Abstract
We report to fabricate functional three-dimensional (3D) tissue constructs by using an inkjet based bio-prototyping method. With the use of the modified inkjet printers, contractile cardiac hybrids that exhibit the forms of the 3D rectangular sheet and even the “half heart” (with two connected ventricles) have been fabricated by arranging alternate layers of biocompatible alginate hydrogels and mammalian cardiac cells according to pre-designed 3D patterns. In this study, primary feline adult and H1 cardiomyocytes were used as model cardiac cells. Alginate hydrogels with controlled micro-shell structures were built by spraying cross-linkers in micro drops onto un-gelled alginic acid. The cells remained viable in constructs as thick as 1 cm due to the programmed porosity. Microscopic and macroscopic contractile functions of these cardiomyocytes constructs were observed in vitro. These results suggest that the inkjet bio-prototyping method could be used for hierarchical design of functional cardiac pseudo tissues, balanced with porosity for mass transport and structural support.
1. Introduction
Heart attacks and heart failure remain among the most prominent health challenges despite many breakthroughs in cardiovascular surgery and medicine.[1] In particular, congestive heart failure (CHF) affects an estimated 4.8 million patients in US and 400,000 new cases are diagnosed each year.[2] Mortality is greater than 50% within 5 years of this diagnosis.[3] Moreover, more than 1,000,000 CHF patients will die within two years following their diagnosis.[1] Additionally, CHF is a considerable problem worldwide, affecting approximately 1% of infants and is associated with significant morbidity and mortality.
CHF is characterized by loss or dysfunction of cardiomyocytes, whether because of ischemic heart disease, hypertensive heart disease, or idiopathic cardiomyopathy.[2] Although some stem cells might be present in the heart, the adult heart muscle in unable to regenerate and the damaged myocardial tissue is replaced with non contractile scar tissue.[4, 5] To date, the primary clinical solution for CHF patients is heart tissue transplantation. This approach, however, is limited due to the persistent shortage in donors. At present, one of the alternative therapeutic strategies is the implantation of functional, in vitro-grown cardiac muscle tissues.[3, 6, 7] To perform this challenging task, fabrication of 3D functional cardiac tissue-like constructs have to be addressed.
Furthermore, the need for bioengineered cardiac tissues is not limited to heart tissue; it has the potential of widespread use for in vitro applications such as the use of perfused three-dimensional human cardiac tissues for toxicological research, drug testing and screening or personalized medicine. In these devices, cardiac tissues with specific electrical and electro-mechanical functions are needed as functional transduction elements.
Currently artificial cardiac tissue constructs are mainly produced by the molding[8] and/or layering[9, 10] methods. These methods, however, are limited to low efficiency as well as lacking of effective mechanisms to deliver a variety of cardiac muscle cells into their appropriate sites within 3D scaffolds. The heart tissue consists of approximately 30% cardiac myocytes and 70% non-myocytes.[6, 11] Non-myocytes play a pivotal role in cardiomyocytes development, differentiation, and functionality.[12] Therefore, an effective method to subtly arrange different cardiac cells to generate spatially-organized cardiac constructs has become critically important. The inkjet based bio-prototyping technology may offer a viable solution.
The feasibility of the novel concept of joining cells and biomaterials with the help of inkjet printers into 3D scaffolds and cellular structures has been demonstrated [13-16]. We have shown that commercially available desktop printers can be modified to perform diverse tasks, such as printing self-assembled monolayers, proteins, and other extracellular matrix molecules [17, 18]. In this study we will explain how the inkjet bio-prototyping technology was used to fabricate characterized functional cardiac tissue constructs. Primary feline adult and H1 cardiomyocytes were used as model cardiac cells and alginate gel was used as biocompatible scaffold for the bioengineered cardiac tissues. Functional cardiac constructs with specific forms and structures have been fabricated by arranging alternate layers of alginate hydrogels and mammalian cardiac cells according to pre-designed 3D patterns.
