Abstract
Context:
Many children with idiopathic short stature have a delayed bone age. Idiopathic short stature with advanced bone age is far less common.
Objective:
The aim was to identify underlying genetic causes of short stature with advanced bone age.
Setting and Design:
We used whole-exome sequencing to study three families with autosomal-dominant short stature, advanced bone age, and premature growth cessation.
Results:
Affected individuals presented with short stature [adult heights −2.3 to −4.2 standard deviation scores (SDS)] with histories of early growth cessation or childhood short stature (height SDS −1.9 to −3.5 SDS), advancement of bone age, and normal endocrine evaluations. Whole-exome sequencing identified novel heterozygous variants in ACAN, which encodes aggrecan, a proteoglycan in the extracellular matrix of growth plate and other cartilaginous tissues. The variants were present in all affected, but in no unaffected, family members. In Family 1, a novel frameshift mutation in exon 3 (c.272delA) was identified, which is predicted to cause early truncation of the aggrecan protein. In Family 2, a base-pair substitution was found in a highly conserved location within a splice donor site (c.2026+1G>A), which is also likely to alter the amino acid sequence of a large portion of the protein. In Family 3, a missense variant (c.7064T>C) in exon 14 affects a highly conserved residue (L2355P) and is strongly predicted to perturb protein function.
Conclusions:
Our study demonstrates that heterozygous mutations in ACAN can cause a mild skeletal dysplasia, which presents clinically as short stature with advanced bone age. The accelerating effect on skeletal maturation has not previously been noted in the few prior reports of human ACAN mutations. Our findings thus expand the spectrum of ACAN defects and provide a new molecular genetic etiology for the unusual child who presents with short stature and accelerated skeletal maturation.
Longitudinal bone growth occurs at the growth plate by endochondral ossification. At the growth plate, chondrocyte proliferation, chondrocyte hypertrophy, and cartilage matrix secretion contribute to chondrogenesis and thus to growth (1). The newly formed cartilage is continuously invaded by blood vessels and bone cells that remodel it into new bone tissue. The net result is bone elongation (2). This process of longitudinal bone growth is governed by a complex network of endocrine, paracrine, and intracellular mechanisms (3, 4). Consequently, short stature could potentially be caused by mutations in any of the many genes that directly or indirectly affect the process of chondrogenesis at the growth plate (5, 6).
Currently, only a minority of children with short stature can be provided with a molecular diagnosis such as a genetic defect in the growth hormone/IGF-I axis or a specific skeletal dysplasia. Instead, a large proportion of children with short stature are given the diagnosis, idiopathic short stature (ISS). ISS is defined as a height that is at least two standard deviation scores (SDS) below the mean for age, sex, and population without evidence of systemic, endocrine, nutritional, or genetic abnormalities (7). ISS is a diagnosis of exclusion and thus likely includes many different etiologies. Recently, however, genetic diagnoses have been identified in subgroups of patients with ISS, including mutations and deletions in the short stature homeobox gene (SHOX) (8), heterozygous mutations in the natriuretic peptide receptor-2 (NPR2) gene (9, 10) and protein-tyrosine phosphatase, nonreceptor type, 11(PTPN11) (11).
Bone age, assessed from a standard left hand and wrist radiograph, is useful both in the evaluation and prognosis of short stature (12). Short stature is most commonly associated with a delayed bone age, including in most endocrine diseases, malnutrition, chronic disease, and also frequently in ISS. Conversely, advanced bone age is common with tall stature, occurring in precocious puberty and hyperthyroidism. The combination of short stature and advanced bone age is much less common, and, when it occurs, results in a poor height prediction and marked adult short stature.
Here we describe individuals from three families with ISS marked by accelerated bone maturation and premature growth cessation due to heterozygous aggrecan (ACAN) mutations.
Patients and Methods
Subjects
This study was approved by the Institutional Review Boards of Boston Children's Hospital and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and written informed consent was obtained from all participants or their legal guardians. Height and weights were plotted on the Centers for Disease Control and Prevention growth charts (www.cdc.gov/growthcharts) and z scores were calculated using the NHANES III growth data (US Department of Health and Human Services, National Center for Health Statistics. Third National Health and Nutrition Examination Survey, 1988–1994. Hyattsville: National Center for Health Statistics, 1996).
Sequencing
Whole-exome sequencing of the participants in Families 1 and 3 was conducted from genomic DNA isolated from blood or saliva and was performed at the Broad Institute (Cambridge, MA) while Family 2 underwent whole-exome sequencing at Boston Children's Hospital Genomic Diagnostic Laboratory. Hybrid selection was performed using Agilent's SureSelect Human All Exon Kit (Agilent Technologies). We sequenced the samples using the Illumina HiSeq platform (Illumina) and aligned the resulting reads to the human reference genome version 19 with Burrows-Wheeler Aligner (http://bio-bwa.sourceforge.net/) (13). We then applied the Genome Analysis Toolkit (http://www.broadinstitute.org/gatk) (14) base quality score recalibration, and indel realignment, and performed single nucleotide polymorphism and indel discovery and genotyping using variant quality score recalibration (15). We included in our study only variants that passed all quality filters. Variants were annotated for functional effect using SnpEff 2.0.5 for Families 1 and 3 (http://snpeff.sourceforge.net/) (16) and Annovar (http://www.openbioinformatics.org/annovar/) for Family 2.
