Abstract
Inosine (hypoxanthine 9-beta-D-ribofuranoside), a purine nucleoside with multiple intracellular roles, also serves as an extracellular modulatory signal. On neurons, it can produce anti-inflammatory and trophic effects that confer protection against toxic influences in vivo and in vitro. The protective effects of inosine treatment might also be mediated by its metabolite urate. Urate in fact possesses potent antioxidant properties and has been reported to be protective in preclinical Parkinson’s disease (PD) studies and to be an inverse risk factor for both the development and progression of PD. In this study we assessed whether inosine might protect rodent MES 23.5 dopaminergic cell line from oxidative stress in a cellular model of PD, and whether its effects could be attributed to urate. MES 23.5 cells cultured alone or in presence of enriched murine astroglial cultures MES 23.5-astrocytes co-cultures were pretreated with inosine (0.1–100 uM) for 24 hours before addition of the oxidative stress inducer H2O2 (200uM). Twenty-four hours later, cell viability was quantified by MTT assay or immunocytochemistry in pure and MES 23.5-astrocytes co-cultures, respectively. H2O2-toxic effect on dopaminergic cells was reduced when they were cultured with astrocytes, but not when they were cultured alone. Moreover, in MES 23.5-astrocytes co-cultures, indicators of free radical generation and oxidative damage, evaluated by nitrite (NO2−) release and protein carbonyl content, respectively, were attenuated. Conditioned medium experiments indicated that the protective effect of inosine relies on the release of a protective factor from inosine-stimulated astrocytes. Purine levels were measured in the cellular extract and conditioned medium using HPLC method. Urate concentration was not significantly increased by inosine treatment however there was a significant increase in levels of other purine metabolites, such as adenosine, hypoxanthine and xanthine. In particular, in MES 23.5-astrocytes co-cultures, inosine medium content was reduced by 99% and hypoxanthine increased by 127-fold. Taken together these data raise the possibility that inosine might have a protective effect in PD that is independent of any effects mediated through its metabolite urate.
Keywords: MES 23.5 cells, astrocytes, urate, HPLC, cell viability, oxidative stress
1 Introduction
Inosine is a purine shown to have trophic protective effects on neurons and astrocytes subjected to hypoxia or glucose-oxygen deprivation (Haun et al., 1996) and to induce axonal growth following neuronal insult in vivo and in vitro (Wu et al., 2003; Chen et al., 2002; Zurn and Do, 1988; Benowitz et al., 1998; Petrausch et al., 2000). Moreover, inosine showed antinflammatory effects in the central nervous system (CNS) and periphery (Hasko et al., 2000; Rahimian et al., 2010; Jin et al., 1997; Gomez and Sitkovsky, 2003; Shen et al., 2005). Some (Toncev, 2006; Markowitz et al., 2009) but not all (Gonsette et al., 2010) clinical studies have suggested a possible antioxidant protective effect of inosine in multiple sclerosis patients (Markowitz et al., 2009). In these trials inosine consistently elevated serum urate, which was proposed to mediate any protective effect of inosine (Markowitz et al., 2009; Spitsin et al., 2010).
Oxidative stress is thought to be a key pathophysiological mechanism in Parkinson’s disease (PD) leading to cellular impairment and death (Ross and Smith, 2007). Urate – a major antioxidant circulating in the human body - has emerged as inverse risk factor for PD. Clinical and population studies have found urate level in serum or CSF to correlate with a reduced risk of developing PD in healthy individuals and with a reduced risk of clinical progression among PD patients (Weisskopf et al., 2007; Schwarzschild et al., 2008; Ascherio et al., 2009). Moreover, in cellular and animal models of PD, urate elevation has been shown to reduce oxidative stress and toxicant-induced loss of dopaminergic neurons (Cipriani et al., 2012b; Cipriani et al., 2012a; Zhu et al., 2011; Wang et al., 2010; Gong et al., 2012; Chen et al., 2013). Although inosine can elevate urate concentration in the periphery in animals and humans, little is known about its effect on urate level in the CNS (Rahimian et al., 2010; Spitsin et al., 2010; Ceballos et al., 1994; Scott et al., 2002). A cellular study indicated that inosine added to cortical astroglial (but not neuronal) cultures increases urate concentration in the medium (Ceballos et al., 1994).
In the present study we characterized a protective effect of inosine on oxidative stress-induced dopaminergic cell death in a cellular model of PD and investigated whether urate elevation might mediate the effect.
2 Material and methods
2.1 Animals
C57BL/6 mice were employed to obtain astroglial cultures. All experiments were performed in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals with approval from the animal subjects review board of Massachusetts General Hospital.
2.2 MES 23.5 cell line
The rodent MES 23.5 dopaminergic cell line (Crawford et al., 1992) was obtained from Dr. Weidong Le at Baylor College of Medicine (Houston, USA). MES 23.5 cells were cultured on polyornithine-coated T75 flasks (Corning Co, Corning, NY) in culture medium; Dulbecco modified Eagle medium (DMEM, Invitrogen/Gibco), added with Sato components (Sigma Immunochemicals), and supplemented with 2% newborn calf serum (Invitrogen), 1% fibroblast growth factor (Invitrogen), penicillin 100 U ml−1 and streptomycin 100 μg mL−1 (Sigma), at 37° C in a 95% air–5% carbon dioxide, humidified incubator. Culture medium was changed every 2 days. At confluence, MES 23.5 cells were either sub-cultured new T-75 flasks or used for experiments. For experiments, MES 23.5 cells were seeded at a density of 600 cells per mm2. onto polyornithine-coated plates or flasks (according to the assay, see below) in culture medium. Twenty-four hours later, it was changed to DMEM serum-free medium. At this time, increasing concentrations of inosine (0–100 μM) were added to the cultures for 24 hours and again during toxicant treatment. 200 μM H2O2 were added to the cultures for 24 hours and then cells were used for assays.
2.3 Enriched astroglial cultures
Astroglial cultures were prepared from the brains of 1- or 2-day-old neonatal mice as previously described (Cipriani et al., 2012b). Briefly, cerebral cortices were digested with 0.25% trypsin for 15 min at 37° C. The suspension was pelleted and re-suspended in culture medium (DMEM, fetal bovine serum (FBS) 10%, penicillin 100 U ml−1 and streptomycin 100 μg ml−1 to which 0.02% deoxyribonuclease I was added). Cells were plated at a density of 1,800 cells per mm2 on poly-L-lysine (100 μg ml−1)/DMEM/F12-coated flasks and cultured at 37° C in humidified 5% CO2-95% air for 7–10 days until reaching confluence.