2. Materials and methods
2.1 Cell Preparation
Two cardiomyocytes including primary feline adult cardiomyoctyes and HL1 cardiac muscle cell line were used in this study. The primary feline cardiomyocytes were prepared according to the established isolation protocol[19]. Briefly, ten adult cats of either sex (1.8-4.0 kg) were anesthetized with meperidine (92.2 mg/kg im), acepromazine maleate (5 mg/kg im), ketamine hydrochloride (50 mg/mg im) and heparinized (1,000 U iv). A left thoracotomy was performed, the heart was rapidly removed, the aorta was cannulated, and the coronary arteries were perfused retrogradely for 10 min; first with a recirculating HEPES-Krebs buffer (in mM: 140 NaCl, 4.8 KCl, 2.4 MgSO4, 1.2 NaH2PO4, 4.0 NaHCO3, 0.5 CaCl2, 12 HEPES, and 12.5 glucose), second with a non-recirculating buffer of the same composition without supplemental calcium and third with a recirculating calcium-free buffer supplemented with 1.6 mg/ml collagenase B (Boehringer-Mannheim). Perfusion was terminated when the heart was flaccid. The heart was removed from the cannula, the cardiac tissue was minced and gently sieved through a 210-μm nylon mesh to isolate the cardiomyocytes. After centrifugation the cells were washed with HEPES-Krebs buffer and 1% BSA and re-suspended in the same substances.
The HL-1 cardiac muscle cell line was obtained as a gift from William C. Claycomb (Louisiana State University Health Science Center). The HL-1 cells derived from AT-1 cardiomyocytes are a new cardiac muscle cell line available that continuously divides and spontaneously contracts while maintaining a differentiated cardiac phenotype [20]. The culture of the HL-1 cells was prepared by seeding 100,000 cells in a gelatin/fibrinogen-coated T-75 flask [21] and incubating at 37°C under 95% air / 5% CO2 in Dulbecco's Modified Eagles Media (DMEM) augmented by 10% fetal bovine serum and 1% antibiotic antimycotic (Sigma). The culture medium was replaced every 2 days. When reaching confluence, the cells were removed from the flasks by the aid of trypsin-EDTA (Sigma) and collected by centrifugation (1000 rpm, 5 min).
2.2 Gels preparation
Sodium alginic acid (Acros Chemcicals) and gelatin (Sigma Chemicals) were dissolved in 1× phosphate buffered saline (PBS). The final concentrations of the two chemicals were obtained to be 2.3% and 0.1%, respectively. For sterilization and complete dissolution, the mixture was autoclaved at 121°C for 30 min.
2.3 Inkjet bio-prototyping set-up
HP DeskJet 550 printers were modified with the use of the methods previously described [22]. Moreover, in order to build 3D structures, a z-axis module was added into the modified printers. The z-axis module customized from a stepper motor assembly was built to provide the printer with an electronically controlled elevator chamber. For the elevator stage a 2.5-cm round glass cover-slip (Fisher Scientific, Pittsburgh, PA) was fixed to the tip of a metallic rod, which was connected to the stepper motor. A DC power supply was used to generate a 4V signal to operate the stepper motor through a series of 4 toggle switches. A sterile 50 ml conical tube (Fischer Scientific) was customized and used as the elevator chamber. A commercial inkjet cartridge (HP51626A) was emptied and rinsed thoroughly with double distilled water and 100% ethanol several times until completely free of ink [23]. It was then sonicated using an ultrasonic sonicator to remove any ink residues or blockages. The cartridge was sprayed with 100% ethanol and the whole assembly of the printer along with the cartridge was placed overnight in a Class II hood under ultraviolet light prior to use for extra sterilization.
