Abstract
Objective:
Despite improved short-term outcomes, concerns remain regarding durability of thoracic endovascular aortic repair(TEVAR). The purpose of this analysis was to evaluate the pathology-specific incidence of secondary aortic interventions(SAI) after TEVAR and their impact on survival.
Methods:
Retrospective review was performed of all TEVAR procedures and SAI at one institution from 2004-2011. Kaplan-Meier analysis was used to estimate survival.
Results:
Of 585 patients, 72(12%) required SAI at a median of 5.6 months(interquartile range(IQR):1.4-14.2) with 22(3.7%) requiring multiple SAI. SAI incidence differed significantly by pathology(P=.002): acute dissection(21.3%), post-surgical(20.0%), chronic dissection(16.7%), degenerative aneurysm(10.8%), traumatic transection(8.1%), penetrating ulcer(1.5%), and other etiologies(14.8%). Most common indications after dissection were persistent false lumen flow and proximal/distal extension of disease. For degenerative aneurysms, SAI was performed primarily to treat type I/III endoleaks. SAI patients had a greater mean number of comorbidities(P<.0005), stents placed(P=.0002), and postoperative complications after the index TEVAR(P<.0005) compared to those without SAI. Freedom from SAI at 1 and 5 years(95% CI) was estimated to be 86%(82-90%) and 68%(57-76%), respectively. There were no differences in survival(95% CI) between patients requiring SAI and those who did not: SAI 1-year: 88%(77-93%), 5-year: 51%(37-63%) and no SAI 1-year: 82%(79-85%), 5-year: 67%(62-71%)(Log-rank P=0.2).
Conclusion:
SAI after TEVAR is not uncommon, particularly in patients with dissection, but does not affect long-term survival. Aortic pathology is the most important variable impacting survival and dictated need, timing and mode of SAI. The varying incidence of SAI by indication underscores the need for diligent surveillance protocols that should be pathology-specific.
Introduction
After Food and Drug Administration (FDA) approval in 20051, thoracic endovascular aortic repair (TEVAR) was quickly adopted as the primary treatment strategy for degenerative thoracic aortic aneurysms2. Since that time, this technology has been increasingly utilized to treat a variety of other pathologies such as traumatic injuries, penetrating ulcer/intramural hematoma, as well as acute and chronic dissection3-7 ENREF 3 ENREF 3 ENREF 3. Despite the early benefits of TEVAR in reducing morbidity and mortality, long-term durability is still in question, and uncertainty remains regarding pathology-specific incidence of treatment failure.
Because TEVAR has been available for less than a decade, and there has been a rapid evolution in the applications of this technology, data regarding the incidence and impact of secondary aortic interventions (SAI) are limited. The purpose of this analysis is to describe the incidence of SAI after TEVAR for various clinical indications and to determine the effect of secondary interventions on clinical outcomes, including survival.
Methods
This study was approved by the Institutional Review Board at the University of Florida (IRB#528-2011).
Database and patient cohorts
A prospectively maintained endovascular aortic registry at the University of Florida was queried for all patients undergoing TEVAR from 2004-2011. All subjects underwent retrospective review of the electronic medical record and radiographic imaging to obtain details regarding indications, demographic data, anatomic and procedure specific variables, as well as need and type of reintervention. Acute and chronic dissection with aneurysm was defined as previously described by Hagan et al8. Specifically, aortic dissection treated < 14 days from symptom onset was cataloged as acute while patients treated for dissection ≥ 14 days from symptom onset where characterized as chronic. Comorbidities, complications and adjunctive procedures for TEVAR were classified based on the Society for Vascular Surgery reporting guidelines9. SAI was defined as any unplanned open or endovascular procedure required to treat either the primary pathology (e.g. intended treatment zone) or remote aortic disease (e.g. synchronous or metachronous aortic pathology). This specifically excluded non- aortic procedures performed to address complications of the TEVAR unrelated to the index aortic pathology (e.g. access vessel repair, iliac artery intervention or delayed carotid-subclavian bypass for arm ischemia).
During the study period, 585 patients underwent TEVAR for various indications, including: degenerative descending thoracic aneurysm (TAA), aortic dissection (AD), chronic type B aortic dissection with aneurysm (CTBAD), postsurgical anastomotic pseudoaneurysm, traumatic aortic transection (TAT), penetrating ulcer/intramural hematoma (PAU), thoracoabdominal aneurysm (TAAA), atheroembolic disease and Kommerel’s diverticulum. There were 98 (16.7%) patients who required secondary postoperative procedures, of whom 26 (4.4%) underwent operations for access vessel related complications and/or upper extremity ischemia and were excluded from the SAI subgroup. The remaining 72 patients (12.3%) received open and/or endovascular SAI and were compared to patients not undergoing SAI (n=513).
