Abstract
Context:
A role of lipids in human fecundity is hypothesized as cholesterol is the main substrate for steroid synthesis and has also been shown to affect the hormonal milieu and steroidogenesis in both men and women.
Objective:
The objective of the study was to evaluate the association between male and female serum lipid concentrations and time to pregnancy (TTP).
Design/Setting:
A population-based prospective cohort study recruiting couples from 16 counties in Michigan and Texas (2005–2009) using sampling frameworks allowing for identification of couples planning pregnancy in the near future.
Participants:
Five hundred one couples desiring pregnancy and discontinuing contraception were followed up for 12 months or until a human chorionic gonadotropin pregnancy was detected.
Main Outcome and Measures:
Fecundability odds ratios (FORs) and 95% confidence intervals (CIs) were estimated after adjusting for age, body mass index, race, and education in relationship to female, male, and joint couple lipid concentrations.
Results:
Serum free cholesterol levels were higher on average among male and female partners of couples who did not became pregnant during the study follow-up (female, P = .04; male, P = .009), and levels in female partners were associated with significantly longer TTP in models based on both individual and couples concentrations (individual models: FOR 0.98, 95% CI 0.97, 0.99; couple models: FOR 0.98, 95% CI 0.97, 0.99). Male free cholesterol concentrations were associated with TTP only in the couple-based models (FOR 0.98, 95% CI 0.97, 0.99). Sensitivity analyses suggested that the observed associations are unlikely to be explained by potential unmeasured confounding such as diet.
Conclusions:
Our results suggest that serum free cholesterol concentrations in both men and women have an effect on TTP, highlighting the importance of cholesterol and lipid homeostasis for male and female fecundity.
The worldwide obesity epidemic is accompanied by an increasing prevalence of dyslipidemia in adults, which in turn is associated with multiple disorders such as diabetes, polycystic ovary syndrome, metabolic syndrome, cancer, and cardiovascular disease. A role of lipids in human fecundity is also hypothesized because cholesterol is the main substrate for steroid synthesis (1) and has also been shown to affect the hormonal milieu and steroidogenesis in both men and women (2).
There is a considerable body of literature supporting a role for lipids in male and female fecundity (3–12) because higher high-density lipoprotein (HDL) concentrations have been associated with better oocyte and embryo outcomes (4, 13) as well as effects on spermatogenesis (2). However, we are unaware of any research that has evaluated serum lipid profiles in relation to couple fecundity, as measured by time to pregnancy (TTP) independent of body mass index (BMI). This couple-based measure requires each partner of the couple to be fecund or biologically capable of reproduction. Indirect evidence in support of a relationship between serum lipids and TTP comes from research that reports a negative relation between partners' BMI and TTP (14–20). These findings also have been corroborated among couples undergoing assisted reproductive technologies (21–26). To our knowledge, the interplay between serum lipids, couples' BMIs, and TTP has never been investigated and serves as the impetus for study. We used the recently completed Longitudinal Investigation of Fertility and the Environment (LIFE) study to address this data gap, particularly in light of a growing percentage of reproductive-age couples who are overweight or obese yet desirous of pregnancy.
Materials and Methods
Design and study population
The LIFE study is a prospective cohort study designed to investigate environmental influences on human fecundity and fertility, and its design and methods have been previously described (27). In brief, 501 couples discontinuing contraception for the purposes of becoming pregnant were recruited from 16 counties in Michigan and Texas between 2005 and 2009 using sampling frameworks tailored for each state, allowing for the identification of couples planning pregnancy in the near future. Eligible couples were followed up for 12 months or until a human chorionic gonadotropin (hCG) pregnancy. Inclusion criteria included the following: female aged 18–44 years and male aged 18+ years who were in a committed relationship; an ability to communicate in English or Spanish; the female partner had menstrual cycles between 21 and 42 days and with no injectable hormonal contraception within the past year; and neither partner was surgically or medically sterile. Two percent (n = 1188) of the 51 715 individuals screened met the minimal eligibility criteria, of which 501 (42%) enrolled. Full human subjects' approval was granted prior to obtaining informed consent from all couples.
Data collection
Upon enrollment, in-person interviews were conducted separately with each partner to ascertain health, demographic, and reproductive histories as well as physical activity and medication and supplement use. Couples completed daily journals while attempting to become pregnant until a hCG pregnancy or 12 months of trying to capture lifestyle behaviors relevant to fecundity, sexual intercourse, medication use (including lipid lowering drugs), and menstruation and pregnancy test results for female participants. To maximize all couples' fecundity, female partners were instructed in the use of the commercially available Clearblue Easy fertility monitors (Swiss Precision Diagnostics formerly Unipath). Daily levels of estrone-3-glucoronide and LH were tracked commencing on day 6 of the cycle and up to 20 days thereafter. Monitors indicated low, high, or peak fertility, as determined by the ratio of estrone-3-glucoronide and LH, with peak indicative of impending ovulation. Women also were trained in the use of the digital Clearblue Easy home pregnancy test for detecting hCG pregnancy, and all women's urine samples were tested prior to enrollment to ensure the absence of pregnancy. The fertility monitor is 99% accurate for detecting the LH surge and 91% accurate for peak fertility when compared with the gold standard of ultrasonography (28). For both partners of the couple, the nurse obtained nonfasting blood (∼2 mL) for the quantification of serum lipids and cotinine. Samples were immediately transported to the laboratory, centrifuged, and aliquoted. Samples were frozen at −20°C or colder until shipment on ice to the study's laboratory for analysis of serum lipids. Each partner of the couple was remunerated $75 for complete participation in the study.
