Abstract
Background and aims. This study aimed to investigate release of nickel ion from three types of nickel-titanium-based wires in the as-received state and after immersion in a simulated oral environment.
Materials and methods. Forty specimens from each of the single-strand NiTi (Rematitan "Lite"), multi-strand NiTi (SPEED Supercable) and Copper NiTi (Damon Copper NiTi) were selected. Twenty specimens from each type were used in the as-received state and the others were kept in deflected state at 37ºC for 2 months followed by autoclave sterilization. The as-received and recycled wire specimens were immersed in glass bottles containing 1.8 mL of artificial saliva for 28 days and the amount of nickel ion released into the electrolyte was determined using atomic absorption spectrophotometry.
Results. The single-strand NiTi released the highest quantity of nickel ion in the as-received state and the multi-strand NiTi showed the highest ion release after oral simulation. The quantity of nickelion released from Damon Copper NiTi was the lowest in both conditions. Oral simulation followed by sterilization did not have a significant influence on nickel ion release from multi-strand NiTi and Damon Copper NiTi wires, but single-strand NiTi released statistically lower quantities of nickel ion after oral simulation.
Conclusion. The multi-strand nature of Supercable did not enhance the potential of corrosion after immersion in the simulated oral environment. In vitro use of nickel-titanium-based archwires followed by sterilization did not significantly increase the amount of nickel ion released from these wires.
Keywords: Copper NiTi, ion release, spectrophotometry, supercable
Introduction
Biocompatibility of orthodontic materials has long been considered a concern for clinicians due to the long period of orthodontic treatment and the high incidence of allergic reactions to metal appliances.1 Nickel, a main ingredient of orthodontic materials, can cause severe health hazards in biologic tissues. Hypersensitivity is the most common consequence of exposure to nickel-containing products, with incidence ranging from 4.5% to 20% in the literature.-5 The cytotoxic effect of nickel has been shown in cell culture studies.6-8 Subtoxic levels of nickel are capable of causing DNA strand breaks and DNA base damage9 and inhibition of DNA lesions repair.10 Small quantities of nickel ion are capable of activating monocytes and possibly enhance an inflammatory response in soft tissues.8
Because of their excellent mechanical properties of superelasticity, great working range and shape memory, nickel titanium alloys have widespread use in clinical orthodontics, especially in the early stages of treatment for leveling and aligning malposed teeth. These alloys contain nickel and titanium ions in a relatively similar weight ratio. Despite the high content of nickel in NiTi archwires, nickel release from these wires has been demonstrated to be low and under the required threshold to cause biological effects.6,11-12, This has been attributed to the spontaneous formation of titanium oxide layer on the surface,13 which protects the alloy from corrosion, thus limiting outward ion movement. However, the presence of low pH and high temperature expedite corrosion and nickel ion release from nickel titanium alloys to the levels that are several folds greater than those occurring in higher pH and lower temperature.11,14-15 The protective oxide layer on the surface of NiTi wires is also degraded in fluoride-containing environments, leading to corrosion and degradation in mechanical properties of NiTi wires.16-19 Under these conditions, the amount of nickel released from nickel titanium alloys may be sufficient to induce allergic reactions at least in patients with a clinical history of hypersensitivity to metal appliances. Previous studies have shown that exposure to nickel-containing orthodontic appliances produce nickel hypersensitivity in subjects sensitized to nickel before initiating therapy.4,20
Today, advances in material technology have resulted in the development of superelastic nickel-titanium-based wires with greater range of deflection while exerting dramatically lower force magnitude in the plateau region compared to the conventional NiTi archwires. Supercable is a seven-strand nickel titanium coaxial archwire introduced by Strite Industries and has been advocated for engaging severely crowded teeth and high-buccal canines without undergoing plastic deformation. Damon Copper NiTi was introduced by Ormco for initial tooth alignment, especially when using Damon self–ligating brackets in order to give optimal force range and increase the rate of tooth movement. The superior load-defection properties of Supercable and Damon Copper NiTi archwires have been demonstrated in previous studies,19,21 but no study has evaluated the release of nickel ion from these orthodontic archwires in the as-received state and after exposure to a simulated oral environment. The multi-strand configuration in Supercable lowers the stiffness, but it may make the wire susceptible to corrosion, which in turn would affect the quantity of nickel ion release.