Clinical Practice
The index TEVAR for the various aortic pathologies and subsequent SAI were performed at the discretion of the operating surgeon as were intraoperative adjunctive procedures (e.g. access conduit, subclavian revascularization, and spinal drainage). The following aortic stent grafts were used during the study interval: TAG/C-TAG 45% (W. L. Gore and Associates, Flagstaff, AZ), Zenith/TX2 40% (Cook Inc, Bloomington, IN), Talent/Valiant 9% (Medtronic Inc, Santa Rosa, CA), , and Bolton Relay 5% (Bolton Medical, Sunrise, FL). Previous reports from our group reflect a homogeneous approach to treatment philosophy10 that is based on a multi-disciplinary team of Vascular and Cardiovascular surgeons. The practice patterns at the University of Florida have been previously described in detail regarding anatomic considerations11, characteristics of the TEVAR implantation procedure11-13 and postoperative care of patients with TAA11, AD13, CTBAD14, and TAT12 treated with TEVAR. All patients underwent preoperative computed tomographic angiography (CTA) with centerline reconstruction (TeraRecon, Inc., San Mateo, CA). Postoperative surveillance consisted of imaging at 1-month, 6-months and annually thereafter, unless more frequent imaging was deemed clinically necessary.
Indications and Classification of SAI
Patients who underwent SAI (N=72) were further characterized based on the initial type of secondary aortic reintervention (endovascular vs. open). Mechanical failure, endograft infection, and antegrade/retrograde dissection caused by the TEVAR were categorized as graft-related complications. Endoleak definitions were based on the SVS reporting guidelines9. Type Ia/b and III endoleaks routinely underwent SAI with the rare exception of patients with prohibitive anatomy and/or medical comorbidities. Persistent type II endoleak with continued aneurysm degeneration (≥5mm) beyond 6 months from the index TEVAR also typically underwent reintervention. For dissections, the most common rationale for SAI was a persistent patent false lumen resulting in compromised proximal/distal fixation, visceral/lower extremity malperfusion, persistent pain, or continued false lumen aneurysmal degeneration. Finally, common indications for SAI in TAA patients included remote aortic degeneration (e.g. aortic site not in immediate apposition to the endograft or intended treatment zone) or synchronous multilevel aortic disease.
Study endpoints and statistical analysis
The primary endpoint was the rate of SAI following the index TEVAR. Additional endpoints included complications following SAI and mortality. All deaths were verified by query of the Social Security Death Masterfile.
Statistical analyses were completed using STATA 11 (StataCorp, College Station, Tex) and the R statistical software package (www.r-project.org; V.2.15.0). Categorical variables were summarized using frequencies and percentages while continuous variables were evaluated with means and standard deviations (±SD) if normally distributed and median values with interquartile range (IQR), if not. Comparisons of patient or procedure-related characteristics in subgroup analyses were performed using the Fisher exact test, two-sample t-test, or Wilcoxon rank sum test as appropriate. Patient survival and SAI rates were estimated using Kaplan-Meier methodology and compared between groups using the log-rank test. Statistical significance was assumed at a P-value < .05.
Results
Over the study period, 585 patients (mean age ± SD 65.3 ± 15.2; 32% female, N=185) underwent TEVAR for treatment of 384 (66%) elective and 201(34%) urgent or emergent thoracic aortic pathologies. The most common indication was degenerative descending thoracic aortic aneurysm, which accounted for 46% of all procedures (Figure 1a). Dissection-related pathology was the index TEVAR indication in 27% of cases, which constituted the second largest subgroup. ‘Other’ indications (5%) included mycotic etiologies, atheroembolic disease, thoracoabdominal aneurysms and Kommerel’s diverticulum.
Figure 1.

A) Indications for the initial TEVAR by aortic pathology. Notably, 73% of the index TEVARs were performed for dissections and degenerative aneurysms. 2) Incidence or rate of SAI during follow up stratified by initial TEVAR indication.
The rate of SAI by index TEVAR indication is depicted in Figure 1b. Patients with AD, post-surgical anastomotic pseudoaneurysm, and CTBAD had the highest overall rates of SAI. Patients with dissection-related pathologies had nearly double the SAI rate of those undergoing TEVAR for degenerative aneurysm (P=.02). Focal pathology such as TAT (8.1%) and PAU (1.5%) had the lowest rates of SAI. The demographics and comorbidity profile of patients requiring SAI compared to those who did not are depicted in Table 1. Notably, patients requiring SAI were more likely to be male and had significantly greater prevalence of multiple comorbidities compared to patients who did not (P=.04).
Table 1.
Demographics and comorbidities of patients: No SAI vs. SAI
| Feature, No.(%) | No SAI (N=513) |
+ SAI (N=72) |
P value* |
|---|---|---|---|
| Age (years, mean±SD) | 65.7±15.1 | 63.1±16.0 | .1 |
| Gender (female) | 172(34%) | 13(18%) | .01 |
| Body mass index (mean±SD) | 27.4±5.7 | 28.1±5.0 | .3 |
|
| |||
| Hypertension | 143(28%) | 40(56%) | <.0005 |
| Dyslipidemia | 64(12%) | 21(29%) | .001 |
| Coronary artery disease | 38(7%) | 12(17%) | .02 |
| COPD | 38(7%) | 11(15%) | .04 |
| Congestive heart failure | 12(2%) | 3(4%) | .4 |
| Renal insufficiency (Cr≥1.8) | 45(9%) | 8(11%) | .5 |
| Composite (mean±SD) | 0.7±1.3 | 1.4±1.5 | <.0005 |
t-test for continuous variables or chi-square or Fisher’s exact test for categorical variables when appropriate.
Indications for Secondary Aortic Intervention after TEVAR
Figure 2 provides specific details regarding the SAIs performed after TEVAR for patients with AD, CTBAD and TAA; the three most common index TEVAR pathologies. These three indications accounted for 82% (N=59) of the cases requiring SAI.
