Abstract
Delirium, an acute disorder of attention and cognition, is a common, serious, costly, under-recognized and often fatal condition for seniors. Its diagnosis requires a formal cognitive assessment and history of acute onset of symptoms. Given its typically complex multifactorial etiology, multicomponent nonpharmacologic risk factor approaches have proven to be the most effective strategy for prevention. To date, there is no convincing evidence that pharmacologic prevention or treatment is effective. Drug reduction for sedation and analgesia combined with nonpharmacologic approaches are recommended. Delirium may provide a window to elucidate brain pathophysiology, serving both as a marker of brain vulnerability with decreased reserve and a potential mechanism for permanent cognitive damage. As a potent patient safety indicator, delirium provides a target for system-wide process improvements. Public health priorities will include improvements in coding and reimbursement, improved research funding, and widespread education for clinicians and the public about the importance of delirium.
[Panel]: Case
An 83 year old recently widowed woman who lives alone is brought to her physician by her daughter for evaluation of falling, fever, shortness of breath, and poor oral intake. She has a history of diabetes, hypertension, congestive heart failure, reflux esophagitis, and depression. She is taking metformin, enalapril, digoxin, atenolol, ranitidine, paroxetine, and lorazepam. On examination, she has a low-grade fever, poor skin turgor, dry mucous membranes, and audible wheezing and rhonchi at both lung bases. She is sleepy, withdrawn, and not cooperative with the examination. Her physician is concerned about pneumonia and increased depression. Her cognitive status is not assessed.
Introduction
Despite first being described over 2500 years ago, delirium remains frequently unrecognized and poorly understood. Delirium, an acute decline in cognitive functioning, is a common, serious, and often fatal problem affecting up to 50% of hospitalized seniors, and costing over $164 billion (2011) per year in the United States1 and over $182 billion (2011) per year2, 3 in 18 European countries combined (See Appendix). As a preventable condition in 30–40% of cases,4, 5 delirium holds substantial public health relevance as a target for interventions to prevent its associated burden of downstream complications and costs.6 Accordingly, delirium is now included on the patient safety agenda,7 and has been increasingly targeted as an indicator of healthcare quality for seniors.8, 9
Delirium can be thought of as “acute brain failure,” a multifactorial syndrome analogous to acute heart failure and may provide a novel approach to elucidate brain functioning and pathophysiology. With its acute onset in response to noxious insults, such as major surgery or sepsis, delirium may help to shed light on cognitive reserve; that is, the brain’s resilience to withstand external factors.10 In this context, delirium may serve as a marker of the vulnerable brain with diminished reserve capacity. Recent evidence further suggests that the trajectory of “normal” cognitive aging may not be a smooth linear decline, but rather a series of punctuated declines and recoveries in the face of delirium and major medical insults.11, 12 Finally, in addition to serving as a marker of the vulnerable brain, accumulating evidence (see “Current Controversies” section below) suggests that delirium itself may lead to permanent cognitive decline and dementia in some patients.
The purpose of this report is to provide a state-of-the-art review of the syndrome of delirium to guide clinical practice and to elucidate important areas for future research.
[Panel] Search Strategy and Selection Criteria
Articles for this Review were identified by comprehensive searches of Medline, PubMed and reference lists from relevant original articles and systematic reviews (see Appendix Table 1) using the search terms: “delirium”, “acute confusion”, and “organic brain syndrome”. Original articles published in English between 1990 and 2012 were included. To provide an overview of the areas of epidemiology, etiology, nonpharmacologic and pharmacologic management, reviews were conducted from 2004–2012 to update a previous comprehensive review;13 with the exceptions of validated risk prediction models and nonpharmacologic studies, where we expanded our search to include original articles published between 1990 and 2012. All data presented are taken from those of the original article; no meta-analysis was performed. In all non-ICU settings, the study populations included in the selected articles were generally age 65 years and older. For epidemiologic studies, we required a sample size of ≥100; prospective sampling framework; satisfaction of the STROBE criteria for setting, participants, measurement and statistical methods; and use of a validated delirium instrument. The pathophysiology search used the same search terms with the addition of “etiology”, “pathophysiology”, “physiopathology”, or “pathogenesis”. For nonpharmacologic and pharmacologic prevention and treatment studies, we required a sample size ≥25 in each study arm; prospective sampling framework; use of a validated delirium instrument; and a modified Jadad quality score of ≥4 (range 0–6) that included the following components: randomization or balanced allocation (1 point); appropriate description of randomization or balanced allocation (1 point); blinding (1 point); double blinding required for pharmacologic studies); appropriate description of blinding (1 point); description of dropouts/withdrawals (1 point); and N ≥100 (1 point). Two reviewers rated each article and reached consensus on all ratings. Since the goal of this manuscript was to provide a comprehensive review of primary articles, systematic reviews and meta-analyses were not routinely included; however, all of their reference lists were checked to insure the comprehensive inclusion of primary articles in our review process (Appendix Table 1).
Epidemiology
Based on a systematic literature review from 2004–2012, articles on incidence and outcomes of delirium were selected by the following criteria: sample size of 100 or more; prospective sampling framework; satisfaction of STROBE criteria;14 and use of a validated delirium instrument. The timeframe for this review was chosen to update a previous comprehensive review.13 An additional inclusion criterion for incidence studies was serial delirium assessments at no more than 3-day intervals by trained research staff or clinicians. Table 1 presents the prevalence rates (present on admission) and incidence rates (new onset) of delirium across different patient populations as described in 35 selected studies (See Appendix Table 4 for reference citations of all articles); the sum of both prevalence and incidence yields the overall occurrence rates in each setting. The highest incidence rates were observed in the intensive care unit, postoperative, and palliative care settings. Since many of these studies excluded patients with cognitive impairment or dementia at baseline, these rates likely represent underestimates of true incidence rates. In general medical and geriatric wards, the prevalence of delirium (present on admission) of 18–35% must be added to the incidence rates to yield the overall occurrence rates of delirium in these populations of 29–64% (Table 1). The prevalence of delirium in the community setting is relatively low (1–2%), but its onset usually brings the patient to emergency care. On presentation to the emergency department, delirium is present in 8–17% of all seniors and 40% of nursing home residents.
Table 1.
Incidence of Delirium and Its Outcomes*
| Population | Prevalence (range) †, Incidence (range) | Outcomes (Adjusted Relative Risks‡, RR) |
|---|---|---|
| Surgical | ||
| Cardiac | - - - 11%-46% |
Cognitive Dysfunction (RR=1.7) Functional Decline (RR = 1.9) |
| Non-Cardiac | - - - 13% - 50% |
Functional Decline (RR = 2.1) Cognitive Dysfunction (RR = 1.6) |
| Orthopedic | 17% 12% – 51% |
Dementia/ Cognitive Dysfunction (RR = 6.4 – 41.2) Institutionalization (RR = 5.6) |
| Medical | ||
| General Medical | 18% – 35% 11% – 14% |
Mortality (RR= 1.5 –1.6) Functional decline (RR = 1.5) |
| Geriatric Units | 25% 20% – 29% |
Falls (RR = 1.3) Mortality (RR = 1.9) Institutionalization (RR = 2.5) |
| Intensive Care | 7%–50% 19% – 82% |
Mortality (RR = 1.4 – 13.0) Longer LOS (RR = 1.4 – 2.1) Extended Mechanical Ventilation (RR = 8.6) |
| Stroke | - - - 10% - 27% |
Mortality (RR = 2.0) Any of 3 outcomes: increased LOS, functional impairment, or death (RR= 2.1) |
| Dementia | 18% 56% |
Cognitive Decline (RR = 1.6–3.1) Institutionalization (RR = 9.3) Mortality (RR = 5.4) |
| Palliative Care/Cancer | - - - 47% |
- - - |
| Nursing Home/Postacute Care | 14% 20% - 22% |
Mortality (RR = 4.9) |
| Emergency Department | 8% - 17% - - - |
Mortality (RR = 1.7) |
LOS=length of stay; RR=relative risk. See Appendix Tables 4–5 for complete list of references and further details on all articles. All values in this table were derived from selected articles meeting the following criteria: sample size of ≥100 or more; satisfaction of STROBE criteria for setting, participants, measurement and statistical methods; and using a validated delirium instrument. An additional inclusion criterion for incidence studies was serial delirium assessments at no more than 3 day intervals by trained research staff or clinicians.
The sum of both prevalence and incidence yields the overall occurrence rates of delirium in each setting.
Adjusted relative risks were derived from studies that provided adjustment for at least one covariable.
