Abstract
Context:
Puberty is marked by sleep-associated changes in LH pulse frequency and amplitude. Early pubertal girls with obesity exhibit blunted day-to-night changes in LH secretion; whether this occurs in late pubertal obese girls is unknown.
Objective:
The objective of the study was to test two hypotheses: 1) blunted day-to-night changes in LH secretion occur in both early and late pubertal obese girls, and 2) such alterations are specifically associated with hyperandrogenemia.
Design:
This was a cross-sectional analysis.
Setting:
The study was conducted at a clinical research center.
Patients or Other Participants:
Twenty-seven early pubertal, premenarcheal girls (12 of whom were obese) and 63 late pubertal (postmenarcheal) girls (27 of whom were obese) participated in the study.
Intervention:
Blood samples were taken every 10 minutes from 7:00 pm to 7:00 am.
Main Outcome Measure:
Change in LH pulse frequency [LH interpulse interval (IPI)] from daytime hours (7:00 pm-11:00 pm, while awake) to nighttime hours (11:00 pm to 7:00 am, while generally asleep).
Results:
Both nonobese and obese postmenarcheal girls demonstrated significant day-to-night decreases in LH pulse frequency (IPI increases of 33% and 16%, respectively), but day-to-night changes were blunted in obese girls (P = .004, obese vs nonobese). Day-to-night LH pulse frequency decreased significantly in postmenarcheal obese subjects with normal T concentrations (26% IPI increase) but not in those with hyperandrogenemia. Similar differences were evident for LH pulse amplitude. Nonobese and obese early pubertal girls exhibited nonsignificant differences in day-night LH pulse frequency (day to night IPI increase of 26% vs decrease of 1%, respectively).
Conclusions:
Day-to-night changes in LH pulse secretion are blunted in postmenarcheal obese adolescent girls. This phenomenon may in part reflect hyperandrogenemia.
Pubertal maturation in girls is characterized by evolving patterns of pulsatile GnRH secretion. Early puberty is marked by sleep-associated increases in LH (and by inference GnRH) pulse frequency and amplitude, with little LH release during the day (1–4). As puberty progresses, daytime LH pulse frequency gradually increases, whereas nocturnal frequency appears to remain relatively constant (2, 4). By late puberty, follicular phase LH pulse frequency decreases with sleep, whereas LH pulse amplitude continues to increase overnight (2, 4), similar to findings in adult women (5). Because fast and slow GnRH pulse frequencies differentially influence LH and FSH secretion, day-night differences in GnRH release may be critical for normal LH and FSH secretion across pubertal maturation and the menstrual cycle.
We and others have reported that early pubertal girls who are either overweight (6, 7) or obese (4) demonstrate blunted nocturnal increases in LH secretion. We have hypothesized that obesity and/or hyperandrogenemia (HA) may contribute to high LH pulse frequency with dampened day-night changes in both early and late puberty (8). However, previous studies have been inadequate to address this issue. We therefore performed an analysis to test the following hypotheses: 1) obesity is associated with blunted sleep-to-wake changes in LH pulse frequency and amplitude in both early and late pubertal girls, and 2) altered LH secretion in obese girls is specifically associated with HA.
Subjects and Methods
Subjects
Ninety adolescent girls [aged 7–17 y (mean ± SD, 13.9 ± 2.4 y), Tanner stages 1–5 (4.2 ± 1.2)] were studied at the University of Virginia (UVA) and the University of California, San Diego. Exclusion criteria included congenital adrenal hyperplasia, hyperprolactinemia, and hypothyroidism; we did not include or exclude subjects on the basis of obesity, hyperandrogenism, or menstrual status. With the exception of androgens and insulin, all laboratory values were within normal limits. Subjects were healthy; none was anorectic, overly athletic, sexually precocious, or taking drugs known to affect the reproductive axis within 90 days of study.
Baseline clinical and hormonal characteristics are shown in Table 1 and Table 2. Twenty-seven girls were early pubertal (premenarcheal) and 63 were late pubertal (postmenarcheal). Tanner 1 girls were included only if mean estradiol during frequent blood sampling was greater than 20 pg/mL (n = 5; estradiol 32 ± 12 pg/mL). Fifteen premenarcheal girls had neither obesity nor HA (defined below in Data and statistical analysis), five had obesity without HA, and seven had both obesity and HA. Twenty-seven nonobese postmenarcheal girls had neither HA nor oligomenorrhea (average intermenstrual length > 45 d in girls at least 2 y postmenarche), two had HA without oligomenorrhea, six had oligomenorrhea without HA, and one had both HA and oligomenorrhea. Seven obese postmenarcheal girls had neither HA nor oligomenorrhea, six had HA without oligomenorrhea, two had oligomenorrhea without HA, and 12 had both HA and oligomenorrhea. The 13 subjects with both oligomenorrhea and HA may fulfill the criteria for polycystic ovary syndrome (PCOS) according to recent guidelines (9). Data from 57 subjects were reported in an earlier analysis (4).
Table 1.
