Abstract
The failure of antiviral vaccines is often associated with rapid viral escape from specific immune responses. In the past, conserved epitope or algorithmic epitope selections, such as mosaic vaccines, have been designed to diversify immunity and to circumvent potential viral escape. An alternative approach is to identify conserved stable non–HIV-1 self-epitopes present exclusively in HIV-1–infected cells. We showed previously that human endogenous retroviral (HERV) mRNA transcripts and protein are found in cells of HIV-1–infected patients and that HERV-K (HML-2)–specific T cells can eliminate HIV-1–infected cells in vitro. In this article, we demonstrate that a human anti–HERV-K (HML-2) transmembrane protein Ab binds specifically to HIV-1–infected cells and eliminates them through an Ab-dependent cellular cytotoxicity mechanism in vitro. Thus, Abs directed against epitopes other than HIV-1 proteins may have a role in eliminating HIV-1–infected cells and could be targeted in novel vaccine approaches or immunotherapeutic modalities.
Introduction
Vaccines designed to generate an effective immune response against HIV-1 have had limited success as a result of the frequent mutations of the virus caused by its high rate of replication. As a consequence, the immune response targets obsolete HIV-1 epitopes, and new viral species are allowed to replicate unchecked (1).
Human endogenous retroviruses (HERVs) make up ∼8% of the human genome, but these evolutionary ancient viral sequences are largely considered to be silent (2, 3). HERV-K (HML-2), the most recently integrated HERV, was reported to express proteins in some disease states (4, 5). During HIV-1 infection, HERV-K mRNA transcripts and viral proteins can be detected in serum (6, 7). The mechanisms of interaction between HIV-1 and HERV-K are still under investigation, but the HIV-1 accessory proteins Vif and Tat are thought to play a role in HERV-K protein expression (8, 9).
In this article, we show that the HERV-K (HML-2) envelope transmembrane (TM) protein is expressed on the surface of HIV-1–infected cells. A human anti–HERV-K (HML-2) TM Ab (HA-137) is able to eliminate these infected cells in vitro through an Ab-dependent cell-mediated cytotoxicity (ADCC) mechanism. Eliciting immune responses to HERV-K (HML-2) in vivo might lead to the production of Abs that target HIV-1–infected cells, and passive immunotherapy with an anti–HERV-K (HML-2) Ab could circumvent viral variation by targeting conserved ancestral viral proteins.
Materials and Methods
Cells and sera
Sera and PBMCs were obtained from healthy seronegative volunteers at low risk for contracting HIV infection. Sera from chronically HIV-1–infected individuals were obtained from the SCOPE cohort at the University of California, San Francisco.
Ab purification and labeling
PBMCs from a long-term nonprogressing HIV-1 patient (elite controller) were freshly isolated and infected with cell culture supernatant containing EBV (10). After 4 wk, cells were seeded in a 96-well plate to isolate an anti–HERV-K (HML-2) TM Ab-secreting B cell clone. Screening was done by ELISA. The positive clone was expanded, and the supernatant was used for Ab purification by affinity chromatography. The Ab was given the identifier “HA-137.”
ELISA
Adapted from the method of Michaud et al. (7). A set of HERV-K 102 (AF164610.1) Env peptides was used to map the response. Peptides with single alanine mutations were used to identify the exact epitope.
Viruses and in vitro infection
Stocks of HIV-1LAI, HIV-1Bal, and clade B and C primary isolates 91US_4 (US4), 89BZ_167, and 90SE_364 (SE) were expanded. Infected PBMCs (day 6) were stained for surface protein expression using HERM-1811-5 (TM) or HA-137–Alexa Fluor 488 by immunofluorescence and flow cytometry.