Laboratory analysis
All assays were quantified using the Hitachi model 912 clinical analyzer at the Centers for Disease Control and Prevention laboratory. Samples collected for analysis were stored frozen and thawed only once for lipid analysis. Total cholesterol was analyzed with the Roche Cholesterol/HP method (Roche Diagnostics), an enzymatic colorimetric determination using cholesterol esterase and cholesterol oxidase. Free cholesterol used the Wako Free Cholesterol C method (Wako Chemicals USA, Inc), an enzymatic colorimetric assay, which uses cholesterol oxidase and peroxidase but omits cholesterol esterase. Triglycerides were analyzed with the Roche triglycerides/glycerophosphate oxidase method without blanking, and phospholipids were measured by using the Wako Phospholipids B enzymatic colorimetric method. The Wako phospholipids method uses phospholipase D and choline oxidase for the analysis, so it measures specifically the major choline-containing phospholipids including lecithin, lysolecithin, and sphingomyelin (29). Serum levels of cotinine were quantified using liquid chromatography-isotope dilution tandem mass spectrometry (30) for the assessment of baseline exposure to smoking with cut points based on previous literature (31, 32). All analyses were subjected to standard quality assurance procedures, and all reported results were from runs found to be in control by standard statistical methods. The serum pools for the lipid analysis have remained stable for more than 20 years, suggesting reliable testing of lipid parameters in frozen samples.
Statistical analysis
All data were entered into a web-based data management system capable of handling the study's hierarchical data structure stemming from prospective longitudinal data collection at the partner, cycle, and day level. Descriptive analysis included the inspection of missing data and influential observations. A menstrual cycle was defined as the interval between the onset of bleeding in one cycle as reported in the daily journal with at least 2 days of bleeding with increased intensity to the onset of the next similar bleeding episode using longitudinally collected data from the daily journal and fertility monitors. This definition excluded any episodic noncyclic bleeding, largely using data from fertility monitors that capture the onset of menses when the woman first begins to test her urine. Pregnancy was defined as a positive test on the day of expected menstruation. The TTP was the number of menstrual cycles to a positive pregnancy test or censoring.
The study cohort was assessed by select characteristics for each partner and quartiles of free cholesterol. Differences in characteristics between quartiles of free cholesterol were assessed using an ANOVA and a Fisher's exact test, where appropriate. The distribution of individual lipid concentrations for both partners were compared by pregnancy status (pregnant, not pregnant, no observed pregnancy at the time of censoring), with differences assessed using an ANOVA.
Cox models for discrete left truncated survival time (33) to account for time off contraception prior to enrollment were used to estimate the fecundability odds ratio (FOR) and 95% confidence intervals (CIs), using SAS proportional odds model in SAS software (SAS version 9.2; SAS Institute, Inc). All significance was assessed using an α level of .05. FORs estimate the odds of becoming pregnant each cycle, given preconception baseline lipid concentrations conditional on not being pregnant in the previous cycle. FORs less than 1 denote a reduction in fecundity or a longer TTP, and FORs greater than 1 denote a shorter TTP. Models were first run for female and male concentrations modeled individually and then jointly with both partners' lipid concentrations included, in keeping with the couple-dependent nature of pregnancy. The Pearson's correlation was ρ = 0.09 between male and female total cholesterol and ρ = 0.23 between male and female free cholesterol, easing concerns about collinearity. Based on an a priori biological literature review, we used a directed acyclic graph to find the minimal set of confounders for model adjustment. Models were adjusted for male and female age (years), BMI (kilograms per square meter), race (non-Hispanic white, non-Hispanic black, Hispanic, other), and education (<high school/equivalent, some college or technical school, college graduate, or higher) (8, 14, 34, 35).
Couples who withdrew from the study before achieving pregnancy, largely as a result in a change in pregnancy intention, or who were not pregnant after 12 months of trying were censored in all analyses (n = 114) (27). Models were also adjusted for fish consumption (as a proxy for dietary intake), past use of oral contraception (before baseline), menstrual regularity, physical activity, and use of lipid-lowering drugs (any report on the prospectively ascertained daily journal of taking medications including lipid lowering drugs during the study follow-up), although adjustment for these factors did not appreciably change the results and were not included in the final models for parsimony.
A sensitivity analysis was conducted to evaluate the possible impact of unmeasured confounding by diet (eg, fiber, fat, or red meat intake) in estimating the association between serum lipids and TTP. Fiber and red meat intake were considered as potential unmeasured confounders because their dietary intake is strongly correlated with lipid concentrations and associated with hormone levels in premenopausal women that may influence TTP (36–40). However, although we focus here on potential dietary factors, this could represent any unmeasured factor. As such, we simulated a wide range of situations such as a variable for a range of correlations between the unmeasured factor and TTP (from half to double the observed effect of lipids on TTP) and between the unmeasured factor and lipids (ρ = 0 to ρ = .9) to represent mild to severe potential confounding. We compared the results of our final adjusted models with models adjusted for this simulated dietary factor.
Results
The LIFE study cohort comprised 501 couples of whom 347 (69%) achieved pregnancy, whereas 54 (11%) did not and 100 (20%) withdrew at some point from the study. Mean age of male and female partners was 31.8 ± 4.9 and 30.0 ± 4.1 years, respectively, and most were college-educated non-Hispanic white couples. Age was significantly associated with free cholesterol levels for both men and women (Table 1). Lipids were quantified for 491 male (98.0%) and 489 females (97.6%), with no differences in various characteristics by availability of blood (data not shown). The main reason for the absence of serum was insufficient volume after the analysis of environmental chemicals such as polychlorinated biphenyls. There was a higher percentage of Hispanic men in the upper quartile of free cholesterol levels as compared with all other quartiles. Mean BMI increased across free cholesterol quartiles among both men and women, and a higher percentage of men and women reported vigorous exercise in the lower quartiles of free cholesterol. No women in the study were taking lipid-lowering drugs, and no differences in intake were observed across quartiles of free cholesterol in men. A greater percentage of women reporting a history of irregular than regular menstrual cycles was observed in the highest quartile of free cholesterol.
Table 1.
