Abstract
Mobilization of hematopoietic stem and progenitor cells (HPCs) is induced by treatment with granulocyte-colony stimulating factor, chemotherapy, or irradiation. We observed that these treatments are accompanied by a release of chemotactic activity into the blood. This plasma activity is derived from the bone marrow, liver, and spleen and acts on HPCs via the chemokine receptor CXCR4. A human blood peptide library was used to characterize CXCR4-activating compounds. We identified CXCL12[22–88] and N-terminally truncated variants CXCL12[24–88], CXCL12[25–88], CXCL12[27–88], and CXCL12[29–88]. Only CXCL12[22–88] could effectively bind to CXCR4 and induce intracellular calcium flux and chemotactic migration of HPCs. CXCL12[25–88] and CXCL12[27–88] revealed neither agonistic nor antagonistic activities in vitro, whereas CXCL12[29–88] inhibited CXCL12[22–88]-induced chemotactic migration. Since binding to glycosaminoglycans (GAG) modulates the function of CXCL12, binding to heparin was analyzed. Surface plasmon resonance kinetic analysis showed that N-terminal truncation of Arg22-Pro23 increased the dissociation constant KD by one log10 stage ([22–88]: KD: 5.4±2.6 μM; [24–88]: KD: 54±22.4 μM). Further truncation of the N-terminus decreased the KD ([25–88] KD: 30±4.8 μM; [27–88] KD: 23±1.6 μM; [29–88] KD: 19±5.4 μM), indicating increasing competition for heparin binding. Systemic in vivo application of CXCL12[22–88] as well as CXCL12[27–88] or CXCL12[29–88] induced a significant mobilization of HPCs in mice. Our findings indicate that plasma-derived CXCL12 variants may contribute to the regulation of HPC mobilization by modulating the binding of CXCL12[22–88] to GAGs rather than blocking the CXCR4 receptor and, therefore, may have a contributing role in HPC mobilization.
Introduction
Hematopoietic stem and progenitor cells (HPCs) are a rare population of mainly quiescent cells that self-renew and differentiate into all mature blood cell types to continually reconstitute the hematopoietic and immune systems over an organism's entire lifespan. The ability to mobilize hematopoietic stem cells into the blood is clinically exploited for stem cell apheresis and mobilized peripheral blood transplantation. HPC mobilization is co-regulated by a wide range of stressors, including DNA damage, chemotherapeutic drugs, cytokines, and chemokines such as CXCL8 [interleukine-8 (IL-8)], CXCL1 [growth-regulated oncogene alpha (GROα)], and CXCL12 [stromal-derived factor-1α (SDF-1α)]. These cytokines and chemokines, along with bioactive lipids such as sphingosine-1-phosphate and ceramide-1-phosphate, together with complement factors may also have a significant impact on the homing of circulating and transplanted HPCs and their engraftment in the bone marrow (BM) [1–3].
Stem cell mobilization has been correlated with the disruption of adhesive interactions between HPCs and the BM microenvironment. Mobilization within the BM is affected by the proteolytic degradation of vascular cell adhesion molecule-1 (VCAM-1) and CXCL12 by neutrophil proteases and is effected through the shedding of membrane-bound stem cell factor (SCF) by matrix metalloproteinase 9 (MMP9). The regulation of HPC mobilization and engraftment is dependent on interactions between the HPC ligands VLA-4, α4β7, PSGL-1, and various endothelial adhesion molecules (eg, VCAM-1, MAdCAM-1, P-selectin, and E-selectin). VLA-4 and VCAM-1 have been found to play a major role in hematopoietic progenitor cell homing to the BM, whereas the E- and P-selectins support VLA-4/VCAM-1-mediated homing. Another essential regulator for stem cell targeting to the BM is CD44 and its major receptor, hyaluronic acid [4,5].
CXCL12 and its receptor CXCR4 enable HPCs to migrate along a CXCL12 gradient and activate adhesion of HPCs via VLA-4 and CD44 [5]. CXCL12 is constitutively expressed by human BM endothelial and stromal cells and has been shown to induce arrest of HPCs to the endothelium. Granulocyte-colony stimulating factor (G-CSF) or irradiation regulates the hematopoietic stem cell niche by altering the local CXCL12 concentration by induction of proteolytic degradation and altered expression of CXCL12 in osteoblasts [1]. Blocking or deficiency of CXCR4 or CXCL12 reduces G-CSF-induced mobilization, demonstrating an active role for CXCL12/CXCR4 in mobilization of progenitors [6]. Presentation of CXCL12 to HPCs by endothelial cells is mediated by glycosaminoglycans (GAGs) that are covalently attached to proteoglycans. Chemokines are sequestered by GAGs, causing increased oligomerization, increased local concentrations, and, in turn, resulting in the formation of a chemokine gradient [7,8].
To date, little is known about the biological activity of CXCL12 in blood plasma. Here, we show that the level of functionally active CXCL12 increases during stem cell mobilization and transplantation. We demonstrate that CXCL12 is present in blood plasma by isolating native CXCL12 from a blood plasma filtrate. Furthermore, the co-existence of functionally active and N-terminally truncated CXCL12 variants in blood plasma suggests that the activation status of CXCL12 in blood is subject to regulation by proteases, as derived from thrombocytes and granulocytes. Our results show that the identified N-terminally truncated CXCL12 variants do not bind to the CXCR4 receptor but may modulate the biological properties of active CXCL12 by competing for GAG binding sites.
Materials and Methods
Chemicals, blood samples, and cells
Recombinant mIL-3, rhIL-6, and rhCXCL12[22–89] were purchased from R&D Systems (Minneapolis, MN). Purified anti-human CXCR4 antibody (clone 12G5) was purchased from Pharmingen (Heidelberg, Germany).
Informed consent for participation in the study was obtained from patients undergoing autologous HPC mobilization via G-CSF and/or transplantation, according to the Helsinki Declaration and a procedure approved by the local ethics committee. Peripheral blood samples were obtained from these patients at the time of leukapheresis or approximately 28 days after stem cell transplantation. Samples were anticoagulated in preservative-free heparin (5,000 U/mL). Plasma was separated by centrifugation at 3,000 g for 15 min and used within 6 h or stored at −30°C or less.
FDCP-mix multipotent murine hematopoietic progenitor cells (clone A4) were obtained from C. Heyworth and E. Spooner (Christie Hospital, Paterson Institute, Manchester, United Kingdom) and maintained in Iscove's modified Dulbecco's medium (IMDM) that was supplemented with 20% (vol/vol) horse serum (HS) and 10 ng/mL rmIL-3 (R&D Systems) at 37°C in 5% CO2. The cells were passaged thrice a week to a cell concentration of 2×105 cells/mL and maintained at 37°C/5% CO2. Human primary CD34+ HPCs were isolated from leukapheresis products after HPC mobilization with G-CSF and/or chemotherapy. Mononuclear cells were isolated by density gradient centrifugation in a Ficoll gradient (density 1.077 g/mL; Biochrom KG, Berlin, Germany). CD34+ cells were isolated from mononuclear cells using magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions. The CD34+ cell preparations were >95% viable as determined by flow cytometric analysis of propidium iodide-stained cells on an FACScan. The purity of the CD34+ progenitor cells was assessed by flow cytometry using fluorescein-conjugated monoclonal HPCA-2 anti-CD34 antibodies (Becton Dickinson, Rödermark, Germany) and was found to be 70%–95%. Murine BM Lin− HPCs were prepared by negative selection from murine BM cells that were incubated with rat anti-mouse antibodies for B220 (MI/70, IgG 2b), MAC-1, Gr-1, TER119, CD8, and CD4. Lin− cells were separated using MicroBead columns as described by the manufacturer (Miltenyi Biotec). Human embryonic kidney (HEK) 293T cells (German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany) were cultured and transfected as previously described [9]. Briefly, HEK293T cells were grown at 37°C/5% CO2 in Dulbecco's modified Eagle's medium (DMEM) that was supplemented with 10% fetal bovine serum, penicillin, and streptomycin, and cells were transiently transfected with cDNA encoding CXCR4 (pcDNA3-CXCR4) using linear polyethyleneimine (Mw 25,000; Polysciences, Warrington, PA).
Chemotaxis assay and intracellular Ca2+ measurements
Chemotaxis was assessed in 48-well or 96-well Boyden chambers (Neuroprobe, Cabin John, MD) via polyvinylpyrrolidone-free polycarbonate membranes (Nucleopore; Neuroprobe) with 5-μm pores for 2×104 CD34+ cells or for 1×105 FDCP-mix hematopoietic progenitor cells, respectively. Before the chemotaxis assay, factor-dependent-cell-Paterson (FDCP)-mix cells were washed and cultured in IMDM without HS and rmIL-3 for 4 h. Chemokines, plasma, or peptide fractions were diluted in IMDM and added to the lower wells of the Boyden chambers. Cells were seeded above the membrane and allowed to migrate for 16 to 18 h. Migrated cells were detected by DNA staining with CyQuant cell proliferation assay dye (Molecular Probes, Inc., Eugene, OR). To measure intracellular Ca2+ flux, CXCR4-transfected HEK293T cells were seeded in 96-well black plates (Costar, High Wycombe, United Kingdom) at 200,000 cells/well and cultured overnight. After 30 min at 37°C in loading medium (Hepes-dissolved HBSS, pH 7.4, containing 2.5 mM probenecid and 1 μM Fluo-4 AM; Molecular Probes, Leiden, The Netherlands), cells were washed in loading medium without Fluo-4 AM. The cells were placed in the FLIPR system (Molecular Devices, Munich, Germany), and basal fluorescence was determined at room temperature ( ex=488 nm,
ex=488 nm,  em=540 nm). Changes in cellular fluorescence were recorded online after the addition of 50 μL test compounds that were diluted in wash buffer.
em=540 nm). Changes in cellular fluorescence were recorded online after the addition of 50 μL test compounds that were diluted in wash buffer.
Irradiation and BM transplantation in mice
DBA/2 or BDF1 mice (8–12 weeks old) were obtained from Charles River Laboratories (Sulzfeld, Germany) and housed in standard cages under standard conditions. Experiments were performed with permission from the local animal experimentation supervisory committee. Mice were irradiated with a lethal dose of γ-irradiation (two doses of 5.5 Gy, 6 h apart). After 24 h, 4–5×106 BM Lin− cells from nonirradiated mice were injected via i.v.; for some experiments, the injected cells were retrieved from the lower wells of transmigration chambers. For preparation of organ-conditioned media, mice were killed by CO2 asphyxia at various time intervals after irradiation and BM transplantation. BM and spleen cell suspensions were prepared by flushing femurs or spleens with IMDM. The liver and lungs were cut into slices using razor blades that were fixed especially for this purpose at a distance of 1 mm. BM cells (1×106 cells/mL) and tissues were subsequently incubated in IMDM with 20% fetal calf serum (FCS) at 37°C/5% CO2. After 24 h of incubation, supernatants were collected, filtered through 0.45-μm pores, and stored at −80°C until use for chemotaxis assays.