In order to remove non-astroglial cells, flasks were agitated at 200 rpm for 20 min in an orbital shaker and treated with 10 μM cytosine arabinoside (Ara-C) dissolved in cultured medium for 3 days. After the treatment, astrocytes were subjected to mild trypsinization (0.1 % for 1 min) and then sub-plated (120 cells per mm2) onto poly-L-lysine (100 μg ml−1)/DMEM/F12-coated plates or flasks (according to the assay, see below) in DMEM plus 10% FBS for assays. Astroglial cultures comprised >95% astrocytes, <2% microglial cells and <1% oligodendrocytes; no neuronal cells were detected (Cipriani et al., 2012b).
2.4 MES 23.5-astrocytes co-cultures
MES 23.5 cells were cultured on a layer of enriched astroglial cultures prepared as described above. Briefly, astrocytes were allowed to grow for 48 hours on poly-L-lysine (100 μg ml−1)/DMEM/F12-coated plates or flasks (according to the assay, see below) in DMEM plus 10% FBS. Then, MES 23.5 cells were seeded on top at a concentration of 600 cells per mm2 in MES 23.5 culture medium. An astrocyte:MES 23.5 cell ratio of 1:5 was chosen on the basis of our previous observations (Cipriani et al., 2012b), which indicated this proportion of astrocytes sufficiently low to avoid a direct effect of astrocytes on dopaminergic cell survival. Twenty-four hours later, medium was changed to DMEM serum-free medium and subjected to treatments. Inosine was added to the cultures 24 hours before and during 200 μM H2O2 treatment. In our previous study this H2O2 concentration was shown to have no effect on astrocyte viability (Cipriani et al., 2012b). At the end of treatment, MES 23.5 cells were easily detached from astrocytes and dissociated by gently pipetting up and down the medium before processing for biochemical assays.
2.5 Conditioned media experiments
Enriched-astrocytes cultures were grown on poly-L-lysine (100 μg ml−1)/DMEM/F12-coated 6 well-plates in DMEM plus 10% FBS. Astrocytes were let grow for three days and then medium was changed to MES 23.5 culture medium in order to reproduce co-culture conditions. The day after, medium was changed to DMEM containing 100 μM inosine or vehicle. Twenty-four hours later, conditioned medium was collected and filtered through a 0.2 μM membrane to remove cellular debris. MES 23.5 cells were treated with increasing concentrations of conditioned medium 24 hours before and during H2O2 treatment.
2.6 Drugs
Inosine was dissolved in DMEM as 20X concentrated stocks. H2O2 was dissolved in PBS (0.1 M, pH 7.4) as 100X concentrated stocks. Drugs were obtained from Sigma.
2.7 Cell viability and toxicity assessments
In MES 23.5 cultures, cell viability was measured by the 3-(4,5-dmethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Sigma). This assay is based on the conversion of the yellow tetrazolium salt MTT by mitochondrial dehydrogenase of live cells to the purple formazan (Hansen et al., 1989). Briefly, MES 23.5 cells were cultured in polyornithine-coated 96-well plates (600 cells per mm2) and grown for at least 24 hours. Then, the medium was changed to DMEM serum-free medium for 24 hours before H2O2 was added. In order to assess inosine protection, increasing concentrations of drug (0–100 μM) were loaded 24 hours before and again during toxicant treatment. After washes, 100 μl of MTT solution (0.5 mg ml−1 in DMEM) were added for 3 hours at 37° C. Then, MES 23.5 cells were lysed with 10ul/well of acidic isopropanol (0.01M HCl in absolute isopropanol) to extract formazan that was measured spectrophotometrically at 490 nm with a Labsystems iEMS Analyzer microplate reader.
In MES 23.5-astrocytes co-cultures, surviving MES 23.5 cells were quantified by immunocytochemistry (Lotharius et al., 2005, Dumitriu et al., 2011, Cipriani et al., 2012b). MES 23.5 were grown on top of astrocytes in 96-well plates as described above. Increasing concentrations of drug (0–100 μM) were loaded 24 hours before and again during toxicant treatment. After washing in PBS, cultures were fixed with 4% (wt/vol) paraformaldehyde for 1 hour at room temperature. Then, cells were incubated with an Alexa 488-conjugated antibody specific for neuronal cells, Milli-Mark FluoroPan Neuronal Marker, (Millipore; 1:200, overnight at 4 °C). Fluorescence was read at 535 nm by using a microplate reader.
2.8 High-Performance Liquid Chromatography
To determine purine content in cells and medium samples, MES 23.5, MES 23.5-astrocytes co-cultures or enriched astroglial cultures were prepared as described above and cultured in T75 flasks. Purine content was determined using our previously described HPLC-based analytical methods (Burdett et al., 2013). Briefly, cell medium was collected and added with 30% vol/vol of a buffer containing 150 mM phosphoric acid, 0.2 mM EDTA, and 1 μM 3,4-dihydroxybenzylamine (DHBA; used as internal standard). Cells were collected after washing in ice-cold PBS and purines were extracted in the same buffer used for medium. Samples were then filters through a 0.2 μm Nylon microcentrifuge filter (Spin-X, Corning) at 4° C. Samples were maintained at 4° C and injected using an ESA Biosciences (Chelmsford, MA) autosampler, and chromatographed by a multi-channel electrochemical/UV HPLC system with effluent from the above column passing through a UV-VIS detector (ESA model 528) set at 254 nm and then over a series of electrodes set at −100 mV, + 250mV and + 450 mV. To generate a gradient two mobile phases were used. Mobile phase A consisted of 0.2 M potassium phosphate and 0.5 mM sodium 1-pentanesulfonate; mobile phase B consisted of the same plus 10 % (vol/vol) acetonitrile. Mobile phase B increased linearly from 0 % to 70 % between 6th and 14th min of the run.
2.9 Nitrite (NO2−) release
MES 23.5-astrocytes co-cultures were grown on 96-well plate as described above. After treatments, nitrite release (NO2−), an indicator of free radical generation, was quantified in cell medium by Griess assay. An azo dye is produced in the presence of nitrite by the Griess reaction and colorimetrically detected. Briefly, 100 μl of supernatant collected from treated cultures were added to 100 μl of Griess reagent (Sigma) and absorbance was read at 540 nm with a microplate reader. Blanks were prepared by adding medium containing toxicants and/or protectants to Griess solution.