Sociodemographic Description of Study Cohort by Quartile of Serum Free Cholesterol Concentrations for Male and Female Partners, LIFE Study, 2005–2009
| Characteristics, n, % | Females Free Cholesterol, mg/dL |
Males Free Cholesterol mg/dL |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Q1, <39 | Q2, 39–45 | Q3, 46–51 | Q4, >51 | P Value | Overall | Q1, <42 | Q2, 42–49 | Q3, 49–57 | Q4, >57 | P Value | |
| Age, y, mean (SD) | 30.0 (4.1) | 29.6 (3.9) | 29.2 (3.8) | 29.7 (4.2) | 31.2 (4.3) | .0005 | 31.8 (4.9) | 30.8 (4.6) | 31 (4.5) | 32.1 (5.1) | 33.1 (5.1) | .0008 |
| Age at menarche, y, mean (SD) | 12.6 (1.6) | 12.5 (1.4) | 12.5 (1.6) | 12.7 (1.6) | 12.5 (1.6) | .79 | N/A | |||||
| BMI, kg/m2, mean (SD) | 27.6 (7.3) | 25.3 (5.6) | 26.8 (6.7) | 28 (8.1) | 29.9 (7.7) | <.0001 | 29.8 (5.6) | 28.3 (5.4) | 29.9 (6.2) | 29.9 (5.3) | 30.9 (5.1) | .004 |
| Race/ethnicity | ||||||||||||
| Non-Hispanic white | 385 (79) | 79 (77) | 105 (78) | 96 (79) | 105 (81) | .71 | 384 (78) | 99 (84) | 84 (74) | 107 (83) | 94 (72) | .07 |
| Non-Hispanic black | 24 (5) | 3 (3) | 10 (7) | 8 (7) | 3 (2) | 23 (5) | 5 (4) | 8 (7) | 3 (2) | 7 (5) | ||
| Hispanic | 47 (10) | 12 (12) | 11 (8) | 11 (9) | 13 (10) | 45 (9) | 3 (3) | 13 (11) | 10 (8) | 19 (15) | ||
| Other | 33 (7) | 8 (8) | 9 (7) | 7 (6) | 9 (7) | 39 (8) | 11 (9) | 9 (8) | 9 (7) | 10 (8) | ||
| Education | ||||||||||||
| Less than high school/equivalent | 30 (6) | 6 (6) | 9 (7) | 10 (8) | 5 (4) | .08 | 47 (10) | 9 (8) | 7 (6) | 14 (11) | 17 (13) | .05 |
| Some college or technical school | 92 (19) | 9 (9) | 30 (22) | 23 (19) | 30 (23) | 141 (29) | 24 (20) | 33 (29) | 46 (36) | 38 (29) | ||
| College graduate or higher | 367 (75) | 87 (85) | 96 (71) | 89 (73) | 95 (73) | 303 (62) | 85 (72) | 74 (65) | 69 (53) | 75 (58) | ||
| Household income, $ | ||||||||||||
| <29 999 | 27 (6) | 4 (4) | 7 (5) | 9 (7) | 7 (5) | .46 | 22 (4) | 7 (6) | 4 (4) | 4 (3) | 7 (5) | .56 |
| 30 000 to 49 999 | 64 (13) | 12 (12) | 18 (13) | 16 (13) | 18 (14) | 53 (11) | 13 (11) | 12 (11) | 16 (12) | 12 (9) | ||
| 50 000 to 69 999 | 67 (14) | 11 (11) | 27 (20) | 12 (10) | 17 (13) | 90 (18) | 15 (13) | 18 (16) | 27 (21) | 30 (23) | ||
| ≥70 000 | 331 (68) | 75 (74) | 83 (61) | 85 (70) | 88 (68) | 326 (66) | 83 (70) | 80 (70) | 82 (64) | 81 (62) | ||
| Health insurance | 446 (92) | 95 (93) | 124 (93) | 110 (90) | 117 (91) | .85 | 446 (91) | 109 (92) | 102 (90) | 118 (92) | 117 (91) | .92 |
| Cotinine, ng/mL | ||||||||||||
| No exposure (0, 9.99) | 426 (88) | 95 (94) | 120 (90) | 103 (86) | 108 (83) | .20 | 384 (78) | 100 (85) | 91 (80) | 104 (81) | 89 (68) | .08 |
| Passive smoking (10, 99.99) | 24 (5) | 3 (3) | 5 (4) | 8 (7) | 8 (6) | 22 (4) | 4 (3) | 7 (6) | 4 (3) | 7 (5) | ||
| Active smoking (100, 299.99) | 27 (6) | 3 (3) | 8 (6) | 6 (5) | 10 (8) | 44 (9) | 8 (7) | 6 (5) | 12 (9) | 18 (14) | ||
| Heavy smoking (300, 595.31) | 7 (1) | 0 (0) | 0 (0) | 3 (3) | 4 (3) | 40 (8) | 5 (4) | 10 (9) | 9 (7) | 16 (12) | ||
| Baseline alcohol | ||||||||||||
| Less than once per month | 52 (14) | 10 (13) | 16 (15) | 13 (15) | 13 (13) | .78 | 29 (7) | 7 (7) | 5 (5) | 5 (5) | 12 (11) | .49 |
| Once per month | 72 (20) | 14 (18) | 23 (22) | 16 (18) | 19 (20) | 39 (9) | 10 (10) | 10 (10) | 10 (9) | 9 (8) | ||
| 2–3 d/mo | 88 (24) | 18 (24) | 22 (21) | 22 (25) | 26 (27) | 80 (19) | 17 (17) | 19 (19) | 23 (21) | 21 (19) | ||
| Once a week | 69 (19) | 16 (21) | 19 (18) | 12 (14) | 22 (23) | 104 (25) | 21 (21) | 26 (27) | 36 (33) | 21 (19) | ||
| 2–3 times per week | 74 (20) | 15 (20) | 20 (19) | 23 (26) | 16 (16) | 127 (30) | 39 (38) | 26 (27) | 28 (25) | 34 (31) | ||
| 4–6 times per week | 7 (2) | 3 (4) | 3 (3) | 0 (0) | 1 (1) | 28 (7) | 4 (4) | 8 (8) | 7 (6) | 9 (8) | ||
| Every day | 3 (1) | 0 (0) | 2 (2) | 1 (1) | 0 (0) | 12 (3) | 4 (4) | 4 (4) | 1 (1) | 3 (3) | ||
| Vigorous exercise program during the last 12 mo | 195 (40) | 41 (40) | 51 (38) | 61 (50) | 42 (32) | .04 | 207 (42) | 57 (48) | 51 (45) | 56 (43) | 43 (33) | .09 |
| Lipid-lowering drugs | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | N/A | 18 (4) | 7 (6) | 5 (4) | 5 (4) | 1 (1) | .17 |
| Parity (number of live births) | ||||||||||||
| 0 | 257 (53) | 49 (49) | 71 (53) | 68 (56) | 69 (53) | .77 | ||||||
| 1 | 158 (33) | 35 (35) | 40 (30) | 40 (33) | 43 (33) | |||||||
| 2+ | 71 (15) | 17 (17) | 23 (17) | 13 (11) | 18 (14) | |||||||
| Menstrual regularity | ||||||||||||
| Regular | 408 (83) | 79 (77) | 114 (84) | 104 (85) | 111 (85) | .02 | ||||||
| Not regular | 37 (8) | 16 (16) | 10 (7) | 7 (6) | 4 (3) | |||||||
| Varies | 44 (9) | 7 (7) | 11 (8) | 11 (9) | 15 (12) | |||||||
Abbreviations: N/A, not available; Q, quartile.
