Abstract
Background
This study aimed to develop a methodology for quantifying esophageal bolus retention using a high-resolution esophageal impedance topography (EIT) technique.
Methods
The ability of impedance to quantify bolus retention was validated by comparison with concurrent fluoroscopic imaging (barium bolus) in 10 healthy subjects. High-resolution impedance manometry (HRIM) studies without fluoroscopy were performed in another 15 healthy subjects to define normal values using saline. HRIM data from each subject were analyzed using a MATLAB program customized for calculating the esophageal impedance integral (EII) prior to the contraction wave-front as EII1 and after the contraction as EII2, and presented as a ratio of EII2/EII1, which was compared to the percent of barium areas retained in the esophagus on fluoroscopy determined by a blinded reviewer.
Key Results
In 93% (37/40) of barium swallows, the results from the EIT method were in agreement with fluoroscopy results with one of three patterns: 1) 25 normal bolus transit, 2) 8 bolus stasis, and 3) 4 retrograde escape or reflux. Three swallows (8%) had slight retention identified by EIT, but no retention detected by fluoroscopy. The correlation between percent of bolus retained in the esophagus detected by fluoroscopy and percent of bolus retention (EII2/EII1) after swallows with EIT method was r=0.96 (p<0.001) in supine and r=0.69 (p<0.001) in upright position.
Conclusions & Inferences
The EII ratio (EII2/EII1) is a surrogate for the fraction of retained bolus after a swallow and this metric may be useful in better defining esophageal function.
Keywords: bolus retention, esophageal emptying, esophagus, fluoroscopy, impedance, manometry
INTRODUCTION
Multichannel intraluminal impedance (MII), a technique first described by Silny over two decades ago (1), allows evaluation of bolus transit without radiation exposure. This technique uses differences in resistance to alternating current between air, liquid, and esophageal mucosa to determine intraesophageal bolus transit (2, 3). Studies using combined videofluoroscopy and impedance published by Silny et al. (2) and Simren et al (4) validated the ability of MII to detect bolus movement. Using simultaneous videoesophagram, impedance and manometry measurements, Imam et al. reported that impedance nadir values correlated with barium area measurements on fluoroscopy (5, 6) suggesting a relationship to the quantity of bolus present in the esophagus.
Although up to 18 channels of MII commercial catheters are available (7) and impedance color contour plot views (CCPV) are provided by commercial software (Given Image, Medical Measurement System, and Sandhill Scientific, Inc.), most MII data analysis currently utilizes the impedance line-tracing mode (1–6) and only a few MII studies have analyzed bolus transit using the CCPV mode (7–10). In a line-tracing mode, bolus entry at each impedance sensor is defined by a 50% drop between the pre-swallow impedance baseline and impedance nadir while bolus exit is defined by the return to this 50% point (11, 12). Using this scheme, swallows were classified as: (1) complete bolus transit (CBT), if bolus entry occurred at the most proximal site (e.g., 20 cm above EGJ) and bolus exit points were recorded in all distal impedance-measuring sites (e.g., 15 cm, 10 cm and 5 cm above the EGJ) or; (2) incomplete bolus transit (IBT), if bolus exit was not identified at any of the distal impedance-measuring sites (11, 13). In the CCPV mode, CBT equates to no residual bolus color after each swallow and IBT as a persistent colorized area (7, 9). However, the set-points for defining the impedance color contour range are arbitrary and may have significant variation among individuals and swallows. Hence, both the line-tracing mode and CCPV have limitations in their current formats largely limiting them to a qualitative analysis of bolus transit.
The aim of this study was to develop a methodology for quantifying esophageal bolus retention using a high-resolution impedance topography technique. We hypothesized that esophageal pressure topography and esophageal impedance topography (EIT) could be combined to estimate the fractional bolus clearance using computational algorithms that quantitatively compared impedance data in regions of interest in the pre-contraction and post-contraction domains as demarcated by pressure topography.