Variant filtering and analysis
We hypothesized that the patients had rare, highly penetrant genetic variants causing their short stature. We only considered variants that were not present in the 1000 Genomes Project (November 2012 release) and the National Heart, Lung, and Blood Institute exome variant server (ESP 6500 release). We also limited our analysis to variants that were predicted to have an effect on the protein coding sequence, including nonsynonymous codon changes, frameshift variants, splice site variants, and coding indels.
For missense variants, functional effect was assessed with the PolyPhen2 prediction tool, which estimates the effect of missense variants (17). A score of 0.00 is least likely to perturb protein function, whereas a score of 1.00 suggests a missense variant that is most likely to perturb protein function. Protein function was summarized using information found in the UniProt database (www.uniprot.org) (NM_001135.3; NP_001126.3). The Online Mendelian Inheritance of Man (www.omim.org) database was used to identify any known disease associations.
For all three families, based on the pedigrees, a dominant mode of inheritance was assumed for the analyses.
Family 1
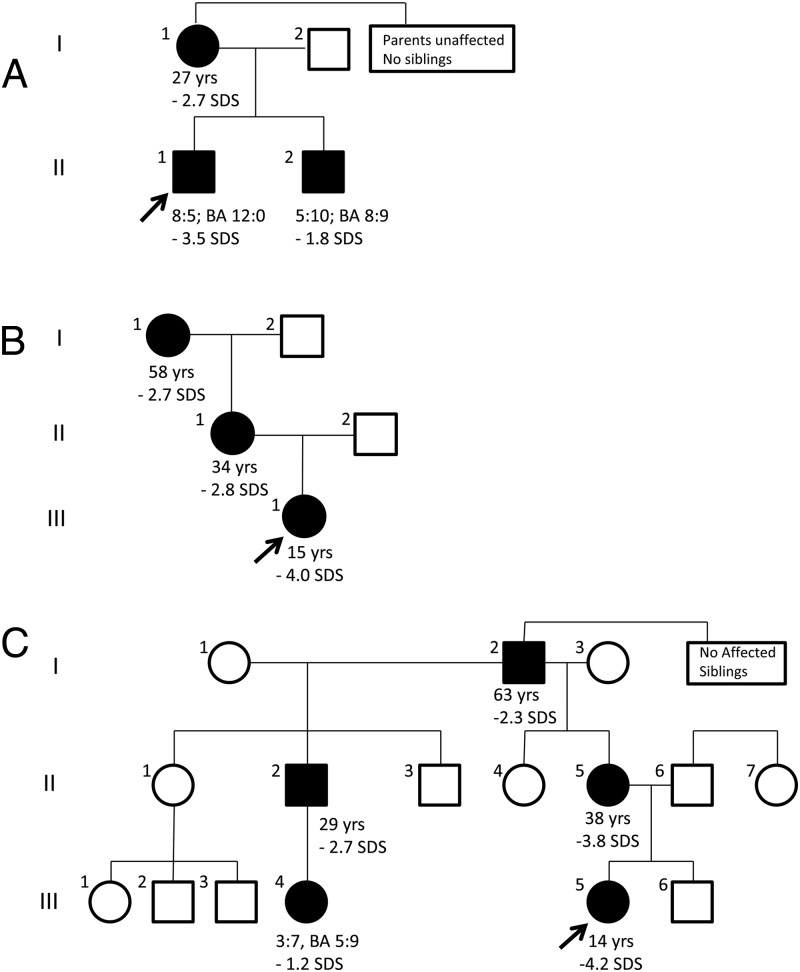
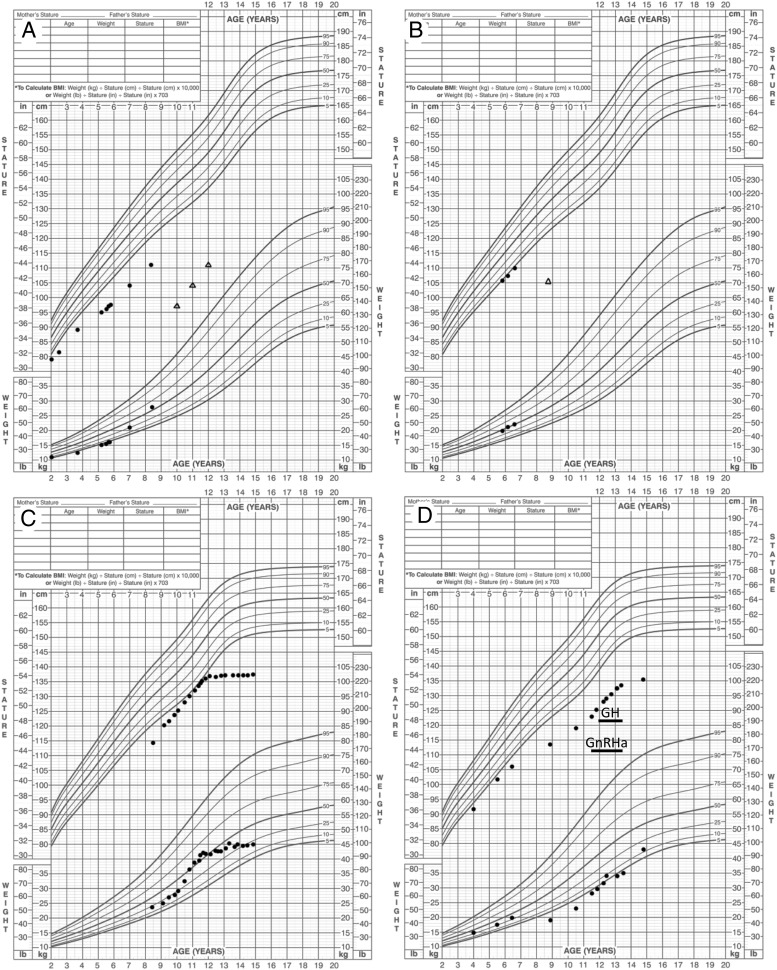
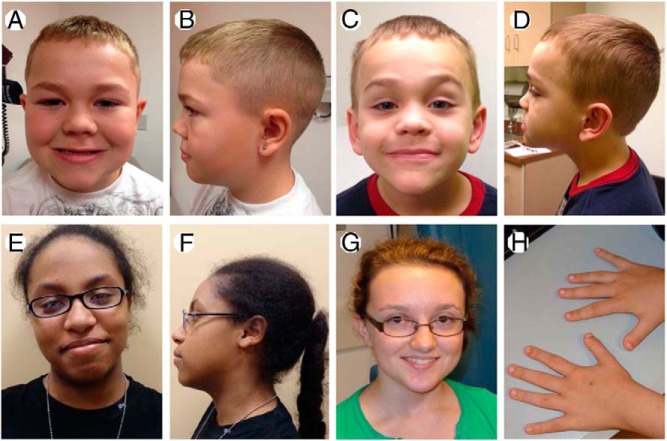
The index case (Figure 1A) is an 8-year-and-5-month-old Caucasian male with proportional short stature and markedly advanced bone age (Figure 2A, Table 1). He was born short for gestational age (birth length, 42.5 cm, −4.2 SDS; birth weight, 2.78 kg, −1.9 SDS) at 40 weeks of gestation. Growth rate has been below normal at least since 2 years of age (Figure 2A). At the time studied he was noted to be a short, proportional, and prepubertal boy with mild midface hypoplasia, flat nasal bridge, mild prognathism, and no signs of sex steroid exposure, and specifically no palpable gynecomastia. (Table 1; Figure 3A–B). Repeated endocrine evaluations have been normal with no indications of GH deficiency, hypothyroidism, early puberty, or condition of increased sex steroid production (http://press.endocrine.org/doi/suppl/10.1210/jc.2013-1332/suppl_file/jc-14-1332.pdf Supplemental Table 1). At chronologic age 5 years 2 months his bone age was markedly advanced at 9.0 years. Since then, his bone age has remained advanced approximately 4 years above chronologic age on repeat bone age assessments (Figure 2A, Supplemental Figure 1). Comparative genomic hybridization microarray did not detect any deletions or duplications. Skeletal survey performed at 7 years and 5 months of age was normal with no signs of a skeletal dysplasia. Bone mineral density assessment using Dual X-ray Absorptiometry revealed bone mineral densities in the normal range (Supplemental Table 1; Lunar iDXA; Combined NHANES/Lunar matched for age, sex, and ethnicity).
