Abstract
Drosophila hemocytes are essential for the animal to resist Staphylococcus aureus infections. Phagocytosis is a central component of the hemocyte-mediated immune response. It involves regulated interaction between the phagocytic and the endocytic compartments. Rab GTPases are pivotal for the membrane trafficking and fusion events, and thus are often targets of intracellular pathogens that subvert phagocytosis. An in vivo screen identified Rab2 and Rab14 as candidates for proteins regulating phagosome maturation. Since Rab14 is often targeted by intracellular pathogens, an understanding of its function during phagocytosis and the overall immune response can give insight into the pathogenesis of intracellular microbes. We generated a Drosophila Rab14 mutant and characterized the resulting immune defects in animals and specifically in hemocytes in response to an S. aureus infection. Hemocyte based immunofluorescence studies indicate that Rab14 is recruited to the phagosome and like Rab7, a well-characterized regulator of the phagocytic pathway, is essential for progression of phagosome maturation. Rab14 mutant hemocytes show impaired recruitment of Rab7 and of a lysosomal marker onto S. aureus phagosomes. The defect in phagocytosis is associated with higher bacterial load and increased susceptibility to S. aureus in the animal.
Introduction
Drosophila hemocytes play an important role during the immune response against the extracellular pathogen Staphylococcus aureus (Nehme et al., 2011). Hemocytes are phagocytic and are functionally analogous to the mammalian macrophage. The uptake of a microbe by a phagocytic cell is followed by fusion of the microbe-containing phagosome with the early endosome, the late endosome and eventually with the lysosome, resulting in microbial clearance (Desjardins et al., 1994). A comparison of the proteomes found that 70% of the mammalian phagosome proteins were also found on Drosophila S2 cell phagosomes (Garin et al., 2001, Stuart et al., 2007). Hence, much of the phagocytic machinery, that includes proteins necessary for signaling and membrane trafficking following uptake, is likely conserved between Drosophila and mammals.
RabGTPases, members of the Ras superfamily of small GTPases, are required to maintain specificity during the fusion processes (Vieira et al., 2002). At different stages of maturation, phagosomes are marked by the transient association of RabGTPases, Rab5 and Rab7 (Desjardins et al., 1994) which in turn are essential for progression of phagosome maturation (Alvarez-Dominguez et al., 1999, Rupper et al., 2001, Harrison et al., 2003). A wide array of RabGTPases are associated with phagosomes (Garin et al., 2001, Stuart et al., 2007), and each presumably contribute to phagolysosome biogenesis. However, apart from Rab5 and Rab7, there is a limited understanding of the function of other RabGTPases in the process. Also it is not known how a defect in phagosome maturation affects the overall immune response of a host. Since hemocytes have been demonstrated to be critical against S. aureus, the Drosophila infection model provides an ideal system to gain such insights.
From a phagosome maturation screen of adults expressing dominant negative (DN) versions of each of the phagosomal RabGTPases, we identify Rab14 as a potential regulator of S. aureus phagosome maturation. Rab14 is known to be targeted by intracellular pathogens (Kyei et al., 2006, Capmany et al., 2010, Huang et al., 2010, Seixas et al., 2012), but its function within the cell is not well understood. Our study examines the role of Rab14 during the cellular responses in Drosophila. Cell biological studies indicated that Rab14 localizes to early endosomes and also, to Rab7-positive late endosomes. The localization is consistent with its likely function, as endosomal progression is impaired in the absence of Rab14. Since endocytic and phagocytic machinery overlaps, we hypothesized that Rab14 actively regulates phagosome maturation at an essential stage and this may explain why it is actively targeted by intracellular pathogens. This is consistent with our finding that Rab14 regulates fusion of phagosmes with the late endosomes and lysosomes Our study also demonstrates that Rab14 function is critical for the animal’s ability to control bacterial loads during a systemic infection, thus demonstrating the central role of phagocytosis during the Drosophila immune response to S. aureus.
Results
A screen identifies Rab14 as a potential regulator of phagosome maturation
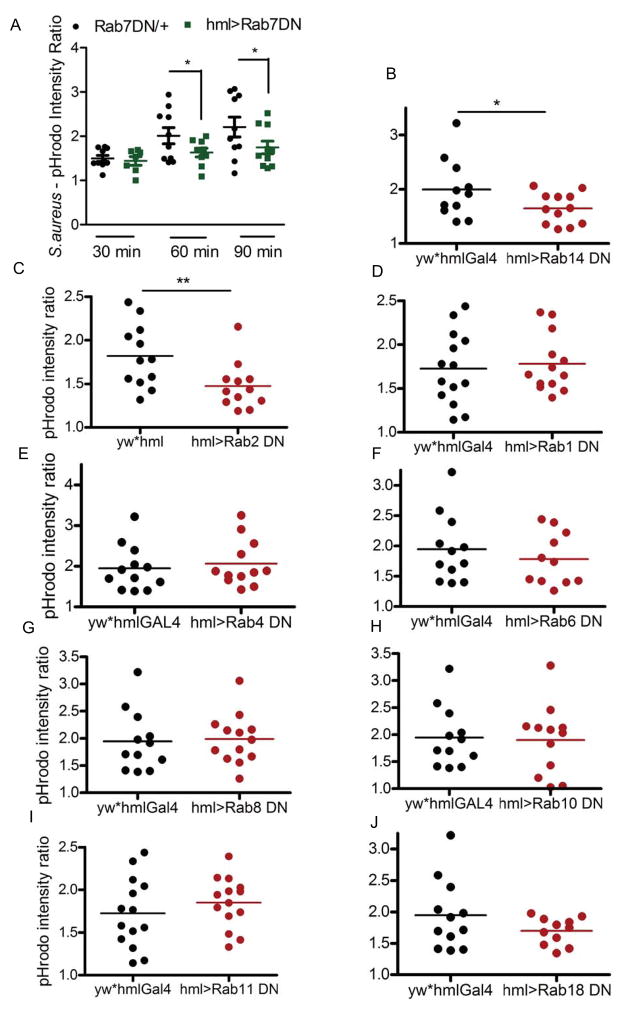
In an attempt to identify the RabGTPases that regulate phagosome maturation, we screened all of the RabGTPases associated with latex bead phagosomes that had not been previously characterized as a regulator of phagocytosis in a metazoan system (Stuart et al., 2007). Drosophila has around 33 Rab genes (Zhang et al., 2007) and the proteomic data indicated the presence of 12 RabGTPases on the phagosome: Rab1, 2, 4, 5, 6, 7, 8, 10, 11, 14, 18, 35. Scott and group (Zhang et al., 2007) have generated transgenic fly stocks of DN- mutants of the individual RabGTPases. Utilizing those lines, we expressed DN-RabGTPases specifically in the hemocytes. Thereafter, the adult flies were assessed for the effect on phagosome maturation of pHrodo-conjugated S. aureus. The pHrodo dye is sensitive to pH: it is non-fluorescent at neutral pH and its fluorescence intensity increases with an increase in acidity. The progression of phagosome maturation involves fusion of a microbe-containing phagosome with increasingly acidic compartments. Thus, following uptake by the hemocytes, as maturation progresses, pHrodo-conjugated microbes show an increase in fluorescence intensity. In Drosophila, the hemocytes along the dorsal vessel can be visualized through the cuticle and provide a means to monitor phagocytosis and maturation in vivo (Elrod-Erickson et al., 2000). Rab7 is a well characterized regulator of maturation; the pHrodo conjugates of S. aureus displayed slower kinetics of maturation, which was notable at 60 and 90 min post-injection in adults expressing a dominant negative (DN) form of Rab7 in hemocytes (Fig. 1A).
Figure 1. Identification of regulators of phagosome maturation.
