Summary
Background
The hypothalamic–pituitary–adrenal (HPA) axis is a critical mediator linking stress to health. Understanding how to modulate its reactivity could potentially help reduce the detrimental health effects of HPA axis activation. Social evaluative threat is a potent activator of this system. Access to control and coping responses can reduce its reactivity to pharmacological activation. Compassionate or affiliative behaviors may also moderate stress reactivity. Impact of these moderators on social evaluative threat is unknown. Here, we tested the hypotheses that interventions to increase control, coping, or compassionate (versus competitive) goals could reduce HPA-axis response to social evaluative threat.
Methods
Healthy participants (n = 54) were exposed to social evaluative threat using the Trier Social Stress Test (TSST). They were randomly assigned to receive one of four different instructions prior to the stressor: Standard TSST instructions (SI), standard instructions with access to “control” (SI Control), or one of two cognitive interventions (CI) that (1) increased familiarity and helped participants prepare coping strategies (CI Coping), or (2) shifted goal orientation from self-promotion to helping others (CI Compassionate Goals). ACTH and Cortisol were obtained before and after stress exposure via intravenous catheter.
Results
Control alone had no effect. CI Compassionate Goals significantly reduced ACTH and Cortisol responses to the TSST; CI Coping raised baseline levels. Compassionate Goals reduced hormonal responses without reducing subjective anxiety, stress or fear, while increasing expression of pro-social intentions and focus on helping others.
Conclusions
Brief intervention to shift focus from competitive self-promotion to a goal orientation of helping-others can reduce HPA-axis activation to a potent psychosocial stressor. This supports the potential for developing brief interventions as inoculation tools to reduce the impact of predictable stressors and lends support to growing evidence that compassion and altruistic goals can moderate the effects of stress.
Keywords: HPA, Cortisol, ACTH, Stress, Psychosocial stress, Trier Social Stress Test, Compassion, Control, Coping
1. Introduction
The hypothalamic—pituitary adrenal (HPA) axis and its end product, Cortisol, critically mediate the negative impact of “stress” on health, impacting the onset, course and pathophysiology of medical (Chrousos and Gold, 1998; McEwen, 1998) and psychiatric disorders (Carroll et al., 1981; Young et al., 2003). They link early life experience to lifelong vulnerability to biobehavioral disturbances that undermine adaptive functioning (Ladd et al., 1999). Understanding HPA psychobiology can help reduce stress-related illness and psychiatric disorders and promote healthy development and adaptive resilience. Despite decades of research, however, we still have not clearly defined what specific aspects of “stress” activate this system or the factors that moderate its activity when it is stimulated. Identification of specific factors that activate and modulate the HPA axis can guide efforts to reduce exposure to the type of stress that damages health and can shape interventions to reduce the deleterious impact of predictable but unavoidable stressors.
Links between psychosocial stress, the HPA axis, and health outcomes have been extensively explored epidemio-logically. Cortisol levels are influenced by socioeconomic status, race/ethnicity, and complex psychosocial constructs like overload, job strain, or burnout (Adam and Kumari, 2009). Cortisol is also linked to onset or course of autoimmune, allergic, infectious and neoplastic diseases (Elenkov et al., 1999), as well as vascular disease (Rosmond et al., 1998), heart disease, diabetes, and stroke (Rosmond and Bjorntorp, 2000). Epidemiological work has the power needed to detect associations and control for covariates (Adam and Kumari, 2009), but it cannot precisely identify key factors and causal pathways that mediate the impact of social and psychological experience on the acute biological responses that are relevant to longer-term health. For this, experimental studies are needed. A meta-analysis of laboratory-based psychosocial stress studies has demonstrated that Cortisol reactivity is not a function of negative affect or subjective distress, but is linked most closely to social evaluative threat or lack of control over perceived stressors (Dickerson and Kemeny, 2004). These two factors may be linked, in that social evaluative threat may be a particularly potent HPA activator because it is inherently uncontrollable. Enhancing sense of control may be one way to modulate system activation, and perhaps enhance health and resilience.
Another moderator of HPA axis activity is social support (Levine, 2000; Uchino et al., 1996), which may moderate general stress effects (Cosley et al., 2010; Kirschbaum et al., 1995) through affiliation-induced release of oxytocin, which can inhibit HPA axis activation (Heinrichs et al., 2003). Affiliation is also experienced through giving social support (Brown et al., 2008; Konrath and Brown, 2012) and giving to others out of concern for their wellbeing (operationalized in constructs like compassion, loving-kindness, altruism, helping, and volunteerism) is attracting scientific interest as a stress reducer and health enhancer (e.g., Hofmann et al., 2011). Endorsing compassionate goals can prospectively enhance perceptions of available social support and reduce focus on self-other competition, and is associated with enhanced emotional well-being (Crocker and Canevello, 2008; Crocker et al., 2010). Giving to others (e.g., volunteering, caregiving) reduces self-focus and can yield psychological and health benefits for the giver (Konrath and Brown, 2012). Cultivating a motivational state related to helping others may activate a neurobehavioral “caregiving system” that promotes social bonds and perhaps enhances health by dampening stress reactivity (Davidson and McEwen, 2012; Konrath and Brown, 2012; Taylor et al., 2000; Wang, 2005). The key psychosocial factors at work may involve shifting focus from self-protection or self-promotion to core values that emphasize concern for the well-being of others and contributing to the greater good (Crocker and Canevello, 2012). Biological mechanisms through which compassionate or altruistic goals and giving to others can enhance emotional well-being and improve health may involve HPA axis modulation (Konrath and Brown, 2012). However, efforts to directly demonstrate modulation of Cortisol release in the laboratory by compassion meditation (Pace et al., 2009) or providing support to a stressed other (Smith et al., 2009) have not been successful. A more direct counter to social evaluative threat (Dickerson and Kemeny, 2004), by shifting focus from self-image goals toward compassionate goals (Crocker and Canevello, 2012), may be more effective.
Our laboratory has explored psychosocial modulation of HPA axis activity using specific psychological manipulations and an externally activated system. Initial studies used direct pharmacological activators to provide a “cleaner” test of psychological modulators. Access to control and/or cognitive coping tools reduced Cortisol or corticotropin (ACTH) output even when the system was activated by direct pituitary stimulation (Abelson et al., 2005, 2008, 2010), suggesting that top-down inhibitory control of the system is “in play” even with pharmacological activation taking place outside the brain. Here we extend these studies to the most widely used laboratory model of psychosocial HPA axis activation — the Trier Social Stress Test (TSST), which allows us to examine the effects of control and coping when activation occurs indirectly, through a psychosocial rather than pharmacological pathway. This allows us to test whether the focus of stress reducing interventions needs to be specifically tailored to the nature of the challenge being faced, or whether control and coping are sufficiently potent modulators that they can ameliorate biological stress responses regardless of activation pathway. Given growing interest in health benefits of support, compassion and giving, and the face-validity of a compassion manipulation in the context of the social evaluative and self-image-focused nature of the TSST, we also included an intervention designed to shift focus from self-promotion to helping others (called here a “compassionate goal intervention”). Evidence that access to controlling or coping responses can reduce HPA reactivity in the TSST would further support the specific importance of these modulators to this stress response system, regardless of mode of activation. Evidence that a compassion intervention can buffer biological stress responses would support the theory that HPA axis effects may mechanistically mediate health benefits of helping others.
