Abstract
Cholinesterase inhibitors (ChEIs) are widely used for the symptomatic treatment of Alzheimer’s disease (AD). In vitro and in animal studies, ChEIs have been shown to influence the processing of Aβ and the phosphorylation of tau, proteins that are the principal constituents of the plaques and neurofibrillary tangles, respectively, in AD brain. However, little is known about the effects of these drugs on Aβ and tau pathology in AD. Using avidin-biotin immunohistochemistry and computer-assisted image analysis, we compared Aβ and tau loads in the frontal and temporal cortices of 72 brains from matched cohorts of AD patients who had or had not received ChEIs. Patients treated with ChEIs had accumulated significantly more phospho-tau in their cerebral cortex than had untreated patients (P = 0.004). Aβ accumulation was reduced but not significantly. These data raise the possibility that increased tau phosphorylation may influence long-term clinical responsiveness to ChEIs.
Keywords: Alzheimer’s disease, Cholinesterase inhibitors, Amyloid, Tau, Neuropathology
Introduction
Progressive cognitive decline in Alzheimer’s disease (AD) correlates closely with loss of cholinergic function. The deficit in acetylcholine-induced activity is associated with marked loss of cholinergic neurons (chiefly from the basal forebrain) [14] and a decrease in specific acetylcholine receptor subunits. Initial reports suggested that the muscarinic receptors are predominantly affected in AD [6, 7] but subsequent studies showed extensive loss of nicotinic receptors as well [8]. Most of the licensed pharmacological treatments for AD are cholinesterase inhibitors (ChEIs). These improve cognition and function in many individuals, but not all patients benefit [11] and the duration of response is unclear.
Data from cell culture studies and rodent models suggest that drugs which interact with muscarinic and nicotinic receptors influence the metabolism of amyloid-β peptide (Aβ) and phosphorylation of tau [3, 12]. These experimental data are supported by post-mortem observations of an increased density of Aβ plaques in patients with Parkinson’s disease who had been treated with anticholinergic drugs [10] and of a reduced Aβ load in patients with dementia with Lewy bodies (DLB) who had been treated with ChEIs [1]. In a case-control study, AD patients receiving the ChEI donepezil had a slowed progression of hippocampal atrophy [4], suggesting a decreased rate of progression of their AD. In a previous autopsy study focusing on DLB, in which a high proportion of patients have concurrent AD pathology, we found that in comparison to untreated patients, those receiving ChEIs had a significant reduction of Aβ but a non-significant increase in tau pathology [1].
In the present study we have compared the extent of Aβ and tau pathology in post-mortem brain tissue from AD patients who had been treated with ChEIs, to that in tissue from patients who had not.
Materials and methods
Patients
Post-mortem brain tissue was examined from 72 patients with AD (56 from South West Dementia Brain Bank, Bristol UK and 16 from the Alzheimer Disease Research Center Neuropathology and Molecular Genetics Core at UCLA, Los Angeles, USA), whose brains had been donated for research. All of the patients had a neuropathologically confirmed diagnosis of probable or definite AD according to Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) criteria, and of Braak tangle stage V–VI [2]. Half (the treatment group, n = 36) had received a course of treatment with one or more ChEIs (donepezil n = 8, galantamine n = 9, tacrine n = 17, rivastigmine n = 1) whilst the other half (the control group) had no history of ChEI treatment. The two groups were approximately matched for age, gender (treatment group: females 15, age range 52–88 years, mean = 73, SD = 9.3; control group: females 15, age range 60–99 years, mean = 76.1, SD = 8.4) and highest educational level. APOE genotypes (*/*, */ε4, ε4/ε4) were 11, 10 and 1 in the treatment group and 6, 11 and 7 in the controls (χ2 = 5.94, P = 0.051). Relevant clinical data including age at death, initial and final Mini-Mental State Examination (MMSE) scores, specific ChEI, frequency and duration of treatment and duration of disease were extracted from the patients’ medical records when available. This study was approved by Frenchay Research Ethics Committee.