METHODS
Subjects
The ability of Impedance to quantify bolus volume retention was validated by comparison with concurrent fluoroscopic imaging using a 50% barium/50% saline mixture in 10 healthy subjects (4 males, mean age 28 years, range 21–47). High-resolution impedance manometry (HRIM) studies without fluoroscopy were performed in another 15 healthy subjects (7 males, mean age 33 years, range 20–50) to define normal values using saline. Volunteers were recruited by advertisement or word of mouth and had no history of gastrointestinal symptoms or surgery. The study protocol was approved by the Northwestern University Institutional Review Board and informed consent was obtained from each subject.
Study protocol
Concurrent HRIM and videofluoroscopy studies were done during 2 barium swallows (5-ml thin barium with 50% saline) in the supine and upright position after at least a 6-hr fast. The HRIM catheter was a 4.2 mm outer diameter solid-state assembly with 36 circumferential pressure sensors at 1-cm intervals and 18 impedance segments at 2 cm intervals (Given Imaging, Los Angeles, CA). Transducers were calibrated at 0 and 300 mmHg using externally applied pressure. The assembly was placed transnasally and positioned to record from the hypopharynx to the stomach with about 3 intragastric pressure sensors. Fluoroscopic imaging and HRIM data were simultaneously displayed on a computer screen and stored on the hard drive for further analysis (Figure 1A). The HRIM protocol done without fluoroscopy included a 5-min baseline recording and ten 5-ml swallows in a supine position using 50% saline for test swallows at 20–30 seconds intervals.
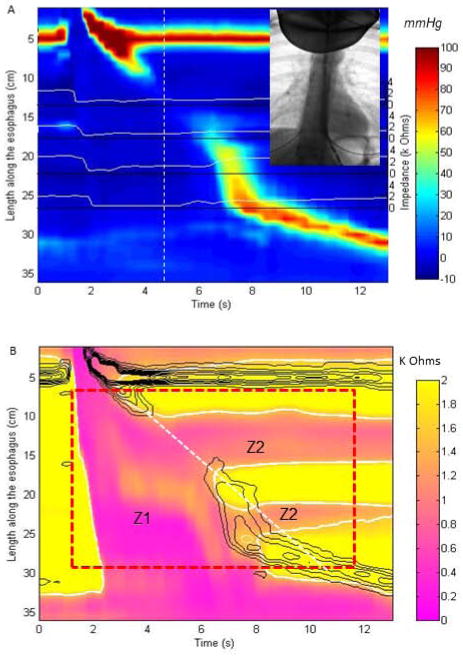
Figure 1.
Methodology for derivation of esophageal impedance topography (EIT) parameters: (A) 4 original Impedance tracings (white lines) are superimposed on esophageal pressure topography plot for a swallow with weak peristalsis. The simultaneous video image at the time point at 5 seconds (white dashed line) was used for the validation component of the study. (B) the pressure contours at 20 mmHg intervals (black solid lines) are overlaid with the enhanced impedance data with the same resolution as pressure data in both time and spatial domain after interpolation, on which a region of interest (ROI, red dashed rectangle) is selected to define the space-time domain into a pre-contraction zone and post-contraction zone (white dashed tangent line defines two areas). The impedance contour (thick white curves) at impedance value of 50% drop from mean baseline impedance to the mean nadir impedance are superimposed on the EIT plot within the ROI for calculation of EIT parameters including bolus volume integral prior to the contraction wave-front at 20 mmHg isobaric contour (Z1) as EII1 and in area 2 after the contraction (Z2) as EII2 divided by a dashed white line. The impedance signals outside of the ROI (red box) are not included in the measurement of EII2.
Data analysis
Validation of HRIM for assessment of bolus volume retention
Video images from the barium swallows were reviewed by two investigators (JEP and FN) using ManoView software (ESO 3.0, Given Imaging, Los Angeles, CA) for synchronously displaying the images with cursor defined time points on the HRIM color topography plots. For each swallow, the areas of barium movement on fluoroscopy and the impedance color contour changes on HRIM data were analyzed independently. If barium was present after the esophageal contraction, the residual barium was traced, localized relative to the impedance rings on the HRIM assembly, and the area was calculated in mm2. The inter-electrode separation on the HRIM catheter was used to correct for fluoroscopic magnification (6). These data on bolus retention were then compared to the analogous EIT metrics.
