Abstract
Astrocyte swelling and brain edema are major complications of the acute form of hepatic encephalopathy (acute liver failure, ALF). While elevated brain ammonia level is a well-known etiological factor in ALF, the mechanism by which ammonia brings about astrocyte swelling is not well understood. We recently found that astrocyte cultures exposed to ammonia activated nuclear factor-kappaB (NF-κB), and that pharmacological inhibition of such activation led to a reduction in astrocyte swelling. Although these findings suggest the involvement of NF-κB in astrocyte swelling in vitro, it is not known whether NF-κB contributes to the development of brain edema in ALF in vivo. Furthermore, pharmacological agents used to inhibit NF-κB may have non-specific effects. Accordingly, we used transgenic (Tg) mice that have a functional inactivation of astrocytic NF-κB and examined whether these mice are resistant to ALF-associated brain edema. ALF was induced in mice by treatment with the hepatotoxin thioacetamide (TAA). Wild type (WT) mice treated with TAA showed a significant increase in brain water content (1.65%) along with prominent astrocyte swelling and spongiosis of the neuropil, consistent with the presence of cytotoxic edema. These changes were not observed in Tg mice treated with TAA. Additionally, WT mice with ALF showed an increase in inducible nitric oxide synthase (iNOS) immunoreactivity in astrocytes from WT mice treated with TAA (iNOS is known to be activated by NF-κB and to contribute to cell swelling). By contrast, Tg mice treated with TAA did not exhibit brain edema, histological changes nor an increase in iNOS immunoreactivity. We also examined astrocytes cultures derived from Tg mice to determine whether these cells exhibit a lesser degree of swelling and cytopathological changes following exposure to ammonia. Astrocyte cultures derived from Tg mice showed no cell swelling nor morphological abnormalities when exposed to ammonia for 24 h. By contrast, ammonia significantly increased cell swelling (31.7%) in cultured astrocytes from WT mice and displayed cytological abnormalities. Moreover, we observed a lesser increment in inducible nitric oxide synthase and NADPH oxidase activity (both are also known to be activated by NF-κB and to contribute to astrocyte swelling) in astrocyte cultures from Tg mice treated with ammonia, as compared to ammonia-treated WT mice astrocytes. These findings strongly suggest that activation of NF-κB is a critical factor in the development of astrocyte swelling/brain edema in ALF.
Keywords: Acute liver failure, ammonia, astrocyte swelling, iNOS, NADPH oxidase, NF-κB
Introduction
Hepatic encephalopathy (HE) is a major neuropsychiatric disorder that occurs in patients with severe liver failure. The acute form of HE (acute liver failure; ALF) is a potentially lethal condition as it may lead to cerebral edema, increased intracranial pressure and brain herniation. While the pathogenesis of HE is incompletely understood, ammonia has been strongly implicated as an important factor [for reviews, see Albrecht and Jones (1999); Hazell and Butterworth (1999)], and astrocytes appear to be the primary target of its neurotoxicity (Norenberg 1996). Astrocyte swelling is the major neuropathologic finding in ALF (Norenberg 1977; Traber et al. 1987; Swain et al. 1991), and both in vivo (Voorhies et al. 1983; Willard-Mack et al. 1996) and in vitro (Norenberg et al. 1991; Zwingmann et al. 2000) studies have shown astrocyte swelling after exposure to a pathophysiological concentration of ammonia. The precise mechanism by which ammonia brings about such swelling remains unclear.
We recently documented that exposure of astrocyte cultures to ammonia stimulated the activation (nuclear translocation) of the transcription factor nuclear factor-kappaB (NF-κB) (Sinke et al., 2008). Ammonia-treated astrocyte cultures have also been shown to increase the activity of inducible nitric oxide synthase (iNOS) and to promote NO generation (Schliess et al. 2002; Sinke et al. 2008), factors known to be associated with cell swelling/brain edema (for review, see Norenberg et al., 2007), while treatment of cultures with the NF-κB inhibitor BAY 11–7082, diminished iNOS protein expression and NO generation (Sinke et al. 2008). Additionally, inhibition of NF-κB activation by BAY 11–7082 reduced astrocyte swelling in culture (Sinke et al. 2008).
Another consequence of NF-κB activation is induction of oxidative stress (OS) (for review, see Bowie and O’Neill 2000). Regulation of NADPH oxidase (NOX) by NF-κB represents a major mechanism for the induction of OS, since NOX is an important source of superoxide generation in most cells (Manea et al., 2007; Luengo-Blanco et al. 2008). OS is known to induce cell swelling/brain edema in different neurological conditions (for review, see Norenberg et al., 2009). Ammonia-treated astrocyte cultures have been shown to increase NOX activity, and treatment of cultures with the NOX inhibitor, apocyanin, reduced ammonia-induced astrocyte swelling (Reinehr et al., 2007; Jayakumar et al., 2009). These studies suggested that activation of NF-κB is involved in the mechanism of ammonia-induced astrocyte swelling, in part through enhanced NOX activation. While these studies implicate NF-κB in cell swelling mechanisms in cultured astrocytes as a consequence of oxidative stress, the role of NF-κB in the brain edema associated with ALF is not known.
Since pharmacological inhibitors often have non-specific effects, the present study examined transgenic (Tg) mice that possess a functional inactivation of astrocytic NF-κB, and determined whether these mice are resistant to thioacetamide (TAA)-induced brain edema. Additionally, we examined whether astrocyte cultures derived from Tg mice leads to a reduction in cell swelling in cultured astrocytes following ammonia treatment, and whether a reduction in iNOS and NOX activities (both are known to be activated by NF-κB) occur in astrocytes derived from Tg mice. Our study demonstrates that Tg mice treated with the hepatotoxin TAA are resistant to the development of brain edema as compared to WT mice. Similarly, cultured astrocytes derived from Tg mice when treated with ammonia showed no significant cell swelling. These findings invoke an important role of NF-κB in the mechanism of the astrocyte swelling/brain edema associated with ALF.
Materials and methods
ALF model
The mouse model used in this study was developed by Brambilla et al., 2005, in which the astroglial NF-κB is inactivated by expressing a mutated form of the I-kappaB-alpha under the control of GFAP promoter (glial fibrillary acidic protein [GFAP]-dn mice). This mutation prevents its phosphorylation and thereby prevents the activation of NF-κB. These transgenic (Tg) mice survive normally to adulthood and do not exhibit any apparent abnormal sensori-motor phenotype. Such astroglial specific NF-κB inactivation has been shown to improve functional recovery in various neurological disorders, including ischemia (Dvoriantchikova et al., 2009), pain and inflammation (Fu et al., 2007, 2010), spinal cord injury (Brambilla et al., 2005, 2009a), improvement in functional outcome following experimental autoimmune encephalomyelitis (Brambilla et al., 2009b) as well as recovery from learning and memory deficits (Bracchi-Ricard et al., 2008).
Tg and wild-type (WT) mice were given the hepatotoxin thioacetamide (TAA, 100 mg/kg body weight, i.p.) for 3 consecutive days. The clinical status and liver function of the TAA mouse model have been previously established (Sarhan et al. 1993; Itzhak et al. 1995). Animals were sacrificed 28 h after the last injection. Control mice received normal saline only. To prevent hypoglycemia, mice were given 12.5 ml/kg body weight of an iso-osmolar solution (at 12 h intervals, s.c.) consisting of 5% dextrose and 0.45% saline with 20 mEq/l of potassium chloride (Zimmermann et al. 1989; Hilgier et al. 1994). Procedures followed guidelines established by the National Institute of Health (Guide for the Care and use of Laboratory Animals) and were approved by the Institutional Animal Care and Use Committee.
Clinical status
TAA-treated mice were clinically monitored twice a day and stages of encephalopathy were graded according to the criteria of Gammal et al. (1990): Grade I: lethargy (generalized reduction in spontaneous activity); Grade II: mild ataxia; Grade III: lack of spontaneous movement but with intact righting reflex; Grade IV: loss of righting reflex but intact pain reflex (measured by reaction to tail pinch); Grade V: coma.
Liver function tests
To assess the extent of liver failure in TAA-treated mice, blood levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were determined 28 h after the last injection of TAA using a Cobes 0501 automatic analyzer (Roche Diagnostics, IN). Plasma ammonia level was measured using a commercially available ammonia assay kit, following the manufacturer’s protocol (Sigma–Aldrich, St. Louis, MO).
