Abstract
A mutation in the IL7Rα locus has been identified as a risk factor for multiple sclerosis (MS), a neurodegenerative autoimmune disease characterized by inflammation, demyelination, and axonal damage. IL7Rα has well documented roles in lymphocyte development and homeostasis, but its involvement in disease is largely understudied. Here we use the experimental autoimmune encephalomyelitis (EAE) model of MS to show that a less severe form of the disease results when IL7Rα expression is largely restricted to thymic tissue in IL7RTgIL7R−/− mice. Compared to wild type (WT) mice, IL7RTgIL7R−/− mice exhibited reduced paralysis and myelin damage that correlated with dampened effector responses, namely decreased TNF production. Furthermore, treatment of diseased WT mice with neutralizing anti-IL7Rα antibody also resulted in significant improvement of EAE. Additionally, chimeric mice were generated by bone marrow transplant to limit expression of IL7Rα to cells of either hematopoietic or non-hematopoietic origin. Mice lacking IL7Rα only on hematopoietic cells develop severe EAE, suggesting that IL7Rα expression in the non-hematopoietic compartment contributes to disease. Moreover, novel IL7Rα expression was identified on astrocytes and oligodendrocytes endogenous to the central nervous system. Chimeric mice that lack IL7Rα only on non-hematopoietic cells also develop severe EAE, which further supports the role of IL7Rα in T cell effector function. Conversely, mice that lack IL7Rα throughout both compartments are dramatically protected from disease. Taken together, these data indicate that multiple cell types utilize IL7Rα signaling in the development of EAE, and inhibition of this pathway should be considered as a new therapeutic avenue for MS.
Introduction
Multiple Sclerosis (MS) is a debilitating autoimmune disease of the central nervous system (CNS) causing severe neurological damage in young adults (1-3). MS and its animal model experimental autoimmune encephalomyelitis (EAE) are characterized by extensive inflammation and axonal injury, ultimately leading to severe neurodegeneration (1-5). Although several immune mediators likely contribute to the detrimental microenvironment within the CNS, it is proposed that T helper cells are a driving force in the disease, namely through the Th1 and Th17 subsets with their production of inflammatory IFNγ and IL17, respectively (6-8). TNF, another effector cytokine, is known to have both pro- and anti-inflammatory effects in EAE and MS (9, 10). These particular cytokines heavily impact pathogenesis, however the specific etiology behind the autoimmune response is still elusive.
Although environmental factors may influence disease susceptibility (11-16), there is substantial evidence coupling a strong genetic component to MS as well (17-25). Multiple risk alleles have been identified through genome wide association studies, many of which are related to the immune system (17-23). In particular, single nucleotide polymorphisms (SNPs) in the gene encoding IL7Rα have emerged through genetic studies of MS patients with differing ethnic backgrounds. The SNP (rs6897932; T244I) most frequently identified in the risk allele is located at an alternative splice site within IL7RA (17, 18, 25-28). Subsequent in vitro studies associated the SNP with low levels of exon 6 skipping, leading to a modest increase in the soluble isoform of IL7Rα (25, 29). This was supported by qRT-PCR analysis of PBMCs from healthy patients, which presented a decrease in IL7RA for carriers of the risk allele (25). However, opposing results have been reported in relapsing-remitting MS patients where IL7RA transcript was increased in PBMCs compared to controls (30). Nevertheless, MS patients also displayed increased soluble IL7Rα in cerebrospinal fluid compared to individuals with other non-inflammatory neurological diseases, suggesting specificity in MS (27). Furthermore, it was recently shown that IL7-mediated stimulation of IL7Rα promotes Th1 differentiation (31), and is also implicated in the survival and expansion of pathogenic Th17 cells (32) in EAE and MS. Collectively, these findings have shifted the focus of IL7Rα biology from its established roles in lymphocyte development, homeostatic survival and proliferation (33-37) to its potential contributions in disease settings.
Aside from the immune system, the IL7/IL7Rα signaling pathway has been proposed to function within the CNS. Indeed, IL7Rα transcripts have been identified in whole mouse brain extracts, as well as rat cultured subventricular zone progenitors and embryonic neurons (38). Furthermore, IL7 was found to promote outgrowth and survival of neuronal cultures (38). With respect to astrocytic expression, no transcript was detected in rat primary cultures (38), however transcript and translated protein have been reported on human primary astrocytes (39). It has also been reported that astrocytes in human MS brain tissue secrete IL7 (40). Taken together, these findings highlight the importance of expanding the experimental scope to include non-T cell lineages during the investigation of IL7Rα in EAE.
The main objective of our studies was to determine how IL7Rα contributes to disease development and progression in the EAE model of MS. Here we show, both with a genetic (IL7RTgIL7R−/− mice) and a pharmacologic approach (anti-IL7Rα antibody administration), that systemic inhibition of IL7Rα signaling greatly reduces the overall severity of EAE. This is accomplished in part by an altered T cell effector response, namely the dramatic loss of TNF-producing T cells. We also show that IL7Rα inhibition in bone marrow-derived cells alone is not sufficient to induce protection from EAE. This suggests that non-hematopoietic lineages expressing IL7Rα must also partake in disease, for which we have identified astrocytes and oligodendrocytes as plausible candidates. These findings significantly enhance our understanding of IL7Rα function and the impact it has in EAE, underscoring the importance of taking this pathway into consideration when developing new MS therapies.
Material and Methods
Mice
Experiments were performed according to protocols approved by the Institutional Animal Care and Use Committee of the University of Miami. All mice used were 2-6 months of age on the C57BL/6 background. Females were predominantly used, however a small proportion of males were included to identify sex-linked differences; none were noted. C57BL/6 control mice were obtained from Charles River Laboratories and Jackson Laboratories. IL7RTgIL7R−/− mice were generated as previously described (34, 41). Briefly, mice containing a wild type IL7Rα transgene driven by the proximal lck promoter were crossed to IL7R−/− mice (B6.129S7-Il7rtm1Imx/J) originally obtained from Jackson Laboratories. TSLPR−/− (B6;129S-Crlf2tm1Wjl/Mmnc) cryo-preserved embryos were obtained from Mutant Mouse Regional Resource Centers, and line resuscitation was carried out by the Transgenic Animal Core Facility at Sylvester, University of Miami. μMT mice (B6.129S2-Igh-6tm1Cgn/J), Rag1−/− mice (B6.129S7-Rag1tm1Mom/J) and CD45.1 congenic wild type mice (B6.SJL-ptprca/BoAiTac) were originally obtained from Jackson Laboratories and Taconic Farms, respectively. IL7Rα-GFP mice were a kind gift from A. Singer at the National Cancer Institute. Animals were housed in a virus/antigen-free facility with a 12-hour light/dark cycle, controlled temperature and humidity, and provided with water and food ad libitum.
Induction of active EAE and assessment of functional recovery
Age-matched mice were immunized with MOG35-55 peptide (Biosynthesis, Lewisville, TX), as previously described (42, 43). Briefly, mice are i.p. injected with pertussis toxin (425 ng/mouse) on days 0 and 2. Additionally, mice are injected s.c. on days 1 and 7 with 150 μg of MOG peptide emulsified in complete Freund's adjuvant. Clinical signs of EAE are assessed daily using a standard scale of 0-6 as follows: 0, no clinical signs; 1, slight loss of tail tone; 1.5 moderate to severe loss of tail tone 2, flaccid tail; 2.5 flaccid tail and difficulty in walking 3, complete hindlimb paralysis; 3.5, complete hindlimb paralysis and partial forelimb paralysis; 4, total paralysis; 4.5 total paralysis and very poor health (incontinence, etc.); 5, moribund; and 6, death. Clinical onset of EAE is considered when mice first achieve a score of 2. The clinical disease index (CDI) is a measure of disease severity and is calculated as the sum of clinical scores for each mouse over the course of the study.
Histology
Animals were perfused with 0.1M PBS followed by 4% paraformaldehyde in PBS, spinal cords removed and post-fixed overnight. Tissues were cryopreserved in PBS + 25% sucrose and cryostat-cut into 15μm thick transverse sections. Myelinated white matter was identified in thoracic spinal cord sections with luxol fast blue stain. Hematoxylin and eosin counterstains further identified inflammatory infiltration and areas of myelin damage. Staining took place at the Histology Core Facility, University of Miami, Miller School of Medicine. Images were obtained with an Olympus BX51 upright microscope.
