Abstract
Context:
Uteroplacental hypoxia has been reported to lower estrogen levels in preeclampsia as the result of reduced aromatase activity.
Objective:
We asked whether the chronic hypoxia of residence at high altitude in the absence of preeclampsia lowered estrogen, whether such effects differed in Andean vs European high-altitude residents, and whether such effects were related to uterine artery diameter or blood flow.
Design, Setting, and Participants:
Studies at weeks 20 and 36 of pregnancy were conducted in 108 healthy Bolivian low- (400 m, n = 53) or high-altitude (3600 m, n = 55) residents of European (n = 28 low and 26 high altitude) or Andean (n = 25 low and 29 high altitude) ancestry. All groups were similar in age, nonpregnant body mass index, and pregnancy weight gain.
Results:
High-altitude residence increased circulating progesterone, cortisol, estrone, 17β-estradiol, and estriol levels (all P < .01). High-altitude Andeans vs Europeans at week 36 had higher progesterone, estrone, 17β-estradiol, and estriol levels as well as product to substrate ratios for the reactions catalyzed by aromatase, whereas week 36 cortisol levels were greater in the European than Andean women (all P < .05). Lower cortisol, higher estriol (both P < .01), and trends for higher progesterone and 17β-estradiol levels were associated with greater uterine artery diameters and blood flow at high altitude.
Conclusions:
Chronic hypoxia does not lower but rather raises estrogen levels in multigenerational Andeans vs shorter-term Europeans, possibly as the result of greater aromatase activity. Because hypoxia alone does not lower estrogen, other attributes of the disease may be responsible for the lower estrogen levels seen previously in preeclamptic women.
Steroid hormone levels during pregnancy reflect the integrated functioning of maternal, placental and fetal systems. Interest in pregnancy steroidogenesis has been rekindled by the observation that mice null for catechol-O-methyl-transferase (COMT), an enzyme involved in estrogen metabolism, develop preeclampsia-like symptoms that can be reversed by administration of the COMT product, 2-methoxyestradiol (2-ME) (1). Lower 2-ME levels in women with severe preeclampsia and HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome, and progressively lower estrone and 17β-estradiol levels with increasing severity of hypertensive complications have also been reported (2, 3), suggesting that persistent placental hypoxia may inhibit aromatase to lower the levels of 17β-estradiol from which 2-ME is derived (4). The idea that persistent placental hypoxia could inhibit aromatase agree with experimental data and prior observations that circulating 17β-estradiol and estriol levels were lower in pregnant women at high compared with low altitudes (2–8). However, the lower levels seen previously at high altitude may have been due to the inclusion of women with preeclampsia or gestational hypertension, and other studies have not found lower placental COMT expression in severe preeclampsia or lower serum 2-ME levels in late-onset preeclampsia vs normotensive women (9, 10), making it unclear as to whether hypoxia alone or some other attribute(s) of the disease is responsible for lowering estrogen levels in hypertensive pregnancies.
We therefore sought to determine whether the chronic hypoxia of residence at high altitude lowered steroid hormone levels in the absence of hypertensive complications of pregnancy. High-altitude studies have the advantage that maternal hypoxia is present throughout pregnancy and thus have fewer confounding factors than disease states in which hypoxia varies in severity and time of onset. Residence at high altitude is known to reduce the pregnancy rise in uterine artery diameter, lower uterine artery blood flow, and decrease infant birth weight, with multigenerational Andeans being relatively protected from such effects compared with European residents (11–16). Because steroid hormones, particularly estradiol and cortisol, influence uterine vascular responses to pregnancy (17), we also sought to determine whether variation in pregnancy hormones was related to uterine artery diameter or blood flow. We considered that such studies would be of value for understanding the role of hormonal characteristics in pregnancy outcomes and especially for the 140 million high-altitude residents worldwide who comprise the largest group at risk for maternal/fetal complications of pregnancy (18).
Materials and Methods
Subjects were 108 Bolivian residents of either low (Santa Cruz, 400 m) or high altitude (La Paz, 3600 m) who comprised a subset of those reported previously (14). Women were recruited through their prenatal care physicians and gave written informed consent to study procedures that had been approved by the human subject review committees of the University of Colorado Denver, Wake Forest University, and the Colegio Médico in Bolivia. Inclusion criteria were that the subject was predominantly of either Andean or European ancestry, in good health, receiving prenatal care, having a singleton pregnancy, and at no known risk for pregnancy complications. Exclusion criteria were visiting low altitude during the current pregnancy, smoking, diagnosis of preeclampsia (ie, two or more blood pressure readings of 140/90 mm Hg at least 6 hours apart and > 1+ proteinuria by dipstick or 300 mg/L in a 24 hour collection) or incomplete infant birth weight, sex, or gestational age data. The final sample consisted of 108 women, specifically 28 European and 25 Andean low-altitude residents and 26 European and 29 Andean high-altitude residents. All high-altitude Andeans were lifelong residents of greater than 2500 m, whereas the Europeans had lived at high altitude 7 ± 1 years, with all but one having been born and raised at low altitude. All the low-altitude residents were born and raised at low altitude.
Ancestry was determined by self-identification as Andean (ie, the Aymára or Quechua indigenous groups of South America) or European and was validated by assessing parental and grandparental surnames and a panel of 81 ancestry-informative genetic markers (AIMs) (13). Andean AIMs were 85% ± 2% Indigenous American, 10% ± 2% European, and 5% ± 1% West African, whereas European AIMs were 30% ± 4% Indigenous American, 62% ± 4% European, and 8% ± 1% West African. Although there was considerable Indigenous American admixture among the Europeans, the inspection of parental and grandparental surnames and residential histories revealed that all the Indigenous American groups were of low-altitude origin (eg, Amazonian, Guaraní, and Nahutl).
