Abstract
In this study, we investigated whether nuclear factor erythroid 2-related factor 2 (Nrf2) activation in astrocytes contributes to the neuroprotection induced by a single hyperbaric oxygen preconditioning (HBO-PC) against spinal cord ischemia/reperfusion (SCIR) injury. In vivo: At 24 h after a single HBO-PC at 2.5 atmospheres absolute for 90 min, the male ICR mice underwent SCIR injury by aortic cross-clamping surgery and observed for 48 h. HBO-PC significantly improved hindlimb motor function, reduced secondary spinal cord edema, ameliorated the reactivity of spinal motor-evoked potentials, and slowed down the process of apoptosis to exert neuroprotective effects against SCIR injury. At 12 h or 24 h after HBO-PC without aortic cross-clamping surgery, Western blot, enzyme-linked immunosorbent assay, realtime-polymerase chain reaction and double-immunofluorescence staining were used to detect the Nrf2 activity of spinal cord tissue, such as mRNA level, protein content, DNA binding activity, and the expression of downstream gene, such as glutamate-cysteine ligase, γ-glutamyltransferase, multidrug resistance protein 1, which are key proteins for intracellular glutathione synthesis and transit. The Nrf2 activity and downstream genes expression were all enhanced in normal spinal cord with HBO-PC. Glutathione content of spinal cord tissue with HBO-PC significantly increased at all time points after SCIR injury. Moreover, Nrf2 overexpression mainly occurs in astrocytes. In vitro: At 24 h after HBO-PC, the primary spinal astrocyte-neuron co-cultures from ICR mouse pups were subjected to oxygen-glucose deprivation (OGD) for 90 min to simulate the ischemia-reperfusion injury. HBO-PC significantly increased the survival rate of neurons and the glutathione content in culture medium, which was mainly released from asctrocytes. Moreover, the Nrf2 activity and downstream genes expression induced by HBO-PC were mainly enhanced in astrocytes, but not in neurons. In conclusion, our findings demonstrated that spinal cord ischemic tolerance induced by HBO-PC may be mainly related to Nrf2 activation in astrocytes.
Key words: : astrocyte, hyperbaric oxygen preconditioning, neuroprotection, Nrf2, oxygen-glucose deprivation, spinal cord ischemia/reperfusion
Introduction
Spinal cord ischemia-reperfusion (SCIR) injury remains a devastating complication of thoracic aortic intervention, both open and endovascular.1,2 Although advancements in surgical adjuncts, such as distal aortic perfusion, cerebrospinal fluid drainage, lesser ischemic duration, localized hypothermia, and evoked potential monitoring appear to modestly reduce risk of spinal cord injury,3–5 these advancements in surgical adjuncts are controversial and limited. Unfortunately, once the injury has clinically manifested, no pharmacological adjuncts have proven clinically efficacious in attenuating this injury.6 Pharmacological adjuncts to reduce this complication have been long sought after, with few proven strategies filtering up to clinical use.
Hyperbaric oxygen preconditioning (HBO-PC) has been found to protect the central nervous system (CNS) from ischemic/reperfusion insult, which suggests that HBO-PC is a safe and feasible method and might be potentially promising to provide neuroprotective benefits for ischemic stroke in clinic.7–9 There are evidences that the production of reactive oxygen species (ROS) at a non-lethal level or up-regulated activity of antioxidant enzymes plays an important role in HBO-PC-induced ischemic tolerance.10,11 The precise mechanism underlying HBO-PC, however, has not been fully elucidated, and more evidence is needed for HBO-PC to be used clinically.
Over the course of evolution, cells have developed complex cellular defense mechanisms and strategies to cope with and defend against oxidative stress. Nuclear factor erythroid 2-related factor 2 (Nrf2) is a key transcriptional factor for antioxidant response element-regulated genes.12 The expression of phase-II detoxification and antioxidant enzymes is governed by a cis-acting regulatory element named the antioxidant response element (ARE). ARE-containing genes are regulated by Nrf2, a member of the Cap ‘n’ Collar basic-leucine-zipper family of transcription factors. ARE-regulated genes are preferentially activated in astrocytes, which consequently have more efficient detoxification and antioxidant defenses than neurons.13,14 Astrocytes closely interact with neurons to provide structural, metabolic, and trophic support, as well as actively participating in the modulation of neuronal excitability and neurotransmission. Therefore, functional alterations in astrocytes can shape the interaction with surrounding cells, such as neurons and microglia. Activation of Nrf2 in astrocytes protects neurons from a wide array of insults in different in vitro and in vivo paradigms, confirming the role of astrocytes in determining the vulnerability of neurons to noxious stimuli.15
In this study, we used an established SCIR injury mouse model by aortic cross-clamping surgery to examine the hypothesis that spinal ischemic tolerance induced by HBO-PC is associated with an increase of Nrf2 transcriptional activity. Moreover, we used the spinal astrocyte-neuron co-culture to investigate whether Nrf2 activation in astrocytes plays an important role in an HBO-PC-induced neuroprotective effect against SCIR injury.
Methods
Animals and groups
The Animal Care and Use Committee of the Second Military Medicine University approved all experiments, and this investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health. Adult male ICR mice (12∼16 weeks; 25∼30 g) were used for all experiments and were housed at a temperature of 22∼24°C and 12:12 h light/dark cycle controlled environment with free access to food and water. In the experiment with surgery treatment, mice were randomly assigned to the following groups: sham group (n=21); HBO-PC plus SCIR group (n=36); SCIR group (n=36). In the experiment only with HBO-PC, mice were randomly assigned to the following groups: CON group (n=24); post-HBO 12 h group (n=36); post-HBO 24 h group (n=39).
HBO-PC in vivo
Mice were exposed to 100% oxygen at 2.5 atmosphere absolute (ATA) for 90 min in a transparent hyperbaric rodent chamber (Type RDC 150-300-6, Second Military Medical University, Shanghai, China). Compression and decompression of HBO were performed at the rate of 0.2 ATA/min. The gas in the chamber was continuously ventilated to prevent the retention of CO2, and the temperature was maintained within the range of 22∼25°C. After the exposure to HBO, the mice were maintained in a normoxic environment for 24 h until aortic cross-clamping surgery.
Surgical procedures
For all surgical procedures, mice were maintained at pre-defined core temperatures (35∼36°C) using a temperature controlled surgical platform. Mice were anesthetized by inhalation of 3% isofluorane for induction, then maintained at 2% isofluorane through a face mask driven by 100% O2 flow. Heparin (400 IU/kg) was administered subcutaneously to all animals before the procedure. A cervicothoracic approach with aseptic technique was used to expose the aortic arch, as previously described.16 Under direct visualization, the aortic arch was cross-clamped between the left common carotid artery and the left subclavian artery, then an additional aneurysm clip was placed on the subclavian artery to minimize collateral flow. The vascular occlusion was confirmed with a laser Doppler blood flow monitor (Moor Instruments, UK) to achieve a greater than 90% decrease in distal aortic flow measured at the femoral artery, and maintained for 4 min. Then the clips were removed, and the chest was closed in layers.
Sham mice had the aortic arch exposed through the same procedure but no aortic cross-clamping. With maintaining their core temperature, mice spontaneously recovered from anesthesia on the surgical platform. Bladders were manually expressed twice per day for the whole duration of the experiment. Mice also were administered 1 mL lactated Ringer subcutaneously twice per day and prophylactic antibiotics once per day for 7 days after surgery.
Functional locomotor scores
After reperfusion, hindlimb function was quantified at 0, 12, 24, 36, and 48 h using the Basso Mouse Scale for Locomotion.17 The Basso Mouse Scale is a 10-point scale (0∼9) that uses operational definitions to quantify the magnitude and rate recovery of hindlimb movements, forelimb-hindlimb coordination, and trunk stability in spinal cord injured mice.
Spinal cord water content
At 48 h after SCIR, the T-10 through L-3 spinal cord sections were harvested, weighed, and heated at 98°C for 48 h and reweighed. Percent water content was calculated as ([wet weight – dry weight]/wet weight×100).18
Electrophysiology
At 1 week after sham or SCIR, spinal cord function was evaluated by motor-evoked potentials (MEPs) as described previously.19,20 Briefly, mice were anesthetized using tribromoethanol, and rectal temperature was maintained at 37°C. For this purpose, three gold electrodes were inserted into the scalp and one into the soft palate to apply electric impulses (MultiPulse Stimulator D185–Mark, Digitimer LTD) to the motor cortex to quantify MEPs. Electrodes were placed in the muscles of the extremities to measure muscular movements caused by the electric impulses. After electric stimulation of the cerebral motor cortex, the muscular answers of the upper and the lower extremities were recorded. Stimulus intensity was 1∼3 mA, and each recorded response was an average of 5 to 10 sweeps. Amplitude was measured as the distance from baseline to the peak of the response. Each data point is the average of two recordings from one side of each mouse. The electrophysiology was performed blinded. We analyzed the amplitude and latency of MEPs to find the difference after SCRI.
