Abstract
OBJECTIVES
To determine whether hyperglycemia is related to prevalent frailty status in older women.
DESIGN
Secondary data analysis of baseline data of a prospective cohort study.
SETTING
Baltimore, Maryland.
PARTICIPANTS
Five hundred forty-three women aged 70 to 79.
METHODS
Research used baseline data from 543 participants in the Women’s Health and Aging Studies I and II aged 70 to 79 who had all variables needed for analyses. The dependent variable was baseline frailty status (not frail, prefrail, frail), measured using an empirically derived model defining frailty according to weight loss, slow walking speed, weakness, exhaustion, and low activity (1–2 characteristics Present = prefrail, ≥3 = frail). Covariates included body mass index (BMI), interleukin-6 (IL-6), age, race, and several chronic diseases. Analyses included descriptive methods and multinomial logistic regression to adjust for key covariates.
RESULTS
A hemoglobin A1c (HbA1c) level of 6.5% or greater in older women was significantly associated with higher likelihood of prefrail and frail status (normal HbA1c <6.0% was reference). The association between HbA1C levels of 6.0% to 6.5% and frailty status was not different from that of normal HbA1c, but HbA1c levels of 6.5% to 6.9% had nearly twice the likelihood of frailty (odds ratio (OR) = 1.96, 95% confidence interval (CI) = 1.47–2.59) as normal HbA1c. A HbA1c level of 9.0% or greater was also strongly associated (OR = 2.57, 95% CI = 1.99,3.32). Significant associations were also seen between baseline prefrail and frail status and low (18.5–20.0 kg/m2) and high (.30.0 kg/m2) body mass index (BMI), interleukin-6, and all chronic diseases evaluated, but controlling for these covariates only minimally attenuated the independent association between HbA1c and frailty status.
CONCLUSION
Hyperglycemia is associated with greater prevalence of prefrail and frail status; BMI, inflammation, and comorbidities do not explain the association. Longitudinal research and study of alternative pathways are needed.
Keywords: hyperglycemia, frailty, older women
As the population ages, type 2 diabetes mellitus is increasingly becoming a disease of older adults. Incidence and prevalence of type 2 diabetes mellitus is rapidly increasing in people aged 65 to 79;1,2 recent Centers for Disease Control and Prevention data show that incident type 2 diabetes mellitus is five times as great in adults aged 65 to 79 as in those younger than 45. Diabetes mellitus is an important contributor to the comorbidity burden,3 geriatric conditions,4 and complex health status5 seen in some older adults.
Frailty is hypothesized to be a geriatric condition of physiological vulnerability and multisystem dysfunction associated with aging that increases the risks of adverse health outcomes such as falls, disability, and death.6 Frailty has been shown to be associated with several major chronic diseases; with disruptions in several physiological systems, including endocrine and inflammatory systems; and with undernutrition and obesity. It has previously been shown, using an empirical model of frailty developed in the Cardiovascular Health Study (CHS) and validated in the Women’s Heath and Aging Studies (WHAS), that frailty is associated with prevalent diabetes mellitus, atherosclerotic vascular disease, and obesity.7 It has also been demonstrated that glucose intolerance in older adults without diagnosed diabetes mellitus is related to prevalent frailty; markers of increased inflammation (C-reactive protein, interleukin (IL)-6) and endocrine dysregulation (insulin-like growth factor-1) are also associated with prevalent frailty.8 Recently, longitudinal analysis from the CHS has demonstrated that insulin resistance as measured according to the homeostasis model assessment of insulin resistance is associated with incident frailty.9
Although glucose intolerance, insulin resistance, and reported diabetes mellitus appear to be associated with frailty, it is not known whether hyperglycemia itself is related to frailty. Research so far is also consistent with the hypothesis that the pathophysiology of frailty involves the obesity7 and inflammatory disruptions associated with insulin resistance9 and type 2 diabetes mellitus, as well as with vascular complications of diabetes mellitus. Therefore, this research was undertaken to test the complementary hypothesis: that hyperglycemia itself would be associated with prevalent frailty independently of complications of diabetes mellitus, obesity, and high IL-6 levels that have also been shown to be associated with frailty.