Study protocol and techniques
Women were scheduled for study at 20 and 36 weeks of pregnancy and 4 months postpartum, with pregnancy week determined by the last menstrual period and confirmed by fetal biometry at the week 20 visit. Actual study times were 21.1 ± 0.3 and 36.2 ± 0.2 weeks of pregnancy and 3.5 ± 0.2 months postpartum. Subjects completed a questionnaire concerning demographic and residential characteristics. Height was measured by stadiometer, body weight by balance scale in lightly clothed subjects, and triceps + subscapular skinfolds at week 20 by Lange caliper and averaged from the right and left sides for an index of body fat during pregnancy. Each visit was scheduled at midmorning at which time blood was withdrawn from quietly resting, unstressed subjects after an at least 2-hour fast from the antecubital vein into collection tubes containing EDTA. Plasma was aliquoted in 1-mL tubes for storage at −80°C.
All hormone assays were performed by LabCorp (Laboratory Corporation of America). Progesterone, cortisol, T, and 17β-estradiol were measured by electrochemiluminescence immunoassay; androstenedione by liquid chromatography/tandem mass spectrometry; estriol by immunochemiluminometric assay; and estrone by RIA with an interassay precision of 2.9% for progesterone, 1.7% for cortisol, 14.8% for T, 6.1% for 17β-estradiol, 10.35% for androstenedione, 10.23% for estriol, and 16.05% for estrone. Reference intervals were 0.2–222.3 ng/mL for progesterone; 23–194 ng/mL for cortisol; 0.08–0.48 ng/mL for T; 0.125->4.300 ng/mL for 17β-estradiol; 0.28–2.30 ng/mL for androstenedione; 2.3->12.0 ng/mL for estriol; and 1.2–22.9 ng/mL for estrone.
Uterine artery diameter and blood flow were measured using an ATL 3000 transabdominal ultrasound unit (Phillips) with a 4-MHz curved linear array probe and configured for obstetric use with color imaging and Doppler as previously described (13, 14). Infant birth weight, length, gestational age, and sex were obtained from medical records completed by hospital personnel at the time of delivery.
Statistics
Data are reported as the mean ± SEM. After confirmation of normality for each hormone using the D'Agostino-Pearson, the Shapiro-Wilk, and the Kolmogorov-Smirnov tests (at an α = .05), hormone levels were compared between ancestry groups and weeks of pregnancy at a given altitude using two-way ANOVA or across altitudes, ancestry groups, and time using three-way ANOVA with Dunnett's multiple comparisons. Weight gain during pregnancy was calculated as the difference between the week 36 and postpartum measurements. Analysis of covariance was used to assess the effect of parity on hormone levels and gestational age and infant sex on birth weight or ponderal index. Pearson product-moment correlation coefficients were computed to assess the relationships between uterine artery (UA) diameter and hormone levels. All statistics were performed using Prism version 5.0 or SPSS version 12.0. Comparisons are reported as significant when the two-tailed P < .05 and as trends when P < .05 and P < .10.
Results
Subject characteristics
The high-altitude Europeans were older; otherwise, all groups were similar in age, nonpregnant body mass index (BMI), and pregnancy weight gain (Table 1). Few women were obese (BMI > 30 kg/m2) in the nonpregnant state (overall frequency 7%). Skinfolds at week 20 were reduced in the high-altitude Europeans compared with their low-altitude counterparts. The high-altitude Andeans were shorter than the Europeans or low-altitude Andeans. Parity was greater at high than low altitude and tended to be greater in the high-altitude Andeans than Europeans (Table 1), but the number of primiparous women was equivalent in the two high-altitude groups (28%).
Table 1.
Subject Characteristics
| Variable | Ancestry | Low Altitude | High Altitude | P Altitude |
|---|---|---|---|---|
| Age, y | European | 25.8 ± 1.0 | 33.1 ± 1.0 | P < .001 |
| Andean | 25.2 ± 1.1 | 25.8 ± 1.2 | NS | |
| P ancestry | NS | P < 0.001 | ||
| Nonpregnant BMI, kg/m2 | European | 25.6 ± 0.8 | 23.7 ± 1.0 | NS |
| Andean | 25.7 ± 0.7 | 26.4 ± 0.9 | NS | |
| P ancestry | NS | NSa | ||
| Pregnancy weight gain, kg | European | 8.2 ± 0.8 | 9.8 ± 1.0 | NS |
| Andean | 6.6 ± 1.5 | 8.5 ± 0.5 | NS | |
| P ancestry | NS | NS | ||
| Skinfolds week 20, mm | European | 46.4 ± 2.3 | 35.8 ± 3.1 | P < .05 |
| Andean | 41.4 ± 2.5 | 41.0 ± 2.2 | NS | |
| P ancestry | NS | NS | ||
| Height, cm | European | 159.1 ± 1.3 | 160.7 ± 1.3 | NS |
| Andean | 155.5 ± 0.8 | 150.3 ± 0.9 | P < .001 | |
| P ancestry | P < 0.05 | P < 0.001 | ||
| Parity, n, live births | European | 1.5 ± 1.2 | 2.0 ± 0.2 | P < .05 |
| Andean | 1.6 ± 0.2 | 2.6 ± 0.3 | P < .01 | |
| P ancestry | NS | NSa | ||
| Infant birth weight, gb | European | 3237 ± 121 | 3143 ± 88 | NS |
| Andean | 3203 ± 143 | 3037 ± 86 | NSa | |
| P ancestry | NS | NS | ||
| Infant ponderal index, kg/(m3)b | European | 27.2 ± 0.6 | 27.4 ± 0.8 | NS |
| Andean | 26.3 ± 0.7 | 26.3 ± 0.8 | NS | |
| P ancestry | NS | NS |
Abbreviation: NS, not significant. Values are mean ± SD.
Designating a trend (.05 < P < .10).
Adjusted for variation in gestational age and sex.
The UA diameter and blood flow at week 20 and week 36 were similar in the two ancestry groups at low altitude but greater in the high-altitude Andeans than Europeans (Table 2). However, unlike the larger group from which this subset was drawn (14), neither infant birth weight nor ponderal index differed between altitudes or ancestry groups (Table 1).
Table 2.