The NiTi wires may remain in the oral cavity for several months in strained conditions, and this might affect the corrosion resistance of the alloy. Due to the relatively high cost of Supercable and Damon Copper NiTi, the release of nickel ion from these wires after simulated clinical use and sterilization is also worthy of investigation. The aim of this study was to determine and compare the quantity of nickel ion released from single-strand Ni-Ti, multi-strand Ni-Ti and Damon Copper Ni-Ti wires in the as-received condition and after storage in a simulated oral environment followed by autoclave sterilization.
Materials and Methods
Three types of nickel-titanium-based archwires, including Rematitan “Lite” (Dentaurum, Ispringen, Germany), Damon Copper NiTi (Ormco Corp., Glendora, CA, USA) and SPEED Supercable (Strite Industries, Cambridge, Ontario, Canada), were selected. The first two archwires are single-strand, whereas the third is a multi-strand wire (Table 1). Forty specimens from each type were obtained by cutting 2-cm lengths from the straight posterior parts of maxillary 0.016-inch round NiTi wires. For each wire, twenty specimens were used in the “as-received” state and the others were kept for 2 months in a simulated oral environment (groups 1 to 6, Table 1). Overall, sixty preformed archwires were used to provide 120 wire specimens.
Table 1. Nickel-titanium-based wires used in this study classified by commercial name, composition, number of strands and test condition .
| Group | Number | Commercial name | Composition | Number of strands | Test condition |
| 1 | 20 | Rematitan “Lite” | NiTi | Single-strand | As-received |
| 2 | 20 | Recycled | |||
| 3 | 20 | Damon Copper NiTi | Copper NiTi | Single-strand | As-received |
| 4 | 20 | Recycled | |||
| 5 | 20 | Supercable | NiTi | Multi-strand | As-received |
| 6 | 20 | Recycled |
For oral simulation, acrylic plates were used on each 3-mm step designed to represent moderate crowding in the anterior segment of the maxillary dentition. Ten rows of brackets were bonded to each acrylic model using self-curing acrylic resin.Figure 1 Each row contained three standard edgewise upper central incisor brackets of 0.018-inch slot size (Dentaurum, Ispringen, Germany), which were positioned at the same line with their long axis parallel. The wire specimens were held on the brackets by using elastomeric ligatures (Ortho Technology, Tampa, Florida, USA). The acrylic models were placed in plastic containers filled with Fusayama Meyer artificial saliva solution and kept in a 37°C incubator for 2 months. The artificial saliva with a pH value of 7.03 consisted of KCl (0.4 g/L), NaCl (0.4 g/L), CaCl2.2H2O (0.906 g/L), NaH2PO4.2H2O (0.690 g/L), Na2S.9H2O (0.005 g/L) and Urea (1 g/L).22 At the end of the 2-month incubation, the specimens were retrieved from the solution, washed and dried. The wires were then subjected to a thermocycling process for 500 cycles, using 5° and 55°C temperatures with a dwell time of 30 seconds per bath. Finally, the specimens were sterilized in an autoclave at 121°C (250°F) and 15 to 20 psi for 20 minutes.
Figure 1.
An acrylic model for holding wire specimens in deflected state over a 2-month period.
Ion Release Determination
For ion release analysis, the specimens in groups 1, 2, 3, 4, 5 and 6 were placed in individual glass bottles containing 1.8 mL of Fusayama Meyer artificial saliva solution. The bottles were kept in an incubator at 37ºC for 28 days and then the nickel ion concentration in the electrolyte was detected using a graphite-furnace atomic absorption spectrophotometer (Perkin Elmer, Model 4100 ZL, Norwalk, CT, USA).