Adverse outcomes associated with delirium, drawn from selected studies that included adjustment for confounders, are presented in Table 1. Delirium is consistently associated with an increased mortality rate across all nonsurgical patient populations, including general medical, geriatric, intensive care unit (ICU), stroke, dementia, nursing home, and emergency department. Patients who develop delirium in the ICU are at 2–4 fold increased risk of death both in and out of the hospital;15–18 those who develop delirium on general medical or geriatric wards are at 1.5-fold increased risk for death in the year following hospitalization;19–21 and patients with delirium in the emergency department have an approximately 70% increased risk of death during the first six months after the visit.22 Cognitive impairment is common among surgical patients who develop delirium, with impairments lasting up to one year postoperatively;12, 23, 24 and physical function is impaired for 30 days or more after discharge among surgical and non-surgical patients who develop delirium.20, 25, 26 Delirium at admission to post-acute care is associated with a five-fold increased risk of six-month mortality.27 Among older patients with dementia, delirium is associated with increased rates of cognitive decline,28–30 institutionalization,29 and mortality.29
Diagnosis
Delirium is a clinical diagnosis, which is often unrecognized and easily overlooked. Recognition requires a brief cognitive screening and astute clinical observation. Key diagnostic features include an acute onset and fluctuating course of symptoms, inattention, impaired level of consciousness, and disturbance of cognition (e.g., disorientation, memory impairment, alteration in language).31, 32 Supportive features include disturbance in sleep-wake cycle, perceptual disturbances (hallucinations or illusions), delusions, psychomotor disturbance (hypo- or hyper-activity), inappropriate behavior, and emotional lability. The current reference standard diagnostic criteria are the Diagnostic and Statistical Manual of the American Psychiatric Association (DSM-IV TR)33 and the International Classification of Diseases (ICD-10) from the World Health Organization34 [Appendix Table 2]. Over 24 delirium instruments have been used in published studies.35, 36 The most widely used instrument for identification of delirium is the Confusion Assessment Method (CAM) [Appendix Table 3],6, 31, 36, 37 validated in high quality studies including over 1000 patients with sensitivity of 94%, specificity of 89%, and high inter-rater reliability. Cognitive testing and training are recommended for optimal use of the CAM. The CAM, which has been used in over 4,000 published studies to date and translated into at least 12 languages, has been adapted for use in the ICU,38 emergency department,39 and nursing home, where it is now included as part of the Minimum Data Set,40 a standardized comprehensive assessment of all residents in U.S. long-term care facilities. Behavioral checklists for delirium symptoms, such as DOS,41 NuDESC,42 and NEECHAM,43 are used particularly in nursing-based studies. For measuring delirium severity, the most widely used tools include the Delirium Rating Scale (DRS and DRS-98)44, 45 and Memorial Delirium Assessment Scale (MDAS).46 Summation of CAM items has been used as a severity indicator.4, 47, 48 A validated chart review method for identification of delirium has been developed for retrospective identification,49 but its sensitivity is more limited. The Family Confusion Assessment Method (FAM-CAM) has been developed to identify delirium symptoms from reports of family and informal caregivers, which holds promise to assist with early recognition of delirium.50
Etiology
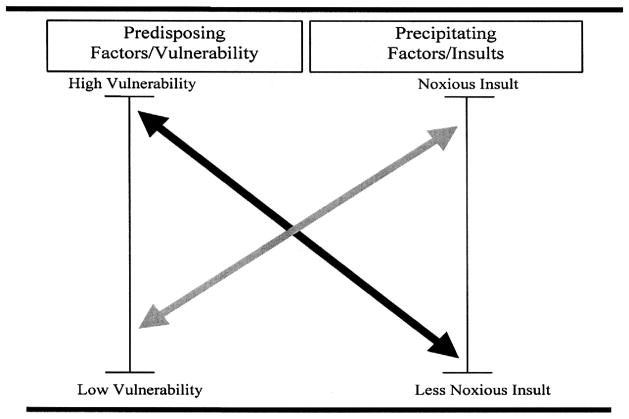
While a single factor may lead to delirium, more commonly delirium is multifactorial in older persons. The multifactorial model for the etiology of delirium has been well-validated and widely accepted.51 The development of delirium involves the complex inter-relationship between a vulnerable patient with multiple predisposing factors and exposure to noxious insults or precipitating factors (Figure 1). Thus, in patients who are highly vulnerable to delirium, such as those with underlying dementia and multimorbidity, a relatively benign insult--such as a single dose of sleeping medication--may be enough to precipitate delirium. Conversely, in a young healthy patient, delirium will develop only after exposure to a series of noxious insults, such as general anesthesia, major surgery, multiple psychoactive medications, ICU stay, and sleep deprivation. Clinically, the implications of this multifactorial etiology are that addressing a single risk factor is unlikely to resolve the delirium, and that multicomponent approaches will be most effective for both prevention and treatment.
Figure 1. Multifactorial model of delirium in older persons.
The onset of delirium involves a complex interaction between the patient’s baseline vulnerability (predisposing factors) present on admission, and precipitating factors or noxious insults occurring during hospitalization. See text for details.
To date, many risk factors for delirium have been identified.13, 52 Table 2 presents predisposing and precipitating factors identified from 11 studies with prospectively validated prediction models for delirium across different clinical populations, including medical, surgical (non-cardiac and cardiac), and intensive care. The leading risk factors consistently identified at admission in both medical and non-cardiac surgery populations were dementia or cognitive impairment, functional impairment, vision impairment, history of alcohol abuse, and advanced age (> 70 years). Comorbidity burden or presence of specific comorbidities (e.g., stroke, depression) were associated with an increased risk in all patient populations. In the ICU study, younger patients were included and baseline factors (e.g., dementia, functional impairment) were not significant independent predictors. Precipitating factors varied more across patient populations. In medical patients, polypharmacy, psychoactive medication use, and physical restraints were the leading factors, conferring up to a 4.5 times increased risk. Abnormal laboratory values were risk factors in all populations, conferring between a 40% and 500% increased risk. While a complete listing of the medical and neurologic diseases that may cause or contribute to delirium is beyond the scope of this review, clinicians should remain aware that both common and rare conditions that may present with delirium.
Table 2.
Risk Factors for Delirium from Validated Predictive Models*
| Risk Factors | General Medicine | Surgery | Intensive Care Unit | |
|---|---|---|---|---|
| Non-cardiac | Cardiac | |||
| Relative Risks | ||||
| Predisposing factors | ||||
| Dementia | 2.3–4.7 | 2.8 | ||
| Cognitive impairment | 2.1–2.8 | 3.5–4.2 | 1.3 | |
| History of delirium | 3.0 | |||
| Functional impairment | 4.0 | 2.5–3.5 | ||
| Vision impairment | 2.1–3.5 | 1.1–3.0 | ||
| Hearing impairment | 1.3 | |||
| Comorbidity/severity of illness | 1.3–5.6 | 4.3 | 1.1 | |
| Depression | 3.2 | 1.2 | ||
| History of transient ischemia/stroke | 1.6 | |||
| Alcohol abuse | 5.7 | 1.4–3.3 | ||
| Older age | 4.0 | 3.3–6.6 | 1.1 | |
| Precipitating Factors | ||||
| Medications | ||||
| Multiple medications added | 2.9 | |||
| Psychoactive medication use | 4.5 | |||
| Sedative-hypnotics | 4.5 | |||
| Use of physical restraints | 3.2–4.4 | |||
| Use of bladder catheter | 2.4 | |||
| Physiologic | ||||
| Elevated serum urea | 5.1 | 1.1 | ||
| Elevated BUN/creatinine ratio | 2.0 | 2.9 | ||
| Abnormal serum albumin | 1.4 | |||
| Abnormal sodium, glucose, or potassium | 3.4 | |||
| Metabolic acidosis | 1.4 | |||
| Infection | 3.1 | |||
| Any iatrogenic event | 1.9 | |||
| Surgery | ||||
| Aortic aneurysm | 8.3 | |||
| Non-cardiac thoracic | 3.5 | |||
| Neurosurgery | 4.5 | |||
| Trauma admission | 3.4 | |||
| Urgent admission | 1.5 | |||
| Coma | 1.8–21.3 | |||
See Appendix Table 6 for complete list of references. BUN=blood urea nitrogen
Predictive models for delirium are useful to identify high risk patients for proactive implementation of preventive strategies, for identifying patients who need closer monitoring, for identifying vulnerability factors for intervention, for prognostic decision-making, and for determining clinical trial eligibility. The ability to stratify risk can assist physicians in explaining risks to patients and families, and can help families to better understand the recovery process and potential outcomes.
Pathophysiology
Given the complex multifactorial etiology of delirium, each individual episode of delirium is likely to have a unique set of component contributors, with each set representing a discrete yet sufficient causal mechanism that may differ with each episode. Thus, it is likely that the quest for a single cause or mechanism for delirium--the “final common pathway”--will remain unanswered. Rather, accumulating evidence suggests that several different sets of interacting biological factors result in disruption of large-scale neuronal networks in the brain, leading to acute cognitive dysfunction.53 Some of the leading hypothesized mechanisms contributing to delirium appear in Table 3 including neurotransmitters, inflammation, physiologic stressors, metabolic derangements, electrolyte disorders, and genetic factors. Many biological factors may interfere directly with neurotransmission and/or cellular metabolism,54 including drugs,55 hypercortisolism,56 electrolyte disturbances,57 hypoxia,58 or impaired glucose oxidation.59 The list of potential neurotransmitters involved in delirium is long,60 but a relative cholinergic deficiency and/or dopamine excess are the most commonly inferred,61, 62 correlating with the adverse effects of anticholinergic or dopaminergic drugs.63
Table 3.
Overview of Potential Pathophysiologic Contributors to Delirium
| Biological factor | Experiment/ Observation* | Hypothesis† | Review‡ |
|---|---|---|---|
| Neurotransmitters | |||
| Acetylcholine | E / O | X | |
| Dopamine | E / O | X | |
| Gamma-Aminobutyric-acid (GABA) | E / O | ||
| Melatonin | E / O | X | |
| Tryptophan, serotonin | O | X | |
| Glutamate, N-Methyl-D-aspartate (NMDA) | O | ||
| Epinephrine/Norepinephrine | -- | X | |
| Pro-inflammatory markers | |||
| Interferon (IFN) α/β | E | X | |
| Interleukin 6 (IL-6) | O | X | |
| Interleukin 8 (IL-8) | O | X | |
| Interleukin 10 (IL-10) | O | ||
| Tumor Necrosis Factor (TNF-α) | -- | X | X |
| Interleukin 1-β (IL 1-β) | -- | X | X |
| Prostaglandin E (E2, EP1–4) | -- | X | X |
| Physiologic stressors | |||
| Cortisol | O | ||
| S100B | O | ||
| Neopterin | O | ||
| Hypoxia | O | ||
| Metabolic disorders | |||
| Lactate | E / O | ||
| Glucose | O | ||
| Insulin-like growth factor 1 (IGF-1) | O | X | |
| Hypercapnia | -- | X | X |
| Electrolyte disorders | |||
| Sodium, calcium, magnesium | E / O | ||
| Genetic factors | |||
| Apolipoprotein E (ApoE) | O | X | |
| Glucocorticoid receptor | O | ||
| Dopamine transporter, receptor | O | X | |
| Toll like receptor 4 | -- | X |
See Appendix Table 7 for complete list of references.
Refers to the type of human data available. E=controlled data available in humans, e.g. clinical trials and/or inference from unintended side effects of medications; O=observational data available in humans.