Figure 2.

Flowchart of secondary aortic interventions stratified by the 3 most common TEVAR indications.
Acute Dissection
For AD patients (N=75) treated with TEVAR, 21.3% (N=16) required SAI. Median time to SAI after TEVAR for AD was 1 month (IQR: 0.4, 1.8). Of the 16 patients in the AD SAI cohort, a majority (69%; N=11) had an open operation as their initial remedial procedure and 31% (N=5) underwent multiple SAI (Figure 2a). Initial SAI was urgent or emergent in 14 of 16 patients (87.5%). Types of open SAI after TEVAR for AD included ascending/transverse arch reconstruction (N=4) and proximal/distal cerclage utilizing a Dacron graft (N=6). One patient underwent surgical conversion with device explantation due to false lumen rupture on postoperative day one. The most prevalent indications for the various open surgical revisions were a persistent patent false lumen resulting in continued pain (N=2), false lumen expansion (N=2), retrograde dissection (N=3) and hemothorax/rupture (N=4). Similarly, a persistent patent false lumen with intractable pain (N=1) or communication with a false lumen rupture (N=4) led to endovascular SAI utilizing proximal/distal endograft extension and/or embolization in another 5 patients.
Chronic Dissection with Aneurysm
For CTBAD patients, 17% (N=14) underwent SAI at a median time of 7 months (IQR: 3.7, 13.3) after TEVAR. Of these, initial open and endovascular SAI were evenly distributed with 28.5% (N=4) requiring multiple SAI. Ascending/transverse arch reconstruction was required in 1 patient due to a proximal endoleak resulting in a persistent patent false lumen and continued aneurysm expansion while a second patient experienced retrograde dissection 1.5 months after the initial TEVAR. Cerclage of the thoracic aorta around the endograft with intercostal artery ligation was successful in 2 additional patients who had a persistent patent false lumen. Late open conversion was required in 3 cases due to proximal [Gore TAG (W.L. Gore and Associates, Flagstaff, Ariz)] endograft collapse (1), distal (Gore TAG) endograft in-folding (1), and distal thoracic/visceral aortic degeneration (1) (Figure 2b).
For patients who had initial endovascular SAI (N=7) after TEVAR for CTBAD, the most common indication was a persistent patent false lumen from proximal (N=2) or distal/remote site septal fenestrations (N=5). Reintervention strategies included proximal endograft extension in 2 patients while 2 others underwent distal extension to the level of the celiac artery. Two additional subjects underwent stent graft placement across a large visceral septal fenestration for a persistent patent false lumen. A single patient underwent embolization of the left subclavian artery due to retrograde filling of the false lumen (Figure 2b).
Degenerative Aneurysms
In contrast to dissection related pathology, the majority (76%; N=22) of initial SAI for TAA were endovascular procedures. However, similar to acute and chronic dissection patients, approximately one-third of the SAI patients (34%; N=9) required multiple SAI (Figure 2c). Median time to SAI was significantly longer at 13.1 months (IQR: 4.7, 14.9, P <.0005) for TAA compared to dissection-related pathology. Notably, of the 22 patients undergoing endovascular SAI, 20 (91%) underwent proximal or distal extension for type I endoleak or a bridging stent placement for Type III endoleak. Of the minority of patients requiring open SAI, 2 underwent transverse arch replacement and 2 underwent open repair with either endograft removal (1) or incorporation of the prior endograft into the repair (1).
Type and Outcome of SAI after TEVAR
Operative details of the index TEVAR for patients requiring SAI versus those who did not are highlighted in Table 2. The mean number of stents deployed, incidence of postoperative complications, and overall length of stay with the index TEVAR were all significantly greater in patients who subsequently required SAI. No association between device type used during the index TEVAR and subsequent SAI was noted (P=.1). The types of SAI and indications (N=112 procedures in 72 patients) are outlined in Table 3. Multiple SAIs were required in 31% (n=22) of patients, and notably there were 4 arch reconstructions performed for retrograde aortic dissection. The patients who developed retrograde aortic dissection after TEVAR were initially treated with a Gore TAG device (W.L. Gore and Associates, Flagstaff, Ariz) (N=2) and a Cook Zenith TX2-Proform device (Cook Medical, Inc. Bloomington, Ind) (N=2).
Table 2.
Operative details of the Index TEVAR
| Feature No. (%) | No SAI (N=513) |
+ SAI (N=72) |
P value* |
|---|---|---|---|
| Prior AAA repair | 97(19%) | 10(14%) | .3 |
| Procedure details | |||
| Urgent/emergent | 169(33%) | 32(44%) | .06 |
| ASA III/IV | 398(78%) | 56(78%) | 1 |
| Number stents (±SD) | 2.1±1.0 | 2.6±1.3 | .0002 |
| Left subclavian coverage | 255(50%) | 38(53%) | .7 |
| Adjunct | 174(34%) | 30(42%) | .2 |
| Complication with 1st TEVAR | 165(32%) | 46(64%) | <.0005 |
| Length of stay (days, ±SD) | 8±10 | 13±19 | .005 |
t-test for continuous variables or chi-square or Fisher’s exact test for categorical variables when appropriate.
Table 3.
