Abstract
The activation of the developmental program in mammalian eggs relies on the initiation at the time of fertilization of repeated rises in the intracellular concentration of free calcium ([Ca2+]i), also known as [Ca2+]i oscillations. The ability to mount the full complement of oscillations is only achieved at the end of oocyte maturation, at the metaphase stage of meiosis II (MII). Over the last decades research has focused on addressing the mechanisms by which the sperm initiates the oscillations and identification of the channels that mediate intracellular Ca2+ release. This review will describe the up-to-date knowledge of other aspects of Ca2+ homeostasis in mouse such as the mechanisms that transport Ca2+ out of the cytosol into the endoplasmic reticulum (ER), the Ca2+ store of the oocyte/egg, into other organelles and also those extrude Ca2+. Evidence pointing to channels in the plasma membrane that mediate Ca2+ entry from the extracellular milieu, which is required for the persistence of the oscillations, is also discussed, along with the modifications that these mechanisms undergo during maturation. Lastly, we highlight areas where additional research is needed to obtain a better understating of the molecules and mechanisms that regulate homeostasis in this unique Ca2+ signaling system.
Introduction
Before fertilization, mammalian eggs are arrested at the metaphase stage of the second meiosis (MII). Sperm entry triggers a series of increases in the intracellular free-Ca2+ concentration ([Ca2+]i), termed [Ca2+]i oscillations, which enable exit from the MII and induce egg activation [1]. Importantly, in mammals the Ca2+ signal unfolds in a pattern of brief but periodical rises that last for several hrs after sperm entry. The spatio-temporal information provided by the pattern of these [Ca2+]i responses is decoded by downstream effectors that underpin the distinct cellular events of egg activation, which include cortical granule (CG) exocytosis, extrusion of the second polar body (2PB), pronuclear (PN) formation and entry into first mitosis [2]. Although the presence of long-lasting [Ca2+]i oscillations is a hallmark of mammalian fertilization, the underlying molecular changes that make them possible remain elusive.
Inositol 1,4,5-trisphosphate (IP3)-mediated Ca2+ release from intracellular stores is primarily responsible for the generation of the [Ca2+]i wave and oscillations at fertilization [3]. Although the general properties of Ca2+ release mechanism during oscillations have been described in the context of IP3 production and regulation of its cognate receptor (IP3R), the regulation of the [Ca2+]i responses in these cells may be far more complex. For instance, to continue the long-term [Ca2+]i oscillations without attenuation, after each [Ca2+]i rise [Ca2+]i levels need to be rapidly returned to baseline values, and the Ca2+ content of the endoplasmic reticulum (ER), the main Ca2+ reservoir in the cell [4], needs to be refilled ([Ca2+]ER) in anticipation of the next [Ca2+]i rise. To bring [Ca2+]i to baseline levels, cells either reuptake the free cytosolic Ca2+ into the ER refilling the store and/or remove it to other organelles or to the extracellular space [5, 6]. Nevertheless, to consistently replenish [Ca2+]ER and maintain oscillations, extracellular Ca2+ must enter into the egg/oocyte across the plasma membrane by a variety of channels and mechanisms, one of which is the store operated Ca2+ entry (SOCE) mechanism [7]. Despite the pivotal role of these channels/mechanisms on Ca2+ homeostasis and support of the oscillations, few studies have examined the underlying molecules and their function in mammalian oocytes/eggs.
Prior to ovulation, during oocyte maturation, oocytes undergo nuclear and cytoplasmic modifications in preparation for fertilization. Immature oocytes resume meiosis and transition from the germinal vesicle (GV) stage to the MII stage. Importantly, the precise spatio-temporal pattern of sperm-associated [Ca2+]i responses in eggs is established during maturation. In fact, in vitro fertilized GV oocytes show fewer [Ca2+]i oscillations and each [Ca2+]i rise exhibits lesser duration and amplitude than those observed in fertilized MII eggs [8, 9]. In spite of this knowledge, the mechanisms underlying the enhanced Ca2+ releasing ability of matured eggs vs. GV oocytes are not well understood, although multiple parameters (e.g. IP3R modifications, ER re-organization, increase in [Ca2+]ER) are thought to be involved in this process [10]. In addition, given that several of the parameters of Ca2+ homeostasis progressively change during maturation, for instance [Ca2+]ER steadily increases during maturation, the molecules responsible for these adjustments in Ca2+ homeostasis may experience dynamic modifications during maturation such that some of the mechanisms/channels active at the GV stage may not be so at the MII stage and vice versa.
This review highlights the changes that [Ca2+]ER experiences during maturation and fertilization. The mechanisms that contribute to the regulation of [Ca2+]ER are also discussed, including Ca2+ buffering systems and Ca2+ influx mechanisms as well as the changes that these mechanisms undergo during oocyte maturation.
Ca2+ homeostasis during fertilization
The initiation of [Ca2+]i oscillations during mammalian fertilization is thought to be triggered by the release from the sperm of a male specific Phospholipase C (PLC) enzyme, PLCζ, following fusion of the gametes [11, 12]. More details about PLCζ can be found in another chapter of this special issue. Therefore, our focus will be on other components of the Ca2+ toolkit that regulate Ca2+ homeostasis in oocytes and eggs.
