Abstract
Background and Purpose
Endovascular thrombectomy is an increasingly used treatment for arterial occlusion in acute stroke. Various devices (including most extensively the Mechanical Embolus Removal in Cerebral Ischemia/MERCI Retriever device) have been utilized for this.
Methods
We review the neuropathologic findings in 5 patients (age range 59–87 years) who died acutely or as late as 38 days after procedures using the Merci (4 patients) and Penumbra (1 patient) devices were carried out to remove thrombo-emboli from the middle cerebral artery (MCA). Partial recanalization was achieved by thrombectomy in all 5 patients.
Results
All patients showed extensive cerebral infarcts, 3/5 with clinical hemorrhagic transformations of the infarct or frank intraparenchymal hemorrhage after thrombectomy— in one case this was judged to be at least partly on the basis of concomitant hypertensive microvascular disease. With one exception basal arteries examined in detail by immunohistochemistry, showed prominent, though usually non-occlusive (and generally non-ulcerated) atheromata, often with significant luminal stenosis. One patient showed a subintimal dissection with resultant occlusion of the MCA.
Conclusions
In this highly selected group of patients, the vascular pathologic abnormalities affecting basal arteries were variable, but complicated atherosclerosis was a common finding. Extensive irreversible brain necrosis prior to therapeutic procedures may have contributed to deaths.
Keywords: Thrombectomy, stroke recovery, MERCI device, cerebral infarct, intracerebral hemorrhage
INTRODUCTION
Endovascular thrombectomy procedures are frequently performed to extract thromboemboli that have occluded major branches of the circle of Willis and proximal arteries emerging from it1–7. The design, preclinical testing and practical uses of various devices employed to carry out the procedure have been described in several publications, as have treatment outcomes8–15. The retrieved thromboemboli have been carefully studied for their cellular and biochemical components with the hope that the results of such studies may shed light on why thromboembolectomy is effective in only some patients16.
Autopsy findings have not previously been described in patients who have undergone thrombectomy. Analysis of outcomes following thrombectomy shows that mortality is substantial in both those in whom recanalization of an occluded artery has been achieved (mortality rates of 20–40%) and those in whom recanalization is not achieved (60–80%) 10. Patients who undergo autopsy (following any therapeutic procedure) clearly represent a highly selected population with the worst possible outcome. We undertook this study to investigate vascular and brain parenchymal abnormalities in five patients who died after thrombectomy.
SUBJECTS & METHODS
Subjects included in the study were five individuals (4 females, one male, with an age range of 59–87 years, mean 75.6) who expired and underwent autopsy following thrombectomy after acute stroke. The indication for thrombectomy was to restore blood flow to the neurovasculature by removing thrombus. The attending physician used his judgement to offer the treatment option with the greatest potential for a beneficial outcome.
The MERCI ® Retriever device had been used in 4 individuals, while the fifth patient was treated with a Penumbra ® device. Patient #2 had an angioplasty performed in the affected left MCA M1 segment of the artery, after thrombectomy, while patient #4 was given intravenous tissue plasminogen activator (tPA) in conjunction with thrombectomy. The procedures were performed by GRD (patients #1, 2), ST (patients #3,5) and FV (patient #4). All physicians were involved since the inception of its use in man and are highly trained and experienced with the device and were involved in the pivotal trials leading to FDA clearance of the device.
All autopsies were performed over an 89 month period (May, 2001through October, 2008). Patient demographics and details of their presenting stroke are provided in Table 1. All individuals had presented with a large left or right cerebral hemispheric (MCA territory) infarct—three were thought to have originated from a cardiogenic embolus, one from in situ thrombosis in the right MCA, and in our most recently encountered patient there was significant atherosclerosis of the cerebral arteries as well as multiple potential sources of cardiogenic emboli. The most recently studied individual had developed symptoms during an airplane flight, necessitating an emergency landing and transfer to the hospital. Presenting symptoms and medications patients were receiving are listed. All subjects had undergone thrombectomy with a mild anticoagulant as rapidly as possible after having experienced their ‘stroke.’ Groin puncture was median 5 hours 10 minutes after last known well time, range 1 hour 15 minutes to 7 hours 15 minutes.
Table 1.
Clinical and Pathologic Summary of Study Patients
| Patient ID | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 |
|---|---|---|---|---|---|
| Age/Sex | - 73 yr female | - 87 yr female | - 59 yr female | - 84 yr male | - 75 yr female |
| Presenting Symptoms and Relevant Medications |
|
|
|
|
|
| Ischemic Event Prior to Thrombectomy Device | May 2001:
|
June 2004:
|
October 2007:
|
January 2008:
|
September 2008:
|
| Thrombectomy Procedure & Follow-up |
|
|
|
|
|
| Infarct At Autopsy |
|
|
|
|
|
| Cerebral Arterial Pathology |
|
|
|
|
|
End of procedure Thrombolysis in Cerebral Infarction (TICI) score was 2 in all patients17. Patients survived over a range of 1–38 days following thrombectomy. One patient (subject #3, the longest post-thrombectomy survivor in this series) had undergone hemicraniectomy 12 days following the stroke to relieve rising intracranial pressure.
In all autopsies, brains were removed and processed using conventional techniques. Complete autopsy was carried out on four five patients. Prior to sectioning of each brain, the circle of Willis and its major branches were dissected from its base and cross-sectioned. Multiple segments from major branches of the circle of Willis were studied by routine histology and/or immunohistochemistry (see below). Digital images of fixed brain slices were retained.
Routine paraffin sections of arteries from all cases were stained with hematoxylin and eosin, as well as the elastica van Gieson (EVG) method (Diagnostic Biosystems, Pleasanton, CA) to highlight elastica and intimal thickening. Immunoperoxidase immunohistochemistry was performed on sections from selected blocks using primary antibodies to smooth muscle actin (SMA, to show smooth muscle cells) and CD68 (to demonstrate macrophages). Primary antibodies for these studies were from Dako (Carpinteria, CA) and used at a primary dilution of 1:100 (both SMA and CD68). Digital photographs of EVG-stained arteries were subject to the computer programs Adobe Photoshop CS2 Version 9.0 and Image J 1.38X, allowing for quantification of percentage stenosis of atherosclerotic segments.
RESULTS
Clinicopathologic features for all patients are summarized in Table 1. Figures representing radiographic and neuropathologic findings are intentionally presented sequentially from the earliest patient encountered, to the most recent, using the numbering system given in Table 1. The atheromatous plaque in all examined arteries showed varying collections of SMA-immunoreactive smooth muscle cells and CD68-immunoreactive macrophages—smooth muscle cells often forming a subintimal cap over collections of macrophages. None showed ulceration of the plaque or intra-plaque hemorrhage (Figures 1, 3, 6, 8 C-E and 11). Lumina of MCA segments examined from patients #2, #4 and #5 were patent. Athero-emboli were not found in the distal circulation of any case though rare small arteries showed occlusion by platelet-fibrin thromboemboli. The estimated degree of arterial stenosis varied from virtually none to 73%. Patient #3, thought to have experienced a cardiogenic embolus (Figures 5 and 6), showed advanced organization of the right MCA thrombo-embolus with ingrowth of smooth muscle cells into the occlusive material, consistent with extended (38 day) survival. Figures 4, 7 and 9 illustrate relevant neuroimaging studies (pre- and/or post-thrombectomy) for patients #3, #4 and #5, respectively; Figures 2, 3A, 5, 8A & B, and 10 illustrate brain parenchymal and vascular abnormalities. Patient #1 was the only individual to have suffered a subintimal dissection of the right MCA (Figure 1). This dissection occurred over a comparatively non-atherosclerotic portion of that artery. Arterial segments from 4 of the 5 patients (the exception being patient #3) showed significant complicated atherosclerosis with varying degrees of atherosclerotic stenosis of arterial segments that had been traversed during thrombectomy..