2. Methods
2.1. Design
Healthy participants (n = 54) were exposed to social evaluative threat — using the Trier Social Stress Test (TSST) – in a single laboratory visit. They were randomly assigned to receive one of four different instruction sets upon arrival: Standard TSST instructions (SI), standard instructions with perceived “control” (SI Control), or one of two cognitive interventions (CI) that (1) increased familiarity and helped participants prepare coping strategies (CI Coping), or (2) shifted goal orientation from self-promotion to helping others (CI Compassionate Goals). Analyses focused on effects of instruction group on neuroendocrine responses (ACTH and Cortisol), but also examined effects on subjective measures. Procedures were IRB approved.
2.2. Participants
Healthy adults were recruited through multi-media advertising. After phone screening, qualifying individuals received face-to-face evaluation to assess eligibility using self-report measures and a Structured Clinical Interview for DSM-IV (SCID). Qualifying participants were 18–45 years old, medically healthy, within 30% of ideal body weight, with no recent exposure to psychoactive medication (2 months), no history of substance dependence or recent abuse (6 months), low levels of tobacco and alcohol use (mean 2.3 drinks/week), negative urine drug screens, and normal screening laboratory results. They had no psychiatric disorders and no first-degree family history of anxiety (except specific phobia) or affective disorders. Females were premenopausal, not pregnant or lactating, not using birth control pills, and studied between days 18 and 27 of the menstrual cycle (luteal phase). They signed written consent and were paid $100 for their participation.
Three participants were excluded due to hormonal values greater than two standard deviations above group means (two from the SI group and one from CI Compassionate Goals). Two participants were excluded due to missing values (one each from CI Coping and CI Compassionate Goals). The final sample analyzed included 54 participants.
2.3. Procedures
Participants reported at 1:00 p.m., sat in a reclining chair in an accommodation room, and listened to assigned instructions, delivered via tape recording and discussion with the principal investigator (see below). Instructions were completed at least 60 min prior to start of the TSST and 45— 55 min before the first “baseline” (pre-TSST) sample. All participants received an identical, standard introduction to the TSST itself at its initiation around 2:30 p.m. (see below). Intravenous access was established no later than 1:30 p.m., using an 18–20 gauge angiocatheter in an antecubital vein, kept open with a normal saline drip. Participants then rested comfortably for 1 h, to accommodate to the research setting and IV. Pre-TSST blood samples were drawn at 2:10 and 2:25 p.m. (20 and 5 min prior to TSST initiation). Participants moved to a second room for the TSST at 2:30 p.m. Additional samples were obtained between speech and arithmetic tasks of the TSST and at 10, 20, 30, 45, and 60 min following its completion (back in the accommodation room). Participants were debriefed and dismissed after the last sample was collected.
2.3.1. TSST challenge
After accommodation, participants moved to a “test room” for the TSST, first meeting a panel consisting of one male and one female observer. A video camera and tape recorder were evident. Participants were told that they would assume the role of a job applicant invited for a personal interview with a selection committee. A written job description (developed individually for each participant based on employment aspirations elicited at screening) was handed to them and they were told to prepare a 5 min speech to convince the panel that they were the best candidates for the job. They were encouraged to promote themselves (e.g., by enumerating accomplishments and explaining why they might stand out over other applicants). Panel members were described as faculty or graduate students trained to evaluate non-verbal manifestations of stress and anxiety during the talk, as well as in subsequent reviewing of video recordings with other experts. Participants had 3 min to prepare the speech while standing at a table in front of the panel. They could take notes, but were not permitted to use them during the talk. After 3 min, the panel leader asked participants to stand in front of the panel and camera and to begin their 5 min talk. They were encouraged to utilize the entire 5 min and questioned in a standardized fashion if they did not. Panelists scrutinized them during their presentation and provided no feedback. After the presentation, participants sat briefly for a blood draw and then stood again and were told to serially subtract 13 from 1022 as accurately and rapidly as possible. If they made errors, they had to start over. This continued for 5 min, after which participants were escorted back to the accommodation room. The video camera was remotely operated to focus on participants as they began the task and flash a red light as actual recordings were made.
2.3.2. Instructions (delivered an hour prior to introduction of the TSST)
Standard Instructions (SI, n = 15) included a brief description of data collection procedures and indication that participants would later engage in a speaking task in front of a panel and a video camera, followed by a brief thinking task. To make it parallel to the enhanced control condition (see below), they were also informed that being under observation in a speaking task could be stressful to some, and that there was a curtain in the room that could be drawn to block off the observers and camera. They were told that an indicator light would be lit if they had permission to use the curtain to shelter themselves from observation. If the light was not lit, they would not have this option. They were told that the data would be more useful if they did not close the curtain, but that if the light was lit, the decision was in their hands. For participants in the SI group, the indicator light remained off – informing them that they could not use the curtain and would have to complete the task in front of the panel and on camera.
The enhanced control condition (SI Control, n = 16) was identical to the SI condition except the indicator light was lit when they entered the test room, giving participants permission to use the curtain if they wished to do so. Just prior to start of the TSST they received a written reminder that closing the curtain could reduce distress if any was generated by scrutiny of the panel and camera. They were told not to feel embarrassed if they wanted to use this option and that others had done so. To close the curtain, they handed a yellow card marked “CLOSE” to the nurse sitting behind them.
Subjects in the Coping Intervention group (CI Coping, n = 12) received the Standard Instructions modified to remove discussion of the curtain, indicator and light, immediately followed by additional, supplemental information designed to facilitate cognitive coping. This information mirrored techniques used in the coping intervention that modulated HPA response to pharmacological activation (Abelson et al., 2008) – normalizing expectable emotional and physical responses to the social challenge and providing cognitive coping tools to make the experience feel less threatening, unexpected, or unusual. Personal experiences were elicited and used as a context in which to normalize negative thoughts and anxiety-related bodily sensations and discuss ways to prevent these from triggering an escalating cycle of anxiety. Participants were reassured that physical and emotional sensations were normal and expectable responses in this task, and that there were no real “threats” involved.
The intervention designed to shift goal orientation from self-promotion to helping others (CI Compassionate Goals, n = 11) started with Standard Instructions as in the CI Coping condition, now followed by additional instructions highlighting the self-promoting, competitive cognitive set that a job interview usually elicits and suggesting that an alternative approach to getting a job could focus on the good one could do for others if given this employment opportunity. It suggested that rather than trying to show superiority to other applicants and protecting themselves by focusing on strengths while hiding weaknesses, they might feel more comfortable and do a better job by talking about ways to use the job to contribute to a larger mission beyond their own accomplishments and promote a greater good, emphasizing other-focused or compassionate values (see Burson et al., 2012). Personal experiences were elicited and used as a context to highlight the difference between proving oneself (a defensive set) and focusing on something larger and more important than oneself (a compassionate or altruistic set). To help participants sustain this shift, they were asked to spend a few minutes during the 60 min accommodation period (when all subjects were reading or doing homework) thinking about and perhaps creating a list of their own pro-social or self-transcendent values (Schwartz and Boehnke, 2004) and how they would like to live them out in their future work. To help them reframe the goal of the cognitive (arithmetic) challenge in a self-transcendent way, they were given a brief written note, just before it commenced, reminding them that they were a part of our scientific team and could help us and promote science by trying as hard as they could – their goal was to help the researchers by doing their best, not to prove their competence or outperform others.