Figure 1.
Pedigrees of the families studied: A) Family 1; B) Family 2; C) Family 3. The arrows indicate the proband. Individuals carrying heterozygous ACAN mutations are indicated by solid symbols whereas unaffected individuals are indicated as open symbols. The age (years) and adult height SDS is indicated for all affected individuals that have reached adult height and age (years:months), bone age (BA; years:months), and height SDS at most recent visit for all affected children who are still growing.
Figure 2.
Growth charts of patients with heterozygous ACAN mutations. A) Proband of family 1 (II:1); B) Younger brother of proband Family 1 (II:2); C) Proband of Family 2 (III:1); D) Proband of Family 3 (III:5). Bone age assessments are indicated by triangles (A, B).
Table 1.
Clinical Characteristics of Selected Individuals With Heterozygous ACAN Mutations
| Individual | Family 1 |
Family 2 |
Family 3 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| II:1a | II:2 | I:1 | I:1 | II:1 | III:1a | II:5 | III:5a | III:4 | |
| Sex | Male | Male | Female | Female | Female | Female | Female | Female | Female |
| Age, y:mo | 8:5 | 5:10 | 27 | 58 | 34 | 14:1 | 38 | 14:9 | 3:7 |
| Height, cm (SDS) | 111.3 (−3.5) | 105.7 (−1.8) | 145.9 (−2.7) | 145.8 (−2.7) | 144.9 (−2.8) | 137.2 (−4.0b) | 138.2 (−3.8) | 135.8 (−4.2b) | 93.3 (−1.2) |
| Weight, kg (SDS) | 27.9 (±0.0) | 19.9 (−0.5) | 81.5 (+1.6) | 86.2 (+1.8) | 44.5 (−0.7) | 41.3 (−2.7) | 43.5 (−1.1) | 15.1 (±0.0) | |
| Arm span (cm) | 110.7 | 104.4 | 147.3 | 156.0 | 147.0 | 146.5 | |||
| Sitting height index (SDS) | 0.55 (+1.7) | 0.57 (+1.7) | 0.56 (+2.1) | 0.52 (−0.6) | 0.54 (+1.5) | ||||
| Head circumference, cm | 53.0 | 53.0 | 57.2 | 58 | 54.5 | 56.0 | |||
| Bone age, y:mo (SDS) | 12:0 (+4.5) | 8:9 (+4.0) | N/A | N/A | N/A | N/A | N/A | N/A | 5:9 (+3.5) |
| Predicted adult height, cm | 138 cm | 148 cm | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Age at growth cessation, y | N/A | N/A | 10 | Unknown | 12 − 13 | 12 | 12 | 12 | N/A |
| Midface hypoplasia | Yes | Yes | Yes | Yes | Yes | Yes | No | No | No |
| Short thumbs | No | No | No | No | No | No | Yes | Yes | Yes |
| Brachydactyly | Yes | Yes | Yes | Yes | Yes | Yes | No | No | No |
| Early-onset osteoarthritis | No | No | No | No | No | No | Yes | OD | N/A |
OD, osteochondritis dissecans.