Statistical Analysis
One-sample Kolmogorov-Smirnov test showed that the data distribution was normal; therefore, two-way ANOVA was used to compare nickel release from the as-received and recycled specimens of different NiTi wires. The analysis was performed with SPSS 16.0 (SPSS Inc, Chicago, Ill, USA), and the significance level was predetermined at P < 0.05.
Results
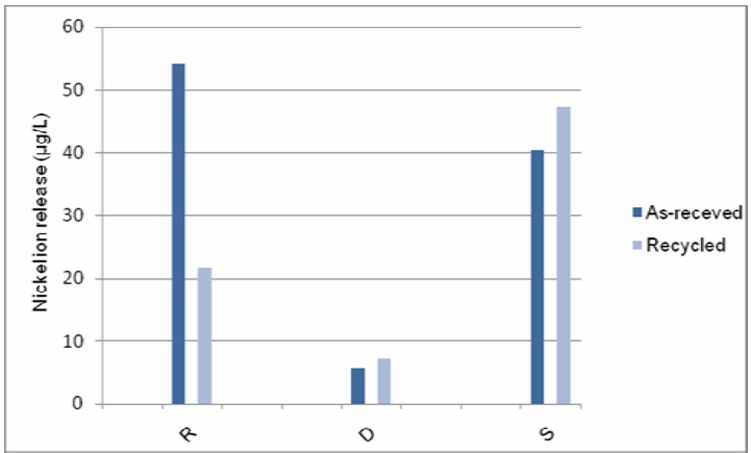
Table 2 displays the concentrations of nickel ions released into the electrolyte for the three types of wires in the as-received state and after oral simulation. Statistical analysis exhibited a significant interaction between the wire type and wire treatment (P<0.001), making it necessary to evaluate these variables separately. Therefore, one-way ANOVA was run, which delineated statistically significant differences between the release of nickel ion from the three types of wires in the as-received state (P<0.001), and after oral simulation (P<0.001) (Table 2). Pairwise comparisons by Tukey tests revealed that among the as-received wires, Rematitan “Lite” released significantly greater quantities of nickel ion compared to Supercable (P<0.05), and Supercable released significantly greater amounts of nickel ion compared to Damon Copper NiTi wire (P<0.05). After oral simulation, the highest and the lowest nickel concentrations in the electrolyte were observed with Supercable and Damon copper NiTi wires, respectively (P<0.05) (Table 2).
Table 2. Descriptive statistics and comparison of nickel ion concentration in the electrolyte (µg/L) between the three wires in the as-received state and after oral simulation .
| Type of wire | As-received state | After oral simulation | P-value (t-test) | ||||
| Mean | SD | Pairwise Comparisons** | Mean | SD | Pairwise Comparisons** | ||
| Rematitan “Lite” | 53.96 | 16.81 | a | 21.7 | 11.90 | b | < 0.001* |
| Damon Copper NiTi | 5.7 | 1.42 | c | 7.17 | 3.60 | c | 0.104 |
| Supercable | 40.3 | 21.18 | b | 47.23 | 17.09 | a | 0.262 |
| P-value (ANOVA) | < 0.001* | < 0.001* | |||||
| * Statistically significant difference at p < 0.05 | |||||||
| ** Different letters indicate statistically significant differences at p < 0.05 | |||||||
Student’s t-test revealed that the nickel ion concentrations in the electrolyte were not statistically different between the as-received state and after oral simulation in Supercable and Damon Copper NiTi groups, whereas Rematitan “Lite” released significantly lower quantities of nickel after immersion in the simulated oral environment (Table 2). Figure 2 compares the quantities of nickel ion released from the tested wires in the as-received state and after oral simulation.
Figure 2.
Comparison of nickel ion release (μg/L) from different nickel-titanium-based wires in the as-received state and after oral simulation.