Characteristics of All Subjects Grouped by Menarcheal and Obesity Statuses
| Premenarcheal |
Postmenarcheal |
|||
|---|---|---|---|---|
| Nonobese (n = 15) | Obese (n = 12) | Nonobese (n = 36) | Obese (n = 27) | |
| Age, y | 11.4 ± 2.1 (12.1) | 11.5 ± 1.6 (11.5) | 14.7 ± 1.8 (14.4) | 15.4 ± 1.5 (15.8) |
| Gynecological age, y | Not applicable | Not applicable | 2.3 ± 1.5 (2) | 2.8 ± 1.8 (3) |
| Tanner stage | 2.7 ± 1.4 (2) | 3.0 ± 1.5 (3) | 4.6 ± 0.6 (5) | 4.9 ± 0.3 (5) |
| BMI z-score | 0.02 ± 1.0 (0.16) | 2.36 ± 0.3 (2.37)a | 0.72 ± 0.7 (0.98) | 2.25 ± 0.3 (2.3)a |
| Mean LH, IU/L | 3.0 ± 3.2 (2.5) | 2.4 ± 1.9 (2.8) | 6.1 ± 3.3 (5.1) | 6.2 ± 0.6 (5.9) |
| Mean FSH, IU/L | 3.0 ± 1.7 (3.0) | 2.5 ± 1.4 (2.5) | 4.4 ± 1.6 (4.0) | 4.0 ± 1.1 (3.8) |
| Total T, ng/dL | 12.6 ± 10.4 (6.9) | 37.7 ± 33.9 (30.4) | 22.0 ± 14.8 (17.9) | 46.3 ± 24.6 (40.7)a |
| SHBG, nmol/L | 57.3 ± 18.4 (51.4) | 17.9 ± 10.6 (14.4)a | 34.4 ± 15.0 (33.9) | 15.6 ± 7.2 (13.1)a |
| Free T, pg/mL | 1.8 ± 2.0 (0.9) | 11.2 ± 11.4 (7.6) | 4.1 ± 3.1 (2.9) | 12.6 ± 7.1 (12.3)a |
| Estradiol, pg/mL | 39.2 ± 14.3 (40.7) | 35.5 ± 20.6 (30.0) | 58.9 ± 28.9 (55.7) | 56.5 ± 23.9 (56.6) |
| Progesterone, ng/mL | 0.3 ± 0.1 (0.3) | 0.5 ± 0.3 (0.3) | 0.5 ± 0.2 (0.4) | 0.5 ± 0.2 (0.5) |
| DHEAS, μg/dL | 58.2 ± 57.9 (41.3) | 83.4 ± 56.1 (64.6) | 148.2 ± 88.2 (132.5) | 142.1 ± 72.6 (139.0) |
| Insulin, μIU/mL | 11.6 ± 9.4 (10.8) | 29.2 ± 17.1 (25.6)a | 17.1 ± 9.1 (16.3) | 27.6 ± 13.8 (22.8)a |
| IGF-I, ng/mL | 334 ± 114 (388) | 320 ± 126 (317) | 373 ± 105 (363) | 286 ± 145 (306) |
Abbreviations: DHEAS, dehydroepiandrosterone sulfate. Results are shown as mean ± SD (median). Each pairwise comparison (obese vs nonobese, HA obese vs non-HA obese) within pre- and postmenarcheal groups was performed using the Wilcoxon rank-sum test, a nonparametric test based on ranks of observations and requiring no assumptions about the underlying distribution of data. The method of normal approximation was used for pairwise comparisons involving at least 10 observations per group. To convert conventional units to SI units: total T (nanograms per deciliter) × 0.03467 (nanomoles per liter); free T (picograms per milliliter) × 3.467 (picomoles per liter); estradiol (picograms per milliliter) × 3.671 (picomoles per liter); progesterone (nanograms per milliliter) × 3.18 (picomoles per liter); DHEAS (micrograms per deciliter) × 27.211 (nanomoles per liter); insulin (microunits per milliliter) × 7.175 (picomoles per liter); IGF-I (nanograms per milliliter) × 0.1307 (nanomoles per liter).
P < .05 after Bonferroni correction for multiple comparisons.
Table 2.
Characteristics of Obese Subjects Only Grouped by Menarcheal and HA Statuses
| Premenarcheal |
Postmenarcheal |
|||
|---|---|---|---|---|
| Non-HA (n = 5) | HA (n = 7) | Non-HA (n = 9) | HA (n = 18) | |
| Age, y | 10.3 ± 1.0 (10.4) | 12.5 ± 1.2 (11.8)a | 15.0 ± 1.8 (15.6) | 15.6 ± 1.4 (15.8) |
| Gynecological age, y | Not applicable | Not applicable | 1.9 ± 1.2 (2) | 3.3 ± 1.9 (3) |
| Tanner stage | 2.2 ± 1.1 (2) | 3.6 ± 1.5 (3) | 4.8 ± 0.4 (5) | 4.9 ± 0.2 (5) |
| BMI z-score | 2.19 ± 0.3 (2.1) | 2.48 ± 0.3 (2.37) | 2.22 ± 0.2 (2.22) | 2.26 ± 0.3 (2.35) |
| Mean LH, IU/L | 0.5 ± 0.6 (0.2) | 3.7 ± 1.1 (3.2)a | 4.8 ± 2.7 (4.5) | 6.9 ± 3.0 (6.6) |
| Mean FSH, IU/L | 1.5 ± 0.8 (1.5) | 3.2 ± 1.2 (2.8) | 4.5 ± 1.5 (4.1) | 3.8 ± 0.8 (3.8) |
| Total T, ng/dL | 7.3 ± 4.1 (5.5) | 59.4 ± 27.8 (56.5)a | 21.8 ± 6.8 (21.1) | 58.5 ± 20.6 (52.3)a |
| SHBG, nmol/L | 24.8 ± 11.9 (23.8) | 13.0 ± 6.4 (12.6) | 20.7 ± 9.3 (19.2) | 13.0 ± 4.1 (12.7) |
| Free T, pg/mL | 1.7 ± 1.3 (1.3) | 17.9 ± 10.4 (18.2)a | 5.1 ± 1.5 (4.9) | 16.4 ± 5.6 (14.8)a |
| Estradiol, pg/mL | 26.6 ± 9.4 (23.3) | 41.8 ± 24.6 (31.5) | 43.4 ± 24.9 (29.6) | 63.1 ± 21.1 (61.7) |
| Progesterone, ng/mL | 0.3 ± 0.1 (0.3) | 0.6 ± 0.4 (0.6) | 0.4 ± 0.1 (0.4) | 0.6 ± 0.2 (0.6) |
| DHEAS, μg/dL | 52.2 ± 28.0 (61.0) | 105.7 ± 62.2 (95.9) | 130.1 ± 50.7 (131.5) | 148.1 ± 82.1 (141.3) |
| Insulin, μIU/mL | 20.4 ± 9.8 (21.0) | 36.5 ± 19.2 (30.9) | 33.3 ± 14.3 (34.4) | 24.8 ± 13.0 (22.1) |
| IGF-I, ng/mL | 277 ± 134 (244) | 351 ± 121 (333) | 369 ± 86 (347) | 249 ± 152 (225) |
Abbreviations: DHEAS, dehydroepiandrosterone sulfate. Results are shown as mean ± SD (median). Each pairwise comparison (obese vs nonobese, HA obese vs non-HA obese) within pre- and postmenarcheal groups was performed using the Wilcoxon rank-sum test, a nonparametric test based on ranks of observations and requiring no assumptions about the underlying distribution of data. Since pairwise comparisons involved fewer than 10 observations per group, exact Wilcoxon tests were used. To convert conventional units to SI units: total T (nanograms per deciliter) × 0.03467 (nanomoles per liter); free T (picograms per milliliter) × 3.467 (picomoles per liter); estradiol (picograms per milliliter) × 3.671 (picomoles per liter); progesterone (nanograms per milliliter) × 3.18 (picomoles per liter); DHEAS (micrograms per deciliter) × 27.211 (nanomoles per liter); insulin (microunits per milliliter) × 7.175 (picomoles per liter), IGF-I (nanograms per milliliter) × 0.1307 (nanomoles per liter).
P < .05 after Bonferroni correction for multiple comparisons.
Study procedures
Study procedures were approved by the Institutional Review Boards at UVA and the University of California, San Diego. Informed assent and consent were obtained from subjects and custodial parents, respectively. As previously described (4), each volunteer underwent a detailed medical history, physical examination (including pubertal staging using the Tanner scale for breast development), and fasting laboratory evaluation (including 17-hydroxyprogesterone, TSH, and prolactin) to ensure general health and the absence of unexpected hormonal parameters.