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
The vertebral column was removed en bloc from T-10 through L-3, and the spinal cord was harvested by forcefully injecting phosphate-buffered saline (PBS, pH 7.4) into the spinal column. Spinal cords were preserved in 10% formalin for at least 24 h before paraffin embedding and sectioning. TUNEL assay was performed on paraffin-embedded sections using the in-situ cell death detection kit (Roche Diagnostics Corp., Indianapolis, IN). According to standard protocols, the slides were incubated with 3% H2O2 for 5 min, rinsed, and then incubated in terminal transferase (TdT) buffer for 15 min at 22°C. The TdT reaction mixture was added and incubated for 30 min at 37°C. After blocking with bovine serum albumin and incubation with avidin-biotin complex, the TUNEL reaction was visualized by chromogenic staining with 3,3′ diaminobenzidine. The numbers of TUNEL-positive cells were counted by a pathologist at 200× magnification, 30 fields per section blinded to the study group.
Immunohistochemistry
Spinal cord sections were blocked by 10% normal goat serum with 0.1% Triton×100 for 1 h at room temperature. After rinsing with PBS, the sections were incubated with anti-Nrf2 (Sigma-Aldrich) overnight at 4°C, double stained with anti-glial fibrillary acidic protein (GFAP) or anti-neuronal nuclei (NeuN) (Millipore) to label astrocytes or neurons. Sections were subsequently rinsed and incubated with alexa fluor (Alexa) or fluorescein isothiocyanate (FITC) conjugated secondary antibodies (Invitrogen) for 1 h at room temperature. All sections were mounted with Vectashield media (Vector Laboratories) with 4′,6-diamidino-2-phenylindole (DAPI) and a cover slip applied. Tissue sections were viewed with a microscope, and images were captured using a camera.
Caspase activity assay
The activities of caspase-3 and caspase-9 were measured with a caspase-3/CPP32 Fluorometric Assay Kit, and a caspase-9/CPP32 Fluorometric Assay Kit (Biovision Research Products, Mountain View, CA). Briefly, animals were euthanized at 1 week after SCIR, and their spinal cords were harvested for analysis. Spinal cord samples were homogenized in ice-cold cell lysis buffer and kept at 4°C for 1 h, and the homogenate was centrifuged at 12,000 g for 15 min at 4°C. Protein content was measured by an enhanced BCA protein assay kit. Equal amounts of the protein samples were incubated in a 96-well plate with 50 mL of 2×reaction buffer. Reactions were initiated by adding 5 mL of 1 mM DEVD-AFC substrate. After incubation in the dark at 37°C, the plate was read in a fluorometer equipped with a 400 nm excitation filter and a 505 nm emission filter.
Quantitative real-time polymerase chain reaction (RT-PCR)
For evaluation of Nrf2, glutamate-cysteine ligase (GCL), γ-glutamyltransferase (γGT), multidrug resistance protein 1 (MRP1), and β-actin gene expression, real-time TaqMan RT-PCR was performed. RNA was isolated at 12 h and 24 h after HBO-PC using Trizol (Invitrogen, Carlsbad, CA) according to the manufacturer's instruction. First-strand cDNA was synthesized using Superscript II. cDNA was quantified in duplicate on a Rotor-Gene RG3000 (Corbett Research, Sydney, Australia) using a SYBRgreen core reagent kit (Molecular Probes) according to the manufacturer's instructions. Expression of each sample was normalized on the basis of its β-actin mRNA content.
PCR reactions were performed in 25 μL volumes with 2.5 μL of the appropriate RT reaction mixture. The following sequence-specific primers were used in RT-PCR. For Nrf2, forward, 5-TCA GCGACGGAAAGAGTA-3; reverse, 5-TGGGCAACCTGGGAG TAG-3. For GCL, forward, 5-CGTGGTGGATGGGTGTAGC-3; reverse, 5-TAAAGCCTGATCCAAGTAACTCTG-3. For γGT, forward, 5-AAAGTGGCACAGGAGCGAGAT-3; reverse, 5-GGG ATGAGTCCAGGAAACACG-3. For MRP1, forward, 5-CAAT GCTGTCATGGCGATG-3; reverse, 5-GATCCGATTGTCTTTG CTCTTCA-3. For β-actin, forward, 5-CCAGCCGAGCCACATC GCTC-3; reverse, 5-ATGAGCCCCAGCCTTCTCCAT-3. Reactions were run in duplicate, and real-time data were analyzed with Rotor-Gene Real-Time Analysis Software 6.0.
Western blot analysis
Isolated spinal cord tissues or primary cell cultures were placed into lysis buffer containing 10 mmol/L Tris-HCl (pH 7.6), 100 mmol/L NaCl, 1 mmol/L ethylenediaminetetraacetic acid, 1% (w/vol), Triton X-100, and protease inhibitor cocktail. Lysates were then sonicated and centrifuged for 15 min at 10,000 g at 4°C. Supernatants were collected and protein concentrations determined using the Bradford method. Each sample was separated by SDS-PAGE and electroblotted onto a polyvinylidene fluoride membrane. After blocking, membranes were blotted overnight at 4°C with anti-Nrf2 (1:2500), anti-GCL (1:2500), anti-γGT (1:2500), anti-MRP1 (1:2500), and anti-β-actin (1:2500) antibodies (Sigma-Aldrich). After washing, blots were incubated for 1 h at room temperature with horseradish peroxidase (HRP) conjugated secondary antibody (1:2000) (Santa Cruz Biotechnology). Membranes were re-probed with β-actin to confirm equal loading. Primary antibodies were visualized by enhanced chemiluminescence. Bands were semi-quantified using densitometry.
Nrf2 DNA-binding assay
To measure Nrf2 DNA-binding activity, nuclear extracts were obtained 12 h and 24 h after HBO-PC using a nuclear extraction kit (Active Motif). Nrf2 DNA-binding activity of the nuclear fraction was determined by using an enzyme-linked immunosorbent assay-based kit (Active Motif). The assay uses a 96-well plate to which oligonucleotide containing an antioxidant response element has been immobilized. Nrf2 contained in nuclear extracts then bind specifically to this oligonucleotide and is detected through the use of an antibody directed against Nrf2. Addition of a secondary antibody conjugated to HRP provides a sensitive colorimetric readout that is easily quantified by spectrophotometry. The specificity of the assay was confirmed by adding wild-type competitor oligonucleotide (20 pmol) to positive-control nuclear extracts. The precision of the assay was determined by statistical analysis of the quantification results for wild-type competitor oligonucleotide that was measured three times in threefold, respectively.
Cell culture
Primary astrocyte cultures and neuronal cultures were prepared from spinal cords of ICR mouse pups.21 Astrocyte cultures were then seeded at a low density (15,000/mL) on Primaria plates (BD Falcon) and grew for 2 weeks to confluency in MEM (Invitrogen) supplemented with 10% horse serum and 25 U/mL penicillin plus 25 g/mL streptomycin. On reaching confluency, the astrocytes were treated with 8 μM cytosine-D-arabinofuranoside, a mitotic inhibitor for 3 days to kill off contaminating cells. The astrocytes were used for experiments at 2 to 3 weeks in culture. GFAP staining confirmed greater than 95% purity of the astrocyte cultures. Neurons were purified by centrifugation on an Optiprep cushion, followed by isolation of p75NTR expressing motor neurons by immunoaffinity selection with the IgG monoclonal antibody, then plated in MEM supplemented with 10% horse serum, 2.5% fetal bovine serum, and 25 U/mL penicillin plus 25 g/mL streptomycin. For co-culture experiments, we developed a “sandwich” culture system where spinal neurons are grown suspended above a feeder layer of mouse spinal astrocytes, as previously described by Banker and Goslin.22 Astrocyte monolayers were washed twice with PBS, and neurons were plated on top at a density of 350 cells/cm2. Co-cultures were maintained for 48 h in L15 medium supplemented with 0.63 mg/mL sodium bicarbonate, 5 μg/mL insulin, 0.1 mg/mL conalbumin, 0.1 mM putrescine, 30 nM sodium selenite, 20 nM progesterone, 20 mM glucose, 100 IU/mL penicillin, 100 μg/mL streptomycin, and 2% horse serum.