2.4. Assays
ACTH and Cortisol were assayed using commercial kits. Cortisol was assayed using Coat-a-Count Cortisol kits (Siemens, USA), a well-validated radioimmunoassay (RIA) with analytical sensitivity of 0.2 mcg/dl and inter-assay and intra-assay variabilities of less than 5%. ACTH was assayed using a chemiluminescent assay (Immulite 1000 ACTH, Siemens, USA) with an analytical sensitivity of 9 pg/mL and inter-assay and intra-assay variability of less than 5%.
2.5. Measures
Baseline self-report measures included the Spielberger State-Trait Anxiety Inventory (STAI; Spielberger and Gorsuch, 1970) and Beck Depression Inventory-II (BDI-II; Beck et al., 1996). Subjective states were recorded before, during and after the TSST using Visual Analog Scales (VASs), administered upon arrival, at the time of each blood sample, and after the 3 min speech preparation period. These quantified emotions or cognitions on 100-mm visual analog lines (“not at all” to “most ever”). A primary dependent variable was subjective anxious distress (sum of VAS ratings of “anxious”, “nervous”, and “fearful”).
2.6. Statistical analysis
Primary analyses focused on hormonal time series using repeated measures analyses of variance (RM-ANOVAs) with particular interest in group-by-time interactions, which capture differential stress responses to the TSST between instruction groups. Planned follow-up tests included one-way ANOVAs examining group differences on calculated measures to quantify mean levels prior to the TSST (average of two pre-TSST samples), peak value (maximum after TSST initiation), and peak response (maximum after initiation minus mean pre-TSST levels). Subjective anxious distress (SAD) and other emotional and cognitive responses were analyzed similarly.
ACTH and Cortisol RM-ANOVAs were all repeated using log transformed data (to improve normality). Results were identical. Hormonal responses were also analyzed using repeated measures analysis within a mixed model framework examining the impact of group, sex, time and their interactions. First-order autoregressive covariance structure was the best fit for the data and all analyses were conducted using maximum likelihood estimation (ML). Findings mirrored analyses with RM-ANOVA, and all key findings remained significant with sex included in the models. For clarity of presentation and ease of interpretation, we present only the standard ANOVAs and untransformed hormonal data.
3. Results
3.1. Demographic and hormonal data
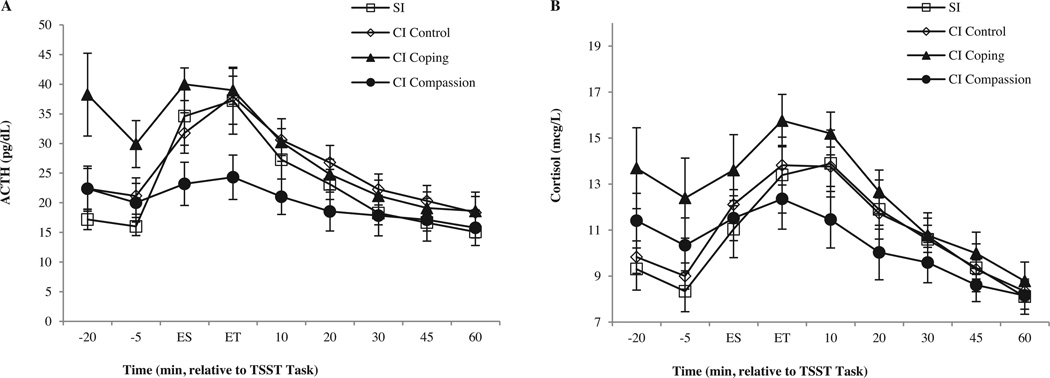
The four instruction groups did not differ in age, sex, BMI, alcohol consumption, aerobic exercise or self-reported measures of depressive symptoms or trait anxiety (Table 1). As expected, ACTH and Cortisol levels increased significantly in response to the TSST (Fig. 1; time F8,400 = 27.39, p < .0001, ηp2 = .35; F8,400 = 34.42, p < .0001, ηp2 = .41, respectively). Instruction groups did not differ in overall ACTH or Cortisol levels (group F3,50 = 2.03, p=.12, ηp2=.11; F3,50 = 1.15, p = .34, ηp2 = .07), but instruction groups did differ significantly in ACTH and Cortisol responses to the stressor over time (group × time interaction F24,400 = 2.75, p<.0001, ηp2=.14; F24,400= 1–95, p=.005, ηp2=.11). The data (Fig. 1) show that SI and SI Control groups were identical in hormonal responses, whereas the cognitive interventions altered pre-TSST levels of ACTH and Cortisol (CI Coping) or challenge reactivity (CI Compassionate Goals).
Table 1.
Group means (±SD) for demographics and self-report questionnaires.
| SI | SI Control | CI Coping | CI Compassion | |
|---|---|---|---|---|
| Age (years) | 24.27 (6.73) | 25.13 (8.46) | 21.83 (2.48) | 21.45 (1.97) |
| Sex (percent female) | 46.7 | 31.3 | 33.3 | 45.5 |
| BMI | 23.12 (3.66) | 23.25 (3.12) | 24.11 (2.12) | 22.21 (1.88) |
| Alcohol use (d/w) | 2.18 (2.55) | 3.31 (5.14) | 1.50 (1.29) | 1.80 (2.59) |
| Aerobic exercise (hrs/w) | 2.90 (2.24) | 2.25 (1.82) | 3.67(1.89) | 2.68 (2.63) |
| BDI-II | 1.93 (2.84) | 2.31 (2.27) | 2.83 (2.25) | 1.73 (3.52) |
| STAI-Trait | 27.80 (5.73) | 28.44 (4.93) | 30.50 (6.33) | 31.00 (9.39) |
Note. One-way ANOVAs comparing the four groups showed no significant differences (all p’s > .10). SI, standard instructions; SI Control, SI with access to control intervention; CI Coping, cognitive intervention to facilitate coping; CI Compassion, cognitive intervention to promote compassionate goal orientation; BMI, body-mass-index; Alcohol use (drinks per week); Aerobic exercise (hours per week); BDI-II, Beck Depression Inventory; STAI-Trait, State-Trait Anxiety Inventory – Trait Subscale.
Figure 1.
Mean (±SE) ACTH (Panel A) and Cortisol (Panel B) levels in response to the TSST over time (minutes relative to the TSST challenge; ES, End of Speech; ET, End of Task). Four different groups received standard instructions (SI), enhanced control (SI Control), cognitive intervention to facilitate coping (CI Coping), or cognitive intervention to promote compassionate goal orientation (CI Compassion). CI Control elevated baseline levels and CI Compassion reduced task responses (see text).