Discussion
The present investigation evaluated the quantity of nickel ion released from different nickel-titanium-based wires in the as-received state and after oral simulation. In order to replicate the clinical conditions, acrylic models were used to keep wires in the crowded space during a 2-month interval and the specimens were subjected to thermocycling and sterilization processes. Jia et al6 demonstrated that cycling straining caused a significant increase in nickel ion release from NiTi wires immersed in an artificial saliva solution. Another option used in some studies is to determine the nickel ion release of retrieved wires, which had been engaged in the oral environment for 1 month or more.10,23 The nickel ion content was measured over 28 days because a previous study found that the metal ion release from orthodontic appliances is almost complete within 4 weeks.24
Among the tested wires, Rematitan “Lite” released the highest amount of nickel in the as-received state and Supercable exhibited the highest release after oral simulation. In both conditions, the lowest quantity of nickel ion in the electrolyte was found in Damon Copper NiTi group, which might be attributed to the addition of a small amount of copper into the alloy structure, which has been shown to lower the reactivity of titanium, thus increasing corrosion resistance and biocompatibility of copper-containing NiTi wires.14 The findings of this study indicated that in nickel-sensitive patients, use of Copper NiTi wires should be preferred to nickel-titanium archwires.
The results of this study showed no significant difference in nickel ion release from the as-received and recycled Damon Copper NiTi specimens. Despite its multi-strand nature, which may increase the possibility of corrosion, Supercable did not show a significant increase in nickel concentration in the electrolyte after immersion, either. On the other hand, oral simulation had a significant influence on the quantity of nickel ion released from single-strand NiTi wire, in such a way that Rematitan “Lite” released lower amounts of nickel after oral simulation compared to the as-received condition. Poosti et al23 also found a smaller amount of nickel ion release after clinical use and wet sterilization of a superelastic nickel titanium wire (Rematitan “Lite”) compared to the as-received specimens, but the difference was negligible and not statistically significant.
Several studies have shown that the nickel-titanium archwires release greater amounts of nickel ion into the electrolyte in the first day or week of immersion and the release is significantly slowed down during the following immersion periods.11,15,25-26, This has been attributed to the growing layer of titanium oxide on the surface of NiTi wires, which prevents further diffusion of the alloy constituents into the aqueous environment.11,15 Therefore, it can be assumed that the nickel ion release from Rematitan “Lite” mainly occurs over the oral simulation period; therefore, when the wires were immersed in the electrolyte for ion detection analysis, the amount of release significantly decreased compared to that of the as-received specimens. Although this study did not evaluate the net effect of sterilization on nickel ion release from recycled archwires, previous authors have demonstrated that sterilization of NiTi wires by dry heat or steam autoclave had no significant influence on surface parameters or the amount of nickel ion released from NiTi wires.23,27
