Abstract
Context:
Insufficient sleep is associated with increased cardiometabolic risk. Alterations in hypothalamic-pituitary-adrenal axis may underlie this link.
Objective:
Our objective was to examine the impact of restricted sleep on daytime profiles of ACTH and cortisol concentrations.
Methods:
Thirteen subjects participated in 2 laboratory sessions (2 nights of 10 hours in bed versus 2 nights of 4 hours in bed) in a randomized crossover design. Sleep was polygraphically recorded. After the second night of each session, blood was sampled at 20-minute intervals from 9:00 am to midnight to measure ACTH and total cortisol. Saliva was collected every 20 minutes from 2:00 pm to midnight to measure free cortisol. Perceived stress, hunger, and appetite were assessed at hourly intervals by validated scales.
Results:
Sleep restriction was associated with a 19% increase in overall ACTH levels (P < .03) that was correlated with the individual amount of sleep loss (rSp = 0.63, P < .02). Overall total cortisol levels were also elevated (+21%; P = .10). Pulse frequency was unchanged for both ACTH and cortisol. Morning levels of ACTH were higher after sleep restriction (P < .04) without concomitant elevation of cortisol. In contrast, evening ACTH levels were unchanged while total and free cortisol increased by, respectively, 30% (P < .03) and 200% (P < .04). Thus, the amplitude of the circadian cortisol decline was dampened by sleep restriction (−21%; P < .05). Sleep restriction was not associated with higher perceived stress but resulted in an increase in appetite that was correlated with the increase in total cortisol.
Conclusion:
The impact of sleep loss on hypothalamic-pituitary-adrenal activity is dependent on time of day. Insufficient sleep dampens the circadian rhythm of cortisol, a major internal synchronizer of central and peripheral clocks.
Insufficient sleep duration, due to voluntary sleep restriction, insomnia, or shift work, is increasingly common in industrialized countries (1, 2). In addition to the adverse effects on alertness, performance, and safety (1, 2), epidemiological and experimental evidence indicates that recurrent sleep loss may adversely affect human health. Habitual short sleep, usually 6 hours or less, has been linked in epidemiological studies to increased mortality and increased prevalence and incidence of numerous pathological conditions including obesity, diabetes, cardiovascular diseases, vulnerability to infection, depression, and anxiety (3–10). The mechanisms by which sleep loss promotes these conditions remain unclear (3–10). Because the hypothalamic-pituitary-adrenal (HPA) axis regulates a wide range of physiologic processes and is affected by experimental sleep restriction, it is a putative key mediator of the adverse health effects of insufficient sleep (3–11).
Multiple studies have observed that sleep loss, either from acute partial or total sleep deprivation or from recurrent sleep curtailment or insomnia, results in elevated evening cortisol levels (12–19). The role of ACTH levels in this evening elevation of cortisol secretion has not been elucidated. A complete assessment of the HPA axis requires the measurement of both ACTH and cortisol levels at frequent intervals across the day. Indeed, not only the levels of cortisol but also its variation across the day play a role for health and disease (20, 21). Two studies have assessed ACTH and cortisol at different times of day in sleep-deprived subjects, but they have yielded inconsistent results. One study found increased evening cortisol levels in the face of unchanged ACTH levels after 5 nights of 4 hours in bed in comparison with 2 nights of 10 hours in bed (19). The other examined the impact of 2 nights of 4 hours in bed vs 2 nights of 8 hours in bed and found no differences in either ACTH or cortisol (22). To elucidate the impact of insufficient sleep on HPA activity, the present study used a randomized cross-over design to compare daytime and pulsatile variations of ACTH, total cortisol and free cortisol in healthy young subjects after 2 nights of 4 hours vs 10h in bed.
Subjects and Methods
Participants
Volunteers were 13 healthy young men (age, 21 [20; 23] years; body mass index, 24.6 [21; 25] kg/m2) who took no medication and did not smoke. They all had regular nocturnal bedtimes of 7 to 9 hours. Subjects with insomnia complaints or having traveled across time zones less than 4 weeks before the study were excluded.