ADCC and NK cell degranulation
Six days after HIV-1 infection, NK cells were isolated from frozen autologous PBMCs. A total of 105 infected PBMCs (targets) was plated, and NK cells were added at various ratios for 6 h at 37°C in duplicate. ADCC was determined by flow cytometry. Cells were stained with LIVE/DEAD cell dye (Life Technologies) and with T cell and HIV-1 core markers. Target cells were defined as AmCyan− CD3+ CD8− HIV-1 Gag+. The killing mediated by the Abs was defined as the ratio of target cells (without Ab) − target cells (Ab)/target cells (without Ab). For the degranulation assay [adapted from Thobakgale et al. (11)], CD107a-FITC or FITC isotype control was added with GolgiStop (BD Biosciences) and brefeldin A. Purified human IgG (10 μg/ml), HA-137 (10 μg/ml), b12 (3 μg/ml), and Z13 (3 μg/ml), used as positive controls, were added. Unstained and PHA-L–blasted PBMCs were used as controls.
Results and Discussion
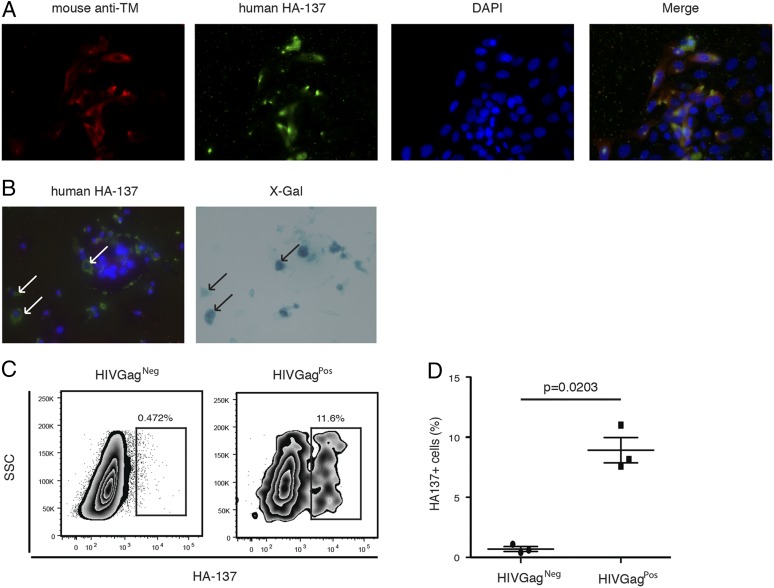
We showed previously that HERV mRNA transcripts and protein are present in the cells of HIV-1–infected patients and that HERV-K (HML-2)–specific T cells can eliminate HIV-1–infected cells in vitro (6, 11). In this study, we used a human Ab (HA-137), which recognizes the HIV-1–induced HERV-K (HML-2) TM protein (7), to test for ADCC activity on HIV-1–infected cells. To determine whether HA-137 recognizes HIV-1–infected cells, we used HIVLAI-infected TZMbl cells, which contain a β-gal reporter gene whose expression is induced by HIV-1 Tat. Using this system and a commercially available mouse anti–HERV-K TM Ab, we first established that HA-137 reacts with TM protein present on the surface of infected cells (Fig. 1A). Next, we confirmed that HA-137 specifically marked the surface of productively HIV-infected TZMbl cells (Fig. 2B).
FIGURE 1.
Identification of linear Ab epitopes in HERV-K (HML-2) TM expressed by infected cells. (A and B) Immunofluorescence. Representative images of HERV-K TM surface expression on infected TZMbl cells. Original magnification ×40. The detection of TM protein surface expression was performed by immunofluorescence on in vitro HIV-1LAI–infected TZMbl cells 2 d postinfection. (A) Red: mouse anti–HERV-K (HML-2) TM; green: HA-137; blue: nucleus. (B) Green: HA-137; blue: nucleus (left panel). Blue: X-gal staining (right panel). Arrows represent coexpression of HIV-1 Tat protein (right panel) and HA-137 epitope (left panel). (C) Representative flow cytometry plots depicting HA-137 extracellular binding on infected PBMCs. HA-137 recognition was assessed by measuring the frequency of HA-137Alexa Fluor 488+ cells gated on live HIVGag+ or HIVGag− cells from infected PBMCs. (D) Cumulative data from three independent experiments. The horizontal line represents the median in each group. The p value was derived by the Mann–Whitney T test.