1) Ca2+ buffering mechanisms
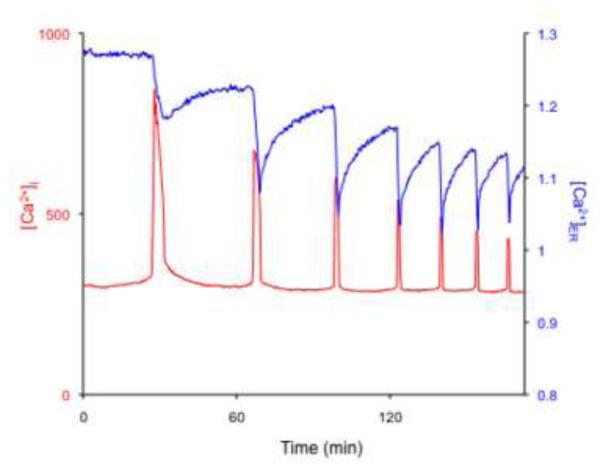
To bring [Ca2+]i to baseline levels after a [Ca2+]i rise, cells either reuptake the free cytosolic Ca2+ into the ER by the action of the sarco-endoplasmic reticulum Ca2+ ATPases (SERCA) and/or remove it to the surrounding environment by the action of plasma membrane Ca2+ ATPase (PMCA) and Na+/Ca2+ exchanger [5, 6]. Few studies have addressed the function of these molecules in mammalian oocytes/eggs. Three different SERCA genes (ATP2A1-3) encode three main isoforms (SERCA1–3), each of which undergoes tissue-specific splicing, further increasing the diversity of these pumps [13]. SERCA1a and SERCA1b variants are expressed in skeletal muscle. SERCA2a variant is expressed in cardiac muscle, whereas SERCA2b variant is expressed nearly ubiquitously and is thus considered the housekeeping isoform. SERCA3 is instead expressed in a limited number of non-muscle cells [13]. In Xenopus oocytes, expression of the SERCA2 protein was documented using immunofluorescence and it was shown to undergo reorganization similar to that described for IP3R during oocyte maturation, which is consistent with the fact that both proteins are ER-resident proteins and with the reorganization that the ER undergoes during maturation [14]. The molecular presence and cellular distribution of SERCA2b has not yet been characterized in mammalian oocytes, although transcripts and proteins have been reported [15, 16]. The functional importance of SERCA can be surmised by the alteration of [Ca2+]i levels caused by exposure to thapsigargin, an specific inhibitor of SERCA [17]. For example, exposure of MII eggs to thapsigargin causes a slow and steady rise in [Ca2+]i followed by a protracted decline while in fertilized eggs, addition of thapsigargin or other SERCA inhibitors, prematurely terminates [Ca2+]i oscillations [17, 18]. Nonetheless, the impact that these inhibitors have in [Ca2+]ER has not been investigated, and therefore the regulatory role of [Ca2+]ER on the pattern and/or persistence of oscillations is unknown in this species. Here, we made use D1ER, a FRET-based Ca2+ sensor [19], to directly examine [Ca2+]ER levels during oscillations. To initiate [Ca2+]i oscillations, we injected mouse eggs with mouse PLCζ cRNA, a procedure that we and others have shown to cause long-lasting and fertilization-like oscillations (Fig. 1) [11]. As depicted in the figure, each [Ca2+]i rise is accompanied by a rapid decline in [Ca2+]ER that is followed by a slow recovery. Compared to the quick return to baseline that cytosolic [Ca2+]i transients experience, [Ca2+]ER levels return to near basal levels in a very gradual manner, suggesting that other Ca2+ buffering systems contribute to cytosolic [Ca2+]i clearance. Moreover, the upstroke of the next [Ca2+]i increase occurs before [Ca2+]ER levels are fully recovered, and for approximately the first 3 [Ca2+]i rises [Ca2+]ER levels progressively decrease, although after that they seemingly reach a plateau, where each [Ca2+]i increase occurs from the same [Ca2+]ER level.
Fig. 1.
[Ca2+]ER and [Ca2+]i undergo simultaneous but opposite changes in concentration during oscillations in mouse MII eggs. Ca2+ responses were induced by injection of 0.05 μg/μl mouse PLCζ cRNA into MII eggs. In vitro transcribed D1ER RNA was injected into eggs 5 hr before the initiation of [Ca2+]i measurements. The emission ratio of D1ER (YFP/CFP) was used to estimate relative changes in [Ca2+]ER (right axis, blue trace). [Ca2+]i (left axis, red trace) was recorded using Rhod-2. Rhod-2 is generally used to measure mitochondrial Ca2+, although given that it fails to target into mitochondria in mouse eggs, it is possible to use it as a reported of [Ca2+]i (Dumollard, R. et al.).
