Abstract
Incomplete spinal cord injury (iSCI) often results in significant motor impairments that lead to decreased functional mobility. Loss of descending serotonergic (5HT) input to spinal circuits is thought to contribute to motor impairments, with enhanced motor function demonstrated through augmentation of 5HT signaling. However, the presence of spastic motor behaviors in SCI is attributed, in part, to changes in spinal 5HT receptors that augment their activity in the absence of 5HT, although data demonstrating motor effects of 5HT agents that deactivate these receptors are conflicting. The effects of enhancement or depression of 5HT signaling on locomotor function have not been thoroughly evaluated in human iSCI. Therefore, the aim of the current study was to investigate acute effects of 5HT medications on locomotion in 10 subjects with chronic (>1 year) iSCI. Peak overground and treadmill locomotor performance, including measures of gait kinematics, electromyographic (EMG) activity, and oxygen consumption, were assessed before and after single-dose administration of either a selective serotonin reuptake inhibitor (SSRI) or a 5HT antagonist using a double-blinded, randomized, cross-over design. Results indicate that neither medication led to improvements in locomotion, with a significant decrease in peak overground gait speed observed after 5HT antagonists (from 0.8±0.1 to 0.7±0.1 m/s; p=0.01). Additionally, 5-HT medications had differential effects on EMG activity, with 5HT antagonists decreasing extensor activity and SSRIs increasing flexor activity. Our data therefore suggest that acute manipulation of 5HT signaling, despite changes in muscle activity, does not improve locomotor performance after iSCI.
Key words: : locomotor function, neurotransmitters, rehabilitation, spinal cord injury
Introduction
Incomplete spinal cord injury (iSCI) results in significant impairments in volitional and reflexive motor function that can lead to loss of independent functional mobility. Though the time course and extent of motor recovery after SCI vary substantially, initial depression of reflex and volitional motor output (i.e., “spinal shock”) is thought to result, in part, to the loss of descending serotonergic (5HT) input from brainstem pathways. In animal models of chronic SCI, structural changes in 5HT receptors on motoneurons below the lesion can result in supersensitivity to residual 5HT input1 and constitutive activity (receptor activity in the absence of 5HT).2 Ultimately, this leads to poorly regulated motoneuron excitability and responsiveness to afferent input or residual descending commands. Such changes may also to contribute to the development of spastic motor behaviors in both animals and humans with SCI.2,3 Spastic motor behaviors in human SCI consist of spastic hypertonia, which includes both spasticity (velocity-dependent increases in stretch reflex excitability4 and exaggerated tendon-tap reflexes5) and spasms, which manifest as sustained, involuntary, multi-joint muscle activation in response to multiple sensory inputs.3,6,7 The presence of spastic motor behaviors is thought by many to negatively affect locomotor function in patients with iSCI,8,9 primarily through resultant abnormal muscle activation patterns during gait.10 Conversely, other studies have reported no effect of spastic motor behaviors on gait performance.11,12
The potential effect of spastic behaviors on motor function has driven the use of pharmacological therapies to reduce spastic motor activity.13 This long-standing practice continues despite conflicting data on the effectiveness of antispastic treatments to improve functional performance, including walking ability, in those with iSCI.14,15 Inconsistent evidence for the effect of antispastic treatments on function may be secondary to the pharmacological agents tested, as those often used in the clinical setting (baclofen and benzodiazapines)16 depress excitability throughout the neuraxis.17–19 Such widespread suppression of neural excitability may affect volitional performance of functional tasks.16,20 However, the use of selective pharmacological agents that target 5HT receptor subtypes thought to underlie spastic motor behaviors may more specifically reduce spastic motor activity and improve walking ability. For example, administration of cyproheptadine, a 5HT2c antagonist and inverse agonist, has been shown to improve walking in iSCI and hemiplegia.21–23 Specific changes in human iSCI include improved walking without assistance,22–25 increase preferred gait speed,22,24,26 and more-normalized muscle activity patterns.23,24,26 In contrast, however, more recent studies in animal models and humans with SCI indicate that administration with 5HT antagonists may suppress spastic activity27 as well as volitional strength28 and locomotor function.2,29 This is consistent with the suggestion that the presence of spastic motor behaviors may promote the recovery of locomotor activity30 and the functional use of these involuntary, reflexive mechanisms during locomotion to compensate for volitional weakness.
Despite conflicting evidence for the effect of 5HT antagonists on locomotor function, 5HT agonists have been shown to facilitate locomotor output and modulate locomotor kinematics, including joint angle excursion, step length, foot placement, and intralimb coordination.31–33 Similarly, agents that increase endogenous 5HT signaling in humans have been shown to be effective modulators of volitional and reflexive motor output, leading to increases in clinical measures of strength and spasticity.28,34 Though the role of spastic motor activity on locomotion is unclear, increases in volitional strength with augmented 5HT signaling may potentiate locomotor performance, because selective lower extremity strength has been positively correlated to locomotor function in humans with SCI.11,35–37
Current data detailing the effects of pharmacological modulation of 5HT signaling on locomotor performance is unclear. The present study was designed to evaluate acute effects of 5HT medications on locomotor performance in subjects with iSCI. Acute responses to single doses of 5HT medications were utilized in this study because previous data have suggested rapid changes in neural excitability and motor function in patients with neurological injury.28 Acute single-dose administration was also employed to circumvent the desensitization of 5HT receptors that occurs with chronic dosing.38 In light of recent data,28 the primary hypotheses were that a 5HT agent that increases endogenous 5HT would enhance locomotor performance, whereas an agent that blocks 5HT receptor activity would suppress locomotor performance. Improving our current understanding of how these agents affect locomotor performance after SCI may affect how these agents are utilized clinically to promote the recovery of functional ambulation.
Methods
Ten ambulatory subjects with iSCI, classified by the American Spinal Injury Association Impairment Scale (AIS) as D,39 participated in a double-blinded, randomized, cross-over design to assess the effects of acute pharmacological manipulation of 5HT transmission on various measures of locomotor performance. A total of 10 subjects were considered sufficient to elicit changes in locomotor behavior based on our preliminary estimates.28 The agents used were overencapsulated, orally administered doses of a selective serotonin reuptake inhibitor (SSRI; 10 mg of escitalopram oxalate; Forest Pharmaceuticals, Inc., Earth City, MO) and a 5HT antagonist (8 mg of cyproheptadine, Periactin; Merck & Co. Inc., West Point, PA), with dosages chosen based on previously published and preliminary data.2,28,40 Escitalopram acts on presynaptic terminals to inhibit the reuptake of 5HT, thereby increasing the concentration of endogenous 5HT in the synaptic cleft. Cyproheptadine acts on postsynaptic neurons to block ligand-mediated and constitutive 5HT receptor activity.
Inclusion criteria for participation were subject age between 18 and 75 years, history of iSCI >1 year, and spinal lesions above neurologic level T10 (although no individuals with thoracic injuries were enrolled). Exclusion criteria consisted of concurrent illness that may limit exercise or walking performance, including the following conditions: unhealed decubiti; uncontrolled cardiopulmonary disease, including orthostatic hypotension and recurrent autonomic dysreflexia; active heterotopic ossification; other peripheral or central neurologic injury; and previous sensitivity to either medication. Recent use (<2 weeks previous) of antispastic agents, antidepressants, or other agents known to interact with study medications also excluded subjects from participation. Further, subjects who were receiving physical therapy or were enrolled in a training study less than 3 months previous were also excluded. All subjects obtained medical clearance to participate and provided written informed consent before participation. All procedures were conducted in accord with the Declaration of Helsinki and approved by the local institutional review board.