Levels of Steroid Hormones and Their Ratios in the Various Study Groups
| Variable | Altitude | Group | Week 20 | Week 36 | P, Time | P, Altitude | P, Ancestry | P, Ancestry × Altitude |
|---|---|---|---|---|---|---|---|---|
| Progesterone, ng/mL | Low | European | 49.6 ± 13.7 | 196.8 ± 16.8 | <.001 | <.01 | NS | NS |
| Low | Andean | 44.1 ± 15.9 | 203.4 ± 17.8 | |||||
| High | European | 86.6 ± 16.9 | 200.5 ± 16.8 | |||||
| High | Andean | 66.2 ± 14.2 | 283.3 ± 14.8 | |||||
| Cortisol, ng/mL | Low | European | 142.6 ± 11.1 | 198.7 ± 13.6 | <.001 | <.001 | NS | <.05 |
| Low | Andean | 173.6 ± 12.9 | 210.5 ± 14.5 | |||||
| High | European | 212.8 ± 13.2 | 272.8 ± 13.2 | |||||
| High | Andean | 205.9 ± 11.6 | 234.0 ± 12.0 | |||||
| Androstenedione, ng/mL | Low | European | 1.7 ± 0.2 | 1.9 ± 0.2 | NS | NS | <.01 | NS |
| Low | Andean | 1.2 ± 0.2 | 1.1 ± 0.2 | |||||
| High | European | 1.4 ± 0.2 | 1.7 ± 0.2 | |||||
| High | Andean | 1.2 ± 0.2 | 1.1 ± 0.2 | |||||
| Estrone, ng/mL | Low | European | 1.8 ± 0.7 | 2.6 ± 0.8 | <.001 | <.001 | <.05 | NS |
| Low | Andean | 1.8 ± 0.8 | 5.4 ± 0.9 | |||||
| High | European | 2.8 ± 0.8 | 6.4 ± 0.8 | |||||
| High | Andean | 3.1 ± 0.7 | 7.8 ± 0.7 | |||||
| Testosterone, ng/mL | Low | European | 0.50 ± 0.05 | 0.70 ± 0.06 | <.001 | NS | NS | NS |
| Low | Andean | 0.40 ± 0.06 | 0.60 ± 0.07 | |||||
| High | European | 0.48 ± 0.06 | 0.70 ± 0.06 | |||||
| High | Andean | 0.41 ± 0.05 | 0.79 ± 0.06 | |||||
| 17β-Estradiol, ng/mL | Low | European | 8.6 ± 1.9 | 23.0 ± 2.4 | <.001 | <.001 | <.05 | <.05 |
| Low | Andean | 9.2 ± 2.2 | 23.8 ± 2.5 | |||||
| High | European | 11.4 ± 2.3 | 26.1 ± 2.3 | |||||
| High | Andean | 13.2 ± 2.0 | 38.6 ± 2.1 | |||||
| Estriol, ng/mL | Low | European | 1.5 ± 0.8 | 9.3 ± 0.9 | <.001 | <.001 | <.015 | <.05 |
| Low | Andean | 2.2 ± 0.9 | 9.6 ± 1.0 | |||||
| High | European | 3.3 ± 0.9 | 10.5 ± 0.9 | |||||
| High | Andean | 3.6 ± 0.8 | 16.9 ± 0.8 | |||||
| Reactions catalyzed by aromatase | ||||||||
| Estrone/androstenedione | Low | European | 1.2 ± 0.7 | 2.0 ± 0.9 | <.001 | <.05 | <.01 | NS |
| Low | Andean | 1.8 ± 0.9 | 6.3 ± 0.9 | |||||
| High | European | 2.2 ± 0.9 | 4.6 ± 0.9 | |||||
| High | Andean | 2.8 ± 0.7 | 6.9 ± 0.8 | |||||
| 17β−Estradiol/ Testosterone | Low | European | 19.6 ± 2.7 | 34.0 ± 3.3 | <.001 | <.01 | <.001 | NS |
| Low | Andean | 24.2 ± 3.1 | 44.1 ± 3.5 | |||||
| High | European | 26.8 ± 3.2 | 40.1 ± 3.3 | |||||
| High | Andean | 35.6 ± 2.9 | 50.0 ± 2.9 | |||||
| Reactions catalyzed by 17βHSD | ||||||||
| Testosterone/androstenedione | Low | European | 0.30 ± 0.03 | 0.43 ± 0.04 | <.001 | <.05 | <.001 | NS |
| Low | Andean | 0.34 ± 0.04 | 0.57 ± 0.04 | |||||
| High | European | 0.35 ± 0.04 | 0.49 ± 0.04 | |||||
| High | Andean | 0.36 ± 0.03 | 0.71 ± 0.04 | |||||
| 17β-Estradiol/estrone | Low | European | 8.0 ± 3.1 | 23.0 ± 3.8 | NS | <.01 | NS | NS |
| Low | Andean | 14.8 ± 3.6 | 12.2 ± 4.0 | |||||
| High | European | 4.6 ± 3.7 | 5.0 ± 3.8 | |||||
| High | Andean | 7.4 ± 3.2 | 6.7 ± 3.4 | |||||
| UA diameter, cm | Low | European | 0.50 ± 0.01 | 0.50 ± 0.02 | NS | <.001 | <.001 | <.001 |
| Low | Andean | 0.48 ± 0.01 | 0.48 ± 0.02 | |||||
| High | European | 0.56 ± 0.02 | 0.54 ± 0.01 | |||||
| High | Andean | 0.65 ± 0.01 | 0.65 ± 0.01 | |||||
| UA blood flow (unilateral), mL/min | Low | European | 328 ± 37 | 369 ± 45 | <.05 | <.001 | <.05 | <.001 |
| Low | Andean | 274 ± 44 | 346 ± 48 | |||||
| High | European | 392 ± 48 | 445 ± 47 | |||||
| High | Andean | 642 ± 58 | 805 ± 51 |
Abbreviation: NS, not significant. Values are mean ± SEM. P values are shown for the effects of time (week 20 vs week 36), altitude (low vs high), and ancestry (European vs Andean) using three-way ANOVA.