Analysis of the chromatographic elution characteristics of chemotactic activity in mobilized plasma
Aliquots (20 mL) of blood plasma from patients receiving chemotherapy and G-CSF treatment were subjected to a preparative reverse-phase (RP)-C18 chromatography using a Bakerbond PrepPak RP-C18 cartridge [300×47 mm inner diameter (i.d.), 15–30 μm, 300 Å; Waters, Milford, MA]. Separation was performed at a flow rate of 40 mL/min using a linear binary gradient of 100% solvent A (10 mM HCl) to 60% solvent B (10 mM HCl in 80% acetonitrile) in 50 min and from 60% solvent B to 100% solvent B in 2.5 min. Fractions of 50 mL were collected. Chemotactic activity was assayed by subjecting 0.4 mL plasma equivalents to the chemotaxis chamber. CXCL12 levels in chromatographic fractions with chemotactic activity were analyzed by an enzyme-linked immunosorbent assay (ELISA) using antibodies and protocols from R&D Systems.
Preparation of a peptide library
A 10,000 l volume of human hemofiltrate (HF) for large-scale recovery of plasma peptides was obtained from patients with renal failure. The ultrafilters used for hemofiltration had a specified molecular mass cut-off of 50 kDa. On collection, the sterile filtrate was immediately cooled to 4°C and acidified to pH 3 to prevent bacterial growth and proteolysis. Subsequently, the filtrate was conditioned to pH 2.7 and applied onto a strong cation exchanger (Fractogel TSK SP 650(M), 100×250 mm; Merck, Darmstadt, Germany) using an Autopilot chromatography system (PerSeptive Biosystems, Wiesbaden, Germany). Bound peptides were eluted using seven buffers of increasing pH, resulting in seven pH pools. The seven buffers were composed as follows: I: 0.1 M citric acid monohydrate, pH 3.6; II: 0.1 M acetic acid+0.1 M sodium acetate, pH 4.5; III: 0.1 M malic acid, pH 5.0; IV: 0.1 M succinic acid, pH 5.6; V: 0.1 M sodium dihydrogen phosphate, pH 6.6; VI: 0.1 M disodium hydrogen phosphate, pH 7.4; and VII: 0.1 M ammonium carbonate, pH 9.0. The seven pools (pH pools) were collected, individually loaded onto an RP column (125×100 mm i.d., Source RPC, 15 μm; Pharmacia), and eluted in a gradient of 100% solvent A (0.01 M HCl in water) to 60% solvent B (0.01 M HCl in 80% acetonitrile). Fractions of 200 mL were collected. The final peptide library contained approximately 500 different fractions. Aliquots of each fraction were used for the biological tests.
Purification of chemotactic activity and mass spectroscopic analysis
Fractions were screened for intracellular Ca2+ flux induction in CXCR4-transfected HEK293T cells and for chemotactic activation of FDCP-mix cells. The identified activity was further purified to homogeneity by four consecutive chromatographic steps.
Matrix-assisted laser desorption/ionization–mass spectrometry (MALDI-MS) was performed using a LaserTec RBT MALDI-MS (PerSeptive/Vestec, Houston, TX). Isolated material was applied to a stainless steel multiple sample tray as an admixture with sinapinic acid using the dried drop technique [10]. Measurements were performed in linear mode. The instrument was equipped with a 1.2 m flight tube and a 337 nm nitrogen laser. Positive ions were accelerated at 30 kV, and 64 laser shots were automatically accumulated per sample position. The time-of-flight data were externally calibrated for each sample plate and sample preparation. Data acquisition and analysis were performed using the GRAMS 386 software (version 3.04) supplied by the manufacturer. Sequence analyses of the isolated peptides were performed by stepwise Edman degradation using a gas-phase automated sequencer (Model 473 A; Applied Biosystems, Inc., Weiterstadt, Germany). The resulting PTH-amino acids were identified by integrated high-pressure liquid chromatography (HPLC). High-resolution mass spectrometry was performed by linear ion trap Fourier transform ion cyclotron resonance mass spectrometry (LTQ-FT-ICR-MS/MS; Proteome Factory AG, Berlin, Germany). A 2-μL aliquot of each acidified peptide fraction in 50% acetonitrile was applied to the MS equipment in offline mode via an Advion NanoMate 100 chip-electrospray system (Advion, Ithaca, NY), and detection was performed on a Finnigan LTQ-FT mass spectrometer (ThermoFisher, Schwerte, Germany) that was equipped with a 6T magnet. MS overview spectra were taken in FT mode according to the manufacturer's instrument settings for offline mode with a mass tolerance of 3 ppm. Peptide fragmentation and detection were accomplished in the instrument's LTQ ion trap.
Synthesis of CXCL12 variants
CXCL12 molecules were prepared by solid-phase peptide synthesis as previously described [10]. The purified products were characterized by HPLC, capillary zone electrophoresis, electrospray mass spectrometry, and sequence analysis. The net peptide content was determined by amino-acid analysis.
Processing of full-length CXCL12[22–89] by blood cells
Peripheral blood mononuclear cells (PBMCs), polymorphonuclear neutrophils (PMNs), and thrombocytes were isolated according to established methods [10]. PBMCs and PMNs were isolated from 30 mL venous blood mixed with 10 mL hydroxyethyl starch (Plasmasteril; Fresenius, Bad Homburg, Germany). Erythrocytes were sedimented for 45 min at RT. The supernatant was centrifuged at 200 g for 20 min. The cell pellet was resuspended in 20 mL FCS-free RPMI 1640, layered onto 15 mL of Ficoll–Paque (Pharmacia Biotech, Uppsala, Sweden), and centrifuged at 1,000 g for 15 min at RT with no braking to slow the centrifuge. PBMCs were taken from the interface, diluted with two volumes of RPMI 1640, and centrifuged at 200 g for 20 min. The cell pellet was resuspended in RPMI 1640 to a concentration of 1×107 cells/mL. PMNs were isolated from the cell pellet of the Ficoll–Paque gradient. The pellet was resuspended in RPMI 1640 and centrifuged at 200 g for 20 min. Erythrocytes were lysed by resuspending the pellet in 24 mL of double-distilled water for 30 s, followed by the addition of 8 mL of 3.6% NaCl solution. Cells were centrifuged at 500 g for 8 min and resuspended in FCS-free RPMI 1640 medium at a concentration of 2×107 cells/mL. Platelets were isolated from 10 mL blood using sodium citrate as the anticoagulant. Platelet-rich plasma was obtained by centrifuging blood at 800 g for 5 min. The supernatant was diluted with 10% citrate-citric acid-dextrose solution containing 2.94 g/100 mL trisodium citrate, 2.10 g/100 mL citric acid, and 2.45 g/100 mL glucose. The platelet-rich plasma was subsequently centrifuged at 2,000 g for 12 min, the supernatant was discarded, and the platelet pellet was gently resuspended in FCS-free RPMI 1640 medium. For processing experiments, 100 μL of thrombocytes, 2×107 cells/mL of PBMCs, and 2×107 cells/mL of PMNs were incubated at 37°C with 20 μg of CXCL12[22–89] for 30 min. Subsequently, the cells were sedimented by centrifugation at 400 g for 5 min. The supernatant was withdrawn and acidified with 0.5 M acetic acid. Chromatography of the supernatants was performed on an RP-HPLC column [250×4 mm i.d., Source RPC 15; Pharmacia, Uppsala, Sweden]. CXCL12 variants were identified via its elution position in the RP-HPLC as well as by MALDI-MS and Edman sequencing.
Receptor binding studies
HEK293T cells transiently transfected with cDNA encoding CXCR4 or CXCR7 were harvested at 48 h after transfection. Membranes were prepared and bound with [125I]-CXCL12 (PerkinElmer Life and Analytical Sciences, Boston, MA) as previously described [9]. Briefly, membranes (10 μg) were incubated for 2 h at room temperature in 96-well plates in binding buffer (50 mM Hepes, pH 7.4, 1 mM CaCl2, 5 mM MgCl2, 100 mM NaCl, and 0.5% bovine serum albumin) in the presence of ∼50 pM of [125I]-CXCL12[22–89] and increasing concentrations of indicated CXCL12 peptides. Membranes were harvested via filtration with Unifilter GF/C plates (PerkinElmer Life and Analytical Sciences) that were pretreated with 1% polyethyleneimine and washed thrice with ice-cold wash buffer (50 mM Hepes, pH 7.4, 1 mM CaCl2, 5 mM MgCl2, and 500 mM NaCl). Radioactivity was assayed via liquid scintillation with a MicroBeta counter (PerkinElmer Life and Analytical Sciences).
Rate constants of CXCL12 variants binding to biotinylated low-molecular-weight heparin
The measurements were performed as described by Amara et al. using a Biacore™ X100 instrument (GE Healthcare, Buckinghamshire, United Kingdom) [11]. Briefly, low-molecular-weight heparin (enoxaparin, 80 mg/mL) was biotinylated using EZ-Link® Hydrazide Biotin following the manufacturer's instructions. After dialysis against PBS, biotinylated heparin was injected with running buffer (HBS-EP+) on the second cell of an SA Chip. Approximately 200 resonance units of material were immobilized. For each CXCL12 variant, six different concentrations (0–200 nM) were injected onto the chip for 3 min (at 30 μL/min) followed by a dissociation phase of 20 min. The sensor chip was regenerated with two pulses for 1 min with 1.5 M NaCl in HBS-EP+ buffer. Sensorgrams were normalized from the baseline signal, and kinetic constants were determined using Biacore X100 evaluation 2.0 software.
Mobilization of HPC by CXCL12 variants
The application of CXCL12 variants in Balb/C mice was performed retroorbitally. In all experiments, retro-orbital application was performed with a total volume of 100 μL of 0.9% NaCl, with or without 10 μg of CXCL12[22–88], and/or 25 μg of each N-terminally truncated CXCL12 variant. After 20 min, mice were sacrificed and blood was withdrawn by cardiac puncture. Peripheral blood mononuclear cells (MNCs) were isolated after centrifugation over a discontinuous gradient using Biocoll Separation Solution (Biochrom, Berlin, Germany). MNCs (105) were plated in triplicate in 1 mL of Methocult® GF for murine cells (M3434; Stem Cell Technologies, Grenoble, France) containing 50 ng/mL of recombinant murine SCF (Stem Cell Technologies), rmIL-3 (R&D Systems), 10 ng/mL of rhIL-6 (R&D Systems), and 3 U/mL of rh erythropoietin (Sandoz, Basel, Switzerland) in 35-mm suspension culture dishes (Nunc, Naperville, IL). Cultures were incubated at 37°C in 100% humidity and 5% CO2 for 7 days. Scoring was performed with an inverted microscope at 340× magnification on day 7.