HBO-PC in vitro
The plated primary cultured cells were cultured in OxyCure 3000 hyperbaric incubator (OxyHeal Health Group) at 100% O2 at 2.4 ATA, at 37°C for 90 min. To maintain a physiological pH, culture media was replaced with CO2-independent media (Gibco). Immediately after treatment, cells were either returned to normal complete media and culture conditions for recovery or harvested at 12 and 24 h for further analysis.
Oxygen and glucose deprivation (OGD) treatment
OGD was performed as described previously.23,24 Briefly, ischemia was introduced by a buffer exchange to Hanks solution, which is an ischemia-mimetic solution (in mmol/L: 140 NaCl, 3.5 KCl, 0.43 KH2PO4, 1.25 MgSO4, 1.7 CaCl2, 5 NaHCO3, 20 HEPES, pH 7.3). The buffered Hanks solution was previously gassed with 95% N2/5% CO2 for 30 min. Subsequently, the culture dishes were placed in a hypoxic incubator chamber (Billups-Rothenberg) equilibrated with 95% N2/5% CO2 at 37°C for 1 h. Then the cell culture medium was replaced by MEM media with glucose and serum, in which the cells were incubated for 24 h.
Cell viability assay
Briefly, the culture medium was changed to a medium containing 0.5 g/L MTT and incubated for 4 h at 37°C. Then the supernatant was discarded, and the cells were mixed thoroughly with dimethylsulfoxide (100 μL/well). When the crystals were dissolved, the optical density absorbance values of 10 wells in each group were measured with an Elx800 plate reader at 570 nm. Cell viability is directly proportional to the absorbance value.
Measurement of glutathione (GSH)
GSH of spinal cord tissues and cell culture medium was measured by spectrophotometry. GSH was measured by adding the standard or sample to 100 μL of a 1:1 mixture of three units/mL GSH reductase with 0.67 mg/mL 5,5′-dithiobis (2-nitrobenzoic acid). The reaction was initiated by the addition of 20 μL of 0.67 mg/mL nicotinamide adenine dinucleotide phosphate (NADP) reduced and the increase in absorbance at 450 nm was monitored. GSH values in tissues are normalized to protein content. All measurements were performed in triplicate.
Statistical analysis
For all analyses, the individual performing the analysis was blinded to the study group. All quantitative data are expressed as mean±standard deviation. Statistical analysis was performed by using the SPSS Statistics 17.0 (SPSS, Chicago, IL). The significance of differences between means was verified by analysis of variance (ANOVA) followed by Tukey test. For the analysis of cell count results, a nonparametric Kruskal-Wallis ANOVA was used, followed by the Dunn test. Statistical significance was placed at p<0.05.
Results
HBO-PC improves neurological outcome and spinal cord edema in mouse SCIR model
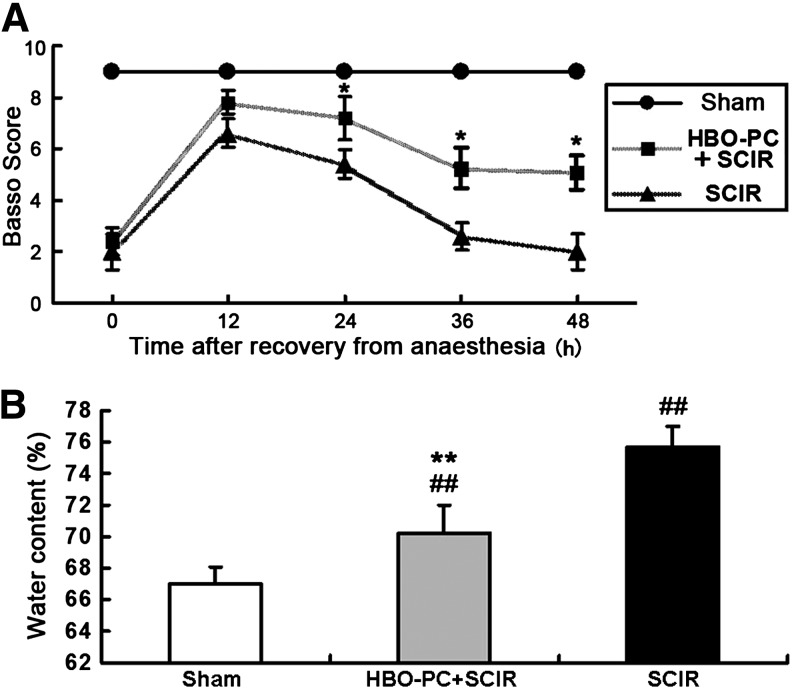
All mice with 4 min of SCIR injury exhibited a functional neurologic deficit after recovery from anesthesia. Over the following 48-h observation period, once function was lost in this progressive manner, no animals were observed to regain function after 24 h of reperfusion. At 24 h, 36 h, 48 h after SCIR injury, mice treated with HBO-PC had significantly preserved neurological function compared with the SCIR group (Fig. 1A). Moreover, water content of the spinal cord significantly increased at 48 h after SCIR injury, but HBO-PC before aortic occlusion palliated the spinal cord edema caused by SCIR injury (Fig. 1B).
FIG. 1.
Beneficial effect of hyperbaric oxygen preconditioning (HBO-PC) on hindlimb function and spinal cord water content after spinal cord ischemia/reperfusion (SCIR) injury. (A) There is no neurologic deficit in the Sham group. Basso scores were significantly higher in the HBO-PC+SCIR group compared with the SCIR group without HBO-PC at 24 h, 36 h, and 48 h after aortic cross-clamping (mean±standard deviation [SD], n=6, *p<0.05 vs. SCIR group). (B) The water content of the spinal cord was significantly higher in the HBO+SCIR group and SCIR group at 48 h after SCIR injury, but that was much lower in the HBO+SCIR group compared with the SCIR group (mean±SD, n=6, ##p<0.01 vs. the Sham group, **p<0.01 vs. the SCIR group).
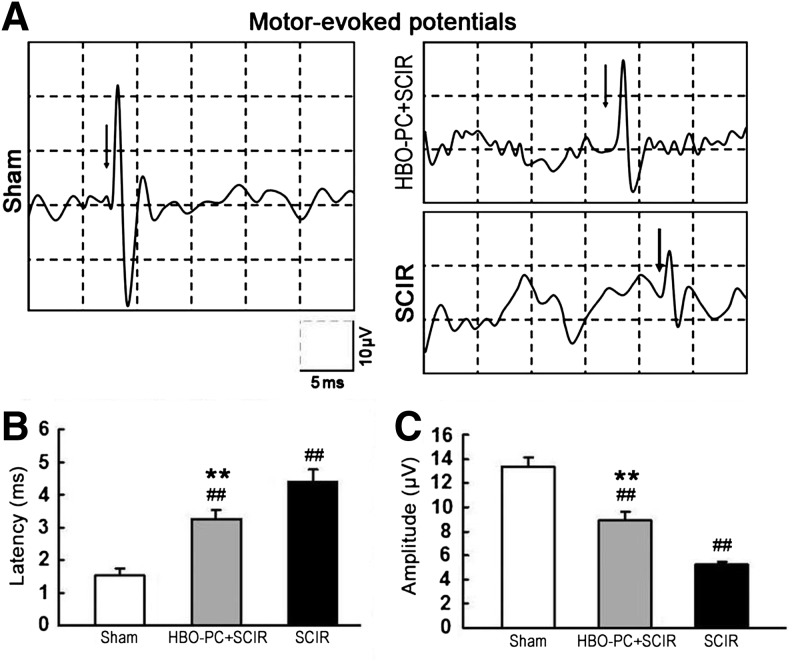
HBO-PC improves electrophysiologic abnormalities in mouse SCIR model
As shown in Figure 2, there are representative tracings of recorded MEPs at 1 week after sham or SCIR treatment. The latency of MEPs was significantly prolonged, and the amplitude of MEPs was significantly reduced in all mice with SCIR injury. HBO-PC, however, can induce a significant improvement in electrophysiological abnormalities.
FIG. 2.
Hyperbaric oxygen preconditioning (HBO-PC)-induced improvements on motor-evoked potentials (MEPs) after spinal cord ischemia/reperfusion (SCIR) injury. (A) Representative tracings of recorded MEPs from mice at 1 week after sham or SCIR. (B) The latency of MEPs was significantly prolonged at 1 week after SCIR injury and was longer in the SCIR group than the HBO-PC+SCIR group. (C) The amplitude of MEPs was reduced at 1 week after SCIR injury and was smaller in the SCIR group than the HBO-PC+SCIR group (mean±standard deviation, n=4, ##p<0.01 vs. Sham group, **p<0.01 vs. SCIR group).