Esophageal Impedance Topography (EIT) method for quantifying bolus volume
HRIM data from each subject were exported from ManoView™ software (Given Imaging, Los Angeles, CA) to MATLAB™ (The MathWorks Inc., Natick, MA, U.S.A.) for further analysis using a MATLAB program customized for calculating EIT parameters developed for quantifying bolus volume retention (Figure 1B). Four functional steps were performed for the analysis:
Optimizing resolution of impedance and manometry. For each test swallow, original pressure and impedance data (Figure 1A) were plotted in color contours and enhanced in the spatial dimension by interpolation using a cubic Hermite polynomial function to increase the spatial data by a factor of 10 for pressure data and 20 for impedance data giving the impedance data the same resolution in both temporal and spatial domain as the pressure data (Figure 1B);
Creating the space-time domain regions for assessing bolus retention. From the overlapped pressure and impedance color contour plots, a space-time region of interest (ROI) was defined from the lower limit of the upper esophageal sphincter (UES) to the upper limit of the EGJ and from onset of swallowing (UES relaxation, UESR) to 12 seconds later or to completion of peristalsis (red dashed rectangle in Figure 1B). The best fit straight line that demarcated the peristaltic wave front was defined and utilized to divide the swallow ROI into the swallow impedance area (Z1) and the post-swallow impedance area (Z2).
Defining domains of bolus presence. At all positions within the ROI the times of nadir impedance were identified. Thereafter, the mean baseline impedance and the mean nadir impedance were calculated to derive the 50% drop impedance value and to define domains where a 50% drop occurred. These areas were highlighted and outlined by the solid white line that is overlapped on the EIT plot in Figure 1B.
Deriving a quantitative measure of bolus retention using impedance pixel integrals. The retained bolus volume was estimated as the ratio of the summated esophageal impedance integral (EII) post-swallow (EII2 in ROI Z2) compared to EII1 in ROI Z1. Each pixel in the bolus domain (<50% drop from the mean baseline value) was assigned an impedance integral calculated as its impedance value multiplied by pixel time resolution (0.01 s) and by spatial resolution (0.1 cm), expressed as Ohm-s-cm. All impedance volume integrals in ROI Z1 prior to the contraction onset at 20 mmHg isobaric contour were totaled as EII1 value and compared to those (EII2) in ROI Z2 after the contraction (offset at 20 mmHg) and presented as a ratio of EII2/EII1.
High resolution impedance manometry (HRIM) analysis using line tracing mode
HRIM studies were analyzed to define esophageal pressure topography features associated with incomplete bolus transit (IBT). Bolus transit was assessed using the impedance tracing mode on ManoView™. Impedance tracings of all instances of IBT were reviewed to verify that they failed to meet established criteria for complete bolus transit (≥50% decrease in impedance from the baseline) (7, 12).
Statistical analysis
All results were expressed as mean (range). Pearson’s linear correlation was used for correlation analysis. A Student t-test was used for comparison of EIT parameters between two groups. Statistical significance was accepted when p value < 0.05.
RESULTS
Validation of EIT methodology against videofluoroscopy
A total of 40 swallows (4 per subject, 2 supine and 2 upright) were analyzed from the 10 healthy subjects with concurrent fluoroscopic imaging and HRIM data. In 92.5% (37/40) of swallows, the results from EIT method were in agreement with fluoroscopy results with one of three patterns: 1) normal bolus transit, 2) bolus stasis, and 3) retrograde escape or bolus reflux (Table 1). The three episodes of disagreement were consistent with modest drops in impedance with nadir values of 862 ohms, 968 ohms, and 965 ohms. These nadir values would be associated with trivial barium area based on previous work by Imam et al (6).
Table 1.
Comparison of videofluoroscopy and esophageal impedance topography (EIT) method in detecting bolus volume retention in 40 barium swallows from 10 subjects
| Videofluoroscopy | |||||
|---|---|---|---|---|---|
| normal | stasis | Retrograde | Not seen | ||
| EIT method | normal | 25 | |||
| stasis | 8 | 3 | |||
| retrograde | 4 | ||||
| Not seen | 0 | ||||
Most swallows (62.5%, 25/40) had complete bolus transit (Figure 2), 8 swallows had bolus stasis (6 with absent peristalsis and 2 with weak contraction) (Figure 3), and 4 swallows had retrograde escape or reflux detected by both methods (Figure 4). Three of 40 (8%) swallows had divergent results by these two methods. These three swallows had small retention at transition zone or distal esophagus (EII2/EII1<0.3) identified by the EIT method, but no retention detected by videofluoroscopy.