Results
Identification and characterization of HPC chemotactic activity in human blood plasma
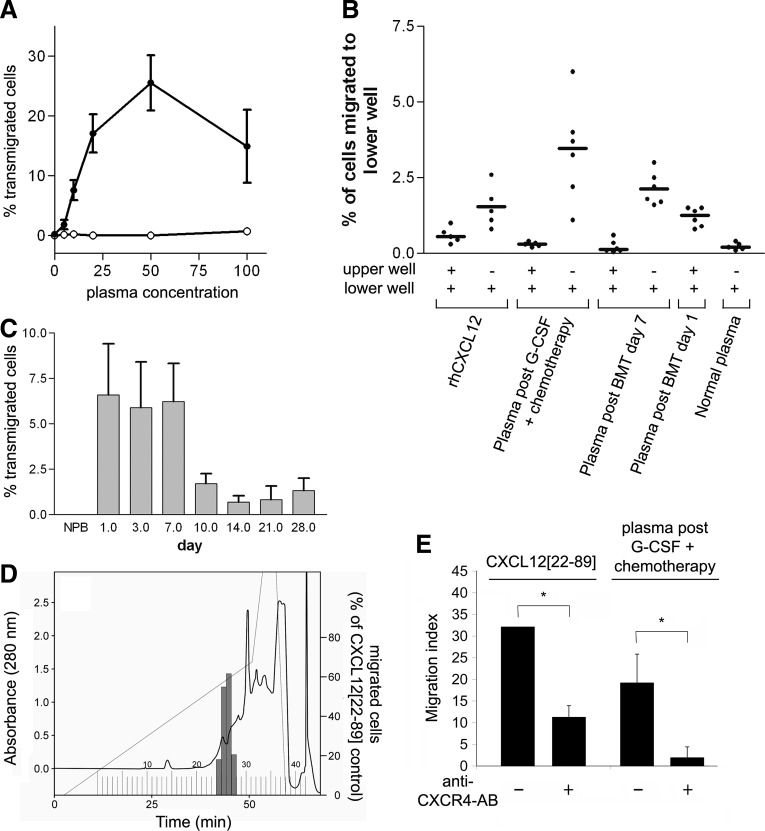
To identify potential chemotactic activity during HPC mobilization, plasma samples were obtained from patients who received G-CSF or high-dose chemotherapy combined with G-CSF concurrent with leukapheresis. Mobilized plasma induced a bell-shaped dose-response curve of FDCP-mix HPCs, typical for chemotactic substances. Maximum chemotactic activity was observed when 50% of mobilized blood plasma was mixed with 50% medium (Fig. 1A). Therefore, all further chemotaxis assays were performed with a concentration of 50% mobilized plasma. In these assays, plasma from normal individuals was inactive. The plasma also induced transwell migration of human CD34+ HPCs (Fig. 1B, C). The co-application of patient plasma and HPCs in the upper wells of the chemotaxis chamber did not induce migration of HPCs, indicating the predominantly chemotactic nature of the activity (Fig. 1B). In addition to analyzing blood plasma during the mobilization phase, we analyzed blood plasma from patients undergoing HPC transplantation immediately after an intensive conditioning regimen. As early as 1 day after transplantation, blood plasma exhibited chemotactic activity. This activity was present for the first 7 days after HPC transplantation, whereas this activity declined in the latter phase (Fig. 1C). Overall, 14/14 blood plasma samples from patients who received G-CSF plus chemotherapy for HPC mobilization, 6/10 blood plasma samples from voluntary stem cell donors who received G-CSF alone before leukapheresis, and 3/3 blood plasma samples from patients after autologous stem cell transplantation were found to contain HPC chemotactic activity.
FIG. 1.
Chemotactic activity for HPC in blood plasma. (A) Plasma from healthy individuals (○) or patients who received G-CSF and chemotherapy (•) was added to the lower wells of transwell chemotaxis chambers at different concentrations. Approximately 105 FDCP-mix hematopoietic progenitor cells were seeded into the upper wells. After 16 h, the number of cells that had migrated into the lower well was determined. The given values are the mean±SEM of three experiments performed with plasma from three different donors. (B) Plasma from patients treated with chemotherapy plus G-CSF or chemotherapy plus bone marrow transplantation was added into the lower well only or to both wells. Approximately 2×104 CD34+ cells were seeded into the upper wells of chemotaxis chambers. CXCL12[22–89] (100 ng/mL) was used as a control. Mean values are indicated by ( ), individual values by (•). (C) Chemotactic activity in plasma from patients treated with high-dose chemotherapy plus autologous stem cell transplantation was determined by the addition of 50% plasma from patients before or at different time points after transplantation to the lower wells. The given values are the mean±SEM of three experiments performed with plasma from different donors. (D) Identification of HPC chemotactic activity in chromatographic fractions obtained from mobilized plasma. Each 20 mL aliquot of patient plasma was subjected to preparative RP-C18 HPLC chromatography as described in the “Materials and Methods” section. Fractions of 50 mL were collected. The equivalent of 0.4 mL plasma was added to the lower wells of a chemotaxis chamber to determine the chemotactic activity. (E) The effect of functional blocking of the anti-CXCR4 receptor on the migration of CD34+ HPCs. Blood plasma was obtained from a patient on day 8 after mobilization with G-CSF/chemotherapy. CD34+ cells were preincubated with anti-CXCR4-AB (12G5, 2.5 μg/mL) for 30 to 60 min and then added to the chemotaxis chamber. The given values are the mean±SD; *P<0.05; Student's t-test. G-CSF, granulocyte-colony stimulating factor; HPC, hematopoietic stem and progenitor cells; HPLC, high-pressure liquid chromatography; RP, reverse phase; SEM, standard error of the mean; SD, standard deviation.
), individual values by (•). (C) Chemotactic activity in plasma from patients treated with high-dose chemotherapy plus autologous stem cell transplantation was determined by the addition of 50% plasma from patients before or at different time points after transplantation to the lower wells. The given values are the mean±SEM of three experiments performed with plasma from different donors. (D) Identification of HPC chemotactic activity in chromatographic fractions obtained from mobilized plasma. Each 20 mL aliquot of patient plasma was subjected to preparative RP-C18 HPLC chromatography as described in the “Materials and Methods” section. Fractions of 50 mL were collected. The equivalent of 0.4 mL plasma was added to the lower wells of a chemotaxis chamber to determine the chemotactic activity. (E) The effect of functional blocking of the anti-CXCR4 receptor on the migration of CD34+ HPCs. Blood plasma was obtained from a patient on day 8 after mobilization with G-CSF/chemotherapy. CD34+ cells were preincubated with anti-CXCR4-AB (12G5, 2.5 μg/mL) for 30 to 60 min and then added to the chemotaxis chamber. The given values are the mean±SD; *P<0.05; Student's t-test. G-CSF, granulocyte-colony stimulating factor; HPC, hematopoietic stem and progenitor cells; HPLC, high-pressure liquid chromatography; RP, reverse phase; SEM, standard error of the mean; SD, standard deviation.
Characterization of chemotactic activity and identification of tissue origin
To better characterize the nature of the chemotactic activity, blood plasma from two patients who received chemotherapy and G-CSF treatment was subjected to an RP HPLC column elution with an acetonitrile gradient. Chemotactic activity was present in two adjacent fractions, 26 and 27, eluting in a single peak, indicating that the HPC chemotactic activity was likely induced by a single compound (Fig. 1D). Subsequently, we determined by ELISA that the CXCL12 concentrations in fractions 26 and 27 were ∼50 ng/mL in the blood. For the detection of CXCL12 IR, a polyclonal IgG (AF-310-NA; R&D systems) immunized against CXCL12[22–89] was used, indicating that in addition to intact CXCL12[22–88] the N-terminally truncated CXCL12 variants were also detected. Furthermore, the chemotactic activity was efficiently neutralized by functional inhibition of CXCR4 using the anti-CXCR4 antibody 12G5, supporting the fact that chemotactic activity is via CXCL12 which specifically stimulates HPCs through the chemokine receptor CXCR4 (Fig. 1E). A murine HPC transplant model was established to investigate the tissue source of the chemotactic activity. We observed a time-dependent increase in HPC chemotactic activity after murine BM transplantation, similar to that seen in patients (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/scd). Supernatants were prepared from different mouse tissues at the time of maximal plasma chemotactic activity. The chemotactic activity for HPCs was significantly induced in the BM, liver, and spleen from mice after BM transplantation compared with conditioned media from tissues of untreated mice (Supplementary Table S1).
Purification of stem cell chemotactic activity in human blood filtrate
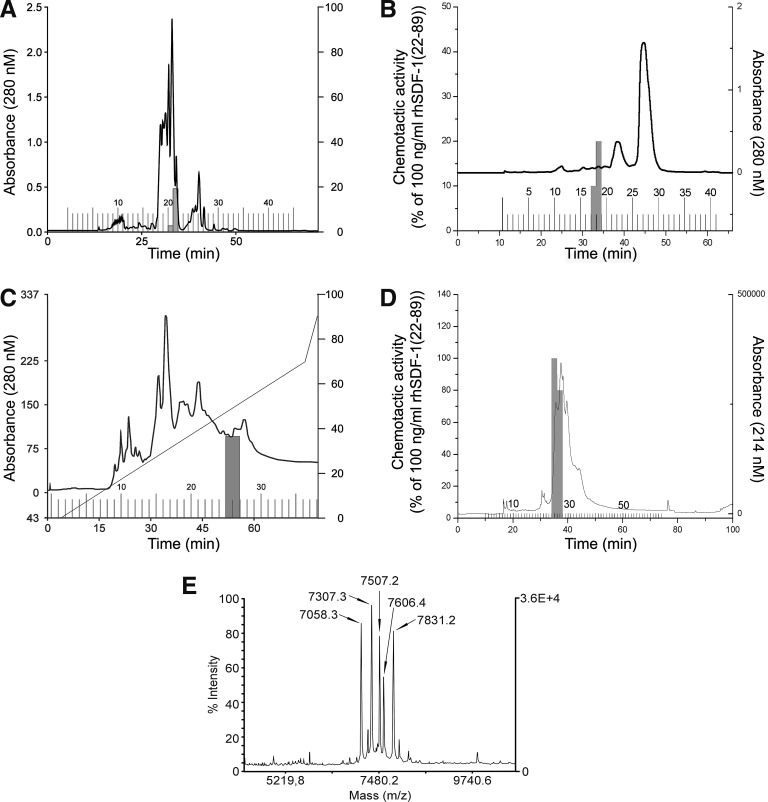
To determine the molecular characterization in human blood plasma for CXCR4-inducing activity, we screened peptide libraries produced from 10,000 L of HF from patients with renal insufficiency. Significant CXCR4-activating material was found in one peptide library within the fractions containing basic peptides (peptide library 030120; pH pool 7, fractions 21 and 22, and pH pool 8, fraction 21). Activation of the CXCR4 receptor was confirmed by a screening assay using FDCP-mix cells and cross-desensitization experiments, which showed that recombinant CXCL12[22–89] blocked the CXCR4 receptor response (data not shown). At each individual chromatographic step, chemotactic activity was consistently eluted in a single biologically active peak. Figure 2 shows the purification steps that were applied to the CXCR4-activating material from the peptide library (preparative RP-HPL chromatography, analytical cation exchange, and analytical RP-HPL chromatography). The same fractions consistently showed CXCR4 activation and HPC-migration stimulatory activity (Fig. 2). With this method, the HPC chemoattractant activity was purified >106-fold; ∼50 μg CXCL12 was obtained from 400 g of starting material. MALDI-MS and Edman degradation showed that the active fractions contained different variants of CXCL12: CXCL12[22–88], CXCL12[24–88], CXCL12[25–88], CXCL12[27–88], and CXCL12[29–88] (Fig. 2E; Table 1).
FIG. 2.
