Abstract
PURPOSE
Young adults (YAs; ages 18–39) with cancer face interrupted developmental milestones and increased stressors that can adversely influence psychosocial adjustment. Transitioning from active treatment to post-treatment survivorship can be particularly challenging. The purpose of this study is to describe the health-related quality of life (HRQL) and psychological adaptation of YAs post-treatment relative to young adults without cancer.
METHODS
Three cohorts of YAs of mixed cancer diagnoses (N=120, 0–12 months post-treatment; N=102, 13–24 months post-treatment; and N=113, 25–60 months post-treatment; combined M=31.8 years old, combined gender=68% women) and an age, education, gender, and partner-status matched group of healthy control participants (HCs; N=335) were recruited via an online research panel. All participants completed measures assessing demographic and clinical characteristics, HRQL (physical, emotional, social, and spiritual), and psychological adaptation (anxiety, depression, positive affect, posttraumatic growth). Measure content was slightly modified for applicability to HCs without a cancer history.
RESULTS
Multivariate analysis of covariance found a significant main effect for group (YAs versus HCs) and a significant group-by-cohort interaction. YAs reported poorer physical (p=.005, d=.22) and emotional well-being (p=.011, d=.20) but better social well-being (p<.001, d=.49). YAs reported comparatively stable scores (p=.74) for posttraumatic growth compared to HCs, who reported greater posttraumatic growth across cohorts (p=.01, d=16).
CONCLUSIONS
Findings underscore the negative and positive sequelae for YAs and highlight the need for comprehensive assessment among YA survivors of cancer. A matched, HC group allows the HRQL and psychological adaptation of YAs to be placed in context, enabling a more precise determination of the impact of cancer on YAs.
Keywords: survivorship, quality of life, young adults, controlled comparison study, posttraumatic growth
INTRODUCTION
The five-year cancer survival rate for adolescents and young adult (YAs) aged 15 to 39 years old has not improved in almost three decades and contrasts markedly with improvements observed in the five-year survival rates of younger and older age groups.1 Accordingly, studies of older cancer survivors 2, 3 or adult survivors of childhood cancer4, 5 represent the majority of psychosocial oncology survivorship research. The limited studies available on YAs have focused on healthcare needs,6, 7 positive and negative life impact of cancer,8 and fertility concerns.9 More recently, research has included the post-diagnosis (6–14 months) health-related quality of life (HRQL) of adolescents and YAs with cancer,10 but additional work is needed to further understand their post-treatment HRQL and general psychological adaptation as they transition from the end of treatment to a stage of monitoring (i.e., re-entry) and beyond.
Cancer survivors of all ages likely experience common life disruptions secondary to cancer (e.g., goal interference and altered interpersonal relationships and body-sexual image); however, the specific impact and meaning attributed to these disruptions may vary across developmental life stages.11 Given the unique emotional and social life changes that take place during young adulthood (e.g., developing a positive body image and sexual identity, dating and building social networks, making decisions about higher education, careers, and family), a cancer diagnosis and treatment for YAs may be especially disruptive.12 Furthermore, similar to other medically underserved groups such as racial and ethnic minorities, YA cancer survivors face challenges related to healthcare access, including restricted or delayed medical care due to having the highest uninsured rate of any age group in the U.S.13
The National Cancer Policy Board and Institute of Medicine suggest the phase of cancer following primary treatment is particularly important for survivors.14 Understanding the challenges for survivors as they navigate re-entry and the later phases of the cancer survivor trajectory is critical in order to facilitate healthy adaptation. Despite the lack of improvement in 5-year survival rates for YAs with cancer, studies have not fully investigated the general psychological adjustment and HRQL among this group as they transition from treatment completion to long-term survivorship (e.g., 5–10 years post treatment). To that end, we build on and extend the important work by Smith et al.,10 by recruiting a large sample of YA cancer survivors stratified across three cohorts based on time since active treatment completion (0–12 months, 13–24 months, 25–60 months) and comparing them to a sample of age-, education-, gender-, and partner status-matched healthy controls (HCs). The three cohort timeframes were selected in advance to capture variability in the cancer survivorship re-entry period (generally 1–2 years post-treatment) and longer survivorship (3–5 years post-treatment).15 We designed the study and specific assessments to capture the breadth of experiences among cancer survivors, an approach that was informed by a well-known research and measurement model of cancer survivorship.16 Single-item or global indices of HRQL are insufficient to increase our understanding of the experience of YAs with cancer given the multiple psychosocial and developmental challenges they encounter. A strength of the model we used is the range of HRQL outcomes included: physical, emotional, social, and spiritual. We did not have a priori expectations for differences in outcomes by cancer type, thus we did not stratify participants based on these characteristics. Recent findings on young adults with cancer have not found significant associations among cancer type or severity and distress.17 Importantly, the matched healthy comparison group enabled us to situate our findings in the appropriate context by distinguishing between HRQL and psychosocial adjustment due to the experience of cancer versus HRQL and psychosocial adjustment due to normal developmental changes. We hypothesized YAs would report poorer HRQL (physical, emotional, social, spiritual) and negative psychosocial adaptation (anxiety, depression) but also more positive psychosocial adaptation (positive affect, posttraumatic growth) relative to HCs.
