Abstract
Context:
Despite common use of supplemental vitamin D2 in clinical practice, the associations of serum vitamin D2 concentrations with other vitamin D metabolites and total vitamin D are unclear.
Objective:
The aim of the study was to measure vitamin D2 and D3 levels and examine their associations with each other and with total vitamin D.
Design:
We performed a cross-sectional analysis of 679 randomly selected participants from the Osteoporotic Fractures in Men Study. 25-Hydroxyvitamin D2 [25(OH)D2], 25(OH)D3, 1,25-dihydroxyvitamin D2 [1,25(OH)2D2], and 1,25(OH)2D3 were measured using liquid chromatography-tandem mass spectrometry and were summed to obtain total 25(OH)D and 1,25(OH)2D. Associations between all metabolites (D2, D3, and total levels) were examined using Wilcoxon rank-sum tests and Spearman correlations.
Results:
25(OH)D2 and 1,25(OH)2D2 were detectable in 189 (27.8%) and 178 (26.2%) of the men, respectively. Higher 25(OH)D2 levels did not correlate with higher total 25(OH)D (r = 0.10; P = .17), although median total 25(OH)D was slightly higher in those with detectable vs undetectable 25(OH)D2 (25.8 vs 24.3 ng/mL; P < .001). 25(OH)D2 was not positively associated with total 1,25(OH)2D levels (r = −0.11; P = .13), and median 1,25(OH)2D level was not higher in those with detectable vs undetectable 25(OH)D2. Higher 25(OH)D2 was associated with lower 25(OH)D3 (r = −0.35; P < .001) and 1,25(OH)2D3 (r = −0.32; P < .001), with median levels of both D3 metabolites 18–35% higher when D2 metabolites were undetectable.
Conclusions:
In a cohort of older men, 25(OH)D2 is associated with lower levels of 25(OH)D3 and 1,25(OH)2D3, suggesting that vitamin D2 may decrease the availability of D3 and may not increase calcitriol levels.
Vitamin D is important for skeletal health and calcium homeostasis. Total 25-hydroxyvitamin D [25(OH)D] levels are measured in clinical settings to assess the adequacy of vitamin D stores and to ensure sufficient substrate for conversion to the biologically active form, 1, 25-dihydroxyvitamin D [1,25(OH)2D]. Although the optimal level of 25(OH)D has been debated (1–3), total 25(OH)D levels correlate with important clinical outcomes such as hip fracture (4), bone loss at the hip (5), and falls (6, 7). When vitamin D is insufficient, practitioners commonly recommend supplementation with ergocalciferol (D2) or cholecalciferol (D3). Although some data suggest that cholecalciferol is a more potent supplement for increasing total vitamin D levels (8), it is uncertain whether ergocalciferol and cholecalciferol and their metabolites are biologically equivalent at the vitamin D receptor. Furthermore, the way one metabolite affects the levels of other vitamin D metabolites is unknown and may have implications for which form of supplementation is most effective.
Using liquid chromatography-tandem mass spectrometry (LC-MS/MS) assays that allow for separate quantification of vitamin D2 and vitamin D3 metabolites, we sought to quantify and examine the associations among 25(OH)D2, 25(OH)D3, 1,25(OH)2D2, 1,25(OH)2D3, and total levels of both forms of vitamin D in a large cohort of older men to better understand how vitamin D2 relates to the other vitamin D measures.
Subjects and Methods
Study participants
The study design and cohort characteristics of the Osteoporotic Fractures in Men Study (MrOS) have been previously described (9, 10). Briefly, 5994 community-dwelling men were enrolled from six clinical centers in the United States (Birmingham, Alabama; Minneapolis, Minnesota; Palo Alto, California; Monongahela Valley near Pittsburgh, Pennsylvania; Portland, Oregon; and San Diego, California) from March 2000 through April 2002. Men were eligible to participate if they were ≥65 years old, were able to walk without assistance of another person, and did not have bilateral hip replacements. The Institutional Review Board at each center approved the study, and written consent was obtained from all participants. From the MrOS cohort, 679 men with baseline serum available were randomly selected to evaluate the relationships of vitamin D metabolites.
Study measurements
Vitamin D metabolite measurements.
Fasting morning blood samples were initially collected in 2000–2002, and serum was prepared and stored at −70°C until thawed for assays. LC-MS/MS methods were used to measure levels of vitamin D2 and D3 metabolites [25(OH)D2, 25(OH)D3, 1,25(OH)2D2, 1,25(OH)2D3] in archived serum obtained in approximately equal numbers in each of the four seasons.
Measures of 25(OH)D2 and 25(OH)D3 were performed at the Mayo Medical Laboratories in Rochester, Minnesota, using LC-MS/MS (11). These levels were then added together to obtain total 25(OH)D levels. The lower limit of quantification (LLQ) was 4 ng/mL for 25(OH)D2 and 2 ng/mL for 25(OH)D3. Aliquots of a single serum pool were included in alternate assay runs. Using the pooled serum, the interassay coefficients of variation (CVs) for 25(OH)D2 and 25(OH)D3 were 6.1 and 4.4%, respectively, and the intra-assay CVs were 4.4 and 4.9%, respectively (11).
1,25(OH)2D2 and 1,25(OH)2D3 were measured at the University of Leuven in Belgium using LC-MS/MS (12). These levels were then added together to obtain total 1,25(OH)2D levels. The LLQ was 4.3 pg/mL for 1,25(OH)2D2 and 6 pg/mL for 1,25(OH)2D3. Interassay CVs of pooled serum at low and high serum concentrations, respectively, were 10.1% for serum with a mean concentration of 7.16 pg/mL and 5.9% for serum with a mean concentration of 55.8 pg/mL (12). If values were below the LLQ, those men were classified as having an undetectable value of that metabolite. The percentage of total 25(OH)D that was 25(OH)D2 was calculated, as was the percentage of total 1,25(OH)2D that was 1,25(OH)2D2.
