Abstract
Background:
Subclinical thyroid dysfunction is common in the elderly, yet its relationship with hip fracture and bone mineral density (BMD) is unclear.
Objective:
We examined the association between endogenous subclinical hyper- and hypothyroidism and hip fracture and BMD in older adults.
Methods:
A total of 4936 US individuals 65 years old or older enrolled in the Cardiovascular Health Study and not taking thyroid preparations were included. Analyses of incident hip fracture were performed by thyroid status, over a median follow-up of 12 years. A cross-sectional analysis of thyroid status and BMD was performed in a subset of 1317 participants who had dual-energy x-ray absorptiometry scans. Models were adjusted for risk factors and stratified by sex.
Results:
No association was found between subclinical hypothyroidism and incident hip fracture compared with euthyroidism, when assessed at a single time point or persisting at two time points, in either women [hazard ratio (HR) 0.91, 95% confidence interval (CI) 0.69–1.20 for a single and HR 0.79, 95% CI 0.52–1.21 for two time points] or men (HR 1.27, 95% CI 0.82–1.95 for a single and HR 1.09, 95% CI 0.57–2.10 for two time points). Likewise, no association was found between subclinical hyperthyroidism and incident hip fracture in either sex (HR 1.11, 95% CI 0.55–2.25 in women and HR 1.78, 95% CI 0.56–5.66 in men). No association was found between subclinical thyroid dysfunction and BMD at the lumbar spine, total hip, or femoral neck sites.
Conclusions:
Our data suggest no association between subclinical hypothyroidism or subclinical hyperthyroidism and hip fracture risk or BMD in older men and women. Additional data are needed to improve the precision of estimates for subclinical hyperthyroidism and in men.
Osteoporosis is common in both women and men with increasing age (1). Fragility fractures are associated with increased mortality (2) and osteoporosis medication use reduces mortality risk in men and women (3). Although overt hyperthyroidism (4–6) and overt hypothyroidism (5) have been linked to osteoporosis in both women and men, it is unclear whether subclinical thyroid dysfunction, in which TSH levels are altered but thyroid hormone levels are in the reference range, has a negative effect on bone. The finding that TSH acts directly on bone (7, 8) supports the idea that subclinical thyroid dysfunction may also confer risk of low bone density and fracture.
Some studies of older or middle-aged adults suggest that TSH concentration is associated with bone mineral density (BMD) (9–14) or fracture risk (13, 15–17), but other reports found discrepant results (18–22). Subclinical thyroid dysfunction occurs in approximately 16% of those aged 65 years and older (23), and TSH has been shown to increase with age (24). If subclinical thyroid dysfunction has a negative effect on bone, the effects would largely be seen in the elderly.
In the present study, we aimed to use data from the Cardiovascular Health Study (CHS), a large cohort study of community-dwelling men and women aged 65 years and older, to determine whether endogenous subclinical thyroid dysfunction, assessed at either one time point or persistent, is an independent risk factor for low bone density or incident hip fracture in older men and women.
Subjects and Methods
Study participants
The CHS is a population-based, longitudinal study of 5888 adults aged 65 years and older (25). Enrollment of the original cohort of 5201 adults occurred between May 1989 and June 1990, and an additional cohort of 687 African Americans was enrolled in 1992–1993. Eligible individuals were identified from an age- and sex-stratified random sample of the Medicare eligibility rosters in four US communities: Washington County, Maryland; Allegheny County, Pennsylvania; Sacramento County, California; and Forsyth County, North Carolina. To be eligible, individuals had to be noninstitutionalized, expecting to remain in the area for the following 3 years, not in active treatment for cancer, not wheelchair bound at home, not requiring a proxy respondent at entry, and capable of providing medical consent. Household members of the sampled individuals were recruited, if eligible. The institutional review boards of all four sites and the coordinating center at the University of Washington in Seattle approved the study. All participants gave informed consent.
Annual study visits through 1998–1999 included a detailed medical history, physical examination, and assessment of health status. Blood was drawn after a 12-hour fast, and serum was processed and stored in −70°C freezers for future testing. Follow-up for events, including hip fracture, is ongoing.
Thyroid function testing
TSH testing was performed on 3678 baseline samples from the original cohort using a third-generation assay with a functional sensitivity of 0.008 mU/L (LumaTag hTSH; Nichols Institute) as previously described (23) and in participants from both cohorts with available serum at the 1992–1993 (n = 3996), 1994–1995 (n = 4005), and 1996–1997 (n = 3371) visits using a third-generation assay with a functional sensitivity of 0.005 mU/L (Elecsys 2010 analyzer; Roche Diagnostics) as previously described (26). A validation study was performed across the range of TSH values using both assays (27). Free T4 (FT4) concentrations were assessed at all time points in participants with abnormal TSH levels, using a direct monoclonal antibody assay (Amerlex-MAB; Amersham International) at the 1989–1990 visit and a chemiluminescent immunoassay (Elecsys 2010; Roche Diagnostics) at all other time points. Total T3 concentrations were measured using the Elecsys chemiluminescent assay in individuals with serum TSH levels below 0.10 mU/L at the 1992–1993, 1994–1995, and 1996–1997 visits.