Subjects with regular menses were admitted to the Clinical Research Center between cycle days 8 and 10. Subjects with irregular menses were admitted between cycle days 8 and 10 or 60 or more days after menses. A forearm iv catheter was placed, and hemoglobin and β-human chorionic gonadotropin were obtained to exclude anemia and pregnancy, respectively. Progesterone concentrations were less than 1.5 ng/mL in all subjects at the time of the study.
Blood samples were obtained from 7:00 pm to 7:00 am to allow assessments of LH every 10 minutes; FSH every hour; and estradiol, progesterone, and T every 2 hours. Subjects were offered meals at standard mealtimes. Lights were extinguished at 11:00 pm to facilitate sleep, which was recorded by trained observers. Eighteen subjects (14 postmenarcheal, 11 obese) wore actigraphs (Motionlogger Basic-L; Ambulatory Monitoring, Inc) on their nondominant wrists. This watch-like device records movement and allows estimation of sleep periods via validated data analysis protocols (10). Fasting samples were drawn at 7:00 am for SHBG, dehydroepiandrosterone sulfate, IGF-I, and insulin.
Hormonal measurements
Samples were analyzed at the UVA Center for Research in Reproduction Ligand Core Laboratory. Samples from an individual were analyzed in duplicate in the same assay for each hormone. Manufacturer, assay sensitivity, and intra- and interassay coefficients of variation (CVs) for all hormone measurements have been reported previously (4). Briefly, LH was measured by chemiluminescence (sensitivity, 0.1 IU/L; intraassay CV < 4%; interassay CV < 7%). Total T was measured by RIA (sensitivity 10 ng/dL; intraassay CV < 5%; interassay CV < 8%), and SHBG was measured by chemiluminescence (sensitivity 0.2 nmol/L; intraassay CV < 3%; interassay CV < 5%). Free T was calculated using total T and SHBG (11).
Data and statistical analysis
Menarcheal status was used to assign girls into early and late pubertal groups. Exact body mass index (BMI)-for-age percentiles and BMI z-scores were calculated as previously described (12); obesity was defined as BMI-for-age percentile of 95 kg/m2 or greater. Hyperandrogenemia was defined by a morning free T exceeding the maximum value observed in our cohort of normal weight (BMI-for-age percentile < 85 kg/m2) girls of the same Tanner stage with neither clinical hyperandrogenism (eg, hirsutism) nor oligomenorrhea (defined above) (13). Specific cutoffs (picograms per milliliter) were 1.4 (Tanner 1), 3.2 (Tanner 2), 4.3 (Tanner 3), 8.6 (Tanner 4), and 9.0 (Tanner 5), based on data in 12, nine, nine, nine, and 14 girls, respectively.
LH pulses were identified using the computerized pulse detection algorithm Cluster 7 (14) using previously described methods (4, 15). LH interpulse intervals (IPIs) were calculated as previously described (16) and used as the primary measure of LH pulse frequency. Compared with pulse counts, IPIs may provide more precise estimates of LH pulse frequency within discrete and relatively short time blocks. The average IPI during the 7:00–11:00 pm time block (while awake) was designated daytime IPI; the average IPI during the 11:00 pm to 7:00 am time block, when subjects were primarily asleep, was designated nighttime IPI. Lower IPI values represent higher LH pulse frequencies and vice versa. Thus, day-to-night decreases in LH IPI denote day-to-night increases in LH pulse frequency, whereas increases in LH IPI indicate decreases in LH pulse frequency.
Primary statistical analyses
The primary outcome variable was day-to-night change in LH IPI (nighttime IPI minus daytime IPI). Data were analyzed via two ANOVA procedures. One ANOVA assessed the effects of menarcheal status (pre- vs postmenarche) and obesity status (nonobese vs obese) on day-to-night changes in LH IPI. We hypothesized that such changes are blunted in both pre- and postmenarcheal girls with obesity. A second ANOVA was conducted in obese girls to assess the effects of menarcheal status and HA status (non-HA vs HA) on day-to-night changes in LH IPI. We hypothesized that HA predicts blunted day-night changes in LH IPI. (We were unable to perform a three factorial ANOVA because we did not recruit any nonobese premenarcheal subjects with HA.) To satisfy the normality assumption of ANOVA, LH IPI data were log transformed prior to analysis; thus, within-group and between-group comparisons of day-night IPI changes specifically relate to these log transformed data. Day-to-night changes in LH IPI are reported as percentage changes in geometric mean (GM) LH IPI with Bonferroni-adjusted lower and upper 95% confidence limits. (The GM is a location parameter similar to arithmetic mean, computed as the antilog of the mean of log transformed values).
In the setting of the ANOVA tests, tests for equal means at baseline (daytime values) were conducted using the Welch test, a version of the Student's t test that remains valid when the homogenous variance assumption is deemed to be violated. Subject characteristics shown in Table 1 were compared using the Wilcoxon rank-sum test, a nonparametric test based on ranks of observations and requiring no assumptions about the underlying distribution of data.
For all tests, a two-sided value of P ≤ .05 was used as the null hypothesis rejection rule. Correction for multiple comparisons was performed using the highly rigorous Bonferroni method, and only corrected P values are reported. All statistical analyses were performed using SAS version 9.2 (SAS Institute Inc).
Secondary analyses
ANOVA was used to assess the effects of menarcheal and obesity statuses on day-to-night changes in the average LH pulse amplitude (pulse peak minus preceding trough). We hypothesized that such changes are blunted in girls with obesity. A second ANOVA in obese girls assessed the effects of menarcheal and HA statuses on day-night LH pulse amplitude changes. Log transformation was not required to satisfy the assumptions of ANOVA, so results are expressed as mean differences (nighttime minus daytime values) with Bonferroni-adjusted 95% confidence limits.
Multiple linear regression was performed to assess predictors of daytime (7:00–11:00 pm) LH IPI (model 1) and nighttime (11:00 pm to 7:00 am) LH IPI (model 2). Predictor variables included age, menarcheal status, BMI z-score, and free T concentration. Regression models were estimated via ordinary least squares, and restricted cubic-spline functions of the predictor variables were used to assess nonlinear trends. Conventional F tests were used to test for predictor variable vs outcome variable association.
As described in Supplemental Materials, additional analyses were performed to assess (a) the effects of menarcheal and obesity statuses on day-night changes in mean LH and FSH concentrations, and (b) the time since most recent menses as a possible predictor of day-night LH pulse frequency changes among postmenarcheal subjects.