As mentioned, the lag time between the return of [Ca2+]i rises to baseline and the recovery of [Ca2+]ER suggests the involvement of other Ca2+ buffering mechanisms for clearing [Ca2+]i out of the cytosol. To this end, the functional activity of Na+/Ca2+ exchanger was demonstrated in mouse eggs [20, 21]. It was shown that elimination of Na+ from the external media caused [Ca2+]i responses, or accelerated existing ones, and these responses were ascribed to a reverse mode of Na+-Ca2+ exchange. Importantly, in spite of the initial changes in [Ca2+]i levels, even in the absence of external Na+, [Ca2+]i returned to baseline levels, implying that the action of other mechanisms, possibly plasma membrane Ca2+ ATPase (PMCA) may be more physiologically relevant [21]. In spite of those initial studies, not until recently was the possible involvement PMCA in mammalian oocytes tested. It was shown that inhibition of PMCA by millimolar concentrations of gadolinium (Gd3+), generating a Ca2+ insulation system, as both the influx and efflux of Ca2+ are greatly reduced, increased the amplitude and duration of the initial [Ca2+]i rise and oscillations [22], suggesting the active presence of this mechanism in mouse eggs. Nonetheless, the molecular identification of PMCA was not documented in that study and thus the molecular presence of PMCAs in mammalian oocytes/eggs needs confirmation. In contrast, in Xenopus oocytes, both the molecular presence and function of PMCA1 were demonstrated [23].
Besides the aforementioned mechanisms, the mitochondria may also contribute to shape [Ca2+]i rises during oscillations [24, 25], as these organelles can uptake Ca2+ into their matrix, thereby alleviating the overall cytosolic Ca2+ load [26]. In support of this function, inhibition of mitochondrial function disrupted [Ca2+]i oscillations and caused a sustained increase in [Ca2+]i in mouse eggs [27, 28]. Nonetheless, this does not seem to be its main function in terms of Ca2+ homeostasis, as inhibition of mitochondrial depolarization did not immediately terminate sperm-initiated oscillations [28]. Instead, and possibly due to its vicinity to the IP3R1/ER, the Ca2+-driven ATP output may be the mitochondria’s most critical contribution to Ca2+ homeostasis in MII eggs, as ATP production maintains SERCA activity, which is required to maintaining [Ca2+]ER and to sustain sperm-triggered Ca2+ oscillations [17]. Importantly, direct assessment of mitochondrial Ca2+ uptake has not been performed because the conventional inhibitors, such as ruthenium red, are somehow ineffective in eggs. Recently, however, an RNAi screen showed that a protein named MICU1 is required for mitochondrial Ca2+ uptake [29] and its discovery led to the molecular identification of the mitochondrial Ca2+ unipoter [30, 31]. Thus, using knockdown approaches it will be worth investigating the role of these molecules on [Ca2+]i oscillations in mammalian eggs.
2) Ca2+ influx mechanisms
Given that only a fraction of Ca2+ from each [Ca2+]i rise is deposited back into the ER Ca2+ store by SERCA, extracellular Ca2+ must be taken in to replenish [Ca2+]ER. Support for the notion that Ca2+ influx plays a pivotal role in fertilization is long-standing, as sperm-initiated [Ca2+]i oscillations cease prematurely in the absence of external Ca2+ [22, 32, 33]. Nonetheless, the molecular mechanisms underlining Ca2+ influx in mammalian eggs are poorly understood. The cells input extracellular Ca2+ across the plasma membrane through a variety of mechanisms, including receptor-operated channels (ROCs), voltage-operated channels (VOCs) [34] and store-operated Ca2+ channels (SOCs) [7, 34, 35]. The changes in membrane potential associated with [Ca2+]i responses in fertilized hamster eggs are of very low magnitude to suggest activation of VOCs during this process [36]. Further, several additional findings suggest that the participation of these channels in mammalian fertilization is likely minor, as [Ca2+]i rises precede changes in membrane potential [33], Ca2+ influx continues between [Ca2+]i rises [37] and, in the mouse, the changes in membrane potential are nearly imperceptible [33]. These findings raise the prospect that Ca2+ influx in eggs may be attained, at least in part, by a different mechanism(s). Instead, SOCE, which is associated with [Ca2+]ER levels, may fulfill at least in part this role in mammalian eggs. Towards this end, exposure of MII eggs to thapsigargin evoked a Ca2+ rise after addition of extracellular Ca2+, suggesting that SOCE is active in these cells [17]. Subsequent studies implicated SOCE during [Ca2+]i oscillations, as using the manganese-quenching technique it was found that in mouse eggs the initiation of each [Ca2+]i rise coincided with divalent cation influx [37, 38]. Nevertheless, additional demonstration of the participation of this mechanism in mammalian fertilization was prevented by lack of knowledge of the molecular components of this mechanism.