Proband.
Adult-height SDS.
Figure 3.
Patients with short stature, advanced bone age, and/or history of early growth cessation. A and B) Proband of Family 1 at 9 years 3 months of age; C and D) Younger brother of Family 1 proband at 6 years 8 months of age; E and F) Proband of Family 2 at 15 years of age; G and H) Proband of Family 3 at 15 years of age. Affected individuals of Family 3 has short thumbs and distal phalanges (H).
The mother (Figure 1A), is 145.9 cm and was, by history, of normal height until her linear growth ceased at the age of 10 years (2 years before menarche). At 15 years of age a bone age x-ray revealed that all growth plates were fused. She has midface hypoplasia with a flat nasal bridge. The younger brother (Figures 1A, and 3, C and D, Table 1) of the index case has similar features and a stature at the lower end of the normal range (Table 1, Figure 2B). He has mild midface hypoplasia, flat nasal bridge, and prognathism (Figure 3D). At the time studied, he was 5 years 10 month old, prepubertal with no signs of sex steroid exposure, and a height of 105.7 cm (−1.8 SDS). His bone age was markedly advanced at 8 years and 9 months resulting in a predicted adult height of 148 cm (−4.0 SDS). His endocrine evaluation including GH stimulation testing was normal (Supplemental Table 1).
Family 2
The proband (Figures 1B and 3, E–F) is an African American girl who was born full term after an uncomplicated pregnancy with a birth weight of 3.06 kg (−1.0 SDS) and her height has been close to the third percentile during most of her childhood (Figure 2C). Her growth ceased at approximately 12 years of age despite a normally timed puberty. Thelarche occurred at approximately 9.5 years of age, and menarche at 11 years and 5 months. At a chronologic age of 12 years and 7 months, her bone age was 14 years and 0 months. She has well-controlled type 1 diabetes mellitus, diagnosed at age 9 years of age. She has only very mild dysmorphic features with a somewhat broad forehead, mild midface hypoplasia, posteriorly rotated ears, exaggerated lumbar lordosis, brachydactyly, and broad great toes (Figure 3E–F). Her adult height is 137.2 cm (−4.0 SDS). Laboratory evaluation has not revealed a hormonal or other systemic explanation for her short stature (Supplemental Table 1).
The mother is 144.9 cm (−2.8 SDS) and grandmother is 145.8 cm (−2.7 SDS) and both have similar features including exaggerated lumbar lordosis and brachydactyly (Figure 1B, Table 1). The father of the proband was not available for study.
Family 3
The index case (Figures 1C and 3, G and H) was born full term at a birth weight of 2.64 kg (−2.1 SDS) and birth length of 47 cm (−2.0 SDS). Height was close to −3 SDS (first percentile) until the age of 6.5 years when her growth rate declined (Figure 2D). Thelarche occurred at 10 years of age. She was evaluated for short stature at the age of 11.5 years of age, at which time she was at midpuberty (Tanner stage B3/Ph4) and still premenarcheal. She was noted to have relative macrocephaly (Head circumference >2 SDS), mild bilateral genu valgum, and short thumbs (Figure 3H). Laboratory evaluation was normal (Supplemental Table 1). A skeletal survey was normal with no signs of a skeletal dysplasia. A bone age assessment at age 11 years and 7 months revealed an advanced bone age of 13 years and 0 months (+1.4 SDS) resulting in a predicted adult height of 130 cm. She was started on a long-acting GnRH agonist (leuprolide acetate, 11.25 mg i.m. every 4 weeks) at age 11 years 7 months and GH was added at 12 years of age (Figure 2D). Repeat bone age assessments during GnRH analog treatment at chronologic age 12 years 5 months, and at 13 years 5 months were read as 13 years and 0 months, confirming that GnRH agonist treatment successfully blocked bone age advancement. Both GnRH agonist and GH treatments were discontinued at 13.5 years and she stopped growing shortly thereafter at a final adult height of 135.8 cm (−4.2 SDS, Figure 2D). Menarche occurred at 14.5 years of age. In addition, she has a history of persistent pain in both knees and a knee x-ray and magnetic resonance imaging detected multiple osteochondral lesions along the weight-bearing parts of the medial femoral condyles consistent with osteochondritis dissecans.
The proband's mother (Figure 1C; II:5) has proportional short stature and similar features including short, broad thumbs. Her height is 138.2 cm (−3.8 SDS) and she stopped growing at 12 years of age despite a late puberty with menarche at age 18 years. The proband's maternal uncle (height: 157.5 cm; −2.7 SDS) and maternal grandfather (height:160.0 cm; −2.3 SDS) also have a history of early growth cessation. Both the mother and uncle (Figure 2C; II:2) have severe bilateral knee arthritis and the mother has been referred for knee replacement surgery at the age of 38 years. The proband's first cousin (Figure 1C; III:4) was born full term with a normal birth weight (3.57 kg, −0.4 SDS) and birth length (48 cm, −1.6 SDS). Since a young age, she had been growing at the 25th percentile for length/height. At the age of 3 years and 7 months her growth rate is decelerating and she was found to have an advanced bone age of 5 years 9 months (Table 1). She does not have any history or clinical signs or symptoms of sex steroid exposure.