In Vivo Neutralization
Wild type mice were induced with EAE and i.p. injected with anti-IL7Rα (A7R34, BioXcell), or IgG control (Sigma) at 20μg/g bodyweight upon disease onset, which was determined once the majority of mice displayed a clinical score of 2 or greater. A total of 10 injections were administered at 48-hour intervals starting from day 19.
Leukocyte isolation from spleen and spinal cord
Individual spleens or spinal cords (SC) were harvested at specified time points. Tissue homogenates were passed through 70μm cell strainers. Red blood cells from spleens were lysed with Tris-buffered ammonium chloride (140mM NH4Cl, 17mM Tris, pH 7.65). Immune cells from SC were enriched by negative selection with myelin removal magnetic beads and columns according to manufacturer protocol (Miltenyi Biotec). Cell numbers were assessed by trypan blue staining.
Flow cytometry
Non-specific staining was prevented with an FcR block (2.4G2) (44) prior to adding the following surface marker antibody conjugates: CD45-FITC (1:300), CD8-PerCP-Cy5.5 (1:80), CD25-PE-Cy7 (1:80), CD127-Biotin (1:100) from eBioscience; CD45.1-biotin (1:200), CD45.2-FITC (1:200), Streptavidin-PE (1:50) from Biolegend; CD8a-APC-H7 (1:100) from BD Pharmingen. Cells were then fixed overnight with 1% paraformaldehyde in flow cytometry buffer, or fixed and permeablized (FoxP3 staining kit, eBioscience) prior to intracellular staining with FoxP3-eFlour450 (1:100) from eBioscience. All incubations were performed at 30 minutes, 4°C. Cells were analyzed using an Accuri3 or LSRII flow cytometer and FACSDiva software (BD Biosciences).
Ex vivo restimulation
Tissues were harvested and processed as above. Prior to staining, cells were incubated at 105-106 cells/mL with Phorbol 12-myristate 13-acetate (50ng/mL; Sigma), Ionomycin calcium salt (0.75μg/mL; Sigma), and 1μL Golgi Plug protein transport inhibitor (BD Biosciences) for 4 hours at 37°C, 5% CO2. Cells were stained, fixed and permeablized as above using TNFα-PerCP-eFluor710 (1:100), IFNγ-eFluor450 (1:100), and IL17A-AlexaFluor647 (1:100) intracellular antibodies from eBioscience.
Mice Chimeras
Bone marrow cells were isolated as follows: femur and tibia were collected from both hind limbs of donor mice. Bone marrow (BM) was flushed out with sterile Hank's Balanced Salt Solution and single cell-suspensions obtained following repeated cycles of aspiration through a syringe with a 26 gage needle. T cells were depleted by incubating suspensions with anti-CD4 (RL-172) and anti-CD8 (HO2.2) monoclonal antibodies, and fresh Low Tox-M Rabbit Complement (Cedar Lane Lab Ltd.) for 45 minutes at 37°C, 5% CO2. Donor cells were washed with HBSS, and then intravenously injected (tail vein, 5×105 cells/mouse) into recipient mice that were γ-irradiated (300-900 rad; Gammacell 40, Cs-137 source) one day in advance. Recipients were maintained on oral antibiotics for 2 weeks post BM transplant, and allowed at least 9 weeks recovery before EAE induction.
Immunofluorescence Staining
Animals were perfused with 0.1M PBS followed by 4% paraformaldehyde in PBS, spinal cords removed and post-fixed overnight. Tissues were cryopreserved in PBS + 25% sucrose and cryostat-cut into 15μm thick transverse sections. After blocking for 1 hour with 5% normal goat serum in 0.1M phosphate-buffered saline + 0.4% Triton-X, sections were incubated overnight at 4°C with primary antibodies against glial fibrillary acidic protein (GFAP; mouse, 1:500, BD Biosciences), CC1 (mouse, 1:500, Calbiochem), and GFP (rabbit, 1:50, Santa Cruz). Immunoreactivity was visualized either with secondary species-specific fluorescent antibodies (AlexaFluor-594 and -488, Invitrogen). Images were obtained with an Olympus FluoView 1000 confocal microscope.
Statistical analysis
Statistical analysis of the clinical course of EAE was carried out with the Mann-Whitney test. For single comparisons, Student's t-test was applied. P values equal to or less than 0.05 were considered statistically significant.
Results
IL7Rα deficiency yields EAE protection
Although IL7R−/− mice would be the obvious choice to directly address the role of IL7Rα in EAE pathophysiology, the use of this genetic model has significant limitations due to the severely impaired T cell development, which is driven by IL7Rα signaling under normal conditions (45, 46). As a result, IL7R−/− mice lack the cellular repertoire necessary for normal EAE pathogenesis (47, 48). To circumvent this issue, we turned to the use of IL7RTgIL7R−/− mice, which were engineered to express IL7Rα in the thymus of IL7R−/− mice. The rescued IL7Rα expression allows for sufficient T cell development while maintaining the IL7Rα deficient phenotype in extra-thymic tissues (34, 41; Fig. 1A). Analysis of total T cell number in the spleens of naïve IL7RTgIL7R−/− mice revealed that CD4 T cells were restored to WT levels and CD8 T cells improved by over 50%, all of which were negative for IL7Rα expression by flow cytometry as expected (Fig. 1A). This was in stark contrast to IL7R−/− mice where total T cell numbers amounted to less than 5% of WT (Fig. 1A). Notably, B cell maturation was stunted in both IL7R−/− and IL7RTgIL7R−/− mice (Fig. 1A) due to the lack of IL7Rα signaling in the bone marrow (49). Although B cells do contribute to EAE/MS pathology (50), they are not required for disease onset or progression in the C57BL/6 background following induction by the MOG35-55 peptide (51) and therefore are not expected to interfere with the EAE paradigm of IL7RTgIL7R−/− mice in these studies. To further confirm this, we induced EAE in B cell deficient μMT mice with the MOG35-55 peptide as previously described (42, 43) and found indeed that they developed EAE similar to WT mice (Fig. 1B).
Figure 1. Lymphocyte distribution in IL7RTgIL7R−/− mice.
(A) Quantification of total cell numbers are shown for the indicated mice following flow cytometry analysis of CD4, CD8, B220, and IL7Rα expression after gating on the total lymphocyte population from the spleen. Results are expressed as the mean ± SEM with n ≥ 5 per group from 3 independent experiments. # indicates p<0.05; * indicates p<0.0005; unpaired t test. (B) Clinical course of EAE in indicated mice after induction with the MOG35-55 peptide. Daily scores from one representative experiment are expressed as the mean ± SEM with n=4 per group. ns, indicates p>0.05; Mann Whitney t test.
We proceeded to induced EAE in IL7RTgIL7R−/− mice with the MOG35-55 peptide and found that IL7RTgIL7R−/− mice developed significantly less severe EAE compared to WT controls. Although onset of disease was similar between groups (Table 1), clinical scores were significantly lower in IL7RTgIL7R−/− mice and showed steady improvement through the chronic state while WT developed worse EAE. Indeed, the average clinical score of IL7RTgIL7R−/− mice corresponded to 1.5 at peak disease (20 days post injection; dpi) and recovered to a score of 1 at the chronic state (40 dpi), signifying only mild flaccidity of the tail and no hind limb paralysis (Fig. 2A). On the contrary, WT mice reached clinical scores above 2 from peak disease (20 dpi) onward showing varying degrees of hind limb and forelimb paralysis (Fig. 2A). The difference in EAE severity was also reflected in the significantly reduced CDI and incidence of disease in IL7RTgIL7R−/− mice compared to WT (Table 1). Histological analysis of myelin with luxol fast blue on thoracic SC cross-sections revealed that IL7RTgIL7R−/− mice had fewer and smaller lesions caused by infiltrating leukocytes (Fig. 2B), hence reduced myelin damage. This paralleled the improved clinical scores of IL7RTgIL7R−/− mice compared to WT controls. To further confirm the involvement of IL7Rα in EAE and complement our genetic studies, we administered anti-IL7Rα neutralizing antibody (A7R34) following induction of EAE. Antibody administration was started at peak disease for a more therapeutically relevant treatment paradigm. Mice that received anti-IL7Rα showed significant recovery compared to IgG-treated controls (Fig. 2C), with an EAE profile similar to IL7RTgIL7R−/− mice (Fig. 2A). The anti-IL7Rα mediated recovery correlated with reduced levels of myelin damage in thoracic SC sections at 55 dpi (Fig. 2D). The EAE recovery was not simply due to T cell depletion by antibody-dependent cell-mediated cytotoxicity, since T cells bound to anti-IL7Rα were readily detected during treatment (Supplemental Fig. 1), opening the possibility that instead T cell function may be altered.