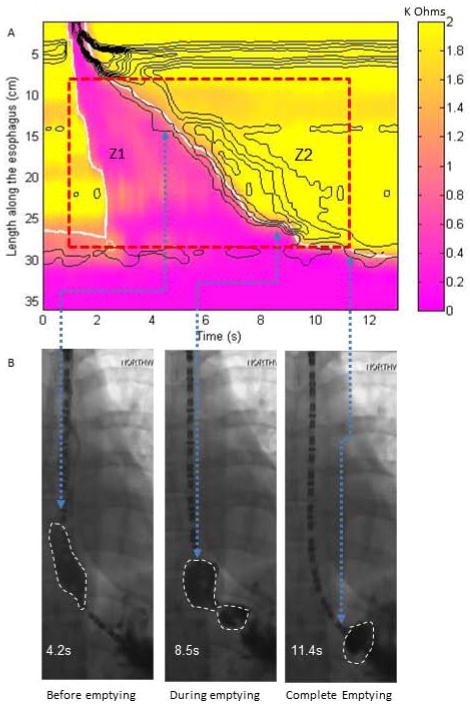
Figure 2.
Example plots and fluoroscopic images for a normal swallow in a supine position with complete bolus transit. (A) EIT plot for derivation of bolus volumes in Z1 and Z2 area (EII2/EII1 =0). (B) Fluoroscopic images taken at various time points along the swallow with the arrows delineating the time point on the EIT plot and the simultaneous location on the fluoroscopy spot film. The last image reveals no bolus remaining in the esophageal body at the end of the swallow coinciding with the end of the ROI. The bolus shown on the last image is below the diaphragm.
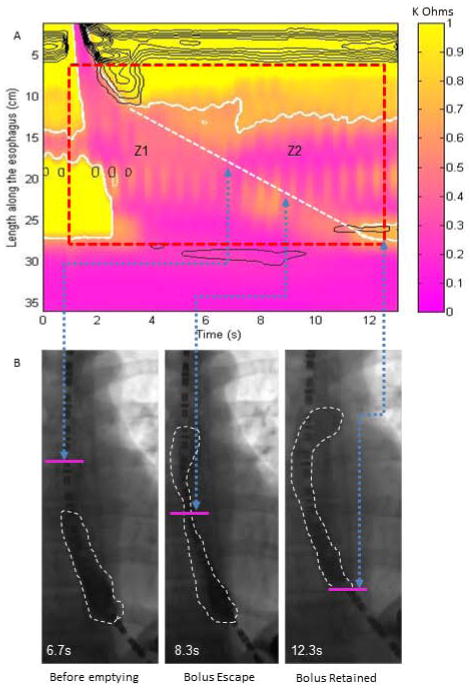
Figure 3.
Example plots for an absent swallow in a supine position with incomplete bolus transit validated by fluoroscopic imaging. (A) EIT plot for derivation of bolus volume integrals in Z1 and Z2 area (EII2/EII1 =0.46). (B) Fluoroscopic images taken during esophageal filling with the arrows delineating the time point on the EIT plot and the simultaneous location on the fluoroscopy spot film. The pink line marks the position of the Z1/Z2 interface separating the two distinct areas. Note that the barium presence at 8.3 seconds is similar to the value at the 12.3 seconds, however, the bolus above the pink line is counted within the Z2 domain and the bolus below is considered part of the Z1 domain.