Vitamin D intake.
Dietary and supplemental calcium and vitamin D intake were assessed at baseline with the Block Food Frequency questionnaire (13, 14). Vitamin D intake from supplements did not include whether the vitamin D supplement was ergocalciferol or cholecalciferol. Participants brought in all medications used within the last 30 days. A computerized dictionary, based on the original Established Populations for Epidemiologic Studies of the Elderly (EPESE) coding system (14), was used to categorize the medications. Each medication was matched to its ingredient(s) based on the Iowa Drug Information Service (IDIS) Drug Vocabulary (College of Pharmacy, University of Iowa, Iowa City, Iowa).
Other measures.
Questionnaires were administered at baseline to obtain information regarding smoking history, alcohol consumption, self-reported medical/surgical history, and demographic factors. The Physical Activity Score for the Elderly (PASE) (15) was used to assess physical activity. Standard balance beam or digital scales were used to obtain weight (kilograms) and Harpenden stadiometers for height (centimeters). These data were then used to calculate body mass index (BMI) as kilograms per meter squared. Serum creatinine was measured on baseline serum using a variation of the Jaffe enzymatic method, and renal function was expressed as estimated glomerular filtration rate (eGFR) in mL/min/1.73 m2 using a standardized serum creatinine-based formula (5, 16). Participants' ability to rise from a chair (without assistance or using their arms) was used to assess lower extremity performance. Information on how these measures, including height, weight, BMI, serum creatinine, and assessment of lower extremity performance, were obtained has been previously described (5, 11).
Statistical analyses
The distribution of each vitamin D measure was examined. Spearman correlations were used to examine the associations among vitamin D metabolites, including total vitamin D levels, when values were in the detectable range. Proportions of men with detectable 25(OH)D2 and 1,25(OH)2D2 were calculated, and differences in the medians of the other vitamin D measures by detectability of each D2 metabolite were tested using Wilcoxon rank sum tests. The proportions of men with detectable 25(OH)D2 according to reported vitamin D supplement use were calculated, and a χ2 test was performed. For 25(OH)D2, 1,25(OH)2D2, and total 1,25(OH)2D, three different participants with extreme values of one of the above metabolites were identified on histograms, and analyses were repeated after they were removed. Analyses were also repeated for the 284 men that reported no vitamin D supplement use and the 169 men that had detectable levels of all metabolites to see whether the relationships among vitamin D metabolites were different in these subgroups. To evaluate for differences by participant characteristics such as obesity and supplement use, similar analyses were performed in those subgroups. For those who reported a history of gastric surgery, analyses with D3 and total metabolites were performed. The number with detectable D2 metabolites in those with a history of gastric surgery was too small for analysis. For all analyses described above, results were not adjusted for season; however, similar analyses stratified by season of collection (winter vs summer) were also performed, and differences in medians by season were tested using the Kruskal-Wallis test. All analyses were conducted using SAS version 9.3 (SAS Institute).
Results
Characteristics of participants
Baseline characteristics of the participants are shown in Table 1. Men were between the ages of 65 and 91 years at baseline (mean age, 74.1 y) and were predominantly non-Hispanic Caucasian (90.7%). Most participants had normal renal function (83.7% with eGFR ≥60 mL/min/1.73 m2), reported good or excellent health status (85.0%), and were nonsmokers (97.2%). The average BMI of the participants was 27.4 kg/m2 (range, 17.2–45.1 kg/m2); 20.6% were obese (BMI ≥30 kg/m2). Approximately 8.2% reported previous surgery to remove part of the stomach or intestines. Although specific medical conditions, indications, and surgical history are not available, we believe this higher than expected figure could be the result of partial gastrectomies performed for peptic ulcer disease before the widespread use of proton pump inhibitors. Overall, 58.1% of men reported using vitamin D supplements at baseline [vs 57.6% of men who had 25(OH)D3 <20 ng/mL]. The average vitamin D intake from supplements and food was 384 IU/d. The characteristics of the randomly selected 679 participants that comprise this dataset are similar to the entire MrOS cohort (Table 1).
Table 1.