2.10 Protein carbonyl protein assay
MES 23.5-astrocytes co-cultures were grown in 6-well plates as described above. After treatments, cultures were washed with ice-cold PBS and oxidized proteins were detected in MES 23.5 cells, using the Oxyblot assay kit (Chemicon). MES 23.5 cells were detached from astrocytes in ice-cold PBS, spun to form a pellet at 4° C and resuspended in ice-cold RIPA buffer containing 50 mM DTT. Cells were allowed to lyse on ice for 15′. For the assay, 20 ug of protein were derivatized in 10 μL of 2,4-dinitrophenylhydrazine (DNPH). After derivatization samples were subjected to sodium dodecyl sulfate (SDS)-polyacrylamide gel (10% [wt/vol] acrylamide, 0.1% [wt/vol] SDS) and transferred electrophoretically onto 0.2 μ nitrocellulose membranes. Membranes were loaded with an antibody specific to dinitrophenylhydrazone moiety of the proteins and reaction visualized by chemiluminescence.
2.11 Protein detection
After treatment, cells were washed in ice-cold PBS, collected and resuspended in ice-cold Ripa buffer. Cells were incubated on ice for 15′, followed by sonication for complete lysis. Proteins were quantified in 4 μl of each sample using Bio-Rad Protein Assay reagent (Biorad Laboratories) and measured at 600 nm with a microplate reader.
2.12 Statistical analysis
Statistical analysis was performed by GraphPad Prism version 4.00 (GraphPad Software Inc.). Unpaired Student’s t-test was used when two group samples were compared. ANOVA analysis followed by Newman-Keuls was used when more than two group samples were compared. Values were expressed as mean ± SEM. Differences with a P< 0.05 were considered significant and indicated in figures by symbols explained in legends.
3 Results
3.1 Astrocytes mediated protective effect of inosine on dopaminergic cells
Previously we showed that urate protected a dopaminergic cell line (MES 23.5) against oxidative stress when cells were cultured with astrocytes (Cipriani et al., 2012b). To assess whether inosine protected the dopaminergic cell line in a similar way we tested inosine on MES 23.5 cells cultured alone or with cortical astrocytes (MES 23.5-astrocyte co-cultures) treated with 200 μM H2O2.
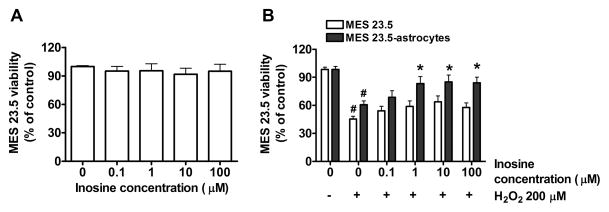
Inosine on its own had no effect on MES 23.5 viability (one-way ANOVA, P > 0.05) (Fig. 1A), and showed only a trend toward modest protection with increasing concentrations from 0.1 to 100 μM against H2O2 toxicity (one-way ANOVA, P > 0.05) in pure MES 23.5 cultures. However, in the presence of a relatively low density of astrocytes (plated at a density of 120 cells per mm2), MES 23.5 cell viability significantly increased in comparison to inosine-untreated cells (P < 0.05; Fig. 1B).
Figure 1. Astrocytes potentiated the protective effect of inosine.
on 200 μM H2O2-treated MES 23.5 cells. A) Viability of MES 23.5 cells treated for 24 hours with increasing concentrations of inosine (0–100 μM). B) Effect of inosine treatment (0–100 μM) at 24 hours of toxic treatment with 200 μM H2O2 on viability of MES 23.5 cells cultured alone (white bars) or in presence of astrocytes (gray bars). Cultures were treated with inosine 24 hours before and during toxic treatment. Data represent the means ± SEM of values from four experiments, each of which yielded a mean of triplicate determinations for each condition. One-way ANOVA analysis (P< 0.001) followed by Newman-Keuls multiple comparison test (#P< 0.01 vs respective ‘0 inosine/-H2O2‘ value; *P< 0.05 vs ‘0 inosine/+H2O2’ value).
3.2 Inosine decreased toxicant-induced oxidative stress
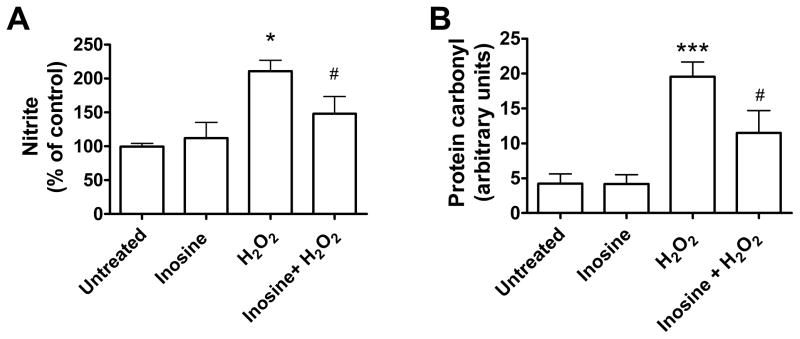
To determine whether protection was associated with reduced oxidative stress and protein damage, we measured the effect of inosine on oxidative stress markers in H2O2-treated co-cultures of MES 23.5 cells and astrocytes. At 24 hours, inosine decreased the level of NO2− (nitrite), an indicator of free radical generation, from 2-fold to 1.4-fold of control value in cell medium (P = 0.00139, Fig. 2A). Moreover, at 3 hours inosine decreased protein oxidation, measured as protein carbonyl content in MES 23.5 cells (after removal from astrocytes), from 4.6- to 2.7-fold of control value (P = 0.002) (Fig. 2B).
Figure 2. Inosine reduced oxidative stress in MES 23.5 cells cultured with astrocytes.
(A) Effect of inosine treatment (0–100 μM) on 200 μM H2O2-induced NO2− release in the medium of MES 23.5-astrocytes co-cultures at 24 hours of toxic treatment. Cultures were treated with inosine 24 hours before and during toxic treatment. Data represent the means ± SEM of three triplicate experiments. (B) Effect of inosine treatment (0–100 μM) on 200 μM H2O2-induced protein carbonylation in MES 23.5 cells cultured with astrocytes at 3 hours of toxic treatment. Cultures were treated with inosine 24 hours before and during toxic treatment. Data represent the means ± SEM of six replicates over three independent experiments. One-way ANOVA: *P< 0.05 and ***P< 0.001 vs untreated and inosine (alone) values; #P< 0.05 vs H2O2 (alone) value.