METHODS
Subjects
The analytical cohort for this study consists of women aged 70 to 79 living in Baltimore who participated in WHAS I and II, two complementary, population-based studies designed to evaluate the causes and course of physical disability in older women living in the community, and who had blood available for measurement of hemoglobin A1c (HbA1c) and free of conditions that mimic frailty (see below, N = 543). WHAS I participants were recruited from an age-stratified random sample of women aged 65 years and older selected from Medicare enrollees residing in 12 contiguous ZIP code areas in Baltimore between 1992 and 2000.10 The initial sample included 5,300 women, from which the one-third most-disabled women (n = 1,400) were selected based on reported disability. Finally, 1,002 women agreed to participate in WHAS I. The initial standardized questionnaires, physical performance measures, and a directed physical examination were performed in the women’s homes; approximately 75% of the women also consented to blood testing. Previous studies have shown that there were no significant differences in race or body mass index (BMI) between those who did and did not participate in the blood drawing, but women who did and did not participate in the blood drawing differed in age (77.4 vs 80.7, P<.001).11
WHAS II, begun in 1994, was specifically designed to be a companion study for WHAS I and includes a cohort of women aged 70 to 79 selected to be representative of the two-thirds least-disabled women living in the community.12 Participants were selected from age-stratified random samples from the same sampling frame as in WHAS I; 436 women participated. An interview, directed physical examination, and physical performance measures standardized to those performed in WHAS I were administered in the Johns Hopkins Functional Status Laboratory. Phlebotomy was performed in 93% of WHAS II participants following the same protocol as that used in WHAS I. Details on the study methods and sampling design of the WHAS studies are published elsewhere.12–14 For the current analyses, a combined sample linking the two WHAS studies was used using a methodology that has been developed by the WHAS research team and has been used in several published studies.9,15 The analytical sample for this research consists of women participating in WHAS I or WHAS II who were aged 70 to 79 at baseline, who had all variables available, and who did not have stroke or Parkinson’s disease (nearly all with these conditions are frail) or BMI less than 18.5 kg/m2 (part of the definition of frailty). Appropriate sampling weights have been calculated to adjust for differential selection probability with respect to age and disability status from the sampling frame.16
Variables
The dependent variable, frailty status, was measured using the model of the frailty phenotype developed previously. This empirical model is a composite variable developed in the CHS and validated in WHAS.13 Although the original measures were developed in the CHS study, similar or identical measures are present in WHAS I and II. Frailty status is based on five indicators: weight loss, weakness, exhaustion, slowness, and low physical activity. The frailty phenotype was considered present if three or more of the indicators are present; the presence of one or two indicates a prefrail state. Grip strength was measured in WHAS according to the CHS protocol: level of maximal grip strength in the stronger hand. Weakness was the grip strength in the lowest 20% of women. Speed in WHAS was based on a 4-m measured walk at usual pace. The subject could use a walking aide but not the aid of another person. Slowness was defined as the walking speed of the slowest 20% of women. To measure energy expenditure, WHAS used a subset of the Minnesota Leisure Time Activities Questionnaire used in CHS14 that was condensed from the original 18 activities to assess participation in six: walking, doing strenuous household chores, doing strenuous outdoor chores, dancing, bowling, and exercise.15 Again, low physical activity was defined as the activity level of the lowest 20%. Exhaustion in WHAS was defined as a positive response to at least one of three relevant questions: felt unusually tired in the last month, felt unusually weak in the last month, or had an unusually low energy level. Frailty-eligible weight loss criteria was applied if a woman’s weight as measured at baseline represented a self-reported decrease of at least 10% from weight at age 60 or if a women’s baseline BMI was less than 18.5 kg/m2, the lowest World Health Organization (WHO) BMI risk category.
Only 27 women in this sample had a BMI less than 18.5 kg/m2; these women therefore had one frailty criterion by definition, and all had at least two more criteria and were thus frail. As noted previously, because of the confounding according to definition in this group, these 27 women were excluded from further analyses.
Nonfasting blood samples were obtained using venipuncture between 9:00 a.m. and 2:00 p.m. Processing, aliquoting, and freezing were performed at the Core Genetics Laboratory of the Johns Hopkins University School of Medicine following a standardized protocol. Tubes of whole blood, serum, and plasma were frozen for each woman who had her blood drawn. IL-6 was measured from frozen serum within a few weeks using a commercial enzyme-linked immunosorbent assay (Quantikine Human, R & D Systems, Minneapolis, MN). The major independent variable of interest was HbA1c, measured from frozen whole blood by the Johns Hopkins General Clinical Research Center using standardized methods and also run within a several week period. All HbA1c values were remeasured, because the glycosylated hemoglobin (GHB) assay used for 95% of round 1 of WHAS I was an ion exchange technique that cannot be compared with current HbA1c levels. It was measured using low-pressure cation-exchange chromatography (Ciba Corning 765 Glycomat; reference range 5–8.6%). Some women had another GHB assay type, a bromate affinity high-performance liquid chromatography assay (Primus). By late 1998, all women had a BioRad HbA1c assay, which can be compared with current HbA1c methods. Before this research, a pilot was run to determine the correlation between these older GHB assays and current standardized HbA1c tests that demonstrated poor correlation (r = 0.57). To assure comparability of all HbA1c values, all available frozen blood samples for all women in WHAS I and II from all blood draw waves and techniques were rerun. A subset of the frozen samples was not measurable because of freeze–thaw damage, and another subset did not have frozen samples available. This problem mainly occurred in the WHAS I baseline sample, which was the oldest. Analyses demonstrated that these data were missing at random (there was no association between missing data and relevant covariates).17 Because women with missing data had measures of several related covariates, including the older GHB values, model-based multiple imputation was used.18 Covariates included in the imputation model were those used in the analytical models: “outdated” GHB measures, age, race, BMI, and chronic diseases. In WHAS I, at baseline 743 of 1,002 women had blood drawn; 463 of 743 had frozen whole blood that gave good results. Of those missing, 203 had the covariates available for multiple imputation, so the sample available for WHAS I was 666. WHAS II had 383 or 434 valid frozen blood samples available. When WHAS I women aged 70 to 79 were combined with WHAS II women, the number available was 659, but as previously noted, women with stroke, Parkinson’s disease, or BMI less than 18.5 kg/m2 were dropped, leaving 543 in the analytical sample.