HBO-PC slowed down the apoptotic process in mouse spinal cord with SCIR injury
TUNEL staining revealed that the apoptosis process in the spinal cord (as evidenced by an increase in TUNEL-positive cells) was significantly reduced by HBO-PC at 24 h before aortic occlusion (Fig. 3A). In addition, the levels of caspase-9 and caspase-3 activity in the spinal cord were all increased at 1 week after SCIR injury. After normalization with the activities in sham-operated mice, the caspase-9 activity of the HBO-PC+SCIR group was lower than that of the SCIR group (Fig. 3B). Similarly, the caspase-3 activity of the HBO-PC+SCIR group was lower than that of the SCIR group (Fig. 3C).
FIG. 3.
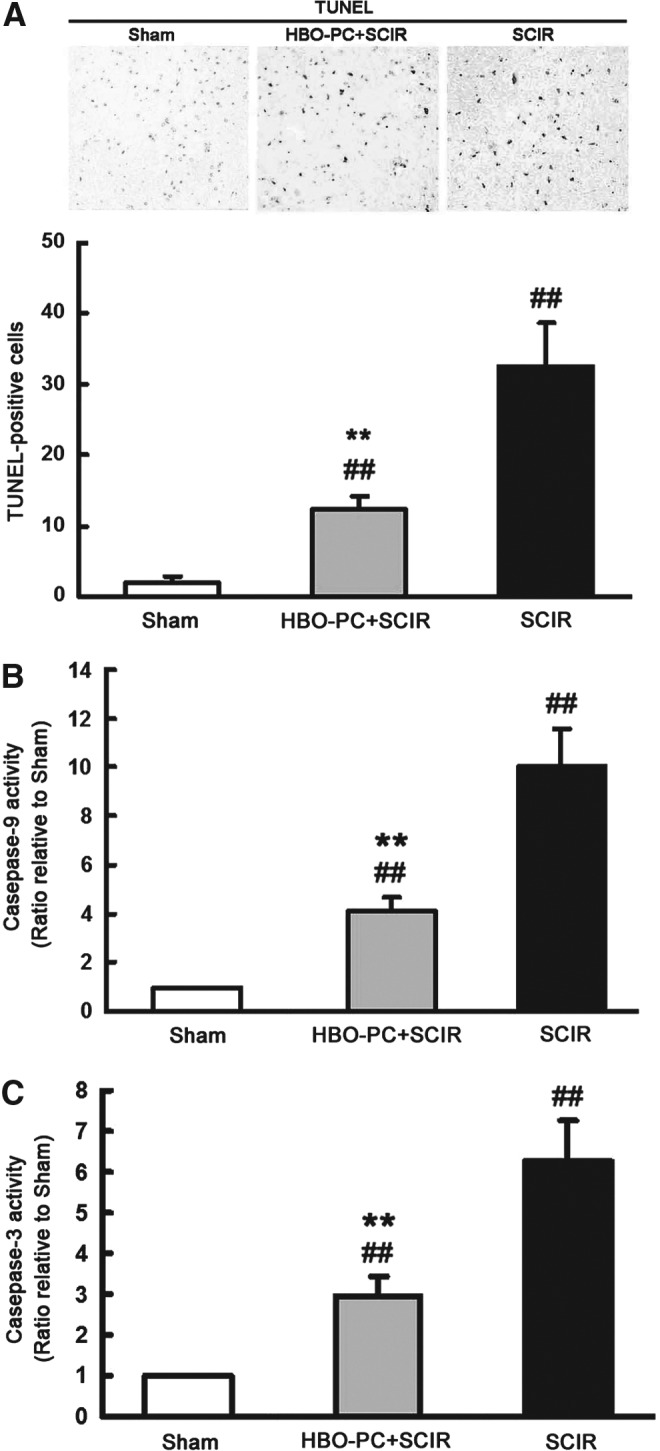
Hyperbaric oxygen preconditioning (HBO-PC) slowed down the apoptotic process in spinal cord ischemia/reperfusion (SCIR) injury. (A) The number of TUNEL-positive cells significantly increased in the spinal cord at 1 week after SCIR injury, and HBO-PC significantly reduced the SCIR-induced increase in TUNEL-positive cells (mean±standard deviation [SD], n=6, ##p<0.01 vs. Sham group, **p<0.01 vs. SCIR group). (B, C) The enzymatic activity of caspase-9 and caspase-3 at 1 week increased in the spinal cord of mice with SCIR injury. The enzymatic activity of caspase-9 or caspase-3 at 1 week after SCIR injury, however, was significantly reduced in the HBO-PC+SCIR group compared with the SCIR group (mean±SD, n=6, ##p<0.01 vs. the Sham group, **p<0.01 vs. the SCIR group).
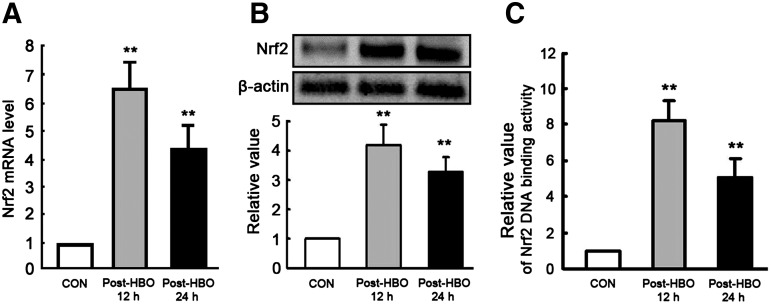
HBO-PC induces Nrf2 activation and the up-regulation of Nrf2 downstream gene expression in mouse spinal cord
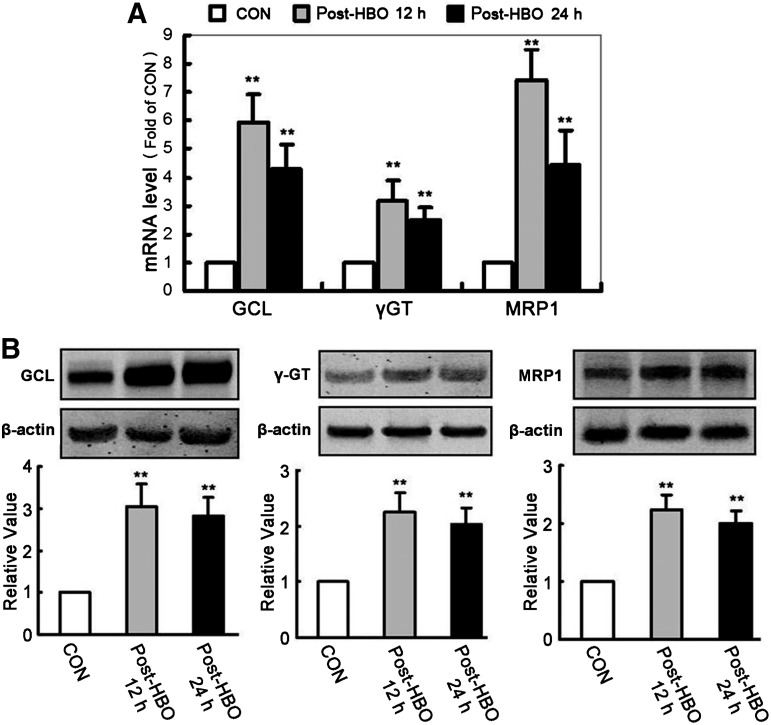
To determine whether HBO-PC induced Nrf2 activation before the experimental modeling of SCIR injury, we analyzed the mRNA (Fig. 4A) and protein (Fig. 4B) levels of Nrf2 in the spinal cord. At 12 h and 24 h after HBO-PC, the Nrf2 mRNA and protein levels of the spinal cord without SCIR injury were significantly increased compared with the CON group. Moreover, the DNA-binding activity of nuclear Nrf2 significantly increased at 12 h (8.22±1.1 fold) and 24 h (5.03±1.09 fold) after HBO-PC (Fig. 4C). Nrf2 downstream genes, such as GCL, γGT, and MRP1, were all key proteins for intracellular GSH synthesis and transit, which mRNA (Fig. 5A) and protein (Fig. 5B) expression levels are all significantly increased in spinal cord tissues at 12 h and 24 h after a single HBO-PC.
FIG. 4.
Hyperbaric oxygen preconditioning (HBO-PC) induces nuclear factor erythroid 2-related factor 2 (Nrf2) activation in mouse spinal cord (mean±standard deviation, n=6, **p<0.01 vs. CON group). (A) The Nrf2 mRNA level of spinal cord measured by real-time polymerase chain reaction was significantly enhanced at 12 h and 24 h after a single HBO-PC. (B) Using the housekeeping gene β-actin as an internal standard, the Nrf2 protein expression of spinal cord was also significantly increased measured by Western blot at 12 h and 24 h after a single HBO-PC. (C) Add 10 μg nuclear extract/well from the mice of each group, the relative DNA-binding activity of Nrf2 was determined using an enzyme-linked immunosorbent assay-based kit. The DNA-binding activity of nuclear Nrf2 significantly increased at 12 h and 24 h after a single HBO-PC.