. Hypothesis: indicates that studies in humans are not yet available to support the mechanism
Review: indicates that a review of the mechanism has been published
Other causal mechanisms interfere with neurotransmission more indirectly. For instance, the systemic inflammatory response seen in sepsis may result in a cascade of local (brain) neuroinflammation triggered by inflammatory cytokines, leading to endothelial activation, impaired blood flow, and neuronal apoptosis. Neuroinflammation can lead to microglial over-activation, resulting in a neurotoxic response with further neuronal injury.64 Peripheral inflammation can also activate the central nervous system by several routes, including vagal afferents, circulating pro-inflammatory cytokines,65 endothelial activation with disruption of the blood-brain barrier,66 and microglial activation.67 The distinction between local and distant pathologies may be artificial, however, since the different inflammatory factors and neurotransmitters are closely intertwined.68
Advanced neuroimaging techniques may shed additional light on pathophysiology. Local and distant factors together account for overall and regional perfusion abnormalities observed in the delirious brain.69, 70 Total cerebral and regional perfusion are decreased with impaired cardiac output71 and with loss of cerebral autoregulation in the damaged brain;72 both mechanisms may be at play during sepsis.73 In addition, rapidly evolving functional imaging techniques may provide a powerful means to help differentiate preexisting changes and more newly acquired structural damage related to delirium.74
Although delirium can occur at any age, the young and the old carry the highest risks. In the young, neuronal networks that are underdeveloped and less complex might be more easily perturbed.75 In the old, gradual accumulation of permanent damage to neurons, dendrites, receptors, and microglia,76 as well as the impact of cerebrovascular disease or head trauma, may render the old, particularly those with underlying cognitive impairment, more susceptible to delirium when biologically stressed.77 Depending on the underlying causal mechanism, patients may overcome a delirious state without any residual effects or, alternatively, develop permanent neurological sequelae.78, 79 Understanding the pathophysiologic basis for the stressors and the substrates leading to permanent damage from delirium will advance the concept of cognitive reserve, opening new avenues for risk stratification and therapeutic approaches.80
Evaluation and Work-Up
The most important step in the evaluation is to establish the diagnosis of delirium by obtaining a history from an informed observer (e.g., family member, caregiver, or staff member) and by performing a brief cognitive assessment. To differentiate delirium from dementia, obtaining the history is critical to establish the patient’s baseline, determine the acuity of mental status change and fluctuations typical of delirium, and to search for etiologic clues. Brief cognitive screening should be conducted with formal cognitive screening tests, such as the Short Portable Mental Status Questionnaire,81 the Mini-Cog,82 or the Montreal Cognitive Assessment.83 If time is extremely limited, then assessment of orientation along with an attention task, such as naming days of the week (allow 0 errors) or months of the year (allow 1 error) backwards, serial 7’s (allow 1 error on 5 subtractions), or reciting digit spans backwards (normal: ≥3 digits backwards) can provide a basic screening. With this cognitive testing, fulfillment of screening criteria for delirium can be determined.
Given the high rates of adverse outcomes and mortality, any suspected or uncertain case (including those with lethargy or who are unable to complete an interview) should be treated as delirium until proven otherwise. The initial management focuses on three simultaneous priorities: (1) maintaining patient safety; (2) searching for the causes; and (3) managing delirium symptoms. For maintaining patient safety, efforts should focus on protecting the airway and preventing aspiration; maintaining hydration and nutrition; preventing skin breakdown; providing safe mobility while preventing falls; and avoiding restraints and bed alarms which have been shown to increase risk and persistence of delirium, and of injury.84, 85
Table 4 outlines the suggested work-up and initial management for delirium. Several fundamental points in the evaluation of delirium are worthy of special emphasis. First, because delirium can be the harbinger of a medical emergency, every patient presenting with delirium should be screened for acute physiologic disturbance such as hypoxemia, low blood glucose, and high arterial carbon dioxide. Another challenging aspect is the occult or atypical presentation of disease in older persons; for instance, an octogenarian with myocardial infarction presents more often as delirium than with classic symptoms of chest pain or shortness of breath. Thus, a nonspecific complaint from a family member that the patient “is just not him/herself” should never be taken lightly. Another important principle is that the diagnostic evaluation (e.g., laboratory testing, neuroimaging) must be targeted based on the history and physical examination; an untargeted battery of testing is likely to be low-yield.86
Table 4.
Evaluation and Management of Suspected Delirium*
| Evaluation of Delirium | |
| History |
|
| Vital signs |
|
| Physical and neurological examination |
|
| Targeted laboratory evaluation (selected tests based on clues from history and physical) | Based on history and physical examination, consider:
|
| Targeted neuroimaging (selected patients) |
|
| Electroencephalography (selected patients) |
|
| Management of Delirium | |
| Medication adjustments |
|
| Address acute medical issues |
|
| Reorientation strategies |
|
| Maintain safe mobility |
|
| Normalize sleep-wake cycle |
|
| Pharmacologic management (severe agitation or psychosis only) |
|
BID=twice daily; CBC=complete blood count; IM=intramuscular; mgs=milligrams; po=by mouth; PRN=as needed medication.
The electroencephalogram (EEG) has limited sensitivity and specificity in diagnosis of delirium. However, delirium does result in a characteristic pattern of diffuse slowing with increased theta and delta activity and poor organization of background rhythm that correlates with severity of delirium. EEG can be particularly useful to differentiate organic etiologies from functional or psychiatric disorders in difficult-to-assess patients, to evaluate deteriorating mental status in patients with dementia, and to identify occult seizures (e.g., nonconvulsive status epilepticus or atypical complex partial seizures).87, 88 Quantitative and spectral EEG may further assist in evaluation of delirium, but their performance characteristics need further investigation.
Neuroimaging, including noncontrast head computed tomography (CT) scans and magnetic resonance imaging (MRI), are low-yield in unselected patients, and are recommended for the following targeted indications: acute focal neurologic findings (since stroke or hemorrhage may present with delirium), history of or signs of recent fall or head trauma, fever with suspicion of encephalitis, or decreased level of consciousness with no identified etiology.89, 90 In patients with an identified medical etiology of delirium or with preexisting dementia,91 over 98% will have a normal brain scan. Lumbar puncture should be considered92 in cases where the suspicion of meningitis, encephalitis, or subarachnoid hemorrhage is high. It may also be indicated in cases where delirium is persistent or where no etiology of delirium can be identified.
For initial management of delirium symptoms, nonpharmacologic approaches are the first-line management strategy (see below), including removing or minimizing anticholinergic and psychoactive medications; family or companion involvement for reorientation and comfort; nonpharmacologic approaches to sleep and relaxation;93 creating a quiet, soothing, warm environment; and attending to pain. Pharmacologic management should be reserved for patients with severe agitation which would result in the interruption of essential medical therapies (such as mechanical ventilation or dialysis catheters) or result in self-harm, or for patients with extremely distressing psychotic symptoms (such as hallucinations or delusions).
Nonpharmacologic Prevention and Treatment
Primary prevention of delirium with nonpharmacologic multicomponent approaches have gained widespread acceptance as the most effective strategy for delirium.6, 13, 37 Nonpharmacologic approaches for prevention and treatment of delirium are summarized in Table 5; this table presents 13 studies which included ≥25 patients each in intervention and control groups, applied a prospective sampling framework; used a validated delirium assessment, and achieved a modified Jadad score94 of at least 4 points. Of these, the most widely disseminated approach is the Hospital Elder Life Program (HELP),4, 95, 96 a multicomponent intervention strategy with proven effectiveness and cost-effectiveness for prevention of delirium and functional decline97, 98 through targeting risk factors for delirium. The interventions include reorientation, therapeutic activities, reduction of psychoactive medications, early mobilization, promoting sleep, maintaining hydration and nutrition, and providing vision and hearing adaptations. The program is implemented by a skilled interdisciplinary team, assisted by either nursing staff or trained volunteers. While originally evaluated in a largescale controlled clinical trial, over 10 follow-up studies have demonstrated HELP to be effective in diverse settings and populations.99–101 The program is now implemented in over 200 hospitals worldwide, but adaptations and alternatives may be required in some settings due to constraints on resources or availability of skilled interdisciplinary geriatric professionals. Critical factors for initiating and sustaining HELP include: gaining internal support; ensuring effective champions; maintaining program fidelity while adapting to local circumstances; documenting positive outcomes; and obtaining long-term funding and resources.102, 103 The savings in healthcare costs per HELP patient are approximately $9,000 (USD) per year.
Table 5.