Experimental protocol
The protocol was approved by the University of Chicago Institutional Review Board, and all participants gave written informed consent. As shown in Figure 1, the subjects participated in 2 laboratory sessions in randomized order. These sessions were conducted in the Clinical Research Center and spaced at least 6 weeks apart. During the week preceding each session, the subjects were asked to conform to fixed bedtimes (11:00 pm to 7:00 am). They were asked not to deviate from this schedule by more than 30 minutes. Naps were not allowed. Wrist activity was monitored continuously to verify compliance. We obtained valid actigraphic recordings for at least 6 prestudy nights in 11 of the 13 subjects. Median time in bed (TIB) was 8 hours 19 minutes (8 hours 2 minutes; 8 hours 35 minutes) and median total sleep time (TST) was 7 hours 13 minutes (6 hours 49 minutes; 7 hours 27 minutes). Prestudy TIB and TST did not differ between the 2 experimental conditions (P = .16 and P = .42, respectively). One experimental session involved 2 nights of 10 hours in bed (10:00 pm to 8:00 am; rested sleep condition), and the other involved 2 nights of 4 hours in bed (1:00–5:00 am; short sleep condition). Sleep was polygraphically recorded during each night. For each of the 2 sessions, the first overnight stay began at 7:00 pm with a standard hospital dinner and the subjects were discharged the next morning after breakfast, which was served at 8:00 am, to attend to their habitual activities. They were readmitted in the early evening and, after receiving a standard hospital dinner at 7:00 pm, they remained on bed rest until midnight the next day. We examined whether the subjects had taken naps while out of the Clinical Research Center by analyzing actigraphic recordings: only 4 naps, lasting 121 minutes in total, were detected in the 4-hours-in-bed condition in a single subject. After the second night and for both sleep conditions, bedtimes were at midnight, blood and salivary samples were obtained at 20-minute intervals from 9:00 am to midnight and from 2:00 pm to midnight, respectively, while meals were replaced by constant glucose infusion at a rate of 5 g/kg/24 hours. The subjects remained recumbent throughout the sampling procedure. Blood samples were obtained through a sterile heparin-lock catheter that was kept patent with a slow drip of heparinized saline. Sampling started 1 hour after catheter insertion to avoid observing the effect of the venipuncture stress on ACTH and cortisol levels. Measures of perceived stress were obtained at hourly intervals during blood sampling using the tense and calm subscales of the Visual Analog Scales for Global Vigor and Affect (23) and the 5-point nervous subscale of the Positive Affect and Negative Affect Scale (24). Scores of hunger and appetite were obtained at hourly intervals using validated scales (25, 26). Light intensity at eye level was below 50 lux.
Figure 1.
Schematic representation of the experimental protocol. Subjects participated in 2 experimental sessions presented in randomized order: one session involved 2 nights of 10 hours in bed (dark gray bars), and the other session involved 2 nights of 4 hours in bed (light gray bars). Sleep was polygraphically recorded during each night. After the second night of each experimental session, bedtimes were at midnight, blood and salivary samples were obtained at 20-minute intervals from 9:00 am to midnight and from 2:00 pm to midnight, respectively, while meals were replaced by constant glucose infusion at a rate of 5 g/kg/24 hours and measures of perceived stress, hunger, and appetite were obtained at hourly intervals.
The morning profiles of glucose and insulin concentrations and the daytime profiles of appetite scores, hunger scores, and leptin and ghrelin concentrations in both sleep conditions have been previously reported (27, 28).
Sleep recording
Sleep was polygraphically recorded using a digital electroencephalogram acquisition system (Digitrace recording system; SleepMed Inc). The recordings were scored at 30-second intervals in stages wake; N1, N2, and N3; and rapid eye movement (REM) according to standard criteria (29). Sleep onset and final awakening were defined as the time corresponding to the first and last 30-second interval scored N2, N3, or REM. The following variables were determined: TIB (ie, time interval separating lights off from lights on), sleep period (SP, ie, time interval separating sleep onset from morning awakening), TST (ie, sleep period − duration of intrasleep wake periods), sleep efficiency (ie, TST/TIB × 100), sleep maintenance (ie, TST/sleep period × 100), duration of REM sleep, duration of non-REM sleep (ie, stages N1 + N2 + N3), duration of light non-REM sleep (ie, stages N1 + N2), duration of slow-wave sleep (SWS) (ie, stage N3), and duration of wake after sleep onset (WASO).
Assays
Blood drawn from the indwelling catheter was collected in prechilled EDTA tubes for ACTH and in serum separator tubes for cortisol. EDTA tubes were immediately centrifuged at 4°C. Serum separator tubes were allowed to clot at ambient temperature and were then centrifuged at 4°C within 30 minutes. Plasma and serum aliquots were frozen at −20°C until assay. Saliva samples were collected using Salivettes (Sarstedt Inc). After being kept in a refrigerator until the end of the sampling period (<11 hours), they were centrifuged and frozen at −20°C, until assay. Plasma ACTH and serum cortisol were measured by immunochemiluminometric assay (Immulite; Siemens). The limit of sensitivity of the ACTH assay was 1 pg/mL, and the coefficient of intra-assay variation was ≤9.6%. The limit of sensitivity of the cortisol assay was 1.0 μg/dL, and the coefficient of intra-assay variation was 5% on average (21). Free cortisol in saliva was measured by RIA (Coat-A-Count; Diagnostic Products Corporation) following the manufacturer's directions. The limit of sensitivity of the assay was 0.025 μg/dL, and the coefficient of intra-assay variation averaged 4%. For each hormone, all samples from the same subject were measured in the same assay.
Analyses of ACTH and cortisol profiles
For plasma ACTH and serum cortisol, we calculated the areas under the curve (AUC) over the entire sampling period (overall AUC, 9:00 am to midnight), the morning (morning AUC, 9:00 am to 1:00 pm) and the evening (evening AUC, 8:00 pm to midnight). For salivary cortisol, we calculated the AUC over the entire sampling period (2:00 pm to midnight) and the evening AUC (8:00 pm to midnight). The rate of decline of hormonal levels across the day was calculated as the slope of the linear regression of concentrations vs time between 9:00 am and midnight for serum cortisol and plasma ACTH and between 2:00 pm and midnight for salivary cortisol. Significant pulses of individual plasma ACTH, serum cortisol, and salivary cortisol profiles were identified and characterized using the ULTRA algorithm included in the Chronobiological series analyzer software (CSA; http://www.ibridgenetwork.org/uctech/chronobiological-series-analyzer-csa). A pulse was considered significant if both its increment and its decline exceeded, in relative terms, twice the intra-assay coefficient of variation.