FIG. 5.
Hyperbaric oxygen preconditioning (HBO-PC) induces the up-regulation of nuclear factor erythroid 2-related factor 2 (Nrf2) downstream gene expression. (A) The mRNA expression levels of glutamate-cysteine ligase (GCL), γ-glutamyltransferase (γGT), and multidrug resistance protein 1 (MRP1) measured by real-time polymerase chain reaction were significantly enhanced at 12 h and 24 h after a single HBO-PC (**p<0.01 vs. the CON group). (B) Using the housekeeping gene β-actin as an internal standard, the protein expression levels of GCL, γGT, and MRP1 in spinal cord tissues measured by Western blot were also significantly increased at 12 h and 24 h after a single HBO-PC (**p<0.01 vs. the CON group).
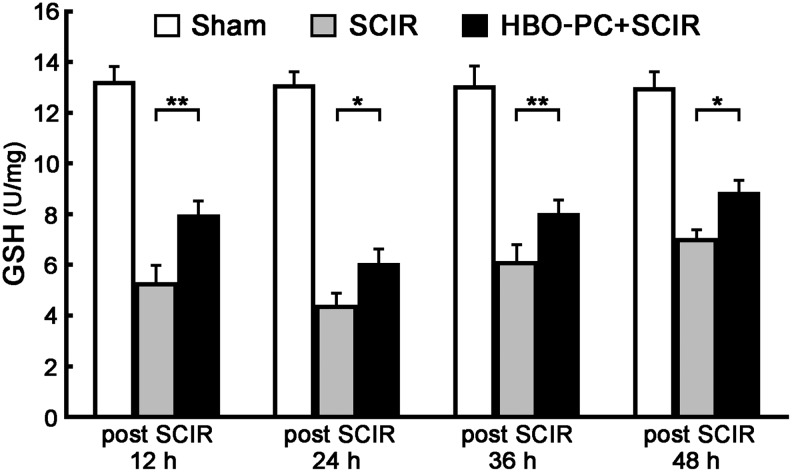
HBO-PC increases GSH content of the spinal cord with SCIR injury
In addition to its role in peroxide detoxification, GSH is the main cysteine storage in the cell, the major function of which is to maintain the cellular redox homeostasis. Compared with Sham group, the GSH content of the spinal cord significantly decreased at all time points (12, 24, 36, 48 h) after SCIR injury (Fig. 6). The GSH content of the HBO-PC+SCIR group, however, was much higher than that of the SCIR group. These results show that the higher GSH content induced by HBO-PC gave more antioxidant capacity to the spinal cord.
FIG. 6.
Hyperbaric oxygen preconditioning (HBO-PC) increases glutathione (GSH) content of the spinal cord with spinal cord ischemia/reperfusion (SCIR) injury. The GSH content of the spinal cord significantly decreased in the HBO-PC+SCIR and SCIR groups at 12, 24, 36, and 48 h after SCIR injury. Compared with the SCIR group, the GSH content of the spinal cord in the HBO-PC+SCIR group was much higher (mean±standard deviation, n=6, *p<0.05 or **p<0.01 vs. the SCIR group).
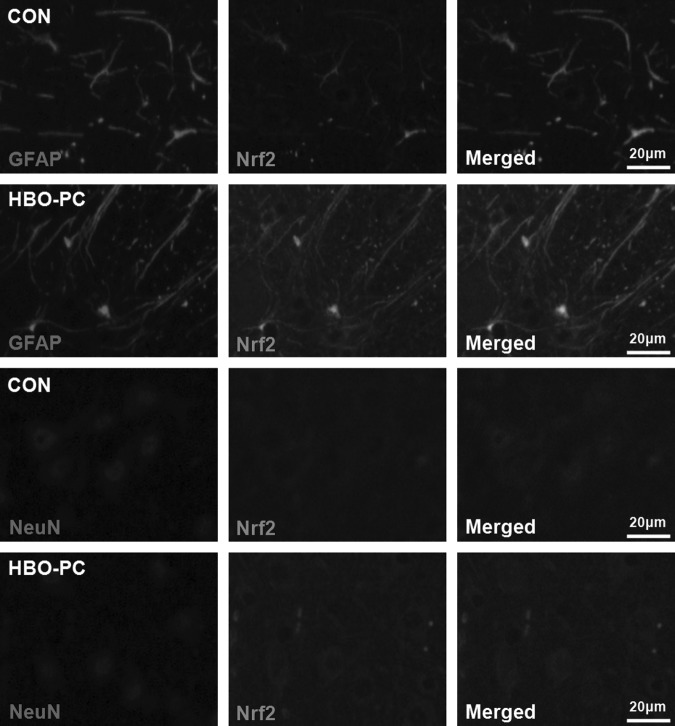
Nrf2 overexpression induced by HBO-PC mainly localized in astrocytes of mouse spinal cord
To explore the main location of Nrf2 expression induced by HBO-PC, double-immunofluorescence staining was performed. Sections from mice treated with post-HBO 24 h or controls were stained for anti-Nrf2 (green), the neuronal marker anti-NeuN (red), and the astrocyte marker anti-GFAP (red). As Figure 7 shows, the green fluorescence intensity of Nrf2 immunoreactivity was significantly increased in GFAP positive astrocytes, and there is only a slight enhancement of Nrf2 immunoreactivity in NeuN positive neurons. Therefore, these results suggested that Nrf2 overexpression induced by HBO-PC in the spinal cord mainly occurs in astrocytes.
FIG. 7.
Representative images of spinal cord sections stained with anti-nuclear factor erythroid 2-related factor 2 (Nrf2) and the astrocyte marker glial fibrillary acidic protein (GFAP) or the neuronal marker anti-neuronal nuclei (NeuN) by double-immunofluorescence labeling. Scale bar=20 μm. At 24 h after hyperbaric oxygen preconditioning (HBO-PC), the fluorescence intensity of Nrf2 immunoreactivity was significantly increased in GFAP positive astrocytes, and there is only a slight enhancement of Nrf2 immunoreactivity in NeuN positive neurons.
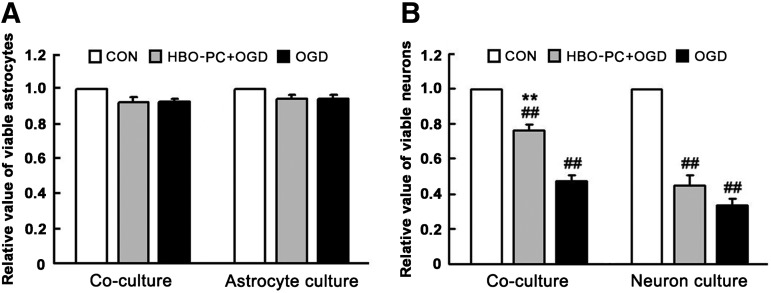
HBO-PC improves the cell viability of neurons in co-cultures treated with OGD
The MTT assay was used to evaluate the cell viability of OGD-insulted primary astrocytes and neurons from mouse spinal cord. As shown in Figure 8A, the cell viability of OGD-insulted astrocytes in co-culture or only astrocyte culture has no significant change compared with the CON group without OGD treatment. As shown in Figure 8B, the cell viability of OGD-insulted neurons in co-culture or only neuron culture significantly decreased, but the neuronal viability of the HBO-PC+OGD group is higher than that of the OGD group in co-culture. Thus, HBO-PC significantly enhanced the neuronal viability in the presence of astrocytes. These results show that HBO-PC exerts a significant neuroprotective effect on OGD-insulted neuron cultures. Moreover, the astrocytes may play an important role in HBO-PC-induced neuroprotection.
FIG. 8.
The cell viability of primary astrocytes and neurons from spinal cord was evaluated by MTT assay at 24 h after 60 min of oxygen-glucose deprivation (OGD) treatment. (A) In co-culture or only astrocyte culture, there was no significant reduction in astrocyte viability of the hyperbaric oxygen preconditioning (HBO-PC)+OGD group and the OGD group compared with the CON group. (B) In co-culture or only neuron culture, the neuronal viability of the HBO-PC+OGD group and the OGD group significantly decreased (**p<0.01 vs. CON group). The neuronal viability of the HBO-PC+OGD group, however, is significantly higher than that of the OGD group in co-culture (##p<0.01 vs. OGD group).