Indications and descriptions of SAI (N=112) in all TEVAR patients (N=72)
| Type of SAI procedure | |||||
|---|---|---|---|---|---|
| Rationale for reintervention | No.(%) (N=112) |
Open repair (N=46) |
Endovascular (N=65) |
||
| Endoleak | |||||
| Type 1a | 15(13.4%) | Arch reconstruction | 1 | Proximal extension | 11 |
| Cerclage | 1 | ||||
| Surgical conversion | 2 | ||||
| Type 1b | 7(6.3%) | Cerclage | 1 | Distal extension | 5 |
| Type IV TAAA | 1 | ||||
| Type II | 14(12.5%) | Embolization | 11 | ||
| Proximal extension | 2 | ||||
| Relining | 1 | ||||
| Type III | 4(3.6%) | Bridge graft | 4 | ||
| Type IV | 2(1.8%) | Relining | 2 | ||
|
| |||||
| Persistent false lumen flow | |||||
| Proximal fixation | 7(6.2%) | Arch reconstruction | 4 | Proximal extension | 2 |
| Surgical conversion | 1 | ||||
| Distal fixation | 14(12.5%) | Cerclage | 5 | Distal extension | 7 |
| Surgical conversion | 2 | ||||
| Proximal and distal fixation | 2(1.8%) | Cerclage | 2 | ||
| Remote site fenestration | 8(7.1%) | Surgical conversion | 1 | Visceral/renal stent graft | 3 |
| Distal extension | 2 | ||||
| Chimney EVAR | 1 | ||||
| Left subclavian embolization | 1 | ||||
| Graft complication* | 14(12.5%) | Surgical conversion | 5 | Proximal extension | 4 |
| Arch reconstruction | 1 | Distal extension | 1 | ||
| Cerclage | 1 | Proximal + distal extension | 1 | ||
| Retrograde dissection | 4(3.6%) | Arch replacement | 4 | ||
| Disease progression and/or remote aortic procedure |
20(17.9%) | Visceral and/or infrarenal | 10 | Hybrid repair | 3 |
| Ascending arch replacement | 1 | Infrarenal aortic stent graft | 3 | ||
| Proximal thoracic extension | 1 | ||||
| Chimney EVAR | 1 | ||||
| Fenestrated EVAR | 1 | ||||
| Aortobronchial fistula | 1(<1%) | Partial graft excision | 1 | ||
| Aortoesophageal fistula | 1(<1%) | Graft removal | 1 | ||
Graft complication includes arch pseudoaneurysm post-TEVAR, enfolding, retraction, infection, and acute precipitation of false lumen rupture; Hybrid repair = visceral aortic debranching with endovascular aortic stent graft(EVAR)
Outcomes following the initial SAI, based on whether this was an endovascular or open procedure, are reported in Tables 4 and 5, respectively. Specifically, for patients that underwent SAI after TEVAR, outcomes were examined with respect to the initial SAI (as several patients required multiple open or endovascular SAI). Length of stay, 30-day mortality, and postoperative complications following open SAI were comparable to reported rates after open thoracic aortic surgery5, 15-17. Similarly, clinical outcomes following endovascular SAI were not worse than that after the index TEVAR.
Table 4.
Outcomes after Open SAI for TEVAR
| Feature, No. (%) | Open (N=30) |
|---|---|
| Length of stay (days, ±SD) | 20±16 |
| 30-day mortality | 7% |
| Complication | 53% |
| Neurologic | 23% |
| Spinal cord ischemia | 17% |
| Stroke | 7% |
| Pulmonary | 33% |
| Cardiac | 3% |
| Bleeding/wound | 13% |
| Renal (25%↓eGFR) | 17% |
SD, standard deviation; eGFR, estimated glomerular filtration rate*
Table 5.
Outcomes after Endovascular SAI for TEVAR
| Feature, No. (%) | Endo SAI (N=42) |
Index TEVAR (N=585) |
P value* |
|---|---|---|---|
| Length of stay (days, ±SD) | 6±7 | 9±12 | .14 |
| 30-day mortality | 0% | 6% | .2 |
| Complication | 14% | 36% | .004 |
| Neurologic | 5% | 15% | .06 |
| Spinal cord ischemia | 2% | 9% | .1 |
| Stroke | 0% | 8% | .06 |
| Pulmonary | 2% | 8% | .2 |
| Cardiac | 7% | 4% | .3 |
| Bleeding/wound | 2% | 5% | .4 |
| Renal (25%↓eGFR) | 5% | 6% | .8 |
SD, standard deviation; eGFR, estimated glomerular filtration rate
t-tests for continuous variables or chi-square or Fisher’s exact tests for categorical variables when appropriate.
Incidence and Survival Impact of SAI after TEVAR
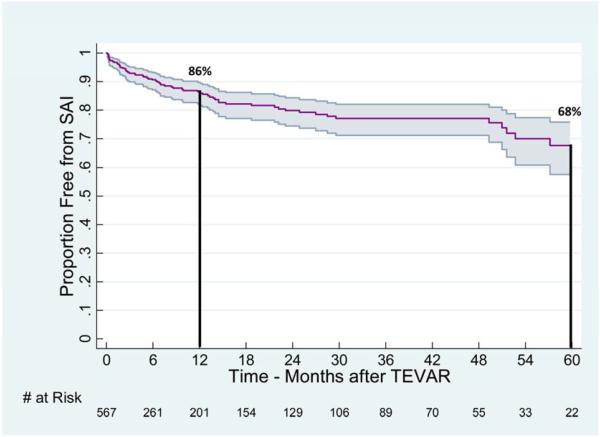
At a median follow-up of 5.8 months (IQR: 0.9, 25.8), the 1 and 5-year freedom from SAI after TEVAR for the entire cohort were estimated to be 86% (95% CI: 82-90%) and 68% (57-76%), respectively (Figure 3). Stratification by pathologic indication for TEVAR reveals significant differences (Figure 4), in freedom from SAI between TAA, CTBAD with aneurysm, and AD (P <.0005).