Recently, the proteins responsible for SOCE have been identified; stromal interaction molecule 1 (STIM1) is located mostly in the ER where it acts as a Ca2+ sensor, as its downregulation was shown to decrease Ca2+ influx in response to thapsigargin [39, 40]. Given that STIM1 lacks an obvious channel, the search was on to find the required channel partner protein, one of which was quickly identified as Orai1 [41-43]. The expression and functional analysis using siRNA knockdown of Stim1 and Orai1 were performed using porcine eggs and the downregulation of them prevented persistent [Ca2+]i oscillations [44, 45]. Although the involvement of Stim1 and Orai1 on [Ca2+]i oscillations has been demonstrated, their regulation, for instance, the spatio-temporal patterns at each [Ca2+]i response, and whether or not these molecules overlap during Ca2+ influx, remains obscure. In mouse oocytes, studies have also reported the presence of these molecules and their possible involvement in Ca2+ homeostasis both during maturation and fertilization. Nevertheless, the cellular organization, re-distribution and expression of these proteins during maturation as well their contribution to fertilization-associated oscillations needs to be clarified [46]. Further, transient receptor potential (TRP) ion channels [47], which are associated with both SOCs and ROCs and are expressed in mammalian eggs [15], may also participate in Ca2+ influx during oscillations and their role needs to be further explored.
Although the biological significance of [Ca2+]i oscillations in mammalian eggs seems to be ambiguous in terms of embryo development, it might be that episodic [Ca2+]i responses are important for the stepwise completion of the events of egg activation [10, 48]. Generally, different events of egg activation show differential susceptibility to [Ca2+]i increases, and their initiation and completion also have differential Ca2+ requirements. For instance, early events such as CG exocytosis and 2PB extrusion require fewer [Ca2+]i responses than later events such as PN formation and maternal recruitment of mRNAs [49]. While these results associated the completion of egg activation events to the number of [Ca2+]i rises, Miao and colleagues recently demonstrated using the Ca2+ insulation system with millimolar levels of extracellular Gd3+ that even if [Ca2+]i oscillations occur, eggs fails to extrude 2PB. Given that Ca2+ influx is obliterated under those conditions, the conclusion was drawn that, besides replenishing [Ca2+]ER, Ca2+ influx is required for completion of certain events of egg activation [22]. Up to now, the role of [Ca2+]i rises on egg activation events have been considered in the context of activation of the Ca2+/Calmodulin-dependent protein kinase II (CaMKII). It is well established that each sperm-induced [Ca2+]i rise is accompanied by a parallel increase in Ca2+/Calmodulin-dependent protein kinase II (CaMKII) activity [50]. Further, the role of CaMKII on egg activation in mammals was documented first by a series of studies where expression of constitutive active forms of CaMKII initiated all events of egg activation, except CG exocytosis, and promoted development to the blastocyst stage [51, 52], and second by genetic studies whereby depletion of CaMKIIγ isoform abrogated the ability of these eggs to exit MII in response to [Ca2+]i stimulation [53, 54] and caused infertility [54]. Thus, the authors speculated that Ca2+ influx activates critical signaling pathways upstream of CaMKIIγ that are required for 2PB emission [22]. Since the detection of Ca2+ by target molecules can be highly selective due to space, time and concentration constrains, as it should be when the signal involved regulates the completion of specific cellular events, the regulation of 2PB extrusion by Ca2+ influx could be viewed in the context of local vs. global Ca2+ sensing [55]. Regardless, further studies are needed to address the signals linking [Ca2+]i increases and each egg activation event. Further, while the suggested role of Ca2+ influx on 2PB extrusion is an important observation, the effects of high Gd3+ on 2PB extrusion should also be examined after use of treatments that induce egg activation and 2PB extrusion without increasing [Ca2+]i and presumably without inducing Ca2+ influx, such as the case after addition of the CDK1 And MAPK inhibitors [56].
Ca2+ homeostasis during oocyte maturation
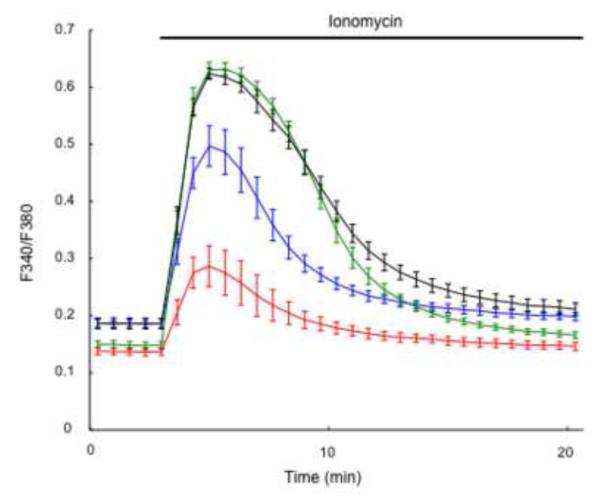
As described above, mouse eggs acquire fertilization-competence during oocyte maturation. Multiple factors are involved in the increased Ca2+ releasing ability of MII eggs, including IP3R1 phosphorylation and increased [Ca2+]ER content [18], although more in-depth studies are needed to determine the contribution of each of these changes to the overall increase in Ca2+ responsiveness. Following the theme of this review, here we continue the discussion of factors that regulate the increase in [Ca2+]ER. The increase in [Ca2+]ER during maturation is a well-documented phenomenon [8, 9, 18] and here we show that addition of ionomycin induces [Ca2+]i responses of increasing magnitude as maturation progresses (Fig. 2). How the increase in [Ca2+]ER contributes to [Ca2+]i oscillations in MII eggs was investigated by using cyclopiazonic acid (CPA), a reversible SERCA inhibitor [18]. CPA-treated GV oocytes advanced to the MII stage without delay or gross abnormalities, although without increasing their [Ca2+]ER. In this condition, CPA-matured eggs injected with PLCζ cRNA showed a shortened first [Ca2+]i rise even though CPA was washed away before initiating the oscillations. These results suggest that the increase in [Ca2+]ER directly impact [Ca2+]i oscillations, especially the robust first [Ca2+]i rise.