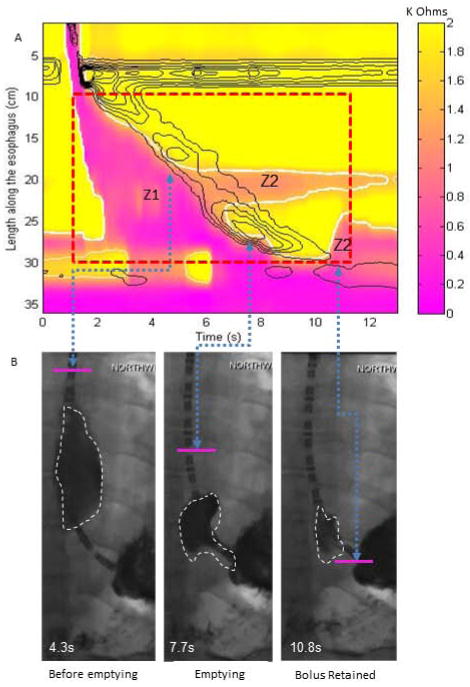
Figure 4.
Example plots for a normal swallow in a supine position with incomplete bolus transit identified by both fluoroscopic imaging and esophageal impedance topography (EIT) parameters. (A) EIT plot for derivation of bolus volumes in Z1 and Z2 area (EII2/EII1 =0.31). In this example the two Z2 zones are added together, however, the middle Z2 zone represents minimal to trivial bolus retention whereas the distal Z2 zone is consistent with significant bolus retention related to a small defect at the distal node. (B) Fluoroscopic images taken during esophageal filling with the arrows delineating the time point on the EIT plot and the simultaneous location on the fluoroscopy spot film. The pink line marks the position of the Z1/Z2 interface separating the two distinct areas. Note the correlation between presence of bolus at the distal Z2 zone and fluoroscopy, but no bolus on fluoroscopy at the middle Z2 zone consistent with low impedance integrals.
A summary of the EIT parameters for all 40 test swallows (28 CBT (13 supine, 15 upright) and 12 IBT (7 supine, 5 upright)) based on videofluoroscopy (area in Z2 ≥10%) are shown in Table 2. There was a strong correlation between percent of areas retained in the esophagus detected by fluoroscopic imaging and percent of bolus volume integral remained (EII2/EII1) after swallows with EIT method (r=0.96 for supine and r=0.69 for upright position, p<0.001).
Table 2.
Mean (range) esophageal impedance topography (EIT) parameters in 40 test swallows (28 CBT and 12 IBT) based on videofluoroscopy (area in Z2 ≥ 10%).
| Supine | upright | |||
|---|---|---|---|---|
| 13 swallows with CBT | 7 swallows with IBT | 15 swallows with CBT | 5 swallows with IBT | |
| Bolus volume in area 2 (EII2) | 0.5 (0–6.0) | 16.9 (1–39)* | 2.5 (0–28) | 26.3 (8.5–47)* |
| Bolus volume ratio (EII2/EII1) | 0.01 (0–0.14) | 0.4 (0.1–0.8)* | 0.04 (0–0.3) | 0.3 (0.2–0.5)* |
Note:
P<0.01 vs. swallows with CBT.
Normal values of EII2/EII1 ratio in asymptomatic controls
Based on the impedance tracing mode with the threshold of 50% impedance change, CBT was seen in all test swallows in 6/15 (40%) control subjects; one to four swallows were associated with IBT in the other 9 subjects. Applying Chicago Classification definitions of these swallows, 12 swallows had failed peristalsis, 8 had large breaks (6 at the proximal and 2 at the distal pressure troughs), 20 had small breaks (4 IBT and 16 CBT), and the remainder of 110 swallows had normal peristalsis. Table 3 details the EIT parameters in these four swallow phenotypes. As expected, the Z2 EII2 values and EII2/EII1 ratios for the failed peristalsis or swallows with large breaks were significantly greater than esophageal pressure topography with small breaks. These parameters were also significantly smaller for normal peristalsis than for the other three swallow phenotypes, indicating greater bolus retention for failed peristalsis and weak swallows with large and small breaks (Table 3).
Table 3.