2.4 Fabrication of 3D cardiac constructs
3D cardiac constructs with specific “half heart” forms of 1 cm inner diameter with two connected ventricles and rectangular sheet of 3×0.8×0.5 cm dimension were printed layer-by-layer using cardiomyocytes micro-entrapped alginate/gelatin gels, and crosslinked on demand with calcium chloride (CaCl2). Cell pellets were collected by centrifugation in a conical tube. After aspirating the supernatant, the cell pellets were re-suspended in the alginate/gelatin solution to obtain a final concentration of 2×106 or 7×106 cells/ml for isolated feline cardiomyocytes or HL1 cells in the un-crosslinked alginate/gelatin gel, respectively. The 3D rectangular sheet pattern was designed in a Word document (Microsoft, Redmond, WA) by using a 2D square pattern. The pattern for the 3D “half heart” is composed of a series of consecutive elliptic or circular patterns with different dimensions, also designed in a Word document. The inkjet cartridge was filled with 1.5 ml of 0.25M CaCl2 solution. Hybond® paper (Amersham Pharmacia Biotech, Ireland) was placed on the cover slip of the z-stage and the 3D cardiac constructs were printed on this paper. After every print action a re-coating procedure was performed by lowering the elevator platform into the alginate filled chamber and brought back 100 microns below the original position utilizing the stepper motor assembly. This step was done to ensure the gelling of the printed structure. In order to maximize the amount of cardiomyocytes used 0.25ml of the cell suspensions were manually placed onto the gelled rings after each re-coating procedure. The stacked rings (for 3D “half heart”) or rectangular forms (for 3D sheet) gelled atop each other to create a 3D construct with pre-designed form and structure. The constructs were carefully detached from the Hybond paper and placed in 1× PBS for 5 min to remove excessive un-crosslinked alginate. After treateding with 0.25M CaCl2 solution for 5 min for further crosslinking, the 3D cardiac constructs were moved into polystyrene dishes (Fisher Scientific) filled with cell culture medium. As controls, 3D printed constructs with no cellular components were made by the same printing method.
2.5 Modification of hydrogel formula
To improve cellular attachment on the printed alginate gel scaffolds, gel formulations that would allow for attachment and alignment of the cardiomyocytes were tested. The tested formulations were pure alginate and alginate augmented by 1% laminin (Sigma-Aldrich). The parallel alginate channels were fabricated by directly printing CaCl2 onto the pure alginate gel or alginate/laminin gel. In all experiments the primary feline cardiomyocytes were introduced under laminar flow into the channels.
2.6 Scanning electron microscopy
3D printed sheets without the entrapment of living cells were observed with SEM to evaluate the microstructures of printed alginate scaffolds. The samples were fixed with 4% formaldehyde in 1× PBS (pH7.4) for 20-30 min at room temperature. After dehydrating through a graded series of ethanol rinses, the samples were critically dried under vacuum and were sputter-coated with a thin layer of Chromium using a Hummer® 6.2 Sputter Coater (Anatech, Ltd) in a 100 m Torr vacuum argon environment for a 3 min period and 10 mA of current. Images were taken using a Hitachi S4700N Field Emission Scanning Electron Microscope (FESEM) at a 5 kV accelerating voltage.
2.7. Characterizations of 3D cardiac pseudo tissues
The tensile properties of the cardiac tissue samples were determined at room temperature by stretching the sample at a constant deformation rate of 5mm/min. The unaxial tensile testing was performed by using the MTS electromechanical testing system (MTS Systems Corporation, Eden Prairie, MN) and original data were acquired and analyzed by the software of TestWorks® (MTS System Corporation). The resulting stress-strain data were used to calculate the linear modulus (LM) and ultimate tensile stress (UTS). The LM was defined as the slope of the linear portion of the stress-strain curve, which occurred in the range of 0-100% of the UTS. The peak stress achieved during mechanical testing was taken as the UTS.
Mechano-electrical evaluations of the printed constructs were performed using a commercial product computer assisted videomicroscopy and video edge-detection protocols (IonOptix®). The Grass S-88 electrical stimulator used in these experiments provides an alternating polarity square pulse of 5 ms with amplitude of 140 V at a frequency of 1 Hz. To be more specific, the voltage across the platinum electrodes inside the study chamber was measured to be approximately 2 V.