Brain ammonia estimation
Brain ammonia was measured as described previously (Rama Rao et al., 2010). Briefly, at the end of treatment, mice were decapitated, brains quickly removed and cortices from control and experimental animals were homogenized. After centrifugation, ammonia levels in the supernatants were measured using an ammonia assay kit, following the manufacturer’s protocol (Sigma–Aldrich, St. Louis, MO).
Liver and brain histopathology
For histopathologic correlation, samples of liver and brain from TAA-treated mice were fixed in 10% formalin for 24 h, processed routinely for paraffin sections and stained with Hematoxylin and Eosin (H&E).
Brain water measurement
The gravimetric method of Marmarou et al. (1978) was employed to estimate brain edema. In brief, cortical tissue (10 mg) was placed in a bromobenzine-kerosine density gradient column. The equilibration points of the brain samples were read at 2 min. Conversion of specific gravity to brain water was performed as previously described (Marmarou et al., 1978).
Astrocyte cultures
Astrocyte cultures were prepared from brains of 1-day-old WT and Tg mice pups using the method of Ducis et al. (1990). Briefly, cerebral cortices were freed of meninges, minced, dissociated by trituration and vortexing, passed through sterile nylon sieves and placed in Dulbecco's modified Eagle medium (Life Technologies, Gaithersburg, MD) containing penicillin, streptomycin, and fetal bovine serum. Cultures were maintained at 37°C in a humidified incubator provided with 5% CO2 and 95% air. After 10 days in culture, bovine serum was replaced with 10% horse serum. On day 14, cultures were treated and maintained with dibutyryl cyclic-AMP (Sigma, St. Louis, MO) to enhance cell differentiation (Juurlink and Hertz 1985). Cultures consisted of at least 98% astrocytes as determined by glial fibrillary acidic protein and glutamine synthetase immunocytochemistry. The remaining cells consisted of microglia. Experiments were carried out in 3- to 4-week-old cultures.
Cell volume determination
Cell volume was estimated by measuring the intracellular water space using the method of Kletzien et al. (1975), as modified for cell cultures by Kimelberg (1987) and Norenberg et al. (1991). Briefly, 1 mmol/L of 3-O-methylglucose and 0.5 µCi/ml [3H]-3-O-methylglucose (Sigma, St. Louis, MO) were added to the cultures 6 hours before the volume assay. At the end of the incubation period, culture medium was aspirated, and an aliquot was saved for radioactivity determination. Cells were rapidly washed 6 times with ice-cold buffer containing 229 mmol/L of sucrose, 1 mmol/L of Tris-nitrate, 0.5 mmol/L of calcium nitrate, and 0.1 mmol/L of phloretin, pH 7.4. Cells were harvested into 0.5 ml of 1N sodium hydroxide. Radioactivity was determined in the cell extracts and medium, and an aliquot of the cell extract was used for protein estimation by bicinchoninic acid method (BCA, Pierce, Rockford, IL). Values were normalized to protein level, and cell volume was expressed as a percent change over control.
Immunoblots
Samples of astrocytes were analyzed for iNOS protein content by Western blots as described previously (Jayakumar et al. 2006). In brief, equal amounts of protein were subjected to gel electrophoresis and immunoblotting. An antibody against iNOS (1:750; Santa Cruz Biotechnology Inc, Santa Cruz, CA) was used to detect iNOS protein expression. Anti-α-tubulin antibody was obtained from Oncogene (San Diego, CA, USA). Horseradish peroxidase-conjugated anti-mouse and anti-rabbit secondary antibodies (Vector Laboratories, Burlingame, CA) were used at 1:1000 dilutions.
Immunohistochemistry of inducible nitric oxide synthase (iNOS)
WT and Tg mice (4 animals each from control and TAA-treated) were decapitated, brains rapidly removed and fixed in 10% buffered formalin. The tissue was processed routinely for paraffin embedding and 10 µm thick sections were prepared. Fluoroscence immunohistochemistry was performed to visualize iNOS in cortical astrocytes, as previously described (Stanislaus et al., 1999). Since glial fibrillary acidic protein is known to be down-regulated in ALF (Sobel et al., 1981; Kretzschmar et al., 1985; Kimura and Budka, 1986), as well as in ammonia-treated astrocyte cultures (Norenberg et al., 1990), we instead used an antibody to glutamine synthetase (GS), an astrocyte-specific enzyme, to visualize iNOS in astrocytes. Sections were blocked with 10% goat serum and incubated with specific antibodies to iNOS (N-20, sc-651 Santa Cruz Biotechnology) and to GS (sc-74430 Santa Cruz Biotechnology) overnight at 4°C. Sections were washed with TBS containing 1% Tween-20 (TBS-T) and then incubated for 2 h with fluorescence-conjugated secondary antibodies (1:500; AlexaFlour-546 for GS and AlexaFlour-488 for iNOS, Molecular Probes, Oregon). Sections were covered with mounting media (Vector Laboratories) and examined with a confocal laser-scanning microscope. Random collection of images from sections of control and TAA-treated mice was achieved by systematically capturing each image in a blinded manner by moving the microscope stage by approximately 5 mm in four different directions. At least 15 fluorescent images were captured per mouse, and the images were merged to localize astrocytic iNOS expression.
Measurement of nitric oxide generation
Nitric oxide production was analyzed in cultured astrocytes using DAF-2DA, a green fluorescence probe specific for intracellular NO (Sinke et al. 2008). Briefly, control and ammonia-treated culture plates were washed three times with serum-free culture medium and incubated with DAF-2DA (10 µM) for 15 min at 37°C. Cultures were then washed two to three times with PBS, scraped into 500 µL of 0.2% Triton X-100 and sonicated for 5 s. A small aliquot of the cell extract was removed for protein estimation and 250 µL of the extract was transferred to a 96-well microtiter plate (Fluorolite 1000, Dynex Technologies Inc, Chantilly, VA). Fluorescence was measured at an excitation wavelength of 492 nm and the emission fluorescence at 515 nm. The results were expressed as fluorescence intensity units/mg protein.
NADPH oxidase activity
Cells exposed to different experimental conditions were washed 3 times with Hanks’ balanced salt solution, trypsinized, resuspended in 300 µl homogenization buffer [phosphate buffer (50 mmol/L), EDTA (0.01 mmol/L), leupeptin (2 µmol/L), pepstatin A (2 µmol/L), phenylmethylsulfonyl fluoride or phenylmethanesulfonyl fluoride (1 mmol/L) pH 7.4], and homogenized by sonication (15 s pulses x3). NADPH oxidase activity was measured by a chemiluminescence assay in a 50 mmol/L phosphate buffer pH 7.4, containing 1.0 mmol/L EGTA, 5 µmol/L lucigenin, and 100 µmol/L NADPH as substrate. The assay was initiated by the addition of 20 µl homogenate. OOV0254;2 production was measured by means of lucigenin chemiluminescence (Jaimes et al. 2004), and adjusted for protein content. The results were expressed as luminescence intensity/min/mg protein. Protein content in homogenates was measured by the bicinchoninic acid method (BCA, Pierce, Rockford, IL).
Statistical analysis
All experiments were performed and repeated 6–7 times using cells derived from different batches of astrocyte cultures. The number of individual culture plates used in each experimental group was 5 for astrocyte swelling, and 2–4 for Western blot studies. Seven animals from each group were used for in vivo studies. The data of all experiments were subjected to analysis of variance followed by Tukey’s post hoc comparisons. A value of p<0.05 was considered significant.
Results
Treatment of mice with TAA
Clinical grading of encephalopathy in mice (7/group) treated with TAA was as follows: Day 1 - All mice from both groups (WT and Tg) were in Grade 1. Day 2 - In WT mice, 1 was in Grade I, and 6 were in Grade II, whereas in Tg mice 5 were in Grade I, and 2 in Grade II. Day 3 - In WT mice, 5 were in Grade IV, and 2 in Grade V, whereas in Tg mice 4 were in Grade III, and 3 in Grade IV. This data is presented in graphical form in Figure 1.
Figure 1.
Stages of encephalopathy over the course of TAA treatment in WT and Tg mice.