Mean (range) of EIT parameters in 15 healthy subjects (150 swallows) with 4 types of swallows
| Normal peristalsis (n=110) | Small break (2–5 cm) (n=20) | Large break (>5 cm) (n=8) | Failed peristalsis (n=12) | |
|---|---|---|---|---|
| EII2 | 6.0 (0–106)* | 28.8 (0–111) | 61.6 (11–122)* | 74.1 (12–118)* |
| EII2/EII1 | 0.03 (0–0.25)* | 0.15 (0–0.49) | 0.4 (0.31–0.58)* | 0.43 (0.3–0.69)* |
Note:
p<0.05 vs. swallows with small break at the 20 mmHg isobaric contour
Correlation of EII2/EII1 ratio with length of breaks in isobaric contour plots
As shown in Table 3, 28 swallows had either small (2–5 cm) or large (>5 cm) breaks in the 20 mmHg isobaric contour (mean 4.0, range 2–8.9 cm). A previous study demonstrated that IBT only occurred in association with ≥2 cm breaks at the 20 mmHg isobaric contour (7). In line with this, the EII2/EII1 ratio values for these 28 swallows showed a significant correlation with break length (r=0.70, p<0.001) indicating that large breaks were associated with a greater volume of bolus retention.
DISCUSSION
The aim of this study was to determine if computational algorithms could be utilized to calculate quantitative estimates of bolus transit from HRIM studies. We found that an EIT metric summating pixel impedance volumes prior to and after peristalsis and expressing this as a ratio exhibited a strong correlation with retained bolus area (mm2) calculated from simultaneous videofluoroscopic images in the supine position and a moderate correlation in the upright position. Additionally, the EII2/EII1 ratio showed a consistent trend of increased values with increasing degrees of peristaltic dysfunction according to Chicago Classification definitions. Thus, the EII2/EII1 ratio may provide a more quantitative method to gauge impaired bolus transit beyond the dichotomous CBT/IBT distinction currently utilized in conventional impedance evaluation.
The most important function of the esophagus is to transport ingested food to the stomach. Conventionally, the barium videoesophagram was the gold standard for examining esophageal bolus transit whereas manometry was the gold standard for defining the contractile function of the esophagus. However, the simultaneous application of these techniques requires considerable technical support and exposure to radiation (14). These issues were the impetus for the development of multichannel intraluminal impedance (MII) to assess bolus transit and this technology was combined with manometry to provide a comprehensive single test of esophageal function. Combined impedance-manometry studies revealed the heterogeneity of bolus transit abnormalities amongst esophageal motor disorders and also refined our understanding of the relationship between bolus transit and peristaltic function (13). However, the analysis of bolus transit was relegated to a paradigm where bolus presence was assessed as an all or none event and, although there was some ability to determine whether bolus retention was present at multiple levels, there was no parameter or criteria to objectively define degrees of IBT.
Previous investigators have reported that nadir impedance is an important surrogate for bolus volume on esophageal impedance recordings (6, 10). Imam et al reported on the usefulness of nadir impedance in quantifying retained barium bolus on esophagrams and their results suggested a correlation with impedance nadir values (6). However, that study was limited by a small number of impedance channels and we hypothesized that this approach could be extended to HRIM to provide a more accurate and quantitative measure of bolus retention. By using the spatial resolution of both the impedance and manometry data during HRIM, we defined an appropriate region of interest in which we could quantify bolus presence based on an impedance pixel volume before and after the contraction. Using computational algorithms, we developed a metric expressing bolus retention after a swallow as a ratio of after/before impedance integrals. That ratio had a strong correlation with barium bolus area measured from simultaneous videofluoroscopy in the supine position. Although it is tempting to extrapolate absolute volumes based on the fact that the ingested volume was controlled, that would depend on knowing the percentage of the ingested volume that remained in the esophagus in the Z1 ROI, which is likely variable.
Whether calculating the EII2/EII1 ratio will improve our ability to define a symptomatic correlate for dysphagia will require further analysis in a heterogeneous patient population and likely some refinement to determine appropriate cutoff values. In a previous study using combined impedance and manometry (15), Lazarescu et al reported a very poor positive predictive value for abnormal impedance in predicting symptom perception during swallowing and proposed that visceral sensitivity was likely a more important determinant of symptom perception. However, there may be levels of bolus retention that are more likely to induce symptoms and future studies should include mechanisms to address the potential confounding effect of hypersensitivity. In fact, our control data revealed that a substantial number of control subjects had impaired bolus transit and varying degrees of peristaltic dysfunction. The range of EII2/EII1 in healthy subjects with failed peristalsis ranged from 0.3 to 0.69 suggesting that a large proportion of the bolus was retained and, yet, these subjects were asymptomatic. Thus, we suspect that the EII2/EII1 ratio will not be a predictor in and of itself, but may stratify symptom severity in patients with dysphagia or GERD.