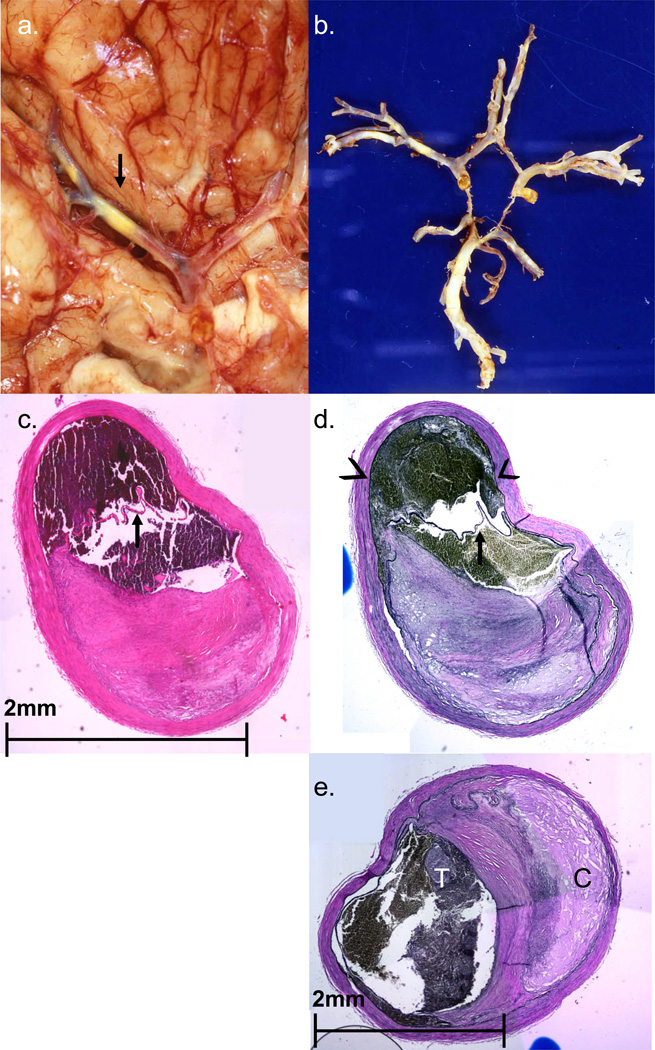
FIGURE 1.
Case #1. A. Basal view of brain reveals severe multifocal atherosclerotic plaque in the M1 segment of the right MCA (arrow). B. Circle of Willis dissected away from the brain reveals multifocal atherosclerosis. C,D. Closely adjacent histologic sections from the right MCA (C from a section stained with H&E, D from a section stained with elastica van Gieson/EVG). Arrows in both panels show subintimal dissection. Note large amount of subintimal blood (arrowheads in D) which has led to severe compromise of the lumen. Dissection was in a cross-sectional portion of the artery not visibly involved by significant atheroma (lower half of the section). E. Photomicrograph of the right MCA (M1 segment) containing extensive plaque and cholesterol accumulation (C) and thrombus formation (T). (EVG stain).
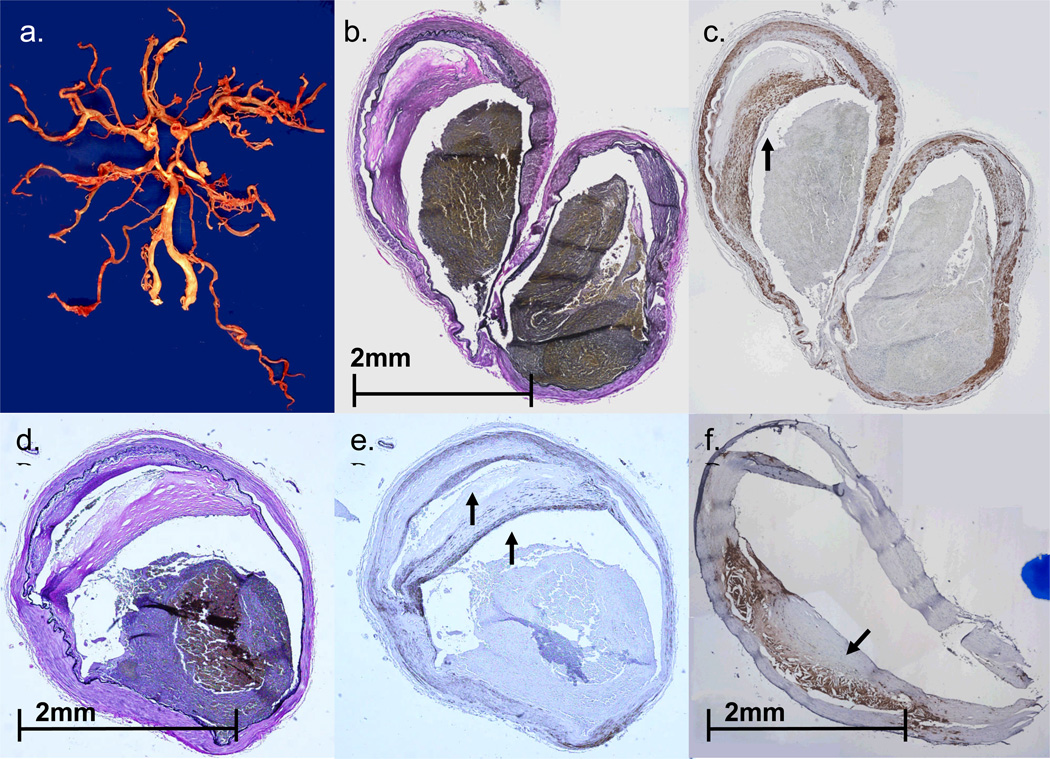
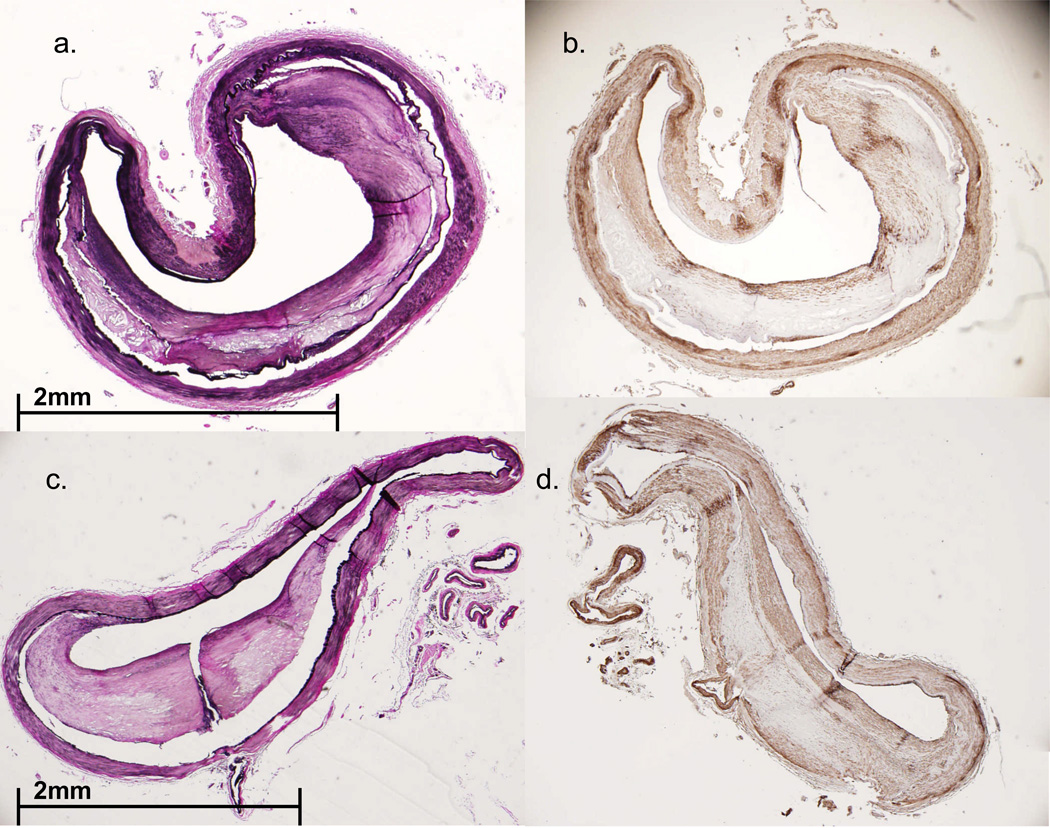
FIGURE 3.