Results
Family 1
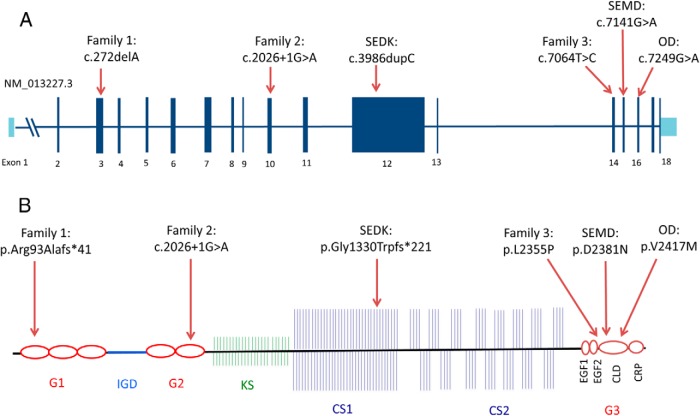
Whole-exome sequencing was performed in the three affected individuals: the proband, his brother, and mother. We filtered for variants present in a heterozygous state in all three patients and identified 19 variants meeting all of our filtering criteria. After review of the functions and disease associations for the 19 variants, we identified a novel frameshift mutation in exon 3 (c.272delA) in ACAN as the most likely causal variant. The mutation is predicted to cause early truncation of the aggrecan protein (Figure 4). Sanger sequencing was performed in all members of the family and confirmed that the three affected individuals (Figure 2: I:1, II:1, II:2) were all heterozygous for the variant, whereas the unaffected father (I:2) was homozygous for the reference allele (Supplemental Figure 2A).
Figure 4.
Structure of the ACAN gene and aggrecan protein and the locations of current and previously reported mutations. A, Top panel shows the gene structure (RefSeq NM_013227.3) of ACAN and the locations of the variants identified in Families 1, 2, and 3, as well as the previously identified pathogenic variants (SEDK, spondyloepiphyseal dysplasia type Kimberly; SEMD, spondyloepimetaphyseal dysplasia; OD, osteochondritis Dissecans); B) Organization of the aggrecan proteoglycan (G1, 2, 3, globular domain 1, 2, 3; IGD, interglobular domain; KS, keratan sulfate; CS1, 2, chondroitin sulfate 1, 2; CLD, C-type lectin domain; CRP, complement regulatory like domain; EGF1, 2, epidermal growth factor–like domain 1, 2). Protein coordinates are based on RefSeq NP_037359.3. Figures are modified from (29) and are drawn approximately to scale.
Family 2
For Family 2, we conducted whole-exome sequencing in the affected proband and her affected mother. We filtered for novel variants present in the heterozygous state in both individuals and identified 60 variants meeting all filtering criteria. We nominated a novel splice donor site variant in ACAN (c.2026+1G>A) as the causal variant, because the variant replaces a highly conserved residue in a splice donor site (Figure 4, S2). Disruption of a highly conserved splice donor site is likely to result in incorrect splicing and will thus alter the down-stream amino acid sequence. We confirmed via Sanger sequencing that the variant was present in the proband, her mother, and her affected grandmother (Supplemental Figure 2B).
Family 3
For family 3, we conducted whole-exome sequencing in the proband, her unaffected brother, and her affected first cousin. We filtered for variants present in the heterozygous state in both affected individuals, but homozygous reference in the unaffected brother. The only identified variant meeting all filtering criteria was a missense variant (c.7064T>C) in exon 14 of ACAN that leads to an amino acid substitution in a highly conserved (Supplemtnal Figure 2E) residue (p.L2355P) in the C-type lectin domain (Figure 4), which is predicted by PolyPhen2 to perturb protein function with a score of 1.00. Subsequent Sanger sequencing revealed that all available affected individuals (Figure 1C: II:2, II:5, III:4, III:5) were heterozygous for this variant, and all tested unaffected individuals (Figure 2C: II:1, II:3, II:4, III:6) were homozygous for the reference allele (Supplemental Figure 2D).
ACAN was the only gene with a novel nonsynonymous variant that segregated with the phenotype in all three families. Because Family 3 only had a single candidate variant, we also looked for overlap of any candidate genes between Families 1 and 2 only. Again, ACAN was the only gene with a variant meeting our criteria that was shared between these two families.
Discussion
In this study we describe three families with a rare autosomal-dominant form of ISS with advanced bone age, early growth cessation, but only subtle other skeletal abnormalities. Using whole-exome sequencing, we identified novel heterozygous nonsynonymous genetic variants in the aggrecan gene (ACAN) with perfect segregation within three families, i.e. the ACAN variants were detected in all affected, but in none of the unaffected family members.
The clinical features of short stature, advanced bone age, and early osteoarthritis observed in our subjects are readily explained by the known biological roles of aggrecan. Aggrecan is a critical proteoglycan component of the cartilage matrix, both in articular and growth plate cartilage, thus accounting for the dual phenotypic effects on both these types of cartilage. Consistent with this explanation, homozygous mutations in ACAN in both mice and chicks severely disrupt growth plate function and impair bone elongation (18, 19). In both species, chondrocytes are tightly packed, with little intervening matrix. In chicks, there is evidence for abnormal Indian hedgehog, fibroblast growth factor, and bone morphogenetic protein signaling, leading to premature hypertrophic chondrocyte maturation (18). Because hypertrophic differentiation induces vascular invasion and ossification of growth cartilage (3), these findings provide a likely explanation for the advanced bone age, premature growth cessation, and early epiphyseal fusion observed in the current study.