(A) Rab7 was used to validate the pHrodo-based phagosome maturation assay. The Rab7DN line was outcrossed to either w1118 (control) or hmlGAL4 for hemocyte-specific expression of the mutant protein. Adult flies were injected with pHrodo-S. aureus and examined at 30, 60 or 90 min post-injection. The fluorescence intensity in an area around the dorsal vessel and background fluorescence was quantified, and the ratio was used to plot the scatterplot. Each dot represents the intensity associated with one fly. For the screen, adult flies were injected with pHrodo S. aureus and imaged after 60 min. The DN lines were crossed to hmlGAL4. The parental line of the dominant-negative expressing line (yw) outcrossed to the hemolectin driver line was used as the control. Representative dot plots for: (B) Rab14, n=6 (C) Rab2, n=4 (D) Rab1, n=3 (E) Rab4, n=3 (F) Rab6, n=3 (G) Rab8, n=5 (H) Rab10, n=3 (I) Rab11, n=3 and (J) Rab18, n=3 are shown. n= number of experimental replicates * p < 0.05 ** p < 0.01.
Rab14 and Rab2 (Fig. 1B, C) were identified as potential regulators of phagocytic uptake and/or phagosome maturation in the screen. Earlier RNAi-based studies (Shim et al., 2010) found no effect on phagocytic uptake of E. coli particles by S2 cells, after downregulation of any of the Rabs that we tested. This suggests that the demonstrated decreased pHrodo intensity in Rab2 and Rab14 DN mutants could be due to defects in maturation. Rab2 and Rab14 have been shown to regulate apoptotic cell phagosome maturation in C. elegans (Guo et al., 2010). We chose to pursue Rab14 as little is known about is function and it has been proposed to be involved with the pathogenesis of the intracellular pathogen M. tuberculosis (Kyei et al., 2006).
Rab14 positively regulates maturation of phagosomes containing Staphylococcus aureus
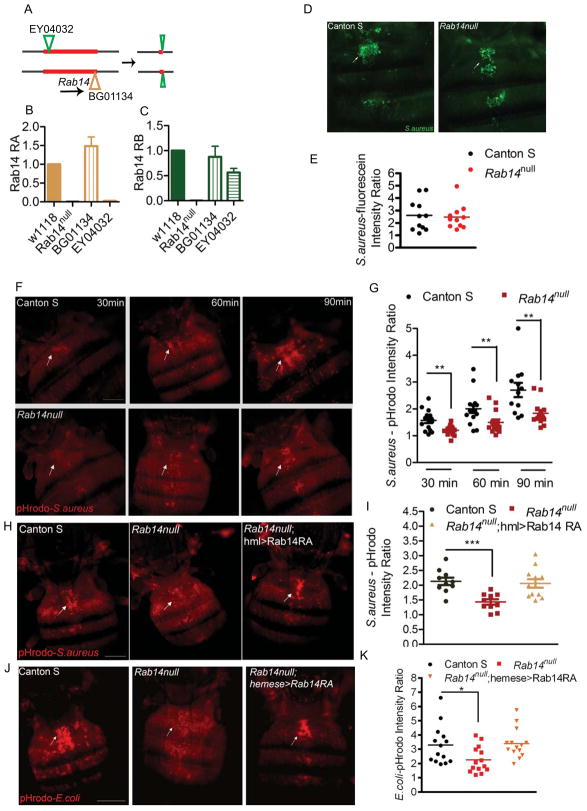
The Drosophila genome encodes three different Rab14 isoforms; RA, RB and RC. RNA expression analyses detected only the RA and RB isoforms in adults (Fig. S1A). The three isoforms show 81% identity to human Rab14 and 100% identity to each other in a core region shared among all isoforms. To investigate the role of Rab14 during phagosome maturation, we generated a Rab14 mutant. Two transposon lines, EY04032 and BG01134 conveniently flanked the Rab14 gene, allowing the generation of a Rab14 mutant using transposase-induced recombination (Fig. 2A). Sequencing of the interval in the mutant chromosome indicated two excision sites: one within the first transposon EY04032 and the other at the end of the second transposon BG01134. The joining of these two ends resulted in a deletion of the intervening sequence and generation of a Rab14null allele (Fig. 2B, C). The Rab14 mutants showed no apparent morphological defects and were fertile.
Figure 2. Rab14 is required for phagosome maturation of S. aureus.
(A) Transposase-induced recombination between the transposons EY04032 and BG01134 in males led to the generation of a Rab14 mutant. Flies carrying the two Rab14 transposon insertions, EY04032 and BG01134, and a transposase-expressing transgene were generated. Transposase-induced mitotic recombination in males (Chen et al., 1998) led to the generation of a chromosome lacking Rab14. After mating, male flies were screened to select for recombined chromosomes using phenotypic markers associated with the two transposons. The potential null mutants were screened and a Rab14 mutant was identified. The genomic DNA between the two transposons was lost from the mutant along with the transposon BG01134. However a small segment of EY04032 was retained in the mutant. (B, C) The Rab14 expression is not detectable in the Rab14 mutant indicating it is a null allele. Quantitative-real time PCR was used to examine expression of Rab14 isoforms (B) RA and (C) RB in wildtype, Rab14null, BG01134 and EY04032 mutant adults. (D, E) Rab14 mutants are not affected for phagocytic uptake of S. aureus. Representative images and quantification of fluorescence intensity of wildtype and the Rab14 mutant to examine the uptake of fluorescein-conjugated S. aureus 30 min post injection. (F, G) Rab14 is a positive regulator of phagosome maturation. The phagosome maturation assay was carried out in wildtype and Rab14 mutants as described for Rab7DN mutants. (F) A representative image for each timepoint and (G) a scatterplot of the quantified images is shown. Rab14 function in the hemocyte is essential for phagosome maturation. Rescue of (H, I) S. aureus or (J, K) E. coli phagosome maturation in the Rab14 mutant was examined upon hemocyte-specific expression of Rab14 (Rab14null; hml>Rab14RA or Rab14null; hemese>Rab14RA). For rescue, the flies were imaged 60 min postinjection. Phagocytosis experiments were repeated at least 3 times with 8–12 flies in each experiment. The arrows indicate dorsal vessel-associated hemocytes which were examined and quantified. Data was analyzed by unpaired t-test and asterisks on the graphs indicate p-values of significance: * p<0.05, ** p<0.01, *** p<0.001. Scale bar, 0.2mm
To assess what role Rab14 might be playing in hemocytes, we examined phagocytic uptake and phagosome maturation in adult Rab14 mutant flies. To examine phagocytic uptake, flies were injected with fluorescein-conjugated S. aureus and the intracellular fluorescence along the dorsal vessel was evaluated after 30 min. The uptake of fluorescein-conjugated S. aureus (Fig. 2D) or E. coli (Fig. S1B) was comparable in wildtype and the Rab14 mutant. Quantification of the fluorescence associated with the dorsal vessel (Fig. 2E, S1C) further confirmed that Rab14 is not required for initial phagocytic uptake. This result is consistent with the work of Kim and colleagues, who carried out a phagocytosis screen to identify RabGTPases involved in the uptake of E. coli in Drosophila S2 cells (Shim et al., 2010). They found that down-regulation of Rab14 does not affect the uptake of E. coli.
Next, we examined phagosome maturation in Rab14 mutant flies using pHrodo-conjugated microbial particles (Cuttell et al., 2008). Rab14 mutants showed defect at 30, 60 and 90 min post-injection of pHrodo-S. aureus (Fig. 2F, G). The mutant displayed slower kinetics of maturation instead of a complete block which could be due to redundancy with other RabGTPases. The defect in maturation of S. aureus phagosomes observed in Rab14 mutants could be rescued with hemocyte-specific expression of Rab14 (Fig. 2H, I). Rab14 also plays an essential role in maturation of E. coli phagosomes (Fig. 2J, K). Hence the phagocytic machinery seems to be conserved for these different classes of bacteria.
The rescue of the maturation defect in Rab14 mutants by hemocyte-specific expression of Rab14 indicates its function in the hemocyte is critical for phagosome maturation. To determine if there might be differences between the Rab14 isoforms RA and RB, we examined the localization of the fluorophore-tagged proteins in larval hemocytes (Fig. S1D). Both the isoforms showed similar localization patterns suggestive of overlapping functions.