Light microscopic examination of livers taken from WT mice treated with TAA revealed a moderate degree of degeneration, necrosis and slight lobular collapse. Similar findings were observed in Tg mice treated with TAA (figures not shown).
The basal levels of plasma aspartate aminotransferase (AST) as well as alanine aminotransferase (ALT) in untreated transgenic animals were higher than those in WT controls (92 and 90.1% for AST and ALT, respectively). On the other hand, Tg mice treated with TAA showed a several-fold increase in AST and ALT, that was not statistically different from TAA-treated WT mice (Table 1). Plasma and brain ammonia level increased 5.7- and 3.6-fold, respectively, in TAA-treated WT mice (Table 2). Similar findings were observed in Tg mice treated with TAA.
TABLE 1.
Liver enzymes of mice treated with TAA
| WT-Control | WT-TAA | Tg-Control | Tg-TAA | |
|---|---|---|---|---|
| AST (units/liter) | 150.3 ± 17.4 | 3220.0 ± 1312.0* | 288.00 ± 86.0† | 4399.00 ± 845.0* § |
| ALT (units/liter) | 27.0 ± 5.0 | 3740.0 ± 1258.0* | 51.33 ± 9.7† | 4881.00 ± 1138.0* § |
Mean values ± SD. Control n = 7; TAA n = 7 (28 h after the third injection)
Statistically significant difference from respective controls (p < 0.05)
Statistically different from WT-Control.
Not statistically different from WT-TAA.
TABLE 2.
Blood and brain ammonia of mice treated with TAA
| Ammonia level | ||
|---|---|---|
| Groups | Blood (µmol/L) | Brain (µmol/0.2 gm tissue) |
| WT-Control | 72.6 ±16.8 | 309.8 ± 41.1 |
| WT-TAA | 411.9 ± 24.2* | 1106.3 ± 172.14* |
| Tg-Control | 67.4 ± 19.5 | 276.1 ± 31.4 |
| Tg-TAA | 357.8 ± 29.6*† | 1012.6 ± 143.5*† |
Control n = 4; TAA n = 4. Ammonia levels were determined 28 h after the third injection of TAA.
Statistically significant difference from respective controls (p < 0.05).
Not statistically different from WT-TAA.
Brain ammonia concentrations in WT and Tg mice controls were 1.55 and 1.38 mmoles/gm tissue, respectively; in TAA-treated WT and Tg mice the values were 5.54 and 5.06 mmoles/gm tissue, respectively. Taking into account the 80% brain water content in mice, the latter values translate to approximately 5 mM brain ammonia. Similar values have been reported in various models of acute liver failure in rats (Mans et al., 1979; Swain et al., 1992; Rama Rao et al., 2010).
Histologic findings
Astrocytes from cerebral cortex of WT mice treated with TAA possessed enlarged, vacuolated nuclei, while the cytoplasm was pale and expanded (Figure 2). The neuropil was highly vacuolated (spongiotic), especially around blood vessels and neurons. No significant changes were observed in other cellular elements of the CNS. By contrast, astrocytes and the neuropil from Tg mice treated with TAA were normal with only an occasional astrocyte displaying slight nuclear pallor. Control (untreated) Tg and WT mice showed no histological abnormalities (Figure 2).
Figure 2.
Histopathological changes in cerebral cortex of WT and Tg mice treated with TAA. A. Section from cerebral cortex of WT control mice (arrows denote astrocyte nuclei). B. WT mice treated with TAA show prominently enlarged and vacuolated astrocyte nuclei (long arrows); some of the astrocytes display swollen, clear cytoplasm. There is extensive vacuolization (spongiosis) of the neuropil (crossed short arrows), prominent perivascular (short arrows) and perineuronal vacuolization (crossed long arrows), all features highly characteristic of swollen astrocytic processes. C. Cerebral cortex from control Tg mice show no lesions. D. Cortex from TAA-treated Tg mice is intact except for the occasional presence of astrocytes showing slight nuclear pallor and enlargement (arrows). Scale bar, 20 µm.
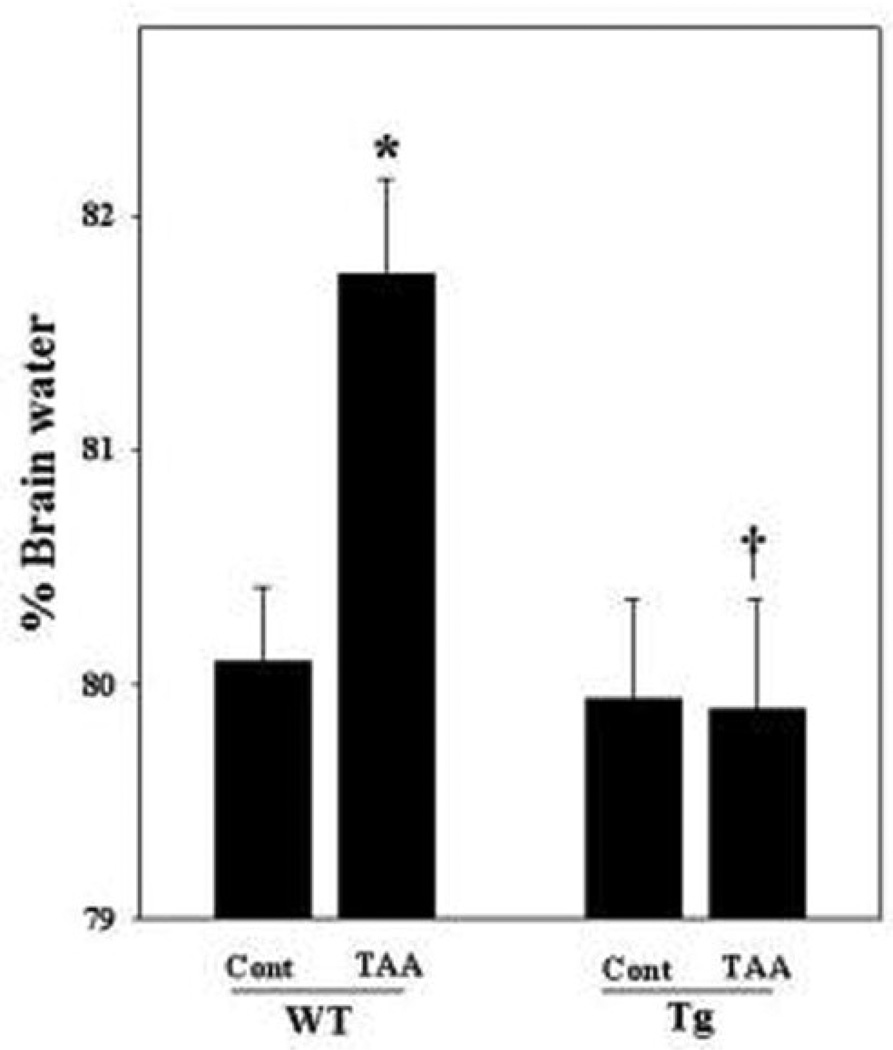
Brain water content
Animals were sacrificed 28 h after the last injection of TAA. WT mice treated with TAA showed a 1.65% increase in water content as compared to WT saline-treated controls. No increase in brain water content was detected in TAA-treated Tg mice (Figure 3). Brain water content in control Tg mice (79.6%) was similar to the WT controls (80.1%).
Figure 3.
Brain edema after acute liver failure in Tg mice. WT mice treated with TAA showed swelling (1.65%) as compared to control (WT control). No brain edema was detected in Tg mice. ANOVA, n=7. *p<0.05 vs. WT control. †p<0.05 vs. WT TAA. WT, wild type; Tg, transgenic; Cont, control, TAA, thioacetamide. Error bars represent mean ± SEM.