The research staff measured weight and height according to standardized protocols (these protocols were identical in WHAS I and II), and BMI was calculated. BMI was categorized according to the WHO criteria as normal (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), and obese (>30.0 kg/m2). Demographic information was obtained from the standardized questionnaire, which was nearly identical for WHAS I and II.
WHAS I and II determined the prevalence of major chronic diseases of aging, including coronary artery disease (CAD), osteoarthritis, stroke, diabetes mellitus, and chronic obstructive pulmonary disease (COPD) at baseline. WHAS investigators adjudicated these diseases based on the questionnaire, physical examination measures, physician contact, and medical records. Seventeen diseases have been ascertained; the methodology and algorithms have been published.10 The adjudication of diabetes mellitus for baseline WHAS I missed many women who would be classified as having diabetes mellitus according to present-day standards (adjudication used results from an older GHB assay and relied on physician diagnosis). Because the research question concerned the relationship between HbA1c and frailty, secular changes in diabetes mellitus diagnosis did not influence the results.
Statistical Analysis
As described above, model-based multiple imputation was used to increase the numbers of women with HbA1c available, increasing the analytical sample size. The distribution of HbA1c and BMI was explored. Because analysis suggested nonlinear effects, HbA1c and BMI were both categorized. The characteristics of women with different levels of HbA1c were studied using standard descriptive statistics; the relationship between frailty status and HbA1c level and BMI was studied graphically. Multivariable models used multinomial logistic regression to investigate the association between independent variables with prefrailty and frailty (using not frail as the reference group). First, the association between HbA1c and frailty status was investigated controlling for age and demographic variables. Then, additional variables were added in sequential models: BMI, then IL-6, then the adjudicated chronic diseases that have been shown to be associated with frailty (osteoarthritis, CAD, heart failure, depressive symptoms, and COPD).6 As noted above, HbA1c and BMI were categorized to account for potential nonlinear relationships. Finally, two key interactions were tested in the multivariable model: HbA1c with BMI and HbA1c with IL-6.
To appropriately interpret inferences derived from the combined data back to the sampling population of community- dwelling women aged 70 to 79, study-specific probability weights were used for all analyses. Construction of the weights has been detailed previously.16 Probability weights were incorporated into all of the descriptive and regression analyses. The statistical program used was SAS, version 9 (SAS Institute, Inc., Cary, NC).
RESULTS
Table 1 shows the baseline characteristics of women with different levels of HbA1c categorized into five groups (<6.0%, 6.0–6.4%, 6.5–6.9%, 7.0–8.9%, and ≥9.0%). Fifty-one percent of the women had HbA1c levels less than 6.0%, which is a nondiabetic HbA1c; 16% had HbA1c of 7.0% and greater. Age and most diseases did not differ according to level of HbA1c in this sample of older women. Results did not show a stepwise increase in mean IL-6 level according to HbA1c group, although the mean value of IL-6 for subjects with HbA1c less than 6.0% and those with HbA1c of 6.0% and greater was significantly different (3.66 ± 3.37 pg/mL vs 4.41 ± 3.74 pg/mL, P<.02, data not shown). Several other characteristics tended to increase in frequency as HbA1c level increased, although in several cases, this trend did not continue at HbA1c levels of 9.0% or greater. Race, educational level, proportion prefrail, and BMI followed this pattern, with decreasing proportion of white race, decreasing education, and increasing BMI until the highest level of HbA1c. Two indicators of mobility difficulty (difficulty walking one-quarter of a mile and low walking speed), as well as prevalence of CAD, increased in proportion with higher levels of HbA1c. (COPD had the opposite relationship with HbA1c.) The proportion with frailty generally increased with higher HbA1c (with the exception of HbA1c of 7.0–8.9%).
Table 1.