METHODS
Subjects and Procedures
All procedures were implemented after approval for use of human subjects from the local institutional review board. U.S. community-dwelling Internet panel samples of YAs and HCs were consented and recruited by Toluna, an Internet survey company (http://www.toluna-group.com) over the course of 12-months in 2010–2011 and 2-months in 2012, respectively. Our scientific team has found that recruiting clinical samples from Internet panels can be a cost-effective, efficient, and valid means of data collection as evidenced by our experience with two large-scale NIH-funded efforts.18, 19 To recruit study participants from the general population, Toluna sent e-mails to invite potential participants from their databases to enroll in the current study following a screening process to ensure eligibility. Eligible participants had access to the Internet and were able to read and understand English. YAs were eligible if they were diagnosed with cancer (excluding basal cell skin carcinoma), between the ages of 18–39, and within 0–60 months post-treatment. Exclusion criteria included a recurrent diagnosis of cancer, history of multiple primary cancers, and receipt of palliative or hospice care. Of the 30,520 individuals who accessed this survey, 3,944 were eligible (12.9%), a number that is larger than but generally congruent with prevalence rates of cancer in this age group.20
HCs were eligible if they did not have a past diagnosis of cancer, and shared the same age, gender, education level, and partner status of a YA group participant. Of the 16,333 individuals who accessed this survey, 2,383 were eligible and individually matched to a YA group participant (14.6%). YAs and HCs completed demographic and medical information items (history of acute and chronic health conditions) along with other study measures. YAs completed items asking about their cancer history. Participants who completed the survey were eligible for prize or incentive-based compensation through Toluna.
As a standard approach to reduce the potential for fraudulent data, cases were excluded by Toluna for participants who skipped >20 items or whose completion time for their survey was less than one-third of the median survey length. After survey completion, we excluded data for YAs who did not provide an identifiable cancer diagnosis and HCs who indicated a past history of cancer when reporting comorbid health conditions. We also excluded participants from both groups who engaged in straight-line responding (i.e., selecting the same response option for all items within a given questionnaire that included reverse-scored items). During data cleaning, 3,609 and 2,048 suspicious and potentially invalid cases were excluded from the YA and HC groups, respectively. Compared to participants that we retained, YA participants that we excluded were more likely to describe themselves as white, male, older, married, employed full-time, having a college degree or higher, or having a poorer performance status. HC participants that we excluded were more likely to describe themselves as younger, single, female, unemployed, or having some college education or less. We analyzed responses from the remaining 335 participants in each group. Additional procedures for data quality control and fraud prevention are described at: http://www.toluna-group.com/toluna-difference/data-quality/.
Study Measures
All participants completed a battery of self-report measures via Toluna’s secure, web-based platform assessing: (a) demographic and medical information, (b) HRQL, and (c) general psychological adjustment variables.
Demographic and Medical Information
Demographic information included gender, age, race, ethnicity, marital status, education, and annual household income. Medical information included history of any significant medical conditions (arthritis, migraines, insomnia, etc.). In addition, YAs were asked to provide information about their cancer history (i.e., disease type, stage, and ECOG performance status).21
HRQL
YAs completed the Functional Assessment of Cancer Therapy-General (FACT-G)22 and the Functional Assessment of Chronic Illness Therapy-Spiritual Well-Being (FACIT-Sp).23 HCs completed parallel versions of the above measures, the Functional Assessment of Cancer-Therapy General Population (FACT-GP)24 and the Functional Assessment of Chronic Illness Therapy-Spiritual Well-Being Non-Illness (FACIT-Sp-NI).25 These are psychometrically sound instruments and they provide global and domain-specific assessments of HRQL for the past 7 days. Global HRQL is captured by a single-item from the FACT-G and FACT-Gp, “I am content with the quality of my life right now,” whereas core HRQL domains are represented by multi-item subscales for emotional, physical, social, and spiritual well-being. Higher scores indicate greater HRQL. Internal consistency reliability was high for these subscales across both groups (physical well-being: YA=.93, HC=.86; emotional well-being: YA=.86, HC=.80; social well-being: YA=.81, HC=.83; spiritual well-being: YA=.87, HC=.90).