Classification by thyroid status
Study participants were classified into one of three groups based on their first available thyroid function test: 1) subclinical hyperthyroidism was defined as a TSH concentration less than 0.45 mU/L with FT4 and T3 concentrations in the reference range; 2) subclinical hypothyroidism was defined as a TSH concentration of greater than 4.50 mU/L and less than 20 mU/L with a normal FT4 concentration; and 3) euthyroidism was defined as a TSH concentration of 0.45–4.50 mU/L. Due to small numbers, participants with overt hyper- or hypothyroidism were excluded from analysis.
Assessment of outcome variables
Incident hip fractures were assessed every 6 months by patient report, with confirmation by hospital medical record reviews. The Centers for Medicare and Medicaid Services (CMS) inpatient data of hip fractures were used in participants who were lost to follow-up. Diagnoses were ascertained using International Classification of Diseases, ninth revision, codes and included nontraumatic hip fractures through June 2011. Dual-energy x-ray absorptiometry (DXA) was performed in 1994–1995 at the Pennsylvania and California sites only. Hip and whole-body DXA scans were obtained using Hologic QDR-2000 densitometers (Hologic, Inc) with a protocol similar to that in the Study of Osteoporotic Fractures and the Fracture Intervention Trial (28, 29). Scans were read blindly at the University of California, San Francisco, Reading Center using Hologic software, version 7.10.
Assessment of covariates
Demographic and anthropometric factors were obtained at baseline and during follow-up. Covariates included age, sex, self-reported race, weight, smoking status (never, former, or current), alcohol use, and activity level. Usual leisure time activity was assessed using the modified Minnesota leisure-time activities questionnaire that evaluated frequency and duration of 15 different activities during the prior 2 weeks (30). Use of thyroid medications (thyroid hormones, antithyroid medications), bisphosphonates, thiazide diuretics, oral steroids, and estrogens was assessed at baseline and annually via examination of medication bottles.
Statistical analysis
Analyses included participants who at the time of their first thyroid tests (out of the four time points with measurements, hereafter identified as baseline) were not taking thyroid medication and who were in the subclinically hypothyroid, subclinically hyperthyroid, or euthyroid group. Baseline characteristics between participants who were subclinically hypothyroid, subclinically hyperthyroid, and euthyroid were compared using an ANOVA or χ2 test. Years of observation at risk were defined from baseline to date of a hip fracture, death, or last contact in those without data from CMS, whichever occurred first. Participants with a hip fracture due to pathology or a motor vehicle accident were censored at the time of their hip fracture. Incidence rates of hip fracture were calculated by dividing the number of participants who developed a hip fracture by the person-years of observation at risk.
Sex-stratified Kaplan-Meier plots were used to display the cumulative incidence of fracture by thyroid status. Sex-stratified multivariable Cox proportional hazards regression models were used to estimate the hazard ratios (HRs) associated with subclinical hypothyroidism and subclinical hyperthyroidism compared with euthyroidism for incident hip fracture. The proportional hazards assumption was tested using Schoenfeld residuals and was not rejected. However, visual examination of the Kaplan-Meier plots showed crossing lines. We explored this by considering only the first 12 years of follow-up. Results were unchanged, and thus, full follow-up is presented. Persistent subclinical thyroid disease was examined as a fixed exposure in which participants with persistent or transient subclinical thyroid disease were compared with persistently euthyroid participants. Participants were classified as being persistently subclinically hypothyroid, persistently subclinically hyperthyroid, or persistently euthyroid if they met criteria at their first and second TSH measurements. All analyses were sex stratified and were adjusted for age, race, weight, physical activity level, smoking, alcohol use, oral corticosteroid and thiazide use, and estrogen use (in women).
In the subgroup of participants who had DXA scans at the 1994–1995 visit, ANOVA and multivariable linear regression were used in cross-sectional analyses of categorical thyroid status with lumbar spine and hip (total and femoral neck) BMD. Persistence at the 1994–1995 visit was determined by thyroid status at the 1992–1993 and 1994–1995 visits or at the 1989–1990 and 1994–1995 visits if missing TSH at the 1992–1993 visit. P values for the model adjusting for age, race, weight, alcohol use, smoking, activity level, oral corticosteroid and thiazide diuretic use, and estrogen and bisphosphonate use (women only) are shown. No men were taking bisphosphonates.
All statistical analyses were performed using STATA version 11 (StataCorp LP).
Results
Baseline characteristics
At the time of first TSH measurement, there were a total of 4936 participants not taking thyroid medications who had thyroid testing results within the subclinically hypothyroid, subclinically hyperthyroid, and euthyroid ranges. This cohort included 678 individuals (418 women, 260 men) with subclinical hypothyroidism, 82 individuals (50 women, 32 men) with subclinical hyperthyroidism, and 4176 (2297 women, 1879 men) euthyroid participants. Characteristics of participants are described in Table 1. Significant differences by thyroid status were found for race in both men and women and age, smoking, alcohol consumption, and corticosteroid use in men only. There were a total of 564 hip fractures (404 women, 160 men) over a median of 12 years of follow-up.
Table 1.