Table I Clinical parameters of EAE in IL7RTgIL7R-/- mice.
| Genotype | Incidence | Day of Onset1 | CDI1,2 |
|---|---|---|---|
| WT | 93% (14/15) | 17.0±0.3 | 52.4±6.7 |
| IL7RTgIL7R-/- | 62% (8/13)* | 16.1±0.8 | 30.5±8.3* |
Results represent the mean ±SEM.
CDI was calculated as the sum of clinical scores for each animal between days 0 and 40.
p<0.05 with respect to WT; Student's t test.
Figure 2. MOG-induced EAE in IL7Rα deficient mice.
(A) Clinical course of EAE in WT and IL7RTgIL7R−/− mice after induction with the MOG35-55 peptide. Daily scores are expressed as the mean ± SEM with n ≥ 13 per group from 3 independent experiments. * indicates p<0.0005; Mann Whitney t test. (B) Assessment of thoracic SC lesions in naïve WT (left) or 25dpi WT (center) and IL7RTgIL7R−/−(right) mice by the myelin stain luxol fast blue with hematoxylin & eosin counterstains. Lower panels correspond to boxes drawn above. (C) Clinical course of EAE in WT mice treated with anti-IL7Rα or IgG control from days 19-37. Daily scores are expressed as the mean ± SEM with n ≥ 9 per group from two independent experiments. * indicates p<0.0001; Mann Whitney t test. (D) Assessment of thoracic SC lesions in naïve WT (left), anti-IL7Rα treated (center), or IgG treated (right) mice at 55 dpi by the myelin stain luxol fast blue with hematoxylin & eosin counterstains. Representative sections were selected to display the general differences observed over 5 mice/group. Scale bars represent 100μm.
The clinical scores and resulting EAE pathology in IL7RTgIL7R−/− and anti-IL7Rα treated WT mice support the hypothesis that IL7Rα signaling does in fact contribute to disease. However, IL7Rα must heterodimerize with either the γc or the thymic stromal lymphopoietin receptor (TSLPR) chain in order to propagate downtstream signaling stimulated by IL7 or TSLP, respectively (52-54). Consequently, signaling mediated by both ligands is defective in IL7RTgIL7R−/− and anti-IL7Rα treated mice. However, known functions of TSLP-mediated signaling result in the induction of a Th2 response, and therefore are not likely to play a role in EAE. To confirm this, we employed a TSLPR−/− mouse line whose IL7/IL7Rα signaling axis remains intact. Under naïve conditions, TSLPR−/− mice did not appear to have any deficiencies in IL7Rα expression, nor in lymphocyte distribution as detected by flow cytometry with a panel of leukocyte markers in the spleen (Supplemental Fig. 2A). We then induced EAE in the TSLPR−/− mice and WT controls using the MOG35-55 peptide as before, and found that a deficit in the TSLP-mediated pathway has no effect on the onset or progression of EAE (Supplemental Fig. 2B). Furthermore, flow cytometry analysis on the SC and spleen showed that the proportions of lymphocytes infiltrating the CNS (Supplemental Fig. 2C) as well as total numbers in the spleen (Supplemental Fig. 2D) were comparable between WT and TSLPR−/− during the chronic phase (40 dpi). Therefore IL7Rα contributions to EAE do not require TSLPR heterodimerization, suggesting that IL7-mediated signaling plays a predominant role in the disease, while TSLP-mediation does not.
IL7Rα deficiency alters T cell activation and cytokine expression
Once cognate antigen is recognized by T cells, activation and rapid amplification of the T cell pool occurs to facilitate the immune response (55). Since this response and subsequent CNS infiltration are paramount to EAE pathophysiology, we evaluated T cell numbers and functional state in IL7RTgIL7R−/− and WT mice at the acute phase of EAE (25 dpi). Significantly fewer CD4 and CD8 T cells were found in the spleens of IL7RTgIL7R−/− mice compared to WT (Fig. 3A), while T cells infiltrating the SC only showed significant reductions in the CD4 subpopulation (Fig. 3B). Recently it has been suggested that IL7Rα signaling may also contribute to T cell function, particularly with respect to Th1 and Th17 subclasses, which are integral to EAE development (31, 32). Therefore, we investigated how the T cell response from IL7RTgIL7R−/− mice might be affected during EAE. Ex vivo restimulation of splenocytes with PMA and ionomycin showed that IL7RTgIL7R−/− mice had dramatic changes in both distribution and total number of functional T cell subsets. First, IL7RTgIL7R−/− mice showed a significant reduction (about 50%) in the relative percentage and overall number of splenic T cells producing TNF compared to WT mice (Fig. 3C, D, F, G). Second, the percentage of splenic IFNγ producing CD4 T cells increased in IL7RTgIL7R−/− mice, although the actual cell number was similar to WT (Fig. 3C, D). In contrast, the percentage of splenic CD8 T cells producing IFNγ was comparable between WT and IL7RTgIL7R−/− mice, yet total numbers were significantly lower in the IL7RTgIL7R−/− mice (Fig. 3F, G). Ex vivo restimulation of SC infiltrating cells showed significant reductions in both TNF-producing and IFNγ-producing CD4 T cells in IL7RTgIL7R−/− mice (Fig. 3E), however no significant differences were detected in CD8 T cells (Fig. 3H). Interestingly, IL17 producing T cells were minimal to absent during acute disease, both in spleen and spinal cord (Fig. 3C-H).
Figure 3. IL7Rα deficiency dampens T cell effector responses in acute EAE.
Quantification of total T cell numbers are shown for the spleen (A, D, G) and SC (B, E , H) of indicated mice at acute EAE (25dpi) following ex vivo stimulation and flow cytometry analysis. Total CD4 and CD8 T cell populations (A, B) were analyzed after gating on the CD45 leukocytes. Representative dot plots for TNF, IFN and IL17 expression within the CD4 (C) and CD8 (F) gates are shown. Cytokine producing CD4 (D, E) and CD8 (G, H) T cells were quantified for spleen and SC. Results are expressed as the mean ± SEM with n ≥ 7 per group from 3 independent experiments. B. *, p<0.05; **, p<0.005; ***, p<0.0005; unpaired t test.
The overall lack of IL17 producing T cells at acute disease was unexpected. Nevertheless, Th17 function has been shown to precede that of Th1 in EAE, and could occur even prior to the appearance of clinical symptoms (56). To test whether this was the case in our model, we analyzed T cell effector function upon ex-vivo stimulation at the pre-disease time point of 12 dpi (Fig. 4). Similar to acute disease, marked reductions were seen in the overall amount of T cells in IL7RTgIL7R−/− spleens (Fig. 4A). As predicted, IL17 producing CD4 T cells were higher than in acute disease, and were more abundant in WT compared to IL7RTgIL7R−/− mice (Fig. 4B, C; Fig. 3C, D). Interestingly though, splenic TNF and IFNγ T cell profiles mirrored that of 25dpi with robust TNF production in WT mice that was dramatically reduced in CD4 and CD8 T cells of IL7RTgIL7R−/− mice (Fig. 4B-E). Likewise, IFNγ producing CD8 T cells were also significantly diminished in IL7RTgIL7R−/− mice (Fig. 4D, E). In the SC, infiltrating immune cells were extremely scarce at 12 dpi and reliable comparisons could not be made (data not shown). Taken together, IL7Rα deficiency reduced the classical Th17 phenotype early on in the periphery. This however did not persist through acute disease, unlike the effects on TNF or IFNγ producing T cells, which were impacted far more and for a longer period of time. Overall we show that IL7Rα blockade reduces the T cell response and represents one contributing factor to less severe disease.
Figure 4. IL7Rα deficiency alters T cell responses prior to EAE clinical symptoms.