Steroid hormones
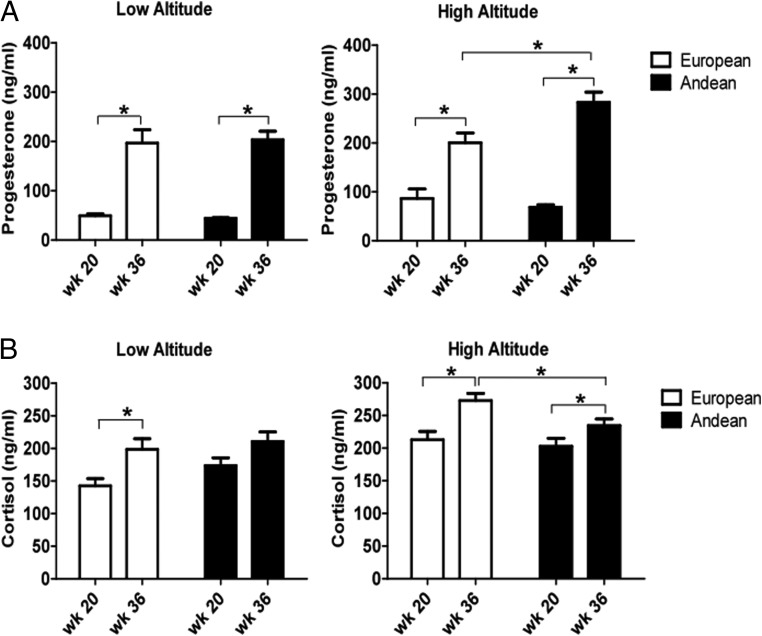
Circulating levels of all hormones but androstenedione rose with advancing gestation (Figures 1 and 2 and Table 2). Progesterone and cortisol levels were similar in the two ancestry groups at low altitude (Figure 1, A and B). High-altitude residence increased both hormone levels (P < .01 and P < .001, respectively; Table 2). Altitude affected the ancestry groups differently such that week 36 progesterone values were lower and week 36 cortisol levels were higher in the European than Andean women (Figure 1, A and B).
Figure 1.
A, Progesterone levels rose with advancing pregnancy and, for the two ancestry groups combined, were increased at high vs low altitude (P < .01). Whereas levels were similar in the two ancestry groups at low altitude, high-altitude Andeans had higher progesterone levels than the Europeans at week 36. B, Cortisol levels rose with advancing pregnancy and, for the two ancestry groups combined, were greater at high than low altitude (P < .001). No ancestry group differences were present at low altitude but at high altitude, Europeans had higher cortisol values than Andeans at week 36. Brackets with asterisks designate significant differences between groups at P < .05.
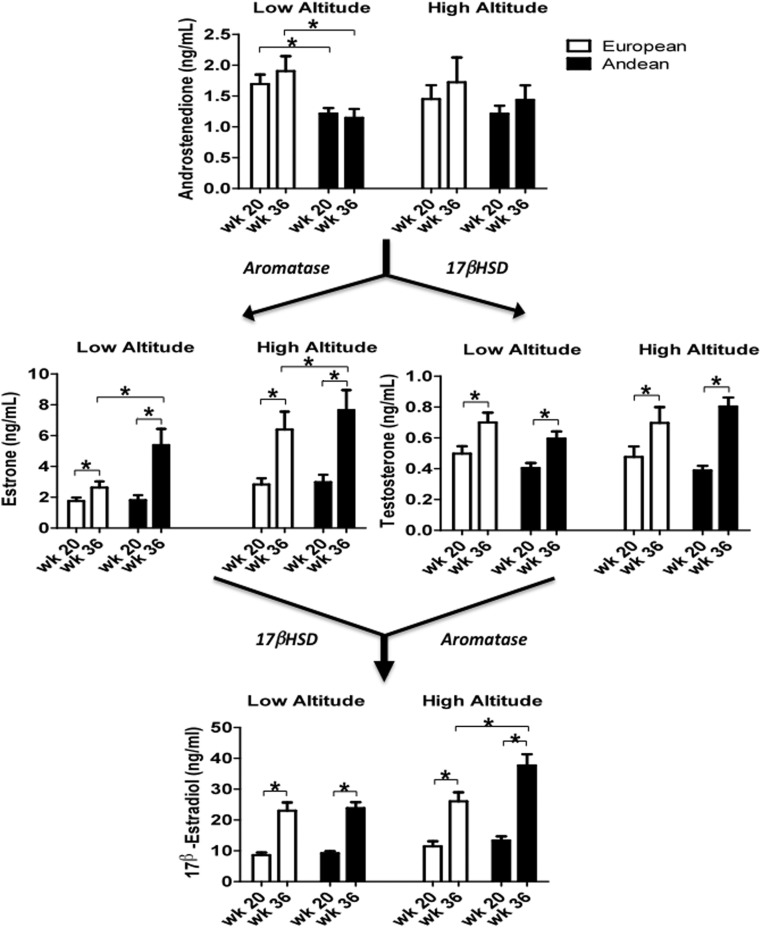
Figure 2.
Estrone, T, and 17β-estradiol rose with advancing gestation. Androstenedione levels were higher in the European than the Andean women at low altitude but similar in the two ancestry groups at high altitude. High-altitude residence and Andean ancestry raised estrone levels (P < .001 and P < .05, respectively) with the result that values were higher in the Andean than European women at week 36 both at low and at high altitude. T, levels were unaffected by ancestry or altitude. 17β-Estradiol levels were increased by residence at high altitude and Andean ancestry (P < .001 and P < .05, respectively). The effect of ancestry interacted with that of altitude (P < .05) such that 17β-estradiol levels were higher in Andean than European high-altitude residents at week 36. Brackets with asterisks designate significant differences between groups at P < .05.