Characteristics of participants according to level of hemoglobin A1c (HbA1c) at baseline
| Characteristic | HbA1c Level | |||||
|---|---|---|---|---|---|---|
| <6.0 (n = 276) | 6.0–6.4 (n = 130) | 6.5–6.9 (n = 50) | 7.0–8.9 (n = 61) | ≥9.0 (n = 26) | P | |
| Age, mean ± SEM | 74.1 ± 0.2 | 74.6 ± 0.2 | 73.8 ± 0.4 | 74.0 ± 0.3 | 73.7 ± 0.6 | 0.15 |
| White, % | 85.5 | 75.7 | 67.5 | 65.7 | 67.2 | 0.004 |
| Education, years, mean ± SEM | 12.1 ± 0.3 | 11.3 ± 0.3 | 11.8 ± 1.5 | 10.4 ± 0.5 | 11.2 ± 0.6 | 0.62 |
| Prefrail, % | 39.8 | 39.6 | 49.1 | 54.8 | 54.2 | <0.001 |
| Frail, % | 9.5 | 9.2 | 20.6 | 11.8 | 19.6 | <0.001 |
| Walk one-quarter of a mile with difficulty, % | 25.8 | 33.2 | 49.5 | 45.9 | 56.9 | <0.001 |
| Slowness, %* | 28.7 | 28.1 | 39.6 | 44.7 | 53.4 | <0.001 |
| Interleukin-6, pg/mL, mean ± SEM | 4.5 ± 0.4 | 4.7 ± 0.6 | 4.8 ± 0.5 | 4.3 ± 0.5 | 4.6 ± 0.5 | 0.97 |
| BMI, kg/m2, mean ± SEM | 26.9 ± 0.3 | 27.8 ± 0.5 | 29.8 ± 0.9 | 31.5 ± 0.9 | 30.5 ± 1.1 | <0.001 |
| BMI, kg/m2, % | ||||||
| 18.5–19.9 | 5.6 | 2.8 | 0 | 0 | 0 | <0.001 |
| 20.0–24.9 | 34.0 | 33.7 | 26.5 | 14.4 | 14.4 | |
| 25.0–29.9 | 38.7 | 30.8 | 31.4 | 33.5 | 40.3 | |
| ≥30 | 21.7 | 32.7 | 42.1 | 52.2 | 45.2 | |
| Osteoarthritis, % | 69.9 | 66.4 | 62.2 | 65.8 | 69.0 | 0.59 |
| Coronary artery disease, % | 21.3 | 19.6 | 28.2 | 24.8 | 32.2 | 0.01 |
| Chronic obstructive pulmonary disease, % | 28.5 | 31.0 | 22.4 | 23.6 | 27.7 | 0.03 |
| Hemoglobin, g/dL, mean ± SEM | 13.2 ± 0.1 | 13.2 ± 0.1 | 13.2 ± 0.2 | 13.1 ± 0.2 | 13.0 ± 0.3 | 0.82 |
4-m walking speed ≤0.65 m/s for height ≤159 cm and ≤0.76 m/s for height >159 cm.
SEM = standard error of the mean; BMI = body mass index
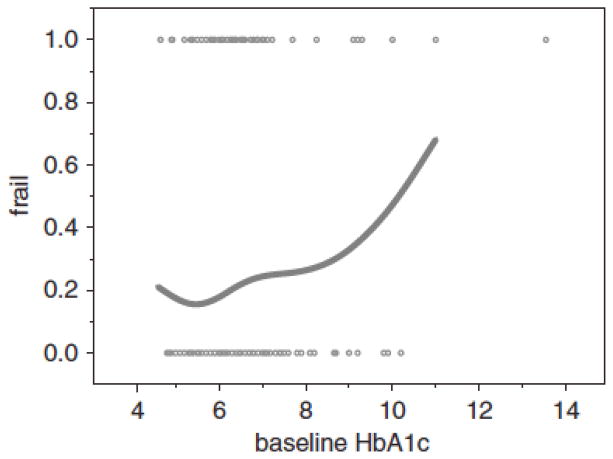
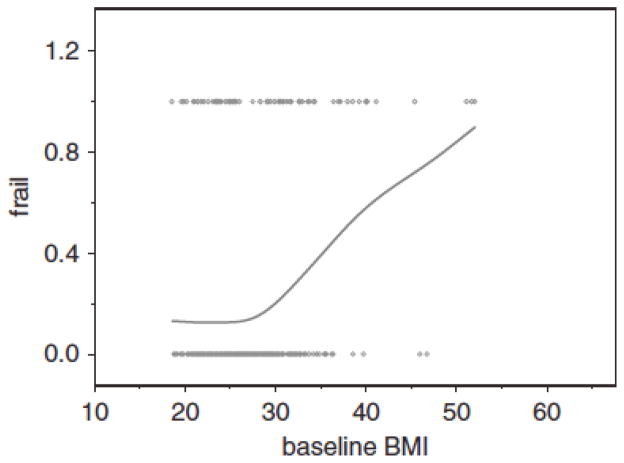
Figures 1 and 2 illustrate the crude cross-sectional association between HbA1c and BMI and frailty by plotting HbA1c (%) and BMI (kg/m2) against the proportion of women with frailty. The HbA1c plot suggests a complex, nonlinear relationship; low HbA1c is associated with a slightly higher proportion of women with frailty and normal HbA1c with the lowest proportions, and a rapid rise until a HbA1c level of approximately 7% with a leveling off and then a rapid rise above 8%. The BMI plot (Figure 2) shows a flat association until a BMI of between 25.0 and 30.0 kg/m2 and then a rapid, essentially linear rise with increasing BMI. Below 20.0 kg/m2, there is a suggestion of greater frailty, consistent with research that has demonstrated the association between frailty and low BMI, although this plot was truncated at a BMI of 18.5 kg/m2 because that is part of the frailty definition, so the association between frailty and low BMI could not be fully examined in this study.
Figure 1.
The association between baseline frail status (does not include not frail or prefrail) and baseline hemoglobin A1c (HbA1c) (unadjusted). The x-axis is HbA1c (%). The y-axis is the proportion of women who are frail.
Figure 2.
The association between baseline frail status (does not include not frail or prefrail) and baseline body mass index (BMI) (unadjusted). The x-axis is BMI (kg/m2). The y-axis is the proportion of women who are frail.