Prior to unpacking the significant group-by-time interactions, we examined the two SI groups more closely. They differed only in status of the signal light (on for those with control) indicating permission to close a curtain to shelter themselves from observation. No participants utilized the curtain. On debriefing, most indicated that they had not noted whether the signal light was on or off. When directly compared to each other, they showed no differences in Cortisol or ACTH (RM-ANOVA, group F1,29 = 0.07, p=.79, ηp2 = .003; group × time interaction F8,232 = 0.26, p = .98, ηp2 = .009; and group F1,29 = 0.72, p = .40, ηp2 = .02; group -× time interaction I8,232 = 0.65, p = .73, ηp2 = .02, respectively). Nor did they differ in age, sex, race, body-mass index (BMI), alcohol consumption, aerobic exercise, depression symptoms, and trait anxiety (all p’s > .30). Because the SI Control intervention had no impact, and in order to more directly and powerfully examine the impacts of the altered instructions used in our two cognitive interventions, we combined the two standard instruction groups into a single, enlarged SI group (n = 31). This more cleanly isolated the effects of the Coping and Compassionate Goal interventions, relative to standard instructions, in follow-up analyses of the significant interactions found in the primary ANOVAs.
Repeating the overall RM-ANOVAs with three groups produced identical results and comparable effect sizes to the four group analyses, with significant time effects (p < .0001) and significant group-by-time interactions (p<.0002) for both hormones. Follow-up analyses and summary variables comparing CI Coping, CI Compassionate Goals and the enlarged SI group (Table 2) confirmed the overall ANOVAs and the graphical impression that CI Coping raised pre-TSST levels of HPA axis activity and CI Compassionate Goals reduced stress reactivity. The groups differed in mean pre-TSST ACTH levels (F2,51 = 6.00, p= .005, ηp2 = .19), and post hoc tests (Fisher’s PLSD) verified that the CI Coping group was significantly elevated at this “baseline” relative to the other two groups (p = .001 and .02), which did not differ from each other (p = .67). The groups also differed in peak ACTH levels following challenge (F2,51 = 3.45, p=.04, ηp2=.12), with post-hoc’s verifying that the CI Compassionate Goals group had significantly lower post-challenge ACTH peaks relative to the other two groups (p = .02), which did not differ from each other (p=.65). The impact of CI Compassionate Goals instructions on ACTH was also seen in the ACTH peak response measure (F2,51 = 3.88, p = .03, ηp2 = .13), with post hoc tests verifying that the CI Compassionate Goals group had significantly lower ACTH response to the TSST challenge relative to the Standard Instruction group (p= .02). The CI Coping group also appeared reduced in ACTH response relative to SI (p = .052), primarily due to their elevated pre-TSST levels. Since pre-TSST “baseline” measures of ACTH also reflect anticipation of the coming challenge, true resting levels are not evident for some subjects until the end of the experiment, when all procedures are over. We repeated peak response analyses using as our basal measure the mean of the last two samples obtained (+45 and +60 min after the TSST), when more of the subjects are at a truer resting level (as reflected in (a) the loss of group differences that were created at baseline by the interventions and (b) the reduced variance at these time points, see Fig. 1). This confirmed that CI Compassionate Goals significantly reduced the ACTH response relative to SI (p = .025) and CI Coping (p< .04), while the latter two groups did not differ from each other (p=.80).
Table 2.
Group means (±SD) for hormonal and subjective data.
| Sl | CI Coping | CI Compassion | ||
|---|---|---|---|---|
| Hormonal and subjective anxious distress (SAD) measures | ||||
| ACTH | Mean baseline | 19.26 (10.36) | 34.08 (18.42) | 21.19 (11.47) |
| Post TSST peak level | 39.31 (19.36) | 41.84(8.38) | 25.82 (12.08)* | |
| Peak response | 20.05 (20.17) | 7.77(17.39) | 4.64(11.54)* | |
| Cortisol | Mean baseline | 9.14 (2.95) | 13.04(6.01)* | 10.87 (3.92) |
| Post TSST peak level | 14.76 (4.74) | 16.38 (4.06) | 13.12 (4.17) | |
| Peak response | 5.63 (4.41) | 3.35 (3.92) | 2.25 (4.62)* | |
| SAD | Mean baseline | 16.11 (15.11) | 26.78 (10.48) | 17.16 (21.65) |
| Post TSST peak level | 83.77(61.22) | 74.08 (42.85) | 73.91 (48.70) | |
| Peak response | 67.66 (52.76) | 47.30 (38.69) | 56.75 (42.27) | |
| Visual Analog Scales (VAS) ratings | ||||
| Proving yourself | 65.08 (25.15) | 64.91 (33.80) | 55.00 (20.40) | |
| Fear of negative evaluation | 31.53 (30.28) | 32.83 (22.22) | 30.73 (22.23) | |
| Helping others | 41.64(29.41) | 47.83 (31.97) | 68.91 (23.28)* | |
| Making a meaningful contribution | 49.28 (32.19) | 45.25 (31.86) | 78.60 (12.17)* | |
Note. SI, standard instructions; CI Coping, cognitive intervention to facilitate coping; CI Compassion, Cognitive intervention to promote compassionate goal orientation.
p < .05 compared to SI.
Follow-up analyses of Cortisol results mirrored ACTH findings – though less robustly. Variance was greater and not all comparisons reached significance. The three groups differed in mean pre-TSST Cortisol levels (F2,51=4.30, p=.02, ηp2 = .14), with post hoc tests showing that CI Coping instructions elevated anticipatory Cortisol levels relative to SI (p=.006). Groups differed marginally in Cortisol peak response to the TSST (F2,51 =2.91, p= .06, ηp2 = .10), but not in peak levels (p= .23, ηp2 = .06). Post hoc tests confirmed that CI Compassionate Goals lowered Cortisol responses relative to the SI group (p = .03).
In summary, ACTH and Cortisol findings show that CI Coping instructions – modeled after a cognitive intervention that reduced HPA axis responses to pharmacological activators – failed to reduce HPA responses to the TSST and actually increased anticipatory levels, whereas CI Compassionate Goals instructions substantially dampened HPA responses to the TSST challenge.