General psychological adjustment
General psychological adjustment was assessed by two psychometrically-sound measures: the Mental Health Inventory (MHI-18)26 and the Posttraumatic Growth Inventory- Short Form (PTGI-SF).27, 28 The MHI-18 is a measure of general distress over the past 4 weeks and includes subscales of depression, anxiety, and positive affect. Higher scores indicate better mental health (less depression or anxiety, and more positive affect). Posttraumatic growth is a term used to describe positive life changes following a stressful event,27 and the PTGI is a well-researched measure frequently used with cancer patients and survivors. Higher scores indicate higher levels of psychosocial growth. As in previous research investigating posttraumatic growth29 after cancer diagnosis and treatment, the YA group completed the PTGI-SF with reference to their cancer diagnosis and treatment whereas the HC group completed the PTGI-SF with reference to change occurring over the same span of time since cancer diagnosis for their matched counterpart in the YA group. To enhance the validity of their recall, matched HC group participants were asked to describe any major life changes that occurred during that time. The five most frequently described events were: birth of child(ren), 17.6%; physical illness, 15.5%; loss of employment, 14.6%; new employment, 12.5%;, and death of a family member or friend, 12.2%. To assess global perceptions of psychological impact, YA participants were asked an item created as part of the Patient-Reported Outcomes Measurement Information System (PROMIS) cancer supplement and originally administered with the PROMIS illness impact item banks,30 “How positive or negative has the overall impact of your illness been on your views about yourself and your life?” HC participants were asked a parallel question with a variable recall period that corresponded to the timeframe of their matched YA counterpart, “How positive or negative have your experiences during the last X year(s) been on your views about yourself and your life?” Both groups responded using a 7-point Likert scale ranging from “completely negative” to “completely positive.” Internal consistency reliability was also high for these subscales across both groups (depression: YA=.92, HC=.86; anxiety: YA=.85, HC=.81; positive affect: YA=.80, HC=.83; posttraumatic growth: YA=.92, HC=.93).
Statistical Analysis
Descriptive statistics and distributions of demographic, clinical, HRQL, and psychological adaptation measure scores were evaluated. Using chi-square and t-tests, we examined differences in demographic and clinical data. Significant covariates determined in the univariate analysis were included in subsequent multivariate analyses of covariance (MANCOVAs). Our sample size was sufficient (two groups of N=335) to adequately detect small effect sizes (Cohen’s d=0.2 to 0.3) in a MANCOVA using an alpha level of .05 and a convention of 0.80 for statistical power.
RESULTS
The most common cancers affecting adolescents and young adults are breast, thyroid, melanoma, cervical and uterine, Hodgkin Lymphoma, Non-Hodgkin Lymphoma, colorectal, and germ cell tumors.31 Table 1 presents clinical characteristics of YAs with cancer from this current sample. These data are on par with SEER data as seven of the top eight cancers affecting this age group are represented by the current sample. Table 2 presents demographic data for the YAs and HCs. Due to the matching of participants by age, gender, education, and partner status, there were no significant differences for these categories nor were there differences by race or employment status. However, there were significant differences for income (X2(11)=44.045, p<.001) and ethnicity (X2(2)=6.682, p=.035), with the YA sample demonstrating higher income and less ethnic diversity. Income was not significantly associated with any of our outcome variables but ethnicity was and was therefore included as a covariate in subsequent MANCOVAs along with age, gender, and education.
Table 1.
Clinical Characteristics for Young Adult Cancer Survivors
| N= 335 | ||
|---|---|---|
| N | % | |
| Cohort | ||
| 0–12 months post treatment | 120 | 35.8 |
| 13–24 months post treatment | 102 | 30.4 |
| 25–60 months post treatment | 113 | 33.7 |
| ECOG Performance Status | ||
| Normal activity, without symptoms | 181 | 54.0 |
| Some symptoms, not requiring bed rest during waking day | 127 | 37.9 |
| Require bed rest for < 50% of waking day | 24 | 7.2 |
| Require bed rest for > 50% of waking day | 3 | 0.9 |
| Cancer Type | ||
| Breast | 80 | 23.9 |
| Gynecologic (Cervical, Uterine, Ovarian) | 54 | 16.1 |
| Melanoma | 37 | 11.0 |
| Lung | 23 | 6.9 |
| Colorectal | 21 | 6.3 |
| Thyroid | 21 | 6.3 |
| Testicular | 20 | 6.0 |
| Hematologic (NHL, Hodgkin, Leukemia) | 17 | 5.1 |
| Stomach | 12 | 3.6 |
| Hepatobiliary (liver, pancreas, bile duct) | 11 | 3.3 |
| Head and neck | 10 | 3.0 |
| Bone and Sarcomas (soft tissue and bone) | 10 | 3.0 |
| Esophageal | 7 | 2.1 |
| Urinary bladder, Kidney, Renal pelvis | 7 | 2.1 |
| Brain and Central Nervous System | 5 | 1.5 |
| Stage | ||
| Local | 225 | 67.2 |
| Regional | 83 | 24.8 |
| Distal | 15 | 4.5 |
Table 2.
