and express effector molecules in hosts for protracted periods. The evolution of teratocytes offers a second way for wasps to deliver effector molecules, but these cells only form if eggs are able to hatch. One strategy, therefore, would be for BV-carrying wasps to initially rely on venom as a source of effector molecules, followed by sustained production of these factors via the virus and/or teratocytes. That venom and viruses produce related molecules has also been suggested because in some species antigenic similarities have been detected between venom and virus products (Webb & Summers 1990).
Our transcriptome and proteomic profiling data show that venom and teratocytes secrete large amounts of a small repertoire of products. M. demolitor larvae in contrast did not secrete any proteins in amounts that were detectable by Coomassie staining. We thus recognize that M. demolitor larvae may secrete small quantities of some proteins but these products are much lower in abundance than the secretory products from venom glands and teratocytes. Our results also show that almost no overlap exists between MdBV genes and the proteins in M. demolitor venom and teratocyte secretions, which clearly argues against MdBV sustaining the production of venom-like products. Instead our results more strongly suggest that MdBV, venom glands, and teratocytes have evolved to produce proteins with functionally partitioned roles in parasitism of hosts. An especially striking example of functional partitioning is the dichotomy between many of the gene products delivered by MdBV and the high level expression of two hymenoptaecin antimicrobial peptide genes by teratocytes. Prior studies show that several MdBV genes expressed in hosts have immunsuppressive functions that include the disruption of hemocyte function, the phenoloxidase cascade and the Toll and Imd signalling pathways which rely on NF-kB transcription factors to differentially regulate a diversity of immune genes (Beck & Strand 2005; 2007; Lu et al. 2008; Bitra et al. 2012). Disruption of NF-kB signalling contributes to the inability of virus-infected hosts to encapsulate wasp offspring but it also results in significantly reduced expression of antimicrobial peptides by the host, which potentially increases the susceptibility of parasitized larvae to secondary infection by bacteria and other microbes. Yet, our finding that M. demoltor teratocytes express high levels of hymenoptaecins suggests a key function of teratocytes is to compensate for MdBV-mediated immunosuppression of the host and impart protection against opportunistic pathogens during wasp development.
Previous analysis of MdBV and other BV genomes indicates that many of the genes expressed in hosts have diversified into multimember families (Kroemer & Webb 2004; Burke & Strand 2012b). This study expands this picture by showing that: 1) many genes expressed in venom glands and teratocytes also belong to gene families and 2) some MdBV, venom gland, and teratocyte products evolved from genes with normal physiological functions in the wasp. These findings are interesting because genes with physiological functions in a number of other venom-producing organisms have also duplicated to produce new family members that are selectively expressed in venom glands (Casewell et al. 2013). In the case of the Ci-48a and the reprolysin metalloprotease-like genes, our results indicate both families are related to non-venom genes in other insect species, and that each has diversified in M. demolitor and been recruited for expression in either venom glands or teratocytes. Our results also indicate the MdBV egf gene family evolved from another egf-like gene in the M. demolitor genome. Studies of BVs from wasps in the genus Glyptapanteles similarly indicate a family of sugar transporter genes evolved from a wasp gene (Desjardins et al. 2008). In contrast, other gene families present in MdBV and other BVs show evidence of acquisition by horizontal gene transfer from other organisms or are of ancient and uncertain origin (Moreau et al. 2009; Burke & Strand 2012b).
Signatures of positive selection among BV virulence gene family members together with the presence of pseudogenes reflects arms race dynamics with hosts and a ‘birth and death’ model for gene evolution (Nei et al. 1997; Burke & Strand 2012b; Jancek et al. 2013). Arms race interactions have also been proposed to underlie the evolution of venom proteins into multiple family members with different activities (Casewell et al. 2013). We speculate that Ci-48a and reprolysin-like metalloprotease family members expressed in venom glands from M. demolitor have specialized for functions in the early phases of parasitism while those expressed by teratocytes have roles in later stages of parasitism. However, we currently have no insights about what the precise functions of these factors might be. Ci-48a like proteins are known from several species of Hymenoptera and Diptera, whereas reprolysin-like metalloproteases are mosaic proteins that are produced by many animals and have roles in many processes. Several reprolysin-like metalloproteases have also been identified as venom components including one from the parasitoid wasp Eulophus pennicornis, which is implicated in slowing host development (Rawlings & Barrett 1995; Seals & Courtneidge 2003; Price et al. 2009). Taken together, our results highlight the importance of gene family duplication as a key process of not only BV genes but other components of the parasitism arsenal that wasps rely upon to parasitize hosts.
