Abstract
Methods for the construction of thiazolo-, thiazino-, and thiazepino-2H-indazoles from o-nitrobenzaldehydes or o-nitrobenzyl bromides and S-trityl-protected 1°-aminothioalkanes are reported. The process consists of formation of the requisite N-(2-nitrobenzyl)(tritylthio)alkylamine, subsequent deprotection of the trityl moiety with TFA, and immediate treatment with aq. KOH in methanol under Davis–Beirut reaction conditions to deliver the target thiazolo-, thiazino-, or thiazepino-2H-indazole in good overall yield. Subsequent S-oxidation gives the corresponding sulfone.
Introduction
2H-Indazoles are of considerable interest due to their medicinal chemistry applications, which include anticancer,1,2 imidazoline I2 receptor,3 and antiangiogenic4 activities (Figure 1a). Addressing this need, we recently developed the Davis–Beirut reaction,5−16 which is characterized by a noteworthy N,N-bond forming heterocyclization step, for the construction of a wide variety of 2H-indazoles and indazolones (Figure 1b).
Figure 1.
(a) Bioactive 2H-indazoles. (b) A typical Davis–Beirut reaction proceeding through the proposed nitrosoimine intermediate.6
In connection with ongoing research activities addressing cystic fibrosis (CF),17,18 our interest in the development of myeloperoxidase inhibitors19,20 led us to explore introduction of a thioether moiety at C3 of the 2H-indazole scaffold. While attempts to employ RSH in the base-mediated Davis–Beirut reaction failed, we have found that a thiol tethered to the 1°-aminoalkane7,21 used in the reductive amination step to construct the o-nitrobenzylamine intermediate (in turn, prepared from the appropriate o-nitrobenzaldehyde or o-nitrobenzyl bromide precursor) can deliver C3 S-substituted 2H-indazoles. We report here the details of this investigation.
Results and Discussion
Sulfur-containing molecules are quite relevant in medicine and in human biology.22−25 Thioethers also offer an intriguing point of diversification through easy access to higher oxidation states at sulfur. The potential of sulfur-containing 2H-indazoles piqued the interest of Corsi and Palazzo, leading them to develop a limited and somewhat circuitous route to thiazoloindazole 1a that proceeds by pyrolysis of 1-benzyl-3-((2-chloroethyl)thio)-1H-indazole (Scheme 1; 40% yield).26 Attempts to employ external thiols under a variety of Davis–Beirut reaction conditions (generically iPrOH + EtSH + aq. KOH) failed to deliver the thioether-containing 2H-indazole (e.g., the 2H-indazole in Figure 1b where the −OEt moiety would be replaced with the sought after −SEt). These reaction attempts resulted primarily in nitroaryl reduction27 to the corresponding aniline with only trace formation of the thia-containing 2H-indazole.
Scheme 1. Thiazolo-2H-indazole 1a by Pyrolysis of 1-Benzyl-3-((2-chloroethyl)thio)-1H-indazole.
With this setback as a backdrop, we decided to explore the formation of 2,3-dihydrothiazolo[3,2-b]indazole (1a; Scheme 2) from 2-nitrobenzaldehyde and cysteamine before abandoning the thia-based Davis–Beirut reaction. Condensation of these two commercially available reagents in methanol provided the requisite imine 2, but LCMS analysis of the reaction mixture indicated the formation of a second product that was determined to be thioaminal 3. Varied reaction times, reaction conditions, and solvents (MeOH and iPrOH) all resulted in 3 being the major product. Also, attempts at reduction of this crude mixture of 2 and 3 (NaCNBH3 in MeOH), followed immediately by the Davis–Beirut reaction, resulted in a complex mixture of products (TLC).
Scheme 2. Unwanted Aminal Formation using 2-Aminoethanethiol.
These difficulties led us to pursue an alternate route wherein the sulfur moiety was initially protected. While there are a number of effective thiol-protecting groups, trityl protection was selected for all of the aminothiols employed in this study for three reasons (i) the S-trityl group is easy to deprotect; (ii) the S-trityl group is stable to reductive amination conditions; and (iii) the UV activity of the S-trityl protected aminothiols, as well as their markedly different solubility characteristics, makes them easier to isolate and manipulate than their unprotected analogues. In the event (Scheme 3), 2-nitrobenzaldehyde in methanol reacted quickly with 2-(tritylthio)ethanamine (synthesized from cysteamine·HCl and trityl chloride in DMF)28 to give an imine intermediate (not isolated) and subsequent addition of sodium cyanoborohydride delivered 4. This crude o-nitrobenzylamine was treated with TFA and triethylsilane29 in methylene chloride to cleave the trityl protecting group (→ 5), and then the resulting crude thiol was immediately (to minimize disulfide formation) subjected to the Davis–Beirut reaction—treatment with KOH in methanol plus 10% water at 90 °C—to deliver 1a in 77% overall yield from 2-nitrobenzaldehyde.
Scheme 3. Davis–Beirut Route to Thiazolo-2H-indazole 1a.
It is interesting to note that Davis–Beirut reaction on the alcohol analogue of 5 (−SH replaced with −OH, 5a) leads transiently to 2,3-dihydrooxazolo[3,2-b]indazole (6), but base-mediated opening of the dihydrooxazolo ring under the reaction conditions produces 1H-indazol-3(2H)-one 7.12 While this ring-opening of 6 can be avoided by increasing steric hindrance at the electrophilic −CH2– through placement of alkyl substituents at the C2 and/or C3 positions of 6 and by conducting the heterocyclization at 60 °C,12 these modifications are not required in the formation of thiazoloindazole 1a.
With the gratifying result of 4 leading to stable 1a in hand, we next set out to prepare homologues (Scheme 4a) of 1a with six- (thiazino; 1b) and seven-membered (thiazepino; 1c) sulfur-containing heterocycles. Applying Scheme 3 sequence of reactions to 3-(tritylthio)propan-1-amine delivered thiazinoindazole 1b in 82% overall yield, whereas 4-(tritylthio)butan-1-amine delivered 1c in 71% overall yield. This last result led us to prepare the unknown oxygen analogue of 1c for comparison (e.g., 8; Scheme 4b), which we obtained from 4-aminobutan-1-ol (→ 8) in 44% overall yield. Indeed, we observe that the O-analogues of 1a, 1b, and 1c are obtained in lower yield than the corresponding S-analogues: O-analogues of 1a–c in 24% (see 6 in Scheme 3), 84%, and 44% yields (respectively) vs S-analogues 1a–c in 77%, 82%, and 71% yields (respectively).12
Scheme 4. (a) Thiazino and Thiazepino Analogues of 1a. (b) Oxazepino Analogue of 1c.