This apparent association between frailty and low HbA1c was further investigated by categorizing the 22.0% of women (n = 118) who had HbA1c less than 5.5% (data not shown). In unadjusted descriptive analyses, these women had a greater proportion with frailty (13.5%) than those with HbA1c of 5.5 to 6.0 (10.2%), although there were no other clear associations; BMI and hemoglobin level were not significantly different. A higher proportion of the women with HbA1c levels less than 5.5% were Caucasian and had osteoarthritis, but the numbers were small and not statistically significant. Multivariate models (data not shown) did not demonstrate a significantly stronger association between frailty and low HbA1c (<5.5%). Therefore, all multivariate models categorize HbA1c as was done in the descriptive analyses in Table 1 (<6.0%, 6.0–6.4%, 6.5–6.9%, 7.0–8.9%, and ≥9.0%).
Table 2 shows sequential multinomial regression models describing the cross-sectional association between frailty and HbA1c level, as well as with other key covariates. HbA1c level less than 6.0% (normal) is the reference group. All models are controlled for demographics; sequential models add BMI, IL-6, and chronic diseases.
Table 2.
Multivariate Model Describing the Cross-Sectional Association Between Baseline Hemoglobin A1c (HbA1c) Levels and Baseline Prefrail and Frailty Status
| Characteristic | Odds Ratio (95% Confidence Interval) | |||||||
|---|---|---|---|---|---|---|---|---|
| Prefrail | Frail | Prefrail | Frail | Prefrail | Frail | Prefrail | Frail | |
| HbA1c | ||||||||
| <6.0 | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| 6.0–6.4 | 0.81 (0.48–1.35) | 0.89 0.72–1.10 | 0.71 (0.42–1.23) | 0.85 (0.68–1.07) | 0.65 (0.38–1.13) | 0.79 (0.63–0.98) | 0.65 0.41–1.06 | 0.82 (0.66–1.02) |
| 6.5–6.9 | 3.01 (1.64–5.52) | 2.05 1.55–2.70 | 2.75 (1.43–5.28) | 1.96 (1.47–2.59) | 2.47 (1.32–4.64) | 1.72 (1.27–2.32) | 2.64 (1.47–4.73) | 1.81 (1.32–2.49) |
| 7.0–8.9 | 1.64 (0.76–3.52) | 2.22 (1.59–3.11) | 1.38 (0.64–2.97) | 1.89(1.32–2.69) | 1.31 (0.60–2.88) | 1.78 (1.19–2.64) | 1.15 (0.56–2.37) | 1.77 (1.17–2.67) |
| ≥9.0 | 4.59 (3.16–6.68) | 2.89 (2.22–3.76) | 4.47 (2.88–6.95) | 2.57 (1.99–3.32) | 4.40 (2.79–6.94) | 2.41 (1.85–3.12) | 3.80 (2.42–5.94) | 2.34 (1.80–3.04) |
| Age | 1.12 (1.09–1.15) | 1.01 (1.00–1.03) | 1.17 (1.01–1.57) | 1.03 (1.01–1.05) | 1.13 (1.10–1.16) | 1.03 (1.10–1.05) | 1.16 (1.13–1.20) | 1.03 (1.01–1.05) |
| Race | 1.04 (0.88–1.05) | 1.01 (0.95–1.08) | 1.07 (0.98–1.18) | 1.08 (1.02–1.16) | 1.11 (1.01–1.22) | 1.10 (1.03–1.17) | 1.07 (1.04–1.18) | 1.04 (0.97–1.11) |
| Education | 0.80 (0.78–0.82) | 0.87 (0.86–0.88) | 0.81 (0.79–0.83) | 0.88 (0.87–0.90) | 0.80 (0.78–0.82) | 0.88 (0.87–0.90) | 0.82 (0.80–0.84) | 0.89 (0.88–0.90) |
| BMI, kg/m2 | ||||||||
| 18.5–19.9 | 1.45 (0.95–2.22) | 2.04 (1.61–2.58) | 1.67(1.08–2.56) | 2.11 (1.67–2.67) | 1.94 1.24–3.05 | 2.26 1.77–2.87 | ||
| 20.0–24.9 | — | — | — | — | — | — | ||
| 25.0–29.9 | 0.49 (0.39–0.60) | 1.33 (1.19–1.49) | 0.40 (0.32–0.50) | 1.21 (1.08–1.36) | 0.48 (0.38–0.61) | 1.25 (1.11–1.41) | ||
| ≥30.0 | 2.22 (1.83–2.70) | 2.57 (2.26–2.93) | 2.03 (1.66–2.48) | 2.36 (2.06–2.96) | 2.61 (2.12–3.21) | 2.53(2.21–3.90) | ||
| Interleukin-6 | 1.24 (1.21–1.27) | 1.18 (1.16–1.21) | 1.20 (1.17–1.23) | 1.15 1.12–1.17 | ||||
| OA | 1.33 (1.22–1.45) | 1.26 (1.19–1.34) | ||||||
| CAD | 1.69 (1.54–1.85) | 1.27 (1.19–1.34) | ||||||
| CHF | 2.47 (2.13–2.88) | 1.56 (1.39–1.75) | ||||||
| Depressive symptoms | 2.17 (1.90–2.46) | 1.37 (1.24–1.52) | ||||||
| COPD | 1.59 (1.45–1.75) | 1.25 (1.18–1.32) | ||||||
Prefrail and frail were jointly modeled using multinomial logistic regression; model was controlled for age, race, education level, interleukin-6, osteoarthritis (OA), coronary artery disease (CAD), chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), and depression. (Women with a body mass index (BMI) <18.5 kg/m2 were excluded, because BMI <18.5 kg/m2 is included in the definition of frailty.)