Baseline Characteristics of MrOS Participants With Vitamin D Measures and Overall MrOS Cohort
| MrOS Participants With Vitamin D Measures | Overall MrOS Cohort | |
|---|---|---|
| n | 679 | 5994 |
| Age, y | 74.1 (6.0) | 73.7 (5.9) |
| Race | ||
| Non-Hispanic white | 616 (90.7%) | 5362 (89.5%) |
| Black | 22 (3.2%) | 244 (4.1%) |
| Hispanic | 17 (2.5%) | 126 (2.1%) |
| Asian | 16 (2.4%) | 191 (3.2%) |
| Other | 8 (1.2%) | 71 (1.2%) |
| Site | ||
| Birmingham | 99 (14.6%) | 969 (16.2%) |
| Minneapolis | 111 (16.5%) | 1005 (16.8%) |
| Pittsburgh | 116 (17.1%) | 1005 (16.8%) |
| Palo Alto | 118 (17.4%) | 995 (16.6%) |
| Portland | 121 (17.8%) | 1007 (16.8%) |
| San Diego | 114 (16.8%) | 1013 (16.9%) |
| Season of blood drawa | ||
| Winter | 134 (19.7%) | |
| Spring | 174 (25.6%) | |
| Summer | 198 (29.2%) | |
| Fall | 173 (25.5%) | |
| PASE score | 146.7 (65.9) | 146.5 (68.3) |
| Excellent or good health status | 576 (85.0%) | 5135 (85.7%) |
| Current smoker | 19 (2.8%) | 206 (3.4%) |
| Moderate to high alcohol use (≥7 drinks/wk) | 173 (25.5%) | 1549 (25.9%) |
| BMI, kg/m2 | 27.4 (3.7) | 27.4 (3.8) |
| eGFR | ||
| <30 mL/min/1.73 m2 | 4 (0.6%) | 28 (0.5%) |
| 30–59 mL/min/1.73 m2 | 101 (15.7%) | 931 (16.8%) |
| ≥60 mL/min/1.73 m2 | 538 (83.7%) | 4574 (82.7%) |
| Surgery to remove part of stomach or intestines | 56 (8.2%) | 447 (7.5%) |
| Inability to rise from chair | 12 (1.8%) | 56 (0.9%) |
| Total calcium intake, mg/d | 788.4 (381) | 797.9 (387.1) |
| Vitamin D supplement use | 393 (58.1%) | 3529 (59.3%) |
| Daily vitamin D from supplement use, IU | 222.8 (211) | 226.5 (208.6) |
| Daily vitamin D intake, IU | 161.1 (119) | 164.6 (117.6) |
| Vitamin D from diet and supplement, IU | 383.9 (252) | 391.2 (245.5) |
| Serum vitamin D measuresa | ||
| Total 25(OH)D, ng/mL | 24.9 (8.0) | |
| 25(OH)D2 [mean if detectable] | 8.2 (3.2) | |
| Undetectable 25(OH)D2 | 490 (72.2%) | |
| 25(OH)D3, ng/mL | 22.7 (8.3) | |
| Total 1,25(OH)2D, pg/mL | 64.2 (17.7) | |
| 1,25(OH)2D2 [mean if detectable] | 10.1 (5.1) | |
| Undetectable 1,25(OH)2D2 | 501 (73.8%) | |
| 1,25(OH)2D3, pg/mL | 61.6 (18.1) |
Data are expressed as mean (SD) or number (percentage).
No data for overall MrOS cohort (n = 5994) because vitamin D measures were not obtained in everyone.
Vitamin D metabolite levels
The median total 25(OH)D level was 24.8 ng/mL (range, 3.1–55.8 ng/mL), and the median total 1,25(OH)2D level was 61.9 pg/mL (range, 8.7–138.0 pg/mL). Total 25(OH)D was moderately correlated with total 1,25(OH)2D (r = 0.37; P < .001; Figure 1A). All participants had detectable levels of 25(OH)D3 and 1,25(OH)2D3 (median, 22.2 and 59.4 pg/mL, respectively). 25(OH)D2 (median, 7.4 ng/mL) and 1,25(OH)2D2 (median, 8.3 pg/mL) were detectable in only 189 men (27.8%) and 178 men (26.2%), respectively; 169 men (24.9%) had detectable levels of both D2 metabolites. Most men (58.1%) reported using vitamin D supplements at baseline, and they more often had detectable levels of 25(OH)D2 than those that did not report supplement use (46.3 vs 2.5%; P for χ2 < 0.001). Levels of 25(OH)D3 and 1,25(OH)2D3 were positively correlated (r = 0.50; P < .001; Figure 1B), and among the 169 men who had detectable levels of both D2 metabolites, 25(OH)D2 levels were positively correlated with 1,25(OH)2D2 (r = 0.63; P < .001; Figure 1C).
Figure 1.
Associations among 25(OH)D and 1,25(OH)2D metabolites. Spearman correlations (r) between total levels of 25(OH)D and 1,25(OH)2D (A), 25(OH)D3 and 1,25(OH)2D3 (B), and 25(OH)D2 and 1,25(OH)2D2 (C).
Associations of D2 and D3 metabolites with total 25(OH)D and 1,25(OH)2D
Men with higher 25(OH)D3 levels had higher total 25(OH)D concentrations (r = 0.85; P < .001; Figure 2A) and higher total 1,25(OH)2D (r = 0.38; P < .001; Figure 2B). Despite the fact that levels of total 25(OH)D were slightly higher in those with detectable levels of 25(OH)D2 than in those with undetectable 25(OH)D2 (median, 25.8 vs 24.3 ng/mL; P < .001), higher 25(OH)D2 levels were not correlated with higher total 25(OH)D (r = 0.10; P = .17; Figure 2C) among those with detectable 25(OH)D2. Moreover, although median total 1,25(OH)2D was similar in those with undetectable vs detectable 25(OH)D2 (62.0 vs 61.5 pg/mL, respectively), among those with detectable 25(OH)D2, 25(OH)D2 was not positively associated with total 1,25(OH)2D. In fact, those with higher 25(OH)D2 had slightly (but not significantly) lower total 1,25(OH)2D levels (r = −0.11; P = .13; Figure 2D), and there was an inverse correlation (r = −0.32; P < .0001; n = 189) between the ratio of 25(OH)D2/total 25(OH)D and total 1,25(OH)2D such that 1,25(OH)2D levels were lower when 25(OH)D2 represented a greater proportion of total 25(OH)D. Compared to the strong correlation between 1,25(OH)2D3 and total 1,25(OH)2D (r = 0.95; P < .001), in participants with detectable 1,25(OH)2D2 there was a weak, but significant, positive correlation with total 1,25(OH)2D (r = 0.19; P = .01), and the median total 1,25(OH)2D levels were slightly (but not significantly) higher in those with detectable vs undetectable 1,25(OH)2D2 (median, 63.1 vs 61.5 pg/mL, respectively; P = .60) (Table 2). For men in whom it was detectable, 25(OH)D2 comprised 31.8%, on average, of their total 25(OH)D level, although the range was wide: 10.1 to 77.7%. In contrast, for men in whom 1,25(OH)2D2 was detectable, 1,25(OH)2D2 comprised only 16.5% of their total 1,25(OH)2D (range, 4.2–56.3%).