Case #2. A. Circle of Willis dissected from base of the brain. Note patchy, focally accentuated atherosclerosis in major branches of the circle of Willis. B. Transverse section of the moderately atherosclerotic left MCA bifurcation (EVG stain). C. Section of left MCA bifurcation, parallel to that shown in Figure 3B, immunostained with primary antibody to smooth muscle actin (SMA). Note ‘cap’ composed of SMA-immunoreactive cells overlying atheromatous material. D,E. Parallel sections of M2 segment of the left MCA, stained with EVG (D) and primary antibodies to SMA (E). Arrows (in E) indicate two dense layers composed of smooth muscle cells. F. Section of basilar artery from the same patient, immunostained with primary antibodies to CD68- arrow highlights dense accumulation of macrophages in the atheroma.
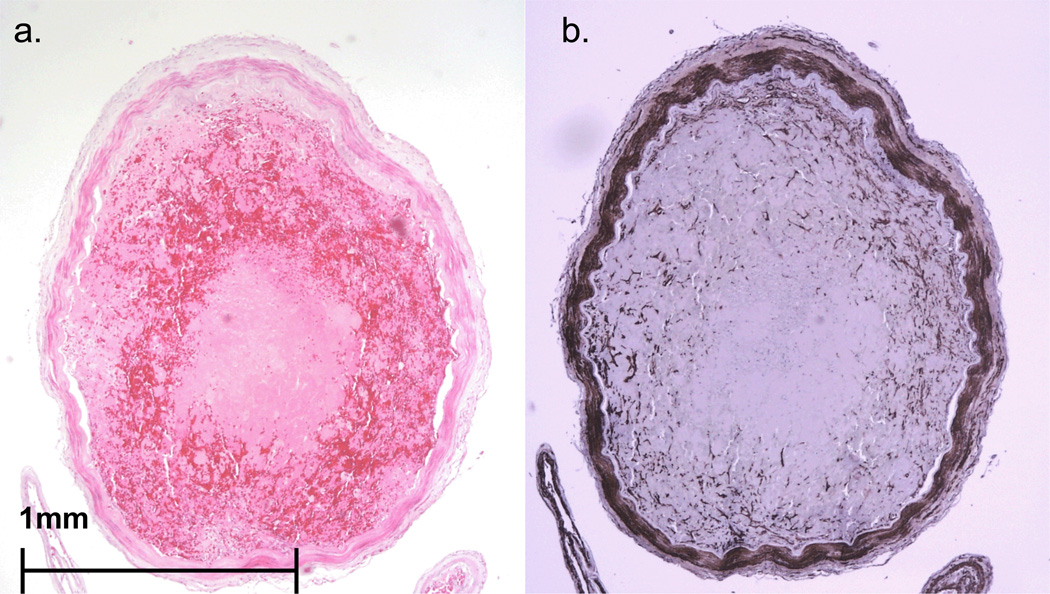
FIGURE 6.
Case #3. Representative cross section of right MCA (A from section stained with H&E, B from a parallel section immunostained with primary antibody to SMA) showing a well organized thromboembolus in a relatively non-atherosclerotic artery. Panel B highlights ingrowth of smooth muscle cells into the thromboembolus.
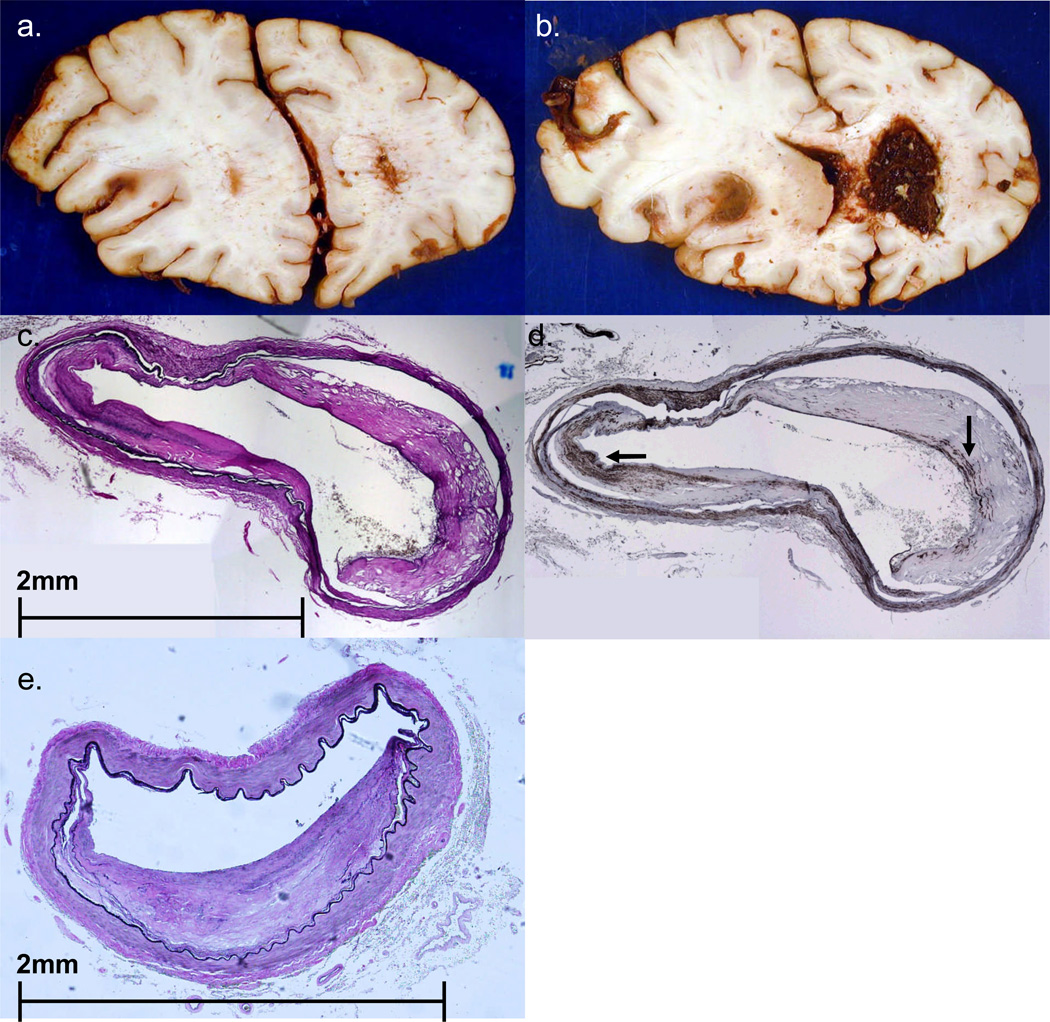
FIGURE 8.