Results
Primary analyses
Day-to-night changes in LH pulse frequency as a function of menarcheal and obesity statuses
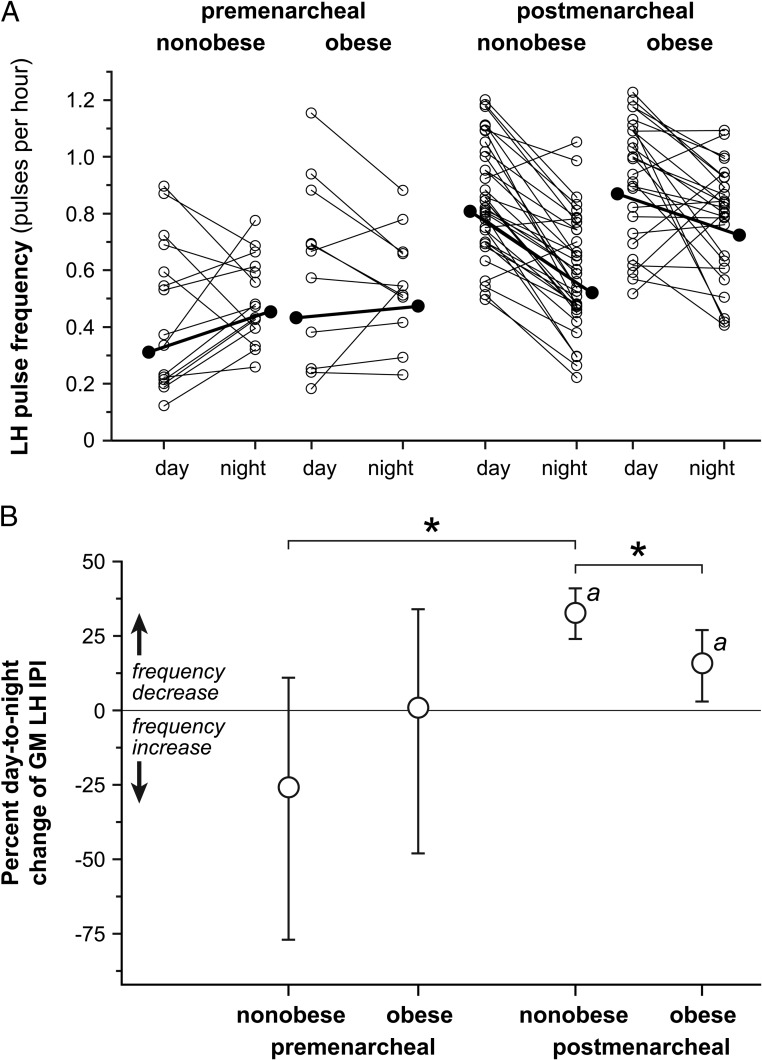
Day-to-night changes in LH pulse frequency are shown in Figure 1. In premenarcheal subjects, obese and nonobese subgroups had similar daytime (baseline) LH pulse frequency. Nonobese premenarcheal girls exhibited a nonsignificant overnight increase in LH pulse frequency [IPI change −26% (95% confidence limit −77, +11)], whereas obese premenarcheal girls demonstrated no day-night change [IPI change +1% (−48, +34)]. The difference between nonobese and obese groups was not significant.
Figure 1.
Day-to-night changes in LH pulse frequency in peripubertal girls partitioned by menarcheal and obesity statuses. A, Daytime (7:00–11:00 pm) and nighttime (11:00 pm to 7:00 am) LH pulse frequency. For clarity of presentation, LH pulse frequency is expressed as the number of pulses per hour, derived from the IPI as follows: 1/IPI × 60. Values derived from individual subjects are shown as open circles, and values derived from the GM are shown as solid circles. B, Day-to-night changes in LH pulse frequency, expressed as the percentage change in GM IPI with Bonferroni-adjusted 95% confidence limits. Importantly, day-to-night increases in the GM IPI represent decreases in pulse frequency and vice versa. a, Bonferroni-corrected P < .05 for day-to-night change within a group; *, Bonferroni-corrected P < .05 for comparison of day-to-night changes between groups.
Nonobase and obese postmenarcheal girls had similar daytime LH frequencies, and both demonstrated significant overnight decreases [IPI change, nonobese: +33% (+24, +41), P < .001; IPI change, obese: +16% (+3, +27), P = .014]. However, nonobese girls had a more pronounced day-to-night decrease in LH pulse frequency compared with obese girls (P = .016).
When performed, wrist actigraphy suggested adequate sleep. Sleep duration (time from first to last sleep) was 7.7 ± 1.0 hours (mean ± SD), and sleep efficiency was 0.87 ± 0.11.
Day-to-night changes in LH pulse frequency in obese girls partitioned by menarcheal and hyperandrogenemia statuses
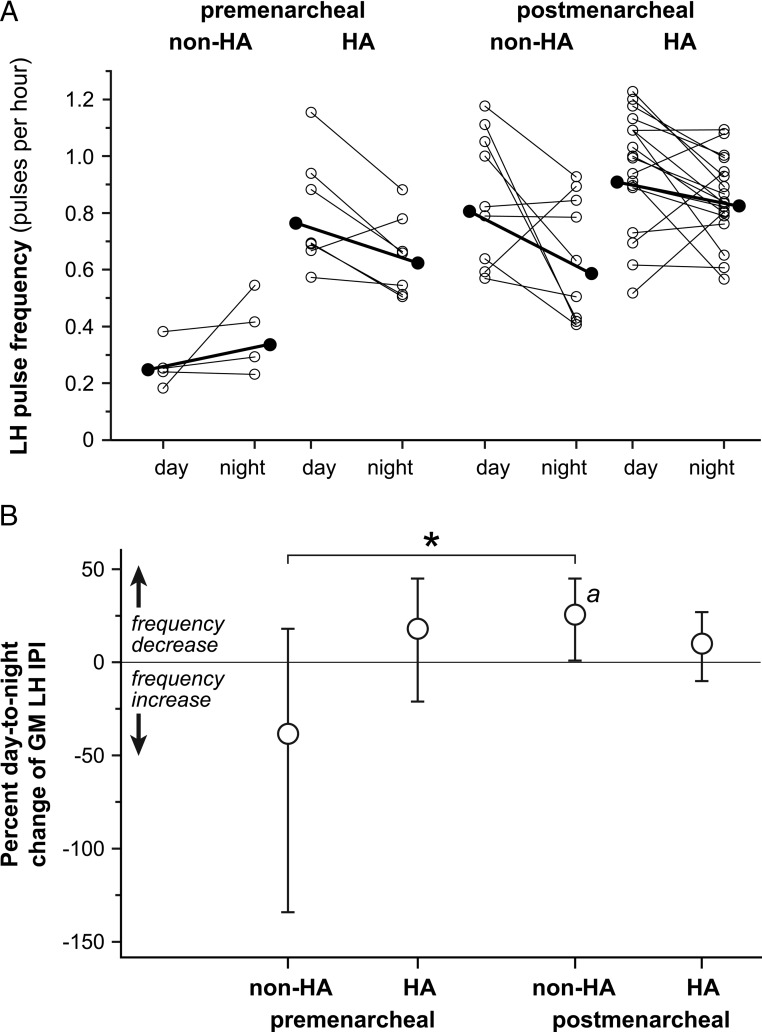
Premenarcheal obese girls with and without HA exhibited a nonsignificant day-to-night decrease and increase, respectively, in LH pulse frequency [IPI change: +18% (−21, +45) vs −38% (−234, +18), respectively] (Figure 2). However, premenarcheal obese girls with HA had significantly higher daytime (ie, baseline) LH pulse frequency compared with their non-HA counterparts (P < .001 by Welch test). In addition, these subjects were older and had higher mean LH levels (Table 1), suggesting unequal developmental maturity.