Demographic Characteristics for YA and HC Groups
| YA N = 335 | HC N = 335 | |||
|---|---|---|---|---|
| Age | 31.8(M) | 5.4(SD) | 31.8(M) | 5.4(SD) |
| N | % | N | % | |
| Female | 229 | 68.4 | 229 | 68.4 |
| Ethnicity | ||||
| Hispanic Origin | 18 | 5.4 | 33 | 9.9 |
| Race | ||||
| White | 281 | 83.9 | 257 | 76.7 |
| Black/African America | 17 | 5.1 | 24 | 7.2 |
| Asian or Pacific Islander | 20 | 6.0 | 26 | 7.8 |
| Native American or Alaskan Native | 5 | 1.5 | 2 | .6 |
| Mixed racial background | 8 | 2.4 | 16 | 4.8 |
| Education | ||||
| Some high school | 5 | 1.5 | 5 | 1.5 |
| High school of equivalent | 40 | 11.9 | 40 | 11.9 |
| Some college | 86 | 25.7 | 86 | 25.7 |
| College | 135 | 40.3 | 135 | 40.3 |
| Some graduate school | 20 | 6.0 | 20 | 6.0 |
| Graduate school | 48 | 14.3 | 49 | 14.6 |
| Marital Status | ||||
| Married | 188 | 56.1 | 188 | 56.1 |
| Single, never married | 95 | 28.4 | 95 | 28.4 |
| Divorced | 19 | 5.7 | 19 | 5.7 |
| Living with partner | 23 | 6.9 | 23 | 6.9 |
| Separated | 10 | 3.0 | 10 | 3.0 |
| Employment Status | ||||
| Employed full-time | 192 | 57.3 | 169 | 50.4 |
| Employed part-time | 27 | 8.1 | 36 | 10.7 |
| Self-employed | 15 | 4.5 | 19 | 5.7 |
| Not employed, but looking for work | 35 | 10.4 | 36 | 10.7 |
| Retired | 6 | 1.8 | 1 | 0.3 |
| Student | 26 | 7.8 | 26 | 7.8 |
| Homemaker | 34 | 10.1 | 48 | 14.3 |
| Income | ||||
| Less than $24,999 | 45 | 13.4 | 59 | 17.6 |
| $25,000 to $49,999 | 71 | 21.2 | 96 | 28.7 |
| $50,000 to $99,999 | 96 | 28.7 | 124 | 37.0 |
| $100,000 to $149,999 | 70 | 20.9 | 30 | 9.0 |
| $150,000 to $249,999 | 35 | 10.4 | 12 | 3.6 |
| $250,000 or more | 8 | 2.4 | 4 | 1.2 |
HRQL Outcomes
We examined responses from both groups to the global HRQL item “I am content with the quality of my life right now.” A Mann-Whitney U test revealed that YAs have better overall perceptions of HRQL (p=.038). To examine specific dimensions of HRQL, a group (YA vs. HC) by cohort (0–12 months, 13–24 months, 25–60 months) MANCOVA revealed a significant main effect for group (Wilks’ Lambda, p<.001) on three indicators of HRQL: Physical Well-Being (F=8.02, p=.005), Emotional Well-Being (F=6.52, p=.011), and Social Well-Being (F=40.64, p<.001). There were no differences between the groups for Spiritual Well-Being (F=1.39, p=.24). Follow-up comparisons revealed the YA group reported significantly worse Physical and Emotional Well-Being scores compared to the HC group (ps<.05; Cohen’s d=.22 and .20, respectively), yet significantly better Social Well-Being scores (ps<.001; Cohen’s d=.49) (See Table 3). There was a >3 point difference in scores suggesting a meaningful difference.32, 33 There were no significant effects for cohort or the group-by-cohort interaction. Secondary analyses using an age group (18–24, 25–29, and 30–39 years old) MANCOVA revealed significant differences (Wilks’ Lambda, p<.001) on three indicators of HRQL: Physical Well-Being (F=3.95, p=.020), Emotional Well-Being (F=5.91, p=.003), and Spiritual Well-Being (F=3.55, p=.030). There were no differences among the YA age groups for Social Well-Being (F=0.82, p=.442). Follow-up comparisons revealed the 30–39 year olds reported significantly better Physical and Emotional Well-Being compared to the 25–29 year olds (p=.016 and p=.003, respectively; See Figure 1). Although there was a significant difference for Spiritual Well-Being, this difference appeared to be due to the effect of two, non-significant trends; 30–39 year olds reported higher scores than 18–24 year olds (p=.100) and 25–29 year olds (p=.120).
Table 3.
Adjusted Means and Standard Errors for Quality of Life and General Psychological Adjustment Outcomes
| Outcome | Young Adult Group N=335 M (SE) | Healthy Control Group N=335 M (SE) | SD σ | Mean Difference | p | Cohen’s d |
|---|---|---|---|---|---|---|
| Quality of Life | ||||||
| Physical Well- being | 19.52(.36) | 20.97(.36) | 6.59 | −1.45 | <.01 | 0.22 |
| Emotional Well- being | 15.92(.30) | 17.00(.30) | 5.49 | −1.08 | <.05 | 0.20 |
| Social Well- being | 18.52(.36) | 15.30(.36) | 6.59 | 3.22 | <.001 | 0.49 |
| Spiritual Well- being | 29.07(.53) | 28.17(.53) | 9.70 | 0.90 | .24 | 0.09 |
| General Psychological Adjustment | ||||||
| Anxiety | 43.97(1.13) | 43.49(1.13) | 20.68 | 0.48 | .76 | 0.02 |
| Depression | 38.38(1.26) | 35.76 (1.26) | 23.06 | 2.62 | .15 | 0.11 |
| Positive Affect | 55.37(1.09) | 53.50(1.09) | 19.95 | 1.87 | .23 | 0.09 |
| Posttraumatic Growth | 27.75(.66) | 27.22(.66) | 12.08 | 0.53 | .58 | 0.04 |
Figure 1.