Purification and identification of CXCL12 as chemotactic activator for HPCs in human plasma. To screen for CXCR4 stimulating activities, aliquots of fractions from different peptide libraries were screened for calcium flux-inducing activity on CXCR4-transfected HEK293T cells. The calcium flux-inducing activity was purified in four chromatographic steps. (A) Preparative RP chromatography using a Bakerbond cartridge RP-C18 column (47 mm i.d.×300 mm, 15–30 μm, 300 Å; Vydac, Hesperia, CA) with an acetonitrile gradient. (B) RP chromatography using a Bakerbond cartridge (47 mm i.d.×300 mm) with an acetonitrile gradient from 100% solvent A (10 mM HCl) to 28% solvent B (10 mM HCl in 80% acetonitrile) over 2 min, from 28% to 55% solvent B over 50 min, and from 55% to 100% solvent B over 1 min. Fractions with a volume of 50 mL were collected. (C) Analytical cation exchange chromatography using a Parcosil ProKat column (4 mm i.d.×50 mm, 7 μm, 300 Å; Biotek, Östringen, Germany) at a flow rate of 1 mL/min using a linear binary gradient of 0% solvent A (10 mM Na2 HPO4, pH 4.5) to 70% solvent B (10 mM Na2 HPO4, pH 4.5, 1 M NaCl) over 70 min, and from 70% to 100% solvent B over 5 min. Two-milliliter fractions were collected. (D) Analytical RP chromatography using a YMC RP-C18 column (4.6 mm i.d.×250 mm) at a flow rate of 0.5 mL/min using a linear binary gradient of 80% solvent A (10 mM HCl) to 60% solvent B (10 mM HCl in 80% acetonitrile) over 60 min. Fractions with a volume of 0.5 mL were collected. (E) Identification of CXCL12 in the biologically active fraction 27 of purification step D by MALDI-MS. The mass peaks marked by the arrows correspond to molecules that represent C-terminally (Lys89 truncated) and N-terminally truncated CXCL12 molecules. The suggested CXCL12 molecules were confirmed by N-terminal Edman degradation. HEK, human embryonic kidney; i.d., inner diameter; MALDI-MS, matrix-assisted laser desorption/ionization–mass spectrometer.
Table 1.
Results of Edman Sequencing of CXCL12 Variants Purified from Human Plasma
| Variant | Theoretical Mw | Detected Mw by MALDI-MS | Determined N-terminal amino-acid sequence |
|---|---|---|---|
| CXCL12[22–88] | 7831.2 | 7831.2 | KPVSLSYRCPCRFF |
| CXCL12[24–88] | 7606.0 | 7606.4 | VSLSYRCPCRFF |
| CXCL12[25–88] | 7506.8 | 7507.2 | SLSYRCPCRFF |
| CXCL12[27–88] | 7306.6 | 7307.3 | SYRCPCRFF |
| CXCL12[29–88] | 7056.3 | 7058.3 | RCPCRFF |
Shown are the calculated theoretical average molecular masses of each molecule, the molecular masses detected by MALDI-MS, and the N-terminal amino-acid sequence determined by Edman sequencing.
MALDI-MS, matrix-assisted laser desorption/ionization–mass spectrometer.
CXCL12 processing by leukocytes and thrombocytes
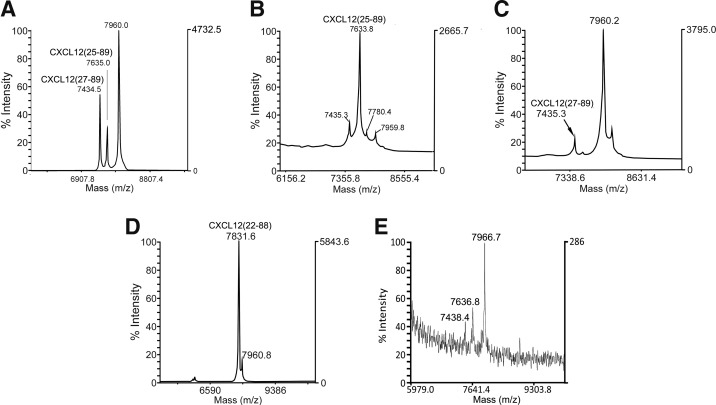
Since intact CXCL12[22–89] is the predominant variant secreted by cells in the body, the identification of CXCL12[22–88] and N-terminally truncated variants in blood plasma suggests that CXCL12 is processed in mature blood cells. Therefore, we investigated whether CXCL12[22–89] could be processed by PBMCs, PMNs, platelets, or platelet microparticles. Twenty micrograms CXCL12[22–89] was incubated with the purified cell populations for 30 min. The cell supernatants were purified by analytical RP chromatography, and the collected fractions were analyzed by MALDI-MS and Edman sequencing. For the endogenous molecules, analytical RP chromatography of the cell supernatants gave identical elution times for the CXCL12 molecules. Incubation of CXCL12[22–89] with isolated PMNs resulted in predominantly N-terminally truncated CXCL12[25–89] and CXCL12[27–89] (Fig. 3A). Neutrophil elastase was found to be responsible for generating CXCL12[25–89] (Fig. 3B and Table 2), whereas neutrophil cathepsin G generated CXCL12[27–89] (Fig. 3C and Table 2). N-terminal processing between amino acids Pro23 and Val24 occurred after incubation with dipeptidylpeptidase IV (DPPIV) as described by Christopherson et al. [12] (Supplementary Fig. S2). In contrast, the low-density PBMC fraction exhibited no significant proteolytic processing activity in CXCL12[22–89] under these experimental conditions. Incubation experiments with platelets revealed quantitative processing of CXCL12[22–89] to CXCL12[22–88] (Fig. 3D). The exclusive detection of molecular mass peaks for CXCL12[22–89] and CXCL12[22–88] by MALDI-MS suggests that thrombocytes contain a protease, which specifically processes the C-terminal Lys89 of CXCL12 (Fig. 3D and Table 2). Platelet microparticle levels have been shown to increase during HPC mobilization after the addition of G-CSF [13]. Therefore, we investigated whether platelet microparticles can process CXCL12[22–89]. MALDI-MS revealed peptides with an Mw of 7438.4 and 7636.8, corresponding to CXCL12[27–89] and CXCL12[25–89], respectively. The platelet-derived microparticles did not produce C-terminally truncated CXCL12[22–88] (Fig. 3E and Table 2). Together, the results of these degradation experiments in the presence of thrombocytes or neutrophilic granulocytes are consistent with the observed chromatically purified CXCL12 moieties.
FIG. 3.
C-terminal and N-terminal proteolytic processing of CXCL12 by platelet and granulocyte proteases. Proteolysis was investigated by incubating 10 μg CXCL12[22–89] in 500 μL with 2×107 isolated PMNs, 1 mU of elastase or cathepsin G, or with 1×109 thrombocytes or ∼1010 platelet microparticles for 30 min. The samples were subsequently centrifuged, and the supernatant was acidified with 0.1 M acetic acid. The samples were evaluated with an analytical RP chromatographic column, and the collected fractions were analyzed by MALDI-MS. CXCL12-containing fractions were sequenced by Edman degradation. (A-E) MALDI-MS traces of CXCL12-containing fractions were subjected to (A) isolated PMNs, (B) granulocyte elastase, (C) granulocyte cathepsin G, (D) isolated platelets, and (E) platelet microparticles. PMNs, polymorphonuclear neutrophils.
Table 2.
Analysis of the CXCL12-Directed Proteolytic Activity of Polymorphonuclear Neutrophils, Platelets, Neutrophil Elastase, and Cathepsin G
| Cells | CXCL12 variant | Theoretical Mw | Detected Mw | N-term. sequence | Protease identified |
|---|---|---|---|---|---|
| Platelets | [22–88] | 7831.2 | 7833.6 | KPVSLSYRCPCRFF | Not known |
| Platelet microparticles | [25–89] | 7635.1 | 7636.8 | Not known | |
| Platelet microparticles | [27–89] | 7435.0 | 7438.4 | Not known | |
| PMNs | [25–89] | 7635.1 | 7635.0 | SLSYRCPCRFF | Elastase |
| PMNs | [27–89] | 7435.0 | 7434.5 | SYRCPCRFF | Elastase Cathepsin G |
Isolated platelets, platelet microparticles, PMNs, 5 MIU cathepsin G, or 5 μg elastase purified from neutrophils were incubated with 20 μg of CXCL12 [22–89] in 500 μL for 60 min. The proteolyzed CXCL12 was chromatographed on an analytical RP column and analyzed by MALDI-MS and N-terminal Edman sequencing. CXCL12[25–89] was produced by neutrophil elastase, whereas CXCL12[27–89] was produced by neutrophil elastase and cathepsin G.
PMNs, polymorphonuclear neutrophils; RP, reverse phase.
Binding of CXCL12 variants to the receptor CXCR4 and CXCR7
To measure the binding of the CXCL12 variants to the CXCR4 and CXCR7 receptor, competition binding experiments were performed using [125I]-CXCL12[22–89] as a tracer for CXCR4 and CXCR7. We found that CXCL12[22–89] and CXCL12[22–88] displayed an almost identical affinity for CXCR4, with IC50 values of 0.131±0.088 and 0.164±0.085 nM, respectively (Table 3). In contrast, CXCL12[25–88], CXCL12[27–88], and CXCL12[29–88] did not compete for binding with [125I]-CXCL12 (Table 3). On the CXCR7 receptor, CXCL12[22–89] revealed a slightly stronger affinity than CXCL12[22–88]. In competition experiments, the IC50 value for CXCL12[22–89] was 0.069±0.024 nM and for CXCL12[22–88], the IC50 was 0.201±0.097 nM (Table 3). Interestingly, at concentrations of 100 nM, CXCL12[25–88] revealed some binding affinity for the CXCR7 receptor, displacing 30% of the [125I]-CXCL12 from the receptor. In contrast, CXCL12[27–88] and CXCL12[29–88] did not show affinity for the CXCR7 receptor. Taken together, CXCL12[22–88] and CXCL12[22–89] strongly bind both CXCR4 and CXCR7; whereas for the other isoforms, only CXCL12[25–88] showed some affinity for CXCR7.
Table 3.
Binding and Functional Parameters for Chemokine Receptors CXCR4 and CXCR7 for Identified CXCL12 Variants
| CXCR4 | CXCR7 | ||
|---|---|---|---|
| Binding parameters | Binding parameters | ||
| Calcium mobilization | Competition with CXCL12[22–89] | Competition with CXCL12[22–89] | |
| CXCL12 variant | EC50±SEM [nM] | IC50±SEM [nM] | IC50±SEM [nM] |
| [22–89] | 91.9±31.1 | 0.131±0.088 | 0.069±0.024 |
| [22–88] | 59.2±13.8 | 0.164±0.085 | 0.201±0.097 |
| [25–88] | n.e.d. | n.b.d. | 19.7±55.4 |
| [27–88] | n.e.d. | n.b.d. | n.b.d. |
| [29–88] | n.e.d. | n.b.d. | n.b.d. |
Values are the mean±SEM for at least three independent determinations.
n.e.d., no effect detectable; n.b.d., no binding detectable (EC50 or IC50>500 nM); SEM, standard error of the mean.