3. Results
3.1 Microstructures of printed alginate scaffolds
Fabrication will affect structure and function of designed hydrogels; thus, the mechanism by which these materials are constructed needed to be investigated. We examined the fabricated alginate hydrogel layers macroscopically and microscopically. Figure 1a shows the overall structure of a 3D alginate scaffold in the form of the “half heart” with two connected ventricles and figure 1b shows SEM images of a cross section of the printed 3D sheet. In the “half heart” constructs, two hollow ventricular chambers were generated to mimic the chamber-wall macroscopic structure of a heart. Meanwhile, the SEM images revealed that the droplets are hollow shells with a diameter of about 25 μm that contain no hydrogel material. The 3D printed alginate scaffolds exhibit thus a unique micro-shell structure, brought about by the printed patterns composed of micro scale droplets. In addition, a few satellite dots are seen around the micro-shell pattern and some collapsed micro-shells were seen in the lower layers of the printed scaffolds.
Figure 1.
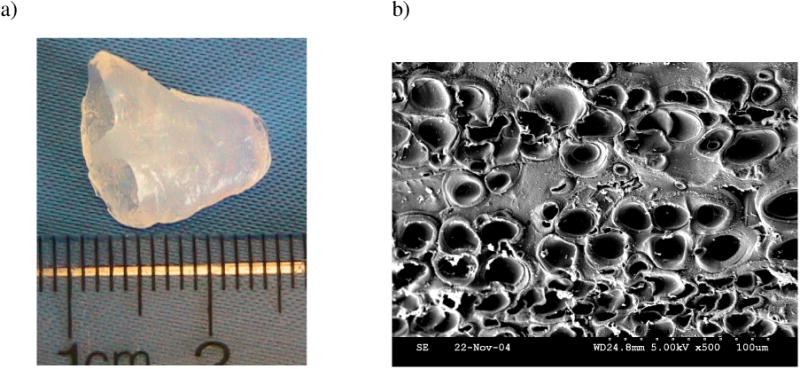
(a) Photography of the printed 3D “half-heart” scaffold with connected ventricles; (b) SEM image of the cross-section of the printed 3D cardiac constructs.
3.2 Mechanical and mechno-electrical characterizations of the printed 3D cardiac constructs
Tensile properties of alginate scaffolds fabricated by the inkjet printing method and the manual method (manually mixing alginic acid and CaCl2 solution) were shown in Table 1. Compared to the manually made scaffold, the printed s cardiac constructs had a lower LM and UTS. One possible reason is the differences in micro-structures between the printed and the manual samples. The printed scaffolds demonstrated the micro-shell structure and had a higher ratio of the void to the matrix than the manual samples. The materials with more voids usually show lower mechanical properties.
Table 1. Tensile properties of alginate scaffolds.
| Printed | Manual | |
|---|---|---|
| LM (kPa) | 113.3±17.5 | 380.0±118.5 |
| UTS (kPa) | 43.9±12.2 | 232.3±88.9 |
| n | 5 | 6 |
Under electrical stimulations, functional excitation-contraction coupling of the printed 3D cardiac pseudo constructs was demonstrated. In the constructs, not only were the individual adult feline cardiomyocytes observed to contract rhythmically within 3D printed constructs, but also the whole constructs were beating periodically. Figure 3 shows the representative beat and velocity transients of the edges of the printed sheets at day 1 of the culture, recorded by the IonOptix® video edge-detection system. Electrically stimulated at 1Hz, the shortening extent of the printed constructs was up to 3.5% of the total length of the constructs, and the shortening and relaxing velocities of the printed constructs entrapped with adult feline cardiomyocytes were about -403.9 ± 23.9μm/s and 437.5 ± 58. 9μm/s (both, n=3), respectively. After incubation in static culture without stimulation for five days, beating upon stimulation was also observed but with weaker activities.
Figure 3.