Characteristics of the Study Sample at Baseline
| Women |
Men |
|||||||
|---|---|---|---|---|---|---|---|---|
| SHypo (n = 418) | Euthyroid (n = 2297) | SHyper (n = 50) | P Value | SHypo (n = 260) | Euthyroid (n = 1879) | SHyper (n = 32) | P Value | |
| Mean age, y | 73.2 (5.6) | 73.0 (5.6) | 73.7 (6.8) | .52 | 75.5 (6.1) | 73.9 (5.7) | 73.8 (6.6) | <.001 |
| Caucasian | 378 (90) | 1835 (80) | 43 (86) | <.001 | 240 (92) | 1577 (84) | 24 (66) | <.001 |
| Weight, kg | 68.1 (14.7) | 67.8 (14.1) | 71.0 (15.6) | .27 | 78.2 (12.2) | 79.4 (12.7) | 76.3 (11.4) | .14 |
| BMI, kg/m2 | 27.0 (5.5) | 26.8 (5.2) | 28.5 (5.5) | .07 | 26.3 (3.9) | 26.4 (3.8) | 25.6 (3.5) | .46 |
| Activity level, kcal | 945 (337–1837) | 862 (276–2010) | 996 (371–1845) | .79 | 1072 (487–2227) | 1195 (450–2490) | 1121 (549–1689) | .91 |
| Ever-smoker | 172 (41.2) | 966 (42.1) | 21 (42.0) | .95 | 153 (58.9) | 1317 (70.2) | 24 (75.0) | .001 |
| Alcohol use | 177 (42.6) | 968 (42.2) | 17 (34.0) | .50 | 128 (49.2) | 1078 (57.5) | 15 (46.9) | .02 |
| Estrogen use | 48 (11.5) | 279 (12.2) | 7 (14.0) | .86 | ||||
| Corticosteroid use | 3 (0.72) | 47 (2.0) | 1 (2.0) | .18 | 2 (0.77) | 39 (2.1) | 3 (9.4) | .005 |
| Thiazide use | 92 (22.1) | 514 (22.4) | 8 (16.0) | .56 | 33 (12.7) | 275 (14.6) | 5 (15.6) | .71 |
| TSH, mU/L | 6.7 (2.7) | 2.2 (1.0) | 0.24 (0.13) | n/aa | 6.6 (2.5) | 2.1 (0.94) | 0.28 (0.13) | n/aa |
Abbreviation: n/a, not available. Entries show number (percentage), except for age, weight, BMI, and TSH, which show mean (SD), and activity level, which shows median (interquartile range).
Groups defined by this measure.
Risk of hip fracture by category of subclinical thyroid dysfunction
Neither women nor men with subclinical thyroid dysfunction had a greater incidence of hip fracture compared with euthyroid participants (Figure 1 and Table 2). Results did not change with adjustment for covariates [subclinical hypothyroidism HR 0.91 and 95% confidence interval (CI) 0.69–1.20 in women and HR 1.27 and 95% CI 0.82–1.95 in men; subclinical hyperthyroidism HR 1.11 and 95% CI 0.55–2.25 in women and HR 1.78 and 95% CI 0.56–5.66 in men]. Although the point estimate suggested that men with subclinical hyperthyroidism may have a higher incidence of hip fracture compared with euthyroid men, power was limited to assess this risk, with only three hip fractures in this group.
Figure 1.
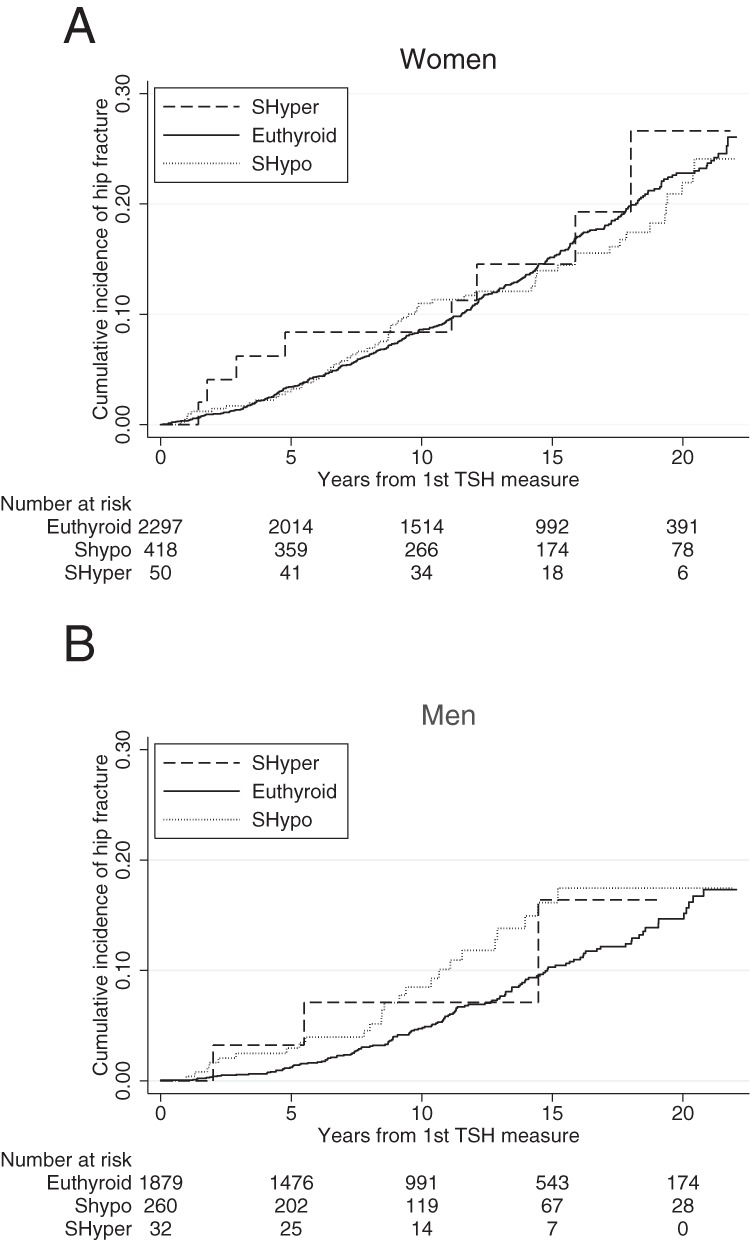
Sex-stratified Kaplan-Meier plots of incident hip fracture in participants not taking thyroid medication at first thyroid test, in women (A) and men (B).