Non-Pharmacologic Prevention and Treatment Studies for Delirium*
| Author, Yr | Type (P,T) | Study Population, | Intervention N | Study Results | Jadad Score†, Design & Limitations |
|---|---|---|---|---|---|
| Martinez 2012 | P | 287 Medical patients (144 I/143 C) | Multicomponent intervention delivered by family members | Lower incidence of delirium (6% vs. 13%, p<.03) and falls (4% vs. 0%, NS). No impact on duration | 6 Randomized single-blind trial |
| Deschodt 2012 | P | 171 Orthopedic patients (94 I/ 77 C) | Preoperative multidisciplinary geriatric consultation | Lower incidence of delirium 37% vs. 53% (p=.04); lower incidence cognitive decline--23% vs. 38%; OR 2.2 (CI 1.1, 4.2) | 4 Parallel group trial (−2) Not balanced allocation |
| Van Rompaey 2012 | P | 136 ICU patients (69 I/ 69 C) | Use of earplugs at night | Lower incidence subsyndromal delirium only (15% vs. 40%); adjusted HR= 0.47 (CI 0.27, 0.82) | 5 Randomized single-blind trial (−1) Dropouts not described |
| Yoo 2012 | P | 518 Medical patients (262 I/ 256 C) | Interdisciplinary team-based geriatric care addressing functioning, medications, sleep | Less transition to nursing home (16% vs. 22%, p=0.005); adjusted OR 0.52 (CI 0.16, 0.94) | 4 Prospective matched cohort design (−2) No blinding |
| Chen 2011 | P | 179 Abdominal surgery patients (102 I/ 77 C) | Modified Hospital Elder Life program (HELP) implemented by nurses | Lower incidence of delirium in Intervention group (0% vs. 17%). No difference in hospital LOS. Lower physical and cognitive decline in Intervention |
4 Before-after study (−2) Not balanced allocation |
| Marcantonio 2010 | P + T | 457 Post-acute care patients (175 I/ 282 C) | Multicomponent intervention: assessment, causes, complications | Improved detection of delirium by RNs (41% vs. 12%, p<0.001) | 6 Cluster-randomized single- blind trial |
| Schweickert 2009 | P | 104 Mechanically ventilated medical ICU patients (49 I / exercises, transfer training and pre-gait 55 C) | Physical and occupational therapy (e.g., passive range of motion, bed mobility exercises) with interruption of sedatives. | Less time in ICU with delirium (2 days vs. 4, p=0.03; 33% of days vs. 57%, p=0.02), less hospital days with delirium (2 days vs. 4, p=0.02; 28% of days vs. 41%, p=0.01), | 6 Randomized single-blind trial |
| Caplan 2006 | P | 104 Medical patients (70 I/ 34 C) | Rehab-at-home by multidisciplinary outreach team | Lower incidence of delirium (0.6% vs. 3.2%, p<.01), shorter rehab (16 days vs. 23 days, p=0.02), lower costs ($6,259 vs. $15,134, p<0.01) | 4 Randomized trial (−2) No blinding |
| Pitkala 2006 | I | 174 Medical patients (87 I /87 C) | Multicomponent, comprehensive geriatric assessment to identify etiology of delirium and make tailored recommendations. | Faster improvement of symptoms of delirium (p=0.002) and higher cognitive performance at 6-months (MMSE=18.4 vs. 15.8 on, p=0.047). | 4 Randomized trial (−2) No blinding |
| Cole 2002 | I | 227 Medical patients (113 I/ 114 C) | Multidisciplinary consult by geriatrician or psychiatrist to determine etiology and make recommendations; daily follow-up by study nurse. Nurse protocol included modifications to environment, orientation and communication. | No difference in time to recovery from delirium. | 6 Randomized single-blind trial |
| Milisen 2001 | P | 120 Hip fracture patients (60 I/60 C) | Enhanced nursing care with delirium screening, geriatric consultation, and pain management | Shorter duration and severity of delirium (p<0.05). Among patients who developed delirium, cognitive function was higher. | 4 Before-after study (−2) Not balanced allocation |
| Marcantonio 2001 | P | 126 Hip fracture patients (62 I/ 64 C) | Proactive geriatric consultation with recommendations from 10 modules for hydration, pain, nutrition, mobilization) | Lower incidence of delirium (RR = 0.64, CI = .37-.98) and severe delirium (RR = 0.40, CI =.18-.89) | 6 Randomized single-blind trial |
| Inouye 1999 | P | 852 Medical patients (426 I/ 426 C) | Hospital Elder Life Program (HELP) targeting 6 factors: cognition, immobility, hydration, sleep, hearing, vision) | Lower incidence of delirium (OR = 0.60, CI = .39-.92); decreased total delirium days (105 vs. 161, p=0.02) and number of delirium episodes (62 vs. 90; p=0.03) | 6 Prospective matched cohort design with balanced allocation |
See Appendix Table 8 for complete list of references. All studies included had modified Jadad quality scores of 4 or greater. C=control patients; CI=95% confidence interval; HR=hazard ratio; I=intervention patients; ICU=intensive care unit; LOS=hospital length of stay; NS=not significant; OR=odds ratio; P=prevention trial; RN=registered nurse; T=treatment trial.
The modified Jadad score (6 points) included: randomization or balanced allocation (1 point); description of method for balanced allocation (1); double blinding (1); description of double-blinding (1); description of withdrawals/dropouts (1); sample size ≥100 (1)
Proactive geriatric consultation is another successful approach, evaluated in a randomized controlled trial,5 with recommendations made by a geriatrician consult before and after surgery based on 10 structured modules (e.g., hydration, pain management, nutrition, mobilization). The success of this strategy, however, is integrally linked to the adherence with the consult recommendations. Other nonpharmacologic studies (Table 5; see references for included articles in Appendix Table 8) have included multifactorial targeted interventions, delirium screening and intervention on geriatric units, staff training or educational programs, and interdisciplinary consultation. Recent approaches include interventions delivered by family members and mobility or rehabilitation interventions, both of which were demonstrated to be effective for prevention of delirium. The use of earplugs at night in one study had modest effectiveness in an ICU trial,104 and may be a useful adjunct to a nonpharmacologic sleep protocol.93 The Delirium Room105 is another intriguing concept to provide specialized management for delirium patients, but has not yet been evaluated in a controlled trial. Unfortunately, many of the nonpharmacologic studies to date have been hampered by methodologic limitations, such as lack of prospective balanced allocation to study groups, lack of a comparison group, or unblinded outcome assessment.
Pharmacologic Prevention and Treatment
Pharmacologic approaches for prevention and treatment of delirium are summarized in Table 6 (see references for included articles in Appendix Table 9). This table presents 16 studies which included at least 25 patients each in intervention and control groups, applied a prospective sampling framework, used a validated delirium assessment, and achieved a modified Jadad score94 of at least 4 points. While these clinical trials have used a variety of pharmacologic approaches, at present there is no convincing, reproducible evidence that any of these treatments are clearly effective for either prevention or treatment of delirium. In six of these trials, there was no difference in delirium rates. In eight of these trials, the target treatment did reduce delirium rates, but the observed reduction either had no impact on clinical outcomes (such as intensive care unit (ICU), hospital length of stay, hospital complications, or mortality), or clinical outcomes were not measured. In two trials, the treatment resulted in potentially worse outcomes: olanzapine reduced incidence, but resulted in greater duration and severity of delirium (without reported clinical outcomes); and rivastigmine resulted in higher delirium duration and mortality. Notably, all of these trials used different approaches to the assessment of delirium and evaluated diverse patient populations; thus, generalizing findings is difficult. Given the preponderance of evidence, however, pharmacologic approaches to prevention and treatment are not recommended at this time.6, 106
Table 6.
Drug Trials for Prevention and Treatment of Delirium*
| Author, Yr | Type (P,T) | Study Population, N | Intervention/ Control | Study Results | Jadad Score†, Limitations |
|---|---|---|---|---|---|
| Prevention Trials | |||||
| Wang 2012 | P | 457 noncardiac surgery patients in ICU 65+ (229 I/ 228 C) | Haloperidol/ placebo | Reduced incidence of delirium (haloperidol 15.3% vs. placebo 23.2%, p=.03). No difference in LOS, post-op complications, or mortality | 6 |
| Kalisvaart 2005 | P | 430 hip-surgery patients 70+ (212 I/ 218 C) | Haloperidol/ placebo | No difference in delirium (15.1% vs. 16.5%, NS); but decreased duration and severity; decreased LOS | 6 |
| Larsen 2010 | P | 400 knee- or hip-replacement patients (196 I/ 204 C) | Olanzapine/placebo | Reduced incidence of delirium (14% vs. 40%, p<.0001), but greater duration and severity in olanzapine | 6 |
| Prakanrattana 2007 | P | 126 cardiac surgery patients (63 I/ 63 C) | Risperidone (single dose)/placebo | Lower incidence of delirium (11.1% vs. 31.7%, RR: 0.35, 95% CI: 0.16–0.77, p=.009). No difference in LOS, ICU days, or post-op complications | 6 |
| Al-Aama 2011 | P | 145 medical patients 65+ (72 I/ 73 C) | Melatonin/placebo | Reduced incidence of delirium (12% vs. 31%, p=.014); no difference in MDAS, LOS, sitters, restraints | 6 |
| Gamberini 2009 | P | 120 cardiopulmonary bypass patients 65+ (59 I/ 61 C) | Rivastigmine/placebo | No difference in delirium rates (rivastigmine 32%, placebo 30%, p=.8). No difference in cognition | 6 |
| Hudetz 2009 | P | 58 cardiopulmonary bypass patients (29 I/ 29 C) | Ketamine/placebo | Lower delirium rate in ketamine (3% vs. placebo 31%, p=.01) | 5 (-1) N<100 |
| Mouzopoulos 2009 | P | 207 hip fracture patients 70+ (102 I/ 105 C) | Fascia iliaca compartment block (FICB)/placebo | Reduced delirium rate (FICB 10.78% vs. placebo 23.8%. RR: 0.45, 95% CI: 0.23–0.87), reduced delirium duration and severity | 4 (-2) Only participants blinded |
| Shehabi 2009 | P | 306 pump cardiac surgery patients 60+ (154 I/152 C) | Dexmedetomidine/ morphine | No difference in delirium (8.6% vs. 15%, RR: 0.57, 95% CI: 0.26–1.1, p=.09); reduced duration; less hypotension | 6 |
| Treatment Trials | |||||
| Girard 2010 | T | 101 mechanically ventilated ICU patients (35 haloperidol/ 30 ziprasidone/36 placebo) | Haloperidol/ziprasi- done/ placebo | No difference in delirium-free or coma-free days (haloperidol 14, ziprasidone 15, placebo 12.5 days, p=.66). No difference in mortality | 6 |
| Hakim 2012 | T | 101 on-pump cardiac surgery patients 65+ (51 I/ 50 C) | Risperidone/ placebo | Lower delirium rate (risperidone 13.7% vs. placebo 34%, p=.031). No difference in LOS in ICU or hospital | 6 |
| Sultan 2010 | P+T | 203 hip surgery patients with spinal anesthesia 65+ (53 Melatonin/ 49 placebo/ 50 midazolam/ 51 clonidine) | Melatonin/midazolam/ clonidine/placebo | Lower delirium rate (melatonin 9.43% vs. placebo 32.65% vs. midazolam 44% vs. clonidine 37.25%). No clinical outcomes reported | 6 |
| van Eijk 2010 | T | 104 ICU patients 54 Intervention; 50 Control | Rivastigmine/placebo | Greater delirium duration (rivastigmine 5.0 vs. placebo 3.0 days, p=.06) and mortality (rivastigmine 22% vs. placebo 8%, p=.07) | 6 |
| Liptzin 2005 | P+T | 80 knee or hip arthoplasty patients 50+ (39 I/ 40 C) | Donepezil/placebo | No difference in delirium rates (donepezil 21%, placebo 17%, p=.69) | 4 (-1) dropouts not defined; (-1) N<100 |
| Riker 2009 | T | 375 mechanically ventilated ICU patients (250 I/ 125 C) | Dexmedetomidine/ midazolam | Lower delirium rate (dexmedetomidine 54% vs. midazolam 77%, p<.001). Longer delirium free days in dexmedetomidine group | 6 |
| Pandharipande 2007 | T | 103 mechanically ventilated ICU patients (52 I/ 51 C) | Dexmedetomidine/ lorazepam | No difference of delirium rate (79% vs. 82%, p=.65), median delirium days (dexmedetomidine 9 vs. lorazepam 7 days, p=.09), or mortality | 6 |
See Appendix Table 9 for complete list of references. C=Control; CI=confidence Interval; FICB=fascia iliaca compartment block; I=Intervention; ICU= LOS: length of stay; MDAS= OR=odds ratio; N=number; P=Prevention Trial; post-op=post-operative; RR=relative risk; T=Treatment Trial.