Quantification of total T cell numbers are shown for the spleen of indicated mice at acute EAE (25dpi) following ex vivo stimulation and flow cytometry analysis. Total CD4 and CD8 T cell populations were analyzed after gating on the CD45 leukocytes (A). Representative dot plots for TNF, IFN and IL17 expression within the CD4 (B) and CD8 (D) gates are shown. Cytokine producing CD4 (C) and CD8 (E) T cells were quantified. Results are expressed as the mean ± SEM with n = 13 per group from 3 independent experiments. *, p<0.05; **, p<0.005; ***, p<0.0005; unpaired t test.
EAE pathology is dependent upon IL7Rα expressed on both hematopoietic and non-hematopoietic cells
Although IL7Rα is most commonly associated with lymphocytes, it has also been identified on dendritic cells, lymphatic microvascular endothelium, and stromal cells (33, 57, 58). Additionally, IL7Rα has been detected in cultured rat hippocampal neurons and mouse subventricular zone progenitors (38), as well as human neuronal and astrocyte cultures (39), warranting the possibility that IL7Rα may contribute to EAE by signaling directly in the CNS. For that reason, we generated a series of chimeric mice to identify whether or not IL7Rα expression on non-bone marrow derived cells contributes to EAE. Since we showed that IL7RTgIL7R−/− mice have lower T cell-dependent inflammatory responses, we first wanted to determine if IL7Rα deficiency on BM derived cells alone would be sufficient for a reduced EAE phenotype. Therefore, we generated chimeras by transplanting BM cells from IL7RTgIL7R−/− mice or CD45.1 congenic WT mice into lethally irradiated Rag1−/− recipients that retain normal IL7Rα expression in non-hematopoietic cells, but lack lymphocyte competition. Upon reconstitution of the immune compartment (Supplemental Fig. 3), EAE was induced in both chimeras and non-irradiated WT controls. Surprisingly, animals that received IL7RTgIL7R−/− donor BM, hence lacking IL7Rα in hematopoietic cells, were not protected from disease. No significant differences were seen in overall clinical scores (Fig. 5A) or incidence of disease between all three groups (Table 2). However, chimeric mice receiving the IL7RTgIL7R−/− BM displayed an earlier onset of disease and modest increase in CDI (Table 2). Flow cytometry analysis of spinal cords at acute disease (18 dpi) showed substantial CD45 leukocyte infiltration in all three groups (Fig. 5B). No significant differences were seen for total CD4 T cells, though Tg→Rag1−/− chimeras showed fewer numbers of CD4+CD25+FoxP3+ T regulatory cells (Fig. 5B), which could explain why this group had a slightly enhanced disease phenotype (Table 2). Additionally, CD8 T cell numbers were significantly increased in WT→Rag1−/− chimeric mice (Fig. 5B). In the spleen, non-irradiated WT mice had significantly more CD45 leukocyte expansion in comparison to either of the chimeric groups (Fig. 5B). In contrast to the SC, peripheral CD4 and CD8 T cells were drastically reduced in Tg→Rag1−/− chimeras (~4-fold) and moderately reduced in WT→Rag1−/− chimeras (~2-fold) compared to WT. Reductions in splenic CD4+CD25+FoxP3+ T regulatory cells were only seen in Tg→Rag1−/− chimeric mice (Fig. 5B). Therefore, hindering IL7Rα signaling specifically on BM-derived cells may alter peripheral expansion in the chimeric model, but has lesser effect on leukocyte infiltration of the SC and is not sufficient to protect from disease.
Figure 5. Selective IL7Rα expression through bone marrow chimerism is not sufficient for EAE protection.
Quantification of total cell numbers are shown for the spleen and SC of indicated mice at acute EAE (18-24 dpi) following flow cytometry analysis (B, D). Total CD45 cells were analyzed after gating on the live cell population. Total CD4 and CD8 T cell populations were analyzed after gating on the CD45 leukocytes, while Tregs were identified by CD25+FoxP3+ expression within the CD4 gate. Results are expressed as the mean ± SEM with n ≥ 8 per group from 3 independent experiments. ns, P>0.05; #, p<0.05; *, p<0.0005; ^, p<0.0001; Mann Whitney t test (A, C) and unpaired t test (B, D).
Table II Clinical parameters of EAE in Ragl-/- chimera mice.
| Genotype | Incidence | Day of Onset1 | CDI1,2 |
|---|---|---|---|
| WT | 74% (14/19) | l4.l±0.4 | 8.7±l.5 |
| WT → Ragl-/- | 88% (l4/16) | l4.9±0.4 | 9.3±l.5 |
| IL7RTgIL7R-/- → Ragl-/- | 9l% (l0/ll) | ll.9±l.6* | 15.1±3.1† |
Results represent the mean ±SEM.
CDI was calculated as the sum of clinical scores for each animal between days 0 and 18.
p<0.05 with respect to WT→Ragl-/-; Student's t test.
p<0.05 with respect to WT; Student's t test.
It is important to note however that IL7Rα signaling remains functional on all host-derived cell types in these chimeric mice and therefore signaling on non-BM derived lineages is still capable of contributing to EAE progression. For that reason, we wanted to identify if IL7Rα deficiency on non-BM derived cells would be sufficient for disease reduction, or if the IL7Rα deficiency is required on cells from both compartments in order to achieve protection from EAE. Hence BM cells from IL7RTgIL7R−/− or CD45.1 congenic WT were transplanted into irradiated IL7R−/− recipients to generate additional chimeric mice. Upon EAE induction, we saw again that chimeric mice receiving WT BM showed no differences in clinical score (Fig. 5C), incidence of disease (Table 3) or immune cell infiltration into the SC (Fig. 5D) compared to WT controls through acute disease (24 dpi). However, the chimeric mice generated to have IL7Rα deficiency in both BM and non-BM derived cell types, hence resembling the phenotype of IL7RTgIL7R−/− mice, were dramatically protected from EAE (Fig. 5C; Table 3) and had little immune infiltration into the SC overall (Fig. 5D). Similarly, peripheral T cell numbers were significantly fewer in Tg→IL7R−/− chimeric mice for most T cell subsets (Fig. 5D). Notably, the only peripheral cell type identified as similar between all three groups was CD4+CD25+FoxP3+ regulatory T cells (Fig. 5D), suggesting that differences in effector T cell expansion may be independent of regulatory T cell function. Overall, these data show that blocking IL7Rα in both hematopoietic and nonhematopoietic cell types is required for EAE protection. It is possible then that cells endogenous to the CNS may directly contribute to disease pathogenesis via IL7Rα signaling, as well as facilitate pro-inflammatory function of immunogenic T cells.
Table III.
Clinical parameters of EAE in IL7R-/- chimera mice.
| Genotype | Incidence | Day of Onset1 | CDI1,2 |
|---|---|---|---|
| WT | 91% (20/22) | 15.6±0.6 | 25.4±3.5 |
| WT → IL7R-/- | 83% (10/12) | 16.0±0.8 | 27.25±6.0 |
| IL7RTgIL7R-/- → IL7R-/- | 8% (1/13) | 23.03 | 0.4±0.4*,† |
Results represent the mean ±SEM.
CDI is calculated as the sum of clinical scores for each animal between days 0 and 24.
No statistics due to n=1.
p<0.0001 with respect to WT→IL7R-/-; Student‘s t test.
p<0.0001 with respect to WT; Student's t test.
IL7Rα expression in the CNS
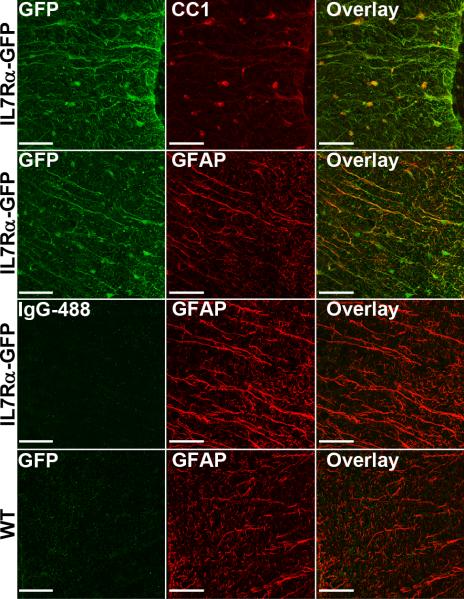
Although there are multiple potential sources for the non-hematopoietic contributions of IL7Rα signaling in EAE, we chose to focus on the CNS directly since this is the affected target tissue, and because the signaling pathway has been implicated in neurons and astrocytes using in vitro model systems (39, 59). Using reporter mice expressing GFP under the IL7Rα promoter and immunofluorescence staining of thoracic SC sections, we show that the IL7Rα-GFP colocalizes with the majority of CC1+ mature oligodendrocytes and a large proportion of GFAP+ astrocytes (Fig. 6). IL7Rα-GFP colocalization was not observed for NeuN+ neurons or CD11b+ microglial cells (data not shown).