Couple-specific select serum lipid components were associated with pregnancy status. Free cholesterol levels in men and women were significantly higher among couples who did not become pregnant in comparison with those becoming pregnant (Table 2). In addition, the male but not female total cholesterol levels also were associated with the absence of pregnancy.
Table 2.
Distribution of Serum Lipid Compartments by Pregnancy Status, LIFE Study, 2005–2009
| Overall (n = 501)a Median (IQR) | Observed Pregnancy in 12 Months of Follow-Up (n = 347) Median (IQR) | Not Pregnant (n = 154)b Median (IQR) | P Value | |
|---|---|---|---|---|
| Female | ||||
| Cholesterol, mg/dL | 180 (42) | 177 (43) | 182 (40) | .25 |
| Free cholesterol, mg/dL | 45 (12) | 44 (11) | 46 (14) | .04 |
| Phospholipids, mg/dL | 222 (44) | 221 (44) | 223 (45) | .59 |
| Triglycerides, mg/dL | 101 (73) | 100 (68) | 101 (87) | .90 |
| Total lipids, mg/dL | 606 (145) | 601 (139) | 612 (158) | .21 |
| Male | ||||
| Cholesterol, mg/dL | 190 (49) | 185 (46) | 198 (50) | .002 |
| Free cholesterol, mg/dL | 49 (15) | 48 (15) | 52 (15) | .009 |
| Phospholipids, mg/dL | 222 (51) | 219 (50) | 226 (53) | .07 |
| Triglycerides, mg/dL | 175 (143) | 168 (146) | 189 (151) | .19 |
| Total lipids, mg/dL | 692 (222) | 679 (220) | 708 (244) | .19 |
Abbreviation: IQR, interquartile range.
Lipid concentrations were measured for a total of 489 females and 491 males.
Not pregnant at censoring time.
FORs were reduced, indicative of a longer time to pregnancy, for each of the five lipid components when modeling female serum lipids individually (Table 3). However, only free cholesterol and total lipids remained significant after adjustment for relevant covariates (FOR 0.983; 95% CIs 0.969, 0.997, and 0.999; 95% CIs 0.998, 1.000, respectively). No significant FORs were observed for male serum lipid components and TTP. When both partners' concentrations were jointly modeled, four of five lipid components quantified in women were associated with FORs less than 1, indicative of a longer TTP. Only free cholesterol remained significant after adjustment (FOR 0.984; 95% CIs 0.970, 0.994). Of note is a significant association for male free cholesterol and TTP as well (FOR 0.984; 95% CIs 0.970, 0.999) for couple-based models.
Table 3.
Serum Lipid Component Concentrations and FORs, LIFE Study, 2005–2009a
| Serum Concentrations | Male and Female Concentrations Modeled Separately |
Couple-Based Concentration Models |
||
|---|---|---|---|---|
| Unadjusted | Adjustedb | Unadjusted | Adjustedc | |
| FOR (95% CI) | FOR (95% CI) | FOR (95% CI) | FOR (95% CI) | |
| Female concentrations | ||||
| Cholesterol | 0.996 (0.992, 0.999) | 0.997 (0.993,1.000) | 0.996 (0.992, 1.000) | 0.997 (0.993, 1.001) |
| Free cholesterol | 0.979 (0.966, 0.992) | 0.983 (0.969, 0.997) | 0.981 (0.967, 0.994) | 0.984 (0.970, 0.999) |
| Phospholipids | 0.997 (0.993, 1.000) | 0.997 (0.993, 1.000) | 0.997 (0.993, 1.000) | 0.997 (0.993, 1.001) |
| Triglycerides | 0.998 (0.996, 1.000) | 0.999 (0.997, 1.000) | 0.998 (0.996, 1.000) | 0.999 (0.997, 1.001) |
| Total lipids | 0.999 (0.998, 1.000) | 0.999 (0.998, 1.000) | 0.999 (0.998, 1.000) | 0.999 (0.998, 1.013) |
| Male concentrations | ||||
| Cholesterol | 0.998 (0.995, 1.001) | 1.000 (0.996, 1.003) | 0.998 (0.995, 1.001) | 0.999 (0.996, 1.002) |
| Free cholesterol | 0.992 (0.983, 1.001) | 0.995 (0.986, 1.005) | 0.996 (0.986, 1.005) | 0.984 (0.970, 0.999) |
| Phospholipids | 0.998 (0.995, 1.001) | 0.999 (0.996, 1.002) | 0.998 (0.996, 1.001) | 0.999 (0.996, 1.002) |
| Triglycerides | 1.000 (0.999, 1.000) | 1.000 (0.999, 1.001) | 1.000 (0.999, 1.001) | 0.999 (0.997, 1.001) |
| Total lipids | 1.000 (0.999, 1.000) | 1.000 (0.999, 1.000) | 1.000 (0.999, 1.000) | 1.000 (0.999, 1.044) |
Bolded values indicate P < .05.