Figure 2.
Associations among 25(OH)D metabolites and total levels of 25(OH)D and 1,25(OH)2D. A, Spearman correlation (r) between 25(OH)D3 and total 25(OH)D. Line of identity represents the men without detectable 25(OH)D2. B–D, Spearman correlations (r) between 25(OH)D3 and total 1,25(OH)2D (B), 25(OH)D2 and total 25(OH)D (C), and 25(OH)D2 levels and total 1,25(OH)2D levels (D).
Table 2.
Median (Interquartile Range) Levels of Vitamin D Metabolites by Detectability of 25(OH)D2 and 1,25(OH)2D2
| 25(OH)D, ng/mL |
1,25(OH)2D, pg/mL |
|||||
|---|---|---|---|---|---|---|
| 25(OH)D2 | 25(OH)D3 | Total 25(OH)D | 1,25(OH)2D2 | 1,25(OH)2D3 | Total 1,25(OH)2D | |
| Median | 0 | 22.2 | 24.8 | 0 | 59.4 | 61.9 |
| Range | 0–29.2 | 3.1–55.8 | 3.1–55.8 | 0–34.2 | 8.7–138.0 | 8.7–138.0 |
| Detectable 25(OH)D2 | 7.4 (6.0–10.0) | 18.3 (13.8–22.1) | 25.8 (22.5–30.3) | 8.1 (6.0–12.1) | 52.4 (40.5–65.9) | 61.5 (49.0–75.2) |
| Undetectable 25(OH)D2 | N.A. | 24.3 (18.8–29.0) | 24.3 (18.8–29.0) | 0.0 (0.0–0.0)b | 61.8 (52.7–75.2) | 62.0 (52.7–75.2) |
| P valuea | N.A. | <.001 | <.001 | N.A. | <.001 | .37 |
| Detectable 1,25(OH)2D2 | 7.5 (6.1–10.3) | 17.8 (13.2–21.5) | 25.3 (21.8–29.8) | 8.3 (6.6–12.5) | 52.6 (40.9–67.3) | 63.1 (49.9–75.6) |
| Undetectable 1,25(OH)2D2 | 0.0 (0.0–0.0)c | 24.3 (19.1–29.1) | 24.6 (19.1–29.6) | N.A. | 61.5 (52.2–74.8) | 61.5 (52.2–74.8) |
| P valuea | N.A. | <.001 | .04 | N.A. | <.001 | .60 |
Abbreviation: N.A., not applicable.
P value for difference between median values according to detectability of specified D2 metabolite.
96% of observations are zero; maximum value of 1,25(OH)2D2 when 25(OH)D2 is undetectable is 10.0 pg/mL.
96% of observations are zero; maximum value of 25(OH)D2 when 1,25(OH)2D2 is undetectable is 8.3 ng/mL.
Associations of D2 and D3 metabolites
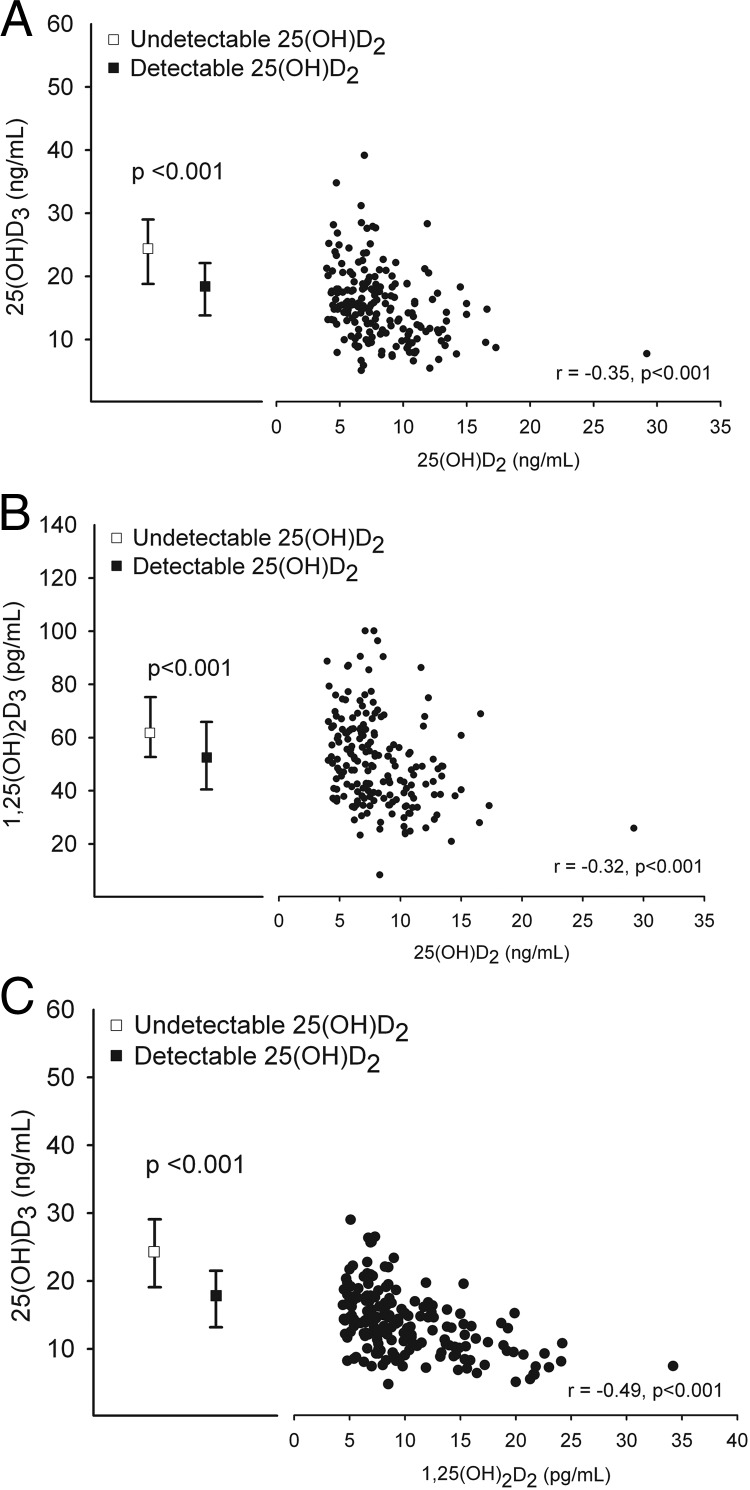
In those with detectable 25(OH)D2, higher levels of 25(OH)D2 were associated with lower levels of 25(OH)D3 (r = −0.35; P < .001; Figure 3A) and lower levels of 1,25(OH)2D3 (r = −0.32; P < .001; Figure 3B). Moreover, median levels of both D3 metabolites were 18–35% higher in those with undetectable D2 metabolites (Table 2). Similarly, men with higher 25(OH)D3 levels had lower levels of 1,25(OH)2D2 (r = −0.49; P < .001; Figure 3C). There was no correlation between 1,25(OH)2D2 and 1,25(OH)2D3 levels (Table 3). A complete listing of all correlations among 25(OH)D and 1,25(OH)2D metabolites can be found in Table 3.
Figure 3.
Associations among D2 and D3 metabolites. Spearman correlations (r) between 25(OH)D2 and 25(OH)D3 (A), 25(OH)D2 and 1,25(OH)2D3 (B), and 25(OH)D3 and 1,25(OH)2D2 (C).
Table 3.
Spearman Correlations Among Vitamin D Metabolite Levels
| 25(OH)D3 | Total 25(OH)D | 1,25(OH)2D2b | 1,25(OH)2D3 | Total 1,25(OH)2D | |
|---|---|---|---|---|---|
| 25(OH)D2a | −0.35 (<.001) | 0.10 (.17) | 0.63 (<.001)c | −0.32 (<.001) | −0.11 (.13) |