Functional activities of the identified CXCL12 molecules
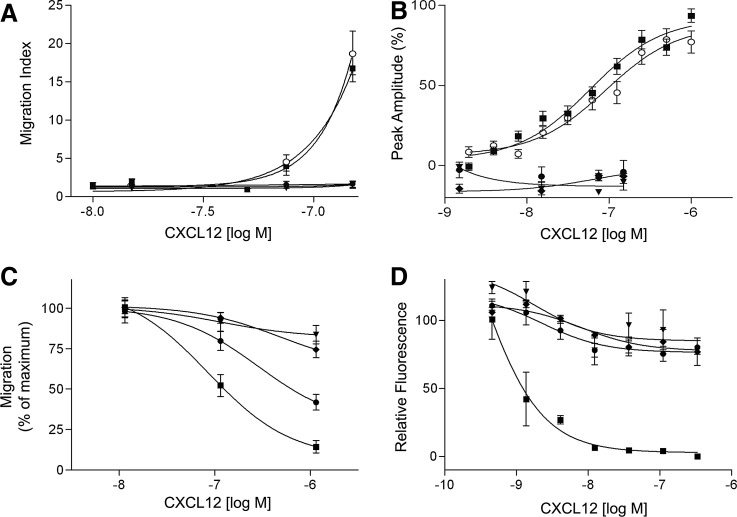
Chemotaxis assays using FDCP-mix HPCs as indicator cells were performed to characterize the biological activities of the identified and isolated CXCL12 variants. CXCL12[22–89] and CXCL12[22–88] demonstrated similar chemotactic activities. At concentrations of 150 nM, both CXCL12[22–89] and CXCL12[22–88] increased the chemotactic index by factors of 18.8±2.8 and 16.8±1.7 (mean±standard error of the mean [SEM]), respectively. In contrast, the N-terminally truncated molecules CXCL12[25–88], CXCL12[27–88], and CXCL12[29–88] did not generate chemotactic activity (Fig. 4A). Similarly, both CXCL12[22–89] and CXCL12[22–88] induced a dose-dependent, rapid, and transient flux of intracellular calcium in CXCR4-transfected HEK293T cells (Fig. 4B). The EC50 values for CXCL12[22–89] and CXCL12[22–88] were 91.9±31.1 and 54.5±13.8 nM, respectively (Fig. 4B). In contrast, the N-terminally truncated molecules CXCL12[25–88], CXCL12[27–88], and CXCL12[29–88] did not elicit calcium flux-inducing activity. At high nanomolar concentrations, CXCL12[22–89] and CXCL12[22–88] also generated significant chemokinetic activity compared with controls, thereby increasing the migration of FDCP-mix progenitor cells by 40% and 30%, respectively (data not shown).
FIG. 4.
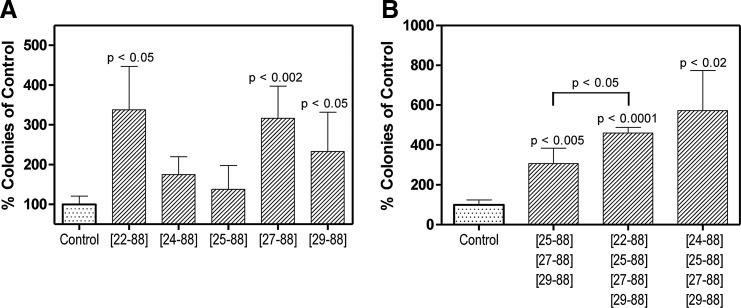
Biological characterization of CXCL12[22–88] and the N-terminally truncated molecules CXCL12[25–88], CXCL12[27–88], and CXCL12[29–88]. (A–D: CXCL12[22–89],  ; CXCL12[22–88], ■; CXCL12[25–88], ▼; CXCL12[27–88], ♦;CXCL12[29–88], ●) (A) Chemotactic responses of hematopoietic FDCP-mix progenitor cells to the different CXCL12 variants were investigated in Boyden chamber chemotaxis assays. CXCL12[22–89] and CXCL12[22–88] induced chemotactic migration at statistically indistinguishable levels. CXCL12[25–88], CXCL12[27–88], and CXCL12[29–88] did not induce chemotactic migration of FDCP-mix progenitor cells. The results of four different experiments were pooled. In each experiment, three replicates were performed for each concentration. The presented values are the mean±SEM. (B) Calcium flux measurements were performed in CXCR4-transfected HEK293T cells using the FLIPR assay system. The results of five different experiments were pooled. Each experiment was performed in triplicate for each concentration. The presented values are the mean±SEM. (C) Inhibition of chemotactic migration induced by 125 nM CXCL12[22–88] by N-terminally truncated variants of CXCL12. CXCL12[25–88], CXCL12[27–88], or CXCL12[29–88] were added to the upper wells of the chemotaxis chamber at concentrations of 12.5, 125, and 1250 nM, respectively. Each experiment was conducted in triplicate for each concentration. The results of three independent experiments were pooled. The presented values are the mean±SEM. (D) Inhibition of the CXCL12[22–88]-induced calcium flux by N-terminally truncated CXCL12 variants. Experiments were performed in CXCR4-transfected HEK293T cells using the FLIPR assay system. CXCL12[22–88], CXCL12[25–88], CXCL12[27–88], and CXCL12[29–88] were applied to the cells at concentrations between 0 and 333 nM. Subsequently, 10 nM CXCL12[22–88] was applied to the cells as a second stimulus. The diagram shows the correlation between the CXCL12 concentration of the first stimulus (X-axis) and the calcium flux induced by the second stimulus (Y-axis). The results from one experiment are representative of three independent experiments with the same results. Each CXCL12 variant and concentration were analyzed in triplicate. The given values are the mean±SEM.
; CXCL12[22–88], ■; CXCL12[25–88], ▼; CXCL12[27–88], ♦;CXCL12[29–88], ●) (A) Chemotactic responses of hematopoietic FDCP-mix progenitor cells to the different CXCL12 variants were investigated in Boyden chamber chemotaxis assays. CXCL12[22–89] and CXCL12[22–88] induced chemotactic migration at statistically indistinguishable levels. CXCL12[25–88], CXCL12[27–88], and CXCL12[29–88] did not induce chemotactic migration of FDCP-mix progenitor cells. The results of four different experiments were pooled. In each experiment, three replicates were performed for each concentration. The presented values are the mean±SEM. (B) Calcium flux measurements were performed in CXCR4-transfected HEK293T cells using the FLIPR assay system. The results of five different experiments were pooled. Each experiment was performed in triplicate for each concentration. The presented values are the mean±SEM. (C) Inhibition of chemotactic migration induced by 125 nM CXCL12[22–88] by N-terminally truncated variants of CXCL12. CXCL12[25–88], CXCL12[27–88], or CXCL12[29–88] were added to the upper wells of the chemotaxis chamber at concentrations of 12.5, 125, and 1250 nM, respectively. Each experiment was conducted in triplicate for each concentration. The results of three independent experiments were pooled. The presented values are the mean±SEM. (D) Inhibition of the CXCL12[22–88]-induced calcium flux by N-terminally truncated CXCL12 variants. Experiments were performed in CXCR4-transfected HEK293T cells using the FLIPR assay system. CXCL12[22–88], CXCL12[25–88], CXCL12[27–88], and CXCL12[29–88] were applied to the cells at concentrations between 0 and 333 nM. Subsequently, 10 nM CXCL12[22–88] was applied to the cells as a second stimulus. The diagram shows the correlation between the CXCL12 concentration of the first stimulus (X-axis) and the calcium flux induced by the second stimulus (Y-axis). The results from one experiment are representative of three independent experiments with the same results. Each CXCL12 variant and concentration were analyzed in triplicate. The given values are the mean±SEM.
To investigate the potential antagonistic activities of the N-terminally truncated molecules, different concentrations of CXCL12[25–88], CXCL12[27–88], and CXCL12[25–88] were loaded in the upper wells of a chemotaxis chamber, and chemotactic migration was induced with 1000 ng/mL CXCL12[22–88]. Neither CXCL12[25–88] nor CXCL12[27–88] displayed any significant antagonism of CXCL12[22–88] chemotactic activity, even at a 10-fold excess (Fig. 4C). In contrast, CXCL12[25–88] antagonized 50% of the chemotactic activity of CXCL12[22–88] when a 10-fold excess was present (Fig. 4C). Chemotactic HPC migration was inhibited by 55% after pretreatment with CXCL12[22–88] at equimolar concentrations. Pretreatment of cells with a 10-fold excess of CXCL12[22–88] exhibited an almost total inhibition of migration (Fig. 4C). Similarly, in CXCR4-transfected HEK293T cells, pretreatment with CXCL12[25–88], CXCL12[27–88], and CXCL12[25–88] inhibited the calcium flux by a maximum of 25% when simultaneously pretreated with 10 nM CXCL12[22–88] (Fig. 4D). In contrast to the high concentrations of CXCL12[22–88] required to induce CXCL12[22–88] antagonistic activity in the chemotaxis assays, equimolar concentrations of CXCL12[22–88] were sufficient to induce an almost complete inhibition of the calcium flux induced by the second stimulus (Fig. 4D).
Binding of CXCL12 variants to heparin
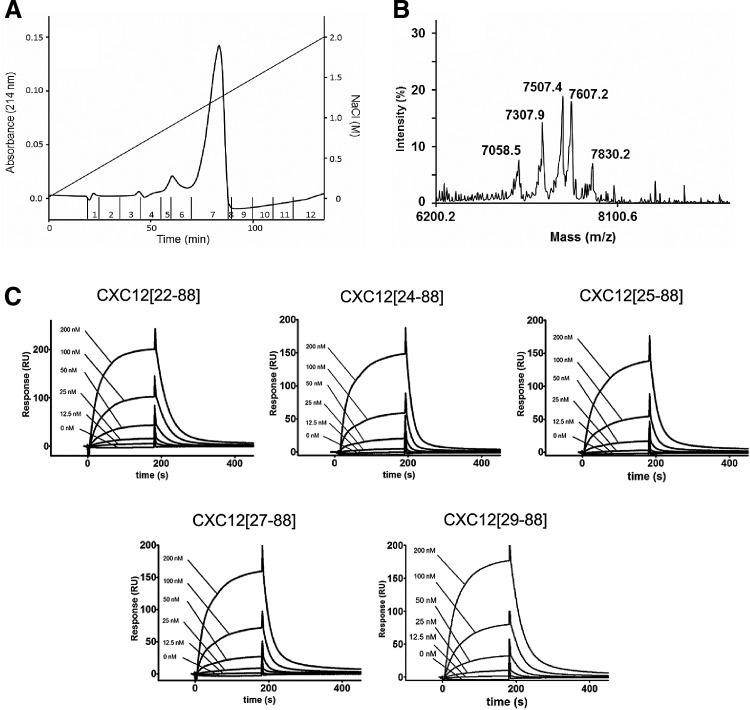
To compare the affinities of the different CXCL12 variants to heparin, a mixture of the five synthesized CXCL12 variants was applied to a heparin sepharose column and subsequently eluted with an NaCl gradient. The collected fractions were subsequently analyzed by MALDI-MS. The elution profile revealed a single peak at ∼1.2 M NaCl, containing the five CXCL12 variants, indicating similar affinities to heparin for all of the five tested variants (Fig. 5). For determination of the dissociation constants for heparin binding, the CXCL12 variants were injected over the Biacore heparin surface in a concentration range 0–200 nM. Afterward, 200 resonance units of biotinylated heparin were immobilized, and the flow rate was maintained at 30 μL/min. All sensorgrams could be fitted to the bimolecular binding reaction model and analyzed by linear transformation. Association rate constants (ka), dissociation rate constants (kd), and dissociation constants (KD) for each variant are given in Table 4. The determined KD for CXCL12[22–88] was 5.41±2.62 μM. The KD of CXCL12[24–88] was elevated by one log10 stage (54±22.4 μM). Consecutive N-terminal truncations were associated with a decrease of the dissociation constants, with a KD for CXCL12[25–88] of 18.7±5.41 μM (Table 4).