Next, the scope of the sulfur-based Davis–Beirut reaction was expanded to include substituents on the carbocyclic ring of these 2H-indazoles. These thia-based Davis–Beirut reactions proceed well with both electron-withdrawing (−COOH → 9, −Br → 10, −Cl → 11, and pyridyl → 14a,b; Scheme 5a) and electron-donating (−OCH2O– → 15a–c and 8,9 di-OMe → 16; Scheme 5b) substituents as well as with both o-nitrobenzaldehyde and o-nitrobenzyl bromide precursors (Scheme 5a–c). Also, substitution ortho to the aldehyde does not dramatically affect the reaction (ortho-Cl → 11). o-Nitrobenzaldehydes can also be replaced with heteroaryl nitroaldehydes (e.g., 12 and 13) to give interesting sulfur-containing heteroaryl analogues (14a,b and 15a–c) in good yields. Benzo-fused analogue 17 was synthesized via trityl-protected 2-aminothiophenol (Scheme 5c).30 In earlier unpublished work, we found that the oxygen analogue of 17 (e.g., where “S” is replaced with “O”) was not similarly accessible with 2-aminophenol; 2-(3-methoxy-2H-indazol-2-yl)phenol was formed instead.
Scheme 5. Versatility of the Thia-Based Davis–Beirut Reaction.
Diverse aminothiols are not readily available, and the literature provides few examples of diverse S-trityl aminoalkanes. Therefore, the method devised to prepare the S-trityl aminoalkanes employed in this study is presented in Scheme 6a (exemplified with the preparation of 22; note that 2-(tritylthio)ethanamine,28 3-(tritylthio)propan-1-amine,31 and 4-(tritylthio)butan-1-amine31 were prepared as reported in the literature). Since the protecting group used for the amine must be orthogonally removed vis-à-vis the S-trityl protecting group, phthalimide protection was selected as both its introduction [amine + phthalic anhydride (PAN) in refluxing xylenes] and its deprotection (phthalimide + hydrazine monohydrate) are well established S-trityl-compatible protocols. From the requisite amino alcohol, amine protection (18 → 19; 80%), alcohol-to-iodide conversion (19 → 20; 76%),32S-alkylation with triphenylmethanethiol (20 → 21; 55%), and hydrazinolysis (21 → 22; 44%) delivers the targeted S-trityl aminoalkane. DL-1-(Tritylthio)propan-2-amine (22) was subsequently employed in the Davis–Beirut sequence with o-nitrobenzaldehye (imine formation and reduction, trityl cleavage, and then aq. KOH in MeOH) to deliver 23—the C3 methyl analogue of 1a—in 66% yield (Scheme 6b).
Scheme 6. (a) Route to S-Trityl Aminoalkanes (Exemplified with 22). (b) Synthesis of an Alkyl-Substituted Thiazoloindazole 23.
Having successfully introduced a thia moiety into the 2H-indazole scaffold, a contrast between these thia- (cf., 1a) and oxo-bridged (cf., 6) systems in terms of Davis–Beirut reaction yields is informative. In fact, whereas some 2,3-dihydrooxazolo[3,2-b]indazoles (cf., 6) and 3,4-dihydro-2H-[1,3]oxazino[3,2-b]indazoles (e.g., oxa analogues of 1b) exhibit base and light sensitivity, the thia-2H-indazoles reported here are quite stable. Also, while the oxo analogues can generally undergo further reaction with the alcohol solvent to form indazolones (cf., 6 → 7 in Scheme 3), the sulfur analogues reported here do not—even at higher reaction temperatures and extended reaction times.
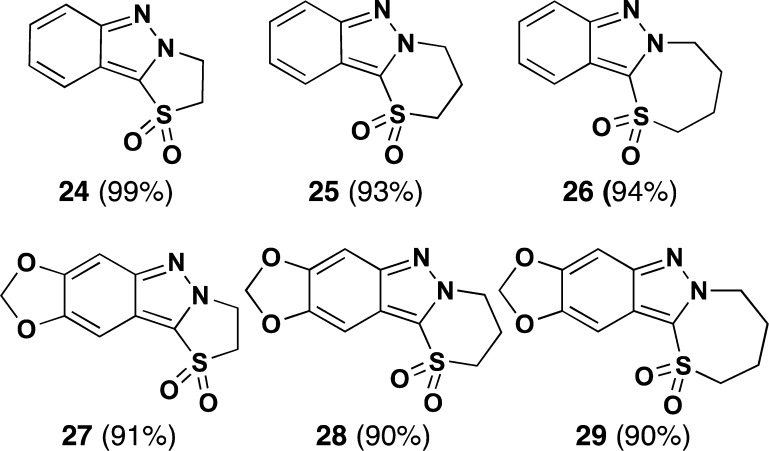
It is also noteworthy that oxo-2H-indazole functionalization is generally limited to conversion to the corresponding indazolone, whereas thia-2H-indazoles present new diversification pathways via, for example, S-oxidation to the corresponding sulfone. Indeed, as summarized in Figure 2, oxidation33 of the thioether moiety in these thiazolo-, thiazino-, and thiazepino-2H-indazoles with Na2WO4·2H2O/30% aq. H2O2/EtOAc leads cleanly and in high yield to the corresponding sulfones (24–29).
Figure 2.
Sulfones obtained by oxidation of the corresponding thia-2H-indazoles.
Conclusion
In summary, we have shown that the Davis–Beirut reaction affords thiazolo-, thiazino-, and thiazepino-2H-indazoles from o-nitrobenzaldehyde and S-trityl-protected 1°-aminoalkanes in good yields. The process consists of reductive amination of the o-nitrobenzaldehyde-derived imine, S-trityl deprotection, and immediate refluxing with aqueous base in methanol. The resulting thia-2H-indazoles are generally more stable than the corresponding oxo-2H-indazoles, and they can also be oxidized to the corresponding sulfones.
Experimental Section
General Experimental
All solvents and reagents were purchased from commercial suppliers and used without further purification. Analytical thin-layer chromatography was carried out on precoated plates (Silica gel 60 F254, 0.50 mm thickness) and visualized with UV light. Flash chromatography was performed with 60 Å, 35–70 mm silica gel. Concentration refers to rotary evaporation under reduced pressure.