Data collection
Subjects participated in 2 days of testing separated by at least 1 week. Before initial locomotor testing, a licensed physical therapist assessed standardized measures of strength, spastic motor activity, and walking ability. Lower-extremity strength was assessed through the summed AIS Lower Extremity Motor Scores of key lower-extremity muscles.41 Spastic motor activity was evaluated using the Spinal Cord Assessment Tool for Spastic Reflexes6 and modified Ashworth testing of bilateral knee flexors and extensors.42 Both ordinal measures capture the magnitude of spastic motor behaviors (spasms and spasticity, respectively) while the subjects are at rest. The Walking Index for Spinal Cord Injury II43,44 was used to quantify walking ability through the use of braces, assistive devices, and therapist assistance for overground locomotion. Walking speed was collected over a 3.85-m instrumented walkway (1.8 m on each end for acceleration/deceleration; GaitMat II; Equitest, Chalfont, PA) with instructions to participants to walk their normal, comfortable pace.
After this initial evaluation, each visit consisted of two locomotor testing sessions that were performed before and 4.5 h after randomized administration of either 5HT medication. The timing of the postmedication testing was chosen to coincide with the mean peak plasma concentrations of the two 5HT agents.44,45 Testing sessions included quantitative evaluation of peak locomotor performance overground and on a motorized treadmill, performed by a physical therapist blinded to drug condition. To evaluate peak overground locomotor performance, subjects were cued to walk “as fast as safely possible” over the instrumented walkway with assistive devices and braces below the knee as required. Approximately 30 min after overground walking trials, peak treadmill locomotor performance was evaluated during a modified graded-intensity treadmill testing paradigm. Subjects were fitted with an overhead safety harness without weight support and instructed to walk on the treadmill at 0.1 m/sec for 2 min, with the speed increased by 0.1 m/sec every 2 min until they required support from the safety harness or voluntary test termination. Primary measures included peak treadmill speed, with secondary measures of gait kinematics, electromyographic (EMG) activity of selected lower-extremity muscles, and metabolic activity.
During treadmill testing, kinematic data (spatiotemporal and angular joint excursions) were collected using 32 spherical reflective markers affixed to the subjects' lower extremities using a previously described modified Cleveland Clinic marker set46 and sampled at 100 Hz with a six-camera motion capture system (Motion Analysis, Santa Rosa, CA). Lower-extremity EMG activity was assessed with bilateral surface recordings from rectus femoris (RF), vastus lateralis (VL), medial hamstrings (MH), tibialis anterior (TA), medial gastrocnemius (MG), and soleus (SOL) using a 32-channel dynamic EMG system (Noraxon Inc., Scottsdale, AZ). EMG data were sampled at 1000 Hz and collected synchronously with kinematic data using Cortex software (Motion Analysis). Data were collected in two intervals of 30 sec for a maximum collection interval of 1 min at each speed. To allow accommodation to each treadmill speed, data collection was initiated 30 sec after each speed increase. Oxygen consumption (VO2; mL/kg/min) was determined using a portable metabolic system (CosMed USA Inc., Chicago, IL) calibrated before testing using room air and a reference gas mixture (16% oxygen and 5% carbon dioxide). Metabolic data were collected on a breath-by-breath basis and stored for subsequent analysis.
Data analysis
All data processing and analyses were performed before the data were unblinded. For overground ambulation, primary (peak gait speed) and secondary (stride length and cadence) measures of gait performance were determined by averaging 3 trials. During treadmill testing, the primary assessment was also peak gait speed, defined as the highest speed at which the subject could walk for at least 1 min. For secondary measures derived from kinematic data, a bilateral 6-degree-of-freedom model of each subject's lower limbs (pelvis, thighs, shanks, and feet) was created from the reflective marker data during static standing on each testing day using Visual 3D (three-dimensional) software (C-Motion Inc., Germantown, MD). This model was applied to filtered marker data (low-pass, second-order recursive Butterworth filter; cut-off frequency, 10 Hz) from walking trials, and 3D joint positions and intersegmental angles were calculated from transformations between model segments.
Kinematic metrics were averaged across multiple normalized gait cycles (heel strike to heel strike, defined by the maximum anterior position of the calcaneal marker) during the two 30-sec collection windows. A minimum of 10 full gait cycles were utilized for all analyses, with the exception of 3 subjects who walked at very slow gait speeds after 5HT antagonists; in these cases, only 5–7 full gait cycles were available for analysis. Kinematic analyses focused on bilateral limbs, with EMG activity analyzed from the more impaired limb as determined by strength scores (or spasticity scores, if strength was equal bilaterally). Specific metrics of interest included stride length, cadence, and sagittal plane joint kinematics, including peak angular excursions and total range of motion at the hip, knee, and ankle (note: 2 participants wore ankle foot orthoses and data were included in analysis). In addition, the average coefficient of correlation (ACC) was calculated to quantify the intralimb consistency of coordination between hip and knee joint across multiple normalized gait cycles. The ACC uses a vector coding technique to analyze the consistency of sagittal-plane hip and knee angles on an angle-angle plot, as described by others,46,47 and performed in our previous work.48 Values of 1.0 represent perfectly consistent coordination of the joint trajectories between gait cycles, whereas values of 0.0 indicate no consistency. Individuals without neurological injury demonstrate ACC values from 0.94 to 0.97.47,49
Additional secondary measures included EMG activity during graded-intensity treadmill ambulation, which were post-processed using custom software (Visual 3D; C-Motion Inc.; and MATLAB; The MathWorks, Inc., Natick, MA). Raw EMG signals were filtered (fourth-order recursive Butterworth; band pass, 30–450 Hz; band stop, 58–62 Hz), rectified, and smoothed using a low-pass filter (fourth-order recursive Butterworth; 20 Hz) to create linear envelopes. EMG data were normalized to gait cycle as a percent from heel strike to heel strike. These data were averaged over all step cycles completed within the collection windows to create and average step profile.
EMG measures from the more impaired limb included the average integrated area throughout the gait cycle for specific muscles. Data from specific muscle groups were pooled together according to their functional synergistic actions during walking, as defined previously in a healthy and neurologically impaired population.50 Generally, flexor and extensor muscle groups were grouped separately, with specific biarticular muscles with both flexor and extensor actions analyzed depending on their expected activity during predetermined phases of the gait cycle.50,51 Specific functional groupings for extensor muscles included SOL, MG, VL, RF (during stance; 0–50% of gait cycle), and MH (during stance; 0–65% of gait cycle). Although MG is biarticular, recent studies have demonstrated limited function as a knee flexor during upright locomotion; MG was therefore considered only as an extensor.50,51 For flexors, TA, RF (during preswing through swing; 50–100% of gait cycle), and MH (during swing; 65–100% of gait cycle) were grouped. Same-day pre- and postdrug testing sessions with EMG electrodes secured in position throughout the testing sessions allowed for pre- to postdrug comparisons of integrated EMG area in flexor and extensor musculature.
In addition, the Spastic Locomotor Disorder Index (SLDI) was calculated to evaluate muscle timing throughout the gait cycle before and after 5HT modulation. Briefly, integrated EMG area during predetermined normative activation (“on”) and inactivation (“off”) times for each recorded muscle throughout the gait cycle52 were calculated. The SLDI was determined for each muscle as the ratio of the integrated EMG area during the summed off time to the area of the summed on time for that muscle: SLDI values closer to 0.0 represent appropriate muscle timing consistent with normative data from intact subjects, whereas higher values indicate abnormal muscle activation patterns during gait (i.e., the presence of muscle activity during the portion of the gait cycle when the muscle should be off ).10
Kinematic and EMG metrics were averaged over all steps taken within the collection window at the fastest speed reached in each testing session as well as the fastest speed met in both testing sessions within 1 day (fastest matched speed). As an example, if a subject reached 0.7 m/sec on the predrug assessment and 0.5 m/sec on the postdrug assessment, metrics were compared between 0.7 m/s predrug and 0.5 m/sec postdrug, as well as between 0.5 m/sec pre- and postdrug. Comparison of metrics at the fastest matched speed allowed for the determination of the effects of 5HT medications without the confounding influence of velocity.