Fig. 2.
[Ca2+]ER content increases during mouse oocyte maturation. [Ca2+]ER was estimated from the [Ca2+]i responses induced by the addition of 2 μM ionomycin under Ca2+ free conditions. The mean fluorescent peak (Fura-2) was compared at 0 (red), 4 (blue), 8 (green) and 12 (black) hr of in vitro maturation, which corresponded with GV, GVBD, MI and MII stages of meiotic progression (n=5).
Little is known about how oocytes accumulate Ca2+ in the stores during maturation, although our preliminary results indicate that external Ca2+ is the source of the increased [Ca2+]ER during maturation (unpublished observations). Further, how Ca2+ loading occurs under basal [Ca2+]i levels is also unclear as well as the mechanisms/channels that might mediate this influx. Nevertheless, it is noteworthy that GV oocytes display spontaneous [Ca2+]i oscillations [57]. Importantly, these oscillations cease around the GV breakdown (GVBD) stage, which is when the most drastic increase in [Ca2+]ER is observed [8]. The temporal coincidence of these observations implies the low levels of [Ca2+]ER at the GV stage, possibly caused by a constitutive Ca2+ leak out of the ER, could promote Ca2+ influx. While additional studies are needed to clarify how spontaneous oscillations are terminated and the mechanism(s) that keeps low levels of [Ca2+]ER in GV mouse oocytes, given that Ca2+ influx is required for the GV-stage [Ca2+]i oscillations and the known regulation of Ca2+ influx by Ca2+ store content [58], it stands to reason that Ca2+ influx might be regulated during oocyte mouse maturation. In this regard, in Xenopus oocytes Ca2+ homeostasis and molecules associated with it undergo profound modifications during maturation and more precisely at the GVBD stage. For example, the function of the Ca2+ influx, SOCE, and efflux, PMCA, pathways is significantly downregulated at the GVBD stage [14, 59]. One of the key mechanisms underlying these downregulation is the dynamic reorganization of the plasma membrane during maturation, as channels and pumps are specifically internalized [14, 60], depriving these oocytes from quick shuttling of Ca2+ to and from the external milieu; seemingly, the membrane trafficking mechanism appear, at least in part, to be conserved between Xenopus and mammalian oocytes [61, 62]. Nonetheless, the contribution of plasma membrane reorganization as a regulatory element of Ca2+ homeostasis in mouse oocytes needs additional investigation. Further, the similarities between mouse and Xenopus oocytes must end at some point during maturation, as mouse eggs at the MII stage undergo oscillations after fertilization and, as noted before, these oscillations rely on Ca2+ influx. Therefore, important questions remain unresolved regarding the regulation of Ca2+ influx in mouse oocytes and eggs. For example, is the same mechanism that mediates Ca2+ influx at the GV stage, but that is inactivated at GVBD, capable of mediating influx in MII eggs following fertilization? Are there other Ca2+ channels that undergo differential regulation during maturation and fertilization? Is it possible that some Ca2+ channels are synthesized/incorporated into the plasma membrane after the GV stage? How conserved are the Ca2+ influx mechanisms during maturation and fertilization among mammals, considering that other elements of the Ca2+ oscillation toolkit such as IP3R1 sensitivity and PlCζ specific activity are widely different among these species. Therefore, studies to answer these questions should occupy researchers interested in this field for some time. Besides providing a deeper understanding of oocyte physiology, identification of these mechanisms is likely to have clinical applications both to improve developmental competence of in vitro matured oocytes as well as in the design of novel egg activation procedures.
Acknowledgements
The authors want to thank Dr. Roger Tsien’s lab, UCD, for sharing the D1ER construct. These studies were completed thanks to grant HD051872 from NIH to R.A.F. We thank Dr. Nan Zhang and Banyoon Cheon for useful discussions. We apologize to those researchers whose work was not cited due to space constraints
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement All authors must disclose any financial and personal relationships with other people or organisations that could inappropriately influence (bias) their work, all within 3 years of beginning the work submitted. If there are no conflicts of interest, authors should state that there are none.
There is no conflict of interests of Dr. R Fissore or Dr. T Wakai.