3.2. Subjective and observational data
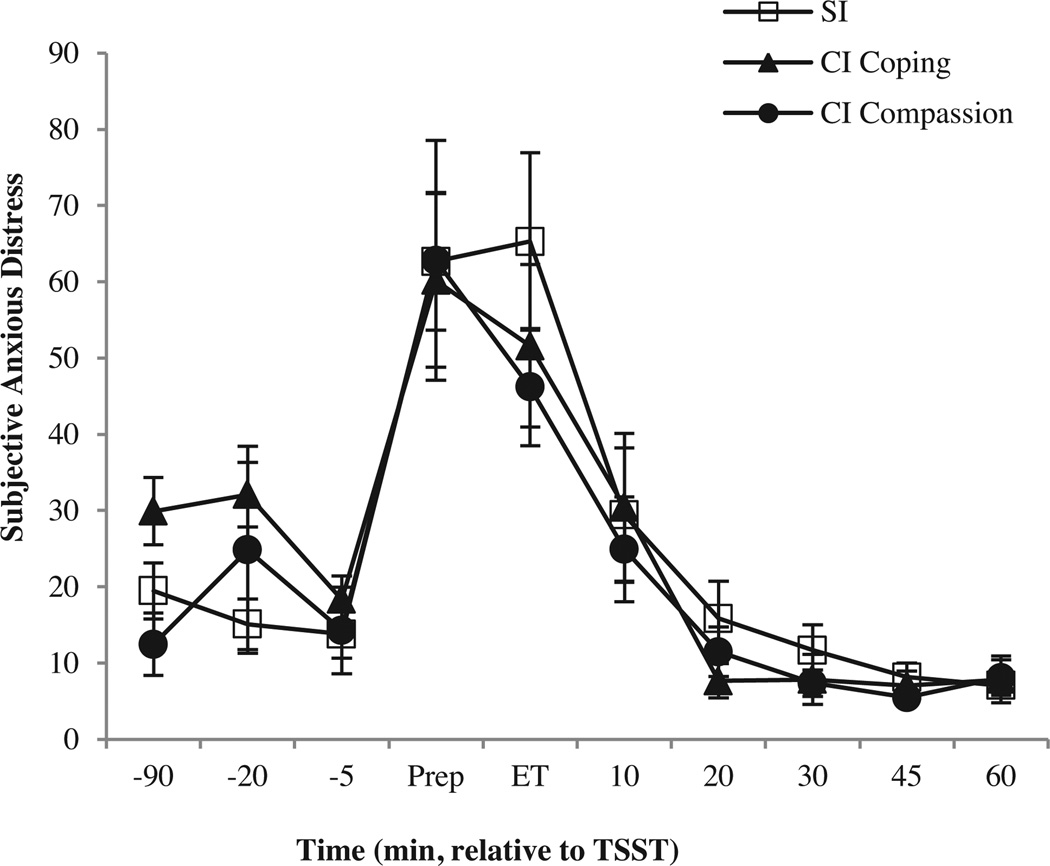
Subjective anxious distress (SAD) rose significantly, as expected, in response to the TSST challenge (time F9,459 = 28.07, p< .0001, ηp2 = .36). Despite hormonal differences, instruction groups did not differ in SAD level (group F2,51 = .12, p=.89, ηp2 = .005) or response (group × time interaction F18,459 = .82, p = .68, ηp2 = .03; Fig. 2). No group differences were detected for mean baseline (F2,51 = 2.04, p=.14, ηp2 = .07), peak value (F2,51 = .21, p=.82, ηp2 = .008), or peak response (F2,51 = .83, p = .44, ηp2 = .03; Table 2). Similarly, VAS rating of “feeling stressed” increased as expected (time F9,459 = 17.60, p<.0001, ηp2=.26), but there were no differences between instruction groups (group F2,51 = −17, p = .84, ηp2 = .007; group × time interaction F18,459 = .30, p=.99, ηp2=.01). VAS measures of “feeling stressed” did not predict any ACTH or Cortisol dependent measures (mean baseline, peak levels, or peak responses; all p’s > .40). No significant relationships could be found between hormonal variables and any subjective measure of emotional states (e.g., fears of negative evaluation, perception of control or coping preparedness, social support or novelty experience; data available upon request).
Figure 2.
Mean (±SE) subjective anxious distress levels in response to the TSST over time (minutes relative to the TSST challenge; Prep, Speech preparation; ET, End of Task). SI and SI Control groups were combined into a single SI group, to enhance detection of potential cognitive intervention effects (CI Coping and CI Compassion). Despite differential hormonal responses, there were no significant group differences in subjective anxious distress.
We conducted a number of manipulation checks, using VAS and observer rating data. VASs assessed ego engagement with the job speech and strategies utilized in pursuing the job (Table 2). Compassion and Coping instructions did not reduce the degree to which participants reported that they were trying to prove that they were the best person for the job (F2,44 = 0.61, p = .55, ηp2 = .03) and did not reduce fears of negative evaluation (F2,50 = 0.02, p= .98, ηp2 = .001). However, Compassionate Goal instructions did lead participants receiving them to “think more about ways to make a positive difference for others at the job” (F2,45 = 3.44, p = .04, ηp2 = .13) and to focus more on “why the job would be meaningful beyond their own accomplishments” (F2,44 = 4.43, p=.02, ηp2=.17).
As an additional check, video recordings of the job talks were coded by observers (blind to experimental manipulations), who counted the total number of participants’ verbal self-references (I, me, my) and rated participants’ compassionate goals (e.g., trying to support others or to make a difference to others in the job) and affiliativeness during the talk (methodological details available upon request). Groups did not differ in total number of self-references (F2,48 = 0.50, p = .61, ηp2 = .02) but did differ in display of compassionate goals (F2,48 = 11.74, p < .001, ηp2 = .33) and affiliative behavior (F2, 48 = 6.45, p = .003, ηp2 = .21). Post hoc tests showed that observers rated the CI Compassionate Goals group as displaying greater compassionate goals and affiliation than the SI (p < .004) and CI Coping groups (p < .009).
4. Discussion
Social evaluative threat, as delivered in the TSST, is a potent and reliable psychosocial activator of the HPA axis (Dickerson and Kemeny, 2004). We have previously shown that brief cognitive interventions targeting sense of control, cognitive coping and novelty reduction can moderate HPA axis response to pharmacological activation (Abelson et al., 2005, 2008, 2010). Here, we tested the ability of parallel interventions, targeting the same psychological constructs, to moderate HPA response to the TSST. We also examined the impact of an additional intervention rooted in growing literature on stress-reducing effects of compassionate goals (Crocker and Canevello, 2012; Davidson and McEwen, 2012; Konrath and Brown, 2012) and designed to shift participants from the competitive, self-promoting orientation typically elicited by the TSST to an other-focused, pro-social orientation. The compassionate goals intervention substantially reduced stress hormone responses to the TSST, whereas control and coping interventions did not.
The failure of coping and control interventions to moderate HPA activity in the TSST was unexpected. Both animal and human data suggest that the factors manipulated – familiarity (reduced novelty), sense of control, and access to coping responses – can reduce HPA reactivity to a variety of threats or challenges (Levine, 2000). Cognitive interventions targeting these factors reduce HPA axis activity in humans even when the system is directly activated pharmacologically at the level of the pituitary (Abelson et al., 2005, 2008, 2010), suggesting that these factors are particularly potent inhibitors of HPA activation, and that psychosocial factors may actively shape HPA reactivity even in laboratory studies designed to probe its pharmacological sensitivities. Nevertheless, these interventions did not reduce HPA reactivity to the TSST challenge. This perhaps supports the psychosocial potency of the TSST as an HPA activator. However, our alternative cognitive manipulation (compassionate goals) did reduce ACTH and Cortisol responses, indicating that despite failure of our control and coping interventions, HPA reactivity to the TSST’s social evaluative threat can be psychologically modulated, and suggesting that there is some psychological specificity to what “works” in different threat contexts.