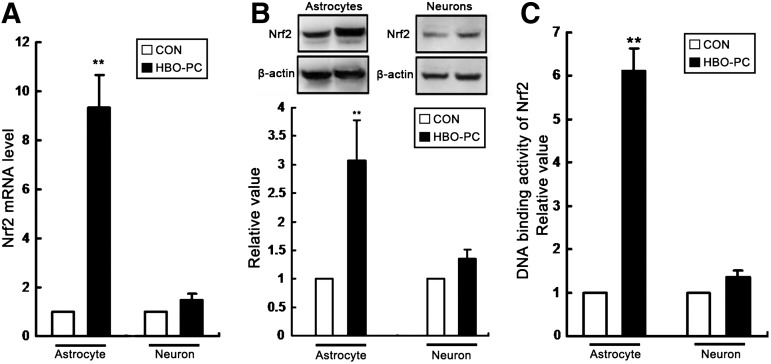
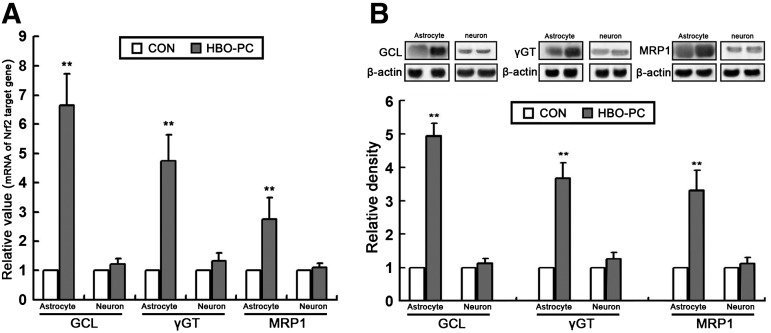
HBO-PC induces Nrf2 activation in astrocytes of co-culture
To determine whether HBO-PC induced Nrf2 activation in cultures, we detected the mRNA level, protein content, and DNA-binding activity of Nrf2 in astrocytes and neurons, respectively, at 24 h and 24 h after HBO-PC. As shown in Figure 9, all indicators related to Nrf2 activation significantly increased in astrocytes of co-culture, but there is no significant change in neurons of co-culture system. These results show that Nrf2 activation induced by HBO-PC in spinal cord mainly occurs in astrocytes.
FIG. 9.
Hyperbaric oxygen preconditioning (HBO-PC) induces anti-nuclear factor erythroid 2-related factor 2 (Nrf2) activation in astrocytes of co-culture. (A) The Nrf2 mRNA level of astrocytes measured by real-time polymerase chain reaction was significantly enhanced at 24 h after HBO-PC (**p<0.01 vs. CON group), but there is no change in neurons. (B) Using the housekeeping gene β-actin as an internal standard, the Nrf2 protein expression of astrocytes measured by Western blot also significantly increased at 24 h after HBO-PC (**p<0.01 vs. CON group), but there is no change in neurons. (C) Using an enzyme-linked immunosorbent assay-based kit, the relative DNA-binding activity of nuclear Nrf2 significantly increased in astrocytes at 24 h after HBO-PC (**p<0.01 vs. CON group), but there is no change in neurons.
HBO-PC induces the up-regulation of Nrf2 downstream gene expression in astrocytes of co-culture
As shown in Figure 10, HBO-PC also significantly increased the mRNA level and protein expression of Nrf2 downstream genes (GCL, γGT, and MRP1) in astrocytes, but not in neurons of the co-culture system. These results show that Nrf2 activation in astrocytes may contribute to the neuroprotective effect induced by HBO-PC.
FIG. 10.
Hyperbaric oxygen preconditioning (HBO-PC) induces the up-regulation of anti-nuclear factor erythroid 2-related factor 2 (Nrf2) downstream gene expression in astrocytes of co-culture. (A) The mRNA levels of glutamate-cysteine ligase (GCL), γ-glutamyltransferase (γGT), and multidrug resistance protein 1 (MRP1) in astrocytes measured by real-time polymerase chain reaction were all significantly enhanced at 24 h after HBO-PC (**p<0.01 vs. CON group), but there is no change in neurons. (B) Using the housekeeping gene β-actin as an internal standard, the protein levels of GCL, γGT, and MRP1 measured by Western blot were significantly increased in astrocytes at 24 h after HBO-PC (**p<0.01 vs. CON group), but there is no change in neurons.
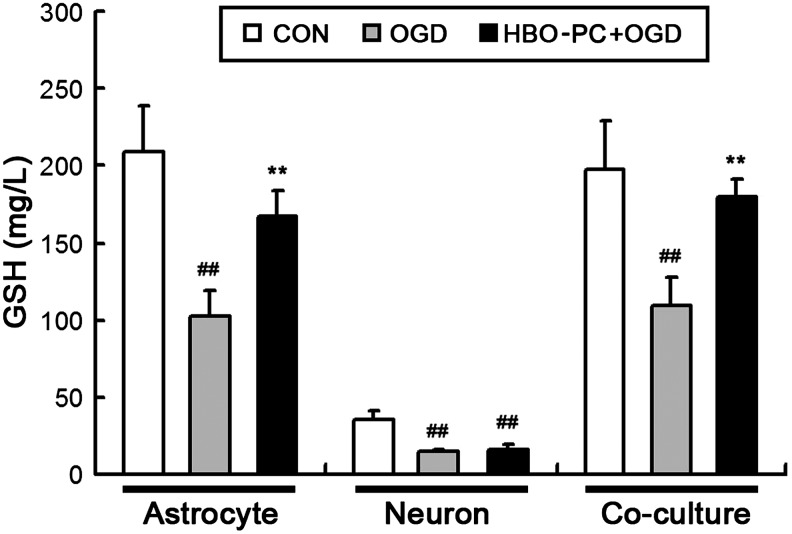
HBO-PC increases GSH content of culture medium with astrocytes after OGD treatment
As shown in Figure 11, the GSH levels in medium of all culture system were measured by spectrophotometric methods at 24 h after OGD treatment. In only neuron culture, the medium GSH level of the HBO+OGD and the OGD group significantly decreased compared with the CON group (##p<0.01 vs. CON group), and there was no significant difference between the HBO+OGD group and the OGD group. In only astrocyte culture or co-culture, the medium GSH level of the HBO+OGD group was much higher than that of the OGD group (**p<0.01 vs. OGD group). Thus, when cultured in the presence of astrocytes, HBO-PC increased the GSH content of the culture medium after OGD treatment. These results further illustrate the possibility that Nrf2 activation in astrocytes may play a critical role in the neuroprotection induced by HBO-PC.
FIG. 11.
Hyperbaric oxygen preconditioning (HBO-PC) increases glutathione (GSH) content of culture medium with astrocytes after oxygen-glucose deprivation (OGD) treatment. In only neuron culture, the medium GSH level of the HBO-PC+OGD and the OGD group significantly decreased compared with the CON group (##p<0.01 vs. CON group), and there was no significant difference between the HBO-PC+OGD group and the OGD group. In only astrocyte culture or co-culture, the medium GSH level of the HBO-PC+OGD group was much higher than that of the OGD group (**p<0.01 vs. OGD group).
Discussion
In the in vivo section of this study, we show that a single HBO-PC (2.5 ATA, 90 min) plays a significant neuroprotective role against SCIR injury. HBO-PC significantly improved hindlimb motor function missing and secondary spinal cord edema in the acute stage after SCIR injury. HBO-PC significantly ameliorated the reactivity of the spinal cord MEPs and inhibited or slowed down the process of apoptosis after SCIR injury in the recovery stage after SCIR injury.
To explore the mechanism of neuroprotection induced by HBO-PC, we measured the mRNA and protein expression levels of Nrf2 and its target genes, such as GCL, γGT, MRP1 (key proteins for intracellular GSH synthesis and transit), which were all enhanced in normal mouse spinal cord after a single administration of HBO-PC. Nrf2 activation confers neuroprotection in various models of neurological diseases by the regulation of multiple downstream genes with detoxification, antioxidant, and anti-inflammatory capacity.13, 25 It is certain that the coordinated expression of these genes plays an important role in cytoprotection, but one group of enzymes involved in GSH synthesis and utilization, such as GCL, γGT, and MRP1, is particularly important for the prevention of oxidative-mediated damage in the CNS.
GSH is the most abundant mammalian thiol-containing antioxidant and synthesized in two consecutive steps.26 In the first step, GSH synthesis is rate-limiting and catalyzed by GCL; in the second step, GSH is formed by γGC binding to glycine. Nrf2 also regulates the expression of cysteine–glutamate exchange transporter, such as MRP1, that maintains intracellular GSH levels by regulating cysteine influx.27–29 In this study, as we expected, HBO-PC significantly increased the GSH content of spinal cord tissue at all time points after SCIR injury, which enhanced total antioxidant capacity.