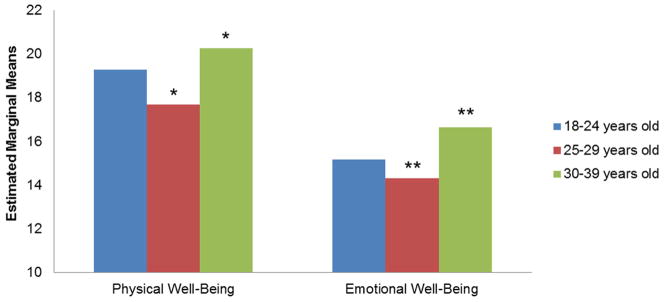
HRQL Score by Age Cohort
18–24 years old, 25–29 years old, 30–39 years old
Note: Mean scores were adjusted for gender, ethnicity, and education. Higher scores indicate better HRQL. *=p<.05, **=p<.01.
General Psychological Adjustment Outcomes
We examined responses from both groups to the global psychological adjustment item. A Mann-Whitney U test revealed that YAs have a greater sense of positive psychological impact on their lives than HCs (p<.001). To examine specific dimensions of psychological adjustment, a group-by-cohort (YA vs. HC; 0–12 months, 13–24 months, 25–60 months) MANCOVA revealed a significant group-by-cohort interaction (Wilks’ Lambda, p=.002). An examination of univariate effects revealed a significant interaction effect for posttraumatic growth (F=3.24, p=.04), and an examination of the simple slopes illustrated that YAs reported comparatively stable posttraumatic growth scores across cohorts (p=.74) relative to HCs, who reported higher scores across cohorts (p=.01, d=.16). No significant differences were found between YA’s and HC’s posttraumatic growth scores within cohorts (See Figure 2). No significant differences in anxiety, depression, or positive affect scores were found for group, cohort, or group-by-cohort interaction (all ps>.05). Secondary analyses using an age group (18–24, 25–29, and 30–39 years old) MANCOVA revealed significant differences (Wilks’ Lambda, p<.001) on two indicators of psychological adjustment: Anxiety (F=5.83, p=.006) and Depression (F=7.05, p=.001). There were no differences among the YA age groups for Positive Affect (F=0.985, p=.375) or Posttraumatic Growth (F=1.66, p=.192). Follow-up comparisons revealed the 30–39 year olds reported significantly less Anxiety and Depression compared to the 25–29 year olds (p=.014 and p=.001, respectively; See Figure 3).
Figure 2.
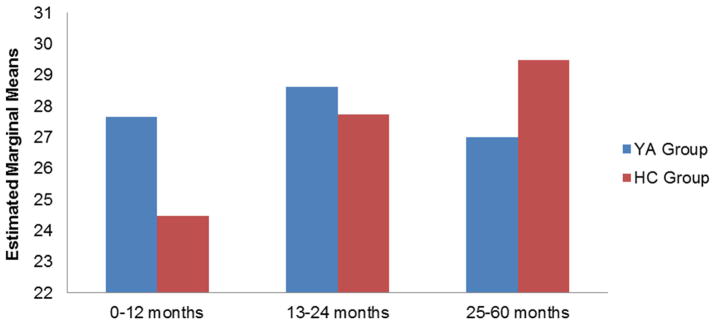
Posttraumatic Growth Scores by Group and Cohort
YA Group, HC Group
Note: Mean scores were adjusted for age, gender, ethnicity, and education. HCs reported higher scores across cohorts (p=.01).
Figure 3.
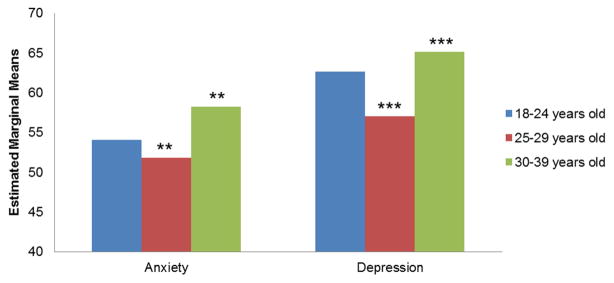
Psychological Adjustment Scores by Age Cohort
18–24 years old, 25–29 years old, 30–39 years old
Note: Mean scores were adjusted for gender, ethnicity, and education. Higher scores indicate better psychological adjustment (i.e., less depression and anxiety). **=p<.01, ***=p<.001.