3.3 Protection mediated by astrocytes does not require their physical contact with dopaminergic cells
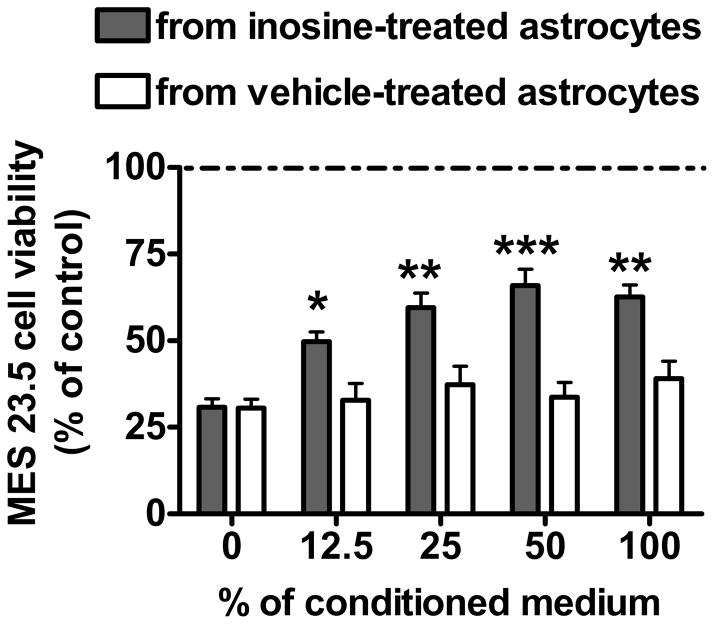
We previously observed that astrocytes mediate urate’s protective effect through release of protective factor(s). To assess if astrocytes mediated inosine’s protective effect in the same fashion, MES 23.5 cells were treated with increasing percentages of medium collected from untreated or inosine-treated astrocytes. Medium from untreated astrocytes did not show a statistically significant effect on H2O2-treated MES 23.5 viability at any given concentration (P > 0.05). On the other hand, conditioned medium from astrocytes treated for 24 hours with 100 μM inosine improved MES 23.5 viability in a concentration-dependent manner (P < 0.001). This observation was confirmed by two-way ANOVA analysis that showed significant effect of conditioned medium (F1, 151 = 46.28, P < 0.0001) and conditioned medium percentage (F1, 151 = 7.31, P < 0.0001) and significant interaction between these two factors (F1, 151 = 3.59, P = 0.0079; Figure 3).
Figure 3. Inosine-conditioned medium from astrocytes increased viability of H2O2-treated MES 23.5 cells.
Effect of increasing concentration of cell medium collected from control (white bars) or 100 μM inosine-treated astrocytes (gray bars) on 200 μM H2O2-induced cell death in MES 23.5 pure cultures. Cultures were treated with conditioned medium 24 hours before and during toxic treatment. Data represent the means ± SEM of thirteen independent experiments. Two-way ANOVA analysis (P = 0.0003) followed by Newman-Keuls multiple comparison test (*P< 0.05, **P< 0.01 and ***P< 0.001 vs respective control value).
3.4 Inosine treatment did not affect urate concentration
Extracellular inosine breakdown has been reported in astroglial cultures (Ceballos et al., 1994). To determine whether inosine degradation occurred in our cultures, purine metabolites of inosine were measured in the medium of MES 23.5-astroglial co-cultures treated with inosine. For this experiment we selected two time points: 0, when inosine was added to the cultures, and 24 hours, when the cultures would be treated with toxicant. Over 24 hours inosine concentration, reflecting both endogenous plus exogenous contributions, was reduced by 99% (P < 0.0001); over the same time period hypoxanthine and xanthine increased by 127-fold (P < 0.0001) and 1.5-fold (P < 0.0001), in comparison to time zero, respectively (Table 1). Thus, the hypoxanthine increment was 1.6 fold greater than the amount of inosine added. Moreover, adenosine, an inosine ‘precursor’, increased by 4-fold (P = 0.0001, Table 1) over the 24 hours. By contrast, urate content was not changed in the medium over the same time period (P = 0.46, Table 1), indicating that extracellular urate unlikely mediated inosine’s effects.
Table 1.
Extracellular purine content at time zero (0) and 24 hours in 100 μM-inosine-treated MES 23.5-strocytes co-cultures.
| Analites | Concentration (μM) | |
|---|---|---|
| 0 | 24 hours | |
| Adenosine | 0.16 ± 0.01 | 0.82 ± 0.04*** |
| Inosine | 104 ± 8 | 0.74 ± 0.09*** |
| Hypoxanthine | 1.25 ± 0.26 | 160 ± 28*** |
| Xanthine | 0.34 ± 0.03 | 0.85 ± 0.05*** |
| Urate | 0.89 ± 0.04 | 0.83 ± 0.05 |
Purine content of co-cultures cell medium was analyzed by high-performance liquid chromatography. Data are expressed as μM. Significance was determined by Student’s t test:,
P < 0.001 vs 0 time point value. Data are presented as means ± SEM of eight experiments.
In our previous studies we found evidence that urate’s protective effect on dopaminergic cells was correlated with its increase within astrocytes (Cipriani et al., 2012b). To assess whether inosine treatment increased intracellular urate in astroglial cells its concentration was measured in inosine treated astrocyte-enriched cultures at time 0 and 24 hours of treatment. Although adenosine increased 2-fold, intracellular concentrations of urate and other purines were not changed at 24 hours in comparison to time 0 (Table 2) and vehicle-treated cells (data not shown). Similarly, no effect was seen on extracellular urate, where inosine induced an approximately 5-fold increase in hypoxanthine concentration (P < 0.01, Table 3). Thus despite the expression of functional xanthine oxidase, the enzyme that converts hypoxanthine to xanthine and in turn to urate in cortical astrocytes (Ceballos et al., 1994), we did not find evidence of the conversion of inosine to urate.
Table 2.
Intracellular purine content at time zero (0) and 24 hours in 100 μM-inosine-treated enriched astroglial cultures.
| Analites | Concentration (nmol/g of protein) | |
|---|---|---|
| 0 | 24 hours | |
| Adenosine | 130 ± 48 | 390 ± 137* |
| Inosine | 607 ± 230 | 477 ± 204 |
| Hypoxanthine | 320 ± 77 | 400 ± 199 |
| Xanthine | 3 ± 1 | 6 ± 3 |
| Urate | 38 ± 5 | 26 ± 2 |
Purines were extracted from enriched astroglial cultures by cell trituration in extracting buffer (see methods) and measured by high-performance liquid chromatography. Data are expressed as nmol/g of protein. Significance was determined by Student’s t test:
P = 0.012. Data are presented as means ± SEM of four experiments.
Table 3.