Rab14 mutant phagosomes show decreased fusion with lysosomes
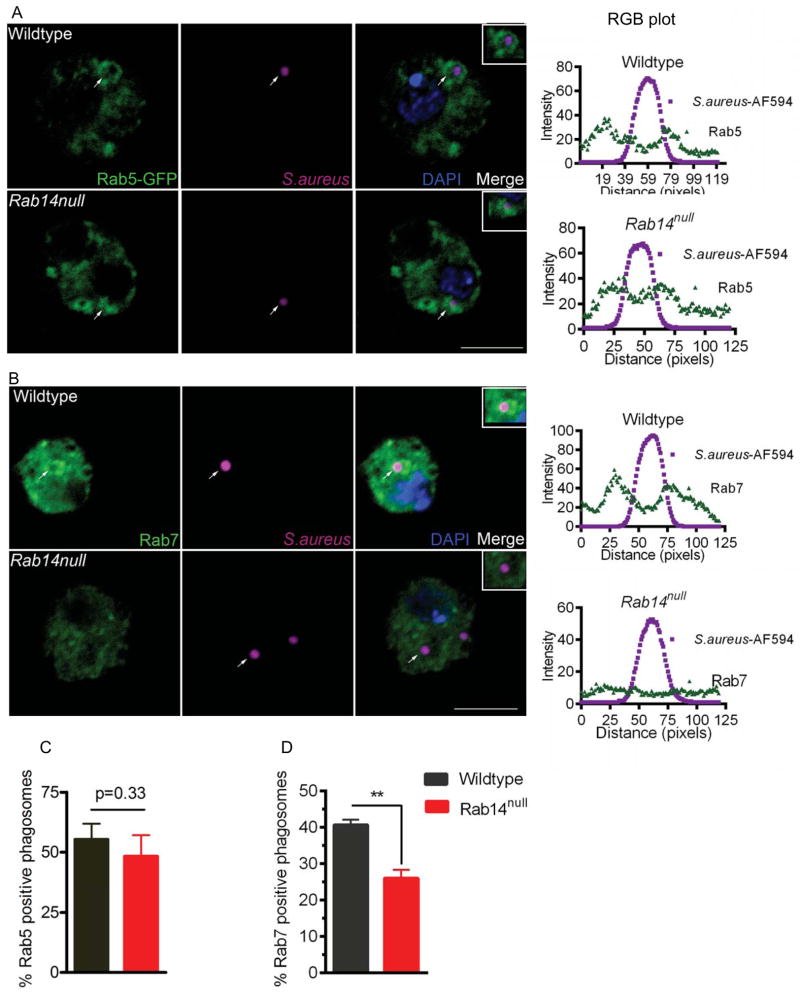
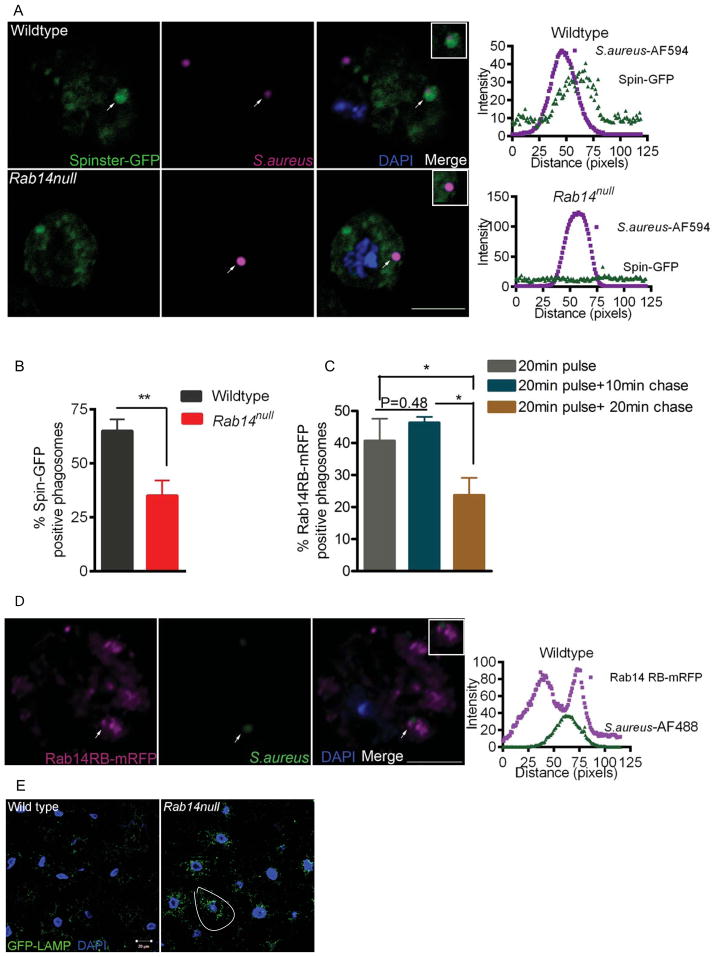
The newly formed phagosome interacts with the early endosome, late endosome and then the lysosome, which leads to acidification of the phagosome (Vieira et al., 2002). The recruitment of the early endosomal marker Rab5 and late endosomal marker Rab7 are essential for this sequential maturation. To understand the role of Rab14, we asked at what stage is phagosome maturation affected in Rab14 mutant hemocytes. We examined the recruitment of Rab5, Rab7 and the lysosomal marker Spinster (Sweeney et al., 2002) onto the S. aureus phagosome in hemocytes from wildtype and Rab14 mutants. The recruitment of Rab5 or Rab7 is characterized by the presence of the protein on the phagosomal membrane surrounding the bacteria and can be examined by immunofluorescence. An analysis of the fluorescence intensity 3 an RGB plot of the image, wherein the endosomal marker signal is observed relative to the microbial signal, provides an unbiased means to assess co-localization. Additionally, the percent recruitment for an individual marker was calculated by taking the ratio of the number of S. aureus phagosomes showing recruitment of the marker over the total number of phagosomes assessed. After a 20 min pulse, phagosomes from both wildtype and Rab14 mutant hemocytes recruited Rab5 in a comparable manner, as assessed by RGB plots of Rab5-S. aureus co-localization (Fig. 3A) and by the fraction of S. aureus phagosomes containing Rab5 (Fig. 3C). These data suggest that phagosome maturation is not affected at the early stages in the absence of Rab14. Next, we looked at Rab7-positive phagosomes after a 10 min chase following the 20 min pulse. When compared to wildtype, Rab14 mutant cells showed significantly lower levels of Rab7 recruitment to S. aureus phagosomes (40.6±1.4% and 26.0±2.3% recruitment, respectively) (Fig. 3B, D). Lastly, the phagosome-lysosome fusion was assessed by examining localization of Spinster onto the phagosomes containing S. aureus after a 20 min chase following the 20 min pulse. Whereas relatively high numbers of bacteria showed co-localization with the lysosomal marker Spinster in wildtype hemocytes, significantly less Spinster-positive phagosomes were observed in Rab14 mutant cells (65.0±5.4% and 35.0±7.1% recruitment, respectively) (Fig. 4A, B). These data suggest that Rab14 plays an important role in formation of phagolysosomes. The recruitment of Rab7 onto phagosomes is essential for its subsequent fusion with late endosomes and lysosomes (Rupper et al., 2001). This indicates that the defect in maturation in the Rab14 mutant could be due to the reduced fusion of phagosomes with late endosomes and lysosomes
Figure 3. Rab14 mutant phagosomes demonstrate reduced recruitment of a late phagosomal marker.