1H NMR spectra were recorded at 600 MHz at ambient temperature with DMSO-d6, MeOD-d4, or CDCl3 as solvents. 13C NMR spectra were recorded at 150 MHz at ambient temperature with DMSO-d6, MeOD-d4 or CDCl3 as solvents. Data for 1H NMR are recorded as follows: chemical shift (δ, ppm), multiplicity (s, singlet; d, doublet; t, triplet; q, quartet; quint, quintet; m, multiplet), coupling constant (Hz), integration. Chemical shifts are reported in parts per million relative to DMSO-d6 (1H, δ 2.50; 13C, δ 39.52), MeOD-d4 (1H, δ 3.31; 13C, δ 49.99), or CDCl3 (1H, δ 7.26; 13C, δ 77.16). Infrared spectra were recorded on an ATI-FTIR spectrometer. High-resolution mass spectra were acquired on an LTQ Orbitrap XL mass spectrometer equipped with an electrospray ionization source operating in the positive ion mode. Samples were introduced into the source via loop injection at a flow rate of 200 ul/min, in a solvent system of 1:1 acetonitrile:water with 0.1% formic acid.
General Procedure A: Preparation of Thiazolo-, Thiazino-, and Thiazepino-2H-indazoles
2-Nitrobenzaldehyde (1.1 equiv) was dissolved in MeOH (0.4 M). The requisite S-trityl-protected aminothioalkane (1.0 equiv) was added, and the solution was stirred until TLC showed consumption of the starting amine. NaCNBH3 (2.0 equiv) was added portionwise, and the solution was stirred until consumption of the imine was observed. The solution was evaporated, and the resulting residue was redissolved in methylene chloride. The organic layer was washed twice with 1 M HCl, dried over Na2SO4, filtered, and concentrated to ∼10 mL. An equal volume of TFA was added, followed by addition of triethylsilane (3.0 equiv). This solution was left to stir for 30 min and then concentrated (care was taken to vent the rotary evaporator to nitrogen to prevent disulfide formation). To the remaining residue was added MeOH (0.1 M), KOH (15 equiv), and 10% v/v H2O. The solution was heated at reflux in a 90 °C oil bath for 3–6 h. Upon reaction completion, the MeOH was evaporated and the remaining aqueous mixture was partitioned with EtOAc. The aqueous layer was extracted with EtOAc twice, and the resulting organic extracts were combined, washed with brine, dried over Na2SO4, filtered, and concentrated. Purification with silica gel chromatography provided the desired indazole.
2,3-Dihydrothiazolo[3,2-b]indazole (1a)
Prepared from 216 mg (0.68 mmol) of 2-(tritylthio)ethan-1-amine using General Procedure A. Purification by silica gel chromatography (20 → 50% EtOAc in hexanes) provided 1a as a white amorphous solid in 77% yield (92 mg); 1H NMR (600 MHz, CDCl3) δ 7.60 (dt, J = 8.9, 1.0 Hz, 1H), 7.47 (dt, J = 8.4, 0.9 Hz, 1H), 7.25 (ddd, J = 8.4, 6.6, 1.0 Hz, 1H), 7.00 (ddd, J = 8.4, 6.6, 0.9 Hz, 1H), 4.67 (t, J = 7.6 Hz, 2H), 3.97 (t, J = 7.6 Hz, 2H); 13C NMR (150 MHz, CDCl3) δ 154.0, 133.1, 126.6, 120.8, 120.0, 118.0, 116.1, 50.4, 34.4. HRMS (ESI) m/z: [M + H]+ calcd for C9H8N2SH 177.0486; Found 177.0482.
3,4-Dihydro-2H-[1,3]thiazino[3,2-b]indazole (1b)
Prepared from 200 mg (0.60 mmol) of 3-(tritylthio)propan-1-amine using General Procedure A. Purification by silica gel chromatography (20 → 50% EtOAc in hexanes) provided 1b as an amorphous tan solid in 82% yield (94 mg). 1H NMR (600 MHz, CDCl3) δ 7.58 (m, 1H), 7.49 (m, 1H), 7.27 (m, 1H), 6.99 (m, 1H), 4.55 (app. t, J = 6.4 Hz, 2H), 3.23 (app. t, J = 6.4 Hz, 2H), 2.54 (app p, J = 6.4 Hz, 2H); 13C NMR (150 MHz, CDCl3) δ 147.8, 127.0, 120.2, 119.8, 119.2, 117.0, 105.2, 48.8, 25.2, 24.8. HRMS (ESI) m/z: [M + H]+ calcd for C10H10N2SH 191.0643; Found 191.0648.
2,3,4,5-Tetrahydro-[1,3]thiazepino[3,2-b]indazole (1c)
Prepared from 199 mg (0.57 mmol) of 4-(tritylthio)butan-1-amine using General Procedure A. Purification by silica gel chromatography (20 → 50% EtOAc in hexanes) provided 1c as an amorphous solid in 71% yield (83 mg). 1H NMR (600 MHz, CDCl3) δ 7.68 (dt, J = 8.8, 1.0 Hz, 1H), 7.65 (dt, J = 8.5, 1.1 Hz, 1H) 7.33 (ddt, J = 8.8, 6.8, 1.1 Hz, 1H), 7.14 (ddt, J = 8.5, 6.8, 1.0 Hz, 1H), 4.84 (m, 2H), 2.78 (m, 2H), 2.25 (m, 2H), 1.90 (m, 2H); 13C NMR (150 MHz, CDCl3) δ 144.6, 128.5, 126.5, 124.0, 123.1, 120.4, 116.2, 53.7, 34.1, 32.2, 26.7. HRMS (ESI) m/z: [M + H]+ calcd for C11H12N2SH 205.0778; Found 205.0772.
2,3,4,5-Tetrahydro-[1,3]oxazepino[3,2-b]indazole (8)
4-Amino-1-butanol (160 mg, 1.8 mmol) and 2-nitrobenzaldehyde (250 mg, 1.65 mmol) were dissolved in iPrOH (5 mL) and stirred until TLC showed consumption of the starting material. NaCNBH3 (310 mg, 4.94 mmol) was added portionwise, and the mixture was stirred for 20 min, at which time TLC showed consumption of the imine. Isopropanol (10 mL) was added to the solution. KOH (1.40 g, 25 mmol) was dissolved in a portion of water (2 mL) and added to the iPrOH solution, which changed to a dark orange color. This solution was heated at 60 °C for 8 h. The organic layer was washed twice with water and once with brine, dried over Na2SO4, filtered, and concentrated. Purification by silica gel chromatography (50% EtOAc in hexanes) provided 8 as a tan amorphous solid in 44% yield (142 mg). 1H NMR (600 MHz, CDCl3) δ 7.54 (dt, J = 8.5, 1.2 Hz, 1H), 7.49 (dt, J = 8.8, 1.0 Hz, 1H), 7.22 (ddd, J = 8.8, 6.6, 1.2 Hz, 1H), 6.97 (ddd, J = 8.5, 6.6, 1.0 Hz, 1H), 4.51 (m, 2H), 4.20 (m, 2H), 2.16 (m, 2H), 1.96 (m, 2H); 13C NMR (150 MHz, CDCl3) δ 148.4, 146.8, 126.5, 120.3, 118.9, 117.1, 110.0, 74.6, 52.8, 31.7, 26.1; HRMS (ESI) m/z: [M + H]+ calcd for C11H12N2OH 189.1028; Found 189.1027.