FIG. 5.
The identified plasma-derived CXCL12 variants CXCL12[22–88], CXCL12[24–88], CXCL12[25–88], CXCL12[27–88], and CXCL12[29–88] bind to heparin. (A) A mixture of synthesized CXCL12 variants (5 μg of CXCL12[22–88], and CXCL12[29–88], 15 μg of CXCL12[24–88], CXCL12[25–88], and CXCL12[27–88]) were subjected to a Heparin Sepharose column (10×125 mm; Amersham Pharmacia, Freiburg, Germany). Elution was performed with a gradient from 100% solvent A [10 mM NaCl/25 mM Tris–HCl (pH 8.0)] to 100% solvent B [2 M NaCl/25 mM Tris–HCl (pH 8.0)] over 80 min with a flow rate of 0.5 mL/min. Fractions were collected manually. (B) Subsequently, the fractions were desalted and subjected to MALDI-MS. The identified Mw corresponds to the CXCL12 variants CXCL12[22–88] (7830.2 Da), CXCL12[24–88] (7607.2 Da), CXCL12[25–88] (7507.4 Da), CXCL12[27–88] (7307.9), and CXCL12[29–88] (7058.5 Da). (C) Surface plasmon resonance sensograms of CXCL12 variants binding to biotinylated low-molecular-weight heparin. Approximately 200 resonance units of material were immobilized. Sensograms were obtained by injecting six different concentrations (0–200 nM) for each CXCL12 variant. Analytes were infused on the chip for 3 min (at 30 μL/min) followed by a dissociation phase of 20 min. The sensor chip was regenerated with two pulses of 1 min of 1.5 M NaCl in HBS-EP+ buffer. Sensorgrams were normalized based on the baseline signal, and kinetic constants were determined using Biacore X100 evaluation 2.0 software.
Table 4.
Rate Constants of CXCL12 Variants Binding to Biotinylated Low-Molecular-Weight Heparin
| CXCL12 variant | ka (M−1s−1) | kd (s−1) | KD (μM) |
|---|---|---|---|
| [22–88] | 6395.5±3044.5 | 0.026±0.0003 | 5.41±2.62 |
| [24–88] | 942.5±376.5 | 0.042±0.0007 | 54±22.4 |
| [25–88] | 1228.5±147.5 | 0.036±0.0011 | 30±4.77 |
| [27–88] | 1295±63 | 0.029±0.0003 | 23±1.36 |
| [29–88] | 1651.5±569.5 | 0.028±0.001 | 18.7±5.41 |
The measurements were performed using a Biacore™ X100 instrument (GE Healthcare). Approximately 200 resonance units of biotinylated heparin were immobilized. For each CXCL12 variant, six different concentrations (0–200 nM) were injected onto the chip. Sensorgrams were normalized from the baseline signal, and kinetic constants were determined using Biacore X100 evaluation 2.0 software.
ka, association rate constant; kd, dissociation rate constant; KD, dissociation constant.
Systemic application of CXCL12 variants results in mobilization of colony-forming unit into blood
To examine the effect of CXCL12 variants on the mobilization of HPC into the peripheral blood, the different variants were injected separately or in different combinations. Twenty minutes after intraorbital application, mice were sacrificed and MNCs were separated. Ten micrograms of CXCL12[22–88] induced an increase of colony-forming cells (CFU-C) to 338%±109% (mean±SEM; P<0.05). Retro-orbital application of 25 μg CXCL12[24–88] or 25 μg CXCL12[25–88] induced a moderate increase of blood CFUs with 175%±45% and 138%±60% (mean±SEM), respectively. However, retroorbital application of 25 μg CXCL12[27–88] or 25 μg CXCL12[25–88] significantly increased the amount of blood CFUs to 316%±82% (P<0.002) and 233%±98% (P<0.05), respectively (Fig. 6A).
FIG. 6.
Detection of increased numbers of circulating colony-forming cells in the CFU-C assay subsequent to the application of CXCL12 variants. Ten micrograms of CXCL12[22–88] and/or 25 μg of each N-terminally truncated CXCL12 variant were injected into Balb/C mice retroorbitally. After 20 min, mice were sacrificed and blood was withdrawn by cardiac puncture. (A) Each CXCL12 variant was separately evaluated, and (B) combinations of CXCL12 variants were evaluated for the potency to mobilize colony-forming cells into the peripheral blood. CFU-C, colony forming cells.
We subsequently tested whether the combination of the different CXCL12 variants revealed an even larger effect on blood CFU mobilization. The combination of CXCL12[25–88], CXCL12[27–88], and CXCL12[25–88] (25 μg each) induced an increase of blood CFUs to 307%±77%. The additional application of 10 μg CXCL12[22–88] to this combination increased the blood CFUs to 573%±201% of the control. The combination of CXCL12[24–88], CXCL12[25–88], CXCL12[27–88], and CXCL12[25–88] (25 μg each) induced an increase of blood CFUs to 460%±29% (Fig. 6B).
Discussion
HPC mobilization is a complex multi-step process which involves intrinsic motility mechanisms, activities of adhesion molecules, various cytokines, chemoattractants, proteolytic enzymes, and other extrinsic factors that enable egress of HSCs into the blood. Release of the chemokine CXCL12 into the circulation is associated with a significant mobilization of HPCs. The intravenous injection of an adenoviral vector expressing CXCL12 (AdSDF1) into severe combined immunodeficient mice, application of CXCL12-binding compounds (eg, fucoidan or GAG mimetics), and catecholamines induce a release of CXCL12 into the blood plasma that is accompanied by a mobilization of HPCs [14–17]. However, it is not known whether CXCL12 in blood plasma is immediately inactivated by proteolytic degradation, or whether the liberated CXCL12 stays functional for a significant time period, given that proteolytic inactivation of CXCL12 by DPPIV in blood plasma takes place with a half life of less than 1 min and that physiological CXCL12 concentrations in blood plasma are proteolytically degraded within 1–2 min [18,19].
Here, we show that G-CSF application or irradiation and HPC transplantation in humans and mice induce the release of biologically relevant HPC chemotactic activity in blood plasma. Using different methods, we identified the chemokine CXCL12 as the HPC chemoattractant in blood plasma. In neutralizing experiments with an anti-CXCR4 antibody, the chemotactic migration of CD34+ and FDCP-mix cells induced by patient plasma was blocked. Furthermore, the separation of patient blood plasma by RP chromatography generated one activity peak containing CXCL12-IR, strongly suggesting that during mobilization, active CXCL12 is liberated into the blood plasma.
The release of CXCL12 into the blood plasma after irradiation is supported by studies investigating CXCL12 expression after irradiation. In irradiated mice, we showed that the liver, spleen, and BM are tissue sources for the chemotactic activity. Other studies demonstrated that irradiation increases the expression levels of CXCL12 mRNA in the BM [20], liver [21], skin [22], and intestine [23]. In addition, an irradiation-induced CXCL12-dependent engraftment of HPC into the spleen and BM was identified [24]. CXCL12 expression was identified in endothelial cells of the BM, which have contact with the blood and are a relevant source of circulating CXCL12 [25].
To further characterize the molecular structure of CXCL12 in blood plasma, we isolated functional CXCR4-reactive activity from a peptide library obtained from a blood hemofiltrate of renal-insufficient patients. After the final purification, mass spectrometric analysis and sequencing revealed that the chemotactically active fractions contained highly purified CXCL12 with molecular heterogeneity at the N-terminus. In addition to chemotactically active CXCL12[22–88], four N-terminally truncated CXCL12 variants (CXCL12[24–88], CXCL12[25–88], CXCL12[27–88], and CXCL12[25–88]) were identified.
These results confirm that in blood plasma, CXCL12 is processed by different proteases. CXCL12[24–88] is a proteolytic product of the metalloprotease DPPIV, which has been suggested to degrade CXCL12 in the circulation with high efficacy [18,19]. CXCL12[25–88] and CXCL12[27–88] are proteolytic products that are generated by neutrophils [5]. Production of CXCL12[25–88] and CXCL12[27–88] in the blood filtrate is most likely dependent on the activation of neutrophils, which are induced on the semipermeable hemofilter membranes used for hemofiltration of patients with chronic renal failure [26]. In stem cell mobilization, granulocytes are activated by G-CSF application, chemotherapy, or irradiation, which convert the BM in a highly proteolytic environment [3,27,28]. The association of neutrophils and increasing elastase concentrations during mobilization of HPC, and the failure of HPC mobilization during neutropenia, suggest that neutrophils and neutrophil proteases are required for HPC mobilization [29,30]. A role for neutrophil elastase in HPC mobilization is also supported by experiments showing that the application of a peptidergic elastase inhibitor reduces G-CSF-induced HPC mobilization [31]. However, studies by Levesque et al. also demonstrated that elastase and cathepsin G are not essential for HPC mobilization [32]. They showed a similar induction of HPC mobilization by G-CSF treatment in elastase- and cathepsin G-deficient mice as in wild-type mice. These results indicate that redundant proteolytic activities are involved in HPC mobilization. MMP-8 is another protease released from granulocytes, which is capable of producing CXCL12[25–88] and is suggested to be involved in stem cell mobilization [33].
Additional proteases with the capability of N-terminal CXCL12 processing include the MMP-1, -2, -3, -9, -13, and -14 (Membrane Type 1-MMP) [34,35]. Importantly, these MMPs produce CXCL12[26–88] [34], a CXCL12 variant that was also identified in a study by Antonsson subsequent to an in vivo application of CXCL12[22–89] in mice [19]. In contrast, CXCL12[26–88] was not identified in our in vitro CXCL12-processing experiments performed with human granulocytes and was not identified in the purified material from blood filtrate, although the CXCL12[26–88]-producing protease MMP-9 is primarily expressed in granulocytes. This discrepancy may be correlated to insufficient proteolytic activation, as MMP-9 is released as an inactive zymogen [36,37]. Although human neutrophil elastase is suggested to prime activation of proMMP-9 [38], the activation of MMP-9 in the context of granulocyte activation on the hemofilter membranes may not be sufficient for the generation of CXCL12[26–88] in humans. However, with the application of G-CSF, the BM progresses to a highly proteolytic microenvironment that is characterized by MMP-2, MMP-9, MMP-14 (MT1-MMP), cathepsin G, neutrophil elastase, and C-terminal processing cathepsin K and carboxypeptidase M [35,39]. In this situation, CXCL12[26–88] may be produced as an important proteolytic CXCL12 variant for stem cell mobilization, which has been suggested to switch receptor specificity to CXCR3 [40,41].
In the study of Antonsson et al. mentioned earlier, all of the CXCL12 variants identified in our study were also found with the exception of CXCL12[27–88] [19]. The absence of CXCL12[27–88] might be related to species-specific differences in the neutrophil serine protease activities [42,43].
Furthermore, Antonsson et al. and our results suggest that CXCL12[25–88] is the shortest proteolytically processed form found in blood plasma. They showed that s.c. or i.v. adminstration of CXCL12[25–88] revealed no additional proteolytic product but instead showed slow elimination of CXCL12[25–88] within 6–8 h postadministration [19].