Psychological explanations of the failure of control and coping and success of compassionate goals in modulating HPA reactivity in this study can only be speculative, but a number of factors can be identified that warrant evaluation in follow-up studies. The control and coping interventions used in this study were designed to parallel as precisely as possible those used successfully in pharmacological models, but they may have had less specific psychological relevance to the psychosocial activating factors at play in the TSST. Generic efforts to convey a sense of control and capacity to cope may have less value if not specifically targeted at the psychological salience of the particular threat encountered. Our control manipulation did not translate well from the pharmacological paradigms and had no effect whatsoever. Gaining control over a social evaluative threat is clearly more complicated than having the power to end or slow drug exposure. Deciding to reduce social scrutiny by closing a curtain might have its own social repercussions, and might not truly end the evaluative threat. Coping is also different in this context. The novelty of a drug infusion paradigm and the potential for specific and predictable drug side effects can be very directly addressed in a coping intervention designed to make the paradigm seem less novel and the drug side effects less threatening. Psychosocial performance, like that posed by the TSST, is less novel, making novelty reduction less relevant; and social evaluative threat is far less concrete than potential drug side effects, making coping enhancement more difficult. The TSST’s impact comes from the observing panel’s high scrutiny, low feedback demeanor and efforts to prepare participants for this appeared to just alert them that a “stressor” was coming, elevating pre-TSST ACTH and Cortisol without reducing reactivity.
Alternatively, there are substantial differences in cognitive/attentional load between the drug and TSST paradigms. In the TSST, where substantial frontal cortical activity is required to keep attention and cognitive resources focused on completion of the speech and arithmetic tasks, competition for limited cognitive control resources may have made it more difficult to recall, access, and utilize the coping tools provided. In the pharmacological paradigms, the task itself, to passively receive a drug infusion, imposed no cognitive demands. Available cognitive and attentional resources could be devoted to utilizing the coping tools (“I am in control, I can cope”) to facilitate a re-appraisal process that successfully shifted the subjective meaning of the novel drug exposure and associated drug side effects.
The failure of our Control and Coping interventions to reduce endocrine responses to the TSST stands somewhat in contrast to evidence that more prolonged training in stress management techniques can reduce salivary Cortisol responses to a subsequently administered TSST (Gaab et al., 2005; Storch et al., 2007). The interventions used in these studies had parallels to our control and coping intervention content in their use of concepts like “stress appraisals,” “control expectancies,” “activated controllability,” “goal achievement,” and “volitional control of unwanted emotions, cognitions, or behaviors.” This evidence supports the relevance of these cognitive phenomena to stress system reactivity, even though our application of similar concepts had no impact in this study. However, the design and goals of these studies were substantially different from ours, making direct comparisons difficult. Their focus was on general stress reduction training, delivered over a substantially longer period of time, with the stress test administered after a significant time delay, trying to show that stress management training can produce sustained reductions in biological stress reactivity. Our focus, in contrast, was on acute manipulation of stress system reactivity to determine immediate, psychological modulators of its activity levels, using very brief interventions to directly alter cognitive sets in a stress test administered immediately after the interventions.
The effectiveness of the compassionate goals intervention demonstrates that the HPA response to TSST is amenable to acute cognitive modulation, suggests that effective coping tools might be more effective if they are threat specific, and perhaps helps identify factors that are more salient to this type of stressor, though follow-up work is clearly needed to empirically identify the active ingredients. It did not work by reducing subjective anxious distress, fear of negative evaluation, efforts to prove oneself, or observed self-focus, since there were no significant group differences on these variables. The measures that were impacted were those consistent with the aim of the intervention – it increased subjectively reported focus on “helping others” and “making a meaningful contribution” and increased behaviors that blind observers rated as more affiliative and other-oriented. The intervention was based on evidence that pursuing compassionate goals in daily life (striving to support others in interpersonal contexts) is linked to increased belief in interconnectedness, reduced competitiveness, and heightened perception of available social support (Crocker and Canevello, 2008). Both reduced competitiveness (e.g., Edwards et al., 2006) and enhanced perceptions of social support can moderate HPA axis activity (Heinrichs et al., 2003; Kirschbaum et al., 1995; Levine, 2000; Rosal et al., 2004; Turner-Cobb et al., 2000). In either case, thinking about others may have particular psychological salience in the context of social evaluative threat, and may activate pathways with inhibitory input to the HPA axis. This may involve direct inhibitory input from prefrontal brain areas that process social information. It could also involve an hypothesized “caregiving system” (Numan, 2006) that facilitates social bonding and dampens HPA axis reactivity (Brown et al., 2011; Davidson and McEwen, 2012; Konrath and Brown, 2012; Taylor et al., 2000; Wang, 2005).
Supportive evidence comes from neuroimaging studies of charitable decision making. Decisions to give to others in these paradigms involve social cognitions that shift attention from one’s own states to the needs of others, activating brain regions associated with empathy (anterior insula) and attention control (posterior superior temporal cortex), integrated through valuation areas (ventral medial prefrontal cortex – vmPFC; Hare et al., 2010). The vmPFC, which integrates conceptual/emotional information important for contextual valuation, has regulatory control projections to affective, autonomic, and endocrine effector systems including the hypothalamus (Roy et al., 2012), inhibiting brain areas activated by aversive cues, while increasing activity in reward regions (Quirk et al., 2000; Roy et al., 2012; Wager et al., 2008). Similarly, our studies of brain regions involved in HPA axis regulation have shown that rostral mPFC processes social emotions (Britton et al., 2006) and also inhibits ACTH responses to stressful stimuli (King et al., 2009). Less direct pathways could involve effects of “other orientation” on subgenual PFC, which modulates septo-hypothalamic function in social attachment (Moll et al., 2006) and elicits oxytocin release (Depue and Morrone-Strupinsky, 2005; Young and Wang, 2004), which in turn has been shown to reduce Cortisol response to the TSST, in interaction with social support (Heinrichs et al., 2003). Thus, there is documentation of neural pathways through which social processing regions can directly inhibit regions involved in stress response activation, and through which attention, appraisal, and valuation may be integrated to attach reward value to giving to others in ways that would down-regulate endocrine stress responses. The fact that our compassionate goals intervention reduced ACTH and Cortisol, while enhancing affiliative emotions and behavior without reducing subjective anxious distress, fear of negative evaluation, efforts to “prove oneself”, or observed self-focus is quite striking and suggests that the creation of a pro-social orientation can be stress reducing even in the face of the anxiety, subjective stress, self-focus, self-promotion, and fear of social evaluation that is purposefully elicited by the TSST. Further work is needed to empirically test speculations about mechanisms and identify the specific psychological factors and neural pathways through which compassion can reduce stress system activation. Further work is also needed to determine whether such stress system effects produce health benefits even when subjectively experienced stress is not reduced.
Several limitations of the present study bear noting. The sample was small and replication is clearly needed. However, the cell sizes were planned on the basis of power analyses on preliminary data. Calculated effect sizes for main analyses (see Section 3) were in the moderate to large range, as predicted, so we had enough power to confirm the key hypothesis about the Compassionate Goals Intervention. The small sample may account for the non-significant result for the Coping Intervention, but the effect size here suggests that an inordinately large sample would be needed to achieve statistical significance (groups of 80 or more). A replication attempt with a sample of this magnitude would not be feasible, though testing a redesigned Coping Intervention (to make it more powerful) would be worthwhile before concluding that coping manipulation cannot impact TSST responses. There are sex differences in various aspects of TSST responding, including the impact of social support (Kirschbaum et al., 1995), but our study was not adequately powered to test for sex-by-group effects. However, studies examining the impact of compassionate goals on other outcomes have not found consistent moderating effects of sex (Crocker and Canevello, 2012); and our central findings held when we controlled for it. “Compassionate Goals” may be stress reducing in the TSST for both men and women, but our data are highly preliminary in that regard and better-powered studies are needed to definitively test for sex differences. Also, as noted, the present report cannot definitively identify mediators of the effect of compassionate goals on HPA responses. Future studies directed toward this aim should experimentally control for alternative explanations such as threats to social dominance (Virgin and Sapolsky, 1997), generic positive emotions (Fredrickson et al., 2000), or cognitive load (distraction). Lastly, effects of compassionate goals may depend upon or be amplified by particular personality traits or perceptions (e.g., Cosley et al., 2010) so moderation or moderated mediation effects should be explored.