Case #4. A,B. Coronal sections of fixed brain show a large left MCA territory subacute infarct, with associated edema and left to right shift of midline structures. There is also extensive intraventricular hemorrhage, especially in the right lateral ventricle. C,D. Cross sections of left MCA (C stained with EVG, D stained immunohistochemically using primary antibody to SMA) show relatively mild atheroma with a smooth muscle cell cap (arrows in D). At another level (panel E) the MCA shows significant atheroma, with estimated 55–60% stenosis of the lumen. None of the lumina shows a thrombo-embolus. (Panel E from a section stained with EVG).
FIGURE 11.
Case #5. Parallel segments of left MCA (A,B; C,D) stained with EVG (A,C) and primary antibody to SMA (B,D) show extensive atheroma of the artery.
FIGURE 5.
Case #3. A-C: Panels represent selected coronal slices of fixed brain, showing right MCA territory infarct undergoing cystic cavitation (arrows in B, C). Arrow in panel A shows extension of infarct into the right basal ganglia, with hemorrhagic transformation. Panel D shows a ‘saddle thromboembolus’ in the right MCA bifurcation (arrows).
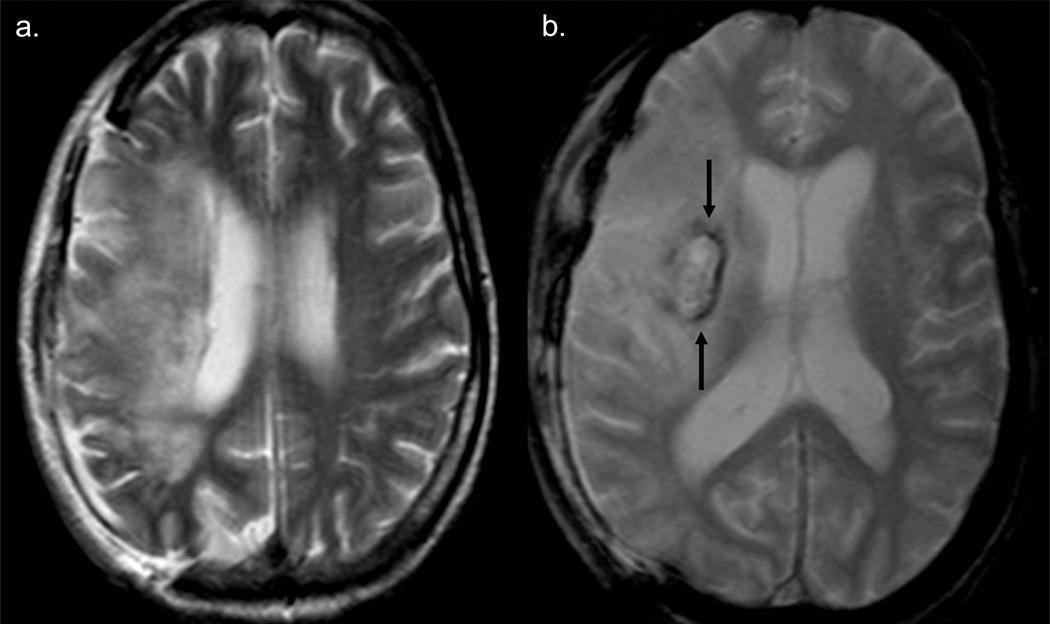
FIGURE 4.
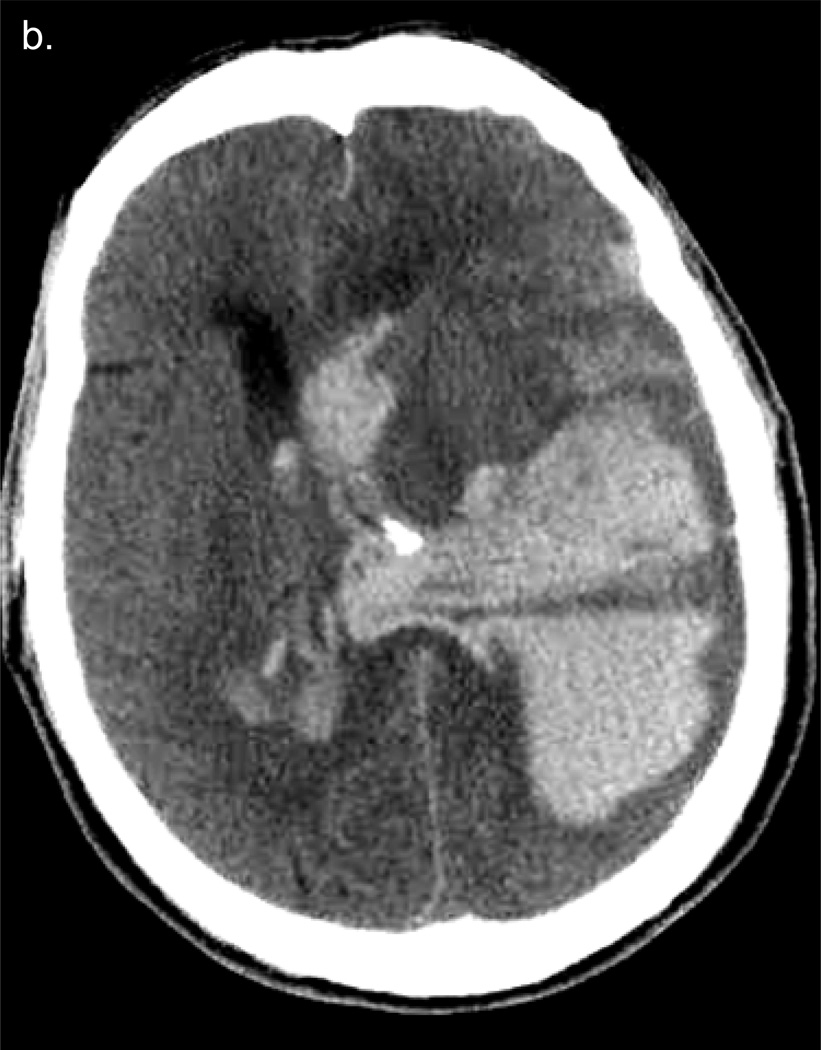
Case #3. A. T2 axial MRI scan (obtained 3 weeks post-thrombectomy) shows right sided craniectomy and extensive right MCA territory infarct. B. GRE axial MRI image shows extensive right MCA territory infarct and a right basal ganglia hemorrhage (arrows).