Among BV-carrying species at-large, few functions have been identified for any venom component. A protein named Vn50 from Cotesia rubecula inhibits the melanisation of host hemolymph (Asgari et al. 2003) while whole venom from two other Cotesia sp. has been implicated in protecting wasp eggs from encapsulation and facilitating entry of BVs into host cells (Stoltz et al. 1988; Hayakawa & Yazaki 1997). In contrast, we detected no Vn50-like protein in M. demolitor venom, while prior studies show that MdBV readily infects C. includens in the absence of venom (Beck & Strand 2007; Pruijssers et al. 2009). In the case of teratocytes, TSP was previously identified from Microplitis croceipes as a protein that adversely affects host protein synthesis (Dahlman et al. 2003), which in turn suggests a possible function for the TSP ortholog we identified from M. demolitor teratocytes. Beyond this protein, however, we have no insights about whether other products we identified from M. demolitor teratocytes are produced by other species because no other teratocyte transcriptome or proteome data are currently available.
The venoms of predatory animals often vary between even closely related species due to host range differences and previously discussed arms race interactions with prey (Casewell et al. 2013). Consistent with this scenario, the virulence genes that BVs from different wasp species deliver to hosts exhibit considerable divergence (Burke & Strand 2012b). The limited overlap we observe between venom components from Chelonus inanitus and M. demolitor suggest host range differences have also favored divergence in microgastroid venom composition. Among all parasitoid species, a recent survey reported 11 functional classes of proteins in venoms (Dorémus et al. 2013) of which 6 are represented in M. demolitor. However, very few direct orthologs of venom proteins have thus far been identified between species, which is also consistent with selection favouring the diversification of venom products. Finally, recent reviews note that the venoms produced by predatory organisms are often complex, whereas the venoms produced by species that function as defensive secretions tend to consist of more streamlined, conserved components (Casewell et al. 2013). Among the best examples of the latter are the venoms of non-parasitic (Aculeate) Hymenoptera such as bees and ants whose defensive venoms cause immediate, localized pain (Kuhn-Nentwig 2003; Hoffman 2006; de Graaf et al. 2010). We did not identify any proteins in M. demolitor venom that are related to components in aculeate hymenopteran venoms, yet our results also show that the composition of M. demolitor venom is relatively simple. Thus, it is possible the composition of hymenopteran venoms generally may be less complex than previously assumed.
Supplementary Material
Acknowledgments
The authors would like to thank Juber Patel for assistance with computational analyses, Kavita Bitra and Jena Johnson for sample preparation, Roger Nilsen at the Georgia Genomics Facility at UGA for sequencing library preparation, and Dennis Phillips and Chau-Wen Chou at the UGA Proteomics and Mass Spectrometry core facility for technical assistance with proteomic analyses.
This work was supported by the National Institutes of Health (F32 AI096552) (GRB), US National Science Foundation (IOS-1261328) (MRS) and US Department of Agriculture (2009-35302-05250) (MRS).
Footnotes
Data accessibility: Transcript sequences: NCBI TSA database accession number GANH00000000, Raw proteomics data: online supplemental material, filename Raw_proteomics_data.xlsx, Sequence alignment files: online supplemental material, filenames Ci-48a-like.phy and Reprolysin-like_metalloprotesases.phy, Tree files: online supplemental material, filenames Ci-48a-like.tre and Reprolysin-like_metalloprotesases.tre
Author contributions: GRB and MRS designed research, performed research, analyzed data, and wrote the manuscript.