Statistical analysis
All group values are expressed as median (quartile 1; quartile 3). Analyses were performed using the R software (http://www.r-project.org). We compared variables obtained in the 2 laboratory sessions using the Wilcoxon exact rank test. In addition, to take advantage of the repeated measures of hormonal levels across the day, we performed mixed-effects ANOVA on serial ACTH and serum cortisol data with condition (4 hours in bed or 10 hours in bed), time of day, and the interaction condition × time of day as factors. Correlations between changes in HPA variables and changes in sleep variables during the preceding night were examined using the Spearman coefficient. Statistical significance is assumed at P values < .05.
Results
Sleep quantity and quality
Table 1 summarizes the sleep variables during the 2 nights of each experimental session. The difference in TST between the 2 sleep conditions averaged 5 hours 16 minutes (−5 hours 27 minutes; −4 hours 49 minutes) (P < .0003). Sleep curtailment was achieved by a 3-hour 28-minute (3-hour 3-minute; 3-hour 56-minute) decrease in the lighter stages of non-REM sleep (stages N1 + N2, P < .0003) and a 1-hour 35-minute [1-hour 28-minute; 1-hour 50-minute) decrease in REM sleep (P < .0003). In contrast, the time spent in deep non-REM sleep, ie, SWS, was not enhanced by sleep restriction. As expected, variables quantifying sleep consolidation were all higher after bedtime restriction. Sleep efficiency and maintenance were enhanced (+5% [1%; 8%], P < .06; and +3% [1%; 6%], P < .03), the time spent in WASO was decreased (−24 [−32; −12] minutes, P < .003) and sleep latency was reduced (−14 [−29; −11] minutes, P < .002).
Table 1.
Mean Sleep Characteristics of the 2 Nights of the 2 Experimental Sessionsa
| 10 h in Bed | 4 h in Bed | P Valueb | |
|---|---|---|---|
| TST, min | 536 (514; 546) | 226 (225; 230) | .0002 |
| Sleep latency, min | 24 (22; 37) | 12 (8; 15) | .001 |
| Sleep efficiency, % | 89 (86; 91) | 94 (92; 95) | .06 |
| Sleep maintenance, % | 94 (94; 97) | 99 (98; 99) | .02 |
| Time spent in sleep stages, min | |||
| WASO | 28 (14; 33) | 3 (2; 4) | .0002 |
| Light non-REM | 329 (278; 358) | 118 (69; 146) | .0002 |
| SWS | 82 (33; 90) | 65 (43; 120) | .72 |
| REM | 138 (118; 155) | 35 (30; 48) | .0002 |
Light non-REM indicates the lighter stages of non-REM sleep (ie, stages N1 + N2). SWS is the deepest stage of non-REM sleep (ie, stage N3).
P values are from the Wilcoxon exact rank test.
ACTH and cortisol profiles
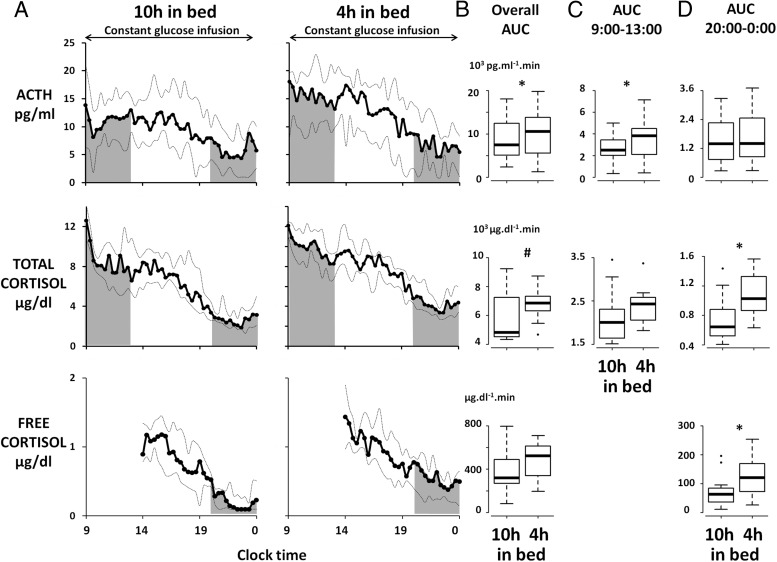
Figure 2A shows the plasma ACTH, serum total cortisol, and salivary free cortisol profiles in the 10-hours-in-bed and in the 4-hours-in-bed conditions. In comparison with the rested condition, overall daytime ACTH levels during short sleep were 19% (10%; 24%) higher (AUC 9:00 am to midnight, 10 551 [5575; 13 821] vs 7481 [5104; 12418] pg/mL/min, P < .03), and there was a trend for a similar increase (ie, +21% [−6%; +41%]) in total cortisol (6844 [6308; 7324] vs 4826 [4519; 7240] μg/dL/min, P = .10) (Figure 2B). Although the rate of decline of serum or salivary cortisol was unaffected by the sleep condition (P = .84 and P = .64, respectively), the rate of ACTH decline across the day tended to be faster (−0.6 [−0.9; −0.4] vs −0.4 [−0.7; −0.3] pg/mL/h, P < .06) in the short sleep condition. Mixed-effects ANOVA confirmed these findings; short sleep was associated with increased overall ACTH (F = 5.6, P < .04) and with a trend for increased overall serum cortisol (F = 3.0, P = .11), and a condition × time of day interaction was found for plasma ACTH (F = 1.4, P < .05) but not for serum cortisol (F = 0.9, P = .67). Subsequent analyses therefore sought to determine whether the impact of sleep loss on ACTH