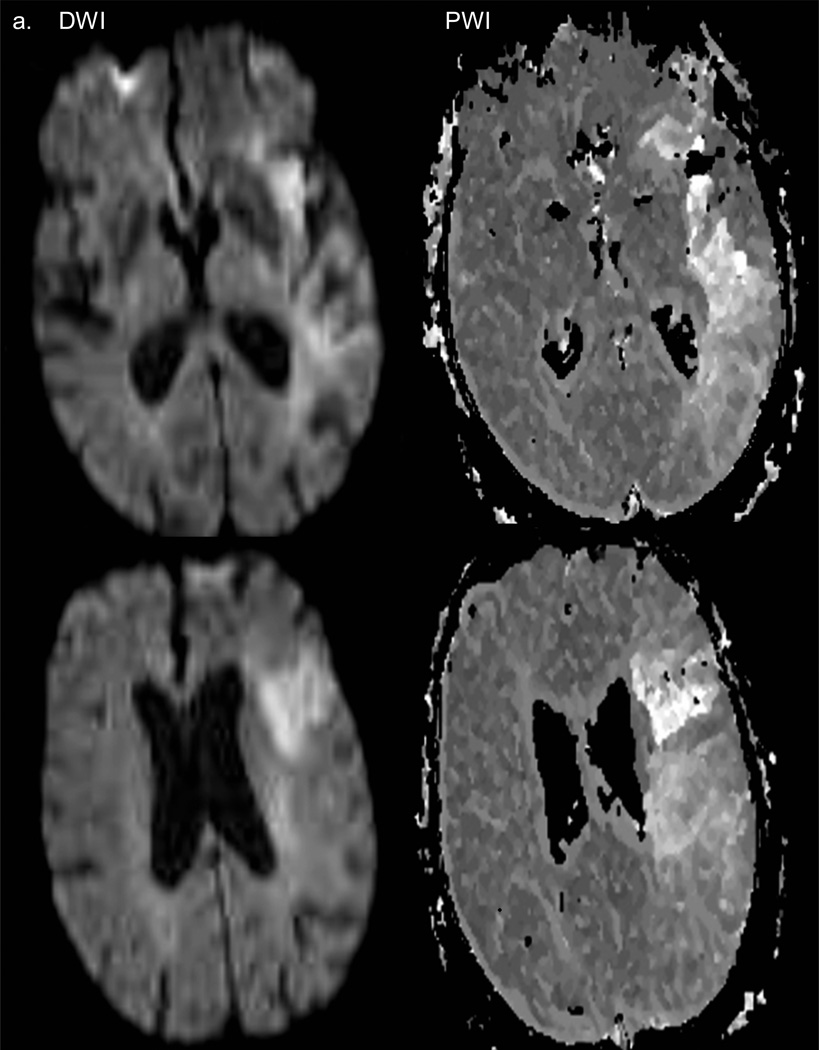
FIGURE 7.
Case #4. A. Axial sections of cerebral hemispheres, taken 3 hours after stroke symptom onset, but immediately before thrombectomy. DWI (diffusion weighted) images show acute infarct in the left MCA territory (panels at left). The perfusion weighted image (PWI) abnormalities (right hand panels) are considerably larger than those shown on the DWI images. B. Large left parieto-occipital intraparenchymal hematoma (post-thromboembolectomy) extending into the lateral ventricles.
FIGURE 9.
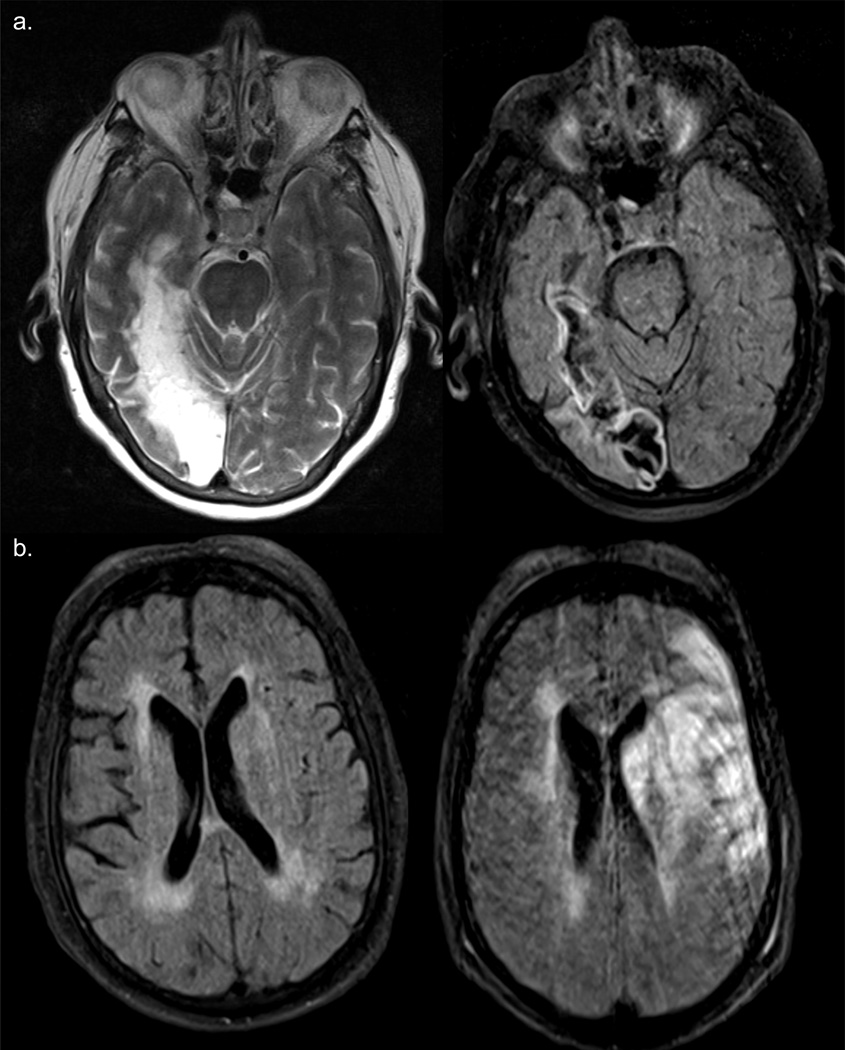
A. Case #5. T2 and FLAIR images (obtained at the time of the patient’s admission to hospital, 1:00 p.m., pre-thrombectomy) show old infarct in the right occipital lobe. B. FLAIR images obtained pre-thrombectomy (at left) and 7 hours later (at right, approx. 5 hours post-thrombectomy) show a new infarct in the left MCA territory.