Despite limitations and the need for follow-up work, this study provides experimental evidence that enhancing compassionate goals can modulate HPA responses to the TSST. These data lend support to recent scientific interest in compassion meditation and loving-kindness meditation (Davidson and McEwen, 2012; Fredrickson et al., 2000; Pace et al., 2009), interventions that foster compassionate thoughts and feelings over the course of many (e.g., 10) weeks and reduce psychological stress. However, our compassionate goals intervention exerted noteworthy stress-buffering effects despite requiring only a few minutes of coaching, whereas studies of longer-term interventions have not yet detected TSST effects (Pace et al., 2009). In addition to being shorter, our intervention also differed from meditation interventions studied by others in its concrete focus on a goal shift toward helping others, rather than on eliciting a general feeling of compassion or loving-kindness toward others. Behavioral intention toward helping, affiliation, and cooperation may be more salient to the HPA axis than emotional states of caring. Our data suggest that brief stress inoculation tools could potentially be developed to reduce Cortisol release in the face of certain predictable stressors, but also suggest that generic stress reducers may be less effective than tools that are designed to address the specific psychological salience of a given threat and/or that are focused on behavioral intentions that foster a sense of connection or affiliation. These data are also consistent with the hypothesis that the health benefits of volunteerism (Konrath and Brown, 2012) may be at least partly mediated through the buffering effects of giving to others on neuroendocrine stress activation. Finally, although the present study employed healthy participants, it has potential relevance to stress-related illnesses by illuminating potential ways to moderate biological effects of stressful experiences. It may also have additional psychiatric relevance. HPA axis hyperactivity is well-established in depression (Pariante and Lightman, 2008) and embracing compassionate goals can reduce symptoms associated with depression (e.g., Crocker et al., 2010), supporting exploration of the hypothesis that interventions designed to foster compassionate goal orientations might reduce both symptoms and hypercortisolemia in patients with depression.
Acknowledgements
These data could not have been collected without the skilled assistance of Claudia White and Erin McRobert, the numerous volunteers who served on the TSST panel, and the expert help of the Michigan Clinical Research unit and its superb staff.
Role of the funding source
This work was supported by the National Institute of Mental Health (R01 MH074852) and by the National Center for Research Resources (UL1RR024986), and is now at the National Center for Advancing Translational Sciences (2UL1TR000433). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest statement
None of the authors have any actual or potential conflict of interest related to the findings of this study.
References
- Abelson JL, Khan S, Liberzon I, Erickson TM, Young EA. Effects of perceived control and cognitive coping on endocrine stress responses to pharmacological activation. Biol. Psychiatry. 2008;64:701–707. doi: 10.1016/j.biopsych.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abelson JL, Khan S, Young EA, Liberzon I. Cognitive modulation of endocrine responses to CRH stimulation in healthy subjects. Psychoneuroendocrinology. 2010;35:451–459. doi: 10.1016/j.psyneuen.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abelson JL, Liberzon I, Young EA, Khan S. Cognitive modulation of the endocrine stress response to a pharmacological challenge in normal and panic disorder subjects. Arch. Gen. Psychiatry. 2005;62:668–675. doi: 10.1001/archpsyc.62.6.668. [DOI] [PubMed] [Google Scholar]
- Adam EK, Kumari M. Assessing salivary Cortisol in large-scale, epidemiological research. Psychoneuroendocrinology. 2009;34:1423–1436. doi: 10.1016/j.psyneuen.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Britton JC, Phan KL, Taylor SF, Welsh RC, Berridge KC, Liberzon I. Neural correlates of social and nonsocial emotions: an fMRI study. Neuroimage. 2006;31:397–409. doi: 10.1016/j.neuroimage.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Brown SL, Brown RM, House JS, Smith DM. Coping with spousal loss: potential buffering effects of self-reported helping behavior. Pers. Soc. Psychol. Bull. 2008;34:849–861. doi: 10.1177/0146167208314972. [DOI] [PubMed] [Google Scholar]
- Brown SL, Brown RM, Preston SD. A model of human caregiving motivation. In: Brown SL, Brown RM, Penner LA, editors. Moving Beyond Self Interest: Perspectives from Evolutionary Biology, Neuroscience, and the Social Sciences. New York: Oxford University Press; 2011. pp. 75–88. [Google Scholar]
- Burson A, Crocker J, Mischkowski D. Two types of value-affirmation implications for self-control following social exclusion. Soc. Psychol. Person. Sci. 2012;3:510–516. [Google Scholar]
- Carroll BJ, Feinberg M, Greden JF, Tarika J, Albala AA, Haskett RF, James NM, Kronfol Z, Lohr N, Steiner M, de Vigne JPEY. A specific laboratory test for the diagnosis of melancholia: standardization, validation, and clinical utility. Arch. Gen. Psychiatry. 1981;38:15–22. doi: 10.1001/archpsyc.1981.01780260017001. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW. Editorial: a healthy body in a healthy mind - and vice versa - the damaging power of uncontrollable stress. J. Clin. Endocrinol. Metab. 1998;83:1842–1845. doi: 10.1210/jcem.83.6.4908. [DOI] [PubMed] [Google Scholar]
- Cosley BJ, McCoy SK, Saslow LR, Epel ES. Is compassion for others stress buffering? Consequences of compassion and social support for physiological reactivity to stress. J. Exp. Soc. Psychol. 2010;46:816–823. [Google Scholar]
- Crocker J, Canevello A. Creating and undermining social support in communal relationships: the role of compassionate and self-image goals. J. Pers. Soc. Psychol. 2008;95:555–575. doi: 10.1037/0022-3514.95.3.555. [DOI] [PubMed] [Google Scholar]
- Crocker J, Canevello A. Consequences of self-image and compassionate goals. In: Devine PG, Plant A, editors. Advances in Experimental Social Psychology. New York: Elsevier; 2012. pp. 229–277. [Google Scholar]
- Crocker J, Canevello A, Breines JG, Flynn H. Interpersonal goals and change in anxiety and dysphoria in first-semester college students. J. Pers. Soc. Psychol. 2010;98:1009–1024. doi: 10.1037/a0019400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ, McEwen BS. Social influences on neuroplasticity: stress and interventions to promote well-being. Nat. Neu-rosci. 2012;15:689–695. doi: 10.1038/nn.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depue RA, Morrone-Strupinsky JV. A neurobehavioral model of affiliative bonding: implications for conceptualizing a human trait of affiliation. Behav. Brain Sci. 2005;28:313–349. doi: 10.1017/S0140525X05000063. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and Cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol. Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Edwards DA, Wetzel K, Wyner DR. Intercollegiate soccer: saliva Cortisol and testosterone are elevated during competition, and testosterone is related to status and social connectedness with teammates. Psychol. Behav. 2006;87:135–143. doi: 10.1016/j.physbeh.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Webster EL, Torpy DJ, Chrousos GP. Stress, corticotropin-releasing hormone, glucocorticoids, and the immune/inflammatory response: acute and chronic effects. Ann. N. Y. Acad. Sci. 1999;876:1–11. doi: 10.1111/j.1749-6632.1999.tb07618.x. [DOI] [PubMed] [Google Scholar]