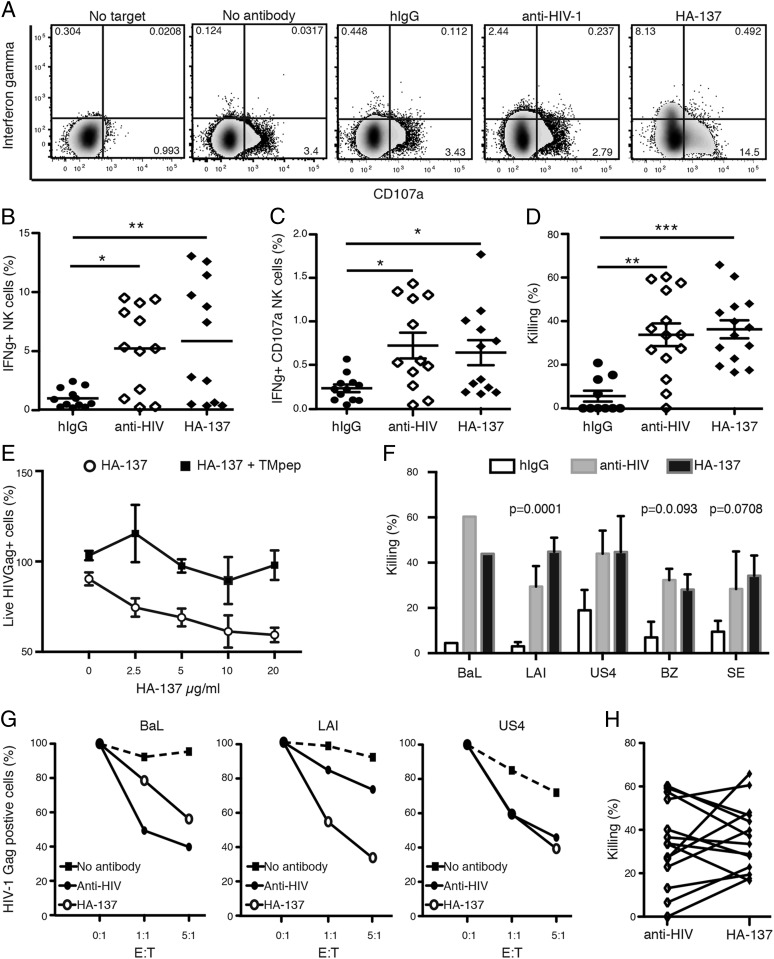
FIGURE 2.
HA-137 increases NK cell reactivity and mediates ADCC in the presence of HIV-1–infected cells. NK cell reactivity was assessed by CD107a and IFN-γ intracellular staining and ADCC by measuring the frequency of live HIVGag+ cells. (A) Representative flow cytometry plots depicting NK activation and degranulation. Cells were gated on live NK cells based on CD56 and CD16 expression. CD107a and IFN-γ staining were compared among NK cells without HIVLAI-infected cells (no target), with target cells without Abs (no Ab), or with the addition of purified hIgG, anti-HIV Abs, or HA-137 (10 μg/ml) after 6 h of incubation. (B and C) Cumulative data from four independent experiments using PBMCs from two healthy seronegative volunteers at low risk for contracting HIV infection and three strains of virus (n = 12). Each symbol represents the mean of duplicates. The frequency of IFN-γ (A), CD107a single-positive (B), and IFNγ+CD107+ double-positive (C) NK cells were compared among NK cells incubated with purified hIgGs, anti-HIV Ab, or HA-137. (D) Killing assay. Plot represents cumulative data of killing at the 5:1 (E:T) ratio from five independent experiments using eight donors (n = 14). The readout for the killing assays was HIV-1–infected cell elimination calculated as [(% live HIV+ cells without effectors − % live HIV+ cells mixed with effectors and/or Ab)/% live HIV+ cells without effectors] × 100. Effect of Ab on killing mediation was compared among purified hIgGs, anti-HIV-1 Abs, and HA-137. Experiments were performed in duplicate for each condition. (E) Dose response and competition assay. Graph represents the ratio of (% live HIV+ cells without effector cells/live HIV+ cells in presence of effector cells and HA137) × 100, in the presence of TMpep (HA-137 + TMpep) or its absence (HA-137). TMpep is a peptide with the amino acid sequence recognized by HA-137 (7). The ability of HA-137 to mediate ADCC is dose dependent and is inhibited when HA-137 is preincubated with TMpep (100 μg/ml) for 30 min. Data are the average of two independent experiments done in duplicate. (F) Killing mediated by either HA-137 or anti–HIV-1 varied with the viral strain used to infect PBMCs. (G) Three representative killing assays with variations in Ab efficiency. ADCC mediated by anti–HIV-1 Abs was higher than (left panel), lower than (middle panel), or equal to (right panel) killing mediated by HA-137 on PBMCs infected with HIVBAL, HIVLAI, or HIVUS4, respectively. (H) Individual and paired killing mediated by HA-137 and HIV-1 Abs for each single combination donor/viral strain. A Spearman test revealed a trend, but no correlation (p = 0.1178, r = +0.4373). *p < 0.05, **p < 0.01, ***p < 0.001, Kruskal–Wallis and Dunn multiple-comparison test (B, C, and D) or the Spearman test (H). The p values < 0.05 were considered significant.