Figure 6. IL7Rαexpression on astrocytes and mature oligodendrocytes.
Representative immunofluorescence staining against IL7Rα-GFP, CC1, and GFAP in thoracic SC sections taken from IL7Rα-GFP reporter mice (top 2 panels). Anti-rabbit IgG-488 staining of IL7Rα-GFP SC sections, and anti-GFP staining of WT SC sections (bottom 2 panels) were used as negative controls. Scale bars represent 50μm.
Discussion
Our study demonstrates that IL7Rα signaling contributes to EAE through mechanisms involving not only modulation of T cell effector function, but also the participation of non-BM-derived cell types that utilize this pathway to drive disease, in addition to showing novel expression of IL7Rα directly in the CNS. The attenuation of EAE clinical scores and myelin damage seen in our IL7RTgIL7R−/− model, in conjunction with recovery after in vivo neutralization of IL7Rα, supports the hypothesis that IL7Rα signaling plays a role in EAE pathogenesis. The improved outcome is partially due to select subpopulations of T cells being sensitive to a lack of IL7Rα signaling, diminishing the overall effect of inflammatory cytokine production, particularly TNF. However, it is evident that the scope of IL7Rα expands beyond T cells with respect to EAE pathogenesis, encompassing non-lymphocyte populations, potentially astrocytes and oligodendrocytes local to the CNS. Our findings build upon the genome wide association studies linking IL7Rα to MS, as well as data accumulated in other models of IL7Rα inhibition in EAE, further highlighting the potential of IL7Rα as a target in treatment strategies for MS.
As with most functional studies of signaling pathways, we turned to an IL7Rα deficient model to further dissect the role of IL7Rα in EAE. However, due to the versatility in downstream effects of IL7Rα signaling, a traditional IL7R−/− line is not suitable for use in the EAE system. It is well known that T cells play a key role in EAE pathogenesis, and IL7R−/− mice are highly deficient in T cell development (3, 45, 46). Walline et al. have shown that IL7R−/− mice are in fact resistant to EAE, associating the effect with reduced IL17 and IFNγ production in T cells (60). Nevertheless, the conclusions were based on the relatively rare production of T cells in IL7R−/− mice (45; Fig. 1) making it difficult to assess whether the EAE resistance was truly due to altered T cell function or simply the result of insufficient T cell numbers to elicit an immune response in EAE. Therefore we chose to take advantage of IL7RTgIL7R−/− mice in these studies, which retain ample T cell development while maintaining the IL7Rα deficient phenotype in non-thymic tissues. Hence, the IL7RTgIL7R−/− mice allow us to more accurately determine how the T cell pool is affected in vivo. Using these unique IL7RTgIL7R−/− mice we show they too are resistant to EAE, albeit to a lesser extent than IL7R−/− mice, highlighting the importance of T cell number alongside effector function.
Neutralizing antibodies have also proven useful as an approach to specifically target signaling pathways and inhibit their function. Here we show that a unique anti-IL7Rα monoclonal antibody, A7R34, yields therapeutic effects by reducing EAE clinical scores along with the associated myelin damage. Recently, it was shown that two additional anti-IL7Rα monoclonal antibodies, SB/14 or S8G9, were also able to attenuate the clinical scores of MOG-induced EAE by in vivo neutralization (31, 32). The general reduction in EAE phenotype following all three modes of treatment further confirms the involvement of IL7Rα signaling in this model. Moreover, these results also support the assumption that a defective B cell compartment in the IL7RTgIL7R−/− mice is not the cause of their reduced disease, for EAE-induced WT mice treated with anti-IL7Rα have substantial B cell numbers available prior to and during treatment, yet average clinical scores resemble that of IL7RTgIL7R−/− mice.
For many years IL7Rα signaling was ignored with respect to its contributions in the effector phase of the T cell response, as its expression is down regulated upon activation (61). However, it is becoming more evident that the IL7 signaling axis can influence select effector subsets prior to receptor down regulation, although to what extent is still unclear. For example, in previous studies neutralizing IL7Rα in vivo with antibodies, conclusions differed in that the SB/14 clone alters the Th17 response while clone 28G9 modifies the Th1 response (31, 32). These conflicting results highlight the complexity of IL7Rα signaling, suggesting the possibility that this pathway may differentially affect multiple effector populations. It is also possible that these discordant outcomes could simply be a result of clonal variations in the anti-IL7Rα antibodies, which may result in different binding affinities towards the receptor, hence altering the capacity to neutralize signal. Therefore, we chose to utilize the IL7RTgIL7R−/− mice to further investigate the effect of IL7Rα inhibition on the entire T cell population and within the various subsets that contribute to the immune response in EAE.
In these studies, we observed that IL7RTgIL7R−/− mice have fewer total T cell numbers in the spleen compared to WT at both pre-clinical and acute disease time points. Thus despite having an equivalent CD4 T cell reserve prior to EAE induction, the IL7Rα deficiency dampens the initial T helper response. Likewise, EAE-induced IL7RTgIL7R−/− mice also have fewer CD8 T cells compared to WT, however the initial differences in naïve splenic CD8 T cell number means that the correlation between IL7Rα deficiency and fewer CD8 T cells at 12 dpi and 25 dpi is not necessarily a function of disease. Indeed, studies under homeostatic conditions show that CD8 T cells appear more sensitive to a block in IL7Rα signaling in comparison to CD4 T cells (35, 62), although the reasons behind this general difference have yet to be determined. In fact, one might argue that the lower baseline number of CD8 T cells could in itself contribute to the overall reduction in EAE of IL7RTgIL7R−/− mice. However, it is important to note that this lower number in the spleen did not correlate with reduced CD8 T cell infiltration into the SC of IL7RTgIL7R−/− mice. Since there were relatively few significant differences between IL7RTgIL7R−/− and WT mice with respect to the CD8 T cell population in the SC, the negative contributions of IL7Rα signaling are more likely to be of consequence in the CD4 helper subsets.
It is known that Th1 and Th17 cells contribute to the damaging microenvironment in EAE by secreting inflammatory cytokines such as TNF, IFNγ, and IL-17 that both directly and indirectly lead to destruction of myelin and axonal injury (10, 63-66). Therefore we went on to examine how T cell function was being affected in EAE-induced IL7RTgIL7R−/− mice. Here we show that deficiency in IL7Rα signaling has the most profound effect on TNF producing CD4 and CD8 T cells. Compared to WT mice, TNF is strikingly less abundant in both peripheral and SC infiltrating cells of IL7RTgIL7R−/− mice, which is in stark contrast to the comparatively small reductions seen in T cell IFNγ and IL17.
The profound reduction of TNF producing T cells is particularly interesting since we have recently shown that the functional outcome of EAE is improved by selectively blocking the secreted form of TNF, while permitting transmembrane TNF to signal normally (10). This is because the soluble form of TNF favors TNF receptor 1 binding which supports chronic inflammation (67), while tmTNF preferentially binds TNF receptor 2, promoting anti-inflammatory responses as well as myelin maintenance in the CNS (68, 69). Therefore, the striking drop in TNF-secreting T cells infiltrating into the CNS and in the periphery of IL7RTgIL7R−/− is functionally relevant to the observed EAE protection, and is likely affecting the detrimental soluble form of TNF rather than beneficial transmembrane TNF.