The modified Jadad score (6 points) included: randomization or balanced allocation (1 point); description of method for balanced allocation (1); double blinding (1); description of double-blinding (1); description of withdrawals/dropouts (1); sample size 100 (1)
Current Controversies
While research in the field of delirium has been booming with the number of research articles on delirium increasing from fewer than 30 per year in 1980 to over 400 per year in 2011, many key aspects of delirium remain poorly understood. While some biomarkers associated with delirium have been identified, the fundamental pathophysiologic basis of delirium remains obscure. Thus, important knowledge gaps will need to be addressed to move the field ahead.
Does delirium lead to dementia?
A major area of controversy is whether delirium is simply a marker of vulnerability to dementia, or whether delirium itself leads to dementia. Ultimately, it is likely that both hypotheses are true. There is little doubt that occurrence of an episode of delirium can signal vulnerability of the brain with decreased cognitive reserve and increased risk for future dementia. In some cases, delirium may bring previously unrecognized cognitive impairment to medical attention. Delirium and dementia commonly coexist, with dementia being a leading risk factor for delirium, i.e., increasing delirium risk by 2–5 fold on hospital admission (Table 2). Moreover, the evidence for delirium leading to permanent cognitive impairment and dementia is increasing, ranging from epidemiologic evidence to tissue culture and animal models. A recent meta-analysis107 involving two studies with 241 total patients demonstrated that delirium was associated with an increased rate of incident dementia, (adjusted relative risk, RR, 5.7, 95% confidence interval, CI, 1.3–24.0). In a sample of 225 cardiac surgery patients, delirium resulted in a severe punctuated decline in cognitive functioning, followed by recovery over 6–12 months in most patients; however, a substantial proportion, particularly those with prolonged delirium, never return to baseline.12 In 263 patients with Alzheimer’s disease, delirium resulted in a fundamental alteration in the trajectory of cognitive decline with a 2-fold acceleration in rate of decline over the year following hospitalization, and accelerated decline persisting over the 5-year follow-up period.30
Additional evidence supports a more direct role for delirium in dementia. An important study with neuropathological confirmation78 demonstrated that in 553 individuals who were 85 years and older at baseline, delirium increased the risk of incident dementia (odds ratio 8.7, 95% CI 2.1–35). In patients without delirium, Alzheimer’s pathology was significantly associated with dementia, whereas no such relationship was seen in those with delirium, raising the possibility of alternative pathologic mechanisms for dementia following delirium. This study was limited, however, by a high rate of missing follow-up observations. Previous studies in animal models and human neuronal cell culture have demonstrated that exposure to inhalational anesthetics may induce neurotoxicity, including apoptosis, caspase activation, A-beta oligomerization and accumulation, neuroinflammation, and mitochondrial dysfunction.108, 109 Preliminary results in humans110 suggest some inhalational anesthetic agents (e.g., isoflurane) may be more neurotoxic than others. Important recent work involving animal models of delirium have demonstrated that in vulnerable animals, systemic inflammatory insults can cause punctuated cognitive decline typical of delirium, followed by acceleration in disease progression typical of dementia.111 Furthermore, a single dose of lipopolysaccharide, inducing an inflammatory insult comparable to a moderate infection in humans, has been shown to induce neuronal death, microglial activation, decreased regional blood flow, and loss of cholinergic activation.112 This accumulating evidence, therefore, lends strong support for the impact of delirium itself contributing to and/or being a mediator of permanent cognitive impairment. Future human studies with careful baseline characterization of cognitive function, control for confounding factors, and long-term follow-up, including neuropsychological testing and neuroimaging, will be helpful to address this important area.
Is delirium primarily a disorder of cognition or arousal?
Historically, delirium was first categorized as a “mental status” problem, a disorder of arousal with varying degrees of obtundation. However, with advances in the field and more sophisticated observation, delirium is now considered to be primarily a disorder of cognition with attention and global cognitive impairments as the key features, rather than a primary disorder of arousal alone.31, 112 This distinction is important to identify delirium that is most associated with poor long-term outcomes. Clearly, delirium includes impairments in both cognition and arousal in many cases. While distinguishing an over-sedated patient from a delirious patient can be challenging, this distinction is in fact clinically relevant. Delirium lasting for 2–3 days or more has been associated with poorer outcomes than more transient episodes, which are often due to psychoactive medications.32, 113 Sedation scales alone (such as the Richmond Agitation and Sedation Scale, RASS),38, 114 which are neither sensitive nor specific for delirium, should not be used alone but rather in conjunction with tests of attention and cognition (in verbal patients) or other diagnostic evaluations. Moreover, while carrying its own prognostic risks, the etiology, pathophysiology, and management of over-sedation should be considered quite distinct from the management of delirium.
Are there pathophysiologic or prognostic differences in the forms of delirium or in specific clinical manifestations?
Delirium has two major psychomotor forms: hypoactive and hyperactive. Patients with acute alcohol withdrawal are more likely to present with the hyperactive form. The predominantly hypoactive form is more common in older patients, and has been generally associated with a worse prognosis.52 While these two forms are distinctive clinically, patients can wax and wane between the two forms during the course of a day or the course of their delirium. EEG manifestations are not reliably different between the two forms.115 Current delirium severity instruments (e.g., DRS98 and MDAS) tend to have more hyperactive symptoms represented in their summative scores than hypoactive symptoms, thus tending to weight hyperactive delirium as more severe. In addition, it is unclear whether different causal mechanisms can be separated by clinical signs and symptoms; that is, are there different, recognizable phenotypes of delirium well beyond the two forms described above?116, 117 Do specific clinical manifestations, such as hallucinations, indicate a separate pathophysiology or prognosis? Clarification of these issues with improved delirium measurement methods and application of sophisticated neuroimaging and pathophysiologic approaches holds substantial ramifications for understanding both the phenomenology and treatment of delirium.
What are appropriate treatment strategies for delirium?
Current clinical trials for delirium management have focused primarily on antipsychotic or sedating medications. While these treatments may reduce the agitation and behavioral symptoms associated with delirium, which are often vexing to healthcare professionals, there is no evidence that these treatments are effective for improving outcomes from delirium. Given the limitations of our measurement instruments, a distinct possibility is that these treatments may convert hyperactive to hypoactive delirium (which is then not measured), contributing to these poor outcomes. Increasing evidence suggests that these treatments may prolong the duration of delirium, prolong associated cognitive impairments, and worsen clinical outcomes. Thus, consideration of other approaches is critical at this juncture, including nonpharmacologic strategies, cognitive rehabilitation, drug reduction or drug-sparing approaches (i.e., substituting less toxic alternatives), and treatments targeted towards inflammation, neuroprotection, sleep enhancement (e.g., melatonin), and reduction of pain and stress including complementary and alternative medicine. Our current approaches for management of delirium must focus on treatments that enhance recovery, maximize functional status, and improve clinical outcomes.
Future Directions and Recommendations
While many knowledge gaps remain, the groundwork laid by the current evidence in delirium highlights a clear path to move forward. Table 7 outlines some of the research priorities in delirium research, and the concomitant public health priorities that will be needed to move the field ahead. Each research domain must be coupled with translation into practice and policy to impact on the problem of delirium. Important public health and policy priorities should include more logical coding and reimbursement strategies for delirium. Currently, there are at least 11 codes for delirium in ICD-9 CM and 23 codes in ICD-10, yet only about 3% of delirium cases are coded in medical records.49 Without a more logical system to record delirium that is occurring in our healthcare systems, large-scale public health efforts will be severely limited. In addition, comprehensive efforts to educate clinicians and the public about delirium, including its importance, recognition, risk factors, prevention and management strategies, will be critical to change the current state of under-recognition and mismanagement. Delirium serves as a potent and well-recognized indicator of healthcare quality across many settings, and creating incentives for system-wide process improvement to address delirium will result in high quality geriatric care more generally. Given that delirium is highly multifactorial and linked to many other common geriatric syndromes (such as falls, pressure ulcers, functional decline, and incontinence), addressing delirium provides a highly practical and effective strategy to improve outcomes, decrease costs, and raise the quality of healthcare system-wide.
Table 7.
Research and Public Health Priorities for Delirium
| Area | Research Priorities | Public Health Priorities |
|---|---|---|
| Recognition |
|
|
| Epidemiology |
|
|
| Pathophysiology |
|
|
| Prevention and Treatment |
|
|
Adapted with permission from: Inouye SK et al. JAMA 1996; 275:852-857 Copyright © 1996 American Medical Association. All Rights Reserved
[Panel] Summary Messages for Clinicians
As the case demonstrates, delirium is easy to overlook without formal cognitive assessment. A brief cognitive examination would have assisted in identification of delirium, hastened appropriate management, and helped to reduce its associated adverse outcomes. In addition, seniors are often on multiple psychoactive medications which increase risk for delirium in the face of stressors such as acute infection. Falling and loss of appetite are often warning signs for delirium. Helpful take-home messages are summarized below (See: www.hospitalelderlifeprogram.org):
Assess for delirium in all older hospitalized patients: use simple cognitive screening and the Confusion Assessment Method. Be sure to get the history or timecourse of any cognitive changes from an informed proxy.
Evaluating medications is a high-yield procedure (the medication list “biopsy”). Reduce psychoactive medications as a first step wherever possible.
Use nonpharmacologic approaches to manage sleep, anxiety, and agitation.
Reserve pharmacologic approaches for patients with severe agitation, which will result in interruption of essential medical therapies (e.g., intubation) or poses a danger for self-injury; or for cases with severe, distressing psychotic symptoms (e.g., hallucinations, delusions).
Involve family members in care, particularly for reorientation and prevention of self-harm.
Avoid bedrest orders; encourage mobility and self-care.
Make sure that patients have their glasses, hearing aids, and dentures. Being able to see, hear, and eat are important in all healthcare settings.
Let patients know their schedule and keep them involved in their care. Communicate regularly with patients and their families.
Supplementary Material
Acknowledgments
Grant funding: Dr. Inouye's time was supported in part by Grant No. P01AG031720 from the National Institute on Aging and by the Milton and Shirley F. Levy Family Chair. Dr. Westendorp's time was supported in part by the Leyden Academy on Vitality and Ageing. Dr. Saczynski's time was supported in part by Grants No. K01AG033643 and U01HL105268 from the National Institutes of Health. The funding sources had no role in the writing of the manuscript or the decision to submit it for publication.