3,4-Dihydro-2H-[1,3]thiazino[3,2-b]indazole-8-carboxylic acid (9)
4-(Bromomethyl)-3-nitrobenzoic acid (150 mg, 0.54 mmol 1.0 equiv) and DIPEA (0.250 mL, 1.40 mmol, 2.5 equiv) were dissolved in MeOH. 3-(Tritylthio)propan-1-amine (480 mg, 1.40 mmol, 2.5 equiv) was dissolved in MeOH and added dropwise over 6 h to the stirring benzyl bromide solution. After addition was complete, the solution was left to stir overnight. The solvent was evaporated, and the remaining residue was redissolved in 10 mL of methylene chloride. To this solution was added 10 mL of TFA, followed quickly by addition of triethylsilane (0.225 mL, 1.40 mmol, 2.5 equiv). The solution was left to stir for 30 min. The solvent was evaporated, and the residue was redissolved in MeOH. KOH (484 mg, 8.63 mmol, 15 equiv) was dissolved in 10% v/v water and added to the MeOH solution. This mixture was heated at reflux in a 90 °C bath for 4 h. After the solution cooled, the MeOH was evaporated and EtOAc was added to the remaining aqueous residue. The aqueous layer was extracted once with EtOAc and then acidified. The aqueous layer was then extracted twice more with EtOAc (these extracts were not combined with the first). The organic layer was then washed with brine, dried over Na2SO4, filtered, and concentrated. Purification by silica gel chromatography (AcOH in 99% EtOAc → 1% AcOH, 10% EtOH in 89% EtOAc) provided 9 as an amorphous pale yellow solid in 73% yield (98 mg). IR (neat) νmax 3250, 1740 cm–1; 1H NMR (600 MHz, DMSO) δ 12.04 (s, 1H), 8.17 (s, 1H), 7.55 (d, J = 8.7 Hz, 1H), 7.47 (d, J = 8.7 Hz, 1H), 4.53 (app. t, J = 5.9 Hz, 2H), 3.33 (app. t, J = 5.5, 5.5 Hz, 2H), 2.57 (app. p, J = 6.3 Hz, 2H); 13C NMR (150 MHz, DMSO) δ 167.7, 146.0, 128.9, 124.6, 120.8, 119.7, 119.2, 48.8, 24.3, 23.9; HRMS (ESI) m/z: [M + H]+ calcd for C11H10N2O2SH 235.0541; Found 235.0540.
9-Bromo-3,4-dihydro-2H-[1,3]thiazino[3,2-b]indazole (10)
Prepared from 132 mg (0.40 mmol) of 3-(tritylthio)propan-1-amine using General Procedure A. Purification by silica gel chromatography (50% EtOAc in hexanes) provided 10 as a yellow amorphous solid in 66% yield (70 mg). 1H NMR (600 MHz, CDCl3) δ 7.79 (s, 1H), 7.55 (d, J = 9.3 Hz, 1H), 7.32 (d, J = 9.3 Hz, 1H), 4.44 (dd, J = 8.3, 7.1 Hz, 2H), 3.66 (app. t, J = 6.2 Hz, 2H), 2.10 (app. p, J = 7.1 Hz, 2H); 13C NMR (150 MHz, CDCl3) δ 147.1, 129.5, 122.9, 122.3, 122.2, 119.0, 115.1, 62.0, 29.4, 27.1; HRMS (ESI) m/z: [M + H]+ calcd for C10H9BrN2SH 268.9748; Found 268.9739.
10-Chloro-3,4-dihydro-2H-[1,3]thiazino[3,2-b]indazole (11)
Prepared from 200 mg (0.60 mmol) of 3-(tritylthio)propan-1-amine using General Procedure A. Purification by silica gel chromatography (50% EtOAc in hexanes) provided 11 as a tan amorphous solid in 60% yield (81 mg). 1H NMR (600 MHz, CDCl3) δ 7.44 (d, J = 8.7 Hz, 1H), 7.12 (dd, J = 8.7, 7.2 Hz, 1H), 6.90 (d, J = 7.2 Hz, 1H), 4.51 (app. t, J = 6.1 Hz, 2H), 3.18 (m, 2H), 2.49 (m, 2H); 13C NMR (150 MHz, CDCl3) δ 148.7, 127.1, 126.0, 125.7, 120.0, 118.5, 115.6, 49.2, 25.2, 24.0; HRMS (ESI) m/z: [M + H]+ calcd for C10H9ClN2SH 225.0253; Found 225.0253.
2,3-Dihydrothiazolo[3′,2′:1,5]pyrazolo[4,3-b]pyridine (14a)
Prepared from 198 mg (0.62 mmol) of 2-(tritylthio)ethan-1-amine using General Procedure A. Purification by silica gel chromatography (50 → 70% EtOAc in hexanes) provided 14a as an orange amorphous solid in 80% yield (88 mg). 1H NMR (600 MHz, CDCl3) δ 8.47 (dt, J = 4.1, 1.2 Hz, 1H), 7.95 (dt, J = 8.8, 1.2 Hz, 1H), 7.19 (ddd, J = 8.8, 4.1, 1.2 Hz, 1H), 4.72 (app. t, J = 7.7 Hz, 2H), 4.00 (app. t, J = 7.7 Hz, 2H); 13C NMR (150 MHz, CDCl3) δ 146.8, 134.6, 133.2, 126.4, 121.8, 105.2, 51.1, 34.4; HRMS (ESI) m/z: [M + H]+ calcd for C8H7N3SH 178.0439; Found 178.0444.
3,4-Dihydro-2H-pyrido[3′,2′:3,4]pyrazolo[5,1-b][1,3]thiazine (14b)
Prepared from 200 mg (0.60 mmol) of 3-(tritylthio)propan-1-amine using General Procedure A. Purification by silica gel chromatography (50 → 70% EtOAc in hexanes) provided 14b as an amorphous yellow solid in 76% yield (87 mg). 1H NMR (600 MHz, CDCl3) δ 8.45 (dt, J = 4.1, 0.5 Hz, 1H), 7.92 (dt, J = 8.8, 0.5 Hz, 1H), 7.19 (ddd, J = 8.8, 4.1, 0.5 Hz, 1H), 4.56 (app. t, J = 4.9 Hz, 2H), 3.27 (m, 2H), 2.57 (m, 2H); 13C NMR (150 MHz, CDCl3) δ 146.1, 140.9, 136.5, 126.5, 125.1, 122.2, 49.3, 24.8, 24.5; HRMS (ESI) m/z: [M + H]+ calcd for C9H9N3SH 192.0595; Found 192.0605.