There are some technical limitations to our approach. Although HRIM displays data in CCPV, there currently is no protocol to calibrate the impedance color contour range and our data suggest that this should be performed with the upper limit being the 50% threshold of baseline and the lower threshold at the nadir impedance value. This is in line with the conventional line-tracing mode paradigm and also provides a means to discern trivial bolus retention from large amounts of bolus retention. In our 15 controls, there was significant overlap between EII2/EII1 ratio in subjects with large peristaltic breaks and failed swallows, while swallows with small peristaltic breaks had levels ranging from 0 to 0.4 suggesting a range from no abnormality to significant. The EII2/EII1 ratio may better define the severity of peristaltic dysfunction and may be complementary to the standard manometric definitions of ineffective esophageal motility.
An additional limitation of the proposed EII2/EII1 method relates to patients with low baseline impedance because of retained food, secretions, or bolus from the previous swallow. Although this may be circumvented by the use of baseline impedance to define the areas of 50% drop during the swallow, we speculate that this technique will probably not be useful in achalasia and an assessment of esophageal emptying after a large volume challenge may be a better measure in that patient group. Although the correlation with fluoroscopy was very good, it does appear that this methodology is more useful in the supine position as the correlation was weaker in the upright position. This is likely related to the increase esophageal emptying in the upright position related to gravity. More bolus will remain in the esophagus after a weak or failed swallow in the supine position and this suggests that the EII2/EII1 has a better correlation when higher volumes of retention are present.
In conclusion, we present a new technique to stratify the degree of bolus retention using HRIM. Although there is no direct volume measurement correlate, our results support that the EII2/EII1 ratio is a surrogate for the fraction of retained bolus after a swallow and this metric may be useful in better defining esophageal function. Given the complexity of symptom generation in dysphagia and reflux, the EII2/EII1 ratio will likely not be predictive of symptoms and complications by itself. However, it may be a useful biomarker for esophageal function specifically in the development of promotility drugs developed to improve esophageal contractility. Future studies using a prospective design should be performed in well-defined patient groups comparing this technique to that described using conventional impedance line-tracings.
Key Messages.
The esophageal impedance integral (EII) ratio expressed as the ratio of EII after peristalsis to that before peristalsis is a surrogate for the fraction of retained bolus after a swallow and this metric may be useful in better defining esophageal function.
The goal of this research was to develop a methodology for quantifying esophageal bolus retention with high-resolution impedance manometry (HRIM).
This study validated the ability of impedance to quantify bolus retention by comparison with concurrent fluoroscopic imaging (barium bolus) in 10 healthy subjects and analyzed HRIM studies in another 15 healthy subjects with a customized MATLAB program to define normal values using saline.
The EII ratio exhibited a strong correlation with retained bolus area calculated from simultaneous fluoroscopic images in the supine position and a moderate correlation in the upright position. Additionally, the EII ratio showed a consistent trend of increased values with increasing degrees of peristaltic dysfunction according to Chicago Classification definitions.
Acknowledgments
FUNDING
This work was supported by R01 DK079902 (JEP) and R01 DK56033 (PJK) from the Public Health Service.
Footnotes
CONFLICT OF INTERESTS
John E. Pandolfino [Given imaging (consulting, educational)]. No other conflicts for remaining authors (ZL, FN, CL, BM, LF, PJK)
AUTHOR CONTRIBUTIONS
ZL contributed to analysis and interpretation of data, drafting of the manuscript, approval of the final version; FN Analysis and interpretation of data, approval of the final version; CL, BM and LF contributed to acquisition and analysis of data, and approval of the final version; PJK contributed to study concept and design, revising of the manuscript critically, and approval of the final version; JEP contributed to study concept and design, analysis and interpretation of data, revising the manuscript critically, and approval of the final version.