The findings suggest that increased Nrf2 activation induced by HBO-PC in the spinal cord plays an important role in protecting against SCIR injury. In addition, the results of double-immunofluorescence staining also suggest that Nrf2 overexpression induced by HBO-PC mainly occurs in astrocytes, which may exert beneficial effects against subsequent SCI injury. Moreover, we found that HBO-PC induced a significant enhancement of Nrf2 expression in astrocytes of the spinal cord by means of double-immunofluorescence staining with a conventional wide-field fluorescence microscope rather than a confocal fluorescence microscopy. Compared with the previous study,30 we did not provide the position of Nrf2. So we further investigated the Nrf2 DNA-binding activity and downstream gene expression in the spinal cord with HBO-PC, that indirectly verified the significant enhancement of Nrf2 transcriptional activity.
In the in vitro section of this study, we show that a single HBO-PC (2.5 ATA, 90 min) plays a significant neuroprotective role in co-culture (mixed spinal neuron/astrocyte) system against OGD injury. The mRNA and protein expression of Nrf2 and its target genes (GCL, γGT, MRP1), were all increased significantly in astrocytes, but not in neurons. The CNS is normally equipped with antioxidant activity to prevent high ROS mediated damage, but neurons express them at a very low activity.31–35 Therefore, it is imperative that neurons co-operate with neighboring astrocytes for a complete cycle of ROS detoxification and neuroprotection.
In our study, HBO-PC induced a slight elevation of Nrf2 mRNA and protein levels in neurons, but there is no statistically significant difference. So the HBO-PC induced effects in Nrf2 signaling mainly take place in astrocytes, which exerted neuroprotection against consequent ischemia-reperfusion injury. Because spinal cord injury induces oxidative stress that contributes to progression of secondary injury pathomechanisms, activation of the Nrf2/ARE pathway may reduce oxidative stress leading to neuroprotection.30
Many studies elegantly highlight the importance of astrocytic Nrf2 for neuronal protection against bioenergetic and oxidative stress-mediated damage. It remains unclear, however, whether neurons necessarily require astrocyte antioxidant protection, even though the vast majority of Nrf2 activation seems to occur in astrocytes.36,37 Moreover, the GSH concentration of co-culture medium increased, which was mainly released from astrocytes. These data are consistent with the results of the in vivo experiment, thereby further supporting our deduction that Nrf2 activation in astrocytes may mediate the spinal cord ischemic tolerance induced by a single HBO-PC.
As the principal housekeeping cells of the nervous system, astrocytes play an integral role in the CNS by providing metabolic intermediates to neurons, modulating synaptic activity, and regulating blood flow in response to neuronal activity.38,39 Moreover, astrocytes are critically important for normal nervous function because of their ability to release neuroprotective factors. Transcription factor represents a critical aspect of adaptive cellular events that allow cells' adaptation to environmental changes and facilitate an array of specific downstream gene expression. Nrf2 is an integral transcription factor that facilitates astrocytic neuroprotection by promoting the expression of genes involved in GSH synthesis and release in astrocytes.13
Because we used the HBO-PC protocol, clinical hyperbaric oxygen protocols are relatively brief, and internal antioxidant defenses are adequate so that biochemical stresses are reversible.40 With the increased ROS induced by HBO-PC, a critical cysteine residue in Keap1 is oxidized, the Nrf2 is released, which promotes the nuclear transcription of a battery of antioxidant enzymes. Nrf2 target genes are preferentially activated in astrocytes, which consequently have more efficient detoxification and antioxidant defenses than neurons.41 The coordinated expression of these enzymes, such as GCL, MRP1, and γGT, allows higher levels of GSH biosynthesis, release, and extracellular cleavage into Cys-Gly. Then neurons take up Cys-Gly in the form of cysteine and glycine. More importantly, astrocytes supply glutamine to neurons, where it is transformed into glutamate.
Thus, the de novo neuronal biosynthesis of GSH depends on the supply of GSH precursors from astrocytes.15 Understanding the adaptive responses that astrocytes undergo in response to acute stress or neurodegenerative conditions will contribute to the development of novel therapies. Our finding indicates that HBO-PC is an effective protocol to prevent the ischemia/reperfusion injury of the CNS.
It is clear that Nrf2 activation represents an exceptional defense mechanism for many cell types. Particularly in the CNS, astrocytic Nrf2 activation has a major role in protecting neurons from noxious stimuli. In our study, we try to verify the possibility that Nrf2 activation in astrocytes may mediate the spinal cord ischemic tolerance induced by a single episode of HBO-PC. Therefore, HBO-PC has potential as a safe and feasible method to provide neuroprotective benefits in aortic surgery. In the future, we will develop experimental tools to manipulate and monitor dynamic changes in the supportive function of astrocytes to define their roles in neuroprotection induced by HBO-PC.
The present study does have limitations. We cannot determine that the neuroprotective effect of HBO-PC in vivo is entirely because of Nrf2 activation in astrocytes, to some extent, which may come from other glial cell populations. Future studies will better elucidate the precise relationship between Nrf2 activation and HBO-PC induced protection. We cannot find a method to specifically inhibit the astrocytic Nrf2 activation, but we can improve the cell culture experimental design. For example, the cell types of co-culture should be added, such as other glial cell populations, and the cell source should be considered to use the Nrf2 knockout mice. Through different combinations of co-cultures, it will be closer to the precise mechanism underlying HBO-PC-induced protection in SCIR model.
Acknowledgment
This work was supported by grants from National Natural Science Foundation of China (No. 81171873) and Youth Foundation of Second Military Medical University (No. 2010QN08).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Svensson L.G., Crawford E.S., Hess K.R., Coselli J.S., and Safi H.J. (1993). Experience with 1509 patients undergoing thoracoabdominal aortic operations. J. Vasc. Surg. 8, 357–370 [PubMed] [Google Scholar]
- 2.Wan I.Y., Angelini G.D., Bryan A.J., Ryder I., and Underwood M.J. (2001). Prevention of spinal cord ischaemia during descending thoracic and thoracoabdominal aortic surgery. Eur. J. Cardiothorac. Surg. 19, 203–213 [DOI] [PubMed] [Google Scholar]
- 3.Conrad M.F., Ergul E.A., Patel V.I., Cambria M.R., Lamuraglia G.M., Simon M., and Cambria R.P. (2011). Evolution of operative strategies in open thoracoabdominal aneurysm repair. J. Vasc. Surg. 53, 1195–1201 [DOI] [PubMed] [Google Scholar]
- 4.Fehrenbacher J.W., Siderys H., Terry C., Kuhn J., and Corvera J.S. (2010). Early and late results of descending thoracic and thoracoabdominal aortic aneurysm open repair with deep hypothermia and circulatory arrest. J. Thorac. Cardiovasc. Surg. 140, Suppl 6, 154–160 [DOI] [PubMed] [Google Scholar]
- 5.Acher C.W., and Wynn M. (2009). A modern theory of paraplegia in the treatment of aneurysms of the thoracoabdominal aorta: an analysis of technique specific observed/expected ratios for paralysis. J. Vasc. Surg. 49, 1117–1124 [DOI] [PubMed] [Google Scholar]
- 6.Smith P.D., Puskas F., Meng X., Lee J.H., Cleveland J.C., Jr., Weyant M.J., Fullerton D.A., and Reece T.B. (2012). The evolution of chemokine release supports a bimodal mechanism of spinal cord ischemia and reperfusion injury. Circulation 126, Suppl 1, S110–S117 [DOI] [PubMed] [Google Scholar]
- 7.Gu G.J., Li Y.P., Peng Z.Y., Xu J.J., Kang Z.M., Xu W.G., Tao H.Y., Ostrowski R.P., Zhang J.H., and Sun X.J. (2008). Mechanism of ischemic tolerance induced by hyperbaric oxygen preconditioning involves upregulation of hypoxia-inducible factor-1alpha and erythropoietin in rats. J. Appl. Physiol. 104, 1185–1191 [DOI] [PubMed] [Google Scholar]