Adjusted for age, BMI, race, and education.
Adjusted for female age, age difference, female BMI, male BMI, female race, male race, female education, and male education.
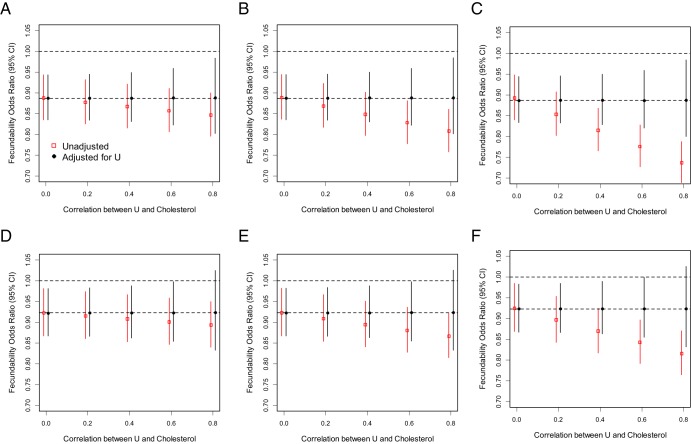
Our sensitivity analysis demonstrated that an unmeasured factor would need to have twice as large an effect as the observed association with lipids and be very highly correlated with lipids (ρ = .6 or larger) to completely explain away the observed association between free cholesterol levels in women and TTP (Figure 1).
Figure 1.
Sensitivity analyses of lipid concentrations (X) and TTP unadjusted and adjusted for a hypothesized unmeasured confounding factor U (eg, standardized dietary factor that could influence both cholesterol levels and TTP). Simulations are data driven based on results from models of male and female standardized free cholesterol (ie, mean 0, SD 1) levels on TTP. b/a is the ratio of the strength of the association between the unmeasured factor and TTP compared with the association between free cholesterol levels and TTP. Top, Female (A, b/a = 0.5; B, b/a = 1; C, b/a = 2); bottom, male (D, b/a = 0.5; E, b/a = 1; F, b/a = 2).
Discussion
Our findings are the first to demonstrate that select serum lipids are associated with reduced couple fecundity as measured by a longer TTP. Of the five lipid components evaluated, free cholesterol was robustly associated with reduced fecundity when modeling female serum lipids individually or in a couple-based approach independent of BMI. Male free cholesterol concentrations were also an independent risk factor for reduced fecundability, irrespective of female lipid levels. These findings fill an important data gap regarding the association between lipid concentrations and couple fecundity, particularly in light of the increased prevalence of overweight couples attempting pregnancy.
TTP is a measure of couples' fecundability (41) and one that does not inform about whether the diminished fecundity arises from the female or male partner or whether it is a function of both. Of interest is the observation that when modeled as a couple-based approach, both male and female free cholesterol levels were significantly associated with reduced fecundability. Interpretation of our findings is limited by the absence of published studies to our knowledge comparing lipid concentrations and TTP. Despite the absence of previous data for preconception couple-based cohort studies, our findings are consistent with previous animal studies that support an association between dyslipidemia and infertility (8). These results are in line with research in humans showing effects of lipids on oocyte quality (3) as well as semen quality (42), underscoring the need for a couple-based approach. This research is also consistent with findings of increased rates of infertility among men with metabolic syndrome (7) and among women with polycystic ovary syndrome and accompanying symptoms of metabolic syndrome (43, 44).
The observation that both total cholesterol and free cholesterol are significantly associated with TTP is not surprising, given that it has been known for many years that there is a highly significant correlation between serum total and free cholesterol (45, 46). With respect to reproductive outcomes, knockout of the scavenger receptor BI−/− in mice leads to a large increase in the unesterified cholesterol to total cholesterol ratio, abnormally large HDL particles, and infertility in females (47). Treatment of these mice with probucol reduces the unesterified cholesterol to total cholesterol ratio and restores fertility (48). Moreover, recent evidence points to the importance of the liver X receptors (LXR; LXRα and LXRβ). LXRs are nuclear transcription factors that are important regulators of cholesterol, fatty acid, and glucose homeostasis. LXR pathways have been shown to be important in reproductive health for both males and females because their deletions have been shown to induce multiple reproductive phenotypes (12, 49).
In addition, the oocyte is cholesterol biosynthesis incompetent and relies on the surrounding cumulus granulosa for many factors including cholesterol, and there is evidence that the communication between the oocyte and granulosa cells is bidirectional (50). Serum and lipoprotein cholesterol may thus ultimately affect the activity of sterodogenic cells. Granulosa cells accumulate cholesteryl esters and use these stores preferentially for steroid synthesis (51). HDL is also the sole source of lipoprotein cholesterol for granulosa cells within the developing follicle, and their steroidogenic potential is dependent on the supply of HDL from the blood via the follicular fluid (52). Total serum cholesterol levels are associated with changes in HDL particle dynamics (53) that may alter the ability of HDL to cross the blood follicle barrier and thereby supply cholesterol for steroidogenesis and ultimately influence fertility. Combined, these findings provide plausible mechanisms for our observed independent associations between the female and male lipid concentrations and TTP.
The differences between the individual and couple-based models highlight that free cholesterol levels are important for fertility for both members of the couple when considered together. It is important to note that although the individual models for males alone were nonsignificant, the point estimates were of a similar direction and magnitude and borderline significant. In addition, the association between total lipids in the female partner and time to pregnancy was observed only in the individual models. In the couple-based models, a larger sample size would be needed to detect small effects on total lipids, given that the model is less parsimonious and precision is affected in nonlinear models. The association observed among male partners is biologically plausible, given that cholesterol is the main substrate for steroid synthesis, and also has been shown to play a crucial role in steroidogenesis and associated downstream effects including spermatogenesis (2). Moreover, there is ample evidence from animal studies linking cholesterolemia, steroidogenesis, and male fertility (54–57).