Figure 2.
Day-to-night changes in LH pulse frequency in obese peripubertal girls partitioned by menarcheal and hyperandrogenemia statuses. A, Daytime (7:00–11:00 pm) and nighttime (11:00 pm to 7:00 am) LH pulse frequency, with data expressed as described in the legend for Figure 1. B, Percentage day-to-night changes in GM IPI with Bonferroni-adjusted 95% confidence limits. Day-to-night increases and decreases in GM IPI represent pulse frequency decreases and increases, respectively. a, Bonferroni-corrected P < .05 for day-to-night change within a group; *, Bonferroni-corrected P < .05 for comparison of day-to-night changes between groups.
Postmenarcheal obese girls with and without HA had similar daytime LH pulse frequency. Obese girls without HA exhibited significant day-to-night decreases in LH pulse frequency [IPI change: +26% (+1, +45); P = .039], whereas obese girls with HA did not [IPI change: +10% (−10, +27)]. The between-group difference was not statistically significant. Time since most recent menses correlated with HA status and therefore represents a potential confounder in these particular analyses (Supplemental Materials).
Secondary analyses
Day-to-night changes in LH pulse amplitude
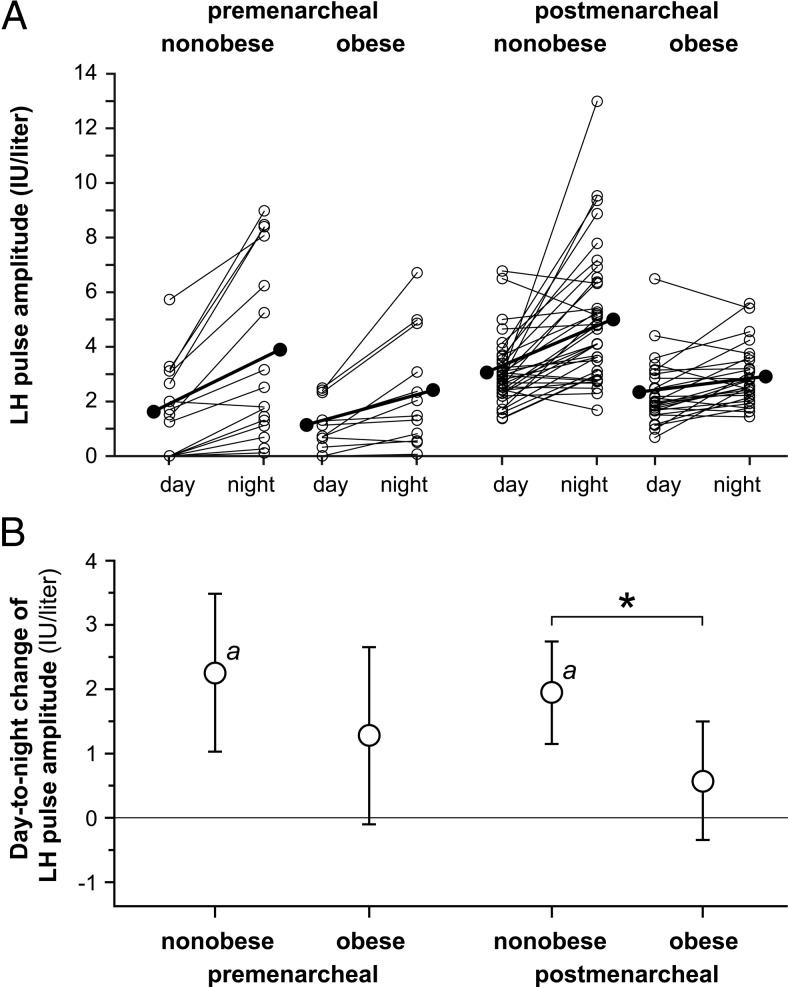
Nonobese premenarcheal girls exhibited a significant overnight increase in mean LH pulse amplitude [+2.26 IU/L (+1.03, +3.49); P < .001] (Figure 3). Obese counterparts exhibited a nonsignificant overnight increase [+1.28 IU/L (−0.10, +2.66)], but the between-group difference was not statistically significant. In postmenarcheal subjects, nonobese girls had a significant overnight increase in mean LH pulse amplitude [+1.95 IU/L (+1.15, +2.74), P < .001], but obese girls did not [+0.58 IU/L (−0.34, +1.50)]. The between-group difference was statistically significant (P = .021).
Figure 3.
Day-to-night changes in LH pulse amplitude in peripubertal girls partitioned by menarcheal and obesity statuses. A, Daytime and nighttime LH pulse amplitude, with individual subject data shown as open circles and means shown as solid circles. B, Absolute day-to-night change in LH pulse amplitude with Bonferroni-adjusted 95% confidence limits. a, Bonferroni-corrected P < .05 for day-to-night change within a group; *, Bonferroni-corrected P < .05 for comparison of day-to-night changes between groups.
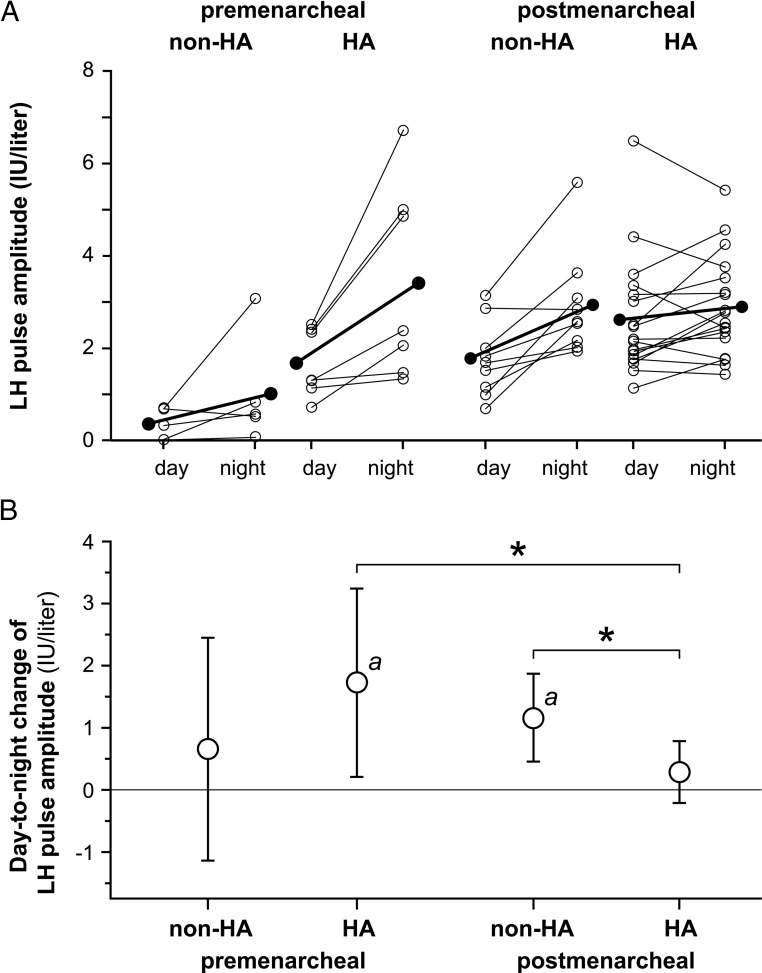
Obese premenarcheal girls without HA did not exhibit a significant overnight increase in mean LH pulse amplitude [+0.66 IU/L (−1.13, +2.45)], but those with HA did [+1.73 IU/L (+0.21, +3.24), P = .024] (Figure 4). The between-group difference was not significant. As described above, these groups exhibited evidence of unequal developmental maturity; this includes higher daytime (ie, baseline) LH pulse amplitude in the HA group (P = .015 by Welch test). Postmenarcheal obese girls without HA had a significant overnight increase in mean LH pulse amplitude [+1.16 IU/L (+0.46, +1.87), P = .001], but those with HA did not [+0.29 IU/L (−0.21, +0.79)]. The between-group difference was statistically significant (P = .046).