and cortisol levels varied according to time of day. The shaded areas in Figure 2A illustrate the morning (9:00 am to 1:00 pm) and evening (8:00 pm to midnight) AUC of plasma ACTH and serum total and salivary free cortisol in the 2 sleep conditions. Sleep restriction was associated with a 10% (+3%; +45%) increase in morning ACTH levels (3828 [2094; 4491] vs 2504 [2008; 3444] pg/mL/min, P < .04) (Figure 2C), without significant elevation of morning cortisol levels (2429 [2059; 2580] vs 2006 [1642; 2310] μg/dL/min, P = .27) (Figure 2C). In contrast, in the evening, sleep loss was associated with increased levels of total cortisol (1026 [865; 1326] vs 642 [522; 880] μg/dL/min, P < .03) (Figure 2D) and salivary cortisol (120 [73; 170] vs 63 [36; 85] μg/dL/min, P < .04), whereas ACTH levels were unaffected (1396 [854; 2458] vs 1389 [734; 2256] pg/mL/min, P = .22) (Figure 2D). As a consequence, the amplitude of the circadian decline of total cortisol levels across the daytime, estimated as the ratio (morning mean levels/evening mean levels), was decreased by 21% (−45%; −4%) when the subjects were sleep-restricted (P < .05), whereas this estimate of circadian amplitude was unchanged for ACTH.
Figure 2.
A, Plasma ACTH, serum total cortisol, and salivary free cortisol profiles after 2 nights of 10 hours in bed (left panels) and after 2 nights of 4 hours in bed (right panels). The solid lines represent the median, the dotted lines represent quartiles 1 and 3. B, AUC of plasma ACTH, serum total cortisol, and salivary free cortisol for the entire sampling period (overall AUC, 9:00 am to midnight for plasma ACTH and serum total cortisol and 2:00 pm to midnight for salivary free cortisol). C, AUC of plasma ACTH and serum total cortisol between 9:00 am and 1:00 pm (morning AUC). D, AUC of plasma ACTH, serum total cortisol, and salivary free cortisol between 8:00 pm and midnight (evening AUC). *, P < .05; #, P < .10.
Table 2 shows the results of the pulse analysis. Over the entire sampling period, the number of ACTH pulses as well as the number, mean trough, and peak and absolute increment of pulses of total and free cortisol were unaffected by the sleep condition. The trough and peak values of the ACTH pulses were enhanced in the sleep restriction condition as compared with the rested condition (+17% [−12%; +49%], P < .05; and +21% [−0.3%; +33%], P < .05, respectively). It is possible that the 20-minute sampling frequency used in our study was insufficient to detect changes in ACTH pulse frequency (30).
Table 2.
Pulse Characteristics of Plasma ACTH, Serum Total Cortisol, and Salivary Free Cortisol and During the Overall Sampling Perioda
| Overall Daytime Period |
P Valueb | ||
|---|---|---|---|
| 10 h in Bed | 4 h in Bed | ||
| Plasma ACTH | |||
| Number of peaks | 8 (5; 9) | 9 (5; 9) | .86 |
| Trough value, pg/mL | 5 (3; 8) | 10 (4; 13) | .04 |
| Peak value, pg/mL | 11 (9; 17) | 15 (9; 21) | .05 |
| Absolute increment, pg/mL | 7 (4; 7) | 7 (6; 8) | .64 |
| Serum total cortisol | |||
| Number of peaks | 6 (5; 7) | 7 (6; 8) | .23 |
| Trough value, μg/dL | 5 (4; 7) | 6 (5; 7) | .31 |
| Peak value, μg/dL | 7 (7; 11) | 9 (9; 10) | .22 |
| Increment, μg/dL | 3 (2; 3) | 3 (3; 4) | .24 |
| Salivary free cortisol | |||
| Number of peaks | 5 (3; 7) | 5 (4; 6) | .74 |
| Trough value, μg/dL | 0.6 (0.4; 0.8) | 0.6 (0.4; 0.8) | .97 |
| Peak value, μg/dL | 1.0 (0.7; 1.2) | 0.9 (0.7; 1.1) | .83 |
| Increment, μg/dL | 0.3 (0.2; 0.5) | 0.3 (0.2; 0.4) | .58 |
Overall sampling period was from 9:00 am to midnight for ACTH and total cortisol and from 2:00 pm to midnight for free cortisol, in the 10-hours-in-bed and 4-hours-in-bed conditions. Data are expressed as median (quartile 1; quartile 3).
P values are from the Wilcoxon exact rank test.