Table 2.
Incidence of Hip Fractures by Baseline Thyroid Status
| Women |
Men |
|||||
|---|---|---|---|---|---|---|
| Events per Number at Risk, n | Incidence Rate (95% CI)a | HR (95% CI)b | Events per Number at Risk, n | Incidence Rate (95% CI)a | HR (95% CI)b | |
| Euthyroid | 337/2297 | 11.2 (10.1, 12.5) | 1.00 (Referent) | 132/1879 | 6.4 (5.4, 7.6) | 1.00 (Referent) |
| SHypo | 59/418 | 10.8 (8.4, 14.0) | 0.91 (0.69, 1.20) | 25/260 | 9.3 (6.3, 13.7) | 1.27 (0.82, 1.95) |
| SHyper | 8/50 | 13.0 (6.5, 26.0) | 1.11 (0.55, 2.25) | 3/32 | 9.5 (3.1, 29.5) | 1.78 (0.56, 5.66) |
| Total | 404/2765 | 11.2 (10.1, 12.3) | 160/2171 | 6.4 (5.4, 7.6) | ||
Abbreviations: SHyper, subclinical hyperthyroidism; SHypo, subclinical hypothyroidism.
Incidence rate is the number of fractures per 1000 person-years.
Adjusted for age, race, weight, alcohol use, smoking, activity level, oral corticosteroid and thiazide use, and estrogen use (women only).
Approximately 75% of the participants had data available to assess hip fracture risk from persistent subclinical thyroid dysfunction (Table 3). One woman and five men progressed to overt hypothyroidism, and two women progressed to overt hyperthyroidism. There was no increased risk of incident hip fracture in those with persistent subclinical hypothyroidism (HR 0.79 and 95% CI 0.52–1.21 in women and HR 1.09 and 95% CI 0.57–2.10 in men) or transient subclinical hypothyroidism (HR 1.10 and 95% CI 0.69–1.76 in women and HR 1.51 and 95% CI 0.65–3.48 in men) compared with euthyroidism (Table 3). We were unable to model risk from persistent or transient subclinical hyperthyroidism due to small numbers.
Table 3.
Incidence of Hip Fractures by Persistent Thyroid Status
| Women |
Men |
|||||
|---|---|---|---|---|---|---|
| Events per Number at Risk, n | Incidence Rate (95% CI)a | HR (95% CI)b | Events per Number at Risk, n | Incidence Rate (95% CI)a | HR (95% CI)b | |
| Persistently euthyroid | 245/1751 | 12.4 (11.0, 14.1) | 1.00 (Referent) | 105/1399 | 7.7 (6.3, 9.3) | 1.00 (Referent) |
| Transiently SHypo | 19/125 | 14.5 (9.3, 22.8) | 1.10 (0.69, 1.76) | 6/53 | 12.4 (5.6, 27.6) | 1.51 (0.65, 3.48) |
| Persistently SHypo | 24/186 | 11.3 (7.5, 16.8) | 0.79 (0.52, 1.21) | 10/124 | 8.6 (4.7, 16.1) | 1.09 (0.57, 2.10) |
| Transiently SHyper | 0/12 | 0 | 1/9 | 10.4 (1.5, 74.1) | ||
| Persistently SHyper | 4/11 | 37.0 (13.9, 98.5) | 1/11 | 11.7 (1.6, 82.7) | ||
| Total | 292/2085 | 12.5 (11.1, 14.0) | 123/1596 | 7.9 (6.6, 9.5) | ||
Abbreviations: SHyper, subclinical hyperthyroidism; SHypo, subclinical hypothyroidism.
Person-time incidence rate is the number of fractures per 1000 population.
Adjusted for age, race, weight, alcohol use, smoking, activity level, oral corticosteroid and thiazide use, and estrogen use (women only).