Measurements of VO2 were averaged across the last 1 min of each speed interval. Baseline metabolic values collected during 2 min of sitting were subtracted from each VO2 measurement during walking.
Statistical analysis
All statistical analyses were performed in StatView software (version 5.0.1; SAS Institute Inc., Cary, NC). Multiple comparisons for each drug (SSRI and 5HT antagonist) necessitated Bonferroni's corrections for all primary and secondary measures, such that the corrected α was set to 0.025. Primary statistical analysis tested the differences in fastest speed of overground and treadmill locomotion before and after the administration of each 5HT agent using paired t-tests. Paired t-tests were also utilized to evaluate differences in overground spatiotemporal measures (cadence and stride length) at fastest speed. For cadence, stride length, and sagittal-plane joint kinematics collected on the treadmill, comparisons were made between pre- to postdrug measurements at fastest speed and fastest matched speeds. Pre- to postdrug comparisons of lower-extremity EMG activity and timing were assessed using Wilcoxon's nonparametric signed-rank test given the limited sample size and variability of EMG. Potential correlations between changes in fastest speed versus spatiotemporal patterns during overground and treadmill testing as well as baseline clinical measures of spastic motor behavior were assessed with Pearson's correlation. Potential correlations between changes in fastest speed and oxygen consumption versus lower-extremity EMG activity during treadmill testing (normalized to predrug values) were assessed using Spearman's rank (α=0.05).
Results
Ten male subjects with chronic motor iSCI participated in this study, with average age of 44±10 years and an average duration of injury of 95±87 months, and all with cervical lesions classified as AIS D (summed AIS lower extremity motor score range, 36–49). Clinical characteristics of these participants and baseline walking values are provided in Table 1.
Table 1.
Subjects' Clinical and Demographic Characteristics
| Subject No. | LOI | DOI (months) | Gait speed (m/sec) | LEMS | mAsh | SCATS | WISCI-II |
|---|---|---|---|---|---|---|---|
| 1 | C5 | 301 | 0.78 | 36 | 6 | 7 | 19 |
| 2 | C5 | 156 | 0.59 | 49 | 9 | 6 | 16 |
| 3 | C3 | 43 | 0.11 | 37 | 5 | 13 | 6 |
| 4 | C2 | 12 | 0.57 | 44 | 3 | 5 | 13 |
| 5 | C5 | 133 | 0.89 | 38 | 6 | 16 | 13 |
| 6 | C3 | 53 | 0.68 | 47 | 8 | 3 | 13 |
| 7 | C5 | 96 | 0.52 | 36 | 11 | 10 | 13 |
| 8 | C6 | 27 | 0.24 | 46 | 14 | 12 | 16 |
| 9 | C6 | 102 | 0.58 | 47 | 0 | 3 | 20 |
| 10 | C7 | 30 | 0.23 | 23 | 9 | 8 | 9 |
Categories include: neurologic level of injury (LOI), duration of injury (DOI), gait speed, Lower Extremity Motor Score (LEMS; range, 0–50), modified Ashworth assessment (mAsh; range, 0–16), the Spinal Cord Assessment Tool for Spastic Reflexes (SCATS; range, 0–18), and the Walking Index for Spinal Cord Injury-II (WISCI-II; range, 0–20). Measures that capture scores for individual muscle strength or spastic motor activity are provided as the summation of scores from each tested muscle on bilateral lower extremities. For all measures, larger scores indicate increased strength, spastic motor behavior, or independence with ambulation.
Effect of serotonergic agents on overground locomotor performance
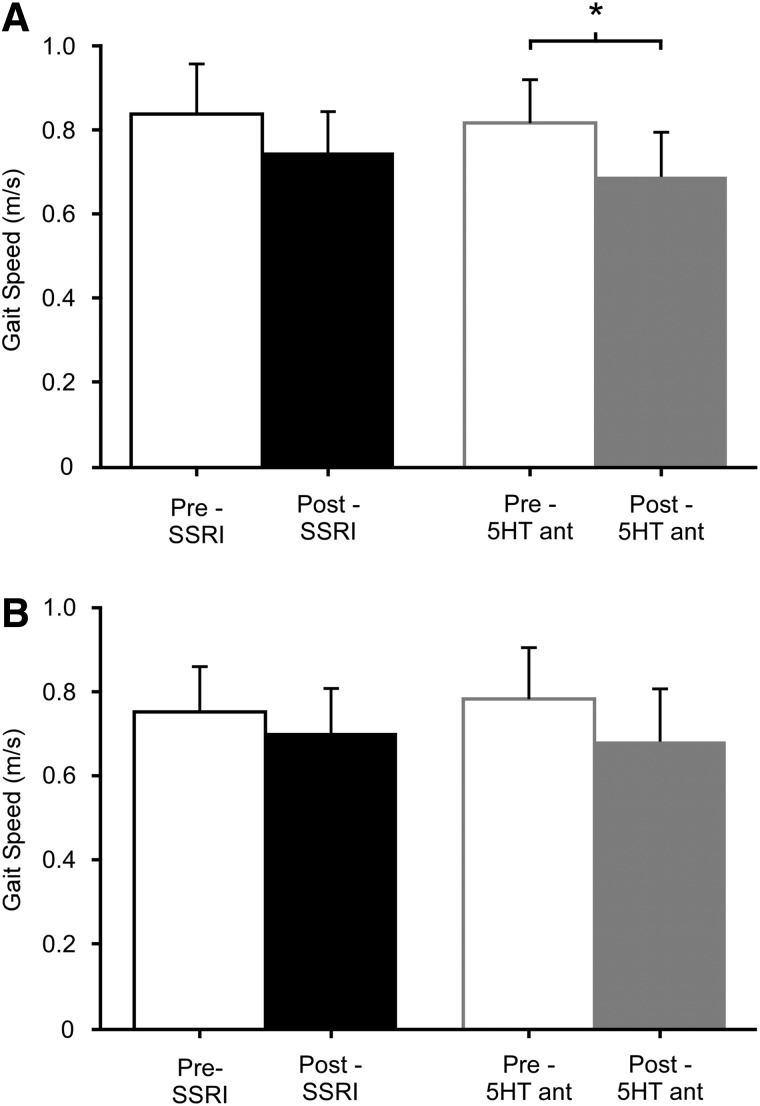
After either 5HT agent, participants demonstrated a small decrease in fastest overground gait speed (Fig. 1A), although only significantly after 5HT antagonists (p=0.01), but not SSRIs (p=0.15). Changes with 5HT antagonists were associated with a significant decrease in stride length (1.1±0.13 to 1.0±0.21 m; p=0.01) and a decrease in cadence that approached significance (84±30 to 77±32 steps/min; p=0.06). Greater correlation was observed between changes in gait speed and cadence (r=0.90; p<0.001), as opposed to stride length (r=0.85; p<0.01). With SSRIs, significant decreases in stride length (1.1±0.15 to 1.0±0.15 m; p=0.01) were observed with no change cadence (85±32 to 86±34 steps/min; p=0.90). Accordingly, changes in speed were better correlated with stride length (r=0.81; p<0.01) than cadence (r=0.72; p<0.05). Baseline clinical characteristics of spasticity or strength were not associated with changes in walking performance after either drug (all r<0.55; p>0.10).
FIG. 1.
Pre- and postdrug assessments of gait speed during overground (A) and treadmill (B) ambulation. Trends for decreased peak gait speed are observed across drug conditions during both overground (A) and treadmill (B) ambulation, with a significant decrease only demonstrated during overground ambulation after 5HT antagonist (5HT ant) dosing. *Significant difference from predrug measures; p<0.025. SSRI, selective serotonin reuptake inhibitor; 5HT, serotonergic.