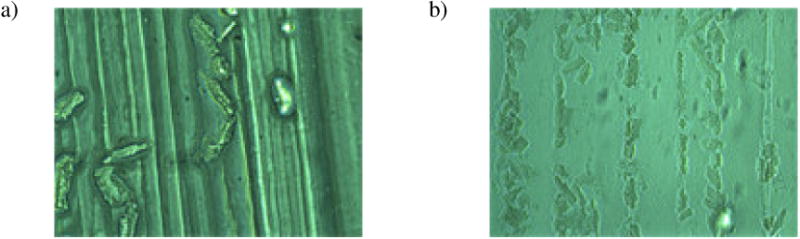
Phase-contrast microscopic images of feline adult cardiomyocytes on the printed alginate channels. Poor attachment was seen on pure alginate channels (a); on the contrary, many cardiomyocytes were found to align and attach to alginate/laminin channels (b).
3.3 The effects of hydrogel formula on cellular attachment and electrical properties
The cellular attachment of the printed parallel channels was evaluated by using a phase-contrast microscope. As shown in Figure 3a, deficient attachment to pure alginate channels was observed. However, as seen in Figure 3b, many cardiomyocytes were found to align and attach to alginate/laminin channels.
The mechano-electrical properties of the printed channels were also evaluated. The whole patches with printed channels were stimulated at 1Hz and contractility was recorded. Pure alginate constructs did not show much contractility; however the individual cardiomyocytes contracted about 9.8%, thus confirming viable, functional cells on the printed channels. Alginate/laminin constructs with channels and aligned cardiomyocytes contracted 7% and individual cardiomyocytes inside the channels contracted on average 7%. One possible reason is that compared to pure alginate scaffold, the somewhat lower contractility of the individual cells on the alginate/alumina scaffold is due to the attachment of cardiomyocytes to the hydrogel construct. This will be further investigated.
4. Discussion
Cardiac muscle tissues play a significant role in the contraction of the atria and ventricles of the heart and endure static and dynamic loads during the beating of the heart. To fabricate cardiac muscles, appropriate scaffold materials are needed. Alginate, a negatively charged polysaccharide from seaweed, can form hydrogel in the presence of divalent ions (e.g. Ca2+).[24, 25] In its hydrogel form, the alginate bears resemblance to glycol-components of the ECM.[1] Because of their gentle gelling properties, alginates are widely used for cell encapsulation in vitro[26] and in vivo[27] as well as for tissue engineering applications [24, 28]. Recently, the feasibility of bioengineering cardiac tissues by using alginate scaffolds has been demonstrated.[29] After grafting into rat infracted myocardium, the alginate cardiac bio-grafts stimulated intense neovascularization from the neighboring coronaries and attenuated left ventricular dilatation and failure[29]. In this study, the alginate was used as s printable hydrogel to fabricate 3D functional cardiac tissue-like constructs
Novel printable hydrogels with adequate mechanical properties are needed to engineer cardiac “pseudo-tissues”. In this study printed alginate gels demonstrated elastic modulus and ultimate tensile strength of 113.3±17.5 kPa and 43.9±12.2 kPa, respectively, correlating well with the modulus of cardiomyocytes that was found to be 100.3+/-10.7 kPa [30]. Most mammalian tissue-derived cells; including feline adult cardiomyocytes are anchorage-dependent and require attachment to a solid surface for viability, growth, and function [31]. Cell attachment is correlated to many factors; such as the stiffness or modulus of the materials, the attachment areas and the surface properties of the materials [32, 33]. It has been demonstrated that most mammalian cells are unable to specifically interact with and attach on cast alginate polysaccharides or their hydrogels [34]. In this study adult cat cardiomyocytes attached to the alginate scaffolds, judged by the harmonic contractile response of matrix and the cells. One possible explanation lies in that the linear modulus of the scaffolds is close to that of the cardiomyocytes.