- Fredrickson BL, Mancuso RA, Branigan C, Tugade MM. The undoing effect of positive emotions. Motiv. Emotion. 2000;24:237–258. doi: 10.1023/a:1010796329158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaab J, Rohleder N, Nater U, Ehlert U. Psychological determinants of the Cortisol stress response: the role of anticipatory cognitive appraisal. Psychoneuroendocrinology. 2005;30:599–610. doi: 10.1016/j.psyneuen.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Knoepfle DT, O’Doherty JP, Rangel A. Value computations in ventral medial prefrontal cortex during charitable decision making incorporate input from regions involved in social cognition. J. Neurosci. 2010;30:583–590. doi: 10.1523/JNEUROSCI.4089-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress Cortisol and subjective responses to psychosocial stress. Biol. Psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Grossman P, Hinton DE. Loving-kindness and compassion meditation: potential for psychological interventions. Clin. Psychol. Rev. 2011;31:1126–1132. doi: 10.1016/j.cpr.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AP, Abelson JL, Britton JC, Phan KL, Taylor SF, Liberzon I. Medial prefrontal cortex and right insula activity predict plasma ACTH response to trauma recall. Neuroimage. 2009;47:872–880. doi: 10.1016/j.neuroimage.2009.05.088. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Klauer T, Filipp S-H, Hellhammer DH. Sex-specific effects of social support on Cortisol and subjective responses to acute psychological stress. Psychosom. Med. 1995;57:23–31. doi: 10.1097/00006842-199501000-00004. [DOI] [PubMed] [Google Scholar]
- Konrath S, Brown SL. The effects of giving on givers. In: Roberts N, Newman M, editors. Handbook of Health and Social Relationships. Washington, DC: American Psychological Association; 2012. [Google Scholar]
- Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Meaney MJ, Plotsky PM. Long-term behavioral and neuroendocrine adaptations to adverse early experience. Prog. Brain Res. 1999;122:81–103. doi: 10.1016/s0079-6123(08)62132-9. [DOI] [PubMed] [Google Scholar]
- Levine S. Influence of psychological variables on the activity of the hypothalamic-pituitary-adrenal axis. Eur. J. Pharmacol. 2000;405:149–160. doi: 10.1016/s0014-2999(00)00548-3. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. N. Engl. J. Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- Moll J, Krueger F, Zahn R, Pardini M, de Oliveira-Souza R, Grafman J. Human fronto-mesolimbic networks guide decisions about charitable donation. Proc. Natl. Acad. Sci. U. S. A. 2006;103:15623–15628. doi: 10.1073/pnas.0604475103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numan M. Hypothalamic neural circuits regulating maternal responsiveness toward infants. Behav. Cogn. Neurosci. Rev. 2006;5:163–190. doi: 10.1177/1534582306288790. [DOI] [PubMed] [Google Scholar]
- Pace TWW, Negi LT, Adame DD, Cole SP, Sivilli TI, Brown TD, Issa MJ, Raison CL. Effect of compassion meditation on neuroendocrine, innate immune and behavioral responses to psychosocial stress. Psychoneuroendocrinology. 2009;34:87–98. doi: 10.1016/j.psyneuen.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008;31:464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J. Neurosci. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosal MC, King J, Ma Y, Reed GW. Stress, social support, and Cortisol: inverse associations? Behav. Med. 2004;30:11–22. doi: 10.3200/BMED.30.1.11-22. [DOI] [PubMed] [Google Scholar]
- Rosmond R, Bjorntorp P. The hypothalamic-pituitary-adrenal axis activity as a predictor of cardiovascular disease, type 2 diabetes and stroke. J. Intern. Med. 2000;247:188–197. doi: 10.1046/j.1365-2796.2000.00603.x. [DOI] [PubMed] [Google Scholar]
- Rosmond R, Dallman MF, Bjorntorp P. Stress-related Cortisol secretion in men: relationships with abdominal obesity, endocrine, metabolic, and hemodynamic abnormalities. J. Clin. Endocrinol. Metab. 1998;83:1853–1859. doi: 10.1210/jcem.83.6.4843. [DOI] [PubMed] [Google Scholar]
- Roy M, Shohamy D, Wager TD. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends Cogn. Sci. 2012;16:147–156. doi: 10.1016/j.tics.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz SH, Boehnke K. Evaluating the structure of human values with confirmatory factor analysis. J. Res. Pers. 2004;38:230–255. [Google Scholar]
- Smith AM, Loving TJ, Crockett EE, Campbell L. What’s closeness got to do with it? Men’s and women’s Cortisol responses when providing and receiving support. Psychosom. Med. 2009;71:843–851. doi: 10.1097/PSY.0b013e3181b492e6. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL. STAI Manual. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- Storch M, Gaab J, Kuttel Y, Stussi AC, Fend H. Psy-choneuroendocrine effects of resource-activating stress management training. Health Psychol. 2007;26:456. doi: 10.1037/0278-6133.26.4.456. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Gurung RAR, Updegraff JA. Biobehavioral responses to stress in females: tend-and-befriend, not fight-or-flight. Psychol. Rev. 2000;107:411–429. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- Turner-Cobb JM, Sephton SE, Koopman C, Blake-Mortimer J, Spiegel D. Social support and salivary Cortisol in women with metastatic breast cancer. Psychosom. Med. 2000;62:337–345. doi: 10.1097/00006842-200005000-00007. [DOI] [PubMed] [Google Scholar]
- Uchino BN, Cacioppo JT, Kiecolt-Glaser JK. The relationship between social support and physiological processes: a review with emphasis on underlying mechanisms and implications for health. Psychol. Bull. 1996;119:488–531. doi: 10.1037/0033-2909.119.3.488. [DOI] [PubMed] [Google Scholar]
- Virgin CE, Sapolsky RM. Styles of male social behavior and their endocrine correlates among low-ranking baboons. Am. J. Primatol. 1997;42:25–39. doi: 10.1002/(SICI)1098-2345(1997)42:1<25::AID-AJP2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. A conceptual framework for integrating research related to the physiology of compassion and the wisdom of Buddhist teachings. In: Gilbert P, editor. Compassion: Conceptualisations, Research, and Use in Psychotherapy. New York: Routledge; 2005. pp. 75–120. [Google Scholar]
- Young EA, Lopez JF, Murphy-Weinberg V, Watson SJ, Akil H. Mineralocorticoid receptor function in major depression. Arch. Gen. Psychiatry. 2003;60:24–28. doi: 10.1001/archpsyc.60.1.24. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z. The neurobiology of pair bonding. Nat. Neurosci. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]