References
- Asgari S, Reineke A, Beck M, Schmidt O. Isolation and characterization of a neprilysin-like protein from Venturia canescens virus-like particles. Insect Molecular Biology. 2002;11:477–485. doi: 10.1046/j.1365-2583.2002.00356.x. [DOI] [PubMed] [Google Scholar]
- Asgari S, Rivers DB. Venom proteins from endoparasitoid wasps and their role in host-parasite interactions. Annual Reviews of Entomology. 2011;56:313–335. doi: 10.1146/annurev-ento-120709-144849. [DOI] [PubMed] [Google Scholar]
- Asgari S, Zhang G, Zareie R, Schmidt O. A serine proteinase homolog venom protein from an endoparasitoid wasp inhibits melanization of the host hemolymph. Insect Biochemistry and Molecular Biology. 2003;33:1017–1024. doi: 10.1016/s0965-1748(03)00116-4. [DOI] [PubMed] [Google Scholar]
- Beck MH, Strand MR. Glc1.8 from Microplitis demolitor bracovirus induces a loss of adhesion and phagocytosis in insect high five and S2 cells. Journal of Virology. 2005;79:1861–1870. doi: 10.1128/JVI.79.3.1861-1870.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck MH, Strand MR. A novel polydnavirus protein inhibits the insect prophenoloxidase activation pathway. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:19267–19272. doi: 10.1073/pnas.0708056104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bèzier A, Annaheim M, Herbinière J, et al. Polydnaviruses of braconid wasps derive from an ancestral nudivirus. Science. 2009;323:926–930. doi: 10.1126/science.1166788. [DOI] [PubMed] [Google Scholar]
- Bitra K, Suderman RJ, Strand MR. Polydnavirus Ank proteins bind NF-kB homodimers and inhibit processing of Relish. PLoS Pathogens. 2012;8:e1002722. doi: 10.1371/journal.ppat.1002722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitra K, Zhang S, Strand MR. Transcriptomic profiling of Microplitis demolitor bracovirus reveals host, tissue and stage-specific patterns of activity. Journal of General Virology. 2011;92:2060–2071. doi: 10.1099/vir.0.032680-0. [DOI] [PubMed] [Google Scholar]
- Burke GR, Strand MR. Deep sequencing identifies viral and wasp genes with potential roles in replication of Microplitis demolitor Bracovirus. Journal of Virology. 2012a;86:3293–3306. doi: 10.1128/JVI.06434-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke GR, Strand MR. Polydnaviruses of parasitic wasps: domestication of viruses to act as gene delivery vectors. Insects. 2012b;3:91–119. doi: 10.3390/insects3010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke GR, Thomas SA, Eum JH, Strand MR. Mutualistic polydnaviruses share essential replication gene functions with pathogenic ancestors. PLoS Pathogens. 2013;9:e1003348. doi: 10.1371/journal.ppat.1003348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casewell NR, Wuster W, Vonk FJ, et al. Complex cocktails: the evolutionary novelty of venoms. Trends in Ecology and Evolution. 2013;28:219–229. doi: 10.1016/j.tree.2012.10.020. [DOI] [PubMed] [Google Scholar]
- Colinet D, Dubuffet A, Cazes D, et al. A serpin from the parasitoid wasp Leptopilina boulardi targets the Drosophila phenoloxidase cascade. Developmental and Comparative Immunology. 2009;33:681–689. doi: 10.1016/j.dci.2008.11.013. [DOI] [PubMed] [Google Scholar]
- Colinet D, Deleury E, Anselme C, et al. Extensive inter- and intraspecific venom variation in closely related parasites targeting the same host: the case of Leptopilina parasitoids of Drosophila. Insect Biochemistry and Molecular Biology. 2013;43:601–11. doi: 10.1016/j.ibmb.2013.03.010. [DOI] [PubMed] [Google Scholar]
- Consoli FL, Lewis D, Keeley L, et al. Characterization of a cDNA encoding a putative chitinase from teratocytes of the endoparasitoid Toxoneuron nigriceps. Entomologia Experimentalis et Applicata. 2007;122:271–278. [Google Scholar]
- Crawford AM, Brauning R, Smolenski G, et al. The constituents of Microctonus sp. parasitoid venoms. Insect Molecular Biology. 2008;17:313–324. doi: 10.1111/j.1365-2583.2008.00802.x. [DOI] [PubMed] [Google Scholar]
- Dahlman DL, Rana RL, Schepers EJ, et al. A teratocyte gene from a parasitic wasp that is associated with inhibition of insect growth and development inhibits host protein synthesis. Insect Molecular Biology. 2003;12:527–534. doi: 10.1046/j.1365-2583.2003.00439.x. [DOI] [PubMed] [Google Scholar]