5. Conclusions
3D functional cardiac tissues with specific forms and structures can be fabricated by the inkjet based bio-prototyping method. The printed cardiac tissues exhibited contractile properties under mild electrical stimuli in vitro. The microstructures of the printed alginate scaffolds exhibited ordered micro-shell arrays in each layer. The cardiac cells remained viable in the printed constructs thick as 1 cm due to the programmed porosity. The printed scaffolds demonstrated adequate elastic moduli and tensile strengths for harmonic response to cardiomycyte contractility. With the obvious advantages of high throughput and flexibility, this inkjet bio-prototyping method offers researchers a cost-effective tool for hierarchical design and fabrication of functional cardiac pseudo tissues.
Figure 2.
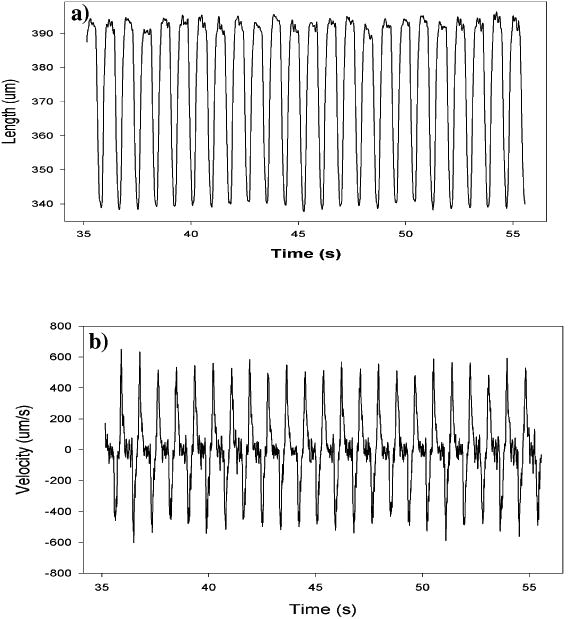
Representative beating transient (a) and velocity (b) of the edges of the cardiac constructs.
References
- 1.Shachar M, Cohen S. Cardiac tissue engineering, ex-vivo: Design principles in biomaterials and bioreactors. Heart Failure Reviews. 2003 Jul;8(3):271–276. doi: 10.1023/a:1024729919743. [DOI] [PubMed] [Google Scholar]
- 2.Kessler PD, Byrne BJ. Myoblast cell grafting into heart muscle: Cellular biology and potential applications. Annual Review of Physiology. 1999;61:219–242. doi: 10.1146/annurev.physiol.61.1.219. [DOI] [PubMed] [Google Scholar]
- 3.Fuchs JR, Nasseri BA, Vacanti JP. Tissue engineering: A 21st century solution to surgical reconstruction. Annals of Thoracic Surgery. 2001;72(2):577–591. doi: 10.1016/s0003-4975(01)02820-x. [DOI] [PubMed] [Google Scholar]
- 4.Sakai T, Li RK, Weisel RD, Mickle DAG, Kim EJ, Tomita S, et al. Autologous heart cell transplantation improves cardiac function after myocardial injury. Annals of Thoracic Surgery. 1999 Dec;68(6):2074–2080. doi: 10.1016/s0003-4975(99)01148-0. [DOI] [PubMed] [Google Scholar]
- 5.Fraser JK, Schreiber RE, Zuk PA, Hedrick MH. Adult stem cell therapy for the heart. International Journal of Biochemistry & Cell Biology. 2004 Apr;36(4):658–666. doi: 10.1016/j.biocel.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 6.Zimmermann WH, Melnychenko I, Eschenhagen T. Engineered heart tissue for regeneration of diseased hearts. Biomaterials. 2004 Apr;25(9):1639–1647. doi: 10.1016/s0142-9612(03)00521-0. [DOI] [PubMed] [Google Scholar]
- 7.Papadaki M, Bursac N, Langer R, Merok J, Vunjak-Novakovic G, Freed LE. Tissue engineering of functional cardiac muscle: molecular, structural, and electrophysiological studies. American Journal of Physiology-Heart and Circulatory Physiology. 2001 Jan;280(1):H168–H178. doi: 10.1152/ajpheart.2001.280.1.H168. [DOI] [PubMed] [Google Scholar]
- 8.Kofidis T, Akhyari P, Boublik J, Theodorou P, Martin U, Ruhparwar A, et al. In vitro engineering of heart muscle: Artificial myocardial tissue. Journal of Thoracic and Cardiovascular Surgery. 2002 Jul;124(1):63–69. doi: 10.1067/mtc.2002.121971. [DOI] [PubMed] [Google Scholar]