| 25(OH)D3 | 1.0 | 0.85 (<.001) | −0.49 (<.001) | 0.50 (<.001) | 0.38 (<.001) |
| 1,25(OH)2D2 | −0.49 (<.001) | −0.12 (.11) | 1.0 | −0.07 (.34) | 0.19 (.01) |
| 1,25(OH)2D3 | 0.50 (<.001) | 0.35 (<.001) | −0.07 (.34) | 1.0 | 0.95 (<.001) |
| Total 1,25(OH)2D | 0.38 (<.001) | 0.37 (<.001) | 0.19 (.01) | 0.95 (<.001) | 1.0 |
Data are presented as correlation coefficient (P value). All correlations are for n = 679, unless otherwise specified below.
For 25(OH)D2, correlations are among the 189 men with detectable 25(OH)D2.
For 1,25(OH)2D2, correlations are among the 178 men with detectable 1,25(OH)2D2.
Correlation is for the 169 men with detectable levels of both D2 metabolites.
Subset analyses
Statistical analyses were repeated among the 284 men who reported no vitamin D supplement use; the associations were unchanged or slightly stronger, but there was no change in the statistical significance or direction of the relationships described (data not shown). No substantial changes were noted when analyses were repeated after excluding three extreme values [one man with high 25(OH)D2 of 29.2 ng/mL; one with high 1,25(OH)2D2 of 34.2 pg/mL; and one with low total 1,25(OH)2D of 8.7 pg/mL] (data not shown). When statistical analyses were repeated among the 169 men with detectable levels of all metabolites, Spearman correlations involving D3 metabolites and total levels were generally stronger (data not shown). As might be expected, those who had their vitamin D measured in the winter, compared to those with measurements in the summer, had slightly lower median values of 25(OH)D3 (19.1 vs 24.8 ng/mL; PKruskal-Wallis < .0001) and total 25(OH)D (23.3 vs 26.3 ng/mL; P < .0001) but similar median 25(OH)D2 (7.9 vs 6.9 ng/mL; P = .22). Although most of the associations were similar, the relationships between 25(OH)D2 and both D3 metabolites were slightly stronger in the winter than in the summer [25(OH)D2 and 25(OH)D3–r = −0.59, P = .0001; vs r = −0.27, P = .05; 25(OH)D2 and 1,25(OH) 2D3–r = −0.46, P < .01; vs r = −0.24, P = .08). Median levels of all metabolites were slightly lower in obese compared to nonobese participants [largest difference in total 1,25(OH)2D–56.6 vs 63.2 pg/mL, PWilcoxon rank-sum = .0006; smallest difference in 25(OH)D2–6.9 vs 7.5 ng/mL, P = .20). These differences were smaller in all 25(OH)D metabolites than they were with all 1,25(OH)2D metabolites. These analyses were generally the same as those described in the whole group except that the relationships for 25(OH)D2 to 1,25(OH)2D2 and both D2 metabolites with 25(OH)D3 were weaker in the obese group (data not shown). Similarly, in the small subset of participants who reported previous gastric surgery, there was no significant difference in the direction or magnitude of the relationships described for total or D3 metabolites. These minor differences in the seasonal variation and obese participants did not materially affect the overall conclusions.