Extracellular purine content at time zero (0) and 24 hours in 100 μM-inosine-treated enriched astroglial cultures.
| Analites | Concentration (μM) | |
|---|---|---|
| 0 | 24 hours | |
| Adenosine | 1.7 ± 0.1 | 1.6 ± 0.4 |
| Inosine | 105 ± 1 | 9.0 ± 0.1*** |
| Hypoxanthine | 22.6 ± 0.1 | 127 ± 21** |
| Xanthine | 8.5 ± 0.1 | 13 ± 2 |
| Urate | 20.3 ± 0.1 | 24 ± 4 |
Purine content of astrocyte medium was measured by high-performance liquid chromatography. Data are expressed as μM. Significance was determined by Student’s t test:
P < 0.01 and
P < 0.001 vs 0 time point value. Data are presented as means ± SEM of four experiments.
Purine increase induced by inosine in mixed-cultures might play a role in inosine protective effect. To assess whether this effect was selective for mixed-cultures, inosine metabolite concentration was also measured in the medium of MES 23.5 cultures after inosine treatment. Similarly to mixed cultures, in MES 23.5 cultures inosine concentration decreased to about 30% (P < 0.0001) and 3% (P < 0.0001) of control at 6 and 24 hours, respectively. Hypoxanthine increased over the time up to 4-fold (P < 0.0001) at 24 hours in comparison to time zero and xanthine by 1.8-fold in comparison to 6 hours (Table 4). Moreover, adenosine increased about 9-fold (P < 0.0001) in comparison to time zero. Urate concentration did not change at any tested time (Table 4). These data exclude a direct effect of inosine metabolites on MES 23.5 cells since no protective effect was found in these experimental conditions.
Table 4.
Extracellular purine content in 100 μM inosine-treated MES 23.5 cells over 24 hours of treatment.
| Analites | Concentration (μM) | ||
|---|---|---|---|
| 0 | 6 hours | 24 hours | |
| Adenosine | 0.28 ± 0.09 | 2.28 ± 0.08 | 2.8 ± 0.4** |
| Inosine | 89 ± 20 | 30 ± 2** | 2.6 ± 0.1*** |
| Hypoxanthine | 149 ± 10 | 680 ± 43*** | 780 ± 81*** |
| Xanthine | N.D. | 0.10 ± 0.04 | 0.28 ± 0.07* |
| Urate | N.D. | N.D. | N.D. |
Purine content of astrocyte medium was measured by high-performance liquid chromatography. Data are expressed as μM. Student’s t test, n = 8,
P < 0.05 vs 6h value. One-way ANOVA followed by Newman-Keuls test:
P < 0.01 and
P < 0.001 vs 0 time point value. Data are presented as means ± SEM of eight experiments.
4 Discussion
We report that inosine prevented oxidative stress induced-cell death in dopaminergic MES 23.5 cells cultured with astrocytes. This effect appeared to be independent of increased intracellular urate, an inosine metabolite and established antioxidant.
As a close structural homolog of adenosine, inosine may confer protection by direct mechanisms, activating multiple subtypes of adenosine receptors that are known to modulate cell death. Several studies have implicated A1, A2A or A3 receptors as mediator of inosine effects in the setting of inflammatory or ischemic injury (Rahimian et al., 2010; Jin et al., 1997; Gomez and Sitkovsky, 2003; Shen et al., 2005). For example, inosine was found to reduce ischemic brain injury in rats likely via an adenosine A3 receptor-dependent pathway (Shen et al., 2005).
In vitro studies showed inosine to be protective in models of hypoxia (Litsky et al., 1999) and glucose-oxygen deprivation (Haun et al., 1996) where it mediated adenosine protective effects. Inosine has been shown to protect neurons with a neurotrophic effect, promoting axonal regeneration in vivo and in vitro (Wu et al., 2003; Chen et al., 2002; Zurn and Do, 1988) and inducing the expression of axonal growth-associated genes (Benowitz et al., 1998; Petrausch et al., 2000). This neuroprotective effect can be exerted with a receptor-independent mechanism, for example, activating the cytoplasmic protein kinase Mst3b as shown in the setting of stroke or traumatic brain injury in rodents (Zai et al., 2011). In vitro and in vivo studies showed inosine to have anti-inflammatory effects in inflammatory or ischemic injury (Hasko et al., 2000; Rahimian et al., 2010; Jin et al., 1997; Gomez and Sitkovsky, 2003; Shen et al., 2005). Moreover, a clinical study raised the possibility that inosine may have antioxidant properties improving structural and neurological impairment in multiple sclerosis patients (Markowitz et al., 2009).
A previous study reported that inosine protection against chemical hypoxia was dependent on the presence of astrocytes in cultures (Litsky et al., 1999). Similarly, we show that inosine’s protective effect on dopaminergic cells was mediated by astrocytes, suggesting a mechanism more complex than a direct protective effect exerted by inosine. Moreover, the rapid inosine degradation occurring in cultures would suggest more of an indirect effect of inosine, which would be consistent with stimulated production and release of an astrocytic protective factor(s) (Imamura et al., 2008).
The rapid elimination of exogenous inosine and increase in its precursor and metabolites are also consistent with the possibility that a purine related to inosine mediates its protective effect. Treatment with inosine at a high concentration relative to endogenous levels increased the concentration of its precursor adenosine in co-cultures, suggesting either conversion of inosine into adenosine (Murray, 1971) or feedback inhibition of adenosine deaminase (Meyskens and Williams, 1971) leading to reduced degradation of endogenous adenosine. Extracellular adenosine in turn may act on its own receptors to enhance survival of dopaminergic neurons in cultures (Michel et al., 1999) or it can be taken up by neurons (Hertz and Matz, 1989).