(A) Wildtype and Rab14 mutant phagosomes show comparable recruitment of the early endosomal marker Rab5. Hemocytes expressing Rab5-GFP (hmlGAL4) were bled out and fixed from wildtype and Rab14 mutant larvae after a 20 min pulse with S. aureus conjugated to AF-594 (magenta). Hemocytes were evaluated for Rab5 (green) recruitment onto phagosomes. The merged image includes Rab5 (green), S. aureus (magenta) and DNA/DAPI (blue). Enrichment of marker-associated fluorescence in the vicinity of bacteria was considered positive for recruitment. An RGB plot for a 120 pixel line across the phagosome was plotted using image analysis software (ImageJ). The arrows on the merged image indicate the phagosome of interest utilized to make the RGB plot. (B) Rab14 mutant phagosomes show a reduced presence of Rab7. A 20 min pulse with S. aureus-AF-594 followed by a 10 chase with Schneider’s media was given to wildtype and Rab14 mutant hemocytes which were then immunostained for Rab7. Rab7 recruitment onto the phagosomes was analyzed. (C, D) Percentages of phagosomes showing recruitment of (C) Rab5 and (D) Rab7 are plotted for wildtype and Rab14null. The experiments were conducted 3 times with 10–12 larvae in each experiment. Images were obtained using confocal microscopy and a total of 70–90 phagosomes were examined for quantification. Error bars indicate standard error of the mean (SEM), n=3. Data was analyzed by two tailed, paired t-test. ** p<0.01. Scale bar, 5μm
Figure 4. Rab14 is recruited to the phagosome and is required for efficient phagolysosome biogenesis.
(A) Rab14 mutant phagosomes show reduced fusion with lysosomes. Hemocytes expressing the lysosomal marker Spin-GFP (hmlGAL4) were bled out and fixed from wildtype and Rab14 mutant larvae after a 20 min pulse with S. aureus conjugated to AF-594 (magenta) followed by a 20 min chase. Hemocytes were evaluated for co-localization between Spinster-GFP and phagosomes as described previously. (B) Percentage of phagosomes showing recruitment of Spin-GFP is plotted for wildtype and Rab14null (n=4). (C) Rab14 is recruited to the phagosome. Transgenic UAS-Rab14-mRFP flies were generated and the transgene was expressed in hemocytes using cgGal4. Recruitment of Rab14-mRFP (magenta) after different chase times following an initial 20 min pulse with S. aureus-AF488 (green) was examined in wildtype hemocytes (n=3). (D) A representative image and RGB plot for the 20 min pulse + 10min chase time point is shown. The experiments were conducted at least 3 times with 10–12 larvae in each experiment. A total of 70–90 phagosomes were examined for quantification. Error bars indicate SEM. Data was analyzed by two tailed, paired t-test. ** p<0.01 * p<0.05. Scale bar, 5 μm. (E) Rab14 mutants show impaired late endosome to lysosome trafficking. Fat body from wildtype and Rab14 mutant larvae expressing GFP-LAMP (tubulin GAL4) were dissected out and fixed. Rab14 mutant fat body cells accumulate perinuclear GFP-LAMP puncta which indicates a defect in late endosome to lysosome trafficking. A single cell showing increased perinuclear puncta has been circled. The experiments were repeated at least 3 times with 6–7 larvae for each experiment. Scale bar, 20 μm.
Next, we examined the recruitment of Rab14 to S. aureus phagosomes in wildtype hemocytes (Fig. 4C, D). Rab14 could be found associated with S. aureus phagosomes just after a 20 min phagocytosis pulse or with a pulse followed by a 10 min chase. However, the presence of Rab14 was significantly reduced on phagosomes following a 20 min chase (Fig. 4C). This timeline of Rab14 recruitment onto phagosomes is consistent with a broad role of this GTPase in regulating the maturation of phagosomes prior to the terminal fusion events that give rise to the phagolysosome.
Following uptake, similar to phagocytosis, endocytosis also involves trafficking to lysosomes, and Rab7 recruitment is essential during both endocytosis and phagocytosis (Feng et al., 1995, Harrison et al., 2003). Hence we asked whether Rab14 also plays a role during endosome to lysosome trafficking. We examined the expression of GFP-LAMP in the Rab14 mutant. Following post-translational modification in the Golgi, the LAMP1-derived cytoplasmic tail is sufficient to target this fusion protein to late endosomes and then to lysosomes (Rohrer et al., 1996). The GFP portion of the transmembrane fusion protein faces the lumen of the compartment. Hence the fluorescence of the fusion protein is lost as the GFP is degraded by hydrolases in the highly acidified lysosomal compartment. An impediment at any step of targeting from the Golgi to the lysosome will lead to accumulation of fluorescent vesicles in the cytoplasm (Pulipparacharuvil et al., 2005). When compared to wildtype, Rab14 mutant fat body cells accumulated significant GFP-LAMP perinuclear puncta, indicating a defect in late endosomal to lysosomal trafficking (Fig. 4E). Together with the reduced recruitment of Spinster to bacterial phagosomes in Rab14 hemocytes, these data suggest that Rab14 activity is important for regulating lysosomal trafficking in the context of both phagosomal and endosomal pathways. This raises the possibility that the primary function of Rab14 is may be in endosomal maturation. Since the phagocytic pathway interacts with the endocytic pathway, a defect in endosomal maturation could affect phagosomal maturation.
Rab14 can be localized both to early and late endosomal compartments
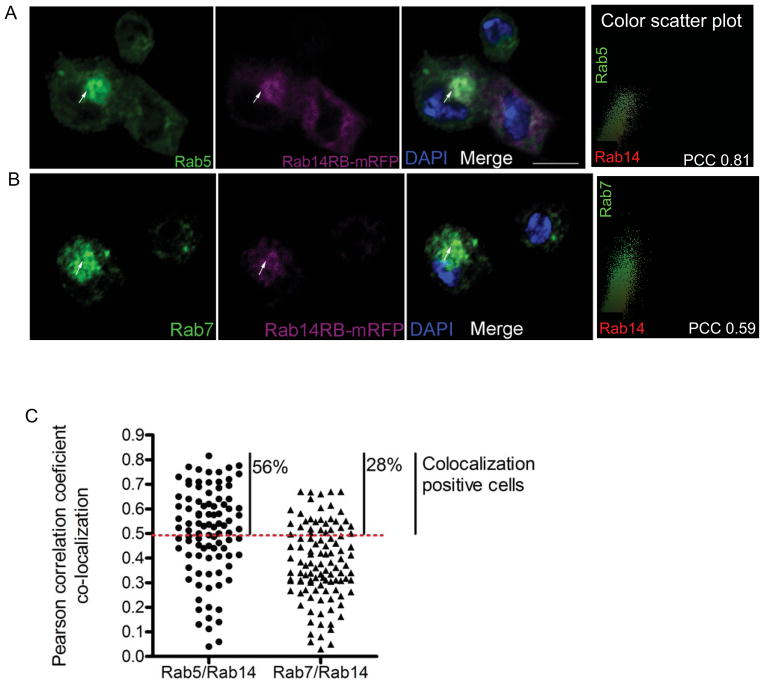
To determine the identity of the Rab14 endosomal compartment, we examined the co-localization of Rab14 with Rab5 and Rab7 across the whole cell. Since a Rab14 antibody is not available, Rab14-mRFP was expressed in hemocytes and immunofluorescence studies were carried out using Rab5, Rab7 and dsRed-conjugated antibody which detects mRFP. Real-time PCR studies were done to examine overall expression of Rab14 after overexpression using CgGal4 or hmlGal4; the expression data indicates Rab14 levels in these lines were comparable to wildtype (Fig. S2). Co-localization analysis on immunofluorescence images were carried out utilizing the Intensity correlation analysis software for Image J (Li et al., 2004). This analysis generates a Pearson coefficient of co-localization for each cell. Pearson Co-localization Coefficient (PCC) values range from −1 to +1, corresponding to degrees of co-localization that range from exclusion (−1) to complete co-localization (+1). PCC values from 0.5 to 1 indicate the presence of co-localization (Zinchuk et al., 2009). The early endosomal marker Rab5 and mRFP-tagged Rab14 showed co-localization in 56% of examined larval hemocytes with PCC reaching a maximum of +0.81 (Fig. 5A, C). A second early endosomal marker, FYVE-GFP also showed similar co-localization with Rab14, further confirming its early endosomal localization (Fig. S3). However the co-localization between Rab14 and the early endosomal markers was not ubiquitous and 44% of examined hemocytes showed low PCC values, indicating that Rab14 and Rab5 also exist in independent compartments (Fig. 5C). This is consistent with our finding that Rab14 could also be found on Rab7-containing compartments in around 28% of hemocytes, with PCC values reaching a maximum of +0.67 (Fig. 5B, C). Hence, like its mammalian ortholog, Drosophila Rab14 is found on early endosomes (Junutula et al., 2004). But unlike a previous report (Proikas-Cezanne et al., 2006), we find that Rab14 is also found on Rab7-positive late endosomal compartments. This discrepancy could be attributed to the partial Rab14 co-localization with both markers at different stages of the maturation process in different cells. Hence, its presence on late endosomes might have been overlooked in the earlier study.