CONCLUSIONS
During the five-year period immediately following completion of active treatment, compared to their HC peers, YAs reported experiencing a better global and social HRQL, and more positive impact on their life, but poorer physical and emotional HRQL. Poorer physical and emotional HRQL among YAs is consistent with our hypothesis and mirrors the challenges of other groups of cancer survivors who describe problems related to long-term effects from treatment such as fatigue, sexual dysfunction, and fears of a recurrence that can influence physical and emotional health.34 There were no group differences for spiritual HRQL. Our finding that YAs had better social HRQL was somewhat surprising given the changes that frequently occur in YAs’ social networks due to normal developmental transitions.13 Indeed, the magnitude of the difference for social HRQL between YAs and HCs was particularly noteworthy. For YAs, this suggested a meaningful difference and may underscore the vital resource social networks can serve to help buffer post-treatment challenges faced by YAs with cancer.12
Results were mixed for positive psychosocial adjustment among YAs relative to HCs. YAs described more global positive psychosocial impact of cancer on their lives but there were no significant group differences for positive affect. Greater posttraumatic growth was relatively stable across YA cohorts. Although the cancer experience may be a catalyst for posttraumatic growth among YAs and suggestive of adaptive coping processes,35 the comparable levels of posttraumatic growth reported among HCs across the three cohorts suggests that self-enhancement bias may possibly contribute to this phenomenon.36 Individuals are often highly motivated to find meaning in their life experiences and to report “having grown” from difficult life experiences.
Although there were no differences by group for anxiety and depression when scores were combined across cohorts, significantly higher anxiety and depression scores were observed among YAs who were in the 25–29 year-old age group compared to those in the 30–39 year-old age group. This is somewhat consistent with recent study findings that survivors of adolescent and YA cancer (diagnosed between the ages of 15 and 29) were significantly more likely to report poorer mental health days in the past month compared to middle-aged adults with no history of cancer.37 Our results should be interpreted with caution given the cross-sectional nature of the data, but it is possible that YAs in the 25–29 year-old age group experience greater psychosocial challenges secondary to interrupted developmental milestones whereas YAs in the 30–39 year-old age group may have relatively fewer interpersonal, vocational, or even financial challenges. Although the social well-being scores do not reflect this, it is plausible that older YAs have more stable social networks that can buffer significant life stressors. In this subsample, 30–39 year-old YAs also reported better physical and emotional well-being scores than the 25–29 year-old YAs, underscoring the relatively better psychosocial outcomes for this subgroup of YAs.
This study is not without limitations. First, by recruiting participants from an online research panel, we obtained two samples with relatively higher education and income relative to population-based samples. This may have introduced a degree of participation bias. In spite of the perceived advantages of our participants with greater resources, we still observed significant differences in HRQL and psychosocial adjustment. With greater variability in socio-economic status, larger and more clinically significant differences in patient-reported outcomes may be apparent. Second, we screened out a large number of respondents who provided “suspicious” response patterns. While this approach reduced the potential for fraudulent data affecting our findings, it may have contributed to some selection bias, affecting the generalizability of our findings. Third, this study was based on a cross-sectional sample which limits our assumptions about causality. That said, descriptive or observational studies serve a vital role in building a knowledge base where research is relatively sparse. 38 To our knowledge, there are no five-year longitudinal studies examining psychosocial aspects of HRQL among YA cancer survivors and, given the relative lack of research in this area, the purposive sampling approach with this descriptive data served as a suitable strategy for approximating change over time.
There are important implications for future research based on these findings. The transient nature of YAs with cancer has typically been considered a challenge for behavioral research studies, yet YAs represent a technologically savvy subgroup. Recruiting participants through web-based means may prove to be a particularly useful approach for engaging this “hard to reach” subgroup and examining important patient-reported outcomes over time. Data collected from the Internet are comparable to data from probability-based general population samples based on recent findings,39 and online research panels can be low-cost and efficient means of data collection given the widespread availability of the Internet among diverse samples. Future research priorities should include using prospective, longitudinal strategies for tracking and recording HRQL and distress trajectories over time, from pre-treatment through longitudinal post-treatment time points. In addition, since YAs represent a wide range of individuals navigating a number of significant and different developmental challenges, examining the impact of cancer within individual age groups may highlight areas of need for supportive services. Given the increased demand for survivorship care plans and age-appropriate psychosocial support,14, 40 providing tailored strategies for YAs may minimize the negative HRQL and psychosocial impacts while leveraging the strength of their social networks. Much work remains, however, to better understand and support this under-served group of cancer survivors.
Acknowledgments
Funding Source: Research reported in this publication was supported by the American Cancer Society-Illinois Division under award number PSB-08-15, the National Cancer Institute of the NIH under award number K07CA158008, and the National Center for Complementary and Alternative Medicine and the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award number U54AR057951-S1. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
The authors have no financial disclosures or conflicts of interest to report.