Effect of serotonergic agents on treadmill locomotor performance
Consistent with results from overground walking, subjects exhibited similar decreases in speed during graded treadmill testing, although changes were not significant for either 5HT antagonists (p=0.04) secondary to the strict α-level used or SSRIs (p=0.09; Fig. 1B). Changes in stride length and cadence during fastest speeds also paralleled changes in overground performance, but were not statistically significant (Table 2). Correlation analysis revealed moderate-to-good associations between changes in peak treadmill speed and stride length (r=0.83) as well as cadence (r=0.77) after SSRIs (both p<0.01). In contrast, very strong correlations were observed between peak treadmill speed and cadence (r=0.94; p<0.001) after 5HT antagonists, with slightly less association to stride length (r=0.72; both p<0.05). Spatiotemporal parameters at fastest matched speeds were not different after either 5HT agent (Table 2).
Table 2.
Pre- and Postdrug Measurements of Spatiotemporal Parameters of Gait at Fastest and Fastest Matched Speeds During Treadmill Ambulation
| SSRI | 5HT antagonist | |||||
|---|---|---|---|---|---|---|
| Pre | Post | p value | Pre | Post | p value | |
| Fastest speed | ||||||
| Stride length (m) | 1.17±0.16 | 1.09±0.16 | 0.04 | 1.19±0.16 | 1.08±0.23 | 0.07 |
| Cadence (steps/min) | 75.2±28.6 | 72.7±28.3 | 0.30 | 76.3±29.0 | 68.9±32.0 | 0.05 |
| Fastest matched speed | ||||||
| Stride length (m) | 1.12±0.19 | 1.09±0.16 | 0.16 | 1.08±0.22 | 1.09±0.22 | 0.71 |
| Cadence (steps/min) | 70.6±28.0 | 72.2±28.0 | 0.21 | 69.2±31.5 | 69.6±30.2 | 0.84 |
Comparisons of spatiotemporal measures at fastest matched speeds demonstrated no differences with either 5HT medication. At fastest speeds, decrease in stride length after SSRIs and cadence after 5HT antagonists approach significance (α=0.025). Values are means±standard deviation.
Detailed analysis of lower-extremity kinematics during fastest treadmill performance revealed minimal differences after either 5HT agent for the less- and more-impaired limb. For peak joint ranges of motion, only peak ankle dorsiflexion range of motion in the more-impaired limb was found to increase after administration of SSRI (p=0.01; Table 3A), without changes at the knee and hip. Total joint excursion did not change at any joint (Table 4). Evaluation of joint kinematics at fastest matched speeds revealed similarly limited changes, with differences demonstrated only for total ankle range of motion after SSRIs in the more-impaired limb (p=0.02; Table 4).
Table 3A.
Pre- and Postdrug Measurements of Peak Sagittal-Plane Joint Range of Motion of the Hip, Knee, and Ankle at Fastest Speed
| SSRI | 5HT antagonist | |||||
|---|---|---|---|---|---|---|
| Fastest | Pre | Post | p value | Pre | Post | p value |
| More impaired limb | ||||||
| Ankle dorsiflexion | 15±8 | 22±10 | 0.01 | 19±7 | 19±9 | 0.82 |
| Ankle plantarflexion | −23±11 | −17±10 | 0.12 | −22±11 | −17±15 | 0.19 |
| Knee flexion | −60±16 | −56±16 | 0.53 | −64±23 | −61±15 | 0.61 |
| Knee extension | −7±16 | −5±18 | 0.9 | −9±16 | −6±12 | 0.34 |
| Hip flexion | 37±15 | 35±11 | 0.91 | 39±12 | 42±12 | 0.45 |
| Hip extension | −17±17 | −11±9 | 0.16 | −10±7 | −6±8 | 0.3 |
| Hip-knee ACC | 0.88±0.04 | 0.88±0.04 | 0.61 | 0.86±0.07 | 0.86±0.08 | 0.36 |
| Less impaired limb | ||||||
| Ankle dorsiflexion | 15±5.7 | 18±8.6 | 0.23 | 17±6.5 | 17±9.3 | 0.95 |
| Ankle plantarflexion | −18±14 | −10±14 | 0.06 | −17±14 | −15±12 | 0.47 |
| Knee flexion | −57±7.5 | −62±9.9 | 0.04 | −60±8.4 | −61±6.3 | 0.79 |
| Knee extension | −3.1±15 | −7.6±16 | 0.02 | −4.3±16 | −3.9±13 | 0.85 |
| Hip flexion | 35±16 | 42±6.7 | 0.24 | 39±9.0 | 44±9.9 | 0.34 |
| Hip extension | −16±17 | −7.9±9.1 | 0.12 | −11±12 | −7.3±8.1 | 0.58 |
| Hip-knee ACC | 0.88±0.08 | 0.90±0.07 | 0.33 | 0.89±0.07 | 0.88±0.09 | 0.88 |
Negative values represent plantarflexion at the ankle, flexion at the knee, and extension at the hip. Values are presented in degrees (mean±standard deviation).
SSRI, selective serotonin reuptake inhibitor; 5HT, serotonergic; ACC, average coefficient of correlation.
Table 4.
Pre- and Postdrug Measurements of Total Joint Range of Motion in the Sagittal Plane of the Hip, Knee, and Ankle at Fastest and Fastest Matched Speeds
| SSRI | 5HT antagonist | |||||
|---|---|---|---|---|---|---|
| Fastest | Pre | Post | p value | Pre | Post | p value |
| More impaired | ||||||
| Ankle | 38±11 | 39±11 | 0.39 | 40±11 | 36±9 | 0.11 |
| Knee | 53±13 | 51±19 | 0.62 | 55±18 | 55±15 | 0.72 |
| Hip | 54±16 | 47±13 | 0.10 | 49±9 | 49±11 | 0.64 |
| Less impaired | ||||||
| Ankle | 31±13 | 27±7.4 | 0.58 | 33±11 | 32±9.3 | 0.45 |
| Knee | 54±14 | 55±17 | 0.98 | 56±13 | 57±14 | 0.48 |
| Hip | 51±10 | 49±13 | 0.28 | 50±8.3 | 51±12 | 0.47 |
| Fastest matched | ||||||
| More impaired | ||||||
| Ankle | 40±8 | 34±11 | 0.02 | 42±14 | 43±10 | 0.76 |
| Knee | 54±13 | 51±19 | 0.41 | 54±17 | 55±15 | 0.59 |
| Hip | 51±16 | 47±8 | 0.13 | 47±8 | 48±12 | 0.35 |
| Less impaired | ||||||
| Ankle | 28±8 | 27±8 | 0.64 | 31±12 | 32±10 | 0.47 |
| Knee | 49±19 | 48±16 | 0.95 | 48±18 | 52±16 | 0.28 |
| Hip | 49±10 | 47±12 | 0.36 | 48±9 | 50±12 | 0.31 |
Values are presented as degrees of joint motion (mean±standard deviation).
SSRI, selective serotonin reuptake inhibitor; 5HT, serotonergic.
Table 3B.