Androstenedione levels were higher in the Europeans than Andeans at low altitude, unaffected by altitude, and similar in the two ancestry groups at high altitude (Figure 2). Residence at high vs low altitude and Andean vs European ancestry increased the levels of estrone, which is produced from androstenedione by aromatase (Figure 2 and Table 2). T, produced by 17β-hydroxysteroid dehydrogenase (17βHSD) from androstenedione, was unaffected by altitude or ancestry (Figure 2).
Estrone and T can be metabolized by either 17βHSD or aromatase to generate 17β-estradiol. High-altitude residence and Andean ancestry increased 17β-estradiol levels and interacted such that the Andeans had higher values than the Europeans at week 36 (Figure 2). There was no difference in 17β-estradiol levels between mothers of male vs. female babies (data not shown).
Comparing the product to substrate ratios for the two hormones produced by aromatase showed that high-altitude residence and Andean ancestry increased both ratios and resulted in Andeans having a higher estrone to androstenedione ratio than Europeans at week 36 at low altitude, nonsignificant but marginally higher estrone/androstenedione ratios at week 36 at high altitude (P = .13) and a higher 17β-estradiol to T ratio at week 20 at high altitude (Figure 3 and Table 2). Less consistent ancestry-group differences were seen in the substrate to product ratios for the reactions catalyzed by 17βHSD; namely, Andeans had higher T to androstenedione ratios than Europeans at week 36 at low and high altitude, and the 17β-estradiol to estrone ratios were similar in the two ancestry groups but lowered by high-altitude residence (Table 2).
Figure 3.
The ratios of estrone to androstenedione (A) and 17β-estradiol to testosterone (B) are used as indices of the amount of substrate converted to metabolite by aromatase. Both ratios increase with advancing pregnancy, high-altitude residence, and Andean ancestry (P < .001, P < .05, and P < .01, respectively) with the result that Andeans have higher estrone to androstenedione ratios than Europeans at week 36 at low altitude and higher 17β-estradiol to T ratios at week 20 at high altitude. Brackets with asterisks designate significant differences between groups at P < .05.
Estriol levels were increased by residence at high altitude and Andean ancestry (P < .001 and P < .015, respectively). There was significant interaction between the effects of ancestry and altitude such that the week 36 levels were greater in the high- compared with low-altitude Andeans but similar in the Europeans (Table 2).
Steroid hormones and UA blood flow
There were trends for higher progesterone and higher 17β-estradiol levels at week 36 to be associated with greater UA diameter and blood flow at high altitude (Table 3). Lower cortisol levels at week 20 were correlated with higher UA blood flow for the two ancestry groups combined (Table 3) or either group alone (r = −0.51, P < .05, and −0.71, P < .01 in the Europeans and Andeans, respectively). Higher estriol levels at week 36 were also associated with greater UA diameter and tended to be associated with greater blood flow (Table 3).
Table 3.
Correlation Coefficients Between Levels of Steroid Hormones (x) and Uterine Artery Diameter or Blood Flow (y)
| Hormone | Low Altitude |
High Altitude |
||||||
|---|---|---|---|---|---|---|---|---|
| UA dia week 20 | UA flow week 20 | UA dia week 36 | UA flow week 36 | UA dia week 20 | UA flow week 20 | UA dia week 36 | UA flow week 36 | |
| Androstenedione | NS | NS | NS | NS | NS | NS | NS | NS |
| Cortisol | NS | NS | NS | NS | NS | −0.62a | NS | NS |
| 17β-Estradiol | NS | NS | NS | NS | NS | NS | 0.29b | 0.32b |
| Estriol | NS | NS | NS | NS | NS | NS | 0.51a | 0.33b |
| Estrone | NS | NS | NS | NS | NS | NS | NS | NS |
| Progesterone | NS | NS | NS | NS | NS | NS | 0.30b | 0.38b |
| T | NS | NS | NS | NS | NS | NS | NS | NS |
Abbreviations: dia, diameter; NS, not significant.
P < .01.
.05 < P < .10.
Discussion
Consistent with our previous study (6), high-altitude residence increased progesterone levels, but unlike our prior report, estrogen levels were not reduced. Rather the levels of all three estrogens, estrone, 17β-estradiol, and estriol, were higher at high than low altitude at week 36 due chiefly to greater altitude-associated increases in the Andean than European women. The product to substrate ratios for the two reactions catalyzed by aromatase, estrone to androstenedione and 17β-estradiol to T, were higher in the Andeans than the Europeans, whereas the corresponding ratios for the reactions catalyzed by 17βHSD did not consistently differ, suggesting that greater aromatase activity may have contributed to the Andeans' higher estrogen levels.
Our study benefitted from certain strengths but also had some weaknesses. Its strengths included the multiple hormones measured, larger sample sizes than in previous reports (2, 6), and the availability of uterine artery diameter and blood flow data in the same women. The groups were well matched in terms of body size; specifically, few were obese, and nonpregnant BMI as well as pregnancy weight gain were similar in all groups with the sole exception that European skinfold values were lower at week 20 at high altitude. Although the Andeans had been born and raised at high altitude and the Europeans only lived there approximately 7 years, we believe the subject's own duration of high-altitude residence was not likely to have been responsible for the ancestry-group differences observed given that lifelong high-altitude residence does not confer protection from altitude-associated reductions in UA blood flow or fetal growth (19). It also seemed unlikely that differences in fat mass and its potential contribution to steroid metabolism were responsible because nonpregnant BMI and pregnancy weight gain were similar in all study groups and few women were obese. Nor were the modest differences in parity likely responsible as the differences in estrone, estriol, and 17β-estradiol levels between altitudes and ancestry groups at week 36 remained significant when parity was included as a covariate (all P < .05). However, neither this nor previous studies (2, 3) were able to measure aromatase activity directly given the lack of maternal and placental tissue samples. Although we standardized blood collection by drawing all blood samples at midmorning after a 2-hour fast, it was not possible to maintain rigorous dietary control, likely increasing variation in study results.