- 9.Shimizu T, Yamato M, Isoi Y, Akutsu T, Setomaru T, Abe K, et al. Fabrication of pulsatile cardiac tissue grafts using a novel 3-dimensional cell sheet manipulation technique and temperature-responsive cell culture surfaces. Circulation Research. 2002 Feb 22;90(3):E40–E48. doi: 10.1161/hh0302.105722. [DOI] [PubMed] [Google Scholar]
- 10.Shimizu T, Yamato M, Kikuchi A, Okano T. Cell sheet engineering for myocardial tissue reconstruction. Biomaterials. 2003 Jun;24(13):2309–2316. doi: 10.1016/s0142-9612(03)00110-8. [DOI] [PubMed] [Google Scholar]
- 11.Manabe I, Shindo T, Nagai R. Gene expression in fibroblasts and fibrosis - Involvement in cardiac hypertrophy. Circulation Research. 2002 Dec 13;91(12):1103–1113. doi: 10.1161/01.res.0000046452.67724.b8. [DOI] [PubMed] [Google Scholar]
- 12.Harada M, Itoh H, Nakagawa O, Ogawa Y, Miyamoto Y, Kuwahara K, et al. Significance of ventricular myocytes and nonmyocytes interaction during cardiocyte hypertrophy - Evidence for endothelin-1 as a paracrine hypertrophic factor from cardiac nonmyocytes. Circulation. 1997 Nov 18;96(10):3737–3744. doi: 10.1161/01.cir.96.10.3737. [DOI] [PubMed] [Google Scholar]
- 13.Varghese D, Deshpande M, Xu T, Kesari P, Ohri S, Boland T. Advances in Tissue Engineering: Cell Printing. Journal of Thoracic and Cardiovascular Surgery. 2005 doi: 10.1016/j.jtcvs.2004.06.050. In press. [DOI] [PubMed] [Google Scholar]
- 14.Wilson WC, Boland T. Cell and organ printing 1: Protein and cell printers. Anatomical Record Part a-Discoveries in Molecular Cellular and Evolutionary Biology. 2003;272A(2):491. doi: 10.1002/ar.a.10057. [DOI] [PubMed] [Google Scholar]
- 15.Xu T, Gregory CA, Molnar P, Cui X, Jalota S, Bhaduri SB, et al. Viability and electrophysiology of neural cell structures generated by the inkjet printing method. Biomaterials. 2006 Jul;27(19):3580–3588. doi: 10.1016/j.biomaterials.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 16.Xu T, Jin J, Gregory C, Hickman JJ, Boland T. Inkjet printing of viable mammalian cells. Biomaterials. 2005 Jan;26(1):93–99. doi: 10.1016/j.biomaterials.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 17.Roth EA, Xu T, Das M, Gregory C, Hickman JJ, Boland T. Inkjet printing for high-throughput cell patterning. Biomaterials. 2004;25(17):3707. doi: 10.1016/j.biomaterials.2003.10.052. [DOI] [PubMed] [Google Scholar]
- 18.Roth EA, Xu T, Das M, Gregory C, Hickman JJ, Boland T. Inkjet printing for high-throughput cell patterning. Biomaterials. 2004 Aug;25(17):3707–3715. doi: 10.1016/j.biomaterials.2003.10.052. [DOI] [PubMed] [Google Scholar]
- 19.Zile MR, Cowles MK, Buckley JM, Richardson K, Cowles BA, Baicu CF, et al. Gel stretch method: a new method to measure constitutive properties of cardiac muscle cells. American Journal of Physiology-Heart and Circulatory Physiology. 1998 Jun;43(6):H2188–H2202. doi: 10.1152/ajpheart.1998.274.6.h2188. [DOI] [PubMed] [Google Scholar]
- 20.White SM, Constantin PE, Claycomb WC. Cardiac physiology at the cellular level: use of cultured HL-1 cardiomyocytes for studies of cardiac muscle cell structure and function. Am J Physiol Heart Circ Physiol. 2004;286:H823–H829. doi: 10.1152/ajpheart.00986.2003. [DOI] [PubMed] [Google Scholar]