Correlations between hormones of the HPA axis, sleep variables, and hunger and appetite
We sought to determine whether the subjects who had the largest difference in TST between the 2 sleep conditions also experienced the largest changes in the HPA axis. The reduction in TST from the 10-hours-in-bed to the 4-hours-in-bed condition was correlated with the increase in the overall daytime levels of ACTH (rSp = 0.64, P < .02) but not of total (rSp = −0.27, P = .36) or free cortisol (rSp = −0.20, P = .52). We also explored potential associations between the activation of the HPA axis and the previously reported elevation in hunger and appetite scores (short sleep vs rested condition; hunger, +19% [14%; 45%], P < .01; and appetite, +16% [9%; 25%], P < .02) (28). The increases in hunger and appetite correlated with the increase in total serum cortisol levels during the same time period (hunger, rSp = 0.60, P < .06; appetite, rSp = 0.67, P < .04) but not with the increase of plasma ACTH (hunger, rSp = 0.10, P = .92; appetite, rSp = 0.09, P = .77).
Measures of perceived stress
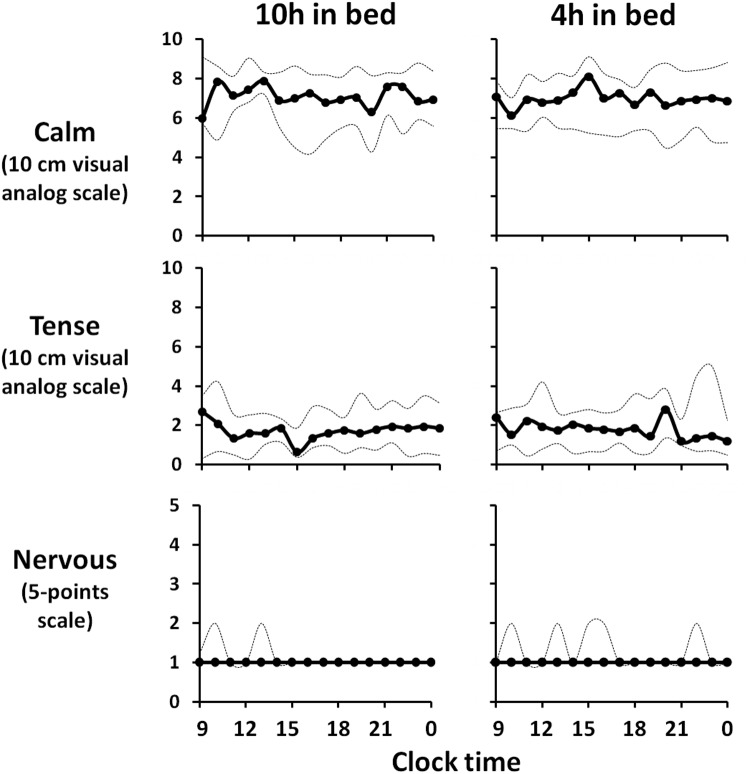
Figure 3 illustrates the profiles of hourly scores of self-perceived stress in the 2 experimental sessions. The overall mean scores for calm, tense and nervous were similar in both sleep conditions (7 [5; 8] vs 7 [6; 8], P = .42; 2 [1; 3] vs 2 [1;3], P = .15; and 1 [1; 1] vs 1 [1; 1[, P = .34, respectively). Similar results were obtained when we analyzed scores obtained in the morning (9:00 am to 1:00 pm, 7 [6; 8] vs 7 [6; 8], P = .27, 2 [1; 3] vs 2 [1; 3], P = .27; and 1 [1; 2] vs 1 [1; 2], P = .50) or in the evening (8:00 pm to midnight; P > .34).
Figure 3.
Hourly scores on the 10-cm visual analog scales for calm and tense and the 5-point scale for nervous after 2 nights of 10 hours in bed (left panels) and after 2 nights of 4 hours in bed (right panels). The solid lines represent the median and the dotted lines represent quartiles 1 and 3. There were no significant effects of bedtime duration on any of these 3 markers of self-perceived stress measures.
Discussion
The present study examined the effects of restricted sleep, as compared with a rested condition, on the spontaneous activity of the HPA axis in healthy subjects under controlled laboratory conditions. By simultaneously assessing the levels of ACTH, total cortisol, and free cortisol at frequent intervals during the daytime period, this study provides, to the best of our knowledge, the most complete assessment of human HPA functioning during experimental sleep loss so far. We found that 2 nights of 4 hours in bed were associated with spontaneous HPA axis hyperactivity, because overall daytime levels of ACTH were increased by 19% and total cortisol tended to increase by 21%. Sleep loss therefore acts as a mild physiological, but not psychological, stressor, because measures of perceived stress remained unaffected by sleep loss. In addition to this overall mild hyperactivity, we found that sleep loss differently affected ACTH and cortisol levels according to the time of day, suggesting that sleep loss alters the circadian modulation of the sensitivity of the adrenals to ACTH. The resulting daily variations of cortisol are characterized by an increase in evening total (+30%) and free cortisol levels (+200%). These elevated cortisol levels at a time when cortisol is usually low are likely to adversely affect the numerous physiological processes modulated by the final product of the HPA axis. Importantly, this elevation of evening cortisol levels after sleep restriction resulted in a more than 20% dampening of the daytime variation of this major internal synchronizing signal of the peripheral circadian clock.