Thyroid status and BMD
A subgroup of 1317 participants had a DXA scan and thyroid laboratory testing at the 1994–1995 visit. No association was found between thyroid classification based on thyroid function testing at the 1994–1995 visit and spine or hip BMD in men or women (Table 4). More than 93% of the participants in this subgroup had thyroid function testing prior to 1994–1995, which allowed for the assessment of persistence (Table 5). No participants progressed from subclinical hypothyroidism to overt hypothyroidism in this subgroup. There was no difference in the spine and hip BMD between those with transient or persistent subclinical hypothyroidism compared with euthyroidism (Table 5). We were unable to assess the relationship between persistent or transient subclinical hyperthyroidism and BMD due to the low number of participants in these groups.
Table 4.
Spine and Hip BMD (Grams per Square Centimeter) by Thyroid Status, Mean (SD)
| Women |
Men |
|||||||
|---|---|---|---|---|---|---|---|---|
| n | Lumbar Spine | Total Hip | Femoral Neck | n | Lumbar Spine | Total Hip | Femoral Neck | |
| Euthyroid | 638 | 0.93 (0.23) | 0.75 (0.14) | 0.65 (0.13) | 533 | 1.13 (0.24) | 0.95 (0.16) | 0.78 (0.14) |
| SHypo | 71 | 0.92 (0.24) | 0.73 (0.14) | 0.63 (0.13) | 53 | 1.16 (0.33) | 0.92 (0.17) | 0.76 (0.16) |
| SHyper | 10 | 1.04 (0.29) | 0.70 (0.12) | 0.63 (0.10) | 12 | 1.14 (0.24) | 0.95 (0.16) | 0.81 (0.12) |
| P valuea | .53 | .29 | .60 | .49 | .64 | .70 | ||
Abbreviations: SHyper, subclinical hyperthyroidism; SHypo, subclinical hypothyroidism.
Adjusted for age, race, weight, alcohol use, smoking, activity level, oral corticosteroid and thiazide use, and estrogen and bisphosphonate use (women only).
Table 5.
Spine and Hip BMD (Grams per Square Centimeter) by Persistence of Subclinical Hypothyroidism, Mean (SD)
| Women |
Men |
|||||||
|---|---|---|---|---|---|---|---|---|
| n | Lumbar Spine | Total Hip | Femoral Neck | n | Lumbar Spine | Total Hip | Femoral Neck | |
| Euthyroid | 533 | 0.92 (0.22) | 0.75 (0.14) | 0.64 (0.13) | 446 | 1.14 (0.24) | 0.95 (0.16) | 0.79 (0.14) |
| Transient SHypo | 40 | 0.93 (0.20) | 0.75 (0.12) | 0.63 (0.12) | 21 | 1.06 (0.23) | 0.89 (0.18) | 0.72 (0.14) |
| Persistent SHypo | 54 | 0.94 (0.25) | 0.74 (0.13) | 0.64 (0.12) | 35 | 1.14 (0.25) | 0.92 (0.18) | 0.77 (0.18) |
| P valuea | .66 | .70 | .68 | .57 | .47 | .42 | ||
Abbreviations: SHyper, subclinical hyperthyroidism; SHypo, subclinical hypothyroidism.
Adjusted for age, race, weight, alcohol use, smoking, activity level, oral corticosteroid and thiazide use, and estrogen and bisphosphonate use (women only).
Discussion
We did not find an association between endogenous subclinical hypothyroidism or subclinical hyperthyroidism and increased risk of hip fracture or lower BMD at the spine or hip in elderly men or women in a large, population-based cohort. To our knowledge, we are the first to examine the persistence of subclinical hypothyroidism and the risk of fracture or low BMD. Confirmation of subclinical hypothyroidism on repeat testing not only mimics recommendations for clinical practice (31), but it is also important in appropriate classification of thyroid status. We have previously shown that within 2 years, 35% of older people with subclinical hypothyroidism revert to euthyroidism (26). However, even with the additional refinement of requiring persistence, we still found no associations of subclinical hypothyroidism with hip fracture or BMD in men or women.
In an analysis of 3567 participants from the CHS original cohort, Lee et al (32) similarly did not detect associations between either type of subclinical thyroid dysfunction and hip fracture in women, although, in contrast to our findings, found associations between endogenous subclinical hypothyroidism and subclinical hyperthyroidism and hip fracture in men. A major difference between the previous analysis and ours is our expansion of the study population to 4936 participants through thyroid function testing in additional participants. Nevertheless, both subclinical thyroid dysfunction and hip fracture are far less common in men than women, which challenges the statistical power to assess this association in men. In the previous analysis, there was a lower incidence of hip fractures in euthyroid men who were not taking thyroid medication (4.9 vs 6.4 fractures per 1000 person-years), which led to a higher HR for subclinical hypothyroidism (1.84, 95% CI 0.98–3.43) in analyses comparable with ours. Longer follow-up and the addition of CMS data likely contributed to the higher euthyroid incidence rate reported in our analyses.