- de Graaf DC, Aerts M, Brunain M, et al. Insights into the venom composition of the ectoparasitoid wasp Nasonia vitripennis from bioinformatic and proteomic studies. Insect Molecular Biology. 2010;19 Suppl 1:11–26. doi: 10.1111/j.1365-2583.2009.00914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins CA, Gundersen-Rindal DE, Hostetler JB, et al. Comparative genomics of mutualistic viruses of Glyptapanteles parasitic wasps. Genome Biology. 2008;9:R183. doi: 10.1186/gb-2008-9-12-r183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorémus T, Urbach S, Jouan V, et al. Venom gland extract is not required for successful parasitism in the polydnavirus-associated endoparasitoid Hyposoter didymator (Hym. Ichneumonidae) despite the presence of numerous novel and conserved venom proteins. Insect Biochemistry and Molecular Biolology. 2013;43:292–307. doi: 10.1016/j.ibmb.2012.12.010. [DOI] [PubMed] [Google Scholar]
- Edgar RC. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics. 2004;5:113. doi: 10.1186/1471-2105-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn RD, Clements J, Eddy SR. HMMER web server: interactive sequence similarity searching. Nucleic Acids Research. 2011;39:W29–37. doi: 10.1093/nar/gkr367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goecks J, Mortimer NT, Mobley JA, et al. Integrative approach reveals composition of endoparasitoid wasp venoms. PLoS ONE. 2013;8:e64125. doi: 10.1371/journal.pone.0064125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfray HCJ. Parasitoids: Behavioral and Evolutionary Ecology. Princeton University Press; Princeton, The United States of America: 1994. [Google Scholar]
- Gopalapillai R, Kadono-Okuda K, Okuda T. Molecular cloning and analysis of a novel teratocyte-specific carboxylesterase from the parasitic wasp, Dinocampus coccinellae. Insect Biochemistry and Molecular Biology. 2005;35:1171–1180. doi: 10.1016/j.ibmb.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Grabherr MG, Haas BJ, Yassour M, et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nature Biotechnology. 2011;29:644–652. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen-Rindal D, Dupuy C, Huguet E, Drezen JM. Parasitoid polydnaviruses: evolution, pathology and applications. Biocontrol Science and Technology. 2013;23:1–61. [Google Scholar]
- Herniou EA, Huguet E, Thézé J, et al. When parasitic wasps hijacked viruses: genomic and functional evolution of polydnaviruses. Philosophical Transactions of the Royal Society of London B Biological Sciences. 2013;368:20130051. doi: 10.1098/rstb.2013.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy HL, Dahlman DL. Extended in vitro culture of Microplitis croceipes teratocytes and secretion of TSP14 protein. Journal of Insect Physiology. 2002;48:401–409. doi: 10.1016/s0022-1910(02)00054-9. [DOI] [PubMed] [Google Scholar]
- Jancek S, Bèzier A, Gayral P, et al. Adaptive selection on bracovirus genomes drives the specialization of Cotesia parasitoid wasps. PloS ONE. 2013;8:e64432. doi: 10.1371/journal.pone.0064432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa Y, Yazaki K. Envelope protein of parasitic wasp symbiont virus, polydnavirus, protects the wasp eggs from cellular immune reactions by the host insect. European Journal of Biochemistry. 1997;246:820–826. doi: 10.1111/j.1432-1033.1997.00820.x. [DOI] [PubMed] [Google Scholar]
- Heavner ME, Gueguen G, Rajwani R, et al. Partial venom gland transcriptome of a Drosophila parasitoid wasp, Leptopilina heterotoma, reveals novel and shared bioactive profiles with stinging Hymenoptera. Gene. 2013;526:195–204. doi: 10.1016/j.gene.2013.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman DR. Hymenoptera venom allergens. Clinical Reviews in Allergy and Immunology. 2006;30:109–128. doi: 10.1385/criai:30:2:109. [DOI] [PubMed] [Google Scholar]
- Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Beck MH, Jiang H, et al. The viral protein Egf1.0 is a dual activity inhibitor of prophenoloxidase activating proteinases 1 and 3 from Manduca sexta. Journal of Biological Chemistry. 2008;283:21323–21333. doi: 10.1074/jbc.M801593200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltenpoth M, Engl T. Defensive microbial symbionts in Hymenoptera. Functional Ecology. 2013 epub ahead of print. [Google Scholar]
- Kroemer JA, Webb BA. Polydnavirus genes and genomes: emerging gene families and new insights into polydnavirus replication. Annual Reviews of Entomology. 2004;49:431–456. doi: 10.1146/annurev.ento.49.072103.120132. [DOI] [PubMed] [Google Scholar]
- Kuhn-Nentwig L. Antimicrobial and cytolytic peptides of venomous arthropods. Cellular and Molecular Life Sciences. 2003;60:2651–2668. doi: 10.1007/s00018-003-3106-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddison WP, Maddison DR. Mesquite: a modular system for evolutionary analysis. [accessed January 2012];2011 Available from http://mesquiteproject.org.