Figure 4.
Day-to-night changes in LH pulse amplitude in obese peripubertal girls partitioned by menarcheal and hyperandrogenemia statuses. A, Daytime and nighttime LH pulse amplitude, with individual subject data shown as open circles and means shown as solid circles. B, Absolute day-to-night change in LH pulse amplitude with Bonferroni-adjusted 95% confidence limits. a, Bonferroni-corrected P < .05 for day-to-night change within a group; *, Bonferroni-corrected P < .05 for comparison of day-to-night changes between groups.
Predictors of daytime and nighttime LH pulse frequency
Multiple regression model 1 predicted greater than 60% of observed variability in daytime LH IPI (P < .001; adjusted R2 = 0.63). Lower daytime IPI (ie, higher daytime LH frequency) was significantly and uniquely associated with postmenarcheal status (partial R2 = 0.093; P = .023), increased age (partial R2 = 0.051; P = .023), and higher free T concentrations (partial R2 = 0.046; P = .034). BMI z-score was not a significant independent predictor of daytime LH IPI.
Multiple regression model 2 was significant (P = .002), but it explained relatively little of the observed variability in nighttime LH IPI (adjusted R2 = 0.22). None of the independent variables was a significant independent predictor of nighttime LH IPI.
Discussion
Earlier reports described blunted day-to-night changes in LH secretion in early pubertal girls who were either overweight (6, 7) or obese (4). This analysis extends these observations to late pubertal obese girls. In particular, nocturnal decreases in LH pulse frequency and increases in LH pulse amplitude were blunted in postmenarcheal obese girls. These data also suggest that such alterations in postmenarcheal obese girls are related to HA.
Although previous reports suggested blunted nocturnal changes in LH secretion in overweight premenarcheal subjects (6, 7), such differences did not reach statistical significance in our current analysis. Nocturnal increases in LH pulse frequency diminish across puberty, with day-night equalization circa midpuberty and nocturnal slowing thereafter (4); we believe that heterogeneity of neuroendocrine maturity within pubertal but premenarcheal groups may have lessened our ability to resolve differences. In our prior studies, subjects were grouped by Tanner stages, which may have improved within-group homogeneity. In the present work, an a priori decision was made to designate pubertal status (early vs late) on the basis of menarcheal status, primarily because this is an objective (albeit historical) parameter.
The mechanisms underlying sleep-wake changes in LH pulse frequency, and changes thereof across puberty, remain unclear. We have recently proposed a working model in which progesterone-negative feedback contributes to low daytime GnRH pulse frequency in early puberty, with sleep-associated pulse frequency being relatively unaffected by progesterone (8, 17). By this model, daytime inhibition by progesterone is gradually dampened across puberty in tandem with the physiological increase in androgens, which can antagonize progesterone-negative feedback (18, 19). If this model is correct, one might expect girls entering neuroendocrine puberty in the setting of HA to exhibit high daytime LH pulse frequency. This would be consistent with data suggesting that the transition from sleep-predominant to wake-predominant LH concentrations occurs approximately 2 years early in hyperandrogenemic girls (20). In the current analysis, the comparison between premenarcheal obese girls with and without HA was hampered by apparent differences in developmental maturity (eg, nonhyperandrogenemic girls were younger and exhibited lower LH concentrations).
Obese postmenarcheal girls with and without HA were of comparable developmental stages, making comparisons more reliable. Obese postmenarcheal girls without HA exhibited significant day-to-night decreases in LH frequency, similar in degree to those in nonobese postmenarcheal girls. However, obese girls with HA did not demonstrate a significant nocturnal decrease in LH pulse frequency, despite having twice as many subjects as the non-HA group. These data suggest that blunted day-to-night decreases in LH pulse frequency in obese girls are related to HA.
Other potential reasons for blunted overnight changes in LH pulse frequency in postmenarcheal obese girls are unclear. Supplemental analyses suggest that time since most recent menses may predict nocturnal LH pulse frequency slowing in postmenarcheal girls, although it does not appear to be a better predictor than HA status. Altered sleep architecture is also a possible contributor to blunted overnight changes in LH pulse frequency (21). Available studies of pubertal children suggest that LH pulses are usually initiated during nonrapid eye movement (REM) sleep (1), slow-wave sleep in particular (21). However, in women studied during the early follicular phase, LH pulses were uncommon during slow-wave and REM sleep but instead tended to follow brief wake episodes (5). Thus, fragmented sleep (frequent awakenings) may contribute to the elevated nighttime LH pulse frequency observed in older adolescents with HA, obesity, or both (4, 20, 22–24). In this regard, de Sousa et al (25) reported that late pubertal obese girls with PCOS had lower sleep efficiency compared with both age-matched obese girls without PCOS and normal-weight controls and a lower percentage of REM sleep compared with normal-weight girls. Because formal polysomnography was not performed in our study, the potential influence of undetected sleep disruption in our postmenarcheal obese girls with HA remains unclear.
Postmenarcheal obese girls demonstrated blunted nocturnal increases in LH pulse amplitude, a phenomenon that was more prominent in those with HA. This finding may partly reflect blunted nocturnal slowing of LH pulse frequency because LH amplitude is inversely associated with the preceding interpulse interval (26). Blunted day-night changes in LH amplitude may also partly relate to an inverse relationship between adiposity (eg, BMI) and LH pulse amplitude and mean LH, as described in obese women with and without PCOS (27–30). Mechanisms underlying this phenomenon in PCOS include reduced pituitary responses to GnRH (29) and enhanced metabolic clearance of endogenous LH (31). However, such studies have not disclosed obesity-associated alterations in LH pulse frequency, consistent with the notion that altered LH pulse frequency reflects HA more so than obesity per se.