Pre- and Postdrug Measurements of Peak Sagittal-Plane Joint Range of Motion of the Hip, Knee, and Ankle at Fastest Matched Speed
| SSRI | 5HT antagonist | |||||
|---|---|---|---|---|---|---|
| Fastest matched | Pre | Post | p value | Pre | Post | p value |
| More impaired limb | ||||||
| Ankle dorsiflexion | 18±7 | 18±5 | 0.98 | 20±10 | 20±10 | 0.84 |
| Ankle plantarflexion | −21±10 | −16±13 | 0.07 | −22±14 | −23±14 | 0.70 |
| Knee flexion | −60±13 | −56±16 | 0.16 | −62±19 | −61±16 | 0.75 |
| Knee extension | −6±16 | −5±18 | 0.84 | −8±15 | −6±12 | 0.44 |
| Hip flexion | 37±14 | 35±11 | 0.75 | 38±11 | 42±12 | 0.29 |
| Hip extension | −14±15 | −11±9 | 0.38 | −9±7 | −6±8 | 0.48 |
| Hip-knee ACC | 0.88±0.04 | 0.88±0.04 | 0.61 | 0.86±0.07 | 0.86±0.08 | 0.36 |
| Less impaired limb | ||||||
| Ankle dorsiflexion | 16±6.2 | 18±7.8 | 0.16 | 18±6.2 | 17±10 | 0.57 |
| Ankle plantarflexion | −12±11 | −8.2±9.3 | 0.06 | −14±14 | −17±15 | 0.43 |
| Knee flexion | −58±8.5 | −62±10 | 0.09 | −59±8.9 | −60±6.6 | 0.51 |
| Knee extension | −6.8±18 | −11±19 | 0.07 | −8.2±19 | −6.9±16 | 0.39 |
| Hip flexion | 35±16 | 41±6.7 | 0.27 | 38±7.4 | 42.6±7.4 | 0.18 |
| Hip extension | −15±16 | −7.6±8.9 | 0.18 | −11±12 | −7.3±7.4 | 0.32 |
| Hip-knee ACC | 0.88±0.08 | 0.90±0.06 | 0.27 | 0.88±0.07 | 0.88±0.10 | 0.79 |
Negative values represent plantarflexion at the ankle, flexion at the knee, and extension at the hip. Values are presented in degrees (mean±standard deviation).
SSRI, selective serotonin reuptake inhibitor; 5HT, serotonergic; ACC, average coefficient of correlation.
For muscle activity at fastest speeds, comparisons between pre- and post-5HT agent administration demonstrated no significant changes in pooled flexor or extensor EMG activity. Specifically, SSRI administration led to nonsignificant increases in flexor (34±68%; p=0.20) and extensor (26±43%; p=0.37) activity, whereas 5HT antagonists led to nonsignificant decreases of EMG activity in both flexor (−15±40%; p=0.08) and extensor (−20±18%; p=0.05) activity that approached significance. Very weak correlations were observed between changes in gait speed and normalized flexor or extensor activity (all rho <0.30; p>0.40).
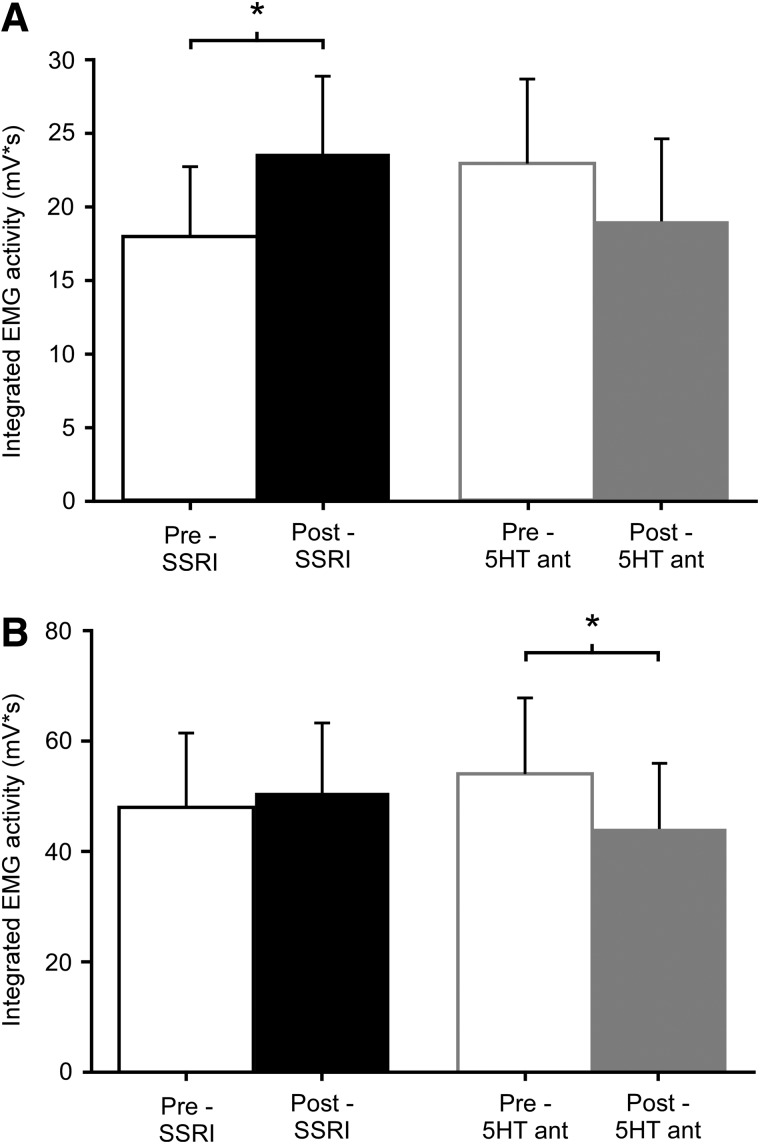
Comparisons of muscle activity at fastest matched speeds were also performed to evaluate 5HT medication effects on walking without influence of treadmill speed. Interestingly, SSRI administration was found to significantly augment flexor activity by 40±40% (p=0.01), with no significant difference in extensor activity (17±22%; p=0.11; Fig. 2A,B). In contrast, 5HT antagonist administration led to significant decreases in EMG activity in extensors (−14±15%; p=0.01), but not flexors (−15±24%; p=0.04; Fig. 2A,B). Additionally, calculations of the SLDI for the six lower-extremity muscles of interest revealed a range of values previously reported to occur in individuals with spastic paresis,10 although there were no significant changes in any individual lower-extremity muscle with either SSRI or 5HT antagonists (Table 5).
FIG. 2.
Pre- and postdrug assessments of average integrated area of EMG activity for lower-extremity flexor (A) and extensor (B) musculature at fastest matched speeds. 5HT medications resulted in divergent effects on lower-extremity muscle activity. SSRIs led to significant increases in pooled flexor EMG activity (A) and 5HT antagonists led to significant decreases in pooled extensor EMG activity (B), with similar trends in overall muscle activity. *Significant difference from predrug measures; p<0.025. EMG, electromyography; SSRI, selective serotonin reuptake inhibitor; 5HT, serotonergic.
Table 5.
SLDI Values for Each Muscle at the Fastest Matched Speeds
| SSRI | 5HT antagonist | |||||
|---|---|---|---|---|---|---|
| Muscle | Pre | Post | p value | Pre | Post | p value |
| Rectus femoris | 0.90±0.37 | 0.87±0.27 | 0.89 | 0.97±0.21 | 1.1±0.20 | 0.18 |
| Vastus lateralis | 0.74±0.29 | 0.75±0.22 | 0.92 | 0.90±0.29 | 0.92±0.25 | 0.21 |
| Medial hamstring | 0.51±0.33 | 0.54±0.3 | 0.89 | 0.37±0.12 | 0.38±0.13 | 0.75 |
| Medial gastrocnemius | 0.37±0.14 | 0.43±0.12 | 0.33 | 0.39±0.18 | 0.43±0.27 | 0.58 |
| Soleus | 0.29±0.17 | 0.35±0.27 | 0.60 | 0.40±0.29 | 0.38±0.31 | 0.58 |
| Tibialis anterior | 1.1±1.9 | 0.49±0.25 | 0.89 | 0.49±0.20 | 0.53±0.22 | 0.26 |
SLDI, Spastic Locomotor Disorder Index; SSRI, selective serotonin reuptake inhibitor; 5HT, serotonergic.