We were also limited in our ability to evaluate hormonal influences on UA diameter or blood flow, given that all samples were obtained at mid- or late gestation by which time most of the enlargement in UA diameter has already occurred (20, 21), and not all subjects were able to be studied at both weeks 20 and 36, limiting our ability to assess the relationship between change in hormone levels and UA blood flow.
In keeping with our prior report (6), we found higher progesterone levels at high than low altitude. We also found that cortisol values were elevated at high vs low altitude, especially in the Europeans, which was not due to corticotherapy because no women were being so treated. It was not possible to measure anxiety or depression levels, but whatever levels of stress present did not seem sufficient to affect the hypothalamic-pituitary axis functioning because no group differences were seen in proinflammatory cytokines (22). Higher cortisol levels have been reported in pregnant sheep or humans under conditions of short-term exposure to high altitude (23, 24), suggesting that the higher cortisol levels in Europeans than Andeans may have reflected less complete acclimatization to high altitude.
However, unlike our prior study in which 17β-estradiol and estriol levels were approximately 30% lower at high altitude (6), we found higher levels of all three estrogens, 17β-estradiol, estriol, and estrone, at 36 weeks of pregnancy in the high- vs low-altitude women. The higher estrogen levels were due chiefly to greater altitude-associated increases in the Andeans, but levels were also higher in the European women. Although study times were matched, the present subjects lived higher (3600 vs 3100 m) and lower (400 vs 1600 m) than in the previous study, but the approximately 15 mm Hg greater difference in arterial oxygen tension would be expected to have exaggerated the effects of altitude. It is possible that differences in the methods used for measuring hormones may have contributed to the variation between studies insofar as 17β-estradiol was measured using electrochemiluminescence immunoassay, estrone by RIA, and estriol by immunochemiluminometric assay in the present study, whereas a RIA was used previously, but it is not clear why the type of assay explains the opposite direction of the changes in estrogen levels observed. Because women with preeclampsia or gestational hypertension were included previously but excluded here, given reports that hypertensive complications of pregnancy themselves lower estrogen levels (2, 3, 25), we concluded that the variation in study results was most likely due to the inclusion of hypertensive women in the prior report and that the chronic hypoxia of residence at high altitude in the absence of preeclampsia raises, not lowers, estrogen levels.
The higher 17β-estradiol, estriol, and estrone levels seen during normal pregnancy at high vs low altitude was counter to our hypothesis that chronic hypoxia inhibited aromatase to lower estrogen levels. The similarity between altitudes in androstenedione levels indicated that the higher estrogen levels were not due to greater substrate availability. Using the approach of Hertig et al (2) we compared hormone levels and the product to substrate ratios for the reactions catalyzed by aromatase (ie, estrone/androstenedione and 17β-estradiol/T) vs those catalyzed by 17βHSD (ie, T/adrostenedione and 17β-estradiol/estrone). Because high altitude and Andean ancestry raised 17β-estradiol, estriol, and estrone levels and consistently raised the aromatase but not the 17βHSD product to substrate ratios, we concluded that residence at high altitude likely increased aromatase activity. Finding higher, not lower, estrogen levels in normotensive women at high vs low altitude suggests that the progressive reductions in estrogen hormone levels seen with increasing severity of preeclampsia (2, 3) are not due to hypoxia per se but rather to impaired placental function or some other attribute of the disease.
The higher 17β-estradiol, estriol, and estrone levels seen at week 36 in the Andean than European high-altitude residents and the Andeans' higher aromatase product to substrate ratios at both altitudes was consistent with the possibility of greater aromatase activity in the Andean than European subjects. Hormone measurements were not obtained in the nonpregnant state but higher 17β-estradiol levels throughout the menstrual cycle have been reported from high (4340 m) vs low (150 m) altitudes in Peru (26). Aromatase polymorphisms are known to be associated with steroid hormone levels (27) and various functional outcomes [eg, (28)], but there have not been any studies to the best of our knowledge of aromatase polymorphisms in Andeans. Future studies are therefore recommended for determining whether aromatase polymorphisms exist in Andeans and, if so, are related to the higher estrogen levels observed.
Estrogens and other steroid hormones are known to play vital roles in maternal uterine vascular adaptations to pregnancy. For example, 17β-estradiol stimulation of endothelial nitric oxide synthase production and activity accounts for approximately 70% of uterine artery vasodilation in the pregnant ewe (29, 30). Endothelium-independent up-regulation of neuronal nitric oxide synthase and large conductance Ca++-activated K+ channels (BkCA) activity, which can be induced ex vivo by incubation of uterine arteries from nonpregnant sheep with 17β-estradiol and progesterone, also contribute to uterine artery vasodilation and the greater than 10-fold rise in blood flow during pregnancy (31–33). Furthermore, chronic hypoxia disrupts normal nitric oxide-dependent uterine artery vasodilator and vascular smooth muscle proliferative responses to pregnancy (34, 35), and a recent series of elegant studies by Zhang and coworkers (36, 37) suggests that alterations in steroid hormones play important roles in mediating such effects. Specifically vessels from sheep exposed chronically to high altitude or from low-altitude animals incubated in hypoxia ex vivo failed to show pregnancy-associated reductions in uterine artery myogenic tone which were due, in turn, to the inhibition of the normal pregnancy-associated up-regulation of BkCAβ1 subunit expression and increased BkCA activity (37).
Given these important effects of steroid hormones and hypoxia on uterine vascular responses to pregnancy and the differences in uterine artery diameter and blood flow seen in pregnant Andean vs European high-altitude residents (11, 12, 14), we asked whether pregnancy hormones were related to UA diameter or blood flow. We found that higher 17β-estradiol, estriol, and progesterone levels were or tended to be associated with larger UA diameter or blood flow at week 36 of pregnancy at high altitude, suggesting that the Andeans' higher hormone levels could have contributed to raising their UA blood flow. No such associations were seen with estrone, but its vascular effects are less potent than those of 17β-estradiol (38). Our observations are also consistent with prior studies in which 17β-estradiol levels progressively declined with increasing severity of hypertensive disease and likely worsening of uteroplacental ischemia (2, 3). The strong association between higher cortisol levels and lower UA blood flow also suggested that the Europeans' higher cortisol levels might have acted to lower UA blood flow via α1-adrenergic stimulated vasoconstriction (39). We therefore concluded that greater aromatase activity in the Andeans may have contributed to raising their estrogen hormone levels and UA blood flow. Additional feed-forward mechanisms could also be involved whereby greater UA blood flow and oxygen delivery helped maintain high aromatase activity, augment fetal estriol production, and raise estrogen levels.