The combination of short stature with advanced bone age is rare and has to our knowledge only been reported in eight syndromes. Interestingly, most known causative mutations either impair proteoglycan synthesis, as in our patients and in inactivating mutations of CANT1 causing Desbuquois dysplasia type 1 (20, 21) and inactivating mutations of XYLT1 causing Desbuquois dysplasia type 2 (22), or decrease signaling through the cAMP-protein kinase A signaling pathway, as in Albright's hereditary osteodystrophy (23), inactivating mutations of PRKAR1A causing Acrodysostosis type 1 (24) and activating mutations of PDE4D causing Acrodysostosis type 2 (25), thus indicating that abnormal extracellular matrix or decreased PTHrP signaling in the growth plate result in accelerated bone age progression, premature growth cessation, and hastened epiphyseal fusion (26).
Before this report, only three families with skeletal dysplasias due to ACAN mutations had been reported. One reported family manifested with a skeletal dysplasia, which was termed spondyloepimetaphyseal dysplasia, aggrecan type (SEMD). The disorder was characterized by extreme short stature (adult height stature −14 to −15 SDS), severe midface hypoplasia, low-set, posteriorly rotated ears, prognathism, short neck, platyspondyly, brachydactyly, broad thumbs, and barrel chest, and was caused by a homozygous missense mutation (p.D2343N) in the G3 C-type lectin domain of the ACAN gene (Figure 4) (27). Heterozygous carriers of the mutation exhibited short stature with mean adult heights of approximately −2 to −4 SDS. Neither homozygous patients nor heterozygous carriers exhibited early-onset osteoarthritis. In contrast, one family with familial osteochondritis dissecans presented with early-onset, severe osteoarthritis and osteochondritis dissecans (28) due to a heterozygous missense mutation (p.V2379M), also in the C-type lectin domain (Figure 4) (29). These patients, similar to the heterozygous carriers of the SEMD mutation, exhibited short stature with mean adult heights of approximately −2 to −4 SDS (28). In a third reported family affected members showed a mild skeletal dyplasia, which was termed spondyloepiphyseal dysplasia, Kimberly type (SEDK) and was characterized by proportional short stature (adult heights approximately −2.0 to −4.9 SDS), with vertebral body deformities and end plate irregularities, and progressive generalized osteoarthropathy (30). The causal variant for SEDK was a frameshift mutation in exon 12 (Figure 4) in the chondroitin sulfate binding domain of ACAN (31). Here, we show evidence that heterozygous mutations in ACAN also can cause a mild skeletal dysplasia characterized by short stature and advanced bone age. Our patients have short stature, variable, mild facial dysmorphism, and brachydactyly, but do not exhibit the vertebral body deformities detected in SEDK and SEMD. It is interesting to note that heterozygous carriers of the SEMD mutation, as well as the individuals with SEDK, and familial osteochondritis dissecans all have adult heights similar to that of the affected individuals in our families, ie, −2 to −4 SDS (27, 28, 30). In addition, individuals in the family with osteochondritis dissecans were reported to grow at a low normal percentile (height SDS, ±0 to −2) during childhood with poor pubertal growth spurts resulting in adult heights below the normal range (height SDS, −2 and −4) (28). This growth pattern is similar to that of our patients, indicating that accelerated bone maturation may contribute to the short stature of patients with familial osteochondritis dissecans as well.
All known heterozygous aggrecan mutations detected to date cause short stature of similar severity, indicating that they similarly affect growth plate cartilage. However, only some mutations seem to affect the articular cartilage and cause early-onset osteoarthritis. Families 1 and 2 have early-frameshift or splice-site mutation (Figure 4), which are likely to cause early truncation of the protein and may therefore functionally represent haploinsufficiency of ACAN. These families have short stature but no evidence of early-onset osteoarthritis, suggesting that ACAN haploinsufficiency primarily affects growth plate cartilage. Family 3 and the previously reported familial osteochondritis dissecans family have missense mutations in the C-type lectin domain (Figure 4) and articular disease, suggesting that the presence of aggrecan protein with a dysfunctional C-type lectin domain causes a more severe phenotype affecting both growth plate and articular cartilage function. This hypothesis might also explain SEDK, which shows osteoarthritis, because the frameshift is predicted to eliminate the c-type lectin domain but leaves a more intact protein than the early mutations of Familes 1 and 2. However, SEMD is also caused by a missense mutation in the C-type lectin domain, but heterozygous carriers do not exhibit early-onset articular disease (27). This is possibly because this particular mutation may only partially disrupt the important interactions with Tenascins, fibulins, and other matrix proteins (32, 33).
In our patients, accelerated bone maturation occurs well before puberty and may thus be estrogen independent. However, the fact that affected females seem to experience sudden growth cessation early in puberty taken together with the finding that GnRH analog treatment essentially froze bone age progression in the proband of Family 3 suggest an important role for estrogen. Taken together, the findings suggest that, in this disorder, accelerated bone age progression occurs during childhood independent of estrogen but that, as in normal adolescents, estrogen plays an important role in driving bone age progression and linear growth deceleration to completion. Whether the same pathways that are implicated in ACAN deficiency, i.e. Indian hedgehog, fibroblast growth factor, and bone morphogenetic protein signaling (18), also mediate the effects of estrogen on bone age acceleration and early growth cessation is not known. Interestingly, our subjects seemed to undergo growth cessation even earlier than their advanced bone ages would suggest, highlighting the inherent inaccuracy of height prediction by bone age assessment in children with underlying genetic growth disorders.