Immunohistochemistry of iNOS in cerebral cortex of mice
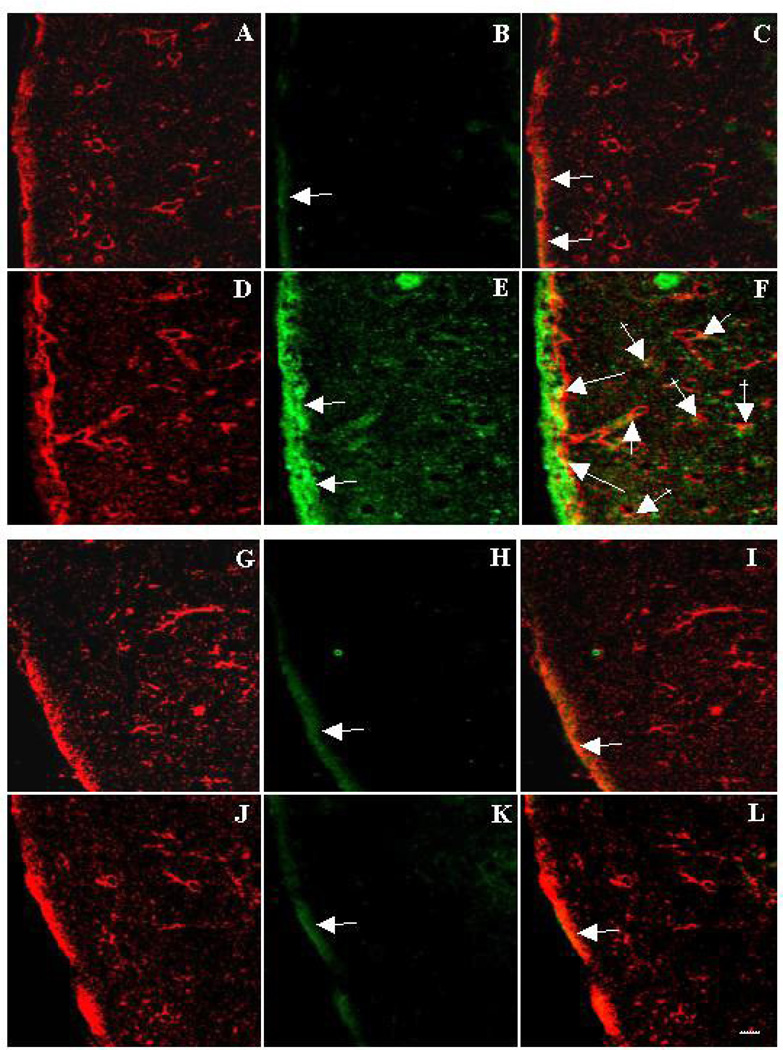
To examine for iNOS expression in astrocytes of WT and Tg mice, immunofluorescence double-labeling experiment was performed using an antibody to iNOS, together with an antibody to glutamine synthaste (GS), an astrocyte-specific enzyme. As shown in Figure 4 B, iNOS expression in WT mice controls was faint, and appeared mostly associated with the pial surface, and partly colocalized with GS (Figure 4 C). However, in TAA-treated brain sections from WT mice, the expression of iNOS was markedly increased over controls (Figure 4 E,F). By contrast, Tg mice treated with TAA (Figure 4 K,L) showed no increase in iNOS expression as compared to controls.
Figure 4.
Immunohistochemistry of inducible nitric oxide synthase (iNOS) and glutamine synthatase (GS) in cerebral cortex of mice. Figures A–F were prepared from WT mice, while G–L are from Tg mice. Green represents iNOS and red is GS. Control sections from WT mice showing GS (A), iNOS (B) and merged image (C). Note a faint green fluorescence (B) indicating a minimal amount of iNOS expression (arrow), which is mostly associated with the pial surface, and partly colocalizes with GS (C) (orange, arrows). Section from TAA-treated WT mice shows GS (red, D) and iNOS (green, E) and a merged image (F). A marked increase in iNOS expression is noted (E, arrows), which strongly colocalizes with GS (orange, F) in pial regions (long arrows), around blood vessels (short arrows) and in the neuropil (crossed arrows), indicating that the astrocytic iNOS expression is upregulated in TAA-treated WT mice. Sections from control Tg mice (G–I) and TAA-treated Tg mice (J–L) showed a similar staining pattern to that of WT control mice (A–C), indicating that astrocytic iNOS expression is not over-expressed in Tg mice after ALF. Scale bar = 50 µm.
Cytopathology of ammonia-treated astrocyte cultures
WT mice astrocytes exposed to NH4Cl (5 mM) for 72 h showed extensive cytoplasmic vacuolization, enhanced stellation and dark-staining cytoplasm, which occasionally contained dense bodies (Figure 5 D,E). These findings were similar to that observed in our earlier investigations (Gregorios et al., 1985; Norenberg et al., 2004). No morphological abnormalities were detected in Tg mice astrocytes exposed to ammonia (Figure 5 B).
Figure 5.
Light microscopy (Giemsa stain) of cultured astrocytes from WT and Tg mice treated with ammonia. A. Astrocytes from control Tg mice show a monolayered sheet of cells possessing a moderate amount of light-staining cytoplasm, fine cytoplasmic processes and round to oval nuclei with evenly distributed chromatin. B. Astrocytes from Tg mice treated with NH4Cl (5 mM) for 72 h show no significant cytopathological changes as compared to Tg control. C. Astrocytes from control WT mice are similar to control Tg mice astrocytes. D. Cultured astrocytes from WT mice treated with NH4Cl (5 mM) for 72 h display slightly shrunken nuclei along with a more prominent and darker cytoplasm, which has a more stellated appearance. Many cells contain numerous vacuoles (long arrows) as well as occasional dense bodies (short arrows). E. WT mice astrocytes exposed to ammonia display similar feature as in D. The cytoplasm, however, is darker and the vacuolization is more intense. All x300.
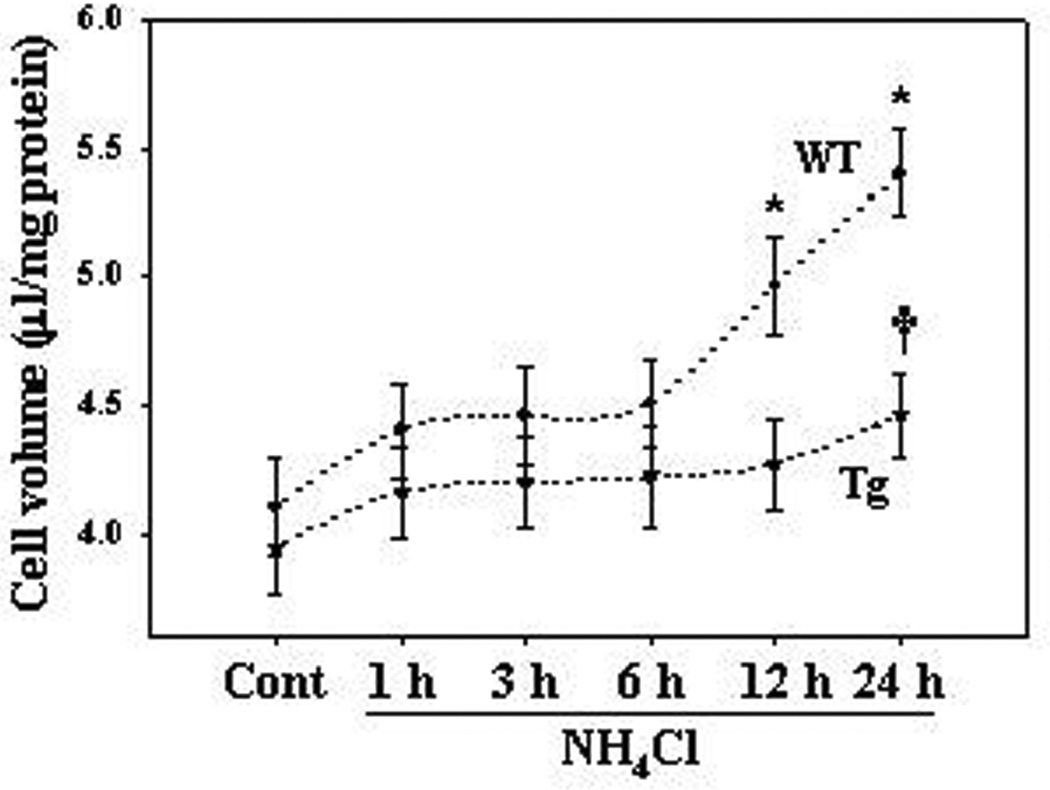
Cell swelling in cultured astrocytes exposed to ammonia
Astrocyte cultures from WT and Tg mice were exposed to a pathophysiological concentration of ammonia (5 mM NH4Cl) for 24 h.; i.e., levels found in brain in experimental ALF (see above for references). The intracellular water space of control cultured astrocytes from WT mice was 4.1 ± 0.1 µl/mg protein. Astrocytes from WT mice treated with ammonia showed no effect on cell volume from 1–6 h (Figure 6). However, treatment with ammonia showed a significant increase in cell swelling (21 and 31.7% at 12 and 24 h, respectively) as compared to WT control (Figure 6). Cultures from Tg mice exhibited a slight increase in cell swelling when exposed to ammonia (13.2% at 24 h), but that increase was not statistically significant different from WT control (Figure 6).