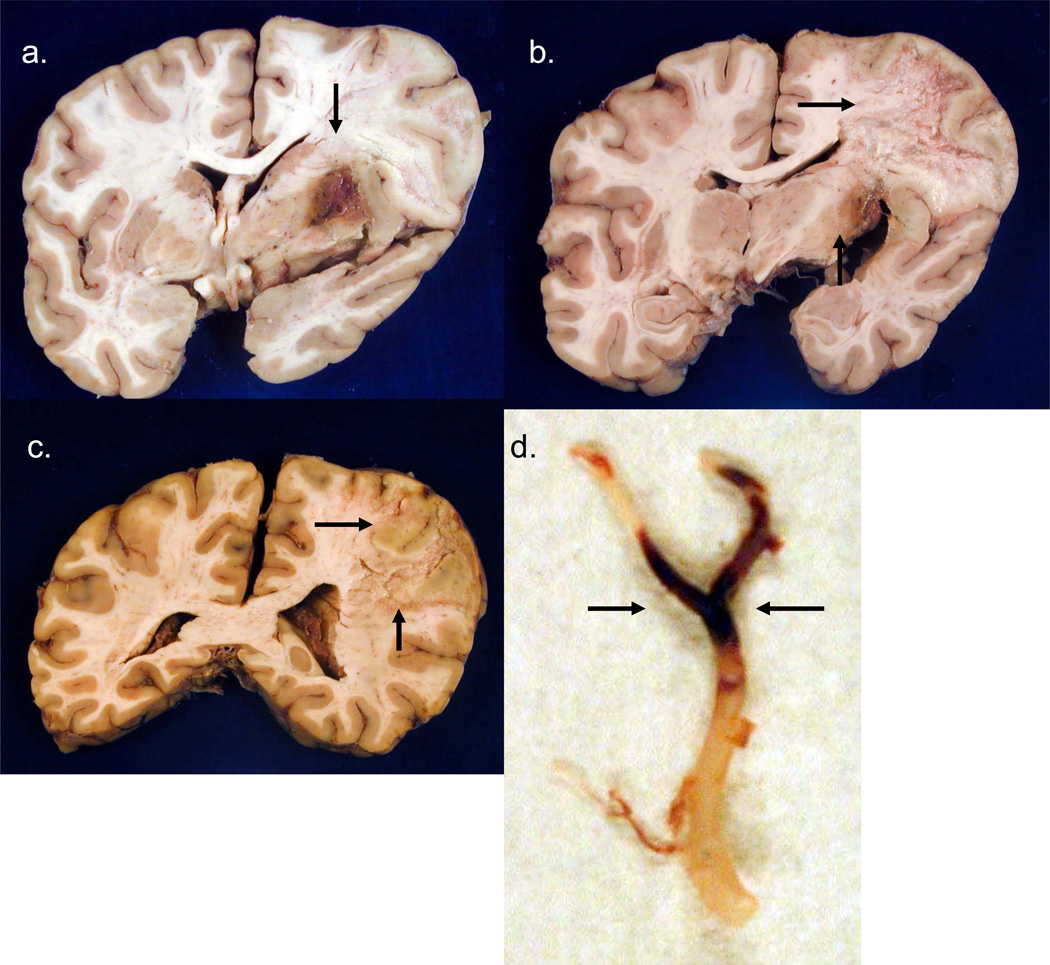
FIGURE 2.
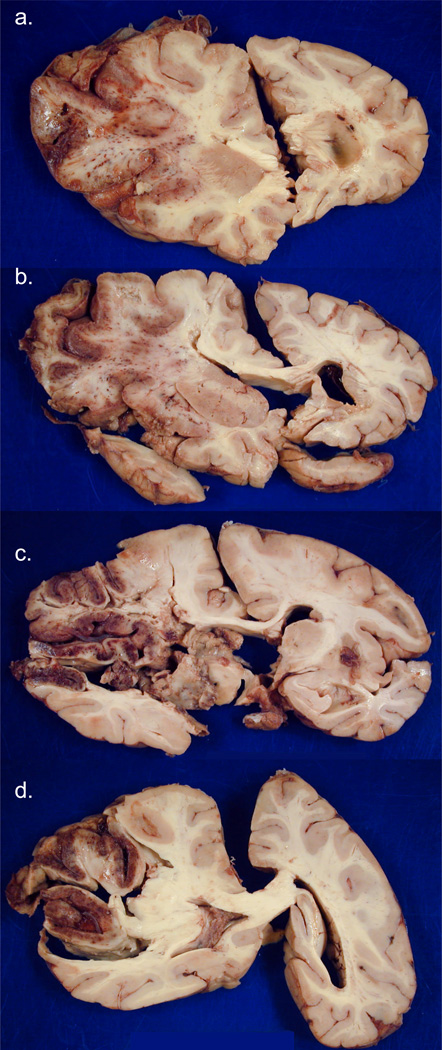
Case #2. [Photographs of brain slices in this and subsequent figures follow the neuropathology convention of left side of the brain appearing on the left of the image.] Coronal sections of fixed slices from cerebral hemispheres arranged rostral to caudal in a-d show subacute necrosis and edema on the left, with significant left to right shift of midline structures. The infarct is extensively hemorrhagic and involves most of the left MCA territory..
All brains examined showed hemispheric infarcts, which were consistent in appearance (grossly and microscopically) with the clinical onset of stroke symptoms and corresponding neuroimaging studies (Figures 2, 4, 5, 7, 8, 9 and 10). In patients #1, 2 and 4 the infarcts were associated with massive, fatal cerebral edema. In patient #3 (who survived the procedure by over a month), edema had subsided and the right cerebral hemispheric infarct showed cystic cavitation. In patient #5, an extensive, substantially hemorrhagic cortical infarct, was noted in the left cerebral hemisphere (Figure 10), while an acute hematoma in the left basal ganglia was attributed to significant hypertensive arteriopathy.
FIGURE 10.
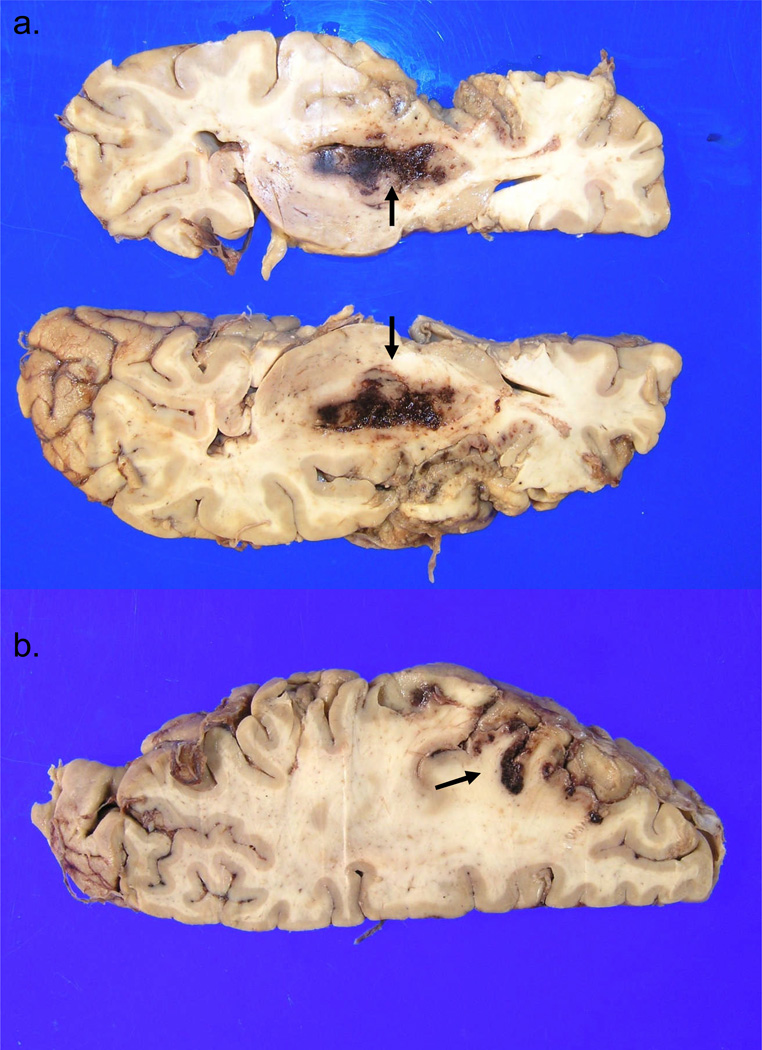
Case #5. Panel A. Axial section of fixed left cerebral hemisphere shows large acute hematoma in the left basal ganglia (arrows), the site of severe hypertensive microvascular disease. Panel B (left cerebral hemisphere) shows extensive left MCA territory subacute infarct. Arrow indicates a prominently hemorrhagic segment of the infarct. Note relative preservation of the left ACA territory (bottom right of the photograph).