Figure 5. Rab14 co-localizes with early and late endosomal markers.
(A, B) Hemocytes expressing Rab14-mRFP (cgGAL4) were bled out, fixed and immunostained for either (A) Rab5 or (B) Rab7. The arrows indicate the intracellular compartments showing co-localization. The color scatterplot for the marked cell was generated using Intensity Correlation analysis (Image J). (C) Pearson correlation coefficients (PCC) for co-localization between Rab5 and Rab14, or Rab7 and Rab14 were obtained using Intensity Correlation analysis (Image J) for each cell and plotted. PCC values from 0.5–1 indicate positive co-localization and the percentage of cells showing co-localization is indicated in the graph. A total of 90–100 hemocytes were examined for co-localization.
Endocytic/phagocytic machinery mutants are susceptible to S. aureus
To evaluate the consequences of Rab14 loss on the ability of the fly to withstand infections, we looked at the survival of Rab14 mutants over a time period following infection with S. aureus. For these experiments, adult flies were injected with a log phase culture of S. aureus and compared to buffer-injected flies as wounding controls. Rab14 mutant flies were significantly more susceptible to S. aureus, while there was no increased mortality in response to wounding (Fig. 6A, B). Also, Rab14 mutants were not susceptible to E. coli infection (Fig. 6C). We also examined survival of flies upon hemocyte-specific expression of Rab7DN and observed no increase in susceptibility to E. coli (Fig. S4). The differential response to the two microbes could be due to the fact that E. coli is not pathogenic to Drosophila. It has been shown that a block of both the cellular and humoral responses is required to see an increase in susceptibility to E. coli (Elrod-Erickson et al., 2000). In one instance, a complete block in maturation does result in susceptibility to E. coli (Akbar et al., 2011), however the delayed phagosome maturation as seen in Rab14 mutants or hemocyte-expressing Rab7DN may not be enough to completely block clearance of the microbe. Expression of either Rab14 isoform RA or RB specifically in the hemocytes rescued the susceptibility of the Rab14 mutant to S. aureus (Fig. 6B). Hence both isoforms can fulfill the immune function of Rab14 in hemocytes, suggesting that they have similar activities. Furthermore, our rescue data indicate that the increased susceptibility in Rab14 mutants is due to Rab14 function specifically in hemocytes. To determine whether the increased death of Rab14 mutants following S. aureus infection was due to defective resistance or decreased tolerance (Schneider et al., 2008), bacterial loads were examined following infection by comparing colony forming units (cfu) 24h after infection. Rab14 mutants had significantly higher S. aureus loads after infection and, as was the case for survival, this could be rescued with hemocyte-specific expression of either isoform (Fig. 6D). Hence the increased susceptibility of the Rab14 mutant to S. aureus is due to the inability of Rab14 deficient hemocytes to limit bacterial growth.
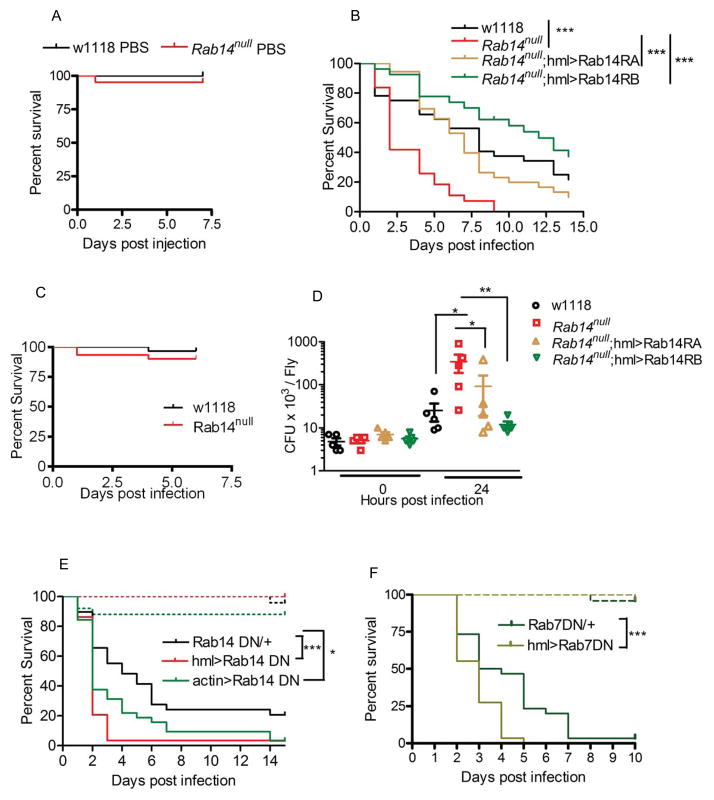
Figure 6. Phagocytic machinery mutants show increased susceptibility to S. aureus.
(A) Rab14 mutants show increased mortality following S. aureus infection. Wildtype and Rab14 mutant adults were injected with (A) sterile PBS (B) a log phase culture of S. aureus or (C) E. coli and survival was examined over a period of time. (D) Rab14 mutants have high bacterial loads following infection. Adults were injected with a log phase culture of S. aureus and bacterial counts were evaluated by plating homogenized adults and counting colony forming units (cfu) immediately or 24h post infection. (B, D) The increased susceptibility of Rab14 mutants to S. aureus is due to a hemocyte-specific role of Rab14. (B) Survival and (D) bacterial count rescues were carried out by hemocyte-specific expression (hml) of Rab14 RA or RB in Rab14 mutants. (E) A functionally active Rab14 in hemocytes is essential to resist S. aureus infection. Adults expressing Rab14DN either ubiquitously (actinGAL4) or in hemocytes (hmlGAL4) were injected with a log phase culture of S. aureus for survival studies. The Rab14DN line outcrossed to w1118 was used as a control. (F) Rab7 is essential for the immune response to S. aureus. Adults with hemocyte-specific expression of Rab7DN were injected with S. aureus and survival over a time period was examined. The Rab7DN line outcrossed to w1118 was used as a control. The dashed and solid lines in the survival curve represent wounding control (PBS injection) and infection respectively. Each survival study was repeated at least 3 times with a minimum of 30 adults /line for individual experiments. The survival data and bacterial count data were analyzed by log rank tests and Mann Whitney tests respectively. * p<0.5, ** p<0.01, *** p<0.001.
Hemocyte-specific expression of a Rab14DN (S49N) (Zhang et al., 2007) in adults increased its susceptibility to S. aureus infection, confirming that the active GTPase function in hemocytes is important for the immune response against S. aureus (Fig. 6E). Furthermore, ubiquitous downregulation of Rab14 activity did not increase susceptibility compared to downregulation in the hemocytes alone (Fig. 6E). This corroborates that the role of Rab14 in hemocytes alone accounts for the decreased ability of Rab14 flies to control an infection with pathogenic S. aureus.
Rab7 is a known regulator of phagosomal maturation. To compare the consequences of hemocyte-specific loss of Rab7 function with that of Rab14, we used hemocyte-specific expression of Rab7DN, and found that it also increased susceptibility to pathogenic S. aureus.(Fig. 6F). Hence our study demonstrates that the function of phagocytic machinery associated RabGTPases, including Rab14, is essential for mounting an effective immune response against pathogenic S. aureus.