- 21.Claycomb WC, Lanson NA, Jr, Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, et al. HL-1 cells: A cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci U S A. 1998;95:2979–2984. doi: 10.1073/pnas.95.6.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pardo LF, Boland T. Characterization of Patterned Self-Assembled Monolayers and Protein Arrays Generated by the Ink-Jet Method. Langmuir. 2003;19:1462–1466. [Google Scholar]
- 23.Xu T, Petridou S, Lee EH, Roth EA, Vyavahare N, Hickman JJ, et al. Construction of High-Density Bacterial Colony Arrays and Patterns by the Ink Jet Method. Biotechnol Bioeng. 2004;85:29–33. doi: 10.1002/bit.10768. [DOI] [PubMed] [Google Scholar]
- 24.Rowley JA, Madlambayan G, Mooney DJ. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999 Jan;20(1):45–53. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 25.Rowley JA, Mooney DJ. Alginate type and RGD density control myoblast phenotype. Journal of Biomedical Materials Research. 2002 May;60(2):217–223. doi: 10.1002/jbm.1287. [DOI] [PubMed] [Google Scholar]
- 26.Fremond B, Malandain C, Guyomard C, Chesne C, Guillouzo A, Campion JP. Correction of bilirubin conjugation in the gunn rat using hepatocytes immobilized in alginate gel beads as an extracorporeal bioartificial liver. Cell transplantation. 1993;2:453–460. doi: 10.1177/096368979300200603. [DOI] [PubMed] [Google Scholar]
- 27.Chang SCN, Rowley JA, Tobias G, Genes NG, Roy AK, Mooney DJ, et al. Injection molding of chondrocyte/alginate constructs in the shape of facial implants. Journal of Biomedical Materials Research. 2001;55:503–511. doi: 10.1002/1097-4636(20010615)55:4<503::aid-jbm1043>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 28.Shapiro L, Cohen S. Novel alginate sponges for cell culture and transplantation. Biomaterials. 1997 Apr;18(8):583–590. doi: 10.1016/s0142-9612(96)00181-0. [DOI] [PubMed] [Google Scholar]
- 29.Leor J, Aboulafia-Etzion S, Dar A, Shapiro L, Barbash IM, Battler A, et al. Bioengineered cardiac grafts - A new approach to repair the infarcted myocardium? Circulation. 2000 Nov 7;102(19):56–61. doi: 10.1161/01.cir.102.suppl_3.iii-56. [DOI] [PubMed] [Google Scholar]
- 30.Mathur AB, Collinsworth AM, Reichert WM, Kraus WE, Truskey GA. Endothelial, cardiac muscle and skeletal muscle exhibit different viscous and elastic properties as determined by atomic force. J Biomech. 2001;34:1545–1553. doi: 10.1016/s0021-9290(01)00149-x. [DOI] [PubMed] [Google Scholar]
- 31.Saltzman W. Cell interactions with polymers. R.G. Landes Company; 1997. [Google Scholar]
- 32.Burg KJL, Boland T. Minimally invasive tissue engineering composites and cell printing. IEEE Eng Med Biol Mag. 2003;(22):84–89. doi: 10.1109/memb.2003.1256277. [DOI] [PubMed] [Google Scholar]
- 33.Rowley JA, Madlambayan G, Mooney DJ. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20:45–53. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 34.Rowley JA, Mooney DJ. Alginate type and RGD density control myoblast phenotype. J Biomed Mater Res. 2002;60:217–223. doi: 10.1002/jbm.1287. [DOI] [PubMed] [Google Scholar]