Major general autopsy findings included the following: Patient #1: Neurogenic pulmonary edema and pleural effusions; Patient #3: Moderate coronary artery atherosclerosis, evidence of ischemic cardiomyopathy with a left ventricular aneurysm and diffuse alveolar damage. Patient #4: Coronary artery disease with past stenting of several arteries, and atherosclerosis of the abdominal aorta; Patient #5: Severe generalized and coronary atherosclerosis with ischemic changes in the myocardium, a history of bioprosthetic aortic valve replacement and cardiomegaly with left ventricular and bi-atrial dilatation. The cerebral abnormalities, especially infarct with edema and/or cerebral hemorrhage, were deemed major factors contributing to death in all cases, but especially in three individuals.
DISCUSSION
To our knowledge, this is the first report describing the vascular and neuropathologic abnormalities in a group of patients who died following endovascular thrombectomy procedures, all carried out to extract thromboemboli from MCA segments. The authors recognize that this small series represents a highly select group of patients —who contrast in striking terms with the numerous patients that have received benefit from innovative thromboembolectomy procedures1–15. In a large multi-center trial of 164 patients who underwent thrombectomy within 8 hours of stroke symptom onset, 57.3% of treated patients showed successful recanalization in ‘treatable’ arteries and 69.5% recanalized vessels after adjunctive therapy18. Favorable clinical outcomes were observed in 36% with an overall mortality of 34%. Outcome may to some extent be dependent upon the nature of the material occluding an affected artery, a detailed morphologic analysis of which has previously been published16. Further detailed analysis may well shed light on molecular components within a thromboembolus that render a patient more or less likely to have a good outcome.
Systemic disease, usually complications of atherosclerosis involving the coronary and other arteries (including the aorta) and pulmonary lesions, almost certainly contributed to death in all patients. In at least 3/5 patients, large MCA territory infarcts with associated mass effect from edema were judged to have significantly contributed to fatal outcomes. In every case, microscopic features of cerebral infarcts were consistent with their having occurred at the time of symptom onset. These five patients did not survive their strokes: three developed hemorrhages after the procedure. Patient #2 had a small subarachnoid hemorrhage, likely caused by the procedure. Patient #4 had a large hemorrhage. In patients #4 and #5 significant parenchymal hemorrhage was superimposed on ischemic lesions in the same cerebral hemisphere. In patient #4 the parenchymal hemorrhage had extended into the lateral ventricle, parenchymal hematomas Berger type 2 and a subarachnoid hemorrhage, due to the tPA19, while patient #5 developed a hemorrhagic infarct type 1, unlikely due to the procedure. The ganglionic hematoma observed in this patient was deemed in part to be on the basis of concomitant hypertensive cerebral microvascular disease. The first patient in our series had experienced a subintimal arterial dissection in a severely atherosclerotic segment of her MCA20, though the dissection occurred in a portion of the arterial wall that did not show significant atheroma. The immediate causes of death for patients #1 and 5 were the families’ decisions to withdraw care. All care was withdrawn except for the ventilator for patient #3, and comfort care protocols were initiated for the remaining patients.
In addition to the one patient who appeared to have a procedure-related MCA dissection, one other (the longest survivor among the five) showed complete occlusion of the MCA segment, with advanced organization of the thromboembolus, consistent with its having developed at or around the time of the presenting stroke and the endovascular procedure. Other arterial segments examined in detail (from patients #1,2,4 and 5) showed variably advanced, usually complicated atheromata (confirmed by immunohistochemistry) with stenosis—though no evidence of acute intra-plaque hemorrhage or intimal ulceration21. The lumens in such vessels were patent. Atheroemboli—sometimes encountered in the distal arteries of patients with severe atherosclerosis in the cervical arteries—were not found in any of these patients despite extensive sampling of brain parenchyma.
The presence of substantial intracranial atherosclerosis in these cases has important implications for patient selection and further device development. Thrombectomy devices are designed to retrieve or aspirate thrombi from relatively normal arterial beds rather than severely atherosclerotic arteries. The loops of the Merci Retriever can snag and unwind on fixed atheroma, with risk of impaired thrombus removal, local vessel dissection, and remote vessel traction injury22. The Penumbra system is too large and bulky to pass easily through stenotic atherosclerotic segments, limiting its ability to aspirate more distally located thrombi. When the target occlusive lesion is known to consist substantially of in situ atherosclerosis, primary angioplasty and stenting -- cracking, stretching, and maintaining radial force on the plaque – could be a treatment option rather than embolectomy23. In the 5 cases in this series, the interventional team believed at the time of treatment that the vascular occlusions were largely thrombi with only minimal in situ atherosclerosis. All patients had normal neck arteries, according to angiography. Patients 2, 4, and 5 had atrial fibrillation and came in with a normal International Normalized Ratio, INR, leading to the presumption that the source of the clots was cardiac. Patient 5 also had a heart valve replacement, increasing the likelihood of a cardiac embolus. Workup for patient 1 was not done due to the severity of patient’s stroke but she had a history of severe ischemic heart disease and previous myocardial infarctions, indicating that the source of clot was presumably cardiac. Patient 3 had ischemic cardiomyopathy with congestive heart failure- her stroke was likely caused by a cardiogenic embolus. The untoward outcome in these 4 cases that actually harbored moderate or extensive local atherosclerosis highlights the need for improved methods to diagnose target occlusion composition pretreatment, such as making intravascular ultrasound catheter tips small and navigable enough for deployment in the intracranial circulation.
Acknowledgments
Source of Funding: Several authors (HVV, RJ, JAS, PMV) are supported in part by UCLA SPOTRIAS grant (PHS # P50 NS 044378). DSL supported by PHS Grant K23 NS054084. HVV supported by the Daljit S. & Elaine Sarkaria Chair in Diagnostic Medicine. This study was supported in part by a generous grant from the Translational Research Fund of the UCLA Department of Pathology & Laboratory Medicine.