For measures of metabolic activity, both study medications led to nonsignificant decreases in VO2 at fastest speeds, with changes after 5HT antagonists approaching significance (5HT antagonist, 15±5.3 to 11±4.4 mL/kg/min; p=0.03 and SSRI, 17±6.6 to 16±5.2 mL/kg/min; p=0.16). Change in peak VO2 was not significantly correlated to the change in flexor (rho=0.19; p=0.57) or extensor (rho=−0.43; p=0.20) muscle activity (postdrug normalized to predrug activity). Comparisons of VO2 at fastest matched speeds demonstrated significantly less oxygen consumption with 5HT antagonist administration (13±4.9 to 11±4.4 mL/kg/min; p=0.02) and no difference in VO2 with SSRI dosing (16±6.7 to 16±5.3 mL/kg/min; p=0.71).
Discussion
This pilot investigation evaluated the effects of acute 5HT modulation on locomotor performance in subjects with chronic iSCI. Administration of a 5HT antagonist resulted in significantly decreased peak overground speed, whereas SSRI resulted in no significant changes. Detailed evaluation of muscle activity and gait kinematics demonstrated few differences at fastest treadmill speeds, whereas comparisons at fastest matched speeds revealed increased flexor muscle activity after SSRI and decreased extensor activity with 5HT antagonists. No significant drug-dependent effect on lower-extremity muscle timing was observed.
The changes in peak gait speed demonstrated here are consistent with recent data,28 with alterations in specific spatiotemporal parameters appearing to account for speed changes in both drug conditions. After SSRI administration, differences in stride length appear to better account for trends in gait speed, whereas changes in cadence better account for changes in speed after 5HT antagonists, with relatively consistent spatiotemporal changes overground and treadmill ambulation (although changes during treadmill ambulation were not significant secondary to the strict α-level). Despite these changes, individual joint kinematics were not substantially altered across testing conditions. Previous reports have demonstrated that treatment of spastic motor behaviors with 5HT antagonists leads to changes in joint angular displacement.10,22,24 These studies describe more-upright posture and decreased hip and knee flexion during ambulation after administration of 5HT antagonist, which was interpreted as a consequence of decreased flexor spasms. In contrast, the only significant change demonstrated in this study was a decrease in total ankle range of motion on the more-impaired limb with SSRIs in fastest matched conditions, apparently mediated largely by the decrease in peak plantarflexion range of motion (Table 3B). The limited changes in hip and knee excursion bilaterally do not readily account for the trend for decreasing stride length, possibly suggesting that the combined effects of minor differences in all joint excursions contributed to altered spatiotemporal patterns.
Despite limited changes in kinematics, the 5HT agents utilized demonstrated opposite effects on overall lower-extremity muscle activity. SSRI administration increased lower-extremity flexor EMG activity, with nonsignificant increases in extensor activity, whereas 5HT antagonists decreased extensor EMG, with nonsignificant decreases in flexor EMG. The greater effect of SSRIs on flexors versus extensors is consistent with recently published data suggesting preferential effects of 5HT signaling in a rodent model.53 Another possibility is that augmented 5HT transmission with SSRIs had less of an effect on extensor musculature secondary to pronounced increases in extensor tone and spastic motor behaviors characteristic in iSCI, such that further increases may be limited.7,54 However, further investigation into the mechanisms that underlie this potentially selective effect 5HT signaling on flexor versus extensor activity is necessary.
Changes in muscle activity after 5HT agents tested at either fastest or fastest matched speed may account for observed differences in VO2. Specifically, the trend of 5HT antagonists to decrease muscle activity may contribute to the reduction of VO2 at either fastest or fastest matched conditions. Conversely, after SSRIs, increases in EMG activity with reduced peak speeds at fastest conditions could account for the unchanged VO2 measurements.
Despite net changes in lower-extremity muscle activity, the present results indicate that the timing of muscle activity (as measured by the SLDI; shown in Table 5) was unaffected by either medication. In contrast, previous studies highlight improved activation patterns as a primary effect of both 5HT agonists33 and antagonists.22,24 Regardless, the present finding may help explain why administration of either 5HT agent did not translate into improvements in locomotor function. Despite greater EMG activity after SSRIs, subjects may have been unable to utilize this acutely increased motor output to achieve higher walking speeds secondary to continuous abnormal muscle timing. Similarly, the combined effects of maintained abnormal muscle activation patterns and the trend for decreased activity in both flexors and extensors after 5HT antagonists may explain the decrease in peak gait speed. These hypotheses are consistent with the recent findings of these 5HT agents on nonwalking, clinical assessments of strength and spastic motor behavior in this population.28 However, future research is required to determine the relationship between locomotor function and lower-extremity strength and muscle activity (magnitude and timing), because the current evidence is unclear.11,36,37,55–58
Little change or reduction in peak gait speed demonstrated in this study after administration of both 5HT agents in humans with iSCI varies substantially from previous animal and human studies assessing locomotor effects of 5HT agents.22,24,26,31–33 Differences between the present study and published results may be, in part, because of differences in experimental designs. Using single-subject investigations, Wainberg and colleagues previously reported beneficial effects of 5HT antagonists on locomotor function using extended, unblinded dosing and provided some indication that the effects may be specific to the clinical presentation of any particular patient.22 Specifically, subjects with a higher level of locomotor function at baseline were noted to demonstrate minimal changes with 5HT antagonists, whereas subjects with severely limited locomotor capacity secondary to spastic motor behaviors exhibited significant walking improvements. Our findings, however, indicate no relationship between baseline clinical measures of spasticity and changes in locomotor function with ether 5HT medication in this pool of subjects who are less severely injured (AIS D). Differences between studies may be because of differences in baseline function or spastic motor behaviors in the subject pools, although there are little data to suggest a differential effect of these medications based on clinical characteristics, and future studies are necessary.
Another possible explanation for the contrasting effects of 5HT antagonists from previous studies22,24 may be the chronicity of the medication administration. The current investigation measured locomotor performance at the peak plasma concentration after a single dose of 5HT medication to evaluate acute effects on locomotor function and circumvent the effects of accommodation and desensitization with repeated dosage. However, improvements in locomotion observed with chronic antispastic treatment,22,24,27 might also be explained by the continuation of therapy allowed during treatment and, possibly, not the medication itself.
The finding of primary clinical importance was that neither 5HT medication led to improvement in locomotor function with single-dose administration, but rather resulted in a decreased or no change in locomotor function with 5HT antagonist and SSRI dosing, respectively. These findings with 5HT antagonists are consistent with more-recent evidence in human and animal models for the suppression of locomotor function.2,28 Such findings with SSRIs, however, are disparate from other single-dose investigations demonstrating improved motor ability and function in both intact59 and neurologically impaired60 individuals. Importantly, SSRI use has also been shown to promote motor recovery after neurologic injury,61 an effect that is potentiated when pharmacological and physical interventions are paired.32,62,63 The current and previous data highlight the need for additional research to determine the effect of pairing pharmacological agents with specific interventions in humans with iSCI.