Alternatively, increased metabolism of inosine may have mediated its protective effect. Inosine breakdown protected cells subjected to glucose deprivation or hypoxia-reoxygenation preserving cellular ATP content (Jurkowitz et al., 1998, Shin et al., 2002, Szoleczky et al., 2012, Modis et al., 2009). Intracellular inosine (and adenosine by way of inosine) was shown to be transformed to hypoxanthine and ribose 1-phosphate by purine nucleotides phosphorylase (Jurkowitz et al., 1998). In turn, ribose 1-phosphate was converted to an intermediate that can enter the anaerobic glycolytic pathway providing the ATP necessary to maintain cell integrity (Jurkowitz et al., 1998). Inhibition of the enzyme purine nucleoside phosphorylase notably prevented the neuroprotective effect of inosine in glial cells and mixed astrocyte-neuronal cultures (Jurkowitz et al., 1998, Litsky et al., 1999). Moreover, this pathway can represent the primary energy source for erythrocytes lacking functional glucose transporters (Young et al., 1985). In our study we found that the hypoxanthine increment was about 24-times higher in MES 23.5-astrocytes co-cultures than in MES 23.5 cells alone after inosine treatment. Purine nucleoside phosphorylase is highly expressed in astrocytes (Ceballos et al., 1994); thus the presence of astrocytes in cultures might provide conditions sufficient for enhanced ATP production during the toxic insult. This raises the possibility that the anaerobic glycolytic pathway might contribute to the protective effect of inosine on dopaminergic cells during oxidative stress. A role for this pathway and the associated production of hypoxanthine by increased purine nucleoside phosphorylase activity in astrocytes may also account for the observed hypoxanthine increase in molar excess of exogenous inosine introduced. Regardless of whether altered cellular energy metabolism induced by inosine breakdown or a specific metabolite of inosine is protective, these scenarios support the hypothesis that inosine treatment might induce release of factor(s) from astrocytes to protect dopaminergic cells.
Inosine has been shown to be converted to urate in cultures (Ceballos et al., 1994) and to elevate urate serum level in rodents and humans (Rahimian et al., 2010; Spitsin et al., 2010; Ceballos et al., 1994; Scott et al., 2002). Although we observed higher extracellular concentrations of inosine’s metabolites, such as hypoxanthine, we did not find increased urate levels in media or in astrocytes. It is unlikely that an earlier increase in urate was missed due to its being metabolized to allantoin since we have already shown that cortical astrocytes and MES 23.5 cells do not express urate oxidase, the enzyme that converts urate to allantoin (Cipriani et al., 2012b). Together these observations argue against a role for urate as the mediator of inosine’s protective effects in this cellular model of oxidative stress in PD. However, purine metabolism is of course different in intact humans versus murine culture models and the present findings of a urate-independent protective effect in culture do not preclude protective effect of urate, which can be substantially elevated in people treated with inosine (The Parkinson Study Group SURE-PD Investigators et al., 2014).
In PD the degeneration of dopaminergic neurons is thought to be induced by accumulation of oxidative damage that leads to mitochondrial impairment and protein aggregation. The finding that inosine prevents oxidant-induced dopaminergic cell loss may be of substantial epidemiological and therapeutic significance for PD. A phase II clinical trial of inosine in early PD showed that inosine was safe, tolerable and effective in raising CSF and serum urate levels (The Parkinson Study Group SURE-PD Investigators et al., 2014). Our results suggest that if CNS inosine itself were elevated in the CNS of treated individuals it could produce a neuroprotective effect independent of urate.
5 Conclusions
Inosine had antioxidant and protective effects on dopaminergic cells with a mechanism that does not require increased urate concentration. This finding further supports inosine as a candidate for PD therapy.
Highlights.
Inosine protected dopaminergic cells subjected to oxidative stress.
Inosine attenuated inductions of indicators of free radical generation and oxidative damage in this model.
Protection by inosine required astrocytes in cultures.
Protection by inosine appeared to be independent of urate.
Acknowledgments
This work was supported by the American Parkinson Disease Association, US National Institutes of Health grants R21NS058324, K24NS060991 and the US Department of Defense grant W81XWH-11-1-0150. MES 23.5 cells and technical advice were kindly provided by Weidong Le, Ken Rock and Hajime Kono. S.C. designed and performed the experiments, analyzed the data and wrote the manuscript. R.B performed the experiments and analyzed the data. M.A.S. designed the experiments and wrote the manuscript. We thank Cody Desjardins, Thomas Burdett and Robert Logan for excellent technical assistance.
Abbreviations used
- Ara-c
cytosine arabinoside
- FBS
fetal bovine serum
- ROS
reactive oxygen species
- DMEM
Dulbecco’s modified Eagle’s medium
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- EDTA
ethylenediaminetetraacetic acid
- DHBA
3,4-dihydroxybenzylamine
Footnotes
Authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ascherio A, LeWitt PA, Xu K, Eberly S, Watts A, Matson WR, Marras C, Kieburtz K, Rudolph A, Bogdanov MB, Schwid SR, Tennis M, Tanner CM, Beal MF, Lang AE, Oakes D, Fahn S, Shoulson I, Schwarzschild MA Parkinson Study Group DATATOP Investigators. Urate as a predictor of the rate of clinical decline in Parkinson disease. Arch Neurol. 2009;66:1460–1468. doi: 10.1001/archneurol.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz LI, Jing Y, Tabibiazar R, Jo SA, Petrausch B, Stuermer CA, Rosenberg PA, Irwin N. Axon outgrowth is regulated by an intracellular purine-sensitive mechanism in retinal ganglion cells. J Biol Chem. 1998;273:29626–29634. doi: 10.1074/jbc.273.45.29626. [DOI] [PubMed] [Google Scholar]