7,8-Dihydro-[1,3]dioxolo[4,5-f]thiazolo[3,2-b]indazole (15a)
Prepared from 211 mg (0.66 mmol) of 2-(tritylthio)ethan-1-amine using General Procedure A. Purification by silica gel chromatography (20 → 50% EtOAc in hexanes) provided 15a as a tan solid in 84% yield (122 mg). mp decomposed at 250 °C; 1H NMR (600 MHz, CDCl3) δ 6.87 (s, 1H), 6.66 (s, 1H), 5.93 (s, 2H), 4.55 (app. t, J = 7.6 Hz, 2H), 3.88 (app. t, J = 7.6 Hz, 2H); 13C NMR (150 MHz, CDCl3) δ 151.3, 149.4, 144.8, 132.1, 111.1, 101.0, 95.0, 94.8, 50.2, 34.5 HRMS (ESI) m/z: [M + H]+ calcd for C10H8N2O2SH 221.0384; Found 221.0384.
8,9-Dihydro-7H-[1,3]dioxolo[4,5-f][1,3]thiazino[3,2-b]indazole (15b)
Prepared from 201 mg (0.60 mmol) of 3-(tritylthio)propan-1-amine using General Procedure A. Purification by silica gel chromatography (20 → 50% EtOAc in hexanes) provided 15b as a tan amorphous solid in 80% yield (113 mg). 1H NMR (600 MHz, CDCl3) δ 7.03 (s, 1H), 6.77 (s, 1H), 6.06 (s, 2H), 4.75 (app. t, J = 5.9 Hz, 2H) 3.27 (m, 2H), 2.55 (m, 2H); 13C NMR (150 MHz, CDCl3) δ 154.0, 146.7, 137.7, 133.6, 114.0, 102.6, 95.4, 91, 47.6, 24.6, 22.9; HRMS (ESI) m/z: [M + H]+ calcd for C11H10N2O2SH 235.0541; Found 235.0523.
7,8,9,10-Tetrahydro-[1,3]dioxolo[4,5-f][1,3]thiazepino[3,2-b]indazole (15c)
Prepared from 180 mg (0.52 mmol) using General Procedure A. Purification by silica gel chromatography (20 → 50% EtOAc in hexanes) provided 15c as an amorphous solid in 73% yield (94 mg). 1H NMR (600 MHz, CDCl3) δ 6.91 (s, 1H), 6.82 (s, 1H), 5.93 (s, 2H), 4.64, (m, 2H), 2.69 (m, 2H), 2.18 (m, 2H), 1.82 (m, 2H); 13C NMR (150 MHz, CDCl3) δ 149.1, 145.7, 144.5, 126.7, 120.3, 101.0, 95.3, 94.2, 53.6, 34.4, 32.5, 27.4; HRMS (ESI) m/z: [M + H]+ calcd for C12H12N2O2SH 249.0697; Found 249.0698.
8,9-Dimethoxy-3,4-dihydro-2H-[1,3]thiazino[3,2-b]indazole (16)
1-(Bromomethyl)-4,5-dimethoxy-2-nitrobenzene (276 mg, 1 mmol, 1.0 equiv) and DIPEA (0.521 mL, 3 mmol, 3.0 equiv) were dissolved in MeOH. 3-(Tritylthio)propan-1-amine (666 mg, 2 mmol, 2 equiv) was dissolved in MeOH and added dropwise over 6 h to the stirring benzyl bromide solution. After addition was complete, the solution was left to stir overnight. The solvent was evaporated, and the remaining residue was redissolved in 10 mL of methylene chloride. To this solution was added 10 mL of TFA, followed quickly by addition of triethylsilane (0.470 mL, 3 mmol, 3.0 equiv). The solution was left to stir for 30 min. The solvent was evaporated, and the residue was redissolved in MeOH. KOH (841 mg, 15 mmol 15 equiv) was dissolved in 10% v/v water and added to this MeOH solution. The mixture was heated at reflux in a 90 °C bath for 4 h. After the solution cooled, MeOH was evaporated and EtOAc was added to the remaining aqueous residue. The aqueous layer was extracted once with EtOAc and then acidified. The aqueous layer was then extracted twice more with EtOAc (these extracts were not combined with the first). The organic layer was then washed with brine, dried over Na2SO4, filtered, and concentrated. Purification by silica gel chromatography (10% EtOH in EtOAc) provided 16 as an amorphous yellow solid in 75% yield (188 mg). 1H NMR (600 MHz, MeOD) δ 6.84 (s, 1H), 6.73 (s, 1H), 4.40 (app. t, J = 5.9 Hz, 2H), 3.88 (s, 3H), 3.84 (s, 3H), 3.25 (dd, J = 8.0, 3.2 Hz, 2H), 2.49 (m, 2H); 13C NMR (150 MHz, MeOD) δ 153.6, 148.1, 145.0, 125.6, 119.3, 117.3, 114.7, 98.1, 96.0, 56.6, 56.4, 25.8; HRMS (ESI) m/z: [M + H]+ calcd for C12H14N2O2SH 251.0854; Found 251.0848.
Benzo[4,5]thiazolo[3,2-b]indazole (17)
Prepared from 178 mg (0.49 mmol) of 2-(tritylthio)aniline using General Procedure A. Purification by silica gel chromatography (50% EtOAc in hexanes) provided 17 as an amorphous solid in 65% yield (71 mg). 1H NMR (600 MHz, CDCl3) δ 8.35 (d, J = 8.2 Hz, 1H), 7,87 (m, 2H), 7.81 (d, J = 8.4 Hz, 1H), 7.63 (t, J = 7.2 Hz, 1H), 7.51 (t, J = 7.3 Hz, 1H), 7.46 (t, J = 7.6 Hz, 1H), 7.17 (t, J = 8.3 Hz, 1H) 13C NMR (150 MHz, CDCl3) δ 162.4, 153.6, 149.0, 135.9, 132.4, 131.8, 131.0, 128.0, 126.6, 125.9, 124.5, 123.9, 121.6. HRMS (ESI) m/z: [M + H]+ calcd for C13H8N2SH 225.0486; Found 225.0482.