We thank Ms. Margaret Puelle for extensive assistance with literature searches and manuscript preparation, Dr. Jirong Yue for review and ratings of prevention and treatment studies, Ms. Jacquelyn Hyde and Dr. Benham Sabayan for assistance with literature searches, and Ms. Margaret Demille for editorial assistance. This work is dedicated to the memory of Joshua Bryan Inouye Helfand and Dr. Mitsuo Inouye.
Footnotes
Author contributions
All authors contributed to the search strategy, selection of articles, synthesis of information identified in the search, drafting and editing the manuscript or relevant sections thereof. Dr. Westendorp focused on the section on pathophysiology. Dr. Saczynski focused on the sections on epidemiology, etiology, and nonpharmacologic management. All authors have seen and approved the final version.
Conflicts of interest
The authors have no conflicts of interest to disclose.
References
- 1.Leslie DL, Marcantonio ER, Zhang Y, Leo-Summers L, Inouye SK. One-year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008 Jan 14;168(1):27–32. doi: 10.1001/archinternmed.2007.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization Regional Office for Europe. European hospital morbidity database. World Health Organization; Copenhagen: 2012. [Google Scholar]
- 3.Organization for Economic Co-operation and Development. OECD Health Data 2012. Organization for Economic Co-operation and Development; Paris: 2012. [Google Scholar]
- 4.Inouye SK, Bogardus ST, Jr, Charpentier PA, Leo-Summers L, Acampora D, Holford TR, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999 Mar 4;340(9):669–76. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 5.Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001 May;49(5):516–22. doi: 10.1046/j.1532-5415.2001.49108.x. [DOI] [PubMed] [Google Scholar]
- 6.O'Mahony R, Murthy L, Akunne A, Young J. Synopsis of the National Institute for Health and Clinical Excellence guideline for prevention of delirium. Ann Intern Med. 2011 Jun 7;154(11):746–51. doi: 10.7326/0003-4819-154-11-201106070-00006. [DOI] [PubMed] [Google Scholar]
- 7.Wachter RM. Understanding patient safety. 2. New York, NY: McGraw-Hill Medical; 2012. [Google Scholar]
- 8.Agency for Healthcare Research and Quality (AHRQ) National Quality Clearinghouse Measure: Delirium: proportion of patients meeting diagnostic criteria on the Confusion Assessment Method (CAM) 2003 [cited 2013 3 Jan]; Available from: http://www.qualitymeasures.ahrq.gov/content.aspx?id=27635.
- 9.Shekelle PG, MacLean CH, Morton SC, Wenger NS. Acove quality indicators. Ann Intern Med. 2001 Oct 16;135(8 Pt 2):653–67. doi: 10.7326/0003-4819-135-8_part_2-200110161-00004. [DOI] [PubMed] [Google Scholar]
- 10.Jones RN, Fong TG, Metzger E, Tulebaev S, Yang FM, Alsop DC, et al. Aging, brain disease, and reserve: implications for delirium. Am J Geriatr Psychiatry. 2010 Feb;18(2):117–27. doi: 10.1097/JGP.0b013e3181b972e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson RS, Hebert LE, Scherr PA, Dong X, Leurgens SE, Evans DA. Cognitive decline after hospitalization in a community population of older persons. Neurology. 2012 Mar 27;78(13):950–6. doi: 10.1212/WNL.0b013e31824d5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saczynski JS, Marcantonio ER, Quach L, Fong TG, Gross A, Inouye SK, et al. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012 Jul 5;367(1):30–9. doi: 10.1056/NEJMoa1112923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inouye SK. Delirium in older persons. N Engl J Med. 2006 Mar 16;354(11):1157–65. doi: 10.1056/NEJMra052321. [DOI] [PubMed] [Google Scholar]
- 14.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007 Oct 20;370(9596):1453–7. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 15.Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE, Jr, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004 Apr 14;291(14):1753–62. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 16.Lin SM, Liu CY, Wang CH, Lin HC, Huang CD, Huang PY, et al. The impact of delirium on the survival of mechanically ventilated patients. Crit Care Med. 2004 Nov;32(11):2254–9. doi: 10.1097/01.ccm.0000145587.16421.bb. [DOI] [PubMed] [Google Scholar]
- 17.van den Boogaard M, Schoonhoven L, van der Hoeven JG, van Achterberg T, Pickkers P. Incidence and short-term consequences of delirium in critically ill patients: A prospective observational cohort study. Int J Nurs Stud. 2012 Jul;49(7):775–83. doi: 10.1016/j.ijnurstu.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 18.Veiga D, Luis C, Parente D, Fernandes V, Botelho M, Santos P, et al. Postoperative delirium in intensive care patients: risk factors and outcome. Rev Bras Anestesiol. 2012 Jul;62(4):469–83. doi: 10.1016/S0034-7094(12)70146-0. [DOI] [PubMed] [Google Scholar]
- 19.Pitkala KH, Laurila JV, Strandberg TE, Tilvis RS. Prognostic significance of delirium in frail older people. Dement Geriatr Cogn Disord. 2005;19(2–3):158–63. doi: 10.1159/000082888. [DOI] [PubMed] [Google Scholar]
- 20.Buurman BM, Hoogerduijn JG, de Haan RJ, Abu-Hanna A, Lagaay AM, Verhaar HJ, et al. Geriatric conditions in acutely hospitalized older patients: prevalence and one-year survival and functional decline. PLoS One. 2011;6(11):e26951. doi: 10.1371/journal.pone.0026951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leslie DL, Zhang Y, Holford TR, Bogardus ST, Leo-Summers LS, Inouye SK. Premature death associated with delirium at 1-year follow-up. Arch Intern Med. 2005 Jul 25;165(14):1657–62. doi: 10.1001/archinte.165.14.1657. [DOI] [PubMed] [Google Scholar]
- 22.Han JH, Shintani A, Eden S, Morandi A, Solberg LM, Schnelle J, et al. Delirium in the emergency department: an independent predictor of death within 6 months. Ann Emerg Med. 2010 Sep;56(3):244–52. e1. doi: 10.1016/j.annemergmed.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bickel H, Gradinger R, Kochs E, Forstl H. High risk of cognitive and functional decline after postoperative delirium. A three-year prospective study. Dement Geriatr Cogn Disord. 2008;26(1):26–31. doi: 10.1159/000140804. [DOI] [PubMed] [Google Scholar]
- 24.Krogseth M, Wyller TB, Engedal K, Juliebo V. Delirium is an important predictor of incident dementia among elderly hip fracture patients. Dement Geriatr Cogn Disord. 2011;31(1):63–70. doi: 10.1159/000322591. [DOI] [PubMed] [Google Scholar]
- 25.Rudolph JL, Inouye SK, Jones RN, Yang FM, Fong TG, Levkoff SE, et al. Delirium: an independent predictor of functional decline after cardiac surgery. J Am Geriatr Soc. 2010 Apr;58(4):643–9. doi: 10.1111/j.1532-5415.2010.02762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oldenbeuving AW, de Kort PL, Jansen BP, Algra A, Kappelle LJ, Roks G. Delirium in the acute phase after stroke: incidence, risk factors, and outcome. Neurology. 2011 Mar 15;76(11):993–9. doi: 10.1212/WNL.0b013e318210411f. [DOI] [PubMed] [Google Scholar]
- 27.Marcantonio ER, Kiely DK, Simon SE, John Orav E, Jones RN, Murphy KM, et al. Outcomes of older people admitted to postacute facilities with delirium. J Am Geriatr Soc. 2005 Jun;53(6):963–9. doi: 10.1111/j.1532-5415.2005.53305.x. [DOI] [PubMed] [Google Scholar]
- 28.Fong TG, Jones RN, Shi P, Marcantonio ER, Yap L, Rudolph JL, et al. Delirium accelerates cognitive decline in Alzheimer disease. Neurology. 2009 May 5;72(18):1570–5. doi: 10.1212/WNL.0b013e3181a4129a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fong TG, Jones RN, Marcantonio ER, Tommet D, Gross AL, Habtemariam D, et al. Adverse outcomes after hospitalization and delirium in persons with Alzheimer disease. Ann Intern Med. 2012 Jun 19;156(12):848–56. doi: 10.7326/0003-4819-156-12-201206190-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gross AL, Jones RN, Habtemariam DA, Fong TG, Tommet D, Quach L, et al. Delirium and Long-term Cognitive Trajectory Among Persons With Dementia. Archives of internal medicine. 2012;172(17):1–8. doi: 10.1001/archinternmed.2012.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990 Dec 15;113(12):941–8. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 32.American Psychiatric Association. Task force on DSM-IV, diagnostic and statistical manual of mental disorders: DSM-IV. 4. Washington, DC: The Association; 1994. [Google Scholar]
- 33.American Psychiatric Association. Task force on DSM-IV, diagnostic and statistical manual of mental disorders: DSM-IV (text revision) 4. Washington, DC: The Association; 2000. [Google Scholar]
- 34.World Health Organization. The ICD-10 classification of mental and behavioural disorders: diagnostic criteria for research. World Health Organization; 1993. [Google Scholar]
- 35.Adamis D, Sharma N, Whelan PJ, Macdonald AJ. Delirium scales: A review of current evidence. Aging Ment Health. 2010 Jul;14(5):543–55. doi: 10.1080/13607860903421011. [DOI] [PubMed] [Google Scholar]
- 36.Wong CL, Holroyd-Leduc J, Simel DL, Straus SE. Does this patient have delirium?: value of bedside instruments. JAMA. 2010 Aug 18;304(7):779–86. doi: 10.1001/jama.2010.1182. [DOI] [PubMed] [Google Scholar]