- Burdett TC, Desjardins CA, Logan R, McFarland NR, Chen X, Schwarzschild MA. Efficient determination of purine metabolites in brain tissue and serum by high-performance liquid chromatography with electrochemical and UV detection. Biomed Chromatogr. 2013;27:122–9. doi: 10.1002/bmc.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceballos G, Tuttle JB, Rubio R. Differential distribution of purine metabolizing enzymes between glia and neurons. J Neurochem. 1994;62:1144–1153. doi: 10.1046/j.1471-4159.1994.62031144.x. [DOI] [PubMed] [Google Scholar]
- Chen P, Goldberg DE, Kolb B, Lanser M, Benowitz LI. Inosine induces axonal rewiring and improves behavioral outcome after stroke. Proc Natl Acad Sci U S A. 2002;99:9031–9036. doi: 10.1073/pnas.132076299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Burdett TC, Desjardins CA, Logan R, Cipriani S, Xu Y, Schwarzschild MA. Disrupted and transgenic urate oxidase alter urate and dopaminergic neurodegeneration. Proc Natl Acad Sci U S A. 2013;110:300–305. doi: 10.1073/pnas.1217296110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriani S, Desjardins AC, Burdett CT, Xu Y, Xu K, Schwarzschild AM. Urate and its transgenic depletion modulate neuronal vulnerability in a cellular model of Parkinson’s disease. PloS one. 2012a;7:e37331. doi: 10.1371/journal.pone.0037331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriani S, Desjardins CA, Burdett TC, Xu Y, Xu K, Schwarzschild MA. Protection of dopaminergic cells by urate requires its accumulation in astrocytes. J Neurochem. 2012b;123:172–181. doi: 10.1111/j.1471-4159.2012.07820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford GD, Jr, Le WD, Smith RG, Xie WJ, Stefani E, Appel SH. A novel N18TG2 x mesencephalon cell hybrid expresses properties that suggest a dopaminergic cell line of substantia nigra origin. J Neurosci. 1992;12:3392–3398. doi: 10.1523/JNEUROSCI.12-09-03392.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitriu A, Pacheco CD, Wilk JB, Strathearn KE, Latourelle JC, Goldwurm S, Pezzoli G, Rochet JC, Lindquist S, Myers RH. Cyclin-G-associated kinase modifies α-synuclein expression levels and toxicity in Parkinson’s disease: results from the GenePD Study. Hum Mol Genet. 2011;20:1478–87. doi: 10.1093/hmg/ddr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez G, Sitkovsky MV. Differential requirement for A2a and A3 adenosine receptors for the protective effect of inosine in vivo. Blood. 2003;102:4472–4478. doi: 10.1182/blood-2002-11-3624. [DOI] [PubMed] [Google Scholar]
- Gong L, Zhang QL, Zhang N, Hua WY, Huang YX, Di PW, Huang T, Xu XS, Liu CF, Hu LF, Luo WF. Neuroprotection by urate on 6-OHDA-lesioned rat model of Parkinson’s disease: linking to Akt/GSK3beta signaling pathway. J Neurochem. 2012;123:876–885. doi: 10.1111/jnc.12038. [DOI] [PubMed] [Google Scholar]
- Gonsette RE, Sindic C, D’hooghe MB, De Deyn PP, Medaer R, Michotte A, Seeldrayers P, Guillaume D ASIIMS study group. Boosting endogenous neuroprotection in multiple sclerosis: the ASsociation of Inosine and Interferon beta in relapsing-remitting Multiple Sclerosis (ASIIMS) trial. Mult Scler. 2010;16:455–462. doi: 10.1177/1352458509360547. [DOI] [PubMed] [Google Scholar]
- Hansen MB, Nielsen SE, Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Method. 1989;119:203–10. doi: 10.1016/0022-1759(89)90397-9. [DOI] [PubMed] [Google Scholar]
- Hasko G, Kuhel DG, Nemeth ZH, Mabley JG, Stachlewitz RF, Virag L, Lohinai Z, Southan GJ, Salzman AL, Szabo C. Inosine inhibits inflammatory cytokine production by a posttranscriptional mechanism and protects against endotoxin-induced shock. J Immunol. 2000;164:1013–1019. doi: 10.4049/jimmunol.164.2.1013. [DOI] [PubMed] [Google Scholar]
- Haun SE, Segeleon JE, Trapp VL, Clotz MA, Horrocks LA. Inosine mediates the protective effect of adenosine in rat astrocyte cultures subjected to combined glucose-oxygen deprivation. J Neurochem. 1996;67:2051–2059. doi: 10.1046/j.1471-4159.1996.67052051.x. [DOI] [PubMed] [Google Scholar]
- Hertz L, Matz H. Inhibition of adenosine deaminase activity reveals an intense active transport of adenosine into neurons in primary cultures. Neurochem Res. 1989;14:755–760. doi: 10.1007/BF00964954. [DOI] [PubMed] [Google Scholar]
- Imamura K, Takeshima T, Nakaso K, Ito S, Nakashima K. Pramipexole has astrocyte-mediated neuroprotective effects against lactacystin toxicity. Neurosci Lett. 2008;440:97–102. doi: 10.1016/j.neulet.2008.05.067. [DOI] [PubMed] [Google Scholar]
- Jin X, Shepherd RK, Duling BR, Linden J. Inosine binds to A3 adenosine receptors and stimulates mast cell degranulation. J Clin Invest. 1997;100:2849–2857. doi: 10.1172/JCI119833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurkowitz MS, Litsky ML, Browning MJ, Hohl CM. Adenosine, inosine, and guanosine protect glial cells during glucose deprivation and mitochondrial inhibition: correlation between protection and ATP preservation. J Neurochem. 1998;71:535–48. doi: 10.1046/j.1471-4159.1998.71020535.x. [DOI] [PubMed] [Google Scholar]
- Litsky ML, Hohl CM, Lucas JH, Jurkowitz MS. Inosine and guanosine preserve neuronal and glial cell viability in mouse spinal cord cultures during chemical hypoxia. Brain Res. 1999;821:426–432. doi: 10.1016/s0006-8993(99)01086-0. [DOI] [PubMed] [Google Scholar]
- Lotharius J, Falsig J, van Beek J, Payne S, Dringen R, Brundin P, Leist M. Progressive degeneration of human mesencephalic neuron-derived cells triggered by dopamine-dependent oxidative stress is dependent on the mixed-lineage kinase pathway. J Neurosci. 2005;25:6329–42. doi: 10.1523/JNEUROSCI.1746-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz CE, Spitsin S, Zimmerman V, Jacobs D, Udupa JK, Hooper DC, Koprowski H. The treatment of multiple sclerosis with inosine. J Altern Complement Med. 2009;15:619–625. doi: 10.1089/acm.2008.0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyskens FL, Williams HE. Adenosine metabolism in human erythrocytes. Biochim Biophys Acta. 1971;240:170–179. doi: 10.1016/0005-2787(71)90654-x. [DOI] [PubMed] [Google Scholar]
- Michel PP, Marien M, Ruberg M, Colpaert F, Agid Y. Adenosine prevents the death of mesencephalic dopaminergic neurons by a mechanism that involves astrocytes. J Neurochem. 1999;72:2074–2082. doi: 10.1046/j.1471-4159.1999.0722074.x. [DOI] [PubMed] [Google Scholar]