This study has several strengths. In particular, this study is the first prospective population-based TTP study with preconception enrollment of both partners. Both male and female concentrations were evaluated, enabling a couple-based approach for studying fecundity. This study was limited in that serum samples obtained were nonfasting, thus increasing the variability in measurement, particularly for triglycerides. However, the fasting status is not likely to be related to TTP, and therefore, any introduced bias would be nondifferential. There were also some possible unmeasured confounders because no assessment of dietary intake was available. However, sensitivity analyses showed that the associations we observed are unlikely to be explained by other dietary factors, such as fiber intake, and are robust in the presence of mild to moderate unmeasured confounding. Although a strong unmeasured confounding factor (twice the association observed with lipids and highly correlated with lipids, ρ = .6 or larger) could explain the association, a degree of confounding this extreme seems implausible, especially given that the correlation between the cholesterol levels in men and women was less than 0.2. However, this cannot be entirely ruled out. We have also limited our analyses to examine associations of single lipids on TTP and did not have measures of HDL or calculated or direct measurement of low-density lipoprotein.
In conclusion, our findings are the first to report that serum lipids may be associated with diminished couple fecundity and a longer TTP. The exact mechanisms remain elusive, but both male and female lipid concentrations were shown to be independent predictors of couple fecundity, after accounting for the role of body adiposity. These findings are of particular relevance, given the increased prevalence of obesity and dyslipidemia worldwide (58), coupled with evolving data suggesting temporal declines in human fecundity (59–61). In fact, the United States has recently launched an action plan for infertility (http://www.cdc.gov/reproductivehealth/infertility/publichealth.htm). Currently infertility in the United States may affect 15% and 17% of reproductive-aged men and women, respectively, underscoring the significance of lipids and fecundity at the population level (62, 63). If corroborated, our data suggest that earlier clinical and public health intervention may be warranted to ensure acceptable ranges of serum lipids in children, teenagers, and young adults to maximize their fecundity and minimize the later onset adult disease.
Acknowledgments
Serum lipids were measured through a Memo of Understanding with the Division of Laboratory Sciences, the Centers for Disease Control and Prevention, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Contributors to the study included the following: E.F.S., S.L.M., R.W.B., D.B.B., Z.C., and G.M.B.L. contributed substantially to the conception and design of the study and analysis and interpretation of the data. E.F.S. wrote the first draft of the manuscript, which was critically revised by S.L.M., R.W.B., D.B.B., Z.C., and G.M.B.L. All the authors had full access to all the data, take responsibility for the integrity of the data and the accuracy of the data analysis, and approved the final manuscript. E.F.S. is the guarantor of this work.
Ethical approval and human subjects' approval were received from all collaborating institutions. All participants provided written informed consent.
Data sharing included the following: statistical code is available from the corresponding author.
Other e-mail addresses include the following: Enrique F. Schisterman, PhD (schistee@mail.nih.gov); Sunni L. Mumford, PhD (mumfords@mail.nih.gov); Richard W. Browne, PhD (rwbrowne@buffalo.edu); Dana Boyd Barr, PhD (dbbarr@emory.edu); Zhen Chen, PhD (chenzhe@mail.nih.gov); and Germaine M. Buck Louis, PhD (louisg@mail.nih.gov).
This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development and National Institutes of Health Contracts N01-HD-3-3355, N01-HD-3-3356, and N01-HD-3-3358.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- body mass index
- CI
- confidence interval
- FOR
- fecundability odds ratio
- hCG
- human chorionic gonadotropin
- HDL
- high-density lipoprotein
- LIFE
- Longitudinal Investigation of Fertility and the Environment
- LXR
- liver X receptor
- TTP
- time to pregnancy.
References
- 1. Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011;32:81–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gwynne JT, Strauss JF., III The role of lipoproteins in steroidogenesis and cholesterol metabolism in steroidogenic glands. Endocr Rev. 1982;3:299–329 [DOI] [PubMed] [Google Scholar]
- 3. Cardozo E, Pavone ME, Hirshfeld-Cytron JE. Metabolic syndrome and oocyte quality. Trends Endocrinol Metab. 2011;22:103–109 [DOI] [PubMed] [Google Scholar]
- 4. Fujimoto VY, Kane JP, Ishida BY, Bloom MS, Browne RW. High-density lipoprotein metabolism and the human embryo. Hum Reprod Update. 2010;16:20–38 [DOI] [PubMed] [Google Scholar]
- 5. Grummer RR, Carroll DJ. A review of lipoprotein cholesterol metabolism: importance to ovarian function. J Anim Sci. 1988;66:3160–3173 [DOI] [PubMed] [Google Scholar]
- 6. Kaplan SA, Meehan AG, Shah A. The age related decrease in testosterone is significantly exacerbated in obese men with the metabolic syndrome. What are the implications for the relatively high incidence of erectile dysfunction observed in these men? J Urol. 2006;176:1524–1527 [DOI] [PubMed] [Google Scholar]
- 7. Kasturi SS, Tannir J, Brannigan RE. The metabolic syndrome and male infertility. J Androl. 2008;29:251–259 [DOI] [PubMed] [Google Scholar]