References
- 1.Bleyer A. Latest Estimates of Survival Rates of the 24 Most Common Cancers in Adolescent and Young Adult Americans. Journal of Adolescent and Young Adult Oncology. 2011;1:37–42. doi: 10.1089/jayao.2010.0005. [DOI] [PubMed] [Google Scholar]
- 2.Bellizzi KM, Aziz NM, Rowland JH, et al. Double Jeopardy? Age, Race, and HRQOL in Older Adults with Cancer. Journal of Cancer Epidemiology. 2012;2012:478642. doi: 10.1155/2012/478642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avis NE, Deimling GT. Cancer survivorship and aging. Cancer. 2008;113:3519–3529. doi: 10.1002/cncr.23941. [DOI] [PubMed] [Google Scholar]
- 4.Kazak AE, Derosa BW, Schwartz LA, et al. Psychological outcomes and health beliefs in adolescent and young adult survivors of childhood cancer and controls. Journal of Clinical Oncology. 2010;28:2002–2007. doi: 10.1200/JCO.2009.25.9564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. New England journal of medicine. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 6.Casillas J, Syrjala KL, Ganz PA, et al. How confident are young adult cancer survivors in managing their survivorship care? A report from the LIVESTRONG Survivorship Center of Excellence Network. Journal of Cancer Survivorship. 2011;5:371–381. doi: 10.1007/s11764-011-0199-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zebrack B. Information and service needs for young adult cancer survivors. Supportive Care in Cancer. 2009;17:349–357. doi: 10.1007/s00520-008-0469-2. [DOI] [PubMed] [Google Scholar]
- 8.Bellizzi KM, Smith A, Schmidt S, et al. Positive and negative psychosocial impact of being diagnosed with cancer as an adolescent or young adult. Cancer. 2012;118:5155–5162. doi: 10.1002/cncr.27512. [DOI] [PubMed] [Google Scholar]
- 9.Gorman JR, Malcarne VL, Roesch SC, Madlensky L, Pierce JP. Depressive symptoms among young breast cancer survivors: the importance of reproductive concerns. Breast Cancer Research and Treatment. 2010;123:477–485. doi: 10.1007/s10549-010-0768-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith AW, Bellizzi KM, Keegan THM, et al. Health-related Quality of Life of Adolescent and Young Adult Cancer Patients in the United States: the AYA HOPE study. Journal of Clinical Oncology. 2013;31:2136–2145. doi: 10.1200/JCO.2012.47.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rowland JH. Developmental stage and adaptation: adult model. In: Holland JC, Rowland JH, editors. Handbook of psychooncology. New York: Oxford University Press; 1990. pp. 25–43. [Google Scholar]
- 12.Zebrack BJ. Psychological, social, and behavioral issues for young adults with cancer. Cancer. 2011;117:2289–2294. doi: 10.1002/cncr.26056. [DOI] [PubMed] [Google Scholar]
- 13.National Cancer Institute. Closing the gap: a strategic plan. Addressing the recommendations of the Adolescent and Young Adult Oncology Progress Review Group; [accessed October 29, 2012]. LIVESTRONG™ Young Adult Alliance. Available from URL: http://planning.cancer.gov/library/2007_ayao_SP.pdf. [Google Scholar]
- 14.Hewitt M, Greenfield S, Stovall E Institute of Medicine (U.S.), American Society of Clinical Oncology. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, D.C: National Academies Press; 2006. [Google Scholar]
- 15.Stanton AL. What happens now? Psychosocial care for cancer survivors after medical treatment completion. Journal of Clinical Oncology. 2012;30:1215–1220. doi: 10.1200/JCO.2011.39.7406. [DOI] [PubMed] [Google Scholar]
- 16.Ferrell BR, Dow KH, Grant M. Measurement of the quality of life in cancer survivors. Quality of Life Research. 1995;4:523–531. doi: 10.1007/BF00634747. [DOI] [PubMed] [Google Scholar]
- 17.Kwak M, Zebrack BJ, Meeske KA, et al. Trajectories of Psychological Distress in Adolescent and Young Adult Patients With Cancer: A 1-Year Longitudinal Study. Journal of Clinical Oncology. 2013;31:2160–2166. doi: 10.1200/JCO.2012.45.9222. [DOI] [PubMed] [Google Scholar]
- 18.Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. Journal of Clinical Epidemiology. 2010;63:1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gershon R, Lai J, Bode R, et al. Neuro-QOL: quality of life item banks for adults with neurological disorders: item development and calibrations based upon clinical and general population testing. Quality of Life Research. 2012;21:475–486. doi: 10.1007/s11136-011-9958-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mariotto AB, Robin Yabroff K, Shao Y, Feuer EJ, Brown ML. Projections of the Cost of Cancer Care in the United States: 2010–2020. Journal of the National Cancer Institute. 2011;103:117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oken MM, Creech RH, Tormey DC, et al. Toxicity And Response Criteria Of The Eastern Cooperative Oncology Group. American Journal of Clinical Oncology. 1982;5:649–655. [PubMed] [Google Scholar]