Figure 6.
Cell swelling in cultured astrocytes from WT and Tg mice. Astrocyte cultures from WT mice exposed to 5 mM NH4Cl significantly increased cell swelling by 12 and 24 h. Cultures from Tg mice exhibited a lesser increment in cell swelling when exposed to ammonia. ANOVA, n=7. *p<0.05 vs. WT control; †p<0.05 vs. Tg control and WT NH4Cl. WT, wild type; Tg, transgenic; Cont, control. Error bars represent mean ± SEM.
iNOS protein expression and nitric oxide (NO) generation in cultured astrocytes
One possible mechanism by which activation of NF-κB may lead to astrocyte swelling is through the upregulation of iNOS resulting in the generation of NO, an agent known to cause swelling in brain slices and in cultured astrocytes (Zielinska et al. 2003; Jayakumar et al. 2006). We recently showed that the ammonia-induced activation of NF-κB stimulates iNOS protein expression and NO generation in rat brain cultured astrocytes (Sinke et al. 2008). The present study examined iNOS protein expression and NO generation in astrocyte cultures from Tg and WT mice treated with ammonia. Astrocyte cultures from WT mice treated with ammonia showed an approximately two-fold increase in iNOS protein expression as compared to non-treated controls (control, 1126 ± 114; ammonia treatment, 3144 ± 317 intensity units/mg protein) (Figure 7). By contrast, cultures from Tg mice treated with ammonia showed a lesser increment in iNOS protein expression (control, 782 ± 64; ammonia treatment, 1410 ± 112) (Figure 7). Similar findings were observed relative to NO after cultures were exposed to ammonia for 24 h (Figure 8).
Figure 7.
Inducible nitric oxide synthase (iNOS) protein expression in cultured astrocytes from WT and Tg mice. A. Western blots display significant increase in iNOS protein expression in astrocytes from WT mice exposed to NH4Cl for 24 h. iNOS protein expression in Tg mice astrocytes after exposure to ammonia was significantly less as compared to WT astrocytes. B. Quantification of ammonia-induced iNOS protein expression. Levels of iNOS protein were normalized to α-tubulin. *p<0.05 vs. WT control; †p<0.05 vs. WT NH4Cl. §p<0.05 vs. WT control. Error bars represent mean ± SEM.
Figure 8.
Nitric oxide (NO) generation in cultured astrocytes from WT and Tg mice. Astrocyte cultures from WT mice exposed to NH4Cl significantly increased NO generation (1 fold by 24 h). Cultures from Tg mice treated with ammonia showed a lesser rise in NO level as compared to WT astrocytes. *p<0.05 vs. WT control; † p<0.05 vs. WT NH4Cl. §p<0.05 vs. WT control. Error bars represent mean ± SEM.
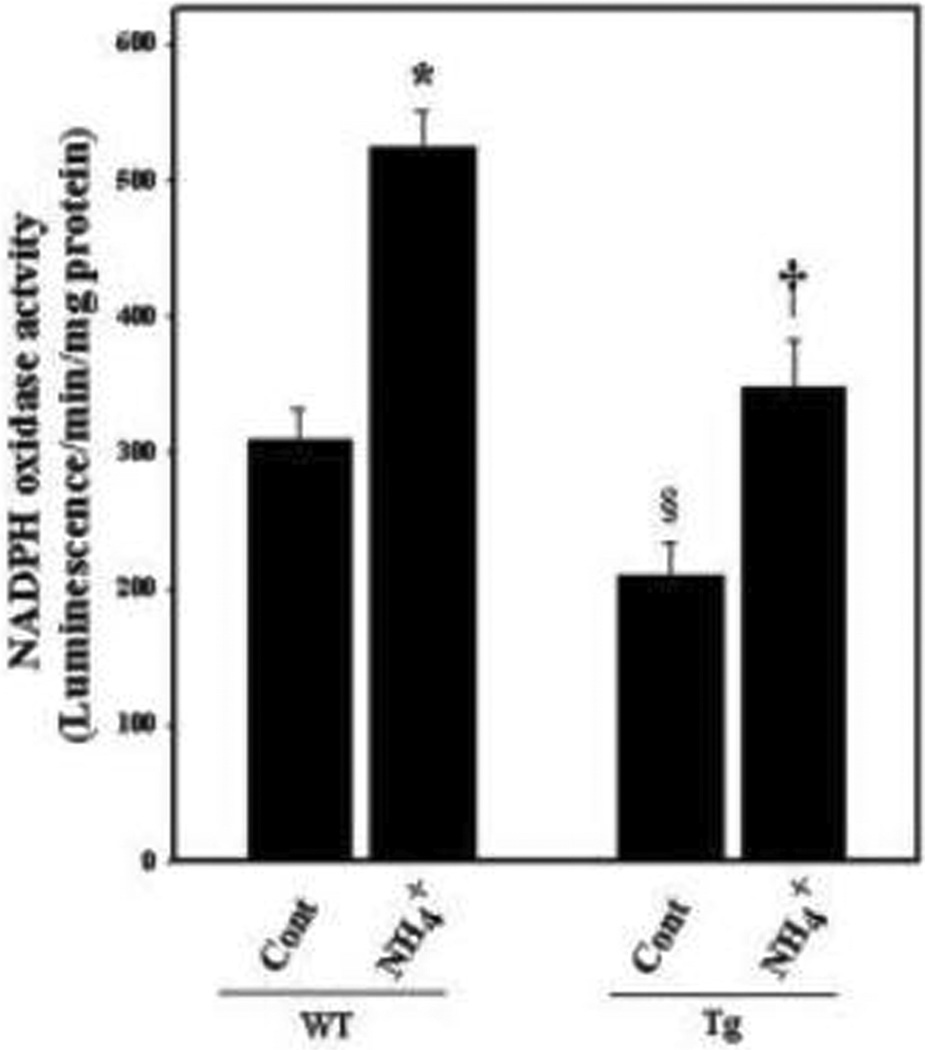
Ammonia-induced NADPH oxidase (NOX) in cultured astrocytes
NF-κB is known to induce NOX activity (Manea et al. 2007; Luengo-Blanco et al. 2008), which is a major source of superoxide generation (Manea et al. 2007). Recently, ammonia was shown to increase NOX activity in cultured astrocytes (Reinehr et al. 2007; Jayakumar et al. 2009), and inhibition of NOX activity significantly attenuated ammonia-induced astrocyte swelling (Jayakumar et al. 2009), implicating NF-κB-mediated NOX activation in the mechanism of ammonia-induced astrocyte swelling. The present study showed an increase (70.1%) in NOX activity following 24 h treatment with ammonia in cultured astrocytes from WT mice (Figure 9). By contrast, astrocyte cultures from Tg mice showed a lesser increment (12.7%) in NOX activity, which however was not statistically different from WT controls.
Figure 9.
NADPH oxidase (NOX) activity in cultured astrocytes from WT and Tg mice. Astrocyte cultures from WT mice exposed to NH4Cl significantly increased NOX activity (1-fold by 24 h). Cultures from Tg mice treated with ammonia showed a lesser rise in NOX activity. *p<0.05 vs. WT control; † p<0.05 vs. WT NH4Cl. WT, wild type; Tg, transgenic; Cont, control, NH4+, NH4Cl. §p<0.05 vs. WT control. Error bars represent mean ± SEM.