- Miller MA, Pfeiffer W, Schwartz T. Proceedings of the Gateway Computing Environments Workshop (GCE) Curran Associates; Red Hook, The United States of America: 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees; pp. 1–8. [Google Scholar]
- Moreau SJM, Huguet E, Drezen JM. Polydnaviruses as tools to deliver wasp virulence factors to impair lepidopteran host immunity. In: Reynolds S, editor. Insect Infection and Immunity: Evolution, Ecology and Mechansisms. Oxford University Press; Oxford, The United Kingdom: 2009. pp. 137–158. [Google Scholar]
- Mortazavi A, Williams BA, McCue K, et al. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nature Methods. 2008;5:621–628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- Murphy N, Banks JC, Whitfield JB, et al. Phylogeny of the parasitic microgastroid subfamilies (Hymenoptera: Braconidae) based on sequence data from seven genes, with an improved time estimate of the origin of the lineage. Molecular Phylogenetics and Evolution. 2008;47:378–395. doi: 10.1016/j.ympev.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Nei M, Gu X, Sitnikova T. Evolution by the birth-and-death process in multigene families of the vertebrate immune system. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:7799–7806. doi: 10.1073/pnas.94.15.7799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda T, Kadono-Okuda K. Perilitus coccinellae teratocyte polypeptide: evidence for production of a teratocyte-specific 540 kDa protein. Journal of Insect Physiology. 1995;41:819–825. doi: 10.1016/s0022-1910(98)00063-8. [DOI] [PubMed] [Google Scholar]
- Pennacchio F, Strand MR. Evolution of developmental strategies in parasitic hymenoptera. Annual Reviews of Entomology. 2006;51:233–258. doi: 10.1146/annurev.ento.51.110104.151029. [DOI] [PubMed] [Google Scholar]
- Periquet G, Bigot Y, Doury G. Physiological and biochemical analysis of factors in the female venom gland and larval salivary secretions of the ectoparasitoid wasp Eupelmus orientalis. Journal of Insect Physiology. 1997;43:69–81. doi: 10.1016/s0022-1910(96)00053-4. [DOI] [PubMed] [Google Scholar]
- Price DR, Bell HA, Hinchliffe G, et al. A venom metalloproteinase from the parasitic wasp Eulophus pennicornis is toxic towards its host, tomato moth (Lacanobia oleracae) Insect Molecular Biology. 2009;18:195–202. doi: 10.1111/j.1365-2583.2009.00864.x. [DOI] [PubMed] [Google Scholar]
- Pruijssers AJ, Falabella P, Eum JH, et al. Infection by a symbiotic polydnavirus induces wasting and inhibits metamorphosis of the moth Pseudoplusia includens. Journal of Experimental Biology. 2009;212:2998–3006. doi: 10.1242/jeb.030635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punta M, Coggill PC, Eberhardt RY, et al. The Pfam protein families database. Nucleic Acids Research. 2012;40:D290–301. doi: 10.1093/nar/gkr1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quicke DL. Parasitic Wasps. Chapman & Hall; New York, The United States of America: 1997. [Google Scholar]
- Rawlings ND, Barrett AJ. Evolutionary families of metallopeptidases. Methods in Enzymology. 1995;248:183–228. doi: 10.1016/0076-6879(95)48015-3. [DOI] [PubMed] [Google Scholar]
- Richards EH, Dani MP. Biochemical isolation of an insect haemocyte anti-aggregation protein from the venom of the endoparasitic wasp, Pimpla hypochondriaca, and identification of its gene. Journal of Insect Physiology. 2008;54:1041–1049. doi: 10.1016/j.jinsphys.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Richards EH, Edwards JP. Parasitization of Lacanobia oleracea (Lepidoptera: Noctuidae) by the ectoparasitc wasp, Eulophus pennicornis: effects of parasitization, venom and starvation on host haemocytes. Journal of Insect Physiology. 1999;45:1073–1083. doi: 10.1016/s0022-1910(99)00091-8. [DOI] [PubMed] [Google Scholar]