Discussion
RabGTPases like Rab5 and Rab7 regulate phagosome fusion and perinuclear transport essential for progression of phagosome maturation (Vieira et al., 2002). However the contribution of RabGTPases in vivo during the immune response has not been examined. We have identified Rab14 as an integral component of the phagocytic machinery and thereby essential for the Drosophila immune response. Rab14 mutants show defects in S. aureus phagosome maturation and cell biological studies indicate that Rab14 is required for efficient recruitment of Rab7 on to the bacterial phagosome and is itself recruited to phagosome on early and late phagosomal compartments. A similar temporal profile for Rab14 recruitment, following Rab5 and preceding Rab7 has been shown in C. elegans during apoptotic cell phagosome maturation (Guo et al., 2010). This finding points to a role for Rab14 during the Rab5 to Rab7 transition. In contrast to our findings, a prior study in murine macrophages indicated that Rab14 is a negative regulator of phagosome maturation, as overexpression of wildtype Rab14 or a GTPase-deficient, constitutively active (CA) Rab14 inhibited maturation of phagosomes containing dead Mycobacteria (Kyei et al., 2006). The contradiction may be due to the nature of those experiments. The appropriate regulation of the active-inactive switch of RabGTPases is critical for its function, so a constitutively active (CA) mutant might result in a gain-of-function phenotype. For instance, although Rab5 regulates early endosome fusion and is essential for the endocytic pathway (Bucci et al., 1992), overexpression of wildtype or CA Rab5, leads to continuous fusion between early endosomes, resulting in large endosomes with early endosome characteristics (Roberts et al., 1999). Also, continuous association of active Rab5 with phagosomes results in maturation arrest (Vieira et al., 2003). Thus a loss-of-function mutant provides a more accurate assessment of the overall role of a Rab GTPase.
A timeline proteomic study of latex bead phagosomes indicated biphasic enrichment of Rab14 unlike the single phase enrichments of Rab5 and Rab7 onto the phagosomes (Rogers et al., 2007). Another proteomic study utilizing murine macrophages indicates the presence of a common Rab7 and Rab14-positive compartment (Duclos et al., 2011). Our data fits the biphasic model of enrichment as we could localize Rab14 on both Rab5 and Rab7 positive endosomes. The functional implication could be that Rab14 is working with both Rab5 and Rab7 to maximize the efficiency of endosomal and phagosomal maturation. This is also consistent with the observation that in the absence of Rab14, maturation is not completely abolished, but instead displays slower kinetics. Also, the viability of Rab14null animals is not compromised, while a more severe defect in endocytic machinery would be expected to be lethal.
Due to their role in membrane trafficking, RabGTPases are often targeted by intracellular pathogens which actively arrest maturation to avoid the lysosomal killing process (Brumell et al., 2007). For instance, Rab5 (Kelley et al., 2003) and Rab14 (Kyei et al., 2006) are actively retained for an extended period of time on mycobacterial phagosomes which do not mature into Rab7-positive compartments (Via et al., 1997). Since Rab7 is excluded from the Mycobacterium phagosome, the extended recruitment of Rab14 could lead to homotypic early phagosome fusion leading to a delay in the formation of the late phagosome, thus aiding maturation arrest. A Rab14-related RabGTPase, RabD regulates phagosome-phagosome fusion in Dictyostelium, (Harris et al., 2002). Homotypic phagosome fusion is unique to Dictyostelium phagosome maturation (Duhon et al., 2002). However in mammalian cells, intracellular pathogens promote homotypic phagosome fusion as a survival strategy (Brumell et al., 2007). Our data indicate that Rab14 is recruited to the early phagosomes and regulates Rab7 recruitment during phagosome maturation. It is possible that Mycobacteria interact with Rab14 to limit Rab7 recruitment, and in the absence of Rab7, Rab14 promotes early phagosome fusion leading to maturation arrest. Our study demonstrates the functional importance of Rab14 during phagosome maturation and the immune response and this may explain why it is specifically targeted by some pathogens.
Lastly, there is a limited understanding of the process and function of phagosome maturation in Drosophila. The removal (Charroux et al., 2009, Defaye et al., 2009) or the functional disabling of hemocytes (Nehme et al., 2011) both increased fly susceptibility to S. aureus, but the underlying mechanism behind the observation was not understood. In this study, we demonstrate that the hemocyte exerts its influence by regulating phagosome maturation which is essential for bacterial clearance.
Materials and Methods
Fly genetics
The Rab14 mutant was generated by transposase-induced recombination in males (Chen et al., 1998, Parks et al., 2004). Genomic DNA from the Rab14 locus was amplified by PCR using the following primers:
Forward (For): 5′-ACTGTGCGTTAGGTCCTGTTCA-3′
Reverse (Rev): 5′-ATGCCACCGAAGATGCTAGCTCAG-3′
The sequencing of the fragments indicated a deletion between +207bp and +3402bp within the 3617bp long Rab14 gene.
For the rescue experiments, Rab14 RB was amplified from cDNA and a fusion with mRFP at the N-terminus was cloned into pUASt. Transgenic flies were generated in a w1118 background (BestGene Inc). Plasmids containing mRFP were a gift from Henry Chang (Purdue University).
Flies used in the experiments: Rab14EY04032, Rab14BG01134, Rab14RA-YFP, RabGTPaseDN, hmlΔGAL4, cgGAL4, actinGAL4 were obtained from Bloomington. Other lines Rab5-GFP, (M. Gonzáles-Gaitán), tubulin>GFP-LAMP (H. Kramer), Spinster-GFP (G. Davis) were also generous contributions.
Bacteria strains
Staphylococcus aureus – ATCC12600
Adult phagocytosis and phagosome maturation experiments
Phagocytosis
5–7 day old adult flies were injected with fluorescein-conjugated S. aureus resuspended in PBS (Invitrogen: S2851, 1.6mg/ml) using a Pneumatic PicoPump PV820 (World Precision Instruments). After 30 min, flies were again injected with Trypan Blue to quench extracellular fluorescence. Images of the dorsal vessel were obtained using a Zeiss stereomicroscope (Discovery V8) with AxioCam Hc camera. Quantification of fluorescence intensity in an area around the dorsal vessel was carried out using Axiovision 4.7. Background fluorescence was also quantified and the ratio was used to plot the graph. The unpaired t-test was used to calculate p-values.
Phagosome maturation
5–7 day old adult flies were injected with pHrodo-conjugated S. aureus resuspended in PBS (Invitrogen: A10010, 8mg/ml). pHrodo conjugates were stored in small aliquots at −20°C. Single aliquots were used for one line to minimize light associated sensitivity. Images were taken at different time points after injection as described above.
Phagosomal marker recruitment and co-localization experiments
Phagosomal marker recruitment
Around 8–10 wildtype and Rab14 mutants feeding 3rd instar larvae were injected with Alexa-fluor (AF) conjugated S. aureus (Invitrogen: S23371, S23372). The pulse of 20 min included 13 min in vivo and 7 min ex vivo phagocytosis. It included 3 min for the bleeding of hemocytes onto poly-lysine coverslips containing Schneider’s media (SM) followed by 4 min of ex vivo incubation. Following the pulse, media was replaced with fresh media and hemocytes were incubated for the required chase time. Afterwards hemocytes were fixed in 4% paraformaldehyde and mounted in Prolong containing DAPI (Invitrogen: P36935). The hemocyte drivers hmlΔGAL4 (Goto et al., 2001) or cgGAL4 (Asha et al., 2003) were used to drive expression of tagged phagosomal markers. For Rab7, rabbit anti-Rab7 (1: 3000, a gift from P. Dolph, Dartmouth College, Chinchore et al., 2009) and a secondary goat anti-rabbit antibody labeled with AF-488 were used.