DISCUSSION
Our study demonstrates that Tg mice in which the astrocytic NF-κB had been inactivated, displayed no brain edema after TAA-induced ALF, in contrast to its WT control. Tg mice treated with TAA also displayed a delay in the development of encephalopathy when compared to WT mice treated with TAA. Additionally, Tg mice treated with TAA did not exhibit an increase in iNOS immunoreactivity. By contrast a marked increase in iNOS immunoreactivity was observed in astrocytes from WT mice treated with TAA. Histological analysis of brain sections from WT mice treated with TAA showed evidence consistent with cytotoxic edema (astrocyte swelling), whereas such changes were not detected in TAA-treated Tg mice. Moreover, astrocyte cultures derived from WT mice exhibited cell swelling as well as morphological abnormalities after ammonia treatment, while these changes were not detected in ammonia-treated astrocytes derived from Tg mice. Collectively, these studies strongly suggest that activation of NF-κB is a crucial factor in the mechanism of astrocyte swelling/brain edema in ALF.
It should be noted that in untreated transgenic animals the levels of AST and ALT were significantly higher than those in WT controls. While the reason for the difference in liver enzymes between WT and Tg mice is unclear, it is possible that a reduction in NF-κB activity in GFAP-containing cells present in the liver (i.e., cells of Ito or hepatic stellate cells), might have negatively impacted on the integrity of hepatocytes in Tg mice, thereby resulting in higher blood levels of “liver enzymes”. However, the rise of AST and ALT levels in TAA-treated WT mice was not statistically different from each other.
NF-κB is a ubiquitous transcription factor that activates the transcription of many genes, especially those involved in immune responses and inflammation (for review, see Baldwin, 1996). The means by which activation of NF-κB contributes to cell swelling in ammonia-treated astrocyte cultures is unclear. NF-κB activation is generally considered a major transcriptional factor responsible for iNOS protein expression and subsequent NO generation (for reviews, see Xie et al. 1994; Kleinert et al. 2004). We recently showed that the NO donors, SNAP and SIN-1, caused astrocyte swelling, and that treatment of cultures with the non-specific nitric oxide synthase (NOS) inhibitor, N-nitro-L-arginine methyl ester (L-NAME), significantly diminished ammonia-induced astrocyte swelling (Jayakumar et al. 2006). Additionally, BAY 11–7082, an inhibitor of NF-κB, significantly abolished ammonia-induced iNOS protein expression as well as NO generation (Jayakumar et al. 2006). These findings, along with the present observation that astrocytes from NF-κB Tg mice treated with ammonia display less iNOS expression and exhibited a decrease in NO production, strongly support a vital role of NF-κB-mediated iNOS in the astrocyte swelling/brain edema associated with ALF.
In addition to nitrosative stress, oxidative stress (OS) is a major consequence of NF-κB activation (for review, see Bowie and O’Neill 2000). Among other mechanisms, activation of NADPH oxidase (NOX) by NF-κB represents an important factor in OS induction, as NOX is one of the most important sources of superoxide generation (Manea et al., 2007; Luengo-Blanco et al. 2008). We recently demonstrated the activation of NOX in ammonia-treated astrocyte cultures, and that inhibition of such activation by apocyanin or dyphenyliodonium significantly reduced astrocyte swelling induced by ammonia (Jayakumar et al. 2009). Additionally, we found a significant decline in NOX activity when astrocyte cultures were treated with BAY 11–7082 after ammonia exposure (unpublished observation).
Since pharmacological inhibitors often have non-specific effects, the present study examined whether cultures from transgenic (Tg) mice with a functional inactivation of astrocytic NF-κB had a similar effect on NOX activity as observed in WT astrocytes treated with the NF-κB inhibitor BAY 11–7082 (Sinke et al., 2008). Our results demonstrate that astrocytes from WT mice treated with ammonia showed an increase in NOX activity, while similarly treated cultures from Tg mice showed a lesser increment in NOX activity. These findings strongly support the view that NF-κB, and associated OS, also contributes to the development of astrocyte swelling/edema in ALF.
The mechanism by which iNOS and NOX mediate cell swelling/brain edema is unclear. However, it is known that activation of these enzymes generate NO and the superoxide anion, both of which react to form the potent oxidant peroxynitrite (Beckman, 1991). We recently showed that treatment of astrocyte cultures with the peroxynitrite scavenger uric acid blocked ammonia-induced astrocyte swelling (Norenberg et al. 2007), suggesting the involvement of iNOS and NOX in the process of cell swelling. It is therefore possible that some key proteins involved in cell volume regulation may be affected by the activation of iNOS and NOX. Relevant to this concept, we previously reported that cultured astrocytes exposed to ammonia increased the activation of the ion transporter Na+-K+-Cl− cotransporter-1 (NKCC1) (Jayakumar et al. 2008), and that inhibition of such activation led to a reduction in astrocyte swelling. Further, we demonstrated that the effect of ammonia on NKCC1 activation and subsequent cell swelling was mediated, in part, through oxidation and nitration of NKCC1, as oxidants and NO donors were able to oxidize and nitrate NKCC1, while antioxidants and NOS inhibitors significantly reduced oxidation/nitration of NKCC1, and cell swelling induced by ammonia (Jayakumar et al. 2008). It is therefore likely that in ammonia-treated WT astrocytes, the activation of NKCC1 by oxidative/nitrosative stress results in the intracellular accumulation of Na+, K+, Cl− leading to cell swelling. Taken together, oxidation/nitration of key membrane proteins that are critical to cell volume regulation may become impaired and result in cell swelling. This may occur following the stimulation of iNOS and NOX; the later very likely occurring through the activation of NF-κB.
While NF-kB mediated ONS seems to play an important role in the mechanism of ammonia-induced astrocyte swelling, other factors that are known to be activated by NF-κB such as phospholipase A2 (PLA2) and cyclooxygenase-2 (COX-2) may also involved in astrocyte swelling. We recently reported that ammonia activates PLA2 and COX-2, and that blocking such activation led to a reduction in astrocyte swelling (Jayakumar et al., 2009). It is therefore possible that factors activated by NF-κB (e.g., PLA2 and COX-2, and possibly other factors) may also contribute to ammonia-induced astrocyte swelling.
Hypothermia is known to reduce brain edema in several neurological conditions (Linares and Mayer, 2009; Rosomoff et al., 1996). To examine whether a reduction in brain edema after TAA treatment in Tg mice may have been due to a reduction in body temperature, the latter was monitored in WT and Tg mice treated with TAA. While TAA-treated Tg mice showed a reduction in body temperature (32.9°C), a similar reduction in body temperature was found in WT mice treated with TAA (32.6°C), indicating that the decreased edema observed in Tg mice was not due to a reduction in body temperature.
While ammonia continues to play a prominent role in the mechanism of brain edema in ALF, our findings regarding the role of NF-κB are highly relevant to an emerging concept that inflammation and cytokines are also important in the mechanism of brain edema in ALF (O'Grady and Williams 1986; Jalan and Williams, 2001; Shawcross et al, 2005; Detry et al., 2006; O’Beirne et al., 2006; Wright et al., 2007; Jiang et al., 2009; Bemeur et al., 2010). As inflammatory mediators are well-known to contribute to NF-κB activation (Stancovski and Baltimore, 1997; Verma and Stevenson. 1997), it is likely that these mediators, whether generated in brain or from the systemic circulation, converge with ammonia to activate the astrocytic NF-κB, culminating in its excessive activation and in an enhancement of astrocyte swelling and brain edema in the setting of ALF.
In summary, this study demonstrates that astrocytes from Tg mice displaying an inactivation of NF-κB showed no cell swelling nor cytopathological changes when exposed to ammonia. By contrast, prominent cell swelling and morphological abnormalities were observed in astrocytes from WT mice after ammonia. Similarly, brain edema was not detected in Tg mice that were treated with the hepatotoxin TAA, whereas a significant increase in brain water content was observed in TAA-treated WT mice. These results parallel morphological studies, which showed evidence of brain edema in WT mice treated with TAA, but not in TAA-treated Tg mice. These findings, along with our previous reports that pharmacological inhibition of NF-κB reduces astrocyte swelling following ammonia exposure, highlight the critical involvement of NF-κB in the mechanism of astrocyte swelling and brain edema associated with acute liver failure.
Acknowledgments
This work was supported by a Merit Review from the Department of Veterans Affairs and by National Institutes of Health Grants DK063311. ARJ is supported by the American Association for the Study of Liver Disease/American Liver Foundation Grants. We thank Alina Fernandez-Revuelta for the preparation of cell cultures.