- Seals DF, Courtneidge SA. The ADAMs family of metalloproteases: multidomain proteins with multiple functions. Genes and Development. 2003;17:7–30. doi: 10.1101/gad.1039703. [DOI] [PubMed] [Google Scholar]
- Shiomi K, Midorikawa S, Ishida M, et al. Plancitoxins, lethal factors from the crown-of-thorns starfish Acanthaster planci, are deoxyribonucleases II. Toxicon. 2004;44:499–506. doi: 10.1016/j.toxicon.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Smith MA, Rodriguez JJ, Whitfield JB, et al. Extreme diversity of tropical parasitoid wasps exposed by iterative integration of natural history, DNA barcoding, morphology, and collections. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:12359–12364. doi: 10.1073/pnas.0805319105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- Strand MR. Polydnaviruses. In: Asgari S, Johnson KN, editors. Insect Virology. Caister Academic Press; Norwich, United Kingdom: 2010. pp. 216–241. [Google Scholar]
- Strand MR, Burke GR. Polydnaviruses as symbionts and gene delivery systems. PLoS Pathogens. 2012;8:e1002757. doi: 10.1371/journal.ppat.1002757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand MR, Burke GR. Polydnavirus-wasp associations: evolution, genome organization, and function. Current Opinion in Virology. 2013;3 doi: 10.1016/j.coviro.2013.06.004. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Strand MR, Pech LL. Immunological basis for compatibility in parasitoid-host relationships. Annual Reviews of Entomology. 1995;40:31–56. doi: 10.1146/annurev.en.40.010195.000335. [DOI] [PubMed] [Google Scholar]
- Strand MR, Meola SM, Vinson SB. Correlating pathological symptoms in Heliothis virescens eggs with development of the parasitoid Telenomus heliothidis. Journal of Insect Physiology. 1986;32:389–402. [Google Scholar]
- Strand MR, Noda T. Alterations in the haemocytes of Pseudoplusia includens after parasitism by Microplitis demolitor. Journal of Insect Physiology. 1991;37:839–850. [Google Scholar]
- Strand MR, Wong EA. The growth and role and Microplitis demolitor teratocytes in parasitism of Pseudoplusia includens. Journal of Insect Physiology. 1991;37:503–515. [Google Scholar]
- Stoltz DB, Guzo D, Belland ER, et al. Venom promotes uncoating in vitro and persistent in vivo of DNA from a braconid polydnavirus. Journal of General Virology. 1988;69:903–907. [Google Scholar]
- Vincent B, Kaeslin M, Roth T, et al. The venom composition of the parasitic wasp Chelonus inanitus resolved by combined expressed sequence tags analysis and proteomic approach. BMC Genomics. 2010;11:693. doi: 10.1186/1471-2164-11-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinson SB, Iwantsch GF. Host regulation by insect parasitoids. Quarterly Review of Biology. 1980;55:143–165. [Google Scholar]
- Webb BA, Strand MR. The Biology and Genomics of Polydnaviruses. In: Gilbert LI, Iatrou K, Gill SS, editors. Comprehensive Molecular Insect Science. Vol. 6. Elsevier; San Diego, The United States of America: 2005. pp. 323–360. [Google Scholar]
- Webb BA, Strand MR, Dickey SE, et al. Polydnavirus genomes reflect their dual roles as mutualists and pathogens. Virology. 2006;347:160–174. doi: 10.1016/j.virol.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Webb BA, Summers MD. Venom and viral expression products of the endoparasitic wasp Campoletis sonorensis share epitopes and related sequences. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:4961–4965. doi: 10.1073/pnas.87.13.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JY, Fang Q, Wang L, Hu C, Ye GY. Proteomic analysis of the venom from the endoparasitoid wasp Pteromalus puparum (Hymenoptera: Pteromalidae) Archives of Insect Biochemistry and Physiology. 2010;75:28–44. doi: 10.1002/arch.20380. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.