The nickel ion release from new and used nickel-titanium archwires has been investigated in previous studies. It is believed that the release of metal ions from the alloys is enhanced by surface corrosion.10,14 Several studies have found surface defects, pitting and crevice corrosion on the surface of NiTi wires immersed in the electrolyte or exposed to the oral environment.11,28-29, However, Grimsdottir and Hensten-Pettersen30 found no detectable difference in surface topography or composition between used and as-received NiTi archwires. Gursoy et al24 found that despite the presence of pitting corrosion on the surface of recycled NiTi wires, the recycling process did not result in significantly greater amount of metal ion release from NiTi archwires. Eliades et al10 indicated that nickel content of as-received and retrieved NiTi archwires were similar, implying that no release took place in vivo.10
The average range of daily dietary intake of nickel is about 200-300 µg,15,31-32, but eating some foods like legumes, spinach, lettuce, grains, baking powder, and cocoa can provide excessive amounts of nickel for the body.31 In the present study, the mean quantities of nickel ion released from the 2-cm length archwires over a 28-day interval ranged from 5.7 µg/L (Damon Copper NiTi) to 54 µg/L (Rematitan “Lite”) for the as-received specimens. For recycled wires, the highest and the lowest quantities of accumulated nickel ion were 7.2 µg/L (Damon Copper NiTi) and 47.2 µg/L (Supercable) after 28 days, respectively. In a full-mouth appliance, including upper and lower jaws, the overall length of NiTi archwires exposed to the saliva is around 28 cm and thus the cumulative nickel release from the two as-received nickel-titanium-based archwires would be in the range of 79.8-756 µg/L. The corresponding value for recycled wires is estimated to be 100.8-660.8 µg/L. If we assume that nickel ion release occurs evenly during the 28-day interval, the daily amount of nickel ion released from new and recycled wires would be modest in comparison with the amounts ingested during daily food intake, which is well under the critical threshold of 600‒2500 µg/L over a 24-hour period required to cause allergic reactions.23 However, if these levels are added to the nickel ions from dietary sources and that released from other parts of fixed orthodontic appliances, such as bands and brackets, the nickel ion concentration may come close to the critical level required for induction of nickel hypersensitivity. Bass et al33 noticed the risk of sensitizing patients to nickel following long-term exposure to nickel-containing orthodontic appliances. Furthermore, in the clinical conditions, the wires are subjected to loads of mastication and tooth brushing and the temperature and chemical conditions are variable. These may contribute to degradation of the protective oxide layer on the surface of nickel titanium archwires, leading to more ion release than that of in vitro conditions. Other predisposing factors such as oral bacterial flora, salivary enzymes, ingested fluids, physical and chemical properties of foods and liquids, acidic environment, and flow rate of saliva can also accelerate metal corrosion and subsequent ion release from orthodontic archwires in the oral environment.32,34 It is difficult to encompass all predisposing factors in experimental conditions; therefore, the results of this study should be interpreted within the limitations of in vitro studies. Further studies should be designed to measure the nickel content in oral tissues and its possible adverse cellular interactions.
Conclusion
1. In the as-received condition, the single-strand NiTi wire (Rematitan “Lite”) produced the highest concentration of nickel in the electrolyte. After oral simulation, the greatest quantity of nickel pertained to the multi-strand NiTi wire (Supercable).
2. Damon Copper NiTi showed the lowest amount of nickel in the electrolyte in both the as-received state and after oral simulation, which may be due to the presence of copper in the alloy structure.
3. The prolonged use of the tested nickel-titanium-based wires followed by sterilization would not increase the risk of corrosion under the conditions used in this study.
References