For subclinical hyperthyroidism, the effect in the previous analysis was magnified by a higher incidence of hip fracture in subclinically hyperthyroid men, although this was based on three hip fractures in 16 men, with a HR of 4.56 (95% CI 1.06–19.64). Of note, the estimates from our analyses are included in each of these 95% CIs. Despite improved precision from a larger cohort, more thorough assessment of fractures through inclusion of CMS data, and a longer duration of follow-up, we are unable to exclude a clinically relevant association between subclinical hypo- or hyperthyroidism and hip fracture in men, as shown by the width of the CIs for these estimates (0.82–1.95 and 0.56–5.66, respectively). However, we also found no association between either type of subclinical thyroid dysfunction and BMD in men, demonstrating consistency across outcomes. In addition, our findings are consistent with a recent case-cohort analysis from the Osteoporotic Fractures in Men cohort, in which baseline subclinical hypothyroidism or subclinical hyperthyroidism was not associated with a change in total hip or femoral neck BMD or a risk of hip or nonspine fractures (15). Additional investigation of this area is warranted, perhaps through the pooling of data across studies to improve the statistical power, as has been effectively performed for analyses of subclinical thyroid dysfunction and cardiovascular events (33–35).
Studies that have examined TSH as a continuous variable across the range of values have found an increased risk of hip fracture (15, 16, 22), nonspine fracture (13), and vertebral fracture (16, 17) at lower TSH levels, although participants with overt thyroid disease were included in several of these analyses. Most studies have found TSH assessed as a continuous variable to be directly associated with spine, femoral neck, total hip, or ultradistal radius BMD (9–12, 18), although this has not been seen in all studies (13, 18–20).
The mechanisms by which thyroid dysfunction may affect bone are complex. Initial reports suggested that thyroid hormone excess causes increased bone turnover (36, 37), supported by a study that demonstrated increases in BMD and decreased bone turnover after a reduction in T4 dose in those inappropriately overtreated (38). Conversely, in overt hypothyroidism, bone biopsies reveal a low bone turnover state (39). Both abnormal bone structure and increased proclivity to fall have been hypothesized to contribute to fractures in overt hypothyroidism (5). However, recent studies indicate that TSH may also be involved in bone remodeling. TSH inhibits osteoblasts and osteoclasts (8) through TSH receptors found on both cell types (7). Administration of TSH and overexpression of TSHR suppresses bone resorption in ovariectomized rodents (40). Knockout mice who lack the TSH receptor demonstrate osteopenia and have greater bone loss than wild-type mice do when both are rendered hyperthyroid and have undetectable TSH concentrations (41). This suggests that the lack of TSH signaling is responsible for increased bone loss in the TSH receptor knockout mice, which may be due to the absence of a TSHβ splice variant that is produced in bone marrow (41). TNFα production, which stimulates osteoclastogenesis, is also increased in TSH deficiency (8, 42). However, the relevance of these studies to humans is unclear because BMD is normal in boys with isolated TSH deficiency who are treated with levothyroxine (43).
A major strength of our study is the use of a large, prospective, community-based cohort of older men and women with a median of 12 years of follow-up for incident fractures. Assessment of thyroid function at more than one time point allowed the analysis of fracture risk in those not taking thyroid medications who were persistently subclinically hypothyroid. However, we were unable to assess the persistence of subclinical hyperthyroidism due to the low number of participants in this group. Persistent subclinical hyperthyroidism has not been found to be associated with osteoporotic fracture in a previous study (21). Our analyses of BMD were cross-sectional, and the analyses of subclinical hyperthyroidism and BMD in men and women were limited by small numbers. Despite the presence of comorbidity, nonthyroidal illness was unlikely to have affected our results because mean FT4 and total T3 were higher in subclinically hyperthyroid compared with euthyroid participants, in a subset who had a full panel of thyroid function tests at the 1992–1993 visit. We limited our examination to endogenous thyroid dysfunction, and we are also unable to comment on the relationship between thyroid function and hip fracture in populations younger than age 65 years. This study was observational and did not examine the risks and benefits of treating either subclinical hypothyroidism or subclinical hyperthyroidism.
In conclusion, our data suggest no relationship between subclinical hypothyroidism, at a single time point or persistent, and fracture risk or BMD in older men or women. We also found no association between subclinical hyperthyroidism and fracture risk or BMD, although we were unable to exclude a clinically meaningful effect of persistent subclinical hyperthyroidism on risk of hip fracture due to limited power. In the absence of randomized clinical trial data, our study offers the best evidence to date that subclinical thyroid dysfunction should not be treated to specifically preserve bone health or prevent fracture. However, the number of men with subclinical thyroid dysfunction was relatively small, and additional data are required to refine risk estimates in this population.
Acknowledgments
This work was supported by Grants R01AG032317 from the National Institute on Aging, T32DK007314 from the National Institute of Diabetes, Digestive, and Kidney Disorders; Contracts HHSN268201200036C, HHSN268200800007C, N01 HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and Grant HL080295 from the National Heart, Lung, and Blood Institute, with an additional contribution from the National Institute of Neurological Disorders and Stroke. Additional support was provided by Grant AG023629 from the National Institute on Aging. A full list of principal Cardiovascular Health Study investigators and institutions can be found at http://www.chs-nhlbi.org.
Disclosure Summary: The authors have nothing to disclose.