References
- [1].Miyazaki S, Hashimoto N, Yoshimoto Y, Kishimoto T, Igusa Y, Hiramoto Y. Temporal and spatial dynamics of the periodic increase in intracellular free calcium at fertilization of golden hamster eggs. Dev Biol. 1986;118:259–267. doi: 10.1016/0012-1606(86)90093-x. [DOI] [PubMed] [Google Scholar]
- [2].Schultz RM, Kopf GS. Molecular basis of mammalian egg activation. Curr Top Dev Biol. 1995;30:21–62. doi: 10.1016/s0070-2153(08)60563-3. [DOI] [PubMed] [Google Scholar]
- [3].Miyazaki S, Yuzaki M, Nakada K, Shirakawa H, Nakanishi S, Nakade S, Mikoshiba K. Block of Ca2+ wave and Ca2+ oscillation by antibody to the inositol 1,4,5-trisphosphate receptor in fertilized hamster eggs. Science. 1992;257:251–255. doi: 10.1126/science.1321497. [DOI] [PubMed] [Google Scholar]
- [4].Berridge MJ. The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium. 2002;32:235–249. doi: 10.1016/s0143416002001823. [DOI] [PubMed] [Google Scholar]
- [5].Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- [6].Bootman MD, Collins TJ, Peppiatt CM, Prothero LS, MacKenzie L, De Smet P, Travers M, Tovey SC, Seo JT, Berridge MJ, Ciccolini F, Lipp P. Calcium signalling--an overview. Semin Cell Dev Biol. 2001;12:3–10. doi: 10.1006/scdb.2000.0211. [DOI] [PubMed] [Google Scholar]
- [7].Smyth JT, Dehaven WI, Jones BF, Mercer JC, Trebak M, Vazquez G, Putney JW., Jr. Emerging perspectives in store-operated Ca2+ entry: roles of Orai, Stim and TRP. Biochimica et biophysica acta. 2006;1763:1147–1160. doi: 10.1016/j.bbamcr.2006.08.050. [DOI] [PubMed] [Google Scholar]
- [8].Jones KT, Carroll J, Whittingham DG. Ionomycin, thapsigargin, ryanodine, and sperm induced Ca2+ release increase during meiotic maturation of mouse oocytes. The Journal of biological chemistry. 1995;270:6671–6677. doi: 10.1074/jbc.270.12.6671. [DOI] [PubMed] [Google Scholar]
- [9].Mehlmann LM, Mikoshiba K, Kline D. Redistribution and increase in cortical inositol 1,4,5-trisphosphate receptors after meiotic maturation of the mouse oocyte. Developmental biology. 1996;180:489–498. doi: 10.1006/dbio.1996.0322. [DOI] [PubMed] [Google Scholar]
- [10].Wakai T, Vanderheyden V, Fissore RA. Ca2+ signaling during mammalian fertilization: requirements, players, and adaptations. Cold Spring Harbor perspectives in biology. 2011;3 doi: 10.1101/cshperspect.a006767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Saunders CM, Larman MG, Parrington J, Cox LJ, Royse J, Blayney LM, Swann K, Lai FA. PLC zeta: a sperm-specific trigger of Ca(2+) oscillations in eggs and embryo development. Development. 2002;129:3533–3544. doi: 10.1242/dev.129.15.3533. [DOI] [PubMed] [Google Scholar]
- [12].Nomikos M, Yu Y, Elgmati K, Theodoridou M, Campbell K, Vassilakopoulou V, Zikos C, Livaniou E, Amso N, Nounesis G, Swann K, Lai FA. Phospholipase Czeta rescues failed oocyte activation in a prototype of male factor infertility. Fertility and sterility. 2012 doi: 10.1016/j.fertnstert.2012.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Brini M, Carafoli E. The plasma membrane Ca(2)+ ATPase and the plasma membrane sodium calcium exchanger cooperate in the regulation of cell calcium. Cold Spring Harbor perspectives in biology. 2011;3 doi: 10.1101/cshperspect.a004168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].El-Jouni W, Jang B, Haun S, Machaca K. Calcium signaling differentiation during Xenopus oocyte maturation. Developmental biology. 2005;288:514–525. doi: 10.1016/j.ydbio.2005.10.034. [DOI] [PubMed] [Google Scholar]
- [15].Su YQ, Sugiura K, Woo Y, Wigglesworth K, Kamdar S, Affourtit J, Eppig JJ. Selective degradation of transcripts during meiotic maturation of mouse oocytes. Developmental biology. 2007;302:104–117. doi: 10.1016/j.ydbio.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wang S, Kou Z, Jing Z, Zhang Y, Guo X, Dong M, Wilmut I, Gao S. Proteome of mouse oocytes at different developmental stages. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:17639–17644. doi: 10.1073/pnas.1013185107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kline D, Kline JT. Thapsigargin activates a calcium influx pathway in the unfertilized mouse egg and suppresses repetitive calcium transients in the fertilized egg. The Journal of biological chemistry. 1992;267:17624–17630. [PubMed] [Google Scholar]
- [18].Wakai T, Vanderheyden V, Yoon SY, Cheon B, Zhang N, Parys JB, Fissore RA. Regulation of inositol 1,4,5-trisphosphate receptor function during mouse oocyte maturation. Journal of cellular physiology. 2012;227:705–717. doi: 10.1002/jcp.22778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Palmer AE, Jin C, Reed JC, Tsien RY. Bcl-2-mediated alterations in endoplasmic reticulum Ca2+ analyzed with an improved genetically encoded fluorescent sensor. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:17404–17409. doi: 10.1073/pnas.0408030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pepperell JR, Kommineni K, Buradagunta S, Smith PJ, Keefe DL. Transmembrane regulation of intracellular calcium by a plasma membrane sodium/calcium exchanger in mouse ova. Biology of reproduction. 1999;60:1137–1143. doi: 10.1095/biolreprod60.5.1137. [DOI] [PubMed] [Google Scholar]