- 1.Sidebottom AJ, Mistry K. Prospective analysis of the incidence of metal allergy in patients listed for total replacement of the temporomandibular joint. Br J Oral Maxillofac Surg. 2014;52:85–6. doi: 10.1016/j.bjoms.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 2.Peltonen L. Nickel sensitivity in the general population. Contact Dermatitis. 1979;5:27–32. doi: 10.1111/j.1600-0536.1979.tb05531.x. [DOI] [PubMed] [Google Scholar]
- 3.Savolainen H. Biochemical and clinical aspects of nickel toxicity. Rev Environ Health. 1996;11:167–73. doi: 10.1515/reveh.1996.11.4.167. [DOI] [PubMed] [Google Scholar]
- 4.Kerosuo H, Kullaa A, Kerosuo E, Kanerva L, Hensten-Pettersen A. Nickel allergy in adolescents in relation to orthodontic treatment and piercing of ears. Am J Orthod Dentofacial Orthop. 1996;109:148–54. doi: 10.1016/s0889-5406(96)70175-0. [DOI] [PubMed] [Google Scholar]
- 5.Menne T. Prevention of nickel allergy by regulation of specific exposures. Ann Clin Lab Sci. 1996;26:133–8. [PubMed] [Google Scholar]
- 6.Jia W, Beatty MW, Reinhardt RA, Petro TM, Cohen DM, Maze CR. et al. Nickel release from orthodontic arch wires and cellular immune response to various nickel concentrations. J Biomed Mater Res. 1999;48:488–95. doi: 10.1002/(sici)1097-4636(1999)48:4<488::aid-jbm14>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 7.McKay GC, Macnair R, MacDonald C, Grant MH. Interactions of orthopaedic metals with an immortalized rat osteoblast cell line. Biomaterials. 1996;17:1339–44. [PubMed] [Google Scholar]
- 8.Wataha JC, Lockwood PE, Marek M, Ghazi M. Ability of Ni-containing biomedical alloys to activate monocytes and endothelial cells in vitro. J Biomed Mater Res. 1999;45:251–7. doi: 10.1002/(sici)1097-4636(19990605)45:3<251::aid-jbm13>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 9.Liang R, Senturker S, Shi X, Bal W, Dizdaroglu M, Kasprzak KS. Effects of Ni(II) and Cu(II) on DNA interaction with the N-terminal sequence of human protamine P2: enhancement of binding and mediation of oxidative DNA strand scission and base damage. Carcinogenesis. 1999;20:893–8. doi: 10.1093/carcin/20.5.893. [DOI] [PubMed] [Google Scholar]
- 10.Eliades T, Zinelis S, Papadopoulos MA, Eliades G, Athanasiou AE. Nickel content of as-received and retrieved NiTi and stainless steel archwires: assessing the nickel release hypothesis. Angle Orthod. 2004;74:151–4. doi: 10.1043/0003-3219(2004)074<0151:NCOAAR>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 11.Huang HH, Chiu YH, Lee TH, Wu SC, Yang HW, Su KH. et al. Ion release from NiTi orthodontic wires in artificial saliva with various acidities. Biomaterials. 2003;24:3585–92. doi: 10.1016/s0142-9612(03)00188-1. [DOI] [PubMed] [Google Scholar]
- 12.Ryhanen J, Niemi E, Serlo W, Niemela E, Sandvik P, Pernu H. et al. Biocompatibility of nickel-titanium shape memory metal and its corrosion behavior in human cell cultures. J Biomed Mater Res. 1997;35:451–7. doi: 10.1002/(sici)1097-4636(19970615)35:4<451::aid-jbm5>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 13.Wever DJ, Veldhuizen AG, de Vries J, Busscher HJ, Uges DR, van Horn JR. Electrochemical and surface characterization of a nickel-titanium alloy. Biomaterials. 1998;19:761–9. doi: 10.1016/s0142-9612(97)00210-x. [DOI] [PubMed] [Google Scholar]
- 14.Ahn HS, Kim MJ, Seol HJ, Lee JH, Kim HI, Kwon YH. Effect of pH and temperature on orthodontic NiTi wires immersed in acidic fluoride solution. J Biomed Mater Res B Appl Biomater. 2006;79:7–15. doi: 10.1002/jbm.b.30505. [DOI] [PubMed] [Google Scholar]
- 15.Kuhta M, Pavlin D, Slaj M, Varga S, Lapter-Varga M. Type of archwire and level of acidity: effects on the release of metal ions from orthodontic appliances. Angle Orthod. 2009;79:102–10. doi: 10.2319/083007-401.1. [DOI] [PubMed] [Google Scholar]
- 16.Schiff N, Dalard F, Lissac M, Morgon L, Grosgogeat B. Corrosion resistance of three orthodontic brackets: a comparative study of three fluoride mouthwashes. Eur J Orthod. 2005;27:541–9. doi: 10.1093/ejo/cji050. [DOI] [PubMed] [Google Scholar]