Figure 3.
Freedom from SAI after the index TEVAR. One and 5 year freedom from SAI (with 95% CI) were estimated at 86% (82-90%) and 68% (57-76%), respectively for the entire cohort of patients (N=585).
Figure 4.
Freedom from SAI when stratified for the 3 most common indications for the index TEVAR. Rate of SAI was highest for the acute dissection, followed by chronic dissection with aneurysm and then degenerative aneurysms.
Survival in the entire TEVAR cohort at 1 and 5 years was estimated to be 83% (95% CI: 80-86%) and 64% (95% CI: 60-69%), respectively. No significant difference in mortality was found between patients requiring SAI and those who did not (P=.2; Figure 5). Furthermore, within each major pathologic indication for TEVAR (AD, CTBAD, and TAA), SAI did not influence patient survival. In addition, neither the mode of the initial SAI, whether open or endovascular, nor if the patient ever underwent open SAI, had an impact on long-term survival (P=.9 and P=.1, respectively). However, survival was significantly different when patients were stratified by aortic pathology (P=.03, Figure 6).
Figure 5.
Survival impact of SAI following TEVAR. No difference in survival was noted between patients who underwent SAI and those who did not.
Figure 6.
Survival when stratified for the 3 most common indications for the index TEVAR. Survival was lowest following TEVAR for acute dissection, followed by degenerative aneurysms, and then chronic dissection with aneurysm.
Discussion
This study is consistent with the existing literature and demonstrates that SAI after TEVAR is common, and these results emphasize the importance of long-term surveillance after thoracic endovascular procedures for any indication. For the three most common indications for TEVAR (AD, CTBAD with aneurysm, and TAA), significant differences in the timing and mode of reintervention were noted, with the highest rate of SAI found in dissection patients. Not surprisingly, focal pathologies requiring a short length of aortic coverage, such as penetrating ulcers or traumatic transections, had relatively low rates of SAI. Both the mode of SAI as well as the time to the first SAI was strongly associated with aortic pathology. Despite the frequent need for repeat intervention after the index TEVAR, neither the need for SAI nor the mode of reintervention impacted long-term survival which was primarily dictated by the pathologic indication for the index TEVAR.
TEVAR has been increasingly utilized for a number of acute and chronic aortic conditions due to reduced morbidity and mortality when compared to open repair4, 18-22. Although there is a lower risk of perioperative morbidity and mortality after endovascular therapy, lifelong monitoring is required due to the ongoing risk of treatment failure. Reintervention after TEVAR is not uncommon and has previously been reported to be as high as 38-72% at 3 years19, 23, 24 for AD patients, 13-41% for CTBAD patients25, 26 and 8-22% for TAA patients27, 28, which are all comparable to results found in this series. Notably, no specific devices were found to be associated with an increased risk of SAI in this series, which is also consistent with other reports27, 28.
In many cases, treatment of dissection with TEVAR violates the basic premise of endovascular repair due to the inherent lack of seal when the device lands in the dissected aorta. Therefore, the lack of durability in the dissection patients is not particularly surprising given the fragile nature of the tissues involved in the repair, especially in an acutely dissected aorta29. At a median time of only one month, 21% of AD patients underwent a remedial procedure, with a majority of these (69%) undergoing an open intervention. The most frequent indications were rupture and malperfusion from a persistent patent false lumen. Notably, six patients underwent aortic banding/cerclage around the endograft, however this strategy has since been abandoned due to a high rate of failure and at the present time, we perform direct aortic reconstruction when TEVAR failure occurs in the management of AD.
SAI occurred in 17% of our CTBAD patients, which was similar to that of AD patients. However, the interventions occurred later, at a median of 7 months, and were equally distributed between open and endovascular interventions, unlike AD patients who were more often repaired with an open approach. The rate of SAI for CTBAD patients in our series is comparable to the 22% reported by Kang et al26, as was the time to reintervention, which was a median time of 10.1 months in their study. Similarly, approximately half of the procedures were distal to the initial TEVAR and performed to treat a persistent patent false lumen and aneurysm expansion analogous to the 43% of SAI performed to address distal false lumen aneurysm formation in patients with CTBAD in other reports26, 30. Further, Sayer and colleagues24 reported 3-year freedom from SAI in CTBAD patients as 45% which is comparable to the 63% in our series. These reported rates of reintervention after TEVAR for dissection demonstrates that SAI may eventually become an expected part of the treatment paradigm for these patients.