We found that thrombocytes reveal a proteolytic activity which liberates Lys89 from CXCL12[22–89]. This proteolytic activity is most likely not related to proteases that are known to process Lys89 from CXCL12. These proteases include carboxypeptidase N [44] and M [45], and Cathepsin X [46,47]. These exoproteases are not present in thrombocytes. Carboxypeptidase N is a soluble protease found in blood plasma; the membrane-bound carboxypeptidase M is expressed in hematopoietic cells, CD34+ cells, myeloid-, erythroid-, megakaryocytic progenitors, mesenchymal stem cells, and fibroblastic cells. Carboxypeptidases N and M exclusively process Lys89 [44], whereas Cathepsin X is secreted by human osteoblasts and reveals a C-terminal exo-peptidase activity for CXCL12[22–89], which cleaves 15 amino acids up to proline P74 [47]. A putative carboxypeptidase for C-terminal processing of CXCL12 represents a thrombin-activatable fibrinolysis inhibitor that is expressed in thrombocytes and is able to process C-terminal Lys residues [48].
To assess the biological relevance of N-terminally truncated CXCL12 variants, we first tested synthesized CXCL12[22–88] and the N-terminally truncated forms for agonistic and antagonistic activity in the chemotaxis and calcium flux-inducing assays. In accordance with other studies, the N-terminally truncated CXCL12 variants revealed no agonistic activities in the chemotaxis and calcium flux assays. In contrast, CXCL12[25–88] displayed significant antagonistic activity in the chemotaxis assays when a 10-fold excess of CXCL12[25–88] was subjected to the HPCs; whereas in the calcium flux assays, only a marginal antagonistic activity was identified. Although Loetscher et al. demonstrated the importance of Arg29 for CXCR4 receptor binding [49], the antagonistic activity of CXCL12[25–88] cannot be explained by competition for the CXCR4 receptor, because CXCL12[25–88] does not bind to CXCR4. In addition, CXCL12[25–88] does not bind to CXCR7, another receptor that mediates CXCL12 activity. However, CXCL12[22–88] and CXCL12[25–88] reveal similar heparin binding affinities, suggesting a competition for GAG binding of both CXCL12 variants, which may be relevant for presentation of CXCL12 to CXCR4. Murphy et al. suggested that GAGs support dimerization and the presentation of CXCL12 dimers to CXCR4 [50]. Since CXCL12 has been observed as a dimer in several crystal structures, CXCL12[25–88] along with CXCL12[22–88] might be able to dimerize in the migrating cells; whereas CXCL12[25–88] might compete with CXCL12[22–88] for GAG binding and, therefore, might reduce the concentration of active CXCL12 in the HPCs [51]. The absence of CXCL12[25–88]-antagonistic activity in the calcium mobilization assay may be correlated with different mechanisms to induce calcium mobilization and chemotaxis via the CXCR4 receptor. Studies of Veldkamp et al. demonstrate that monomeric CXCL12 induces chemotactic activity; whereas a locked CXCL12 dimer, in which the CXCL12 subunits are linked through a disulfide bond, retains Ca2+ mobilization yet loses chemotactic activity, instead preventing endogenous CXCL12-induced chemotactic activity [52,53].
We next investigated whether the application of different CXCL12 variants induced mobilization of HPCs in a mouse model. In our experiments, retro-orbital application of CXCL12[22–88] resulted in mobilization of HPCs into the circulation. Due to the short half life of biologically active CXCL12, mobilization was determined 20 min after retro-orbital application, which reached a threefold increase above control. In addition, application of N-terminally truncated CXCL12 variants induced HPC mobilization, with CXCL12[27–88] and CXCL12[25–88] demonstrating a significant effect on HPC mobilization. In addition to the putative antagonistic mechanism for CXCL12[25–88] as mentioned earlier, competition with biologically active CXCL12 for binding to GAG chains of proteoglycans along the endothelial surface might be responsible for the mobilization of HPCs [7,54]. The excess of N-terminally processed CXC12, although demonstrating a reduced affinity for Heparin, would be sufficient for a relevant displacement of active CXCL12. Netelenbos et al. have shown that CXCL12 presented by GAGs induced haptotactic migration [55]. Displacement of active CXCL12 from GAG binding sites by N-terminally truncated CXCL12 variants may abolish binding and haptotactic migration, and it may induce release of HPCs into the blood.
In conclusion, we have demonstrated the induction of HPC chemotactic activity in several tissues and in blood plasma during HPC mobilization and after irradiation. We have identified CXCL12 in blood plasma during HPC mobilization and subsequently isolated CXCL12 from a blood plasma filtrate. Our results confirmed that different CXCL12 variants exist in blood plasma, with an abundance of N-terminally truncated CXCL12 variants. A balance between the active and the N-terminally truncated CXCL12 variants in the circulation may be involved in regulation of the CXCL12/CXCR4 axis of HPC mobilization. The investigated N-terminally truncated CXCL12 variants revealed no agonistic activity mediated by the CXCR4 receptor, whereas CXCL12[25–88] demonstrates significant antagonistic activity in the chemotaxis assay. In addition, retroorbital application of CXCL12[22–88] or the N-terminal truncated variants in mice induced mobilization of HPCs. Since the investigated N-terminally truncated CXCL12 variants revealed no affinity for the CXCR4 receptor but significant affinity for heparin, the antagonistic activity of CXCL12[25–88] and the HPC mobilizing activity might be correlated to a competition between active and N-terminally truncated CXCL12 variants for GAG binding. Our findings confirm that the CXCL12 activity in blood plasma is regulated by proteolytic mechanisms with relevance for mobilization of HPCs. Further studies are warranted to explore and characterize the relative contributions of plasma- and tissue (eg, BM)-derived CXCL12 variants and their roles in HPC mobilization and engraftment.
Supplementary Material
Acknowledgments
This study was supported in part by a grant from the German Federal Ministry of Education and Research (Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie, grant FKZ 0311815) and the Dutch Organization for Scientific Research (to H.F.V. and M.J.S.).
The authors thank Hongda Shen for his essential contributions, and Jutta Barras-Akhnoukh, Susanne Hollrieder, Susann Busch, Rolf Koppitke, Wilfried Hahn, Tamarah de Jong, and Michael Lai for their excellent technical assistance.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Lapid K, Glait-Santar C, Gur-Cohen S, Canaani J, Kollet O. and Lapidot T. (2012). Egress and mobilization of hematopoietic stem and progenitor cells: a dynamic multi-facet process. StemBook [Internet]. Cambridge, MA: Harvard Stem Cell Institute, 2008–2012 [PubMed] [Google Scholar]
- 2.Bonig H. and Papayannopoulou T. (2013). Hematopoietic stem cell mobilization: updated conceptual renditions. Leukemia 27:24–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim CH, Wu W, Wysoczynski M, Abdel-Latif A, Sunkara M, Morris A, Kucia M, Ratajczak J. and Ratajczak MZ. (2012). Conditioning for hematopoietic transplantation activates the complement cascade and induces a proteolytic environment in bone marrow: a novel role for bioactive lipids and soluble C5b-C9 as homing factors. Leukemia 26:106–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ratajczak MZ, Kim CH, Abdel-Latif A, Schneider G, Kucia M, Morris AJ, Laughlin MJ. and Ratajczak J. (2012). A novel perspective on stem cell homing and mobilization: review on bioactive lipids as potent chemoattractants and cationic peptides as underappreciated modulators of responsiveness to SDF-1 gradients. Leukemia 26:63–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sahin AO. and Buitenhuis M. (2012). Molecular mechanisms underlying adhesion and migration of hematopoietic stem cells. Cell Adh Migr 6:39–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christopher MJ, Liu F, Hilton MJ, Long F. and Link DC. (2009). Suppression of CXCL12 production by bone marrow osteoblasts is a common and critical pathway for cytokine-induced mobilization. Blood 114:1331–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Proudfoot AE. (2006). The biological relevance of chemokine-proteoglycan interactions. Biochem Soc Trans 34:422–426 [DOI] [PubMed] [Google Scholar]
- 8.Blanchet X, Langer M, Weber C, Koenen RR. and von Hundelshausen P. (2012). Touch of chemokines. Front Immunol 3:175–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verzijl D, Storelli S, Scholten DJ, Bosch L, Reinhart TA, Streblow DN, Tensen CP, Fitzsimons CP, Zaman GJ, et al. (2008). Noncompetitive antagonism and inverse agonism as mechanism of action of nonpeptidergic antagonists at primate and rodent CXCR3 chemokine receptors. J Pharmacol Exp Ther 325:544–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richter R, Bistrian R, Escher S, Forssmann W-G, Vakili J, Henschler R, Spodsberg N, Frimpong-Boateng A. and Forssmann U. (2005). Quantum proteolytic activation of chemokine CCL15 by neutrophil granulocytes modulates mononuclear cell adhesiveness. J Immunol 175:1599–1608 [DOI] [PubMed] [Google Scholar]
- 11.Amara A, Lorthioir O, Valenzuela A, Magerus A, Thelen M, Montes M, Virelizier JL, Delepierre M, Baleux F, Lortat-Jacob H. and Arenzana-Seisdedos F. (1999). Stromal cell-derived factor-1alpha associates with heparan sulfates through the first beta-strand of the chemokine. J Biol Chem 274:23916–23925 [DOI] [PubMed] [Google Scholar]
- 12.Christopherson KW, 2nd, Hangoc G, Mantel CR. and Broxmeyer HE. (2004). Modulation of hematopoietic stem cell homing and engraftment by CD26. Science 305:1000–1003 [DOI] [PubMed] [Google Scholar]
- 13.Nomura S, Inami N, Kanazawa S, Iwasaka T. and Fukuhara S. (2004). Elevation of platelet activation markers and chemokines during peripheral blood stem cell harvest with G-CSF. Stem Cells 22:696–703 [DOI] [PubMed] [Google Scholar]
- 14.Hattori K, Heissig B, Tashiro K, Honjo T, Tateno M, Shieh JH, Hackett NR, Quitoriano MS, Crystal RG, Rafii S. and Moore MA. (2001). Plasma elevation of stromal cell-derived factor-1 induces mobilization of mature and immature hematopoietic progenitor and stem cells. Blood 97:3354–3360 [DOI] [PubMed] [Google Scholar]
- 15.Frenette PS. and Weiss L. (2000). Sulfated glycans induce rapid hematopoietic progenitor cell mobilization: evidence for selectin-dependent and independent mechanisms. Blood 96:2460–2468 [PubMed] [Google Scholar]
- 16.Albanese P, Caruelle D, Frescaline G, Delbe J, Petit-Cocault L, Huet E, Charnaux N, Uzan G, Papy-Garcia D. and Courty J. (2009). Glycosaminoglycan mimetics-induced mobilization of hematopoietic progenitors and stem cells into mouse peripheral blood: structure/function insights. Exp Hematol 37:1072–1083 [DOI] [PubMed] [Google Scholar]