Immunohistochemistry
Sections 7 µm in thickness were cut from paraffinembedded, formalin-fixed blocks of frontal lobe in the coronal plane of the head of the caudate nucleus (i.e. including BA 6 and 24) and of temporal lobe in the coronal plane of the lateral geniculate body (including BA 21 and 22). Sections were immunolabelled with a pan-Aβ antibody (1:2000, M07872, DAKO, Glostrup, Denmark; sections were pre-treated with formic acid) and antibody to Ser202 phospho-tau (1:3000, AT8, Zymed, CA, USA), by a standard streptavidin-biotin-peroxidase method and visualised with diaminobenzadine (DAB).
Quantitation of immunolabelling
The extent of parenchymal Aβ deposition and phosphotau accumulation within the frontal and temporal neocortex was quantitated, as previously described [1], using computer-assisted image analysis. Histometrix software (Kinetic Imaging, Nottingham, UK) driving a Leica DM microscope with a motorised stage was used to demarcate three, approximately 5 mm lengths of cortical ribbon in BA 24 and 6 of the frontal and BA 21 and 22 of the temporal lobe. Within each of the three delineated regions, unbiased selection of ten ×20-objective fields was made by the software and the percentage area immunopositive for the antigen of interest (Aβ or phospho-tau) was determined for each section. By interactive editing of the captured images, vascular Aβ was excluded from the analysis, as were any artefacts.
Statistical analyses
Independent t tests with the help of SPSS version 12.0 were used to compare demographic data between treated and untreated cases and Mann–Whitney U tests were used to compare tau measurements in the treated and untreated cases. A P < 0.05 was considered statistically significant.
Results
The demographic characteristics of the treated and untreated cases were very similar, as were the initial MMSE scores; no statistical differences between the two groups were evident for any of these parameters (Table 1). The APOE genotypes for the two cohorts were not significantly different (P = 0.051); however, it is clear that there are more ε4 homozygotes in the untreated cohort than the treated. The genotypes of the cases were not found to affect either the Aβ or phosphorylated tau loads (data not shown). Unfortunately only one-third of each group had a record of a final MMSE being completed within 2 years prior to the individual’s death. For this reason the data for final MMSE scores are not shown in Table 1.
Table 1.
Demographic, clinical and neuropathological data for untreated and cholinesterase inhibitor-treated cases