Our series demonstrates that degenerative pathology has a lower incidence of SAI and a higher probability of successful remediation with endovascular techniques compared to dissection. Similar to other series27, 28, the most common indication for reintervention after TEVAR for TAA was type 1 endoleak requiring endograft extension (Table 3). The overall occurrence of reintervention after TEVAR for TAA was 10.8% and the vast majority (76%) underwent endovascular intervention. The open surgical conversion rate in our series (1.1%) is similar to other reports where conversion rates approach 2-6%27, 28.
Notwithstanding the finding that SAI patients generally had a higher number of comorbidities compared to the non-SAI cohort, the postoperative complications following endovascular (14%) and open SAI (53%) after TEVAR were similar to those following primary endovascular and open surgical repair of a variety of thoracic and thoracoabdominal disease indications5, 27, 31-33. Interestingly, despite the risk of additional morbidity and mortality after undergoing SAI, there was no difference in survival between patients who required aortic remediation after TEVAR and those who did not. Furthermore, there was also no difference in survival based on the initial mode of SAI required, whether open or endovascular, or whether the patient ever underwent open SAI. The primary factors determining survival following TEVAR in this study were aortic pathology and patient comorbidities. The significance of these two factors is demonstrated in Figure 5 where patients treated for CTBAD had the best survival, followed by TAA and then AD patients who had the worst. This mortality trend by pathology is consistent with data from Patterson et al., who reported on the midterm outcomes from the Medtronic Thoracic Endovascular Registry (MOTHER)34. Although CTBAD patients more frequently required SAI compared to TAA patients, these patients had fewer risk factors previously reported by our group to be independent predictors of mortality compared to their TAA counterparts (1.8 ± 0.8 vs. 2.2 ± 0.9, P =.002)10.
The differential rate of SAI by pathology demonstrates that it may be inappropriate to have a standard surveillance protocol that includes all indications. Although this study was not designed to determine the optimal surveillance imaging protocol for a variety of thoracic aortic pathologies, the relatively high rate and early occurrence of SAI in AD patients suggests that the optimal timing of the first postoperative CT might be earlier than that for focal pathologies, and more frequent imaging may be warranted in the early postoperative period. Furthermore, given the rate of multiple SAIs among dissection and TAA patients (~30%), patients requiring a secondary aortic procedure may benefit from increased imaging frequency.
The limitations of this study include its retrospective nature and the relatively small patient numbers within each index TEVAR indication cohort. While multiple comorbidities and certain pathologies were identified to be associated with SAI, no predictive modeling for SAI was possible given the small patient numbers within each SAI cohort which prevented robust statistical modeling. Additionally, no standardized prospective algorithm was in place to control for patient selection, device implantation or rationale for reintervention. The influence of selection bias on timing and need for reintervention, particularly in non-emergent presentations, impacts the results of this study.
An important consideration in this analysis is that less than 25% of these cases were performed within the constraints of a clinical trial and therefore endografts initially designed, tested and approved for chronic aneurysmal pathology were implanted ‘off-label’. These results underscore the current limitations of stent-graft design, particularly for acute and chronic dissection. The endograft frequently must adapt to significant differences in lumen diameter between the proximal and compressed distal true lumen and the next generation of stent-graft design will likely have pathology-specific device modifications to address these challenges (e.g. PETTICOAT35 ENREF 1 [provisional extension to induce complete attachment] technique now being evaluated for acute dissection). Finally, given that the index TEVAR was performed at a tertiary referral center, there is the possibility of missed SAIs performed at other institutions which may influence the findings.
SAI after TEVAR is common, particularly in patients treated for dissection-related pathology, but does not appear to negatively affect long-term survival. Aortic pathology was the most important variable impacting survival as well as dictating the need, timing and mode of SAI. The prevalence of these secondary procedures underscores the need for diligent surveillance protocols that perhaps should be pathology-specific.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 41st Annual Symposium of the Society for Clinical Vascular Surgery, Miami, FL, Friday, March 15th, 2013