- 22.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. Journal of Clinical Oncology. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 23.Peterman AH, Fitchett G, Brady MJ, Hernandez L, Cella D. Measuring spiritual well-being in people with cancer: The Functional Assessment of Chronic Illness Therapy--Spiritual Well-being Scale (FACIT-Sp) Annals of Behavioral Medicine. 2002;24:49–58. doi: 10.1207/S15324796ABM2401_06. [DOI] [PubMed] [Google Scholar]
- 24.Cella D, Zagari MJ, Vandoros C, Gagnon DD, Hurtz HJ, Nortier JW. Epoetin alfa treatment results in clinically significant improvements in quality of life in anemic cancer patients when referenced to the general population. Journal of Clinical Oncology. 2003;21:366–373. doi: 10.1200/JCO.2003.02.136. [DOI] [PubMed] [Google Scholar]
- 25.Bredle JM, Salsman JM, Debb SM, Arnold BJ, Cella D. Spiritual well-being as a component of health-related quality of life: The Functional Assessment of Chronic Illness Therapy—Spiritual Well-Being Scale (FACIT-Sp) Religions. 2011;2:77–94. [Google Scholar]
- 26.Veit CT, Ware JE., Jr The structure of psychological distress and well-being in general populations. Journal of Consulting and Clinical Psychology. 1983;51:730–742. doi: 10.1037//0022-006x.51.5.730. [DOI] [PubMed] [Google Scholar]
- 27.Tedeschi RG, Calhoun LG. The Post-traumatic Growth Inventory: Measuring the positive legacy of trauma. Journal of Traumatic Stress. 1996;9:455–471. doi: 10.1007/BF02103658. [DOI] [PubMed] [Google Scholar]
- 28.Cann A, Calhoun LG, Tedeschi RG, et al. A short form of the Posttraumatic Growth Inventory. Anxiety, Stress and Coping. 2010;23:127–137. doi: 10.1080/10615800903094273. [DOI] [PubMed] [Google Scholar]
- 29.Andrykowski MA, Bishop M, Hahn EA, et al. Long-term health-related quality of life, growth, and spiritual well-being after hematopoietic stem-cell transplantation. Journal of Clinical Oncology. 2005;23:599–608. doi: 10.1200/JCO.2005.03.189. [DOI] [PubMed] [Google Scholar]
- 30.Lai JS, Garcia SF, Salsman JM, Rosenbloom S, Cella D. The psychosocial impact of cancer: evidence in support of independent general positive and negative components. Quality of Life Research. 2012;21:195–207. doi: 10.1007/s11136-011-9935-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations) based on November 2011 SEER data submission, posted to the SEER web site. 2012 Apr; Available from URL: http://seer.cancer.gov/csr/1975_2009_pops09/
- 32.Yost KJ, Sorensen MV, Hahn EA, Glendenning GA, Gnanasakthy A, Cella D. Using multiple anchor-and distribution-based estimates to evaluate clinically meaningful change on the Functional Assessment of Cancer Therapy-Biologic Response Modifiers (FACT-BRM) Instrument. Value in Health. 2005;8:117–127. doi: 10.1111/j.1524-4733.2005.08202.x. [DOI] [PubMed] [Google Scholar]
- 33.Yost KJ, Eton DT. Combining distribution- and anchor-based approaches to determine minimally important differences: The FACIT experience. Evaluation and the Health Professions. 2005;28:172–191. doi: 10.1177/0163278705275340. [DOI] [PubMed] [Google Scholar]
- 34.Stein KD, Syrjala KL, Andrykowski MA. Physical and psychological long-term and late effects of cancer. Cancer. 2008;112:2577–2592. doi: 10.1002/cncr.23448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park CL, Folkman S. Meaning in the context of stress and coping. Review of General Psychology. 1997;1:115–144. [Google Scholar]
- 36.Helgeson VS, Reynolds KA, Tomich PL. A meta-analytic review of benefit finding and growth. Journal of Consulting and Clinical Psychology. 2006;74:797–816. doi: 10.1037/0022-006X.74.5.797. [DOI] [PubMed] [Google Scholar]
- 37.Tai E, Buchanan N, Townsend J, Fairley T, Moore A, Richardson LC. Health status of adolescent and young adult cancer survivors. Cancer. 2012;118:4884–4891. doi: 10.1002/cncr.27445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacobsen PB. Present and Future Research for Survivorship. American Psychosocial Oncology Society - 8th Annual Conference; Anaheim, California. 2011. [Google Scholar]
- 39.Liu H, Cella D, Gershon R, et al. Representativeness of the PROMIS Internet Panel. Journal of Clinical Epidemiology. 2010;63:1169–1178. doi: 10.1016/j.jclinepi.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.American College of Surgeons, Commission on Cancer. Cancer Program Standards 2012:Ensuring Patient-Centered Care. Chicago, IL: American College of Surgeons; 2012. [Google Scholar]