Our previous data suggest that daytime and nighttime LH pulse frequencies change differentially across puberty, with daytime frequency increasing and nighttime frequency remaining relatively stable (4). In keeping with these observations, multiple regression analysis predicted a majority (>60%) of observed variability in daytime LH pulse frequency, with a number of variables (eg, menarcheal status, age, free T) independently predicting daytime LH pulse frequency. In contrast, the regression model for nighttime LH pulse frequency was a relatively poor predictor of observed variability, and no single variable independently predicted nighttime LH pulse frequency. A recent study suggested that daytime but not nighttime LH pulse frequency is acutely altered by progesterone administration (17), and we have previously suggested that, in contrast to waking GnRH pulse frequency, GnRH pulse frequency during sleep is relatively resistant to sex steroid feedback (8, 17). The current analysis is consistent with this notion.
The physiological relevance of nocturnal changes in LH pulse frequency remains uncertain. Higher and lower GnRH pulse frequencies favor LH and FSH secretion, respectively (32–36). Increasing GnRH pulse frequency in early puberty is important for increased LH synthesis/release and hence increased ovarian sex steroid production. However, episodic reductions in GnRH pulse frequency may be important for maintenance of early pubertal FSH synthesis and follicular development. Alternating episodes of high and low GnRH pulse frequency may allow proper production of both gonadotropins prior to the development of monthly cycles. Sleep-related slowing of GnRH pulse frequency has been similarly hypothesized to be important in adult women (5). Supporting this concept, FSH concentrations are highest in the early follicular phase, when sleep-related reductions in LH pulse frequency are most prominent. Also, urinary FSH concentrations correlate with sleep duration in normally cycling women (37).
Importantly, some of our analyses were likely hampered by relatively low statistical power, especially given our very conservative approach to statistical analysis. To our knowledge, though, this is the largest available study that addresses relationships among day-night LH secretion, obesity, and HA in adolescent girls. We also recognize a paucity of normative data to define peripubertal HA. Although we could have used normative data provided by reference laboratories, we believed it best to use normative data specific to our assay system.
In conclusion, day-to-night changes in LH pulse secretion are blunted in postmenarcheal obese adolescent girls. This phenomenon may be in part related to hyperandrogenemia. We suggest that blunted nocturnal slowing of GnRH pulse frequency contributes to increased LH and impaired FSH production, which in turn augments ovarian androgen production and limits follicular development, thus contributing to a PCOS phenotype.
Acknowledgments
We gratefully acknowledge the nurses and staff of the Clinical Research Units at the University of Virginia and University of California, San Diego, for implementation of these sampling protocols and the Center for Research in Reproduction Ligand Core Laboratory for the performance of all assays.
This work was supported by National Institutes of Health Grant R01 HD058671 (to C.R.M.); the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health through Cooperative Agreements U54 HD28934 (to C.B.S., J.C.M., C.R.M.) and U54 HD12303 (to R.J.C.) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research; National Institutes of Health Grant F32 HD066855 (to J.S.C.); National Institutes of Health Grant T32 DK007646 (to J.P.B.); and General Clinical Research Center Grants M01 RR00847 (to the University of Virginia) and M01 RR00827 (to the University of California, San Diego).
Disclosure Summary: J.S.C., J.P.B., C.B.S., J.T.P., R.J.C., and C.R.M. have nothing to declare. J.C.M. is a consultant for AstraZeneca.
Footnotes
- BMI
- body mass index
- CV
- coefficient of variation
- GM
- geometric mean
- HA
- hyperandrogenemia
- IPI
- interpulse interval
- PCOS
- polycystic ovary syndrome
- REM
- rapid eye movement
- UVA
- University of Virginia.
References
- 1. Boyar R, Finkelstein J, Roffwarg H, Kapen S, Weitzman E, Hellman L. Synchronization of augmented luteinizing hormone secretion with sleep during puberty. N Engl J Med. 1972;287:582–586 [DOI] [PubMed] [Google Scholar]
- 2. Apter D, Butzow TL, Laughlin GA, Yen SS. Gonadotropin-releasing hormone pulse generator activity during pubertal transition in girls: pulsatile and diurnal patterns of circulating gonadotropins. J Clin Endocrinol Metab. 1993;76:940–949 [DOI] [PubMed] [Google Scholar]
- 3. Cemeroglu AP, Foster CM, Warner R, Kletter GB, Marshall JC, Kelch RP. Comparison of the neuroendocrine control of pubertal maturation in girls and boys with spontaneous puberty and in hypogonadal girls. J Clin Endocrinol Metab. 1996;81:4352–4357 [DOI] [PubMed] [Google Scholar]
- 4. McCartney CR, Prendergast KA, Blank SK, Helm KD, Chhabra S, Marshall JC. Maturation of luteinizing hormone (gonadotropin-releasing hormone) secretion across puberty: evidence for altered regulation in obese peripubertal girls. J Clin Endocrinol Metab. 2009;94:56–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hall JE, Sullivan JP, Richardson GS. Brief wake episodes modulate sleep-inhibited luteinizing hormone secretion in the early follicular phase. J Clin Endocrinol Metab. 2005;90:2050–2055 [DOI] [PubMed] [Google Scholar]
- 6. Bordini B, Littlejohn E, Rosenfield RL. Blunted sleep-related luteinizing hormone rise in healthy premenarcheal pubertal girls with elevated body mass index. J Clin Endocrinol Metab. 2009;94:1168–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rosenfield RL, Bordini B, Yu C. Comparison of detection of normal puberty in girls by a hormonal sleep test and a gonadotropin-releasing hormone agonist test. J Clin Endocrinol Metab. 2013;98:1591–1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McCartney CR. Maturation of sleep-wake gonadotrophin-releasing hormone secretion across puberty in girls: potential mechanisms and relevance to the pathogenesis of polycystic ovary syndrome. J Neuroendocrinology. 2010;22:701–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565–4592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sadeh A, Acebo C. The role of actigraphy in sleep medicine. Sleep Med Rev. 2002;6:113–124 [DOI] [PubMed] [Google Scholar]
- 11. Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–3672 [DOI] [PubMed] [Google Scholar]