Acknowledgments
Funding for the present work was provided through a Promotion of Doctoral Studies–I scholarship from the Foundation for Physical Therapy (to K.A.L.) and National Institutes of Health (R21-HD046876-01A1) and Craig H. Neilson Foundation grants (to T.G.H.).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Li X., Murray K., Harvey P.J., Ballou E.W., and Bennett D.J. (2007). Serotonin facilitates a persistent calcium current in motoneurons of rats with and without chronic spinal cord injury. J. Neurophysiol. 97, 1236–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray K.C., Nakae A., Stephens M.J., Rank M., D'Amico J., Harvey P.J., Li X., Harris R.L., Ballou E.W., Anelli R., Heckman C.J., Mashimo T., Vavrek R., Sanelli L., Gorassini M.A., Bennett D.J., and Fouad K. (2010). Recovery of motoneuron and locomotor function after spinal cord injury depends on constitutive activity in 5-HT2C receptors. Nat. Med. 16, 694–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorassini M.A., Knash M.E., Harvey P.J., Bennett D.J., and Yang J.F. (2004). Role of motoneurons in the generation of muscle spasms after spinal cord injury. Brain 127, 2247–2258 [DOI] [PubMed] [Google Scholar]
- 4.Lance J. (1980). Symposium synopsis, in: Spasticity: Disordered Motor Control. Year Book Medical Publishers: Chicago, IL [Google Scholar]
- 5.Katz R.T., and Rymer W.Z. (1989). Spastic hypertonia: mechanisms and measurement. Arch. Phys. Med. Rehabil. 70, 144–155 [PubMed] [Google Scholar]
- 6.Benz E.N., Hornby T.G., Bode R.K., Scheidt R.A., and Schmit B.D. (2005). A physiologically based clinical measure for spastic reflexes in spinal cord injury. Arch. Phys. Med. Rehabil. 86, 52–59 [DOI] [PubMed] [Google Scholar]
- 7.Little J.W., Micklesen P., Umlauf R., and Britell C. (1989). Lower extremity manifestations of spasticity in chronic spinal cord injury. Am. J. Phys. Med. Rehabil. 68, 32–36 [DOI] [PubMed] [Google Scholar]
- 8.Scivoletto G., Romanelli A., Mariotti A., Marinucci D., Tamburella F., Mammone A., Cosentino E., Sterzi S., and Molinari M. (2008). Clinical factors that affect walking level and performance in chronic spinal cord lesion patients. Spine 33, 259–264 [DOI] [PubMed] [Google Scholar]
- 9.Krawetz P., and Nance P. (1996). Gait analysis of spinal cord injured subjects: effects of injury level and spasticity. Arch. Phys. Med. Rehabil. 77, 635–638 [DOI] [PubMed] [Google Scholar]
- 10.Fung J., and Barbeau H. (1989). A dynamic EMG profile index to quantify muscular activation disorder in spastic paretic gait. Electroencephalogr. Clin. Neurophysiol. 73, 233–244 [DOI] [PubMed] [Google Scholar]
- 11.Saraf P., Rafferty M.R., Moore J.L., Kahn J.H., Hendron K., Leech K., and Hornby T.G. (2010). Daily stepping in individuals with motor incomplete spinal cord injury. Phys. Ther. 90, 224–235 [DOI] [PubMed] [Google Scholar]
- 12.Norton B.J., Bomze H.A., Sahrmann S.A., and Eliasson S.G. (1975). Correlation between gait speed and spasticity at the knee. Phys. Ther. 55, 355–359 [DOI] [PubMed] [Google Scholar]
- 13.Gracies J.M., Nance P., Elovic E., McGuire J., and Simpson D.M. (1997). Traditional pharmacological treatments for spasticity. Part II: general and regional treatments. Muscle Nerve Suppl. 6, S92–S120 [PubMed] [Google Scholar]
- 14.Taricco M., Pagliacci M.C., Telaro E., and Adone R. (2006). Pharmacological interventions for spasticity following spinal cord injury: results of a Cochrane systematic review. Eura. Medicophys. 42, 5–15 [PubMed] [Google Scholar]
- 15.Domingo A., Al-Yahya A.A., Asiri Y., Eng J.J., and Lam T. (2012). A systematic review of the effects of pharmacological agents on walking function in people with spinal cord injury. J. Neurotrauma 29, 865–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adams M.M., and Hicks A.L. (2005). Spasticity after spinal cord injury. Spinal Cord 43, 577–586 [DOI] [PubMed] [Google Scholar]
- 17.Benarroch E.E. (2012). GABAB receptors: structure, functions, and clinical implications. Neurology 78, 578–584 [DOI] [PubMed] [Google Scholar]
- 18.Sieghart W., and Sperk G. (2002). Subunit composition, distribution and function of GABA(A) receptor subtypes. Curr. Top. Med. Chem. 2, 795–816 [DOI] [PubMed] [Google Scholar]
- 19.Sigel E., and Steinmann M.E. (2012). Structure, function, and modulation of GABA(A) receptors. J. Biol. Chem. 287, 40224–40231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burchiel K.J., and Hsu F.P. (2001). Pain and spasticity after spinal cord injury: mechanisms and treatment. Spine 26, S146–S160 [DOI] [PubMed] [Google Scholar]
- 21.Francisco G.E., and Boake C. (2003). Improvement in walking speed in poststroke spastic hemiplegia after intrathecal baclofen therapy: a preliminary study. Arch. Phys. Med. Rehabil. 84, 1194–1199 [DOI] [PubMed] [Google Scholar]
- 22.Wainberg M., Barbeau H., and Gauthier S. (1990). The effects of cyproheptadine on locomotion and on spasticity in patients with spinal cord injuries. J. Neurol. Neurosurg. Psychiatry 53, 754–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fung J., Stewart J.E., and Barbeau H. (1990). The combined effects of clonidine and cyproheptadine with interactive training on the modulation of locomotion in spinal cord injured subjects. J. Neurol. Sci. 100, 85–93 [DOI] [PubMed] [Google Scholar]
- 24.Norman K.E., Pepin A., and Barbeau H. (1998). Effects of drugs on walking after spinal cord injury. Spinal Cord 36, 699–715 [DOI] [PubMed] [Google Scholar]
- 25.Azouvi P., Mane M., Thiebaut J.B., Denys P., Remy-Neris O., and Bussel B. (1996). Intrathecal baclofen administration for control of severe spinal spasticity: functional improvement and long-term follow-up. Arch. Phys. Med. Rehabil. 77, 35–39 [DOI] [PubMed] [Google Scholar]
- 26.Wainberg M., Barbeau H., and Gauthier S. (1986). Quantitative assessment of the effect of cyproheptadine on spastic paretic gait: a preliminary study. J. Neurol. 233, 311–314 [DOI] [PubMed] [Google Scholar]
- 27.Barbeau H., Richards C.L., and Bedard P.J. (1982). Action of cyproheptadine in spastic paraparetic patients. J. Neurol. Neurosurg. Psychiatry 45, 923–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson C.K., and Hornby T.G. (2013). Divergent modulation of clinical measures of volitional and reflexive motor behaviors following serotonergic medication in human incomplete spinal cord injury. J. Neurotrauma 30, 498–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fouad K., Rank M.M., Vavrek R., Murray K.C., Sanelli L., and Bennett D.J. (2010). Locomotion after spinal cord injury depends on constitutive activity in serotonin receptors. J. Neurophysiol. 104, 2975–2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edgerton V.R., Tillakaratne N.J., Bigbee A.J., de Leon R.D., and Roy R.R. (2004). Plasticity of the spinal neural circuitry after injury. Annu. Rev. Neurosci. 27, 145–167 [DOI] [PubMed] [Google Scholar]
- 31.Barbeau H., Rossignol S. (1990). The effects of serotonergic drugs on the locomotor pattern and on cutaneous reflexes of the adult chronic spinal cat. Brain Res. 514, 55–67 [DOI] [PubMed] [Google Scholar]
- 32.Fong A.J., Cai L.L., Otoshi C.K., Reinkensmeyer D.J., Burdick J.W., Roy R.R., Edgerton V.R. (2005). Spinal cord-transected mice learn to step in response to quipazine treatment and robotic training. J. Neurosci. 25, 11738–11747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Antri M., Orsal D., and Barthe J.Y. (2002). Locomotor recovery in the chronic spinal rat: effects of long-term treatment with a 5-HT2 agonist. Eur. J. Neurosci. 16, 467–476 [DOI] [PubMed] [Google Scholar]