We identified three novel loss-of-function variants mutations in ACAN in three families, all with the rare combination of short stature, advanced bone age, and premature growth cessation. Although we did not study the effects of the mutations on aggrecan or growth plate function, several lines of evidence suggest that these mutations are likely to have caused the disorder. First, the variants cosegregate precisely with the disorder in all three families. Second, there is strong biological plausibility. Aggrecan is the major proteoglycan in cartilage. Mutations in this gene cause premature hypertrophic differentiation of growth plate chondrocytes (18), and mutations in other genes affecting proteoglycan synthesis, also present with short stature and advanced bone age (20–22). Third, the mutations, an early frameshift, a substitution in a highly conserved base-pair of a splice donor site, and a missense mutation in a highly conserved residue, are all strongly predicted to affect protein function. Fourth, the growth pattern of our patients as well as the early-onset osteoarthritis and osteochondritis dissecans of Family 3 are largely consistent with previously reported cases of heterozygous, loss-of-function mutations in ACAN. Thus, taken together, our findings strongly suggest that the identified variants are causative. However, further mechanistic studies to elucidate the effect of the genetic variants on mRNA splicing, protein expression, protein function, as well as studies using genetically modified mouse models to elucidate the downstream mechanisms by which the specific variants affect growth plate chondrogenesis, skeletal maturation, and articular cartilage would provide more rigorous tests of causality and could clarify the mechanisms by which ACAN mutations alter growth plate and articular cartilage development and function.
In summary, we report three families with novel heterozygous variants in the ACAN gene associated with short stature, advanced bone age, variable mild dysmorphic features, and early-onset osteoarthritis. These families indicate that ACAN mutations, in addition to presenting to the clinician as an evident skeletal dysplasia or with early-onset osteoarthritis, also can present as autosomal-dominant short stature with advanced bone age and early growth cessation despite normally timed puberty. Our findings thus double the number of reported families with ACAN mutations, expand the clinical spectrum, and provide a novel molecular genetic etiology for the unusual child with short stature and accelerated skeletal maturation.
Acknowledgments
We thank the patients and their families for their participation in the study.
This work was supported by Harvard Catalyst The Harvard Clinical and Translational Science Center (National Institutes of Health Award No. UL1 TR001102 and financial contributions from Harvard University and its affiliated academic health care centers). This work was also supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development at the National Institutes of Health [1K23HD073351 to A.D.] and the Pediatric Endocrine Society Clinical Scholar Award to A.D. Genomic sequencing for family 2 was performed by Boston Children's Hospital Genomic Diagnostic Laboratory, now called Claritas Genomics through the Research Connection of Boston Children's Hospital.
The work of O.N., J.C.L., and J.B. was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health. O.N. was supported by an ESPE Research Fellowship Grant and a grant from the Swedish Research Council (K2012-99X-21998-01-3), the Swedish Society of Medicine, Her Royal Highness Crown Princess Lovisa's Foundation for Pediatric Care, Wera Ekstrom's Foundation for Pediatric Research, Märta och Gunnar V Philipson's Foundation, Sällskapet Barnavård, Stiftelsen Frimurare Barnhuset i Stockholm, and Karolinska Institutet.
The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the National Center for Research Resources, or the National Institutes of Health.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ISS
- idiopathic short stature
- SDS
- standard deviation score
- SEDK
- spondyloepiphyseal dysplasia, Kimberly type
- SEMD
- spondyloepimetaphyseal dysplasia, aggrecan type.
References
- 1. Hunziker EB, Schenk RK. Physiological mechanisms adopted by chondrocytes in regulating longitudinal bone growth in rats. J Physiol. 1989;414:55–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hunziker EB. Mechanism of longitudinal bone growth and its regulation by growth plate chondrocytes. Microsc Res Tech. 1994;28(6):505–519 [DOI] [PubMed] [Google Scholar]
- 3. Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423(6937):332–336 [DOI] [PubMed] [Google Scholar]
- 4. Nilsson O, Marino R, De Luca F, Phillip M, Baron J. Endocrine regulation of the growth plate. Horm Res. 2005;64(4):157–165 [DOI] [PubMed] [Google Scholar]
- 5. Lui JC, Nilsson O, Chan Y, et al. Synthesizing genome-wide association studies and expression microarray reveals novel genes that act in the human growth plate to modulate height. Hum Mol Genet. 2012;21(23):5193–5201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Warman ML, Cormier-Daire V, Hall C, et al. Nosology and classification of genetic skeletal disorders: 2010 revision. Am J Med Genet A. 2011;155A(5):943–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cohen P, Rogol AD, Deal CL, et al. Consensus statement on the diagnosis and treatment of children with idiopathic short stature: a summary of the Growth Hormone Research Society, the Lawson Wilkins Pediatric Endocrine Society, and the European Society for Paediatric Endocrinology Workshop. J Clin Endocrinol Metab. 2008;93(11):4210–4217 [DOI] [PubMed] [Google Scholar]