References
- 1.Makaroun MS, Dillavou ED, Kee ST, Sicard G, Chaikof E, Bavaria J, et al. Endovascular treatment of thoracic aortic aneurysms: Results of the phase ii multicenter trial of the gore tag thoracic endoprosthesis. J Vasc Surg. 2005;41:1–9. doi: 10.1016/j.jvs.2004.10.046. [DOI] [PubMed] [Google Scholar]
- 2.Scali ST, Goodney PP, Walsh DB, Travis LL, Nolan BW, Goodman DC, et al. National trends and regional variation of open and endovascular repair of thoracic and thoracoabdominal aneurysms in contemporary practice. J Vasc Surg. 2011;53:1499–1505. doi: 10.1016/j.jvs.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hormann M, Pavlidis D, Brunkwall J, Gawenda M. Long-term results of endovascular aortic repair for thoracic pseudoaneurysms after previous surgical coarctation repair. Interact Cardiovasc Thorac Surg. 2011;13:401–404. doi: 10.1510/icvts.2011.271312. [DOI] [PubMed] [Google Scholar]
- 4.Patel HJ, Sood V, Williams DM, Dasika NL, Diener AC, Deeb GM. Late outcomes with repair of penetrating thoracic aortic ulcers: The merits of an endovascular approach. Ann Thorac Surg. 2012;94:516–522. doi: 10.1016/j.athoracsur.2012.03.074. discussion 522-513. [DOI] [PubMed] [Google Scholar]
- 5.Sachs T, Pomposelli F, Hagberg R, Hamdan A, Wyers M, Giles K, et al. Open and endovascular repair of type b aortic dissection in the nationwide inpatient sample. J Vasc Surg. 2010;52:860–866. doi: 10.1016/j.jvs.2010.05.008. discussion 866. [DOI] [PubMed] [Google Scholar]
- 6.Dake MD, Kato N, Mitchell RS, Semba CP, Razavi MK, Shimono T, et al. Endovascular stent-graft placement for the treatment of acute aortic dissection. N Engl J Med. 1999;340:1546–1552. doi: 10.1056/NEJM199905203402004. [DOI] [PubMed] [Google Scholar]
- 7.Patel HJ, Williams DM, Upchurch GR, Jr., Dasika NL, Deeb GM. The challenge of associated intramural hematoma with endovascular repair for penetrating ulcers of the descending thoracic aorta. J Vasc Surg. 2010;51:829–835. doi: 10.1016/j.jvs.2009.11.050. [DOI] [PubMed] [Google Scholar]
- 8.Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, et al. The international registry of acute aortic dissection (irad): New insights into an old disease. Jama. 2000;283:897–903. doi: 10.1001/jama.283.7.897. [DOI] [PubMed] [Google Scholar]
- 9.Fillinger MF, Greenberg RK, McKinsey JF, Chaikof EL. Reporting standards for thoracic endovascular aortic repair (tevar) J Vasc Surg. 2010;52:1022–1033. doi: 10.1016/j.jvs.2010.07.008. 1033 e1015. [DOI] [PubMed] [Google Scholar]
- 10.Scali ST, Chang CK, Feezor RJ, Hess PJ, Jr., Beaver TM, Martin TD, et al. Preoperative prediction of mortality within 1 year after elective thoracic endovascular aortic aneurysm repair. J Vasc Surg. 2012;56:1266–1272. doi: 10.1016/j.jvs.2012.04.018. discussion 1272-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feezor RJ, Martin TD, Hess PJ, Jr., Daniels MJ, Beaver TM, Klodell CT, et al. Extent of aortic coverage and incidence of spinal cord ischemia after thoracic endovascular aneurysm repair. Ann Thorac Surg. 2008;86:1809–1814. doi: 10.1016/j.athoracsur.2008.09.022. discussion 1814. [DOI] [PubMed] [Google Scholar]
- 12.Hong MS, Feezor RJ, Lee WA, Nelson PR. The advent of thoracic endovascular aortic repair is associated with broadened treatment eligibility and decreased overall mortality in traumatic thoracic aortic injury. J Vasc Surg. 2011;53:36–42. doi: 10.1016/j.jvs.2010.08.009. discussion 43. [DOI] [PubMed] [Google Scholar]
- 13.Feezor RJ, Martin TD, Hess PJ, Jr., Beaver TM, Klodell CT, Lee WA. Early outcomes after endovascular management of acute, complicated type b aortic dissection. J Vasc Surg. 2009;49:561–566. doi: 10.1016/j.jvs.2008.09.071. discussion 566-567. [DOI] [PubMed] [Google Scholar]
- 14.Scali ST, Feezor RJ, Chang CK, Stone DH, Hess PJ, Martin TD, et al. Efficacy of thoracic endovascular stent repair for chronic type b aortic dissection with aneurysmal degeneration. J Vasc Surg. 2013;58:10–17. doi: 10.1016/j.jvs.2012.12.071. e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Luozzo G, Geisbusch S, Lin HM, Bischoff MS, Schray D, Pawale A, et al. Open repair of descending and thoracoabdominal aortic aneurysms and dissections in patients aged younger than 60 years: Superior to endovascular repair? Ann Thorac Surg. 2013;95:12–19. doi: 10.1016/j.athoracsur.2012.05.071. discussion 19. [DOI] [PubMed] [Google Scholar]
- 16.Estrera AL, Rubenstein FS, Miller CC, 3rd, Huynh TT, Letsou GV, Safi HJ. Descending thoracic aortic aneurysm: Surgical approach and treatment using the adjuncts cerebrospinal fluid drainage and distal aortic perfusion. Ann Thorac Surg. 2001;72:481–486. doi: 10.1016/s0003-4975(01)02679-0. [DOI] [PubMed] [Google Scholar]
- 17.Coselli JS, LeMaire SA, Conklin LD, Adams GJ. Left heart bypass during descending thoracic aortic aneurysm repair does not reduce the incidence of paraplegia. Ann Thorac Surg. 2004;77:1298–1303. doi: 10.1016/j.athoracsur.2003.10.033. discussion 1303. [DOI] [PubMed] [Google Scholar]