- 17.Dar A, Schajnovitz A, Lapid K, Kalinkovich A, Itkin T, Ludin A, Kao WM, Battista M, Tesio M, et al. (2011). Rapid mobilization of hematopoietic progenitors by AMD3100 and catecholamines is mediated by CXCR4-dependent SDF-1 release from bone marrow stromal cells. Leukemia 25:1286–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lambeir AM, Proost P, Durinx C, Bal G, Senten K, Augustyns K, Scharpe S, Van Damme J. and De Meester I. (2001). Kinetic investigation of chemokine truncation by CD26/dipeptidyl peptidase IV reveals a striking selectivity within the chemokine family. J Biol Chem 276:29839–29845 [DOI] [PubMed] [Google Scholar]
- 19.Antonsson B, De Lys P, Dechavanne V, Chevalet L. and Boschert U. (2010). In vivo processing of CXCL12alpha/SDF-1alpha after intravenous and subcutaneous administration to mice. Proteomics 10:4342–4351 [DOI] [PubMed] [Google Scholar]
- 20.Ponomaryov T, Peled A, Petit I, Taichman RS, Habler L, Sandbank J, Arenzana-Seisdedos F, Magerus A, Caruz A, et al. (2000). Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J Clin Invest 106:1331–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swenson ES, Kuwahara R, Krause DS. and Theise ND. (2008). Physiological variations of stem cell factor and stromal-derived factor-1 in murine models of liver injury and regeneration. Liver Int 28:308–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim JH, Kolozsvary A, Jenrow KA. and Brown SL. (2012). Plerixafor, a CXCR4 antagonist, mitigates skin radiation-induced injury in mice. Radiat Res 178:202–206 [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Gong JF, Zhang W, Zhu WM. and Li JS. (2008). Effects of transplanted bone marrow mesenchymal stem cells on the irradiated intestine of mice. J Biomed Sci 15:585–594 [DOI] [PubMed] [Google Scholar]
- 24.Kollet O, Spiegel A, Peled A, Petit I, Byk T, Hershkoviz R, Guetta E, Barkai G, Nagler A. and Lapidot T. (2001). Rapid and efficient homing of human CD34(+)CD38(−/low)CXCR4(+) stem and progenitor cells to the bone marrow and spleen of NOD/SCID and NOD/SCID/B2m(null) mice. Blood 97:3283–3291 [DOI] [PubMed] [Google Scholar]
- 25.Ding L. and Morrison SJ. (2013). Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature 495:231–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Todeschini M, Macconi D, Fernandez NG, Ghilardi M, Anabaya A, Binda E, Morigi M, Cattaneo D, Perticucci E, Remuzzi G. and Noris M. (2002). Effect of acetate-free biofiltration and bicarbonate hemodialysis on neutrophil activation. Am J Kidney Dis 40:783–793 [DOI] [PubMed] [Google Scholar]
- 27.Bonig H. and Papayannopoulou T. (2012). Mobilization of hematopoietic stem/progenitor cells: general principles and molecular mechanisms. Methods Mol Biol 904:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levesque JP, Hendy J, Takamatsu Y, Simmons PJ. and Bendall LJ. (2003). Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamide. J Clin Invest 111:187–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marquez-Curtis LA, Turner AR, Sridharan S, Ratajczak MZ. and Janowska-Wieczorek A. (2011). The ins and outs of hematopoietic stem cells: studies to improve transplantation outcomes. Stem Cell Rev 7:590–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levesque JP, Takamatsu Y, Nilsson SK, Haylock DN. and Simmons PJ. (2001). Vascular cell adhesion molecule-1 (CD106) is cleaved by neutrophil proteases in the bone marrow following hematopoietic progenitor cell mobilization by granulocyte colony-stimulating factor. Blood 98:1289–1297 [DOI] [PubMed] [Google Scholar]
- 31.Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, Ponomaryov T, Taichman RS, Arenzana-Seisdedos F, et al. (2002). G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol 3:687–694 [DOI] [PubMed] [Google Scholar]
- 32.Levesque JP, Liu F, Simmons PJ, Betsuyaku T, Senior RM, Pham C. and Link DC. (2004). Characterization of hematopoietic progenitor mobilization in protease-deficient mice. Blood 104:65–72 [DOI] [PubMed] [Google Scholar]
- 33.Steinl C, Essl M, Schreiber TD, Geiger K, Prokop L, Stevanovic S, Potz O, Abele H, Wessels JT, Aicher WK. and Klein G. (2013). Release of matrix metalloproteinase-8 during physiological trafficking and induced mobilization of human hematopoietic stem cells. Stem Cells Dev 22:1307–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McQuibban GA, Butler S, Gong JH, Bendall L, Power C, Clark-Lewis I. and Overall CM. (2001). Matrix metalloproteinase activity inactivates the CXC chemokine stromal cell-derived factor-1. J Biol Chem 276:43503–43508 [DOI] [PubMed] [Google Scholar]
- 35.Levesque JP, Hendy J, Takamatsu Y, Williams B, Winkler IG. and Simmons PJ. (2002). Mobilization by either cyclophosphamide or granulocyte colony-stimulating factor transforms the bone marrow into a highly proteolytic environment. Exp Hematol 30:440–449 [DOI] [PubMed] [Google Scholar]
- 36.Vandooren J, Van den Steen PE. and Opdenakker G. (2013). Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9): the next decade. Crit Rev Biochem Mol Biol 48:222–272 [DOI] [PubMed] [Google Scholar]
- 37.Shimanovich I, Mihai S, Oostingh GJ, Ilenchuk TT, Brocker EB, Opdenakker G, Zillikens D. and Sitaru C. (2004). Granulocyte-derived elastase and gelatinase B are required for dermal-epidermal separation induced by autoantibodies from patients with epidermolysis bullosa acquisita and bullous pemphigoid. J Pathol 204:519–527 [DOI] [PubMed] [Google Scholar]
- 38.Jackson PL, Xu X, Wilson L, Weathington NM, Clancy JP, Blalock JE. and Gaggar A. (2010). Human neutrophil elastase-mediated cleavage sites of MMP-9 and TIMP-1: implications to cystic fibrosis proteolytic dysfunction. Mol Med 16:159–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ponte AL, Ribeiro-Fleury T, Chabot V, Gouilleux F, Langonne A, Herault O, Charbord P. and Domenech J. (2012). Granulocyte-colony-stimulating factor stimulation of bone marrow mesenchymal stromal cells promotes CD34+ cell migration via a matrix metalloproteinase-2-dependent mechanism. Stem Cells Dev 21:3162–3172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Denoyer A, Godefroy D, Celerier I, Frugier J, Degardin J, Harrison JK, Brignole-Baudouin F, Picaud S, Baleux F, et al. (2012). CXCR3 antagonism of SDF-1(5–67) restores trabecular function and prevents retinal neurodegeneration in a rat model of ocular hypertension. PLoS One 7:e37873-e37884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jinquan T, Quan S, Jacobi HH, Jing C, Millner A, Jensen B, Madsen HO, Ryder LP, Svejgaard A, et al. (2000). CXC chemokine receptor 3 expression on CD34(+) hematopoietic progenitors from human cord blood induced by granulocyte-macrophage colony-stimulating factor: chemotaxis and adhesion induced by its ligands, interferon gamma-inducible protein 10 and monokine induced by interferon gamma. Blood 96:1230–1238 [PubMed] [Google Scholar]
- 42.Wiesner O, Litwiller RD, Hummel AM, Viss MA, McDonald CJ, Jenne DE, Fass DN. and Specks U. (2005). Differences between human proteinase 3 and neutrophil elastase and their murine homologues are relevant for murine model experiments. FEBS Lett 579:5305–5312 [DOI] [PubMed] [Google Scholar]
- 43.Raymond WW, Trivedi NN, Makarova A, Ray M, Craik CS. and Caughey GH. (2010). How immune peptidases change specificity: cathepsin G gained tryptic function but lost efficiency during primate evolution. J Immunol 185:5360–5368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davis DA, Singer KE, De La Luz SM, Narazaki M, Yang F, Fales HM, Yarchoan R. and Tosato G. (2005). Identification of carboxypeptidase N as an enzyme responsible for C-terminal cleavage of stromal cell-derived factor-1alpha in the circulation. Blood 105:4561–4568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marquez-Curtis L, Jalili A, Deiteren K, Shirvaikar N, Lambeir AM. and Janowska-Wieczorek A. (2008). Carboxypeptidase M expressed by human bone marrow cells cleaves the C-terminal lysine of stromal cell-derived factor-1alpha: another player in hematopoietic stem/progenitor cell mobilization?. Stem Cells 26:1211–1220 [DOI] [PubMed] [Google Scholar]
- 46.Staudt ND, Aicher WK, Kalbacher H, Stevanovic S, Carmona AK, Bogyo M. and Klein G. (2010). Cathepsin X is secreted by human osteoblasts, digests CXCL-12 and impairs adhesion of hematopoietic stem and progenitor cells to osteoblasts. Haematologica 95:1452–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Staudt ND, Maurer A, Spring B, Kalbacher H, Aicher WK. and Klein G. (2012). Processing of CXCL12 by different osteoblast-secreted cathepsins. Stem Cells Dev 21:1924–1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin JH, Garand M, Zagorac B, Schadinger SL, Scipione C, Koschinsky ML. and Boffa MB. (2011). Identification of human thrombin-activatable fibrinolysis inhibitor in vascular and inflammatory cells. Thromb Haemost 105:999–1009 [DOI] [PubMed] [Google Scholar]
- 49.Loetscher P, Gong JH, Dewald B, Baggiolini M. and Clark-Lewis I. (1998). N-terminal peptides of stromal cell-derived factor-1 with CXC chemokine receptor 4 agonist and antagonist activities. J Biol Chem 273:22279–22283 [DOI] [PubMed] [Google Scholar]
- 50.Murphy JW, Cho Y, Sachpatzidis A, Fan C, Hodsdon ME. and Lolis E. (2007). Structural and functional basis of CXCL12 (stromal cell-derived factor-1 alpha) binding to heparin. J Biol Chem 282:10018–10027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ohnishi Y, Senda T, Nandhagopal N, Sugimoto K, Shioda T, Nagal Y. and Mitsui Y. (2000). Crystal structure of recombinant native SDF-1alpha with additional mutagenesis studies: an attempt at a more comprehensive interpretation of accumulated structure-activity relationship data. J Interferon Cytokine Res 20:691–700 [DOI] [PubMed] [Google Scholar]
- 52.Murphy JW, Yuan H, Kong Y, Xiong Y. and Lolis EJ. (2010). Heterologous quaternary structure of CXCL12 and its relationship to the CC chemokine family. Proteins 78:1331–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Veldkamp CT, Seibert C, Peterson FC, De la Cruz NB, Haugner JC, III, Basnet H, Sakmar TP. and Volkman BF. (2008). Structural basis of CXCR4 sulfotyrosine recognition by the chemokine SDF-1/CXCL12. Sci Signal 1:ra4.- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ziarek JJ, Veldkamp CT, Zhang F, Murray NJ, Kartz GA, Liang X, Su J, Baker JE, Linhardt RJ. and Volkman BF. (2013). Heparin oligosaccharides inhibit chemokine (CXC motif) ligand 12 (CXCL12) cardioprotection by binding orthogonal to the dimerization interface, promoting oligomerization, and competing with the chemokine (CXC motif) receptor 4 (CXCR4) N terminus. J Biol Chem 288:737–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Netelenbos T, Zuijderduijn S, van den Born J, Kessler FL, Zweegman S, Huijgens PC. and Drager AM. (2002). Proteoglycans guide SDF-1-induced migration of hematopoietic progenitor cells. J Leukoc Biol 72:353–362 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.