We then assessed the ability of HA-137 to bind HIVLAI-infected cells using fluorescently labeled HA-137. HIVLAI-infected cells were identified by intracellular HIVLAI Gag expression (HIV-1 Gag+) (Fig. 1C). We detected a subpopulation of infected PBMCs (HIV-1 Gag+) expressing TM, which was not present in the HIV-1 Gag− population (Fig. 1C). However, the cumulative results clearly showed that the frequency of HIV-1 Gag+ TM+ double-positive cells was significantly increased (p = 0.0203) in live HIV-1 Gag+ cells compared with HIV-1 Gag− cells, with a mean of 8.9 and 0.7%, respectively (Fig. 1D). This suggests that HA-137 specifically binds HIV-1–infected cells and that the binding is not due to cross-reactivity between the Ab and HIV-1 proteins.
To investigate whether the cellular immunocomplexes (cICs) formed with HA-137 and HIV-1–infected cells increased NK cell recognition and reactivity, we incubated NK cells with HIV-1–infected autologous PBMCs in the presence of HA-137, b12, and Z13 anti–HIV-1 Abs (anti–HIV-1) or purified human IgGs (hIgGs) or without Abs (no Ab). In the absence of target cells, no NK reactivity was observed. Basal NK activity was detected in the absence of Ab or in the presence of hIGgs (Fig. 2A). In the presence of anti–HIV-1 Abs or HA-137, we observed an increased frequency of CD107a+ NK cells and IFN-γ production (Fig. 2A). Cumulative data did not show significant differences with regard to the expression of CD107a+ NK cells between different Abs (data not shown). However, a significant increase in IFNγ+ and IFNγ+ CD107a+ NK cells was observed when HA-137 or anti–HIV-1 Abs were added (Fig. 2B, 2C). These data suggest that HA-137 forms cICs with HIV-1–infected cells, increasing NK reactivity and degranulation and potentially mediating ADCC.
To assess whether HA-137 directed NK-mediated killing of HIV-1–infected target cells, we infected PBMCs with laboratory-adapted viruses or primary isolates. We measured the frequency of live HIV-1 Gag+ cells after 6 h of incubation with autologous NK cells, with or without HA-137. A basal level of killing activity was detected in the absence of Abs (data not shown) or with hIgGs (Fig. 2D). Anti–HIV-1 or HA-137 Abs significantly increased NK cell–mediated killing of infected cells, with a mean killing of ∼33 and 36%, respectively, versus 10% (hIgGs) (Fig. 2D). No difference between hIgGs and the absence of Ab was detected. To assess HA-137 specificity, we performed a dose-dependent assay and showed that HA-137 efficiency is decreased when its concentration is decreased, as well as in the presence of an excess of TM peptide (TMpep) (Fig. 2E). HA-137 has low polyreactivity (Supplemental Fig. 1).