Discussion
Measures of vitamin D metabolites in older, community-dwelling men reveal that higher levels of 25(OH)D2, a form of vitamin D derived exclusively from food and supplements, are associated with lower levels of both 25(OH)D3 and 1,25(OH)2D3 and are not associated with higher total levels of 25(OH)D or 1,25(OH)2D.
To understand possible reasons for these associations, it is helpful to remember that vitamin D metabolism involves several reactions from a cholesterol precursor to result in the more biologically active forms that are measured in clinical practice. These include the substrate-dependent formation of 25(OH)D from vitamin D by 25-hydroxylase and the subsequent highly regulated 1α-hydroxylation to 1,25(OH)2D in the kidney and extrarenal tissues. 24-Hydroxylase metabolizes 25(OH)D and 1,25(OH)2D to forms without clear biological function. Although commonly considered to be biologically equivalent, ergocalciferol and cholecalciferol and their metabolites differ in their binding affinities for vitamin D binding protein (VDBP). Cholecalciferol has about a 2-fold higher affinity for VDBP compared to ergocalciferol (17, 18), and 25(OH)D3 has a higher affinity than does 25(OH)D2 (19, 20), likely yielding different amounts of free vitamin D metabolite with different serum half-lives (D3 >D2) (8) available for hydroxylation. In addition to relative substrate abundance, the rates of synthesis of 25(OH)D2 vs 25(OH)D3 and 1,25(OH)2D2 vs 1,25(OH)2D3 may also depend on enzymatic preference for substrate and/or positive and negative feedback mechanisms, whereby one metabolite (eg, D2) possesses the ability to inhibit the formation and/or induce the degradation of another metabolite (eg, D3). Although many of these enzymatic reactions may be tissue- and species-specific, previous studies have shown that 25-hydroxylation of vitamin D2 and D3 by the enzyme CYP2R1 occurs at equal rates with high affinity but low Km, so vitamin D2 and D3 may well be truly competitive at physiological substrate levels (21–24). Essentially, the vitamin D 25-hydroxylase is saturable at physiological concentrations of vitamin D, so lower 25(OH)D3 levels in the presence of higher 25(OH)D2 may in part be the result of competition for enzymatic activity. Moreover, recent literature suggests that there are likely additional enzymes responsible for 25-hydroxylation (eg, CYP2J2, CYP3A4) that may have different activity levels and prefer D2 over D3 as substrate (24–26). CYP3A4 has demonstrated 24-hydroxylase activity toward both 1α-metabolites and vitamin D3 (24), leaving the possibility for an enzyme that preferentially forms 25(OH)D2 while preferentially degrading 25(OH)D3. The finding that 25(OH)D2 comprises a higher fraction of total 25(OH)D than 1,25(OH)2D2 does of total 1,25(OH)2D (31.8 vs 16.5%, respectively) suggests either a lower rate of 1α-hydroxylation of 25(OH)D2 (unlikely because the renal 1α-hydroxylase does not discriminate between D2 and D3 substrates) or a more rapid catabolism (perhaps more likely due to lower binding of D2 to VDBP). Regulation of vitamin D2 vs D3 formation could allow the organism to adapt to changing external inputs such as dietary and supplemental intake (including extremes of deficiency/excess) and sun exposure within the larger context of metabolic conditions that influence enzymatic activity. In sum, there may be mechanisms by which the abundance of D2 and its metabolites could affect the metabolism and abundance of D3.
Our cross-sectional results demonstrate inverse associations of vitamin D2 and vitamin D3 metabolite levels that are compatible with, and complement, those described in other recent publications. Demetriou et al (27) and Nimitphong et al (28) reported that D2 supplementation decreased 25(OH)D3, and Biancuzzo et al (29) reported decreased 1,25(OH)2D3 levels with D2 supplementation. Our findings are also consistent with a study in which patients were randomized to oral placebo, 2000 IU/d ergocalciferol, or 2000 IU/d cholecalciferol (30). After 8 weeks of therapy, the level of total 25(OH)D was higher in the cholecalciferol supplement group, whereas 25(OH)D3 levels decreased in the group that received ergocalciferol and to a greater degree than those on placebo, indicating that the effect is due to more than just the difference in the half-life of D2 and D3. Similarly, some have noted cholecalciferol to be more effective in raising total 25(OH)D levels compared to ergocalciferol (8, 28). These findings suggest that increasing intake of vitamin D2 results in decreased levels of D3.
Our results and those of others suggest that vitamin D2 supplementation may reduce vitamin D3 availability and contribute proportionately less to overall vitamin D nutrition. Higher 25(OH)D2 levels were associated with lower 25(OH)D3 and 1,25(OH)2D3 concentrations and did not correlate with higher levels of either total 25(OH)D or calcitriol. In contrast, higher levels of 25(OH)D3 were strongly related to higher total 1,25(OH)2D concentrations. A stronger association was seen between 1,25(OH)2D2 and total 1,25(OH)D levels than between 25(OH)D2 and total 25(OH)D levels, perhaps due to the overall lower circulating concentrations of 1,25(OH)2D (measured in picograms per milliliter) compared to 25(OH)D (measured in nanograms per milliliter). The positive association between 1,25(OH)2D2 and 1,25(OH)2D was much weaker than the relationship between 1,25(OH)2D3 and total 1,25(OH)2D and may reflect the relative abundance of 1,25(OH)2D3 compared to 1,25(OH)2D2, and/or the relatively shorter half-life of D2 vs D3 (8). These findings, like other reports in the vitamin D supplementation literature, are compatible with a greater effectiveness of cholecalciferol in raising levels of total 25(OH)D and 1,25(OH)2D (8). Many supplements today are in the form of D3; however, the only high-dose, prescription vitamin D formulation currently available in the United States is ergocalciferol.