In general, HbA1c level 6.0% to 6.5% did not have a significantly different association with frailty from the normal group. HbA1c levels from 6.5% to 6.9% and 7.0% to 8.9% had a similar and significantly higher association with frailty than the reference group; HbA1c levels of 9.0% and greater had a stronger association with frailty than all other groups. For prefrail, the association between the group with HbA1c levels from 7.0% to 8.9% and frailty was sometimes nonsignificant compared with the reference group, but the associations between the groups with HbA1c levels from 6.5% to 6.9% and 9.0% and greater was highly significant. Sequential adjustment for covariates also known to be associated with HbA1c or frailty status – BMI, IL-6, and several chronic diseases – attenuated the associations somewhat, although the associations remained significant and strong.
Several other covariates were also significantly associated with frailty status. Older age was associated with greater likelihood of prefrail and frail status; higher education was always associated with lower likelihood of both. BMI from 18.5 to 20.0 kg/m2 was associated with greater likelihood of frailty and prefrailty after adjustment than normal BMI (20.0–25 kg/m2). Obesity (BMI ≥30.0 kg/m2) was associated with higher likelihood of being prefrail and frail. Overweight (BMI 25.0–29.9 kg/m2) was associated with lower odds of being prefrail but greater odds of being frail. Higher IL-6 level was associated with higher likelihood of being prefrail and frail, as were all of the adjudicated diseases that were included in the model. Interactions between HbA1c and BMI and with IL-6 were not significant.
DISCUSSION
These results confirm the hypothesis that higher HbA1c levels in older women are associated with higher odds of prevalent frailty, although HbA1c levels of 6.0% to 6.5%, just above normal, were not associated with greater likelihood of prefrail and frail status, at least in cross-section. With HbA1c in the range of 6.5% to 6.9%, the association was significantly greater and consistent for HbA1c levels up to 8.9%, although HbA1c levels of 9.0% and greater had a markedly stronger association. Controlling for BMI or IL-6, a measure of inflammation, did not substantially alter any of these associations. These results suggest that HbA1c, as a marker of high glucose and correlated with advanced glycosylation products,19 may be part of a potential pathway to frailty that is at least partially independent of obesity and the inflammatory pathway activation associated with type 2 diabetes mellitus.
The association between hyperglycemia and frailty may be due to hyperglycemia’s role as part of the insulin resistance syndrome; insulin resistance has been shown to be associated with incident frailty.9 In addition, hyperglycemia may have an additive or independent effect. The current study cannot distinguish between these alternative hypotheses because there was no measure of insulin resistance, and the study was not longitudinal. In the current study, the effect of BMI, higher or lower than normal, on frailty was independent of the effect of hyperglycemia. Although this finding may hint that there is an additive effect of insulin resistance and hyperglycemia, data with true measures of insulin resistance are necessary to evaluate the relative association between insulin resistance and hyperglycemia and frailty.
The effect of inflammation was also independent of hyperglycemia and BMI; adding IL-6 to the model did not change the independent associations between hyperglycemia or lower and higher BMI and frailty. Inflammation20,21 and oxidative stress22,23 are among the key physiological components that underlie frailty. Multiple lines of evidence point to a causal role for glucose-mediated cellular oxidative stress in the pathophysiology of microvascular complications that result from insulin resistance and diabetes mellitus.24 Even though the current study demonstrated that hyperglycemia and inflammation (as indicated by IL-6) had independent associations with frailty, the precise inflammatory and oxidative pathways that are associated with the tissue damage that mediates frailty are unclear and probably multiple.25 The glycoxidation pathway caused by glucose auto-oxidation and oxidation of glycated proteins may be one of several key pathways leading to frailty.
Accumulating evidence also suggests an association between hyperglycemia and muscle weakness and poor muscle quality;26 such a relationship could explain part of the association between hyperglycemia and frailty. Recent data suggest that poor muscle quality in patients with diabetes mellitus becomes even poorer with longer duration of diabetes mellitus and higher levels of HbA1c.27 Presumably, inflammation and oxidative stress pathways may be related to poor muscle quality. Finally, there are older adults who have diabetes mellitus that is not related to insulin resistance, for example, the few who have type 1 diabetes mellitus, rarer types of diabetes mellitus, or secondary diabetes mellitus. Future research using data with measures of insulin resistance and hyperglycemia could determine whether such patients also have greater risk for frailty.
Although not the focus of this article, another finding deserves mention. Higher HbA1c levels remained associated with greater odds of prefrailty and frailty even when major chronic disease categories (CAD, COPD, osteoarthritis, depression, and heart failure, all adjudicated by physician researchers as described in the Methods) were considered. Despite several published research papers suggesting that diabetes mellitus is related to frailty, HbA1c had not yet been studied in a multivariate model of frailty status that included some potential complications and comorbidities associated with diabetes mellitus. Given that CAD is a common atherosclerotic complication of diabetes mellitus, the current results suggest that atherosclerotic complications may not fully explain the association between HbA1c and frailty status, although women with stroke, another atherosclerotic complication, were excluded from the analyses because virtually all women with stroke met frailty criteria. Similarly, some other chronic conditions related to high HbA1c levels, particularly peripheral vascular occlusive disease,28 peripheral neuropathy,29 and chronic kidney disease,30 were not included in these models and could be related to frailty. These conditions have not commonly been evaluated in studies of frailty, but future research should be designed to consider these additional comorbidities.