| Treated (n = 36) | Untreated (n = 36) | Statistical analyses | |||
|---|---|---|---|---|---|
| Mean ± SD | Median (IQR) | Mean ± SD | Median (IQR) | ||
| Age (years) | 73 ± 9.3 | 74 (66.25–80) | 76.1 ± 8.4 | 76.5 (69.25–81) | t = 1.302, P = 0.197 |
| Education (years) | 13.4 ± 4.3 | 11 (10–16.25) | 12.46 ± 3.8 | 11 (9–16.25) | t = −0.663, P = 0.32 |
| Initial MMSE score | 15.6 ± 5.3 | 15 (12–20) | 16.8 ± 8.4 | 16.5 (11–23.75) | t = 0.583, P = 0.565 |
| Duration of disease (years) | 7.9 ± 2.9 | 8 (6.38–10) | 8.1 ± 2.9 | 8 (6–10) | t = 0.287, P = 0.775 |
| Pretreatment MMSE scorea | 15.5 ± 5.5 | 15 (12–21) | |||
| Pretreatment disease duration (years)a | 4.2 ± 1.2 | 4 (3–5) | |||
| Length of treatment (months) | 18.4 ± 15.3 | 14.5 (5.25–29.5) | |||
| Parenchymal Aβ load (%) | 2.65 ± 1.64 | 2.45 (1.52–3.59) | 3.11 ± 2.2 | 2.5 (1.48–4.39) | U = 585, P = 0.478 |
| Tau load (%) | 6.21 ± 3.53 | 5.51 (3.75–9.33) | 4.01 ± 3.2 | 3.0 (1.72–5.69) | U = 378, P = 0.004 |
Data available for 19 cases
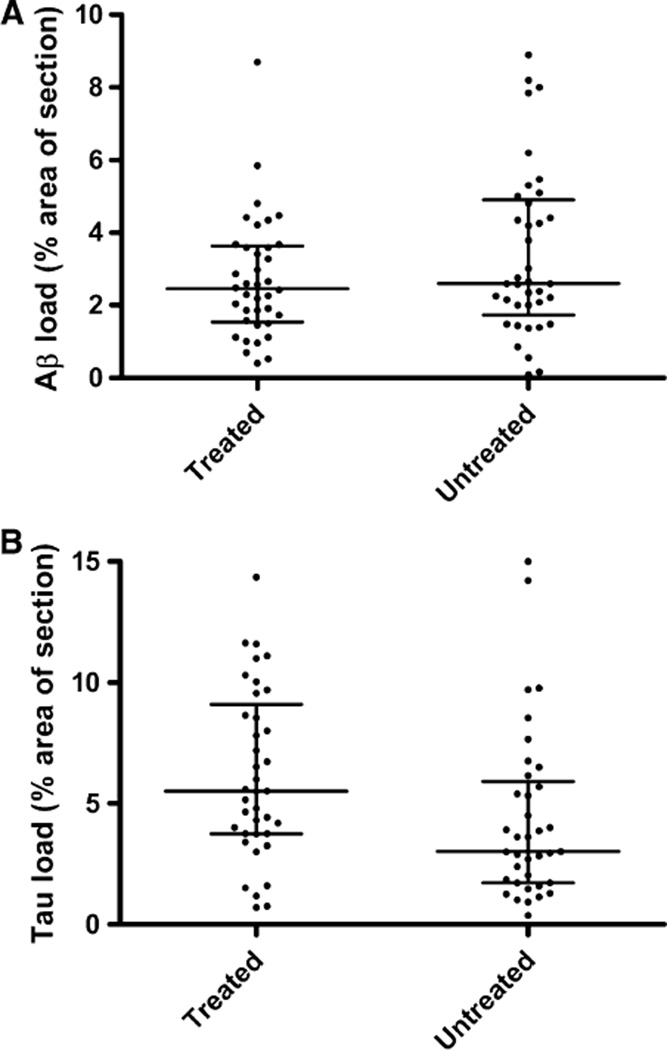
The extent of parenchymal Aβ deposition did not differ significantly between treated and untreated patients (P = 0.48) (Table 1; Fig. 1a) and further analysis showed that Aβ loads did not differ significantly between treatment subgroups. However, the extent of phospho-tau immunolabelling was significantly greater in those who had been treated with a ChEI than those who had not (P = 0.004) (Table 1; Fig. 1b). Phospho-tau load did not differ significantly between treatment subgroups. There was no significant relationship between Aβ or phospho-tau load and either MMSE score at commencement of treatment or duration of treatment.
Fig. 1.
Phosphorylated tau is increased in cholinesterase inhibitor-treated AD cases compared to non-treated AD cases. Frontal and temporal cortical sections were immunolabelled with antibodies to Aβ and Ser 202-phospho tau and visualised with avidin-biotin-peroxidase and DAB. Computer-assisted image analysis software was used to assess the percentage area of section occupied by Aβ and phosphorylated tau. Scatterplots show the distribution of Aβ (a) and phosphorylated tau (b). The horizontal bars indicate the median values and the interquartile ranges
Discussion
Although ChEIs have been used for treatment of many AD patients over several years and despite in vitro and animal studies indicating that these drugs affect the metabolism of Aβ and tau, to our knowledge this is the first post-mortem examination of the possible consequences of ChEI treatment on Aβ and tau pathology in AD in man.