In summary, our study results supported prior observations that the chronic hypoxia of residence at high altitude raised progesterone and cortisol levels during pregnancy but showed that estrogen levels were increased as well. The increases in progesterone and estrogen were more pronounced in Andean than European high-altitude residents and were accompanied in the Andeans by differences in product to substrate ratios suggestive of greater aromatase activity. Given prior observations linking the actions of 17β-estradiol to pregnancy-associated changes in UA vasoreactivity and the associations present in the current study, we speculate that ancestry-group differences in hormone levels and aromatase activity during pregnancy may have conferred evolutionary benefit to the Andeans by raising their UA blood flow. Further studies are required to corroborate these findings in larger groups of subjects with control for the subject's own duration of high-altitude residence, inclusion of genetic testing for population differences in aromatase polymorphisms, and at earlier time points when UA enlargement is occurring. Especially valuable will be placental and myometrial tissue samples in which direct measurement of aromatase activity, myogenic tone, and expression levels of relevant substances could be obtained.
Acknowledgments
We express our appreciation to all the women who generously participated in this project and the many physicians and laboratory personnel in La Paz and Santa Cruz (Bolivia) who helped with subject recruitment and study conduct. We also thank Dr Pooja Mujumdar and Dr David L. Mount for their statistical consultation and the Wake Forest University Translational Science Institute for their generous financial support and assistance with performing the steroid hormone assays.
This work was supported by Grants HLBI-60131, HLBI-079647, and TW-01188 from the National Institutes of Health.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AIM
- ancestry-informative genetic marker
- BkCA
- Ca++-activated K+ channel
- BMI
- body mass index
- COMT
- catechol-O-methyl-transferase
- 17βHSD
- 17β-hydroxysteroid dehydrogenase
- 2-ME
- 2-methoxyestradiol
- UA
- uterine artery.
References
- 1. Kanasaki K, Palmsten K, Sugimoto H, et al. Deficiency in catechol-O-methyltransferase and 2-methoxyoestradiol is associated with pre-eclampsia. Nature. 2008;453:1117–1121 [DOI] [PubMed] [Google Scholar]
- 2. Hertig A, Liere P, Chabbert-Buffet N, et al. Steroid profiling in preeclamptic women: evidence for aromatase deficiency. Am J Obstet Gynecol. 2010;203:477 e471–e479 [DOI] [PubMed] [Google Scholar]
- 3. Jobe SO, Tyler CT, Magness RR. Aberrant synthesis, metabolism, and plasma accumulation of circulating estrogens and estrogen metabolites in preeclampsia implications for vascular dysfunction. Hypertension. 2013;61:480–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Perez-Sepulveda A, Espana-Perrot PP, Norwitz ER, Illanes SE. Metabolic pathways involved in 2-methoxyestradiol synthesis and their role in preeclampsia. Reprod Sci. 2013;20:1020–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kautsky MP, Hagerman DD. Kinetic properties of steroid 19-hydroxylase and estrogen synthetase from porcine ovary microsomes. J Steroid Biochem. 1980;13:1283–1290 [DOI] [PubMed] [Google Scholar]
- 6. Zamudio S, Leslie KK, White M, Hagerman DD, Moore LG. Low serum estradiol and high serum progesterone concentrations characterize hypertensive pregnancies at high altitude. J Soc Gynecol Investig. 1994;1:197–205 [DOI] [PubMed] [Google Scholar]
- 7. Sobrevilla LA, Romero I, Kruger F, Whittembury J. Low estrogen excretion during pregnancy at high altitude. Am J Obstet Gynecol. 1968;102:828–833 [DOI] [PubMed] [Google Scholar]
- 8. Sobrevilla LA, Romero I, Kruger F. Estriol levels of cord blood, maternal venous blood, and amniotic fluid at delivery at high altitude. Am J Obstet Gynecol. 1971;110:596–597 [DOI] [PubMed] [Google Scholar]
- 9. Palmer K, Saglam B, Whitehead C, Stock O, Lappas M, Tong S. Severe early-onset preeclampsia is not associated with a change in placental catechol O-methyltransferase (COMT) expression. Am J Pathol. 2011;178:2484–2488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Seol HJ, Cho GJ, OH, MJ, Kim HJ. 2-Methoxyoestradiol levels and placental catechol-O-methyltransferase expression in patients with late-onset preeclampsia. Arch Gynecol Obstet. 2013;287:881–886 [DOI] [PubMed] [Google Scholar]
- 11. Julian CG, Galan HL, Wilson MJ, et al. Lower uterine artery blood flow and higher endothelin relative to nitric oxide metabolite levels are associated with reductions in birth weight at high altitude. Am J Physiol. 2008;295:R906–R915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zamudio S, Postigo L, Illsley NP, et al. Maternal oxygen delivery is not related to altitude- and ancestry-associated differences in human fetal growth. J Physiol. 2007;582:883–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wilson MJ, Lopez M, Vargas M, et al. Greater uterine artery blood flow during pregnancy in multigenerational (Andean) than shorter-term (European) high-altitude residents. Am J Physiol. 2007;293:R1313–R1324 [DOI] [PubMed] [Google Scholar]