- Módis K, Gero D, Nagy N, Szoleczky P, Tóth ZD, Szabó C. Cytoprotective effects of adenosine and inosine in an in vitro model of acute tubular necrosis. Br J Pharmacol. 2009;158:1565–78. doi: 10.1111/j.1476-5381.2009.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray AW. The biological significance of purine salvage. Annu Rev Biochem. 1971;40:811–826. doi: 10.1146/annurev.bi.40.070171.004115. [DOI] [PubMed] [Google Scholar]
- Petrausch B, Tabibiazar R, Roser T, Jing Y, Goldman D, Stuermer CA, Irwin N, Benowitz LI. A purine-sensitive pathway regulates multiple genes involved in axon regeneration in goldfish retinal ganglion cells. J Neurosci. 2000;20:8031–8041. doi: 10.1523/JNEUROSCI.20-21-08031.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimian R, Fakhfouri G, Daneshmand A, Mohammadi H, Bahremand A, Rasouli MR, Mousavizadeh K, Dehpour AR. Adenosine A2A receptors and uric acid mediate protective effects of inosine against TNBS-induced colitis in rats. Eur J Pharmacol. 2010;649:376–381. doi: 10.1016/j.ejphar.2010.09.044. [DOI] [PubMed] [Google Scholar]
- Ross CA, Smith WW. Gene-environment interactions in Parkinson’s disease. Parkinsonism Relat Disord. 2007;13(Suppl 3):S309–15. doi: 10.1016/S1353-8020(08)70022-1. [DOI] [PubMed] [Google Scholar]
- Schwarzschild MA, Schwid SR, Marek K, Watts A, Lang AE, Oakes D, Shoulson I, Ascherio A, Hyson C, Gorbold E, Rudolph A, Kieburtz K, Fahn S, Gauger L, Goetz C, Seibyl J, Forrest M, Ondrasik J Parkinson Study Group PRECEPT Investigators. Serum urate as a predictor of clinical and radiographic progression in Parkinson disease. Arch Neurol. 2008;65:716–723. doi: 10.1001/archneur.2008.65.6.nct70003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott GS, Spitsin SV, Kean RB, Mikheeva T, Koprowski H, Hooper DC. Therapeutic intervention in experimental allergic encephalomyelitis by administration of uric acid precursors. Proc Natl Acad Sci U S A. 2002;99:16303–16308. doi: 10.1073/pnas.212645999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Chen GJ, Harvey BK, Bickford PC, Wang Y. Inosine reduces ischemic brain injury in rats. Stroke. 2005;36:654–659. doi: 10.1161/01.STR.0000155747.15679.04. [DOI] [PubMed] [Google Scholar]
- Shin CY, Jang ES, Choi JW, Ryu JR, Kim WK, Kim HC, Choi CR, Ko KH. Adenosine and purine nucleosides protect rat primary astrocytes from peroxynitrite-potentiated, glucose deprivation-induced death: preservation of intracellular ATP level. Exp Neurol. 2002;176:175–82. doi: 10.1006/exnr.2002.7913. [DOI] [PubMed] [Google Scholar]
- Spitsin S, Markowitz CE, Zimmerman V, Koprowski H, Hooper DC. Modulation of serum uric acid levels by inosine in patients with multiple sclerosis does not affect blood pressure. J Hum Hypertens. 2010;24:359–362. doi: 10.1038/jhh.2009.83. [DOI] [PubMed] [Google Scholar]
- Szoleczky P, Módis K, Nagy N, Dóri Tóth Z, DeWitt D, Szabó C, Gero D. Identification of agents that reduce renal hypoxia-reoxygenation injury using cell-based screening: purine nucleosides are alternative energy sources in LLC-PK1 cells during hypoxia. Arch Biochem Biophys. 2012;517:53–70. doi: 10.1016/j.abb.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzschild MA, Ascherio A, Beal MF, Cudkowicz ME, Curhan GC, Hare JM, Hooper DC, Kieburtz KD, Macklin EA, Oakes D, Rudolph A, Shoulson I, Tennis MK, Espay AJ, Gartner M, Hung A, Bwala G, Lenehan R, Encarnacion E, Ainslie M, Castillo R, Togasaki D, Barles G, Friedman JH, Niles L, Carter JH, Murray M, Goetz CG, Jaglin J, Ahmed A, Russell DS, Cotto C, Goudreau JL, Russell D, Parashos SA, Ede P, Saint-Hilaire MH, Thomas CA, James R, Stacy MA, Johnson J, Gauger L, Antonelle de Marcaida J, Thurlow S, Isaacson SH, Carvajal L, Rao J, Cook M, Hope-Porche C, McClurg L, Grasso DL, Logan R, Orme C, Ross T, Brocht AF, Constantinescu R, Sharma S, Venuto C, Weber J, Eaton K The Parkinson Study Group SURE-PD Investigators, . Inosine to Increase Serum and Cerebrospinal Fluid Urate in Parkinson Disease: A Randomized Clinical Trial. JAMA Neurol. 2014;71:141–50. doi: 10.1001/jamaneurol.2013.5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toncev G. Therapeutic value of serum uric acid levels increasing in the treatment of multiple sclerosis. Vojnosanit Pregl. 2006;63:879–882. doi: 10.2298/vsp0610879t. [DOI] [PubMed] [Google Scholar]
- Wang LJ, Luo WF, Wang HH, Ni GH, Ye Y, Li D, Liu CF. Protective effects of uric acid on nigrostriatal system injury induced by 6-hydroxydopamine in rats. Zhonghua Yi Xue Za Zhi. 2010;90:1362–1365. [PubMed] [Google Scholar]
- Weisskopf MG, O’Reilly E, Chen H, Schwarzschild MA, Ascherio A. Plasma urate and risk of Parkinson’s disease. Am J Epidemiol. 2007;166:561–567. doi: 10.1093/aje/kwm127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MM, You SW, Hou B, Jiao XY, Li YY, Ju G. Effects of inosine on axonal regeneration of axotomized retinal ganglion cells in adult rats. Neurosci Lett. 2003;341:84–86. doi: 10.1016/s0304-3940(03)00151-4. [DOI] [PubMed] [Google Scholar]
- Young JD, Paterson AR, Henderson JF. Nucleoside transport and metabolism in erythrocytes from the Yucatan miniature pig. Evidence that inosine functions as an in vivo energy substrate. Biochim Biophys Acta. 1985;842:214–24. doi: 10.1016/0304-4165(85)90205-3. [DOI] [PubMed] [Google Scholar]
- Zai L, Ferrari C, Dice C, Subbaiah S, Havton LA, Coppola G, Geschwind D, Irwin N, Huebner E, Strittmatter SM, Benowitz LI. Inosine augments the effects of a Nogo receptor blocker and of environmental enrichment to restore skilled forelimb use after stroke. J Neurosci. 2011;31:5977–5988. doi: 10.1523/JNEUROSCI.4498-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu TG, Wang XX, Luo WF, Zhang QL, Huang TT, Xu XS, Liu CF. Protective effects of urate against 6-OHDA-induced cell injury in PC12 cells through antioxidant action. Neurosci Lett. 2012;506:175–9. doi: 10.1016/j.neulet.2011.10.075. [DOI] [PubMed] [Google Scholar]
- Zurn AD, Do KQ. Purine metabolite inosine is an adrenergic neurotrophic substance for cultured chicken sympathetic neurons. Proc Natl Acad Sci U S A. 1988;85:8301–8305. doi: 10.1073/pnas.85.21.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]