Our finding that the amount of hyperphosphorylated tau was greater in ChEI-treated than in untreated cases was unexpected and needs verification. Since increased tau pathology (higher Braak stage) correlates well with cognitive decline, one possible confounder that we considered was that the treated and untreated patients might have differed in cognitive performance. However, at least as measured by their MMSE scores at commencement of treatment, cognitive status did not differ between the treated and untreated patients. MMSE scores obtained during treatment with ChEIs might have shown whether or not the drugs were having any beneficial effects on cognition, but these data were unfortunately not available for many of the cases. The results are, however, consistent with the experimental literature. The treatment of cell lines and synaptosomes with nicotine increased tau phosphorylation [5, 13] and chronic treatment of SH-SY5Y cells with tacrine, galantamine or donepezil increased the amount of phosphorylated tau [5]. Chronic administration of nicotine to triple transgenic mice (PS1M146V, APPSwe, and tauP301L) produced a marked increase in the aggregation and phosphorylation of tau at the AT8-specific epitope [9].
The exact mechanism by which nicotinic agonists stimulate the phosphorylation of tau is not clear. One study found that the level of p38-MAP kinase, capable of phosphorylating tau in vivo, was increased in transgenic mice that had received nicotine whilst the level of GSK3β, also capable of phosphorylating tau, was unchanged [9].
Limitations of the present study include the modest sample sizes, retrospective design and potential bias related to the patient characteristics in the two cohorts. The study was also hindered by the absence of dosing information for many of the cases. Current guidelines and practice are such that it would be unethical to conduct this type of study prospectively. The present cohorts were well matched with respect to demographic characteristics and initial MMSE scores. As far as sample size is concerned, we were constrained by the availability of archival cases suitable for inclusion in this study (i.e. matched cohorts of patients with treatment records and clinical data) but it will, of course, be important to see whether or not the present findings can be confirmed in other cohorts.
Our study suggests that treatment with ChEIs influences the pathological changes in the brain in AD. In this preliminary investigation the amount of phospho-tau appeared to be increased by ChEIs. These actions are likely to be important in influencing the long-term clinical responses to ChEIs and the strategy of combining a ChEI with a drug that reduces phosphorylation of tau may prove to be a particularly attractive treatment option in the future.
Acknowledgments
We should like to thank Professor Jeff Cummings for allowing us access to clinical data from his patients and Justine Pomakian and Carein Todd-Downing for their help in organising the databases. This work was supported by Janssen Pharmaceuticals and NIH P50 AG 16570 (HVV). The analysis used equipment provided by Alzheimer’s Research Trust (ART) and Bristol Research into Alzheimer’s and Care of the Elderly (BRACE). KAC is supported by an ART research fellowship. HVV is supported in part by the Daljit S. and Elaine Sarkaria Chair in Diagnostic Medicine at the David Geffen School of Medicine at UCLA.
Contributor Information
Katy A. Chalmers, Email: katy.chalmers@bristol.ac.uk, Dementia Research Group, Department of Clinical Science at North Bristol, University of Bristol, Bristol, UK; Dementia Research Group, John James Laboratories, Frenchay Day Hospital, Frenchay Hospital, Bristol BS16 1LE, UK.
Gordon K. Wilcock, Dementia Research Group, Department of Clinical Science at North Bristol, University of Bristol, Bristol, UK
Harry V. Vinters, Department of Pathology and Laboratory Medicine (Neuropathology), David Geffen School of Medicine at UCLA, Los Angeles, USA
Elaine K. Perry, Institute for Ageing and Health, Newcastle General Hospital, Newcastle upon Tyne, UK
Robert Perry, Institute for Ageing and Health, Newcastle General Hospital, Newcastle upon Tyne, UK.
Clive G. Ballard, Wolfson Centre for Age-Related Disease, King’s College London, London, UK
Seth Love, Dementia Research Group, Department of Clinical Science at North Bristol, University of Bristol, Bristol, UK.