This study had several limitations in addition to the above. As previously discussed, the data had no fasting blood tests or oral or intravenous glucose tolerance test, so there was no criterion-standard diagnostic measure of the presence or absence of diabetes mellitus nor any estimate of insulin resistance status. Thus, the relationship between insulin resistance per se and prevalent frailty could not be studied. By measuring HbA1c directly, glycemic status was determined as well as possible, although HbA1c is not sufficient to diagnose diabetes mellitus. Further research into the relationship between insulin resistance and frailty status is needed using data with appropriate variables available. Another limitation was the lack of information on medications. Medications might confound or modify the association between HbA1c and frailty. Some questions, such as whether more-aggressive management of diabetes mellitus may occur in healthier people and thus lead to a spurious association between HbA1c and frailty, cannot be disentangled in these data, although the data demonstrate an association between HbA1c and frailty regardless of treatment or type of diabetes.
Despite these limitations, this research can help understand the contribution of hyperglycemia to frailty in older women living in the community. It was found that there is an increase in associated frailty with HbA1c levels as low as 6.5% that increases as HbA1c increases. This effect is independent of obesity and at least some markers of inflammatory disruption. The effect is also independent of some common chronic diseases that are complications of diabetes mellitus. These findings suggest that hyperglycemia may contribute to the geriatric syndrome of frailty, in addition to or as part of its role in insulin resistance, and that moderate glycemic control may be important in older women beyond its role in contributing to well-established complications. Longitudinal studies are an important next step to determine potential causal relationships between hyperglycemia itself, along with insulin resistance, comorbidities, and management of diabetes mellitus, and the development of frailty.
Acknowledgments
Sponsor’s Role: None.
Footnotes
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this manuscript. Dr. Blaum was supported by National Institute on Aging (NIA) Grant R01 AG021493A and the Ann Arbor Department of Veterans Affairs Geriatric Research, Education, and Clinical Center. This work was supported by NIA Grant RO1 AG11703-01A1, National Institutes of Heath National Center for Research Resources, Outpatient General Clinical Research Center Grant RR00722, R01 AG027012, and NIA Contract NO1-AG12112.
Author Contributions: Drs. Blaum, Xue, Semba, Walston, and Fried contributed to the study concept and design, methods, data analysis and interpretation of data, and preparation of the manuscript. Drs. Fried and Walston contributed to data acquisition. Dr. Tian contributed to study methods, data analysis, and interpretation of data.
References
- 1.Centers for Disease Control and Prevention. [Accessed on June 25, 2008];Crude and Age-Adjusted Prevalence of Diagnosed Diabetes per 100 Population, United States, 1980–2004 [on-line] Available at http://www.cdc.gov/DIABETES/statistics/prev/national/figage.htm.
- 2.Centers for Disease Control and Prevention. [Accessed on June 25, 2008];Crude and Age-Adjusted Incidence of Diagnosed Diabetes per 1000 Population Aged 18–79 Years, United States, 1997–2004. 2005 [on-line]. Available at http://www.cdc.gov/DIABETES/statistics/prev/national/figage.htm.
- 3.Blaum CS, Ofstedal MB, Langa KM, et al. Functional status and health outcomes in older Americans with diabetes mellitus. J Am Geriatr Soc. 2003;51:745–753. doi: 10.1046/j.1365-2389.2003.51256.x. [DOI] [PubMed] [Google Scholar]
- 4.Brown AF, Mangione CM, Saliba D, et al. Guidelines for improving the care of the older person with diabetes mellitus. J Am Geriatr Soc. 2003;51(5 suppl):S265–S280. doi: 10.1046/j.1532-5415.51.5s.1.x. [DOI] [PubMed] [Google Scholar]
- 5.Piette JD, Kerr EA. The impact of comorbid chronic conditions on diabetes care. Diabetes Care. 2006;29:725–731. doi: 10.2337/diacare.29.03.06.dc05-2078. [DOI] [PubMed] [Google Scholar]
- 6.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56A:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 7.Blaum CS, Xue QL, Michelon E, et al. The association between obesity and the frailty syndrome in older women: The Women’s Health and Aging Studies. J Am Geriatr Soc. 2005;53:927–934. doi: 10.1111/j.1532-5415.2005.53300.x. [DOI] [PubMed] [Google Scholar]
- 8.Walston J, McBurnie MA, Newman A, et al. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities. Arch Intern Med. 2002;162:2333–2341. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- 9.Barzilay JI, Blaum C, Moore T, et al. Insulin resistance and inflammation as precursors of frailty: The Cardiovascular Health Study. Arch Intern Med. 2007;167:635–641. doi: 10.1001/archinte.167.7.635. [DOI] [PubMed] [Google Scholar]