- [21].Carroll J. Na+-Ca2+ exchange in mouse oocytes: modifications in the regulation of intracellular free Ca2+ during oocyte maturation. Journal of reproduction and fertility. 2000;118:337–342. doi: 10.1530/jrf.0.1180337. [DOI] [PubMed] [Google Scholar]
- [22].Miao YL, Stein P, Jefferson WN, Padilla-Banks E, Williams CJ. Calcium influx-mediated signaling is required for complete mouse egg activation. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:4169–4174. doi: 10.1073/pnas.1112333109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].El-Jouni W, Haun S, Machaca K. Internalization of plasma membrane Ca2+-ATPase during Xenopus oocyte maturation. Developmental biology. 2008;324:99–107. doi: 10.1016/j.ydbio.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Duchen MR. Mitochondria and calcium: from cell signalling to cell death. The Journal of physiology. 2000;529(Pt 1):57–68. doi: 10.1111/j.1469-7793.2000.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Rizzuto R, Bernardi P, Pozzan T. Mitochondria as all-round players of the calcium game. The Journal of physiology. 2000;529(Pt 1):37–47. doi: 10.1111/j.1469-7793.2000.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- [27].Liu L, Hammar K, Smith PJ, Inoue S, Keefe DL. Mitochondrial modulation of calcium signaling at the initiation of development. Cell calcium. 2001;30:423–433. doi: 10.1054/ceca.2001.0251. [DOI] [PubMed] [Google Scholar]
- [28].Dumollard R, Marangos P, Fitzharris G, Swann K, Duchen M, Carroll J. Sperm-triggered [Ca2+] oscillations and Ca2+ homeostasis in the mouse egg have an absolute requirement for mitochondrial ATP production. Development. 2004;131:3057–3067. doi: 10.1242/dev.01181. [DOI] [PubMed] [Google Scholar]
- [29].Perocchi F, Gohil VM, Girgis HS, Bao XR, McCombs JE, Palmer AE, Mootha VK. MICU1 encodes a mitochondrial EF hand protein required for Ca(2+) uptake. Nature. 2010;467:291–296. doi: 10.1038/nature09358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, Koteliansky V, Mootha VK. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].De Stefani D, Raffaello A, Teardo E, Szabo I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Winston NJ, McGuinness O, Johnson MH, Maro B. The exit of mouse oocytes from meiotic M-phase requires an intact spindle during intracellular calcium release. Journal of cell science. 1995;108(Pt 1):143–151. doi: 10.1242/jcs.108.1.143. [DOI] [PubMed] [Google Scholar]
- [33].Igusa Y, Miyazaki S. Effects of altered extracellular and intracellular calcium concentration on hyperpolarizing responses of the hamster egg. The Journal of physiology. 1983;340:611–632. doi: 10.1113/jphysiol.1983.sp014783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Barritt GJ. Receptor-activated Ca2+ inflow in animal cells: a variety of pathways tailored to meet different intracellular Ca2+ signalling requirements. The Biochemical journal. 1999;337(Pt 2):153–169. [PMC free article] [PubMed] [Google Scholar]
- [35].Putney JW., Jr. Capacitative calcium entry: sensing the calcium stores. The Journal of cell biology. 2005;169:381–382. doi: 10.1083/jcb.200503161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Miyazaki S, Igusa Y. Ca-mediated activation of a K current at fertilization of golden hamster eggs. Proceedings of the National Academy of Sciences of the United States of America. 1982;79:931–935. doi: 10.1073/pnas.79.3.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].McGuinness OM, Moreton RB, Johnson MH, Berridge MJ. A direct measurement of increased divalent cation influx in fertilised mouse oocytes. Development. 1996;122:2199–2206. doi: 10.1242/dev.122.7.2199. [DOI] [PubMed] [Google Scholar]
- [38].Mohri T, Shirakawa H, Oda S, Sato MS, Mikoshiba K, Miyazaki S. Analysis of Mn(2+)/Ca(2+) influx and release during Ca(2+) oscillations in mouse eggs injected with sperm extract. Cell calcium. 2001;29:311–325. doi: 10.1054/ceca.2000.0196. [DOI] [PubMed] [Google Scholar]
- [39].Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr., Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Current biology : CB. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. The Journal of cell biology. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- [42].Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zhang SL, Yeromin AV, Zhang XH, Yu Y, Safrina O, Penna A, Roos J, Stauderman KA, Cahalan MD. Genome-wide RNAi screen of Ca(2+) influx identifies genes that regulate Ca(2+) release-activated Ca(2+) channel activity. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:9357–9362. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wang C, Lee K, Gajdocsi E, Papp AB, Machaty Z. Orai1 mediates store-operated Ca2+ entry during fertilization in mammalian oocytes. Developmental biology. 2012;365:414–423. doi: 10.1016/j.ydbio.2012.03.007. [DOI] [PubMed] [Google Scholar]