2-(1-Hydroxypropan-2-yl)isoindoline-1,3-dione (19)
dl-alaninol (1.00 g, 13.30 mmol, 1 equiv) and phthalic anhydride (2.37 g, 16.00 mmol, 1.2 equiv) were dissolved in xylenes and heated at 140 °C for 3 h. The solution was concentrated, and the resulting solid was purified by silica gel chromatography (20% EtOAc in hexanes) to give the desired phthalimido alcohol. Isolated in 80% yield (2.18 g). Spectral data were in accordance with literature values.34
2-(1-Iodopropan-2-yl)isoindoline-1,3-dione (20)
Triphenylphosphine (2.55g, 9.70 mmol, 2 equiv) and imidazole (67 mg, 0.99 mmol, 0.2 equiv) were dissolved in dry methylene chloride. Iodine (2.50g, 9.84 mmol, 2 equiv) was added, and the solution was left to stir for 10 min. 19 (1.00 g, 4.87 mmol, 1 equiv) was dissolved in dry methylene chloride and added dropwise over 20 min. After addition, the solution was left to stir overnight. The solution was concentrated and then redissolved in EtOAc. The organic layer was washed with saturated sodium thiosulfate and brine, dried over Na2SO4, filtered, and concentrated. Purification by column chromatography (5% EtOAc in hexanes) provided the desired product. Isolated in 76% yield (1.16g). Spectral data were in accordance with literature values.35
2-(1-(Tritylthio)propan-2-yl)isoindoline-1,3-dione (21)
Trityl mercaptan (965 mg, 3.50 mmol, 1.1 equiv) was dissolved in dry DMF and cooled to 0 °C. Sodium hydride (60% dispersion in mineral oil, 152 mg, 3.80 mmol, 1.2 equiv) was added, and the solution was stirred for 20 min. 20 (1.00 g, 3.17 mmol, 1 equiv) was dissolved in dry DMF and added dropwise to the stirring solution over 10 min. After addition, the reaction was allowed to warm to room temperature and tracked by TLC to completion. The solution was partitioned between water and EtOAc. The organic layer was washed with water, dried over Na2SO4, filtered, and concentrated. Purification by silica gel chromatography (30% EtOAc in hexanes) provided 21 as a white amorphous solid in 55% yield (809 mg). IR (neat) νmax 1711, 1600 cm–1; 1H NMR (600 MHz, CDCl3) δ 7.79 (m, 2H), 7.69 (m, 2H), 7.38–7.20 (m, 15H), 4.08 (m, 1H), 3.12 (dd, J = 12.2, 9.8 Hz, 1H), 2.51 (dd, J = 12.2, 9.8 Hz, 1H), 1.30 (d, J = 6.9, 3H); 13C NMR (150 MHz, CDCl3) δ 168.1, 144.7, 134.0, 132.0, 129.7, 128.0, 126.8, 123.3, 67.1, 46.9, 35.4, 18.8. HRMS (ESI) m/z: [M + H]+ calcd for C30H25NO2SH 464.1684; Found 464.1680.
3-Methyl-1-(tritylthio)butan-2-amine (22)
Isoindolinedione 21 (500 mg, 1.07 mmol, 1.0 equiv) was suspended in a 4:1 solution of EtOH:BuOH. To this solution was added hydrazine monohydrate (6.0 equiv). The mixture was heated at reflux overnight. Upon cooling, a white precipitate formed. The precipitate was filtered off, and the filtrate was concentrated. The resulting residue was redissolved in chloroform, and the solution was left to stir for 1 h. The resulting precipitate was filtered off, and the filtrate was concentrated. Purification of the residue by silica gel chromatography (10% EtOAc in methylene chloride →10% MeOH in methylene chloride) provided 22 as a white crystalline solid in 44% yield (160 mg). mp 151–153 °C; IR (neat) νmax 3379 cm–1; 1H NMR (600 MHz, CDCl3) δ 7.32–7.28 (m, 15H), 2.60 (m, 1H), 2.21 (m, 1H), 2.08 (m, 1H), 0.89 (d, J = 6.4 Hz, 3H); 13C NMR (150 MHz, CDCl3) δ 144.9, 129.5, 128.5, 127.2, 66.4, 48.6, 46.6, 22.0. HRMS (ESI) m/z: [M-CPh3+H]+ calcd for C3H9NSH 92.0534; Found 92.0524.
3-Methyl-2,3-dihydrothiazolo[3,2-b]indazole (23)
Prepared from 160 mg (0.47 mmol) of 22 using General Procedure A. Purification by silica gel chromatography (20 → 50% EtOAc in hexanes) provided 23 as an amorphous yellow solid in 66% yield (60 mg). 1H NMR (600 MHz, CDCl3) δ 7.46 (d, J = 9.0 Hz, 2H), 7.20 (m, 1H), 6.89 (m, 1H), 5.58 (m, 1H), 4.66 (ddd, J = 9.5, 8.3 1H), 4.17 (dd, J = 9.5, 8.0, 1H), 1.71 (d, J = 6.4 Hz, 3H); 13C NMR (150 MHz, CDCl3) δ 153.7, 151.4, 126.8, 119.4, 119.0, 117.6, 101, 85.4, 52.7, 20.9. HRMS (ESI) m/z: [M + H]+ calcd for C10H10N2SH 191.0643; Found 191.0644.
General Procedure B: Preparation of Sulfones
The indazole (1.0 equiv) was dissolved in EtOAc to 0.8 M. Na2WO4·2H2O (0.1 equiv) was dissolved in a small amount of water and added to the EtOAc solution. A solution of 30% hydrogen peroxide (3.0 equiv) was added dropwise. After addition, the mixture was stirred until starting material was consumed (tracked by LCMS and TLC). The solution was cooled to 0 °C, and saturated sodium bisulfite (2 mL) was added dropwise. The solution was extracted with EtOAc, and then the organic layer was dried over Na2SO4, filtered, and concentrated.
2,3-Dihydrothiazolo[3,2-b]indazole 1,1-Dioxide (24)
Prepared from 1a (50 mg, 0.28 mmol) by General Procedure B. Note that the sulfone precipitated out of solution and was analyzed without further purification. Sulfone 24 was isolated as a white amorphous solid in 99% yield (58 mg). IR (neat) νmax 1305, 1124 cm–1; 1H NMR (600 MHz, CDCl3) δ 7.83 (dd, J = 8.5, 0.9 Hz, 1H), 7.78 (d, J = 8.8 Hz, 1H), 7.83, 7.43 (ddd, J = 8.8, 6.8, 1.0 Hz, 1H) 7.33 (ddd, J = 8.5, 6.8, 0.8 Hz, 1H), 5.01 (dd, J = 7.3, 6.1 Hz, 2H), 4.15 (dd, J = 7.3, 6.1, 2H); 13C NMR (150 MHz, CDCl3) δ 153.2, 131.1, 127.8, 125.5, 118.7, 118.6, 115.6, 55.3, 46.3; HRMS (ESI) m/z: [M + H]+ calcd for C9H8N2O2SH 209.0384; Found 209.0385.