In addition to expanding the role of IL7Rα within the T cell pool, our data shed light on other cellular compartments whose IL7Rα function contributes to EAE. Until now, T cells have been the only targets of IL7Rα investigation in this model, and only in the context of systemic IL7Rα inhibition (i.e. genetic manipulation or neutralizing antibody). In our studies, using the BM-chimera model system abled further dissection of how IL7Rα is functioning in the disease setting. Indeed, we show that IL7Rα+ cell types other than leukocytes are likely contributing to the pathogenesis of EAE. The severe disease phenotype in IL7RTgIL7R−/−→Rag1−/− chimeric mice, which have functioning IL7Rα on nonhematopoietic cells, denotes that there is at least one additional IL7Rα+ cell type in the periphery contributing to EAE. However, since WT→IL7R−/− chimeric mice display a classic disease progression, we can also conclude that eliminating IL7Rα signaling within the non-BM derived compartment alone is insufficient in attenuating EAE. Furthermore, these results support our data showing that normal IL7Rα signaling contributes to the T cell effector response in EAE.
Since only IL7RTgIL7R−/−→IL7R−/− chimeric mice have reduced EAE, it appears that IL7Rα, at the very least, functions through two cellular paths to collectively drive disease. One speculation is that these cells would also produce downstream effector cytokines, such as TNF, to propagate disease. Compensatory function such as this could explain why EAE still occurs in IL7RTgIL7R−/−→Rag1−/− chimeric mice where T cell function is impaired. It has been shown that microglia and astrocyctes are capable of producing TNF during inflammatory responses in the CNS (70, 71), so we hypothesized that IL7Rα may also be expressed on these cells to promote endogenous TNF production during the inflammatory response of EAE. Using IL7Rα-GFP reporter mice, we positively identified IL7Rα on GFAP+ astrocytes, but not microglia, making astrocytes a strong candidate for further investigation in the IL7Rα deficient models of EAE. Additionally, we identified IL7Rα expression on the cell bodies of CC1+ mature oligodendrocytes. To our knowledge though, there are no reports of oligodendrocyte TNF production, and therefore other known functions of IL7Rα, such as cell survival, may play a role in this cell type.
Overall, our data further support the current hypothesis that IL7Rα signaling does contribute to EAE. Although there is some effect on classical IFNγ producing Th1 and IL17 producing Th17 subsets, IL7Rα predominantly drives TNF-producing T cells in the EAE model. Additionally, these effector cells appear to work in conjunction with non-hematopoietic cell types, potentially astrocytes, which also utilize IL7Rα to drive disease. It is now apparent that the IL7Rα signaling pathway functions in many cellular subsets intricately involved in the detrimental aspects of EAE/MS and therefore serves as an optimal target for MS therapies.
Supplementary Material
Acknowledgements
We would like to thank the Diabetes Research Institute flow cytometry core facility, as well as the Miami Project histology and imaging core facilities at the University of Miami for their advice and technical expertise.
This work was supported by NIH grants 5-R01-NS065479-03 to JRB and 5-T32-NS007492-08 to JLB; and by the Lois Pope LIFE Fellows Program.
Abbreviations
- BM
bone marrow
- CDI
cumulative disease index
- CNS
central nervous system
- DPI
days post immunization
- EAE
experimental autoimmune encephalomyelitis
- GFAP
glial fibrillary acidic protein
- MS
multiple sclerosis
- SC
spinal cord
- SNP
single nucleotide polymorphism
- TSLPR
thymic stromal lymphopoietin receptor
- WT
wild type
References
- 1.Perry VH, Anthony DC. Axon damage and repair in multiple sclerosis. Philos Trans R Soc Lond B Biol Sci. 1999;354:1641–1647. doi: 10.1098/rstb.1999.0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lassmann H, Bruck W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17:210–218. doi: 10.1111/j.1750-3639.2007.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trapp BD, Nave KA. Multiple sclerosis: an immune or neurodegenerative disorder? Annu Rev Neurosci. 2008;31:247–269. doi: 10.1146/annurev.neuro.30.051606.094313. [DOI] [PubMed] [Google Scholar]
- 4.Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- 5.Flugel A, Berkowicz T, Ritter T, Labeur M, Jenne DE, Li Z, Ellwart JW, Willem M, Lassmann H, Wekerle H. Migratory activity and functional changes of green fluorescent effector cells before and during experimental autoimmune encephalomyelitis. Immunity. 2001;14:547–560. doi: 10.1016/s1074-7613(01)00143-1. [DOI] [PubMed] [Google Scholar]
- 6.Issazadeh S, Mustafa M, Ljungdahl A, Hojeberg B, Dagerlind A, Elde R, Olsson T. Interferon gamma, interleukin 4 and transforming growth factor beta in experimental autoimmune encephalomyelitis in Lewis rats: dynamics of cellular mRNA expression in the central nervous system and lymphoid cells. J Neurosci Res. 1995;40:579–590. doi: 10.1002/jnr.490400503. [DOI] [PubMed] [Google Scholar]
- 7.Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 8.Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 9.Kassiotis G, Kollias G. Uncoupling the proinflammatory from the immunosuppressive properties of tumor necrosis factor (TNF) at the p55 TNF receptor level: implications for pathogenesis and therapy of autoimmune demyelination. J Exp Med. 2001;193:427–434. doi: 10.1084/jem.193.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brambilla R, Ashbaugh JJ, Magliozzi R, Dellarole A, Karmally S, Szymkowski DE, Bethea JR. Inhibition of soluble tumour necrosis factor is therapeutic in experimental autoimmune encephalomyelitis and promotes axon preservation and remyelination. Brain : a journal of neurology. 2011;134:2736–2754. doi: 10.1093/brain/awr199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cantorna MT, Hayes CE, DeLuca HF. 1,25-Dihydroxyvitamin D3 reversibly blocks the progression of relapsing encephalomyelitis, a model of multiple sclerosis. Proc Natl Acad Sci U S A. 1996;93:7861–7864. doi: 10.1073/pnas.93.15.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cathala F, Brown P. The possible viral aetiology of disseminated sclerosis. J Clin Pathol Suppl (R Coll Pathol) 1972;6:141–151. [PMC free article] [PubMed] [Google Scholar]
- 13.Haire M, Fraser KB, Millar JH. Measles and other virus-specific immunoglobulins in multiple sclerosis. Br Med J. 1973;3:612–615. doi: 10.1136/bmj.3.5881.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winer S, Astsaturov I, Cheung RK, Schrade K, Gunaratnam L, Wood DD, Moscarello MA, O'Connor P, McKerlie C, Becker DJ, Dosch HM. T cells of multiple sclerosis patients target a common environmental peptide that causes encephalitis in mice. J Immunol. 2001;166:4751–4756. doi: 10.4049/jimmunol.166.7.4751. [DOI] [PubMed] [Google Scholar]
- 15.Elian M, Dean G. Multiple sclerosis among the United Kingdom-born children of immigrants from the West Indies. J Neurol Neurosurg Psychiatry. 1987;50:327–332. doi: 10.1136/jnnp.50.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller DH, Hammond SR, McLeod JG, Purdie G, Skegg DC. Multiple sclerosis in Australia and New Zealand: are the determinants genetic or environmental? J Neurol Neurosurg Psychiatry. 1990;53:903–905. doi: 10.1136/jnnp.53.10.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffjan S, Akkad DA. The genetics of multiple sclerosis: an update 2010. Mol Cell Probes. 2010;24:237–243. doi: 10.1016/j.mcp.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Hafler DA, Compston A, Sawcer S, Lander ES, Daly MJ, De Jager PL, de Bakker PI, Gabriel SB, Mirel DB, Ivinson AJ, Pericak-Vance MA, Gregory SG, Rioux JD, McCauley JL, Haines JL, Barcellos LF, Cree B, Oksenberg JR, Hauser SL. Risk alleles for multiple sclerosis identified by a genomewide study. N Engl J Med. 2007;357:851–862. doi: 10.1056/NEJMoa073493. [DOI] [PubMed] [Google Scholar]