It might be expected that total 25(OH)D and total 1,25(OH)2D would have a stronger positive correlation. However, the relationship described in our cohort is similar in magnitude to that recently reported in the European Male Aging Study (EMAS) cohort (12). Prior to the EMAS cohort, 1,25(OH)2D concentrations were never determined on a large scale with the accurate methods used in these analyses. Further studies should investigate whether factors like PTH, VDBP, calcium, fibroblast growth factor-23, obesity, race, renal function, and/or age may attenuate the association between 25(OH)D and 1,25(OH)2D via their effects on the complex regulation of 1α-hydroxylase and 24-hydroxylase. The strength of this relationship may also depend on the sufficiency of 25(OH)D stores, with a stronger relationship observed in states of 25(OH)D deficiency when its availability functions as the rate-limiting step in 1,25(OH)2D formation.
Strengths of this study include a substantial sample size and the use of sensitive and specific LC-MS/MS vitamin D metabolite assays that separately analyze D2 and D3 concentrations. It also has several potential limitations. Because our analyses are cross-sectional, causal relationships cannot be determined. However, our observational findings are consistent with recent interventional studies (see earlier in Discussion) that revealed similar inverse associations between metabolites of ergocalciferol and cholecalciferol. Although our finding of a negative association between 25(OH)D2 and 25(OH)D3 levels could be due to “confounding by indication” with vitamin D-insufficient men being treated with D2 supplementation, we believe this to be unlikely because 25(OH)D levels were not commonly measured in clinical practice in men in 2000–2002, the amounts of supplementation reported by our participants were not typical of those used to treat vitamin D insufficiency (average, 384 IU/d), and compared to those with higher 25(OH)D3 levels there was not a disproportionate number of men with 25(OH)D3 <20 ng/mL using supplements. Our vitamin D measurements were made in mass units and therefore do not account for the slightly different molecular weights of vitamin D2 and D3 metabolites. However, the magnitude of this error is small and unlikely to affect the relationships described in this paper. Our study included only older, community-dwelling, predominantly Caucasian men and may not be applicable to other populations, and information concerning the forms of vitamin D supplementation (ergocalciferol or cholecalciferol) was not collected from study participants. Finally, it may be important to remember that we examined serum levels that may not accurately reflect those in tissues, and these relationships may differ from tissue to tissue because the availability and/or activity of the hydroxylase enzymes and tissue preferences for D2 or D3 may differ.
Conclusions
In summary, in a large cohort of older men, 25(OH)D2 was associated with lower levels of 25(OH)D3 and 1,25(OH)2D3 and was not associated with higher total levels of 25(OH)D or 1,25(OH)2D. Conversely, total 25(OH)D and 1,25(OH)2D were positively correlated with 25(OH)D3 levels. These associations raise the question of whether ergocalciferol and cholecalciferol are equally effective in maintaining or raising biologically active vitamin D levels. Additional studies are needed to further evaluate these relationships and their implications for the roles of ergocalciferol and cholecalciferol supplementation.
Acknowledgments
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the National Institute on Aging, the National Center for Research Resources, and NIH Roadmap for Medical Research under the following grant numbers: U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01 AG027810, and UL1 TR000128.
This work was supported in part by an independent investigator grant (SRA-12-009) (to E.S.O.) from Merck & Co, Inc. C.M.S. is supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant T32DK007674-20. D.V. is a senior clinical investigator supported by the University Hospital in Leuven.
Complimentary graphic design services for figures were provided by Brian D. Swanson.
Disclosure Summary: C.M.S., C.M.N., S.S., C.G.L., E.B.-C., I.J., J.A.C., S.B., and D.V. have nothing to disclose. E.S.O. consults for and has received research support from Amgen, Lilly, and Merck and serves on the advisory board of Wright Medical Tech. R.B. received lecture fees from Amgen, Novartis, Novo Nordisk, Chugai, and Teijin and gave a license to a university patent on vitamin D analogs to Hybrigenix (France).
Footnotes
- BMI
- body mass index
- CV
- coefficient of variation
- eGFR
- estimated glomerular filtration rate
- LC-MS/MS
- liquid chromatography-tandem mass spectrometry
- LLQ
- lower limit of quantification
- 1,25(OH)2D
- 1,25-dihydroxyvitamin D
- 1,25(OH)2D2
- 1,25-dihydroxyvitamin D2
- 1,25(OH)2D3
- 1,25-dihydroxyvitamin D3
- 25(OH)D
- 25-hydroxyvitamin D
- 25(OH)D2
- 25-hydroxyvitamin D2
- 25(OH)D3
- 25-hydroxyvitamin D3
- VDBP
- vitamin D binding protein.