Abbreviations
- ALF
acute liver failure
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- iNOS
nitric oxide synthase
- mitogen-activated protein kinases
MAPKs
- NF-κB
nuclear factor-kappaB
- NOX
NADPH oxidase
- TAA
thioacetamide
- Tg
transgenic
- WT
wild type.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albrecht J, Jones EA. Hepatic encephalopathy: molecular mechanisms underlying the clinical syndrome. J. Neurol. Sci. 1999;170:38–146. doi: 10.1016/s0022-510x(99)00169-0. [DOI] [PubMed] [Google Scholar]
- Baldwin AS., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu. Rev. Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- Beckman JS. The double-edged role of nitric oxide in brain function and superoxide-mediated injury. J. Dev. Physiol. 1991;15:53–59. [PubMed] [Google Scholar]
- Bémeur C, et al. IL-1 or TNF receptor gene deletion delays onset of encephalopathy and attenuates brain edema in experimental acute liver failure. Neurochem. Int. 2010;56:213–215. doi: 10.1016/j.neuint.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Bowie A, O’Neill LA. Oxidative stress and nuclear factor-kappaB activation: a reassessment of the evidence in the light of recent discoveries. Biochem. Pharmacol. 2000;59:13–23. doi: 10.1016/s0006-2952(99)00296-8. [DOI] [PubMed] [Google Scholar]
- Bracchi-Ricard V, et al. Astroglial nuclear factor-kappaB regulates learning and memory and synaptic plasticity in female mice. J. Neurochem. 2008;104:611–623. doi: 10.1111/j.1471-4159.2007.04993.x. [DOI] [PubMed] [Google Scholar]
- Brambilla R, et al. Inhibition of astroglial nuclear factor kappaB reduces inflammation and improves functional recovery after spinal cord injury. J. Exp. Med. 2005;202:145–156. doi: 10.1084/jem.20041918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla R. Transgenic inhibition of astroglial NF-kappa B leads to increased axonal sparing and sprouting following spinal cord injury. J. Neurochem. 2009a;110:765–778. doi: 10.1111/j.1471-4159.2009.06190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla R. Transgenic inhibition of astroglial NF-kappa B improves functional outcome in experimental autoimmune encephalomyelitis by suppressing chronic central nervous system inflammation. J. Immunol. 2009b;182:2628–2640. doi: 10.4049/jimmunol.0802954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detry O, et al. Brain edema and intracranial hypertension in fulminant hepatic failure: pathophysiology and management. World. J. Gastroenterol. 2006;12:7405–7412. doi: 10.3748/wjg.v12.i46.7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducis I, et al. The benzodiazepine receptor in cultured astrocytes from genetically epilepsy prone rats. Brain. Res. 1990;531:318–321. doi: 10.1016/0006-8993(90)90793-b. [DOI] [PubMed] [Google Scholar]
- Dvoriantchikova G, et al. Inactivation of astroglial NF-kappa B promotes survival of retinal neurons following ischemic injury. Eur. J. Neurosci. 2009;30:175–185. doi: 10.1111/j.1460-9568.2009.06814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu ES, et al. Transgenic glial nuclear factor-kappa B inhibition decreases formalin pain in mice. Neuroreport. 2007;18:713–717. doi: 10.1097/WNR.0b013e3280d9e869. [DOI] [PubMed] [Google Scholar]
- Fu ES, et al. Transgenic inhibition of glial NF-kappa B reduces pain behavior and inflammation after peripheral nerve injury. Pain. 2010;148:509–518. doi: 10.1016/j.pain.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammal S, et al. Reversal of the behavioral and electrophysiological abnormalities of an animal model of hepatic encephalopathy by benzodiazepine receptor ligands. Hepatology. 1990;11:371–378. doi: 10.1002/hep.1840110307. [DOI] [PubMed] [Google Scholar]
- Gregorios JB, et al. Morphologic effects of ammonia on primary astrocyte cultures. I: Light microscopic studies. J. Neuropathol. Exp. Neurol. 1985;44:397–403. doi: 10.1097/00005072-198507000-00003. [DOI] [PubMed] [Google Scholar]
- Hazell AS, Butterworth RF. Hepatic encephalopathy: an update of pathophysiologic mechanisms. Proc. Soc. Exp. Biol. Med. 1999;222:99–112. doi: 10.1046/j.1525-1373.1999.d01-120.x. [DOI] [PubMed] [Google Scholar]
- Hilgier W, Olson JE. Brain ion and amino acid contents during edema development in hepatic encephalopathy. J. Neurochem. 1994;62:197–204. doi: 10.1046/j.1471-4159.1994.62010197.x. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, et al. Acute liver failure and hyperammonemia increase peripheral-type benzodiazepine receptor binding and pregnenolone synthesis in mouse brain. Brain. Res. 1995;705:345–348. doi: 10.1016/0006-8993(95)01244-3. [DOI] [PubMed] [Google Scholar]
- Jaimes EA, et al. Stable compounds of cigarette smoke induce endothelial superoxide anion production via NADPH oxidase activation. Arterioscler. Thromb. Vasc. Biol. 2004;24:1031–1036. doi: 10.1161/01.ATV.0000127083.88549.58. [DOI] [PubMed] [Google Scholar]
- Jalan R, Williams R. The inflammatory basis of intracranial hypertension in acute liver failure. J. Hepatol. 2001;34:940–942. doi: 10.1016/s0168-8278(01)00038-1. [DOI] [PubMed] [Google Scholar]
- Jayakumar AR, et al. Na-K-Cl cotransporter-1 in the mechanism of ammonia-induced astrocyte swelling. J. Biol. Chem. 2008;283:33874–33882. doi: 10.1074/jbc.M804016200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayakumar AR, et al. Oxidative stress and mitogen-activated protein kinase phosphorylation mediate ammonia-induced cell swelling and glutamate uptake inhibition in cultured astrocytes. J. Neurosci. 2006;26:4774–4784. doi: 10.1523/JNEUROSCI.0120-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayakumar AR, et al. Calcium in the mechanism of ammonia-induced astrocyte swelling. J. Neurochem. 2009;109(Suppl 1):252–257. doi: 10.1111/j.1471-4159.2009.05842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, et al. Direct evidence for central proinflammatory mechanisms in rats with experimental acute liver failure: protective effect of hypothermia. J. Cereb. Blood. Flow. Metab. 2009;29:944–952. doi: 10.1038/jcbfm.2009.18. [DOI] [PubMed] [Google Scholar]
- Juurlink BH, Hertz L. Plasticity of astrocytes in primary cultures: an experimental tool and a reason for methodological caution. Dev. Neurosci. 1985;7:263–277. doi: 10.1159/000112295. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK. Anisotonic media and glutamate-induced ion transport and volume responses in primary astrocyte cultures. J. Physiol (Paris) 1987;82:294–303. [PubMed] [Google Scholar]
- Kimura T, Budka H. Glial fibrillary acidic protein and S-100 protein in human hepatic encephalopathy: immunocytochemical demonstration of dissociation of two glia-associated proteins. Acta. Neuropathol. 1986;70:17–21. doi: 10.1007/BF00689509. [DOI] [PubMed] [Google Scholar]
- Kleinert H, et al. Regulation of the expression of inducible nitric oxide synthase. Eur. J. Pharmacol. 2004;500:255–266. doi: 10.1016/j.ejphar.2004.07.030. [DOI] [PubMed] [Google Scholar]
- Kletzien RF, et al. A method using 3-O-methyl-D-glucose and phloretin for the determination of intracellular water space of cells in monolayer culture. Ann. Biochem. 1975;68:537–544. doi: 10.1016/0003-2697(75)90649-1. [DOI] [PubMed] [Google Scholar]
- Kretzschmar HA, et al. Measurement of GFAP in hepatic encephalopathy by ELISA and transblots. J. Neuropathol. Exp. Neurol. 1985;44:459–471. doi: 10.1097/00005072-198509000-00002. [DOI] [PubMed] [Google Scholar]