- 10.Guralnik J, Fried L, Simonsick E, et al. The Women’s Health and Aging Study: Health and Social Characteristics of Older Women with Disability. Bethesda, MD: National Institute on Aging; 1995. NIH Pub. No. 95-4009. [Google Scholar]
- 11.Michelon E, Blaum C, Semba RD, et al. Vitamin and carotenoid status in older women: Associations with the frailty syndrome. J Gerontol A Biol Sci Med Sci. 2006;61A:600–607. doi: 10.1093/gerona/61.6.600. [DOI] [PubMed] [Google Scholar]
- 12.Fried LP, Bandeen-Roche K, Chaves PH, et al. Preclinical mobility disability predicts incident mobility disability in older women. J Gerontol A Biol Sci Med Sci. 2000;55A:M43–M52. doi: 10.1093/gerona/55.1.m43. [DOI] [PubMed] [Google Scholar]
- 13.Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: Characterization in the Women’s Health and Aging Studies. J Gerontol A Biol Sci Med Sci. 2006;61A:262–266. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- 14.Siscovick DS, Fried L, Mittelmark M, et al. Exercise intensity and subclinical cardiovascular disease in the elderly. The Cardiovascular Health Study. Am J Epidemiol. 1997;145:977–986. doi: 10.1093/oxfordjournals.aje.a009066. [DOI] [PubMed] [Google Scholar]
- 15.Simonsick EM, Phillips C, Skinner E, et al. The daily lives of disabled older women. In: Guralnik J, Fried LP, Simonsick EM, Kasper J, Lafferty M, editors. The Women’s Health and Aging Study: Health and Social Characteristics of Older Women with Disability. Bethesda, MD: National Institute on Aging; 1995. pp. 50–69. Vol NIH Pub. No. 95-4009. [Google Scholar]
- 16.Guralnik J, Fried LP, Simonsick EM, et al. Screening the community-dwelling population for disability. In: Guralnik J, Fried LP, Simonsick EM, editors. The Women’s Health and Aging Study: Health and Social Characteristics of Older Women with Disability. Bethesda, MD: National Institute on Aging; 1995. pp. 9–18. Vol NIH Pub. No. 95-4009. [Google Scholar]
- 17.Little RJ, Schenker N. Missing data. In: Arminger G, Clogg CC, Sobel ME, editors. Handbook of Statistical Modeling for the Social and Behavioral Sciences. New York: Plenum Press; 1995. pp. 39–68. [Google Scholar]
- 18.Raghunathan TE, Lepkowski JM, Van Hoewyk J, et al. A multivariate techniques for multiply imputing missing values using a sequence of regression models. Surv Methodol. 2001;25:85–95. [Google Scholar]
- 19.Cipollone F, Iezzi A, Fazia M, et al. The receptor RAGE as a progression factor amplifying arachidonate-dependent inflammatory and proteolytic response in human atherosclerotic plaques: Role of glycemic control. Circulation. 2003;108:1070–1077. doi: 10.1161/01.CIR.0000086014.80477.0D. [DOI] [PubMed] [Google Scholar]
- 20.Franceschi C, Capri M, Monti D, et al. Inflammaging and anti-inflammaging: A systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128:92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 21.Leng SX, Xue QL, Tian J, et al. Inflammation and frailty in older women. J Am Geriatr Soc. 2007;55:864–871. doi: 10.1111/j.1532-5415.2007.01186.x. [DOI] [PubMed] [Google Scholar]
- 22.Semba RD, Ferrucci L, Sun K, et al. Oxidative stress and severe walking disability among older women. Am J Med. 2007;120:1084–1089. doi: 10.1016/j.amjmed.2007.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Semba RD, Lauretani F, Ferrucci L. Carotenoids as protection against sarcopenia in older adults. Arch Biochem Biophys. 2007;458:141–145. doi: 10.1016/j.abb.2006.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 25.Pennathur S, Heinecke JW. Mechanisms of oxidative stress in diabetes: Implications for the pathogenesis of vascular disease and antioxidant therapy. Front Biosci. 2004;9:565–574. doi: 10.2741/1257. [DOI] [PubMed] [Google Scholar]
- 26.Park SW, Goodpaster BH, Strotmeyer ES, et al. Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: The Health, Aging, and Body Composition Study. Diabetes Care. 2007;30:1507–1512. doi: 10.2337/dc06-2537. [DOI] [PubMed] [Google Scholar]
- 27.Park SW, Goodpaster BH, Strotmeyer ES, et al. Decreased muscle strength and quality in older adults with type 2 diabetes: The Health, Aging, and Body Composition Study. Diabetes. 2006;55:1813–1818. doi: 10.2337/db05-1183. [DOI] [PubMed] [Google Scholar]
- 28.Newman AB, Gottdiener JS, McBurnie MA, et al. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci. 2001 Mar;56A:M158–M166. doi: 10.1093/gerona/56.3.m158. [DOI] [PubMed] [Google Scholar]
- 29.Volpato S, Blaum C, Resnick H, et al. Comorbidities and impairments explaining the association between diabetes and lower extremity disability: The Women’s Health and Aging Study. Diabetes Care. 2002;25:678–683. doi: 10.2337/diacare.25.4.678. [DOI] [PubMed] [Google Scholar]
- 30.Shlipak MG, Stehman-Breen C, Fried LF, et al. The presence of frailty in elderly persons with chronic renal insufficiency. Am J Kidney Dis. 2004;43:861–867. doi: 10.1053/j.ajkd.2003.12.049. [DOI] [PubMed] [Google Scholar]