Co-localization experiments
Hemocytes expressing Rab14RB-mRFP (CgGAL4) were bled out from feeding third instar larvae followed by fixing and antibody staining. The following primary antibodies were used: rabbit anti-Rab5 (1:500, ab31261), rabbit anti-Rab7, goat anti-DsRed, (also detects mRFP, 1:500, sc-33354). The following secondary antibodies were used: donkey anti-rabbit IgG-CFL 488 (1:200, sc-362261), mouse anti-goat IgG-TR (1:200, sc-3916).
Endosomal maturation
Fat body was dissected out from wildtype and Rab14 mutant larvae expressing tubulin>GFP-LAMP. The dissections were carried out in cold PBS and fat body was fixed for 30 min in 4% paraformaldehyde. Thereafter the tissue was washed three times for 10 min and mounted in Prolong containing DAPI.
Image acquisition
Images were acquired using a Zeiss LSM 710 (100x/1.4 Oil Plan Apochomat lens or 40x/1.3 Oil Plan NeoFluar). Acquisition software Zen 2000 / LSM for Zeiss 710 was used. For publication, pictures were imported into Adobe Photoshop and adjusted for gain and contrast settings. Images kept in the same panel for comparison were treated alike.
Reverse Transcription and Quantitative Real-Time PCR
RNA was isolated by homogenizing tissue in STAT-60 buffer according to the manufacturer’s protocol (Isotex Diagnostics). The RNA was digested with RNase-free DNase, then subjected to reverse transcription (Fermentas, K1622) and quantitative real-time PCR using LUX probes (Invitrogen) or SYBR green on an ABI 7300 following the manufacturers’ protocols (Fermentas: K0232, K0252). Paired t-test was used to calculate p-values for differences in induction.
Primer Sequence
SYBR primers
Rab14RA: For :5′CAAACAACAATACGCACACATAC3′,
Rev: 5′GAATGTGTAACGTAGGGCGGTTA3′
Rab14 RB: For: 5′ GGACATTCAAATGAGGAGCTGAT3′,
Rev: 5′TCATCTTGACACCGGCAGAA3′
Rab14 RC: For: 5′CTATATACTCAATGACTCTGCAATGTAATA,
Rev: 5′TATGGCGCTGCAGTCATGT
Control RP49: For: 5′GCAAGCCCAAGGGTATCGA3′,
Rev: 5′TAACCGATGTTGGGCATCAG3′
Survival and bacterial load experiments
Survival
Adult flies, 5–7 day old were injected with logarithmic phase culture of S. aureus or E. coli (final OD 0.5). Flies injected with PBS served as a wounding control. The survival after injection was assessed every 24 h. At least thirty flies were injected for each experiment, and the experiments were repeated at least 3 times. Log rank tests were used to test for significant differences in survival curves, and p-values of < 0.05 were deemed significant.
Bacterial load
Adult flies, 5–7 day old were injected with a log phase culture of S. aureus (final OD 0.2) resuspended in PBS. For sample collection, flies were surface sterilized with 70% ethanol, washed in PBS and homogenized with a pestle in Luria-bertani media containing 1% Triton X-100. Five sets of 2–3 flies were collected and pooled for each time point. cfu/fly was calculated by serial dilution and plating on Luria-bertani agar plates. The experiment was repeated at least 3 times. The Mann-Whitney test was used to calculate p-values.
Supplementary Material
Acknowledgments
We thank David Schneider for advice on phagocytosis assays; Graeme Davis, Marcos Gonzáles-Gaitán, Helmut Kramer, and the Bloomington Drosophila Stock Center for fly stocks; Jun Zhang and Matthew Scott for generating tagged Rab14 and Rab14DN lines; Patrick Dolph for the Rab7 antibody; Ashley Nazario for Rab14 transgene expression data; Melek Erdinc for initial characterization of the Rab14 transposon mutant phenotype; Javier Robalino for critical review of manuscript; and Amy Beaven and the core microscopy facility of the Department of Cell Biology and Molecular Genetics for support with confocal microscopy. The work was supported by the National Institutes of Health GM073701 and AI076564.
References
- Akbar MA, Tracy C, Kahr WH, Kramer H. The full-of-bacteria gene is required for phagosome maturation during immune defense in Drosophila. J Cell Biol. 2011;192:383–390. doi: 10.1083/jcb.201008119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Dominguez C, Stahl PD. Increased expression of Rab5a correlates directly with accelerated maturation of Listeria monocytogenes phagosomes. Journal of Biological Chemistry. 1999;274:11459–11462. doi: 10.1074/jbc.274.17.11459. [DOI] [PubMed] [Google Scholar]
- Asha H, Nagy I, Kovacs G, Stetson D, Ando I, Dearolf CR. Analysis of Ras-induced overproliferation in Drosophila hemocytes. Genetics. 2003;163:203–215. doi: 10.1093/genetics/163.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumell JH, Scidmore MA. Manipulation of rab GTPase function by intracellular bacterial pathogens. Microbiology and molecular biology reviews : MMBR. 2007;71:636–652. doi: 10.1128/MMBR.00023-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci C, Parton RG, Mather IH, Stunnenberg H, Simons K, Hoflack B, Zerial M. The small GTPase Rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992;70:715–728. doi: 10.1016/0092-8674(92)90306-w. [DOI] [PubMed] [Google Scholar]
- Capmany A, Damiani MT. Chlamydia trachomatis Intercepts Golgi-Derived Sphingolipids through a Rab14-Mediated Transport Required for Bacterial Development and Replication. Plos One. 2010:5. doi: 10.1371/journal.pone.0014084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charroux B, Royet J. Elimination of plasmatocytes by targeted apoptosis reveals their role in multiple aspects of the Drosophila immune response. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:9797–9802. doi: 10.1073/pnas.0903971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Chu T, Harms E, Gergen JP, Strickland S. Mapping of Drosophila mutations using site-specific male recombination. Genetics. 1998;149:157–163. doi: 10.1093/genetics/149.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuttell L, Vaughan A, Silva E, Escaron CJ, Lavine M, Van Goethem E, et al. Undertaker, a Drosophila Junctophilin, Links Draper-Mediated Phagocytosis and Calcium Homeostasis. Cell. 2008;135:524–534. doi: 10.1016/j.cell.2008.08.033. [DOI] [PubMed] [Google Scholar]
- Defaye A, Evans I, Crozatier M, Wood W, Lemaitre B, Leulier F. Genetic Ablation of Drosophila Phagocytes Reveals Their Contribution to Both Development and Resistance to Bacterial Infection. Journal of Innate Immunity. 2009;1:322–334. doi: 10.1159/000210264. [DOI] [PubMed] [Google Scholar]
- Desjardins M, Huber LA, Parton RG, Griffiths G. Biogenesis of phagolysosomes proceeds through a sequential series of interactions with the endocytic apparatus. Journal of Cell Biology. 1994;124:677–688. doi: 10.1083/jcb.124.5.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duclos S, Clavarino G, Rousserie G, Goyette G, Boulais J, Camossetto V, et al. The endosomal proteome of macrophage and dendritic cells. Proteomics. 2011;11:854–864. doi: 10.1002/pmic.201000577. [DOI] [PubMed] [Google Scholar]
- Duhon D, Cardelli J. The regulation of phagosome maturation in Dictyostelium. Journal of Muscle Research and Cell Motility. 2002;23:803–808. doi: 10.1023/a:1024435913949. [DOI] [PubMed] [Google Scholar]
- Elrod-Erickson M, Mishra S, Schneider D. Interactions between the cellular and humoral immune responses in Drosophila. Current Biology. 2000;10:781–784. doi: 10.1016/s0960-9822(00)00569-8. [DOI] [PubMed] [Google Scholar]