An important and novel result of our study is the increased overall daytime ACTH levels in a state of sleep loss. The only 2 studies that previously assessed ACTH at regular intervals during a prolonged period of time in humans yielded negative results. Blood levels of ACTH and cortisol were measured hourly from 8:00 am to 11:00 pm in the first study (22) and at 9:00 am and then every 2 hours from 10:00 am to 8:00 pm in the second study (19). Although the first study reported no change for both cortisol and ACTH levels when the subjects were allowed 2 nights of 4 hours in bed or 2 nights of 8 hours in bed (22), the second study reported increased cortisol levels, but unchanged ACTH levels, after 5 nights of 4 hours in bed in comparison with 2 nights of 10 hours in bed (19). The fact that these studies sampled ACTH 3 to 6 times less frequently than our study is a likely explanation of the contradictory results (19, 22). Moreover, the elevated ACTH levels reported here during sleep loss in healthy men are in line with rodent studies that showed increased ACTH after total sleep deprivation or recurrent partial sleep restriction (31).
The mechanisms by which sleep loss activates ACTH release are likely to involve increased brain CRH levels rather than increased pituitary sensitivity to CRH, because rodents studies have reported enhanced brain synthesis and release of CRH (32–34) but also reduced CRH binding in the pituitary (32) after acute and chronic sleep restriction. This stimulation of CRH release when wakefulness is extended could be mediated by upregulation of neuropeptides involved in the regulation of both energy homeostasis and HPA axis activity (35). Among these, the orexinergic system is of particular interest in the context of this study. Orexins are synthesized by neurons in the lateral hypothalamus and stimulate arousal and feeding (36). The orexin system has been shown to be overactive when sleep deprivation is behaviorally enforced (36). In animals, intracerebroventricular injection of orexins activates the HPA axis by increasing CRH release in the paraventricular nucleus of the hypothalamus (35). Interestingly, we showed that the increase in overall cortisol after restricted sleep correlated with the increases in hunger and appetite. Taken together, the data suggest that an upregulation of the systems linking arousal and energy homeostasis regulation is likely to be an important contributor of the activation of the HPA axis after sleep restriction. Consistent with our previous study of 6 nights of sleep restriction (15), we found that levels of perceived stress in the present study remained low and were not affected by sleep loss. This finding suggests that the enhanced ACTH levels observed after restricted sleep in our healthy young participants is more likely to be the consequence of a physiological stress rather than of a psychological stress, possibly because in the laboratory environment, the only challenge that our participants had to meet was to comply with the protocol. Under real-life conditions, it is quite possible that sleep restriction may involve an increased psychological response to the daily life stressors.
Another new finding of the present study is that the impact of sleep loss on ACTH and cortisol levels varied according to the time of day; ACTH was increased in the morning in the face of unchanged cortisol levels, whereas total and free cortisol were increased in the evening in the face of unchanged ACTH levels. These results suggest that adrenal sensitivity to ACTH may be reduced in the morning and, as previously suggested (19), enhanced in the evening in a state of sleep loss. Although we and others have hypothesized that the elevated evening cortisol levels may reflect altered HPA axis recovery from the circadian-driven morning stimulation (12, 13, 15), the present findings suggest that changes in the circadian modulation of the sensitivity of the adrenals to ACTH may also be involved (37). As a consequence, although the amplitude of the circadian decline of ACTH levels across the daytime was unaffected by short-term sleep loss, the circadian decline of total cortisol was decreased by 21%. The increase in evening cortisol levels indicates that insufficient sleep may have an impact on the multitude of physiological processes modulated by circulating glucocorticoids (11). As such, the modest but recurrent elevations in cortisol when cortisol levels are usually low are likely to favor, especially in vulnerable subjects, mental or metabolic illnesses (21, 38). The dampening of the circadian rhythmicity of circulating glucocorticoids is also likely to play an important role in the deleterious impact of insufficient sleep. Indeed, the robust rhythm in circulating cortisol levels is a major internal signal synchronizing the central circadian pacemaker in the suprachiasmatic nucleus and the peripheral clocks identified in many, if not all, peripheral tissues. Evidence is rapidly accumulating to indicate that misalignment between central and peripheral clocks has a host of adverse effects, including an increased risk of obesity and type 2 diabetes (37, 39). The dampening of the cortisol rhythm after 2 nights of sleep loss is consistent with recent studies that have shown that lack of sleep alters the phase and the amplitude of circadian-driven rhythms (40, 41).