References
- 1.Ballard CG, Chalmers KA, Todd C, McKeith IG, O’Brien JT, Wilcock G, Love S, Perry EK. Cholinesterase inhibitors reduce cortical A-beta in dementia with Lewy bodies. Neurology. 2007;68:1726–1729. doi: 10.1212/01.wnl.0000261920.03297.64. [DOI] [PubMed] [Google Scholar]
- 2.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 3.Fisher A, Pittel Z, Haring R, Bar-Ner N, Kliger-Spatz M, Natan N, Egozi I, Sonego H, Marcovitch I, Brandeis R. M1 muscarinic agonists can modulate some of the hallmarks in Alzheimer’s disease: implications in future therapy. J Mol Neurosci. 2003;20:349–356. doi: 10.1385/JMN:20:3:349. [DOI] [PubMed] [Google Scholar]
- 4.Hashimoto M, Kazui H, Matsumoto K, Nakano Y, Yasuda M, Mori E. Does donepezil treatment slow the progression of hippocampal atrophy in patients with Alzheimer’s disease? Am J Psychiatry. 2005;162:676–682. doi: 10.1176/appi.ajp.162.4.676. [DOI] [PubMed] [Google Scholar]
- 5.Hellstrom-Lindahl E, Moore H, Nordberg A. Increased levels of tau protein in SH-SY5Y cells after treatment with cholinesterase inhibitors and nicotinic agonists. J Neurochem. 2000;74:777–784. doi: 10.1046/j.1471-4159.2000.740777.x. [DOI] [PubMed] [Google Scholar]
- 6.Mash DC, Flynn DD, Potter LT. Loss of M2 muscarine receptors in the cerebral cortex in Alzheimer’s disease and experimental cholinergic denervation. Science. 1985;228:1115–1117. doi: 10.1126/science.3992249. [DOI] [PubMed] [Google Scholar]
- 7.Mulugeta E, Karlsson E, Islam A, Kalaria R, Mangat H, Winblad B, Adem A. Loss of muscarinic M4 receptors in hippocampus of Alzheimer patients. Brain Res. 2003;960:259. doi: 10.1016/s0006-8993(02)03542-4. [DOI] [PubMed] [Google Scholar]
- 8.Nordberg A, Alafuzoff I, Winblad B. Nicotinic and muscarinic subtypes in the human brain: changes with aging and dementia. J Neurosci Res. 1992;31:103–111. doi: 10.1002/jnr.490310115. [DOI] [PubMed] [Google Scholar]
- 9.Oddo S, Caccamo A, Green KN, Liang K, Tran L, Chen Y, Leslie FM, LaFerla FM. Chronic nicotine administration exacerbates tau pathology in a transgenic model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2005;102:3046–3051. doi: 10.1073/pnas.0408500102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perry EK, Kilford L, Lees AJ, Burn DJ, Perry RH. Increased Alzheimer pathology in Parkinson’s disease related to antimuscarinic drugs. Ann Neurol. 2003;54:235–238. doi: 10.1002/ana.10639. [DOI] [PubMed] [Google Scholar]
- 11.Takeda A, Loveman E, Clegg A, Kirby J, Picot J, Payne E, Green C. A systematic review of the clinical effectiveness of donepezil, rivastigmine and galantamine on cognition, quality of life and adverse events in Alzheimer’s disease. Int J Geriatr Psychiatry. 2006;21:17–28. doi: 10.1002/gps.1402. [DOI] [PubMed] [Google Scholar]
- 12.Verhoeff NP. Acetylcholinergic neurotransmission and the beta-amyloid cascade: implications for Alzheimer’s disease. Expert Rev Neurother. 2005;5:277–284. doi: 10.1586/14737175.5.2.277. [DOI] [PubMed] [Google Scholar]
- 13.Wang HY, Li W, Benedetti NJ, Lee DH. Alpha 7 nicotinic acetylcholine receptors mediate beta-amyloid peptide-induced tau protein phosphorylation. J Biol Chem. 2003;278:31547–31553. doi: 10.1074/jbc.M212532200. [DOI] [PubMed] [Google Scholar]
- 14.Wilcock GK, Esiri MM, Bowen DM, Smith CC. The nucleus basalis in Alzheimer’s disease: cell counts and cortical biochemistry. Neuropathol Appl Neurobiol. 1983;9:175–179. doi: 10.1111/j.1365-2990.1983.tb00105.x. [DOI] [PubMed] [Google Scholar]