- 17.Cioffi M, Gilliland D, Ceccone G, Chiesa R, Cigada A. Electrochemical release testing of nickel-titanium orthodontic wires in artificial saliva using thin layer activation. Acta Biomater. 2005;1:717–24. doi: 10.1016/j.actbio.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Toumelin-Chemla F, Rouelle F, Burdairon G. Corrosive properties of fluoride-containing odontologic gels against titanium. J Dent. 1996;24:109–15. doi: 10.1016/0300-5712(95)00033-x. [DOI] [PubMed] [Google Scholar]
- 19.Ahrari F, Ramazanzadeh BA, Sabzevari B, Ahrari A. The effect of fluoride exposure on the load-deflection properties of superelastic nickel-titanium-based orthodontic archwires. Aust Orthod J. 2012;28:72–9. [PubMed] [Google Scholar]
- 20.Park HY, Shearer TR. In vitro release of nickel and chromium from simulated orthodontic appliances. Am J Orthod. 1983;84:156–9. doi: 10.1016/0002-9416(83)90180-x. [DOI] [PubMed] [Google Scholar]
- 21.Ramazanzadeh BA, Ahrari F, Sabzevari B, Zebarjad SM, Ahrari A. Effects of a simulated oral environment and sterilization on load-deflection properties of superelastic nickel titanium-based orthodontic wires. Int J Orthod Milwaukee. 2011;22:13–21. [PubMed] [Google Scholar]
- 22.Poosti M, Ahrari F, Moosavi H, Najjaran H. The effect of fractional CO laser irradiation on remineralization of enamel white spot lesions. Lasers Med Sci 2013. DOI: 10.1007/s10103-013-1290-9. [DOI] [PubMed] [Google Scholar]
- 23.Poosti M, Rad HP, Kianoush K, Hadizadeh B. Are more nickel ions released from NiTi wires after sterilisation? Aust Orthod J. 2009;25:30–3. [PubMed] [Google Scholar]
- 24.Gursoy S, Acar AG, Sesen C. Comparison of metal release from new and recycled bracket-archwire combinations. Angle Orthod. 2005;75:92–4. doi: 10.1043/0003-3219(2005)075<0092:COMRFN>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 25.Staffolani N, Damiani F, Lilli C, Guerra M, Staffolani NJ, Belcastro S. et al. Ion release from orthodontic appliances. J Dent. 1999;27:449–54. doi: 10.1016/s0300-5712(98)00073-6. [DOI] [PubMed] [Google Scholar]
- 26.Hwang CJ, Shin JS, Cha JY. Metal release from simulated fixed orthodontic appliances. Am J Orthod Dentofacial Orthop. 2001;120:383–91. doi: 10.1067/mod.2001.117911. [DOI] [PubMed] [Google Scholar]
- 27.Pernier C, Grosgogeat B, Ponsonnet L, Benay G, Lissac M. Influence of autoclave sterilization on the surface parameters and mechanical properties of six orthodontic wires. Eur J Orthod. 2005;27:72–81. doi: 10.1093/ejo/cjh076. [DOI] [PubMed] [Google Scholar]
- 28.Petoumeno E, Kislyuk M, Hoederath H, Keilig L, Bourauel C, Jager A. Corrosion susceptibility and nickel release of nickel titanium wires during clinical application. J Orofac Orthop. 2008;69:411–23. doi: 10.1007/s00056-008-0808-4. [DOI] [PubMed] [Google Scholar]
- 29.Eliades T, Eliades G, Athanasiou AE, Bradley TG. Surface characterization of retrieved NiTi orthodontic archwires. Eur J Orthod. 2000;22:317–26. doi: 10.1093/ejo/22.3.317. [DOI] [PubMed] [Google Scholar]
- 30.Grimsdottir MR, Hensten-Pettersen A. Surface analysis of nickel-titanium archwire used in vivo. Dent Mater. 1997;13:163–7. [PubMed] [Google Scholar]
- 31.Grandjean P. Human exposure to nickel. IARC Sci Publ 1984:469-85. [PubMed] [Google Scholar]
- 32.Barrett RD, Bishara SE, Quinn JK. Biodegradation of orthodontic appliancesPart IBiodegradation of nickel and chromium in vitro. Am J Orthod Dentofacial Orthop. 1993;103:8–14. doi: 10.1016/0889-5406(93)70098-9. [DOI] [PubMed] [Google Scholar]
- 33.Bass JK, Fine H, Cisneros GJ. Nickel hypersensitivity in the orthodontic patient. Am J Orthod Dentofacial Orthop. 1993;103:280–5. doi: 10.1016/0889-5406(93)70009-D. [DOI] [PubMed] [Google Scholar]
- 34.Shahabi M, Jahanbin A, Esmaily H, Sharifi H, Salari S. Comparison of some dietary habits on corrosion behavior of stainless steel brackets: an in vitro study. J Clin Pediatr Dent. 2011;35:429–32. doi: 10.17796/jcpd.35.4.m17j2h5827861m55. [DOI] [PubMed] [Google Scholar]