Our study has some limitations. First, the sample size is small. Second, we studied only young men and the data cannot, therefore, be extrapolated to the general population. Third, our participants received a constant glucose infusion instead of 3 meals, an experimental condition that does not mimic real-life conditions. Lastly, sleep restriction was applied for only 2 nights, and the results may not translate to states of chronic sleep debt, although in a well-documented prospective epidemiologic study in 2802 older adults, short sleep predicted higher daytime cortisol levels (42). This study also has several strengths. First, we used a randomized crossover design with rigorously controlled experimental conditions. Second, we assessed both ACTH and total as well as free cortisol, therefore allowing a complete assessment of the HPA axis. Third, we collected blood and saliva samples at frequent intervals throughout the day, allowing us to evaluate the effects of sleep loss on both the pulsatile and circadian variations. Lastly, we complemented our hormonal analysis with repeated assessments of self-reported levels of stress, hunger, and appetite using validated instruments. In conclusion, we found that 2 nights of sleep restriction result in significant disturbances in the spontaneous activity of the HPA axis, including a hyperactivation over the entire daytime period, a dampening of the cortisol daily circadian excursion, and markedly elevated free cortisol levels in the evening, when cortisol secretion is usually quiescent. The increase in cortisol levels was correlated with increased appetite, highlighting the close relationship between the regulation of arousal, energy homeostasis, and the HPA axis. Irrespective of the underlying mechanisms, these alterations in HPA functioning may mediate the association between short sleep and increased risk for various conditions, including obesity, diabetes, cardiovascular diseases, vulnerability to infection, depression, and anxiety.
Acknowledgments
We thank the subjects for participating in the study, the nursing and dietary staff of the University of Chicago Clinical Resource Center for their assistance, and the sleep technologists who performed polysomnographic recordings.
The work described in this article was supported by National Institutes of Health Grants P01 AG-11412, P60 DK-20595, and ULl-TR000430 to the University of Chicago and by the WAKING Team, Lyon Neuroscience Research Center, Inserm U1028-CNRS Unité Mixte de Recherche 5292, Claude Bernard University, Lyon, France. A.G. was supported by a fellowship from the French Society for Research & Sleep Medicine. R.L. is currently a recipient of a grant, “Brains Back to Brussels,” from the Brussels Institute for Research and Innovation (Belgium). The funding sources had no role in the design, conduct, or reporting of this study.
Disclosure Summary: A.G., M.B., L.M., E.T., R.L., M.L.-B., and K.S. have nothing to declare. E.V.C. receives grant support from Philips/Respironics, the ResMed Foundation, and Amylin/Bristol Meyers Squibb; is a consultant for Viropharma/Shire and Vanda Pharmaceuticals, an Associate Editor for the journal SLEEP and for a volume entitled Sleep Loss and Obesity: Intersecting Epidemics published by Springer Science & Business, LLC; and serves as an expert witness for Lamson, Dugan, and Murray, LLP (Omaha, NE).
Footnotes
- AUC
- area under the curve
- HPA
- hypothalamic-pituitary-adrenal
- REM
- rapid eye movement
- SWS
- slow-wave sleep
- TIB
- time in bed
- TST
- total sleep time
- WASO
- wake after sleep onset.
References
- 1. Bliwise DL. Historical change in the report of daytime fatigue. Sleep. 1996;19:462–464 [DOI] [PubMed] [Google Scholar]
- 2. Rajaratnam SM, Arendt J. Health in a 24-h society. Lancet. 2001;358:999–1005 [DOI] [PubMed] [Google Scholar]
- 3. Spiegel K, Tasali E, Leproult R, Van Cauter E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev. 2009;5:253–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33:414–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cappuccio FP, Cooper D, D'Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32:1484–1492 [DOI] [PubMed] [Google Scholar]
- 6. Nielsen LS, Danielsen KV, Sørensen TI. Short sleep duration as a possible cause of obesity: critical analysis of the epidemiological evidence. Obes Rev. 2011;12:78–92 [DOI] [PubMed] [Google Scholar]
- 7. Morselli LL, Guyon A, Spiegel K. Sleep and metabolic function. Pflugers Arch. 2012;463:139–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Faraut B, Boudjeltia KZ, Vanhamme L, Kerkhofs M. Immune, inflammatory and cardiovascular consequences of sleep restriction and recovery. Sleep Med Rev. 2012;16:137–149 [DOI] [PubMed] [Google Scholar]
- 9. Cappuccio FP, Miller MA. Sleep and mortality: cause, consequence, or symptom? Sleep Med. 2013;14:587–588 [DOI] [PubMed] [Google Scholar]
- 10. Alvaro PK, Roberts RM, Harris JK. A systematic review assessing bidirectionality between sleep disturbances, anxiety, and depression. Sleep. 2013;36:1059–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chrousos GP, Kino T. Glucocorticoid action networks and complex psychiatric and/or somatic disorders. Stress. 2007;10:213–219 [DOI] [PubMed] [Google Scholar]
- 12. Leproult R, Copinschi G, Buxton O, Van Cauter E. Sleep loss results in an elevation of cortisol levels the next evening. Sleep. 1997;20:865–870 [PubMed] [Google Scholar]
- 13. Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439 [DOI] [PubMed] [Google Scholar]