There was variation in the killing efficiency for HA-137 and anti–HIV-1 Abs with respect to the strain used to infect cells (Fig. 2F, 2G). For instance, HA-137 seemed to be more efficient against HIVLAI-infected PBMCs than anti–HIV-1 Abs, but the opposite was observed with PBMCs infected with HIVBaL (Fig. 2F, 2G). These observations suggest that the viral strain or the donor is an important factor for the induction of HERV-K (HML-2) protein expression in infected cells and, thus, for the presence of the TM protein at the cell surface. Furthermore, we did not detect any significant correlation when comparing the killing mediated by HA-137 and HIV-1 Abs for individual donor/viral strain combinations, suggesting that HA-137 and anti–HIV-1 Abs target different Ags (Fig. 2H). With respect to the relatively low frequency of HIV-1–infected TM-expressing cells, the unexpectedly strong cytotoxic ability of HA-137 might be explained by the greater amount of Ab used in the killing assay compared with the surface staining experiment. The increased killing might also be explained by a paracrine cytokine production from other cells that interacted with cICs, such as dendritic cells or macrophages (12, 13). Taken together, our results show that HA-137 binds HIV-1–infected cells, forming cICs that induce a polyfunctional and cytotoxic NK cell response that is able to eliminate HIV-1–infected cells in vitro.
Eliminating HIV-1 or HIV-1–infected cells through Ab-mediated targeting of HERV proteins represents a novel approach to anti–HIV-1 therapeutic strategies. Non–HIV-1 Abs, such as anti-GB virus Abs, were reported to have antiviral effects and have increased the interest in non–HIV-1 targets (14). In addition to mediating ADCC, cICs may modulate the immune system by improving B cell responses, dendritic cell activation, and Ag presentation (12).
HERV-K (HML-2) TM is a non–HIV-1 target candidate for a HERV-based vaccine. The safety and immunogenicity of such a vaccine were already demonstrated in nonhuman primates (15). Modulating HERV-K–specific humoral immunity may represent a novel approach in the quest to prevent and eradicate HIV-1 infection.
Acknowledgments
We thank Dennis Burton, Pheroze Joshi, Neil Sheppard, Nancy Haigwood, Anne Hessell, Jeffrey Milush, Andre Raposo, R. Brad Jones, and Mario Ostrowksi for helpful discussions and Pandey Suchitra for help in providing clinical specimens and data. We gratefully acknowledge reagents supplied from the National Institutes of Health AIDS Research and Reference Reagent Program.
This work was supported in part by funds from a Pfizer-sponsored research agreement, the National Institutes of Health (AI076059 and AI084113), the National Center for Research Resources (P51 OD011092), the Bill and Melinda Gates Foundation (OPP108274GCE-II), AmFAR, the Peter and Shelagh Godsoe Family Foundation, and the District of Columbia Developmental Center for AIDS Research (P30-AI087714). S.G.D. and J.N.M. are supported in part by the National Institutes of Health (R24 AI067039, AI069994, and AI071713) and the University of California, San Francisco, Center for AIDS Research (AI027763). SCOPE cohort work was supported by grants from the National Institute for Allergy and Infectious Diseases (K24 AI069994), the Delaney AIDS Research Enterprise (U19AI096109), the Center for AIDS Research (P30 AI027763), the University of California, San Francisco, Clinical and Translational Research Institute Clinical Research Center (UL1 RR024131), and the Center for AIDS Prevention Studies (P30 MH62246).
The online version of this article contains supplemental material.
- ADCC
- Ab-dependent cell-mediated cytotoxicity
- cIC
- cellular immunocomplex
- HERV
- human endogenous retrovirus
- hIgG
- human IgG
- TM
- transmembrane
- TMpep
- TM peptide.
Disclosures
H.-A.M., S.G.D., J.B.S., and D.F.N. are listed as inventors on a patent application related to this work.