- 8.Wang R., Xu J., Xie J., Kang Z., Sun X., Chen N., Liu L., and Xu J. (2010). Hyperbaric oxygen preconditioning promotes survival of retinal ganglion cells in a rat model of optic nerve crush. J. Neurotrauma 27, 763–770 [DOI] [PubMed] [Google Scholar]
- 9.Li Y., Dong H., Chen M., Liu J., Yang L., Chen S., and Xiong L. (2011). Preconditioning with repeated hyperbaric oxygen induces myocardial and cerebral protection in patients undergoing coronary artery bypass graft surgery: a prospective, randomized, controlled clinical trial. J. Cardiothorac. Vasc. Anesth. 25, 908–916 [DOI] [PubMed] [Google Scholar]
- 10.Li Q., Li J., Zhang L., Wang B., and Xiong L. (2007). Preconditioning with hyperbaric oxygen induces tolerance against oxidative injury via increased expression of heme oxygenase-1 in primary cultured spinal cord neurons. Life Sci. 80, 1087–1093 [DOI] [PubMed] [Google Scholar]
- 11.Nie H., Xiong L., Lao N., Chen S., Xu N., and Zhu Z. (2006). Hyperbaric oxygen preconditioning induces tolerance against spinal cord ischemia by upregulation of antioxidant enzymes in rabbits. J. Cereb. Blood Flow Metab. 26, 666–674 [DOI] [PubMed] [Google Scholar]
- 12.Kaspar J.W., Niture S.K., and Jaiswal A.K. (2009). Nrf2: INrf2 (Keap1) signaling in oxidative stress. Free Radic. Biol. Med. 47, 1304–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shih A.Y., Johnson D.A., Wong G., Kraft A.D., Jiang L., Erb H., Johnson J.A., and Murphy T.H. (2003). Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. J. Neurosci. 23, 3394–3406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vargas M.R., and Johnson J.A. (2009). The Nrf2-ARE cytoprotective pathway in astrocytes. Expert. Rev. Mol. Med. 11, e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernandez-Fernandez S., Almeida A., and Bolaños J.P. (2012). Antioxidant and bioenergetic coupling between neurons and astrocytes. Biochem. J. 443, 3–11 [DOI] [PubMed] [Google Scholar]
- 16.Lang-Lazdunski L., Matsushita K., Hirt L., Waeber C., Vonsattel J.P., Moskowitz M.A., and Dietrich W.D. (2000). Spinal cord ischemia: Development of a model in the mouse. Stroke 31, 208–213 [DOI] [PubMed] [Google Scholar]
- 17.Basso D.M., Fisher L.C., Anderson A.J., Jakeman L.B., McTigue D.M., and Popovich P.G. (2006). Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J. Neurotrauma 23, 635–659 [DOI] [PubMed] [Google Scholar]
- 18.Saadoun S., Bell B.A., Verkman A.S., and Papadopoulos M.C. (2008). Greatly improved neurological outcome after spinal cord compression injury in AQP4-deficient mice. Brain 131, 1087–1098 [DOI] [PubMed] [Google Scholar]
- 19.Meylaerts S.A., De Haan P., Kalkman C.J., Jaspers J., Vanicky I., and Jacobs M.J. (2000). Prevention of paraplegia in pigs by selective segmental artery perfusion during aortic cross-clamping. J. Vasc. Surg. 32, 160–170 [DOI] [PubMed] [Google Scholar]
- 20.Simon F., Scheuerle A., Calzia E., Bassi G., Oter S., Duy C.N., Kick J., Brückner U.B., Radermacher P., and Schelzig H. (2008). Erythropoietin during porcine aortic balloon occlusion-induced ischemia/reperfusion injury. Crit. Care. Med. 36, 2143–2150 [DOI] [PubMed] [Google Scholar]
- 21.Cassina P., Peluffo H., Pehar M., Martinez-Palma L., Ressia A., Beckman J.S., Estévez A.G., and Barbeito L. (2002). Peroxynitrite triggers a phenotypic transformation in spinal cord astrocytes that induces motor neuron apoptosis. J. Neurosci. Res. 67, 21–29 [DOI] [PubMed] [Google Scholar]
- 22.Banker G., and Goslin K. (1998). Culturing Nerve Cells, 2nd ed. MIT Press: Cambridge, Mass [Google Scholar]
- 23.Zhan R.Z., Qi S., Wu C., Fujihara H., Taga K., and Shimoji K. (2001). Intravenous anesthetics differentially reduce neurotransmission damage caused by oxygen-glucose deprivation in rat hippocampal slices in correlation with N-methyl-D-aspartate receptor inhibition. Crit. Care Med. 29, 808–813 [DOI] [PubMed] [Google Scholar]
- 24.Furuichi T., Liu W., Shi H., Miyake M., and Liu K.J. (2005). Generation of hydrogen peroxide during brief oxygen-glucose deprivation induces preconditioning neuronal protection in primary cultured neurons. J. Neurosci. Res. 79, 816–824 [DOI] [PubMed] [Google Scholar]
- 25.Vargas M.R., Johnson D.A., Sirkis D.W., Messing A., and Johnson J.A. (2008). Nrf2 activation in astrocytes protects against neurodegeneration in mouse models of familial amyotrophic lateral sclerosis. J. Neurosci. 28, 13574–13581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dringen R. (2000). Metabolism and functions of glutathione in brain. Prog. Neurobiol. 62, 649–671 [DOI] [PubMed] [Google Scholar]
- 27.Hayashi A., Suzuki H., Itoh K., Yamamoto M., and Sugiyama Y. (2003). Transcription factor Nrf2 is required for the constitutive and inducible expression of multidrug resistance-associated protein 1 in mouse embryo fibroblasts. Biochem. Biophys. Res. Commun. 310, 824–829 [DOI] [PubMed] [Google Scholar]
- 28.Sasaki H., Sato H., Kuriyama-Matsumura K., Sato K., Maebara K., Wang H., Tamba M., Itoh K., Yamamoto M., and Bannai S. (2002). Electrophile response element-mediated induction of the cystine/glutamate exchange transporter gene expression. J. Biol. Chem. 277, 44765–44771 [DOI] [PubMed] [Google Scholar]
- 29.Shih A.Y., Erb H., Sun X., Toda S., Kalivas P.W., and Murphy T.H. (2006). Cystine/glutamate exchange modulates glutathione supply for neuroprotection from oxidative stress and cell proliferation. J. Neurosci. 26, 10514–10523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X., de Rivero Vaccari J.P., Wang H., Diaz P., German R., Marcillo A.E., and Keane R.W. (2012). Activation of the nuclear factor E2-related factor 2/antioxidant response element pathway is neuroprotective after spinal cord injury. J. Neurotrauma 29, 936–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raps S.P., Lai J.C., Hertz L., and Cooper A.J. (1989). Glutathione is present in high concentration in cultured astrocytes but not in cultured neurons. Brain. Res. 493, 398–401 [DOI] [PubMed] [Google Scholar]
- 32.Sagara J., Miura K., and Bannai S. (1993). Maintenance of neuronal glutathione by glial cells. J. Neurochem. 61, 1672–1676 [DOI] [PubMed] [Google Scholar]
- 33.Makar T.K., Nedergaard M., Preuss A., Gelbard A.S., Perumal A.S., and Cooper A. J. (1994). Vitamin E, ascorbate, glutathione, glutathione disulfide, and enzymes of glutathione metabolism in cultures of chick astrocytes and neurons: evidence that astrocytes play an important role in antioxidative processes in the brain. J. Neurochem. 62, 45–53 [DOI] [PubMed] [Google Scholar]
- 34.Bolanos J.P., Heales S.J., Land J.M., and Clark J.B. (1995). Effect of peroxynitrite on the mitochondrial respiratory chain: differential susceptibility of neurones and astrocytes in primary culture. J. Neurochem. 64, 1965–1972 [DOI] [PubMed] [Google Scholar]
- 35.Dringen R., Kussmaul L., Gutterer J.M., Hirrlinger J., and Hamprecht B. (1999). The glutathione system of peroxide detoxification is less efficient in neurons than in astrocytes. J. Neurochem. 72, 2523–2530 [DOI] [PubMed] [Google Scholar]
- 36.Calkins M.J., Johnson D.A., Townsend J.A., Vargas M.R., Dowell J.A., Williamson T.P., Kraft A.D., Lee J.M., Li J., and Johnson J.A. (2009). The Nrf2/ARE pathway as a potential therapeutic target in neurodegenerative disease. Antioxid. Redox Signaling 11, 497–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson D.A., Andrews G.K., Xu W., and Johnson J.A. (2002). Activation of the antioxidant response element in primary cortical neuronal cultures derived from transgenic reporter mice. J. Neurochem. 81, 1233–1241 [DOI] [PubMed] [Google Scholar]
- 38.Barres B.A. (2008). The mystery and magic of glia: a perspective on their roles in health and disease. Neuron 60, 430–440 [DOI] [PubMed] [Google Scholar]
- 39.Sofroniew M.V., and Vinters H.V. (2010). Astrocytes: biology and pathology. Acta Neuropathol. 119, 7–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thom S.R. (2011). Hyperbaric oxygen: its mechanisms and efficacy. Plast. Reconstr. Surg. 127, Suppl 1, 131S–141S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Y., and Swanson R.A. (2003). Astrocytes and brain injury. J. Cereb. Blood Flow Metab. 23, 137–149 [DOI] [PubMed] [Google Scholar]