For editorial see page 2654
- BMD
- bone mineral density
- CHS
- Cardiovascular Health Study
- CI
- confidence interval
- CMS
- Centers for Medicare and Medicaid Services
- DXA
- dual-energy x-ray absorptiometry
- FT4
- free T4
- HR
- hazard ratio.
References
- 1. Dawson-Hughes B, Looker AC, Tosteson AN, Johansson H, Kanis JA, Melton LJ., 3rd The potential impact of the National Osteoporosis Foundation guidance on treatment eligibility in the USA: an update in NHANES 2005–2008. Osteoporos Int. 2012;23:811–820 [DOI] [PubMed] [Google Scholar]
- 2. Bliuc D, Nguyen ND, Milch VE, Nguyen TV, Eisman JA, Center JR. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301:513–521 [DOI] [PubMed] [Google Scholar]
- 3. Center JR, Bliuc D, Nguyen ND, Nguyen TV, Eisman JA. Osteoporosis medication and reduced mortality risk in elderly women and men. J Clin Endocrinol Metab. 2011;96:1006–1014 [DOI] [PubMed] [Google Scholar]
- 4. El Hadidy el HM, Ghonaim M, El Gawad S, El Atta MA. Impact of severity, duration, and etiology of hyperthyroidism on bone turnover markers and bone mineral density in men. BMC Endocr Disord. 2011;11:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vestergaard P, Mosekilde L. Fractures in patients with hyperthyroidism and hypothyroidism: a nationwide follow-up study in 16,249 patients. Thyroid. 2002;12:411–419 [DOI] [PubMed] [Google Scholar]
- 6. Karga H, Papapetrou PD, Korakovouni A, Papandroulaki F, Polymeris A, Pampouras G. Bone mineral density in hyperthyroidism. Clin Endocrinol (Oxf). 2004;61:466–472 [DOI] [PubMed] [Google Scholar]
- 7. Abe E, Marians RC, Yu W, et al. TSH is a negative regulator of skeletal remodeling. Cell. 2003;115:151–162 [DOI] [PubMed] [Google Scholar]
- 8. Hase H, Ando T, Eldeiry L, et al. TNFα mediates the skeletal effects of thyroid-stimulating hormone. Proc Natl Acad Sci USA. 2006;103:12849–12854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morris MS. The association between serum thyroid-stimulating hormone in its reference range and bone status in postmenopausal American women. Bone. 2007;40:1128–1134 [DOI] [PubMed] [Google Scholar]
- 10. Kim DJ, Khang YH, Koh JM, Shong YK, Kim GS. Low normal TSH levels are associated with low bone mineral density in healthy postmenopausal women. Clin Endocrinol (Oxf). 2006;64:86–90 [DOI] [PubMed] [Google Scholar]
- 11. Kim BJ, Lee SH, Bae SJ, et al. The association between serum thyrotropin (TSH) levels and bone mineral density in healthy euthyroid men. Clin Endocrinol (Oxf). 2010;73:396–403 [DOI] [PubMed] [Google Scholar]
- 12. Bertoli A, Fusco A, Andreoli A, et al. Effect of subclinical hypothyroidism and obesity on whole-body and regional bone mineral content. Horm Res. 2002;57:79–84 [DOI] [PubMed] [Google Scholar]
- 13. Murphy E, Gluer CC, Reid DM, et al. Thyroid function within the upper normal range is associated with reduced bone mineral density and an increased risk of nonvertebral fractures in healthy euthyroid postmenopausal women. J Clin Endocrinol Metab. 2010;95:3173–3181 [DOI] [PubMed] [Google Scholar]
- 14. van der Deure WM, Uitterlinden AG, Hofman A, Rivadeneira F, Pols HA, Peeters RP, Visser TJ. Effects of serum TSH and FT4 levels and the TSHR-Asp727Glu polymorphism on bone: the Rotterdam Study. Clin Endocrinol (Oxf). 2008;68:175–181 [DOI] [PubMed] [Google Scholar]
- 15. Waring AC, Harrison S, Fink HA, et al. A prospective study of thyroid function, bone loss, and fractures in older men: the MrOS study. J Bone Miner Res. 2013;28:472–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bauer DC, Ettinger B, Nevitt MC, Stone KL. Risk for fracture in women with low serum levels of thyroid-stimulating hormone. Ann Intern Med. 2001;134:561–568 [DOI] [PubMed] [Google Scholar]
- 17. Mazziotti G, Porcelli T, Patelli I, Vescovi PP, Giustina A. Serum TSH values and risk of vertebral fractures in euthyroid post-menopausal women with low bone mineral density. Bone. 2010;46:747–751 [DOI] [PubMed] [Google Scholar]
- 18. Bauer DC, Nevitt MC, Ettinger B, Stone K. Low thyrotropin levels are not associated with bone loss in older women: a prospective study. J Clin Endocrinol Metab. 1997;82:2931–2936 [DOI] [PubMed] [Google Scholar]
- 19. Grimnes G, Emaus N, Joakimsen RM, Figenschau Y, Jorde R. The relationship between serum TSH and bone mineral density in men and postmenopausal women: the Tromso study. Thyroid. 2008;18:1147–1155 [DOI] [PubMed] [Google Scholar]
- 20. Roef G, Lapauw B, Goemaere S, et al. Thyroid hormone status within the physiological range affects bone mass and density in healthy men at the age of peak bone mass. Eur J Endocrinol. 2011;164:1027–1034 [DOI] [PubMed] [Google Scholar]