Abbreviations
- L
Left
- R
Right
- IA-tPA
Intra-arterial Tissue Plasminogen Activator
- MERCI
Mechanical Embolus Removal in Cerebral Ischemia
- TICI
Thrombolysis in Cerebral Ischemia
- ACA
Anterior Cerebral Artery
- BA
Basilar Artery
- ICA
Internal Carotid Artery
- MCA
Middle Cerebral Artery
- PCA
Posterior Cerebral Artery
- SMA
Smooth Muscle Actin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Edgell R, Yavagal DR. Acute endovascular stroke therapy. Current Neurology and Neuroscience Reports. 2006;6:531–538. doi: 10.1007/s11910-006-0057-0. [DOI] [PubMed] [Google Scholar]
- 2.Gupta R, Jovin TG. Endovascular management of acute ischemic stroke: advances in patient and treatment selection. Expert Rev Neurotherapeutics. 2007;7:143–153. doi: 10.1586/14737175.7.2.143. [DOI] [PubMed] [Google Scholar]
- 3.Katz JM, Gobin YP. Merci ® retriever in acute stroke treatment. Expert Rev Med Devices. 2006;3:273–280. doi: 10.1586/17434440.3.3.273. [DOI] [PubMed] [Google Scholar]
- 4.Martinez H, Zoarski GH, Obuchowski AM, Stallmayer B, Papangelou A, Airan-Javia S. Mechanical thrombectomy of the internal carotid artery and middle cerebral arteries for acute stroke by using the Retriever device. AJNR Am J Neuroradiol. 2004;25:1812–1815. [PMC free article] [PubMed] [Google Scholar]
- 5.Molina CA, Saver JL. Extending reperfusion therapy for acute ischemic stroke: emerging pharmacological, mechanical, and imaging strategies. Stroke. 2005;36(10):2311–2320. doi: 10.1161/01.STR.0000182100.65262.46. [DOI] [PubMed] [Google Scholar]
- 6.Schumacher HC, Meyers PM, Yavagal DR, Harel NY, Elkind MSV, Mohr JP, Pile-Spellman J. Endovascular mechanical thrombectomy of an occluded superior division branch of the left MCA for acute cardioembolic stroke. Cardiovasc Intervent Radiol. 2003;26:305–308. doi: 10.1007/s00270-003-2719-5. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki S, Kidwell CS, Starkman S, Saver JL, Duckwiler G, Vinuela F, Ovbiagele B. Use of multimodal MRI and novel endovascular therapies in a patient ineligible for intravenous tissue plasminogen activator. Stroke. 2005;36:e77–e79. doi: 10.1161/01.STR.0000177515.14055.d0. [DOI] [PubMed] [Google Scholar]
- 8.Brekenfeld C, Schroth G, El-Koussy M, Nedeltchev K, Reinert M, Slotboom J, Gralla J. Mechanical thrombectomy for acute ischemic stroke. Comparison of the Catch Thrombectomy Device and the Merci retriever in vivo. Stroke. 2008;39:1213–1219. doi: 10.1161/STROKEAHA.107.495614. [DOI] [PubMed] [Google Scholar]
- 9.Clarencon F, Blanc R, Gallas S, Hosseini H, Gaston A. Thrombectomy for acute basilar artery occlusion by using double Merci retriever devices and bilateral temporary vertebral artery flow reversal. J Neurosurg. 2009;111:53–56. doi: 10.3171/2008.11.JNS081035. [DOI] [PubMed] [Google Scholar]
- 10.Flint AC, Duckwiler GR, Budzik RF, Liebeskind DS, Smith WS for the MERCI and Multi MERCI Writing Committee. Mechanical thrombectomy of intracranial internal carotid occlusion. Pooled results of the MERCI and Multi MERCI Part I trials. Stroke. 2007;38:1274–1280. doi: 10.1161/01.STR.0000260187.33864.a7. [DOI] [PubMed] [Google Scholar]
- 11.Layton KF, White JB, Cloft HJ, Kallmes DF, Manno EM. Expanding the treatment window with mechanical thrombectomy in acute ischemic stroke. Neuroradiology. 2006;48:402–404. doi: 10.1007/s00234-006-0073-4. [DOI] [PubMed] [Google Scholar]
- 12.Lee R, Lui WM, Cheung RTF, Leung GKK, Chan KH. Mechanical thrombectomy in acute proximal middle cerebral artery thrombosis with Alligator retrieval device. Cerebrovasc Dis. 2007;23:69–71. doi: 10.1159/000097031. [DOI] [PubMed] [Google Scholar]
- 13.Liebig T, Reinartz J, Hannes R, Miloslavski E, Henkes H. Comparative in vitro study of five mechanical embolectomy systems: effectiveness of clot removal and risk of distal embolization. Neuroradiology. 2008;50:43–52. doi: 10.1007/s00234-007-0297-y. [DOI] [PubMed] [Google Scholar]
- 14.Penumbra Pivotal Stroke Trial Investigators. The penumbra pivotal stroke trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke. 2009;40(8):2761–2768. doi: 10.1161/STROKEAHA.108.544957. [DOI] [PubMed] [Google Scholar]
- 15.Smith WS, Sung G, Starkman S, Saver JL, Kidwell CS, Gobin P, Lutsep HL, Nesbit GM, Grobelny T, Rymer MM, Silverman IF, Higashida RT, Budzik RF, Marks MP for the MERCI Trial Investigators. Safety and efficacy of mechanical embolectomy in acute ischemic stroke. Results of the MERCI Trial. Stroke. 2005;36:1432–1440. doi: 10.1161/01.STR.0000171066.25248.1d. [DOI] [PubMed] [Google Scholar]
- 16.Marder VJ, Chute DJ, Starkman S, Abolian AM, Kidwell C, Liebeskind D, Ovbiagele B, Vinuela F, Duckwiler G, Jahan R, Vespa PM, Selco S, Rajajee V, Kim D, Sanossian N, Saver JL. Analysis of thrombi retrieved from cerebral arteries of patients with acute ischemic stroke. Stroke. 2006;37:2086–2093. doi: 10.1161/01.STR.0000230307.03438.94. [DOI] [PubMed] [Google Scholar]
- 17.Higashida RT, Furlan AJ, Roberts H, Tomsick T, Connors B, Barr J, Dillon W, Warach S, Brodrick J, Tilley B, Sacks D. Technology Assessment Committee of the American Society of Interventioal and Thrapeutic Neuroradiology; Technology Assessment Committee of the Society of Interventional Radiology. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. 2003;34(8):e109–e137. doi: 10.1161/01.STR.0000082721.62796.09. [DOI] [PubMed] [Google Scholar]
- 18.Smith WS, Sung G, Saver J, Budzik R, Duckwiler G, Liebeskind DS, Lutsep HL, Rymer MM, Higashida RT, Starkman S, Gobin YP for the Multi MERCI Investigators. Mechanical thrombectomy for acute ischemic stroke. Final results of the Multi Merci Trial. Stroke. 2008;39:1205–1212. doi: 10.1161/STROKEAHA.107.497115. [DOI] [PubMed] [Google Scholar]
- 19.Berger C, Fiorelli M, Steiner T, Schäbitz W, Bozzao L, Bluhmki E, Hacke W, von Kummer R. Hemorrhagic transformation of ischemic brain tissue: asymptomatic or symptomatic? Stroke. 2001;32:1330–1335. doi: 10.1161/01.str.32.6.1330. [DOI] [PubMed] [Google Scholar]
- 20.Farrell MA, Gilbert JJ, Kaufmann JCE. Fatal intracranial arterial dissection—clinical pathological correlation. J Neurol Neurosurg Psychiatry. 1985;48:111–121. doi: 10.1136/jnnp.48.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogata J, Yutani C, Otsubo R, Yamanishi H, Naritomi H, Yamaguchi T, Minematsu K. Heart and vessel pathology underlying brain infarction in 142 stroke patients. Ann Neurol. 2008;63:770–781. doi: 10.1002/ana.21401. [DOI] [PubMed] [Google Scholar]
- 22.Liebeskind DS, Saver JL, Jahan R, Sanossian N, Starkman S, Ovbiagele B, Kim D, Buck BH, Ali LK, Yun S, Vespa PM, Salamon N, Villablanca JP, Vinters HV, Vinuela F, Duckwiler GR. Cerebral microbleeds associated with mechanical thrombectomy. Stroke. 2007;38:469. [Google Scholar]
- 23.Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke. 2007;38(3):967–973. doi: 10.1161/01.STR.0000258112.14918.24. [DOI] [PubMed] [Google Scholar]