3,4-Dihydro-2H-[1,3]thiazino[3,2-b]indazole 1,1-Dioxide (25)
Prepared from 1b (50 mg, 0.26 mmol) by General Procedure B. Sulfone 25 was isolated as an off-white crystalline solid in 93% yield (53 mg). mp decomposed at 250 °C; IR (neat) νmax 1342 cm–1; 1H NMR (600 MHz, CDCl3) δ 7.93 (dd, J = 8.5, 0.9 Hz, 1H), 7.72 (d, J = 8.8 Hz, 1H), 7.38 (ddd, J = 8.5, 6.8, 0.9 Hz, 1H), 7.29 (ddd, J = 7.5, 7.0, 0.9 Hz 1H), 4.64 (dd, J = 6.3, 5.6 Hz, 2H), 3.55 (dd, J = 6.3, 5.6 Hz, 2H), 2.83 (app. p, J = 5.9 Hz, 2H); 13C NMR (150 MHz, CDCl3) δ 147.3, 130.7, 127.6, 125.3 119.3, 119.2, 117.9, 51.2, 49.6, 21.3; HRMS (ESI) m/z: [M + H]+ calcd for C10H10N2O2SH 223.0541; Found 223.0531.
2,3,4,5-Tetrahydro-[1,3]thiazepino[3,2-b]indazole 1,1-Dioxide (26)
Prepared from 1c (50 mg, 0.25 mmol) by General Procedure B. Sulfone 26 was isolated as an off-white amorphous solid in 94% yield (54 mg). IR (neat) νmax 1342, 1124, cm–1; 1H NMR (600 MHz, CDCl3) δ 8.11 (dt, J = 8.6, 1.1 Hz, 1H), 7.75 (d, J = 8.6, 1.0 Hz, 1H), 7.38 (ddd, J = 8.7, 6.7, 1.1 Hz, 1H), 7.31 (ddd, J = 8.5, 6.7, 1.0 Hz, 1H), 4.96 (dd, J = 6.1, 5.8 Hz, 2H), 3.39 (dd, J = 6.1, 5.8 Hz, 2H), 2.35 (m, 2H), 1.50 (m, 2H); 13C NMR (150 MHz, CDCl3) δ 147.1, 127.1, 125.8, 123.6, 119.8, 118.2, 105.2, 56.1, 54.8, 27.5 24.3. HRMS (ESI) m/z: [M + H]+ calcd for C11H12N2O2SH 237.0697; Found 237.0681.
7,8-Dihydro-[1,3]dioxolo[4,5-f]thiazolo[3,2-b]indazole 9,9-Dioxide (27)
Prepared from 15a (50 mg, 0.23 mmol) by General Procedure B. Sulfone 27 was isolated as a light tan amorphous solid in 91% yield (52 mg). IR (neat) νmax 1328 cm–1; 1H NMR (600 MHz, DMSO-d6) δ 7.21 (s, 1H), 7.17 (s, 1H), 6.12 (s, 2H), 4.93 (td, J = 6.5, 1.0 Hz, 2H), 4.34 (td, J = 6.5, 1.0 Hz, 2H); 13C NMR (150 MHz, DMSO-d6) δ 149.4, 149.2, 147.5, 130.7, 109.9, 101.8, 95.0, 93.3, 55.0, 46.2. HRMS (ESI) m/z: [M + H]+ calcd for C10H8N2O4SH 253.0283; Found 253.0277.
8,9-Dihydro-7H-[1,3]dioxolo[4,5-f][1,3]thiazino[3,2-b]indazole 10,10-Dioxide (28)
Prepared from 15b (50 mg, 0.21 mmol) by General Procedure B. Sulfone 28 was isolated as a tan amorphous solid in 90% yield (51 mg). IR (neat) νmax 1320 cm–1; 1H NMR (600 MHz, CDCl3) δ 7.15 (s, 1H), 6.97 (s, 1H), 6.02 (s, 2H), 4.54 (app. t, J = 6.0 Hz, 2H), 3.52 (dd, J = 6.3, 5.5 Hz, 2H), 2.81 (app. p, J = 6.0 Hz, 2H); 13C NMR (150 MHz, CDCl3) δ 150.3, 148.2, 145.0, 130.4, 115.7, 101.7, 95.5, 94.4, 51.2, 49.2, 21.4. HRMS (ESI) m/z: [M + H]+ calcd for C11H10N2O4SH 267.0439; Found 267.0437.
7,8,9,10-Tetrahydro-[1,3]dioxolo[4,5-f][1,3]thiazepino[3,2-b]indazole 11,11-Dioxide (29)
Prepared from 15c (50 mg, 0.20 mmol) by General Procedure B. Sulfone 29 was isolated as a yellow amorphous solid in 90% yield (54 mg). IR (neat) νmax 1350 cm–1; 1H NMR (600 MHz, CDCl3) δ 7.28 (s, 1H), 6.94 (s, 1H), 6.00 (s, 1H), 4.79 (dd, J = 5.6, 1.2 Hz, 2H), 3.34 (app. t, J = 6.3 Hz, 2H), 2.34 (m, 2H), 2.00 (m, 2H); 13C NMR (150 MHz, CDCl3) δ 149.8, 148.5, 144.3, 130.0, 120.2, 101.6, 95.0, 94.3, 56.2, 54.3, 27.6, 24.2. HRMS (ESI) m/z: [M + H]+ calcd for C12H12N2O4SH 281.0596; Found 281.0590.
Acknowledgments
We thank the Tara K. Telford Fund for Cystic Fibrosis Research at the University of California-Davis and the National Institutes of Health (Grants DK72517 and GM089153) for generous financial support of this work. We also thank Dr. William Jewell (UC Davis Mass Spectrometry Facility) for assistance with HRMS data collection.