- Linares G, Mayer SA. Hypothermia for the treatment of ischemic and hemorrhagic stroke. Crit. Care. Med. 2009;37:S243–S249. doi: 10.1097/CCM.0b013e3181aa5de1. [DOI] [PubMed] [Google Scholar]
- Luengo-Blanco M, et al. Essential role of nuclear factor-kappaB for NADPH oxidase activity in normal and anhidrotic ectodermal dysplasia leukocytes. Blood. 2008;112:1453–1460. doi: 10.1182/blood-2007-07-099267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manea A, et al. Regulation of NADPH oxidase subunit p22(phox) by NF-kB in human aortic smooth muscle cells. Arch. Physiol. Biochem. 2007;113:163–172. doi: 10.1080/13813450701531235. [DOI] [PubMed] [Google Scholar]
- Mans AM, et al. Correlation of plasma and brain amino acid and putative neurotransmitter alterations during acute hepatic coma in the rat. J. Neurochem. 1979;32:285–292. doi: 10.1111/j.1471-4159.1979.tb00350.x. [DOI] [PubMed] [Google Scholar]
- Marmarou A, et al. A simple gravimetric technique for measurement of cerebral edema. J. Neurosurg. 1978;49:530–537. doi: 10.3171/jns.1978.49.4.0530. [DOI] [PubMed] [Google Scholar]
- Norenberg MD. A light and electron microscopic study of experimental portal-systemic (ammonia) encephalopathy. Progression and reversal of the disorder. Lab. Invest. 1977;36:618–627. [PubMed] [Google Scholar]
- Norenberg MD. Astrocytic-ammonia interactions in hepatic encephalopathy. Semin. Liver. Dis. 1996;16:245–253. doi: 10.1055/s-2007-1007237. [DOI] [PubMed] [Google Scholar]
- Norenberg MD, et al. Ammonia induced decrease in glial fibrillary acidic protein in cultured astrocytes. J. Neuropathol. Exp. Neurol. 1990;49:399–405. doi: 10.1097/00005072-199007000-00004. [DOI] [PubMed] [Google Scholar]
- Norenberg MD, et al. Ammonia-induced astrocyte swelling in primary culture. Neurochem. Res. 1991;16:833–836. doi: 10.1007/BF00965694. [DOI] [PubMed] [Google Scholar]
- Norenberg MD, et al. Oxidative stress in the pathogenesis of hepatic encephalopathy. Metab Brain Dis. 2004;19:313–329. doi: 10.1023/b:mebr.0000043978.91675.79. [DOI] [PubMed] [Google Scholar]
- Norenberg MD, et al. New concepts in the mechanism of ammonia-induced astrocyte swelling. Metab. Brain. Dis. 2007;22:219–234. doi: 10.1007/s11011-007-9062-5. [DOI] [PubMed] [Google Scholar]
- Norenberg MD, et al. Signaling factors in the mechanism of ammonia neurotoxicity. Metab. Brain. Dis. 2009;24:103–117. doi: 10.1007/s11011-008-9113-6. [DOI] [PubMed] [Google Scholar]
- O’Beirne JP, et al. The role of infection and inflammation in the pathogenesis of hepatic encephalopathy and cerebral edema in acute liver failure. Nat. Clin. Pract. Gastroenterol. Hepatol. 2006;3:118–119. doi: 10.1038/ncpgasthep0417. [DOI] [PubMed] [Google Scholar]
- O'Grady JG, Williams R. Management of acute liver failure. Schweiz. Med. Wochenschr. 1986;116:541–544. [PubMed] [Google Scholar]
- Rama Rao KV, et al. Brain edema in acute liver failure: inhibition by L-histidine. Am. J. Pathol. 2010;176:1400–1408. doi: 10.2353/ajpath.2010.090756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinehr R, et al. Hypoosmotic swelling and ammonia increase oxidative stress by NADPH oxidase in cultured astrocytes and vital brain slices. Glia. 2007;55:758–771. doi: 10.1002/glia.20504. [DOI] [PubMed] [Google Scholar]
- Rosomoff HL, et al. Resuscitation from severe brain trauma. Crit. Care. Med. 1996;24:S48–S56. [PubMed] [Google Scholar]
- Sarhan S, et al. Effect of inhibition of ornithine aminotransferase on thioacetamide-induced hepatogenic encephalopathy. Neurochem. Res. 1993;18:539–549. doi: 10.1007/BF00967259. [DOI] [PubMed] [Google Scholar]
- Shawcross D, et al. The pathophysiologic basis of hepatic encephalopathy: central role for ammonia and inflammation. Cell. Mol. Life. Sci. 2005;62:2295–2304. doi: 10.1007/s00018-005-5089-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliess F, et al. Ammonia induces MK-801-sensitive nitration and phosphorylation of protein tyrosine residues in rat astrocytes. FASEB J. 2002;16:739–741. doi: 10.1096/fj.01-0862fje. [DOI] [PubMed] [Google Scholar]
- Sinke AP, et al. NF-kappaB in the mechanism of ammonia-induced astrocyte swelling in culture. J. Neurochem. 2008;106:2302–2311. doi: 10.1111/j.1471-4159.2008.05549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel RA, et al. Glial fibrillary acidic protein in hepatic encephalopathy. An immunohistochemical study. J. Neuropathol. Exp. Neurol. 1981;40:625–632. doi: 10.1097/00005072-198111000-00004. [DOI] [PubMed] [Google Scholar]
- Stancovski I, Baltimore D. NF-kappaB activation: the I kappaB kinase revealed? Cell. 1997;91:299–302. doi: 10.1016/s0092-8674(00)80413-4. [DOI] [PubMed] [Google Scholar]
- Stanislaus R, et al. Amelioration of experimental allergic encephalomyelitis in Lewis rats by lovastatin. Neurosci. Lett. 1999;269:71–74. doi: 10.1016/s0304-3940(99)00414-0. [DOI] [PubMed] [Google Scholar]
- Swain MS, et al. Intracellular pH rises and astrocytes swell after portacaval anastomosis in rats. Am. J. Physiol. 1991;261:R1491–R1496. doi: 10.1152/ajpregu.1991.261.6.R1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain M, et al. Ammonia and related amino acids in the pathogenesis of brain edema in acute ischemic liver failure in rats. Hepatology. 1992;15:449–453. doi: 10.1002/hep.1840150316. [DOI] [PubMed] [Google Scholar]
- Traber PG, et al. Electron microscopic evaluation of brain edema in rabbits with galactosamine-induced fulminant hepatic failure: ultrastructure and integrity of the blood-brain barrier. Hepatology. 1987;7:1272–1277. doi: 10.1002/hep.1840070616. [DOI] [PubMed] [Google Scholar]
- Verma IM, Stevenson J. IkappaB kinase: beginning, not the end. Proc. Natl. Acad. Sci. U. S. A. 1997;94:11758–11760. doi: 10.1073/pnas.94.22.11758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorhies TM, et al. Acute hyperammonemia in the young primate: physiologic and neuropathologic correlates. Pediatr. Res. 1983;17:970–975. doi: 10.1203/00006450-198312000-00009. [DOI] [PubMed] [Google Scholar]
- Willard-Mack CL, et al. Inhibition of glutamine synthetase reduces ammonia-induced astrocyte swelling in rat. Neuroscience. 1996;71:589–599. doi: 10.1016/0306-4522(95)00462-9. [DOI] [PubMed] [Google Scholar]
- Wright G, et al. Brain cytokine flux in acute liver failure and its relationship with intracranial hypertension. Metab. Brain. Dis. 2007;22:375–388. doi: 10.1007/s11011-007-9071-4. [DOI] [PubMed] [Google Scholar]
- Xie QW, et al. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J. Biol. Chem. 1994;269:4705–4708. [PubMed] [Google Scholar]
- Zielinska M, et al. Excitotoxic mechanism of cell swelling in rat cerebral cortical slices treated acutely with ammonia. Neurochem. Int. 2003;43:299–303. doi: 10.1016/s0197-0186(03)00015-9. [DOI] [PubMed] [Google Scholar]
- Zimmermann Z, et al. Hepatic encephalopathy in thioacetamide induced acute liver failure in rats: characterization of an improved model and study of amino acid-ergic neurotransmission. Hepatology. 1989;9:54–60. doi: 10.1002/hep.1840090414. [DOI] [PubMed] [Google Scholar]
- Zwingmann C, et al. Effects of ammonia exposition on glioma cells: changes in cell volume and organic osmolytes studied by diffusion-weighted and high-resolution NMR spectroscopy. Dev. Neurosci. 2000;22:463–471. doi: 10.1159/000017476. [DOI] [PubMed] [Google Scholar]