References
- 1.Walker B. D., Burton D. R. 2008. Toward an AIDS vaccine. Science 320: 760–764 [DOI] [PubMed] [Google Scholar]
- 2.Lander E. S., Linton L. M., Birren B., Nusbaum C., Zody M. C., Baldwin J., Devon K., Dewar K., Doyle M., FitzHugh W., et al. International Human Genome Sequencing Consortium 2001. Initial sequencing and analysis of the human genome [Published errata appear in 2001 Nature 412: 565 and 2001 Nature 411: 720]. Nature 409: 860–921 [DOI] [PubMed] [Google Scholar]
- 3.Subramanian R. P., Wildschutte J. H., Russo C., Coffin J. M. 2011. Identification, characterization, and comparative genomic distribution of the HERV-K (HML-2) group of human endogenous retroviruses. Retrovirology 8: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurth R., Bannert N. 2010. Beneficial and detrimental effects of human endogenous retroviruses. Int. J. Cancer 126: 306–314 [DOI] [PubMed] [Google Scholar]
- 5.van der Kuyl A. C. 2012. HIV infection and HERV expression: a review. Retrovirology 9: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garrison K. E., Jones R. B., Meiklejohn D. A., Anwar N., Ndhlovu L. C., Chapman J. M., Erickson A. L., Agrawal A., Spotts G., Hecht F. M., et al. 2007. T cell responses to human endogenous retroviruses in HIV-1 infection. PLoS Pathog. 3: e165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michaud H. A., de Mulder M., SenGupta D., Deeks S. G., Martin J. N., Pilcher C. D., Hecht F. M., Sacha J. B., Nixon D. F. 2014. Trans-activation, post-transcriptional maturation, and induction of antibodies to HERV-K (HML-2) envelope transmembrane protein in HIV-1 infection. Retrovirology 11: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esnault C., Heidmann O., Delebecque F., Dewannieux M., Ribet D., Hance A. J., Heidmann T., Schwartz O. 2005. APOBEC3G cytidine deaminase inhibits retrotransposition of endogenous retroviruses. Nature 433: 430–433 [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Hernandez M. J., Swanson M. D., Contreras-Galindo R., Cookinham S., King S. R., Noel R. J., Jr., Kaplan M. H., Markovitz D. M. 2012. Expression of human endogenous retrovirus type K (HML-2) is activated by the Tat protein of HIV-1. J. Virol. 86: 7790–7805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tosato, G., and J. I. Cohen. 2007. Generation of Epstein-Barr Virus (EBV)-immortalized B cell lines. Curr. Protoc. Immunol. Chapter 7: Unit 7.22. [DOI] [PubMed]
- 11.Thobakgale C. F., Fadda L., Lane K., Toth I., Pereyra F., Bazner S., Ndung’u T., Walker B. D., Rosenberg E. S., Alter G., et al. 2012. Frequent and strong antibody-mediated natural killer cell activation in response to HIV-1 Env in individuals with chronic HIV-1 infection. J. Virol. 86: 6986–6993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michaud H. A., Gomard T., Gros L., Thiolon K., Nasser R., Jacquet C., Hernandez J., Piechaczyk M., Pelegrin M. 2010. A crucial role for infected-cell/antibody immune complexes in the enhancement of endogenous antiviral immunity by short passive immunotherapy. PLoS Pathog. 6: e1000948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vivier E., Tomasello E., Baratin M., Walzer T., Ugolini S. 2008. Functions of natural killer cells. Nat. Immunol. 9: 503–510 [DOI] [PubMed] [Google Scholar]
- 14.Mohr E. L., Xiang J., McLinden J. H., Kaufman T. M., Chang Q., Montefiori D. C., Klinzman D., Stapleton J. T. 2010. GB virus type C envelope protein E2 elicits antibodies that react with a cellular antigen on HIV-1 particles and neutralize diverse HIV-1 isolates. J. Immunol. 185: 4496–4505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sacha J. B., Kim I. J., Chen L., Ullah J. H., Goodwin D. A., Simmons H. A., Schenkman D. I., von Pelchrzim F., Gifford R. J., Nimityongskul F. A., et al. 2012. Vaccination with cancer- and HIV infection-associated endogenous retrotransposable elements is safe and immunogenic. J. Immunol. 189: 1467–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]