- 12. McCartney CR, Prendergast KA, Chhabra S, et al. The association of obesity and hyperandrogenemia during the pubertal transition in girls: obesity as a potential factor in the genesis of postpubertal hyperandrogenism. J Clin Endocrinol Metab. 2006;91:1714–1722 [DOI] [PubMed] [Google Scholar]
- 13. McCartney CR, Blank SK, Prendergast KA, et al. Obesity and sex steroid changes across puberty: evidence for marked hyperandrogenemia in pre- and early pubertal obese girls. J Clin Endocrinol Metab. 2007;92:430–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Veldhuis JD, Johnson ML. Cluster analysis: a simple, versatile, and robust algorithm for endocrine pulse detection. Am J Physiol. 1986;250:E486–E493 [DOI] [PubMed] [Google Scholar]
- 15. McCartney CR, Gingrich MB, Hu Y, Evans WS, Marshall JC. Hypothalamic regulation of cyclic ovulation: evidence that the increase in gonadotropin-releasing hormone pulse frequency during the follicular phase reflects the gradual loss of the restraining effects of progesterone. J Clin Endocrinol Metab. 2002;87:2194–2200 [DOI] [PubMed] [Google Scholar]
- 16. McCartney CR, Blank SK, Marshall JC. Progesterone acutely increases LH pulse amplitude but does not acutely influence nocturnal LH pulse frequency slowing during the late follicular phase in women. Am J Physiol Endocrinol Metab. 2007;292:E900–E906 [DOI] [PubMed] [Google Scholar]
- 17. Collins JS, Marshall JC, McCartney CR. Differential sleep-wake sensitivity of gonadotropin-releasing hormone secretion to progesterone inhibition in early pubertal girls. Neuroendocrinology. 2012;96:222–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eagleson CA, Gingrich MB, Pastor CL, et al. Polycystic ovarian syndrome: evidence that flutamide restores sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab. 2000;85:4047–4052 [DOI] [PubMed] [Google Scholar]
- 19. Pielecka J, Quaynor SD, Moenter SM. Androgens increase gonadotropin-releasing hormone neuron firing activity in females and interfere with progesterone negative feedback. Endocrinology. 2006;147:1474–1479 [DOI] [PubMed] [Google Scholar]
- 20. Apter D, Butzow T, Laughlin GA, Yen SS. Accelerated 24-hour luteinizing hormone pulsatile activity in adolescent girls with ovarian hyperandrogenism: relevance to the developmental phase of polycystic ovarian syndrome. J Clin Endocrinol Metab. 1994;79:119–125 [DOI] [PubMed] [Google Scholar]
- 21. Shaw ND, Butler JP, McKinney SM, Nelson SA, Ellenbogen JM, Hall JE. Insights into puberty: the relationship between sleep stages and pulsatile LH secretion. J Clin Endocrinol Metab. 2012;97:E2055–E2062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Garcia-Rudaz MC, Ropelato MG, Escobar ME, Veldhuis JD, Barontini M. Augmented frequency and mass of LH discharged per burst are accompanied by marked disorderliness of LH secretion in adolescents with polycystic ovary syndrome. Eur J Endocrinol. 1998;139:621–630 [DOI] [PubMed] [Google Scholar]
- 23. Yoo RY, Dewan A, Basu R, Newfield R, Gottschalk M, Chang RJ. Increased luteinizing hormone pulse frequency in obese oligomenorrheic girls with no evidence of hyperandrogenism. Fertil Steril. 2006;85:1049–1056 [DOI] [PubMed] [Google Scholar]
- 24. Blank SK, McCartney CR, Chhabra S, et al. Modulation of gonadotropin-releasing hormone pulse generator sensitivity to progesterone inhibition in hyperandrogenic adolescent girls—implications for regulation of pubertal maturation. J Clin Endocrinol Metab. 2009;94:2360–2366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. de Sousa G, Schluter B, Buschatz D, et al. A comparison of polysomnographic variables between obese adolescents with polycystic ovarian syndrome and healthy, normal-weight and obese adolescents. Sleep Breathing. 2010;14:33–38 [DOI] [PubMed] [Google Scholar]
- 26. O'Dea LS, Finkelstein JS, Schoenfeld DA, Butler JP, Crowley WF., Jr Interpulse interval of GnRH stimulation independently modulates LH secretion. Am J Physiol. 1989;256:E510–E515 [DOI] [PubMed] [Google Scholar]
- 27. Taylor AE, McCourt B, Martin KA, et al. Determinants of abnormal gonadotropin secretion in clinically defined women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1997;82:2248–2256 [DOI] [PubMed] [Google Scholar]
- 28. Arroyo A, Laughlin GA, Morales AJ, Yen SS. Inappropriate gonadotropin secretion in polycystic ovary syndrome: influence of adiposity. J Clin Endocrinol Metab. 1997;82:3728–3733 [DOI] [PubMed] [Google Scholar]
- 29. Pagan YL, Srouji SS, Jimenez Y, Emerson A, Gill S, Hall JE. Inverse relationship between luteinizing hormone and body mass index in polycystic ovarian syndrome: investigation of hypothalamic and pituitary contributions. J Clin Endocrinol Metab. 2006;91:1309–1316 [DOI] [PubMed] [Google Scholar]
- 30. Jain A, Polotsky AJ, Rochester D, et al. Pulsatile luteinizing hormone amplitude and progesterone metabolite excretion are reduced in obese women. J Clin Endocrinol Metab. 2007;92:2468–2473 [DOI] [PubMed] [Google Scholar]
- 31. Srouji SS, Pagan YL, D'Amato F, et al. Pharmacokinetic factors contribute to the inverse relationship between luteinizing hormone and body mass index in polycystic ovarian syndrome. J Clin Endocrinol Metab. 2007;92:1347–1352 [DOI] [PubMed] [Google Scholar]
- 32. Burger LL, Haisenleder DJ, Dalkin AC, Marshall JC. Regulation of gonadotropin subunit gene transcription. J Mol Endocrinol. 2004;33:559–584 [DOI] [PubMed] [Google Scholar]
- 33. Wildt L, Hausler A, Marshall G, et al. Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology. 1981;109:376–385 [DOI] [PubMed] [Google Scholar]
- 34. Clarke IJ, Cummins JT, Findlay JK, Burman KJ, Doughton BW. Effects on plasma luteinizing hormone and follicle-stimulating hormone of varying the frequency and amplitude of gonadotropin-releasing hormone pulses in ovariectomized ewes with hypothalamo-pituitary disconnection. Neuroendocrinology. 1984;39:214–221 [DOI] [PubMed] [Google Scholar]
- 35. Gross KM, Matsumoto AM, Bremner WJ. Differential control of luteinizing hormone and follicle-stimulating hormone secretion by luteinizing hormone-releasing hormone pulse frequency in man. J Clin Endocrinol Metab. 1987;64:675–680 [DOI] [PubMed] [Google Scholar]
- 36. Spratt DI, Finkelstein JS, Butler JP, Badger TM, Crowley WF., Jr Effects of increasing the frequency of low doses of gonadotropin-releasing hormone (GnRH) on gonadotropin secretion in GnRH-deficient men. J Clin Endocrinol Metab. 1987;64:1179–1186 [DOI] [PubMed] [Google Scholar]
- 37. Touzet S, Rabilloud M, Boehringer H, Barranco E, Ecochard R. Relationship between sleep and secretion of gonadotropin and ovarian hormones in women with normal cycles. Fertil Steril. 2002;77:738–744 [DOI] [PubMed] [Google Scholar]