- 14. Julian CG, Wilson MJ, Lopez M, et al. Augmented uterine artery blood flow and oxygen delivery protect Andeans from altitude-associated reductions in fetal growth. Am J Physiol. 2009;296:R1564–R1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Julian CG, Vargas E, Armaza JF, Wilson MJ, Niermeyer S, Moore LG. High-altitude ancestry protects against hypoxia-associated reductions in fetal growth. Arch Dis Child Fetal Neonatal Ed. 2007;92:F372–F377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Soría R, Julian C, Vargas E, Moore LG, Giussani D. Graduated effects of high-altitude hypoxia and highland ancestry on birth size. Pediatr Res. 2013;74:633–638 [DOI] [PubMed] [Google Scholar]
- 17. Chang K, Lubo Z. Review article: steroid hormones and uterine vascular adaptation to pregnancy. Reprod Sci. 2008;15:336–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Krampl E. Pregnancy at high altitude. Ultrasound Obstet Gynecol. 2002;19:535–539 [DOI] [PubMed] [Google Scholar]
- 19. Julian CG, Hageman JL, Wilson MJ, Vargas E, Moore LG. Lowland origin women raised at high altitude are not protected against lower uteroplacental O2 delivery during pregnancy or reduced birth weight. Am J Hum Biol. 2011;23:509–516 [DOI] [PubMed] [Google Scholar]
- 20. Palmer SK, Zamudio S, Coffin C, Parker S, Stamm E, Moore LG. Quantitative estimation of human uterine artery blood flow and pelvic blood flow redistribution in pregnancy. Obstet Gynecol. 1992;80:1000–1006 [PubMed] [Google Scholar]
- 21. Dickey RP, Hower JF. Ultrasonographic features of uterine blood flow during the first 16 weeks of pregnancy. Hum Reprod. 1995;10:2448–2452 [DOI] [PubMed] [Google Scholar]
- 22. Davila RD, Julian CG, Wilson MJ, et al. Do cytokines contribute to the Andean-associated protection from reduced fetal growth at high altitude? Reprod Sci. 2011;18:79–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harvey LM, Gilbert RD, Longo LD, Ducsay CA. Changes in ovine fetal adrenocortical responsiveness after long-term hypoxemia. Am J Physiol. 1993;264:E741–E747 [DOI] [PubMed] [Google Scholar]
- 24. Braun B, Mawson JT, Muza SR, et al. Women at altitude: carbohydrate utilization during exercise at 4,300 m. J Appl Physiol. 2000;88:246–256 [DOI] [PubMed] [Google Scholar]
- 25. Salas SP, Marshall G, Gutierrez BL, Rosso P. Time course of maternal plasma volume and hormonal changes in women with preeclampsia or fetal growth restriction. Hypertension. 2006;47:203–208 [DOI] [PubMed] [Google Scholar]
- 26. Escudero F, Gonzales GF, Góñez C. Hormone profiling during the menstrual cycle at high altitude. Intl J Gynecol Obstet. 1996;55:49–58 [DOI] [PubMed] [Google Scholar]
- 27. Sowers MR, Wilson AL, Kardia SR, Chu J, Ferrell R. Aromatase gene (CYP 19) polymorphisms and endogenous androgen concentrations in a multiracial/multiethnic, multisite study of women at midlife. Am J Medicine. 2006;119:S23–S30 [DOI] [PubMed] [Google Scholar]
- 28. Van Pottelbergh I, Goemaere S, Kaufman JM. Bioavailable estradiol and an aromatase gene polymorphism are determinants of bone mineral density changes in men over 70 years of age. J Clin Endocrinol Metab. 2003;88:3075–3081 [DOI] [PubMed] [Google Scholar]
- 29. Rosenfeld CR, Cox BE, Roy T, Magness RR. Nitric oxide contributes to estrogen-induced vasodilation of the ovine uterine circulation. J Clin Invest. 1996;98:2158–2166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liao WX, Magness RR, Chen DB. Expression of estrogen receptors-α and –β in the pregnant ovine uterine artery endothelial cells in vivo and in vitro. Biol Reprod. 2005;72:530–537 [DOI] [PubMed] [Google Scholar]
- 31. Rosenfeld CR, Roy T, Cox BE. Mechanisms modulating estrogen-induced uterine vasodilation. Vascul Pharmacol. 2002;38:115–125 [DOI] [PubMed] [Google Scholar]
- 32. Scott PA, Tremblay A, Brochu M, St-Louis J. Vasorelaxant action of 17β-estradiol in rat uterine arteries: role of nitric oxide synthases and estrogen receptors. Am J Physiol. 2007;293:H3713–H3719 [DOI] [PubMed] [Google Scholar]
- 33. Osol G, Moore LG. Maternal uterine vascular remodeling during pregnancy. Microcirculation. 2014;21:38–47 [DOI] [PubMed] [Google Scholar]
- 34. White MM, McCullough RE, Dyckes R, Robertson AD, Moore LG. Chronic hypoxia, pregnancy, and endothelium-mediated relaxation in guinea pig uterine and thoracic arteries. Am J Physiol. 2000;278:H2069–H2075 [DOI] [PubMed] [Google Scholar]
- 35. Rockwell LC, Dempsey EC, Moore LG. Chronic hypoxia diminishes the proliferative response of guinea pig uterine artery vascular smooth muscle cells in vitro. High Alt Med Biol. 2006;7:237–244 [DOI] [PubMed] [Google Scholar]
- 36. Xiao D, Huang X, Yang S, Zhang L. Direct chronic effect of steroid hormones in attenuating uterine arterial myogenic tone: role of protein kinase c/extracellular signal-regulated kinase 1/2. Hypertension. 2009;54:352–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hu XQ, Xiao D, Zhu R, et al. Chronic hypoxia suppresses pregnancy-induced upregulation of large-conductance Ca2+-activated K+ channel activity in uterine arteries. Hypertension. 2012;60:214–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Teoh H, Quan A, Leung SW, Man RY. Vascular effects of estrone and diethylstilbestrol in porcine coronary arteries. Menopause. 2009;16:104–109 [DOI] [PubMed] [Google Scholar]
- 39. Xiao D, Huang X, Ducsay CA, et al. Cortisol-mediated regulation of uterine artery contractility: effect of chronic hypoxia. Am J Physiol. 2004;286:H716–H722 [DOI] [PubMed] [Google Scholar]