Supporting Information Available
1H NMR and 13C NMR spectra for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Yakaiah T.; Lingaiah B. P. V.; Narsaiah B.; Shireesha B.; Ashok Kumar B.; Gururaj S.; Parthasarathy T.; Sridhar B. Bioorg. Med. Chem. Lett. 2007, 17, 3445–3453. [DOI] [PubMed] [Google Scholar]
- Jones P.; Altamura S.; Boueres J.; Ferrigno F.; Fonsi M.; Giomini C.; Lamartina S.; Monteagudo E.; Ontoria J. M.; Orsale M. V.; Palumbi M. C.; Pesci S.; Roscilli G.; Scarpelli R.; Schultz-Fademrecht C.; Toniatti C.; Rowley M. J. Med. Chem. 2009, 52, 7170–7185. [DOI] [PubMed] [Google Scholar]
- Saczewski F.; Hudson A. L.; Tyacke R. J.; Nutt D. J.; Man J.; Tabin P.; Saczewski J. Eur. J. Pharm. Sci. 2003, 20, 201–208. [DOI] [PubMed] [Google Scholar]
- Huang L.-J.; Shih M.-L.; Chen H.-S.; Pan S.-L.; Teng C.-M.; Lee F.-Y.; Kuo S.-C. Bioorg. Med. Chem. 2006, 14, 528. [DOI] [PubMed] [Google Scholar]
- Haddadin M. J.; Conrad W. E.; Kurth M. J. Mini-Rev. Med. Chem. 2012, 12, 1293–1300. [DOI] [PubMed] [Google Scholar]
- Avila B.; El-Dakdouki M. H.; Nazer M. Z.; Harrison J. G.; Tantillo D. J.; Haddadin M. J.; Kurth M. J. Tetrahedron Lett. 2012, 53, 6475–6478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad W. E.; Rodriguez K. X.; Nguyen H. H.; Fettinger J. C.; Haddadin M. J.; Kurth M. J. Org. Lett. 2012, 14, 3870–3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano D. M.; Butler J. D.; Haddadin M. J.; Kurth M. J.; Baucom K. D.; Faul M. M. Org. Synth. 2010, 87, 339–349. [Google Scholar]
- Conrad W. E.; Fukazawa R.; Haddadin M. J.; Kurth M. J. Org. Lett. 2011, 13, 3138–3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila B.; Solano D. M.; Haddadin M. J.; Kurth M. J. Org. Lett. 2011, 13, 1060–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald M. B.; Conrad W. E.; Oakdale J. S.; Butler J. D.; Haddadin M. J.; Kurth M. J. Org. Lett. 2010, 12, 2524–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- In the O-analogue series, solvent competing with the internal nucleophile in the N,N-heterocyclization reaction as well as nucleophilic opening of the oxygen heterocycle results in lower yields of the targeted indazole compared to the S-analogue series where we see no evidence for these side reactions. See:Oakdale J. S.; Solano D. M.; Fettinger J. C.; Haddadin M. J.; Kurth M. J. Org. Lett. 2009, 11, 2760–2763. [DOI] [PubMed] [Google Scholar]
- Butler J. D.; Solano D. M.; Robins L. I.; Haddadin M. J.; Kurth M. J. J. Org. Chem. 2008, 73, 234–240. [DOI] [PubMed] [Google Scholar]
- Mills A. D.; Maloney P.; Hassanein E.; Haddadin M. J.; Kurth M. J. J. Comb. Chem. 2007, 9, 171–177. [DOI] [PubMed] [Google Scholar]
- Mills A. D.; Nazer M. Z.; Haddadin M. J.; Kurth M. J. J. Org. Chem. 2006, 71, 2687–2689. [DOI] [PubMed] [Google Scholar]
- Kurth M. J.; Olmstead M. M.; Haddadin M. J. J. Org. Chem. 2005, 70, 1060–1062. [DOI] [PubMed] [Google Scholar]
- Okiyoneda T.; Veit G.; Dekkers J. F.; Bagdany M.; Soya N.; Xu H.; Roldan A.; Verkman A. S.; Kurth M.; Simon A.; Hegedus T.; Beekman J. M.; Lukacs G. L. Nat. Chem. Biol. 2013, 9, 444–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp J. M.; Wood A. B.; Phuan P.-W.; Lodewyk M. W.; Tantillo D. J.; Verkman A. S.; Kurth M. J. J. Med. Chem. 2012, 55, 1242–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myeloperoxidase, which is expressed in human neutrophil granulocytes, produces hypochlorous acid from hydrogen peroxide and chloride and is thought to be at least partially causative of damage to the CF lung.
- Conese M.; Copreni E.; Di Gioia S.; De Rinaldis P.; Fumarulo R. J. Cystic Fibrosis 2003, 2, 129–135. [DOI] [PubMed] [Google Scholar]
- Previous work has shown that amino alcohols can be employed to deliver oxazolo- and oxazinoindazoles; see refs (12) and (13).
- Mitchard M. Drug Metab. Drug Interact. 1988, 6, 183–202. [DOI] [PubMed] [Google Scholar]
- Rouault T. A. Dis. Models & Mech. 2012, 5, 155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garay A. S. Nature (London) 1956, 177, 91–92. [DOI] [PubMed] [Google Scholar]
- Knappe J. Annu. Rev. Biochem. 1970, 39, 757–776. [DOI] [PubMed] [Google Scholar]
- Corsi G.; Palazzo G. Ann. Chim. 1965, 60, 246–258. [Google Scholar]
- Cope O. J.; Brown R. K. Can. J. Chem. 1961, 39, 1695–1710. [Google Scholar]
- Halkes K. M.; Carvalho de Souza A.; Maljaars C. E. P.; Gerwig G. J.; Kamerling J. P. Eur. J. Org. Chem. 2005, 17, 3650–3659. [Google Scholar]
- Ruggles E. L.; Deker P. B.; Hondal R. J. Tetrahedron 2009, 65, 1257–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noveron J. C.; Olmstead M. M.; Mascharak P. K. J. Am. Chem. Soc. 2001, 123, 3247–3259. [DOI] [PubMed] [Google Scholar]
- Debonis S.; Skoufias D. A.; Marquet B.; Laggner C.; Joseph B.; Kozielski F. J. Med. Chem. 2008, 51, 1115–1125. [DOI] [PubMed] [Google Scholar]
- Goddard C. M. L.; Massah A. R.; Jackson R. F. W. Tetrahedron 2010, 66, 9175–9181. [Google Scholar]
- Bos P. H.; Minnaard A. J.; Feringa B. L. Org. Lett. 2008, 10, 4219–4222. [DOI] [PubMed] [Google Scholar]
- Becker Y.; Eisenstadt A.; Stille J. K. J. Org. Chem. 1980, 45, 2145–2151. [Google Scholar]
- Carocci A.; Catalano A.; Corbo F.; Duranti A.; Amoroso R.; Franchini C.; Lentinia G.; Tortorella V. Tetrahedron: Asymmetry 2000, 11, 3619–3634. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.