- 8. Lobaccaro JM, Gallot D, Lumbroso S, Mouzat K. Liver X receptors and female reproduction: when cholesterol meets fertility! J Endocrinol Invest. 2013;36:55–60 [DOI] [PubMed] [Google Scholar]
- 9. Maqdasy S, Baptissart M, Vega A, Baron S, Lobaccaro JM, Volle DH. Cholesterol and male fertility: what about orphans and adopted? Mol Cell Endocrinol. 2013;368:30–46 [DOI] [PubMed] [Google Scholar]
- 10. Ramirez-Torres MA, Carrera A, Zambrana M. [High incidence of hyperestrogenemia and dyslipidemia in a group of infertile men]. Ginecol Obstet Mex. 2000;68:224–229 [PubMed] [Google Scholar]
- 11. Miettinen HE, Rayburn H, Krieger M. Abnormal lipoprotein metabolism and reversible female infertility in HDL receptor (SR-BI)-deficient mice. J Clin Invest. 2001;108:1717–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mouzat K, Baron S, Marceau G, et al. Emerging roles for LXRs and LRH-1 in female reproduction. Mol Cell Endocrinol. 2013;368:47–58 [DOI] [PubMed] [Google Scholar]
- 13. Browne RW, Bloom MS, Shelly WB, Ocque AJ, Huddleston HG, Fujimoto VY. Follicular fluid high density lipoprotein-associated micronutrient levels are associated with embryo fragmentation during IVF. J Assist Reprod Genet. 2009;26:557–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ramlau-Hansen CH, Thulstrup AM, Nohr EA, Bonde JP, Sorensen TI, Olsen J. Subfecundity in overweight and obese couples. Hum Reprod. 2007;22:1634–1637 [DOI] [PubMed] [Google Scholar]
- 15. Wise LA, Rothman KJ, Mikkelsen EM, Sorensen HT, Riis A, Hatch EE. An internet-based prospective study of body size and time-to-pregnancy. Hum Reprod. 2010;25:253–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gesink L, Maclehose RF, Longnecker MP. Obesity and time to pregnancy. Hum Reprod. 2007;22:414–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wise LA, Palmer JR, Rosenberg L. Body size and time-to-pregnancy in black women. Hum Reprod. 2013;28(10):2856–2864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zaadstra BM, Seidell JC, Van Noord PA, et al. Fat and female fecundity: prospective study of effect of body fat distribution on conception rates. BMJ. 1993;306:484–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jensen TK, Scheike T, Keiding N, Schaumburg I, Grandjean P. Fecundability in relation to body mass and menstrual cycle patterns. Epidemiology. 1999;10:422–428 [DOI] [PubMed] [Google Scholar]
- 20. Bolumar F, Olsen J, Rebagliato M, Saez-Lloret I, Bisanti L. Body mass index and delayed conception: a European multicenter study on infertility and subfecundity. Am J Epidemiol. 2000;151:1072–1079 [DOI] [PubMed] [Google Scholar]
- 21. Maheshwari A, Stofberg L, Bhattacharya S. Effect of overweight and obesity on assisted reproductive technology—a systematic review. Hum Reprod Update. 2007;13:433–444 [DOI] [PubMed] [Google Scholar]
- 22. Colaci DS, Afeiche M, Gaskins AJ, et al. Men's body mass index in relation to embryo quality and clinical outcomes in couples undergoing in vitro fertilization. Fertil Steril. 2012;98:1193–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bakos HW, Henshaw RC, Mitchell M, Lane M. Paternal body mass index is associated with decreased blastocyst development and reduced live birth rates following assisted reproductive technology. Fertil Steril. 2011;95:1700–1704 [DOI] [PubMed] [Google Scholar]
- 24. Keltz J, Zapantis A, Jindal SK, Lieman HJ, Santoro N, Polotsky AJ. Overweight men: clinical pregnancy after ART is decreased in IVF but not in ICSI cycles. J Assist Reprod Genet. 2010;27:539–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Petersen GL, Schmidt L, Pinborg A, Kamper-Jorgensen M. The influence of female and male body mass index on live births after assisted reproductive technology treatment: a nationwide register-based cohort study. Fertil Steril. 2013;99:1654–1662 [DOI] [PubMed] [Google Scholar]
- 26. Pinborg A, Petersen GL, Schmidt L. Recent insights into the influence of female bodyweight on assisted reproductive technology outcomes. Womens Health (Lond Engl). 2013;9:1–4 [DOI] [PubMed] [Google Scholar]
- 27. Buck Louis GM, Schisterman EF, Sweeney AM, et al. Designing prospective cohort studies for assessing reproductive and developmental toxicity during sensitive windows of human reproduction and development—the LIFE Study. Paediatr Perinat Epidemiol. 2011;25:413–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Behre HM, Kuhlage J, Gassner C, et al. Prediction of ovulation by urinary hormone measurements with the home use ClearPlan Fertility Monitor: comparison with transvaginal ultrasound scans and serum hormone measurements. Hum Reprod. 2000;15:2478–2482 [DOI] [PubMed] [Google Scholar]
- 29. Takayama M, Itoh S, Nagasaki T, Tanimizu I. A new enzymatic method for determination of serum choline-containing phospholipids. Clin Chim Acta. 1977;79:93–98 [DOI] [PubMed] [Google Scholar]
- 30. Bernert JT, Jr, Turner WE, Pirkle JL, et al. Development and validation of sensitive method for determination of serum cotinine in smokers and nonsmokers by liquid chromatography/atmospheric pressure ionization tandem mass spectrometry. Clin Chem. 1997;43:2281–2291 [PubMed] [Google Scholar]
- 31. Pirkle JL, Bernert JT, Caudill SP, Sosnoff CS, Pechacek TF. Trends in the exposure of nonsmokers in the U.S. population to secondhand smoke: 1988–2002. Environ Health Perspect. 2006;114:853–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wall MA, Johnson J, Jacob P, Benowitz NL. Cotinine in the serum, saliva, and urine of nonsmokers, passive smokers, and active smokers. Am J Public Health. 1988;78:699–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cox DR. Regression models and life-tables. J R Stat Soc Series B (Methodological). 1972;34:187–220 [Google Scholar]