- 19.Aulchenko YS, Hoppenbrouwers IA, Ramagopalan SV, Broer L, Jafari N, Hillert J, Link J, Lundstrom W, Greiner E, Dessa Sadovnick A, Goossens D, Van Broeckhoven C, Del-Favero J, Ebers GC, Oostra BA, van Duijn CM, Hintzen RQ. Genetic variation in the KIF1B locus influences susceptibility to multiple sclerosis. Nat Genet. 2008;40:1402–1403. doi: 10.1038/ng.251. [DOI] [PubMed] [Google Scholar]
- 20.Comabella M, Craig DW, Camina-Tato M, Morcillo C, Lopez C, Navarro A, Rio J, Montalban X, Martin R. Identification of a novel risk locus for multiple sclerosis at 13q31.3 by a pooled genome-wide scan of 500,000 single nucleotide polymorphisms. PLoS One. 2008;3:e3490. doi: 10.1371/journal.pone.0003490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baranzini SE, Galwey NW, Wang J, Khankhanian P, Lindberg R, Pelletier D, Wu W, Uitdehaag BM, Kappos L, Polman CH, Matthews PM, Hauser SL, Gibson RA, Oksenberg JR, Barnes MR. Pathway and network-based analysis of genome-wide association studies in multiple sclerosis. Hum Mol Genet. 2009;18:2078–2090. doi: 10.1093/hmg/ddp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Consortium T. A. a. N. Z. M. S. G. Genome-wide association study identifies new multiple sclerosis susceptibility loci on chromosomes 12 and 20. Nat Genet. 2009;41:824–828. doi: 10.1038/ng.396. [DOI] [PubMed] [Google Scholar]
- 23.Jakkula E, Leppa V, Sulonen AM, Varilo T, Kallio S, Kemppinen A, Purcell S, Koivisto K, Tienari P, Sumelahti ML, Elovaara I, Pirttila T, Reunanen M, Aromaa A, Oturai AB, Sondergaard HB, Harbo HF, Mero IL, Gabriel SB, Mirel DB, Hauser SL, Kappos L, Polman C, De Jager PL, Hafler DA, Daly MJ, Palotie A, Saarela J, Peltonen L. Genome-wide association study in a high-risk isolate for multiple sclerosis reveals associated variants in STAT3 gene. Am J Hum Genet. 2010;86:285–291. doi: 10.1016/j.ajhg.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zaffaroni M, Di Falco M, Ghezzi A, Rizzolo L, Veneroni G, Marforio S. HLA typing and T-cell subpopulations in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1988;51:1465. doi: 10.1136/jnnp.51.11.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gregory SG, Schmidt S, Seth P, Oksenberg JR, Hart J, Prokop A, Caillier SJ, Ban M, Goris A, Barcellos LF, Lincoln R, McCauley JL, Sawcer SJ, Compston DA, Dubois B, Hauser SL, Garcia-Blanco MA, Pericak-Vance MA, Haines JL. Interleukin 7 receptor alpha chain (IL7R) shows allelic and functional association with multiple sclerosis. Nat Genet. 2007;39:1083–1091. doi: 10.1038/ng2103. [DOI] [PubMed] [Google Scholar]
- 26.Zuvich RL, McCauley JL, Oksenberg JR, Sawcer SJ, De Jager PL, Aubin C, Cross AH, Piccio L, Aggarwal NT, Evans D, Hafler DA, Compston A, Hauser SL, Pericak-Vance MA, Haines JL. Genetic variation in the IL7RA/IL7 pathway increases multiple sclerosis susceptibility. Hum Genet. 2010;127:525–535. doi: 10.1007/s00439-010-0789-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lundmark F, Duvefelt K, Iacobaeus E, Kockum I, Wallstrom E, Khademi M, Oturai A, Ryder LP, Saarela J, Harbo HF, Celius EG, Salter H, Olsson T, Hillert J. Variation in interleukin 7 receptor alpha chain (IL7R) influences risk of multiple sclerosis. Nat Genet. 2007;39:1108–1113. doi: 10.1038/ng2106. [DOI] [PubMed] [Google Scholar]
- 28.O'Doherty C, Kantarci O, Vandenbroeck K. IL7RA polymorphisms and susceptibility to multiple sclerosis. N Engl J Med. 2008;358:753–754. doi: 10.1056/NEJMc0707553. [DOI] [PubMed] [Google Scholar]
- 29.Goodwin RG, Friend D, Ziegler SF, Jerzy R, Falk BA, Gimpel S, Cosman D, Dower SK, March CJ, Namen AE, et al. Cloning of the human and murine interleukin-7 receptors: demonstration of a soluble form and homology to a new receptor superfamily. Cell. 1990;60:941–951. doi: 10.1016/0092-8674(90)90342-c. [DOI] [PubMed] [Google Scholar]
- 30.Ramanathan M, Weinstock-Guttman B, Nguyen LT, Badgett D, Miller C, Patrick K, Brownscheidle C, Jacobs L. In vivo gene expression revealed by cDNA arrays: the pattern in relapsing-remitting multiple sclerosis patients compared with normal subjects. J Neuroimmunol. 2001;116:213–219. doi: 10.1016/s0165-5728(01)00308-3. [DOI] [PubMed] [Google Scholar]
- 31.Lee LF, Axtell R, Tu GH, Logronio K, Dilley J, Yu J, Rickert M, Han B, Evering W, Walker MG, Shi J, de Jong BA, Killestein J, Polman CH, Steinman L, Lin JC. IL-7 promotes T(H)1 development and serum IL-7 predicts clinical response to interferon-beta in multiple sclerosis. Sci Transl Med. 2011;3:93ra68. doi: 10.1126/scitranslmed.3002400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu X, Leung S, Wang C, Tan Z, Wang J, Guo TB, Fang L, Zhao Y, Wan B, Qin X, Lu L, Li R, Pan H, Song M, Liu A, Hong J, Lu H, Zhang JZ. Crucial role of interleukin-7 in T helper type 17 survival and expansion in autoimmune disease. Nat Med. 2010;16:191–197. doi: 10.1038/nm.2077. [DOI] [PubMed] [Google Scholar]
- 33.Akashi K, Kondo M, Weissman IL. Role of interleukin-7 in T-cell development from hematopoietic stem cells. Immunol Rev. 1998;165:13–28. doi: 10.1111/j.1600-065x.1998.tb01226.x. [DOI] [PubMed] [Google Scholar]
- 34.Porter BO, Scibelli P, Malek TR. Control of T cell development in vivo by subdomains within the IL-7 receptor alpha-chain cytoplasmic tail. J Immunol. 2001;166:262–269. doi: 10.4049/jimmunol.166.1.262. [DOI] [PubMed] [Google Scholar]
- 35.Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI, Surh CD. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci U S A. 2001;98:8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seddon B, Tomlinson P, Zamoyska R. Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nat Immunol. 2003;4:680–686. doi: 10.1038/ni946. [DOI] [PubMed] [Google Scholar]
- 37.Guimond M, Veenstra RG, Grindler DJ, Zhang H, Cui Y, Murphy RD, Kim SY, Na R, Hennighausen L, Kurtulus S, Erman B, Matzinger P, Merchant MS, Mackall CL. Interleukin 7 signaling in dendritic cells regulates the homeostatic proliferation and niche size of CD4+ T cells. Nat Immunol. 2009;10:149–157. doi: 10.1038/ni.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michaelson MD, Mehler MF, Xu H, Gross RE, Kessler JA. Interleukin-7 is trophic for embryonic neurons and is expressed in developing brain. Dev Biol. 1996;179:251–263. doi: 10.1006/dbio.1996.0255. [DOI] [PubMed] [Google Scholar]
- 39.Nunnari G, Xu Y, Acheampong EA, Fang J, Daniel R, Zhang C, Zhang H, Mukhtar M, Pomerantz RJ. Exogenous IL-7 induces Fas-mediated human neuronal apoptosis: potential effects during human immunodeficiency virus type 1 infection. J Neurovirol. 2005;11:319–328. doi: 10.1080/13550280500187005. [DOI] [PubMed] [Google Scholar]
- 40.Kremlev SG, Gaurnier-Hausser AL, Del Valle L, Perez-Liz G, Dimitrov S, Tuszynski G. Angiocidin promotes pro-inflammatory cytokine production and antigen presentation in multiple sclerosis. J Neuroimmunol. 2008;194:132–142. doi: 10.1016/j.jneuroim.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 41.Carrio R, Rolle CE, Malek TR. Non-redundant role for IL-7R signaling for the survival of CD8+ memory T cells. Eur J Immunol. 2007;37:3078–3088. doi: 10.1002/eji.200737585. [DOI] [PubMed] [Google Scholar]