- 21. Vadiveloo T, Donnan PT, Cochrane L, Leese GP. The Thyroid Epidemiology, Audit, and Research Study (TEARS): morbidity in patients with endogenous subclinical hyperthyroidism. J Clin Endocrinol Metab. 2011;96:1344–1351 [DOI] [PubMed] [Google Scholar]
- 22. Svare A, Nilsen TI, Asvold BO, et al. Does thyroid function influence fracture risk? Prospective data from the HUNT2 study, Norway. Eur J Endocrinol. 2013;169:845–852 [DOI] [PubMed] [Google Scholar]
- 23. Cappola AR, Fried LP, Arnold AM, et al. Thyroid status, cardiovascular risk, and mortality in older adults. JAMA. 2006;295:1033–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Waring AC, Arnold AM, Newman AB, Buzkova P, Hirsch C, Cappola AR. Longitudinal changes in thyroid function in the oldest old and survival: the cardiovascular health study all-stars study. J Clin Endocrinol Metab. 2012;97:3944–3950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276 [DOI] [PubMed] [Google Scholar]
- 26. Somwaru LL, Rariy CM, Arnold AM, Cappola AR. The natural history of subclinical hypothyroidism in the elderly: the cardiovascular health study. J Clin Endocrinol Metab. 2012;97:1962–1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hyland KA, Arnold AM, Lee JS, Cappola AR. Persistent subclinical hypothyroidism and cardiovascular risk in the elderly: the cardiovascular health study. J Clin Endocrinol Metab. 2013;98:533–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cummings SR, Black DM, Nevitt MC, et al. Appendicular bone density and age predict hip fracture in women. The Study of Osteoporotic Fractures Research Group. JAMA. 1990;263:665–668 [PubMed] [Google Scholar]
- 29. Black DM, Reiss TF, Nevitt MC, Cauley J, Karpf D, Cummings SR. Design of the Fracture Intervention Trial. Osteoporos Int. 1993;3(suppl 3):S29–S39 [DOI] [PubMed] [Google Scholar]
- 30. Taylor HL, Jacobs DR, Jr, Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31:741–755 [DOI] [PubMed] [Google Scholar]
- 31. Surks MI, Ortiz E, Daniels GH, et al. Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. JAMA. 2004;291:228–238 [DOI] [PubMed] [Google Scholar]
- 32. Lee JS, Buzkova P, Fink HA, et al. Subclinical thyroid dysfunction and incident hip fracture in older adults. Arch Intern Med. 2010;170:1876–1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rodondi N, den Elzen WP, Bauer DC, et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 2010;304:1365–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Collet TH, Gussekloo J, Bauer DC, et al. Subclinical hyperthyroidism and the risk of coronary heart disease and mortality. Arch Intern Med. 2012;172:799–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gencer B, Collet TH, Virgini V, et al. Subclinical thyroid dysfunction and the risk of heart failure events: an individual participant data analysis from 6 prospective cohorts. Circulation. 2012;126:1040–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Engler H, Oettli RE, Riesen WF. Biochemical markers of bone turnover in patients with thyroid dysfunctions and in euthyroid controls: a cross-sectional study. Clin Chim Acta. 1999;289:159–172 [DOI] [PubMed] [Google Scholar]
- 37. Harvey RD, McHardy KC, Reid IW, et al. Measurement of bone collagen degradation in hyperthyroidism and during thyroxine replacement therapy using pyridinium cross-links as specific urinary markers. J Clin Endocrinol Metab. 1991;72:1189–1194 [DOI] [PubMed] [Google Scholar]
- 38. Guo CY, Weetman AP, Eastell R. Longitudinal changes of bone mineral density and bone turnover in postmenopausal women on thyroxine. Clin Endocrinol (Oxf). 1997;46:301–307 [DOI] [PubMed] [Google Scholar]
- 39. Mosekilde L, Melsen F. Morphometric and dynamic studies of bone changes in hypothyroidism. Acta Pathol Microbiol Scand A. 1978;86:56–62 [DOI] [PubMed] [Google Scholar]
- 40. Sun L, Vukicevic S, Baliram R, et al. Intermittent recombinant TSH injections prevent ovariectomy-induced bone loss. Proc Natl Acad Sci USA. 2008;105:4289–4294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Baliram R, Sun L, Cao J, et al. Hyperthyroid-associated osteoporosis is exacerbated by the loss of TSH signaling. J Clin Invest. 2012;122:3737–3741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sun L, Zhu LL, Lu P, et al. Genetic confirmation for a central role for TNFα in the direct action of thyroid stimulating hormone on the skeleton. Proc Natl Acad Sci USA. 2013;110:9891–9896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Papadimitriou A, Papadimitriou DT, Papadopoulou A, Nicolaidou P, Fretzayas A. Low TSH levels are not associated with osteoporosis in childhood. Eur J Endocrinol. 2007;157:221–223 [DOI] [PubMed] [Google Scholar]