References
- 1. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2011;96:1911–1930 [DOI] [PubMed] [Google Scholar]
- 2. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bouillon R, Van Schoor NM, Gielen E, et al. Optimal vitamin D status: a critical analysis on the basis of evidence-based medicine. J Clin Endocrinol Metab. 2013;98:E1283–E1304 [DOI] [PubMed] [Google Scholar]
- 4. Cauley JA, Parimi N, Ensrud KE, et al. Serum 25-hydroxyvitamin D and the risk of hip and nonspine fractures in older men. J Bone Miner Res. 2010;25:545–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ensrud KE, Taylor BC, Paudel ML, et al. Serum 25-hydroxyvitamin D levels and rate of hip bone loss in older men. J Clin Endocrinol Metab. 2009;94:2773–2780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281 [DOI] [PubMed] [Google Scholar]
- 7. Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, et al. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. BMJ. 2009;339:b3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Romagnoli E, Mascia ML, Cipriani C, et al. Short and long-term variations in serum calciotropic hormones after a single very large dose of ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3) in the elderly. J Clin Endocrinol Metab. 2008;93:3015–3020 [DOI] [PubMed] [Google Scholar]
- 9. Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study–a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–585 [DOI] [PubMed] [Google Scholar]
- 10. Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS). Contemp Clin Trials. 2005;26:557–568 [DOI] [PubMed] [Google Scholar]
- 11. Orwoll E, Nielson CM, Marshall LM, et al. Vitamin D deficiency in older men. J Clin Endocrinol Metab. 2009;94:1214–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vanderschueren D, Pye SR, O'Neill TW, et al. Active vitamin D (1,25-dihydroxyvitamin D) and bone health in middle-aged and elderly men: the European Male Aging Study (EMAS). J Clin Endocrinol Metab. 2013;98:995–1005 [DOI] [PubMed] [Google Scholar]
- 13. Block G, Hartman AM, Naughton D. A reduced dietary questionnaire: development and validation. Epidemiology. 1990;1:58–64 [DOI] [PubMed] [Google Scholar]
- 14. Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10:405–411 [DOI] [PubMed] [Google Scholar]
- 15. Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–162 [DOI] [PubMed] [Google Scholar]
- 16. Levey AS, Coresh J, Greene T, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53:766–772 [DOI] [PubMed] [Google Scholar]
- 17. Hollis BW. Comparison of equilibrium and disequilibrium assay conditions for ergocalciferol, cholecalciferol and their major metabolites. J Steroid Biochem. 1984;21:81–86 [DOI] [PubMed] [Google Scholar]
- 18. Nilsson SF, Ostberg L, Peterson PA. Binding of vitamin D to its human carrier plasma protein. Biochem Biophys Res Commun. 1972;46:1380–1387 [DOI] [PubMed] [Google Scholar]
- 19. Glendenning P, Chew GT, Inderjeeth CA, Taranto M, Fraser WD. Calculated free and bioavailable vitamin D metabolite concentrations in vitamin D-deficient hip fracture patients after supplementation with cholecalciferol and ergocalciferol. Bone. 2013;56:271–275 [DOI] [PubMed] [Google Scholar]
- 20. Bouillon R. The vitamin D binding protein DBP. In: Feldman D, Pike JW, Adams JS, eds. Vitamin D. 3rd ed Waltham, MA: Academic Press (Elsevier); 2011;57–72 [Google Scholar]
- 21. Cheng JB, Motola DL, Mangelsdorf DJ, Russell DW. De-orphanization of cytochrome P450 2R1: a microsomal vitamin D 25-hydroxilase. J Biol Chem. 2003;278:38084–38093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shinkyo R, Sakaki T, Kamakura M, Ohta M, Inouye K. Metabolism of vitamin D by human microsomal CYP2R1. Biochem Biophys Res Commun. 2004;324:451–457 [DOI] [PubMed] [Google Scholar]
- 23. Strushkevich N, Usanov SA, Plotnikov AN, Jones G, Park HW. Structural analysis of CYP2R1 in complex with vitamin D3. J Mol Biol. 2008;380:95–106 [DOI] [PubMed] [Google Scholar]
- 24. Zhu J, DeLuca HF. Vitamin D 25-hydroxylase - four decades of searching, are we there yet? Arch Biochem Biophys. 2012;523:30–36 [DOI] [PubMed] [Google Scholar]
- 25. Zhu JG, Ochalek JT, Kaufmann M, Jones G, Deluca HF. CYP2R1 is a major, but not exclusive, contributor to 25-hydroxyvitamin D production in vivo. Proc Natl Acad Sci USA. 2013;110:15650–15655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Aiba I, Yamasaki T, Shinki T, et al. Characterization of rat and human CYP2J enzymes as vitamin D 25-hydroxylases. Steroids. 2006;71:849–856 [DOI] [PubMed] [Google Scholar]
- 27. Demetriou ET, Travison TG, Holick MF. Treatment with 50,000 IU vitamin D2 every other week and effect on serum 25-hydroxyvitamin D2, 25-hydroxyvitamin D3, and total 25-hydroxyvitamin D in a clinical setting. Endocr Pract. 2012;18:399–402 [DOI] [PubMed] [Google Scholar]
- 28. Nimitphong H, Saetung S, Chanprasertyotin S, Chailurkit LO, Ongphiphadhanakul B. Changes in circulating 25-hydroxyvitamin D according to vitamin D binding protein genotypes after vitamin D3 or D2 supplementation. Nutr J. 2013;12:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Biancuzzo RM, Clarke N, Reitz RE, Travison TG, Holick MF. Serum concentrations of 1,25-dihydroxyvitamin D2 and 1,25-dihydroxyvitamin D3 in response to vitamin D2 and vitamin D3 supplementation. J Clin Endocrinol Metab. 2013;98:973–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lehmann U, Hirche F, Stangl GI, Hinz K, Westphal S, Dierkes J. Bioavailability of vitamin D(2) and D(3) in healthy volunteers, a randomized placebo-controlled trial. J Clin Endocrinol Metab. 2013;98:4339–4345 [DOI] [PubMed] [Google Scholar]