- 42.Brambilla R, Persaud T, Hu X, Karmally S, Shestopalov VI, Dvoriantchikova G, Ivanov D, Nathanson L, Barnum SR, Bethea JR. Transgenic inhibition of astroglial NF-kappa B improves functional outcome in experimental autoimmune encephalomyelitis by suppressing chronic central nervous system inflammation. J Immunol. 2009;182:2628–2640. doi: 10.4049/jimmunol.0802954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szalai AJ, Nataf S, Hu XZ, Barnum SR. Experimental allergic encephalomyelitis is inhibited in transgenic mice expressing human C-reactive protein. J Immunol. 2002;168:5792–5797. doi: 10.4049/jimmunol.168.11.5792. [DOI] [PubMed] [Google Scholar]
- 44.Unkeless JC. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J Exp Med. 1979;150:580–596. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peschon JJ, Morrissey PJ, Grabstein KH, Ramsdell FJ, Maraskovsky E, Gliniak BC, Park LS, Ziegler SF, Williams DE, Ware CB, Meyer JD, Davison BL. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med. 1994;180:1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maraskovsky E, Teepe M, Morrissey PJ, Braddy S, Miller RE, Lynch DH, Peschon JJ. Impaired survival and proliferation in IL-7 receptor-deficient peripheral T cells. J Immunol. 1996;157:5315–5323. [PubMed] [Google Scholar]
- 47.Bernard CC, Leydon J, Mackay IR. T cell necessity in the pathogenesis of experimental autoimmune encephalomyelitis in mice. Eur J Immunol. 1976;6:655–660. doi: 10.1002/eji.1830060912. [DOI] [PubMed] [Google Scholar]
- 48.Ortiz-Ortiz L, Nakamura RM, Weigle WO. T cell requirement for experimental allergic encephalomyelitis induction in the rat. J Immunol. 1976;117:576–579. [PubMed] [Google Scholar]
- 49.Nagasawa T. Microenvironmental niches in the bone marrow required for B-cell development. Nature reviews. Immunology. 2006;6:107–116. doi: 10.1038/nri1780. [DOI] [PubMed] [Google Scholar]
- 50.Mann MK, Ray A, Basu S, Karp CL, Dittel BN. Pathogenic and regulatory roles for B cells in experimental autoimmune encephalomyelitis. Autoimmunity. 2012;45:388–399. doi: 10.3109/08916934.2012.665523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hjelmstrom P, Juedes AE, Fjell J, Ruddle NH. B-cell-deficient mice develop experimental allergic encephalomyelitis with demyelination after myelin oligodendrocyte glycoprotein sensitization. J Immunol. 1998;161:4480–4483. [PubMed] [Google Scholar]
- 52.Rochman Y, Kashyap M, Robinson GW, Sakamoto K, Gomez-Rodriguez J, Wagner KU, Leonard WJ. Thymic stromal lymphopoietin-mediated STAT5 phosphorylation via kinases JAK1 and JAK2 reveals a key difference from IL-7-induced signaling. Proc Natl Acad Sci U S A. 2010;107:19455–19460. doi: 10.1073/pnas.1008271107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pandey A, Ozaki K, Baumann H, Levin SD, Puel A, Farr AG, Ziegler SF, Leonard WJ, Lodish HF. Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin. Nat Immunol. 2000;1:59–64. doi: 10.1038/76923. [DOI] [PubMed] [Google Scholar]
- 54.Park LS, Martin U, Garka K, Gliniak B, Di Santo JP, Muller W, Largaespada DA, Copeland NG, Jenkins NA, Farr AG, Ziegler SF, Morrissey PJ, Paxton R, Sims JE. Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: Formation of a functional heteromeric complex requires interleukin 7 receptor. J Exp Med. 2000;192:659–670. doi: 10.1084/jem.192.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miyajima A, Miyatake S, Schreurs J, De Vries J, Arai N, Yokota T, Arai K. Coordinate regulation of immune and inflammatory responses by T cell-derived lymphokines. FASEB J. 1988;2:2462–2473. doi: 10.1096/fasebj.2.9.2836253. [DOI] [PubMed] [Google Scholar]
- 56.Murphy AC, Lalor SJ, Lynch MA, Mills KH. Infiltration of Th1 and Th17 cells and activation of microglia in the CNS during the course of experimental autoimmune encephalomyelitis. Brain Behav Immun. 2010;24:641–651. doi: 10.1016/j.bbi.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 57.Dus D, Krawczenko A, Zalecki P, Paprocka M, Wiedlocha A, Goupille C, Kieda C. IL-7 receptor is present on human microvascular endothelial cells. Immunol Lett. 2003;86:163–168. doi: 10.1016/s0165-2478(03)00018-x. [DOI] [PubMed] [Google Scholar]
- 58.Pillai M, Torok-Storb B, Iwata M. Expression and function of IL-7 receptors in marrow stromal cells. Leuk Lymphoma. 2004;45:2403–2408. doi: 10.1080/10428190412331283189. [DOI] [PubMed] [Google Scholar]
- 59.Mehler MF, Rozental R, Dougherty M, Spray DC, Kessler JA. Cytokine regulation of neuronal differentiation of hippocampal progenitor cells. Nature. 1993;362:62–65. doi: 10.1038/362062a0. [DOI] [PubMed] [Google Scholar]
- 60.Walline CC, Kanakasabai S, Bright JJ. IL-7Ralpha confers susceptibility to experimental autoimmune encephalomyelitis. Genes Immun. 2011;12:1–14. doi: 10.1038/gene.2010.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alves NL, van Leeuwen EM, Derks IA, van Lier RA. Differential regulation of human IL-7 receptor alpha expression by IL-7 and TCR signaling. J Immunol. 2008;180:5201–5210. doi: 10.4049/jimmunol.180.8.5201. [DOI] [PubMed] [Google Scholar]
- 62.Mackall CL, Fry TJ, Gress RE. Harnessing the biology of IL-7 for therapeutic application. Nature reviews. Immunology. 2011;11:330–342. doi: 10.1038/nri2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Petermann F, Korn T. Cytokines and effector T cell subsets causing autoimmune CNS disease. FEBS letters. 2011;585:3747–3757. doi: 10.1016/j.febslet.2011.03.064. [DOI] [PubMed] [Google Scholar]
- 64.Young HA, Hardy KJ. Role of interferon-gamma in immune cell regulation. Journal of leukocyte biology. 1995;58:373–381. [PubMed] [Google Scholar]
- 65.Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E, Saeland S, Blanchard D, Gaillard C, Das Mahapatra B, Rouvier E, Golstein P, Banchereau J, Lebecque S. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183:2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Akassoglou K, Bauer J, Kassiotis G, Pasparakis M, Lassmann H, Kollias G, Probert L. Oligodendrocyte apoptosis and primary demyelination induced by local TNF/p55TNF receptor signaling in the central nervous system of transgenic mice: models for multiple sclerosis with primary oligodendrogliopathy. The American journal of pathology. 1998;153:801–813. doi: 10.1016/S0002-9440(10)65622-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Holtmann MH, Neurath MF. Differential TNF-signaling in chronic inflammatory disorders. Curr Mol Med. 2004;4:439–444. doi: 10.2174/1566524043360636. [DOI] [PubMed] [Google Scholar]
- 68.Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- 69.Grell M, Douni E, Wajant H, Lohden M, Clauss M, Maxeiner B, Georgopoulos S, Lesslauer W, Kollias G, Pfizenmaier K, Scheurich P. The transmembrane form of tumor necrosis factor is the prime activating ligand of the 80 kDa tumor necrosis factor receptor. Cell. 1995;83:793–802. doi: 10.1016/0092-8674(95)90192-2. [DOI] [PubMed] [Google Scholar]
- 70.Renno T, Krakowski M, Piccirillo C, Lin JY, Owens T. TNF-alpha expression by resident microglia and infiltrating leukocytes in the central nervous system of mice with experimental allergic encephalomyelitis. Regulation by Th1 cytokines. J Immunol. 1995;154:944–953. [PubMed] [Google Scholar]
- 71.Lieberman AP, Pitha PM, Shin HS, Shin ML. Production of tumor necrosis factor and other cytokines by astrocytes stimulated with lipopolysaccharide or a neurotropic virus. Proc Natl Acad Sci U S A. 1989;86:6348–6352. doi: 10.1073/pnas.86.16.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.