- 37.Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc. 2008 May;56(5):823–30. doi: 10.1111/j.1532-5415.2008.01674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ely EW, Margolin R, Francis J, May L, Truman B, Dittus R, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) Crit Care Med. 2001 Jul;29(7):1370–9. doi: 10.1097/00003246-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 39.Han JH, Zimmerman EE, Cutler N, Schnelle J, Morandi A, Dittus RS, et al. Delirium in older emergency department patients: recognition, risk factors, and psychomotor subtypes. Acad Emerg Med. 2009 Mar;16(3):193–200. doi: 10.1111/j.1553-2712.2008.00339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Center for Medicare & Medicaid Services (CMS) Minimum Data Set, Version 3.0. Centers for Medicare & Medicaid Services; 2010. [Google Scholar]
- 41.Schuurmans MJ, Shortridge-Baggett LM, Duursma SA. The Delirium Observation Screening Scale: a screening instrument for delirium. Res Theory Nurs Pract. 2003 Spring;17(1):31–50. doi: 10.1891/rtnp.17.1.31.53169. [DOI] [PubMed] [Google Scholar]
- 42.Gaudreau JD, Gagnon P, Harel F, Tremblay A, Roy MA. Fast, systematic, and continuous delirium assessment in hospitalized patients: the nursing delirium screening scale. J Pain Symptom Manage. 2005 Apr;29(4):368–75. doi: 10.1016/j.jpainsymman.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 43.Neelon VJ, Champagne MT, Carlson JR, Funk SG. The NEECHAM Confusion Scale: construction, validation, and clinical testing. Nurs Res. 1996 Nov-Dec;45(6):324–30. doi: 10.1097/00006199-199611000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Trzepacz PT, Baker RW, Greenhouse J. A symptom rating scale for delirium. Psychiatry Res. 1988 Jan;23(1):89–97. doi: 10.1016/0165-1781(88)90037-6. [DOI] [PubMed] [Google Scholar]
- 45.Trzepacz PT, Mittal D, Torres R, Kanary K, Norton J, Jimerson N. Validation of the Delirium Rating Scale-revised-98: comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatry Clin Neurosci. 2001 Spring;13(2):229–42. doi: 10.1176/jnp.13.2.229. [DOI] [PubMed] [Google Scholar]
- 46.Breitbart W, Rosenfeld B, Roth A, Smith MJ, Cohen K, Passik S. The Memorial Delirium Assessment Scale. J Pain Symptom Manage. 1997 Mar;13(3):128–37. doi: 10.1016/s0885-3924(96)00316-8. [DOI] [PubMed] [Google Scholar]
- 47.Cole MG, Dendukuri N, McCusker J, Han L. An empirical study of different diagnostic criteria for delirium among elderly medical inpatients. J Neuropsychiatry Clin Neurosci. 2003 Spring;15(2):200–7. doi: 10.1176/jnp.15.2.200. [DOI] [PubMed] [Google Scholar]
- 48.Milisen K, Foreman MD, Abraham IL, De Geest S, Godderis J, Vandermeulen E, et al. A nurseled interdisciplinary intervention program for delirium in elderly hip-fracture patients. J Am Geriatr Soc. 2001 May;49(5):523–32. doi: 10.1046/j.1532-5415.2001.49109.x. [DOI] [PubMed] [Google Scholar]
- 49.Inouye SK, Leo-Summers L, Zhang Y, Bogardus ST, Jr, Leslie DL, Agostini JV. A chart-based method for identification of delirium: validation compared with interviewer ratings using the confusion assessment method. J Am Geriatr Soc. 2005 Feb;53(2):312–8. doi: 10.1111/j.1532-5415.2005.53120.x. [DOI] [PubMed] [Google Scholar]
- 50.Steis MR, Evans L, Hirschman KB, Hanlon A, Fick DM, Flanagan N, et al. Screening for delirium using family caregivers: convergent validity of the family confusion assessment method and interviewer-rated confusion assessment method. J Am Geriatr Soc. 2012 Nov;60(11):2121–6. doi: 10.1111/j.1532-5415.2012.04200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996 Mar 20;275(11):852–7. [PubMed] [Google Scholar]
- 52.Marcantonio ER. Postoperative delirium: a 76-year-old woman with delirium following surgery. JAMA. 2012 Jul 4;308(1):73–81. doi: 10.1001/jama.2012.6857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watt D, Koziol K, Budding D. Delirium and confusional states. In: Noggle C, Dean R, editors. Disorders in Neuropsychiatry. New York: Springer Publishing Company; 2012. [Google Scholar]
- 54.Maclullich AM, Ferguson KJ, Miller T, de Rooij SE, Cunningham C. Unravelling the pathophysiology of delirium: a focus on the role of aberrant stress responses. J Psychosom Res. 2008 Sep;65(3):229–38. doi: 10.1016/j.jpsychores.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alagiakrishnan K, Wiens CA. An approach to drug induced delirium in the elderly. Postgrad Med J. 2004 Jul;80(945):388–93. doi: 10.1136/pgmj.2003.017236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Joels M. Impact of glucocorticoids on brain function: relevance for mood disorders. Psychoneuroendocrinology. 2011 Apr;36(3):406–14. doi: 10.1016/j.psyneuen.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 57.Marcantonio ER, Rudolph JL, Culley D, Crosby G, Alsop D, Inouye SK. Serum biomarkers for delirium. J Gerontol A Biol Sci Med Sci. 2006 Dec;61(12):1281–6. doi: 10.1093/gerona/61.12.1281. [DOI] [PubMed] [Google Scholar]
- 58.Schoen J, Meyerrose J, Paarmann H, Heringlake M, Hueppe M, Berger KU. Preoperative regional cerebral oxygen saturation is a predictor of postoperative delirium in on-pump cardiac surgery patients: a prospective observational trial. Crit Care. 2011;15(5):R218. doi: 10.1186/cc10454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Caplan GA, Kvelde T, Lai C, Yap SL, Lin C, Hill MA. Cerebrospinal fluid in long-lasting delirium compared with Alzheimer's dementia. J Gerontol A Biol Sci Med Sci. 2010 Oct;65(10):1130–6. doi: 10.1093/gerona/glq090. [DOI] [PubMed] [Google Scholar]
- 60.Gaudreau JD, Gagnon P. Psychotogenic drugs and delirium pathogenesis: the central role of the thalamus. Med Hypotheses. 2005;64(3):471–5. doi: 10.1016/j.mehy.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 61.Young BK, Camicioli R, Ganzini L. Neuropsychiatric adverse effects of antiparkinsonian drugs. Characteristics, evaluation and treatment. Drugs Aging. 1997 May;10(5):367–83. doi: 10.2165/00002512-199710050-00005. [DOI] [PubMed] [Google Scholar]
- 62.Hshieh TT, Fong TG, Marcantonio ER, Inouye SK. Cholinergic deficiency hypothesis in delirium: a synthesis of current evidence. J Gerontol A Biol Sci Med Sci. 2008 Jul;63(7):764–72. doi: 10.1093/gerona/63.7.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lauretani F, Ceda GP, Maggio M, Nardelli A, Saccavini M, Ferrucci L. Capturing side-effect of medication to identify persons at risk of delirium. Aging Clin Exp Res. 2010 Oct-Dec;22(5–6):456–8. doi: 10.1007/bf03324944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hughes CG, Patel MB, Pandharipande PP. Pathophysiology of acute brain dysfunction: what's the cause of all this confusion? Curr Opin Crit Care. 2012 Oct;18(5):518–26. doi: 10.1097/MCC.0b013e328357effa. [DOI] [PubMed] [Google Scholar]
- 65.MacLullich AM, Edelshain BT, Hall RJ, de Vries A, Howie SE, Pearson A, et al. Cerebrospinal fluid interleukin-8 levels are higher in people with hip fracture with perioperative delirium than in controls. J Am Geriatr Soc. 2011 Jun;59(6):1151–3. doi: 10.1111/j.1532-5415.2011.03428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008 Jan;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jalleh R, Koh K, Choi B, Liu E, Maddison J, Hutchinson MR. Role of microglia and toll-like receptor 4 in the pathophysiology of delirium. Med Hypotheses. 2012 Dec;79(6):735–9. doi: 10.1016/j.mehy.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 68.van Gool WA, van de Beek D, Eikelenboom P. Systemic infection and delirium: when cytokines and acetylcholine collide. Lancet. 2010 Feb 27;375(9716):773–5. doi: 10.1016/S0140-6736(09)61158-2. [DOI] [PubMed] [Google Scholar]
- 69.Fong TG, Bogardus ST, Jr, Daftary A, Auerbach E, Blumenfeld H, Modur S, et al. Cerebral perfusion changes in older delirious patients using 99mTc HMPAO SPECT. J Gerontol A Biol Sci Med Sci. 2006 Dec;61(12):1294–9. doi: 10.1093/gerona/61.12.1294. [DOI] [PubMed] [Google Scholar]
- 70.Pfister D, Siegemund M, Dell-Kuster S, Smielewski P, Ruegg S, Strebel SP, et al. Cerebral perfusion in sepsis-associated delirium. Crit Care. 2008;12(3):R63. doi: 10.1186/cc6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Siepe M, Pfeiffer T, Gieringer A, Zemann S, Benk C, Schlensak C, et al. Increased systemic perfusion pressure during cardiopulmonary bypass is associated with less early postoperative cognitive dysfunction and delirium. Eur J Cardiothorac Surg. 2011 Jul;40(1):200–7. doi: 10.1016/j.ejcts.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 72.Sabayan B, Jansen S, Oleksik AM, van Osch MJ, van Buchem MA, van Vliet P, et al. Cerebrovascular hemodynamics in Alzheimer's disease and vascular dementia: a meta-analysis of transcranial Doppler studies. Ageing Res Rev. 2012 Apr;11(2):271–7. doi: 10.1016/j.arr.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 73.Zampieri FG, Park M, Machado FS, Azevedo LC. Sepsis-associated encephalopathy: not just delirium. Clinics (Sao Paulo) 2011;66(10):1825–31. doi: 10.1590/S1807-59322011001000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Choi SH, Lee H, Chung TS, Park KM, Jung YC, Kim SI, et al. Neural network functional connectivity during and after an episode of delirium. Am J Psychiatry. 2012 May;169(5):498–507. doi: 10.1176/appi.ajp.2012.11060976. [DOI] [PubMed] [Google Scholar]
- 75.Hatherill S, Flisher AJ. Delirium in children and adolescents: A systematic review of the literature. J Psychosom Res. 2010 Apr;68(4):337–44. doi: 10.1016/j.jpsychores.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 76.Izaks GJ, Westendorp RG. Ill or just old? Towards a conceptual framework of the relation between ageing and disease. BMC Geriatr. 2003 Dec 19;3:7. doi: 10.1186/1471-2318-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cole MG. Delirium in elderly patients. Am J Geriatr Psychiatry. 2004 Jan-Feb;12(1):7–21. [PubMed] [Google Scholar]