- 8. Rao E, Weiss B, Fukami M, et al. Pseudoautosomal deletions encompassing a novel homeobox gene cause growth failure in idiopathic short stature and Turner syndrome. Nat Genet. 1997;16(1):54–63 [DOI] [PubMed] [Google Scholar]
- 9. Olney RC, Bukulmez H, Bartels CF, et al. Heterozygous mutations in natriuretic peptide receptor-B (NPR2) are associated with short stature. J Clin Endocrinol Metab. 2006;91(4):1229–1232 [DOI] [PubMed] [Google Scholar]
- 10. Vasques GA, Amano N, Docko AJ, et al. Heterozygous mutations in natriuretic peptide receptor-B (NPR2) gene as a cause of short stature in patients initially classified as idiopathic short stature. J Clin Endocrinol Metab. 2013;98(10):E1636–E1644 [DOI] [PubMed] [Google Scholar]
- 11. Wang SR, Carmichael H, Andrew SF, et al. Large-scale pooled next-generation sequencing of 1077 genes to identify genetic causes of short stature. J Clin Endocrinol Metab. 2013;98(8):E1428–E1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pyle SI, Waterhouse AM, Greulich WW. Attributes of the radiographic standard of reference for the National Health Examination Survey. Am J Phys Anthropol. 1971;35(3):331–337 [DOI] [PubMed] [Google Scholar]
- 13. Li H, Durbin R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics. 2010;26(5):589–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McKenna A, Hanna M, Banks E, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20(9):1297–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. DePristo MA, Banks E, Poplin R, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43(5):491–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cingolani P, Platts A, Wang le L, et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly (Austin). 2012;6(2):80–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Adzhubei IA, Schmidt S, Peshkin L, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7(4):248–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Domowicz MS, Cortes M, Henry JG, Schwartz NB. Aggrecan modulation of growth plate morphogenesis. Dev Biol. 2009;329(2):242–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Watanabe H, Yamada Y. Chondrodysplasia of gene knockout mice for aggrecan and link protein. Glycoconj J. 2002;19(4–5):269–273 [DOI] [PubMed] [Google Scholar]
- 20. Kim OH, Nishimura G, Song HR, et al. A variant of Desbuquois dysplasia characterized by advanced carpal bone age, short metacarpals, and elongated phalanges: report of seven cases. Am J Med Genet A. 2010;152A(4):875–885 [DOI] [PubMed] [Google Scholar]
- 21. Nizon M, Huber C, De Leonardis F, et al. Further delineation of CANT1 phenotypic spectrum and demonstration of its role in proteoglycan synthesis. Hum Mutat. 2012;33(8):1261–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bui C, Huber C, Tuysuz B, et al. XYLT1 Mutations in Desbuquois Dysplasia Type 2. Am J Hum Genet. 2014;94(3):405–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mantovani G, Ferrante E, Giavoli C, et al. Recombinant human GH replacement therapy in children with pseudohypoparathyroidism type Ia: first study on the effect on growth. J Clin Endocrinol Metab. 2010;95(11):5011–5017 [DOI] [PubMed] [Google Scholar]
- 24. Linglart A, Menguy C, Couvineau A, et al. Recurrent PRKAR1A mutation in acrodysostosis with hormone resistance. N Engl J Med. 2011;364(23):2218–2226 [DOI] [PubMed] [Google Scholar]
- 25. Lindstrand A, Grigelioniene G, Nilsson D, et al. Different mutations in PDE4D associated with developmental disorders with mirror phenotypes. J Med Genet. 2014;51(1):45–54 [DOI] [PubMed] [Google Scholar]
- 26. Hirai T, Chagin AS, Kobayashi T, Mackem S, Kronenberg HM. Parathyroid hormone/parathyroid hormone-related protein receptor signaling is required for maintenance of the growth plate in postnatal life. Proc Natl Acad Sci U S A. 2011;108(1):191–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tompson SW, Merriman B, Funari VA, et al. A recessive skeletal dysplasia, SEMD aggrecan type, results from a missense mutation affecting the C-type lectin domain of aggrecan. Am J Hum Genet. 2009;84(1):72–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stattin EL, Tegner Y, Domellöf M, Dahl N. Familial osteochondritis dissecans associated with early osteoarthritis and disproportionate short stature. Osteoarthritis Cartilage. 2008;16(8):890–896 [DOI] [PubMed] [Google Scholar]
- 29. Stattin EL, Wiklund F, Lindblom K, et al. A missense mutation in the aggrecan C-type lectin domain disrupts extracellular matrix interactions and causes dominant familial osteochondritis dissecans. Am J Hum Genet. 2010;86(2):126–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Anderson IJ, Tsipouras P, Scher C, Ramesar RS, Martell RW, Beighton P. Spondyloepiphyseal dysplasia, mild autosomal dominant type is not due to primary defects of type II collagen. Am J Med Genet. 1990;37(2):272–276 [DOI] [PubMed] [Google Scholar]
- 31. Gleghorn L, Ramesar R, Beighton P, Wallis G. A mutation in the variable repeat region of the aggrecan gene (AGC1) causes a form of spondyloepiphyseal dysplasia associated with severe, premature osteoarthritis. Am J Hum Genet. 2005;77(3):484–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Aspberg A, Binkert C, Ruoslahti E. The versican C-type lectin domain recognizes the adhesion protein tenascin-R. Proc Natl Acad Sci U S A. 1995;92(23):10590–10594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aspberg A. The different roles of aggrecan interaction domains. J Histochem Cytochem. 2012;60(12):987–996 [DOI] [PMC free article] [PubMed] [Google Scholar]