- Feng Y, Press B, Wandingerness A. Rab-7 An important regulator of late endocytic membrane traffic. Journal of Cell Biology. 1995;131:1435–1452. doi: 10.1083/jcb.131.6.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garin J, Diez R, Kieffer S, Dermine JF, Duclos S, Gagnon E, et al. The phagosome proteome: Insight into phagosome functions. Journal of Cell Biology. 2001;152:165–180. doi: 10.1083/jcb.152.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto A, Kumagai T, Kumagai C, Hirose J, Narita H, Mori H, et al. A Drosophila haemocyte-specific protein, hemolectin, similar to human von Willebrand factor. Biochemical Journal. 2001;359:99–108. doi: 10.1042/0264-6021:3590099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo PF, Hu TJ, Zhang JA, Jiang SY, Wang XC. Sequential action of Caenorhabditis elegans Rab GTPases regulates phagolysosome formation during apoptotic cell degradation. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:18016–18021. doi: 10.1073/pnas.1008946107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haine ER, Moret Y, Siva-Jothy MT, Rolff J. Antimicrobial Defense and Persistent Infection in Insects. Science. 2008;322:1257–1259. doi: 10.1126/science.1165265. [DOI] [PubMed] [Google Scholar]
- Harris E, Cardelli J. RabD, a Dictyostelium Rab14-related GTPase, regulates phagocytosis and homotypic phagosome and lysosome fusion. Journal of Cell Science. 2002;115:3703–3713. doi: 10.1242/jcs.00050. [DOI] [PubMed] [Google Scholar]
- Harrison RE, Bucci C, Vieira OV, Schroer TA, Grinstein S. Phagosomes fuse with late endosomes and/or lysosomes by extension of membrane protrusions along microtubules: Role of Rab7 and RILP. Molecular and Cellular Biology. 2003;23:6494–6506. doi: 10.1128/MCB.23.18.6494-6506.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Hubber A, McDonough JA, Roy CR, Scidmore MA, Carlyon JA. The Anaplasma phagocytophilum-occupied vacuole selectively recruits Rab-GTPases that are predominantly associated with recycling endosomes. Cellular Microbiology. 2010;12:1292–1307. doi: 10.1111/j.1462-5822.2010.01468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junutula JR, De Maziere AM, Peden AA, Ervin KE, Advani RJ, van Dijk SM, et al. Rab14 is involved in membrane trafficking between the Golgi complex and endosomes. Molecular Biology of the Cell. 2004;15:2218–2229. doi: 10.1091/mbc.E03-10-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley VA, Schorey JS. Mycobacterium’s arrest of phagosome maturation in macrophages requires Rab5 activity and accessibility to iron. Molecular Biology of the Cell. 2003;14:3366–3377. doi: 10.1091/mbc.E02-12-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyei GB, Vergne I, Chua J, Roberts E, Harris J, Junutula JR, Deretic V. Rab14 is critical for maintenance of Mycobacterium tuberculosis phagosome maturation arrest. Embo Journal. 2006;25:5250–5259. doi: 10.1038/sj.emboj.7601407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Lau A, Morris TJ, Guo L, Fordyce CB, Stanley EF. A syntaxin 1, G alpha(o), and N-type calcium channel complex at a presynaptic nerve terminal: Analysis by quantitative immunocolocalization. Journal of Neuroscience. 2004;24:4070–4081. doi: 10.1523/JNEUROSCI.0346-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehme NT, Quintin J, Cho JH, Lee J, Lafarge M-C, Kocks C, Ferrandon D. Relative Roles of the Cellular and Humoral Responses in the Drosophila Host Defense against Three Gram-Positive Bacterial Infections. Plos One. 2011:6. doi: 10.1371/journal.pone.0014743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks AL, Cook KR, Belvin M, Dompe NA, Fawcett R, Huppert K, et al. Systematic generation of high-resolution deletion coverage of the Drosophila melanogaster genome. Nature Genetics. 2004;36:288–292. doi: 10.1038/ng1312. [DOI] [PubMed] [Google Scholar]
- Proikas-Cezanne T, Gaugel A, Frickey T, Nordheim A. Rab14 is part of the early endosomal clathrin-coated TGN microdomain. Febs Letters. 2006;580:5241–5246. doi: 10.1016/j.febslet.2006.08.053. [DOI] [PubMed] [Google Scholar]
- Pulipparacharuvil S, Akbar MA, Ray S, Sevrioukov EA, Haberman AS, Rohrer J, Kramer H. Drosophila Vps16A is required for trafficking to lysosomes and biogenesis of pigment granules. Journal of Cell Science. 2005;118:3663–3673. doi: 10.1242/jcs.02502. [DOI] [PubMed] [Google Scholar]
- Roberts RL, Barbieri MA, Pryse KM, Chua M, Stahl PD. Endosome fusion in living cells overexpressing GFP-rab5. Journal of Cell Science. 1999;112:3667–3675. doi: 10.1242/jcs.112.21.3667. [DOI] [PubMed] [Google Scholar]
- Rogers LD, Foster LJ. The dynamic phagosomal proteome and the contribution of the endoplasmic reticulum. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:18520–18525. doi: 10.1073/pnas.0705801104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer J, Schweizer A, Russell D, Kornfeld S. The targeting of Lamp1 to lysosomes is dependent on the spacing of its cytoplasmic tail tyrosine sorting motif relative to the membrane. Journal of Cell Biology. 1996;132:565–576. doi: 10.1083/jcb.132.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupper A, Grove B, Cardelli J. Rab7 regulates phagosome maturation in Dictyostelium. Journal of Cell Science. 2001;114:2449–2460. doi: 10.1242/jcs.114.13.2449. [DOI] [PubMed] [Google Scholar]
- Schneider DS, Ayres JS. Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases. Nature Reviews Immunology. 2008;8:889–895. doi: 10.1038/nri2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seixas E, Ramalho JS, Mota LJ, Barral DC, Seabra MC. Bacteria and Protozoa Differentially Modulate the Expression of Rab Proteins. Plos One. 2012:7. doi: 10.1371/journal.pone.0039858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim J, Lee SM, Lee MS, Yoon J, Kweon HS, Kim YJ. Rab35 Mediates Transport of Cdc42 and Rac1 to the Plasma Membrane during Phagocytosis. Molecular and Cellular Biology. 2010;30:1421–1433. doi: 10.1128/MCB.01463-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart LM, Boulais J, Charriere GM, Hennessy EJ, Brunet S, Jutras I, et al. A systems biology analysis of the Drosophila phagosome. Nature. 2007;445:95–101. doi: 10.1038/nature05380. [DOI] [PubMed] [Google Scholar]
- Sweeney ST, Davis GW. Unrestricted synaptic growth in spinster - A late endosomal protein implicated in TGF-beta-mediated synaptic growth regulation. Neuron. 2002;36:403–416. doi: 10.1016/s0896-6273(02)01014-0. [DOI] [PubMed] [Google Scholar]
- Via LE, Deretic D, Ulmer RJ, Hibler NS, Huber LA, Deretic V. Arrest of mycobacterial phagosome maturation is caused by a block in vesicle fusion between stages controlled by rab5 and rab7. Journal of Biological Chemistry. 1997;272:13326–13331. doi: 10.1074/jbc.272.20.13326. [DOI] [PubMed] [Google Scholar]
- Vieira OV, Botelho RJ, Grinstein S. Phagosome maturation: aging gracefully. Biochemical Journal. 2002;366:689–704. doi: 10.1042/BJ20020691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira OV, Bucci C, Harrison RE, Trimble WS, Lanzetti L, Gruenberg J, et al. Modulation of Rab5 and Rab7 recruitment to phagosomes by phosphatidylinositol 3-kinase. Molecular and Cellular Biology. 2003;23:2501–2514. doi: 10.1128/MCB.23.7.2501-2514.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Schulze KL, Hiesinger PR, Suyama K, Wang S, Fish M, et al. Thirty-one flavors of Drosophila Rab proteins. Genetics. 2007;176:1307–1322. doi: 10.1534/genetics.106.066761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinchuk V, Grossenbacher-Zinchuk O. Recent advances in quantitative colocalization analysis: Focus on neuroscience. Progress in Histochemistry and Cytochemistry. 2009;44:125–172. doi: 10.1016/j.proghi.2009.03.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.