- 14. Vgontzas AN, Bixler EO, Lin HM, et al. Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: clinical implications. J Clin Endocrinol Metab. 2001;86:3787–3794 [DOI] [PubMed] [Google Scholar]
- 15. Spiegel K, Leproult R, L'hermite-Balériaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. 2004;89:5762–5771 [DOI] [PubMed] [Google Scholar]
- 16. Nedeltcheva AV, Kessler L, Imperial J, Penev PD. Exposure to recurrent sleep restriction in the setting of high caloric intake and physical inactivity results in increased insulin resistance and reduced glucose tolerance. J Clin Endocrinol Metab. 2009;94:3242–3250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Buxton OM, Pavlova M, Reid EW, Wang W, Simonson DC, Adler GK. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes. 2010;59:2126–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Omisade A, Buxton OM, Rusak B. Impact of acute sleep restriction on cortisol and leptin levels in young women. Physiol Behav. 2010;99:651–656 [DOI] [PubMed] [Google Scholar]
- 19. Reynolds AC, Dorrian J, Liu PY, et al. Impact of five nights of sleep restriction on glucose metabolism, leptin and testosterone in young adult men. PloS One. 2012;7:e41218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chung S, Son GH, Kim K. Circadian rhythm of adrenal glucocorticoid: its regulation and clinical implications. Biochim Biophys Acta. 2011;1812:581–591 [DOI] [PubMed] [Google Scholar]
- 21. Plat L, Leproult R, L'Hermite-Baleriaux M, et al. Metabolic effects of short-term elevations of plasma cortisol are more pronounced in the evening than in the morning. J Clin Endocrinol Metab. 1999;84:3082–3092 [DOI] [PubMed] [Google Scholar]
- 22. Schmid SM, Hallschmid M, Jauch-Chara K, et al. Disturbed glucoregulatory response to food intake after moderate sleep restriction. Sleep. 2011;34:371–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Monk TH. A visual analogue scale technique to measure global vigor and affect. Psychiatry Res. 1989;27:89–99 [DOI] [PubMed] [Google Scholar]
- 24. Clark LA, Watson D, Leeka J. Diurnal variation in the positive affects. Motivation Emotion. 1989;13:205–234 [Google Scholar]
- 25. Stubbs RJ, Hughes DA, Johnstone AM, et al. The use of visual analogue scales to assess motivation to eat in human subjects: a review of their reliability and validity with an evaluation of new hand-held computerized systems for temporal tracking of appetite ratings. Br J Nutr. 2000;84:405–415 [DOI] [PubMed] [Google Scholar]
- 26. Fernstrom MH, Krowinski RL, Kupfer DJ. Appetite and food preference in depression: effects of imipramine treatment. Biol Psychiatry. 1987;22:529–539 [DOI] [PubMed] [Google Scholar]
- 27. Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol. 2005;99:2008–2019 [DOI] [PubMed] [Google Scholar]
- 28. Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–850 [DOI] [PubMed] [Google Scholar]
- 29. Silber MH, Ancoli-Israel S, Bonnet MH, et al. The visual scoring of sleep in adults. J Clin Sleep Med. 2007;3:121–131 [PubMed] [Google Scholar]
- 30. Iranmanesh A, Lizarralde G, Short D, Veldhuis JD. Intensive venous sampling paradigms disclose high frequency adrenocorticotropin release episodes in normal men. J Clin Endocrinol Metab. 1990;71:1276–1283 [DOI] [PubMed] [Google Scholar]
- 31. Meerlo P, Sgoifo A, Suchecki D. Restricted and disrupted sleep: effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep Med Rev. 2008;12:197–210 [DOI] [PubMed] [Google Scholar]
- 32. Fadda P, Fratta W. Stress-induced sleep deprivation modifies corticotropin releasing factor (CRF) levels and CRF binding in rat brain and pituitary. Pharmacol Res. 1997;35:443–446 [DOI] [PubMed] [Google Scholar]
- 33. Fujihara H, Sei H, Morita Y, Ueta Y, Morita K. Short-term sleep disturbance enhances brain-derived neurotrophic factor gene expression in rat hippocampus by acting as internal stressor. J Mol Neurosci. 2003;21:223–232 [DOI] [PubMed] [Google Scholar]
- 34. Koban M, Le WW, Hoffman GE. Changes in hypothalamic corticotropin-releasing hormone, neuropeptide Y, and proopiomelanocortin gene expression during chronic rapid eye movement sleep deprivation of rats. Endocrinology. 2006;147:421–431 [DOI] [PubMed] [Google Scholar]
- 35. Rohleder N, Kirschbaum C. Effects of nutrition on neuro-endocrine stress responses. Curr Opin Clin Nutr Metab Care. 2007;10:504–510 [DOI] [PubMed] [Google Scholar]
- 36. Mieda M, Sakurai T. Overview of orexin/hypocretin system. Prog Brain Res. 2012;198:5–14 [DOI] [PubMed] [Google Scholar]
- 37. Dickmeis T. Glucocorticoids and the circadian clock. J Endocrinol. 2009;200:3–22 [DOI] [PubMed] [Google Scholar]
- 38. de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–475 [DOI] [PubMed] [Google Scholar]
- 39. Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Markwald RR, Melanson EL, Smith MR, et al. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc Natl Acad Sci U S A. 2013;110:5695–5700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moller-Levet CS, Archer SN, Bucca G, et al. Effects of insufficient sleep on circadian rhythmicity and expression amplitude of the human blood transcriptome. Proc Natl Acad Sci U S A. 2013;110:E1132–E1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kumari M, Badrick E, Sacker A, Kirschbaum C, Marmot M, Chandola T. Identifying patterns in cortisol secretion in an older population. Findings from the Whitehall II study. Psychoneuroendocrinology. 2010;35:1091–1099 [DOI] [PubMed] [Google Scholar]