- [45].Lee K, Wang C, Machaty Z. STIM1 is required for Ca2+ signaling during mammalian fertilization. Developmental biology. 2012;367:154–162. doi: 10.1016/j.ydbio.2012.04.028. [DOI] [PubMed] [Google Scholar]
- [46].Gomez-Fernandez C, Lopez-Guerrero AM, Pozo-Guisado E, Alvarez IS, Martin-Romero FJ. Calcium signaling in mouse oocyte maturation: the roles of STIM1, ORAI1 and SOCE. Molecular human reproduction. 2012;18:194–203. doi: 10.1093/molehr/gar071. [DOI] [PubMed] [Google Scholar]
- [47].Venkatachalam K, Montell C. TRP channels. Annual review of biochemistry. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ducibella T, Fissore R. The roles of Ca2+, downstream protein kinases, and oscillatory signaling in regulating fertilization and the activation of development. Developmental biology. 2008;315:257–279. doi: 10.1016/j.ydbio.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ducibella T, Huneau D, Angelichio E, Xu Z, Schultz RM, Kopf GS, Fissore R, Madoux S, Ozil JP. Egg-to-embryo transition is driven by differential responses to Ca(2+) oscillation number. Developmental biology. 2002;250:280–291. [PubMed] [Google Scholar]
- [50].Markoulaki S, Matson S, Abbott AL, Ducibella T. Oscillatory CaMKII activity in mouse egg activation. Dev Biol. 2003;258:464–474. doi: 10.1016/s0012-1606(03)00133-7. [DOI] [PubMed] [Google Scholar]
- [51].Knott JG, Gardner AJ, Madgwick S, Jones KT, Williams CJ, Schultz RM. Calmodulin-dependent protein kinase II triggers mouse egg activation and embryo development in the absence of Ca2+ oscillations. Dev Biol. 2006;296:388–395. doi: 10.1016/j.ydbio.2006.06.004. [DOI] [PubMed] [Google Scholar]
- [52].Madgwick S, Levasseur M, Jones KT. Calmodulin-dependent protein kinase II, and not protein kinase C, is sufficient for triggering cell-cycle resumption in mammalian eggs. J Cell Sci. 2005;118:3849–3859. doi: 10.1242/jcs.02506. [DOI] [PubMed] [Google Scholar]
- [53].Chang HY, Minahan K, Merriman JA, Jones KT. Calmodulin-dependent protein kinase gamma 3 (CamKIIgamma3) mediates the cell cycle resumption of metaphase II eggs in mouse. Development. 2009;136:4077–4081. doi: 10.1242/dev.042143. [DOI] [PubMed] [Google Scholar]
- [54].Backs J, Stein P, Backs T, Duncan FE, Grueter CE, McAnally J, Qi X, Schultz RM, Olson EN. The gamma isoform of CaM kinase II controls mouse egg activation by regulating cell cycle resumption. Proc Natl Acad Sci U S A. 107:81–86. doi: 10.1073/pnas.0912658106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Tadross MR, Dick IE, Yue DT. Mechanism of local and global Ca2+ sensing by calmodulin in complex with a Ca2+ channel. Cell. 2008;133:1228–1240. doi: 10.1016/j.cell.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Phillips KP, Petrunewich MA, Collins JL, Booth RA, Liu XJ, Baltz JM. Inhibition of MEK or cdc2 kinase parthenogenetically activates mouse eggs and yields the same phenotypes as Mos(−/−) parthenogenotes. Developmental biology. 2002;247:210–223. doi: 10.1006/dbio.2002.0680. [DOI] [PubMed] [Google Scholar]
- [57].Carroll J, Swann K. Spontaneous cytosolic calcium oscillations driven by inositol trisphosphate occur during in vitro maturation of mouse oocytes. The Journal of biological chemistry. 1992;267:11196–11201. [PubMed] [Google Scholar]
- [58].Putney JW, Bird GS. Cytoplasmic calcium oscillations and store-operated calcium influx. The Journal of physiology. 2008;586:3055–3059. doi: 10.1113/jphysiol.2008.153221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Machaca K, Haun S. Store-operated calcium entry inactivates at the germinal vesicle breakdown stage of Xenopus meiosis. The Journal of biological chemistry. 2000;275:38710–38715. doi: 10.1074/jbc.M007887200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Yu F, Sun L, Machaca K. Orai1 internalization and STIM1 clustering inhibition modulate SOCE inactivation during meiosis. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:17401–17406. doi: 10.1073/pnas.0904651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].El-Jouni W, Haun S, Hodeify R, Hosein Walker A, Machaca K. Vesicular traffic at the cell membrane regulates oocyte meiotic arrest. Development. 2007;134:3307–3315. doi: 10.1242/dev.005454. [DOI] [PubMed] [Google Scholar]
- [62].Lowther KM, Nikolaev VO, Mehlmann LM. Endocytosis in the mouse oocyte and its contribution to cAMP signaling during meiotic arrest. Reproduction. 2011;141:737–747. doi: 10.1530/REP-10-0461. [DOI] [PubMed] [Google Scholar]