References
- 1.Silny J. Intraluminal multiple electric impedance procedure for measurement of gastrointestinal motility. J Gastrointest Motil. 1991;3:151–62. [Google Scholar]
- 2.Silny J, Knigge KP, Fass J, Rau G, Matern S, Schumpelick V. Verification of the intraluminal multiple electrical impedance measurement for recording of gastrointestinal motility. J Gastrointest Motil. 1993;5:107–122. [Google Scholar]
- 3.Srinivasan R, Vela MF, Katz PO, Tutuian R, Castell JA, Castell DO. Esophageal function testing using multichannel intraluminal impedance. Am J Physiol Gastrointest Liver Physiol. 2001;280:G457–62. doi: 10.1152/ajpgi.2001.280.3.G457. [DOI] [PubMed] [Google Scholar]
- 4.Simren M, Silny J, Holloway R, Tack J, Janssens J, Sifrim D. Relevance of ineffective oesophageal motility during oesophageal acid clearance. Gut. 2003;52:784–90. doi: 10.1136/gut.52.6.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imam H, Shay S, Ali A, Baker M. Bolus transit patterns in healthy subjects: a study using simultaneous impedance monitoring, videoesophagram, and esophageal manometry. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1000–6. doi: 10.1152/ajpgi.00372.2004. [DOI] [PubMed] [Google Scholar]
- 6.Imam H, Marrero F, Shay S. Impedance nadir values correlate with barium bolus amount. Dis Esoph. 2012;25:600–7. doi: 10.1111/j.1442-2050.2011.01302.x. [DOI] [PubMed] [Google Scholar]
- 7.Roman S, Lin Z, Kwiatek MA, Pandolfino JE, Kahrilas PJ. Weak peristalsis in esophageal pressure topography: classification and association with dysphagia. Am J Gastroenterol. 2011;106:349–56. doi: 10.1038/ajg.2010.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bulsiewicz WJ, Kahrilas PJ, Kwiatek MA, Ghosh SK, Meek A, Pandolfino JE. Esophageal pressure topography criteria indicative of incomplete bolus clearance: a study using high-resolution impedance manometry. Am J Gastroenterol. 2009;104:2721–8. doi: 10.1038/ajg.2009.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jung KW, Jung H-Y, Romero Y, Katzka D, Murray JA. Impact of display alternatives in the determination of bolus handling: a study using high-resolution manometry with impedance. Am J Gastroenterol. 2011;106:1854–6. doi: 10.1038/ajg.2011.233. [DOI] [PubMed] [Google Scholar]
- 10.Omari T, Kritas S, Cock C. New insights into pharyngo-esophageal bolus transport revealed by pressure-impedance measurement. Neurogastroenterol Motil. 2012;24:e549–e556. doi: 10.1111/nmo.12007. [DOI] [PubMed] [Google Scholar]
- 11.Tutuian R, Vela MF, Balaji NS, Wise JL, Murray JA, Peters JH, Shay SS, Castell DO. Esophageal function testing with combined multichannel intraluminal impedance and manometry: multicenter study in healthy volunteers. Clin Gastroenterol Hepatol. 2003;1:174–82. doi: 10.1053/cgh.2003.50026. [DOI] [PubMed] [Google Scholar]
- 12.Sifrim D, Castell D, Dent J, Kahrilas PJ. Gastro-oesophageal reflux monitoring: review and consensus report on detection and definitions of acid, non-acid, and gas reflux. Gut. 2004;53:1024–31. doi: 10.1136/gut.2003.033290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tutuian R, Castell DO. Combined multichannel intraluminal impedance and manometry clarifies esophageal function abnormalities: study in 350 patients. Am J Gastroenterol. 2004;99:1011–19. doi: 10.1111/j.1572-0241.2004.30035.x. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen NQ, Silny J, Marters S. Multiple intraluminal electrical impedance-metry for recording of upper gastrointestinal motility: current results and future implications. Am J Gastroenterol. 1999;94:306–17. doi: 10.1111/j.1572-0241.1999.00847.x. [DOI] [PubMed] [Google Scholar]
- 15.Lazarescu A, Karamanolis G, Aprile L, De Oliveira RB, Dantas R, Sifrim D. Perception of dysphagia: lack of correlation with objective measurements of esophageal function. Neurogastroenterol Motil. 2010;22:1292–e337. doi: 10.1111/j.1365-2982.2010.01578.x. [DOI] [PubMed] [Google Scholar]