- 34. Dunson DB, Colombo B, Baird DD. Changes with age in the level and duration of fertility in the menstrual cycle. Hum Reprod. 2002;17:1399–1403 [DOI] [PubMed] [Google Scholar]
- 35. Nelson SM, Fleming R. Obesity and reproduction: impact and interventions. Curr Opin Obstet Gynecol. 2007;19:384–389 [DOI] [PubMed] [Google Scholar]
- 36. Gaskins AJ, Mumford SL, Zhang C, et al. Effect of daily fiber intake on reproductive function: the BioCycle Study. Am J Clin Nutr. 2009;90:1061–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mumford SL, Schisterman EF, Siega-Riz AM, Gaskins AJ, Wactawski-Wende J, VanderWeele TJ. Effect of dietary fiber intake on lipoprotein cholesterol levels independent of estradiol in healthy premenopausal women. Am J Epidemiol. 2011;173:145–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chavarro JE, Rich-Edwards JW, Rosner BA, Willett WC. Protein intake and ovulatory infertility. Am J Obstet Gynecol. 2008;198:210–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chavarro JE, Rich-Edwards JW, Rosner BA, Willett WC. Dietary fatty acid intakes and the risk of ovulatory infertility. Am J Clin Nutr. 2007;85:231–237 [DOI] [PubMed] [Google Scholar]
- 40. Chavarro JE, Rich-Edwards JW, Rosner BA, Willett WC. Iron intake and risk of ovulatory infertility. Obstet Gynecol. 2006;108:1145–1152 [DOI] [PubMed] [Google Scholar]
- 41. Joffe M. Time to pregnancy: a measure of reproductive function in either sex. Asclepios Project. Occup Environ Med. 1997;54:289–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sebastian SM, Selvaraj S, Aruldhas MM, Govindarajulu P. Pattern of neutral and phospholipids in the semen of normospermic, oligospermic and azoospermic men. J Reprod Fertil. 1987;79:373–378 [DOI] [PubMed] [Google Scholar]
- 43. Sartor BM, Dickey RP. Polycystic ovarian syndrome and the metabolic syndrome. Am J Med Sci. 2005;330:336–342 [DOI] [PubMed] [Google Scholar]
- 44. Vignesh JP, Mohan V. Polycystic ovary syndrome: a component of metabolic syndrome? J Postgrad Med. 2007;53:128–134 [DOI] [PubMed] [Google Scholar]
- 45. Leonard PJ, Shaper AG, Jones KW. Relationship between free and total cholesterol values in human serum. Am J Clin Nutr. 1965;17:377–380 [DOI] [PubMed] [Google Scholar]
- 46. Sperry WM. The relationship between total and free cholesterol in human blood serum. J Biol Chem. 1936;114:125 [Google Scholar]
- 47. Rigotti A, Trigatti BL, Penman M, Rayburn H, Herz J, Krieger M. A targeted mutation in the murine gene encoding the high density lipoprotein (HDL) receptor scavenger receptor class B type I reveals its key role in HDL metabolism. Proc Natl Acad Sci USA. 1997;94:12610–12615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Trigatti BL, Krieger M, Rigotti A. Influence of the HDL receptor SR-BI on lipoprotein metabolism and atherosclerosis. Arterioscler Thromb Vasc Biol. 2003;23:1732–1738 [DOI] [PubMed] [Google Scholar]
- 49. El-Hajjaji FZ, Oumeddour A, Pommier AJ, et al. Liver X receptors, lipids and their reproductive secrets in the male. Biochim Biophys Acta. 2011;1812:974–981 [DOI] [PubMed] [Google Scholar]
- 50. Wigglesworth K, Lee KB, O'Brien MJ, Peng J, Matzuk MM, Eppig JJ. Bidirectional communication between oocytes and ovarian follicular somatic cells is required for meiotic arrest of mammalian oocytes. Proc Natl Acad Sci USA. 2013;110:E3723–E3729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hu J, Zhang Z, Shen WJ, Azhar S. Cellular cholesterol delivery, intracellular processing and utilization for biosynthesis of steroid hormones. Nutr Metab (Lond). 2010;7:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Azhar S, Tsai L, Medicherla S, Chandrasekher Y, Giudice L, Reaven E. Human granulosa cells use high density lipoprotein cholesterol for steroidogenesis. J Clin Endocrinol Metab. 1998;83:983–991 [DOI] [PubMed] [Google Scholar]
- 53. Yetukuri L, Soderlund S, Koivuniemi A, et al. Composition and lipid spatial distribution of HDL particles in subjects with low and high HDL-cholesterol. J Lipid Res. 2010;51:2341–2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bataineh HN, Nusier MK. Effect of cholesterol diet on reproductive function in male albino rats. Saudi Med J. 2005;26:398–404 [PubMed] [Google Scholar]
- 55. Gupta RS, Dixit VP. Effect of dietary cholesterol on spermatogenesis. Z Ernahrungswiss. 1988;27:236–243 [DOI] [PubMed] [Google Scholar]
- 56. Purohit A, Daradka HM. Effect of mild hyperlipidaemia on testicular cell population dynamics in albino rats. Indian J Exp Biol. 1999;37:396–398 [PubMed] [Google Scholar]
- 57. Yamamoto Y, Shimamoto K, Sofikitis N, Miyagawa I. Effects of hypercholesterolaemia on Leydig and Sertoli cell secretory function and the overall sperm fertilizing capacity in the rabbit. Hum Reprod. 1999;14:1516–1521 [DOI] [PubMed] [Google Scholar]
- 58. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009–2010. NCHS Data Brief. 2012;1–8 [PubMed] [Google Scholar]
- 59. Joffe M. Time trends in biological fertility in Britain. Lancet. 2000;355:1961–1965 [DOI] [PubMed] [Google Scholar]
- 60. Joffe M, Key J, Best N, Keiding N, Jensen TK. Human fertility decline? Epidemiology. 2006;17:238–239 [DOI] [PubMed] [Google Scholar]
- 61. Sallmen M, Weinberg CR, Baird DD, Lindbohm ML, Wilcox AJ. Has human fertility declined over time? why we may never know. Epidemiology. 2005;16:494–499 [DOI] [PubMed] [Google Scholar]
- 62. Louis JF, Thoma ME, Sorensen DN, et al. The prevalence of couple infertility in the United States from a male perspective: evidence from a nationally representative sample. Andrology. 2013;1:741–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Thoma ME, McLain AC, Louis JF, et al. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril. 2013;99:1324–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]