- 18.Subramanian S, Roselli EE. Thoracic aortic dissection: Long-term results of endovascular and open repair. Semin Vasc Surg. 2009;22:61–68. doi: 10.1053/j.semvascsurg.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Eggebrecht H, Nienaber CA, Neuhauser M, Baumgart D, Kische S, Schmermund A, et al. Endovascular stent-graft placement in aortic dissection: A meta-analysis. Eur Heart J. 2006;27:489–498. doi: 10.1093/eurheartj/ehi493. [DOI] [PubMed] [Google Scholar]
- 20.Patel HJ, Hemmila MR, Williams DM, Diener AC, Deeb GM. Late outcomes following open and endovascular repair of blunt thoracic aortic injury. J Vasc Surg. 2011;53:615–620. doi: 10.1016/j.jvs.2010.09.058. discussion 621. [DOI] [PubMed] [Google Scholar]
- 21.Andersen ND, Barfield ME, Hanna JM, Shah AA, Shortell CK, McCann RL, et al. Intrathoracic subclavian artery aneurysm repair in the thoracic endovascular aortic repair era. J Vasc Surg. 2013;57:915–925. doi: 10.1016/j.jvs.2012.09.074. [DOI] [PubMed] [Google Scholar]
- 22.Cambria RP, Crawford RS, Cho JS, Bavaria J, Farber M, Lee WA, et al. A multicenter clinical trial of endovascular stent graft repair of acute catastrophes of the descending thoracic aorta. J Vasc Surg. 2009;50:1255–1264. doi: 10.1016/j.jvs.2009.07.104. e1251-1254. [DOI] [PubMed] [Google Scholar]
- 23.Schoder M, Czerny M, Cejna M, Rand T, Stadler A, Sodeck GH, et al. Endovascular repair of acute type b aortic dissection: Long-term follow-up of true and false lumen diameter changes. Ann Thorac Surg. 2007;83:1059–1066. doi: 10.1016/j.athoracsur.2006.10.064. [DOI] [PubMed] [Google Scholar]
- 24.Sayer D, Bratby M, Brooks M, Loftus I, Morgan R, Thompson M. Aortic morphology following endovascular repair of acute and chronic type b aortic dissection: Implications for management. Eur J Vasc Endovasc Surg. 2008;36:522–529. doi: 10.1016/j.ejvs.2008.06.023. [DOI] [PubMed] [Google Scholar]
- 25.Parsa CJ, Schroder JN, Daneshmand MA, McCann RL, Hughes GC. Midterm results for endovascular repair of complicated acute and chronic type b aortic dissection. Ann Thorac Surg. 2010;89:97–102. doi: 10.1016/j.athoracsur.2009.09.029. discussion 102-104. [DOI] [PubMed] [Google Scholar]
- 26.Kang WC, Greenberg RK, Mastracci TM, Eagleton MJ, Hernandez AV, Pujara AC, et al. Endovascular repair of complicated chronic distal aortic dissections: Intermediate outcomes and complications. J Thorac Cardiovasc Surg. 2011;142:1074–1083. doi: 10.1016/j.jtcvs.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 27.Lee CJ, Rodriguez HE, Kibbe MR, Chris Malaisrie S, Eskandari MK. Secondary interventions after elective thoracic endovascular aortic repair for degenerative aneurysms. J Vasc Surg. 2013 doi: 10.1016/j.jvs.2012.10.124. [DOI] [PubMed] [Google Scholar]
- 28.Leurs LJ, Harris PL, Buth J. Secondary interventions after elective endovascular repair of degenerative thoracic aortic aneurysms: Results of the european collaborators registry (eurostar) J Vasc Interv Radiol. 2007;18:491–495. doi: 10.1016/j.jvir.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 29.Bhamidipati CM, Ailawadi G. Acute complicated and uncomplicated type iii aortic dissection: An endovascular perspective. Semin Thorac Cardiovasc Surg. 2009;21:373–386. doi: 10.1053/j.semtcvs.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akutsu K, Nejima J, Kiuchi K, Sasaki K, Ochi M, Tanaka K, et al. Effects of the patent false lumen on the long-term outcome of type b acute aortic dissection. Eur J Cardiothorac Surg. 2004;26:359–366. doi: 10.1016/j.ejcts.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 31.Szeto WY, McGarvey M, Pochettino A, Moser GW, Hoboken A, Cornelius K, et al. Results of a new surgical paradigm: Endovascular repair for acute complicated type b aortic dissection. Ann Thorac Surg. 2008;86:87–93. doi: 10.1016/j.athoracsur.2008.04.003. discussion 93-84. [DOI] [PubMed] [Google Scholar]
- 32.Rectenwald JE, Huber TS, Martin TD, Ozaki CK, Devidas M, Welborn MB, et al. Functional outcome after thoracoabdominal aortic aneurysm repair. J Vasc Surg. 2002;35:640–647. doi: 10.1067/mva.2002.119238. [DOI] [PubMed] [Google Scholar]
- 33.Goodney PP, Travis L, Lucas FL, Fillinger MF, Goodman DC, Cronenwett JL, et al. Survival after open versus endovascular thoracic aortic aneurysm repair in an observational study of the medicare population. Circulation. 2011;124:2661–2669. doi: 10.1161/CIRCULATIONAHA.111.033944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patterson B, Holt P, Nienaber C, Cambria R, Fairman R, Thompson M. Aortic pathology determines midterm outcome after endovascular repair of the thoracic aorta: Report from the medtronic thoracic endovascular registry (mother) database. Circulation. 2013;127:24–32. doi: 10.1161/CIRCULATIONAHA.112.110056. [DOI] [PubMed] [Google Scholar]
- 35.Nienaber CA, Kische S, Zeller T, Rehders TC, Schneider H, Lorenzen B, et al. Provisional extension to induce complete attachment after stent-graft placement in type b aortic dissection: The petticoat concept. J Endovasc Ther. 2006;13:738–746. doi: 10.1583/06-1923.1. [DOI] [PubMed] [Google Scholar]