- 34.Stolp-Smith K.A., and Wainberg M.C. (1999). Antidepressant exacerbation of spasticity. Arch. Phys. Med. Rehabil. 80, 339–342 [DOI] [PubMed] [Google Scholar]
- 35.Kim C.M., Eng J.J., and Whittaker M.W. (2004). Level walking and ambulatory capacity in persons with incomplete spinal cord injury: relationship with muscle strength. Spinal Cord 42, 156–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dobkin B., Barbeau H., Deforge D., Ditunno J., Elashoff R., Apple D., Basso M., Behrman A., Harkema S., Saulino M., and Scott M.; Spinal Cord Injury Locomotor Trial Group. (2007). The evolution of walking-related outcomes over the first 12 weeks of rehabilitation for incomplete traumatic spinal cord injury: the multicenter randomized Spinal Cord Injury Locomotor Trial. Neurorehabil. Neural Repair 21, 25–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Field-Fote E.C., Lindley S.D., and Sherman A.L. (2005). Locomotor training approaches for individuals with spinal cord injury: a preliminary report of walking-related outcomes. J. Neurol. Phys. Ther. 29, 127–137 [DOI] [PubMed] [Google Scholar]
- 38.Loubinoux I., Tombari D., Pariente J., Gerdelat-Mas A., Franceries X., Cassol E., Rascol O., Pastor J., and Chollet F. (2005). Modulation of behavior and cortical motor activity in healthy subjects by a chronic administration of a serotonin enhancer. NeuroImage 27, 299–313 [DOI] [PubMed] [Google Scholar]
- 39.Consortium for Spinal Cord Medicine. (2000). Outcomes following traumatic spinal cord injury: clinical practice guidelines for health-care professionals. J. Spinal Cord Med. 23, 289–316 [DOI] [PubMed] [Google Scholar]
- 40.Hornby T.G., Schmit B.D., and Theiss R.D. (2006). Serotonergic modulation of motor function in humans incomplete spinal cord injury, in: Society for Neuroscience Abstracts: Atlanta, GA [Google Scholar]
- 41.El Masry W.S., Tsubo M., Katoh S., El Miligui Y.H., and Khan A. (1996). Validation of the American Spinal Injury Association (ASIA) motor score and the National Acute Spinal Cord Injury Study (NASCIS) motor score. Spine 21, 614–619 [DOI] [PubMed] [Google Scholar]
- 42.Bohannon R.W., and Smith M.B. (1987). Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys. Ther. 67, 206–207 [DOI] [PubMed] [Google Scholar]
- 43.Dittuno P.L., and Ditunno J.F., Jr. (2001). Walking Index for Spinal Cord Injury (WISCI II): scale revision. Spinal Cord 39, 654–656 [DOI] [PubMed] [Google Scholar]
- 44.Gunja N., Collins M., and Graudins A. (2004). A comparison of the pharmacokinetics of oral and sublingual cyproheptadine. J. Toxicol. Clin. Toxicol. 42, 79–83 [DOI] [PubMed] [Google Scholar]
- 45.PDR Staff (2011). Physicians' Desk Reference. 65th ed. PDR Network, LLC: Montvale, NJ [Google Scholar]
- 46.Lewek M.D., Cruz T.H., Moore J.L., Roth H.R., Dhaher Y.Y., and Hornby T.G. (2009). Allowing intralimb kinematic variability during locomotor training poststroke improves kinematic consistency: a subgroup analysis from a randomized clinical trial. Phys. Ther. 89, 829–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Field-Fote E.C., and Tepavac D. (2002). Improved intralimb coordination in people with incomplete spinal cord injury following training with body weight support and electrical stimulation. Phys. Ther. 82, 707–715 [PubMed] [Google Scholar]
- 48.Thompson C.K., Jayaraman A., Kinnaird C., and Hornby T.G. (2011). Methods to quantify pharmacologically induced alterations in motor function in human incomplete SCI. J. Vis. Exp. Apr 18;(50). pii: . doi: 10.3791/2148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pang M.Y., and Yang J.F. (2000). The initiation of the swing phase in human infant stepping: importance of hip position and leg loading. J. Physiol. 528, 389–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clark D.J., Ting L.H., Zajac F.E., Neptune R.R., and Kautz S.A. (2010). Merging of healthy motor modules predicts reduced locomotor performance and muscle coordination complexity post-stroke. J. Neurophysiol. 103, 844–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neptune R.R., Clark D.J., and Kautz S.A. (2009). Modular control of human walking: a simulation study. J. Biomech. 42, 1282–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hornby T.G., Straube D.S., Kinnaird C.R., Holleran C.L., Echauz A.J., Rodriguez K.S., Wagner E.J., and Narducci E.A. (2011). Importance of specificity, amount, and intensity of locomotor training to improve ambulatory function in patients poststroke. Top. Stroke Rehabil. 18, 293–307 [DOI] [PubMed] [Google Scholar]
- 53.Chopek J.W., MacDonell C.W., Power K.E., Gardiner K., and Gardiner P.F. (2013). Removal of supraspinal input reveals a difference in the flexor and extensor monosynaptic reflex response to quipazine independent of motoneuron excitation. J. Neurophysiol. 109, 2056–2063 [DOI] [PubMed] [Google Scholar]
- 54.Barolat G., and Maiman D.J. (1987). Spasms in spinal cord injury: a study of 72 subjects. J. Am. Paraplegia Soc. 10, 35–39 [DOI] [PubMed] [Google Scholar]
- 55.Wirz M., Zemon D.H., Rupp R., Scheel A., Colombo G., Dietz V., and Hornby T.G. (2005). Effectiveness of automated locomotor training in patients with chronic incomplete spinal cord injury: a multicenter trial. Arch. Phys. Med. Rehabil. 86, 672–680 [DOI] [PubMed] [Google Scholar]
- 56.Wirz M., Colombo G., and Dietz V. (2001). Long term effects of locomotor training in spinal humans. J. Neurol. Neurosurg. Psychiatry 71, 93–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wirz M., van Hedel H.J., Rupp R., Curt A., and Dietz V. (2006). Muscle force and gait performance: relationships after spinal cord injury. Arch. Phys. Med. Rehabil. 87, 1218–1222 [DOI] [PubMed] [Google Scholar]
- 58.Winchester P., Smith P., Foreman N., Mosby J.M., Pacheco F., Querry R., and Tansey K. (2009). A prediction model for determining over ground walking speed after locomotor training in persons with motor incomplete spinal cord injury. J. Spinal Cord Med. 32, 63–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Loubinoux I., Pariente J., Boulanouar K., Carel C., Manelfe C., Rascol O., Celsis P., and Chollet F. (2002). A single dose of the serotonin neurotransmission agonist paroxetine enhances motor output: double-blind, placebo-controlled, fMRI study in healthy subjects. Neuroimage 15, 26–36 [DOI] [PubMed] [Google Scholar]
- 60.Zittel S., Weiller C., and Liepert J. (2008). Citalopram improves dexterity in chronic stroke patients. Neurorehabil. Neural Repair 22, 311–314 [DOI] [PubMed] [Google Scholar]
- 61.Dam M., Tonin P., De Boni A., Pizzolato G., Casson S., Ermani M., Freo U., Piron L., and Battistin L. (1996). Effects of fluoxetine and maprotiline on functional recovery in poststroke hemiplegic patients undergoing rehabilitation therapy. Stroke 27, 1211–1214 [DOI] [PubMed] [Google Scholar]
- 62.Pariente J., Loubinoux I., Carel C., Albucher J.F., Leger A., Manelfe C., Rascol O., and Chollet F. (2001). Fluoxetine modulates motor performance and cerebral activation of patients recovering from stroke. Ann. Neurol. 50, 718–729 [DOI] [PubMed] [Google Scholar]
- 63.Chollet F., Tardy J., Albucher J.F., Thalamas C., Berard E., Lamy C., Bejot Y., Deltour S., Jaillard A., Niclot P., Guillon B., Moulin T., Marque P., Pariente J., Arnaud C., and Loubinoux I. (2011). Fluoxetine for motor recovery after acute ischaemic stroke (FLAME): a randomised placebo-controlled trial. Lancet Neurol. 10, 123–130 [DOI] [PubMed] [Google Scholar]