- 78.Davis DH, Muniz Terrera G, Keage H, Rahkonen T, Oinas M, Matthews FE, et al. Delirium is a strong risk factor for dementia in the oldest-old: a population-based cohort study. Brain. 2012 Sep;135(Pt 9):2809–16. doi: 10.1093/brain/aws190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kolanowski AM, Fick DM, Clare L, Therrien B, Gill DJ. An intervention for delirium superimposed on dementia based on cognitive reserve theory. Aging Ment Health. 2010 Mar;14(2):232–42. doi: 10.1080/13607860903167853. [DOI] [PubMed] [Google Scholar]
- 80.Soiza RL, Sharma V, Ferguson K, Shenkin SD, Seymour DG, Maclullich AM. Neuroimaging studies of delirium: a systematic review. J Psychosom Res. 2008 Sep;65(3):239–48. doi: 10.1016/j.jpsychores.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 81.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975 Oct;23(10):433–41. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 82.Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A. The mini-cog: a cognitive 'vital signs' measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry. 2000 Nov;15(11):1021–7. doi: 10.1002/1099-1166(200011)15:11<1021::aid-gps234>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 83.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005 Apr;53(4):695–9. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 84.Inouye SK, Zhang Y, Jones RN, Kiely DK, Yang F, Marcantonio ER. Risk factors for delirium at discharge: development and validation of a predictive model. Arch Intern Med. 2007 Jul 9;167(13):1406–13. doi: 10.1001/archinte.167.13.1406. [DOI] [PubMed] [Google Scholar]
- 85.Park M, Tang JH. Changing the practice of physical restraint use in acute care. J Gerontol Nurs. 2007 Feb;33(2):9–16. doi: 10.3928/00989134-20070201-04. [DOI] [PubMed] [Google Scholar]
- 86.Hirano LA, Bogardus ST, Jr, Saluja S, Leo-Summers L, Inouye SK. Clinical yield of computed tomography brain scans in older general medical patients. J Am Geriatr Soc. 2006 Apr;54(4):587–92. doi: 10.1111/j.1532-5415.2006.00692.x. [DOI] [PubMed] [Google Scholar]
- 87.Jacobson S, Jerrier H. EEG in delirium. Semin Clin Neuropsychiatry. 2000 Apr;5(2):86–92. doi: 10.153/SCNP00500086. [DOI] [PubMed] [Google Scholar]
- 88.Jenssen S. Electroencephalogram in the dementia workup. Am J Alzheimers Dis Other Demen. 2005 May-Jun;20(3):159–66. doi: 10.1177/153331750502000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hardy JE, Brennan N. Computerized tomography of the brain for elderly patients presenting to the emergency department with acute confusion. Emerg Med Australas. 2008 Oct;20(5):420–4. doi: 10.1111/j.1742-6723.2008.01118.x. [DOI] [PubMed] [Google Scholar]
- 90.Lai MM, Wong Tin Niam DM. Intracranial cause of delirium: computed tomography yield and predictive factors. Intern Med J. 2012 Apr;42(4):422–7. doi: 10.1111/j.1445-5994.2010.02400.x. [DOI] [PubMed] [Google Scholar]
- 91.Hufschmidt A, Shabarin V. Diagnostic yield of cerebral imaging in patients with acute confusion. Acta Neurol Scand. 2008 Oct;118(4):245–50. doi: 10.1111/j.1600-0404.2008.01006.x. [DOI] [PubMed] [Google Scholar]
- 92.Marcantonio ER. In the clinic. Delirium. Ann Intern Med. 2011 Jun 7;154(11):ITC6–1–15. doi: 10.7326/0003-4819-154-11-201106070-01006. [DOI] [PubMed] [Google Scholar]
- 93.McDowell JA, Mion LC, Lydon TJ, Inouye SK. A nonpharmacologic sleep protocol for hospitalized older patients. J Am Geriatr Soc. 1998 Jun;46(6):700–5. doi: 10.1111/j.1532-5415.1998.tb03803.x. [DOI] [PubMed] [Google Scholar]
- 94.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled clinical trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 95.Inouye SK, Bogardus ST, Jr, Baker DI, Leo-Summers L, Cooney LM., Jr The Hospital Elder Life Program: a model of care to prevent cognitive and functional decline in older hospitalized patients. Hospital Elder Life Program. J Am Geriatr Soc. 2000 Dec;48(12):1697–706. doi: 10.1111/j.1532-5415.2000.tb03885.x. [DOI] [PubMed] [Google Scholar]
- 96.Inouye SK, Baker DI, Fugal P, Bradley EH. Dissemination of the hospital elder life program: implementation, adaptation, and successes. J Am Geriatr Soc. 2006 Oct;54(10):1492–9. doi: 10.1111/j.1532-5415.2006.00869.x. [DOI] [PubMed] [Google Scholar]
- 97.Rizzo JA, Bogardus ST, Jr, Leo-Summers L, Williams CS, Acampora D, Inouye SK. Multicomponent targeted intervention to prevent delirium in hospitalized older patients: what is the economic value? Med Care. 2001 Jul;39(7):740–52. doi: 10.1097/00005650-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 98.Leslie DL, Zhang Y, Bogardus ST, Holford TR, Leo-Summers LS, Inouye SK. Consequences of preventing delirium in hospitalized older adults on nursing home costs. J Am Geriatr Soc. 2005 Mar;53(3):405–9. doi: 10.1111/j.1532-5415.2005.53156.x. [DOI] [PubMed] [Google Scholar]
- 99.Caplan GA, Harper EL. Recruitment of volunteers to improve vitality in the elderly: the REVIVE study. Intern Med J. 2007 Feb;37(2):95–100. doi: 10.1111/j.1445-5994.2007.01265.x. [DOI] [PubMed] [Google Scholar]
- 100.Chen CC, Lin MT, Tien YW, Yen CJ, Huang GH, Inouye SK. Modified hospital elder life program: effects on abdominal surgery patients. J Am Coll Surg. 2011 Aug;213(2):245–52. doi: 10.1016/j.jamcollsurg.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 101.Rubin FH, Neal K, Fenlon K, Hassan S, Inouye SK. Sustainability and scalability of the hospital elder life program at a community hospital. J Am Geriatr Soc. 2011 Feb;59(2):359–65. doi: 10.1111/j.1532-5415.2010.03243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bradley EH, Schlesinger M, Webster TR, Baker D, Inouye SK. Translating research into clinical practice: making change happen. J Am Geriatr Soc. 2004 Nov;52(11):1875–82. doi: 10.1111/j.1532-5415.2004.52510.x. [DOI] [PubMed] [Google Scholar]
- 103.Bradley EH, Webster TR, Baker D, Schlesinger M, Inouye SK. After adoption: sustaining the innovation. A case study of disseminating the hospital elder life program. J Am Geriatr Soc. 2005 Sep;53(9):1455–61. doi: 10.1111/j.1532-5415.2005.53451.x. [DOI] [PubMed] [Google Scholar]
- 104.Van Rompaey B, Elseviers MM, Van Drom W, Fromont V, Jorens PG. The effect of earplugs during the night on the onset of delirium and sleep perception: a randomized controlled trial in intensive care patients. Crit Care. 2012 May 4;16(3):R73. doi: 10.1186/cc11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Flaherty JH, Steele DK, Chibnall JT, Vasudevan VN, Bassil N, Vegi S. An ACE unit with a delirium room may improve function and equalize length of stay among older delirious medical inpatients. J Gerontol A Biol Sci Med Sci. 2010 Dec;65(12):1387–92. doi: 10.1093/gerona/glq136. [DOI] [PubMed] [Google Scholar]
- 106.Barr J, Fraser GL, Puntillo K, Ely EW, Gelinas C, Dasta JF, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013 Jan;41(1):263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 107.Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010 Jul 28;304(4):443–51. doi: 10.1001/jama.2010.1013. [DOI] [PubMed] [Google Scholar]
- 108.Xie Z, Dong Y, Maeda U, Moir R, Inouye SK, Culley DJ, et al. Isoflurane-induced apoptosis: a potential pathogenic link between delirium and dementia. J Gerontol A Biol Sci Med Sci. 2006 Dec;61(12):1300–6. doi: 10.1093/gerona/61.12.1300. [DOI] [PubMed] [Google Scholar]
- 109.Zhang Y, Xu Z, Wang H, Dong Y, Shi HN, Culley DJ, et al. Anesthetics isoflurane and desflurane differently affect mitochondrial function, learning, and memory. Ann Neurol. 2012 May;71(5):687–98. doi: 10.1002/ana.23536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang B, Tian M, Zhen Y, Yue Y, Sherman J, Zheng H, et al. The effects of isoflurane and desflurane on cognitive function in humans. Anesth Analg. 2012 Feb;114(2):410–5. doi: 10.1213/ANE.0b013e31823b2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cunningham C, Campion S, Lunnon K, Murray CL, Woods JF, Deacon RM, et al. Systemic inflammation induces acute behavioral and cognitive changes and accelerates neurodegenerative disease. Biol Psychiatry. 2009 Feb 15;65(4):304–12. doi: 10.1016/j.biopsych.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cunningham C. Systemic inflammation and delirium: important co-factors in the progression of dementia. Biochem Soc Trans. 2011 Aug;39(4):945–53. doi: 10.1042/BST0390945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chedru F, Geschwind N. Disorders of higher cortical functions in acute confusional states. Cortex. 1972;8(4):395–411. doi: 10.1016/s0010-9452(72)80004-2. [DOI] [PubMed] [Google Scholar]
- 114.Chester JG, Beth Harrington M, Rudolph JL. Serial administration of a modified Richmond Agitation and Sedation Scale for delirium screening. J Hosp Med. 2012 May-Jun;7(5):450–3. doi: 10.1002/jhm.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Koponen H, Partanen J, Paakkonen A, Mattila E, Riekkinen PJ. EEG spectral analysis in delirium. J Neurol Neurosurg Psychiatry. 1989 Aug;52(8):980–5. doi: 10.1136/jnnp.52.8.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Khoury M, Beaty T, Cohen H. Fundamentals of Genetic Epidemiology. Oxford: Oxford University Press; 1993. [Google Scholar]
- 117.Maldonado JR. Pathoetiological model of delirium: a comprehensive understanding of the neurobiology of delirium and an evidence-based approach to prevention and treatment. Crit Care Clin. 2008 Oct;24(4):789–856. ix. doi: 10.1016/j.ccc.2008.06.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.